Abstract
Aims
Tocilizumab, an interleukin-6 (IL-6) receptor (IL-6R) targeting antibody, enhances the anti-tumour effect of conventional chemotherapy in preclinical models of cancer. We investigated the anti-tumour effect of tocilizumab in osteosarcoma (OS) cell lines.
Methods
We used the 143B, HOS, and Saos-2 human OS cell lines. We first analyzed the IL-6 gene expression and IL-6Rα protein expression in OS cells using reverse transcription real time quantitative-polymerase chain reaction (RT-qPCR) analysis and western blotting, respectively. We also assessed the effect of tocilizumab on OS cells using proliferation and invasion assay.
Results
The OS cell lines 143B, HOS, and Saos-2 expressed IL-6R. Recombinant human IL-6 treatment increased proliferation of 143B and HOS cells. Tocilizumab treatment decreased proliferation and invasion of 143B, HOS, and Saos-2.
Conclusion
In conclusion, we confirmed the production of IL-6 and the expression of IL-6R in OS cells and demonstrated that tocilizumab inhibits proliferation and invasion in OS cells.
Cite this article: Bone Joint Res 2020;9(11):821–826.
Keywords: Osteosarcoma, Tocilizumab, Anti-tumour effect, Interleukin-6, Interleukin-6 receptor
Article focus
To investigate interleukin-6 (IL-6) production and IL-6 receptor (IL-6R) expression in osteosarcoma (OS) cells.
To assess the effect of IL-6R-targeting antibody on OS cells.
Key messages
The OS cell lines 143B, HOS, and Saos-2 express IL-6Rα.
Recombinant human IL-6 treatment increases proliferation of 143B, HOS, and Saos-2 cells.
Tocilizumab treatment decreases proliferation and invasion of 143B, HOS, and Saos-2 cells.
Strengths and limitations
The study highlights the potential of using tocilizumab in combination with other therapeutic agents.
Only in vitro analysis was performed; further in vivo research is warranted to confirm the applicability in clinical settings.
Introduction
Osteosarcoma (OS) is the most common primary malignant bone tumour in children and adolescents.1 The overall survival of non-metastatic OS has improved considerably since 1980, owing to the development of chemotherapy and surgical techniques;2 however, the five-year survival rate remains at 60% to 80%.3 The treatment regimen of doxorubicin, cisplatin, methotrexate, and ifosfamide has not changed over the last two decades, thereby necessitating the development of new therapeutic strategies for improvement of survival in patients with OS.4
The cytokine interleukin-6 (IL-6) shows pleiotropic effects on various cell types in the tumour microenvironment and regulates the expression of signal transducer and activator of transcription 3 (STAT3), a pro-oncogenic transcription factor.5 IL-6 is produced in several cells, including fibroblasts, endothelial cells, macrophages, and cancer cells or tissues.6,7 Recently, several studies reported the association of high IL-6 levels with poor prognosis in patients with cancers such as in pancreatic cancer, breast cancer, colorectal cancer, small cell lung cancer, renal cell carcinoma, and soft tissue sarcoma.8-14 Moreover, serum IL-6 levels affected disease-free survival in patients with bone sarcoma.15 Tocilizumab (Chugai Pharmaceutical Co, Tokyo, Japan), an IL-6 receptor (IL-6R) targeting antibody, enhanced the anti-tumour effect of conventional chemotherapy in preclinical models of mucoepidermoid carcinoma.16 However, the effect of IL-6R-targeting antibody on OS has not yet been reported. Therefore, in this study, we investigated the anti-tumour effect of tocilizumab in OS cell lines. The study highlights the potential of using tocilizumab in combination with other therapeutic agents. Further in vivo research is warranted to confirm the applicability in clinical settings.
Methods
Osteosarcoma cell lines
We used the 143B, HOS, and Saos-2 human OS cell lines in this study; 143B and HOS were cultured in minimum essential medium (MEM; Gibco, Carlsbad, California, USA) containing 10% fetal bovine serum (FBS). Saos-2 was cultured in McCoy’s 5A (modified) medium (Gibco) containing 15% FBS. Cells were maintained as attached monolayers and incubated in a humidified atmosphere with 5% CO2 at 37°C.
