Abstract
Objective
To evaluate whether intravenous (IV) golimumab produces improvements in skin and nail symptoms that are concomitant with improvements in quality of life (QoL) and joint symptoms in patients with psoriatic arthritis.
Methods
Patients were randomized to either IV golimumab 2 mg/kg at weeks 0, 4, then every 8 weeks (q8w) through week 52 or placebo at weeks 0, 4, then q8w, with crossover to IV golimumab 2 mg/kg at weeks 24, 28, and then q8w through week 52. Assessments included Psoriasis Area and Severity Index (PASI), modified Nail Psoriasis Severity Index (mNAPSI), Dermatology Life Quality Index (DLQI), and American College of Rheumatology (ACR) rheumatoid arthritis response criteria.
Results
Through week 24, achievement of PASI 75/90/100 responses (P ≤ .0098) and mean improvements in mNAPSI (−11.4 vs −3.7; P < .0001) and DLQI (−9.8 vs −2.9; P < .0001) were significantly greater with golimumab versus placebo. Responses were maintained in patients treated with golimumab through week 52. In placebo‐crossover patients, increases in the proportion of patients achieving PASI 75/90/100 responses were observed from weeks 24 to 52, and mean improvements in mNAPSI (from −3.7 to −12.9) and DLQI (from −2.9 to −7.8) increased from weeks 24 to 52. Simultaneous achievement of PASI and DLQI responses, PASI and ACR responses, and mNAPSI and DLQI responses were also observed. Similar responses were observed for all assessments regardless of concomitant methotrexate use.
Conclusion
Improvements in skin and nail psoriasis symptoms with IV golimumab in patients with psoriatic arthritis were concomitant with improvements in QoL and arthritis disease activity through 1 year.
Keywords: DLQI, intravenous golimumab, mNAPSI, psoriasis, psoriatic arthritis
Introduction
Psoriatic arthritis develops in up to 30% of patients with psoriasis (1), and in 75% to 85% of patients with psoriatic arthritis, wherein joint symptoms are preceded by skin lesions, with an approximate mean delay of 10 years (2, 3). In addition, approximately 80% of patients with psoriatic arthritis have active skin psoriasis (4), and up to 90% have nail involvement (2, 3). Both skin and nail psoriasis are associated with a high burden of illness and have a major impact on quality of life (QoL) (1, 2, 3). Skin psoriasis is associated with physical symptoms, including itching, scaling, and flaking (5, 6). In addition, the visibility of psoriasis can result in embarrassment, self‐consciousness, and depression (2, 5). Nail psoriasis can cause pain and difficulties in daily activities and can lead to anxiety and depression (3). Furthermore, nail psoriasis may be a predictor of joint disease, is often associated with worsening arthritis, and can be challenging to treat (3). Thus, skin and nail psoriasis are both important to consider when treating psoriatic arthritis.
The burden of skin and nail psoriasis in patients with psoriatic arthritis factors prominently in treatment guidelines and needs to be incorporated in the physician’s treatment decision‐making process for psoriatic arthritis (7, 8, 9). According to the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) treatment guidelines, psoriatic arthritis treatment should include assessment of all six domains of psoriatic arthritis, including skin and nail psoriasis (7). In addition, guidelines suggest that patients with psoriatic arthritis should be treated using a “treat‐to‐target” strategy, such as targeting minimal disease activity or very low disease activity (7, 8, 10, 11, 12, 13), both of which include a skin component as part of their criteria (ie, Psoriasis Area and Severity Index [PASI] ≤1) (14, 15).
GO‐VIBRANT was a Phase 3, multicenter, randomized, double‐blind, placebo‐controlled trial of intravenous (IV) golimumab, a fully human anti–tumor necrosis factor‐α agent, in adult patients with active psoriatic arthritis. The primary and major secondary end points of GO‐VIBRANT through week 24 (16) and week 52 (17) have been previously reported. The objectives of the analyses presented here were to evaluate the improvement of skin and nail symptoms through week 52 in patients with psoriatic arthritis treated with IV golimumab, with and without concomitant methotrexate, in the GO‐VIBRANT study and also to evaluate whether improvements in skin and nail symptoms were concomitant with improvements in Dermatology Life Quality Index (DLQI) scores and American College of Rheumatology 20% and 50% improvement in rheumatoid arthritis response criteria (ACR20 and ACR50, respectively).
