Key Points
Question
Does an intervention aimed at improving guideline adherence for the management of acute heart failure, including intensive intravenous nitrate therapy and management of precipitating factors, improve hospital discharge and survival at 30 days?
Findings
In this stepped-wedge cluster randomized trial that included 503 patients 75 years and older who presented to the emergency department with acute heart failure, implementation of an early and comprehensive care bundle compared with usual care improved guideline adherence, but had no significant effect on number of days alive and out of hospital at 30 days (median of 19 d in both groups).
Meanings
This emergency department care bundle did not improve 30-day outcomes among older patients with acute heart failure.
Abstract
Importance
Clinical guidelines for the early management of acute heart failure in the emergency department (ED) setting are based on only moderate levels of evidence, with subsequent low adherence to these guidelines.
Objective
To test the effect of an early guideline-recommended care bundle on short-term prognosis in older patients with acute heart failure in the ED.
Design, Setting, and Participants
Stepped-wedge cluster randomized trial in 15 EDs in France of 503 patients 75 years and older with a diagnosis of acute heart failure in the ED from December 2018 to September 2019 and followed up for 30 days until October 2019.
Interventions
A care bundle that included early intravenous nitrate boluses; management of precipitating factors, such as acute coronary syndrome, infection, or atrial fibrillation; and moderate dose of intravenous diuretics (n = 200). In the control group, patient care was left to the discretion of the treating emergency physician (n = 303). Each center was randomized to the order in which they switched to the “intervention period.” After the initial 4-week control period for all centers, 1 center entered in the intervention period every 2 weeks.
Main Outcomes and Measures
The primary end point was the number of days alive and out of hospital at 30 days. Secondary outcomes included 30-day all-cause mortality, 30-day cardiovascular mortality, unscheduled readmission, length of hospital stay, and kidney impairment.
Results
Among 503 patients who were randomized (median age, 87 years; 298 [59%] women), 502 were analyzed. In the intervention group, patients received a median (interquartile range) of 27.0 (9-54) mg of intravenous nitrates in the first 4 hours vs 4.0 (2.0-6.0) mg in the control group (adjusted difference, 23.8 [95% CI, 13.5-34.1]). There was a significantly higher percentage of patients in the intervention group treated for their precipitating factors than in the control group (58.8% vs 31.9%; adjusted difference, 31.1% [95% CI, 14.3%-47.9%]). There was no statistically significant difference in the primary end point of the number of days alive and out of hospital at 30 days (median [interquartile range], 19 [0- 24] d in both groups; adjusted difference, −1.9 [95% CI, −6.6 to 2.8]; adjusted ratio, 0.88 [95% CI, 0.64-1.21]). At 30 days, there was no significant difference between the intervention and control groups in mortality (8.0% vs 9.7%; adjusted difference, 4.1% [95% CI, −17.2% to 25.3%]), cardiovascular mortality (5.0% vs 7.4%; adjusted difference, 2.1% [95% CI, −15.5% to 19.8%]), unscheduled readmission (14.3% vs 15.7%; adjusted difference, −1.3% [95% CI, −26.3% to 23.7%]), median length of hospital stay (8 d in both groups; adjusted difference, 2.5 [95% CI, −0.9 to 5.8]), and kidney impairment (1% in both groups).
Conclusions and Relevance
Among older patients with acute heart failure, use of a guideline-based comprehensive care bundle in the ED compared with usual care did not result in a statistically significant difference in the number of days alive and out of the hospital at 30 days. Further research is needed to identify effective treatments for acute heart failure in older patients.
Trial Registration
ClinicalTrials.gov Identifier: NCT03683212
This unblinded cluster randomized trial compares the effects of an emergency department (ED)–based comprehensive, guideline-recommended care bundle vs usual care on the number of days alive and out of the hospital at 30 days in older patients with acute heart failure cared for at French hospitals.
Introduction
Acute heart failure is one of the most common syndromes in older patients visiting the emergency department (ED).1 In an international observational study that included patients 75 years and older from 2010 to 2013, acute heart failure was associated with a 10% mortality risk and up to 30% risk of hospital readmission at 30 days.2
International recommendations and clinical guidelines on acute heart failure include the use of moderate doses of diuretics, early initiation of nitrates, and the use of noninvasive ventilation when indicated, along with the early detection and management of precipitating factors, such as acute coronary syndrome, infection, or atrial fibrillation.3,4,5 However, these recommendations are based on low levels of evidence. The cornerstone trials on small samples of patients with acute heart failure reported a clinical benefit with early high-dose intravenous nitrates.6,7 Subsequent large-scale trials testing novel agents with vasodilator properties failed to confirm improved outcomes, possibly because of effects besides vasodilation that might affect mortality risk.8,9 As a potential consequence, previous reports confirmed low adherence to these recommendations, leading to underuse of nitrates in the ED.10,11
The failure of recent acute heart failure studies to report clinical benefits with specific treatments may be attributable to both the delay from presentation to treatment, because recent studies suggest that an early therapy is associated with better prognosis, and the lack of management of precipitating factors, because acute heart failure prognosis has been reported to be associated with the underlying precipitant of worsening heart failure.12,13 Whether early ED management of congestion and precipitating factors improves outcomes is unknown.
