Abstract
Objective
Diagnosis of systemic lupus erythematosus (SLE) made by standard diagnostic laboratory tests (SDLTs) has sensitivity and specificity of 83% and 76%, respectively. A multivariate assay panel (MAP) combining complement C4d activation products on erythrocytes and B cells with SDLTs yields a sensitivity and specificity of 80% and 86%, respectively, presumably enabling earlier SLE diagnosis at lower severity, with associated lower health care costs compared with SDLT diagnoses. We compared the payer budget impact of diagnosing SLE using MAP (incremental cost of $108) versus SDLTs.
Methods
We modeled a health plan of 1 million enrollees. SLE diagnosis among suspected patients was 9.2%. The MAP arm assumed 80%/20% of patients were tested with MAP/SDLTs, versus 100% tested with SDLTs in the SDLT arm. Prediagnosis direct costs were estimated from claims data, and postdiagnosis costs were obtained from the literature. Based on improved MAP performance, the assumed hazard ratio for diagnosis rate compared with SDLTs was 1.74 (71%, 87%, 90%, and 91% of patients who develop SLE are diagnosed in years 1 to 4 compared with 53%, 75%, 84%, and 88% of patients diagnosed with SDLTs).
Results
Total 4‐year pre‐ and postdiagnosis direct costs for patients with suspected SLE tested with MAP were $59 183 666 compared with $61 174 818 tested by SDLTs, with lower costs in the MAP arm due primarily to prediagnosis savings related to reduced hospital admissions.
Conclusion
Incorporating MAP into SLE diagnosis results in estimated 4‐year direct cost savings of $1 991 152 ($0.04 per member per month). By facilitating earlier diagnosis of SLE, MAP may enhance patient outcomes.
INTRODUCTION
Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by alternating periods of disease activity and remission (1). The incidence in the United States is approximately 5.5 to 11.7 per 100 000 person‐years (2, 3). These rates are potentially underestimated and biased toward more severe cases of SLE2. In a rheumatology clinic that sees a group of patients suspected of a rheumatic disease and confirmed to have a positive antinuclear antibody (ANA) and a negative anti–double‐stranded (ds) DNA, 9.2% developed SLE over approximately 10 years of follow‐up (4).
The diagnosis of SLE is complicated as it relies on interpretation of the relationship between widely varying clinical manifestations and laboratory findings (5). There are various limitations to using standard diagnostic laboratory tests (SDLTs) in SLE diagnosis; ANA tests have high diagnostic sensitivity but a high false‐positive rate among healthy individuals (6, 7). Furthermore, it has been suggested that because of variability in test kits, clinical trials using ANA assays should specify the kit used and its performance characteristics (8). Finally, antibodies to dsDNA and/or to Smith antigen have low sensitivity and are negative in many patients with SLE (9). It has been reported that earlier SLE diagnosis is associated with lower flare rates, reduced health care utilization, and lower costs in a commercially insured population (10). These findings, combined with the limitations of SDLTs, support the clinical need for improved SLE diagnostic tools.
Cell‐bound complement activation products (CB‐CAPs) are fragments formed upon complement activation that bind covalently to hematopoietic cells (5). The performance characteristics of CB‐CAPs used in SLE diagnosis have recently been validated in a prospective multicenter clinical validation study (11). A multivariate assay panel (MAP) that combines complement C4d activation products, C4d on erythrocytes and B cells, with antibodies to nuclear antigens, dsDNA IgG (with Crithidia confirmation), Smith, Sjogren's syndrome type‐B (SS‐B/La), topoisomerase I (Scl‐70), centromere protein B (CENP), histidyl t‐RNA synthetase (Jo‐1), and cyclic citrullinated peptites (CCP), has been developed to improve SLE diagnosis. It yields improved overall diagnostic performance with a sensitivity and specificity of 80% and 86%, respectively (11, 12), compared with a sensitivity and specificity of 83% and 76%, respectively, for SDLTs (ANA, antibodies to dsDNA, Smith, SS‐B/La, Scl‐70, CENP, Jo‐1, and CCP) (11). Despite the lower sensitivity, the superior specificity of MAP (86%) over SDLTs (76%) results in a higher positive predictive value associated with MAP (36.75%) compared with SDLTs (26.02%). Furthermore, MAP has a higher positive likelihood ratio compared with SDLT (5.7 vs 3.5) and a higher Youden index (0.66 vs 0.59), which is a combination of sensitivity and specificity that ranges from 0 (if the test reports the same proportion of positive tests in the control and diseased group) to 1 (indicating that there are neither false‐positives nor false‐negatives resulting from the test) (13).
This paper examines the potential budget impact to a US commercial health plan based on the presumed earlier, more accurate SLE diagnosis and improved clinical outcomes associated with MAP testing compared with SDLTs.
PATIENTS AND METHODS
This analysis was conducted in accordance with the principles of the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) task force on budget impact models (14), including, but not limited to, choice of time horizon, recommended static Excel spreadsheet design, and reporting format. All patient‐level data were de‐identified and thus this analysis is exempt from institutional board review and ethics committee approval. All patient‐level data were de‐identified and thus this analysis is exempt from institutional board review and ethics committee approval. Selection of clinical and cost parameters included in this model was based on analysis of longitudinal medical and pharmacy claims data, clinician input, and review of literature to estimate the cost of diagnosing and treating SLE. Base case estimates for clinical and cost parameters are included in Table 1.
Table 1.