PATIENTS AND METHODS
Patients
Included in this study were biologic‐naïve adults with active psoriatic arthritis, defined as five or more swollen and five or more tender joints, C‐reactive protein of 0.6 mg/dL or greater, and active or documented history of plaque psoriasis despite treatment with disease‐modifying antirheumatic drugs and/or nonsteroidal anti‐inflammatory drugs. Full inclusion/exclusion criteria are described elsewhere (16). All patients provided written consent.
Study design
The GO‐VIBRANT study design has been previously published (16). Briefly, patients were randomized 1:1 either to IV golimumab 2 mg/kg at weeks 0 and 4 and then every 8 weeks (q8w) through week 52 or to placebo (normal saline for IV infusion) at weeks 0 and 4 and then q8w, with crossover to IV golimumab 2 mg/kg at weeks 24 and 28 and then q8w through week 52. At week 16, all patients who qualified for early escape (<5% improvement in swollen and tender joint counts) were allowed to receive a protocol‐specified change in concomitant medications at the investigator’s discretion (16). The study protocol was approved by an independent ethics committee or institutional review board for each site, and the study was conducted in accordance with the principles of the Declaration of Helsinki that are consistent with Good Clinical Practices and local regulatory requirements.
Study assessments
In patients with 3% or more body surface area (BSA) psoriatic involvement at baseline, skin response was assessed using PASI (scores of 0‐72) (18), change from baseline in health‐related QoL relating to skin symptoms was assessed using DLQI (scores of 0‐30) (19) in patients with DLQI greater than 1 at baseline, and the activity of peripheral arthritis was assessed using ACR response criteria for improvement in rheumatoid arthritis (20). Simultaneous achievement of a PASI response (≥75%, ≥90%, or 100% improvement in PASI score from baseline [PASI 75/90/100]) and an improvement of five points or more in DLQI score (shown to be a clinically important improvement in DLQI in patients with a variety of dermatologic conditions, including psoriasis) (21), ACR20, or ACR50 was also assessed post hoc in these patients. Skin and nail response was assessed using modified Nail Psoriasis Severity Index (mNAPSI, 0‐130) (22) in patients with mNAPSI score greater than 0 at baseline. The minimal clinically important difference for mNAPSI is not known. For this study, we determined the proportion of patients who achieved 50%, 75%, or 100% improvement in mNAPSI score, assuming that a 50% or greater improvement in mNAPSI score would be clinically meaningful. Simultaneous achievement of 50%, 75%, or 100% improvement in mNAPSI score from baseline and an improvement of 5 points or more in DLQI score from baseline was also assessed post hoc in patients with 3% or more BSA psoriatic involvement, DLQI score greater than 1, and mNAPSI score greater than 0 at baseline.
Statistical analyses
All statistical tests for PASI assessments were performed at an α = .05 (two‐sided), and differences between treatment groups were tested using the Cochran‐Mantel‐Haenszel test for dichotomous end points and mixed‐effects model repeated‐measures methodology using observed data for continuous variables. Analysis of covariance (ANCOVA) was used to test differences in the changes from baseline in mNAPSI and DLQI scores between treatment groups. Because these analyses are post hoc, all statistics are descriptive, and all provided P values are nominal. No treatment comparisons were conducted beyond week 24 after placebo crossover because there was no control group after that time point. For continuous variables, missing data were imputed using last observation carried forward. For binary end points with completely missing components, nonresponder imputation was applied for missing data.
RESULTS
Patient disposition and disease characteristics
A total of 480 patients were randomized to golimumab (n = 241) or placebo (n = 239). Mean age was 46 years, and 52% of all patients were men. Demographic and disease characteristics were well balanced between treatment groups (16). At baseline, 394 patients (placebo, n = 198; golimumab, n = 196) had 3% or more BSA psoriatic involvement, and 367 patients had an mNAPSI score greater than 0 (mean 18.6; placebo, n = 170; golimumab, n = 197). Among patients with 3% or more BSA psoriatic involvement at baseline, mean PASI score was 9.9. Among patients with DLQI score greater than 1 and with 3% or more BSA psoriatic involvement at baseline (n = 283), mean DLQI score was 13.7.
