Abstract
Piglets experience a rapid decrease in body temperature immediately after birth, increasing the risk of mortality. The objective of this study was to determine the effect of drying and/or warming piglets at birth on rectal temperature over the first 24 h after birth. The study was carried out at a commercial sow facility using a completely randomized design with four treatments (applied to piglets at birth): Control (no drying or warming), Desiccant (dried using a desiccant), Warming Box (placed in a box under a heat lamp for 30 min), and Desiccant + Warming Box (both dried and warmed as above). Farrowing pens had one heat lamp, temperatures under which were similar to the warming box (35 °C). A total of 68 litters (866 piglets) were randomly allotted to a treatment at the birth of the first piglet. At birth, each piglet was identified with a numbered ear tag and weighed; rectal temperature was measured at 0, 10, 20, 30, 45, 60, 120, and 1,440 min after birth. Data were analyzed using a repeated-measures model using PROC MIXED of SAS. Litter was the experimental unit, piglet was a subsample of the litter; and the model included the fixed effects of treatment, time (the repeated measure), and the interaction. Rectal temperatures at birth and 1,440 min after birth were similar (P > 0.05) for all treatments. At all times between 10 and 120 min after birth, Control piglets had lower (P ≤ 0.05) temperatures than the other three treatments. The Desiccant and Warming Box treatments had similar (P > 0.05) temperatures at most measurement times, but the Desiccant + Warming Box treatment had the highest (P ≤ 0.05) rectal temperatures at most times between 10 and 60 min. In addition, for all treatments, light (<1.0 kg) birth weight piglets had lower (P ≤ 0.05) temperatures than medium (1.0–1.5 kg) or heavy (>1.5 kg) piglets at all times between 10 and 120 min. In addition, at these measurement times, the deviation in temperature between the Control and the other three treatments was greater for light than medium or heavy piglets. In conclusion, both drying and warming piglets at birth significantly increased rectal temperatures between 10 and 120 min after birth, with the combination of the two interventions having the greatest effect, especially for low birth weight piglets.
Keywords: birth weight, desiccant, hot box, piglet, temperature, warming
INTRODUCTION
Newborn piglets have little body surface insulation and limited capacity for thermoregulatory heat production, resulting in a high critical temperature (around 35 °C; Mount, 1959). Due to the lower thermoneutral zone for sows (Black et al., 1993), farrowing rooms are typically kept at temperatures considerably below the piglets’ critical temperature. The resulting temperature gradient leads to considerable heat loss from the body surface of the piglet, mainly due to convection and radiation. In addition, piglets are born wet and experience heat loss due to evaporation of the amniotic fluids. Therefore, in the absence of any intervention, all piglets will experience some degree of hypothermia under typical farrowing room conditions. This results in decreased mobility and vigor, a diminished ability to compete with littermates during suckling, and, therefore, reduced colostrum intake (Le Dividich and Noblet; 1981). This reduced energy intake and decreased immune status predisposes piglets to mortality from secondary causes, such as starvation, disease, and crushing (Devillers et al., 2011). Low birth weight piglets are at the greatest risk of hypothermia immediately after birth due to a higher body surface:volume ratio and, therefore, relatively greater potential to lose more heat than heavier littermates (Herpin et al., 2002).
One method to limit this heat loss is to reduce the temperature gradient by increasing the environmental temperature that piglets experience after birth. However, increasing the temperature of the farrowing room, although potentially beneficial for the piglets, would lead to heat stress for the sows, resulting in reduced feed intake and milk production (Farmer and Quesnel, 2009). To address this issue, most farrowing pens include a localized area at a higher temperature using, for example, heat lamps. However, newborn piglets are generally not confined to the heated area and are more attracted to the sow (Houbak et al., 2006; Pedersen et al., 2006). Warming boxes (a box placed under the heat source) can be utilized to confine piglets to the heated area for short periods of time after birth to minimize heat loss. Another method of reducing this early postnatal heat loss is through limiting the evaporation of the amniotic fluid from the body surface by drying piglets at birth (removing the source of evaporation). In this regard, Vande Pol et al. (2020) showed that drying piglets with a desiccant was effective at reducing piglet temperature loss in the early postnatal period. In theory, the combination of drying and warming piglets should have a greater effect on reducing postnatal heat loss in the newborn piglet than either approach applied separately because it reduces heat loss via three different routes (evaporation, convection, and radiation).
Although both drying and warming of piglets at or near birth are widely used in commercial practice, there has been little published research on the effects of these approaches. Therefore, the objective of this study was to determine the effects of drying and/or warming piglets at birth on rectal temperatures over the first 24 h after birth.
MATERIALS AND METHODS
This study was conducted in the farrowing facilities of a commercial breed-to-wean farm of The Maschhoffs, LLC, located near Crawfordsville, IN, during the months of January through March 2018. The experimental protocol was approved by the University of Illinois Institutional Animal Care and Use Committee prior to the initiation of the research.
