This economic evaluation assesses the budget impact associated with adding avapritinib for metastatic or unresectable GIST in patients with a platelet-derived growth factor receptor alpha (PDGFRA) exon 18 variant or after 3 or more previous treatments to a hypothetical US health plan formulary.
Key Points
Question
What is the potential budget impact of adding avapritinib to a hypothetical US health plan formulary for metastatic or unresectable gastrointestinal stromal tumors in patients with a platelet-derived growth factor receptor alpha (PDGFRA) exon 18 variant or after 3 or more previous treatments?
Findings
This economic evaluation estimated that adding avapritinib to a formulary for a population with a mixed health plan was associated with total incremental annual plan costs in years 1, 2, and 3 of $0.004, $0.006, and $0.010 per member per month, respectively.
Meaning
Model results suggest that the use of avapritinib for treatment of patients with unresectable or metastatic gastrointestinal stromal tumors and a PDGFRA exon 18 variant or after 3 or more prior treatments would have a minimal budget impact to a US managed care health plan.
Abstract
Importance
With the approval of avapritinib for adults with unresectable or metastatic gastrointestinal stromal tumors (GISTs) harboring a platelet-derived growth factor receptor alpha (PDGFRA) exon 18 variant, including PDGFRA D842V variants, and National Comprehensive Cancer Network guideline recommendations as an option for patients with GIST after third-line treatment, it is important to estimate the potential financial implications of avapritinib on a payer’s budget.
Objective
To estimate the budget impact associated with the introduction of avapritinib to a formulary for metastatic or unresectable GISTs in patients with a PDGFRA exon 18 variant or after 3 or more previous treatments from the perspective of a US health plan.
Design, Setting, and Participants
For this economic evaluation, a 3-year budget impact model was developed in March 2020, incorporating costs for drug acquisition, testing, monitoring, adverse events, and postprogression treatment. The model assumed that avapritinib introduction would be associated with increased PDGFRA testing rates from the current 49% to 69%. The health plan population was assumed to be mixed 69% commercial, 22% Medicare, and 9% Medicaid. Base case assumptions included a GIST incidence rate of 9.6 diagnoses per million people, a metastatic PDGFRA exon 18 mutation rate of 1.9%, and progression rate from first-line to fourth-line treatment of 17%.
Exposures
The model compared scenarios with and without avapritinib in a formulary.
Main Outcomes and Measures
Annual, total, and per member per month (PMPM) budget impact.
Results
In a hypothetical 1-million member plan, fewer than 0.1 new patients with a PDGFRA exon 18 variant per year and 1.2 patients receiving fourth-line therapy per year were eligible for treatment. With avapritinib available, the total increase in costs in year 3 for all eligible adult patients with a PDGFRA exon 18 variant was $46 875, or $0.004 PMPM. For patients undergoing fourth-line treatment, the total increase in costs in year 3 was $69 182, or $0.006 PMPM. The combined total budget impact in year 3 was $115 604, or $0.010 PMPM, including an offset of $3607 in postprogression costs avoided or delayed. The higher rates of molecular testing resulted in a minimal incremental testing cost of $453 in year 3.
Conclusions and Relevance
These results suggest that adoption of avapritinib as a treatment option would have a minimal budget impact to a hypothetical US health plan. This would be primarily attributable to the small eligible patient population and cost offsets from reduced or delayed postprogression costs.
Introduction
Gastrointestinal stromal tumors (GISTs) are a rare type of cancer associated with characteristic activating variants in genes encoding the tyrosine kinase receptors for c-Kit (CD117) (KIT) (approximately 80% of diagnoses) or platelet-derived growth factor receptor alpha (PDGFRA) (5%-10% of diagnoses).1,2 Estimates vary, but the total number of new US GIST cases per year is considered to be less than 3000,3 with PDGFRA exon 18 variants representing the majority of PDGFRA variants.4,5 Approximately 30% of patients with GIST receive treatment in the adjuvant setting, and 64% are eventually receive treatment in the metastatic or unresectable setting.6 Testing for PDGFRA variant status is recommended in clinical guidelines, and it has been estimated (before the introduction of avapritinib) that increasing this testing might result in lower health plan costs7,8; however, molecular testing remains underused during the diagnostic workup. On the basis of a 2018 study of medical records for 403 US patients, only 49% of patients received testing at diagnosis. Before the approval of avapritinib, treatment selection was typically made by line of therapy instead of by variant status.9
Avapritinib is a precision therapy designed to be a selective and potent inhibitor of KIT and PDGFRA variant kinases. The drug is indicated for the treatment of adults with unresectable or metastatic GIST who harbor a PDGFRA exon 18 variant, including PDGFRA D842V.10 Patients with PDGFRA exon 18 have previously been treated similarly to the general patient population and those with metastatic KIT-related GIST, with kinase inhibitors including imatinib, sunitinib, and regorafenib.7 Given that patients with PDGFRA D842V variants are typically resistant to imatinib treatment and that other available treatment options for these patients are reported to have suboptimal tolerability, there has been need for a more effective therapy.11,12
Avapritinib also targets variants in KIT exons 11, 11/17, and 17, with a 50% inhibitory concentration less than 25 nM,10 and the National Comprehensive Cancer Network (NCCN) guidelines, version 2.2020, recommend avapritinib for use in certain circumstances for patients with KIT-related GIST who experienced disease progression after treatment with imatinib, sunitinib, and regorafenib (ie, as fourth-line treatment for GIST).7
Budget impact models (BIMs) are used by health care decision-makers to estimate the incremental cost of including a new treatment in drug formularies. The primary objective of this study was to estimate the budget impact of avapritinib in adult patients with unresectable or metastatic GIST with a PDGFRA exon 18 variant or progression to fourth-line treatment from a US managed care health plan perspective.
