Abstract
Cultures of primary tumors are very useful as a personalized screening system for effective therapeutic options. We here describe an effective method of reproducing human primary colon tumors through primary culture and a mouse xenograft model. A total of 199 primary colon tumor cultures were successfully established under optimized conditions to enrich for tumor cells and to expand it for long-term storage in liquid nitrogen. To examine whether these stored cultures retained original tumor properties, fifty primary cultures were xenografted into NOD–SCID mouse. Histological and tumor marker analysis of four representative tumor xenografts revealed that all of the xenograft retained its primary tumor characteristics. Oncomap analysis further showed no change in the major mutations in the xenografts, confirming that our method faithfully reproduced human colon tumors. A drug sensitivity assay revealed that two of the primary cultures were hypersensitive to oxaliplatin rather than 5-FU, which was used in the patients, suggesting it as an effective therapeutic option. We thus present an effective, reproducible preclinical model for testing various personalized therapeutic options in colon cancer patients.
Keywords: Patient derived xenograft (PDX), Colon cancer, Oncomap analysis, Primary culture, Drug sensitivity
1. Introduction
Colorectal cancer (CRC) is the third most frequent malignancy and the fourth leading cause of deaths from cancer worldwide [1]. Despite significant advances in treatments, CRC the second leading cause of cancer related death in the United States [2]. New and improved generation chemotherapeutic agents and molecular targeted agents for CRC have emerged in recent years [3–8, and reviewed in 9]. Although affected patients diagnosed with early stage disease have high cure rates, relapses (or recurrences) occur that result in a poor five-year survival rate in these cases. To improve treatment strategies and the prognosis for CRC patients, the development of personalized cancer therapies are necessary. Increasing evidences have shown that the responses of particular tumors to selected drugs can be predicted by the molecular signatures of the cancer [10,11]. This suggests that personalized and targeted treatments are likely to be developed in the future [12,13]. However, to achieve this goal, the effectiveness of the targeting agents needs to be tested first using a preclinical model. In this regard, there are currently no efficient preclinical evaluation systems for cancer drugs that could function as a routine test for individualized therapy regimens [14].
In vitro evaluation systems, such as immortalized cancer cell lines, have typically been used in the past for cancer therapeutics. Although in vitro immortalized cancer cell lines have the advantage of being robust and tractable, they have some significant and notable limitations, including the fact that their phenotypic and molecular characteristics are readily altered by environmental factors. One hypothesis in this regard is that cells change their characteristics to survive under culture conditions, and cells with a high capacity for survival adaptation will be selected during passage in culture, leading to a predominance of cells that exhibit very different characteristics from the original tumor [15,16].
To overcome these limitations of in vitro evaluation systems, patient-derived xenograft (PDX) models have been developed and used to test cancer therapeutics as they are suitable for the assessment of chemosensitivity and consequently for the selection of the most effective regimen for an individual cancer patient [17–26]. Fresh patient tumors have been directly inoculated into non-obese diabetic-severely combined immune deficient (NOD–SCID) mice so that they can be amplified into a larger tumor mass. Although this protocol is generally accepted as a very efficient in vivo system for preclinical trials of anti-cancer therapeutics that preserves patient integrity, it also has limitations when specimens are too small. Moreover, primary cells are not then available for further in vitro assessment. To address these limitations, we have developed a new PDX model as a preclinical in vivo evaluation system. We used stably stored primary cancer cells originating from colon cancer patients to produce a mouse xenograft. Pathological and genetic characterizations of the tumors from PDX mice and patients were performed. We found that the xenograft tumors and primary cells exhibited the same characteristics at both the morphological and genetic level as the originating patient tumors, indicating the feasibility of our method for in vitro and in vivo preclinical trials of new drugs. To our knowledge, this is the first description of a xenograft model established via stably stored primary cultures derived from a patient’s colon tumor.
2. Materials and methods
2.1. Patients and colon specimens
Experiments were performed after receiving patient informed consent and approval from the Institutional Review Board and Animal Care Committee of the Asan Medical Center. Human colon samples were excised from four patients during surgery and were obtained through the Asan Bio Resource Center. These tissues were fixed in cold 2% formaldehyde for 4 h and embedded in paraffin at 56 °C. The paraffin embedded (PE) blocks were cut at a 4 μm thickness, and these sections were stained with hematoxylin and eosin (H&E). Pathologic diagnoses and gradings were performed by two pathologists in accordance with the WHO colon cancer classification system [27].
2.2. Primary cell culture from human colon cancer tissue
Prior to formalin fixation of patient colon specimens, a small piece of tumor tissue was removed and cultured to establish primary cancer cell lines. Briefly, tumor tissues were minced with a scissor and subsequently digested using 1 mg/mL of type IV collagenase (Sigma Chemical Co., St. Louis, MO) in DMEM/F12 for 90 min at 37 °C. After incubation, tissues were washed with medium containing 10% fetal bovine serum (Sigma Chemical Co.,). We used two culture methods as follows: (A) to favor the adhesion and growth of epithelial tumor cells, Renal Epithelial cell Basal Media (REBM; Lonza, Walkersville, MD) containing human epidermal growth factor (hEGF), hydrocortisone, epinephrine, insulin, transferrin, GA-1000, FBS and a Triiodothyronine-Single Quots Kit were used to culture primary colon cancer cells plated on collagen type 1 dishes in a humidified incubator at 37 °C under a 5% CO2 atmosphere; and (B) in coated matrigel, we used based REBM (without FBS) with 1 X N2, 1 X B27, 10 μM Rock inhibitor and 10 μM gastrin as colon primary cell culture media. Successfully isolated and cultured cells were then harvested and stored in liquid nitrogen at each passage.
