Abstract
To date, it remains unclear if severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) co-infection exacerbates liver injury in patients with chronic hepatitis B virus (HBV) infection. In this study, we present a retrospective study of 133 hospitalized confirmed mild coronavirus disease 2019 (COVID-19) cases, including 116 patients with COVID-19 with negative serum hepatitis B antigen and 17 HBV inactive carriers with COVID-19. We found that there were no significant differences for the discharge rate or duration of hospitalization between the two groups. However, inactive HBV carriers with SARS-CoV-2 co-infection are at a higher risk of abnormal liver function tests. The enhanced liver injury induced by SARS-CoV-2 and HBV co-infection was identified as the hepatocyte type rather than the cholangiocyte type. Moreover, the inflammatory response, including abnormal lactate dehydrogenase, D-dimer and interleukin-6 production, may contribute to this injury following SARS-CoV-2 co-infection. Collectively, SARS-CoV-2 and HBV co-infection exacerbates liver function of the patients with COVID-19.
Keywords: Abnormal liver function, COVID-19, HBV, Inactive HBV carriers, Liver injury, SARS-CoV-2
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; COVID-19, coronavirus disease 2019; GGT, gamma-glutamyltransferase; HBV, hepatitis B virus; IQR, interquartile range; LDH, lactate dehydrogenase; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TBIL, total bilirubin
Introduction
Chronic hepatitis B virus (HBV) infection is a major cause of chronic liver disease worldwide.1 Especially, co-infections of HBV and other viruses, like human immunodeficiency virus (HIV) and severe acute respiratory syndrome coronavirus (SARS-CoV), are more likely to accelerate liver injury progression and lead to poor clinical outcomes.2,3 Liver injury is prevalent in mild or severe cases of coronavirus disease 2019 (COVID-19), which is caused by SARS-CoV-2 infection.4, 5, 6, 7 Zou et al8 have recently reported that the patients with SARS-CoV-2 and HBV co-infection with liver injury are more likely to develop poor outcome and have a worse prognosis. To date, it remains unclear if SARS-CoV-2 co-infection exacerbates liver injury in patients with chronic HBV infection.
In the present study, we aimed to evaluate the relationship between of SARS-CoV-2/HBV co-infection and liver function and explore possible mechanisms in confirmed COVID-19 patients. Thus, we mainly investigated the dynamic changes of clinical characteristics of liver injury and representative inflammation biomarkers in inactive HBV carriers after SARS-CoV-2 co-infection.
Materials and methods
Study design
Clinical records and laboratory results were obtained from 133 hospitalized confirmed COVID-19 cases from Chongqing Three Gorges Central Hospital and Yongchuan Hospital affiliated with Chongqing Medical University in Chongqing, China from January 23 to March 11, 2020. All 133 hospitalized cases were divided into two groups, including 116 patients with COVID-19 with negative serum hepatitis B antigen (HBsAg) and 17 HBV inactive carriers with COVID-19. All the enrolled cases presented with mild symptoms (i.e., fever, cough, expectoration and other upper respiratory tract symptoms) and without abnormalities.9 None of them had severe comorbidities, such as acute respiratory distress syndrome (ARDS), acute-on-chronic liver failure (ACLF), acute kidney injury, or acute cardiac injury. Individuals with other concurrent chronic liver diseases, hypertension, diabetes, coronary heart disease, cardiovascular disease, malignancy, cerebrovascular disease, chronic kidney disease, autoimmune liver disease, tuberculosis, chronic obstructive pulmonary disease or other pneumonia were excluded. All HBV inactive carriers had a negative serum HBeAg, low serum HBV-DNA (<1000 IU/ml) and maintained normal serum ALT. All the cases had been treated with the medications as confirmed cases recommended by the National Health Commission of the PRC due to the suspected SARS-CoV-2 infection.9
Ethical approval
This study was approved by the Ethics Commission of Chongqing Medical University (reference number: 2020006). Written informed consent was exempted by the Ethics Commission of the designated hospital for emerging infectious diseases.
Virological tests for SARS-CoV-2 and HBV
The presence of SARS-CoV-2 was detected by the RT-PCR assay recommended by the National Centers for Disease Control and Prevention of China (China CDC).6 The levels of serum HBV DNA were measured by high throughput real-time quantitative PCR system (LightCycle 480, Roche), according to the manufacturer's instructions. The levels of serum HBsAg, HBeAg, anti-HBs, anti-HBe and anti-HBc were measured by electrochemical luminescence automatic immune analyzer (Elecsys 2010, Roche).