The aim of this trial was to test the efficacy on clinical outcomes of an early, comprehensive, and guideline-recommended care bundle on older patients with acute heart failure in the ED.14
Methods
Study Design
The protocol and statistical analysis plan of the ELISABETH trial are available in Supplement 1 and have been previously published in detail.14 This was an unblinded, superiority, stepped-wedge, cluster randomized clinical trial in France aimed at testing the effect of an early comprehensive care bundle for acute heart failure on older patients in the ED. Fifteen EDs in France participated in the study. The trial recruited from December 2018 to September 2019, and follow-up ended in October 2019. The study was approved by an institutional review board (Comité de Protection des Personnes SOOM 2, Toulouse academic hospital, France). Written informed consent was sought for all patients before inclusion. If the patient lacked capacity to give consent, the emergency physician sought consent from a relative of the patient. If no such relative was available, research staff were able to proceed to an emergency inclusion. For the latter, as soon as the patient’s clinical condition allowed it, a clinical research technician or a physician informed them about the trial and sought written informed consent. The reporting of this trial followed the Consolidated Standards of Reporting Trials (CONSORT) statement extended to stepped-wedge cluster randomized trials.15,16
Patients
Consecutive patients 75 years and older with a clinical diagnosis of acute heart failure in the ED were eligible for inclusion in the trial. Acute heart failure diagnosis was made on the basis of having acute or worsening dyspnea and/or orthopnea and at least 1 of the following: bilateral pulmonary rales or peripheral edema, signs of pulmonary congestion on chest radiography or cardiac echocardiography, or elevation in level of natriuretic peptides (brain natriuretic peptide or N-terminal pro–brain natriuretic peptide). As a pragmatic study, whether the diagnosis of acute heart failure was confirmed was left to the discretion of the physician, and echocardiography was not mandatory.
Patients were not included if they presented with another obvious cause of acute illness (eg, ST-elevation myocardial infarction, severe sepsis), systolic blood pressure less than 100 mm Hg, any contraindication for nitrate therapy (severe mitral or aortic stenosis or severe aortic regurgitation), or a known chronic kidney impairment that required dialysis. Because this trial focused on early management of acute heart failure, patients in whom the time from ED presentation to inclusion was more than 6 hours were also excluded. Patients with no Social Security or who were incarcerated or under guardianship were also excluded.
All patients were followed up at 30 days via hospital visit or phone interview if already discharged. They were instructed to return to the same ED or hospital in the event of recurrent or worsening symptoms. A local clinical research assistant checked for return visits to the ED or admission to the hospital during the follow-up period. The phone interview was performed using a structured questionnaire that recorded length of initial hospital stay, any return visit to the ED, or admission to the hospital. When patients could not be contacted by phone, the patient’s general practitioner was contacted. If it was not possible to contact the patient or their physician, death records were sought from the patient’s birth town administrative record.
Randomization
After an initial control period (first step) of 4 weeks in all centers, one of the EDs switched to the “intervention period” every 2 weeks. The order in which they switched was randomized. The first 2 weeks of the intervention period in each center was also a training period, in which a clinical research technician presented and detailed the care bundle and assisted emergency physicians in implementing it. After 32 weeks, all EDs were in the “intervention period” for the 8 remaining weeks of inclusion (eFigure 1 in Supplement 2). Randomization of the order of the switch was performed by an independent statistician. At study commencement, EDs were classified on size based on their annual census. Randomization was stratified on cluster size (small, medium, large). Each set of 3 consecutive time periods would contain 1 small, 1 medium, and 1 large site in random order.
Intervention
This study had an unblinded design, which was chosen because the intervention could not be performed in a blinded fashion for either physicians or patients. In participating EDs and in most French EDs, patients are first seen by a triage nurse then seen by a senior emergency physician, with or without the help of a trainee. Only senior emergency physicians could include patients, and consequently the entirety of the ED medical management was completed by a senior emergency physician. In France, regular emergency medical services (EMS) cannot administer treatments, therefore no patients conveyed by regular EMS received any treatment before inclusion in the ED. However, some patients may have been transported by a physician-staffed EMS (service mobile d’urgence-réanimation), where treatment could have been given. These patients are usually directly admitted to an intensive care unit. If that was not the case, patients in whom treatment was already started before inclusion were excluded.
The intervention of this trial consisted of a guideline-recommended care bundle for the early management of acute heart failure in the ED. In the control period, patient care was left to the discretion of the treating emergency physician (usual care). At the beginning of the trial, the recommendations for acute heart failure management were described to all emergency physicians, including moderate-dose diuretics, high-dose intravenous nitrates, search for and early management of the most frequent precipitating factors, and noninvasive ventilation when indicated.
In the intervention period, after a patient’s inclusion, emergency physicians were instructed to follow the early care bundle for acute heart failure, with the help of a handover checklist. This care bundle mandated treatment initiation within 1 hour and completion within 4 hours of early management of pulmonary edema with 40 mg (if not currently receiving diuretics) or a daily dose (if already receiving oral diuretics) of intravenous furosemide or intravenous nitrates in titration (given in 3-mg boluses every 5 minutes for 1 hour, titrated until the patient reached a systolic blood pressure above 100 mm Hg) and detection and management of precipitating factors (specifically acute coronary syndrome, rapid atrial fibrillation, and suspected infection). Suspicion of cardiac injury (based on troponin level measurement, echocardiography, and electrocardiogram analysis) required introduction of antiplatelet therapy and referral to cardiologic intensive care unit for potential admission and coronary angiogram if indicated. Suspicion of respiratory tract infection (based on chest radiograph findings, C-reactive protein, procalcitonin, and leukocytes level) required early introduction of antibiotics. Atrial fibrillation with a heart rate above 100/min required introduction of antiarythmic therapy (amiodarone, digoxin, or β-blockers as indicated). Noninvasive ventilation was administered in the event of respiratory distress or hypercarbia with pH less than 7.35.