2.3. In vivo tumor generation in NOD–SCID mouse
All four primary cultured cell lines recovered from the liquid nitrogen stocks were implanted in 6–8 week-old NOD/SCID mice (Charles River Laboratories, Wilmington, MA). Briefly, 106 patient-derived primary cancer cells were suspended in 100 μl of Matrigel (BD Biosciences, San Jose, CA), and injected into the subcutaneous layer on the backs of NOD/SCID mice. After 2–3 months, when the tumor size reached above 1 cm3, the mice were anesthetized via an intra-peritoneal injection of a 40 mg/kg Zoletil (Virbac, Virbac laboratories BP 27–06511 Carros, France) – 5 mg/kg Rumpum (Bayer Korea, South Korea) mixture, and the tumors were surgically removed.
2.4. Assessment of morphology and immunophenotype
Morphology comparisons between the original and engrafted tumors were performed by two pathologists. On H&E staining, the pathologic type, differentiation grading, and architectures of the tumors were evaluated. For immunophenotypic comparisons between the original and xenograft tumors, immunohistochemical staining of formalin-fixed paraffin-embedded tissue sections was performed using an automated staining device (Benchmark XT; Ventana Medical Systems, Tucson, AZ). Briefly, 4 μm thick whole tissue sections were transferred onto poly-l-lysine coated adhesive slides and dried at 74 °C for 30 min. After epitope retrieval by heating for 1 h in ethylene diaminetetra-acetic acid (pH 8.0) in the autostainer, the samples were incubated with various antibodies including anti-cytokeratin 7 (CK7) (1:400 dilution; DAKO, Glostrup, Denmark) and anti-CK 20 (1:200 dilution; DAKO) as markers of the epithelium; anti-epidermal growth factor receptor (EGFR) (1:100 dilution; Zymed, Carlsbad, CA); anti-MLH1 (1:100 dilution, Novocastra, Newcastle, UK); anti-TP53 (1:1500 dilution, DAKO, Glostrup, Denmark); anti-MSH-2 (1:200 dilution, Pharmingen, NJ) and anti-carcinoembryonic antigen (CEA) (1:200; Novocastra, Newcastle, UK) as adenocarcinoma markers. The sections were subsequently incubated with secondary antibodies, and then visualized using an ultraView Universal DAB Detection kit (Ventana Medical Systems). Nuclei were counterstained with Harris hematoxylin.
2.5. Tumor genotyping by comparison of gene mutation profiles
DNA samples from original tumor tissues, primary cancer cells, and xenograft tumors were obtained from paraffin embedded blocks after a histopathology review. Cancer gene mutation profiling was performed using the “OncoMap system”, a mass spectrometry based genotyping panel. OncoMap includes 440 sites in 41 tumor-related genes, such as KRAS, TP53, STK11, EGFR, and BRAF [28] and is used at the Asan Medical Center for tumor genotype determination.
3. Results
3.1. Colon cancer patient profiles
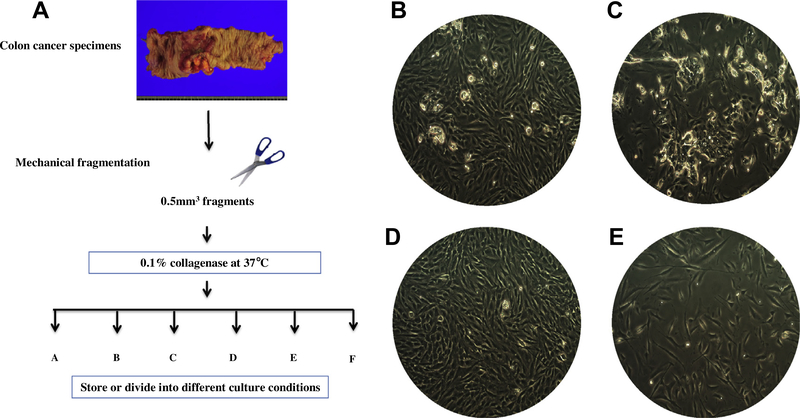
To establish colon primary cultures from tumors, tissue biopsies were treated as shown in Fig. 1A. We have now established 199 primary tumor cultures from 276 patients and representative images of four of these cultures are shown in Fig. 1B–E. The profiles of the four patients (three males) with the corresponding original tumors are summarized in Table 1. We further analyzed the four tumors as these four gave successful and faster xenografts. The ages of these patients ranged from 23 to 79. Pathologically two out of the three male tumors were adenocarcinomas, whereas the remaining male and the female tumor were mucinous adenocarcinomas. Histologically, three out of these four tumors were positive for p53 or MSH2 and all were positive for the known markers of colon cancer, MLH1 and CEA (carcinoembryonic antigen).
Fig. 1.
Establishment of primary cultures from colon tumors with a high success rate. (A) Schematic view of primary colon cultures from patient specimens. Fresh tumor specimens was minced with scissors and subsequently digested with collagenase. Primary cells were cultured under the conditions shown in Table 3. (B–E) Representative images of four primary cultures from colon tumors are shown. (B–E) Cases 1–4, respectively. More information is listed in Table 1. These cells were cultured in REBM (see the Materials and Methods) for 10 days with 3 passages. Pictures were taken at a 100× original magnification.