Reverse transcription real time quantitative-polymerase chain reaction analysis
Total RNA was isolated from the OS cell lines using the mirVANA Isolation Kit (Thermo Fisher Scientific, Waltham, Massachusetts, USA) and reverse transcribed using random primers and the First Strand cDNA Synthesis Kit (Roche, Basel, Switzerland) for reverse transcription real time quantitative-polymerase chain reaction analysis (RT-qPCR). Real-time PCR for IL-6 expression was performed using the TaqMan Gene Expression Assays (Thermo Fisher Scientific) and ABI PRISM 7000 Sequence Detection System (Applied Biosystems, Waltham, Massachusetts, USA). VIC-labelled glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the endogenous control.
Western blot analysis
The 143B, HOS, and Saos-2 cells were treated with 0 ng/ml, 10 ng/ml, or 100 ng/ml of recombinant human IL-6 (PeproTech, Rocky Hill, New Jersey, USA) for 24 hours, and lysed using radioimmunoprecipitation (RIPA) buffer (Millipore, Temecula, California, USA) supplemented with a protease inhibitor cocktail, 0.5 mM phenylmethylsulfonyl fluoride, and 0.2 mM Na3VO4. The 143B, HOS, and Saos-2 cells were treated with 100 ng/ml of recombinant human IL-6 for two hours, followed by 0 μg/ml, 10 μg/ml, or 100 μg/ml tocilizumab for 24 hours and lysed using RIPA buffer supplemented with a protease inhibitor cocktail, 0.5 mM phenylmethylsulfonyl fluoride, and 0.2 mM Na3VO4. Proteins were separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE), and the samples were adjusted to the same protein concentration before loading. Proteins were transferred to a nitrocellulose membrane and blotted using the following antibodies (at the dilutions recommended by the manufacturer): IL-6Rα (1:200 dilution; Santa Cruz Biotechnology, Dallas, Texas, USA), STAT3 (1:2000; Cell Signalling Technology, Beverly, Massachusetts, USA), and phospho-STAT3 (1:2000; Cell Signalling Technology). β-actin was assayed as the loading control.
Cell proliferation assay
The 143B, HOS, and Saos-2 cells were seeded (1 × 104 cells per well) in 96-well plates in 100 μl medium and treated with 10 ng/ml recombinant human IL-6 (using 10 μl recombinant human IL-6 solution) and 0, 10, 100, or 200 μg/ml tocilizumab for 24 hours. Cell viability was measured using the Cell-Titer 96 Aqueous One Solution Cell Proliferation Assay Kit (Promega, Madison, Wisconsin, USA). The IL-6R–targeting antibody tocilizumab was kindly donated by Chugai Pharmaceutical Co. (Tokyo, Japan).
Cell invasion assay
Cell invasion assays were performed using the Corning BioCoat Matrigel Invasion Chambers (Corning, New York, USA). The 143B, HOS, and Saos-2 cells were seeded (3 × 104 cells/well) in the upper chamber, and serum-free medium containing IL-6 (10 ng/ml) with or without tocilizumab (100 μg/ml) was added to the lower chamber. After 24 hours of incubation, non-migrating cells were removed from the upper chamber using a cotton swab, and the cells on the lower surface of the insert were stained with haematoxylin and eosin. Invading cells were recorded and counted using Olympus cellSens ver.1.18 software (Olympus, Tokyo, Japan) and a BX50 microscope (Olympus) equipped with the DP74 camera (Olympus).
Statistical analysis
All in vitro experiments were repeated at least three times to ensure reproducibility. Data are expressed as means ± SD (Figures 1 and 2). Differences between two groups were compared using the Mann-Whitney non-parametric analysis of variance test. A p-value < 0.05 was considered to indicate statistical significance.
Fig. 1.
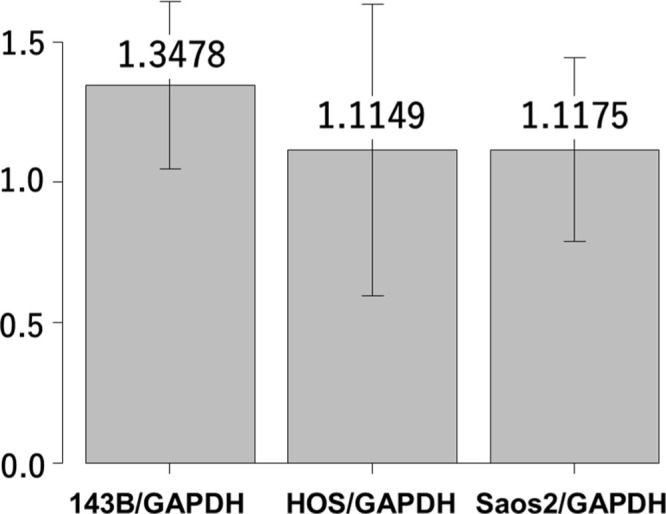
Reverse transcription real time quantitative-polymerase chain reaction (RT-qPCR) analysis showing interleukin-6 (IL-6) gene expression in 143B, HOS, and Saos-2 cells. Y-axis indicates the relative ratio of messenger RNA (mRNA). GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Fig. 2.
