Abstract
Monitoring parasitic contamination in raw vegetables used in salads is an important measure in controlling the occurrence of gastroenterological diseases, which may be life-threatening. This study aimed to inspect the parasitological contamination of some raw vegetables used in salads. Eight commonly consumed vegetable types were purchased from street vendors in the city markets. Vegetables were washed and the sediments were obtained for microscopic examination. Genomic DNA was isolated from contaminated samples. Our result showed that 34.4% of the studied samples were contaminated with one or more species of parasites. Lettuce was the most commonly contaminated vegetable type (29.5%), while tarragon leaves showed the lowest level of contamination (2.3%). The risk of contamination was significantly higher in lettuce samples in comparison with the other samples studied. Giardia duodenalis was the most prevalent parasite detected (38.6%) and was abundantly found in lettuce isolates (23.5%). Molecular typing revealed that all Giardia samples found in the contaminated specimens belonged to Assemblage B. Blastocystis spp. were the second most prevalent parasite in samples (29.5%), they were frequently detected in lettuce leaves (30.8%). Other parasites were found in low frequencies. The high level of parasitic contamination found in our study indicates an urgent need to identify the sources of contamination and to monitor irrigation water and ensure its cleanliness.
Keywords: Vegetables, Contamination, Intestinal parasites, Giardia, Damascus
Highlights
-
•
Lettuce leaves had the highest risk of contamination.
-
•
Giardia and Blastocystis were the most prevalent contaminating parasites found.
-
•
Strict measures are needed to reduce raw vegetables contamination.
1. Introduction
Gastroenterological diseases are mostly seen in developing countries where, contaminated water, poor sanitation, poor hygiene and civil wars contribute to their prevalence, and most certainly have a great impact on global health (Mandeville et al., 2009). According to WHO, no more than 34% of people gain access to proper sanitation facilities in these countries (WHO, 2008). Raw vegetable consumption often contributes to an epidemiological role in the transmission of parasitic food-borne diseases (de W Blackburn and McClure, 2002; Eraky et al., 2014). Studies over the world have shown that many species of enteric helminthes and protozoa can infect humans who consume contaminated, improperly washed fruits and vegetables (Al-Shawa and Mwafy, 2007; Adanir and Tasci, 2013; Ismail, 2016). Vegetables become contaminated with different parasitic phases (cyst, oocyst, ova, larvae) during irrigation by waste water or by direct contamination from animals and humans during harvesting, packing, transport processing, distribution, and marketing (Amoah et al., 2007; Gabre and Shakir, 2016). Many countries around the world have realized the importance of evaluating the role of raw vegetables in the transmission of intestinal parasites (Abougrain et al., 2010; Omowaye and Audu, 2012; Chau et al., 2014; Khan et al., 2017).
To our knowledge, only one previous study has been done in Syria, to evaluate the parasitological contamination of fresh vegetables, sold at different markets in Alqalamon region located at the northwest of the city of Damascus (Alhabbal, 2015). Hence, this study was designed to investigate the level of parasitological contamination of vegetables sold by street vendors in many areas of the city of Damascus, in an effort to help authorities to find out the contamination level and to design comprehensive monitoring and educational programs according to the needs.
2. Materials and methods
2.1. Samples collection
A total of 128 fresh vegetable samples were randomly purchased from street vendors at the most-popular markets in the city of Damascus: Al Zablatani, Saroujah, Al Midan, Mazzeh, Al Shaalan, and Al Ameen neighborhood, between March and June 2019. Eight commonly consumed vegetable types were collected in this study (16 samples for each type): radish, spearmint, lettuce, tarragon, coriander, parsley, watercress, and arugula.
2.2. Laboratory examination
To detect the presence of parasites in the studied samples, we followed the protocol according to Erdoğrul and Şener (2005). Briefly, each fresh vegetable sample was collected into a sterile plastic bag, labeled with a unique number, and transported immediately to the laboratory. Approximately 150 g of each sample was washed by shaking for 15 min with sterile normal saline (0.9% NaCl), then removed and the remaining wash solution was left for 5 h to sediment. The supernatant was discarded and the remaining wash solution (about 20 ml) with the sediments was centrifuged at 112 ×g for 10 min. The supernatant was then discarded and the residual was collected with 5 ml of the saline. Iodine stained smears were prepared from each sample to detect parasite forms using light microscopy at 100× and 400×.
