Abstract
With increasing treatment options available, the management of locally advanced and metastatic prostate cancer (PCa) is growing more complex, nuanced, and individualized. Strategies for combining surgery, radiation, chemotherapy, and androgen deprivation therapy (ADT) continue to evolve, as do ADT and immunotherapy options. Additionally, multiple adjunctive agents for metastatic PCa have been recently approved or are pending approval. As the number of locally advanced and metastatic prostate cancers being diagnosed rises, so does the need to consider patients' clinical situations and personal preferences. This review discusses current and potential future approaches to managing locally advanced and metastatic PCa.
Keywords: Prostate cancer, Metastatic prostate cancer, Locally advanced prostate cancer, Androgen deprivation therapy, Chemotherapy
As diagnostic techniques and treatments for locally advanced and metastatic prostate cancer (PCa) continue to evolve, men with these more advanced prostate cancers are living longer.1,2 Yet PCa remains the second-leading cause of cancer death among American men.3
The National Cancer Institute predicts that in 2020, 191,930 American men will be diagnosed with PCa, and 33,330 men will die of PCa.4 Since 1999, the rate of PCa incidence per 100,000 American men has fallen from approximately 170 to 109.5,5 as organizations began advising against routine prostate-specific antigen (PSA) testing in 2008.6 Although incidence of low-risk prostate cancers fell 37% between 2004 and 2013, incidence of metastatic PCa during those years rose 72%.7 To help clinicians better understand and navigate the treatment landscape for locally advanced and metastatic PCa, this review assesses present and potential future therapies.
In brief, when selecting nonsurgical options for androgen deprivation therapy (ADT), physicians must consider multiple factors, including these drugs' pharmacological profiles, efficacy, and cost. For initial treatment of intermediate- and high-risk localized PCa, guidelines recommend radical prostatectomy (RP) or radiotherapy (RT), with or without ADT. Management of biochemical recurrence (BCR) remains controversial. First-line treatment for newly diagnosed M1 PCa (metastatic hormone-sensitive prostate cancer [mHSPC]) involves ADT alongside guideline considerations for adding approved androgen receptor axis inhibitors as well as docetaxel. For castration-resistant prostate cancer (CRPC), guidelines recommend maintaining castrate testosterone (T) levels through continued ADT.
To discuss current treatments and future concerns in locally advanced and metastatic PCa, the author searched PubMed, meeting abstracts, and clinicaltrials.gov from 1941 through early 2020 using search terms such as locally advanced prostate cancer (LAPC) guidelines, gonadotropin-releasing hormone (GnRH) agonist/antagonist, ADT, and intermediate, high-risk, and metastatic PCa. The author ultimately selected 102 of the most relevant publications for inclusion in this article.
Results
The vast majority of US cancers—77%, according to National Cancer Institute/Surveillance, Epidemiology, and End Results (NCI/SEER) data (2009–2015)—remain localized at diagnosis, whereas 13% and 6% of PCas have spread to regional lymph nodes and distant sites, respectively.4 The aggressiveness of initial PCa treatment generally depends on the risk level with which disease presents (Table 1, Table 2).
TABLE 1.
Prostate Cancer Risk Stratification
| Risk Level | Very Lowa | Lowa | Intermediateb | Highb | Very Highb |
|---|---|---|---|---|---|
| Clinical stage | T1c | T1–T2a | T2b–T2c | T3a | T3b–T4 |
| Gleason score (GS) | ≤6 | ≤6 | 7 | 8–10 | 8–10 |
| Prostate-specific antigen (ng/mL) | <10 | <10 | 10–20 | >20 | >20 |
| Additional findings | <3 cores positive, ≤50% cancer in each core, PSA density <0.15 ng/mL/g | Primary Gleason pattern 5, >4 cores GS 8–10 |
TABLE 2.
International Society of Urological Pathologists Grading System10
| Risk Group | Grade Group | Gleason Score |
|---|---|---|
| Low | 1 | ≤6 |
| Intermediate favorable | 2 | 7 (3 + 4) |
| Intermediate unfavorable | 3 | 7 (4 + 3) |
| High | 4 | 8 |
| High | 5 | 9–10 |
Therapeutic Goals of ADT
Since Huggins and Hodges first elucidated the androgen-dependent nature of PCa growth, reducing serum testosterone levels through ADT has become the first-line strategy for treating advanced and metastatic disease.11,12 The American Urological Association (AUA), the National Comprehensive Cancer Network (NCCN), and the European Association of Urology (EAU) all recommend ADT as primary systemic therapy for advanced and metastatic PCa, and in combination with neoadjuvant or adjuvant radiation therapy in localized or locally advanced PCa.8,13,14 Over the years, the target T level of ADT has arguably evolved from ≤50 ng/dL to below 20 ng/dL based on some association guidelines and consensus papers, suggesting that the latter lower level represents a more effective medical castration.11
Non-surgical ADT Options
The variety of treatment options for LAPC and metastatic PCa allows individualization of choices for selecting ADT. Through shared decision-making (SDM), physicians and patients must consider not only clinical factors, but also patients' lifestyles, injection preferences, and compliance factors, along with accessibility and costs.
Widely used non-surgical ADT options include GnRH agonists and GnRH antagonists (Table 3). Popular extended-release formulations allow dosing at intervals ranging from 1 to 12 months.11
TABLE 3.
Pharmacological Profiles of GnRH Agonists and Antagonists
| Clinical Parameter | GnRH Agonists | GnRH Antagonists |
|---|---|---|
| Initial testosterone (T) surge (which may cause clinical flare) | Yes | No |
| T suppression onset | 3 d | Immediate |
| Castration achieved 28 d | 3–4 d | |
| T microsurges | Yes | No |
| Follicle-stimulating hormone suppression | Partial | Rapid and sustained |
| Prostate-specific antigen suppression | Slower through Day 60 ± | Faster in first 2 mo |
Mechanistically, GnRH agonists bind to GnRH receptors, initially provoking profound stimulation, which leads to substantial increases in GnRH/luteinizing hormone (LH), follicle-stimulating hormone (FSH), and T.15,16 Sustained overstimulation of the pituitary desensitizes GnRH receptors, thus leading to decreased hormone levels.17 To block clinical flare symptoms such as urinary obstruction and bone pain stemming from the initial hormonal surge provoked by GnRH agonists, physicians should prescribe a firstgeneration anti-androgen (bicalutamide, flutamide, or nilutamide) before or along with GnRH agonist therapy (combined androgen blockade [CAB]) and continuing in combination for at least 7 days.8
GnRH antagonists block androgen receptors, immediately halting GnRH/LH production. Results include rapid T suppression without an initial hormonal surge, and prolonged suppression without escapes or microsurges as can occur upon re-administration of GnRH agonist doses.15 Additionally, GnRH antagonists quickly suppress FSH, which contributes to flares associated with GnRH agonist therapy.16 In the CS21 phase 3 trial, degarelix achieved faster T decline in the first month, superior PSA decline in the first 2 months, and slightly better efficacy in metastatic disease than did leuprolide.18 However, this trial did not provide evidence to support a short- or long-term advantage of degarelix over leuprolide in patients with BCR. Disadvantages of GnRH antagonists in clinical practice may include their cost, convenience level (only monthly dosing is available), and administration tolerability.19
Additional SDM considerations include serum T nadir level. Increasing evidence indicates that very low T levels may be associated with improved outcomes, including survival.21–23 Such studies underscore the importance of both monitoring T levels and choosing an ADT that achieves T nadir efficacy.14
Adverse events (AEs) associated with ADT also require discussion. In 2010, the US Food and Drug Administration (FDA) and other organizations warned users of GnRH agonists regarding the statistically significant increased risks of diabetes and cardiovascular (CV) events with these drugs, and recommended that physicians evaluate patients for these risk factors.24,25
An analysis of six phase 3 trials by Albertsen and colleagues showed that patients treated with degarelix in comparison to an LHRH agonist had a 40% reduction in risk of CV event or death during their first year of ADT.26 Among patients with pre-existing cardiovascular disease (CVD), degarelix-treated patients had an HR of 0.44 for CV (95% confidence interval (CI), 0.26–0.74; P = 0.002) event or death. In the first prospective trial to analyze CV outcomes among patients treated with either GnRH agonists or antagonists, Margel and colleagues showed that men in the agonist group experienced more major adverse cardiovascular and cerebrovascular events (MACCE) compared with the GnRH antagonist group (20% vs 3%, respectively; P = 0.013).27
In the ongoing phase 3 PRONOUNCE trial (NCT02663908), investigators will randomize a total of 900 patients to receive either leuprolide 3-month depot or degarelix monthly for 1 year. The primary endpoint is time from randomization to first confirmed occurrence of the composite MACE endpoint (nonfatal myocardial infarction, nonfatal stroke, or death due to any cause).
