Abstract
Aims
Dementia is a major global challenge for health and social care in aging populations. A third of all dementia may be preventable due to cardiovascular risk factors. Intensive multi-domain intervention trials targeting primarily cardiovascular risk factors show improved cognitive function in people at risk. Such interventions will, however, be expensive to implement in all individuals at risk and will represent unrealistic economic tasks for most societies. Therefore, a risk score identifying high-risk individuals is warranted.
Methods and results
In 61 664 individuals from two prospective cohorts of the Danish general population, we generated 10-year absolute risk scores for all-cause dementia from cardiovascular risk factors and genetics. In both sexes, 10-year absolute risk of all-cause dementia increased with increasing age, number of apolipoprotein E (APOE) ɛ4 alleles, number of genome-wide association studies (GWAS) risk alleles, and cardiovascular risk factors. The highest 10-year absolute risks of all-cause dementia seen in smoking women with diabetes, low education, APOE ɛ44 genotype, and 22–31 GWAS risk alleles were 6%, 23%, 48%, and 66% in those aged 50–59, 60–69, 70–79, and 80–100, respectively. Corresponding values for men were 5%, 19%, 42%, and 60%, respectively.
Conclusion
Ten-year absolute risk of all-cause dementia increased with age, APOE ɛ4 alleles, GWAS risk alleles, diabetes, low education, and smoking in both women and men. Ten-year absolute risk charts for dementia will facilitate identification of high-risk individuals, those who likely will benefit the most from an early intervention against cardiovascular risk factors.
Keywords: Cardiovascular risk factors, Dementia, Genetics, Epidemiology
Graphical Abstract
Graphical Abstract.
See page 4034 for the editorial comment on this article (doi: 10.1093/eurheartj/ehaa691)
Introduction
Due to the successes of intervention and prevention in atherosclerotic cardiovascular disease and other common diseases, people now live long enough to develop highly age-dependent dementia disorders. Therefore, dementia is a major global challenge for health and social care in aging populations. A third of old people now die with dementia, and worldwide incidence numbers are projected to be higher than 130 million by 2050.1 There are no available curative treatments. However, recent estimates from the Lancet Commission based on randomized controlled trials like FINGER2 , 3 and preDIVA4 suggest that a third of all dementia may be preventable,1 primarily by treating well-established cardiovascular risk factors such as diabetes, hypertension, smoking, and physical inactivity.5 A key recommendation is to be ambitious about prevention, focusing on interventions to build up resilience and healthier lifestyles,1 because postponing dementia just for a couple of years would enable many more to reach the end of life without developing dementia.1 , 6 The exact nature of prevention and whether it should be applied to all at risk of dementia, or targeted towards high-risk groups, remains unresolved.
Despite drastic increases in dementia prevalence globally, age-standardized incidences are declining in affluent parts of the world,1 most likely explained by better control of cardiovascular risk factors and by a general improvement in educational levels during the last decades.1 , 7 , 8 These findings are supported by a comprehensive intervention trial targeting primarily vascular risk factors leading to improved cognitive function in people at risk of dementia.2 , 3 However, such intensive and staff-requiring interventions will be expensive to implement in all at risk of dementia and will represent unrealistic economic tasks for most societies. Therefore, a combined risk score that can identify high-risk individuals who likely will benefit the most from targeted preventive interventions is warranted. Because the genetic contribution to late-onset dementia is substantial through the apolipoprotein E (APOE) ɛ4 allele,1 and 30 other loci identified more recently in genome-wide association studies (GWAS),9 , 10 a combined risk score should consist of these genetic components as well as the most important modifiable risk factors, in order to identify those at the very highest risk.
In the present study, we generated 10-year absolute risk scores for all-cause dementia combining cardiovascular risk factors and genetics. These algorithms were constructed in 61 664 individuals aged 20–100 from two prospective cohorts of the Danish general population, the Copenhagen General Population Study (CGPS) and the Copenhagen City Heart Study (CCHS) and may serve as tools for comprehensive risk stratification to identify high-risk individuals for targeted prevention.
Methods
The studies were approved by institutional review boards and Danish ethics committees [no (KF) 100.2039/91 and no (KF) 01-144/01] and were conducted according to the Declaration of Helsinki. Written informed consent was obtained from all individuals. Individuals in both studies were white and of Danish descent.
