Summary
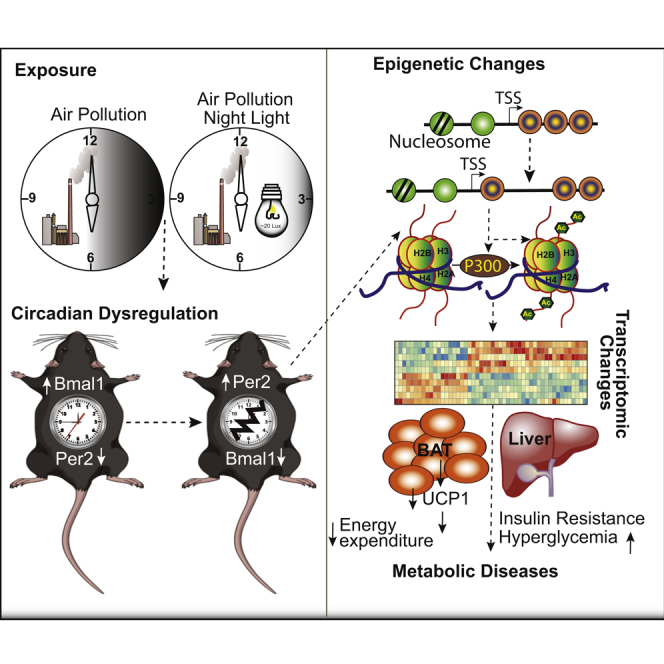
Particulate matter ≤2.5μm (PM2.5) air pollution is a leading environmental risk factor contributing disproportionately to the global burden of non-communicable disease. We compared impact of chronic exposure to PM2.5 alone, or with light at night exposure (LL) on metabolism. PM2.5 induced peripheral insulin resistance, circadian rhythm (CR) dysfunction, and metabolic and brown adipose tissue (BAT) dysfunction, akin to LL (with no additive interaction between PM2.5 and LL). Transcriptomic analysis of liver and BAT revealed widespread but unique alterations in CR genes, with evidence for differentially accessible promoters and enhancers of CR genes in response to PM2.5 by ATAC-seq. The histone deacetylases 2, 3, and 4 were downregulated with PM2.5 exposure, with increased promoter occupancy by the histone acetyltransferase p300 as evidenced by ChIP-seq. These findings suggest a previously unrecognized role of PM2.5 in promoting CR disruption and metabolic dysfunction through epigenetic regulation of circadian targets.
Subject Areas: Environmental Health, Pollution, Transcriptomics, Metabolic Engineering
Graphical Abstract
Highlights
-
•
Air pollution disrupts the circadian rhythm (CR) similar to light at night
-
•
Dysregulated circadian genes result in insulin resistance and metabolic diseases
-
•
PM2.5 alters chromatin structure of circadian genes at regulatory regions
-
•
PM2.5 alters chromatin structure by recruiting histone acetyl transferase (HAT), p300
Environmental Health; Pollution; Transcriptomics; Metabolic Engineering
Introduction
Air pollution is a leading environmental risk factor contributing disproportionately to the global burden of non-communicable diseases (NCDs) (Landrigan et al., 2018). The particulate matter ≤2.5 μm in diameter (PM2.5) component is the most extensively studied component, with evidence from epidemiological and empirical studies implicating it in the development of insulin resistance (IR) and type II diabetes (T2D) (Munzel et al., 2017b; Rajagopalan et al., 2018). Studies to date have implicated inflammation, oxidative stress, and metabolic dysfunction in response to inhaled PM2.5 as broad pathways underlying air pollution effects. However, integrated mechanistic insights are currently lacking (Munzel et al., 2017a).
The circadian system is an evolutionarily conserved system that allows organisms to anticipate predictable environmental changes through coordinated and synchronized changes in behavior and physiology (Bass and Lazar, 2016). The system typically entails entrainment of the internal clock to external environmental cues, so that the rhythmicity of physiological and behavioral processes align with the periodicity of the external environment (Partch et al., 2014). At a molecular level, a central feature of the circadian system is the transcriptional translational feedback loop, involving the core clock genes (Clock, Bmal1, Period 1–3 [Per1, Per2, and Per3] and Cryptochrome 1–2 [Cry1 and Cry2]) that are transcribed rhythmically and are part of this feedback loop (Bass and Lazar, 2016; Koike et al., 2012; Partch et al., 2014). Given the importance of continuous light as a classic circadian disruptor, we were interested in comparing the effect of PM2.5 on continuous light (regular day light at day time and dim light at night time; LL) versus control ambient light conditions (regular day light at day time and dark at night time; LD).
Given that many clock genes are epigenetic regulators, we were particularly interested in comparing the effects of PM2.5 and light exposure on chromatin dynamics as a potential mechanism by which environmental triggers may exert broad influence on a range of transcriptional targets (Aubrecht et al., 2015; Bedrosian et al., 2016; Hayashi et al., 2007; Nelson and Chbeir, 2018; Russart and Nelson, 2018). In this study, we exposed mice to chronic inhalation of PM2.5, concentrated from ambient air pollution in Cleveland, Ohio. The concentration of these ambient particles allowed for exposure levels comparable with air quality in many cities in Asia. Our data provide new evidence on PM2.5 exposure-induced metabolic dysfunction through epigenetic regulation of core clock genes.
Results
PM2.5 Exposure and Circadian Gene Dysregulation: Discovery Dataset
An initial unbiased RNA sequencing analysis of liver tissue (harvested at ZT02) from male C57BL/6J mice exposed to concentrated PM2.5 or filtered air (FA) revealed a significant dysregulation of multiple circadian genes. Exposure to the Versatile Aerosol Concentration Enrichment System (VACES) for 5–6 h a day, 5 days a week for 14 weeks, (n = 12/group) demonstrated downregulation of positive regulators like Bmal1 and upregulation of negative regulators like Id1, Dbp, and Bhlhe4 (Figures S1A and S1B). We conducted a second set of validation experiments to evaluate the comparative and synergistic effects (if any) of PM2.5 versus continual light exposure, using a modified VACES system to allow for simultaneous PM2.5 exposure and light exposure. C57BL/6J mice at the age of 4 weeks were exposed to FA or concentrated PM2.5 for 30 weeks under controlled temperature and humidity as described previously (Figures 1A and S2)Rajagopalan et al., 2020, Sun et al., 2009. The exposure was for 6 h per day with standard light-dark cycle (12 h day light/12 h dark) (LD) or constant light (12 h day light/12 h dim light) (LL), with concomitant ad libitum access to standard chow diet. Mean daily PM2.5 concentrations inside the chambers were 93.9 ± 25.16 μg/m3. Mean daily ambient PM2.5 concentrations during the same period in Cleveland were 12.0 ± 3.76 μg/m3 (Figure S2A). Mean weekly PM2.5 exposure (Figure S2B) and mean elemental concentration (Figure S2D) observed in the VACES system are enumerated. Weekly ambient mean temperature and humidity were also recorded (Figure S2C). Overall, there was approximately an 8-fold enrichment of ambient PM2.5.
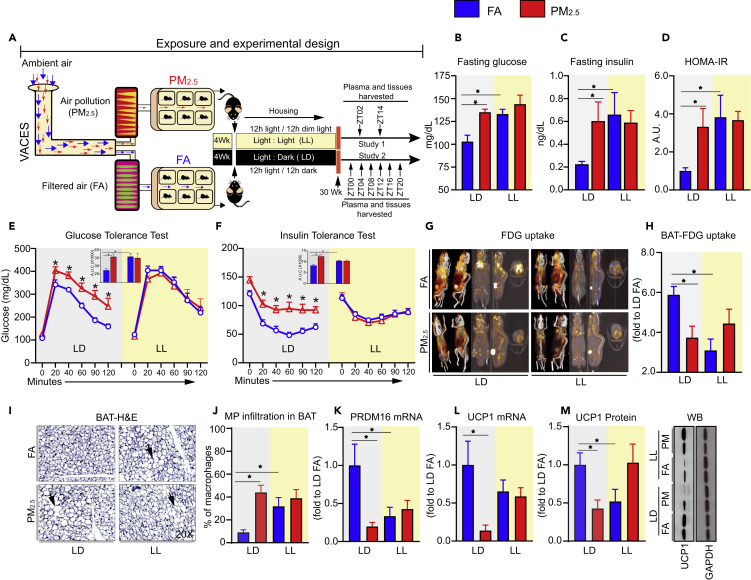
Figure 1.