2.3. Genomic isolation and typing
Total genomic DNA was extracted from all positive contaminated vegetable precipitates examined by microscope, using QiaAmp Stool DNA Mini Kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. Diagnostic PCR using the known sequenced-tagged site (STS)-specific primer sets (F:5′GGA ATC CTC TTA GAG GGA CAC TATACA T 3′, R: 5′ TTA CTA AAA TCC AAA GTG TTC ATCGGA C 3′), were used to detect the presence of Blastocystis spp. (Stensvold et al., 2006). PCR conditions were as follows: initial denaturation at 94 °C for 3 min followed by 30 cycles of 94 °C for 1 min 58 °C for 1 min and 72 °C for 1 min with a final extension at 72 °C for 5 min. PCR products were electrophoresed by 1.5% agarose gel and visualized using a UV trans illuminator. Molecular typing of Giardia duodenalis assemblages using β-giardin gene was done according to the protocol performed by Skhal et al. (2016). The amplified products were digested by HaeIII restriction enzyme (Thermo Fisher Scientific, USA) according to the protocol used by Rabih et al. (2020). Digested products were separated by 2% agarose gel electrophoresis at 100 V for 20 min, in parallel with 100 bp DNA ladder (Sigma) as size standard, and three different assemblage types as controls, then visualized and photographed using a UV trans illuminator.
2.4. Data analysis
Statistical analysis was performed using MedCalc® (Version 14.8.1) program. The Odds ratio (OR) was calculated to compare between vegetable types and the risk of parasites contamination. p < 0.05 value was considered statistically significant.
3. Results
Our study showed that 44 of 128 samples of fresh vegetables obtained and examined from street vendors in Damascus, were contaminated with one or more intestinal parasites, giving a rate of (34.4%). A total of 35 samples were contaminated with protozoa (79.5%) while 9 (20.5%) were contaminated with helminths (ova, larvae). Lettuce samples were the most contaminated vegetable and had the highest number of parasite types [13/44; (29.5%)] while tarragon leaves had the lowest contamination level with only one parasite type [1/44; (2.3%)]. (Table 1).
Table 1.
Prevalence of parasites among fresh vegetables sold by street vendors in Damascus city.
| Parasites | Fresh Vegetables (16 samples for each) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parsley | Arugula | Radish | Lettuce | Spearmint | Watercress | Tarragon | Coriander | ||
| protozoa | Entamoeba spp. | 2 | 0 | 0 | 2 | 1 | 0 | 0 | 0 |
| Giardia duodenalis | 3 | 2 | 2 | 4 | 2 | 2 | 1 | 1 | |
| Blastocystis spp. | 2 | 1 | 1 | 4 | 2 | 2 | 0 | 1 | |
| helminthes | Ascaris lumbricoides | 1 | 1 | 0 | 2 | 1 | 0 | 0 | 0 |
| Strongyloides spp | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Hymenolepis nana | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | |
| Taenia spp. | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | |
| Total number of contaminated samples (44) (%) | 8 | 5 | 4 | 13 | 6 | 5 | 1 | 2 | |
| 18.2% | 11.4% | 9.1% | 29.5% | 13.6% | 11.4% | 2.3% | 4.5% | ||
Giardia duodenalis was the only parasite that contaminated all types of examined vegetables and was abundantly detected in lettuce samples (4/17, 23.5%) followed by parsley (3/17, 17.6%), spearmint, radish, arugula and watercress (2/17, 11.8%).
The comparison between vegetable types and the risk of contamination revealed significant results concerning lettuce. The risk of contamination was 65 times higher in lettuce samples than tarragon (OR: 65, p value = 0.00003) and more than 30 times higher than coriander (Table 2). Moreover, the risk of contamination was also 15 times higher in parsley samples than tarragon (OR: 15, p value = 0.015).