Table 1:
Patient information.
| Case no. | Diagnosis | Sex | Age | IHC |
|---|---|---|---|---|
| Case 1 | Mucinous adenocarcinoma | M | 79 | MLH1: positive MSH2: positive p53: positive (+++) CEA: Diffuse cytoplasmic (++) |
| Case 2 | Adenocarcinoma, MD | M | 48 | MLH1: positive MSH2: positive p53: Negative CEA: Diffuse cytoplasmic (+++) |
| Case 3 | Mucinous adenocarcinoma | F | 23 | MLH1: positive MSH2: Negative p53: positive CEA: Diffuse cytoplasmic (+++) |
| Case 4 | Adenocarcinoma, MD | M | 51 | MLH1: positive MSH2: positive p53: positive (+++) CEA: Apicoluminal (++) |
3.2. Comparative histological analysis of original tumors and mouse xenografts
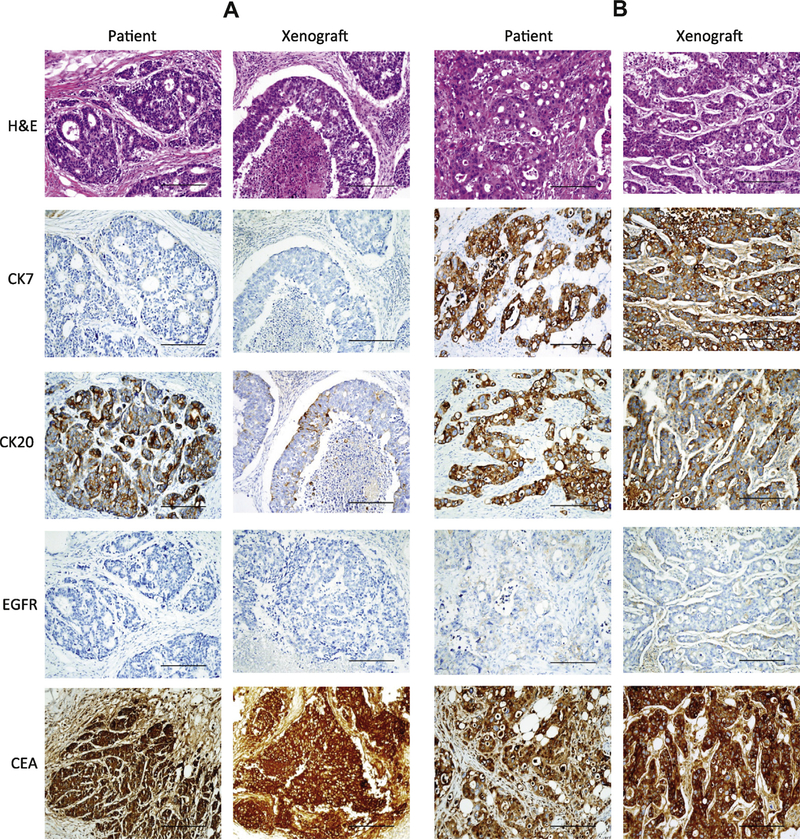
Out of our panel of 199 primary colon cancer cultures, we tested 50 as xenografts and eight xenografts were successfully established. In these eight cases, we observed tumors at 3–4 months after the injection of 106 cells into NOD–SCID mice. Histological analysis was performed to examine whether these xenografts retained the characteristics of its original tumor. The tumor tissues were probed with antibodies against CK7, CK20, CEA and EGFR to assess their histological similarity with the primary tumor. Fig. 2 shows representative pictures of the four primary tumors and corresponding xenografts. H&E staining revealed similarities in terms of the basic cellularity and morphology of these pairs. In case 1, low levels of CK7 and EGFR were detected in both the primary tumor and xenograft, whereas the CK20 level was decreased in xenograft. This pattern was also observed in cases 3 and 4, but the CK20 level was comparable in both tumors. In contrast, case 2 showed strong CK7, CK20 and CEA signals in both of the primary tumors and xenografts. These results demonstrate that our primary culture derived xenografts faithfully reproduce the original human colon tumors from which they were derived.
Fig. 2.
Histological analysis of colon tumors from the original patients and derived mouse xenografts. The histology of representative primary tumors (Patient) and corresponding xenograft tumors (Xenograft) was determined using H&E staining. (A–D) Cases 1–4, respectively. In addition to H&E staining, immunohistological staining was performed using antibodies against CK7, CK20, EGFR5 and CEA. Pictures were taken at a 200× original magnification; scale bar, 200 μm.
3.3. Mutation profile analysis of primary tumors and corresponding xenografts
Based on our histological results, we evaluated whether our xenografts retained the molecular characteristics of the original tumor. We used the OncoMap system that analyzes 460 mutations in 41 cancer genes (Supplementary Table 1). Surprisingly, we found from this mutation analysis with OncoMap that our xenografts retained or even harbored enriched major mutations of the primary tumor in all cases (Table 2). In contrast, the corresponding primary cultures contained either a portion only of the major mutations or none of the mutations of the primary colon tumor. For example, the R175H mutation of Tp53 in case 1 was present in 50% of the original tumor, was substantially reduced in frequency in the primary culture (10.1%), but was evident in 100% of cells in the xenograft. This suggests culturing in conditioned media triggers a considerable amount of cancer cell selection.