Chronic Air Pollution (PM2.5) Induces Insulin Resistance in C57BL/6 Mice
(A–F) (A) Schematic diagram illustrating the exposure and experimental design of the study (wild-type mice were exposed to either filtered air (FA) or PM2.5 (6 h/day for 5 days/week) in standard light dark cycle (LD) or constant light (12 h daylight/12 h dim light) (LL) conditions, with concomitant ad libitum access to standard chow diet for 30 weeks (Cohort 1: n = 12, Cohort 2: n = 24 per group), (B) fasting blood glucose, (C) fasting insulin, (D) HOMA-IR (overnight fasting before collecting blood) levels as a measure of systemic insulin resistance (n = 12), (E) glucose (2 g/kg) and (F) insulin intolerance test (0.75 U/kg) are summarized by the areas under curve (AUC) following FA, PM2.5 exposure (n = 12).
(G–M) (G) representative PET scan images, (H) quantification of standardized FDG uptake in brown fat (SUV), (I) representative image from BAT histology (H&E stain) (n = 5), (J) quantification of percentage of macrophage infiltration in the BAT, (K) mRNA expression of BAT-specific marker prdm16, and (L) UCP1, (M) quantification of UCP1 protein levels (n = 5) and its representative blot. All data are reported as mean ± SEM. For statistical analysis, p values (∗p < 0.05) were calculated relative to DAN-FA mice by a two-way analysis of variance (ANOVA) (C and D), one-way ANOVA (E, F, G, J, L, and M) with Tukey's Bonferroni test and by unpaired two-tailed t test (n).
Chronic Air Pollution (PM2.5) Induces Insulin Resistance and Alterations in Energy Homeostasis
We assessed fasting glucose, insulin, and HOMA-IR in the plasma of male mice exposed to either FA or PM2.5 under LD or LL conditions for 30 weeks (Figures 1B–1D). PM2.5 increased fasting blood glucose levels (Figure 1B) similar to that observed in mice maintained under LL conditions. Importantly LD-PM2.5 and LL-FA-exposed mice demonstrated higher plasma insulin and HOMA-IR (Figures 1C and 1D). No changes in body weight and fat mass were noted between the groups (Figures S3A–S3C). Significant differences in glucose tolerance and insulin sensitivity were observed in both LD-FA versus LD-PM2.5 (Figures 1E and 1F). As anticipated, LL mice exposed to FA had impaired whole-body glucose tolerance and insulin sensitivity but co-exposure to PM2.5 did not result in further exacerbation of phenotype (Figures 1E and 1F). Positron emission tomographic (PET) imaging quantitation of 18F-flurodeoxyglucose (18F-FDG) uptake provides a non-invasive measure of tissue glycolysis. PET imaging revealed ~50% less 18F-FDG uptake within brown adipose tissue (BAT) of LD-PM2.5 mice when compared with LD-FA mice (Figures 1G and 1H). 18F-FDG uptake in other tissues such as epididymal white adipose, liver, heart, kidney, and brain were comparable across groups (data not shown). Histological assessment of BAT showed increased “whitening,” indicative of lipid and macrophage infiltration, in LD-PM2.5 and LL-FA mice, compared with LD-FA mice (Figures 1I and 1J). Expression of the BAT-specific genes Prdm16 and Ucp1 (Figures 1K and 1L) was reduced in LD-PM2.5 and LL-FA mice. Additionally, Ucp1 protein expression was also reduced (Figure 1M).
PM2.5 and LL Alters Stress Hormones, Metabolism, and Circadian Rhythmicity of Core Circadian Genes in Liver and Brown Adipose Tissue
Timed-urinary levels of corticosterone over a 24-h period revealed increased corticosterone at multiple time points with LD-PM2.5 versus LD-FA, with a corresponding increase in the aggregate area under curve measurement (Figures S4A and S4B). Periodic corticosterone levels over 24 h in LL-FA exposed mice, although significantly different compared with LD-FA exposed mice (consistent with a circadian disruptor effect of light), was no different from LL-PM2.5 exposed mice, suggesting no additional effect of concomitant exposures. Plasma levels of corticosterone and urinary epinephrine, norepinephrine, and dopamine levels were significantly higher in PM2.5-exposed mice (Figures S4C–S4F), although these differences were temporally variable, with higher values earlier in the day (zeitgeber time ZT, 02 h) for plasma corticosterone and later in the day for others. Metabolic parameters assessed by indirect calorimetry over 48 h revealed significant reduction in energy expenditure, VO2 and VCO2 in LD-PM2.5 (Figures 2A, 2C, and 2D). Reduced respiratory exchange ratio (RER) was also noted in LD-PM2.5 but only at night (Figure 2B), suggesting that LD-PM2.5 mice oxidized fat preferentially at the expense of carbohydrate. Although PM2.5 and LL independently altered the above metabolic parameters, in combination they exhibited no additional synergistic effect (data not shown). Significant regulation of genes involved in fatty acid oxidation and gluconeogenesis were noted in both PM2.5 and LL exposure, including reduction in PGC1α, PPARα, and CPT1 and upregulation of PEPCK, respectively (Figure S5). LL, but not PM2.5, upregulated Acyl-CoA Carboxylase (ACC). However, PM2.5 in association with LL increased the ACC expression at ZT14 (Figure S5), associated with CPT1 downregulation, which may then restrict fatty acid entry into the mitochondria for further oxidation. Although no significant changes were observed in pyruvate dehydrogenase kinase isozyme 1 (PDK1) by PM2.5, LL, or combination of both (Figure S5) pyruvate dehydrogenase kinase isozyme 4 (PDK4), which also inhibits pyruvate dehydrogenase complex, reducing the conversion of pyruvate from glucose and amino acids to acetyl-CoA, was markedly upregulated by PM2.5 but only in conjunction with LL (Figure S5).
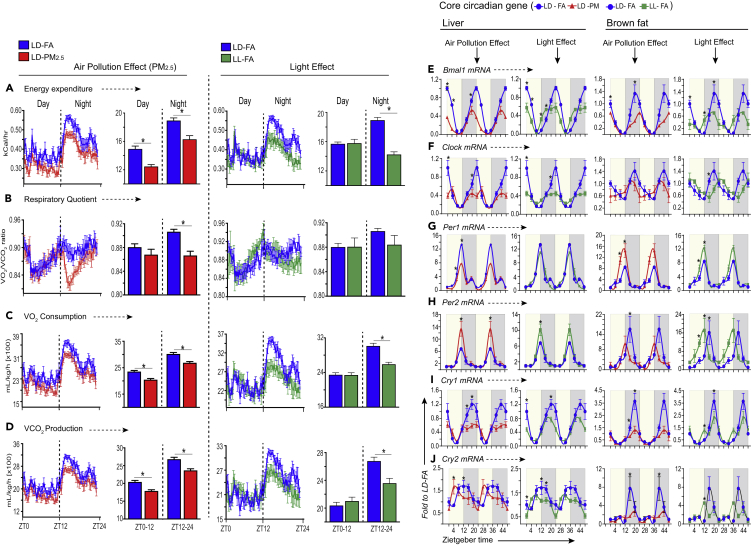
Figure 2.