Methods
Model Structure, Perspective, and Time Horizon
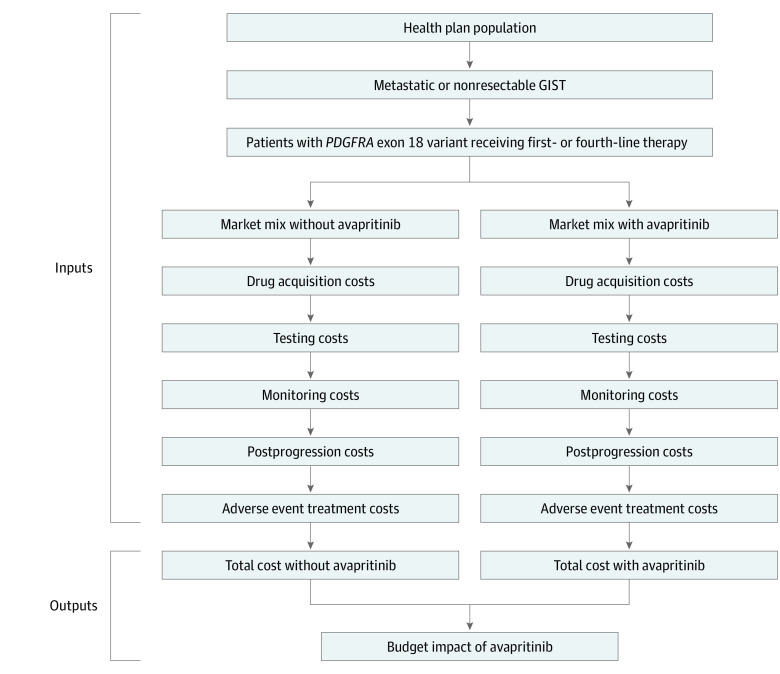
For this economic evaluation, a BIM was developed in Microsoft Excel. The model allocated eligible patients to treatment options based on the projected uptake of avapritinib and compared a scenario with avapritinib with a scenario without avapritinib (Figure 1). The budget impact is the difference between the total costs of the scenario with avapritinib and the scenario without avapritinib (Figure 2). The BIM included information on the number of eligible patients for treatment, depending on the rates of PDGFRA testing, and the current and anticipated market shares of treatment options. The model was finalized on March 31, 2020. Because this research did not include human data, institutional review board approval was not required. The BIM was designed in line with the methodological guidelines from the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) reporting guideline, and the study followed the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) reporting guideline.13,14
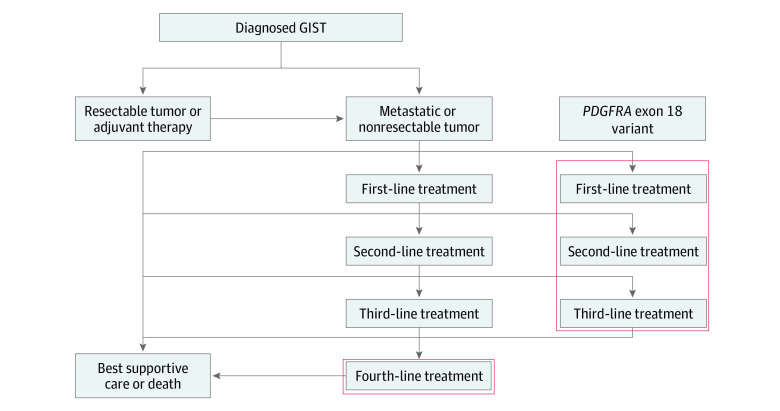
Figure 1. Flow of Treatment of Gastrointestinal Stromal Tumors (GISTs).
Figure 2. Budget Impact Model Calculation Structure.
GIST indicates gastrointestinal stromal tumor; PDGFRA, platelet-derived growth factor receptor alpha.
Costs were calculated based on attributable drug acquisition, monitoring, postprogression, molecular testing, and adverse event management costs. For patients with a PDGFRA exon 18 variant, costs associated with up to 2 subsequent lines of treatment after progression for each treatment option were modeled. For patients who received avapritinib as a fourth-line treatment, costs associated with subsequent best supportive care were accounted for after treatment progression given the lack of approved treatments.