Table 2.
Comparison of vegetable types and the risk of contamination with parasites.
| Parsley |
Arugula |
Radish |
Lettuce |
Spearmint |
Watercress |
Coriander |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Odd | p value | Odd | p value | Odd | p value | Odd | p value | Odd | p value | Odd | p value | Odd | p value | |
| Arugula | 2.2 | 0.472 | – | – | – | – | – | – | – | – | – | – | – | – |
| Radish | 3 | 0.273 | 1.36 | 1.000 | – | – | – | – | – | – | – | – | – | – |
| Lettuce | 0.2 | 0.135 | 0.10 | 0.011⁎ | 0.08 | 0.004⁎ | – | – | – | – | – | – | – | – |
| Spearmint | 1.67 | 0.722 | 0.76 | 1.000 | 0.56 | 0.704 | 7.22 | 0.029⁎ | – | – | – | – | – | – |
| Watercress | 2.2 | 0.472 | 1.000 | 1.000 | 0.73 | 1.000 | 9.53 | 0.011⁎ | 1.32 | 1.000 | – | – | – | – |
| Coriander | 7 | 0.053⁎ | 3.18 | 0.394 | 2.3 | 0.654 | 30.3 | 0.0002⁎ | 4.2 | 0.22 | 3.18 | 0.394 | – | – |
| Tarragon | 15 | 0.015⁎ | 6.8 | 0.172 | 5 | 0.333 | 65 | 0.00003⁎ | 9 | 0.082⁎ | 6.8 | 0.171 | 2.14 | 1.000 |
Statistically significant associations (p < 0.05).
The frequency of isolated parasites in the order of appearance in our study was: G. duodenalis, Blastocystis spp., Entamoeba spp., Ascaris lumbricoides, Hymenolepis nana, Strongyloides spp. and Taenia spp. (Table 3; Fig. 1).
Table 3.
Frequency of parasites isolated from fresh vegetables sold by street vendors in Damascus.
| Parasites | Number of positive samples (%) | |
|---|---|---|
| Giardia duodenalis | 17 | (38.6%) |
| Blastocystis spp. | 13 | (29.5%) |
| Entamoeba spp. | 5 | (11.4%) |
| Ascaris lumbricoides | 5 | (11.4%) |
| Hymenolepis nana | 2 | (4.5%) |
| Strongyloides spp. | 1 | (2.3%) |
| Taenia spp. | 1 | (2.3%) |
| Total | 44 | (100%) |
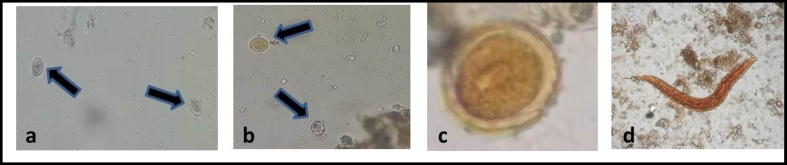
Fig. 1.
Iodine stained parasites found in this survey. a. Giardia cysts (arrows);
b. Entamoeba spp. cysts (arrows); c. Ascaris lumbricoides ova; d. Strongiloides spp. larvae. Original magnification: 400×.
The presence of Giardia and Blastocystis were both confirmed on molecular bases. The amplification of gDNA isolated from drenched contaminated vegetables, using specific primers for Giardia and Blastocystis, gave the expected PCR product sizes 514 bp and 310 bp respectively. Molecular typing of Giardia showed that it belonged to Assemblage B (Fig. 2).
Fig. 2.