PM2.5 Perturbs Resting Energy Expenditure Levels and Peripheral Circadian Rhythm in Wild-Type Mice
Wild-type mice were exposed to FA or PM2.5 and light at night (LL) or light at day (LD) as described earlier (n = 6 per group). (A) Hourly energy expenditure during light (6 am–6 pm) and dark (6 pm–6 am) cycle and total light and dark energy expenditure level, (B) hourly day/night respiratory quotient ratio (RQ) and total average day and night RQ value, (C) hourly day/night oxygen consumption rate and total day and night average O2 consumption rate, (D) hourly day/night CO2 release rate and total day and night average CO2 release rate. All line graphs show average of light and dark cycle of mice over a 48-h period. Total average day and night energy expenditure (A), respiratory quotient (B), oxygen consumption (C), and carbon dioxide production (D) are depicted in bar graphs. Values are shown as mean ± SEM. Data are expressed as ∗p < 0.05 relative to LD-FA mice as determined by a two-way analysis of variance (ANOVA) with Tukey's multiple comparison test. mRNA levels of (E) Bmal1, (F) Clock, (G) Period (Per) 1, (H) Period (Per) 2, (I) Cryptochrome (Cry) 1, and (J) Cryptochrome (Cry) 2 in both liver and brown fat (see also Figure S6, Tables S1 and S2), monitored over 24 h (for all panels graphs (E–J) are double plotted). Pale yellow background for (E)–(J) represents tissues harvested during day (light phase), whereas gray background represents tissues harvested at night (dark phase). At each ZT time, tissues from each group of mice (n = 4) were used for analysis. Data are expressed (fold to basal LD-FA ZT00) as mean ± SEM, ∗p < 0.05 relative to LD-FA ZT00 mice is determined by a two-way analysis of variance (ANOVA) with Tukey's multiple comparison test.
To determine if PM2.5 affects the peripheral circadian clock, mRNA expression of Bmal1, Clock, Per1, Per2, Cry1, and Cry2 was measured in livers and brown adipose tissues at multiple time points. Expression of Bmal1, Clock, Per1, Per2, Cry1, and Cry2 in the liver showed a time-dependent variation in the LD-PM2.5 mice that was different from that of LD-FA mice (Figures 2E–2J). Cosinor analysis was applied to investigate the presence of a 24-h rhythm. Cosinor analysis depicted a significant circadian rhythmicity in the expression of Bmal1, Clock, Per2, Cry1, and Cry2 transcripts in the liver of LD-FA mice. A significant effect of both PM2.5 and LL on cycling as well as other rhythmic parameters like phase, amplitude, and mesor (rhythm adjusted mean) was observed (Figures 2E–2J, Tables S1 and S2). A significant change in response to an intervention was required to have a reduction in amplitude (1/2 the distance between peak and trough) and a change in either mesor and/or acrophase. Using these criteria, we noted changes in cycling in Bmal1, Clock, and Cry1 in liver of LD-PM2.5 and LL-FA mice when compared with liver of LD-FA mice (Table S1). A phase advance was noted in Bmal1 and Cry2 in LD-PM2.5 and LL-FA mice compared with control (LD-FA). Specifically, liver expression of Bmal1 and Cry2 was phase advanced by ~4 h in LD-PM2.5 mice (Figures 2E and 2J). In LL-FA, the phase of Bmal1 and Cry2 was slightly different than LD-PM2.5 compared with LD-FA, the phase was advanced by ~4 h in Bmal1 and ~2 h in Cry2 (Figures 2E and 2J, TableS1). Loss of circadian rhythmicity of Clock, Per2, Cry1, and Cry2 expression was observed in animals exposed to LD-PM2.5; however, in LL-FA only Per2 and Cry2 transcripts lost rhythmicity. A significant decrease in mesor value of several core clock genes (Bmal1, Clock, Per1, Per2, and Cry1) was noted in LD-PM2.5 mice. In response to LL, changes in mesor were limited to only three genes in LL-FA (Bmal1, Clock, andCry1). Furthermore, the amplitudes of Bmal1, Clock, and Cry1 were also significantly reduced in the liver of mice exposed to LD-PM2.5 and LL-FA (Table S1). However, the amplitude of Per2 was significantly increased by PM2.5 and LL-FA in contrast to change observed in other clock genes with these exposures (Table S1). These results are consistent with loss of cycling of the core circadian mechanism by PM2.5 when compared with FA mice. Unexpectedly, the combination of light and PM2.5 (except for the amplitude of Clock in liver), did not impact mesor, amplitude, or acrophase of core clock genes when compared with light only condition (Table S1, Figure S6). Individually both PM2.5 and LL cause a disruption in rhythmicity; however, the combination of light and PM2.5 appears to be a stronger entrainment cue for the expression of Per1 and Cry1(Table S1, Figure S6).
There were notable differences in circadian gene expression comparing BAT with liver. LD-FA mice showed significant circadian rhythm (CR) in expression of most of the genes (Bmal1, Clock, Per2, and cry1) (Table S2). For instance, only a significant circadian rhythm could be detected for Bmal1 and Cry1 transcript in mice exposed to LD-PM2.5 (Figures 2E and 2I, Table S2). None of the other genes exhibited significant cycling according to the zero amplitude test in Cosinor analysis (Table S2). However, analysis of mRNA expression suggests that expression of Bmal1, Per1, Cry1, and Cry2, but not Clock or Per2, is significantly reduced in LD-PM2.5 mice (Figures 2E–2J, Table S2). PM2.5 and LL had complex effects on Per1 and Per2 genes in the BAT, which was diametrically opposite to the pattern observed in the liver, i.e., Per1 was upregulated by both PM2.5 and LL-FA in BAT but downregulated by both in the liver, with mRNA expression highest at ZT12 and lowest at ZT24 (Figures 2G and 2H). Both PM2.5 as well as LL-FA significantly decreased peak levels of Cry1 transcript at ZT20 and Cry2 transcript at ZT16 in BAT (Figures 2I and 2J and Table S2). In contrast to the liver, the light plus PM2.5 stimuli significantly decreased mesor, amplitude, and phase variation of Bmal1 in BAT (Table S2 and Figure S6A). Although the amplitudes of Per1, Per2, and Cry1 with light plus PM2.5 were unchanged, mesor values were significantly different compared with light only condition (Table S2 and Figures S6C–S6E). Phase delay (~4 h) and phase advance (~4 h) were noticed in expression of Clock and Per2 genes, respectively, in LL-FA condition compared with LD-FA (Figures 2F and 2H, Table S2). Like liver, the combination of light and PM2.5 appears to be a stronger entrainment cue as seen for the expression of Per1 and Cry1 in BAT (Table S2, Figure S6). Overall, our data suggest that PM2.5 is comparable with light exposure in causing widespread circadian disruption in the liver and BAT. Our results on circadian gene variations in liver and BAT are different from those recently published (Li et al., 2020; Wang et al., 2020). This could be due to the duration of PM2.5 exposure (10 versus 30 weeks in our study, which truly represents a chronic exposure scenario), inclusion of light at night as a positive control comparator, and the unbiased nature of our analysis.