All treatments were to be initiated in the ED within the first hour of medical management for at least 4 hours. Their discontinuation was left to the discretion of the admitting physician.
Outcomes
The primary objective of the trial was to test the effect of an early comprehensive guideline-recommended care bundle on morbidity and mortality at 30 days in older patients. The primary end point was the number of days alive and out of hospital during the 30-day period after the ED visit. The choice of this end point allowed for capturing the burden of acute heart failure in terms of mortality, hospital length of stay, and early readmission to the hospital, as recommended by the European Society of Cardiology consensus paper.17 Patients who died before day 30 were counted as having zero days alive and out of hospital. A return visit to the ED was considered as 1 day in the hospital. Details on the calculation of this end point were previously detailed.14
The secondary end points included 30-day all-cause mortality, 30-day cardiovascular mortality, hospital readmission within 30 days, length of in-hospital stay truncated at 30 days, and changes of more than 2-fold in creatinine level from inclusion to day 30 or to discharge (whichever came first). Creatinine was measured at day zero in the ED and at discharge or day 30.
Power Analysis
The full statistical plan and sample size calculation are available in Supplement 1. Sample size was calculated under the superiority hypothesis. Based on previous cohort analysis, the mean (SD) number of days alive and out of the hospital at 30 days was estimated at 14 (9).10,12,18 To be clinically relevant and consistent with previous literature, it was estimated that the intervention should improve this end point by 20% (ie, ≥3 days).8,9 For 80% power and an α of 5%, 283 patients needed to be included. Adding the cluster effect for this stepped-wedge design and assuming an intracluster correlation at 0.001, the required sample size was 454 patients. Taking into account a rate of loss to follow-up of 10%, the necessary sample size was 500 patients—2 per cluster for each 2-week period (step). Due to acute heart failure seasonality and fewer cases in the summer period, we had to increase the length of the last inclusion step from 4 weeks to 8 weeks to reach the target.19,20
Statistical Analysis
The primary analysis included all patients who were randomized (Figure 1), with missing outcome data replaced using worst-case imputation (zero days alive and out of the hospital). Baseline characteristics were expressed as numbers and percentages for categorical variables and mean and SD or median and interquartile range (IQR) for continuous variables, depending on their distribution. Unadjusted differences and 95% CIs were calculated using the Wald method with continuity correction for binary variables and using the Brookmeyer and Crowley method for continuous variables. Adjusted differences were calculated using a generalized linear regression mixed model with Bernoulli (binary) distribution and an identity link function with intervention, time period, and cluster size (categorical) as fixed effects and cluster as a random effect for binary variables and using a quantile regression model for continuous variables.21 The 2-week training period for each site was analyzed as part of the intervention period. Primary and secondary outcomes were analyzed in all randomized patients with a primary outcome available. The number of days alive and out of hospital was analyzed using a generalized linear regression mixed model with negative binomial distribution with intervention, time period, and cluster size as fixed effects and cluster as a random effect.22 A sensitivity analysis was performed on all patients who completed the trial. All-cause mortality at 30 days, cardiovascular mortality at 30 days, and hospital readmission were compared between groups by using generalized linear regression mixed model with Poisson distribution. The logarithm of the number of patients was included as an offset term in the model. In surviving patients, the hospital length of stay in days was compared between the 2 groups by using a generalized linear regression mixed model with negative binomial distribution. For secondary outcomes, fixed and random effects were defined as previously described. For nonhospitalized patients, length of hospital stay was counted as zero days. The percentage of patients with a change of more than 2-fold in creatinine between inclusion and 30 days were not analyzed because of the presence of a small number of cases and no or negligible differences between the 2 groups.
Figure 1. Flow of Patients and Clusters in a Study of the Effect of an Emergency Department (ED) Care Bundle Among Older Patients With Acute Heart Failure.
The order in which each of the 15 clusters (EDs) switched to the intervention period was randomized.
Because of the potential for type I error due to multiple comparisons, the findings of analyses of secondary end points should be interpreted as exploratory. The original analysis plan was intended to study different extensions of the models as sensitivity analyses, including a time × cluster interaction as a fixed effect and as a random effect. However, due to the structure of the data (low proportion of events by cluster by period), these sensitivity analyses could not be performed. All superiority tests were 2-sided and P values <.05 were considered significant. SAS V.9.4 software (SAS Institute Inc) and Stata version 16 (Stata Corp), were used for statistical analyses.
Results
Study Population
Fifteen EDs participated in the trial and 503 patients were recruited. Among them, 1 patient was under guardianship and was excluded, 5 patients withdrew their consent, 3 were lost to follow-up, and 1 was missing the primary end point. Therefore, 502 patients were included in the primary analysis: 303 in the control group and 199 in the intervention group. A total of 493 patients completed the trial and were included in the sensitivity analysis (Figure 1; eFigure 1 and eTable 1 in Supplement 2). The mean (SD) number of patients recruited was 34 (12) by center and 31 (20) by step. The median (IQR) age of participants was 87 (81-91) years, 298 (59%) were women, and 269 (54%) had known chronic cardiac failure. Patients’ baseline characteristics were similar between the 2 groups and are reported in Table 1. Pulmonary congestion was similar between intervention and control groups, including mean (SD) oxygen saturation (90.6% [6.7%] vs 91.1% [6.9%]), respiratory rate (26.2 [6.7] per min vs 25.9 [7.3] per min), and systolic blood pressure (155 [29] mm Hg vs 149 [28] mm Hg).
