Abstract
The effect of the functional groups of capping agents was investigated in the synthesis of copper selenide, copper sulphide and copper oxide nanoparticles using oleylamine (OLA) and trioctylphosphine (TOP). These capping molecules have demonstrated their ability to act as reducing agents, surfactants, solvents and enhancement of colloidal stabilization. They also offer electron donating abilities from the two group 5A elements, P and N. Nitrogen atom in an amine group possess stronger surface interactions and higher basicity than P atom in the phosphines. Copper chalcogenide nanoparticles were prepared using Hot-injection method and characterized using UV/Vis spectroscopy, TEM and XRD. The optical and structural properties of the yielded nanoparticles showed dependence on the type of capping interactions from the two agents. Nanoparticles synthesized using TOP produced two phases whereas a single phase was observed from OLA as confirmed by XRD. Although TOP and OLA exhibit similar features, but their affinity to metals differs resulting to significant different morphology and crystallinity of the produced nanoparticles. Amine group has higher affinity for protons than phosphine due to the lone pair of electrons it possesses which it easily donates to H+ compared to phosphine. The high proton affinity of oleylamine makes it interact faster than trioctylphosphine. OLA in overall produced larger particle sizes compared to TOP but generated a wider variety of shapes.
Keywords: Inorganic chemistry, Materials chemistry, Nanotechonology, Oleylamine, Trioctylphosphine, Copper oxide nanocrystals, Copper sulphide nanocrystals, Copper selenide nanocrystals, Capping molecule, Shape and size
Inorganic chemistry; Materials chemistry; Nanotechonology, Oleylamine; Trioctylphosphine; Copper oxide nanocrystals; Copper sulphide nanocrystals; Copper selenide nanocrystals; Capping molecule; Shape and size.
1. Introduction
Colloidal nanocrystals are nanometer-sized inorganic particles that are stabilized by a layer of surfactants that are attached to their surface [1]. Each colloidal nanocrystal is made up of few hundreds atoms and its growth can be easily controlled by varying synthetic parameters such as temperature, time of the reaction, concentration of precursors and capping molecule [2]. Their size range is usually from 2 to about 100 nm which allows their band gap to be tuned hence the Plasmon Resonance shifts [3]. When the material size is physically changed to be in the similar magnitude as the Bohr radii, quantum confinement effects are observed [4, 5] and the new electronic and optical properties [6, 7] emerging at the scale length that can be controlled by tuning the density of their electronic states [8]. During the synthesis of nanocrystals, the capping molecule is usually employed to provide passivation and electronic stabilisation to the nanocrystals as they form [9, 11]. It is therefore important to choose a good capping molecule that does not bond too strongly to the precursors as this would prevent particles from growing. The capping molecule should not have too weak bonds with the precursors either as the particles would grow bigger [9]. Due to this complexity, a general survey of capping agents in nano catalysis is therefore necessary [12]. It is necessary to understand the rate at which a capping molecule attaches and detaches to the surface as this influences the growth rate and the final structure of particles [3].
The capping molecules such as trioctylphosphine (TOP) and oleylamine (OLA) can be used as solvents, reducing agents and stabilizers [9, 11, 13, 14]. They provide good interaction and they can be used at higher temperatures due to their high boiling points [15]. Since OLA is liquid at room temperature, it simplifies the washing procedures that follow the chemical synthesis of nanocrystals [15]. Commercially, OLA has a much lower cost than commonly used pure alkylamines. Nonetheless, some concerns have been raised with regards to its purity and reproducibility [13]. Trioctylphosphine is a long chain alkyl phosphine (C24H51P) that uses its phosphine group (PR3) [16] for interaction whereas oleylamine is a long-chain primary alkyl amine that uses its amine group (NH2-) for interaction [15]. The increased proton affinity of phosphines is due to the stabilization of phenyl phosphonium ion by Л donation from phenyl group to the empty orbitals of phosphorus in the PR3 group, in contrast, the amines are rather stabilized by conjugation of nitrogen lone pair with the aromatic ring [9]. Generally, amine group has higher affinity for protons than phosphine due to the lone pair of electrons it possesses which it easily donates to H+ compared to phosphine. The high proton affinity of oleylamine makes it interact faster than trioctylphosphine. The comparison between the capping agents was done to probe the effect of the interaction between phosphine and the amine influences the particles in their properties (see Scheme 1).