PM2.5 Effects on Circadian Transcriptome
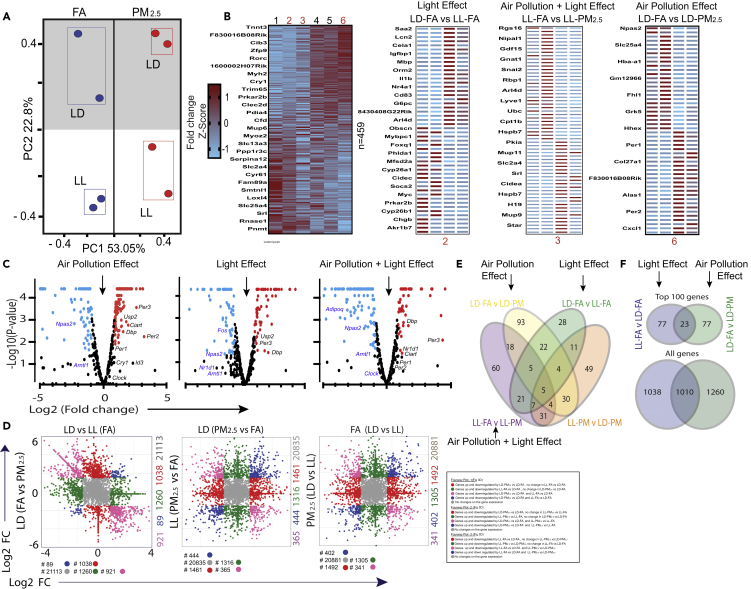
In order to investigate downstream pathways affected by exposure to PM2.5 and light, we performed RNA-seq of liver isolated from animals exposed to LD-FA, LD-PM2.5, LL-FA, and LL-PM2.5. A DESeq analysis was used to compute significant changes in gene expression on exposure to PM2.5 and LL (Anders and Huber, 2010). Principal component analysis (PCA) revealed the variability across biological replicates and similarity between treatments (Figure 3A). Figure 3B depicts a heatmap of all differentially expressed genes (≥2-fold change) and the top 50 candidate genes differentially regulated by PM2.5 as compared with LL. Genes that were significantly different based on p value in at least one pairwise comparison (n = 459) are depicted in the volcano plot (Figure 3C). In order to further isolate the differential impact of PM2.5 versus LL exposure, we plotted DEG's in a four-way plot, comparing two variables (pair) at a time. Pairwise comparisons restrict dimensionality of comparison in gene profiling, allowing one to distinguish unique effects of exposure (light versus PM2.5) and to understand combinatorial impact. Figures 3D and S7 demonstrate combinatorial impact of exposures and depict DEGs that are uniquely expressed in response to light and PM2.5 versus those DEGs that are uniquely responsive to light or PM2.5 individually. Figure 3E depicts a Venn diagram demonstrating unique and overlapping DEGs in response to PM2.5 and light as well as the Top 100 DEGs. Overall, only 30% of DEGs overlapped between PM2.5 and LL, further corroborating our observation on the uniqueness of the phenotypes caused by PM2.5 exposure versus light in the liver of chronic PM2.5 exposed mice. These results support the idea that the genes affected by two independent environmental circadian disruptors exhibit unique transcriptional signatures.
Figure 3.
Differential Effect of PM2.5 and Light at Night (LL) on Liver Transcriptome
Wild-type mice were exposed to FA or PM2.5 (6 h/day for 5 days/week) in standard light dark cycle (LD) or constant light conditions (12 h day light/12 h dim light) (LL) with concomitant ad libitum access to standard chow diet for 30 weeks (n = 2 per group).
(A) PCA plot of normalized sequence counts—each circle of same color represents biological replicates of treatments FA/PM2.5 and light conditions LL/LD.
(B) Heatmaps of DEGs that are significantly different in at least one pairwise comparison normalized and represented as Z scores and each panel represents DEG from one pairwise comparison. Heatmaps of top 50 DEGs based on p values of three different pairwise comparisons in right panel.
(C) Volcano plots showing significantly up- and downregulated differentially expressed circadian/oscillatory genes, labeled in groups that are being compared in the study (see also Figure S7).
(D) Four-way fold change plot shows the DEG in two pairwise comparisons as marked in axes.
(E) Venn diagram indicates the number of overlapping genes between different pairwise comparison.
(F) Venn diagram showing the top 100 DEGs, 30% DEGs overlap between PM2.5 and LL.
Expression Profiling of Circadian Genes and Downstream Pathways with PM2.5 Exposure
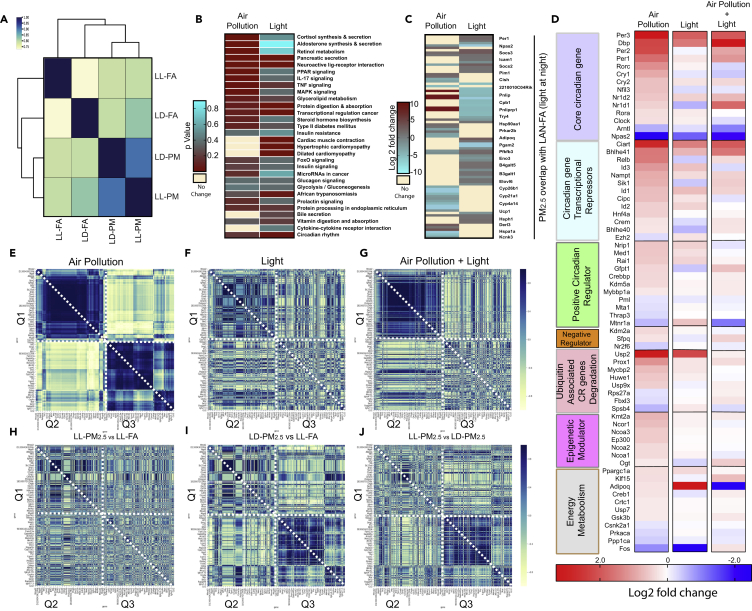
Given the substantial differences in expression profiling in response to two contrasting exposures (light and PM2.5), the 459 DEGs that were significant in at least one pairwise comparison from the four different treatment combinations LD (FA versus PM2.5), LL (FA versus PM2.5) were compared to examine co-regulated genes. In this analysis, the correlation between DEGs reflects the degree of co-regulation, with markedly co-regulated genes depicted in dark blue on heatmaps, with genes with lower co-regulation (correlation) shown in cream yellow (Figure 4A). It is apparent that PM2.5 exerts a distinctive and greater influence compared with light exposure (LL-FA) based on co-regulated genes. We then turned to other significant pathways regulated in response to PM2.5 alone (LD-PM2.5) and with light exposure alone (LL-FA, Figure 4B). We selected the top 25 significantly upregulated pathways from I-pathway analysis based on p value cutoff (0.05) and compared these genes within the two groups, with the heatmap representing significantly regulated genes with PM2.5 (LD-PM2.5 versus LD-FA) and light (LL-PM2.5 versus LL-FA, Figure 4C). The top pathways regulated in response to PM2.5 included cortisol and aldosterone synthesis, retinol metabolism, and inflammatory signaling (IL-17, TNF, MAPK). These were distinct from pathways regulated in response to LL exposure. However, a number of pathways enriched in response to PM2.5 and light also demonstrated concordance and were positively correlated (data not shown). When we analyzed specific genes that comprised the enriched pathways in Figure 4B, many genes in the enriched pathways in both LD-PM2.5 versus FA as well as LL-PM2.5 versus LL-FA were not concordant with the pathway (Figure 4C). This implies that genes belonging to the same pathway may exhibit differential regulation depending on the condition and hierarchy in the pathway. Next, we focused specifically on the effect of PM2.5, light, and the combination on clusters of core circadian genes and seven classes of oscillatory circadian genes (Figure 4D). The expression of multiple core circadian genes as well as other oscillatory genes were highly regulated in response to both PM2.5 and light with no additional impact of the combination. In fact, the combination of LL/PM2.5 exposure seemed to have neutral effects on many core circadian genes and oscillatory circadian gene clusters (Figure 4D). Moreover, an additional correlation analysis (data not shown) revealed a high degree of correlation between PM2.5 and light exposure, suggesting possible similarities between the two exposures in causing CR dysfunction.
Figure 4.
Transcriptomic Signature in the Liver Reveals Positive Correlation between Circadian Disruption, PM2.5, and LL
(A) Correlation between pairwise comparisons: dark blue indicates highly correlated, light yellow indicates least correlated.
(B) Heatmap (p value) of circadian pathways disrupted by air pollution and light. Dark red indicates enriched pathways and yellow indicates depleted pathways.
(C) Correlation plots of all DEGs from pairwise comparisons: blue indicates positive correlation and yellow indicates negative correlation.
(D) Heat maps of log2 fold change expression of CR genes divided into seven functional classes under three different conditions—PM2.5, LL, and a PM2.5 + LL: red indicates high expression (fold change), blue indicates low expression (fold change), whereas cream color indicates no change.
(E–J) Spearman correlation heatmap of DEGs in at least one of the pairwise comparisons: blue indicates high correlation and yellow indicates low correlation to represent the effect of (E) PM2.5, (F) light at night, (G) PM2.5 and light, (H) PM2.5 with light versus FA with light, (I) PM2.5 at dark versus FA at light, (J) PM2.5 with light versus PM2.5 at dark.