The decision to initiate ADT should encompass further detailed discussion with the patient and family regarding the myriad of ADT AEs as well as preventative strategies to diminish or avoid ADT complications.1,28–31
Although T monitoring should be standard practice for men on ADT, the optimal timing of T measurement is still debated. A 3- to 6-month interval has been suggested,14 but the author's clinical experience favors obtaining a baseline T before ADT initiation, then confirming castrate T levels 1 to 3 months following medical or surgical castration. If T >50 (or 20) ng/dL, consider checking GnRH level to differentiate incorrect administration from ineffective castration. If the latter, consider switching to another GnRH agonist/antagonist or bilateral orchiectomy. If T remains elevated, some authors have suggested adding an estrogen or an anti-androgen for further hormonal manipulation, although the clinical benefit remains uncertain.8
ADT 6 Bone-targeted Therapy
Men who initiate ADT may already be at risk for osteoporosis due to advanced age, hypogonadism, and/or other risk factors.32 To combat ADT-associated bone loss, the NCCN advises screening and treatment for osteoporosis according to general-population guidelines from the National Osteoporosis Foundation.33 When fracture risk warrants drug therapy, the NCCN recommends denosumab (60 mg SC every 6 months), zoledronic acid (ZA; 5 mg IV annually), or alendronate (70 mg PO weekly) to boost bone mineral density (BMD).
Clinical Scenarios and Treatment Strategies
Intermediate- and High-risk Localized PCa
Regarding initial treatment, AUA, EAU, and NCCN guidelines recommend RP or RT, with certain caveats, and possible ADT for intermediateor high-risk PCa (Table 4, Figure 1). NCCN recommendations divide intermediate-risk PCa into favorable (1 intermediate risk factor [IRF], Grade Group 1 or 2, and <50% biopsy cores positive) and unfavorable (2 or 3 IRFs and/or Grade Group 3 and/or ≥50% cores positive) strata.
TABLE 4.
| Organization | Intermediate Risk | High Risk | |
|---|---|---|---|
| AUA/ASTRO/SUO | Favorable or unfavorable: RP or RT + ADT | RP or RT + ADT | |
| EAU | RP if life expectancy >10 y, only as part of multimodal therapy ePLND if estimated metastatic risk >5% EBRT + ADT (4–6 months) with low-dose brachytherapy boost |
RP if life expectancy >10 y, only as part of multimodal therapy ePLND EBRT + long-term ADT (2–3 y) |
|
| NCCN | Favorable | Unfavorable | If life expectancy >5 y or symptomatic: EBRT + ADT (1.5–3 y) EBRT + brachytherapy + ADT (1–3 y) RP + PLND: If adverse features without LN metastases: EBRT ± ADT (6 mo) or observation If LN metastasis: ADT ± EBRT, or observation |
| If life expectancy >10y: Active surveillance EBRT or brachytherapy alone RP ± PLND if predicted probability of LN metastases ≥2%: Adverse features without LN metastases: EBRT ± ADT (6 mo) or observation LN metastasis: ADT ±EBRT, or observation |
Life expectancy >10y: RP ± PLND if predicted probability of LN metastases 2%: Adverse features without LN metastases: EBRT ± ADT (6 mo) or observation LN metastases: ADT (category 1) ± EBRT, or observation EBRT ± ADT (4 mo) |
||
| If life expectancy <10 y: Observation (preferred) EBRT or brachytherapy alone |
Life expectancy <10 y: Observation (preferred) EBRT + brachytherapy ± ADT (4 mo) |
ADT, androgen deprivation therapy; ASTRO, American Society for Radiation Oncology; AUA, American Urological Association; EAU, European Association of Urology; EBRT, external beam radiotherapy; ePLND, extended pelvic lymph node dissection; LN, lymph node; NCCN, National Comprehensive Cancer Network; RP, radical prostatectomy; RT, radiotherapy; SUO, Society of Urologic Oncology.
Figure 1.
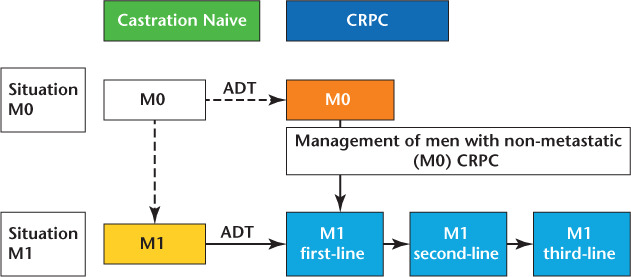
Disease states and strategies.34 ADT, androgen deprivation therapy; CRPC, castration-resistant rostate cancer.
Radiation With Adjuvant ADT
ADT is recommended in patients with unfavorable intermediate- and high-risk PCa considering RT.36,37 Neoadjuvant therapy with a GnRH agonist often reduces overall prostate volume by 25% to 33% within 3 months,38,39 a finding that supports the common practice of beginning ADT 2 to 3 months before starting radiation. Many studies have shown benefits for the combination of radiation with short-term ADT.40–45
Additional studies support use of radiation and long-term ADT in combined populations of intermediate-and high-risk local PCa.46–52 Although Level I evidence supports ADT for all intermediate-risk disease, compelling retrospective and post hoc evidence suggests that favorable intermediate-risk disease may be treated adequately with RT alone.53
RT in High-risk PCa
Approaches to high-risk localized PCa using adjuvant systemic therapies have remained relatively unchanged for the past few decades.54 For patients with nodal metastases, standard-of-care approaches include adjuvant ADT, based largely on the trial by Messing and colleagues.55 Neoadjuvant or adjuvant ADT is also considered standard in high- and intermediate-risk PCa being treated with RT, although optimal ADT duration has not been established.53
Current recommendations for using radiation with long-term ADT in high-risk PCa are based partly on trials showing cancer-specific and overall survival (OS) benefits for 28 to 36 months of ADT in patients with locally advanced disease.49,56 These findings led to 2010 NCCN guideline changes and clinical-practice changes to include 2 to 3 years of ADT for high-risk PCa.57
Considering the AEs associated with ADT, shortening long-term ADT may be desirable. Although the PCS IV phase 3 trial showed an overall survival hazard ratio (OSHR) of 1.02 for 18 versus 36 months ADT,58 it remains unclear whether these results support a conclusion that 18 months ADT is noninferior to 36 months because the trial did not use a noninferiority design. However, Yang and colleagues reported that an HR near 1.0, with a relatively narrow CI, raises the likelihood that the OS difference between 18 and 36 months for this population is small. These authors therefore suggested that these emerging data raise the possibility that 18 months may be sufficient for select high-risk patients.53
RP Plus ADT
AUA/American Society for Radiation Oncology (ASTRO)/Society of Urologic Oncology (SUO) and EAU guidelines recommend against using neoadjuvant ADT for localized PCa in patients who have chosen RP outside of clinical trials.14,36
Moderate evidence suggests that 6 to 8 months of neoadjuvant ADT before RP provides clinical benefit (usually lower positive margin rates after RP and decreased PSA recurrence risk after 2–5 years), but no studies have demonstrated an OS benefit.59 Conversely, evidence including a meta-analysis of 11,149 patients60 shows that long-term ADT immediately after RP can benefit men with high-risk localized PCa, particularly those with positive lymph nodes.59
BCR
BCR definitions differ slightly depending on primary treatment and guideline authors. The EAU and AUA define post-RP relapse as PSA >2 ng/mL, and post-RT >2 ng/mL above nadir (or, for the AUA, 3 consecutive PSA increases).61,62 Because patients with PSA recurrence after primary RP or RT have different risks of subsequent symptomatic metastatic disease, physicians should carefully interpret BCR endpoints when considering treatment initiation, for example, ADTs.14
Once PSA relapse is diagnosed, physicians must determine as accurately as possible whether the recurrence has occurred locally or distantly. Initial clinical and pathologic factors of the recurrence (T category, PSA, and Gleason score) and PSA kinetics (PSA doubling time [PSADT] and interval to PSA failure) help determine the risk of subsequent metastases and PCa-specific mortality (PCSM).14 For specific recommendations regarding BCR after surgery, radiation, high-intensity focused ultrasound (HIFU), or cryoablation and after primary surgery with adjuvant radiation or after localized salvage failure, please see Figures 2, 3, and 4.