Clinical and cost parameters used in MAP arm and SDLTs arm
| Parameter | MAP Arm | SDLTs Arm | Sources/Notes on Derivation |
|---|---|---|---|
| Clinical parameters | |||
| % with SLE among those with suspected SLE | 9.2% | 9.2% | Dijkstra et al (4) |
| % with RA, SS, or other CTD among those with suspected SLE | 58% | 58% | Dijkstra et al (4) |
| Ratio of RA to SS | 1.44 | 1.44 | Dijkstra et al (4); RA and SS represent CTDs with high and low postdiagnosis costs, respectively |
| Sensitivity | 80% | 83% | Putterman et al (11) |
| Specificity | 86% | 76% | Putterman et al (11) |
| Diagnosed with definitive CTD within year 1 | 71% | 53% | Hazard ratio of 1.74 for the assumed diagnosis rate applied a |
| Diagnosed with definitive CTD within year 2 | 87% | 75% | Hazard ratio of 1.74 for the assumed diagnosis rate applied a |
| Diagnosed with definitive CTD within year 3 | 90% | 84% | Hazard ratio of 1.74 for the assumed diagnosis rate applied a |
| Diagnosed with definitive CTD within year 4 | 91% | 88% | Hazard ratio of 1.74 for the assumed diagnosis rate applied a |
| Cost parameter | |||
| Incremental cost of MAP vs SDLTs | $108 | N/A | Medicare allowable CY 2017 |
| Average prediagnosis cost per patient with SLE/year | $24 593 | $24 593 | MarketScan Data Claims Analysis |
| Average prediagnosis cost per patient without SLE/year b | $17 495 | $17 495 | MarketScan Data Claims Analysis |
| Ratio of prediagnosis costs per patient without SLE: prediagnosis costs per patient with SLE | 0.7114 | 0.7114 | Costs for patient with newly diagnosed SLE without nephritis and matched control, Li et al (17) |
| Average per patient cost for mild SLE/year | $7021 | $7021 | Garris et al (19) |
| Average per patient cost for moderate SLE/year | $14 516 | $14 516 | Garris et al (19) |
| Average per patient cost for severe SLE/year | $47 252 | $47 252 | Garris et al (19) |
| Average per‐year postdiagnosis cost for true‐positive c , d | $18 123 | $19 578 | Garris et al (19) |
| Average true‐negative per year postdiagnosis cost d | $10 375 | $10 375 | Kawatkar et al (20) and McDonald et al (21). Assume true‐negative managed as a mix of RA/SS |
| Average false‐positive per‐year postdiagnosis cost d | $14 441 | $14 441 | Garris et al (19). Assume false‐positive managed like SLE, RA managed like mild/moderate/severe SLE, and SS managed like mild SLE |
| Average false‐negative per‐year postdiagnosis cost d | $14 516 | $14 516 | Garris et al (19). Assume false negative managed similarly to Moderate Disease severity |
Abbreviations: CTD, connective tissue disease; MAP, multivariate assay panel; RA, rheumatoid arthritis; SDLT, standard diagnostic laboratory test; SLE: systemic lupus erythematosus; SS, Sjogren’s syndrome.
The diagnosis rate was converted into a base case hazard function to estimate the percent diagnosed for each year in the model (Figure 1).
Prediagnosis costs for patients who are SLE negative are based on the factor of 0.7114 from Li et al (17).
Case severity mix for SDLTs and associated postdiagnosis costs in each severity (ie, mild, moderate, and severe) category were sourced from Garris et al (19). SDLTs: mild SLE: 26% at $7021; moderate SLE: 52% at $14 516; severe SLE: 22% at $47 252. MAP: Costs for all severity grades of SLE are equivalent to those in the SDLTs arm; however, weights are adjusted: mild SLE, 36%; moderate SLE, 45%; and severe SLE, 19%
False‐positives: individuals without SLE who were diagnosed with SLE; false‐negatives: individuals who were incorrectly diagnosed as not having SLE; true‐negative: individuals who were correctly diagnosed as not having SLE; true‐positive: individuals who were correctly diagnosed as having SLE
Clinical inputs
Comparison of MAP and SDLT performance
This model was developed to examine the economic impact of the presumed reduction in the time to SLE diagnosis using MAP versus SDLTs. This analysis assessed pre‐ and postdiagnosis costs for MAP and SDLTs over a 4‐year time horizon, shown yearly and as a 4‐year total. The hypothetical US health plan consisted of one million enrollees, of which 0.1% (1000 individuals) (Figure 1A, 2) are suspected of having SLE, of which 9.2% (92 individuals) are diagnosed with SLE (ie, 92 with SLE among 1 million total enrollees); of the 90.8% (908 individuals) patients with suspected SLE but are not diagnosed with SLE, 58% (527 individuals) are diagnosed with rheumatoid arthritis (RA) or Sjogren syndrome (SS) or another connective tissue disease (CTD) other than SLE over the 4‐year time horizon of the model (Table 1; Figure 1A) (4). The remaining 42% (381 of 908 individuals) of patients with suspected SLE remain undiagnosed with any CTD and continue to incur prediagnosis costs throughout the 4‐year time horizon of the model (Figure 1A). The 9.2% SLE diagnosis rate among those suspected of having SLE included in this model is near the upper limit of the SLE annual incidence range of 5.6 to 11.7 per 100 000 (2, 3). Refer to Figure 1B for calculations pertaining to calculation of 0.1% suspected SLE (A) and diagnosis rates in years 1‐4 for SDLTs and MAP (B).
Figure 1A.
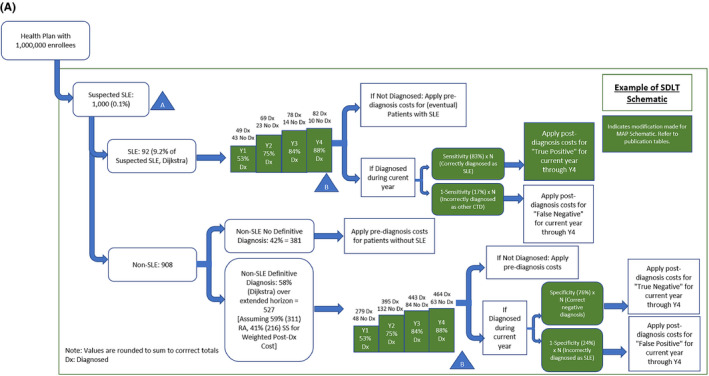
Schematic of commercial plan population subject to MAP vs SDLTs based on calculation of 0.1% suspected SLE (A) and diagnosis rates in years 1 to 4 for MAP and SDLTs (B). The model is for a hypothetical US health plan consisting of one million enrollees, of which 0.1% (1000individuals) are suspected of having SLE and 9.2% (92 individuals) of these are diagnosed with SLE. Refer to Figure 1B for calculations pertaining to (A) and (B).
Figure 2.
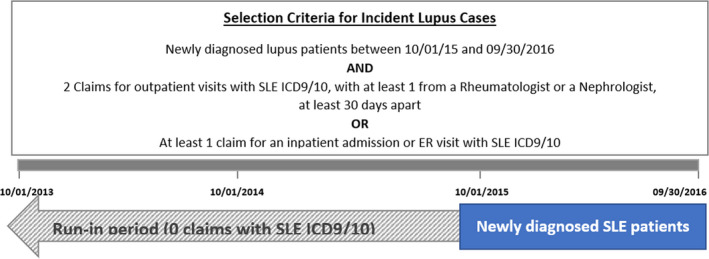
Selection criteria for incident lupus cases to assess prediagnosis costs via a claim analysis.
Figure 1B.
