Abstract
Patient: Male, 32-year-old
Final Diagnosis: COVID-19 • influenza • pneumonia
Symptoms: Cough • fever • shortness of breath
Medication: —
Clinical Procedure: —
Specialty: Infectious Diseases
Objective:
Rare co-existance of disease or pathology
Background:
There was a growing presumption that coinfection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and another viral respiratory illness was nonexistent. Although there has been an increasing number of coinfection cases since the beginning of the SARS-CoV-2 pandemic, there is still a significant lack of information regarding the symptomatology, treatment, prognosis, and reasoning behind coinfection. This raises concern of the possibility of misdiagnosis or delay in treatment.
Case Report:
This case report discusses a coinfection of SARS-CoV-2 and Influenza A in a 32-year-old man to highlight that these viruses can coexist within the same patient. This patient unfortunately died of persistent respiratory failure after several days in the ICU.
Conclusions:
Coinfection of SARS-CoV-2 and Influenza A can occur and lead to a poor prognosis.
MeSH Keywords: Coinfection, Coronavirus, COVID-19, Influenza A virus
Background
Some early studies on severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in China reported the low occurrence of SARS-CoV-2 with other respiratory infections [1]. The Centers for Disease Control and Prevention (CDC) recommends testing for other respiratory pathogens before conducting SARSCoV-2 testing, which can help rule out SARS-CoV-2 infection if the results are positive for another pathogen [2]. This has led to a growing presumption that coinfection with SARS-CoV-2 and another viral respiratory illness is nonexistent. Additionally, the symptomatology of SARS-CoV-2 and influenza are similar. Unfortunately, following this presumption can lead to significant adverse outcomes such as delay in appropriate treatment and increased morbidity and mortality. The similarities in symptomatology may also lead to alternative diagnosis considerations, which may further delay appropriate treatment.
Previously, in the Middle Eastern respiratory syndrome coronavirus (MERS-CoV) epidemic, there were reported cases of coinfections with MERS-CoV with other respiratory viruses, including influenza, respiratory syncytial virus, and human meta-pneumovirus [3,4]. While not all coinfecting respiratory viruses increased disease severity, those that did typically led to an increased oxygen requirement in cases when the infection alone had a significant impact on the patient in the setting of MERS-CoV. Influenza has a range of severity in patients depending on comorbidities, which is well documented. Patients with MERS-CoV and coinfecting influenza had an increased oxygen requirement and a longer hospital stay [3,4]. The MERSCoV infects similarly to SARS-CoV-2 infection because they are in the same viral family. This further contradicts the presumption that coinfection of SARS-CoV-2 and another respiratory pathogen cannot coexist. Cases of coinfections with another respiratory pathogen are likely to occur if the SARS-CoV-2 pandemic continues through the seasons of other respiratory pathogens. This case report discusses a patient with coinfection of SARS-CoV-2 and influenza A, focusing on disease severity, respiratory status, and comorbidities.
Case Report
The patient is a 32-year-old man with no known past medical history, who presented to the emergency department with a fever, cough, and shortness of breath for 2 days. The patient reported having diarrhea 3 days prior to the onset of the fever and cough, but denied diarrhea on admission. He denied any chest pain, abdominal pain, nausea, or vomiting. The patient’s symptoms began shortly after exposure to his father, who had similar symptoms. The father had been experiencing symptoms just prior to the onset of the patient’s symptoms, and was also admitted to the hospital to rule out SARS-CoV-2.
Our patient had no other known sick contacts. He was admitted to our hospital to rule out community acquired pneumonia versus viral syndrome secondary to SARS-CoV-2 or influenza.
Physical exam on admission was unremarkable, showing a regular heart rate and heart rhythm with no murmurs, rubs, or gallops present. There was no jugular venous distension. The lung examination showed that the patient’s lungs were clear to auscultation bilaterally. There was evidence of ecchymosis from cupping marks present on the patient’s back. On admission, the patient was normotensive, and no tachycardia was present. His respiratory rate was 18 breaths per min and he had an oxygen saturation (SpO2) of 95% on room air.
Initial laboratory investigation results showed an unremarkable complete blood count. Results were remarkable for hyper-glycemia (282 mg/dL), elevated hemoglobin A1c level of 14%, elevated lactate dehydrogenase (LDH) of 792 U/L, and elevated C-reactive protein (CRP) of 126.61 mg/L. An initial venous blood gas analysis performed to assess for ketoacidosis related to undiagnosed diabetes showed mild acidemia. An electrocardiogram showed a normal sinus rhythm without QTc prolongation. A chest X-ray showed peripheral patchy opacities in the lower lung zones, which could be seen in viral pneumonia (Figure 1).