Scheme 1.
Chemical structures of tri-n-octylphosphine (a) and oleylamine (b).
In this work, the synthesis of copper chalcogenides including copper selenide, copper sulphide, and copper oxide was explored through usage of trioctylphosphine and oleylamine as capping agents via colloidal hot injection method. Nano-sized semiconductors have been synthesized by a variety of methods for tuning the properties for specific applications. The methods that have been used for their synthesis includes precipitation methods [17], sol gel methods [18], Solid-Liquid discharge [19], electrochemical radiolysis alcohothermal [20], alcohothermal [21], direct thermal [22], sonochemical [23], microwave radiation [24], colloidal-thermal methods [25], Hot injection method [26] etc. Some of these methods are complicated and have drawbacks such as the drastic conditions, difficult control of particle growth, higher energy consumption etc. [25]. The colloidal hot injection method which was employed in this work has been reported to produce nanocrystals with a narrow size distribution, it enables you to control the conductive properties of the material by controlling the size of the crystals, it has got an advantage of separating nucleation and growth stages during the synthesis which leads to high mono dispersed material without having to do post synthesis size-selective technique [27].
Copper (I) chloride salt was reacted with sulphur powder, selenide powder and urea as a source of sulphur, selenide and oxygen respectively to produce copper selenide, copper sulphide and copper oxide nanocrystals. The three chalcogenides behave differently from one another even though they all from the chalcogen group depending on their ionisation potential, electronegativity and electron affinity. These properties tend to decrease with the increase of atomic size in the group (from O to S). These trends are useful in predicting the reactivity of the three towards other elements as their metallic character increases down the group. Oxygen has the smallest size as compared to sulphur and selenium atoms and shows distinct differences between it and the other elements in the group.
2. Materials and method
Oleylamine (OLA), trioctylphosphine (TOP), copper (I) chloride salt, selenium powder, sulphur powder, urea, methanol (99.5%), acetone (99.8%) were purchased Sigma Aldrich. In this study, synthesis of copper oxide, copper sulphide and copper selenide nanoparticles were approached by colloidal hot-injection method. About 5 ml of OLA was placed in a three-neck flask; equipped with a reflux condenser (waterless condenser), thermometer under a nitrogen atmosphere for both reactions. The content was heated to 120 °C under nitrogen gas while stirring using a magnetic stirrer bar. About 330 mg (0.0033 mol) of copper chloride was dispersed into 3 mL of OLA or TOP and injected into hot OLA through the syringe into the three-neck flask. The content was heated up to 220 °C, at which a solution (3 mL in OLA or TOP) of about 26.06 mg (0.0033 mol) of selenium (sulphur or urea) was added via a syringe. The content was heated at 220 °C for about 30 min, followed by cooling to 80 °C, then cleaned twice with ethanol and once with acetone via centrifugation at 5000 rev/min for 10 min. The precipitate was left to dry for 24 h at room temperature. The sample was dispersed into toluene, sonicated for 30 min and then characterised for optical properties using double beam Perkin Elmer lambda 25 UV/Vis spectroscopy with a wavelength range of 0–900 nm and emissions were measured using a single beam Jasco spectrofluorometer FP-8600 with XE lamp at 150W operated at 200–1010 nm (PL). The morphology of the particles was determined by drop casting the particles that were dissolved in toluene to a copper grids then images were taken using Transmission electron microscopy Technai G2 TEM Spirit operated at 200 kV. The solid sample was further characterized with X-Ray diffraction patterns using Bruker D2 Phase analyser, XRD Beam knife 3 mm (5 mm is all the way up), diffracted beam anti-scatter slit 6.6 mm. Transmission electron microscopy (TEM).
3. Results and discussion
3.1. The effect of capping agent on the copper selenide nanocrystals
-
(a)
Optical properties of copper selenide nanocrystals
The base groups found in trioctylphosphine and oleylamine provide surface interaction on nanoparticles that could influence size and shape of nanoparticles and stronger binding molecules tend to direct particle growth better and resulting in one predominant shape and sizes as compared to the lesser binding. This therefore influences particles to have a greater or lesser blue shift than the bulk and from each other. Due to high proton affinity of R3P than NH2 group, greater blue shift is expected than the bulk. To understand the size confinement of the synthesized nanocrystals, their optical properties were generally studied from the light absorption and emission. Such properties include absorption band edge, shift toward lower or higher wavelengths, size estimation and distribution and are compared to the bulk materials [9, 28]. The Ultraviolet Visible and fluorescence spectral data of the synthesized TOP/OLA capped copper selenide, copper sulphide and copper oxide nanocrystals are shown in Figure 1 and Table 1.