Liver test parameters and abnormalities
Alanine aminotransferase (ALT), alkaline phosphatase (ALP), aspartate aminotransferase (AST), D-dimer, gamma-glutamyl transferase (GGT), interleukin-6 (IL-6), lactate dehydrogenase (LDH), and total bilirubin (TBIL) in plasma samples were measured by fully automated biochemical analysis (Cobas 4000, Roche), according to the manufacturer's instructions. Liver test abnormality was defined by the elevation of the following liver enzymes in serum: ALP >125 U/L, ALT >50 U/L, AST >40 U/L, GGT >60 U/L, and TBIL >26 μmol/L. Raised ALT and/or AST more than 3 times the upper limit units (ULN) and/or raised TBIL over 2 ULN were defined as liver injury.6 Thrombocytopenia was defined as a platelet count of less than 125 × 109/L. Elevated serum inflammation biomarkers, including D-dimer > 0.55 mg/L, IL-6 > 5.4 pg/ml, and/or LDH >250 U/L, were defined as abnormal inflammatory response.
Statistical analyses
Continuous variables as median and interquartile range (IQR) or mean and standard deviation (SD). Analysis of variance with independent sample t test or Mann–Whitney U test was used to determine significant differences (Graph Pad Prism 7). Categorical variables were presented as frequency and percentages and compared by the chi-square test or Fisher exact test. Differences were considered as statistically significant when a P <0.05.
Results
Clinical characteristics
A total of 133 cases, including 116 patients hospitalized with COVID-19 with serum HBsAg-negative (SARS-CoV-2 group) and 17 HBV inactive carriers with COVID-19 (HBsAg-positive, HBeAg-negative with undetectable HBV viral load, SARS-CoV-2/HBV co-infection group), were enrolled in the analysis. No patient had hepatitis C virus (HCV) or HIV co-infection. The enrolled cases had a median age of 45 (IQR, 36–51) years and 54.14% (72/133) of male and presented with mild symptoms (Table 1). There were no differences in age and sex distribution (P > 0.05) between these two groups. Throughout the course of their disease progressions, all COVID-19 cases with or without HBV co-infection presented with mild clinical symptoms, including fever, cough, expectoration and other upper respiratory tract symptoms. Moreover, none of them had severe comorbidities, such as ARDS, ACLF, acute kidney injury, or acute cardiac injury.
Table 1.
Demographic and clinical characteristics of patients with SARS-CoV-2 and chronic HBV co-infection.
| Characteristics | All patients (n = 133) |
SARS-CoV-2 (n = 116) |
SARS-CoV-2 + HBV (n = 17) |
P |
|---|---|---|---|---|
| Age, median (IQR), years | 45 (36–51) | 45 (35–51) | 48 (45–51) | 0.153 |
| Sex, male (%) | 72 (54.14) | 61 (52.59) | 11 (64.71) | 0.438 |
| Clinical types, mild/severe | 133 (100.00) | 116 (100.00) | 17 (100.00) | >0.999 |
| Time for detection since the onset of symptoms, median (IQR), days | 6 (3–9) | 6 (4–9) | 4 (3–7) | 0.053 |
| HBsAg, positive N (%) | 17 (12.78) | 0 (0.00) | 17 (100.00) | <0.001 |
| HBeAg, positive N (%) | 0 (0.00) | 0 (0.00) | 0 (0.00) | >0.999 |
| HBV DNA, positive N (%) | 0 (0.00) | 0 (0.00) | 0/17 (0.00) | >0.999 |
| Anti-HBs, positive N (%)a | 71 (54.20) | 71 (62.28) | 0 (0.00) | <0.001a |
| Anti-HBe, positive N (%)a | 76 (58.02) | 74 (64.91) | 2/15 (11.76) | <0.001a |
| Anti-HBc, positive N (%)a | 88 (67.18) | 71 (62.28) | 17 (100.00) | 0.001a |
| Treatments | ||||
| Antiviral | 133 (100.00) | 116 (100.00) | 17 (100.00) | >0.999 |
| Arbidol | 88 (66.17) | 78 (67.24) | 10 (58.82) | 0.585 |
| Lopinavir/ritonavir | 131 (98.50) | 114 (98.28) | 17 (100.00) | >0.999 |
| Interferon | 129 (97.00) | 113 (97.41) | 16 (94.12) | 0.425 |
| Antibiotic | 104 (78.20) | 89 (76.72) | 15 (88.24) | 0.362 |
| Methylprednisolone | 11 (8.27) | 9 (7.76) | 2 (11.76) | 0.621 |
| Discharged, N (%) | 120 (90.23) | 106 (91.38) | 14 (82.36) | 0.373 |
| Hospital stays, median (IQR), days | 15 (12–19) | 15 (13–19) | 14 (10–15) | 0.186 |
Data are median (IQR) or n (%). HBeAg, hepatitis B e antigen; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; IQR, interquartile range; TBIL, total bilirubin.
Two data are missed.