Figure 1.
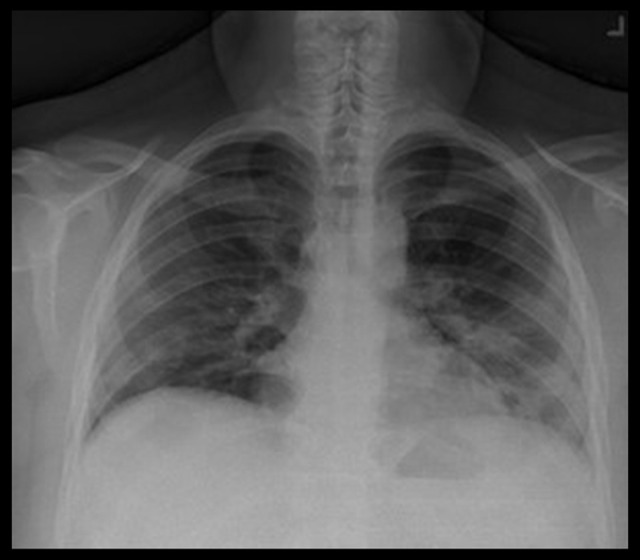
Chest X-ray showing peripheral patchy opacities in the lower lung zones.
Upon admission, the patient was started on 1 g of IV ceftriaxone daily, 500 mg of IV azithromycin daily, 400 mg of IV hydroxychloroquine every 12 h, and 220 mg of oral zinc supplementation daily to cover both community acquired pneumonia and SARS-CoV-2. SARS-CoV-2 RNA and influenza A and influenza-B antigen nasal swabs were sent for reverse transcription polymerase chain reaction (RT-PCR) testing. Within hours of admission, the patient desaturated to 79% SpO2 and required 6 L/min supplemental oxygen via a nasal cannula. This was switched to a high-flow nasal cannula (HFNC) to maintain SpO2 >94% and adequate oxygenation per pulmonology recommendations. Given the patient’s new oxygen requirement, the infectious disease team was consulted, and they recommended adding 600 mg of IV linezolid every 12 h. An insulin regimen was started for the patient’s new diagnosis of diabetes.
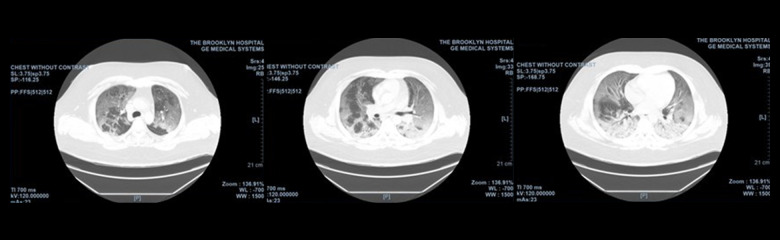
On hospital day 2, the patient’s SARS-CoV-2 RNA and influenza A nasal swab results were positive. Oseltamivir 30 mg was added to his medication regimen. A chest computed tomography (CT) scan (Figure 2) was performed, which showed extensive bilateral ground-glass opacities with areas of confluent consolidation in the posterior lung bases.
Figure 2.
Chest computed tomography (CT) scan without contrast showing extensive bilateral ground-glass opacities with areas of confluent consolidation in the posterior lung bases.
Throughout his hospital stay, the patient had continuous, repeated fever spikes. He remained stable until hospital day 5, when he developed worsening tachypnea with an increased FiO2 requirement and worsening PaO2/FiO2 ratio. The patient’s SpO2 remained at around 87%, despite an FiO2 of 100% on HFNC. His respiratory rate increased to 28 to 32 breaths per min. A repeat chest X-ray was done, which showed worsening of the prior bilateral lower lung opacities. Additional laboratory results revealed an elevated ferritin level of 884 ng/mL and a persistently elevated LDH level of 870 U/L. Arterial blood gas showed normal pH and normocarbia but hypoxia and an elevated bicarbonate level. Due to the new onset of acute respiratory distress syndrome (ARDS) secondary to sepsis, which was secondary to coinfection with SARS-CoV-2 and influenza A, the patient was intubated, placed on mechanical ventilation, and upgraded to the Intensive Care Unit (ICU). The patient was given a course of 800 mg of IV tocilizumab. He also experienced acute kidney injury and was anuric; therefore, hemodialysis was started. Linezolid was discontinued after the respiratory sensitivity culture and completed course of 1000 mg of IV meropenem daily and 400 mg-100 mg per 5 mL IV lopinavir and ritonavir (brand name Kaletra) every 12 h per the infectious disease team’s recommendation. The patient self-extubated during the spontaneous awakening trial and was transitioned to bilevel positive airway pressure (BIPAP), then to HFNC. The patient was downgraded but was found to be hypoxic on the medical floor. A repeat chest X-ray (Figure 3) showed worsening opacities. BIPAP was placed again, but the patient could not tolerate it, and went into respiratory distress. The patient was re-intubated and placed back in the ICU. Unfortunately, the patient died after several additional days in the ICU.
