Abstract
Aims
The risk and burden of cardiovascular disease (CVD) are higher in homeless than in housed individuals but population-based analyses are lacking. The aim of this study was to investigate prevalence, incidence and outcomes across a range of specific CVDs among homeless individuals.
Methods and results
Using linked UK primary care electronic health records (EHRs) and validated phenotypes, we identified homeless individuals aged ≥16 years between 1998 and 2019, and age- and sex-matched housed controls in a 1:5 ratio. For 12 CVDs (stable angina; unstable angina; myocardial infarction; sudden cardiac death or cardiac arrest; unheralded coronary death; heart failure; transient ischaemic attack; ischaemic stroke; subarachnoid haemorrhage; intracerebral haemorrhage; peripheral arterial disease; abdominal aortic aneurysm), we estimated prevalence, incidence, and 1-year mortality post-diagnosis, comparing homeless and housed groups. We identified 8492 homeless individuals (32 134 matched housed individuals). Comorbidities and risk factors were more prevalent in homeless people, e.g. smoking: 78.1% vs. 48.3% and atrial fibrillation: 9.9% vs. 8.6%, P < 0.001. CVD prevalence (11.6% vs. 6.5%), incidence (14.7 vs. 8.1 per 1000 person-years), and 1-year mortality risk [adjusted hazard ratio 1.64, 95% confidence interval (CI) 1.29–2.08, P < 0.001] were higher, and onset was earlier (difference 4.6, 95% CI 2.8–6.3 years, P < 0.001), in homeless, compared with housed people. Homeless individuals had higher CVD incidence in all three arterial territories than housed people.
Conclusion
CVD in homeless individuals has high prevalence, incidence, and 1-year mortality risk post-diagnosis with earlier onset, and high burden of risk factors. Inclusion health and social care strategies should reflect this high preventable and treatable burden, which is increasingly important in the current COVID-19 context.
Keywords: Homeless, Cardiovascular, Electronic health records, Health inequalities, COVID-19
Graphical Abstract
Graphical Abstract.
See page 4021 for the editorial comment on this article (doi: 10.1093/eurheartj/ehaa796)
Introduction
Homelessness affects an estimated 100 million people worldwide.1 Social exclusion (including homeless individuals) and low socioeconomic status are associated with increased mortality and morbidity,2 , 3 but the relationship between homelessness and specific health outcomes remains under-investigated, leading to a dearth of specific targeted healthcare interventions.
Cardiovascular diseases (CVDs) are the leading cause of burden of disease in the UK and worldwide,4 , 5 but the majority of research concerning health and healthcare of homeless people has focused on infectious diseases, mental health, and acute crisis management,2 even though the CVD burden among homeless people may be higher than the general population.6
Existing studies show higher risk of chronic diseases and death, including CVDs2 , 7 , 8 in homeless people, but have considered single or composite measures of CVD, neither looking across different CVDs together, nor the whole population, nor all age categories.9–13 Such data are crucial, whether to inform pathways for treatment and prevention in individuals, or to understand the population burden of homelessness and CVD for planning and implementation of public health interventions and policies, and even more so in the context of COVID-19.14 , 15 If homeless people have elevated burden and risk of CVDs, they are at higher mortality risk with COVID-19.
In housed individuals, studies across different CVDs have been instructive, whether in comparative epidemiology and time trends, or quality of treatment and prioritization in clinical practice and research,16 , 17 increasingly using electronic health records (EHRs),18 but not in homeless populations, which are defined variably across studies and countries.19 Translating these definitions into EHR presents obstacles, but if overcome, an overall view of CVD in homeless people could be gained.
We conducted a retrospective cohort study using national linked EHRs to investigate prevalence, incidence, and outcomes across major CVDs in homeless individuals, compared with housed individuals.
Methods
Data source
The study population used linked data from three sources of EHRs in the UK: Clinical Practice Research Datalink (CPRD), hospital episode statistics, and the Office of National Statistics for mortality data. CPRD is a primary care database that directly collects de-identified data of individuals from general practices (GPs) in the UK and is representative of the UK population in terms of age, sex, and ethnicity.20 This study was part of CALIBER (CArdiovascular disease research using LInked Bespoke studies and Electronic health Records).18
Phenotype algorithm development
The CPRD GOLD database was searched for Read codes relating to homelessness. Supplementary material online, Table S1 provides a full list of the terms. We developed an EHR algorithm for homelessness, which was reviewed by a panel of clinical and academic experts in homeless health, including primary and secondary care.