PASI responses
The mean change from baseline in PASI in patients with 3% or more BSA psoriatic involvement at baseline was significantly greater (P < .001) in golimumab‐treated versus placebo‐treated patients at week 14 (−8.4 vs −1.0, respectively) and week 24 (−8.7 vs −1.3, respectively). At week 52, improvement was maintained in patients treated with golimumab (−9.1) and numerically increased in patients treated with placebo following crossover to golimumab at week 24 (−6.9). In addition, as previously reported, significantly greater proportions of patients treated with golimumab versus placebo achieved a PASI 75, PASI 90, or PASI 100 response at weeks 14 and 24 (Figure 1A) (16). In patients randomized to receive golimumab, PASI responses were maintained from weeks 24 to 52; PASI 75 response was 64.8% and 71.9% at weeks 24 and 52, respectively; PASI 90 was 42.9% and 56.1%, respectively; and PASI 100 was 25.5% and 28.6%, respectively (Figure 1A) (16, 17). Similar results were observed at all time points irrespective of baseline methotrexate use (Figure 1B and C).
Figure 1.
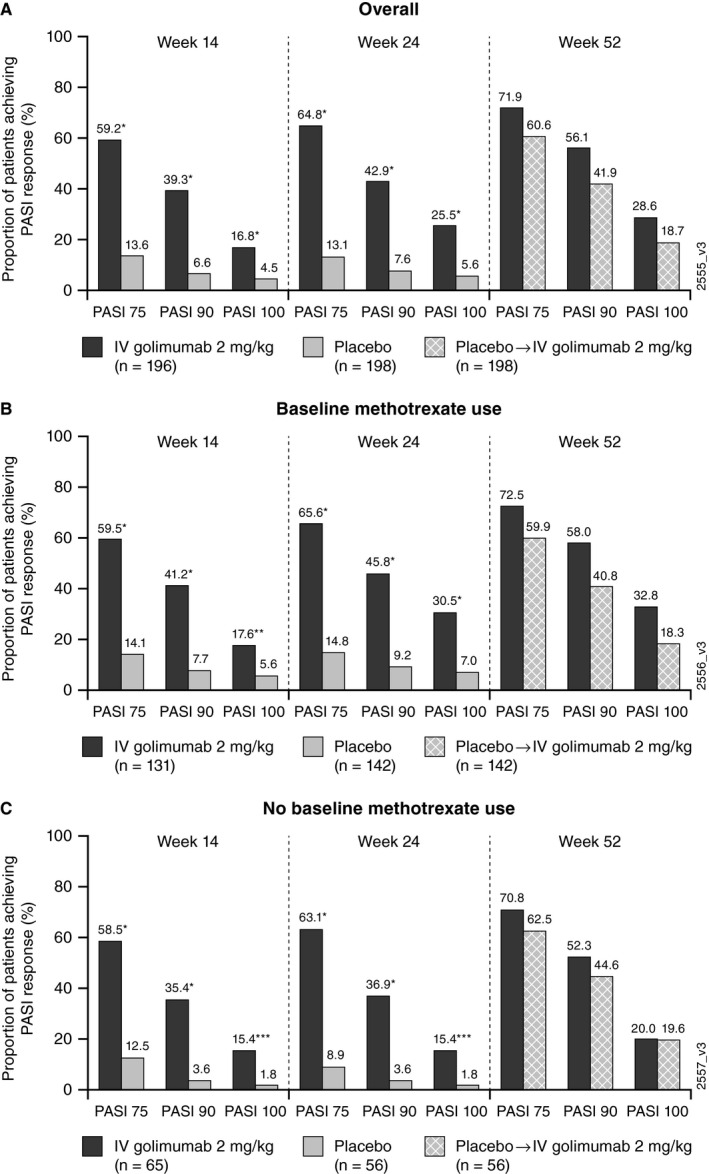
Proportions of patients with 3% or more body surface area of psoriatic involvement at baseline who achieved Psoriasis Area and Severity Index (PASI) 75, PASI 90, and PASI 100 responses overall (A) and in patients with (B) and without (C) baseline methotrexate use. P values are based on Cochran‐Mantel‐Haenszel test with baseline methotrexate use (yes/no) as a stratification variable for all patients, and a χ2 test by baseline methotrexate use (yes/no). *P < .0001, **P = .0020, ***P = .0098. IV, intravenous.