Figure 3.
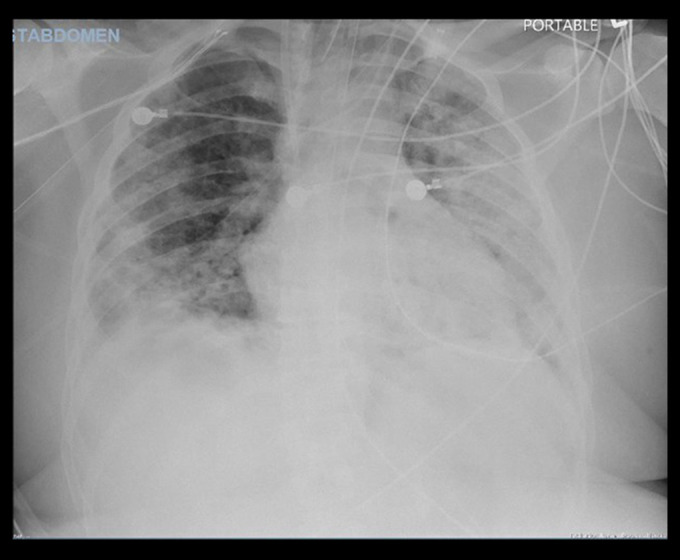
Repeated chest X-ray during recurrent episode of respiratory failure showing worsening opacities.
Discussion
The current guidelines from the CDC suggest initially screening for influenza because SARS-CoV-2 shares common symptomatology with influenza, including fever, dry cough, dyspnea, myalgias, and fatigue [1]. The SARS-CoV-2 pandemic began in the winter months when influenza has a high prevalence. Differentiating between SARS-CoV-2 and influenza during the peak times for both illnesses can be difficult. The influenza test is rapid, inexpensive, and easily accessible in most institutions; therefore, collecting this test is reasonable [2]. Laboratory studies in a SARS-CoV-2 patient will typically show leukopenia, lymphopenia, elevated LDH, and elevated CRP. Chest CT scans show ground-glass opacities with consolidations in the bilateral lung fields [1,5–9]. A variety of influenza strains and other respiratory viruses also present with this pattern of findings [6,7,9]. SARS-CoV-2 testing is limited and results are not rapid. Without appropriate and accessible testing for SARSCoV-2, this can lead to a presumptive misdiagnosis and improper treatment. Given the pandemic’s prevalence and high suspicion of SARS-CoV-2, oseltamivir was not started immediately in the present case. At the time of this patient’s case, there had not been many reports of coinfection with influenza, which contributed to the delay in starting oseltamivir.
The mechanism of infection of SARS-CoV-2 and influenza are different, which is likely the reason that coinfection can occur. Influenza has been known to coinfect patients with other respiratory viruses [10,11]. While there is no definitive mechanism determined yet, there have been many studies performed that evaluate the possible mechanisms of coinfection. This has provided the idea that respiratory viruses with different infection mechanisms may either enhance, slow, or inhibit a coin-fecting virus [12]. Influenza viruses infect the epithelial cells throughout the respiratory tract. They bind through hemagglutinin onto the sialic acid sugars on surfaces of respiratory epithelial cells [13]. SARS-CoV-2 virion has an S-glycoprotein that attaches to the ACE2 receptor on the surface of human lower respiratory tract epithelial cells [14–16]. The differing infection mechanisms likely allow coinfection to be possible, although further studies are needed to determine this.