Table 1. Baseline Characteristics of Participants in a Study of the Effect of an Emergency Department Care Bundle Among Older Patients With Acute Heart Failure.
| Variable | Intervention (n = 199) | Usual care (n = 303) | Normal values |
|---|---|---|---|
| Age, median (IQR), y | 87.0 (81.0-90.0) | 87.0 (81.0-91.0) | |
| Sex, No. (%) | |||
| Women | 112 (56.3) | 186 (61.4) | |
| Men | 87 (43.7) | 117 (38.6) | |
| Comorbidities, No. (%)a | |||
| Chronic pulmonary disease | 39 (19.6) | 46 (15.2) | |
| Chronic heart failure | 111 (55.8) | 158 (52.3) (n = 302) | |
| Chronic kidney disease | 49 (24.6) | 73 (24.1) (n = 302) | |
| Diabetes | 54 (27.1) | 92 (30.4) (n = 302) | |
| Myocardial infarction | 80 (40.2) | 91 (30.0) (n = 302) | |
| Vital signs at randomization, mean (SD) | |||
| Heart rate/min | 86.3 (23.5) | 86.6 (23.8) | |
| Oxygen saturation, % | 90.6 (6.7) (n = 185) | 91.1 (6.9) (n = 286) | |
| Respiratory rate/min | 26.2 (6.7) (n = 187) | 25.9 (7.3) (n = 273) | |
| Systolic blood pressure, mm Hg | 155.3 (28.8) (n = 187) | 148.8 (27.9) (n = 273) | |
| Temperature, °C | 36.8 (0.6) (n = 198) | 36.7 (0.7) (n = 273) | |
| Medication at randomization, No. (%) | |||
| ACE inhibitor | 58 (29.1) | 84 (27.7) | |
| ARB | 41 (20.6) | 79 (26.1) | |
| Antibiotic | 18 (9.0) | 25 (8.3) | |
| Anticoagulant | 96 (48.2) | 147 (48.5) | |
| Antiplatelet | 75 (37.7) | 116 (38.3) | |
| β-Blocker | 123 (61.8) | 165 (54.5) | |
| Diuretic | 129 (64.8) | 222 (73.3) | |
| Nitrates | 13 (6.5) | 17 (5.6) | |
| Laboratory results, median (IQR)b | |||
| BNP, ng/L | 591.0 (251.5-977.0) (n = 81) | 620.0 (319.6-1220.0) (n = 151) | <450 |
| Creatinine, mg/L | 11.2 (8.7-14.7) (n = 81) | 11.5 (8.9-16.0) (n = 301) | 6-12 |
| C-reactive protein, mg/L | 13.7 (5.0-47.1) (n = 155) | 13.4 (5.0-43.7) (n = 236) | <5 |
| Hemoglobin, mean (SD), g/dL | 12.4 (2.0) (n = 155) | 12.1 (1.8) (n = 300) | 12-17 |
| Leukocytes, G/L | 9.0 (6.9-11.9) (n = 155) | 8.3 (6.6-10.8) (n = 301) | 4-10 |
| NT pro-BNP, ng/L | 4.2×103 (1.8×103-7.9×103) (n = 116) | 5.0×103 (2.3×103-9.3×103) (n = 144) | 2×103 |
| Procalcitonin, μg/L | 0.1 (0.1-0.2) (n = 54) | 0.1 (0.1-0.3) (n = 45) | <0.1 |
| Troponin, μg/L | 0.0 (0.0-0.1) (n = 185) | 0.0 (0.0-0.1) (n = 243) | <14 |
| pH, mean (SD) | 7.4 (0.1) (n = 176) | 7.4 (0.1) (n = 210) | 7.35-7.45 |
| Paco2, mm Hg | 40.0 (33.5-47.0) (n = 176) | 39.0 (34.0-45.0) (n = 210) | 35-45 |
| Pao2, mm Hg | 74.0 (65.0-91.5) (n = 176) | 70.0 (60.0-87.0) (n = 210) | 80-100 |
| Bicarbonate, mean (SD), mmol/L | 25.8 (5.2) (n = 150) | 24.7 (4.4) (n = 221) | 22-26 |
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blockade; IQR, interquartile range; NT pro-BNP, N-terminal pro–brain natriuretic peptide.
Comorbidities were recorded by the emergency physician during the ED visit.
Laboratory results were obtained as part of ED care.
Treatment in the ED
In the intervention group, compared with the control group, a statistically significantly higher percentage of patients were treated with intravenous nitrates (96% vs 25%; adjusted difference, 71% [95% CI, 62% to 80%]), were given a higher median dose of intravenous nitrates (27.0 mg vs 4.0 mg at 4 hours of initial management; adjusted difference, 24 mg [95% CI, 13.5 to 34.1]), and were treated with diuretics (98% vs 90%; adjusted difference, 6.8% [95% CI, 0.5% to 13.0%]) (Table 2). A precipitating factor was present in 45% of patients, and these factors were treated in significantly more patients in the ED in the intervention group than in the control group (58.8% vs 31.9%; adjusted difference, 31.1% [95% CI, 14.3% to 47.9%]) (eTable 2 in Supplement 2).