Figure 1.
(i) Absorption and (ii) emission spectra of copper selenide, copper sulphide and copper oxide nanocrystals synthesized using trioctylphosphine (TOP) and oleylamine (OLA) as capping agents.
Table 1.
Optical parameters of copper sulphide, copper selenide and copper oxide nanocrystals synthesized using TOP and OLA as capping agents.
| Np's | Capping |
|||||
|---|---|---|---|---|---|---|
| OLA |
TOP |
|||||
| Band edge (nm: eV) | Emission max (nm) | FWHM (nm) | Band edge (nm: eV) | Emission max (nm) | FWHM (nm) | |
| CuS | 580 (2.14) | 599 | 85 | 318 (3.90) | 339 | 45 |
| CuSe | 560 (2.21) | 415 | 51 | 315 (3.94) | 342 | 37 |
| CuO | 492 (2.49) | 436 | 82 | 528 (2.34) | 502 | 72 |
Both OLA and TOP-capped copper selenide, copper sulphide and copper oxide nanocrystals had a large blue shift in absorption compared to their bulk band gap (1180, 1022, and 1033 nm) respectively. This is an indication of a particle size reduction implying that relatively smaller size particles have been successfully synthesized. The absorption band edges of copper selenide and copper sulphide TOP-capped nanocrystals were observed around 315 nm and 318 nm, respectively. The largest blue shift was observed as compared to OLA-capped nanoparticles with absorption band edges around 560 and 580 nm.
The band edge values suggest that TOP-capped particles were smaller in comparison to those capped with OLA. This could be due to TOP being a stronger coordinating agent than OLA. The absorption curves of the two materials showed different line shapes predicting different optical properties and different crystalline phases. The absorption band edges of OLA and TOP-capped copper oxide nanocrystals were observed at 492 nm and 528 nm, respectively. The absorption band edge of OLA-capped material is blue shifted from that of TOP-capped material suggesting smaller particles for OLA-capped material. Both material produced identical absorption curve suggesting similar optical properties. This was not observed on CuSe or CuS nanocrystals. Similar optical properties of copper oxides nanocrystals might result from its high stability in thermodynamic reactions. The emission peaks of copper selenide and copper sulphide TOP-capped nanocrystals were observed around 342, 599 nm respectively and for OLA-capped nanocrystals were observed at 415 nm and 339 nm respectively. Both TOP and OLA spectra of copper selenide nanocrystals produced narrow emission peaks with a full width at half maximum (FWHM) of 37 nm and 51 nm respectively, suggesting monodispersed materials. This suggests that OLA-capped particles were less monodispersed than those from TOP-capped material. Copper sulphide nanocrystals that are TOP-capped produced a narrow emission peak with FWHM of 45 nm predicting a monodispersed particle distribution for material while its counterpart gave a broader emission peak with a corresponding FWHM of 85 nm suggesting a polydispersed distribution. This is due to TOP being a stronger coordinating agent than OLA which is a moderate coordinating agent.
The emission peaks of both copper selenide and copper sulphide TOP-capped material are red shifted to their absorption peaks whereas a blue shift is observed for their OLA-capped material. The blue shift could be due to the longer-wavelength Localized Surface Plasmon Resonance absorption of the OLA-capped material which is observed in near IR region resulting to an anti-Stokes shift. Rokovich et al. (2008) [29] deduced that the anti-Stokes shift photoluminescence (ASPL) occurs when the material emit light at shorter wavelength than which the material was illuminated because of thermal interactions with the excited atoms. Rokovich further elucidated that ASPL is often observed in semiconductor nanocrystals when the energy gap between the excitation energy and excited electronic level is comparable or larger than the maximum energy in the material hence more energy is released than what was initially absorbed. Xiong et al. 2017 [30] also reported on PbS quantum dots which produced an anti-Stokes shift photoluminescence spectrum and observed a gradual shift towards shorter wavelength when the excitation intensity was increased. This phenomenon was also observed when the excitation of TOP and OLA-capped material was increased (400–450 nm) and (300–350 nm), respectively. Their intensities were drastically reduced.