In patients who crossed over from placebo to golimumab at week 24, PASI responses increased numerically from weeks 24 to 52; PASI 75 response increased from 13.1% at week 24 to 60.6% at week 52; PASI 90 increased from 7.6% to 41.9%, respectively; and PASI 100 increased from 5.6% to 18.7%, respectively (Figure 1A) (16, 17). Similar results were observed in placebo‐crossover patients irrespective of baseline methotrexate use (Figure 1B and C).
mNAPSI response
In patients with mNAPSI score greater than 0 at baseline, the mean improvement from baseline in mNAPSI score was significantly greater in patients treated with golimumab versus those treated with placebo at week 14 (−9.6 vs −1.9, P < .0001) and Week 24 (−11.4 vs −3.7, P < .0001; as previously reported in Husni et al (17)) (Figure 2A). At week 52, mNAPSI response was maintained in patients randomized to receive golimumab (−11.4 at week 24 and −12.1 at week 52) and increased numerically (from −3.7 to −12.9) in patients who crossed over from placebo to golimumab at week 24 (17). Similar patterns of mNAPSI response in patients treated with golimumab and placebo were observed at each time point irrespective of baseline methotrexate use.
Figure 2.

Mean change from baseline (BL) in modified Nail Psoriasis Severity Index (mNAPSI) (A) and Dermatology Life Quality Index (DLQI) (B) scores overall and in patients with and without BL methotrexate use and simultaneous achievement (C) of clinically important improvement from BL in both mNAPSI (≥50%/≥75%/100%) and DLQI (≥5‐point improvement) scores. A, mNAPSI was assessed in all randomized patients with mNAPSI score greater than 0 at BL. P values are based on analysis of covariance (ANCOVA) with BL methotrexate use (yes/no) and mNAPSI score as covariates for all patients and only BL mNAPSI by methotrexate use (yes/no). *P < .0001, **P = .0006. B, DLQI was assessed in all randomized patients with 3% or more body surface area with psoriatic involvement at BL and DLQI score greater than 1 at BL. P values are based on ANCOVA with baseline methotrexate use (yes/no) as a covariate for all patients and on analysis of variance by methotrexate use (yes/no). *P < .0001. C, Assessed in all randomized patients with 3% or more body surface area with psoriatic involvement, mNAPSI score greater than 0, and DLQI score greater than 1 at BL. P values are based on Cochran‐Mantel‐Haenszel test controlling for baseline methotrexate use (yes/no) for all patients. *P ≤ .0002. IV, intravenous.
DLQI response
In patients with 3% or greater BSA psoriatic involvement and DLQI score greater than 1 at baseline, the mean improvement from baseline in DLQI score was significantly greater in patients treated with golimumab versus those treated with placebo at week 14 (−9.3 vs −3.0, P < .0001) and week 24 (−9.8 vs −2.9, P < .0001) (Figure 2B). At week 52, mean DLQI improvement was maintained in patients randomized to receive golimumab (−9.8 at week 24 and −9.5 at week 52) and was increased numerically in patients who crossed over from placebo to golimumab at week 24 (from −2.9 at week 24 to −7.8 at week 52). Similarly, significantly more patients treated with golimumab than those treated with placebo achieved an improvement of 5 points or greater in DLQI score at week 14 (75.3% vs 38.4%, P < .0001) and week 24 (80.0% vs 34.6%, P < .0001) (Supplemental Figure 1). At week 52, the proportion of patients who achieved an improvement of 5 points or greater in DLQI score was maintained in patients treated with golimumab (78.7%) and increased in patients who crossed over from placebo (63.9%). Similar patterns of significance were observed at each time point irrespective of baseline methotrexate use (Figure 2B and Supplemental Figure 1).