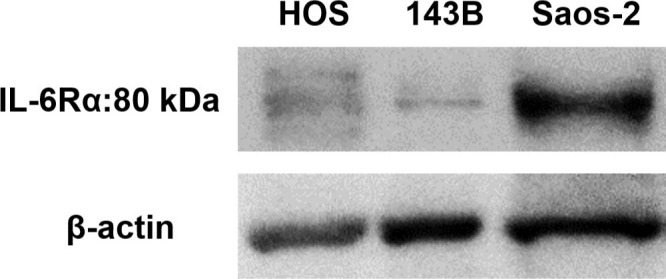
Representative western blot images showing expression of interleukin-6 receptor-alpha (IL-6Rα) in 143B, HOS, and Saos-2 cells.
Results
Effect of recombinant human IL-6 on OS cell lines
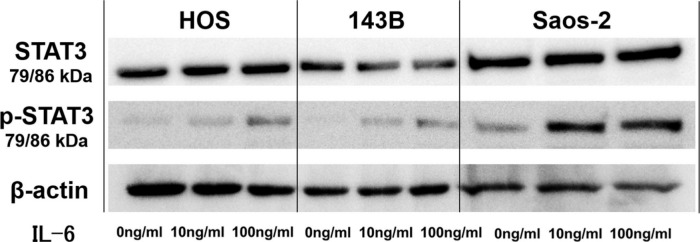
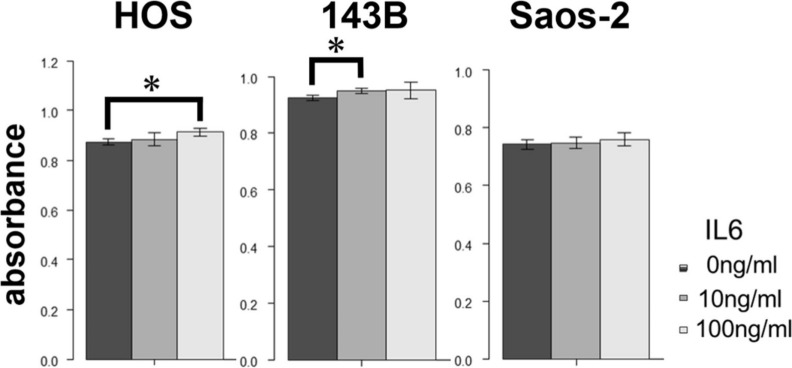
We first analyzed the IL-6 gene expression and IL-6Rα protein expression in the HOS, 143B, and Saos-2 cells using RT qPCR analysis and western blotting, respectively. The three OS cell lines showed different levels of IL-6 messenger RNA (mRNA) (Figure 1) and IL-6Rα (Figure 2). Next, to determine the involvement of the Janus kinase (JAK)-signal transducer and activator of transcription (STAT) pathway in IL-6 production, we treated the cells with recombinant human IL-6. STAT3 is constitutively activated via tyrosine phosphorylation in many human cancers. Therefore, we assayed STAT3 and phospho-STAT3 levels in HOS, 143B, and Saos-2 cells treated with different concentrations of recombinant human IL-6 using western blotting. All three cell lines expressed high levels of STAT3 and showed a dose-dependent increase in phospho-STAT3 levels (Figure 3). We next assessed the effect of different concentrations of recombinant human IL-6 on cell viability. Treatment with 100 ng/ml and 10 ng/ml recombinant human IL-6 significantly increased the proliferation rates of HOS and 143B cells, respectively (p < 0.05, Mann-Whitney U test; Figure 4).
Fig. 3.
Representative western blot images showing effect of interleukin-6 (IL-6) on phosphorylation of signal transducer and activator of transcription 3 (STAT3) in 143B, HOS, and Saos-2 cells. p-STAT3, phospho-STAT3.