For copper oxide nanocrystals, the maximum emission intensity of TOP-capped material was found at 502 nm and was red shifted from that of OLA-capped material located at 436 nm. This is in agreement with the absorption spectra, predicting bigger particles for TOP capped particles. The emission peaks of both materials are blue shifted from their absorption bands. This phenomenon (ASPL) was also observed in copper selenide nanocrystals that are preveously discussed. TOP capped material produced a broader emisson peak compared to OLA-capped material with FWHM of 82 nm while OLA produced an emission peak with FWHM of 72 nm. The broadness of this emission peak can be attributed to a wider size distribution or polydispersed material. The narrower emission peak of the OLA-capped material suggests a well dsitributed size population.
-
(b)
Morphology of the synthesized nanocrystals
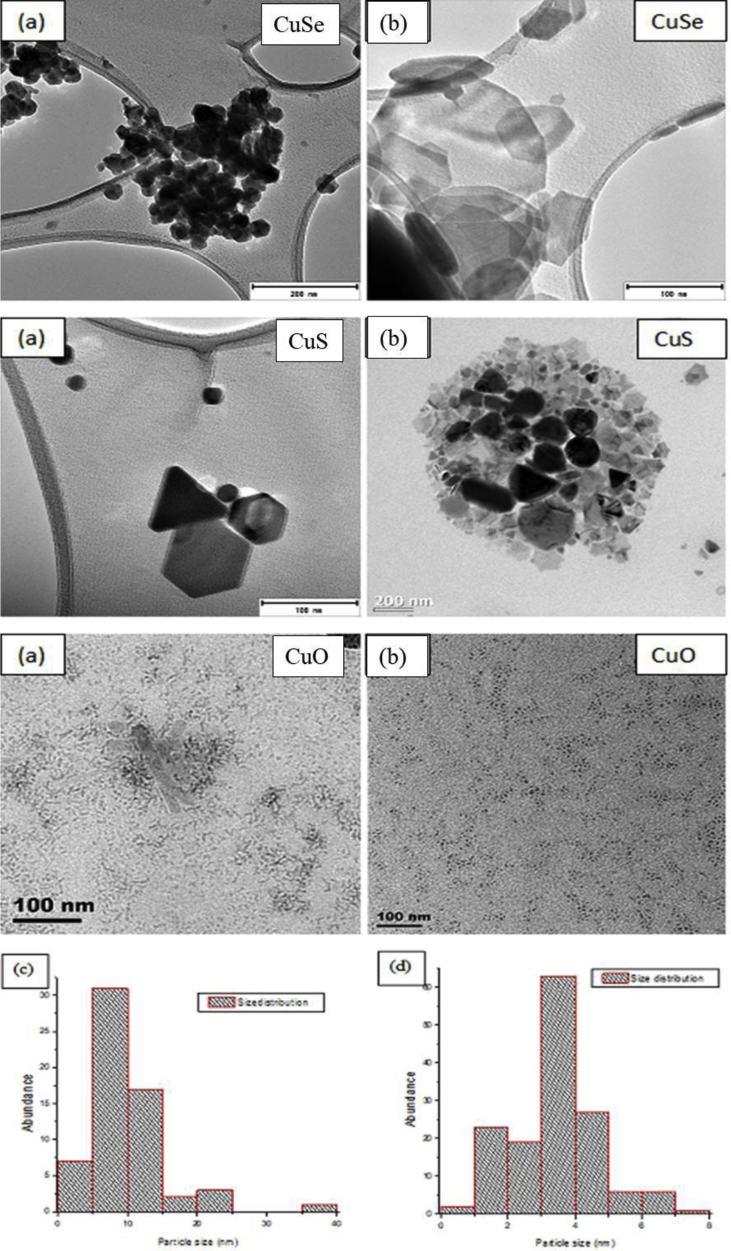
The TEM images of synthesized copper selenide, copper sulphide and copper oxide nanoparticles are shown on Figure 2. Due to high agglomeration and mixed morphology, the particle sizes of copper selenide and copper sulphide were not measured hence their corresponding size distribution graphs are not shown. Both copper selenide and copper sulphide nanocrystals produced mixed morphologies. Copper selenide TEM images revealed that OLA-capped nanomaterials have mixed morphologies including hexagonal, spheres and truncated triangles-like structures. They gave broad size range forming sheet like structures, whilst TOP-capped nanomaterials gave smaller nanoparticles that are highly agglomerated with undefined shapes. The particle size difference between TOP-and OLA-capped nanomaterials explained the large blue shift that was observed between the absorption curves of the two materials.