Study population
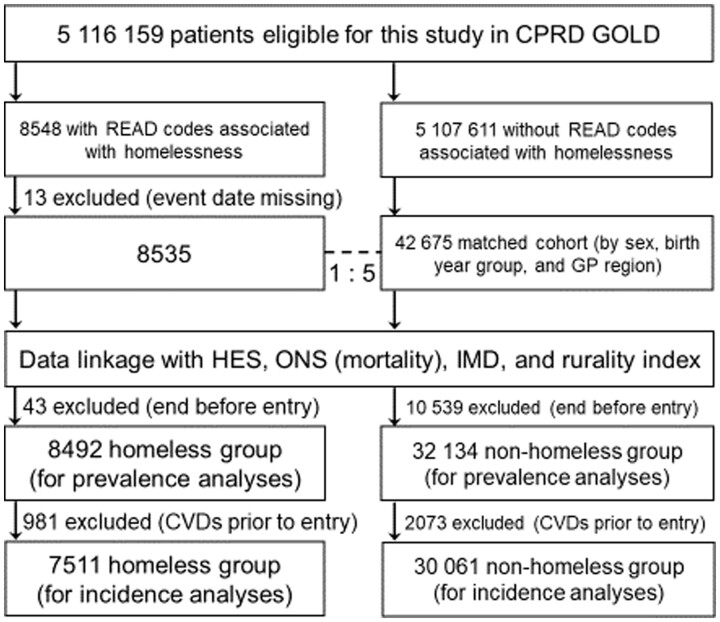
Individuals aged ≥16 years between 1 January 1998 and 31 January 2019 were included based on the EHR homelessness phenotype and had been registered in a practice meeting research data recording standards for at least 1 year. Individuals were excluded if ‘homeless’ status was missing. Controls (matched by age, sex, and GP region) were selected (5:1 for each homeless person) from eligible individuals without the homeless EHR phenotype (Figure 1). The homeless and housed samples were 8492 (7511 for incidence analyses) and 32 134 (30 061 for incidence analyses), respectively.
Figure 1.
Inclusion criteria for study of cardiovascular disease in homeless and housed individuals. CPRD, Clinical Practice Research Datalink; CVDs, cardiovascular diseases; HES, hospital episode statistics; IMD, Index of Multiple Deprivation; ONS, Office of National Statistics.
Baseline characteristics
At baseline (i.e. the first record in the study period 1998–2019), variables included socio-demography [age, gender, ethnicity, rurality, and deprivation level by Index of Multiple Deprivation (IMD)]. Since IMD is a postcode measure of deprivation and the recorded postcode of a homeless individual is unlikely to be a good measure of their level of deprivation (e.g. if a hostel is in an area with comparatively low social deprivation or the address of a relative is given), we did not apply IMD measures to homeless individuals. Clinical data included body mass index (BMI), blood pressure, total serum cholesterol, smoking, and alcohol. Comorbidities included atrial fibrillation, hypertension, diabetes, chronic obstructive pulmonary disease, chronic kidney disease (CKD), dyslipidaemia, and osteoarthritis. Medication usage included statin, antihypertensive medication, angiotensin-converting enzyme inhibitor, angiotensin receptor blocker, and beta-blocker.
Outcomes
Primary outcomes were 12 CVD event types [using code lists and validated algorithms as per previous CALIBER studies18 , 21 , 22: stable angina (SA), unstable angina (UA), myocardial infarction (MI), sudden cardiac death/cardiac arrest, unheralded coronary death, heart failure (HF), transient ischaemic attack, ischaemic stroke or stroke not further specified, subarachnoid haemorrhage, intracerebral haemorrhage, peripheral arterial disease, and abdominal aortic aneurysm]. Diseases were grouped as CVD overall (all 12 subtypes), cardiac (SA, UA, MI, sudden cardiac death/cardiac arrest, and unheralded coronary death), cerebrovascular (transient ischaemic attack, ischaemic stroke, subarachnoid haemorrhage, and intracerebral haemorrhage), and peripheral vascular (peripheral arterial disease and abdominal aortic aneurysm). Mortality from all causes after the first diagnosis of CVD was also investigated.
Statistical analysis
Prevalence was the number of individuals with disease prior to baseline, divided by total number of individuals (per 1000 persons). Incidence rate was the number of individuals (free from the 12 subtypes of CVD, as defined above, at baseline) who developed disease during follow-up, divided by the total person-years at risk (rate per 1000 person-years). Prevalence and incidence rates were estimated for homeless and housed individuals and reported by sex and age (<35, 35–44, 45–54, 55–64, 65–74, and ≥75 years). Overall prevalence rate ratios and incidence rate ratios (IRRs) comparing age- and sex-matched homeless and housed people were calculated. We also provide plots of age- and gender-specific prevalence and incidence for each disease category comparing the homeless and housed population. To explore the additional impact of homelessness above and beyond neighbourhood measures of social deprivation, these plots also include lines for the housed population in IMD1 (least deprived) and IMD5 (most deprived).
We used Kaplan–Meier analysis to estimate 1-year mortality rates after first CVD diagnosis. Patient follow-up was started on the date on which all eligibility criteria were met after 1 January 1998 and was censored on the date of first CVD presentation, death from other causes, last data collection from CPRD (31 January 2019) or deregistration from the practice, whichever occurred first. Cox regression models were used to calculate hazard ratios for homeless compared to housed individuals, adjusted by sex and age at incidence. Survival curves are shown comparing homeless and housed groups with additional curves for housed IMD 1 and IMD 5 population. Proportional hazard assumption was assessed by plotting log–log survival functions, stratified by homelessness. The t-test was used to assess age difference at incidence. We used R 3.4.3, RStudio 1.1414, and Stata 15.1.
Ethical approval and funding
The study was approved by the Independent Scientific Advisory Committee of the Medicines and Healthcare products Regulatory Agency (protocol 18_283). The funder of the study (National Institute of Health Research, NIHR, Programme Development Grant: RP-DG-0117-10003) had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data and had final responsibility for the decision to submit for publication.
Results
Baseline characteristics
The study cohort included 40 626 individuals, 8492 of whom were homeless and 32 134 were housed (Figure 1), with matched sex and age structures between the two groups (Table 1). Homeless individuals were more likely to be in urban areas compared to housed people (92.5% vs. 89.3%, P < 0·001). Homeless individuals had lower BMI, blood pressure, and total cholesterol, but higher rates of previous/current smoking and excess/binge drinking (all P < 0.001). Comorbidities and use of statins and beta-blockers were more common in homeless individuals (all P < 0.001). There was no significant difference in the use of antihypertensive medication between the two groups. Smoking and alcohol use were greater in men than women in both homeless and housed populations (data not shown).
