Abstract
We have known for just over a decade that functional RNA is shuttled between cells (Nat. Cell Biol. (2007) 9, 654–659). In that short time, there have been countless reports of extracellular RNA (exRNA) and extracellular vesicles (EVs) participating in diverse biological processes in development (Dev. Cell (2017) 40, 95–103), homoeostasis (Nature (2017) 542, 450–455) and disease (Nature (2017) 546, 498–503). Unsurprisingly – as these disciplines are still in their infancies – most of this work is still in the ‘discovery biology’ phase. However, exRNA and EVs show promise as disease biomarkers and could be harnessed in novel therapies.
Keywords: microRNA, renal physiology, therapeutics
The renal tubular epithelial cell (rTEC) has been well-characterised with respect to its ability to release and take up extracellular RNA (exRNA) and EVs [1,2]. We also know quite a lot about how these signals can reprogramme rTEC function. For example, Camussi and co-workers have demonstrated that extracellular vesicles (EVs) and exRNA from mesenchymal stem cells confer protection from acute kidney injury (AKI) in rodent models [3–6]. Similarly, hypoxic rTECs release EVs that can protect against AKI [7]; the complete mechanism is not known but in part relies on transfer of HIF-1α [8].
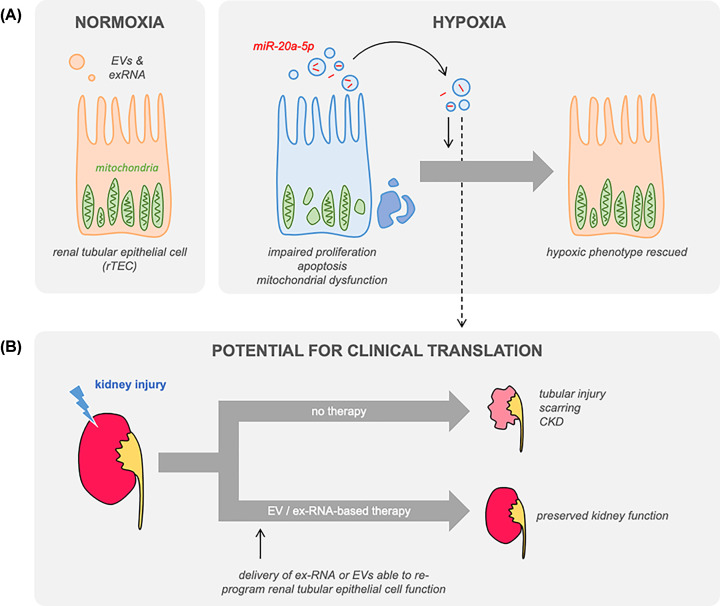
If we are ever going to bridge the chasm between bench and bedside, then we need to develop a thorough understanding of the molecular mechanisms mediating any therapeutic effect, and of the feasibility of delivering RNA-/vesicle-based reagents. In this context, the work reported by Yu and co-workers [9] is a small but significant step in the right direction. This group replicated the previous observation that EVs derived from hypoxic rTECs can protect against hypoxia-induced injury in vitro. In an attempt to determine the causative molecular mechanism, they used small RNA sequencing to detect microRNAs that were enriched in EVs from hypoxic (compared with normoxic) cells. They identified miR-20a-5p as one such miRNA and went on to show – using an agomir and antagomir approach – that this alone was sufficient to protect against hypoxia-induced defects in cell proliferation and mitochondrial function (Figure 1). Furthermore, they showed that the intravenous injection of a miR-20a-5p agomir could protect against tubular injury in a mouse ischaemia–reperfusion model.
Figure 1. Protective role of microRNA in the kidney.
(A) Conclusions from Yu and co-workers. Under hypoxic conditions in vitro, renal tubular cells released EVs enriched in microRNAs that were predicted to target mitochondrial pathways, including miR-20a-5p [9]. These vesicles (or an miR-20a-5p agomir) ameliorated hypoxia-induced changes in mitochondrial function and cell proliferation. In vivo, the miR-20a-5p agomir protected rTECs from injury after ischaemia–reperfusion. (B) Potential for clinical translation. The present study adds to a literature supporting the principle that ex-RNA and EVs could provide effective therapies for kidney disease. ExRNA or EVs could be used to re-programme rTECs, ameliorating the atrophic and fibrotic sequelae of untreated kidney injury.
This reductionist approach – in which one or two critical miRNA species are identified and validated – has been widely followed by other researchers, e.g. [10–13]. The advantage is that one may be able to identify simple nucleic acid reagents that can be produced at scale and delivered safely and effectively to treat disease. For example, the miR-21 inhibitor RG-012 has entered clinical trials in Alport syndrome [14]. The disadvantage of this approach is that it sits at odds with what we know about EV and exRNA biology and is thus not able to answer questions of physiological relevance. We know that EVs contain a complex mix of multiple miRNAs (and other classes of small RNA) that are predicted to regulate thousands of target genes. It seems highly implausible that a single miRNA – at physiological concentrations – should be responsible for the bulk of this effect. Acknowledging that it may be impossible to decipher this complexity, alternative pragmatic strategies rely on attempts to produce therapeutic-grade artificial vesicles [15]. The vesicle ‘coat’ may prove to be critical: siRNA within a cell-derived vesicle is more effective at gene silencing than it is when delivered as naked nucleic acid or packaged within artificial lipid nanoparticles [16]. Ultimately, any significant breakthrough is likely to follow from a combination of both reductionist and pragmatic approaches.
The work reported by Yu and co-workers opens many questions regarding physiological relevance [9]. Does miR-20a-5p play a significant role in maintaining rTEC homoeostasis under physiological conditions? What role is played by the other miRNAs that are differentially regulated in hypoxia? Does the target cell differentiate between exogenous miR-20a-5p delivered in an EV and cell-endogenous miR-20-5p? Do the effects on mitochondrial function cause improved cellular health or are they merely associated?