Fig. 4.
Effect of treatment with recombinant human interleukin-6 (IL-6) on proliferation of 143B, HOS, and Saos-2 cells. *p < 0.05, Mann-Whitney U test.
Effect of IL-6R–targeting antibody on OS cell lines
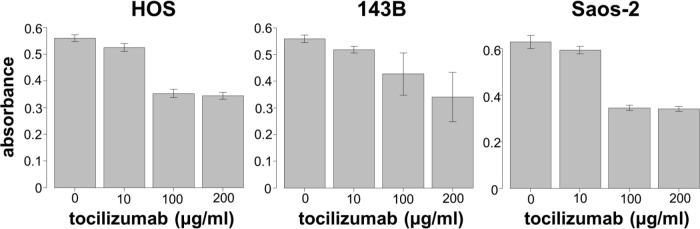
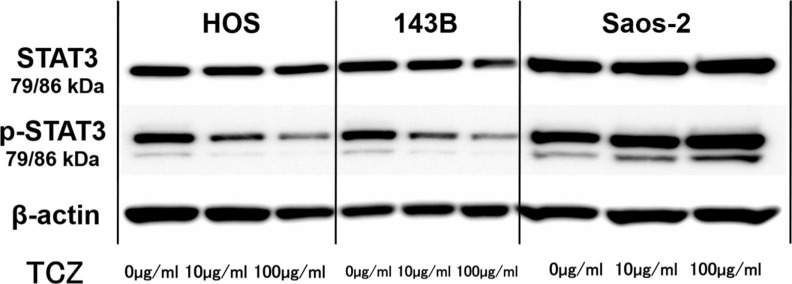
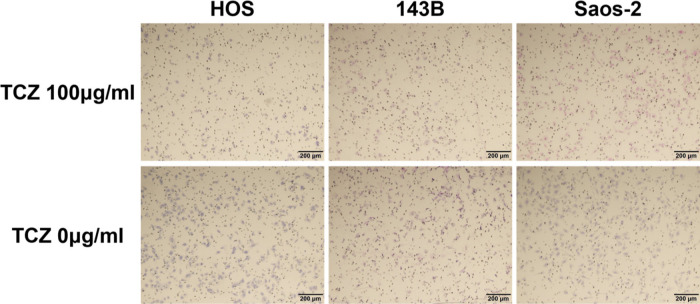
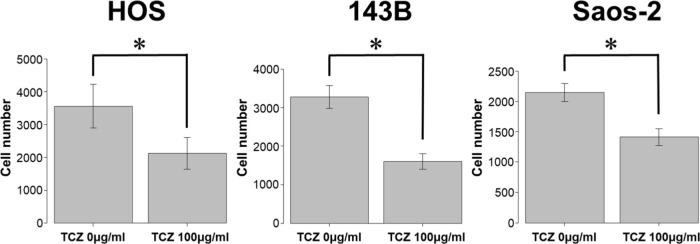
We next assessed the effect of different concentrations of tocilizumab, the IL-6R–targeting antibody, on the proliferation and invasion of HOS, 143B, and Saos-2 cells. Tocilizumab decreased cell viability in all three cell lines (Figure 5). For both HOS and Saos-2 cells, 100 μg/ml and 200 μg/ml tocilizumab significantly inhibited cell proliferation. In 143B cells, tocilizumab significantly decreased cell viability in a dose-dependent manner (Figure 5). We assayed the effect of tocilizumab to the STAT3 and phospho-STAT3 levels in OS cell lines under treatment with recombinant human IL-6 using western blotting. HOS and 143B cells expressed dose-dependent decrease in phospho-STAT3 levels, although there was no relationship between the expression of phospho-STAT3 and administration of tocilizumab (Figure 6). Cell invasion assays showed that the number of cells invading through the membrane was significantly lower for tocilizumab-treated cells, compared to that in the respective controls for all three OS cell lines (p < 0.001, Mann-Whitney U test; Figures 7 and 8).
Fig. 5.
Effect of tocilizumab treatment on proliferation of 143B, HOS, and Saos-2 cells.
Fig. 6.
Representative western blot images showing effect of tocilizumab (TCZ) treatment on phosphorylation of signal transducer and activator of transcription 3 (STAT3) in 143B, HOS, and Saos-2 cells with recombinant human interleukin-6 (IL-6). p-STAT3, phospho-STAT3.