Simultaneous skin, nail, and joint responses
Compared with patients who were treated with placebo, significantly greater proportions of patients treated with golimumab with mNAPSI score greater than 0, DLQI score greater than 1, and 3% or more BSA psoriatic involvement at baseline achieved 50% or more, 75% or more, or 100% improvement in mNAPSI score from baseline and an improvement of 5 points or more in DLQI score from baseline at weeks 14 and 24 (P < .0001) (Figure 2C). At week 24, 57.9% versus 11.2%, 45.9% versus 5.6%, and 25.6% versus 5.6% of patients treated with golimumab versus those treated with placebo simultaneously achieved 50% or greater, 75% or greater, and 100% improvement in mNAPSI score, respectively, as well as an improvement of 5 points or more in DLQI score. At week 52, the proportions of patients in the placebo‐crossover group who achieved simultaneous improvements in mNAPSI and DLQI scores increased compared with week 24 and approached the proportions observed in the golimumab treatment group. Among patients randomized to golimumab, 35.3% had 100% improvement in mNAPSI score and an improvement of 5 points or more in DLQI score compared with 25.2% of patients in the placebo‐crossover group. Results were similar regardless of methotrexate use (Supplemental Figure 2A and B).
Compared with patients treated with placebo, significantly greater proportions of patients treated with golimumab achieved simultaneous PASI responses (PASI 75, PASI 90, or PASI 100) and an improvement of 5 points or more in DLQI score (Figure 3A), an ACR20 response (Figure 3B), or an ACR50 response (Supplemental Figure 3A) at weeks 14 and 24 (P ≤ .0012). These simultaneous responses were maintained through week 52 for all end points in patients randomized to golimumab and increased from week 24 to 52 in patients who crossed over from placebo to golimumab at week 24. Results were similar regardless of methotrexate use (Supplemental Figure 2C‐F and Supplemental Figure 3B and C).
Figure 3.

Proportions of patients who achieved a Psoriasis Area and Severity Index (PASI) 75/90/100 response and an improvement of 5 points or more in Dermatology Life Quality Index (DLQI) score (A) or an American College of Rheumatology 20% response criteria (ACR20) (B). A, Assessed in randomized patients with 3% or more body surface area with psoriatic involvement and DLQI score greater than 1 at baseline. B, Assessed in randomized patients with 3% or more body surface area with psoriatic involvement at baseline. For both panels, P values are based on Cochran‐Mantel‐Haenszel test controlling for baseline methotrexate use (yes/no) for all patients. *P < .0001. IV, intravenous.
PASI 90 and an improvement of 5 or more points in DLQI score were simultaneously achieved by 36.0% of patients treated with golimumab versus 4.5% of those treated with placebo (P < .0001) at week 14 and by 39.3% versus 5.3% of patients (P < .0001), respectively, at week 24 (Figure 3A). At week 52, 51.3% of patients randomized to golimumab and 34.6% of placebo‐crossover patients achieved PASI 90 and an improvement of 5 or more points in DLQI score. Results were similar in patients who did (Supplemental Figure 2C) and did not (Supplemental Figure 2D) have methotrexate use at baseline.
PASI 90 and ACR20 were simultaneously achieved by 33.2% of patients treated with golimumab versus 3.0% of patients treated with placebo (P < .0001) at week 14 and by 38.8% versus 4.5% of patients (P < .0001), respectively, at week 24 (Figure 3B). At week 52, 47.4% of patients randomized to golimumab and 37.4% of placebo‐crossover patients achieved PASI 90 and ACR20 responses. Results were similar in patients who did (Supplemental Figure 2E) and did not (Supplemental Figure 2F) have methotrexate use at baseline. A similar pattern of results was observed for PASI 90 and ACR50 overall (Supplemental Figure 3A) and in patients with (Supplemental Figure 3B) and without (Supplemental Figure 3C) baseline methotrexate use.
DISCUSSION
IV golimumab treatment demonstrated clinically meaningful improvement in skin and nail psoriasis, irrespective of concomitant methotrexate use. Significantly greater proportions of patients treated with golimumab than those treated with placebo achieved PASI 75, PASI 90, or PASI 100 responses at week 14, and these responses were maintained through week 52. Similarly, improvements in mNAPSI and DLQI scores were significantly greater in patients treated with golimumab versus those treated with placebo at week 14, and these improvements were maintained through week 52. In addition, responses were numerically improved from week 24 to week 52 for all assessments in patients treated with placebo who crossed over to golimumab at week 24. All results were consistent in patients who were or were not using methotrexate at baseline.