The model used a 3-year time horizon. Incident cohorts starting each year were tracked until estimated median survival or the model horizon was reached. No discounting of costs was required owing to the short time horizon.
Model Inputs
Target Population
The BIM estimated the population within a 1-million member health plan eligible for treatment for unresectable or metastatic GIST with a PDGFRA exon 18 variant or requiring fourth-line treatment. The model base case plan was a mixed 69% commercial, 22% Medicare, and 9% Medicaid population, believed to be a representative default mix.15 A population increase of 0.6% per year was assumed, consistent with US Census estimates.16
The number of treated patients was calculated depending on the plan type(s) selected, the age and sex distribution of covered lives, and the age- and sex-adjusted incidence of GIST. Other inputs for patient population were the proportion of patients diagnosed with unresectable or metastatic GIST (64%)17 and the proportions of patients with unresectable or metastatic GIST initiating first-line treatment (96%)6 and progressing to fourth-line treatment (17% of those entering first-line treatment based on a US-based medical record review of more than 400 patients). The unadjusted incidence rate of new GIST cases in the US was assumed to be 0.96 per million. For patients with metastatic or unresectable GIST, the PDGFRA exon 18 variant rate was identified as 1.9%.18 For each of the 2 settings modeled, 3 newly incident cohorts entered the model in each year, with the 3 cohorts with the PDGFRA exon 18 variant tracked from year to year until the time horizon of the model was reached, given that median survival was expected to be longer than 3 years, and patient cohorts receiving fourth-line treatment were tracked until median survival.
Treatments and Market Share
For unresectable or metastatic GIST with a PDGFRA exon 18 variant including a D842V variant, in the scenario without avapritinib, the market share of imatinib was assumed to be 80% and the market share of sunitinib, regorafenib, and nilotinib was assumed to be approximately 7% each (based on a US-based medical record review of more than 400 patients). In the scenario with the introduction of avapritinib, the market share of all comparators decreased proportionally as avapritinib increased its market share to 45%, 60%, and 80% in years 1, 2, and 3, respectively. For unresectable or metastatic GIST with progression to fourth-line treatment, market share without avapritinib was assumed to be 30% for regorafenib and sorafenib and 20% for nilotinib and pazopanib for all 3 years based on the same medical record review. In the scenario with avapritinib, the market share of all comparators decreased proportionally as avapritinib increased its market share to 35%, 50%, and 70% in years 1, 2, and 3, respectively.
Cost Estimation
Costs are presented in 2019 US dollars, and any costs from previous years were inflated using the medical care component of the Consumer Price Index from the US Department of Labor.19 Drug costs (both brand and generic) were estimated using a monthly pricing approach (Table 1),2,20,21,22,23,24,25,26 calculated by multiplying the wholesale acquisition cost of each treatment per dose by the duration of treatment based on median progression-free survival (PFS) per each comparator’s respective prescribing label, taking into account treatment cycles. Imatinib is the only treatment for which generic wholesale acquisition cost was used; all other treatments used branded prices. Prices were sourced from Micromedex Redbook.23 On the basis of available data when the model was finalized, median PFS was not reached and not estimable for patients with PDGFRA exon 18. Therefore, a draft estimate of median PFS for patients with PDGFRA exon 18 D842V of 29.5 months was used.20 For patients with the PDGFRA exon 18 variant receiving first-line treatment with imatinib, sunitinib, regorafenib, and nilotinib, owing to the absence of available data and under the assumption that all treatments have a similar (lack of) response for this variant, duration of treatment was based on the median PFS associated with imatinib for first-line treatment of patients with the PDGFRA exon 18 variant (6.4 months).2 For patients receiving fourth-line treatment, a median PFS of 3.7 months was used for avapritinib based on preliminary NAVIGATOR clinical trial data in the subpopulation of patients receiving fourth-line or greater therapy.21 Duration of fourth-line treatment with regorafenib, nilotinib, sorafenib, and pazopanib was based on the median PFS associated with repeat imatinib treatment (1.8 months) from a clinical trial of metastatic GIST after failure of imatinib and sunitinib therapy.22 Because all comparators in the model were orally administered, no cost for administration was included. Real-world median overall survival associated with for first-line treatment of patients with the PDGFRA exon 18 variant was observed to be greater than 36 months in a natural history study.20 For patients who progressed to fourth-line treatment, the model used overall survival of 12.3 months for avapritinib and 8.2 months for all comparators.10,22
Table 1. Drug Acquisition and Treatment Cost Inputs.
| Variable | First-line treatment | Fourth-line treatment | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Avapritinib | Imatinib | Sunitinib | Regorafenib | Nilotinib | Avapritinib | Regorafenib | Nilotinib | Sorafenib | Pazopanib | |
| Duration of treatment, moa | 29.5 | 6.4 | 6.4 | 6.4 | 6.4 | 3.7 | 1.8 | 1.8 | 1.8 | 1.8 |
| Drug acquisition cost per month, $b | 32 000 | 1404 | 15 023 | 19 493 | 27 557 | 32 000 | 19 493 | 27 557 | 20 148 | 13 913 |
| Adverse event cost per month, $c | 407 | 204 | 180 | 219 | 51 | 588 | 219 | 161 | 56 | 389 |
| Monitoring cost per month, $d | 176 | 191 | 195 | 176 | 189 | 176 | 176 | 189 | 183 | 189 |
Abbreviations: GIST, gastrointestinal stromal tumors; PDGFRA, platelet-derived growth factor receptor alpha.