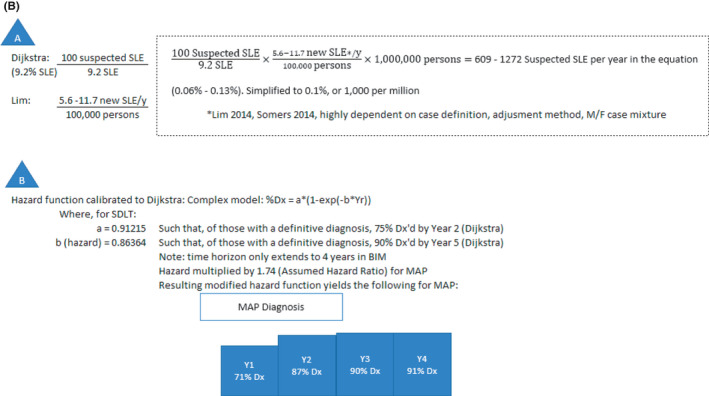
- Dijkstra et al (4) reports 9.2% SLE prevalence among those suspected of having SLE, Lim et al (2) and Somers (3) report 5.5 to 11.7 new cases of SLE per year per 100 000. The inverse of Dijkstra’s estimate was multiplied by Lim’s estimated values and applied to a commercial payer population of 1 000 000 enrollees, giving a range of 0.06% to 0.13% suspected SLE prevalence through a factor‐label calculation. This was simplified to 0.1% suspected SLE prevalence.
- Coefficients a and b of the hazard function–based model (% diagnosed = a × (1‐exp(‐b × year)) were estimated by calibrating to definitive diagnosis data from Dijkstra et al (4) (ie, 75% diagnosed by year 2 and 90% diagnosed by year 5). This yields simultaneous equations: 0.75 = a × (1‐exp(‐2b)) and 0.90 = a × (1‐exp(‐5b)), which, when solved using Excel Solver, yield a = 0.91215 and b = 0.86364.
Budget impact models for diagnostic tests incorporate test sensitivity and specificity for analysis purposes. We utilized a sensitivity and specificity of 83% and 76%, respectively, reported for SDLTs (ANA, antibodies to dsDNA, Smith, SS‐B/La, Scl‐70, CENP, Jo‐1, and CCP) (Table 1) (11). Based on these test performance characteristics, the proportion of the cohort of one million enrollees in the hypothetical US commercial health plan in this model that is in a prediagnosis or a postdiagnosis state varies over time. The proportion of individuals in the postdiagnosis state increases cumulatively over the time horizon of the model as more individuals are diagnosed with SLE and other CTDs. The diagnosis rate of CTDs and the corresponding proportion diagnosed with SLE using SDLTs is based on Dijkstra et al (4), where the diagnosis rate among all individuals eventually receiving a CTD diagnosis was 75% within 2 years and 90% within 5 years. The diagnosis rate was converted into a hazard function (Figure 1B) to estimate the percentage of diagnoses for each year in the model.
The improved overall test performance characteristics for MAP (Table 1) (11) are assumed to result in earlier diagnosis compared with SDLTs, as illustrated by Figure 1A and B. This model utilizes a base hazard ratio of 1.74 to modify the model’s SDLT hazard function to estimate the percent diagnosed for each year in MAP (71%, 87%, 90%, and 91% of the suspected SLE patients diagnosed with MAP in years 1 to 4 compared with 53%, 75%, 84%, and 88% with SDLTs) (Table 1; Figure 1B). The 1.74 hazard ratio was deemed to be a clinically relevant difference in time‐to‐diagnosis based on clinician and payer feedback, and we wanted to assess its impact on a commercial health plan. Thus, the model is developed to simulate the budget impact of clinically meaningful outcomes longitudinally over a 4‐year time horizon.
Prediagnosis costs were estimated from a commercial medical and pharmacy claims analysis that evaluated medical resource use during the 2‐year period preceding the initial SLE diagnosis. Postdiagnosis costs for SLE, RA, and SS were estimated from various published literature sources (Table 1). Average annual pre‐ and postdiagnosis costs were applied to the proportion of individuals in the pre‐ and postdiagnosis stage in each 1‐year time interval over the 4‐year time horizon of the model; this proportion differed between the MAP and SDLT arms.
We used a base case model assumption that in a health plan in the MAP arm, 80% of those suspected to have SLE will be tested with a one‐time MAP (in year 1), and the remaining 20% will be tested with SDLTs alone. This reflects the possibility that 20% would be readily diagnosed on clinical examination with SDLTs, thus not requiring MAP. The SDLT arm assumes that 100% of the suspected SLE population is tested with SDLTs alone.
Cost inputs
The cost year used in this model was 2017, and all costs from literature prior to 2017 were inflated by 3% per year to reflect standardized costs in 2017, consistent with ISPOR guidance and published Consumer Price Index for medical care (14).
Prediagnosis costs
As prediagnosis costs of SLE are not well documented in the literature, we derived estimates of these costs based on a longitudinal analysis of commercial medical and pharmacy claims data obtained from the MarketScan Commercial Claims Database (15). These data were analyzed to document costs preceding diagnosis in patients with newly diagnosed SLE between October 1, 2015, and September 30, 2016. For each newly diagnosed patient, continuous eligibility was confirmed by using a run‐in period of 24 months prior to their first claim associated with a SLE diagnosis. Diagnosis of SLE was confirmed via the following algorithm: at least two separate claims for outpatient visits with SLE ICD9/10, with at least one of them from a rheumatologist or a nephrologist, at least 30 days apart, or at least one claim for an inpatient admission or emergency room visit with SLE ICD9/10 (Figure 2) (16). Average annual prediagnosis payer costs per patient were estimated for the 2 years preceding SLE diagnosis by summing the total of each paid claim amount for each patient and calculating the per patient mean for inclusion in the model. Prediagnosis annual total average costs per patient with and without SLE were $24 593 and $17 495, respectively (Table 1).
The commercial claims analysis only accounted for prediagnosis costs of patients with SLE. To estimate the prediagnosis costs in patients suspected of having SLE but not diagnosed as such ($17 495) as a proportion of costs associated with SLE, first‐year postdiagnosis costs for patients with SLE and without nephritis ($13 014) and matched control patients without SLE ($9258) were used to calculate a ratio of 0.7114 (Table 1) (17). This ratio was applied to prediagnosis costs for patients with SLE obtained from the commercial claims analysis to estimate the prediagnosis cost associated with patients without SLE. The ratio is within the range of other published results, although we do note that published ranges can vary from 0.2217 to 0.8115 (16, 18).