Most of patients received with antibiotic therapy (104, 78.20%) and/or antiviral therapy (133, 100.00%), including arbidol, lopinavir/ritonavir, and interferon. There were no differences in antibiotic and antiviral therapy between the two groups. Besides, there was no patient received anti-HBV therapy with nucleot(s)ide analogues. Up to 21 days after onset to hospital, 106 (91.38%) patients in SARS-CoV-2 group and 14 (82.36%) in SARS-CoV-2/HBV co-infection group had been discharged, respectively. The median interval from onset to hospitalization was 15 (IQR: 13–19) days in SARS-CoV-2 group and 14 (10–15) days in SARS-CoV-2 + HBV co-infection group. Collectively, these two groups had no difference in the discharge rate or length of stay in hospital.
Dynamic changes of liver function during hospitalization
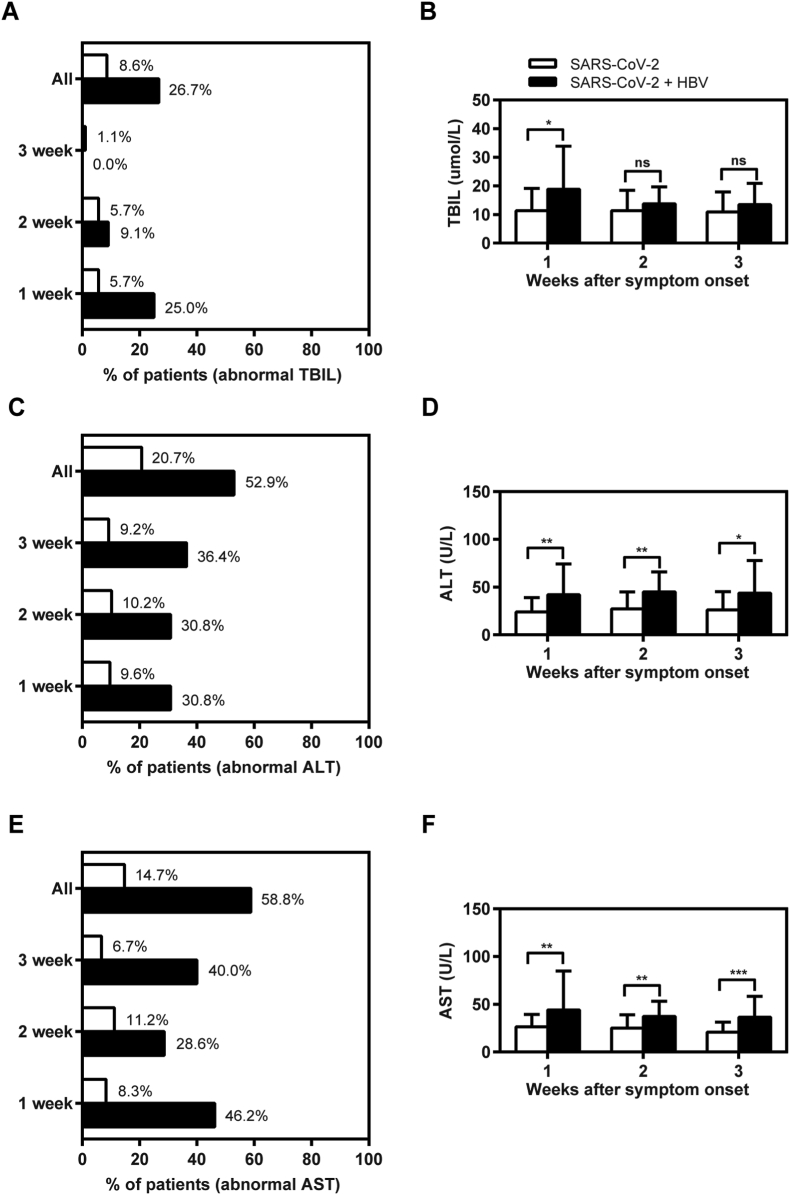
To evaluate the relationship between SARS-CoV-2/HBV co-infection and liver function, we analyzed the dynamic changes in clinical indicators associated with liver injury for 3 weeks after the onset of symptoms. The results revealed that the outlier ratios and mean values of serum TBIL levels in the inactive HBV carriers with COVID-19 are significantly higher than those in COVID-19 cases without pre-existing HBV infection at first week after the onset of symptoms (Fig. 1A and B). However, there was no difference in the outlier ratios and mean values of serum TBIL levels between the two groups at 2–3 week after the onset of symptoms. Moreover, we found that the outlier ratios and the mean values of serum ALT and AST levels in SARS-CoV-2/HBV co-infection group are much higher than those in SARS-CoV-2 group (Fig. 1C–F). Particularly, 64.71% (11/17) inactive HBV carriers with COVID-19 and 20.69% (24/116) COVID-19 cases had abnormal ALT and/or AST levels during the hospitalization (Table 2), respectively. While there were no raised ALT and/or AST more than 3 ULN in the COVID-19 cases without HBV co-infection, 14.29% (2/17) inactive HBV carriers with COVID-19 had liver injury. Thus, a greater proportion of inactive HBV carriers had abnormal liver test results after SARS-CoV-2 co-infection.