There are also questions of clinical relevance. Is miR-20a-5p effective in AKI due to other (toxic, infective, inflammatory, vascular) causes? Does its delivery incite off-target toxicity? Is it effective at protecting rTECs if delivered hours or days after the injury? Would any effect translate into patient-centred clinical outcomes? Nevertheless, the present paper adds to the growing body of work suggesting that EV and exRNA-based therapeutics are possible in principle. It may be a small step on the road from bench to bedside, but every small step is welcome.
Abbreviations
- AKI
acute kidney injury
- EV
extracellular vesicle
- exRNA
extracellular RNA
- rTEC
renal tubular epithelial cell
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
References
- 1.Hunter R.W., Dear J.W. and Bailey M.A. (2020) Chapter 11 - Exosomes in nephrology. In Exosomes(Edelstein L., Smythies J., Quesenberry P. and Noble D., eds), pp. 257–283, Academic Press [Google Scholar]
- 2.Oosthuyzen W., Scullion K.M., Ivy J.R., Morrison E.E., Hunter R.W., Starkey Lewis P.J. et al. (2016) Vasopressin regulates extracellular vesicle uptake by kidney collecting duct cells. J. Am. Soc. Nephrol. 10.1681/ASN.2015050568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruno S., Grange C., Deregibus M.C., Calogero R.A., Saviozzi S., Collino F. et al. (2009) Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J. Am. Soc. Nephrol. 20, 1053–1067 10.1681/ASN.2008070798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collino F., Bruno S., Incarnato D., Dettori D., Neri F., Provero P. et al. (2015) AKI recovery induced by mesenchymal stromal cell-derived extracellular vesicles carrying microRNAs. J. Am. Soc. Nephrol. 26, 2349–2360 10.1681/ASN.2014070710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lindoso R.S., Collino F., Bruno S., Araujo D.S., Sant’Anna J.F., Tetta C. et al. (2014) Extracellular vesicles released from mesenchymal stromal cells modulate miRNA in renal tubular cells and inhibit ATP depletion injury. Stem Cells Dev. 23, 1809–1819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruno S., Tapparo M., Collino F., Chiabotto G., Deregibus M.C., Soares Lindoso R. et al. (2017) Renal regenerative potential of different extracellular vesicle populations derived from bone marrow mesenchymal stromal cells. Tissue Eng. Part A 23, 1262–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang G., Yang Y., Huang Y., Zhang L., Ling Z., Zhu Y. et al. (2017) Hypoxia-induced extracellular vesicles mediate protection of remote ischemic preconditioning for renal ischemia-reperfusion injury. Biomed. Pharmacother. 90, 473–478 [DOI] [PubMed] [Google Scholar]
- 8.Zhang W., Zhou X., Yao Q., Liu Y., Zhang H. and Dong Z. (2017) HIF-1-mediated production of exosomes during hypoxia is protective in renal tubular cells. Am. J. Physiol. Renal Physiol. 313, F906–F913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu W., Zeng H., Chen J., Fu S., Huang Q., Xu Y. et al.. miR-20a-5p is enriched in hypoxia-derived tubular exosomes and protects against acute tubular injury. Clin. Sci. (Lond.) 134, 2223–2234 10.1042/CS20200288 [DOI] [PubMed] [Google Scholar]
- 10.Fong M.Y., Zhou W., Liu L., Alontaga A.Y., Chandra M., Ashby J. et al. (2015) Breast-cancer-secreted miR-122 reprograms glucose metabolism in premetastatic niche to promote metastasis. Nat. Cell Biol. 17, 183–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lv L.-L., Feng Y., Wu M., Wang B., Li Z.-L., Zhong X. et al. (2020) Exosomal miRNA-19b-3p of tubular epithelial cells promotes M1 macrophage activation in kidney injury. Cell Death Differ. 27, 210–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu Y., Shen L., Li F., Yang J., Wan X. and Ouyang M. (2019) microRNA-16-5p-containing exosomes derived from bone marrow-derived mesenchymal stem cells inhibit proliferation, migration, and invasion, while promoting apoptosis of colorectal cancer cells by downregulating ITGA2. J. Cell. Physiol. 234, 21380–21394 10.1002/jcp.28747 [DOI] [PubMed] [Google Scholar]
- 13.Liang Y.-C., Wu Y.-P., Li X.-D., Chen S.-H., Ye X.-J., Xue X.-Y. et al. (2019) TNF-α-induced exosomal miR-146a mediates mesenchymal stem cell-dependent suppression of urethral stricture. J. Cell. Physiol. 234, 23243–23255 10.1002/jcp.28891 [DOI] [PubMed] [Google Scholar]
- 14.Genzyme, a Sanofi Company (2019) A Phase 1, open-label study to evaluate the safety, pharmacodynamics, and pharmacokinetics of RG-012 for injection, including its effect on renal microRNA-21, in subjects with Alport syndrome, Clinical trial registration. clinicaltrials.gov [Google Scholar]
- 15.Tapparo M., Bruno S., Collino F., Togliatto G., Deregibus M.C., Provero P. et al. (2019) Renal regenerative potential of extracellular vesicles derived from mirna-engineered mesenchymal stromal cells. Int. J. Mol. Sci. 20, 2381 10.3390/ijms20102381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reshke R., Taylor J.A., Savard A., Guo H., Rhym L.H., Kowalski P.S. et al. (2020) Reduction of the therapeutic dose of silencing RNA by packaging it in extracellular vesicles via a pre-microRNA backbone. Nat. Biomed. Eng. 4, 52–68 10.1038/s41551-019-0502-4 [DOI] [PubMed] [Google Scholar]