Table 2. Treatment of Participants in a Study of the Effect of an Emergency Department (ED) Care Bundle Among Older Patients With Acute Heart Failure.
| Variable | Intervention (n = 199) | Usual care (n = 303) | Adjusted difference (95% CI), %a |
|---|---|---|---|
| Treatment in the emergency department, No. (%) | |||
| Furosemideb | 195 (98.0) | 274 (90.4) | 6.8 (0.5 to 13.0) |
| Dose, median (IQR), mg | 40.0 (40.0 to 80.0) (n = 195) | 60.0 (40.0 to 80.0) (n = 273) | –13.1 mg (–25.4 to –0.9) |
| IV nitratesc | 191 (96.0) | 74 (24.5) (n = 302) | 71.0 (61.6 to 80.3) |
| Cumulative dosing at hour 1, median (IQR), mg | 18.0 (9.0-30.0) (n = 187) | 3.0 (2.0-4.0) (n = 54) | 14.9 mg (8.9 to 20.8) |
| Cumulative dosing at hour 4, median (IQR), mg | 27.0 (9.0-53.5) (n = 188) | 4.0 (2.0-6.0) (n = 73) | 23.8 mg (13.5 to 34.1) |
| Antibiotics | 39 (19.6) | 38 (12.5) | 10.8 (1.6 to 19.9) |
| Antiplatelet agents | 15 (7.5) | 23 (7.6) | 0.2 (–7.9 to 8.4) |
| Dual antiplatelet agentsd | 4 (2.0) | 3 (1.0) | |
| Antiarrhythmics | 22 (11.1) | 23 (7.6) | 2.6 (–5.7 to 10.8) |
| Noninvasive ventilation | 29 (14.6) | 29 (9.6) | 6.0 (–4.6 to 16.6) |
| ED discharge disposition, No. (%) | |||
| Home | 3 (1.5) | 15 (5.0) | –3.1 (–9.4 to 3.2) |
| ED observation unit | 96 (48.2) | 137 (45.2) | –2.9 (–16.3 to 10.6) |
| Hospital ward | 60 (30.2) | 111 (36.6) | 3.2 (–9.7 to 16.2) |
| Intensive care unite | 40 (20.1) | 38 (12.5) | 9.2 (–1.6 to 20.0) |
Abbreviations: IQR, interquartile range; IV, intravenous.
Differences were adjusted for intervention, time period, and cluster size (categorical) as fixed effects and cluster as a random effect.
Furosemide was the only intravenous diuretic used in participating centers.
Cumulative dose of nitrates over the period that includes boluses (first hour) and infusion (between first hour and fourth hour) among patients who were treated with nitrates.
Given the small numbers, no analysis was performed for the dual antiplatelet agents variable.
Including cardiac intensive care unit.
Study End Points
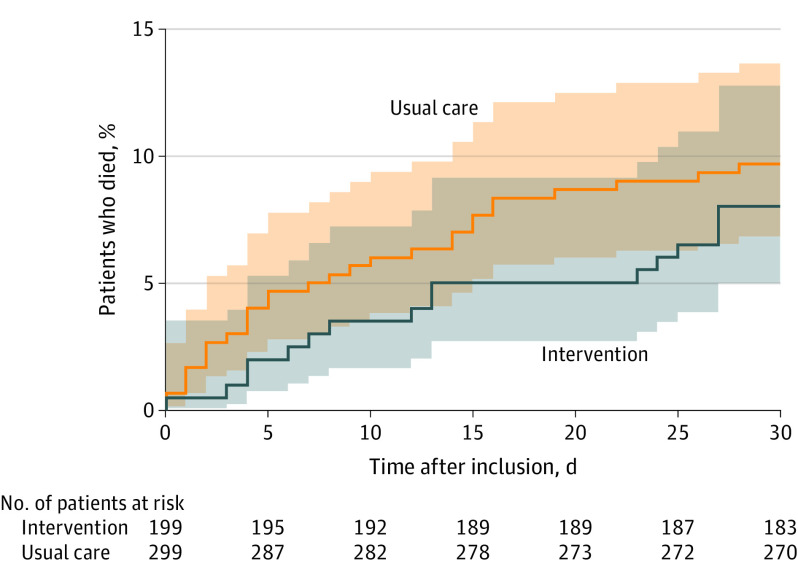
Among the 502 patients included in the primary analysis, the median (IQR) number of days alive and out of hospital at 30 days was 19 (0-24) in the intervention group and 19 (0-24) in the control group (adjusted difference, −1.9 [95% CI, −6.6 to 2.8]); adjusted ratio, 0.88 [95% CI, 0.64-1.21]; P = .44) (Table 3). The intraclass correlation coefficient was 0.057. A sensitivity analysis of patients that completed the trial exhibited similar results (eTable 3 and eTable 4 in Supplement 2). There was no statistically significant difference in the intervention vs control groups in all-cause mortality at day 30 (8.0% vs 9.7%; adjusted difference, 4.1% [95% CI, −17.2% to 25.3%]; adjusted risk ratio, 1.17 [95% CI, 0.53-2.57]; reversal in direction due to the adjustment for covariates; Figure 2), cardiovascular mortality at day 30 (5.0% vs 7.4%; adjusted difference, −1.3% [95% CI, −15.5% to 19.8%]), median (IQR) length of initial hospital stay (8 [5-21] d vs 8 [5-16] d; adjusted difference, 2.5 [95% CI, −0.9 to 5.8]; adjusted ratio, 1.22 [95% CI, 0.94-1.59]), unscheduled readmission (14.3% vs 15.7%; adjusted difference, −1.3% [95% CI, −26.3% to 23.7%]; adjusted risk ratio, 0.96 [95% CI, 0.48-1.95]), or acute kidney injury at 30 days (1.0% vs 1.4%) (Table 3).