The median progression-free survival associated with avapritinib for treatment of PDGFRA exon 18 was not reached in the most recent data cutoff for PDGFRA exon 18. In the absence of PDGFRA exon 18–specific median progression-free survival, the median progression-free survival was assumed to be the same as for PDGFRA exon 18 D842V, for which more recent data are available.20 For first-line treatment, duration of treatment was assumed to be equal to median progression-free survival.2,21,22
Costs presented in this table do not include the post-progression costs associated with each treatment line. Avapritinib drug acquisition for fourth-line treatment was assumed to be the same as for PDGFRA exon 18. Drug acquisition costs per month were extracted from the IBM Micromedex 2020.23
Adverse event incidence rates were converted to monthly incidence rates using the formula: monthly rate = −ln (1 − [fraction of patients with AE/time in months]). Adverse event rates were obtained from prescribing information or clinical trials for GIST. For avapritinib, in accordance with the prescribing information, 44% of individuals were receiving treatment for at least 12 months and 56% for at least 6 months; a median duration of exposure of 6 months was assumed for avapritinib on a conservative basis. Adverse event costs are calculated as the median hospitalization cost from HCUPnet.24
Monitoring requirements were calculated based on National Comprehensive Cancer Network–recommended testing procedures for GIST, with treatment-specific adaptations based on monitoring recommendations reported in the respective prescribing information. Monitoring costs were extracted from the 2019 Centers for Medicare & Medicaid Services Clinical Laboratory Schedule and the 2019 Centers for Medicare & Medicaid Services Physician Fee Schedule.25,26
The model estimated costs attributable to postprogression treatment given the meaningful increase in median PFS among patients with the PDGFRA exon 18 variant.2,21 A weighted monthly postprogression cost was calculated by multiplying the proportion of time of receipt of each line of treatment after progression20 by the costs for each treatment line. Costs at each line were calculated using pharmacy, monitoring, and adverse event costs for treatments observed in a proprietary US-based medical record review of more than 400 patients with GIST, including the portion of patients that switched to best supportive care. Details of postprogression calculations are shown in the eTable in the Supplement.
Molecular testing rates at diagnosis were estimated at 49% in the proprietary medical record review mentioned above and assumed in the base case to increase to 69% over 3 years when avapritinib was introduced. A scenario analysis with rates increasing to 100% was also assessed. Patients having the PDGFRA exon 18 variant but not tested were assumed not to be treated with avapritinib. The increase in testing rates was associated with a corresponding increase in the identified patients with the PDGFRA exon 18 variant. Molecular testing costs were assumed to be for single-gene polymerase chain reaction tests.21
Treatment-specific monthly monitoring procedures and rates were based on NCCN-recommended procedures for GIST. Treatment-specific adaptations were based on monitoring recommendations in each treatment’s prescribing label, and with unit costs for monitoring were sourced from Centers fore Medicare & Medicaid Services laboratory fee schedules and applied for the duration of treatment.7,25
Grade 3 or higher adverse events were included in the model. Adverse event rates were obtained from prescribing information for products approved for GIST or from pivotal clinical trials on GIST.10,12,24,25,27,28,29,30,31,32 The adverse events included in the model were selected on the basis of 2% or greater grade 3 or higher incidence for avapritinib in the NAVIGATOR trial interim safety analysis.33 Adverse event rates were attributable to study drug except for imatinib for which the drug-attributable rate was not available; therefore, all cause incidence was used.28 Annualized adverse event incidence rates were converted to monthly incidence rates using median duration of exposure and the following formula: monthly adverse event rate = −ln (1 − [fraction of patients with adverse events/month of exposure]). Adverse event costs were assumed to be represented by the median hospitalization cost extracted from the Agency for Healthcare Research and Quality HCUPnet tool24 for the following International Statistical Classification of Diseases, Tenth Revision, Clinical Modification codes: R06.00 (dyspnea, unspecified), R11.0 (nausea), R11.10 (vomiting, unspecified), R19.7 (diarrhea, unspecified), R10.9 (unspecified abdominal pain), R60.9 (edema, unspecified), R53.83 (other fatigue), F09 (unspecified mental disorder due to known physiological condition), J90 (pleural effusion, not elsewhere classified), R21 (rash and other nonspecific skin eruption), and R63.0 (anorexia). Other health care resource utilization costs were not included because published data were not available with sufficient information on costs for patients with metastatic or unresectable GIST.