Animals, Experimental Design, Treatments, and Allotment
A total of 68 litters (866 piglets) were used in the study. Sows were from commercial dam lines of Yorkshire and Landrace origin that had been mated to commercial sire lines. The study used a completely randomized design, with litter as the experimental unit (17 litters per treatment) and piglet as a subsample of the litter, to compare four treatments (applied at birth): Control (no drying or warming); Desiccant (piglets were completely dried by repeatedly coating with a commercial cellulose-based desiccant); Warming Box [piglets were placed in a plastic box under a heat lamp (temperature in the box 35.3 ± 3.64 °C) for 30 min]; Desiccant + Warming Box [piglets dried and warmed as above (temperature in the box 35.9 ± 2.94 °C)]. Litters were randomly allotted to treatment at the start of farrowing after the birth of the first piglet, with the restriction that dam genotype and parity were balanced across treatments.
Housing and Management
Sows were housed in individual farrowing crates, each located within a farrowing pen that had either woven metal or perforated plastic flooring. Crate dimensions were 0.55 by 1.95 m, giving a floor space within the crate of 1.07 m2; pen dimensions were 1.52 by 2.07 m, giving a total pen floor space of 3.15 m2. Crates were equipped with a sow-operated feed dispenser attached to a feed trough and a nipple-type water drinker for the sow. An infrared heat lamp was suspended over an insulated rubber mat located in the center of the floor area on one side of the farrowing pen (average temperature under the heat lamp was 36.1 ± 3.15 °C). For the treatments that used a warming box, the lamp was suspended over the plastic box throughout farrowing, with piglets being placed in the warming box after birth and removed after 30 min and returned to the farrowing pen, at the udder. Thermostats to maintain farrowing room temperature were set to 22.5 °C throughout the study period, and temperatures were regulated using fans and heaters.
Management in the farrowing facility was according to unit protocols, which were generally in line with standard commercial practices. Sows that had not farrowed by 116 d of gestation were induced to farrow on the following day using Lutalyse (one injection of 1 mL given at 0600 h; Zoetis, Parsippany, NJ); the identity of each sow induced and the date of induction were recorded. The farrowing process was monitored continuously by the investigators; if the interval between the births of piglets exceeded 60 min, the investigator checked the birth canal for obstructions and assisted the farrowing process as needed.
Procedures and Measurements
Piglet and sow rectal temperatures were measured using an HSTC-TT-K-24S-36 thermocouple attached via an SMPW-K-M connector to a dual input K/J digital thermometer (HH801A; Omega; Stamford, CT). A different thermocouple was used for the piglets and the sows. Thermometers were calibrated each week during the study period by taking measurements in a temperature-controlled chamber that was set at temperatures that encompassed the expected range (i.e., 30, 32, 34, 36, 38, and 40 °C). Measured and set temperatures were used to develop regression equations for both sow and piglet thermocouples, and all rectal temperature measurements taken during the study were adjusted using these regression equations.
Sow rectal temperature was measured (at a depth of 10 cm) at the start and end of the farrowing process and at 24 h after farrowing. Piglet rectal temperature was measured at birth, piglets were given a uniquely numbered ear tag for identification, and treatments were applied. Piglet temperatures were also measured at 10, 20, 30, 45, 60, 120, and 1440 min after birth. After treatments were completed (immediately for the Control and Desiccant treatments and after 30 min for the Warming Box and Desiccant + Warming Box treatments), piglets were returned to the farrowing pen, being placed at the udder. Piglets were weighed on the day of birth using a Brecknell LPS-15 bench scale (Avery Weigh-Tronix; Fairmont, MN). Scales were calibrated daily prior to use with a standard test weight.
Ambient temperatures in each farrowing pen [behind and at either side of the sow (one of these measurements being under the heat lamp)] were measured at the beginning and end of the farrowing process using a digital infrared thermometer [TOOGOO GM320 LCD digital infrared thermometer gun (Shenzhen IMC Digital Technology Co., Shenzhen, China)].
Statistical Analysis
The litter of piglets was the experimental unit for all measurements; piglet was a subsample of litter. The PROC UNIVARIATE procedure of SAS (SAS Inst. Inc., Cary, NC) was used to verify normality and homogeneity of variances of the residuals. All variables conformed to the assumptions of normality and homogeneity and were analyzed using the PROC MIXED procedure of SAS (Littell et al., 1996). The study was carried out using a completely randomized design; the model used for the analysis of sow parameters and litter measurements accounted for the fixed effect of treatment. The model used for the analysis of treatment differences in piglet birth weight also included the random effect of piglet within litter.
Treatment effects on piglet rectal temperatures were analyzed using a repeated-measures analysis, with the model accounting for the fixed effects of treatment, measurement time, and the interaction, and the random effect of piglet within litter. A repeated-measures statement was included in the model with measurement time as the REPEATED term and piglet as the SUBJECT term.
An analysis was carried out to determine if the response to treatments differed according to piglet birth weight. The data set was divided into three birth weight categories: light (<1.0 kg), medium (1.0–1.5 kg), or heavy (>1.5 kg). The maximum weight for the light category (i.e., 1.0 kg) represented the birth weight below which preweaning mortality increases substantially (Zotti et al., 2017). The minimum weight for the heavy category (i.e., 1.5 kg) represented the weight above which preweaning mortality is relatively unaffected by birth weight (Zotti et al., 2017). Piglet rectal temperature data at each measurement time were analyzed using a statistical model that included the fixed effects of birth weight category, treatment, and the interaction and the random effect of piglet within litter.