Table 3. Study End Points in the Primary Analysis in a Study of the Effect of an Emergency Department Care Bundle Among Older Patients With Acute Heart Failure.
| End point | Intervention (n = 199) |
Usual care (n = 303) | Difference (95% CI) | Adjusted ratio (95% CI) | Adjusted risk ratio (95% CI) | |
|---|---|---|---|---|---|---|
| Unadjusted | Adjusteda | |||||
| Primary, median (IQR) | ||||||
| Time alive and out of hospital at 30 d, d | 19.0 (0.0 to 24.0) | 19.0 (0.0 to 24.0) | 0.0 (–4.0 to 4.0) | –1.9 (–6.6 to 2.8) | 0.88 (0.64 to 1.21) | |
| Secondary, No. (%) | ||||||
| 30-day all-cause mortality | 16 (8.0) | 29 (9.7) (n = 299) | –1.7% (–7.1% to 3.8%) | 4.1% (–17.2% to 25.3%) | 1.17 (0.53 to 2.57) | |
| 30-day cardiovascular mortality | 10 (5.0) | 22 (7.4) (n = 299) | –2.3% (–7.0% to 2.3%) | 2.1% (–15.5% to 19.8%) | 1.12 (0.45 to 2.82) | |
| 30-day hospital readmission | 22 (14.3) (n = 154) | 37 (15.7) (n = 235) | –1.5% (–9.2% to 6.3%) | –1.3% (–26.3% to 23.7%) | 0.96 (0.48 to 1.95) | |
| Length of hospital stay, median (IQR), d | 8.0 (5.0 to 21.0) (n = 182) | 8.0 (5 to 16.0) (n = 269) | 0.0 (–1.8 to 1.8) | 2.5 (–0.9 to 5.8) | 1.22 (0.94 to 1.59) | |
| 2-fold rise in creatinine levelb | 2 (1.0) (n = 192) | 4 (1.4) (n = 287) | ||||
Abbreviation: IQR, interquartile range.
Differences, ratios, and risk ratios were adjusted for time period and cluster size (categorical) as fixed effects and cluster as a random effect. The difference is expressed as intervention minus control and the ratio as intervention/control.
Given the small numbers, no analysis was performed for the 2-fold rise in creatinine level end point.
Figure 2. Cumulative Incidence of All-Cause Mortality in a Study of the Effect of an Emergency Department Care Bundle Among Older Patients With Acute Heart Failure.
Shading represents 95% CIs. All patients were observed until date of death or 30 days. Data were missing for vital status in 4 patients.
There was a similar rate of nitrate use in the control group (eFigure 2 in Supplement 2) and management of precipitating factors in the 5 centers randomized to later crossover than those in the usual care period (17% vs 15% respectively).
Discussion
In this stepped-wedge cluster randomized trial that included 502 older adults with acute heart failure, an early and comprehensive guideline-recommended care bundle resulted in a significantly higher use of intravenous nitrates and more frequent management of precipitating factors, but did not significantly improve the number of days alive and out of hospital at 30 days nor any other early outcome.
For the past few decades, intravenous nitrates have been recommended for the early treatment of patients with acute heart failure.6,7 However, the failure of further trials to confirm any clinical benefit of intravenous nitrates in patients with acute heart failure resulted in subsequent moderate level of evidence for this recommendation, hence the reported low adherence in older patients.11 Several studies suggested that a more comprehensive and early treatment of patients with acute heart failure in the ED could improve prognosis, because prognosis could be dependent on underlying conditions and precipitating factors.18,23 The implementation of the tested care bundle resulted in a significantly higher adherence to guideline-recommended therapy both in the management of acute pulmonary congestion and of the precipitating factors. The control group in this study received similar treatments to those reported in a 2019 multicenter observational study.10 In that study, only 34% of 73 patients with acute heart failure received intravenous nitrates and 20% were treated for precipitating factors in the ED. In France, intravenous nitrates are the only nitrates used for patients with acute heart failure, and no other nitrates were used in this study (or in France in general).24 More patients in the intervention group had to be admitted to an intensive care unit. Whether this was the result of adverse effects of the intervention or of a more proactive approach in this group is unknown.
In the present trial, it is possible that there could have been a Hawthorne effect, in that patients in the control group would receive similar treatments to patients in the intervention group because physicians would be aware that the patients were in the trial and that their treatment decisions were being recorded. This was not the case possibly due to the stepped-wedge cluster-randomization methodology.10 There was also a low risk of contamination in sites randomized to later crossover, with a similar rate of nitrate use and management of precipitating factors. Furthermore, these results showed an intraclass correlation coefficient estimation of 0.05, higher than the correlation anticipated, which reduced statistical power. However, it seems unlikely that this would have changed the results of the primary outcome.
Regarding management of acute pulmonary congestion, these results are consistent with those from the GALACTIC trial, in which early and sustained vasodilatation therapy was not significantly associated with improved outcomes. The present trial also tested a comprehensive approach with earlier implementation of the intervention: patients were randomized within 6 hours of ED presentation and the care bundle was initiated during the first hour of medical management. With a significantly higher rate of management of precipitating factors, this trial is complementary to the GALACTIC trial and others that tested intervention during the first days of admission for acute heart failure.8
This trial focused on older patients, and it is likely that in these patients, who often present with comorbidities, the prognosis is not driven by pulmonary edema but rather by precipitating factors and underlying conditions.8,9,12,25Given the study findings, a more specific care pathway for older adults may need to be considered.