Table 1.
Baseline characteristics of homeless and housed individuals in the study population
| Homeless, n (%) | Housed, n (%) | P-values | |
|---|---|---|---|
| Total number | 8492 | 32 134 | – |
| Age (years), mean [SD] | 39.0 [14.6] | 38.8 [14.7] | Matched |
| Gender, male | 4975 (58.6) | 18 650 (58.0) | Matched |
| Social deprivation (IMD) | n/a | ||
| Least deprived | n/a | 6629 (20.6) | – |
| Most deprived | n/a | 5205 (16.2) | – |
| Ethnicity | <0.001 | ||
| White | 6094 (81.2) | 17 670 (81.3) | – |
| South Asian | 163 (2.2) | 742 (3.4) | – |
| Black | 499 (6.6) | 746 (3.4) | – |
| Others | 749 (10.0) | 2578 (11.9) | – |
| Urban area | 7854 (92.5) | 28 711 (89.3) | <0.001 |
| BMI (kg/m2), mean [SD] | 25.8 [6.1] | 26.4 [5.6] | <0.001 |
| SBP (mmHg), mean [SD] | 124.2 [17.3] | 125.7 [17.0] | <0.001 |
| DBP (mmHg), mean [SD] | 76.5 [11.1] | 77.0 [10.7] | 0.002 |
| Total serum cholesterol (mmol/L), mean [SD] | 5.17 [1.20] | 5.26 [1.12] | <0.001 |
| Smoking status | <0.001 | ||
| Non-smoker | 1515 (21.9) | 12 471 (51.7) | – |
| Ex- or current smoker | 5412 (78.1) | 11 635 (48.3) | – |
| Alcohol consumption | <0.001 | ||
| Non-drinker | 1237 (45.4) | 3475 (52.4) | – |
| Ex- or current drinker | 1217 (44.7) | 2992 (45.1) | – |
| Excess or binge drinker | 271 (9.9) | 161 (2.4) | – |
| Comorbidities | |||
| Atrial fibrillation | 115 (1.4) | 238 (0.7) | <0.001 |
| Hypertension | 837 (9.9) | 2752 (8.6) | <0.001 |
| Diabetes | 468 (5.5) | 1000 (3.1) | <0.001 |
| Chronic obstructive pulmonary disease | 1939 (22.8) | 4501 (14.0) | <0.001 |
| CKD | 98 (1.2) | 297 (0.9) | 0.05 |
| Dyslipidaemia | 321 (3.8) | 1103 (3.4) | 0.12 |
| Osteoarthritis | 555 (6.5) | 1478 (4.6) | <0.001 |
| Medication use | |||
| Statin | 1075 (12.7) | 3663 (11.4) | 0.001 |
| Antihypertensive medication | 1118 (13.2) | 4192 (13.0) | 0.77 |
| ACE inhibitor | 762 (9.0) | 3027 (9.4) | 0.21 |
| Angiotensin receptor blocker | 220 (2.6) | 1047 (3.3) | 0.002 |
| Beta-blocker | 1170 (13.8) | 3106 (9.7) | <0.001 |
Proportion of participants with non-missing values: social deprivation 99.9% (40580/40626), ethnicity 72% (29241), BMI 59.8% (24280), blood pressure 68.2% (27713), total cholesterol 26.6% (10789), smoking status 76.4% (31033), alcohol consumption 23% (9353). P -value was for the difference between homeless and housed individuals.
ACE, angiotensin-converting enzyme; BMI, body mass index; CKD, chronic kidney disease; DBP, diastolic blood pressure; IMD, Index of Multiple Deprivation; SBP, systolic blood pressure; SD, standard deviation.
Prevalence
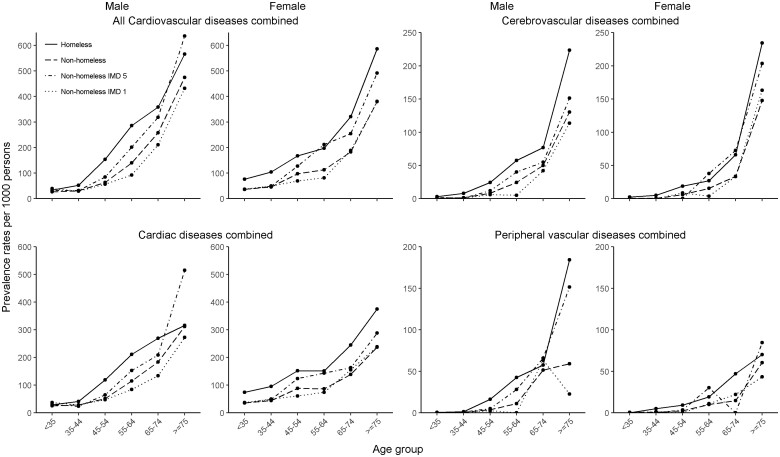
Baseline CVD prevalence was higher in homeless people than in housed people across subtypes: 8.7% and 5.0% (SA); 1.2% and 0.4% (UA); 1.4% and 0.7% (MI); 0.3% and 0.1% (sudden cardiac death/cardiac arrest); 0.9% and 0.6% (HF); 0.6% and 0.3% (transient ischaemic attack); 1.1% and 0.5% (ischaemic stroke); 0.2% and 0.1% (subarachnoid haemorrhage); 0.2% and 0.1% (intracerebral haemorrhage); 1.0% and 0.4% (peripheral arterial disease); and 0.1% and 0.1% (abdominal aortic aneurysm), respectively. Homeless people had higher prevalence of all CVDs than housed people (11.6% and 6.5%), in men and women, and in most age groups (Table 2 and Figures 2 and 3). A gradation from housed IMD 1, housed IMD 5, to homeless people in increasing order was seen in most sub-groups (Supplementary material online, Tables S2 and S3).