Statistical Analysis
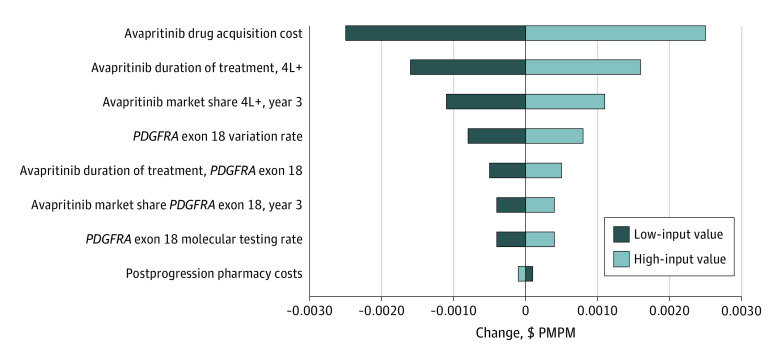
A 1-way deterministic sensitivity analysis for the BIM was conducted by increasing and decreasing key parameter values by a factor of 20%. Key parameters included avapritinib drug price, treatment duration, variant rate, molecular testing rate, postprogression pharmacy costs, and treated market share for avapritinib. All analyses were performed used Microsoft Excel.
Results
In a hypothetical health plan with 1 million members and a mixed plan population (69% commercial, 22% Medicare, and 9% Medicaid), fewer than 0.1 new patients per year with GIST and the PDGFRA exon 18 variant who received first-line therapy and 1.2 patients per year who received fourth-line therapy for GIST were estimated to be eligible for treatment in each of the 3 years, or 1.3 patients for both settings combined. For combined patients with the PDGFRA exon 18 variant receiving first- and fourth-line therapy, the total number of eligible patients was 1.0 for 100% commercial coverage, 2.2 for Medicare, and 0.9 for Medicaid.
Before the introduction of avapritinib as fourth-line therapy for GIST in patients with the PDGFRA exon 18 variant, using a base-case analysis of a 1-million member health plan, the year 3 total cost was $62 205 from a payer perspective. The total cost with the introduction of avapritinib was $177 809 for year 3, resulting in an incremental budget impact of $115 604 and a per member per month (PMPM) impact of $0.010. The incremental budget impact in the first and second years of avapritinib availability was $45 038 and $76 758, respectively, corresponding to a PMPM budget impact of $0.004 in the first year and $0.006 in the second year. These values include cost offsets of $454, $1999, and $3607 in avoided or delayed postprogression costs in years 1, 2, and 3, respectively.
The year 3 budget impact considering both fourth-line treatment and presence of the PDGFRA exon 18 variant included an increase in pharmacy cost of $112 981, increase in monitoring and testing cost of $558, and increase in adverse event cost of $2035. The increased rates of molecular testing resulted in an incremental health plan testing cost of $453 in year 3 of avapritinib availability.
In an analysis of only patients with the PDGFRA exon 18 variant, the total incremental budget impact of introducing avapritinib was $11 007 in year 1, $27 937 in year 2, and $46 875 in year 3, corresponding to a PMPM budget impact of $0.001, $0.002, and $0.004, respectively. Details are presented in Table 2.
Table 2. Budget Impact Model Results for Avapritinib for a 1-Million Member Health Plan by Cost Typea.
| Model | Value | ||
|---|---|---|---|
| Year 1 | Year 2 | Year 3 | |
| GIST, PDGFRA exon 18, and fourth-line treatment | |||
| Eligible patients, No. | 1.22 | 1.24 | 1.26 |
| Total cost, $ | |||
| With avapritinib available | 96 157 | 133 403 | 177 809 |
| Without avapritinib available | 51 120 | 56 646 | 62 205 |
| Incremental budget impact | |||
| Total, $ | 45 038 | 76 758 | 115 604 |
| Owing to change in postprogression costs, $b | −454 | −1999 | −3607 |
| PMPM | 0.004 | 0.006 | 0.010 |
| GIST and PDGFRA exon 18 | |||
| Eligible patients, No. | 0.07 | 0.08 | 0.09 |
| Total cost, $ | |||
| With avapritinib available | 19 200 | 41 398 | 65 636 |
| Without avapritinib available | 8192 | 13 461 | 18 761 |
| Incremental budget impact | |||
| Total, $ | 11 007 | 27 937 | 46 875 |
| Owing to change in postprogression costs, $b | −610 | −2224 | −3924 |
| PMPM | 0.001 | 0.002 | 0.004 |
| GIST and fourth-line treatment | |||
| Eligible patients, No. | 1.15 | 1.16 | 1.17 |
| Total cost, $ | |||
| With avapritinib available | 78 203 | 93 408 | 113 735 |
| Without avapritinib available | 44 024 | 44 288 | 44 553 |
| Incremental budget impact | |||
| Total, $ | 34 179 | 49 121 | 69 182 |
| Owing to change in postprogression costs, $b | 157 | 225 | 317 |
| PMPM | 0.003 | 0.004 | 0.006 |
Abbreviations: GIST, gastrointestinal stromal tumors; PDGFRA, platelet-derived growth factor receptor alpha; PMPM, per member per month.