Figure 2.
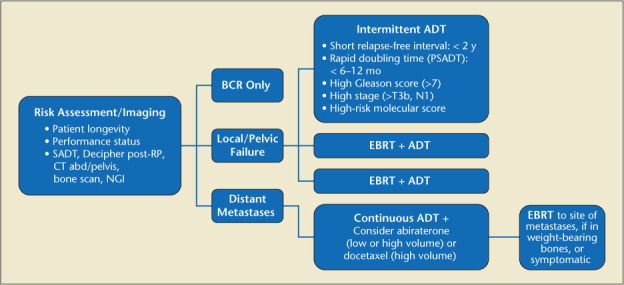
Primary failure after surgery (PSA >0.2). ADT, androgen deprivation therapy; BCR, biochemical recurrence; EBRT, external beam radiotherapy; NGI, next-generation imaging; PSA, prostate-specific antigen; RP, radical prostatectomy; SADT, salvage androgen deprivation therapy.
Figure 3.
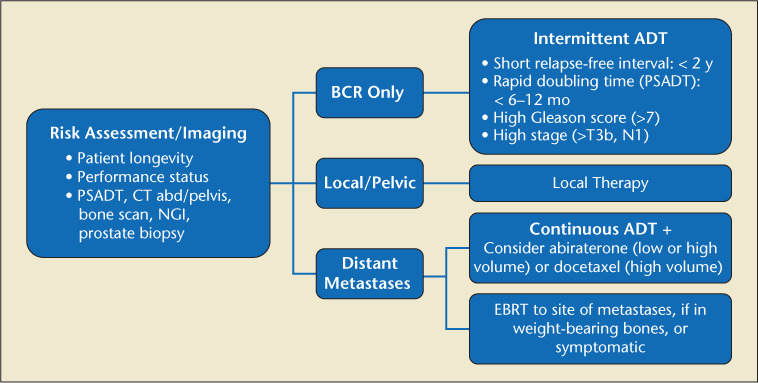
Radiation, HIFU, cryoablation failure (PSA2 + Nadir).103 ADT, androgen deprivation therapy; BCR, biochemical recurrence; EBRT, external beam radiotherapy; NGI, next-generation imaging; PSA, prostate-specific antigen; PSADT, PSA doubling time.
Figure 4.
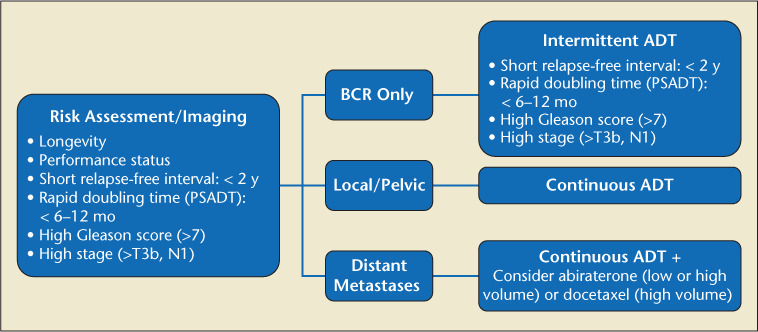
Primary failure after surgery (PSA >0.2).103 ADT, androgen deprivation therapy; BCR, biochemical recurrence; PSA, prostate-specific antigen; PSADT, PSA doubling time.
The management of BCR following primary curative treatment remains controversial, partly because PSAonly recurrence does not consistently correlate with either PCa-specific or overall survival.63 Recommendations regarding post-RP recurrence illustrate subtle differences between guidelines. For example, the EAU strongly recommends treating patients with a PSA rise from undetectable using salvage radiotherapy (SRT), but not offering hormonal therapy to every pN0 patient treated with SRT. AUA guidelines state that clinicians should offer ADT to patients being treated with SRT after RP failure (PSA ≥0.20 ng/mL).64 For low-risk BCR, the EAU strongly recommends offering androgen suppression (AS) and possibly delayed SRT. In 10-year follow-up of the GETUG-AFU 16 trial, progression-free survival for patients treated with RT plus shortterm goserelin (10.8 mg on the first day of RT and 3 months later) was 64%, versus 49% for patients treated with radiotherapy alone (HR 0.54; 95% CI, 0.43–0.68; stratified log-rank test, P < 0.0001). These authors concluded with a recommendation for AS plus RT as salvage treatment in patients with rising PSA concentration after RP.65
For BCR after RT, options include ADT or local procedures such as salvage RP, cryotherapy, interstitial brachytherapy, and HIFU, but weak evidence makes firm recommendations impossible.14 Ongoing phase 3 trials may help guide future practice in BCR (Table 5).
TABLE 5.
Ongoing Phase 3 Trials in Biochemical Recurrence
| Trial | Objectives | Estimated Completion |
|---|---|---|
| RADICALS (NCT00541047) | Assess timing of RT and use of ADT in conjunction with postoperative radiotherapy | September 2021 |
| Determine the impact of RT on quality of life | ||
| RAVES (NCT00860652) | Compare adjuvant RT vs active surveillance with early salvage RT in post-RP patients with positive margins and/or pT3 disease | December 2026 |
ADT, androgen deprivation therapy; RP, radical prostatectomy; RT, radiotherapy.
Newly Diagnosed Metastatic PCa
Median OS for patients who present with mHSPC (Table 6) is approximately 42 months.66 Upon conventional radiographic imaging detecting M1 disease, the clinicians' therapeutic selection choices are now numerous.67
TABLE 6.
Treatment Recommendations for Newly Diagnosed Metastatic Hormone-sensitive Prostate Cancer
| National Comprehensive Cancer Network | European Association of Urology | |
|---|---|---|
| GnRH agonist + first-generation anti-androgen (nilutamide, flutamide, bicalutamide) ± docetaxel GnRH agonist + abiraterone GnRH antagonist ± docetaxel GnRH antagonist ± abiraterone Orchiectomy ± abiraterone |
Immediate systemic treatment (ADT) for symptomatic or asymptomatic disease Orchiectomy or ADT + docetaxel if fit enough for chemotherapy Surgery and or local radiotherapy if evidence of impending complications such as spinal cord compression or bone fracture Castration + abiraterone + prednisone if fit enough for this regimen No anti-androgen monotherapy LHRH antagonists, especially if impending spinal cord compression or bladder outlet obstruction Short-term anti-androgens for patients treated with LHRH agonist to reduce risk of flare |
Strong |
| Weak | ||
ADT, androgen deprivation therapy.
EAU guidelines strongly recommend treating both symptomatic and asymptomatic M1 patients (or discussing deferred castration with well-informed patients). The first-line treatment for newly diagnosed M1 PCa is ADT.66 No Level I evidence favors a specific type of ADT, except in patients with impending spinal-cord compression, for whom either bilateral orchiectomy or GnRH antagonists are preferred, per the EAU (albeit with a “weak” recommendation).14 Offer surgical or medical castration alone (with or without an anti-androgen) only to patients unfit for or uninterested in newer combinations including either docetaxel or abiraterone acetate plus prednisone.66
ADT in mHSPC
ADT remains the gold standard in mHSPC, regardless of metastatic volume (low vs high) with Level I evidence for combining treatments (docetaxel, abiraterone, apalutamide), according to NCCN, EAU, and ESMO guidelines.8,14,68 To avoid castration-dependent pharmacokinetics of docetaxel, physicians commonly start docetaxel 6 to 12 weeks after initiating ADT.
Adding the second-generation AR signaling inhibitor (ASI) abiraterone acetate to ADT represents another standard of care.69 In the LATITUDE and STAMPEDE trials, this combination produced nearly identical OS improvements of 38% (HR 0.62 and 0.63, respectively).70,71
The phase 3 TITAN trial showed that apalutamide plus ADT significantly improved radiographic progression-free survival (rPFS) and OS versus placebo plus ADT in patients with metastatic castration-sensitive PCa.72 In September 2019, the FDA approved apalutamide for use in this population.73 In mid-2019, NCCN guidelines added apalutamide as a category 1 option for M1 castration-naive PCa.8 Additionally, the FDA granted priority review for enzalutamide in mHSPC based on positive results of the ARCHES and ENZAMET phase 3 trials. The ARCHES phase 3 trial showed that at median of 14.4 months, ADT plus enzalutamide significantly improved rPFS over ADT plus placebo.74 In ENZAMET, HR for death in the enzalutamide group was 0.67 (95% CI, 0.52–0.86; P = 0.002) versus ADT alone at a median follow-up of 34 months.75
Four-year follow-up of STAMPEDE, moreover, showed that ADT plus upfront docetaxel or abiraterone produced no significant differences between these two regimens in median OS, metastasis-free survival (MFS), or PCa-specific survival.76 Because castrated patients clear docetaxel roughly twice as quickly as un-castrated patients,77 do not delay docetaxel until patients become castration resistant.78 Clinicians continue to debate which treatment to add to ADT, and then how to best sequence the next line of therapy.