Table 2.
Comparisons of prevalence, incidence, and 1-year mortality (adjusted for age and sex) in homeless people vs. age- and sex-matched controls
| Disease | Prevalence (%) |
PRR (95% CI, P) | Incidence (per 1000 person-years) |
IRR (95% CI, P) | Crude 1-year mortality (%) |
aHR(95% CI, P) | |||
|---|---|---|---|---|---|---|---|---|---|
| Homeless | Housed | Homeless | Housed | Homeless | Housed | ||||
| All CVDs | 11.6 | 6.5 | 1.79 (1.67–1.92, <0.001) | 14.7 | 8.1 | 1.80 (1.62–2.01, <0.001) | 15.3 | 11.6 | 1.64 (1.29–2.08, <0.001) |
| Cardiac | 9.3 | 5.4 | 1.73 (1.60–1.88, <0.001) | 10.3 | 5.8 | 1.76 (1.55–2.01, <0.001) | 11.8 | 10.1 | 1.57 (1.15–2.16, 0.005) |
| Cerebrovascular | 1.9 | 0.8 | 2.25 (1.85–2.74, <0.001) | 2.4 | 1.4 | 1.80 (1.39–2.35, <0.001) | 17.8 | 16.1 | 1.49 (0.9–2.45, 0.12) |
| Peripheral vascular | 1.1 | 0.5 | 2.33 (1.80–3.03, <0.001) | 1.4 | 0.8 | 1.67 (1.19–2.36, 0.003) | 9.5 | 15.1 | 0.90 (0.37–2.21, 0.82) |
aHR, adjusted hazard ratio (adjusted for age and sex); CI, confidence interval; CVDs, cardiovascular diseases; IRR, incidence rate ratio; PRR, prevalence rate ratio.
Figure 2.
Prevalence of all cardiovascular, cardiac, cerebrovascular diseases and peripheral vascular diseases.
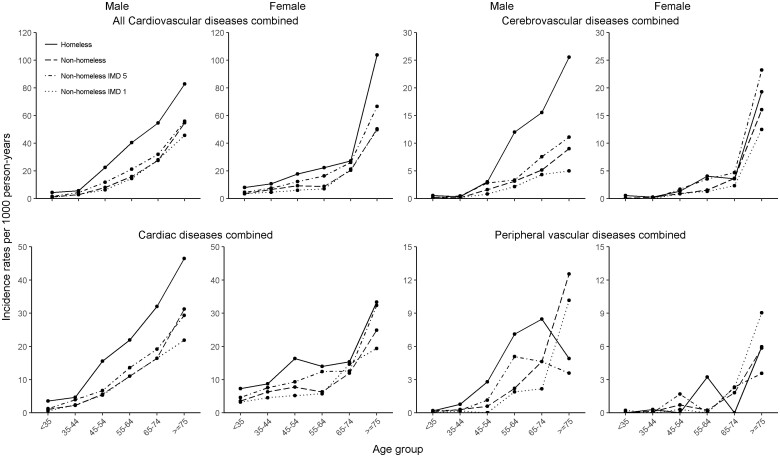
Figure 3.
Incidence of all cardiovascular, cardiac, cerebrovascular diseases and peripheral vascular diseases.
Incidence
As with prevalent CVD, homeless people had higher incidence rate for all CVDs combined, compared to their housed counterparts, in most sex and age categories. Homeless men had more than double the incidence of cardiac diseases combined than that in housed men [IRR 2.76, 95% confidence interval (CI) 2.04–3.55 in the 45–54-year group and 3.35, 1.87–6.03 in the <35-year group] (Table 2). Homeless women also had higher incidence of cardiac diseases compared to housed women (2.20, 1.26–3.85 in the 55–64-year group and 2.04, 1.38–3.00 in the <35-year group). Homeless individuals had earlier age of incidence of all CVDs [difference: 4.6 (95% CI 2.8–6.3) years], cardiac [4.9 (2.8–6.9) years], cerebrovascular [3.7 (−0.2 to 7.7) years], and peripheral vascular diseases [7.9 (2.9–12.9)] (Supplementary material online, Table S4). Other than cerebrovascular and peripheral vascular diseases in women (where numbers of events were small), incidence of CVD in all arterial territories was higher in homeless people than in housed people in most age groups (Figure 3). An additional increase between housed IMD 5 and homeless groups was observed as prevalence. Other than UA, MI, sudden cardiac death/cardiac arrest, unheralded coronary death, ischaemic stroke, intracerebral haemorrhage and peripheral arterial disease in women and transient ischaemic attack, subarachnoid haemorrhage, and abdominal aortic aneurysm in both sexes (where numbers of events were low), incidence for all CVD subtypes was higher in homeless individuals than in housed individuals (Supplementary material online, Table S3).