Incremental cost of MAP compared with SDLTs
The model uses an incremental cost of $108 (Medicare Allowable CY 2017) for MAP compared with SDLTs (Table 1), which encompasses the additional cost of the CB‐CAPs component of MAP testing and represents a benchmark for commercial payers.
Postdiagnosis costs
Postdiagnosis costs for treating mild, moderate, and severe SLE were sourced from Garris et al (19) and calculated as a weighted average based on the distribution of disease severity at diagnosis. Garris et al (19) created algorithms to categorize patients by SLE severity by combining elements of disease activity with elements of cumulative damage and/or use of SLE medications. Costs from Garris et al (19) were applied to individuals correctly diagnosed with SLE, and categories of costs included: inpatient stays, emergency room visits, physician office and hospital outpatient office visits, outpatient lab procedures, outpatient pharmacy prescriptions, and other medical costs.
Among patients correctly diagnosed with SLE (ie, true‐positives), the use of MAP is presumed to result in earlier diagnosis of SLE with a resultant higher likelihood of a patient being in a less severe disease state compared with diagnosis using SDLTs alone. In Garris et al (19), where patients were diagnosed by SDLTs alone, the SLE case mixture was 26% mild, 52% moderate, and 22% severe, yielding an average weighted postdiagnosis cost of $19 578 (Table 1). For the MAP arm, where the improved MAP performance compared with SDLT performance is assumed to lead to earlier diagnosis, we used factors derived from Oglesby et al (10) to shift the severity mixture based on early versus late SLE diagnosis (Table 2). When applied, the Garris case mixture shifts to 36% mild, 45% moderate, and 19% severe, yielding an average weighted postdiagnosis cost of $18 123 (Table 1).
Table 2.
Calculation of shift in case mix distribution for early diagnosed SLE
| Disease status | ||||
|---|---|---|---|---|
| Mild | Moderate | Severe | Total | |
| Assume severity mix for SDLT from Garris (19) a | ||||
| No. | 789 | 1558 | 643 | 2990 |
| % | 26.39 | 52.11 | 21.51 | 100 |
| Use Oglesby (10) to calculate change in patients with early vs. late SLE diagnosis b | ||||
| Early SLE diagnosis | 2703 | 1161 | 302 | 4166 |
| Late SLE diagnosis | 2305 | 1465 | 396 | 4166 |
| No. of individuals displaced (early – late SLE) | 398 | −304 | −94 | NA |
| % of total (4166) displaced | 9.55 | −7.30 | −2.26 | NA |
| Shift Garris (19) based on displacement percentages c | ||||
| Calculation | 789 + (2990 × 9.55%) | 1,558 + (2990 × −7.30%) | 643 + (2990 × −2.26%) | 2990 |
| New total | 1074.65 | 1339.81 | 575.53 | 2990 |
| Severity mix for early diagnosis, % | 35.94 | 44.81 | 19.25 | 100.00 |
Abbreviations: SDLT, standard diagnostic laboratory test; SLE, systemic lupus erythematosus.
Displays the SLE severity mix from Garris (19), which is used for late‐diagnosed SLE in the model.
Case distribution mix for early‐ vs late‐diagnosed SLE from Oglesby (10). Using this table category, for each severity, the number displaced was calculated (early diagnosis – late diagnosis). The percent of total (4166) displaced for each severity was then determined.
This percentage of total displaced was then applied to the Garris (19) case mixture values to shift severity distribution. The resulting case mix is used in the model for early‐diagnosed SLE.
In patients without SLE but in which another CTD was correctly diagnosed (ie, true‐negatives), based on expert opinion, we made a simplifying assumption that 59% of cases would be diagnosed as having RA and 41% would have SS (4) (RA:SS ratio of 1.44 [Table 1;Figure 1A]). This was made on the basis that RA and SS represented 91% of specific non‐SLE CTD diagnoses (4), and the concern that robust cost estimates were not available for the other non‐SLE conditions identified (eg, CREST syndrome). Postdiagnosis costs for RA ($13 012) and SS ($783) were derived from Kawatkar et al (20) and McDonald et al (21), respectively, and adjusted to the analysis year. The resulting postdiagnosis cost of patients who were correctly diagnosed with another CTD was $10 375 per year (Table 1).
The costs of individuals without SLE who were diagnosed with SLE (ie, false‐positives) and those who were incorrectly diagnosed as not having SLE (ie, false‐negatives) were estimated based on cost data reported in Garris et al (19). Individuals without SLE who were diagnosed as having SLE would receive standard SLE treatment as opposed to an individual who was correctly diagnosed as not having SLE. In this cost calculation, individuals who truly had RA but were diagnosed as having SLE would accrue costs as if they had SLE; therefore, the weighted average of the cost associated with a correct diagnosis of mild/moderate/severe SLE was applied. Also, individuals who had SS but were diagnosed as having SLE would be treated as if they had mild SLE (19). This resulted in costs increasing from $10 375, which reflects the cost of those correctly diagnosed as not having SLE, to $14 441, which reflects the cost of those incorrectly diagnosed with SLE (Table 1). The $14 441 cost of individuals without SLE who were diagnosed as having SLE is less than the cost for those correctly diagnosed as having SLE by the SDLT and MAP arms ($19 578 and $18 123, respectively) (Table 1).
We also believe that patients with SLE who were incorrectly diagnosed as not having SLE would be undertreated and would incur lower health care–related costs compared with individuals correctly diagnosed as having SLE. The exact degree of cost reduction is not known; thus, we reduced the aggregate SLE cost of false‐negatives to the inflation‐adjusted cost that is reported in Garris et al (19) for moderate SLE ($14 516) (Table 1).
Sensitivity analysis
A one‐way sensitivity analysis (Figure 3) was performed to examine the impact of varying baseline assumptions in terms of change to the average 4‐year annual incremental savings per person tested with MAP compared with SDLTs. Parameters tested included: the specificity of the MAP, percentage of those receiving MAP in the MAP arm, cost of non‐SLE prediagnosis, percentage of individuals with SLE of those suspected of having SLE, percentage of those with RA, SS, or other CTD among those with suspected SLE, time to diagnosis hazard ratio, the cost of those correctly diagnosed with SLE for SDLTs, cost of those incorrectly diagnosed with SLE with MAP or SDLTs, cost of SLE prediagnosis, cost of those with incorrectly diagnosed SLE who have another CTD with MAP or SDLTs, incremental cost of MAP versus SDLTs, sensitivity of MAP, cost of those correctly diagnosed with SLE with MAP, and the cost of those without SLE where another CTD was correctly diagnosed with MAP or SDLTs. The change to incremental savings per unit increase of clinical variables and the change to incremental savings per $100 increase to cost variables were also examined (Figure 3B and C).