Fig. 7.
Upper row shows the effect of tocilizumab (TCZ) treatment on invasion of 143B, HOS, and Saos-2 cells. Lower row shows the invasion of 143B, HOS, and Saos-2 cells without TCZ treatment.
Fig. 8.
Effect of tocilizumab (TCZ) treatment on invasion of 143B, HOS, and Saos-2 cells. *p < 0.001, Mann-Whitney U test.
Discussion
In the present study, we confirmed the expression of IL-6R in three OS cell lines. We also demonstrated that IL-6 promoted proliferation whereas tocilizumab, the IL-6R-targeting antibody, suppressed proliferation in OS cells. IL-6 plays an important role in the neoplastic process by affecting tumour cell adhesion, motility, proliferation, tumour-specific antigen expression, and thrombopoiesis.17 It regulates the crosstalk between tumour cells, inflammatory cells, and vascular endothelial cells and enhances the survival and plasticity of tumour stem cells.18,19 These findings provided the rationale for clinical trials investigating the efficacy of targeted IL-6 inhibitors in different cancers; these trials showed promising results.20,21 High IL-6 production and IL-6–mediated autocrine proliferation were reported in renal cell carcinoma cell lines.22 Moreover, significant elevation of IL-6 expression in colorectal cancer tissues was associated with tumour invasion depth.23 In the present study, we confirmed the production of IL-6 in 143B, HOS, and Saos-2 OS cell lines using RT-qPCR analysis.
We showed that IL-6 promoted STAT3 phosphorylation in the 143B, HOS, and Saos-2 OS cell lines. Binding of IL-6 to IL-6R induces the homodimerization of the glycoprotein 130 (gp130), an IL-6 transducer, leading to phosphorylation of JAK1, which then induces the phosphorylation and subsequent nuclear translocation of STAT3. STAT3 is a critical mediator of oncogenic signalling and is activated in many human cancers.24,25 Furthermore, activation of STAT3 signalling induces the expression of certain anti-apoptotic genes and several genes involved in promoting cell proliferation and tumour growth.26
Tocilizumab is a fully humanized monoclonal antibody against IL-6R and has been approved for treatment of patients with rheumatoid arthritis.27 In Japan, tocilizumab has also been approved for the treatment of polyarticular-course juvenile idiopathic arthritis, systemic-onset juvenile idiopathic arthritis, and Castleman’s disease.28 Currently, its anti-inflammatory effect and potency as an anti-tumour agent are being widely investigated, as this drug was initially investigated in the field of oncology. Several studies have reported that IL-6 represents a potential therapeutic target for several tumours, including sarcomas. IL-6R-targeting antibody inhibited IL-6 signalling, suppressed tumour angiogenesis and growth, reduced spheroid formation, and inhibited cell cycle progression in colon cancer cell lines.29,30 IL-6R-targeting antibody also decreased tumour progression in ovarian cancer cell lines.31 Consistent with these findings, the present study showed that tocilizumab treatment inhibited cell proliferation and invasion in OS cells. Our study also showed that tocilizumab decreased phospho-STAT3 levels in HOS and 143B cells. These findings indicate that tocilizumab may suppress tumour progression via the STAT3 pathway. However, phospho-STAT3 levels were not decreased by the administration of tocilizumab in Saos-2 cells. The present result may suggest that further study should be performed using HOS or 143B.
Recently, a phase I trial conducted in the Netherlands, involving carboplatin/doxorubicin treatment in combination with tocilizumab for patients with recurrent epithelial ovarian cancer, showed the possibility of providing a survival benefit.32 Similarly, a combination therapy of paclitaxel and tocilizumab was suggested to benefit patients with mucoepidermoid carcinoma.16 Thus, combining tocilizumab with chemotherapeutic agents might enhance the anti-tumour effect in patients with OS. In conclusion, we confirmed the presence of JAK-STAT pathway-mediated production of IL-6 and the expression of IL-6R in OS cells, and demonstrated that tocilizumab inhibited proliferation and invasion in OS cells. Further in vivo research is warranted to confirm the applicability in clinical settings.
Author contributions
T. Hagi: Performed the experiment, validation, formal analysis, investigation, and visualization, Curated the data, Wrote the original draft of the manuscript.