Mortality
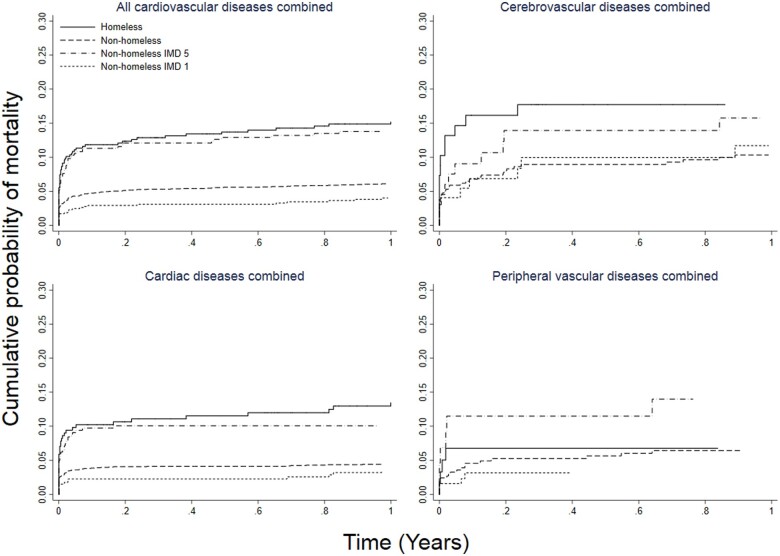
Crude 1-year mortality rates after the first diagnosis of any CVDs were 15.3% and 11.6% in homeless and housed people, respectively. Homeless individuals had higher age- and sex-adjusted mortality than housed individuals for all CVDs, cardiac and cerebrovascular, but not peripheral vascular diseases (Figure 4). Being homeless was associated with higher risk of mortality for all CVDs combined (adjusted hazard ratio 1.64; 95% CI 1.29–2.08, P < 0.001) and cardiac diseases (1.57; 1.15–2.16, P = 0.005), but not for cerebrovascular (1.49; 0.90–2.45, P = 0.12) and peripheral vascular diseases (0.90; 0.37–2.21, P = 0.82) (Table 2).
Figure 4.
Age- and sex-adjusted 1-year mortality for all cardiovascular, cardiac, cerebrovascular diseases and peripheral vascular diseases in homeless and non-homeless individuals. IMD, Index of Multiple Deprivation.
Discussion
This first large-scale population-based study of CVDs in the homeless population has four major findings. First, homeless people have increased burden of comorbidities, particularly smoking, alcohol, diabetes, and hypertension, compared with housed individuals. Second, they are around 1.8 times more likely to have CVDs at baseline, compared with their housed counterparts. Third, homeless people are around 1.8 times more likely to develop new CVD, with important differences by age, sex, and arterial territory. Fourth, homeless individuals are around 1.6 times more likely to die within 1 year of diagnosis, compared with housed individuals.
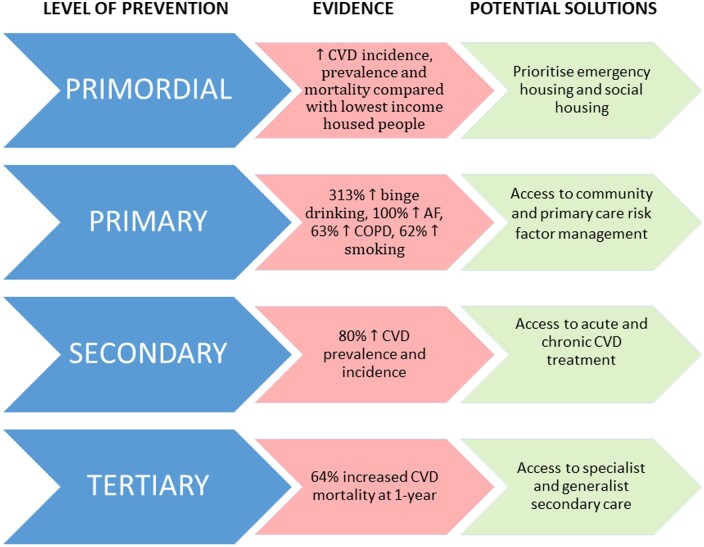
High rates of smoking and comorbidities indicate an unmet need for primary prevention and integrated care of homeless CVD (Take home figure).23 Prior studies corroborate the high burden of hypertension and CVD risk factors,24 , 25 but there are no specific interventions for homeless or inclusion health populations and limited research regarding CVD to-date.26 The relatively high burden of chronic obstructive pulmonary disease, atrial fibrillation, and other chronic conditions highlight that services to prevent and treat CVD cannot be ‘stand-alone’ and must account for the multimorbidity and complex social, psychological, and physical factors encountered by this heterogeneous population. Homeless people have been identified as particularly vulnerable in the current COVID-19 pandemic.27 The UK government has emphasized particular high-risk conditions (including CVD, chronic obstructive pulmonary disease and diabetes), which are associated with high excess COVID-19-related risk of mortality and indicate a greater need for social isolation.28 We now show that homeless individuals have higher prevalence of these same factors, making them high risk for poor outcomes associated with COVID-19 infection.
Take home figure.
Prevention, evidence and potential solutions for cardiovascular disease in homeless people. CVD, cardiovascular disease.