For all analyses, differences between least-squares means were separated using the PDIFF option of SAS, and differences were considered significant at P ≤ 0.05. All P-values were adjusted using a Tukey’s adjustment for multiple comparisons.
RESULTS AND DISCUSSION
Sow parameters and farrowing pen temperatures have been summarized by treatment in Table 1. There were no differences (P > 0.05) between treatments for any of the parameters or measurements. In general, the sows used in the study and the temperature conditions in the farrowing facilities were typical of U.S. commercial production. The majority of sows on the study were between parities 2 and 8. Average sow temperatures before and after farrowing were between 38.2 and 38.7 °C, which is typical for farrowing sows (Littledike et al., 1979). Average farrowing room temperatures (between 21.4 and 22.6 °C; Table 1) were close to the set point (22.5 °C).
Table 1.
Summary of sow parity and rectal temperature and farrowing pen temperatures during the study by treatment
| Treatment1 | ||||||
|---|---|---|---|---|---|---|
| Item | Control | Desiccant | Warming Box | Desiccant + Warming Box | SEM | P-value |
| Average sow parity | 3.8 | 3.5 | 4.1 | 4.4 | 0.71 | 0.84 |
| Number of sows by parity2 | ||||||
| Parity 1 | 0 | 0 | 0 | 0 | – | – |
| Parity 2 | 4 | 5 | 4 | 1 | – | – |
| Parities 3 and 4 | 7 | 5 | 4 | 9 | – | – |
| Parities 5–8 | 4 | 6 | 7 | 5 | – | – |
| Parities 9+ | 2 | 1 | 2 | 2 | – | – |
| Sow rectal temperature, °C | ||||||
| Start of farrowing | 38.2 | 38.2 | 38.2 | 38.3 | 0.13 | 0.89 |
| After farrowing | 38.4 | 38.4 | 38.4 | 38.4 | 0.15 | 0.95 |
| 24 h after farrowing | 38.4 | 38.7 | 38.6 | 38.6 | 0.18 | 0.81 |
| Farrowing pen temperature, °C | ||||||
| Before farrowing | ||||||
| Under heat lamp | 35.9 | 35.9 | 36.2 | 35.1 | 0.79 | 0.79 |
| Side of pen opposite heat lamp | 22.6 | 22.2 | 21.5 | 22.0 | 0.59 | 0.62 |
| Behind sow | 22.3 | 22.1 | 21.7 | 21.4 | 0.49 | 0.57 |
| After farrowing | ||||||
| Under heat lamp | 35.8 | 35.0 | 36.1 | 34.6 | 0.67 | 0.33 |
| Side of pen opposite heat lamp | 22.7 | 22.3 | 22.2 | 22.4 | 0.49 | 0.90 |
| Behind sow | 21.8 | 21.8 | 21.6 | 21.6 | 0.46 | 0.97 |
1Control: piglets were not dried; Desiccant: piglets were dried at birth by coating with a desiccant; Warming Box: piglets were placed in a warming box for 30 min after birth; Desiccant + Warming Box: piglets were dried at birth by coating with a desiccant, then placed in a warming box for 30 min.
2Parity = total number of litters including the one used in the study.
Effect of Treatments on the Temperature Decline of Piglets
Least-squares means for the drying and/or warming treatments for litter size, piglet birth weight, and piglet rectal temperature over the first 24 h after birth are presented in Table 2. The number of piglets born alive (12.3 to 13.3 per litter) were similar (P > 0.05) across treatments and were comparable to values for U.S. herds reported by PigChamp at the time that this study was conducted (13.2 piglets per litter; PigChamp 2017–2018). There were no differences between treatments (P > 0.05) for piglet birth weights (Table 2), which were similar to those reported in recent studies (e.g., Feldspausch et al., 2019).
Table 2.
Least-squares means for the effect of treatment on litter size, birth weight, and rectal temperature of piglets over the first 24 h after birth
| Treatment1 | ||||||
|---|---|---|---|---|---|---|
| Control | Desiccant | Warming Box | Desiccant + Warming Box | SEM | P-value | |
| Number of litters | 17 | 17 | 17 | 17 | – | – |
| Number of piglets born alive | ||||||
| Total | 226 | 209 | 214 | 217 | – | – |
| Average per litter | 13.3 | 12.3 | 12.6 | 12.8 | 0.85 | 0.86 |
| Piglet birth weight (born alive), kg | 1.46 | 1.46 | 1.45 | 1.44 | 0.023 | 0.89 |
| Piglet rectal temperature, °C | ||||||
| Time after birth, min | ||||||
| 0 | 38.9 | 38.9 | 38.9 | 38.9 | 0.03 | 0.98 |
| 10 | 36.7c | 37.1b | 37.4ab | 37.6a | 0.03 | <0.0001 |
| 20 | 35.6c | 36.9b | 37.0b | 37.8a | 0.03 | <0.0001 |
| 30 | 35.2c | 37.2b | 37.2b | 38.1a | 0.03 | <0.0001 |
| 45 | 35.5d | 37.7b | 37.3c | 38.2a | 0.03 | <0.0001 |
| 60 | 36.1c | 38.1a | 37.7b | 38.4a | 0.03 | <0.0001 |
| 120 | 37.7b | 38.5a | 38.3a | 38.6a | 0.03 | <0.0001 |
| 1,440 | 38.7 | 38.7 | 38.6 | 38.7 | 0.03 | 0.14 |
a,b,c,dWithin a row, means with differing superscripts differ at P ≤ 0.05.