Table 2:
Mutation analysis of primary colon tumors, cultures and xenografts using OncoMap.
| Case no. | Sample description | APC_T1556 fs*3_c. 112203856insA_h | BRAF_V600E_V600A_c. 140099605A>G>T_h | BRAF_V600K_605_606delinsNT_h | TP53_R306*_c. 7517747G>A_h | TP53_R175H_c. 7519131C>T_h | TP53_R273H_c. 7517845C>T_h | KRAS_G12D_c. 25289551C>T_h |
|---|---|---|---|---|---|---|---|---|
| Case 1 | PT | wt | wt | wt | wt | TC (51) | wt | wt |
| PCC – A | wt | wt | wt | wt | TC (10.1) | wt | wt | |
| XT | wt | wt | wt | wt | T (100) | wt | wt | |
| Case 2 | PT | DEL.A (24) | AT (35) | TT.AC (32) | GA (34) | wt | wt | wt |
| PCC – A | DEL | AT (2.7) | AC | GA (5) | wt | wt | wt | |
| XT | A (97) | AT (74) | TT.AC (74) | A (100) | wt | wt | wt | |
| Case 3 | PT | DEL.A (24) | wt | wt | wt | wt | wt | TC (29) |
| PCC – A | DEL | wt | wt | wt | wt | wt | TC (5.8) | |
| XT | DEL.A (45) | wt | wt | wt | wt | wt | TC (48) | |
| Case 4 | PT | wt | wt | wt | wt | wt | TC (39) | TC (42) |
| PCC – A | wt | wt | wt | wt | wt | TC (38) | TC (43) | |
| XT | wt | wt | wt | wt | wt | TC (46) | TC (59) |
PT, patient tissue; PCC, primary cancer cells; XT, xenograft tumor.
3.4. Optimization of primary culture conditions
Even though our xenografts showed a histology and molecular profile that were similar to the original tumor, our data suggest that the primary cultures do not retain these characteristics. To identify the optimal conditions for primary cultures so that the major mutations of their primary tumors are preserved, we tested six different culture conditions (denoted A to F; Table 3). The growth media used for these different protocols are summarized below (culture condition A was used for the experiment shown in Figs. 1 and 2 and Table 2):
The addition of 0.5% fetal bovine serum (FBS) or supplement including 1 × N2, 1 × B27, 5 ng/μl Rock inhibitor and gastrin (N2, B27, Rock inhibitor and gastrin constitute the basic growth medium for cancer stem cell cultures).
Coating of culture dishes with collagen isolated from rat-tail (C, D) or matrigel (E, F).
Table 3:
Culture conditions tested for primary colon cancer cells.
| Culture type | Media | Supplement | Culture dish |
|---|---|---|---|
| A | REBM | Basal media | Collagen coated dish by BD |
| B | (−FBS) | ||
| C | (−FBS) 1 × N2 1 × B27 +Rock inhibitor +Gastrin |
Isolated collagen coated dish from rat tail | |
| D | (−FBS) | ||
| E | −FBS 1 × N2 1 × B27 +Rock inhibitor +Gastrin |
Matrigel by BD | |
| F | (−FBS) |
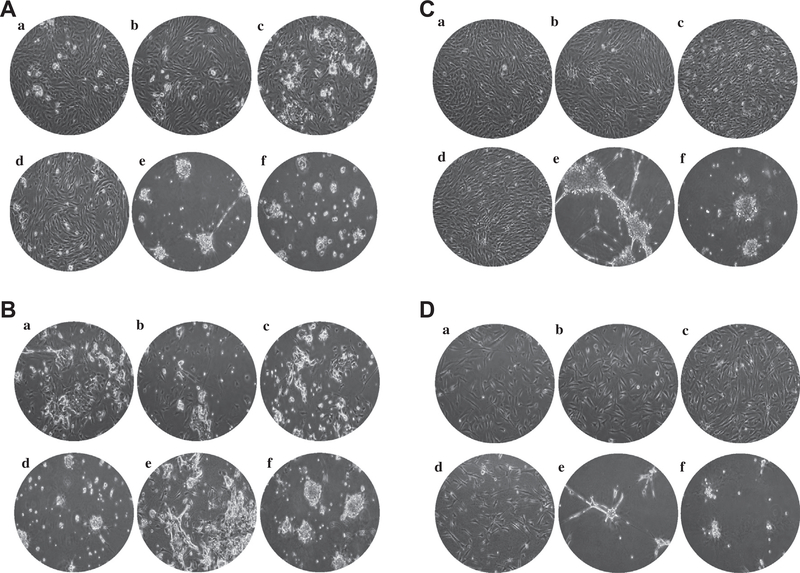
We observed superior, two-dimensional cell attachment for conditions A–D (with an exception in case 2, that showed a different cell morphology), as shown in Fig. 3. In contrast, cultures grown on matrigel (E and F) produced spiky or ball like structures, which were quite distinct from the attached cells obtained under conditions A–D. It appears from this that the matrigel supports anchorage independent cell growth, which can generate three-dimensional structures. Interestingly, we found that condition E was optimal for primary culture retention of the major mutations found in the primary tumor (Table 4). For example, the R175H mutation of Tp53 in case 1 was present in 51% of cells in primary tumor but decreased to 10.1% of the primary culture population (Table 4). Under culture condition E however, this mutation was maintained at 30.1% in primary culture populations. This suggested that anchorage independent cultures with supplements including N2, B27 and gastrin might be the optimal conditions to grow primary tumor cells that retain original tumor molecular characteristics.