The health plan population mix was 69% commercial, 22% Medicare, and 9% Medicaid.
Postprogression costs that have been avoided or delayed.
For the patients with GIST receiving fourth-line treatment, the total incremental budget impact of introducing avapritinib was $34 179 in year 1, $49 121 in year 2, and $69 182 in year 3, corresponding to a PMPM budget impact of $0.003, $0.004, and $0.006, respectively. Results differed by plan type, with the year 3 budget impact for both the presence of the PDGFRA exon 18 variant and fourth-line therapy for 100% commercial coverage estimated at $90 734 ($0.007 PMPM), Medicare at $205 864 ($0.017 PMPM), and Medicaid at $81 802 ($0.007 PMPM).
The sensitivity analysis was generally consistent with base case results and showed that the combined PMPM budget impact was most sensitive to assumptions on avapritinib drug acquisition costs, duration of fourth-line treatment, and fourth-line market share. An increase in postprogression pharmacy costs resulted in a small decrease in the budget impact. (Figure 3). A separate scenario analysis with testing rate increasing over 3 years to 100% with the introduction of avapritinib resulted in incremental testing costs of $1155 and a year 3 budget impact of $65 061 ($0.005 PMPM) for patients with the PDGFRA exon 18 variant.
Figure 3. Sensitivity of Average Budget Impact to Change in Input Values.
4L+ indicates fourth-line or higher therapy; PDGFRA, platelet-derived growth factor receptor alpha; and PMPM, per member per month.
Discussion
Before the availability of avapritinib, effective treatment options for patients with GIST and the PDGFRA exon 18 variant were lacking, leading to a poor prognosis for these patients.2,22 New treatments for rare cancers and cancer subtypes identified with diagnostic tests have become available in recent years, and health plans need tools to assess the economic impact and plan for future spending. In addition, before the recent approval of ripretinib,34 there were no US Food and Drug Administration–approved treatments for patients after 3 therapies, although avapritinib remains listed in the NCCN guidelines as an option that may be useful under certain circumstances.
Population estimates from the model that indicate the number of patients potentially eligible for avapritinib for fourth-line treatment of metastatic or unresectable GIST or treatment of GIST with the PDGFRA exon 18 variant is likely to be small, with approximately 1 new patient per million members annually in commercial and Medicaid plans and approximately 2 patients per million in a Medicare plan. Change in testing costs had a negligible effect on total costs, with a $453 increase in cost for a 1-million member plan based on an increase in testing rates of 20 percentage points, or $1155 if increased to 100%.
The total budget impact for a health plan associated with the addition of avapritinib is on average minimal, with a PMPM of less than $0.01 in a typical mixed plan. The small budget impact is primarily reflective of the small size of the eligible patient population. The main element for the budget increase associated with the introduction of avapritinib is higher pharmacy costs owing in large part by substantially longer PFS compared with that associated with comparators; PFS is an element of efficacy that is associated with increased treatment duration. This increase in treatment duration is partially offset by a reduction in postprogression costs, as evaluated by pharmacy, adverse event, and monitoring costs attributable to treatments taken after disease progression.
Limitations
This study has limitations. Estimates of health care resource utilization (HRU) are not included in the model because published evidence estimating HRU for metastatic or unresectable GIST was not found. If HRU data were available, total costs would be higher and the budget impact of avapritinib introduction would most likely be lower given that patients receiving avapritinib spend less time with disease progression, which is likely to be associated with higher HRU. Further research to evaluate HRU in this context would be useful.
Uncertainties in clinical inputs included lack of data available for multiple therapies, including the use of an estimate of median PFS associated with avapritinib for treatment of patients with the PDGFRA exon 18 D842V variant and use of median PFS inputs extrapolated from an observational imatinib study for all exon 18 comparators. The use of trial results in a repeat third-line setting for all fourth-line comparators was necessary owing to lack of available data in a clinical trial setting for any of the fourth-line competitors, albeit the short 1.8-month treatment duration for comparators was unlikely to lead to underestimation of the budget impact. Although this lack of data creates meaningful uncertainties, sensitivity analyses showed that with 20% changes to individual input values, the PMPM budget impact remained modest at less than $0.012 PMPM in the third year after availability.