Figure 1.
Liver functions abnormality during hospitalization in the patients with SARS-CoV-2 and chronic HBV co-infection. A total of 133 hospitalized confirmed cases for COVID-19 were divided into two groups: COVID-19 cases with negative serum HBsAg (SARS-CoV-2 group, n = 116) and inactive HBV carriers with COVID-19 (SARS-CoV-2 + HBV group, n = 17) in the cross-sectional study. Serum samples were collected during the 3 weeks after the onset of symptoms and tested in parallel for TBIL (A–B), ALT (C–D) and AST (E–F). The positive ratios of serum TBIL (A), ALT (C), and AST (E) outliers were calculated for each group. The mean values of serum TBIL (B), ALT (D), and AST (F) levels were analyzed and compared between the two groups, respectively. ∗P < 0.05; ∗∗P < 0.01∗∗∗P < 0.001; ns, not significant. ALT, alanine aminotransferase; AST, aspartate aminotransferase; TBIL, total bilirubin.
Table 2.
Liver tests of patients with SARS-CoV-2 and chronic HBV co-infection during hospitalization.
| Characteristics | All patients (n = 133) |
SARS-CoV-2 (n = 116) |
SARS-CoV-2 + HBV (n = 17) |
P |
|---|---|---|---|---|
| TBIL, U/L, Median (IQR) | 9.00 (5.75–13.70) | 8.35 (5.38–12.85) | 15.10 (10.20–24.60) | 0.027 |
| Normal, N (%) | 120 (90.23) | 107 (81.03) | 13 (76.47) | 0.001 |
| 1–2 ULN, N (%) | 13 (9.73) | 9 (7.76) | 4 (23.53) | |
| 2–3 ULN, N (%) | 0 (0.00) | 0 (0.00) | 0 (0.00) | |
| >3 ULN, N (%) | 0 (0.00) | 0 (0.00) | 0 (0.00) | |
| ALT, U/L, Median (IQR) | 21.20 (14.70–36.00) | 19.25 (13.93–33.55) | 33.00 (22.00–56.10) | <0.001 |
| Normal, N (%) | 102 (76.69) | 94 (81.03) | 8 (47.06) | 0.001 |
| 1–2 ULN, N (%) | 25 (18.80) | 19 (16.38) | 6 (35.29) | |
| 2–3 ULN, N (%) | 5 (3.76) | 3 (2.59) | 2 (11.76) | |
| >3 ULN, N (%) | 1 (0.75) | 0 (0.00) | 1 (5.88) | |
| AST, U/L, Median (IQR) | 23.20 (17.80–32.00) | 23.00 (17.00–30.60) | 31.20 (22.00–49.10) | <0.001 |
| Normal, N (%) | 106 (79.70) | 98 (84.48) | 8 (47.06) | 0.001 |
| 1–2 ULN, N (%) | 22 (16.54) | 15 (12.93) | 7 (41.18) | |
| 2–3 ULN, N (%) | 4 (3.01) | 3 (2.59) | 1 (5.88) | |
| >3 ULN, N (%) | 1 (0.75) | 0 (0.00) | 1 (5.88) | |
| ALP, U/L, Median (IQR)a | 71.00 (55.00–82.00) | 70.00 (54.00–81.00) | 75.00 (63.00–85.00) | 0.0036 |
| Normal, N (%) | 127 (96.95) | 112 (96.55) | 15 (100.00) | 1.000 |
| 1–2 ULN, N (%) | 4 (3.01) | 4 (3.45) | 0 (0.00) | |
| 2–3 ULN, N (%) | 0 (0.00) | 0 (0.00) | 0 (0.00) | |
| >3 ULN, N (%) | 0 (0.00) | 0 (0.00) | 0 (0.00) | |
| GGT, U/L, Median (IQR)a | 24.00 (16.00–44.00) | 23.00 (15.00–43.25) | 36.00 (19.00–83.00) | 0.409 |
| Normal, N (%) | 106 (80.92) | 96 (82.76) | 10 (66.67) | 0.162 |
| 1–2 ULN, N (%) | 18 (13.74) | 15 (12.93) | 3 (20.00) | |
| 2–3 ULN, N (%) | 3 (2.29) | 2 (1.72) | 1 (6.67) | |
| >3 ULN, N (%) | 4 (3.05) | 3 (2.59) | 1 (6.67) |
Data are median (IQR) or n (%). ALT, alanine aminotransferase; ALP, alkaline phosphatase; AST, aspartate aminotransferase; GGT, gamma-glutamyltransferase; IQR, interquartile range; TBIL, total bilirubin.
Two data are missed.