Fig. 3.
Morphological differences between primary cultures established under various conditions. To optimize the culture conditions to retain the major mutations found in primary cultures, 6 combinations (a–f) of culture matrix and media supplements were tested. (A–D) Cases 1–4, respectively. Each of primary colon culture was cultured for 7 days. Pictures were taken at a 100× original magnification.
Table 4:
Mutation analysis of primary colon cancer cells cultured under different conditions.
| Case no. | Sample description | APC_T1556 fs*3_c.112203856insA_h | BRAF_V600E_V600A_c. 140099605A>G>T_h | BRAF_V600K_605_606delinsNT_h | TP53_R306*_c. 7517747G>A_h | TP53_R175H_c. 7519131C>T_h | TP53_R273H_c. 7517845C>T_h | KRAS_G12D_c. 25289551C>T_h |
|---|---|---|---|---|---|---|---|---|
| Case 1 | PT | wt | wt | wt | wt | TC (51) | wt | wt |
| PCC – A | wt | wt | wt | wt | TC (10.1) | wt | wt | |
| PCC – B | wt | wt | wt | wt | TC(13.2) | wt | wt | |
| PCC – C | wt | wt | wt | wt | TC(23.4) | wt | wt | |
| PCC – D | wt | wt | wt | wt | TC(7.5) | wt | wt | |
| PCC – E | wt | wt | wt | wt | TC(30.1) | wt | wt | |
| PCC – F | wt | wt | wt | wt | TC(24.4) | wt | wt | |
| XT | wt | wt | wt | wt | T (100) | wt | wt | |
| Case 2 | PT | DEL.A (24) | AT (35) | TT.AC (32) | GA (34) | wt | wt | wt |
| PCC – A | DEL | AT (2.7) | AC | GA (5) | wt | wt | wt | |
| PCC – B | DEL | A | AC | GA(4.2) | wt | wt | wt | |
| PCC – C | DEL | A | AC | GA(2.1) | wt | wt | wt | |
| PCC – D | DEL | A | AC | GA(3.7) | wt | wt | wt | |
| PCC – E | DEL.A(9.9) | AT(11.9) | N.D. | GA(13.5) | wt | wt | wt | |
| PCC – F | DEL.A(7.6) | AT(11.1) | AC | GA(8.0) | wt | wt | wt | |
| XT | A (97) | AT (74) | TT.AC (74) | A (100) | wt | wt | wt | |
| Case 3 | PT | DEL.A (24) | wt | wt | wt | wt | wt | TC (29) |
| PCC – A | DEL | wt | wt | wt | wt | wt | TC (5.8) | |
| PCC – B | DEL.A(5.1) | wt | wt | wt | wt | wt | TC(4.3) | |
| PCC – C | DEL.A(10.2) | wt | wt | wt | wt | wt | TC(12.1) | |
| PCC – D | DEL | wt | wt | wt | wt | wt | TC(2.6) | |
| PCC – E | DEL.A(21.2) | wt | wt | wt | wt | wt | TC(18.5) | |
| PCC – F | DEL.A(12.6) | wt | wt | wt | wt | wt | TC(10.7) | |
| XT | DEL.A (45) | wt | wt | wt | wt | wt | TC (48) | |
| Case 4 | PT | wt | wt | wt | wt | wt | TC (39) | TC (42) |
| PCC – A | wt | wt | wt | wt | wt | TC (38) | TC (43) | |
| PCC – B | wt | wt | wt | wt | wt | TC(21.6) | wt | |
| PCC – C | wt | wt | wt | wt | wt | TC(34.5) | wt | |
| PCC – D | wt | wt | wt | wt | wt | TC(35.4) | wt | |
| PCC – E | wt | wt | wt | wt | wt | TC(42.1) | wt | |
| PCC – F | wt | wt | wt | wt | wt | TC(44.2) | wt | |
| XT | wt | wt | wt | wt | wt | TC (46) | TC (59) |
PT, patient tissue; PCC, primary cancer cells; XT, xenograft tumor; N.D., not detected.
3.5. Drug sensitivity assay of primary cultures
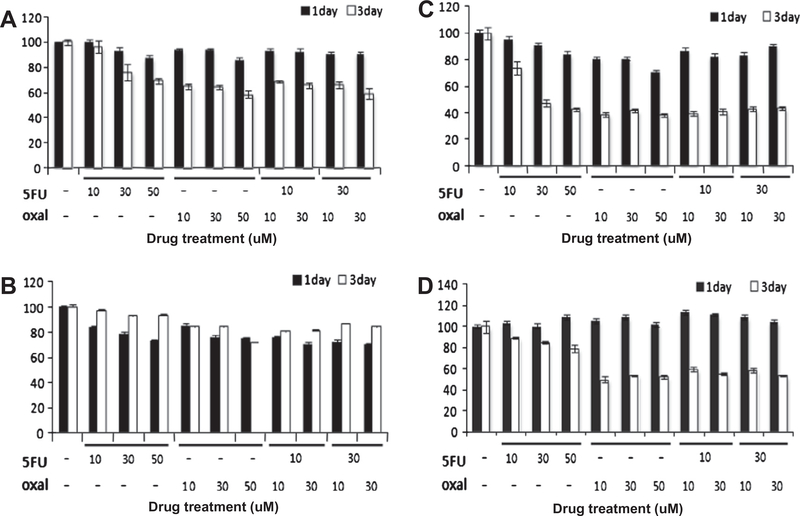
We next investigated the feasibility of using established primary cultures in personalized therapeutic strategies. To this goal, we questioned whether the stably stored primary culture reflects the chemosensitivity of the patients where the cultures were derived from. The four primary cultures assessed in our analysis were treated with two cancer drugs and cell proliferation was measured by MTT assay. The results are shown in Fig. 4. In the case 3, where the patients responded to 5-FU well (panel C), we found the primary culture also showed hypersensitivity to 5-FU. In contrast, for the case 2 where the patient responded to a combinatory chemotherapy (domperidon tab, 10 mg; cozaar tab, 50 mg; and phazyme tab, 95 mg, panel B) did not show such sensitivity to 5-FU, suggesting that the primary culture can reflect selective chemosensitivity of the corresponding patient. Interestingly, for the case 1 (panel A, no chemotherapy), case 3 (panel C, chemotherapy with 5-FU) and case 4 (panel D, chemotherapy with 5-FU and Eloxactin), we found better anti-proliferative effect of oxaliplatin than 5-FU, suggesting this drug might have therapeutic potential to these patients.