Figure 3.
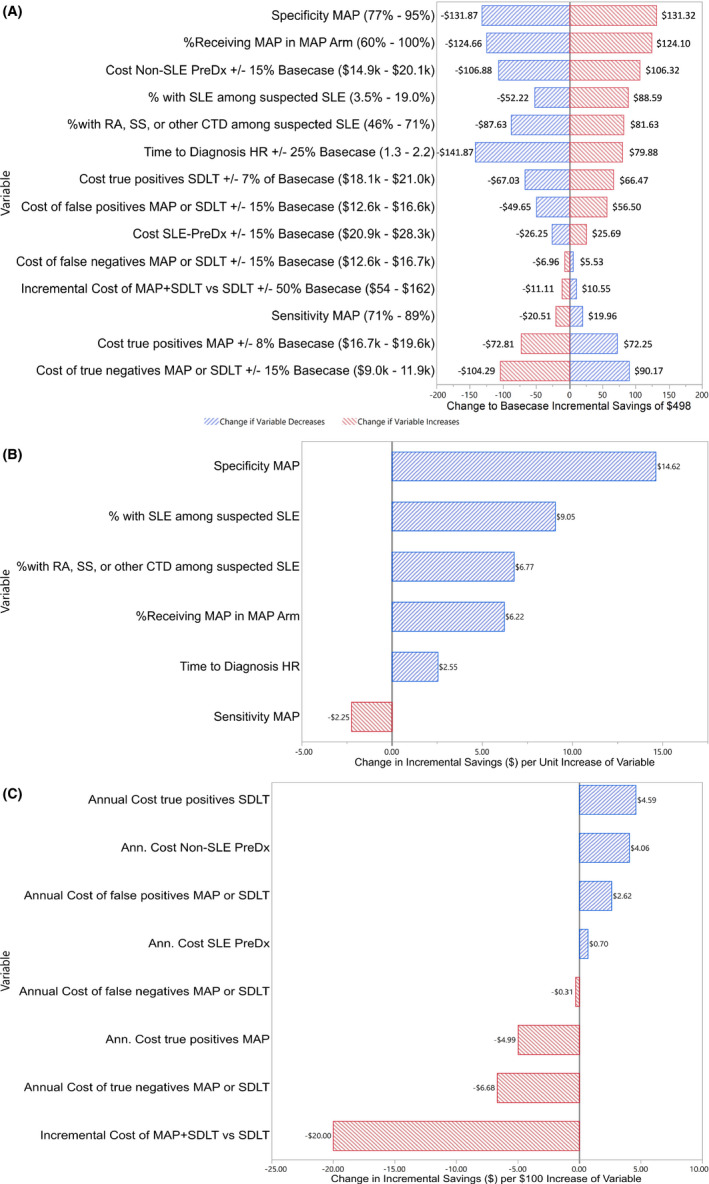
- Results are presented as a shift in the average annual cost savings per individual [Total Cost Savings/(1000 individuals suspected of SLE*4 years)] as a variable is increased/decreased to its upper/lower limit. Abbreviations: Ann, annual; Inc, incremental.
- The change to incremental savings per unit increase of variable was assessed via a one‐way sensitivity analysis. For example, the range in cost savings differences for MAP specificity was (131.32 + |−131.87|) = 263.19 (see Figure 3A). The range of specificity was (95 – 77) = 18 (Figure 3A). For each unit increase in specificity, cost savings increased by (263.19/18) = 14.62 (Figure 3B). The budget impact model is most sensitive to the specificity of the MAP test, with savings of $14.62 per 1% absolute increase in specificity.
- The change to incremental savings per $100 increase in cost was assessed in a one‐way sensitivity analysis. The budget impact model is most sensitive to the cost of the MAP test, with a reduction of cost savings of $20 per $100 increase in cost of the test. This figure can be used to evaluate any number of changes to cost assumptions. For example, if one alternately assumes that the prediagnosis cost with SLE is $23 593 ($1000 less than the base case value of $24 593) and assumes that the prediagnosis cost for non‐SLE is $11 796 ($5699 less than the base case value of $17 495), then the change to prediagnosis costs with SLE would change the average annual savings to −$1000 × $0.70/$100 increase = −$7.00, and the change to prediagnosis costs for non‐SLE would change the average annual savings to −$5699 × $4.06/$100 increase = −$231.38. Thus, the average annual cost savings of $498 would adjust from $498 − $238 = $260.
The lower and upper two‐sided 95% confidence intervals from the corresponding literature were used as plausible high‐low values for the percentage of those with SLE among those with suspected SLE and the percent with RA, SS, or other CTD among those with suspected SLE. The specificity and sensitivity of MAP were set to ±9% of the base case values, the proportion of patients suspected of having SLE who received MAP was set to ±20% of the base case value, the time to diagnosis hazard ratio was set to ±25% of the base case value, and the cost of the MAP was set to ±50% of the base case value. All other costs were subject to ±15% of the base case value in order to reflect plausible values, except for the cost of true‐positives with SDLTs ($19 578) and MAP ($18 123), which were subject to a ±7% and ±8%, respectively. In a best‐case scenario for the SDLT arm, the case mix would be no different than the case mix in the MAP arm, ie, the case mix would result in an SDLT cost of $18 123, which is a 7% decrease from the true‐positive SDLT cost of $19 578. Similarly, in a worst‐case scenario for the MAP arm, the case mix for MAP would be no different than in the SDLT arm, ie, the case mix would result in a MAP cost of $19 578, which is an 8% increase from the true‐positive MAP cost of $18 123.
RESULTS
Based on the analysis of claims data, in the 2‐year period preceding SLE diagnosis, most patients experienced outpatient office visits (99%), other outpatient procedures (99%), outpatient lab procedures (99%), outpatient pharmacy prescriptions (98%), and outpatient radiology procedures (91%) (Figure 4). In contrast, only 55% had emergency room visits and 33% had hospital admissions. Hospital inpatient visits were the largest cost contributor at $52 567 as the mean cost per patient hospitalized. The average number of hospitalizations in the 2 years prior to SLE diagnosis was 1.8 for patients who had any hospitalization; this was the lowest number of units used per patient across all categories analyzed. In contrast, outpatient lab procedures had the highest number of units used per patient in the 2‐year prediagnosis period (57.9) as well as the lowest cost at $2054 on average per patient.