To examine the co-regulation of DEGs in response to PM2.5 and light, we turned to a correlation heatmap that enables pattern recognition in multi-omics assays, by embedding significance of gene expression with gene-gene correlational analysis. In this heatmap, the colors represent the degree of correlation between genes as well as magnitude of expression in response to PM2.5 (Figure 4E), light (Figure 4F), and the combination of PM2.5 and light (Figure 4G). From this analysis, DEGs in response to PM2.5 demonstrated a distinct visual pattern of strongly correlated genes that were confined to three quadrants (Q1, Q2, and Q3). In contrast, light exposure resulted in a pattern that was more diffuse in comparison (Figure 4F). The combination exposure of PM2.5 and light (Figure 4G) provided a unique hybrid pattern restricted to Q2, whereas Q1 and Q3 were reminiscent of the predominant pattern of PM2.5 (Q1 in Figure 4E) and light (Q3 in Figure 4F). All other combinations of treatments (Figures 4H–4J) were representations that were intermediate between the predominant patterns seen with PM2.5 (Figure 4E) and light (Figure 4F), respectively.
PM2.5-Mediated Transcriptional Reprograming Involves Alteration of Chromatin Structure
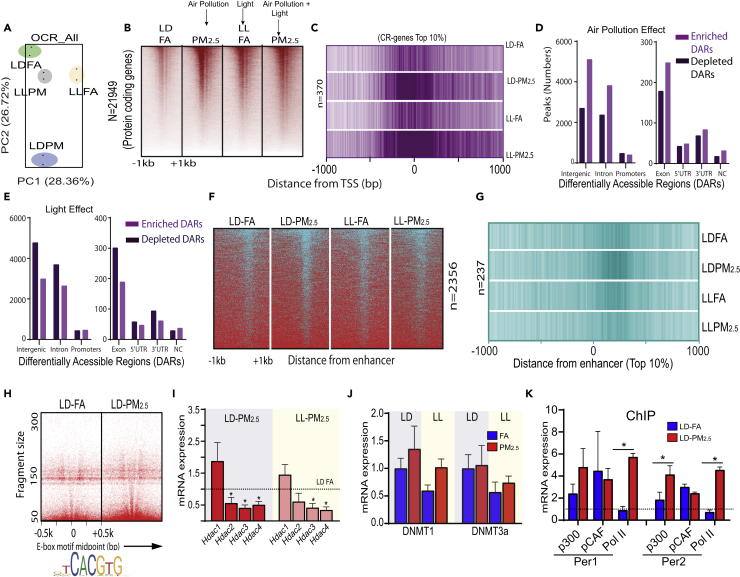
Our transcriptomic analysis suggests that both PM2.5 and light mediate broad transcriptional regulation of a multitude of pathways, potentially invoking epigenetic regulation. We performed a genome-wide omni ATAC (ATAC-seq on purified nuclei) on mouse liver exposed to 30 weeks of PM2.5 (Corces et al., 2017). PCA analysis confirmed the consistency of biological replicates (Figure 5A). Heatmap sorted based on occupancy of uniquely mapped reads, covering all protein coding genes, is depicted in Figure 5B. We depicted the openness of promoters of top 10% (n = 370) of highly “occupied” core and oscillatory circadian genes as a composite heatmap (Figure 5C) under control conditions (LD-FA, where the intensity of magenta is an indication of active transcription, whereas lighter shades suggest closed chromatin structure indicative of repressive transcription). Our results show that irrespective of light, PM2.5 exposure results in an open configuration of the promoter regions of CR genes (Figure 5C). Since differentially accessible open chromatin regions include all classes of cis-regulatory elements (promoters, enhancers, and insulators) we sought to determine if PM2.5 and light affect all cis-regulatory elements uniformly or if their effects are specific to certain cis-regulatory elements (Thurman et al., 2012). Figure 5D depicts the overall distribution of differentially accessible regions (DARs) during PM2.5 and light at night exposure seen with PM2.5 with enriched and depleted DARs particularly in the intergenic, intronic, 5′ UTR, and 3′ UTR regions. PM2.5 had a unique pattern that appeared to be opposite to that observed with LL (Figure 5E). Figure 5F depicts a histogram of all enhancer elements based on exposures. Figure 5G shows that PM2.5 exposure induces an open configuration of enhancers (top 10% from http://cirgrdb.biols.ac.cn/), whereas light exposure does not cause any change in configuration.
Figure 5.
PM2.5 Drives Chromatin Structural Changes via Histone Acetylation
(A) PCA plot of normalized sequence counts from ATAC-seq; each colored circle represents biological replicates of treatments labeled as LD FA/LD PM2.5 and light conditions as LL FA/LL PM2.5; (B) heat maps of normalized sequence tags mapped with respect to transcription start site (TSS) (±1 kb region) of all annotated protein coding gene conditions as mentioned in (A); (C) composite heatmap of top 10% of all oscillatory gene TSS in conditions mentioned in graph with respect to TSS (±1 kb region).
(D–G) (D and E) The distribution of differentially accessible regions (DARs) in different genomic coordinates: dark magenta indicates depleted DARs, whereas light magenta indicates enriched DARs, (F) heatmaps similar to those depicted in (B), but with respect to circadian enhancers, (G) composite map similar to (C), but with respect to top 10% of enhancer midpoints as in the conditions mentioned in axis.
(H–K) (H) V plot mapping the distance from motif mid-point (E-Box) to indicate openness of chromatin structure that is represented by color density of V-line, (I) mRNA expression of HDACs 1–4 in LD and LL groups upon PM2.5 exposures, (J) mRNA expression of DNMT1 and DNMT3a in LD and LL upon PM2.5 exposures, and (K) Chip-qPCR analysis showing binding of circadian genes Per1 and Per2 with p300, PCAF, Pol II.
Chromatin Dynamics in Circadian Genes in Response to PM2.5 Is Facilitated by Repressing HDACs
Given that PM2.5 effected chromatin dynamics of enhancer elements, we evaluated Bmal1 binding upon PM2.5 and light exposure, given its central role in circadian control and its role as a transcription factor reported to bind to the E-Box elements of multiple genes including other circadian targets (Shostak and Brunner, 2019). Figure 5H depicts that the E-Box spanning motif (±500 bp) in response to PM2.5 exposure is enriched with E-box binding proteins, indicating occupancy of E-box binding proteins, mainly Bmal1/Clock (Figure 5H). We investigated the mechanism of altered chromatin dynamics in response to PM2.5 exposure and investigated the role of histone acetyl transferases (HATs) and histone deacetylases (HDACs), hypothesizing a reduction in HDACs, or increase in HATs leading to “openness” of chromatin in circadian gene promoters and enhancers. Figure 5I shows a differential regulation of HDACs 2, 3, and 4 transcripts with PM2.5 exposures. Combination with light exposure had no additional effect on HDACs. Next, we examined enzymes involved in DNA methylation, such as DNA methyltransferase 1 (DNMT1) and 3 alpha (DNMT 3a). Neither PM2.5 nor LL made any significant changes on DNMT (1 and 3a) expression (Figure 5J). To identify the HAT responsible for PM2.5-specific acetylation of chromatin as a potential mechanism, we performed ChIP-qPCR for p300, pCAF, and polymerase II (Pol II) and probed for their presence in two circadian targets (Per 1 and 2), which were upregulated upon PM2.5 exposure with resultant increase in openness of Per1 and Per2. We found that the HAT predominantly responsible for acetylating chromatin upon PM2.5 exposure is p300 and that these genes were transcriptionally active, indicated by the presence of Pol II.
Discussion
This study provides evidence that chronic ambient air pollution causes disruptions in CR that result in metabolic abnormalities. Specifically, PM2.5 exposure induces hyperinsulinemia and BAT dysfunction and results in altered metabolism (including impaired O2 consumption and energy expenditure). These phenotypic changes were associated with reprogramming of pathways involved in inflammation, lipid oxidation, and gluconeogenesis, all without changes in body weight. CR disruption was evidenced by marked changes in the rhythmic synthesis of daily corticosteroids, along with changes in amplitude and desynchronization of Bmal1, Clock, Per1, Per2, Cry1, and Cry2 mRNA oscillations in the liver and BAT. Although there were phenotypic similarities between LL and PM2.5 exposures, there were also distinct transcriptional and epigenomic differences. Importantly, the effect of light on the transcriptome represented only half of the DEGs when compared with PM2.5 or co-exposure to both PM2.5 and light at night. Although the magnitude, directionality, and tissue-specific changes were distinct (liver and BAT) for PM2.5 and light, a degree of correlation was also noted, suggesting possible similarities between the two exposures in causing CR disruption.