The model assumed that the number of patients switching to fourth-line treatment after third-line treatment did not change with the introduction of avapritinib. Estimates of the potential uptake of avapritinib and corresponding market shares were assumption based and were predictions that are hard to base on solid data, albeit the 70% market share prediction for fourth-line treatment in year 3 (80% for PDGFRA exon 18) was determined before the approval of ripretinib for fourth-line treatment of GIST and likely to resulted in a conservative estimate of the budget impact for avapritinib. Given that ripretinib pricing has been set at the same level as avapritinib, if an assumption is made that the 70% market share as fourth-line therapy is split between those 2 agents, the model could be viewed as an indication of the budget impact of introducing both agents.23
Conclusions
The results of this economic modeling study suggest that the use of avapritinib for treatment of patients with unresectable or metastatic GIST with a PDGFRA exon 18 variant or after 3 or more prior treatments would be associated with a minimal budget impact to a US managed care health plan. Although introduction of avapritinib is expected to increase testing costs, these costs were shown to be negligible. The budget impact varied among several plan types evaluated (commercial, Medicare, and Medicaid), but results were robust to wide variation in key model inputs. Results were attributable primarily to the small patient population with this rare cancer type and to cost savings from reduced postprogression costs.
eTable. Weighted Postprogression Cost by Line
References
- 1.Barnett CM, Corless CL, Heinrich MC. Gastrointestinal stromal tumors: molecular markers and genetic subtypes. Hematol Oncol Clin North Am. 2013;27(5):871-888. doi: 10.1016/j.hoc.2013.07.003 [DOI] [PubMed] [Google Scholar]
- 2.Cassier PA, Fumagalli E, Rutkowski P, et al. ; European Organisation for Research and Treatment of Cancer . Outcome of patients with platelet-derived growth factor receptor alpha-mutated gastrointestinal stromal tumors in the tyrosine kinase inhibitor era. Clin Cancer Res. 2012;18(16):4458-4464. doi: 10.1158/1078-0432.CCR-11-3025 [DOI] [PubMed] [Google Scholar]
- 3.Demetri GD, von Mehren M, Antonescu CR, et al. NCCN Task Force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw. 2010;8(suppl 2):S1-S41. doi: 10.6004/jnccn.2010.0116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corless CL, Schroeder A, Griffith D, et al. PDGFRA mutations in gastrointestinal stromal tumors: frequency, spectrum and in vitro sensitivity to imatinib. J Clin Oncol. 2005;23(23):5357-5364. doi: 10.1200/JCO.2005.14.068 [DOI] [PubMed] [Google Scholar]
- 5.Heinrich MC, Maki RG, Corless CL, et al. Primary and secondary kinase genotypes correlate with the biological and clinical activity of sunitinib in imatinib-resistant gastrointestinal stromal tumor. J Clin Oncol. 2008;26(33):5352-5359. doi: 10.1200/JCO.2007.15.7461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y, Call J. Mutational testing in gastrointestinal stromal tumor. Curr Cancer Drug Targets. 2019;19(9):688-697. doi: 10.2174/1568009619666190326123945 [DOI] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology, Soft Tissue Sarcoma, version 2.2020. Accessed June 10, 2020. https://www.nccn.org/professionals/physician_gls/pdf/sarcoma.pdf
- 8.Proudman D, Miller A, Nellesen D, Mankoski R, Norregaard C, Sullivan E. The cost impact of increased molecular testing rates for the treatment of patients with gastrointestinal stromal tumors. Paper presented at: the Virtual Academy of Managed Care Pharmacy Annual Meeting, May 18-20, 2020. [Google Scholar]
- 9.Mamlouk KK, Crouch Z, Lee P, Mendonca C, Gumina M, Rubin B. Understanding US mutational testing patterns and treatment preferences in gastrointestinal stromal tumors (GIST) patients. Poster presented at National Comprehensive Cancer Network Meeting; March 21-23, 2019; Orlando, FL. [Google Scholar]
- 10.Blueprint Medicines Corporation . Avapritinib. Prescribing information. Blueprint Medicines Corporation; January 2020.