The high prevalence of CVD compared with housed individuals shows that the current paradigm for managing homeless and inclusion health, which largely neglects CVD and non-communicable diseases (NCDs), needs a total shift in thinking. As homelessness increases in the UK and globally,29 CVD, which is already known to be a cause of homeless morbidity and mortality,6 is likely to also increase. Part of the neglect is likely to be due to under-diagnosis and under-treatment of risk factors, which require improvements in system approaches to individuals and populations. However, beyond gaps in evidence and implementation, there are clearly gaps in awareness and advocacy. There are parallels with the global neglect of CVD and NCDs in low- and middle-income countries three decades ago.30 A combination of robust epidemiologic data and mobilization of stakeholders was needed to begin to address the global burden of NCDs, and it is likely that the same strategies will be required to improve CVD management in homeless individuals.
Compared with housed populations, increased incidence of cardiac and cerebrovascular disease appears to be greater public health concerns than peripheral vascular disease in homeless people. The observed lower rates of peripheral vascular disease may reflect under-diagnosis rather than true lower rates, which warrants further investigation. Our incidence analyses demonstrate the extreme impact of homelessness beyond the impact of social deprivation. As in previous population-based studies of arterial disease across territories,16 incidence of CVD in women seems to lag behind men by approximately 10 years but catches up in later life. For example, the highest IRR for all CVD among female individuals was seen in the 55–64 age group, while among male individuals, IRR decreased as age increased, with a second peak in 45–54 years. The sex difference in older age can potentially be attributed to the increased risk of cardiac diseases associated with menopause in women.31 It appears that homelessness further increases this risk for older women. There has been no study focusing on the cardiac risk management of women after menopause, which seems worthy of further investigation.
We showed that homeless people had a 1.6-fold higher mortality risk at 1 year, compared to their housed counterparts, if they developed any CVD, even after adjustment for age and sex. This may simply be because homeless people are at higher mortality risk than the housed population,2 but it can also be argued that the elevated mortality risk after CVD onset can be attributed to delayed diagnosis or challenges in disease management, including potentially worse adherence to medications.23 Interestingly, our baseline data do not suggest a difference between homeless and housed individuals with respect to the prescription of antihypertensive medications. However, adherence is likely to be low, which we are not able to analyse with these data. Furthermore, given that incidence rates of CVD are higher in homeless individuals, a greater need for anti-hypertensives may be expected. We also found very poor 1-year survival from CVD beyond the poor rates observed in the most deprived (IMD 5), housed individuals, the reasons for which warrant further investigation, but could include later diagnosis in relation to stage of disease or poor access to interventions.
The feasibility of developing EHR phenotypes for homelessness is an added value of our study. Although Read codes have been replaced by SNOMED-CT in UK clinical practice, the code lists (Supplementary material online, Table S1) can be easily adapted for research and practice. To accelerate health research in this particularly vulnerable population, a more accessible and less costly methodology is needed, which an EHR phenotype (which should be validated) can address.
The age- and disease-specific prevalence in homeless individuals were similar to that seen in a recent cross-sectional (Supplementary material online, Figure S1 and Supplementary material online, Table S5),7 validating the algorithm we have developed. Age-specific incidence rates of territory-specific CVD in housed individuals in this study were similar to rates observed in the Oxford Vascular Study (Supplementary material online, Figure S4).
Limitations
There are several limitations of the current study. Although this is large population-based study, it is observational by design and CPRD is restricted to those registered by GPs. GP registration rates for homeless individuals are likely to be poor, particularly for younger individuals, which might have affected the observed relationship between homelessness and burden of CVDs, which are strongly associated with age.16 In addition, housing status is not measured in the Quality and Outcomes Framework, the presence of which has enhanced the data quality of CPRD.20 As such, GPs may not be incentivized to record this information in a Read code. If this is the case, some potentially homeless people may have been classified as housed. However, we have assumed low rates of misclassification, due to the relative size of the housed population compared to the homeless population. Unmeasured confounders may have impact on the increased risk and worse outcomes for homeless people observed in this study. Potential confounders include smoking, alcohol, hypertension, and other comorbidities, social factors, and more importantly, drug use. We did not adjust for behavioural risk factors because they are likely to be on the causal pathway and we were interested in burden rather than causation. Existing studies suggest a relationship between prevalent drug abuse and CVD risks9; however, this was not measured in the current study, partly due to a lack of established phenotyping for recreational drug use.
Conclusions
Despite limitations, we provide a comprehensive and multi-dimensional picture of the burden and risk across a wide range of CVDs and across the whole age spectrum within the homeless population (Take home figure), compared to a housed matched cohort. In terms of risk factors, sex- and age-specific prevalence and incidence rates, and disease-specific mortality rates, we have quantified the elevated CVD risks among homeless people. Health professionals, policymakers, and the public need to recognize homeless populations as a high-risk group for CVDs and plan appropriate interventions and policies.
Perspectives
Competency in medical knowledge: CVD has higher prevalence, incidence, and mortality in homeless individuals than their housed counterparts.
Competency in patient care: in homeless individuals, screening, and diagnosis of CVD across arterial territories, as well as prevention through optimal management of risk factors, should be prioritized.
Translational outlook 1: although this is a retrospective EHR study, the observations of early onset and high incidence of CVD, and the high-risk factor burden, will inform future studies of how homelessness increases CVD risk, which in turn will guide integrated interventions.
Translational outlook 2: health and social care strategies in inclusion health should reflect this high burden of treatable risk factors and disease, which is increasingly important in the current context of COVID-19.
Data availability
Data for these analyses are not available and require application to and approval by Clinical Practice Research Datalink (CPRD).