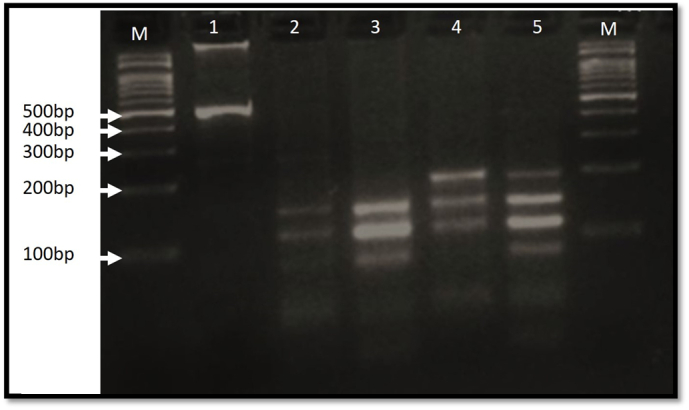
Agarose gel electrophoresis of the PCR products after HaeIII enzyme digestion. Lane 1 uncut β-giardin PCR product, lane 2 represent assemblage B (our study sample) (150, 117–113, 84, 26–24 bp). Lanes 3–5: positive controls [lane 3: assemblage B, lane 4: assemblage A (200, 150, 117–113, 50 bp), lane 5 assemblage (A + B) as mixed control]. M: Molecular marker.
4. Discussion
Vegetables are one of the most essential sources of nourishment. The WHO endorsed the consumption of not less than 400 g of vegetables per day as nutrient supplementation, and to prevent chronic diseases (WHO, 2003). As the realization of the potential for food-borne outbreaks of parasitic infections increases, studies interested in detecting vegetable contamination with parasites are being increased worldwide (Said, 2012; Elmajdoub et al., 2017). In Syria, raw vegetables and green salads are one of the main traditional dishes in homes and restaurants. A limited number of studies have been conducted in Syria to evaluate parasitic contamination on vegetables (Alhabbal, 2015), therefore our study was performed to estimate the extent of parasitic contamination of some commonly consumed raw vegetables, sold by street vendors in the city of Damascus.
The overall parasitic contamination rate of the 128 samples examined in this study was 34.4%. This rate is consistent with the previous study conducted in Alqalamon region 31.40% (Alhabbal, 2015) and approximately similar to some studies conducted in neighboring countries including 39% in Egypt (Etewa et al., 2017), 37% in Palestine (Al-Hindi et al., 2016), 46% in Saudi Arabia (Gabre and Shakir, 2016), 29% in Jordan (Ismail, 2016), and 51% in Iraq (Ghanim et al., 2019).
In our study, lettuce recorded the highest level of contamination among fresh vegetable samples (29.5%) and had the highest risk of contamination compared with other vegetables. These results can be explained by the fact that lettuce leaves have rough surfaces that may facilitate attaching parasites and keeping their cysts and eggs when washing with contaminated water. In addition, lettuce has a very short stem, allowing more contact with soil and irrigation water (Damen et al., 2007; Hassan et al., 2012).
Our results revealed that G. duodenalis was the most prevalent parasite (38.6%), it was found to be significantly higher in lettuce leaves (23.5%) than in other examined samples. This finding was similar to the previous studies conducted in Syria 21.4% (Alhabbal, 2015), Egypt 15.8% (Eraky et al., 2014) and Jordan 23.3% (Ismail, 2016).
Blastocystis spp. were the second prevalent parasite found in contaminated vegetable samples (29.5%) and were detected in a high percentage in lettuce leaves (30.8%). The rate of contamination of this parasite varied between 4% and 13% in previous studies conducted in Saudi Arabia, Egypt, and northern Iran (Al-Binali et al., 2006; Etewa et al., 2017; Esboei et al., 2017 respectively). The presence of Giardia and Blastocystis was confirmed on molecular bases, partly to define their sources. The finding of Giardia Assemblage B in our results indicates that the origin of contamination could be from direct contact with human feces, although a zoonotic source is also possible (Trout et al., 2005; Soliman et al., 2011), or from contaminated irrigation water as an indirect source (Efstratiou et al., 2017). Giardia parasite can be found in healthy people (asymptomatic) as well as in the stools of patients who have gastrointestinal problems. In contrast to our knowledge of Giardia pathogenicity, the clinical significance of Blastocystis and its pathological impacts are still not clear. (Clark et al., 2013). Seventeen sub-types or species of Blastocystis have been described, of which 9 have been reported in humans (Alfellani et al., 2013). Recent studies correlated the presence of Blastocystis infection with irritable bowel syndrome (IBS), (Jimenez-Gonzalez et al., 2012; Krogsgaard et al., 2015). Epidemiological and molecular studies are still in progress by our research team concerning Blastocystis and its pathological correlation (unpublished results).