The relationship between CR disruption and metabolic abnormalities (like glucose intolerance, hyperglycemia, and hyperinsulinemia) are well documented (Bedrosian et al., 2016; Gooley, 2016; Krishnaiah et al., 2017; Stenvers et al., 2019). Indeed, CR alterations in metabolism may represent a common denominator for the development of a wide variety of NCDs including cancer and metabolic and cardiovascular disease (Bass and Lazar, 2016; Crnko et al., 2019; Hernandez-Rosas et al., 2020). Recent studies demonstrate that as much as 50% of mouse liver metabolites are under circadian control (Cho et al., 2012; Krishnaiah et al., 2017). Disruption of CR genes has been noted to cause widespread alterations in acetylation and methylation, and conversely, alterations in acetylation and methylation patterns profoundly affect metabolic homeostasis (Kupers et al., 2019; Lombardi et al., 2019; Xia et al., 2015). DNA methylation or phosphorylation of epigenetic modifier proteins has been shown to accompany metabolic stress in mice and humans (Fiedler and Shaw, 2018; Kupers et al., 2019; Liu et al., 2019; Ma et al., 2019). We utilized high-throughput RNA-seq combined with ATAC-seq correlation analysis to interpret aberrantly expressed circadian and metabolic targets in PM2.5 exposed mice. PM2.5 produced a distinct transcriptional signature compared with LL exposure. There did not appear to be any additive or synergistic effects of LL in addition to PM2.5. The expression studies using timed qPCR analysis in liver also demonstrated similarities with the unbiased RNA-seq data in that Per2 mesor and amplitude increased significantly but mesor and amplitudes of all the other core clock genes (Bmal1, Clock, Per1, and Cry1) were decreased significantly. Expression of Bmal1 and Cry2 was phase advanced in PM2.5-exposed mice. A striking feature of RNA-seq expression data in liver in response to PM2.5 was the downregulation of core components of the Bmal1/Npas2 pathways and the upregulation of the negative feedback regulators: Per1, Per2, Cry1, and Cry2. Despite the observed similarities, the observed differences between differential gene expression profile and cycling analysis of individual core clock genes may be due to the timing differences (multiple time points from ZT0 to ZT24) of tissues harvested for timed PCR analysis, compared with one specific time (ZT02) for harvesting tissues for RNA-seq analysis. In addition, four of the thirteen genes associated with negative regulation of core clock genes, not measured during cycling analysis, were significantly upregulated by PM2.5 (Ciart, Id3, Bhlhe41, and Relb). In particular, Circadian Associated Repressor of Transcription (Ciart) or CHRONO (Computationally highlighted repressor of the network oscillator) transcript was highly upregulated in liver by PM2.5, with prior studies demonstrating that it represses the transcriptional activity of the Clock/Bmal1 heterodimer by abrogating the interaction of Clock/Bmal1 with the transcriptional coactivator Crebbp (Anafi et al., 2014; Annayev et al., 2014; Hatanaka and Takumi, 2017).
Circadian oscillations are subject to multiple layers of control. Casein kinase I proteins (CSNK1D and CSNK1E) and F box and leucine-rich repeat proteins like FBXL3 effect the nuclear accumulation and/or stability of clock components, respectively (Busino et al., 2007). Evidence also highlights the importance of epigenetic modification of histone residues (e.g., HDAC3, p300) and DNA methylation histone modifiers in modulating these feedback loops (Asher and Schibler, 2011; Busino et al., 2007). DNA acetylation and methylation are by far the most frequently evaluated epigenetic mechanism (Liu et al., 2015; Rider and Carlsten, 2019). A growing body of evidence implicates that long- and short-term exposures to ambient PM2.5 are associated with altered histone acetylation and DNA methylation of genes related to inflammation and cytokines, which results in perturbation of circulating cytokines, and fasting blood glucose (Bind et al., 2014; Chen et al., 2016; Li et al., 2018; Liu et al., 2015). Epigenetic programming in response to environmental exposure may be viewed as a critical and necessary buffer against adverse health response through widespread regulation of gene expression and chromosome integrity (Padmanabhan and Billaud, 2017). Chromatin remodeling may be particularly important with respect to environmental exposures and may help buffer and regulate gene expression (Padmanabhan and Billaud, 2017). Important changes in chromatin dynamics were noted with PM2.5, including changes in promoter and enhancer “openness.” E-Box spanning (±500 bp) motifs with PM2.5 were enriched in binding proteins, indicating occupancy of E-box binding proteins Bmal1/Clock. This was accompanied by differential downregulation of HDACs 2, 3, and 4 transcripts with PM2.5 exposure. To identify the HAT responsible for PM2.5 specific acetylation of chromatin, ChIP-qPCR for p300, pCAF and polymerase II in two circadian targets (Per 1 and 2) were performed. Our data demonstrate that p300 is the candidate HAT and Per1 and Per2 were transcriptionally active indicated by the presence of Polymerase II. Thus, increased acetylation could provide an explanation for the metabolic effects of PM2.5 exposure. Notably, in a small clinical study (in four individuals, two of whom were exposed to high and two to low PM2.5), peripheral blood monocytes among highly exposed individuals showed markedly altered global histone 3 lysine 27 acetylation (H3K27ac, known to be an activation histone modification marker) among highly exposed individuals (Liu et al., 2015). Among differentially modified H3K27ac loci, 1,080 loci were induced in the group with high PM2.5, whereas 158 loci were suppressed. The two subjects exposed to high PM2.5 tended to have higher number of peaks. H3K27ac peaks overlapped in promoter and enhancer regions, consistent with a role of H3K27ac as a promoter and enhancer marker. Both the TSS and enhancer peaks were higher in the individuals with high PM2.5 exposure compared with low-exposed individuals (Liu et al., 2015). CHRONO, which was highly upregulated in liver by PM2.5 in our studies, not only represses the transcriptional activity of the Clock/Bmal1 heterodimer but has also been shown to repress histone acetyltransferase (HAT) activity of the Clock/Bmal1 heterodimer, reducing histone acetylation of its target genes (Anafi et al., 2014; Annayev et al., 2014; Hatanaka and Takumi, 2017). The mammalian E3 ubiquitin ligase complexes have been shown to specifically regulate the stability of Per and Cry proteins. FBXL3, a component of the SKP1-CUL1-F-box-protein (SCF) E3 ubiquitin ligase complex, has been reported to target Cry1 for ubiquitination and an additional molecular component of the negative feedback loop that generates circadian rhythmicity (Busino et al., 2007; Siepka et al., 2007). Loss of FBXL3 activity can thus lead to Cry stabilization owing to decreased ubiquitination resulting in downregulation of Bmal1. In our study, Usp2, another E3 deubiquitinating ligase enzyme, was also highly upregulated in the liver by PM2.5. This may represent an additional explanation for the downregulation of the Bmal1 and Npas2 that we observed in our study.
Limitations of the Study
We recognize that our data have several important limitations that warrant further investigation including characterization of sexual dimorphism in response to air pollution. In a related study, we showed that females are resistant to PM2.5 metabolic effects suggesting that these mechanisms may indeed be relevant (Rajagopalan et al., 2020). Moreover, although food intake is an important synchronizing cue for the clocks of various tissues with essential metabolic roles in peripheral tissues, light is the dominant environmental cue for the suprachiasmatic nucleus master clock that synchronizes the phases of all the other molecular clocks in the body. In the absence of light (the primary zeitgeber), feeding is therefore an ineffective zeitgeber for the SCN. However, feeding has been shown to be a potent zeitgeber for the peripheral circadian rhythm regardless of opposing cues from the light-entrained SCN clock (Pickel and Sung, 2020). The primary goal of our study, therefore, was to explore the interaction between light and air pollution. Even though the mice were not completely restricted for food access during the resting phase (day time), during 6 h of the FA/PM2.5 exposure 5 days a week, the mice were unable to access food. However, it will be interesting to determine whether the effects of PM2.5 on the liver or BAT circadian rhythms would be abolished with restricted diet in the future. Furthermore, inclusion of methylation analysis of CR genes and their targets and creation of comprehensive epi-genomic maps for air pollution PM2.5 exposures are clearly warranted. Additionally, these findings should prompt further investigations into the importance of central versus peripheral CR disruption in mediating the effects of air pollution. In summary, we observed that PM2.5 induces hyperinsulinemia and BAT dysfunction and results in altered metabolism and alterations in circadian rhythm genes. These findings suggest a previously unrecognized role of PM2.5 in promoting CR disruption and metabolic dysfunction through epigenetic regulation of circadian targets. Well-designed human studies are needed to evaluate the clinical relevance of these pollution-induced alterations on circadian rhythms and health overall.