Fig. 4.
Drug sensitivity assay of the primary cultures revealing possible alternative therapeutic options. (A–D) Cases 1–4, respectively. Each of the primary cultures was treated with either 5-FU or oxaliplatin or a combination of both at various doses as indicated on the x-axis. The cell proliferation rate was measured by MTT assay at one or 3 days after drug treatment.
4. Discussion
The development of a PDX model provides better tool for personalized cancer research [29–34] than the well-known cancer cell lines that have been widely used. This is because PDX cells often retain the cellular heterogeneity and molecular characteristics of the primary cancer. With these advantages, the use of a PDX system to screen a number of cancer drugs offers an attractive preclinical study model. The direct transplantation of colon tumor tissues into immunocompromised mice has been performed previously with a couple of limitations [35,36]. First, bacterial contamination of the colon cancer xenograft, often results in septic shock in the recipient mouse. Second, substantial amounts of tissue are needed and any transplantation failures will lead to the loss of primary tissue that is impossible to regenerate or replace. Hence, the generation of xenografts using cultured primary cells is desirable. However, as primary cultures can be passaged for only a short time, a serial xenograft is required to maintain the human primary tumor.
We established a PDX model of colon cancer using frozen stocks of primary tumor cultures. We achieved a 70% success rate in establishing these primary cultures. Although the subsequent PDX success rate was only 20%, the four primary cultures we tested in this study produced tumors under mouse xenograft conditions that conserved the histological features of the primary tumor. As the primary cultures were successfully maintained and expanded, we had sufficient material to use as the need arose. Moreover, the Oncomap analysis revealed that the primary tumor mutation profiles were retained or even enriched in all of the xenografts, providing molecular evidence for the suitability of our strategy. In contrast, when the same analysis was done with the primary cultures, 3 out of 4 showed a reduction or loss of primary tumor mutations. This finding suggests that the tumorigenic sub-population of cells was substantially lost during primary culturing and then enriched by unknown selective pressure during in vivo xenograft growth. Hence, we evaluated several culture conditions to optimize the retention of tumor characteristics in primary explants and found that matrigel and culture supplements including N2, B27, Rock inhibitor and gastrin enabled these primary cultures to better retain the major mutations of the original tumor. However, we found also that this culture condition is not suitable for a drug sensitivity assay because it is too expensive and technically difficult for routine use.
The drug sensitivity assay shown in Fig. 4 provides two useful insights. The first is that oxaliplatin had better anti-proliferative activity than 5-FU, suggesting its use as an alternative therapeutic option. The second however was that, the combination of 5-FU and oxaliplatin did not show any synergistic effect. This implies that these two drugs affect the same signaling pathway or related targets. Future studies in this regard will need to focus on the screening of other cancer drugs that might exert synergistic effects [37,38] with preexisting therapeutic agents.
In summary, we demonstrated here an effective method of reproducing human primary colon tumors through stably stored primary cultures and mouse xenograft model. The usefulness of the stable storage of the primary culture would be in two folds. First, there is no need to keep the xenograft that is costly and labor intensive as our data showed stably stored primary culture retain unaltered histological and molecular characteristics. Second, when there is a new drug appear, the stored culture (in addition to the fresh primary culture available from other patients) can be used to examine the feasibility of new drug for each case of patients and figure out which patient can benefit from the drug. This strategy will be a good example of a personalized medicine.
Supplementary Material
Acknowledgements
This study was supported by a grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (A062254), the National Research Foundation of Korea Grant funded by the Korean Government (Ministry of Education, Science and Technology, [NRF-355-2010-1-C00071]), and a grant of the Leading Foreign Research Institute Recruitment Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (MEST) (2011-0030105).
Footnotes
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A. Supplementary material
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.canlet.2013.11.010.