Limitations
This study has several limitations. First, only patients 75 years and older were included, because this trial focused on a more homogeneous phenotype of patients.26 However, this population may still include patients with preserved and reduced systolic cardiac function. This parameter was not assessed in included patients, and it is possible that the intervention may cause different outcomes in different phenotypes of patients. The present trial included mostly “wet and warm” patients, the most common phenotype of acute heart failure.23 Second, a selection bias may also have occurred because some patients may have refused to be included in the study and some eligible patients may have not been screened by the emergency physicians. Because this number was not recorded, the extent of this bias cannot be ascertained.
Third, the intervention included high-intensity intravenous nitrates, with a median dose of 27 mg of isosorbide dinitrate in the intervention group vs 4 mg in the control group in the first 4 hours. The optimal dose may lie somewhere between those 2 numbers. Fourth, the rate of hypotension that may have been caused by this treatment was not recorded, because the objective was to evaluate the overall effect of the bundle. However, this risk is limited because the intervention included close monitoring of blood pressure and withholding of nitrate therapy if the systolic blood pressure dropped below 100 mm Hg. Fifth, the potential use in the ED of oral or topical nitrates was not recorded in this study. However, in France, only intravenous nitrates are used for acute heart failure, and the percentage of patients previously treated with topical nitrates for chronic heart failure was similar in both groups (6%). Sixth, a short-term prognosis end point (30 days) was chosen to capture the overall effect of the tested care bundle, and this end point may have been influenced by post-ED therapy in the ward that is not standardized. Seventh, the rate of management of acute coronary syndrome was low in both groups, suggesting a suboptimal management of this precipitating factor, which may have limited the benefit of the intervention.
Conclusions
Among older patients with acute heart failure, use of a guideline-based comprehensive care bundle in the ED compared with usual care did not result in a statistically significant difference in the number of days alive and out of the hospital at 30 days. Further research is needed to identify effective treatments for acute heart failure in older patients.
Trial protocol and statistical analysis plan
eFigure 1: Study diagram
eFigure 2: Trend of median dose of given iv nitrates in control groups
eTable 1. Study diagram in primary analysis (as-randomized) and sensitivity analysis (patients that completed the trial)
eTable 2: Presence and treatment of suspected precipitating factors in the ED in the primary (as-randomized) analysis
eTable 3: Management in the ED in the sensitivity analysis (patients that completed the trial). IQR, interquartile range
eTable 4: Study endpoints in the sensitivity analysis (patients that completed the trial)
Data sharing statement
References
- 1.Ezekowitz JA, Bakal JA, Kaul P, Westerhout CM, Armstrong PW. Acute heart failure in the emergency department: short and long-term outcomes of elderly patients with heart failure. Eur J Heart Fail. 2008;10(3):308-314. doi: 10.1016/j.ejheart.2008.01.014 [DOI] [PubMed] [Google Scholar]
- 2.Teixeira A, Parenica J, Park JJ, et al. ; GREAT (Global Research on Acute Conditions Team) Network . Clinical presentation and outcome by age categories in acute heart failure: results from an international observational cohort. Eur J Heart Fail. 2015;17(11):1114-1123. doi: 10.1002/ejhf.330 [DOI] [PubMed] [Google Scholar]
- 3.McMurray JJV, Adamopoulos S, Anker SD, et al. ; ESC Committee for Practice Guidelines . ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33(14):1787-1847. doi: 10.1093/eurheartj/ehs104 [DOI] [PubMed] [Google Scholar]
- 4.Mebazaa A, Yilmaz MB, Levy P, et al. . Recommendations on pre-hospital & early hospital management of acute heart failure: a consensus paper from the Heart Failure Association of the European Society of Cardiology, the European Society of Emergency Medicine and the Society of Academic Emergency Medicine. Eur J Heart Fail. 2015;17(6):544-558. doi: 10.1002/ejhf.289 [DOI] [PubMed] [Google Scholar]
- 5.Collins SP, Storrow AB, Levy PD, et al. . Early management of patients with acute heart failure: state of the art and future directions—a consensus document from the SAEM/HFSA acute heart failure working group. Acad Emerg Med. 2015;22(1):94-112. doi: 10.1111/acem.12538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cotter G, Metzkor E, Kaluski E, et al. . Randomised trial of high-dose isosorbide dinitrate plus low-dose furosemide versus high-dose furosemide plus low-dose isosorbide dinitrate in severe pulmonary oedema. Lancet. 1998;351(9100):389-393. doi: 10.1016/S0140-6736(97)08417-1 [DOI] [PubMed] [Google Scholar]
- 7.Sharon A, Shpirer I, Kaluski E, et al. . High-dose intravenous isosorbide-dinitrate is safer and better than Bi-PAP ventilation combined with conventional treatment for severe pulmonary edema. J Am Coll Cardiol. 2000;36(3):832-837. doi: 10.1016/S0735-1097(00)00785-3 [DOI] [PubMed] [Google Scholar]