1Control: piglets were not dried; Desiccant: piglets were dried at birth by coating with a desiccant; Warming Box: piglets were placed in a warming box for 30 min after birth; Desiccant + Warming Box: piglets were dried at birth by coating with a desiccant, then placed in a warming box for 30 min.
There was no effect (P > 0.05) of treatment on rectal temperatures at birth (Table 2) with the means for all treatments being the same (Table 2). This was as expected as birth temperatures were taken before the treatments were applied. Birth temperatures observed in previous research have varied from 37.0 °C (Kammersgaard et al., 2011) to 40.5°C (Pomeroy, 1953). In addition, Kammersgaard et al. (2011) found considerable variation within the same study (between 37.0 and 41.5 °C). Piglet temperatures decline rapidly after birth (Table 2), and variation between studies for birth temperature may reflect differing times of measurement relative to the time of birth.
The decline in rectal temperature of Control piglets after birth, which provides an estimate of changes experienced by undried piglets, was extensive, with the minimum temperature, which was at 30 min, being 3.7 °C lower than at birth (Table 2). Subsequently, temperatures increased and approached the level observed at birth by 1,440 min. A number of studies have also found that the minimum temperature of undried piglets occurred at 30 min after birth; however, values at this time varied between studies ranging from 33.6 °C (Xiong et al., 2018) to 36.6 °C (Pattison et al., 1990). Most studies have found that, on average, temperatures reach levels close to those at birth by 24 h after birth (McGinnis et al., 1981; Xiong et al., 2018; Cooper et al., 2019).
Piglets on the Desiccant and Warming Box treatments had higher (P ≤ 0.05) temperatures than those on the Control treatment at all times between 10 and 120 min after birth (Table 2). In addition, temperatures were similar for the Desiccant and Warming Box treatments at 10, 20, 30, and 120 min after birth but were lower (P ≤ 0.05) for the Warming Box treatment at 45 and 60 min. However, the differences at these two times were relatively small (0.4 °C). Minimum temperatures of piglets on both of these treatments occurred earlier and were higher (P ≤ 0.05) than those on the Control (Table 2). Both drying and warming of piglets at birth have been used in commercial production; however, there has been limited research comparing these approaches. Most studies have shown that drying reduced the extent of piglet temperature decline in the first 60 min after birth; however, the magnitude of the effect varied between studies. This may in part be due to the use of different drying materials and/or the timing of measurement of rectal temperature after birth (e.g., Berbigier et al., 1978; McGinnis et al., 1981). However, studies have also shown variation in the effectiveness of using a desiccant as the drying agent for reducing postnatal temperature decline. Cooper et al. (2019) found that the maximum difference in temperature between undried piglets and those dried with a desiccant was at 45 min and was 2.4 °C, whereas, for Vande Pol et al. (2020), this was at 60 min and was 1.4 °C. In the current study, the maximum difference was 2.2 °C and was at 45 min after birth (Table 2). Further research is required to establish the reasons for this variation in response to similar drying treatments.
Published studies related to the warming of piglets at birth are limited in number and varied considerably in approach. Pedersen et al. (2016) found that confining piglets under a radiant heat source (at 34 °C) for 2 h compared to leaving them at room temperature (at 20.9 °C) increased the minimum temperature by between 1.2 and 1.4 °C, which is similar to the results for the Warming treatment in the current study. In contrast, Pattison et al. (1990) showed a small increase in temperature (0.3 °C at 60 min after birth) from confining piglets in a heated creep area for 45 min. However, the warming treatment in that study started at 15 min after birth, by which time piglet temperatures would have decreased considerably. A number of studies added localized heat sources to the farrowing pen without confining piglets to the heated areas (e.g., McGinnis et al., 1981; Andersen and Pedersen, 2015) and found a smaller effect on rectal temperatures than the current study, suggesting that confining piglets to a heated area was a more effective approach. Instead of providing a localized heat source for warming piglets, some studies have evaluated the impact of increasing the temperature of either the farrowing pen or the entire room. Le Dividich and Noblet (1981) found that the rectal temperature of piglets kept at an ambient temperature of 30–32 °C was 1.6 °C higher (at 20 min after birth) than that of piglets kept at 18–20 °C. Pedersen et al. (2013) found that piglets in rooms at 25 °C had higher temperatures at 30 min after birth (0.9 °C) than those in rooms kept at 15 or 20 °C. In comparison, the current study found a difference between the Warming Box and Control treatments of 2.0 °C at this time.