Figure 2.
TEM images of trioctylphosphine (a) and oleylamine (b) capped copper selenide, copper sulphide and copper oxide nanoparticles together with their size distribution (c) and (d). The XRD patterns (Figure 3) of copper selenide nanocrystals revealed some highly crystalline materials as depicted by the sharp peaks for both TOP and OLA capping agents. Both TOP and OLA capped copper selenide materials produced cubic Cu7.16Se4 phase which is indexed to PDF card no. 01-071-4325. However, an additional phase of tetragonal Cu3Se2 with PDF card no.04-003-6622 was associated to Umangite phase in TOP capped particles. Their crystal planes together with their 2Theta values are listed on Table 2 below respectively.
The agglomeration observed in TOP-capped nanomaterials might be due to high surface energy which resulted in increased forces of attraction between particles. The use of TOP as capping agent provided more steric hindrance compared to OLA and thus having smaller particles compared to the nanoplates produced by OLA. During the synthesis of OLA capped nanoparticles, the nuclei that were produced at the beginning of the reaction combined to form bigger particles through Ostwald ripening mechanism hence nanosheets were produced. TOP gave particles with size distribution ranging from 14 to 76 nm which indicates good population in comparison to OLA-capped nanoparticles in which much broader size range of 14–101 nm was observed. The broad size range observed in OLA-capped particles is inconsistent with its corresponding FWHM which predicted a less monodispersed population than TOP-capped particles. Copper sulphide TOP capped material produced smaller particles with size range from 11 nm to 74 nm whereas those capped with OLA produced a wide particle sizes with sizes ranging from 20 nm to 200 nm. The wide particle size distribution of OLA-capped material aligns with its optical characteristics which showed a broad emission peak and a larger FWHM of 85 nm predicting a polydispersed distribution. Unlike copper selenide nanocrystals, copper sulphide nanocrystals both materials produced mixed morphologies. TOP-capped nanomaterials produced cubes, hexagonals and triangles like structures, while OLA-capped material produced the three above mentioned structures together with rods, truncated triangles and more undefined structures. The wider variety of particle shapes for particles produced by OLA capped material is also justified by its broader emission peak. Wide range of different shapes was also observed on copper selenide particles capped with OLA. This wide variety of shapes that OLA-capped material produced could be due to the preferential binding facet of OLA. Mourdikoudis et al, (2013) [13] also reported the formation of irregular-shaped NiSe nanoparticles when OLA was used in the triple (solvent, reductant, surfactant) role, and more controlled morphology was observed after adding TOP in the system. The difference in morphology and crystallinity of the produced nanoparticles may result from their functional groups, where the lack of the C=C bond in TOP was suggested to limit its coordination with AuCl, thus yielding modified shapes [31]. The variety of morphology is also related to OLA weak bonding on the surface of nanoparticles resulting in various shapes. An agglomeration was observed on OLA-capped material while TOP-capped material showed a well distribution of particles. The agglomeration observed in OLA as a capping agent can be attributed to its longer chain bering NH2 group with less binding ability towards copper chalcogenide materials. OLA-capped material produced both small and big particles. This suggests that with parameter optimization, a variety of structures can be produced with this capping.
TEM images of the yielded copper oxide nanocrystals revealed that OLA-capped material are nearly spherical in shape with size average of 3 nm while TOP-capped material produced flakes like structures with average size of 25 nm. The smaller size of OLA-capped material is consistent with the corresponding optical properties which suggested smaller particles in comparison to TOP-capped nanocrystals. A bit of agglomeration of smaller particles is observed in TOP-capped material and this is consistent with the broadness of its corresponding emission peak. On the other hand OLA-capped material produced particles that are monodispersed and nicely persivised, and this is also in line to what was predicted by the optical properties of the correspondance. This is attributed to the ability of OLA as a good surfactant, solvent and reducing agent. The mixed morphology that was observed on copper selenide and copper sulphide OLA-capped nanocrystals was not observed on OLA-capped copper oxide nanocrystals rather small spheres like structures were observed. This could be due to the small size and high stability of oxides which resulted in sharp nucleation leading to uniform particles obtained.