In selecting which combination for high-volume mHSPC patients, guidelines do not recommend either abiraterone or docetaxel over the other. In high-volume mHSPC, there is unequivocal OS benefit to ADT with docetaxel versus ADT alone,70 according to NCCN and ASCO guidelines.8,79 In lowvolume mHSPC, a recent analysis of STAMPEDE results revealed that docetaxel confers additive benefits regardless of metastatic burden—5-year OS in the low-burden group specifically was 72%, versus 57% for ADT alone (P = 0.107).80 ADT plus abiraterone also represents a reasonable standard of care irrespective of metastatic burden.68
In low-volume mHSPC, NCCN and ESMO 2019 guidelines recommend RT to the prostate for patients without contraindications to radiotherapy. ADT is also required, unless medically contraindicated. In a section of the STAMPEDE trial, however, adding radiotherapy to standard care in patients with low metastatic burden improved failure-free survival but not OS.81 EAU guidelines recommend (although weakly) offering castration plus RT to patients whose first presentation is M1 disease, with low volume as defined by CHAARTED criteria.
For patients with metastasis at first presentation (versus relapse/metastases after definitive local therapy), EAU guidelines cite that docetaxel or abiraterone should be consider as standard therapy in conjunction with ADT.14 However, ASCO recommends ADT plus abiraterone strongly in high-risk de novo mHSPC and moderately in lower-risk cases.79
CRPC
Regardless of ADT modality, nearly all patients with advanced PCa maintained on ADT eventually develop castration resistance, requiring changes in therapeutic strategy. In all patients with rising PSA and/or clinical progression, physicians must evaluate serum T to confirm castrate resistance before considering treatments.13 In CRPC, continuing ADT to maintain castrate T levels (≤20 ng/dL) is strongly recommended and considered the standard of care in both metastatic and nonmetastatic CRPC.8,14,82
In deciding which patients to evaluate for metastatic disease, the EAU suggests using baseline PSA, PSA velocity, and PSADT, which have been associated with time to first bone metastasis, bone MFS, and OS.83,84 The PROSPER and SPARTAN phase 3 trials in high-risk M0 CRPC showed significant MFS benefits for enzalutamide (HR for metastasis or death vs placebo, 0.29) and apalutamide (HR 0.28), respectively.85,86 In the ARAMIS trial, median MFS for patients on darolutamide was 40.4 months, versus 18.4 months for placebo (HR for metastasis or death with darolutamide, 0.41; P < 0.001).87 NCCN, EAU, and AUA guidelines also offer recommendations regarding treatments such as abiraterone, docetaxel, sipuleucel-T, and bone-targeting therapies in mCRPC decision-making.8,14,82
Intermittent Androgen Deprivation Therapy (IADT)
Another clinical option involves stopping ADT in well-informed and requesting patients who have had a strong PSA response (usually defined as PSA <1 ng/dL in metastatic and relapsing disease) yet have significant tolerability concerns.14 Patients should undergo examinations every 3 to 6 months and those without evidence of progression should resume ADT if PSA rises above an empirically chosen threshold (subjectively designated in various trials, 10–20 ng/dL).11,14 Allowing T levels to recover between treatment cycles may reduce ADT-associated AEs (Figure 5).11
Figure 5.
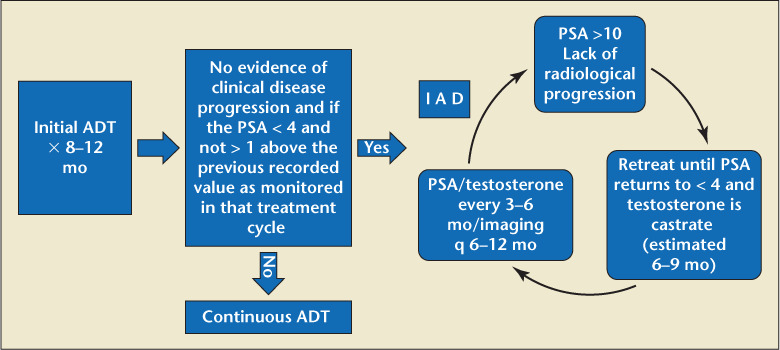
Intermittent androgen deprivation therapy (IADT) pathway.12,14 ADT, androgen deprivation therapy; PSA, prostate-specific antigen; PSADT, PSA doubling time.
Studies in locally advanced, relapsing, and metastatic PCa suggest that IADT is noninferior to continuous ADT (CADT) while offering potentially fewer side effects and better quality of life.88–91 However, not all IADT studies show clear advantages over continuous CADT. Most IADT trials show only modest reductions in AEs during off-treatment phases.92 The ICELAND trial showed no significant differences between IADT and CADT in health-related quality of life or AEs.93
EAU and NCCN guidelines note that although IADT appears to reduce sexual and other side effects of CADT, the largest trial addressing IADT in M1b patients (SWOG 9346) failed to demonstrate noninferiority to CADT.94 Additionally, a recent analysis of a large metastatic PCa trial revealed an increased risk of CV events for patients on IADT.95
Ideal IADT candidates have yet to be defined. NCCN guidelines say IADT may be allowed to reduce toxicity in M0 castration-naive patients with PSA persistence or recurrence after RP or EBRT, or those with castration-naive M1 PCa.8 The EAU recommends offering IADT only to well-informed patients with significant ADT AEs who had strong PSA responses to ADT induction (PSA <4 ng/mL in metastatic disease).14
Metastasis-directed Therapy (MDT)
Oligometastatic PCa denotes an intermediate state between localized disease and widespread metastases, marked by a limited number of metastases (usually 3–5).96 By targeting metastases directly, MDT could potentially delay the need for systemic treatment in patients relapsing after local therapy; however, no data suggest an OS improvement for MDT.14 Research regarding optimal strategies for oligometastatic PCa are lacking, and MDT remains experimental. However, numerous clinical MDT trials are ongoing.97
Discussion
Presently, ADT remains the gold standard for advanced PCa. Regarding the combination of ADT with chemotherapy and/or novel AR antagonist therapy in mHSPC, several ongoing trials will help determine ideal treatment sequencing (Table 7).
TABLE 7.
Ongoing Androgen Deprivation Therapy Plus Chemotherapy Trials
| Study | Phase | Estimated Primary Completion |
|---|---|---|
| Docetaxel Before Degarelix in Newly Diagnosed Metastatic Prostate Cancer (NCT03069937) | 2 | February 2020 |
| PEACE1 (A Phase III Study for Patients with Metastatic Hormone-Naïve Prostate Cancer; NCT01957436) | 3 | 2020 |
| S1216 (ADT + TAK-700 vs ADT + Bicalutamide for Metastatic Prostate Cancer; NCT01809691) | 3 | March 2022 |
| ARASENS (ODM-201/Darolutamide in Addition to Standard ADT and Docetaxel in Metastatic Castration Sensitive Prostate Cancer; NCT02799602) | 3 | August 2022 |
| STAMPEDE (Systemic Therapy in Advancing or Metastatic Prostate Cancer: Evaluation of Drug Efficacy; NCT00268476) | 2/3 | September 2024 |
| Arm A (control: ADT + radiotherapy ± docetaxel ± abiraterone) | ||
| Arm C (ADT + docetaxel + prednisolone) | ||
| Arm E (ADT + zoledronic acid + docetaxel + prednisolone) | ||
| Arm G (ADT + abiraterone + prednisolone) | ||
| Arm J (ADT + abiraterone + enzalutamide + prednisolone) |
However, growing awareness of ADT side effects and availability of novel hormonal agents—along with healthcare payers' concern for value-based care—are impacting the treatment landscape for therapeutic selections.