Furthermore, our study revealed the presence of other types of parasites but in low frequencies (Table 3). This finding indicates the diverse species of parasites contaminating raw vegetables used in salads and highlights the importance of washing vegetables at all stages.
5. Conclusion
Our results point out a high contamination level in salad vegetables sold by street vendors in the city of Damascus. This may be the result of direct human contamination, or either by using sewage water in irrigating crops or through washing it due to the scarceness of freshwater in some areas of Damascus. Further research is needed to identify the precise source of contamination and applying strict control of vegetable cultivation areas, such as keeping animals far from direct contact to agricultural crops, periodic inspection of water sources used in irrigation, in addition to conducting awareness campaigns on parasite contamination and its different sources.
Declaration of competing interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
Acknowledgment
This research was conducted with financial assistance from Damascus University. The authors wish to thank Doctor Imad Alkadi from Damascus University for his contribution to statistical analysis.
References
- Abougrain A.K., Nahais M.H., Madi N.S., Said M.M., Ghenghesh K.S. Parasitological contamination in salad vegetables in Tripoli-Libya. Food Control. 2010;21(5):760–762. doi: 10.1016/j.foodcont.2009.11.005. [DOI] [Google Scholar]
- Adanir R., Tasci F. Prevalence of helminth eggs in raw vegetables consumed in Burdur, Turkey. Food Control. 2013;31(2):482–484. doi: 10.1016/j.foodcont.2012.10.032. [DOI] [Google Scholar]
- Al-Binali A.M., Bello C.S., El-Shewy K., Abdulla S.E. The prevalence of parasites in commonly used leafy vegetables in South Western, Saudi Arabia. Saudi Med J. 2006;27:613–616. [PubMed] [Google Scholar]
- Alfellani M.A., Stensvold C.R., Vidal-Lapiedra A., Onuoha E.S., Fagbenro-Beyioku A.F., Clark C.G. Variable geographic distribution of Blastocystis subtypes and its potential implications. Acta Trop. 2013;126:11–18. doi: 10.1016/j.actatropica.2012.12.011. [DOI] [PubMed] [Google Scholar]
- Alhabbal A.T. The prevalence of parasitic contamination on common sold vegetables in Alqalamoun region. Int. J. Pharm. Sci. Rev. Res. 2015;30(1):94–97. (No. 18). (ISSN 0976-044X) [Google Scholar]
- Al-Hindi A.I., Elmanama A.A., Khalaf S. Prevalence of intestinal parasites and microbial contamination in common edible vegetables used in Gaza Governorate, Palestine. J Food Safe & Hyg. 2016;2(1–2):21–25. [Google Scholar]
- Al-Shawa R.M., Mwafy S.N. The enteroparasitic contamination of commercial vegetables in Gaza Governorates. J. Infect. Dev. Countries. 2007;1:62–66. http://dspace.alazhar.edu.ps/xmlui/handle/123456789/495 (URI:) [Google Scholar]
- Amoah P., Drechsel P., Abaidoo R.C., Klutse A. Effectiveness of common and improved sanitary washing methods in selected cities of West Africa for the reduction of coliform bacteria and helminth eggs on vegetables. Tropical Med. Int. Health. 2007;12:40–50. doi: 10.1111/j.1365-3156.2007.01940.x. [DOI] [PubMed] [Google Scholar]
- Chau H.L.Q., Thong H.T., Chao N.V., Hung P.H.S., Hai V.V., An L.V. Microbial and parasitic contamination on fresh vegetables sold in traditional markets in Hue City, Vietnam. J of Food & Nutrition Res. 2014;2(12):959–964. doi: 10.12691/jfnr-2-12-16. [DOI] [Google Scholar]