References
- [1].Ferlay J, Parkin DM, Steliarova-Foucher E, Estimates of cancer incidence and mortality in Europe, Eur. J. Cancer 46 (2010) (2008) 765–781. [DOI] [PubMed] [Google Scholar]
- [2].American Cancer Society, Colorectal Cancer Facts & Figures 2011–2013, American Cancer Society, Atlanta, GA, 2011. [Google Scholar]
- [3].Bustin SA, Murphy J, RNA biomarkers in colorectal cancer, Methods 59 (2013) 116–125. [DOI] [PubMed] [Google Scholar]
- [4].Chung HH, Jang BI, A perspective: role of targeted therapy in colon cancer, Korean J. Gastroenterol 61 (2013) 128–135. [DOI] [PubMed] [Google Scholar]
- [5].Francescangeli F, Patrizii M, Signore M, Federici G, Di Franco S, Pagliuca A, Baiocchi M, Biffoni M, Ricci Vitiani L, Todaro M, De Maria R, Zeuner A, Proliferation state and polo-like kinase1 dependence of tumorigenic colon cancer cells, Stem Cells 30 (2012) 1819–1830. [DOI] [PubMed] [Google Scholar]
- [6].Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. , Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer, N. Eng. J. Med 350 (2004) 2335–2342. [DOI] [PubMed] [Google Scholar]
- [7].Van Cutsem E, Köhne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, D’Haens G, et al. , Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer, N. Engl. J. Med. 360 (2009) 1408–1417. [DOI] [PubMed] [Google Scholar]
- [8].Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, Humblet Y, et al. , Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study, J. Clin. Oncol 28 (2010) 4697–4705. [DOI] [PubMed] [Google Scholar]
- [9].Hagan S, Orr MC, Doyle B, Targeted therapies in colorectal cancer-an integrative view by PPPM, EPMA J. 4 (2013) 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Dotan E, Goldstein LJ, Optimizing chemotherapy regimens for patients with early-stage breast cancer, Clin. Breast Cancer 10 (Suppl 1) (2010) E8–15. [DOI] [PubMed] [Google Scholar]
- [11].Johnson KA, Brown PH, Drug development for cancer chemoprevention: focus on molecular targets, Semin. Oncol 37 (2010) 345–358. [DOI] [PubMed] [Google Scholar]
- [12].Azzoli CG, Park BJ, Pao W, Zakowski M, Kris MG, Molecularly tailored adjuvant chemotherapy for resected non-small cell lung cancer: a time for excitement and equipoise, J. Thoracic Oncol 3 (2008) 84–93. [DOI] [PubMed] [Google Scholar]
- [13].Hao Y, Wang C, Cao B, Hirsch BM, Song J, Markowitz SD, Ewing RM, Sedwick D, Liu L, Zheng W, Wang Z, Gain of interaction with IRS1 by p110α-helical domain mutants is crucial for their oncogenic functions, Cancer Cell 23 (2013) 583–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Herbst RS, Heymach JV, Lippman SM, Lung cancer, N. Engl. J. Med 359 (2008) 1367–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].De Witt Hamer PC, Van Tilborg AA, Eijk PP, Sminia P, Troost D, Van Noorden CJ, Ylstra B, Leenstra S, The genomic profile of human malignant glioma is altered early in primary cell culture and preserved in spheroids, Oncogene 27 (2008) 2091–2096. [DOI] [PubMed] [Google Scholar]
- [16].Pandita A, Aldape KD, Zadeh G, Guha A, James CD, Contrasting in vivo and in vitro fates of glioblastoma cell subpopulations with amplified EGFR, Genes Chromosomes Cancer 39 (2004) 29–36. [DOI] [PubMed] [Google Scholar]
- [17].Jass JR, Classification of colorectal cancer based on correlation of clinical, morphological and molecular features, Histopathology 50 (2007) 113–130. [DOI] [PubMed] [Google Scholar]
- [18].John T, Kohler D, Pintilie M, Yanagawa N, Pham NA, Li M, Panchal D, Hui F, Meng F, Shepherd FA, Tsao MS, The ability to form primary tumor xenografts is predictive of increased risk of disease recurrence in early-stage non-small cell lung cancer, Clin. Cancer Res.: Official J. Am. Assoc. Cancer Res 17 (2011) 134–141. [DOI] [PubMed] [Google Scholar]
- [19].Julien S, Merino-Trigo A, Lacroix L, Pocard M, Goere D, Mariani P, Landron S, Bigot L, Nemati F, Dartigues P, Weiswald LB, Lantuas D, Morgand L, Pham E, Gonin P, Dangles-Marie V, Job B, Dessen P, Bruno A, Pierre A, De The H, Soliman H, Nunes M, Lardier G, Calvet L, Demers B, Prevost G, Vrignaud P, Roman-Roman S, Duchamp O, Berthet C, Characterization of a large panel of patient-derived tumor xenografts representing the clinical heterogeneity of human colorectal cancer, Clin. Cancer Res 18 (2012) 5314–5328. [DOI] [PubMed] [Google Scholar]
- [20].Jin K, Lan H, Cao F, Han N, Xu Z, Li G, He K, Teng L, Differential response to EGFR- and VEGF-targeted therapies in patient-derived tumor tissue xenograft models of colon carcinoma and related metastases, Int. J. Oncol 41 (2012) 583–588. [DOI] [PubMed] [Google Scholar]
- [21].Dong X, Jin K, Hu X, Du F, Lan H, Han N, Ma Z, Xie B, Cui B, Teng L, Cao F, Antitumor effect of FP3 in combination with cetuximab on patient-derived tumor tissue xenograft models of primary colon carcinoma and related lymphatic and hepatic metastases, Int. J. Mol. Med 30 (2012) 126–132. [DOI] [PubMed] [Google Scholar]