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Sanjay Rajagopalan (sxr647@case.edu).
Materials Availability
This study did not generate new unique reagents.
Data and Code Availability
The datasets/code (sequencing) generated during this study are available at NCBI Gene Expression Omnibus GEO; http://www.ncbi.nlm.nih.gov/geo/ under accession number GSE145566
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We are grateful to Dr. Chris A. Flask, Associate Professor, Department of Radiology, School of Medicine for determining body fat/water composition (MRI) and for conducting the FDG uptake (PET/CT) study and analysis. We thank Dr. Colleen Croniger, Associate Professor, Department of Nutrition School of Medicine for mice metabolic cage study and analysis. We thank Dr. Brendan Eck, Department of Biomedical Engineering for making 3D images for FDG uptake. We acknowledge Jenifer Mikulan, Institute Pathology for BAT histopathological study. We thank Dr. E Ricky Chan, Institute of Computational Biology, Case Western Reserve University for transcriptome and ATAC-seq analysis. This work was supported by the National Institute of Environmental Health Sciences (NIEHS) of the National Institutes of Health (NIH) under Award Numbers U01ES026721 (to S.B. and S.R.) and 5R01ES019616 and 1RO1ES015146 (to S.R.)
Author Contributions
S.Rajagopalan and S.B. conceptualized and initiated PM2.5-based exposure design, edited and approved, wrote, and finalized the final manuscript. J.A.D. designed the light at night exposure experiments, edited the manuscript. R.P. and V.V. performed most experiments. E.A.E.C. conducted the circadian gene expression study. R. Padmanabhan and B.P. conducted bioinformatics analysis. R.P., E.A.E.C., R.S.G., L.D., and G.B. conducted PM exposure and tissue collection. R.P. conducted phenotype assays including BAT histology, microscope imaging, and GTT/ITT. V.V. prepared sequencing libraries and performed OMNI ATAC-seq. J.C.D. performed statistical analysis for all experiments. R.P., V.V., and S.R. wrote a draft manuscript. S. Rajagopalan, S. Rao, M.J.K., Z.F., and A.T. contributed to revising and finalizing the manuscript.
Declaration of Interests
The authors declare no competing financial interests.
Published: November 20, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101728.
Supplemental Information
References
- Anafi R.C., Lee Y., Sato T.K., Venkataraman A., Ramanathan C., Kavakli I.H., Hughes M.E., Baggs J.E., Growe J., Liu A.C. Machine learning helps identify CHRONO as a circadian clock component. PLoS Biol. 2014;12:e1001840. doi: 10.1371/journal.pbio.1001840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders S., Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11:R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annayev Y., Adar S., Chiou Y.Y., Lieb J.D., Sancar A., Ye R. Gene model 129 (Gm129) encodes a novel transcriptional repressor that modulates circadian gene expression. J. Biol. Chem. 2014;289:5013–5024. doi: 10.1074/jbc.M113.534651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asher G., Schibler U. Crosstalk between components of circadian and metabolic cycles in mammals. Cell Metab. 2011;13:125–137. doi: 10.1016/j.cmet.2011.01.006. [DOI] [PubMed] [Google Scholar]
- Aubrecht T.G., Jenkins R., Nelson R.J. Dim light at night increases body mass of female mice. Chronobiol. Int. 2015;32:557–560. doi: 10.3109/07420528.2014.986682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass J., Lazar M.A. Circadian time signatures of fitness and disease. Science. 2016;354:994–999. doi: 10.1126/science.aah4965. [DOI] [PubMed] [Google Scholar]
- Bedrosian T.A., Fonken L.K., Nelson R.J. Endocrine effects of circadian disruption. Annu. Rev. Physiol. 2016;78:109–131. doi: 10.1146/annurev-physiol-021115-105102. [DOI] [PubMed] [Google Scholar]
- Bind M.A., Lepeule J., Zanobetti A., Gasparrini A., Baccarelli A., Coull B.A., Tarantini L., Vokonas P.S., Koutrakis P., Schwartz J. Air pollution and gene-specific methylation in the Normative Aging Study: association, effect modification, and mediation analysis. Epigenetics. 2014;9:448–458. doi: 10.4161/epi.27584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busino L., Bassermann F., Maiolica A., Lee C., Nolan P.M., Godinho S.I., Draetta G.F., Pagano M. SCFFbxl3 controls the oscillation of the circadian clock by directing the degradation of cryptochrome proteins. Science. 2007;316:900–904. doi: 10.1126/science.1141194. [DOI] [PubMed] [Google Scholar]
- Chen R., Meng X., Zhao A., Wang C., Yang C., Li H., Cai J., Zhao Z., Kan H. DNA hypomethylation and its mediation in the effects of fine particulate air pollution on cardiovascular biomarkers: a randomized crossover trial. Environ. Int. 2016;94:614–619. doi: 10.1016/j.envint.2016.06.026. [DOI] [PubMed] [Google Scholar]
- Cho H., Zhao X., Hatori M., Yu R.T., Barish G.D., Lam M.T., Chong L.W., DiTacchio L., Atkins A.R., Glass C.K. Regulation of circadian behaviour and metabolism by REV-ERB-alpha and REV-ERB-beta. Nature. 2012;485:123–127. doi: 10.1038/nature11048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corces M.R., Trevino A.E., Hamilton E.G., Greenside P.G., Sinnott-Armstrong N.A., Vesuna S., Satpathy A.T., Rubin A.J., Montine K.S., Wu B. An improved ATAC-seq protocol reduces background and enables interrogation of frozen tissues. Nat. Methods. 2017;14:959–962. doi: 10.1038/nmeth.4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crnko S., Du Pre B.C., Sluijter J.P.G., Van Laake L.W. Circadian rhythms and the molecular clock in cardiovascular biology and disease. Nat. Rev. Cardiol. 2019;16:437–447. doi: 10.1038/s41569-019-0167-4. [DOI] [PubMed] [Google Scholar]
- Fiedler E.C., Shaw R.J. AMPK regulates the epigenome through phosphorylation of TET2. Cell Metab. 2018;28:534–536. doi: 10.1016/j.cmet.2018.09.015. [DOI] [PubMed] [Google Scholar]
- Gooley J.J. Circadian regulation of lipid metabolism. Proc. Nutr. Soc. 2016;75:440–450. doi: 10.1017/S0029665116000288. [DOI] [PubMed] [Google Scholar]
- Hatanaka F., Takumi T. CHRONO integrates behavioral stress and epigenetic control of metabolism. Ann. Med. 2017;49:352–356. doi: 10.1080/07853890.2016.1276301. [DOI] [PubMed] [Google Scholar]
- Hayashi M., Shimba S., Tezuka M. Characterization of the molecular clock in mouse peritoneal macrophages. Biol. Pharm. Bull. 2007;30:621–626. doi: 10.1248/bpb.30.621. [DOI] [PubMed] [Google Scholar]
- Hernandez-Rosas F., Lopez-Rosas C.A., Saavedra-Velez M.V. Disruption of the molecular circadian clock and cancer: an epigenetic link. Biochem. Genet. 2020;58:189–209. doi: 10.1007/s10528-019-09938-w. [DOI] [PubMed] [Google Scholar]