The simultaneous achievement of clinically important PASI and DLQI responses and mNAPSI and DLQI responses in relatively large proportions of patients treated with IV golimumab at weeks 14 through 52 suggests that there is an association between these assessments and DLQI. A correlation between DLQI and PASI has been previously established in patients with psoriasis alone and in patients with psoriatic arthritis (23, 24, 25, 26). Studies in both psoriasis and psoriatic arthritis have shown that improvements in PASI and DLQI from baseline following biologic therapy are correlated (demonstrated by correlation analysis) (24, 25, 26). A study by Cozzani and colleagues also demonstrated that PASI and DLQI scores in patients with psoriasis or psoriatic arthritis who were receiving unspecified treatment were correlated, as demonstrated by correlation and linear regression analyses (23). A similar correlation between mNAPSI and DLQI has not been established in patients with psoriasis or psoriatic arthritis; however, mNAPSI has been shown to correlate with the physical component summary score of the Medical Outcomes Study Short Form‐36 (27). It has also been established that nail psoriasis can negatively impact QoL (3, 28, 29). Our results suggest that improvements in skin and nail symptoms may result in corresponding improvements in health‐related QoL as measured by DLQI.
The simultaneous achievement of PASI 75/90/100 and ACR20 responses in significantly greater proportions of patients treated with golimumab versus those treated with placebo observed in this study suggests that IV golimumab is effective in simultaneously inducing and maintaining both skin and joint responses in patients with psoriatic arthritis. To our knowledge, a correlation between PASI and ACR similar to that between PASI and DLQI has not been demonstrated; however, concurrent achievement of PASI 75 and ACR20 has been used to evaluate efficacy of adalimumab (30) and infliximab (31) in patients with psoriatic arthritis, and concurrent achievement of these end points has been shown to be associated with improved health‐related QoL (31).
A limitation of this study is that there is no known minimal clinically important difference for mNAPSI. Although we evaluated a range of improvement for this assessment (50%, 75%, and 100%), the lack of a standard for clinically meaningful improvement limits the contextualization of the mNAPSI change from baseline data. Another limitation of this study is that the GO‐VIBRANT study was not designed or sufficiently powered to assess simultaneous achievement of measures (eg, mNAPSI and DLQI or PASI and ACR), so these informal assessments cannot be used to evaluate association or correlation of these measures. Formal correlation or regression analyses, which were outside the scope of this analysis, must be used to confirm whether improvement in measures of skin and nail psoriasis symptoms is related to improvement in measures of QoL and disease activity.
CONCLUSION
These results suggest that IV golimumab results in significant and sustained improvement in skin and nail psoriasis symptoms in patients with psoriatic arthritis, regardless of baseline methotrexate use. The improvements in skin and nail symptoms appear to be accompanied by improvements in QoL and joint symptoms. Treating all domains related to psoriatic arthritis may improve patient outcomes and its importance should be considered in all patients.
Author contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Mease had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Mease, Husni, Chakravarty, Kafka, Parenti, Kim, Lo, Hsia, Kavanaugh.
Acquisition of data
Lilianne Kim.
Analysis and interpretation of data
Mease, Husni, Chakravarty, Kafka, Parenti, Kim, Lo, Hsia, Kavanaugh.
Supporting information
Fig S1‐S3
Acknowledgments
Writing assistance was provided by Holly Capasso‐Harris of Synchrogenix, LLC, a Certara Company, on behalf of Janssen Research & Development, LLC. The authors would also like to acknowledge Diane D. Harrison, formerly of Janssen Research & Development, LLC, for her contributions to the GO‐VIBRANT study, and Stephen Xu, of Janssen Research & Development, LLC, for providing data analyses.
ClinicalTrials.gov identifier: NCT02181673.
This work was supported by Janssen Research & Development, LLC. Authors who were employees of the study sponsor participated in the study design and collection, analysis, and interpretation of the data. A medical writer employed by the study sponsor provided writing and editorial support. All authors reviewed and approved the manuscript for submission.
Dr. Mease has received research grants or served as a consultant or speaker for AbbVie Pharmaceuticals, Inc; Amgen, Inc; Bristol‐Myers Squibb; Boehringer Ingelheim; Celgene; Galapagos; Genentech; Gilead; Janssen Scientific Affairs, LLC; Eli Lilly and Company; Novartis; Pfizer, Inc; Sun; and UCB. Dr. Husni has served as a consultant for AbbVie Pharmaceuticals, Inc; Amgen, Inc; Bristol‐Meyers Squibb; Eli Lilly and Company; Gilead; Janssen Scientific Affairs, LLC; Novartis; Pfizer, Inc; Regeneron Pharmaceuticals; and UCB. Drs. Chakravarty, Kafka, and Parenti own stock in Johnson & Johnson, of which Janssen Scientific Affairs, LLC, is a wholly owned subsidiary. Drs. Kim, Lo, and Hsia own stock in Johnson & Johnson, of which Janssen Research & Development, LLC is a wholly owned subsidiary. Dr. Kavanaugh has served as a consultant for Janssen Scientific Affairs, LLC. No other disclosures relevant to this article were reported.