Figure 4.
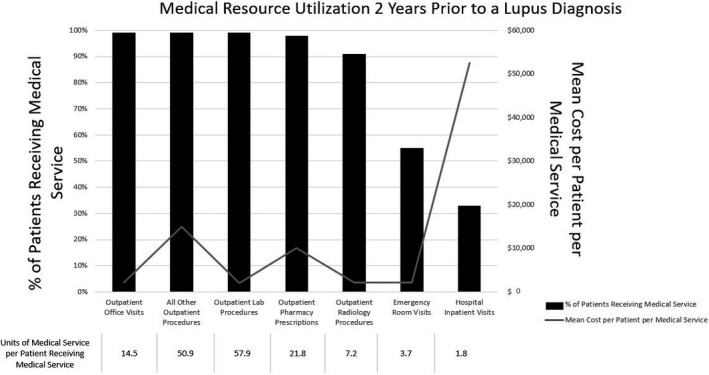
Medical resource usage 2 years prior to systemic lupus erythematosus diagnosis as per claims analysis. The mean cost per patient per medical service refers to among patients using the medical service. Prior to diagnosis, patients with lupus use numerous medical resources; the associated costs and units (number of visits/procedures/prescriptions) per patient were variable between service type (15).
Over the 4‐year time horizon, the total pre‐ and postdiagnosis direct medical costs to a payer for 1000 patients with suspected SLE who were tested with MAP were $59 183 666 versus $61 174 818 assessed by SDLTs, resulting in incremental savings of $1 991 152 (Table 3). Pre‐ and postdiagnosis direct medical costs savings with MAP testing range from $655 403 in year 1 to $326 267 in year 4 for 1000 patients suspected of having SLE. For a US commercial health plan covering one million enrollees, this translates to $0.04 in per member per month (PMPM) savings across years 1 to 4. The largest proportion of savings stem from year 1 in which MAP use versus SDLTs translates to $0.04 PMPM in savings.
Table 3.
Pre‐ and postdiagnosis cost savings, prediagnosis cost savings, and incremental savings between MAP and SDLTs
| Cost Year | |||||
|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 |
Total (years 1‐4) |
|
| Pre‐ and postdiagnosis direct costs a | |||||
| SDLTs | $16 144 023 | $15 298 148 | $14 941 507 | $14 791 139 | $61 174 818 |
| MAP | $15 488 620 | $14 709 163 | $14 521 010 | $14 464 872 | $59 183 666 |
| Cost savings between MAP and SDLT | $655 403 | $588 985 | $420 497 | $326 267 | $1 991 152 |
| Prediagnosis cost savings between MAP and SDLTs | |||||
| Hospital inpatient visits | $581 728 | $375 056 | $187 089 | $85 341 | $1 229 214 |
| Emergency room visits | $41 354 | $26 662 | $13 300 | $6067 | $87 382 |
| Outpatient office visits | $75 668 | $48 785 | $24 336 | $11 101 | $159 890 |
| Outpatient radiology procedures | $66 422 | $42 824 | $21 362 | $9744 | $140 353 |
| Outpatient lab procedures | −$17 787 | $44 397 | $22 146 | $10 102 | $58 858 |
| Outpatient pharmacy prescriptions | $336 716 | $217 090 | $108 291 | $49 397 | $711 493 |
| Other outpatient costs b | $502 990 | $324 292 | $161 766 | $73 790 | $1 062 839 |
| Incremental savings between MAP and SDLT | |||||
| Prediagnosis | $1 587 091 | $1 079 107 | $538 289 | $245 543 | $3 450 029 |
| Postdiagnosis | −$931 688 | −$490 122 | −$117 792 | $80 724 | −$1 458 877 |
Abbreviations: MAP, multivariate assay panel; SDLT, standard diagnostic laboratory test.
Due to the differing sources of pre‐ and postdiagnosis claims data, only prediagnosis costs could be segmented into categories of health services.
Top five other outpatient costs by average payment per patient include: dialysis, therapeutic radiology, specialty drugs other than chemotherapy, chemotherapy, and other major breast surgery.
Across the 4 years, the prediagnosis costs with MAP testing are less than with SDLT testing, with prediagnosis savings ranging from $1 587 091 in year 1 to $245 543 in year 4. However, the postdiagnosis costs with MAP testing exceed those with SDLT testing for years 1 to 3, with the difference ranging between $931 688 in year 1 to $117 792 in year 3. Postdiagnosis savings of $80 724 are realized in year 4. Despite the higher postdiagnosis costs with MAP testing, the much larger prediagnosis cost savings with MAP testing result in net (ie, pre‐ and postdiagnosis combined) positive savings with MAP relative to SDLT testing in each of the 4 years. Reduced inpatient hospital admissions are the biggest driver of prediagnosis cost savings ($581 728 in year 1) (Table 3). The higher postdiagnosis costs with MAP testing are largely due to more individuals in the MAP arm being diagnosed in year 1, resulting in a shift from pre‐ to postdiagnosis costs.
Sensitivity analysis and scenario analysis
Results of the one‐way sensitivity analysis are shown in Figure 3A, and cost and clinical parameters are further analyzed in Figure 3B and C. Results are presented as a shift to the average annual savings per individual of $498 (4‐year incremental savings of $1 991 152 divided by 4 years, divided by 1000 patients). The budget impact model is most sensitive to the specificity of MAP, with average annual savings per individual of $15 per 1% absolute increase in MAP specificity. Second to this, was the proportion of individuals with SLE of those suspected of having SLE, with average annual savings per individual of $9 per 1% absolute increase in percentage of SLE among those suspected of SLE (Figure 3B). In terms of cost inputs, the budget impact model is most sensitive to the cost of the MAP test, with a reduction of average annual cost savings per individual of $20 per $100 increase in the cost of the test (Figure 3C).