T. Nakamura: Conceptualized and supervised the study, Performed the methodology, validation, formal analysis, investigation, and visualization, Collated the resources, Curated the data, Wrote the original draft of the manuscript, Conducted the project administration, Acquired the funding.
K. Kita: Performed the experiment and validation.
T. Iino: Performed the experiment.
K. Asanuma: Performed the validation, Curated the data.
A. Sudo: Wrote, reviewed, and edited the manuscript.
Funding statement
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
ICMJE COI statement
There is no conflict of interest in this article.
Acknowledgements
We thank Chugai Pharmaceutical Co., Ltd. who kindly donated tocilizumab, the IL-6R–targeting antibody. This work was supported by JSPS KAKENHI Grant Number JP15K18429.
© 2020 Author(s) et al. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (CC BY-NC-ND 4.0) licence, which permits the copying and redistribution of the work only, and provided the original author and source are credited. See https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- 1.Picci P. Osteosarcoma (osteogenic sarcoma). Orphanet J Rare Dis. 2007;2:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meyers PA, Heller G, Healey J, et al. Chemotherapy for nonmetastatic osteogenic sarcoma: the Memorial Sloan-Kettering experience. J Clin Oncol. 1992;10(1):5–15. [DOI] [PubMed] [Google Scholar]
- 3.Robl B, Pauli C, Botter SM, Bode-Lesniewska B, Fuchs B. Prognostic value of tumor suppressors in osteosarcoma before and after neoadjuvant chemotherapy. BMC Cancer. 2015;15:379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abdeen A, Chou AJ, Healey JH, et al. Correlation between clinical outcome and growth factor pathway expression in osteogenic sarcoma. Cancer. 2009;115(22):5243–5250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coindre JM, Terrier P, Bui NB, et al. Prognostic factors in adult patients with locally controlled soft tissue sarcoma. A study of 546 patients from the French Federation of cancer centers sarcoma group. J Clin Oncol. 1996;14(3):869–877. [DOI] [PubMed] [Google Scholar]
- 6.Dmitrieva OS, Shilovskiy IP, Khaitov MR, Grivennikov SI. Interleukins 1 and 6 as main mediators of inflammation and cancer. Biochemistry. 2016;81(2):80–90. [DOI] [PubMed] [Google Scholar]
- 7.Chen M-F, Chen P-T, Lu MS, Lin PY, Chen W-C, Lee K-D. Il-6 expression predicts treatment response and outcome in squamous cell carcinoma of the esophagus. Mol Cancer. 2013;12:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mroczko B, Groblewska M, Gryko M, Kedra B, Szmitkowski M. Diagnostic usefulness of serum interleukin 6 (IL-6) and C-reactive protein (CRP) in the differentiation between pancreatic cancer and chronic pancreatitis. J Clin Lab Anal. 2010;24(4):256–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ravishankaran P, Karunanithi R. Clinical significance of preoperative serum interleukin-6 and C-reactive protein level in breast cancer patients. World J Surg Oncol. 2011;9:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimazaki J, Goto Y, Nishida K, et al. In patients with colorectal cancer, preoperative serum interleukin-6 level and granulocyte/lymphocyte ratio are clinically relevant biomarkers of long-term cancer progression. Oncology. 2013;84(6):356–361. [DOI] [PubMed] [Google Scholar]
- 11.Wójcik E, Jakubowicz J, Skotnicki P, Sas-Korczyńska B, Kulpa JK. Il-6 and VEGF in small cell lung cancer patients. Anticancer Res. 2010;30(5):1773–1778. [PubMed] [Google Scholar]
- 12.Kallio JP, Tammela TL, Marttinen AT, Kellokumpu-Lehtinen PL. Soluble immunological parameters and early prognosis of renal cell cancer patients. J Exp Clin Cancer Res. 2001;20(4):523–528. [PubMed] [Google Scholar]
- 13.Hagi T, Nakamura T, Iino T, et al. The diagnostic and prognostic value of interleukin-6 in patients with soft tissue sarcomas. Sci Rep. 2017;7(1):9640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakamura T, Grimer RJ, Carter SR, et al. Outcome of soft-tissue sarcoma patients who were alive and event-free more than five years after initial treatment. Bone Joint J. 2013;95-B(8):1139–1143. [DOI] [PubMed] [Google Scholar]