In the current study, both drying and warming were effective at reducing piglet temperature decline in the early postnatal period; however, the combination of these two approaches was most effective. The Desiccant + Warming Box treatment resulted in the highest (P ≤ 0.05) temperatures compared to all other treatments between 20 and 45 min after birth and the highest minimum temperature at the earliest time after birth (Table 2). This is the first study that we are aware of that combined these treatments. As previously discussed, drying of piglets should minimize evaporative heat loss, whereas warming piglets reduces convective and radiative heat loss by reducing the temperature gradient between the piglet and the environment. Given that these two interventions, applied separately, had a relatively similar effect on postnatal body temperature changes suggests that the magnitude of heat loss by these routes are relatively similar. However, the combination of drying and warming should reduce heat loss by both routes, and the results of this study indicate that this was the most effective method of reducing piglet temperature decline within the first hour after birth. While all of the previous research, including the current study, showed that drying and/or warming piglets increased rectal temperatures within the first hour after birth, most found that the magnitude of this effect subsequently decreased and was minimal by 24 h after birth, when temperatures of piglets on all treatments approached the levels observed at birth.
Effect of Piglet Birth Weight on Responses to Treatments
Least-squares means for the treatment by birth weight category interaction are presented in Table 3. There were interactions (P ≤ 0.05) at all measurement times except at birth. At all other measurement times and for all treatments, light piglets had lower (P ≤ 0.05) temperatures than the other birth weight categories. Medium piglets had lower temperatures than heavy (P ≤ 0.05) at all times between 10 and 60 min for the Control, Desiccant, and Warming Box treatments and at 10 min for the Desiccant + Warming Box treatment (Table 3). At all other times, there were no differences (P > 0.05) between temperatures of medium and heavy piglets for any of the four treatments. Previous research has also shown that the extent and duration of the temperature decline after birth is greater in low birth weight piglets than in heavier littermates (Pattison et al., 1990; Pedersen et al., 2016; Cooper et al., 2019; Vande Pol et al., 2020). Lighter piglets are predisposed to chilling (Muns et al., 2016), having a high body surface area to volume ratio, low body fat for insulation (Curtis, 1974), and limited energy reserves (glycogen and fat) for heat production (Lossec et al., 1998).
Table 3.
Least-squares means for the interaction of treatment (T) and birth weight category (BWC) on the rectal temperature of piglets over the first 24 h after birth
| Item | Treatment (T)1 | P-value | |||||
|---|---|---|---|---|---|---|---|
| Item | Control | Desiccant | Warming Box | Desiccant + Warming Box | SEM | BWC × T interaction | |
| Number of piglets born alive | |||||||
| Light | 15 | 20 | 18 | 33 | – | – | |
| Medium | 101 | 91 | 104 | 77 | – | – | |
| Heavy | 110 | 98 | 92 | 107 | – | – | |
| Piglet rectal temperature, °C | |||||||
| Time after birth, min | |||||||
| 0 | BWC2 | 0.04 | 0.09 | ||||
| Light | 38.7 | 38.7 | 38.6 | 38.7 | – | – | |
| Medium | 38.8 | 38.8 | 38.8 | 38.8 | – | – | |
| Heavy | 39.0 | 38.9 | 39.1 | 38.9 | – | – | |
| 10 | BWC2 | 0.04 | <0.0001 | ||||
| Light | 35.5e | 36.2de | 36.4d | 36.9cd | – | – | |
| Medium | 36.6d | 37.0c | 37.1c | 37.5b | – | – | |
| Heavy | 37.0c | 37.5b | 37.8ab | 37.8a | – | – | |
| 20 | BWC2 | 0.04 | <0.0001 | ||||
| Light | 33.8g | 35.8ef | 36.0ef | 37.1cd | – | – | |
| Medium | 35.4f | 36.7d | 36.9d | 37.7ab | – | – | |
| Heavy | 36.0e | 37.4bc | 37.5bc | 38.1a | – | – | |
| 30 | BWC2 | 0.04 | <0.0001 | ||||
| Light | 33.1g | 35.8e | 36.1e | 37.4cd | – | – | |
| Medium | 35.1f | 36.9d | 37.1d | 38.1ab | – | – | |
| Heavy | 35.7e | 37.7bc | 37.7bc | 38.3a | – | – | |
| 45 | BWC2 | 0.04 | <0.0001 | ||||
| Light | 33.0g | 36.1ef | 35.8ef | 37.2cd | – | – | |
| Medium | 35.4f | 37.4cd | 37.3d | 38.2ab | – | – | |
| Heavy | 36.0e | 38.2ab | 37.7bc | 38.5a | – | – | |
| 60 | BWC2 | 0.04 | <0.0001 | ||||
| Light | 33.1h | 36.6efg | 36.1fg | 37.5cde | – | – | |
| Medium | 36.0g | 37.9bcd | 37.6d | 38.4ab | – | – | |
| Heavy | 36.6f | 38.5a | 38.2abc | 38.6a | – | – | |
| 120 | BWC2 | 0.04 | <0.0001 | ||||
| Light | 35.4f | 37.4e | 37.3e | 38.0cde | – | – | |
| Medium | 37.7e | 38.5abc | 38.3bcd | 38.6ab | – | – | |
| Heavy | 38.0de | 38.8a | 38.6ab | 38.7a | – | – | |
| 1,440 | BWC2 | 0.04 | 0.0002 | ||||
| Light | 38.1abc | 38.0c | 38.5abc | 38.4abc | – | – | |
| Medium | 38.7ab | 38.7ab | 38.5bc | 38.6ab | – | – | |
| Heavy | 38.7ab | 38.9a | 38.6ab | 38.8ab | – | – | |
a,b,c,d,e,f,g,hFor each time, means within the T × BWC interaction with differing superscripts differ, P ≤ 0.05.