REFERENCES
- 1. Rosen CF, Mussani F, Chandran V, Eder L, Thavaneswaran A, Gladman DD. Patients with psoriatic arthritis have worse quality of life than those with psoriasis alone. Rheumatology (Oxford) 2012;51:571–6. [DOI] [PubMed] [Google Scholar]
- 2. Husni ME, Merola JF, Davin S. The psychosocial burden of psoriatic arthritis. Semin Arthritis Rheum 2017;47:351–60. [DOI] [PubMed] [Google Scholar]
- 3. Sobolewski P, Walecka I, Dopytalska K. Nail involvement in psoriatic arthritis. Reumatologia 2017;55:131–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Coates LC, Helliwell PS. Psoriatic arthritis: state of the art review. Clin Med (Lond) 2017;17:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kim WB, Jerome D, Yeung J. Diagnosis and management of psoriasis. Can Fam Physician 2017;63:278–85. [PMC free article] [PubMed] [Google Scholar]
- 6. Lebwohl MG, Kavanaugh A, Armstrong AW, Van Voorhees AS. US perspectives in the management of psoriasis and psoriatic arthritis: patient and physician results from the population‐based multinational assessment of psoriasis and psoriatic arthritis (MAPP) survey. Am J Clin Dermatol 2016;17:87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Coates LC, Kavanaugh A, Mease PJ, Soriano ER, Acosta‐Felquer ML, Armstrong AW, et al. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis 2015 treatment recommendations for psoriatic arthritis. Arthritis Rheumatol 2016;68:1060–71. [DOI] [PubMed] [Google Scholar]
- 8. Gossec L, Smolen JS, Ramiro S, de Wit M, Cutolo M, Dougados M, et al. European League Against Rheumatism (EULAR) recommendations for the management of psoriatic arthritis with pharmacological therapies: 2015 update. Ann Rheum Dis 2016;75:499–510. [DOI] [PubMed] [Google Scholar]
- 9. Husni ME, Fernandez A, Hauber B, Singh R, Posner J, Sutphin J, et al. Comparison of US patient, rheumatologist, and dermatologist perceptions of psoriatic disease symptoms: results from the DISCONNECT study. Arthritis Res Ther 2018;20:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gossec L, McGonagle D, Korotaeva T, Lubrano E, de Miguel E, Østergaard M, et al. Minimal disease activity as a treatment target in psoriatic arthritis: a review of the literature. J Rheumatol 2018;45:6–13. [DOI] [PubMed] [Google Scholar]
- 11. Kavanaugh A, Fransen J. Defining remission in psoriatic arthritis. Clin Exp Rheumatol 2006:24 Suppl 43:S‐83–S‐87. [PubMed] [Google Scholar]
- 12. Singh JA, Guyatt G, Ogdie A, Gladman DD, Deal C, Deodhar A, et al. 2018 American College of Rheumatology/National Psoriasis Foundation guideline for the treatment of psoriatic arthritis. Arthritis Rheumatol 2019;71:5–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Smolen JS, Schöls M, Braun J, Dougados M, FitzGerald O, Gladman DD, et al. Treating axial spondyloarthritis and peripheral spondyloarthritis, especially psoriatic arthritis, to target: 2017 update of recommendations by an international task force. Ann Rheum Dis 2018;77:3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Coates LC, Fransen J, Helliwell PS. Defining minimal disease activity in psoriatic arthritis: a proposed objective target for treatment. Ann Rheum Dis 2010;69:48–53. [DOI] [PubMed] [Google Scholar]
- 15. Coates LC, Helliwell PS. Defining low disease activity states in psoriatic arthritis using novel composite disease instruments. J Rheumatol 2016;43:371–5. [DOI] [PubMed] [Google Scholar]