- 8.Kozhuharov N, Goudev A, Flores D, et al. ; GALACTIC Investigators . Effect of a strategy of comprehensive vasodilation vs usual care on mortality and heart failure rehospitalization among patients with acute heart failure: the GALACTIC randomized clinical trial. JAMA. 2019;322(23):2292-2302. doi: 10.1001/jama.2019.18598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Packer M, O’Connor C, McMurray JJV, et al. ; TRUE-AHF Investigators . Effect of ularitide on cardiovascular mortality in acute heart failure. N Engl J Med. 2017;376(20):1956-1964. doi: 10.1056/NEJMoa1601895 [DOI] [PubMed] [Google Scholar]
- 10.Gorlicki J, Minka F-H, Chouihed T, et al. . Low compliance to guidelines in the management of acute heart failure in emergency elderly patients: a multicenter pilot prospective study. Eur J Emerg Med. 2019;26(5):379-380. doi: 10.1097/MEJ.0000000000000593 [DOI] [PubMed] [Google Scholar]
- 11.Lemachatti N, Philippon A-L, Bloom B, et al. . Temporal trends in nitrate utilization for acute heart failure in elderly emergency patients: a single-centre observational study. Arch Cardiovasc Dis. 2016;109(8-9):449-456. doi: 10.1016/j.acvd.2016.01.014 [DOI] [PubMed] [Google Scholar]
- 12.Arrigo M, Tolppanen H, Sadoune M, et al. ; GREAT Network . Effect of precipitating factors of acute heart failure on readmission and long-term mortality. ESC Heart Fail. 2016;3(2):115-121. doi: 10.1002/ehf2.12083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsue Y, Damman K, Voors AA, et al. . Time-to-furosemide treatment and mortality in patients hospitalized with acute heart failure. J Am Coll Cardiol. 2017;69(25):3042-3051. doi: 10.1016/j.jacc.2017.04.042 [DOI] [PubMed] [Google Scholar]
- 14.Freund Y, Gorlicki J, Cachanado M, et al. . Early and comprehensive care bundle in the elderly for acute heart failure in the emergency department: study protocol of the ELISABETH stepped-wedge cluster randomized trial. Trials. 2019;20(1):95. doi: 10.1186/s13063-019-3188-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hemming K, Taljaard M, Grimshaw J. Introducing the new CONSORT extension for stepped-wedge cluster randomised trials. Trials. 2019;20(1):68. doi: 10.1186/s13063-018-3116-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hemming K, Taljaard M, McKenzie JE, et al. . Reporting of stepped wedge cluster randomised trials: extension of the CONSORT 2010 statement with explanation and elaboration. BMJ. 2018;363:k1614. doi: 10.1136/bmj.k1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zannad F, Garcia AA, Anker SD, et al. . Clinical outcome endpoints in heart failure trials: a European Society of Cardiology Heart Failure Association consensus document. Eur J Heart Fail. 2013;15(10):1082-1094. doi: 10.1093/eurjhf/hft095 [DOI] [PubMed] [Google Scholar]
- 18.Teixeira A, Arrigo M, Tolppanen H, et al. . Management of acute heart failure in elderly patients. Arch Cardiovasc Dis. 2016;109(6-7):422-430. doi: 10.1016/j.acvd.2016.02.002 [DOI] [PubMed] [Google Scholar]
- 19.Barnett AG, de Looper M, Fraser JF. The seasonality in heart failure deaths and total cardiovascular deaths. Aust N Z J Public Health. 2008;32(5):408-413. doi: 10.1111/j.1753-6405.2008.00270.x [DOI] [PubMed] [Google Scholar]
- 20.Kaneko H, Suzuki S, Goto M, et al. . Presentations and outcomes of patients with acute decompensated heart failure admitted in the winter season. J Cardiol. 2014;64(6):470-475. doi: 10.1016/j.jjcc.2014.03.004 [DOI] [PubMed] [Google Scholar]
- 21.Machado JAF, Parente PMDC, Silva JMCS. QREG2: Stata Module to Perform Quantile Regression with Robust and Clustered Standard Errors Boston College Department of Economics; 2020. Accessed June 11, 2020. https://ideas.repec.org/c/boc/bocode/s457369.html
- 22.Hussey MA, Hughes JP. Design and analysis of stepped wedge cluster randomized trials. Contemp Clin Trials. 2007;28(2):182-191. doi: 10.1016/j.cct.2006.05.007 [DOI] [PubMed] [Google Scholar]
- 23.Chioncel O, Mebazaa A, Maggioni AP, et al. ; ESC-EORP-HFA Heart Failure Long-Term Registry Investigators . Acute heart failure congestion and perfusion status: impact of the clinical classification on in-hospital and long-term outcomes; insights from the ESC-EORP-HFA Heart Failure Long-Term Registry. Eur J Heart Fail. 2019;21(11):1338-1352. doi: 10.1002/ejhf.1492 [DOI] [PubMed] [Google Scholar]
- 24.Gorlicki J, Boubaya M, Cottin Y, et al. . Patient care pathways in acute heart failure and their impact on in-hospital mortality, a French national prospective survey. Int J Cardiol Heart Vasc. 2019;26:100448. doi: 10.1016/j.ijcha.2019.100448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Felker GM, Lee KL, Bull DA, et al. ; NHLBI Heart Failure Clinical Research Network . Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med. 2011;364(9):797-805. doi: 10.1056/NEJMoa1005419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Senni M, Tribouilloy CM, Rodeheffer RJ, et al. . Congestive heart failure in the community: a study of all incident cases in Olmsted County, Minnesota, in 1991. Circulation. 1998;98(21):2282-2289. doi: 10.1161/01.CIR.98.21.2282 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol and statistical analysis plan
eFigure 1: Study diagram
eFigure 2: Trend of median dose of given iv nitrates in control groups
eTable 1. Study diagram in primary analysis (as-randomized) and sensitivity analysis (patients that completed the trial)
eTable 2: Presence and treatment of suspected precipitating factors in the ED in the primary (as-randomized) analysis
eTable 3: Management in the ED in the sensitivity analysis (patients that completed the trial). IQR, interquartile range
eTable 4: Study endpoints in the sensitivity analysis (patients that completed the trial)
Data sharing statement