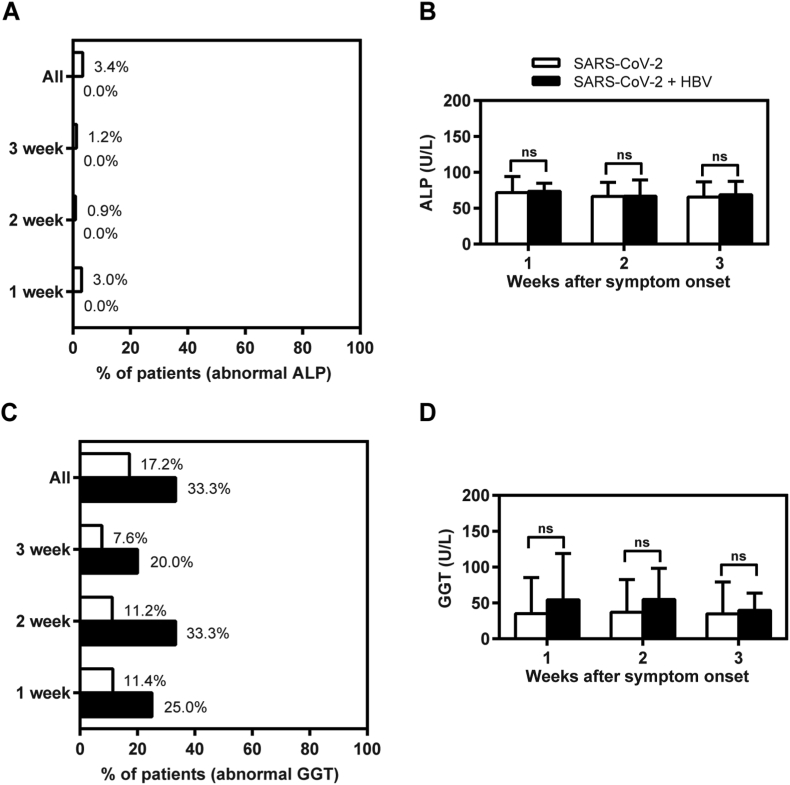
Additionally, we further analyzed the levels of serum ALP and GGT, which are the diagnostic biomarkers for cholangiocyte injury.6 While the COVID-19 cases with HBV co-infection had higher ratios of abnormal GGT than the COVID-19 cases without HBV co-infection, the majority of COVID-19 cases with or without HBV co-infection had normal ALP (Fig. 2A and C). Moreover, there were no significant differences of serum ALP and GGT levels between the two groups during the entire 3-week period (Fig. 2B and D). Thus, these results indicate that direct cytotoxicity to hepatic cholangiocytes is not the major cause of liver injury. Taken together, inactive HBV carriers with SARS-CoV-2 co-infection are at a higher risk of enhanced liver injury of the hepatocyte type.
Figure 2.
Liver functions abnormality with cholangiocyte injury during hospitalization in the patients with SARS-CoV-2 and chronic HBV co-infection. 116 COVID-19 cases with negative serum HBsAg (SARS-CoV-2 group) and 17 inactive HBV carriers with COVID-19 (SARS-CoV-2 + HBV group) were enrolled in this study. Serum samples were collected during the 3 weeks after the onset of symptoms and tested in parallel for ALP (A–B) and GGT (C–D). The positive ratios of serum ALP (A) and GGT (C) outliers were calculated for each group. The mean values of serum ALP (B) and GGT (D) levels were analyzed and compared between the two groups, respectively. ∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001; ns, not significant. ALP, alkaline phosphatase; GGT, gamma-glutamyltransferase.
Dynamic changes of inflammation biomarkers during hospitalization
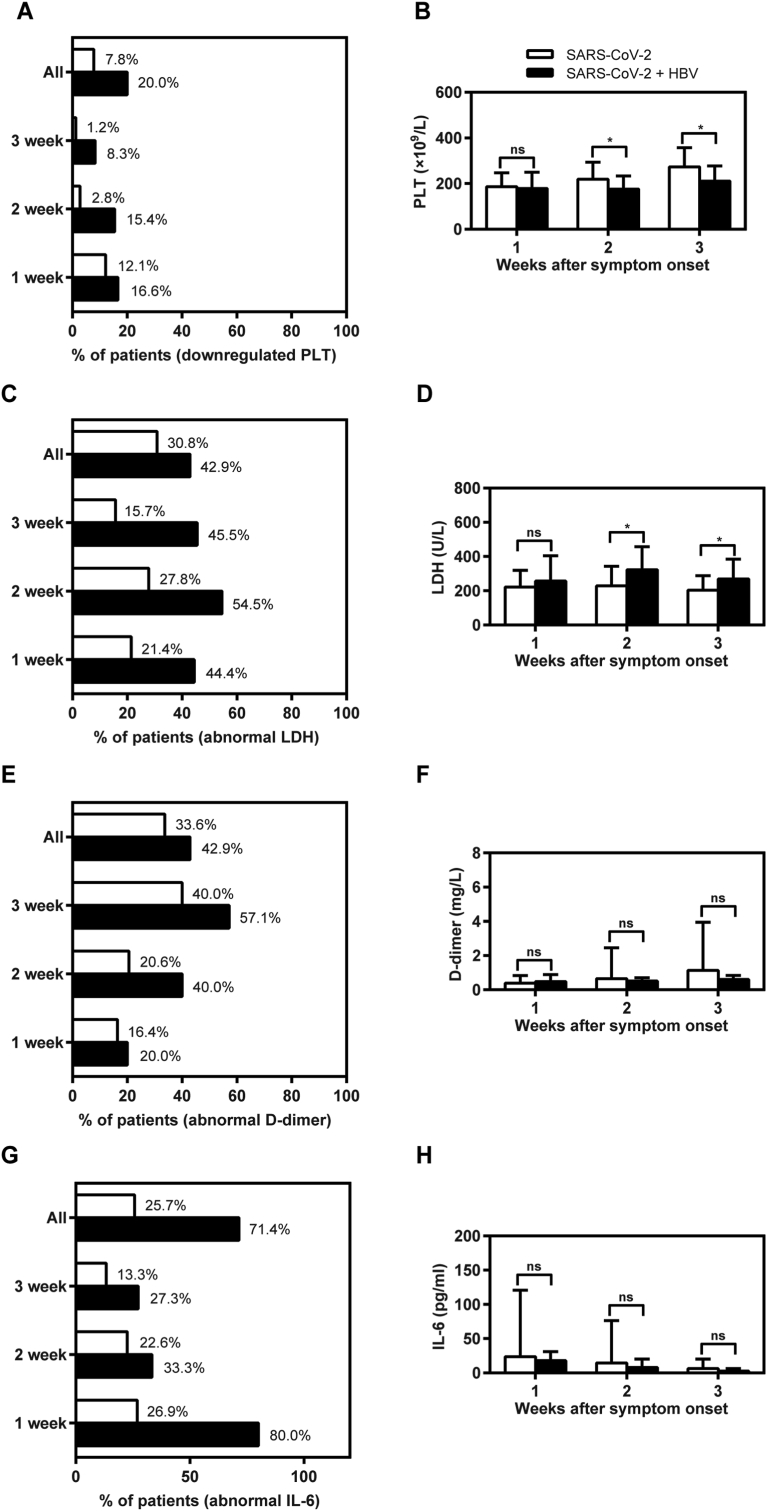
Inflammatory response may play an important role in the liver injury of COVID-19 patients after SARS-CoV-2 infection. We found that thrombocytopenia was more serious in the COVID-19 cases with HBV co-infection at 2–3 week after the onset of symptoms (Fig. 3A and B), suggesting that related inflammatory factors would contribute to their liver injury to some extent.
Figure 3.