1Control: not dried; Desiccant: dried by coating with a desiccant; Warming Box: placed in a warming box for 30 min; Desiccant + Warming Box: dried by coating with a desiccant, then placed in a warming box for 30 min.
2Light = <1.0 kg; medium = 1.0–1.5 kg; heavy = >1.5 kg.
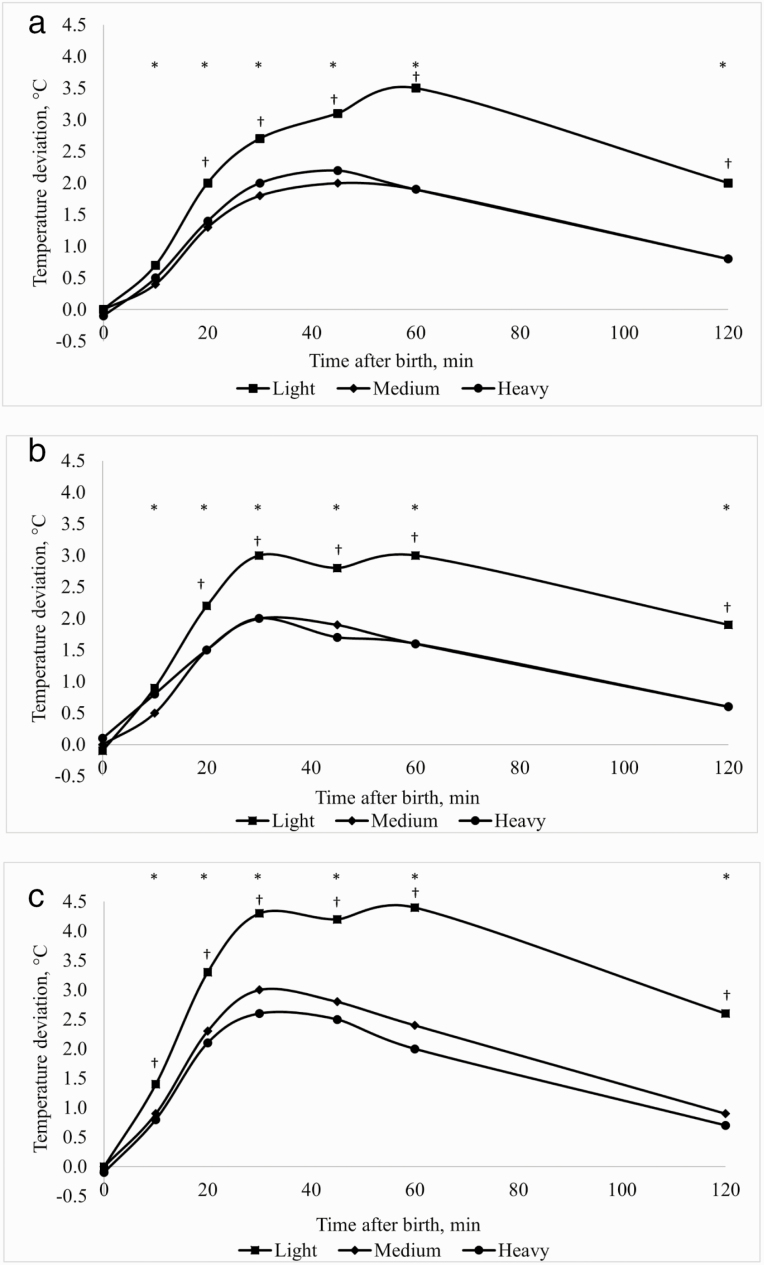
Piglets of all birth weight categories on the three drying and/or warming treatments had higher (P ≤ 0.05) temperatures than those on the Control between 10 and 120 min after birth, with the exception of light piglets on the Desiccant treatment, which had a similar (P > 0.05) temperature to the Control at 10 min after birth (Table 3). Therefore, the treatment by birth weight category interactions were largely due to differences in the magnitude of the temperature deviation between treatments within each birth weight category. This is illustrated by the deviations between the temperatures of the Control and the other three treatments for each birth weight category at each measurement time, which are presented in Fig. 1a–c. For all three treatments, the deviations from the Control treatment were similar (P > 0.05) for medium and heavy piglets at all measurement times from 10 to 120 min but were much greater (P ≤ 0.05) for light piglets between 20 and 120 min after birth. For example, at 30 min after birth, light piglets on the Desiccant + Warming Box treatment had temperatures that were 4.3 °C higher than those on the Control treatment. In comparison, this difference was 3.0 °C for medium and 2.6 °C for heavy piglets at this time (Fig. 1c). For all birth weight categories, the minimum temperature of dried and/or warmed piglets occurred earlier and was greater than the Control. For example, the minimum temperature of light piglets occurred at 10 min after birth for the Desiccant + Warming Box treatment compared to 45 min for the Control (Table 3). These results suggest that drying and warming, either singularly or in combination, reduced the extent and duration of temperature decline for piglets of all birth weights but had a greater effect for those of low birth weight.