- 15.Rutkowski P, Kamińska J, Kowalska M, Ruka W, Steffen J. Cytokine and cytokine receptor serum levels in adult bone sarcoma patients: correlations with local tumor extent and prognosis. J Surg Oncol. 2003;84(3):151–159. [DOI] [PubMed] [Google Scholar]
- 16.Mochizuki D, Adams A, Warner KA, et al. Anti-tumor effect of inhibition of IL-6 signaling in mucoepidermoid carcinoma. Oncotarget. 2015;6(26):22822–22835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang X-P, Yang DC, Elliott RL, Head JF. Down-regulation of expression of interleukin-6 and its receptor results in growth inhibition of MCF-7 breast cancer cells. Anticancer Res. 2011;31(9):2899–2906. [PubMed] [Google Scholar]
- 18.Sansone P, Storci G, Tavolari S, et al. Il-6 triggers malignant features in mammospheres from human ductal breast carcinoma and normal mammary gland. J Clin Invest. 2007;117(12):3988–4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shinriki S, Jono H, Ota K, et al. Humanized anti-interleukin-6 receptor antibody suppresses tumor angiogenesis and in vivo growth of human oral squamous cell carcinoma. Clin Cancer Res. 2009;15(17):5426–5434. [DOI] [PubMed] [Google Scholar]
- 20.Dorff TB, Goldman B, Pinski JK, et al. Clinical and correlative results of SWOG S0354: a phase II trial of CNTO328 (siltuximab), a monoclonal antibody against interleukin-6, in chemotherapy-pretreated patients with castration-resistant prostate cancer. Clin Cancer Res. 2010;16(11):3028–3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coward J, Kulbe H, Chakravarty P, et al. Interleukin-6 as a therapeutic target in human ovarian cancer. Clin Cancer Res. 2011;17(18):6083–6096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alberti L, Thomachot MC, Bachelot T, Menetrier-Caux C, Puisieux I, Blay JY. Il-6 as an intracrine growth factor for renal carcinoma cell lines. Int J Cancer. 2004;111(5):653–661. [DOI] [PubMed] [Google Scholar]
- 23.Zeng J, Tang Z-H, Liu S, Guo S-S. Clinicopathological significance of overexpression of interleukin-6 in colorectal cancer. World J Gastroenterol. 2017;23(10):1780–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bowman T, Garcia R, Turkson J, Jove R. Stats in oncogenesis. Oncogene. 2000;19(21):2474–2488. [DOI] [PubMed] [Google Scholar]
- 25.Darnell JE. Validating STAT3 in cancer therapy. Nat Med. 2005;11(6):595–596. [DOI] [PubMed] [Google Scholar]
- 26.Jing N, Tweardy DJ. Targeting STAT3 in cancer therapy. Anticancer Drugs. 2005;16(6):601–607. [DOI] [PubMed] [Google Scholar]
- 27.Yilmaz S, Simsek I. Early intervention in the treatment of rheumatoid arthritis: focus on tocilizumab. Ther Clin Risk Manag. 2013;9:403–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oldfield V, Dhillon S, Plosker GL. Tocilizumab: a review of its use in the management of rheumatoid arthritis. Drugs. 2009;69(5):609–632. [DOI] [PubMed] [Google Scholar]
- 29.Nagasaki T, Hara M, Nakanishi H, Takahashi H, Sato M, Takeyama H. Interleukin-6 released by colon cancer-associated fibroblasts is critical for tumour angiogenesis: anti-interleukin-6 receptor antibody suppressed angiogenesis and inhibited tumour-stroma interaction. Br J Cancer. 2014;110(2):469–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ying J, Tsujii M, Kondo J, et al. The effectiveness of an anti-human IL-6 receptor monoclonal antibody combined with chemotherapy to target colon cancer stem-like cells. Int J Oncol. 2015;46(4):1551–1559. [DOI] [PubMed] [Google Scholar]
- 31.Isobe A, Sawada K, Kinose Y, et al. Interleukin 6 receptor is an independent prognostic factor and a potential therapeutic target of ovarian cancer. PLoS One. 2015;10(2):e0118080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dijkgraaf EM, Santegoets SJAM, Reyners AKL, et al. A phase I trial combining carboplatin/doxorubicin with tocilizumab, an anti-IL-6R monoclonal antibody, and interferon-α2b in patients with recurrent epithelial ovarian cancer. Ann Oncol. 2015;26(10):2141–2149. [DOI] [PubMed] [Google Scholar]