- [22].Siolas D, Hannon GJ, Patient-derived tumor xenografts: transforming clinical samples into mouse models, Cancer Res. 73 (2013) 5315–5319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Dong X, Guan J, English JC, Flint J, Yee J, Evans K, Murray N, Macaulay C, Ng RT, Gout PW, Lam WL, Laskin J, Ling V, Lam S, Wang Y, Patient-derived first generation xenografts of non-small cell lung cancers: promising tools for predicting drug responses for personalized chemotherapy, Clin. Cancer Res 16 (2010) 1442–1451. [DOI] [PubMed] [Google Scholar]
- [24].Laurent C, Gentien D, Piperno-Neumann S, Némati F, Nicolas A, Tesson B, Desjardins L, Mariani P, Rapinat A, Sastre-Garau X, Couturier J, Hupé P, de Koning L, Dubois T, Roman-Roman S, Stern MH, Barillot E, Harbour JW, Saule S, Decaudin D, Patient-derived xenografts recapitulate molecular features of human uveal melanomas, Mol. Oncol 7 (2013) 625–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kopetz S, Lemos R, Powis G, The promise of patient-derived xenografts: the best laid plans of mice and men, Clin. Cancer Res 18 (2012) 5160–5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Chaudary N, Pintilie M, Schwock J, Dhani N, Clarke B, Milosevic M, Fyles A, Hill RP, Characterization of the tumor-microenvironment in patient-derived cervix xenografts (OCICx), Cancers (Basel) 4 (2012) 821–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].John T, Yanagawa N, Kohler D, Craddock KJ, Bandarchi-Chamkhaleh B, Pintilie M, Sykes J, To C, Li M, Panchal D, Chen W, Shepherd FA, Tsao MS, Characterization of lymphomas developing in immunodeficient mice implanted with primary human non-small cell lung cancer, J. Thoracic Oncol 7 (2012) 1101–1108. [DOI] [PubMed] [Google Scholar]
- [28].MacConaill LE, Campbell CD, Kehoe SM, Bass AJ, Hatton C, Niu L, Davis M, Yao K, Hanna M, Mondal C, Luongo L, Emery CM, Baker AC, Philips J, Goff DJ, Fiorentino M, Rubin MA, Polyak K, Chan J, Wang Y, Fletcher JA, Santagata S, Corso G, Roviello F, Shivdasani R, Kieran MW, Ligon KL, Stiles CD, Hahn WC, Meyerson ML, Garraway LA, Profiling critical cancer gene mutations in clinical tumor samples, PLoS ONE 4 (2009) e7887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zhang XC, Zhang J, Li M, Huang XS, Yang XN, et al. , Establishment of patient-derived non-small cell lung cancer xenograft models with genetic aberrations within EGFR, KRAS and FGFR1: useful tools for preclinical studies of targeted therapies, J. Transl. Med 11 (2013) 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Landis MD, Lehmann BD, Pietenpol JA, Chang JC, Patient-derived breast tumor xenografts facilitating personalized cancer therapy, Breast Cancer Res. 15 (2013) 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Seol HS, Suh YA, Ryu YJ, Kim HJ, Chun SM, et al. , A patient-derived xenograft mouse model generated from primary cultured cells recapitulates patient tumors phenotypically and genetically, J. Cancer Res. Clin. Oncol 139 (2013) 1471–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Bertotti A, Migliardi G, Galimi F, Sassi F, Torti D, Isella C, Corà D, et al. , A molecularly annotated platform of patient-derived xenografts (“xenopatients”) identifies HER2 as an effective therapeutic target in cetuximab-resistant colorectal cancer, Cancer Discov. 1 (2011) 508–523. [DOI] [PubMed] [Google Scholar]
- [33].Krumbach R, Schüler J, Hofmann M, Giesemann T, Fiebig H-H, Beckers T, Primary resistance to cetuximab in a panel of patient-derived tumour xenograft models: activation of MET as one mechanism for drug resistance, Eur. J. Cancer 47 (2011) 1231–1243. [DOI] [PubMed] [Google Scholar]
- [34].Moro M, Bertolini G, Tortoreto M, Pastorino U, Sozzi G, Roz L, Patient-derived xenografts of non-small cell lung cancer: resurgence of an old model for investigation of modern concepts of tailored therapy and cancer stem cells, J. Biomed. Biotechnol (2012) 567–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Fu Y, Besterman JM, Monosov A, Hoffman RM, Models of human metastatic colon cancer in nude mice orthotopically constructed by using histologically intact patient specimens, Proc. Nat. Acad. Sci. USA 88 (1991) 9345–9349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Thalheimer A, Illert B, Bueter M, Gattenlohner S, Stehle D, Gasser M, Thiede A, Waaga-Gasser AM, Meyer D, Feasibility and limits of an orthotopic human colon cancer model in nude mice, Comp. Med 56 (2006) 105–109. [PubMed] [Google Scholar]
- [37].Adiseshaiah PP, Clogston JD, McLeland CB, Rodriguez J, Potter TM, et al. , Synergistic combination therapy with nanoliposomal C6-ceramide and vinblastine is associated with autophagy dysfunction in hepatocarcinoma and colorectal cancer models, Cancer Lett. 337 (2013) 254–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Flis S, Spłwiński J, Inhibitory effects of 5-fluorouracil and oxaliplatin on human colorectal cancer cell survival are synergistically enhanced by sulindac sulfide, Anticancer Res. 29 (2009) 435–441. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.