Although the 2010 warnings from the FDA, AUA, and other organizations regarding the need to evaluate baseline cardiovascular risk in patients being considered for ADT have had less impact than expected, ADT usage appears to be shifting. One study showed that compared with 2008 through 2009, patients with low-risk PCa were significantly less likely to receive ADT in 2011 through 2012 (10.0% vs 14.7%; P < 0.001).98 In the same study, patients with intermediaterisk disease were slightly less likely to receive ADT post-2010 (33.4% vs 35.1%; P < 0.001), and patients with high-risk PCa were slightly more likely to undergo ADT after 2010 (71.1% vs 66.8%; P < 0.001).
Among hormonal agents, 150 mg bicalutamide (now generic) is used for LAPC in the EU and elsewhere, as either adjuvant therapy or a monotherapeutic alternative to surgical or medical castration.99 The oral drug relugolix, now under FDA review, may allow for a oncedaily GnRH antagonist. Relugolix phase 3 results have recently been published.100
Meanwhile, the rise in publications examining T replacement for men who have or have had PCa reflects patients' and providers' interest in minimizing consequences of T suppression.101,102 Immunotherapy trials and other drug-targeted pathways in development may someday obviate the need for ADT for select advanced PCa populations. Examples include SNS-301 (Sensei Biotherapeutics Inc., Gaithersburg, MD) and the novel nanoparticle vaccine candidate INO-5150 (Inovio Pharmaceuticals Inc., Plymouth Meeting, PA), both presently in phase 2. The role of small-molecule or antibodydelivered targeted alpha therapy, which can reach prostate-specific membrane antigen-expressing tumors regardless of metastatic location, may hold promise.103 Also, there is continued interest in MDT to avoid or delay ADT.93,104
Conclusions
The understanding and application with which physicians apply ADT will continue to evolve with new combinatorial approaches and better understanding of AE management. Additional research exploring these combinations and sequencing strategies, as well as the evaluation of novel ADT options and potential alternative approaches, is ongoing and will continue to require educational awareness.
Main Points.
In recent years, incidence of high-risk prostate cancer (PCa) has risen, largely in response to various organizations' 2008 recommendation to avoid routine prostate-specific antigen (PSA) testing.
The landscape of treatments for locally advanced and metastatic PCa continues to expand, as does awareness of treatment side effects and the need to tailor shared decision-making processes to each patient's clinical situation, personal preferences, and lifestyle.
Among non-surgical androgen deprivation therapy (ADT) options, the choice between GnRH agonists and antagonists rests on multiple factors, including these drugs' pharmacological profiles, clinical efficacy, cost, and convenience. A 2010 US Food and Drug Administration (FDA) recommendation to assess baseline cardiovascular (CV) risk in men being considered for GnRH agonist therapy reflects a growing concern over such side effects.
For initial treatment of intermediate- and high-risk localized PCa, American Urological Association (AUA), European Association of Urology (EAU), and National Comprehensive Cancer Network (NCCN) guidelines recommend radical prostatectomy (RP) or radiotherapy (RT), with caveats, with or without ADT. The NCCN recommends ADT for men with high-risk and unfavorable intermediate-risk PCa considering RT. Studies support use of neoadjuvant ADT as well as long-term ADT in appropriate clinical situations.
Management of biochemical recurrence (BCR) remains controversial, as PSA-only recurrence does not consistently correlate with either overall or PCa-specific survival.
First-line treatment for newly diagnosed M1 PCa is ADT; no Level I evidence favors a specific form of ADT, except in cases with impending spinal-cord compression, for which the EAU prefers either bilateral orchiectomy or GnRH antagonists. Additionally, ADT represents the gold standard in metastatic hormone-sensitive prostate cancer (mHSPC), irrespective of metastatic burden; Level I evidence also supports combining ADT with treatments including docetaxel, abiraterone, and apalutamide. Based on results of the ARCHES and ENZAMET phase 3 trials, the FDA has granted priority review status to enzalutamide in mHSPC.
In castrate-resistant prostate cancer (CRPC), whether metastatic or nonmetastatic, continuing ADT to maintain castrate testosterone (T) levels is strongly recommended by multiple organizations. Enzalutimide, apalutamide, and darolutamide have demonstrated positive results in CRPC.
References
- 1.Cherrier MM, Cross DJ, Higano CS, Minoshima S. Changes in cerebral metabolic activity in men undergoing androgen deprivation therapy for non-metastatic estate cancer. Prostate Cancer Prostatic Dis. 2018;21:394–402. doi: 10.1038/s41391-018-0037-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Melloni C, Roe MT. Androgen deprivation therapy and cardiovascular disease. Urol Oncol. 2020;38:45–52. doi: 10.1016/j.urolonc.2019.02.010. [DOI] [PubMed] [Google Scholar]
- 3.American Cancer Society, author. Key Statistics for Prostate Cancer. https://www.cancer.org/cancer/prostate-cancer/about/key-statistics.html. Accessed September 25, 2020.
- 4.National Cancer Institute, author. Cancer Stat Facts: Prostate Cancer. https://seer.cancer.gov/statfacts/html/prost.html. Accessed September 25, 2020.
- 5.Centers for Disease Control and Prevention, author. United States Cancer Statistics: Data Visualizations (1999–2016). https://gis.cdc.gov/Cancer/USCS/DataViz.html. Accessed September 25, 2020.
- 6.American Cancer Society, author. Cancer Facts & Figures 2019. https://www.cancer.org/content/dam/cancerorg/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2019/cancer-facts-and-figures-2019.pdf. Accessed September 25, 2020.
- 7.Weiner AB, Matulewicz RS, Eggener SE, Schaeffer EM. Increasing incidence of metastatic prostate cancer in the United States (2004–2013) Prostate Cancer Prostatic Dis. 2016;19:395–397. doi: 10.1038/pcan.2016.30. [DOI] [PubMed] [Google Scholar]
- 8.National Comprehensive Cancer Network, author. NCCN Clinical Practice Guidelines in Oncology: Prostate Cancer Version 1.2020. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Accessed September 25, 2020. [DOI] [PMC free article] [PubMed]
- 9.Prostate Cancer Foundation, author. Prostate Cancer Patient Guide. https://www.pcf.org/guide/prostate-cancer-patient-guide/. Accessed September 25, 2020.
- 10.Egevad L, Delahunt B, Srigley JR, Samaratunga H. International Society of Urological Pathology (ISUP) grading of prostate cancer—an ISUP consensus on contemporary grading. APMIS. 2016;124:433–435. doi: 10.1111/apm.12533. [DOI] [PubMed] [Google Scholar]
- 11.Crawford ED, Heidenreich A, Lawrentschuk N, et al. Androgen-targeted therapy in men with prostate cancer: evolving practice and future considerations. Prostate Cancer Prostatic Dis. 2019;22:24–38. doi: 10.1038/s41391-018-0079-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shore ND, Abrahamsson PA, Anderson J, et al. New considerations for ADT in advanced prostate cancer and emerging role of GnRH antagonists. Prostate Cancer Prostatic Dis. 2013;16:7–15. doi: 10.1038/pcan.2012.25. [DOI] [PubMed] [Google Scholar]
- 13.Thompson IM, Valicenti R, Albertsen PC, et al. Adjuvant and Salvage Radiotherapy after Prostatectomy: ASTRO/AUA Guideline (2013, amended 2018 & 2019). https://www.auanet.org/guidelines/prostate-cancer-adjuvant-and-salvage-radiotherapy-guideline. Accessed September 25, 2020.
- 14.Mottet N, van den Bergh RCN, Briers E, et al. Prostate Cancer Guidelines 2019. https://uroweb.org/guideline/prostate-cancer/. Accessed September 25, 2020.