The one‐way sensitivity analysis revealed important model sensitivity associated with the assumed hazard ratio. Therefore, a scenario analysis was performed in which the hazard ratio is assumed to be 1.00 (53%, 75%, 84%, and 88% diagnosed in years 1‐4 for both MAP and SDLTs) with no subsequent case‐mix severity shift for MAP from earlier‐diagnosed SLE (cost of true positives for MAP equals the SDLT cost of $19 578). In this scenario analysis, all primary outcomes were regenerated (Table S1), and applicable one‐way sensitivity analyses were repeated (Figure S1). MAP 4‐year cost savings reduces to $465 676. Prediagnosis costs were equivalent between MAP and SDLT in years 2 to 4, and prediagnosis costs were more expensive in year 1 for MAP because of the administration of the MAP test. Postdiagnosis cost savings for MAP increase across years 1 to 4 ($96 978 for year 1 and $162 374 for year 4) were primarily driven by the specificity of MAP, as other drivers of cost savings (earlier diagnosis and case‐mix severity shift of diagnosed SLE) were removed from the model. PMPM savings across years 1 to 4 was $0.01. One‐way sensitivity analysis (Figure S1A) still demonstrates MAP is saves on costs across all ranges of variables except specificity of MAP (cost‐savings threshold is approximately 77% specificity, at which point MAP is no longer cost saving).
DISCUSSION
In this budget impact model, we have demonstrated that the improved specificity of MAP, compared with SDLTs, results in potential total direct cost savings of $1 991 152 to a US commercial plan over a 4‐year time horizon ($0.04 PMPM). The improved performance of MAP compared with SDLTs enables a diagnosis of SLE earlier in the patient’s disease course, which facilitates earlier initiation of appropriate therapy and thus preempts prediagnosis hospitalizations and unnecessary investigations as well as decreases health care costs.
The higher false‐negative rate and lower true‐positive rate associated with the lower sensitivity of MAP versus SDLT is offset by its superior specificity, resulting in fewer false‐positives and more true‐negative diagnoses. Cost savings in the MAP arm compared with those of SDLTs are driven by fewer false‐positives (annual costs of $14 441), which would otherwise be treated as SLE, and more true‐negatives, which do not have costs associated with SLE treatment (annual costs of $10 375). Furthermore, additional cost savings are realized in MAP because a larger proportion of patients transition out of the undiagnosed state (annual costs per patient with and without SLE are $24 593 and $17 495, respectively) and into a diagnosed state with annual costs of $18 123 for those with SLE, $10 375 for those without SLE, $14 441 for those incorrectly diagnosed as having SLE, and $14 516 for those incorrectly diagnosed as not having SLE. Additionally, in MAP, a larger proportion of those correctly diagnosed with SLE transition out of the undiagnosed state at an earlier point in time and thus a larger proportion have milder SLE and lower health care costs (annual costs for lower vs higher case severity mix: $18 123 vs $19 578).
However, there are several important limitations to this analysis. The diagnosis rate of CTDs for SDLTs was based on longitudinal follow‐up in a single rheumatology clinic in the Netherlands (Dijkstra et al) (4). Although sourcing this information from a US‐based study or preferably several studies would be ideal, the Dijkstra study is the only study of its kind in the literature. Although the average time to diagnosis in the Dijkstra study was about 2 years, consistent with other non–US‐based estimates (22), if the proportion diagnosed over time is different than that observed in the United States, model outcomes can be impacted. Other model inputs were also based on this study, including the proportion of patients with SLE among those with suspected SLE and the proportion with RA, SS, or other CTD among those suspected of having SLE. We performed sensitivity analysis to assess the impact of these uncertain model inputs on the model outcomes.
A number of parameters in the model have not been observed directly with the use of MAP testing in clinical practice and therefore are based on assumptions that we believe are reasonable and generalizable. For example, based on the improved performance of MAP versus SDLT, we have assumed that MAP will result in an earlier diagnosis of SLE and hence a higher proportion of patients will be diagnosed with mild (36% vs 26%) disease and a smaller proportion will be diagnosed with moderate (45% vs 52%) and severe (19% vs 22%) disease. The case severity mix for SDLTs, based on Garris (19), was modified using factors describing shifts in severity distribution due to early versus late SLE diagnosis from Ogelsby (10). The extent to which MAP will result in an earlier diagnosis and change the immediate postdiagnosis case severity mix is not known. To address this uncertainty, we performed a scenario analysis assuming MAP does not result in earlier diagnosis of SLE (hazard ratio of 1.0 instead of base case hazard ratio of 1.74) and that there is no subsequent improvement to the SLE severity case mix distribution (Table S1; Figure S1). In this scenario analysis, MAP 4‐year cost savings were reduced to $465 676 from the base case scenario of $1 991 152 and PMPM savings across years 1 to 4 were reduced to $0.01 from $0.04.
The assumption in the MAP arm that 80% of patients will be tested with MAP while the remaining 20% would be readily diagnosed with SDLTs, thus not requiring MAP, is based on clinician feedback and has been evaluated in the sensitivity and scenario analyses (Figure 3A and B; Figure S1A and B). Practice patterns require a threshold of evidence to be shifted and widespread integration of the MAP into clinical practice will require evidence demonstrating its clinical utility.
Further limitations pertain to the impact of using several different sources to estimate pre‐ and post‐diagnosis costs and how those costs were applied in the model. Prediagnosis costs for individuals without SLE were based on an adjustment factor to prediagnosis costs for individuals with SLE. The model assumes that prediagnosis costs, which were based on the average annual prediagnosis costs for 2 years prior to SLE diagnosis from the claims analysis, would carry over across the time horizon of the model while individuals remained undiagnosed. Additionally, commercial claims were used to quantify prediagnosis costs but were not used for postdiagnosis costs because postdiagnosis SLE costs have been extensively characterized in existing literature (19). To account for these limitations, we performed a robust sensitivity analysis for pre‐ and postdiagnosis costs and reported the changes to average annual cost savings per individual per $100 increase in cost. Reporting results in this manner allows average cost savings to be adjusted under any number of alternate cost assumptions.
Despite these limitations, we have developed a budget impact model that is based on a combination of a rigorous claims‐based analysis, well‐designed studies published in the literature, and clinician feedback, all of which are generalizable and applicable to this analysis. In summary, it is postulated that the improved performance of MAP will allow clinicians to make a definitive SLE diagnosis earlier in the patient’s disease trajectory as compared with SDLTs alone. It is therefore expected that by leading to earlier SLE diagnosis, MAP may decrease unnecessary prediagnosis investigations and facilitate earlier and more appropriate treatment, resulting in lower disease activity, reduced inpatient hospital admissions, improved patient outcomes, and decreased health care costs.
AUTHOR CONTRIBUTIONS
All authors drafted or contributed to the critical revision of the manuscript.
Study conception and design
Dr. Clarke, Dr. Weinstein, Wegener, and Powell.
Acquisition of data
Dr. Goss, Heer, and Doshi.
Analysis and interpretation of data
Design of claims analysis. Dr. Goss, Dr. Weinstein, Wegener, and Powell. Design of budget impact model. Dr. Chandra and Piscitello.