Figure 1.
Deviation in piglet rectal temperature between the Control and the Desiccant (a), Warming Box (b), or Desiccant + Warming Box (c) treatments over the first 2 h after birth, for Light (<1.0 kg), Medium (1.0 to1.5 kg), and Heavy (>1.5 kg) Birth Weight Categories. *Within each treatment, deviation from the Control treatment different to 0 (P ≤ 0.05) for all Birth Weight Categories. †Within each treatment, the deviation from the Control treatment for the Light and Medium Birth Weight Categories differed (P ≤ 0.05). There were no differences (P > 0.05) between deviations for Medium and Heavy Birth Weight Categories.
Two studies have evaluated the potential interaction between piglet birth weight and intervention treatments for postnatal temperature changes, and both found similar results to the current experiment. Pedersen et al. (2016) found that adding a radiant heat source to the farrowing pen increased piglet rectal temperatures between 0 and 120 min after birth and reduced the time piglets had temperatures below 35 °C for all weight groups, with these effects being greater for light than heavy piglets. Similarly, Vande Pol et al. (2020) found that drying piglets at birth reduced the magnitude and duration of temperature decline to a greater extent in lower compared to heavier birth weight piglets.
In conclusion, the results of the current study confirm that birth weight is an important factor influencing piglet temperatures in the early postnatal period, with lower birth weight piglets experiencing the greatest extent and duration of temperature decline. Drying or warming piglets at birth were similarly effective at reducing these temperature changes, with the combination being most effective, especially for low birth weight piglets.
Footnotes
Funding, wholly or in part, was provided by the National Pork Checkoff.
LITERATURE CITED
- Andersen H. M., and Pedersen L. J.. . 2015. Effect of radiant heat at the birth site in farrowing crates on hypothermia and behaviour in neonatal piglets. Animal. 10:128–134. doi: 10.1017/S1751731115001913. [DOI] [PubMed] [Google Scholar]
- Berbigier P., Le Dividich J., and Kobilinsky A.. . 1978. Echanges thermiques chez le porcelet nouveau-né: application de la méthode du bilan d’énergie. Ann. Zootech. 27:181–194. doi: 10.1051/animres:19780206. [DOI] [Google Scholar]
- Black J. L., Mullan B. P., Lorschy M. L., and Giles L. R.. . 1993. Lactation in the sow during heat stress. Livest. Prod. Sci. 35:153–170. doi: 10.1016/0301-6226(93)90188-N. [DOI] [Google Scholar]
- Cooper N. C, Vande Pol K. D., Ellis M., Xiong Y., and Gates R.. . 2019. Effect of piglet birth weight and drying on post-natal changes in rectal temperature. J. Anim. Sci. 97:4. doi: 10.1093/jas/skz122.006. [DOI] [Google Scholar]
- Curtis S. 1974. Responses of the piglets to perinatal stressors. J. Anim. Sci. 38:1031–1036. doi: 10.2527/jas1974.3851031x. [DOI] [PubMed] [Google Scholar]
- Devillers N., Le Dividich J., and Prunier A.. . 2011. Influence of colostrum intake on piglet survival and immunity. Animal 5:1605–1612. doi: 10.1017/S175173111100067X. [DOI] [PubMed] [Google Scholar]
- Farmer C., and Quesnel H.. . 2009. Nutritional, hormonal, and environmental effects on colostrum in sows. J. Anim. Sci. 87(13 Suppl.):56–64. doi: 10.2527/jas.2008-1203. [DOI] [PubMed] [Google Scholar]
- Feldspausch, J. A., J. Jourquin, J. R. Bergstrom, J. L. Bargen, C. D. Bokenkroger, D. L. Davis, J. M. Gonzalez, J. L. Nelssen, C. L. Puls, W. E. Trout, and M. J. Ritter. 2019. Birth weight threshold for identifying piglets at risk for preweaning mortality. Transl. Anim. Sci. 3:633–640. doi: 10.1093/tas/txz076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herpin P., Damon M., and Le Dividich J.. . 2002. Development of thermoregulation and neonatal survival in pigs. Livest. Prod. Sci. 78:25–45. doi: 10.1016/S0301-6226(02)00183-5. [DOI] [Google Scholar]
- Houbak B., Thodberg K., Malkvist J., and Pedersen L. J.. 2006. Effect of pen floor heating on piglets use of heated area 0–120 h postpartum. In: M. Mendl, J. W. S. Bradshaw, O. H. P. Burman, A. Butterworth, M. J. Harris, S. D. E. Held, S. M. Jones, K. E. Littin, D. C. J. Main, C. J. Nicol, R. M. A. Parker, E. S. Paul, G. Richards, C. M. Sherwin, P. T. E. Statham, M. J. Toscano, and P. D. Warriss, editors. Proceedings of the 40th International Congress of the ISAE, Bristol, United Kingdom, 8-12 Aug, 2006. Bristol, United Kingdom: International Society for Applied Ethology. p. 156. [Google Scholar]