- 15.Klotz L, Boccon-Gibod L, Shore NS, et al. The efficacy and safety of degarelix: a 12-month, comparative, randomized, open-label, parallel-group phase III study in patients with prostate cancer. BJU Int. 2008;102:1531–1538. doi: 10.1111/j.1464-410X.2008.08183.x. [DOI] [PubMed] [Google Scholar]
- 16.Crawford ED, Rove KO, Schally AV, et al. The role of the FSH system in the development and progression of prostate cancer. Am J Hematol Oncol. 2014;10:5–12. [Google Scholar]
- 17.Van Poppel H. LHRH agonists versus GnRH antagonists for the treatment of prostate cancer. Belgian J Med Oncol. 2010;4:18–22. [Google Scholar]
- 18.Crawford ED, Shore ND, Moul JW, et al. Long-term tolerability and efficacy of degarelix: 5-year results from a phase III extension trial with a 1-arm crossover from leuprolide to degarelix. Urology. 2014;83:1122–1128. doi: 10.1016/j.urology.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 19.Klotz L, Miller K, Crawford ED, et al. Disease control outcomes from analysis of pooled individual patient data from five comparative randomised clinical trials of degarelix versus luteinising hormone-releasing hormone agonists. Eur Urol. 2014;66:1101–1108. doi: 10.1016/j.eururo.2013.12.063. [DOI] [PubMed] [Google Scholar]
- 20.McLeod D, Zinner N, Tomera K, et al. A phase 3, multicenter, open-label, randomized study of abarelix versus leuprolide acetate in men with prostate cancer. Urology. 2001;58:756–761. doi: 10.1016/s0090-4295(01)01342-5. [DOI] [PubMed] [Google Scholar]
- 21.Perachino M, Cavalli V, Bravi F. Testosterone levels in patients with metastatic prostate cancer treated with luteinizing hormone-releasing hormone therapy: prognostic significance? BJU Int. 2010;105:648–651. doi: 10.1111/j.1464-410X.2009.08814.x. [DOI] [PubMed] [Google Scholar]
- 22.Klotz L, O'Callaghan C, Ding K, et al. Nadir testosterone within first year of androgen-deprivation therapy (ADT) predicts time to castration-resistant progression: a secondary analysis of the PR-7 trial of intermittent pursuit continuous ADT. J Clin Oncol. 2015;33:1151–1156. doi: 10.1200/JCO.2014.58.2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kamada S, Sakamoto S, Ando K, et al. Nadir testosterone after long-term follow-up predicts prognosis in patients with prostate cancer treated with combined androgen blockade. J Urol. 2015;194:1264–1270. doi: 10.1016/j.juro.2015.03.120. [DOI] [PubMed] [Google Scholar]
- 24.US Food and Drug Administration, author. FDA Drug Safety Communication: Update to Ongoing Safety Review GnRH Agonists and Notification to Manufacturers of GnRH Agonists to Add New Safety Information to Labeling Regarding Increased Risk of Diabetes and Certain Cardiovascular Diseases. https://www.fda.gov/Drugs/DrugSafety/ucm229986.htm. Accessed September 25, 2020.
- 25.Levine GN, D'Amico AV, Berger P, et al. Androgen-deprivation therapy in prostate cancer and cardiovascular risk. A science advisory from the American Heart Association, American Cancer Society, and American Urological Association. Circulation. 2010;121:833–840. doi: 10.1161/CIRCULATIONAHA.109.192695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Albertsen PC, Klotz L, Tombal B, et al. Cardiovascular morbidity associated with gonadotropin releasing hormone agonists and an antagonist. Eur Urol. 2014;65:565–573. doi: 10.1016/j.eururo.2013.10.032. [DOI] [PubMed] [Google Scholar]
- 27.Margel D, Peer A, Ber Y, et al. Cardiovascular morbidity in a randomized trial comparing GnRH-agonist and GnRH-antagonist among patients with advanced prostate-cancer and pre-existing cardiovascular disease. J Urol. 2019;202(6):1199–1208. doi: 10.1097/JU.0000000000000384. [DOI] [PubMed] [Google Scholar]
- 28.Daniell HW, Dunn SR, Ferguson DW, et al. Progressive osteoporosis during androgen deprivation therapy for prostate cancer. J Urol. 2000;163:181–186. [PubMed] [Google Scholar]
- 29.Smith MR, Finkelstein JS, McGovern FJ, et al. Changes in body composition during androgen deprivation therapy for prostate cancer. J Clin Endocrinol Metab. 2002;87:599–603. doi: 10.1210/jcem.87.2.8299. [DOI] [PubMed] [Google Scholar]
- 30.Smith MR, Lee H, Nathan DM. Insulin sensitivity during combined androgen blockade for prostate cancer. J Clin Endocrinol Metab. 2006;91:1305–1308. doi: 10.1210/jc.2005-2507. [DOI] [PubMed] [Google Scholar]
- 31.Shahani S, Braga-Basaria M, Basaria S. Androgen deprivation therapy in prostate cancer and metabolic risk for atherosclerosis. J Clin Endocrinol Metab. 2008;93:2042–2049. doi: 10.1210/jc.2007-2595. [DOI] [PubMed] [Google Scholar]
- 32.Alibhai S, Zukotynski K, Walker-Dilks C, et al. Bone health and bone-targeted therapies for nonmetastatic prostate cancer: a systematic review and metaanalysis. Ann Intern Med. 2017;167:341–350. doi: 10.7326/M16-2577. [DOI] [PubMed] [Google Scholar]
- 33.Cosman F, de Beur SJ, LeBoff MS, et al. Clinician's guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25:2359–2381. doi: 10.1007/s00198-014-2794-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gillessen S, Omlin A, Attard G, et al. Management of patients with advanced prostate cancer: recommendations of the St Gallen Advanced Prostate Cancer Consensus Conference (APCCC) 2015. Ann Oncol. 2015;26:1589–604. doi: 10.1093/annonc/mdv257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanda MG, Cadeddu JA, Kirkby E, et al. Clinically localized prostate cancer: AUA/ASTRO/SUO guideline. Part one: risk stratification, shared decision-making, and care options. J Urol. 2018;199:683–690. doi: 10.1016/j.juro.2017.11.095. [DOI] [PubMed] [Google Scholar]
- 36.American Urological Association, author. Clinically Localized Prostate Cancer: AUA/ASTRO/SUO Guideline (2017). https://www.auanet.org/guidelines/prostate-cancer-clinically-localized-guideline. Accessed September 28, 2020.
- 37.Roach M. Current trends for the use of androgen deprivation therapy in conjunction with radiotherapy for patients with unfavorable intermediate-risk, high-risk, localized, and locally advanced prostate cancer. Cancer. 2014;120:1620–1629. doi: 10.1002/cncr.28594. [DOI] [PubMed] [Google Scholar]
- 38.Zelefsky MJ, Leibel SA, Burman CM, et al. Neoadjuvant hormonal therapy improves the therapeutic ratio in patients with bulky prostatic cancer treated with three-dimensional conformal radiation therapy. Int J Radiat Oncol Biol Phys. 1994;29:755–761. doi: 10.1016/0360-3016(94)90563-0. [DOI] [PubMed] [Google Scholar]
- 39.Ebara S, Manabe D, Kobayashi Y, et al. The efficacy of neoadjuvant androgen deprivation therapy as a prostate volume reduction before brachytherapy for clinically localized prostate cancer. Acta Med Okayama. 2007;61:335–340. doi: 10.18926/AMO/32878. [DOI] [PubMed] [Google Scholar]
- 40.Roach M, 3rd, Lu J, Pilepich MV, et al. Predicting long-term survival, and the need for hormonal therapy: a meta-analysis of RTOG prostate cancer trials. Int J Radiat Oncol Biol Phys. 2000;47:617–627. doi: 10.1016/s0360-3016(00)00577-0. [DOI] [PubMed] [Google Scholar]
- 41.Jones CU, Hunt D, McGowan DG, et al. Radiotherapy and short-term androgen deprivation for localized prostate cancer. N Engl J Med. 2011;365:107–118. doi: 10.1056/NEJMoa1012348. [DOI] [PubMed] [Google Scholar]
- 42.D'Amico AV, Chen MH, Renshaw A, et al. Long-term follow-up of a randomized trial of radiation with or without androgen deprivation therapy for localized prostate cancer. JAMA. 2015;314:1291–1293. doi: 10.1001/jama.2015.8577. [DOI] [PubMed] [Google Scholar]
- 43.Bolla M, Maingon P, Carrie C, et al. Short androgen-suppression and radiation dose escalation for intermediate- and high-risk localized prostate cancer: results of EORTC trial 22991. J Clin Oncol. 2016;34:1748–1756. doi: 10.1200/JCO.2015.64.8055. [DOI] [PubMed] [Google Scholar]
- 44.D'Amico AV, Manola J, Loffredo M, et al. 6-month androgen suppression plus radiation therapy vs radiation therapy alone for patients with clinically localized prostate cancer: a randomized controlled trial. JAMA. 2004;292:821–827. doi: 10.1001/jama.292.7.821. [DOI] [PubMed] [Google Scholar]