Abnormal inflammatory factors during hospitalization in non-COVID-19 and confirmed COVID-19 cases. All 133 hospitalized confirmed cases for COVID-19 were divided into "SARS-CoV-2" group (n = 116) and "SARS-CoV-2 + HBV" group (n = 17) in this study. Whole blood or serum samples were collected during the 3 weeks after the onset of symptoms and tested in parallel for PLT (A–B), LDH (C–D), D-dimer (E–F), and IL-6 (G–H). The positive ratios of thrombocytopenia (A) from whole blood were calculated for each group. The mean values of PLT count (B) were analyzed and compared between the two groups. The positive ratios of serum LDH (C), D-dimer (E), and IL-6 (G) outliers were also calculated for each group. The mean values of serum LDH (D), D-dimer (F), and IL-6 (H) levels were analyzed and compared between the two groups, respectively. ∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001; ns, not significant. IL-6, interleukin-6; LDH, lactate dehydrogenase; PLT, platelet.
To investigate the possible mechanisms of immune mediated liver injury, we further analyzed the dynamic change of representative inflammation biomarkers, including LDH, D-dimer and IL-6. The outlier ratios of serum LDH, D-dimer and IL-6 levels in the COVID-19 cases with HBV co-infection who were inactive HBV carriers with COVID-19 were much higher than those in COVID-19 cases without HBV co-infection during the 3 weeks after the onset of symptoms (Fig. 3C–H). While there were no significant differences of serum D-dimer and IL-6 levels between the two groups during the entire 3-week period, the mean values of serum LDH levels in the COVID-19 cases with HBV co-infection are significantly higher than those in COVID-19 cases without HBV co-infection at 2–3 week after the onset of symptoms. These results indicated that the immune-mediated liver injury may be a result of the inflammatory response following SARS-CoV-2 co-infection.
Discussion
Currently, there are limited data available regarding liver injury related to SARS-CoV-2 co-infection.5, 6, 7,10,11 A recent study has revealed that the liver injury in the patients with SARS-CoV-2/HBV co-infection was related to disease severity and worse prognosis.8 Our study showed that a greater proportion of inactive HBV carriers had abnormal alterations of liver function parameters after SARS-CoV-2 co-infection, indicating a high risk of liver injury as hepatocyte type.6 The enhanced liver injury of inactive HBV carriers is likely caused by inflammatory factors.