- Koike N., Yoo S.H., Huang H.C., Kumar V., Lee C., Kim T.K., Takahashi J.S. Transcriptional architecture and chromatin landscape of the core circadian clock in mammals. Science. 2012;338:349–354. doi: 10.1126/science.1226339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnaiah S.Y., Wu G., Altman B.J., Growe J., Rhoades S.D., Coldren F., Venkataraman A., Olarerin-George A.O., Francey L.J., Mukherjee S. Clock regulation of metabolites reveals coupling between transcription and metabolism. Cell Metab. 2017;25:961–974 e964. doi: 10.1016/j.cmet.2017.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupers L.K., Monnereau C., Sharp G.C., Yousefi P., Salas L.A., Ghantous A., Page C.M., Reese S.E., Wilcox A.J., Czamara D. Meta-analysis of epigenome-wide association studies in neonates reveals widespread differential DNA methylation associated with birthweight. Nat. Commun. 2019;10:1893. doi: 10.1038/s41467-019-09671-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landrigan P.J., Fuller R., Acosta N.J.R., Adeyi O., Arnold R., Basu N.N., Balde A.B., Bertollini R., Bose-O'Reilly S., Boufford J.I. The Lancet Commission on pollution and health. Lancet. 2018;391:462–512. doi: 10.1016/S0140-6736(17)32345-0. [DOI] [PubMed] [Google Scholar]
- Li H., Chen R., Cai J., Cui X., Huang N., Kan H. Short-term exposure to fine particulate air pollution and genome-wide DNA methylation: a randomized, double-blind, crossover trial. Environ. Int. 2018;120:130–136. doi: 10.1016/j.envint.2018.07.041. [DOI] [PubMed] [Google Scholar]
- Li R., Wang Y., Chen R., Gu W., Zhang L., Gu J., Wang Z., Liu Y., Sun Q., Zhang K. Ambient fine particulate matter disrupts hepatic circadian oscillation and lipid metabolism in a mouse model. Environ. Pollut. 2020;262:114179. doi: 10.1016/j.envpol.2020.114179. [DOI] [PubMed] [Google Scholar]
- Liu C., Xu J., Chen Y., Guo X., Zheng Y., Wang Q., Chen Y., Ni Y., Zhu Y., Joyce B.T. Characterization of genome-wide H3K27ac profiles reveals a distinct PM2.5-associated histone modification signature. Environ. Health. 2015;14:65. doi: 10.1186/s12940-015-0052-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Carnero-Montoro E., van Dongen J., Lent S., Nedeljkovic I., Ligthart S., Tsai P.C., Martin T.C., Mandaviya P.R., Jansen R. An integrative cross-omics analysis of DNA methylation sites of glucose and insulin homeostasis. Nat. Commun. 2019;10:2581. doi: 10.1038/s41467-019-10487-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi A.A., Gibb A.A., Arif E., Kolmetzky D.W., Tomar D., Luongo T.S., Jadiya P., Murray E.K., Lorkiewicz P.K., Hajnoczky G. Mitochondrial calcium exchange links metabolism with the epigenome to control cellular differentiation. Nat. Commun. 2019;10:4509. doi: 10.1038/s41467-019-12103-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J., Nano J., Ding J., Zheng Y., Hennein R., Liu C., Speliotes E.K., Huan T., Song C., Mendelson M.M. A peripheral blood DNA methylation signature of hepatic fat reveals a potential causal pathway for Nonalcoholic fatty liver disease. Diabetes. 2019;68:1073–1083. doi: 10.2337/DB18-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munzel T., Sorensen M., Gori T., Schmidt F.P., Rao X., Brook F.R., Chen L.C., Brook R.D., Rajagopalan S. Environmental stressors and cardio-metabolic disease: part II-mechanistic insights. Eur. Heart J. 2017;38:557–564. doi: 10.1093/eurheartj/ehw294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munzel T., Sorensen M., Gori T., Schmidt F.P., Rao X., Brook J., Chen L.C., Brook R.D., Rajagopalan S. Environmental stressors and cardio-metabolic disease: part I-epidemiologic evidence supporting a role for noise and air pollution and effects of mitigation strategies. Eur. Heart J. 2017;38:550–556. doi: 10.1093/eurheartj/ehw269. [DOI] [PubMed] [Google Scholar]
- Nelson R.J., Chbeir S. Dark matters: effects of light at night on metabolism. Proc. Nutr. Soc. 2018;77:223–229. doi: 10.1017/S0029665118000198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan K., Billaud M. Desynchronization of circadian clocks in cancer: a metabolic and epigenetic connection. Front. Endocrinol. (Lausanne) 2017;8:136. doi: 10.3389/fendo.2017.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partch C.L., Green C.B., Takahashi J.S. Molecular architecture of the mammalian circadian clock. Trends Cell Biol. 2014;24:90–99. doi: 10.1016/j.tcb.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickel L., Sung H.K. Feeding rhythms and the circadian regulation of metabolism. Front. Nutr. 2020;7:39. doi: 10.3389/fnut.2020.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan S., Al-Kindi S.G., Brook R.D. Air pollution and cardiovascular disease: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2018;72:2054–2070. doi: 10.1016/j.jacc.2018.07.099. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S., Park B., Palanivel R., Vinayachandran V., Deiuliis J.A., Gangwar R.S., Das L.M., Yin J., Choi Y., Al-Kindi S. Metabolic effects of air pollution exposure and reversibility. J. Clin. Invest. 2020;130:6034–6040. doi: 10.1172/JCI137315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rider C.F., Carlsten C. Air pollution and DNA methylation: effects of exposure in humans. Clin. Epigenetics. 2019;11:131. doi: 10.1186/s13148-019-0713-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russart K.L.G., Nelson R.J. Light at night as an environmental endocrine disruptor. Physiol. Behav. 2018;190:82–89. doi: 10.1016/j.physbeh.2017.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shostak A., Brunner M. Help from my friends-cooperation of BMAL1 with noncircadian transcription factors. Genes Dev. 2019;33:255–257. doi: 10.1101/gad.324046.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siepka S.M., Yoo S.H., Park J., Song W., Kumar V., Hu Y., Lee C., Takahashi J.S. Circadian mutant Overtime reveals F-box protein FBXL3 regulation of cryptochrome and period gene expression. Cell. 2007;129:1011–1023. doi: 10.1016/j.cell.2007.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenvers D.J., Scheer F., Schrauwen P., la Fleur S.E., Kalsbeek A. Circadian clocks and insulin resistance. Nat. Rev. Endocrinol. 2019;15:75–89. doi: 10.1038/s41574-018-0122-1. [DOI] [PubMed] [Google Scholar]
- Sun Q., Yue P., Deiuliis J.A., Lumeng C.N., Kampfrath T., Mikolaj M.B., Cai Y., Ostrowski M.C., Lu B., Parthasarathy S. Ambient air pollution exaggerates adipose inflammation and insulin resistance in a mouse model of diet-induced obesity. Circulation. 2009;119:538–546. doi: 10.1161/CIRCULATIONAHA.108.799015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurman R.E., Rynes E., Humbert R., Vierstra J., Maurano M.T., Haugen E., Sheffield N.C., Stergachis A.B., Wang H., Vernot B. The accessible chromatin landscape of the human genome. Nature. 2012;489:75–82. doi: 10.1038/nature11232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Li R., Chen R., Gu W., Zhang L., Gu J., Wang Z., Liu Y., Sun Q., Zhang K. Ambient fine particulate matter exposure perturbed circadian rhythm and oscillations of lipid metabolism in adipose tissues. Chemosphere. 2020;251:126392. doi: 10.1016/j.chemosphere.2020.126392. [DOI] [PubMed] [Google Scholar]
- Xia L., Ma S., Zhang Y., Wang T., Zhou M., Wang Z., Zhang J. Daily variation in global and local DNA methylation in mouse livers. PLoS One. 2015;10:e0118101. doi: 10.1371/journal.pone.0118101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets/code (sequencing) generated during this study are available at NCBI Gene Expression Omnibus GEO; http://www.ncbi.nlm.nih.gov/geo/ under accession number GSE145566