Supplementary Material
Acknowledgements
A.N. acquired, analysed, and interpreted the data. A.B. was the principal investigator and had the original research idea. K.D. contributed to the data acquisition and prepared the data. H.E. contributed to the study design. A.B., A.N., H.E., and A.H. contributed to the analysis plan. A.B. and A.N. wrote the initial manuscript and all authors provided critical analysis for the final manuscript.
Funding
National Institute of Health Research, NIHR, Programme Development (Grant RP-DG-0117-10003).
Conflict of interest: A.N. was supported by the Japanese Government Long-Term Overseas Fellowship Programme. A.B. has received grant funding from NIHR, British Medical Association, the European Union, and Astra-Zeneca. Other authors had no conflicts of interest.
Contributor Information
Atsunori Nanjo, Institute of Health Informatics, University College London, 222 Euston Road, London NW1 2DA, UK; Department of Public Health, Imperial College London, London, UK.
Hannah Evans, Institute of Health Informatics, University College London, 222 Euston Road, London NW1 2DA, UK.
Kenan Direk, Institute of Health Informatics, University College London, 222 Euston Road, London NW1 2DA, UK.
Andrew C Hayward, UCL Institute of Epidemiology and Health Care, UCL, London, UK.
Alistair Story, Find and Treat, University College London Hospitals NHS Trust, London, UK.
Amitava Banerjee, Institute of Health Informatics, University College London, 222 Euston Road, London NW1 2DA, UK; Department of Cardiology, University College London Hospitals NHS Trust, UK; Department of Cardiology, Barts Health NHS Trust, London, UK.
References
- 1.Institute of Global Homelessness (IGH). What Is IGH?; 2019. https://www.ighomelessness.org/about (20 September 2020).
- 2. Aldridge RW, Story A, Hwang SW, Nordentoft M, Luchenski SA, Hartwell G, Tweed EJ, Lewer D, Katikireddi SV, Hayward AC. Morbidity and mortality in homeless individuals, prisoners, sex workers, and individuals with substance use disorders in high-income countries: a systematic review and meta-analysis. Lancet 2018;391:241–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Luchenski S, Maguire N, Aldridge RW, Hayward A, Story A, Perri P, Withers J, Clint S, Fitzpatrick S, Hewett N. What works in inclusion health: overview of effective interventions for marginalised and excluded populations. Lancet 2018;391:266–280. [DOI] [PubMed] [Google Scholar]
- 4.GBD 2016 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017;390:1260–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Newton JN, Briggs AD, Murray CJ, Dicker D, Foreman KJ, Wang H, Naghavi M, Forouzanfar MH, Ohno SL, Barber RM, Vos T, Stanaway JD, Schmidt JC, Hughes AJ, Fay DF, Ecob R, Gresser C, McKee M, Rutter H, Abubakar I, Ali R, Anderson HR, Banerjee A, Bennett DA, Bernabé E, Bhui KS, Biryukov SM, Bourne RR, Brayne CE, Bruce NG, Brugha TS, Burch M, Capewell S, Casey D, Chowdhury R, Coates MM, Cooper C, Critchley JA, Dargan PI, Dherani MK, Elliott P, Ezzati M, Fenton KA, Fraser MS, Fürst T, Greaves F, Green MA, Gunnell DJ, Hannigan BM, Hay RJ, Hay SI, Hemingway H, Larson HJ, Looker KJ, Lunevicius R, Lyons RA, Marcenes W, Mason-Jones AJ, Matthews FE, Moller H, Murdoch ME, Newton CR, Pearce N, Piel FB, Pope D, Rahimi K, Rodriguez A, Scarborough P, Schumacher AE, Shiue I, Smeeth L, Tedstone A, Valabhji J, Williams HC, Wolfe CD, Woolf AD, Davis AC. Changes in health in England, with analysis by English regions and areas of deprivation, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aldridge RW, Menezes D, Lewer D, Cornes M, Evans H, Blackburn RM, Byng R, Clark M, Denaxas S, Fuller J, Hewett N, Kilmister A, Luchenski S, Manthorpe J, McKee M, Neale J, Story A, Tinelli M, Whiteford M, Wurie F, Hayward A. Causes of death among homeless people: a population-based cross-sectional study of linked hospitalisation and mortality data in England. Wellcome Open Res 2019;4:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lewer D, Aldridge RW, Menezes D, Sawyer C, Zaninotto P, Dedicoat M, Ahmed I, Luchenski S, Hayward A, Story A. Health-related quality of life and prevalence of six chronic diseases in homeless and housed people: a cross-sectional study in London and Birmingham, England. BMJ Open 2019;9:e025192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Story A. Slopes and cliffs in health inequalities: comparative morbidity of housed and homeless people. Lancet 2013;382:S93. [Google Scholar]
- 9. Jones C, Perera A, Chow M, Ho I, Nguyen J, Davachi S. Cardiovascular disease risk among the poor and homeless—what we know so far. Curr Cardiol Rev 2009;5:69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schinka JA, Bossarte RM, Curtiss G, Lapcevic WA, Casey RJ. Increased mortality among older veterans admitted to va homelessness programs. Psychiatr Serv 2016;67:465–468. [DOI] [PubMed] [Google Scholar]