Our study reported liver injury in mild COVID-19 cases with or without HBV co-infection. Approximately 64.71% of inactive HBV carriers with COVID-19 had abnormal liver tests during the hospitalization, while 14.29% developed liver injury. Similar to the findings reported by Chen et al,12 there were no significant differences in the discharge rate or duration of hospitalization between the COVID-19 cases with or without HBV co-infection. However, we found that a greater proportion of COVID-19 cases with HBV co-infection had abnormal alterations of TBIL, ALT, and AST after the onset of related symptoms, such as nasal congestion, fever, cough, and chills. Several reasons could contribute to the different results in the two studies. First, there is significant heterogeneity in the characteristics of the participants enrolled in these two studies. Besides, we adopted different but stricter criteria for admission and exclusion in our present study.
There are different potential factors that cause liver injury in the COVID-19 cases after SARS-CoV-2 infection. Firstly, SARS-CoV-2 may directly cause liver injury by infecting the targeting cells. SARS-CoV-2 is potential to infect the cholangiocytes with enriched angiotensin-converting enzyme 2 (ACE2) receptor expression in vitro,13,14 indicating that SARS-CoV-2 infection may directly cause ALP and GGT elevation and consequently result in liver damage. Besides, drug-induced liver injury is another critical factor during hospitalism that cannot be ignored.15, 16, 17 Especially, nearly all the patients enrolled in this study have received antibiotic and antiviral drugs, such as arbidol, lopinavir/ritonavir, and interferon, which may cause liver injury.18 However, there were no significant difference in the use of these drugs between the two groups. Considering that thrombocytopenia was more serious in the COVID-19 cases with HBV co-infection, we mainly investigated the relationship between inflammatory factors and liver injury. Immune mediated inflammatory response would be one of main causes of liver injury in the inactive HBV carriers following SARS-CoV-2 co-infection.19,20 In our study, we found that related inflammatory factors, including LDH, D-dimer and IL-6 elevation, may contribute to a greater proportion of abnormal liver function in the COVID-19 cases with HBV co-infection. However, we found that only COVID-19 cases with HBV co-infection had higher levels of serum LDH when compared with those without HBV co-infection at week 2–3 after the onset of symptoms. One of the limitations of our study was the number of patients with co-infection did not match the number of COVID-19 patients. Therefore, this may lead to no significant differences in the serum concentrations of D-dimer and IL-6. If more HBV co-infection patients are investigated, we would see significant differences between the COVID-19 cases with or without HBV co-infection, and the results would be more convincing. Moreover, the exact mechanism of enhanced liver injury caused by inflammatory response needs to be further elucidated.
Possible reasons for the HBV inactive carriers suffering more severe liver injury after SARS-CoV-2 co-infection include but are not limited to the immune status of HBV inactive carriers being disturbed by SARS-CoV-2 co-infection, which may worsen liver injury.20 Besides, inactive HBV carriers may be susceptible to HBV reactivation and liver injury with ALT flares caused by SARS-CoV-2 co-infection in the liver.13,21 Additionally, hospitalized inactive HBV carriers with SARS-CoV-2 co-infection are possibly more sensitive to hepatotoxic antiviral drugs during COVID-19 therapy, leading to more severe liver injury.
Our study also had some limitations. First, the symptoms of 17 HBV inactive carriers with COVID-19 in Chongqing, China, remained mild throughout the course of SARS-CoV-2 infection. It is unclear whether chronic HBV infection may provide some protection from a worse outcome of COVID-19. Second, while all HBV inactive carriers sustained normal serum ALT levels, we did not have the recent baseline values of serum indicators of liver function before the SARS-CoV-2 infection. Finally, as in-patient serum samples were not available from the HBV inactive carriers with SARS-CoV-2 co-infection, we do not know how their serum HBV DNA titer may have changed and whether there was HBV reactivation.
Collectively, inactive HBV carriers with SARS-CoV-2 co-infection are at risk of greater liver injury. The inflammatory response may contribute to this injury following SARS-CoV-2 co-infection. Therefore, these potential threats for the patients with chronic HBV infection should not be underestimated.
Authors contribution
Yong Lin and Ailong Huang conceived and designed the study. Yong Lin, Jun Yuan, Quanxin Long, Jieli Hu, Haijun Deng, and Zhenyu Zhao analyzed and interpreted the data. Yong Lin, Juan Chen and Mengji Lu drafted the manuscript. All authors critically revised the manuscript for important intellectual content.
Conflict of Interests
The authors declare no conflicts of interest that pertain to this work.