The diffraction patterns of copper sulphide material that are capped with OLA and TOP are depicted in Figure 3. The diffraction patterns confirmed that copper sulphide nanocrystals were produced in both methods. Both TOP and OLA capped copper sulphide materials produced properties that were indexed to Cubic Cu1.75S phase which is indexed to PDF card no.04-006-0521. TOP capped material showed a second phase which was indexed to Roxbyite Cu58S32 (Anorthic) phase with PDF card no.00-064-0278.
Table 2.
XRD 2Theta positions and phase composition of copper sulphide, copper selenide and copper oxide nanocrystals synthesized using TOP and OLA as capping agents.
| Nanomaterial | Phase | 2Theta position (°) | Pdf card name |
|---|---|---|---|
| Copper selenide | Cu7.16Se4 (Major phase for TOP and OLA) | 27 | 01-071-4325 |
| 45 | |||
| 53 | |||
| 65 | |||
| 72 | |||
| 83 | |||
| 89 | |||
| Cu3Se2 (Minor phase for TOP) | 25 | 04-003-6622 | |
| 31 | |||
| 40 | |||
| 48 | |||
| 50 | |||
| 51 | |||
| 56 | |||
| Copper sulphide | Cu1.75S (Major phase for TOP and OLA) | 28 | 04-006-0521 |
| 32 | |||
| 47 | |||
| 55 | |||
| 68 | |||
| 74 | |||
| 86 | |||
| Cu58S32 (Minor phase for TOP) | 21 | 00-064-0278 | |
| 25 | |||
| 27 | |||
| 30 | |||
| 37 | |||
| 49 | |||
| 50 | |||
| Cu9S5 (impurities) | 26 | 00-047-1748 | |
| 30 | |||
| Copper oxide | Cu2O (TOP) | 36 | 77–0199 |
| 44 | |||
| 64 | |||
| 78 | |||
| CuO (OLA) | 36 | 48–1548 | |
| 42 | |||
| 47 | |||
| 54 | |||
| 56 | |||
| 64 | |||
| 78 |
Figure 3.
XRD patterns of OLA (a) color black and TOP (b) color red –capped copper sulphide, copper selenide and copper oxide nanoparticles. Indices with exponetial sympol (∗) denotes peaks for the second phase and the symbol (+) denotes impurities.
The TOP-capped material showed some impurities at 2θ values 34°, 36°, 62°, 65° and 71° which are denoted by +. These impurities could be due to unreacted starting materials. The mixture of phases for TOP-capped material was also observed in copper selenide material as well. Impurities of digenite Cu9S5 (Rhombohedral) phase indexed to PDF card no.00-047-1748 were also observed in OLA-capped material with crystalline planes that are obseved at 2θ values 26° and 30° corresponding to the indices [323] and [224], respectively denoted by the symbol ∗. The sharpeness of the diffractogram peaks in OLA capped material confirmed the bigger sizes of particles and good crystallity of copper sulphide nanocrystals than those of TOP capped material.
The XRD patterns of coppper oxide OLA and TOP-capped nanocrystals are displayed Figure 3 as well. The diffraction patterns confirmed the synthesis of copper oxide nanocrystals in both materials. The characteristic peaks of TOP-capped material are mostly found in Cubic phase Cu2O which was indexed to PDF card No. 77-0199. OLA-capped material showed major peaks at 2θ which can be indexed to Monoclinic phase CuO PDF card No. 48-1548. The sharpness of the peaks confirms that highly crystalline particles were prepared in both OLA and TOP-capped materials. Copper oxide nanocrystals that were capped with OLA produced a single phase whereas a mixture of phases was observed in copper selenide and copper sulphide nanocrystals under similar synthetic conditions. This is attributed to high thermodynamic stability of copper oxides nanocrystals which resulted in stronger bonds leading to single phases produced.