It has been noted that influenza coinfection with other respiratory viruses typically occurs in patients with chronic disease or immunosuppression [10,11]. Coinfection with other respiratory viruses also results in worse prognosis, increased hospital stays, and mortality [11]. Our patient had newly diagnosed diabetes mellitus, which is a known cause of immunosuppression. The newly diagnosed diabetes mellitus may have led to an increased risk of coinfection in this patient. Emerging data has shown an increased risk of morbidity and mortality in all patients with a diagnosis of diabetes, which comes from the innate and humoral immune suppression [17]. Patients with diabetes typically have a more severe presentation and quicker respiratory decline than healthy patients and often require admission to the ICU or a longer hospital stay [17]. Our patient had a notable quick decline in respiratory status shortly after admission to the hospital, which required a prolonged ICU stay and intubation. His new onset of diabetes likely contributed to his decline and associated morbidity. This case highlights the relevance of considering coinfection with other respiratory viruses that may alter treatment and outcome when there is concern for SARS-CoV-2 infection.
Conclusions
Influenza A and SARS-CoV-2 coinfection can occur and may lead to a more severe clinical picture. It is important to consider that coinfection can occur, especially in patients with comorbidities. In addition, coinfection treatment will differ, depending on the viruses present. In a patient with a normal influenza, we would treat with oseltamivir within 48 h of symptom presentation. In SARS-CoV-2 infection, however, other medications would be required to treat the patient, based on the current and emerging research, but typically would not include oseltamivir. Having the knowledge that coinfections like this can exist can allow us to appropriately treat the present viruses with hopefulness of an improved morbidity and mortality risk.
Footnotes
Department and Institution where work was done
This work was conducted at the Brooklyn Hospital Center in Brooklyn, NY, U.S.A.
References:
- 1.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet. 2020;395(10223):507–13. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention Evaluating and Testing Persons for Coronavirus Disease 2019 (COVID-19) https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-criteriahtml.
- 3.Alfaraj SH, Al-Tawfiq JA, Alzahrani NA, et al. The impact of co-infection of influenza A virus on the severity of Middle East Respiratory Syndrome Coronavirus. J Infect. 2017;74(5):521–23. doi: 10.1016/j.jinf.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abdulhaq AA, Basode VK, Hashem AM, et al. Patterns of human respiratory viruses and lack of MERS-coronavirus in patients with acute upper respiratory tract infections in southwestern province of Saudi Arabia. Adv Virol. 2017;2017:4247853. doi: 10.1155/2017/4247853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu X, Cai Y, Huang X, et al. Co-infection with SARS-CoV-2 and influenza A virus in patient with pneumonia, China. Emerg Infect Dis. 2020;26(6):1324–26. doi: 10.3201/eid2606.200299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sullivan SJ, Jacobson RM, Dowdle WR, Poland GA. 2009 H1N1 influenza. Mayo Clin Proc. 2010;85(1):64–76. doi: 10.4065/mcp.2009.0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–69. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ding Q, Lu P, Fan Y, et al. The clinical characteristics of pneumonia patients coinfected with 2019 novel coronavirus and influenza virus in Wuhan, China. J Med Virol. 2020 doi: 10.1002/jmv.25781. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stefanska I, Romanowska M, Donevski S, et al. Co-infections with influenza and other respiratory viruses. Adv Exp Med Biol. 2013;756:291–301. doi: 10.1007/978-94-007-4549-0_36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goka E, Vallely P, Mutton K, Klapper P. Influenza A viruses dual and multiple infections with other respiratory viruses and risk of hospitalisation and mortality. Influenza Other Respir Viruses. 2013;7(6):1079–87. doi: 10.1111/irv.12020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Opatowski L, Baguelin M, Eggo RM. Influenza interaction with cocirculating pathogens and its impact on surveillance, pathogenesis, and epidemic profile: A key role for mathematical modelling. PLoS Pathog. 2018;14(2):e1006770. doi: 10.1371/journal.ppat.1006770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dou D, Revol R, Ostbye H, et al. Influenza A virus cell entry, replication, virion assembly and movement. Front Immunol. 2018;9:1581. doi: 10.3389/fimmu.2018.01581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wan Y, Shang J, Graham R, et al. Receptor recognition by the novel corona-virus from Wuhan: An analysis based on decade-long structural studies of SARS coronavirus. J Virol. 2020;94(7):e00127–20. doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–73. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo YR, Cao QD, Hong ZS, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak – an update on the status. Mil Med Res. 2020;7(1):11. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh AK, Gupta R, Ghosh A, Misra A. Diabetes in COVID-19: Prevalence, pathophysiology, prognosis and practical considerations. Diab Metab Syndr. 2020;14(4):303–10. doi: 10.1016/j.dsx.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]