- 11. Asgary R, Sckell B, Alcabes A, Naderi R, Schoenthaler A, Ogedegbe G. Rates and predictors of uncontrolled hypertension among hypertensive homeless adults using New York city shelter-based clinics. Ann Fam Med 2016;14:41–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vijayaraghavan M, Kushel MB, Vittinghoff E, Kertesz S, Jacobs D, Lewis CE, Sidney S, Bibbins-Domingo K. Housing instability and incident hypertension in the CARDIA cohort. J Urban Heal 2013;90:427–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hwang SW, Wilkins R, Tjepkema M, O'Campo PJ, Dunn JR. Mortality among residents of shelters, rooming houses, and hotels in Canada: 11 year follow-up study. BMJ 2009;339:b4036–b4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol 2020;10:1–10. http://www.ncbi.nlm.nih.gov/pubmed/32219363. [DOI] [PubMed] [Google Scholar]
- 15. Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020;5:811–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rothwell PM, Coull AJ, Silver LE, Fairhead JF, Giles MF, Lovelock CE, Redgrave JN, Bull LM, Welch SJ, Cuthbertson FC, Binney LE, Gutnikov SA, Anslow P, Banning AP, Mant D, Mehta Z, Oxford Vascular Study. Population-based study of event-rate, incidence, case fatality, and mortality for all acute vascular events in all arterial territories (Oxford Vascular Study). 2005;366:1773–1783. [DOI] [PubMed] [Google Scholar]
- 17. Rapsomaniki E, Timmis A, George J, Pujades-Rodriguez M, Shah AD, Denaxas S, White IR, Caulfield MJ, Deanfield JE, Smeeth L, Williams B, Hingorani A, Hemingway H. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life-years lost, and age-specific associations in 1.25 million people. Lancet 2014;383:1899–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Denaxas S, Gonzalez-Izquierdo A, Direk K, Fitzpatrick NK, Fatemifar G, Banerjee A, Dobson RJB, Howe LJ, Kuan V, Lumbers RT, Pasea L, Patel RS, Shah AD, Hingorani AD, Sudlow C, Hemingway H. UK phenomics platform for developing and validating electronic health record phenotypes: CALIBER. J Am Med Informatics Assoc 2019:26;1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fazel S, Geddes JR, Kushel M. The health of homeless people in high-income countries: descriptive epidemiology, health consequences, and clinical and policy recommendations. Lancet 2014;384:1529–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Herrett E, Bhaskaran K, Gallagher AM, Mathur R, Smeeth L, Forbes H, Mathur R, van Staa T, Smeeth L. Data Resource Profile: Clinical Practice Research Datalink (CPRD). Int J Epidemiol 2015;44:827–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. George J, Mathur R, Shah AD, Pujades-Rodriguez M, Denaxas S, Smeeth L, Timmis A, Hemingway H. Ethnicity and the first diagnosis of a wide range of cardiovascular diseases: associations in a linked electronic health record cohort of 1 million patients. PLoS One 2017;12:e0178945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shah AD, Langenberg C, Rapsomaniki E, Denaxas S, Pujades-Rodriguez M, Gale CP, Deanfield J, Smeeth L, Timmis A, Hemingway H. Type 2 diabetes and incidence of cardiovascular diseases: a cohort study in 1.9 million people. Lancet Diabetes Endocrinol 2015;3:105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Baggett TP, Liauw SS, Hwang SW. Cardiovascular disease and homelessness. J Am Coll Cardiol 2018;71:2585–2597. [DOI] [PubMed] [Google Scholar]
- 24. Bernstein RS, Meurer LN, Plumb EJ, Jackson JL. Diabetes and hypertension prevalence in homeless adults in the United States: a systematic review and meta-analysis. Am J Public Health 2015;105:e46–e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee TC, Hanlon JG, Ben-David J, Booth GL, Cantor WJ, Connelly PW, Hwang SW. Risk factors for cardiovascular disease in homeless adults. Circulation 2005;111:2629–2635. [DOI] [PubMed] [Google Scholar]
- 26. Al-Shakarchi N, Evans H, Luchenski S, Story A, Banerjee A. Cardiovascular disease in the homeless: a systematic review of observational and interventional studies. Heart 2020;106:1483–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kirkby T. Efforts escalate to protect homeless people from COVID-19 in UK. Lancet Resp Med 2020;8:447–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Banerjee A, Pasea L, Harris S, Gonzalez-Izquierdo A, Torralbo A, Shallcross L, Noursadeghi M, Pillay D, Sebire N, Holmes C, Pagel C, Wong WK, Langenberg C, Williams B, Denaxas S, Hemingway H. Estimating excess 1-year mortality from COVID-19 according to underlying conditions and age in England: a rapid analysis using NHS health records in 3.8 million adults. Lancet 2020;395:1715–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Elsey H, Agyepong I, Huque R, Quayyem Z, Baral S, Ebenso B, Kharel C, Shawon RA, Onwujekwe O, Uzochukwu B, Nonvignon J, Aryeetey GC, Kane S, Ensor T, Mirzoev T. Rethinking health systems in the context of urbanisation: challenges from four rapidly urbanising low-income and middle-income countries. BMJ Glob Heal 2019;4:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Murray CJL, Lopez AD. Mortality by cause for eight regions of the world: global burden of disease study. Lancet 1997;349:1269–1276. [DOI] [PubMed] [Google Scholar]
- 31. Kannel WB, Hjortland MC, McNamara P, Gordon T. Menopause and risk of cardiovascular disease. The Framingham study. Ann Intern Med 1976;85:447–452. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data for these analyses are not available and require application to and approval by Clinical Practice Research Datalink (CPRD).