Funding
This work was supported by the Major National S&T Program (grant numbers 2017ZX10202203 and 2017ZX10302201) from Science & Technology Commission of China, the Emergency Project (grant number cstc2020jscx-fyzx0053) from the Science & Technology Commission of Chongqing, the Natural Science Foundation Project of CQ CSTC (grant number cstc2020jcyj-msxmX0081), the Venture and Innovation Support Program for Chongqing Overseas Returnees (grant number cx2019114), the COVID-19 Emergency Project (grant number CQMUNCP0207) and the Scientific Research Staring Foundation of Chongqing Medical University (grant number X9729) from Chongqing Medical University.
Acknowledgements
We thank all the patients, coordinators and study staff who made this study possible from Wanzhou People's Hospital and Yongchuan Hospital affiliated with Chongqing Medical University in Chongqing, China.
Footnotes
Peer review under responsibility of Chongqing Medical University.
Contributor Information
Mengji Lu, Email: mengji.lu@uni-due.de.
Ailong Huang, Email: ahuang@cqmu.edu.cn.
References
- 1.Fanning G.C., Zoulim F., Hou J., Bertoletti A. Therapeutic strategies for hepatitis B virus infection: towards a cure. Nat Rev Drug Discov. 2019;18(11):827–844. doi: 10.1038/s41573-019-0037-0. [DOI] [PubMed] [Google Scholar]
- 2.Ganesan M., Poluektova L.Y., Kharbanda K.K., Osna N.A. Human immunodeficiency virus and hepatotropic viruses co-morbidities as the inducers of liver injury progression. World J Gastroenterol. 2019;25(4):398–410. doi: 10.3748/wjg.v25.i4.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang Y., Gao Z. Study of the relationship SARS and hepatitis virus B. Chin J Clin Hepatol. 2003;19(6):342–343. [Google Scholar]
- 4.Guan W.J., Ni Z.Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cai Q., Huang D., Yu H. COVID-19: abnormal liver function tests. J Hepatol. 2020;73(3):566–574. doi: 10.1016/j.jhep.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lei F., Liu Y.M., Zhou F. Longitudinal association between markers of liver injury and mortality in COVID-19 in China. Hepatology. 2020;72(2):389–398. doi: 10.1002/hep.31301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zou X., Fang M., Li S. Characteristics of liver function in patients with SARS-CoV-2 and chronic HBV co-infection. Clin Gastroenterol Hepatol. 2020;S1542-3565(20):e30821. doi: 10.1016/j.cgh.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Organization W.H. 2020. Coronavirus Disease 2019 (COVID-19) Weekly Epidemiological Update. [Google Scholar]
- 10.Zhang C., Shi L., Wang F.S. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5(5):428–430. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu L., Liu J., Lu M., Yang D., Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40(5):998–1004. doi: 10.1111/liv.14435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen L., Huang S., Yang J. Clinical characteristics in patients with SARS-CoV-2/HBV co-infection. J Viral Hepat. 2020;27:1504–1507. doi: 10.1111/jvh.13362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chai X.H.L., Zhang Y., Han W. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. bioRxiv. 2020 [Google Scholar]
- 14.Zhao B., Ni C., Gao R. Recapitulation of SARS-CoV-2 infection and cholangiocyte damage with human liver ductal organoids. Protein Cell. 2020;11(10):771–775. doi: 10.1007/s13238-020-00718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bertolini A., van de Peppel I.P., Bodewes F. Abnormal liver function tests in COVID-19 patients: relevance and potential pathogenesis. Hepatology. 2020;72(5):1864–1872. doi: 10.1002/hep.31480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muhovic D., Bojovic J., Bulatovic A. First case of drug-induced liver injury associated with the use of tocilizumab in a patient with COVID-19. Liver Int. 2020;40(8):1901–1905. doi: 10.1111/liv.14516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olry A., Meunier L., Delire B., Larrey D., Horsmans Y., Le Louet H. Drug-induced liver injury and COVID-19 infection: the rules remain the same. Drug Saf. 2020;43(7):615–617. doi: 10.1007/s40264-020-00954-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tillmann H.L., Rockey D.C. Signatures in drug-induced liver injury. Curr Opin Gastroenterol. 2020;36(3):199–205. doi: 10.1097/MOG.0000000000000636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adams D.H., Hubscher S.G. Systemic viral infections and collateral damage in the liver. Am J Pathol. 2006;168(4):1057–1059. doi: 10.2353/ajpath.2006.051296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bangash M.N., Patel J., Parekh D. COVID-19 and the liver: little cause for concern. Lancet Gastroenterol Hepatol. 2020;5(6):529–530. doi: 10.1016/S2468-1253(20)30084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aldhaleei W.A., Alnuaimi A., Bhagavathula A.S. COVID-19 induced hepatitis B virus reactivation: a novel case from the United Arab Emirates. Cureus. 2020;12(6) doi: 10.7759/cureus.8645. [DOI] [PMC free article] [PubMed] [Google Scholar]