- 45.D'Amico AV, Chen MH, Renshaw AA, et al. Androgen suppression and radiation vs radiation alone for prostate cancer: a randomized trial. JAMA. 2008;299:289–295. doi: 10.1001/jama.299.3.289. [DOI] [PubMed] [Google Scholar]
- 46.Widmark A, Klepp O, Solberg A, et al. Endocrine treatment, with or without radiotherapy, in locally advanced prostate cancer (SPCG-7/SFUO-3): an open randomised phase III trial. Lancet. 2009;373:301–308. doi: 10.1016/S0140-6736(08)61815-2. [DOI] [PubMed] [Google Scholar]
- 47.Warde P, Mason M, Ding K, et al. Combined androgen deprivation therapy and radiation therapy for locally advanced prostate cancer: a randomised, phase 3 trial. Lancet. 2011;378:2104–2111. doi: 10.1016/S0140-6736(11)61095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pilepich MV, Winter K, Lawton CA, et al. Androgen suppression adjuvant to definitive radiotherapy in prostate carcinoma: long-term results of phase III RTOG 85-31. Int J Radiat Oncol Biol Phys. 2005;61:1285–1290. doi: 10.1016/j.ijrobp.2004.08.047. [DOI] [PubMed] [Google Scholar]
- 49.Hanks GE, Pajak TF, Porter A, et al. Phase III trial of long-term adjuvant androgen deprivation after neoadjuvant hormonal cytoreduction and radiotherapy in locally advanced carcinoma of the prostate: the Radiation Therapy Oncology Group Protocol 92-02. J Clin Oncol. 2003;21:3972–3978. doi: 10.1200/JCO.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 50.Horwitz EM, Bae K, Hanks GE, et al. 10-year follow-up-up of Radiation Therapy Oncology Group Protocol 92-02: a phase III trial of the duration of elective androgen deprivation in locally advanced prostate cancer. J Clin Oncol. 2008;26:2497–2504. doi: 10.1200/JCO.2007.14.9021. [DOI] [PubMed] [Google Scholar]
- 51.Bolla M, Van Tienhoven G, Warde P, et al. External irradiation with or without long-term androgen suppression for prostate cancer with high metastatic risk: 10-year results of an EORTC randomised study. Lancet Oncol. 2010;11:1066–1073. doi: 10.1016/S1470-2045(10)70223-0. [DOI] [PubMed] [Google Scholar]
- 52.Bolla M, Colette L, Blank L, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet. 2002;360:103–106. doi: 10.1016/s0140-6736(02)09408-4. [DOI] [PubMed] [Google Scholar]
- 53.Yang DD, Nguyen PL. Optimizing androgen deprivation therapy with radiation therapy for aggressive localized and locally advanced prostate cancer. Urol Oncol. 2017 doi: 10.1016/j.urolonc.2017.10.020. [DOI] [PubMed] [Google Scholar]
- 54.Shevach J, Chaudhuri P, Morgans AK. Adjuvant therapy in high-risk prostate cancer. Clin Adv Hematol Oncol. 2019;17:45–53. [PubMed] [Google Scholar]
- 55.Messing EM, Manola J, Yao J, et al. Eastern Cooperative Oncology Group study EST 3886. Immediate versus deferred androgen deprivation treatment in patients with note-positive prostate cancer after radical prostatectomy and pelvic lymphadenectomy. Lancet Oncol. 2006;7:472–479. doi: 10.1016/S1470-2045(06)70700-8. [DOI] [PubMed] [Google Scholar]
- 56.Bolla M, de Reijke TM, Van Tienhoven G, et al. Duration of androgen suppression in the treatment of prostate cancer. N Engl J Med. 2009;360:2516–2527. doi: 10.1056/NEJMoa0810095. [DOI] [PubMed] [Google Scholar]
- 57.Zhao S, Urdaneta AI, Anscher MS. The role of androgen deprivation therapy plus radiation therapy in patients with non-metastatic prostate cancer. Expert Rev Anticancer Ther. 2016;16:929–942. doi: 10.1080/14737140.2016.1218279. [DOI] [PubMed] [Google Scholar]
- 58.Nabid A, Garant MP, Martin AG, et al. Duration of androgen deprivation therapy in high risk prostate cancer: final results of a randomized phase III trial. Eur Urol. 2018;74(4):432–441. doi: 10.1016/j.eururo.2018.06.018. [DOI] [PubMed] [Google Scholar]
- 59.Fang D, Zhou L. Androgen deprivation therapy in nonmetastatic prostate cancer patients: indications, treatment effects, and new predictive biomarkers. Asia Pac J Clin Oncol. 2019;15:108–120. doi: 10.1111/ajco.13108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kumar S, Shelley M, Harrison C, et al. Neo-adjuvant and adjuvant hormone therapy for localised and locally advanced prostate cancer. Cochrane Database Syst Rev. 2006:CD006019. doi: 10.1002/14651858.CD006019.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cornford P, Bellmunt J, Bolla M, et al. EAU-ESTROSIOG guidelines on prostate cancer. part II: treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur Urol. 2017;71:630–642. doi: 10.1016/j.eururo.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 62.American Urological Association, author. PSA Testing for the Pretreatment Staging and Post-treatment Management of Prostate Cancer. https://www.auanet.org/guidelines/prostate-specific-antigen-(psa)-best-practice-statement. Accessed September 28, 2020.
- 63.Artibani W, Porcaro AB, De Marco V, et al. Management of biochemical recurrence after primary curative treatment for prostate cancer: a review. Urol Int. 2018;100:251–262. doi: 10.1159/000481438. [DOI] [PubMed] [Google Scholar]
- 64.Thompson IM, Valicenti R, Albertsen PC, et al. Adjuvant and Salvage Radiotherapy after Prostatectomy: ASTRO/AUA Guideline (2013, amended 2018 & 2019) https://www.auanet.org/guidelines/prostate-cancer-adjuvant-and-salvage-radiotherapy-guideline. Accessed September 28, 2020.
- 65.Carrie C, Magné N, Burban-Provost P, et al. Short-term androgen deprivation therapy combined with radiotherapy as salvage treatment after radical prostatectomy for prostate cancer (GETUG-AFU 16): a 112-month follow-up of a phase 3, randomised trial. Lancet Oncol. 2019;20:1740–1749. doi: 10.1016/S1470-2045(19)30486-3. [DOI] [PubMed] [Google Scholar]
- 66.James ND, Spears MR, Clarke NW, et al. Survival with newly diagnosed metastatic prostate cancer in the “docetaxel era”: Data from 917 patients in the control arm of the STAMPEDE trial (MRC PR08, CRUK/06/019) Eur Urol. 2015;67:1028–1038. doi: 10.1016/j.eururo.2014.09.032. [DOI] [PubMed] [Google Scholar]
- 67.Yu EY, Gillessen S, Mottet N. What do the guidelines say for metastatic prostate cancer starting androgen deprivation therapy? National Comprehensive Cancer Network, European Society for Medical Oncology, and European Association of Urology recommendations. Eur Urol Focus. 2019;5:162–164. doi: 10.1016/j.euf.2018.09.015. [DOI] [PubMed] [Google Scholar]
- 68.Parker C, Gillessen S, Heidenreich A, Horwich A, ESMO Guidelines Committee European Society for Medical Oncology Cancer of the prostate: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(suppl 5):v69–v77. doi: 10.1093/annonc/mdv222. [DOI] [PubMed] [Google Scholar]
- 69.Mottet N, De Santis M, Briers E, et al. Updated guidelines for metastatic hormone sensitive prostate cancer: abiraterone acetate combined with castration is another standard. Eur Urol. 2018;73:316–321. doi: 10.1016/j.eururo.2017.09.029. [DOI] [PubMed] [Google Scholar]
- 70.Fizazi K, Tran N, Fein L, et al. Abiraterone plus prednisone in metastatic, castration sensitive prostate cancer. N Engl J Med. 2017;377:352–360. doi: 10.1056/NEJMoa1704174. [DOI] [PubMed] [Google Scholar]
- 71.James ND, de Bono JS, Spears MR, et al. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med. 2017;377:338–351. doi: 10.1056/NEJMoa1702900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chi KN, Agarwal N, Bjartell A, et al. First results from TITAN: a phase III double-blind, randomized study of apalutamide (APA) versus placebo (PBO) in patients (pts) with metastatic castration-sensitive prostate cancer (mCSPC) receiving androgen deprivation therapy (ADT) J Clin Oncol. 2019;37(15 suppl):5006–5006. [Google Scholar]
- 73.US Food and Drug Administration, author. FDA approves apalutamide for metastatic castration-sensitive prostate cancer. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approve-sapalutamide-metastatic-castration-sensitive-prostate-cancer. Accessed September 28, 2020.