- Clark C.G., van der Giezen M., Alfellani M.A., Stensvold C.R. Recent developments in Blastocystis research. Adv. Parasitol. 2013;82:1–32. doi: 10.1016/B978-0-12-407706-5.00001-0. [DOI] [PubMed] [Google Scholar]
- Damen J.G., Banwat E.B., Egah D.Z., Allanana J.A. Parasitic contamination of vegetables in Jos; Nigeria. Ann. Afr. Med. 2007;6:115–118. doi: 10.4103/1596-3519.55723. [DOI] [PubMed] [Google Scholar]
- de W Blackburn C., McClure P.J. CRC Press; Washington DC: 2002. Foodborne Pathogens: Hazards, Risk Analysis and Control; pp. 18–19. [Google Scholar]
- Efstratiou A., Ongerth J.E., Panagiotis Karanis P. Waterborne transmission of protozoan parasites: review of worldwide outbreaks - an update 2011-2016. Water Res. 2017;114:14–22. doi: 10.1016/j.watres.2017.01.036. [DOI] [PubMed] [Google Scholar]
- Elmajdoub L.O., Mosaab A.O., Mohammed S.A., AboSheba F., Elzwawi S. Prevalence of parasitic contamination of leafy green vegetables in Misurata, Libya. Russian J. of Parasitol. 2017;40(2):197–204. (ISSN 1998-8435) [Google Scholar]
- Eraky M.A., Rashed S.M., Nasr M.E., EL-Hamshary A.M.S., El-Ghannam A.S. Parasitic contamination of commonly consumed fresh leafy vegetables in Benha, Egypt. J. Parasitol. 2014;2014:613960. doi: 10.1155/2014/613960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdoğrul Ö., Şener H. The contamination of various fruit and vegetable with Enterobius vermicularis, Ascaris eggs, Entamoeba histolyca cysts and Giardia cysts. Food Control. 2005;16:557–560. doi: 10.1016/j.foodcont.2004.06.016. [DOI] [Google Scholar]
- Esboei B.R., Sharif M., Daryani A., Hosseini F., Pagheh A.S., Rahimid M., Nasrolahei M. Parasitic contamination in commonly consumed vegetables in Mazandaran Province, Northern Iran. J. Hum. Environ. Health Promot. 2017;2(2):89–95. [Google Scholar]
- Etewa S.E., Abdel-Rahman S.A., Fathy G.M., Abo El-Maaty D.A., Sarhan M.H. Parasitic contamination of commonly consumed fresh vegetables and fruits in some rural areas of Sharkyia Governorate, Egypt. Afro-Egypt J. Infect. Endem. Dis. 2017;7(4):192–202. doi: 10.21608/aeji.2017.17804. [DOI] [Google Scholar]
- Gabre R.M., Shakir A. Prevalence of some human entero parasites in commonly consumed raw vegetables in Tabuk, Saudi Arabia. J. Food Protect. 2016;79(4):655–658. doi: 10.4315/0362-028X.JFP-15-485. [DOI] [PubMed] [Google Scholar]
- Ghanim M.R., Abdul Hussein K.H., Fatehi A.J. Diagnostic study on intestinal parasites isolated from raw consumed vegetables in Misan City/Iraq. Indian J. Public Health Res. & Develop. 2019;10(8):1236–1240. doi: 10.5958/0976-5506.2019.02064.3. [DOI] [Google Scholar]
- Hassan A., Farouk H., Abdul-Ghani R. Parasitological contamination of freshly eaten vegetables collected from local markets in Alexandria, Egypt. A preliminary study. Food Control. 2012;26(2):500–503. doi: 10.1016/j.foodcont.2012.01.033. [DOI] [Google Scholar]
- Ismail Y. Prevalence of parasitic contamination in salad vegetables collected from supermarkets and street vendors in Amman and Baqa'a-Jordan. Polish J. Microbiol. 2016;65(2):201–207. doi: 10.5604/17331331.1204480. (1204480) [DOI] [PubMed] [Google Scholar]