- Kammersgaard T. S., Pedersen L. J., and Jørgensen E.. . 2011. Hypothermia in neonatal piglets: interactions and causes of individual differences. J. Anim. Sci. 89:2073–2085. doi: 10.2527/jas.2010-3022. [DOI] [PubMed] [Google Scholar]
- Le Dividich J., and Noblet J.. . 1981. Colostrum intake and thermoregulation in the neonatal pig in relation to environmental temperature. Biol. Neonate 40:167–174. doi: 10.1159/000241486. [DOI] [PubMed] [Google Scholar]
- Littell R. C., Milliken G. A., Stroup W. W., and Wolfinger R. D.. . 1996. SAS systems for mixed models. SAS Inst. Inc., Cary, NC. [Google Scholar]
- Littledike E. T., Witzel D. A., and Riley J. L.. . 1979. Body temperature changes in sows during the periparturient period. Lab. Anim. Sci. 29:621–624. PMID: 513630. [PubMed] [Google Scholar]
- Lossec G., Herpin P., and Le Dividich J.. . 1998. Thermoregulatory responses of the newborn pig during experimentally induced hypothermia and rewarming. Exp. Physiol. 83:667–678. doi: 10.1113/expphysiol.1998.sp004148. [DOI] [PubMed] [Google Scholar]
- McGinnis R. M., Marple D. N., Ganjam V. K., Prince T. J., and Pritchett J. F.. . 1981. The effects of floor temperature, supplemental heat and drying at birth on neonatal swine. J. Anim. Sci. 53:1424–1432. doi: 10.2527/jas1982.5361424x. [DOI] [PubMed] [Google Scholar]
- Mount L. E. 1959. The metabolic rate of the new-born pig in relation to environmental temperature and age. J. Physiol. 147:333–345. doi: 10.1113/jphysiol.1959.sp006247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muns R., Nuntapaitoon M., and Tummaruk P.. . 2016. Non-infectious causes of pre-weaning mortality in piglets. Livest. Sci. 184:46–57. doi: 10.1016/j.livsci.2015.11.025. [DOI] [Google Scholar]
- Pattison R., English P., MacPherson O., Roden J., and Birnie M.. . 1990. Hypothermia and its attempted control in newborn piglets. Proc. Br. Soc. Anim. Prod. 1990:81–81. doi: 10.1017/S0308229600018626. [DOI] [Google Scholar]
- Pedersen L.J., Jorgensen E., Heiskanen T., and Damm B. I.. 2006. Early piglet mortality in loose-housed sows related to sow and piglet behaviour and to the progress of parturition. Appl. Anim. Behav. Sci. 96: 215–232. doi: 10.1016/j.applanim.2005.06.016. [DOI] [Google Scholar]
- Pedersen L. J., Larsen M. L., and Malmkvist J.. . 2016. The ability of different thermal aids to reduce hypothermia in neonatal piglets. J. Anim. Sci. 94:2151–2159. doi: 10.2527/jas.2015-0219. [DOI] [PubMed] [Google Scholar]
- Pedersen L. J., Malmkvist J., Kammersgaard T., and Jørgensen E.. . 2013. Avoiding hypothermia in neonatal pigs: effect of duration of floor heating at different room temperatures. J. Anim. Sci. 91:425–432. doi: 10.2527/jas.2011-4534. [DOI] [PubMed] [Google Scholar]
- PigChamp 2017–2018. Benchmarking summaries Available from www.pigchamp.com/benchmarking. (Accessed June 11, 2020).
- Pomeroy R. W. 1953. Studies on piglet mortality. I. Effect of low temperature and low plane of nutrition on the rectal temperature of the young pig. J. Agric. Sci. 43:182–191. doi: 10.1017/S0021859600044956. [DOI] [Google Scholar]
- Vande Pol K. D., Tolosa A. F., Shull C. M., Brown C. B., Alencar S. A. S., and Ellis M.. . 2020. Effect of method of drying piglets at birth on rectal temperature over the first 24 hours after birth. Transl. Anim. Sci. doi: 10.1093/tas/txaa183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y., Gates R., Cooper N., and Ellis M.. . 2018. Neonatal piglet core body temperatures model from surface temperature and environment measurements. In: G. Fox, editor. Proceedings of the International Livestock Environment Symposium, Omaha, NE 25–27 Sep, 2018. St. Joseph, MI: American Society of Agricultural and Biological Engineers. p. 1–12. ILES18-128. doi: 10.13031/iles.18-128. [DOI] [Google Scholar]
- Zotti E., Resmini F. A., Schutz L. G., Volz N., Milani R. P., Bridi A. M., Alfieri A. A., and da Silva C. A.. . 2017. Impact of piglet birthweight and sow parity on mortality rates, growth performance, and carcass traits in pigs. R. Bras. Zootec. 46:856–862. doi: 10.1590/s1806-92902017001100004. [DOI] [Google Scholar]