- 16. Kavanaugh A, Husni ME, Harrison DD, Kim L, Lo KH, Leu JH, et al. Safety and efficacy of intravenous golimumab in patients with active psoriatic arthritis: results through week twenty‐four of the GO‐VIBRANT study. Arthritis Rheumatol 2017;69:2151–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Husni ME, Kavanaugh A, Murphy F, Rekalov D, Harrison DD, Kim L, et al. Efficacy and safety of intravenous golimumab through one year in patients with active psoriatic arthritis. Arthritis Care Res (Hoboken) 2020;72:806–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fredriksson T, Pettersson U. Severe psoriasis—oral therapy with a new retinoid. Dermatologica 1978;157:238–44. [DOI] [PubMed] [Google Scholar]
- 19. Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)—a simple practical measure for routine clinical use. Clin Exp Dermatol 1994;19:210–6. [DOI] [PubMed] [Google Scholar]
- 20. Felson DT, Anderson JJ, Boers M, Bombardier C, Furst D, Goldsmith C, et al. American College of Rheumatology preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum 1995;38:727–35. [DOI] [PubMed] [Google Scholar]
- 21. Basra MK, Salek MS, Camilleri L, Sturkey R, Finlay AY. Determining the minimal clinically important difference and responsiveness of the Dermatology Life Quality Index (DLQI): further data. Dermatology 2015;230:27–33. [DOI] [PubMed] [Google Scholar]
- 22. Cassell SE, Bieber JD, Rich P, Tutuncu ZN, Lee SJ, Kalunian KC, et al. The modified Nail Psoriasis Severity Index: validation of an instrument to assess psoriatic nail involvement in patients with psoriatic arthritis. J Rheumatol 2007;34:123–9. [PubMed] [Google Scholar]
- 23. Cozzani E, Linder D, Burlando M, Gallo F, Sampogna F, Bruzzone M, et al. PSOdisk is a reliable, intuitive instrument for the evaluation of psychological distress, which strongly correlates with DLQI: a preliminary study. Eur J Dermatol 2018;28:332–7. [DOI] [PubMed] [Google Scholar]
- 24. Hesselvig JH, Egeberg A, Loft ND, Zachariae C, Kofoed K, Skov L. Correlation between Dermatology Life Quality Index and Psoriasis Area and Severity Index in patients with psoriasis treated with ustekinumab. Acta Derm Venereol 2018;98:335–9. [DOI] [PubMed] [Google Scholar]
- 25. Mattei PL, Corey KC, Kimball AB. Psoriasis Area Severity Index (PASI) and the Dermatology Life Quality Index (DLQI): the correlation between disease severity and psychological burden in patients treated with biological therapies. J Eur Acad Dermatol Venereol 2014;28:333–7. [DOI] [PubMed] [Google Scholar]
- 26. Walsh JA, Arledge T, Nurminen T, Peterson L, Stark J. PGA×BSA: a measure of psoriasis severity tested in patients with active psoriatic arthritis and treated with certolizumab pegol. J Rheumatol 2018;45:922–8. [DOI] [PubMed] [Google Scholar]
- 27. Kavanaugh A, Catalan T, Cassell S. Patient assessments of skin, joint, and nail disease activity in psoriatic arthritis. J Rheumatol 2012;39:653. [DOI] [PubMed] [Google Scholar]
- 28. Baran R. The burden of nail psoriasis: an introduction. Dermatology 2010;221 Suppl 1:1–5. [DOI] [PubMed] [Google Scholar]
- 29. Schons KR, Knob CF, Murussi N, Beber AA, Neumaier W, Monticielo OA. Nail psoriasis: a review of the literature. An Bras Dermatol 2014;89:312–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mease PJ, Signorovitch J, Yu AP, Wu EQ, Gupta SR, Bao Y, et al. Impact of adalimumab on symptoms of psoriatic arthritis in patients with moderate to severe psoriasis: a pooled analysis of randomized clinical trials. Dermatology 2010;220:1–7. [DOI] [PubMed] [Google Scholar]
- 31. Kavanaugh A, Antoni C, Krueger GG, Yan S, Bala M, Dooley LT, et al. Infliximab improves health related quality of life and physical function in patients with psoriatic arthritis. Ann Rheum Dis 2006;65:471–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1‐S3