- Jimenez-Gonzalez D.E., Martinez-Flores W.A., Reyes-Gordillo J., Ramirez-Miranda M.E., Arroyo-Escalante S., Romero-Valdovinos M. Blastocystis infection is associated with irritable bowel syndrome in a Mexican patient population. Parasitol. Res. 2012;110(3):1269–1275. doi: 10.1007/s00436-011-2626-7. [DOI] [PubMed] [Google Scholar]
- Khan W., Mumtaz G.H., Bibi S., Afzal S. Parasitic contamination of fresh vegetables sold at upper and lower Dir districts, Khyber Pakhtunkhwa, Pakistan. Pakistan J. Zool. 2017;49(3):1115–1118. doi: 10.17582/journal.pjz/2017.49.3.sc3. [DOI] [Google Scholar]
- Krogsgaard L.R., Engsbro A.L., Stensvold C.R., Nielsen H.V., Bytzer P. The prevalence of intestinal parasites is not greater among individuals with irritable bowel syndrome: a population-based case-control study. Clin. Gastroenterol. Hepatol. 2015;13(3):507–513. doi: 10.1016/j.cgh.2014.07.065. [DOI] [PubMed] [Google Scholar]
- Mandeville K.L., Krabshuis J., Ladep N.G., Mulder C.J., Quigley E.M., Khan S.A. Gastroenterology in developing countries: issues and advances. World J. Gastroenterol. 2009;15(23):2839. doi: 10.3748/wjg.15.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omowaye O.S., Audu P.A. Parasites contamination and distribution on fruits and vegetables in Kogi, Nigeria. CIBTech J. of Bio-Protocols. 2012;1(1):44–47. [Google Scholar]
- Rabih N., Boutaiba S., Aboualchamat G., Soutton K., Hakem A., Al Nahhas S. Molecular and epidemiological characterization of Giardia intestinalis assemblages detected in Djelfa, Algeria. J. Parasit. Dis. 2020;44(2):281–288. doi: 10.1007/s12639-020-01206-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Said D.E. Detection of parasites in commonly consumed raw vegetables. Alexandria J. of Med. 2012;48:345–352. doi: 10.1016/j.ajme.2012.05.005. [DOI] [Google Scholar]
- Skhal D., Aboualchamat G., Al Nahhas S. Giardia duodenalis in Damascus, Syria: identification of Giardia genotypes in a sample of human fecal isolates using polymerase chain reaction and restriction fragment length polymorphism analyzing method. Acta Trop. 2016;154:1–5. doi: 10.1016/j.actatropica.2015.10.008. [DOI] [PubMed] [Google Scholar]
- Soliman R.H., Fuentes I., Rubio J.M. Identification of a novel assemblage B sub-genotype and a zoonotic assemblage C in human isolates of Giardia duodenalis in Egypt. Parasitol. Int. 2011;60:507–511. doi: 10.1016/j.parint.2011.09.006. [DOI] [PubMed] [Google Scholar]
- Stensvold R., Brillowska-Dabrowska A., Nielsen H.V., Arendrup M.C. Detection of Blastocystis hominis in unpreserved stool specimens by using polymerase chain reaction. J. of Parasitol. 2006;92(5):1081–1087. doi: 10.1645/GE-840R.1. [DOI] [PubMed] [Google Scholar]
- Trout J., Santin M., Greiner E., Fayer R. Prevalence and genotypes of Giardia duodenalis in post-weaned dairy calves. Vet. Parasitol. 2005;130:177–183. doi: 10.1016/j.vetpar.2005.03.032. [DOI] [PubMed] [Google Scholar]
- World Health Organization . Vol. 916. World Health Organization; 2003. Diet, Nutrition and the Prevention of Chronic Diseases. Report of a Joint FAO/WHO Expert Consultation. (May/2019) [PubMed] [Google Scholar]
- World Health Organization . WHO; Geneva: 2008. World Health Statistics 2008.http://www.who.int/whosis/whostat/2008/en/index.html (Available from: URL:) (December/2019) [Google Scholar]