Supporting information
Fig S1A
Fig S1B
Fig S1C
Supplementary Material
Acknowledgments
The authors wish to thank Tyler O’Malley for technical advice, editorial support, proofreading, and submission support.
This study was supported by Exagen Inc. The funding agreement ensured the authors’ independence in designing and performing the claims analysis, designing and analyzing the budget impact model, interpreting results, and writing the report.
Dr. Clarke holds The Arthritis Society Chair in Rheumatic Diseases at the University of Calgary. No other disclosures relevant to this article were reported.
REFERENCES
- 1. Thong B, Olsen NJ. Systemic lupus erythematosus diagnosis and management. Rheumatology (Oxford) 2017;56(suppl_1):i3–i13. [DOI] [PubMed] [Google Scholar]
- 2. Lim SS, Bayakly AR, Helmick CG, Gordon C, Easley KA, Drenkard C. The incidence and prevalence of systemic lupus erythematosus, 2002–2004: The Georgia Lupus Registry. Arthritis Rheumatol 2014;66:357–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Somers EC, Marder W, Cagnoli P, Lewis EE, DeGuire P, Gordon C, et al. Population‐based incidence and prevalence of systemic lupus erythematosus: the Michigan Lupus Epidemiology and Surveillance program. Arthritis Rheumatol 2014;66:369–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dijkstra S, Nieuwenhuys EJ, Swaak AJ. The prognosis and outcome of patients referred to an outpatient clinic for rheumatic diseases characterized by the presence of antinuclear antibodies (ANA). Scand J Rheumatol 1999;28:33–7. [DOI] [PubMed] [Google Scholar]
- 5. Ramsey‐Goldman R, Li J, Dervieux T, Alexander RV. Cell‐bound complement activation products in SLE. Lupus Sci Med 2017;4:e000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Satoh M, Chan EK, Ho LA, Rose KM, Parks CG, Cohn RD, et al. Prevalence and sociodemographic correlates of antinuclear antibodies in the United States. Arthritis Rheumatol 2012;64:2319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Leuchten N, Hoyer A, Brinks R, Schoels M, Schneider M, Smolen J, et al. Performance of antinuclear antibodies for classifying systemic lupus erythematosus: a systematic literature review and meta‐regression of diagnostic data. Arthritis Care Res (Hoboken) 2018;70:428–38. [DOI] [PubMed] [Google Scholar]
- 8. Pisetsky DS, Spencer DM, Lipsky PE, Rovin BH. Assay variation in the detection of antinuclear antibodies in the sera of patients with established SLE. Ann Rheum Dis 2018;77:911–3. [DOI] [PubMed] [Google Scholar]
- 9. Hanly JG, Thompson K, McCurdy G, Fougere L, Theriault C, Wilton K. Measurement of autoantibodies using multiplex methodology in patients with systemic lupus erythematosus. J Immunol Methods 2010;352:147–52. [DOI] [PubMed] [Google Scholar]
- 10. Oglesby A, Korves C, Laliberté F, Dennis G, Rao S, Suthoff ED, et al. Impact of early versus late systemic lupus erythematosus diagnosis on clinical and economic outcomes. Appl Health Econ Health Policy 2014;12:179–90. [DOI] [PubMed] [Google Scholar]
- 11. Putterman C, Furie R, Ramsey‐Goldman R, Askanase A, Buyon J, Kalunian K, et al. Cell‐bound complement activation products in systemic lupus erythematosus: comparison with anti‐double‐stranded DNA and standard complement measurements. Lupus Sci Med 2014;1:e000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. New York State Department of Health . Amended validation report with change of analyte: anti‐CCP2 vs. anti‐MCV as anti‐citrullinated peptide antibody. Project ID: 49893. 2015. https://www.wadsworth.org/exagen‐inc‐3
- 13. Youden WJ. Index for rating diagnostic tests. Cancer 1950;3:32–5. [DOI] [PubMed] [Google Scholar]
- 14. Sullivan SD, Mauskopf JA, Augustovski F, Caro JJ, Lee KM, Minchin M, et al. Budget impact analysis‐principles of good practice: report of the ISPOR 2012 Budget Impact Analysis Good Practice II Task Force. Value Health 2014;17:5–14. [DOI] [PubMed] [Google Scholar]
- 15. The Truven Health MarketScan Databases for life sciences researchers. Truven Health Analytics. IBM Watson Health. https://www.ibm.com/products/marketscan‐research‐databases/databases
- 16. Narayanan S, Wilson K, Ogelsby A, Juneau P, Durden E. Economic burden of systemic lupus erythematosus flares and comorbidities in a commercially insured population in the United States. J Occup Environ Med 2013;55:1262–70. [DOI] [PubMed] [Google Scholar]
- 17. Li T, Carls GS, Panopalis P, Wang S, Gibson TB, Goetzel RZ. Long‐term medical costs and resource utilization in systemic lupus erythematosus and lupus nephritis: a five‐year analysis of a large Medicaid population. Arthritis Rheum 2009;61:755–63. [DOI] [PubMed] [Google Scholar]
- 18. Furst DE, Clarke AE, Fernandes AW, Bancroft T, Greth W, Iorga SE. Incidence and prevalence of adult systemic lupus erythematosus in a large US managed‐care population. Lupus 2013;22:99–105. [DOI] [PubMed] [Google Scholar]
- 19. Garris C, Jhingran P, Bass D, Engel‐Nitz NM, Riedel A, Dennis G. Dennis G: Healthcare utilization and cost of systemic lupus erythematosus in a US managed care health plan. J Med Econ 2013;16:667–77. [DOI] [PubMed] [Google Scholar]
- 20. Kawatkar A, Jacobsen SJ, Levy GD, Medhekar SS, Venkatasubramaniam KV, Herrinton LJ. Direct medical expenditure associated with rheumatoid arthritis in a nationally representative sample from the medical expenditure panel survey. Arthritis Care Res (Hoboken) 2012;64:1649–56. [DOI] [PubMed] [Google Scholar]
- 21. McDonald M, Patel DA, Keith MS, Snedecor SJ. Economic and humanistic burden of dry eye disease in Europe, North America, and Asia: a systematic literature review. Ocul Surf 2016;14:144–67. [DOI] [PubMed] [Google Scholar]
- 22. Feng X, Zou Y, Pan W, Wang X, Wu M, Zhang M, et al. Associations of clinical features and prognosis with age at disease onset in patients with systemic lupus erythematosus. Lupus 2014;23:327–34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1A
Fig S1B
Fig S1C
Supplementary Material