- 74.Armstrong AJ, Szmulewitz RZ, Petrylak DP, et al. Phase 3 study of androgen deprivation therapy (ADT) with enzalutimide (ENZA) or placebo (PBO) in metastatic hormone-sensitive prostate cancer (mHSPC): the ARCHES trial. Presented at: American Society of Clinical Oncology Genitourinary Cancers Symposium; San Francisco. February 14, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sweeney C, Martin AJ, Zielinski RR, et al. Overall survival (OS) results of a phase III randomized trial of standard-of-care therapy with or without enzalutamide for metastatic hormone-sensitive prostate cancer (mHSPC): ENZAMET (ANZUP 1304), an ANZUP-led international cooperative group trial. Presented at: American Society of Clinical Oncology; Chicago. June 2, 2019. Abstract LBA2. [Google Scholar]
- 76.Sydes MR, Spears MR, Mason MD, et al. Adding abiraterone or docetaxel to long-term hormone therapy for prostate cancer: directly randomised data from the STAMPEDE multi-arm, multi-stage platform protocol. Ann Oncol. 2018;29:1235–1248. doi: 10.1093/annonc/mdy072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Franke RM, Carducci MA, Rudek MA, et al. Castration-dependent pharmacokinetics of docetaxel in patients with prostate cancer. J Clin Oncol. 2010;28:4562–4567. doi: 10.1200/JCO.2010.30.7025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Keane T. Update on ADT in advanced prostate cancer. https://www.urotoday.com/journal/everyday-urology-oncology-insights/articles/110209-update-on-adt-in-advanced-prostate-cancer.html. Accessed September 28, 2020.
- 79.Morris MJ, Rumble RB, Basch E, et al. Optimizing anticancer therapy in metastatic non-castrate prostate cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018;36:1521–1539. doi: 10.1200/JCO.2018.78.0619. [DOI] [PubMed] [Google Scholar]
- 80.Clarke NW, Ali A, Ingleby FC, et al. Addition of docetaxel to hormonal therapy in low- and high-burden metastatic hormone sensitive prostate cancer: long-term survival results from the STAMPEDE trial. Ann Oncol. 2019;30:1992–2003. doi: 10.1093/annonc/mdz396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Parker CC, James ND, Brawley CD, et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet. 2018;392:2353–2366. doi: 10.1016/S0140-6736(18)32486-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lowrance WT, Murad MH, Oh WK, et al. Castration-resistant prostate cancer: AUA guideline amendment 2018. J Urol. 2018;200:1264–1272. doi: 10.1016/j.juro.2018.07.090. [DOI] [PubMed] [Google Scholar]
- 83.Smith MR, Kabbinavar F, Saad F, et al. Natural history of rising serum prostate-specific antigen in men with castrate nonmetastatic prostate cancer. J Clin Oncol. 2005;23:2918–2925. doi: 10.1200/JCO.2005.01.529. [DOI] [PubMed] [Google Scholar]
- 84.Smith MR, Cook R, Lee KA, Nelson JB. Disease and host characteristics as predictors of time to first bone metastasis and death in men with progressive castration-resistant nonmetastatic prostate cancer. Cancer. 2011;117:2077–2085. doi: 10.1002/cncr.25762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hussain M, Fizazi K, Saad F, et al. Enzalutamide in men with nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2018;378:2465–2474. doi: 10.1056/NEJMoa1800536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Smith MR, Saad F, Chowdhury S, et al. Apalutamide treatment and metastasis free survival in prostate cancer. N Engl J Med. 2018;378:1408–1418. doi: 10.1056/NEJMoa1715546. [DOI] [PubMed] [Google Scholar]
- 87.Fizazi K, Shore ND, Tammela TL, et al. Darolutamide in nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2019;380:1235–1246. doi: 10.1056/NEJMoa1815671. [DOI] [PubMed] [Google Scholar]
- 88.Abrahamsson PA. Potential benefits of intermittent androgen suppression therapy in the treatment of prostate cancer: a systematic review of the literature. Eur Urol. 2010;57:49–59. doi: 10.1016/j.eururo.2009.07.049. [DOI] [PubMed] [Google Scholar]
- 89.Tunn U. Can intermittent hormone therapy fulfill its promise? Eur Urol Suppl. 2008;7:752–757. [Google Scholar]
- 90.Calais da Silva FE, Bono AV, Whelan P, et al. Intermittent androgen deprivation for locally advanced and metastatic prostate cancer: results from a randomised phase 3 study of the South European Uroncological Group. Eur Urol. 2009;55:1269–1277. doi: 10.1016/j.eururo.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 91.Klotz L, O Callaghan CJ, Ding K, et al. A phase III randomized trial comparing intermittent versus continuous androgen suppression for patients with PSA progression after radical therapy: NCIC CTG PR.7/SWOG JPR.7/CTSU JPR.7/UK Intercontinental Trial CRUKE/01/013. J Clin Oncol. 2011;29(suppl 7):3. [Google Scholar]
- 92.Salonen AJ, Taari K, Ala-Opas M, et al. Comparison of intermittent and continuous androgen deprivation and quality of life between patients with locally advanced and patients with metastatic prostate cancer: a post-analysis of the randomized FinnProstate Study VII. Scand J Urol. 2014;48:513–522. doi: 10.3109/21681805.2014.901410. [DOI] [PubMed] [Google Scholar]
- 93.Schulman C, Cornel E, Matveev V, et al. Intermittent versus continuous androgen deprivation therapy in patients with relapsing or locally advanced prostate cancer: a phase 3b randomised study (ICELAND) Eur Urol. 2016;69:720–727. doi: 10.1016/j.eururo.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 94.Hussein M, Tangen CM, Berry DL, et al. Intermittent versus continuous androgen deprivation in prostate cancer. N Engl J Med. 2013;368:1314–1325. doi: 10.1056/NEJMoa1212299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hershman DL, Unger JM, Wright JD, et al. Adverse health events following intermittent and continuous androgen deprivation in patients with metastatic prostate cancer. JAMA Oncol. 2016;2:453–461. doi: 10.1001/jamaoncol.2015.4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Miura Y, Horie S. The role of hormone therapy and chemotherapy in oligometastatic prostate cancer. ESMO Open. 2019;4:e000471. doi: 10.1136/esmoopen-2018-000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ost P, Bossi A, Decaestecker K, et al. Metastasis-directed therapy of regional and distant recurrences after curative treatment of prostate cancer: a systematic review of the literature. Eur Urol. 2015;67:852–863. doi: 10.1016/j.eururo.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 98.Muralidhar V, Nezolosky MD, Chen YW, et al. National trends in androgen deprivation therapy use for prostate cancer after versus before the controversy surrounding cardiovascular toxicity. Abstract 2589. Int J Radiat Oncol Biol Phys. 2016;96:E241–E242. [Google Scholar]
- 99.Wellington K, Keam SJ. Bicalutamide 150mg: a review of its use in the treatment of locally advanced prostate cancer. Drugs. 2006;66:837–850. doi: 10.2165/00003495-200666060-00007. [DOI] [PubMed] [Google Scholar]
- 100.Shore ND, Saad F, Cookson MS, et al. Oral relugolix for androgen-deprivation therapy in advanced prostate cancer. N Engl J Med. 2020;382:2187–2196. doi: 10.1056/NEJMoa2004325. [DOI] [PubMed] [Google Scholar]
- 101.Kaplan AL, Hu JC, Morgentaler A, et al. Testosterone therapy in men with prostate cancer. Eur Urol. 2016;69:894–903. doi: 10.1016/j.eururo.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bell MA, Campbell JD, Joice G, et al. Shifting the paradigm of testosterone replacement therapy in prostate cancer. World J Mens Health. 2018;36:103–109. doi: 10.5534/wjmh.170007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sartor O, Sharma D. Radium and other alpha inhibitors in prostate cancer. Transl Androl Urol. 2018;7:436–444. doi: 10.21037/tau.2018.02.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Weichselbaum RR, Hellman S. Oligometastases revisited. Nat Rev Clin Oncol. 2011;8:378–382. doi: 10.1038/nrclinonc.2011.44. [DOI] [PubMed] [Google Scholar]