4. Conclusion
Copper chalcogenides (CuSe, CuS and CuO) nanomaterials were synthesized by colloidal hot injection method in both OLA and TOP as capping agents. The phosphorus atoms in TOP provided good stabilization during nanoparticles synthesis to produce monodispersed particles. This contrasts with the amine group on OLA which produced low yield of mixed shapes for CuSe and CuS nanocrystals due to its mild reducing ability. When combined with TOP better control of particles and shapes were obtained. Copper oxide nanoparticles displayed better optical and morphological properties using both capping molecules, OLA and TOP. Generally, TOP gave better controlled particles in size and shape due to stabilization and chemical interaction from the phosphorus atoms with nanoparticles during their synthesis of copper selenide and copper sulphide.
Declarations
Author contribution statement
Makwena Justice Moloto: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Nokhanyo Gloria Mbewana-Ntshanka: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Pierre Kalenga Mubiayi: Analyzed and interpreted the data.
Funding statement
This work was supported by the National Research Foundation (NRF) and the department of chemistry at the Vaal University of Technology.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Yin Y., Alivisatos P.A. Colloidal nanocrystal synthesis and the organic-inorganic interface. Nature. 2005;437:664–670. doi: 10.1038/nature04165. [DOI] [PubMed] [Google Scholar]
- 2.Chang J., Eric R., Waclawik E.R. Colloidal semiconductor nanocrystals: controlled synthesis and surface chemistry in organic media. ASC Adv. 2014;4:23505–23527. [Google Scholar]
- 3.Eustis S., El-Sayed M.A. Why gold nanoparticles are more precious than pretty gold: Noble metal surface plasmon resonance and its enhancement of the radiative and nonradiative properties of nanocrystals of different shape. Chem. Soc. Rev. 2006;35:209217. doi: 10.1039/b514191e. [DOI] [PubMed] [Google Scholar]
- 4.Gaponik N., Rogach A.L. Vol. 120. Springer; Wien, New York: 2008. pp. 73–100. (Semiconductor Nanocrystal Quantum Dots: Synthesis, Assembly, Spectroscopy and Applications). [Google Scholar]
- 5.Verma P., Pandey A.C. Controlled growth of CdS nanocrystals: Core/Shell viz Matrix. J. Biomaterials Nanobiotechnol. 2011;2:409–413. [Google Scholar]
- 6.Haug H., Koch S.W. Vol. 492. World Scientific; 1994. (Quantum Theory of the Optical and Electronic Properties of Semiconductors). [Google Scholar]
- 7.Ramalingam G., Kathirgamanathan P., Ravi G., Elangovan T., Kumar B.A., Manivannan N., Kasinathan K. Quantum confinement effect of 2D nanomaterials: fundamental and applications. Mater. Sci. 2020 [Google Scholar]
- 8.Edvinsson T. Optical quantum confinement and photocatalytic properties in two-, one- and zero-dimensional nanostructures. R. Soc. Open Sci. 2018;5:9. doi: 10.1098/rsos.180387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Onwadiwe D.C., Hrubaru M., Ebenso E.E. Synthesis, structural and optical properties of TOPO and HDA capped Cadmium sulphide nanocrystals, and the effect of capping ligand concentration. J. Nanomater. 2015;7:915. [Google Scholar]
- 11.Liu Y., Yao D., Shen L., Zhang H., Zhang X., Yang B. Alkylthiol-enabled Se powder dissolution in oleylamine at room temperature for the phosphine-free synthesis of copper-based quaternary selenide nanocrystals. J. Am. Chem. Soc. 2012;134:7207–7210. doi: 10.1021/ja300064t. [DOI] [PubMed] [Google Scholar]
- 12.Niu Z., Li Y. Removal and utilization of capping agents in Nanocatalysis. Chem. Mater. 2014;26:72–83. [Google Scholar]
- 13.Mourdikoudis S., Liz-Marzán L.M. Oleylamine in nanoparticle synthesis. Chem. Mater. 2013;25:1465–1476. [Google Scholar]