- 11.Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347(7):472-480. doi: 10.1056/NEJMoa020461 [DOI] [PubMed] [Google Scholar]
- 12.Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006;368(9544):1329-1338. doi: 10.1016/S0140-6736(06)69446-4 [DOI] [PubMed] [Google Scholar]
- 13.Sullivan SD, Mauskopf JA, Augustovski F, et al. Budget impact analysis-principles of good practice: report of the ISPOR 2012 Budget Impact Analysis Good Practice II Task Force. Value Health. 2014;17(1):5-14. doi: 10.1016/j.jval.2013.08.2291 [DOI] [PubMed] [Google Scholar]
- 14.Husereau D, Drummond M, Petrou S, et al. ; CHEERS Task Force . Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. BMJ. 2013;346:f1049. doi: 10.1136/bmj.f1049 [DOI] [PubMed] [Google Scholar]
- 15.US Census Bureau . Current population survey, health insurance. 2018. Accessed August 7, 2019. https://www.census.gov/cps/data/cpstablecreator.html
- 16.US Census Bureau . 2018 National and state population estimates. 2018. Accessed October 18, 2019. https://www.census.gov/newsroom/press-kits/2018/pop-estimates-national-state.html
- 17.Call JW, Wang Y, Montoya D, Scherzer NJ, Heinrich MC. Survival in advanced GIST has improved over time and correlates with increased access to post-imatinib tyrosine kinase inhibitors: results from Life Raft Group Registry. Clin Sarcoma Res. 2019;9:4. doi: 10.1186/s13569-019-0114-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heinrich MC, Owzar K, Corless CL, et al. Correlation of kinase genotype and clinical outcome in the North American Intergroup Phase III Trial of imatinib mesylate for treatment of advanced gastrointestinal stromal tumor: CALGB 150105 Study by Cancer and Leukemia Group B and Southwest Oncology Group. J Clin Oncol. 2008;26(33):5360-5367. doi: 10.1200/JCO.2008.17.4284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.US Department of Labor . Consumer Price Index. March 2020. Accessed April, 2020. https://www.bls.gov/cpi/factsheets/medical-care.htm
- 20.Heinrich M, Jones R, von Mehren M, et al. Clinical activity of avapritinib in ≥fourth-line (4L+) and PDGFRA Exon 18 gastrointestinal stromal tumors (GIST). Paper presented at: 2020 Gastrointestinal Cancers Symposium; San Francisco, California; January 23-25, 2020. [Google Scholar]
- 21.von Mehren M, Heinrich MC, Shi H, et al. A retrospective natural history study of patients (pts) with PDGFRα D842V mutant advanced gastrointestinal stromal tumor (GIST) previously treated with a tyrosine kinase inhibitor (TKI). J Clin Oncol. 2018;36(15)(suppl):11533. doi: 10.1200/JCO.2018.36.15_suppl.11533 [DOI] [Google Scholar]
- 22.Kang YK, Ryu MH, Yoo C, et al. Resumption of imatinib to control metastatic or unresectable gastrointestinal stromal tumours after failure of imatinib and sunitinib (RIGHT): a randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2013;14(12):1175-1182. doi: 10.1016/S1470-2045(13)70453-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.IBM Micromedex. Redbook. March 23, 2020. Accessed March 2020. https://www.micromedexsolutions.com/home/dispatch
- 24.US Department for Health and Human Services . HCUPnet: Healthcare Cost and Utilization Project—free healthcare statistics. 2019. Accessed September 3, 2019. https://hcupnet.ahrq.gov/
- 25.US Centers for Medicare & Medicaid Services . Clinical laboratory fee schedule. 2019. Accessed September 3, 2019. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/index.html
- 26.US Centers for Medicare & Medicaid Services . Medicare physician fee schedule. 2019. Accessed October 4, 2019. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/
- 27.Demetri GD, Reichardt P, Kang YK, et al. ; GRID study investigators . Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381(9863):295-302. doi: 10.1016/S0140-6736(12)61857-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pfizer Inc . Sutent. Prescribing information. Pfizer Inc; 2019.
- 29.Novartis Pharmaceuticals Corporation . Gleevec. Prescribing information. Novartis Pharmaceuticals Corporation; 2018. [Google Scholar]
- 30.Blay JY, Shen L, Kang YK, et al. Nilotinib versus imatinib as first-line therapy for patients with unresectable or metastatic gastrointestinal stromal tumours (ENESTg1): a randomised phase 3 trial. Lancet Oncol. 2015;16(5):550-560. doi: 10.1016/S1470-2045(15)70105-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reichardt P, Blay JY, Gelderblom H, et al. Phase III study of nilotinib versus best supportive care with or without a TKI in patients with gastrointestinal stromal tumors resistant to or intolerant of imatinib and sunitinib. Ann Oncol. 2012;23(7):1680-1687. doi: 10.1093/annonc/mdr598 [DOI] [PubMed] [Google Scholar]
- 32.Mir O, Cropet C, Toulmonde M, et al. ; PAZOGIST study group of the French Sarcoma Groupe-Groupe d’Etude des Tumeurs Osseuses (GSF-GETO) . Pazopanib plus best supportive care versus best supportive care alone in advanced gastrointestinal stromal tumours resistant to imatinib and sunitinib (PAZOGIST): a randomised, multicentre, open-label phase 2 trial. Lancet Oncol. 2016;17(5):632-641. doi: 10.1016/S1470-2045(16)00075-9 [DOI] [PubMed] [Google Scholar]
- 33.Park SH, Ryu MH, Ryoo BY, et al. Sorafenib in patients with metastatic gastrointestinal stromal tumors who failed two or more prior tyrosine kinase inhibitors: a phase II study of Korean gastrointestinal stromal tumors study group. Invest New Drugs. 2012;30(6):2377-2383. doi: 10.1007/s10637-012-9795-9 [DOI] [PubMed] [Google Scholar]
- 34.US Food and Drug Administration . FDA approves ripretinib for advanced gastrointestinal stromal tumor. 2020. Accessed October 26, 2020. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-ripretinib-advanced-gastrointestinal-stromal-tumor
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Weighted Postprogression Cost by Line