- 14.Sharma D., Kanchi S., Bisetty K. Biogenic synthesis of nanoparticles: a review. Arab. J. Chem. 2019;12:3576–3600. [Google Scholar]
- 15.Mourdkoudus S., Liz-Mrzan L.M. Oleylamine in nanoparticle synthesis. Chem. Mater. 2013;25:1465–1476. [Google Scholar]
- 16.Ishizaki T., Yatsugi K., Akedo K. Effect of particle size on the magnetic properties of Ni nanoparticles synthesized with trioctylphosphine as the capping agent. Nanomaterials (Basel) 2016;6:172. doi: 10.3390/nano6090172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suleiman M., Maousa M., Hussein A., Hammouti B., Hadda T.B., Warad I. Copper(II)-Oxide nanostructures: synthesis, Characterizations and their applications. J. Mater. Environ. Sci. 2013;4:792–797. [Google Scholar]
- 18.Zayat M., Levy D. Blue CoAl2O4 particles prepared by the Sol−Gel and Citrate−Gel methods. Chem. Mater. 2000;12:2763–2769. [Google Scholar]
- 19.Yao W., Yu S., Zhou Y., Jiang J., Wu Q., Zhang L. formation of uniform CuO nanorods by Spontaneous Aggregation: selective synthesis of CuO, Cu2O, and Cu nanoparticles by a Solid−Liquid phase Arc discharge process. J. Phys. Chem. B. 2005;109:14011–14016. doi: 10.1021/jp0517605. [DOI] [PubMed] [Google Scholar]
- 20.Son D., You C., Ki T. Structural, optical, and electronic properties of colloidal CuO nanoparticles formed by using a colloid-thermal synthesis process. Appl. Surf. Sci. 2009;255:8794–8797. [Google Scholar]
- 21.Lim W.P., Wong C.T., An S.I., Low H.Y., Chin W.S. Phase-selective synthesis of copper sulfide nanocrystals. Chem. Mater. 2006;18:6170–6177. [Google Scholar]
- 22.Chamsa-Ard W., Brundavanam S., Fung C.C., Fawcett D., Poinern G. Nanofluid types, their synthesis, properties and incorporation in direct solar thermal Collectors: A Review. Nanomaterials (Basel) 2017;7:131. doi: 10.3390/nano7060131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Narongdet W., Piyanut C., Naratip V., Wisanu P. Sonochemical synthesis and Characterization of copper oxide nanoparticles. Energy. Procedia. 2011;29:404–409. [Google Scholar]
- 24.Wang H., Zhu J.J., Zhu J.M., Chen H.Y. Sonochemical method for the preparation of bismuth sulfide nanorods. J. Phys. Chem. B. 2002;106:3848–3854. [Google Scholar]
- 25.Honary S., Barabadi H., Gharaeifathabad E., Naghibi F. Green synthesis of copper oxide nanoparticles using penicillium aurantiogriseum, penicillium Citrinum and Penicillium Waksmanii. Dig. J. Nanomater. Biostruct. 2012;7:999–1005. [Google Scholar]
- 26.Wang D., Li Y. Effective octadecylamine System for nanocrystal synthesis. Inorg. Chem. 2011;50:5196–5202. doi: 10.1021/ic200485v. [DOI] [PubMed] [Google Scholar]
- 27.Mahajan S., Rani M., Dubey R.B., Mahajan J. Synthesis of CdSe crystal using hot injection method. Int. J. Lat. Reas. Sci. Nanotechnol. 2013;1:518–521. [Google Scholar]
- 28.Gracia V.M., Nair P.K., Nair M.T.S. Copper selenide thin films by chemical bath deposition. J. Cryst. Growth. 1999;203:113–124. [Google Scholar]
- 29.Rokovich Y.P., Donegan J.F. Vol. 90. Springer-Verlag Wien; 2008. pp. 257–258. (Anti-Stokes Photoluminescence in Semiconductor Nanocrystal Quantum Dots). [Google Scholar]
- 30.Xiong Y., Liu C., Wang J., Han J., Zhao X. Near-infrared anti-Stokes photoluminescence of PbS QDs embedded in glasses. Optics. Epress. 2017;25:6874–6882. doi: 10.1364/OE.25.006874. [DOI] [PubMed] [Google Scholar]
- 31.Ma Y., Zeng J., Li W., Mckiernan M., Xe Z., Xia Y. Seed-mediated synthesis of truncated gold decahedrons with a AUCl/oleylamine complex as precursor. Adv. Mater. 2010;22:1930–1934. doi: 10.1002/adma.200903930. Mater. 2010, 22, 1930. [DOI] [PubMed] [Google Scholar]