Participants
Copenhagen General Population Study is a prospective study of the Danish general population initiated in 2003 and still recruiting.11–14 Individuals were selected randomly based on the national Danish Civil Registration System to reflect the adult Danish population aged 20–100. Data were obtained from a self-administered questionnaire reviewed together with an investigator at the day of attendance, a physical examination, and from blood samples including DNA extraction. Genotypes were available on 53 546 individuals aged 20–100. Copenhagen City Heart Study is a prospective study of the Danish general population initiated in 1976–78 with follow-up examinations in 1981–83, 1991–94, 2001–03, and 2011–13.11–14 Individuals were recruited and examined as in the CGPS. Genotypes were available on 8118 individuals aged 20–100 from the 1991–94 and 2001–03 examinations. Combining the two studies yielded a total of 61 664 individuals, of whom 2158 developed dementia during a median follow-up of 10 years (range = 1–25 years). No individuals were lost to follow-up. Follow-up began at the time of blood sampling (2003 and onwards for CGPS and 1991–1994 or 2001–2003 for CCHS) and ended at occurrence of a dementia event (n = 2158), death (n = 8788), emigration (n = 334), or on 22 March 2017 (last update of the registry), whichever came first.
Dementia endpoints
In CGPS and CCHS, information on births, deaths, emigrations, and immigrations was collected from the national Danish Civil Registration System. Information on diagnoses of dementia was drawn from the national Danish Patient Registry and the national Danish Registry of Causes of Death. The national Danish Registry of Causes of Death contains data on causes of all deaths in Denmark, as reported by hospitals, forensic medicine, and general practitioners. Alzheimer’s disease was International Classification of Diseases (ICD)8 code 290.10 and ICD10 codes F00 and G30. All-cause dementia further included vascular dementia (ICD10 F01) and unspecified dementia (ICD8 290.18; ICD10 F03).
Genotyping
TaqMan-based (Life Technologies, a part of Thermo Fisher Scientific, Waltham, Massachusetts, USA) or KASP technology-based assays (LGC Genomics, Hoddesdon, Herts, UK) were used to genotype for APOE genotypes p. Cys130Arg (rs429358, legacy name Cys112Arg, c.388T>C) and p. Arg176Cys (rs7412, legacy name Arg158Cys, c.526C>T). The presence of p. Cys130Arg and the absence of p. Arg176Cys on the same allele defines the ε4 allele, whereas the ε2 allele is defined by the absence of p. Cys130Arg and the presence of p. Arg176Cys on the same allele.13 , 15 The rare ε1 allele is defined by the presence of both variants on the same allele. Standard genotyping methods without phasing cannot determine which allele a variant is located on. Consequently, the identified ε4/ε2 individuals can either have the ε1/ε3 or the ε4/ε2 combination. Due to the rarity of the ε1 allele, we assume that most individuals will have the ε4/ε2 combination. TaqMan-based or KASP technology-based assays were also used to genotype for GWAS hits of CR1 rs6656401, BIN1 rs6733839, CD2AP rs10948363, EPHA1 rs11771145, CLU rs9331896, MS4A6A rs983392, PICALM rs10792832, ABCA7 rs4147929, HLA-DRB5-HLA-DRB1 rs9271192, PTK2B rs28834970, SORL1 rs11218343, RIN3 rs10498633, INPP5D rs35349669, MEF2C rs190982, NME8 rs2718058, ZCWPW1 rs1476679, CELF1 rs10838725, FERMT2 rs17125944, and CASS4 rs7274581.9
Cardiovascular risk factors
Cardiovascular risk factors were registered at baseline. Diabetes mellitus was self-reported disease, use of insulin or oral hypoglycaemic agents, non-fasting plasma glucose levels of more than 11 mmol/L (198 mg/dL) and/or a diagnosis of diabetes at baseline from the national Danish Patient Registry. In two sensitivity analyses, diabetes was defined either as (i) non-fasting plasma glucose levels of more than 11 mmol/L (198 mg/dL) at baseline or (ii) self-reported use of insulin or oral hypoglycaemic agents at baseline. Standard hospital assays measured glucose. Hypertension was self-reported use of antihypertensive medication, a systolic blood pressure of 140 mm Hg or greater, and/or a diastolic blood pressure of 90 mm Hg or greater at baseline. In two sensitivity analyses, hypertension was defined either as (i) a systolic blood pressure of 140 mm Hg or greater, and/or a diastolic blood pressure of 90 mm Hg or greater at baseline or (ii) self-reported use of antihypertensive medication at baseline. Smoking was never/ever smoker. Low physical activity was maximum 4 h per week of light physical activity in leisure time. High alcohol intake was self-reported weekly consumption of more than 14 units alcohol/week for women and more than 21 units alcohol/week for men. Low education was <8 years of formal education (equivalent to a maximum of finalized primary school). When analysing midlife cardiovascular risk factors, only individuals aged 40–60 years at baseline were included.
Statistical analysis
We used Stata/S.E. v14.0 (Stata Corp, College Station, TX, USA). Probability values <0.001 were given as powers of 10. P-values fulfilling a Bonferroni corrected criteria of 0.006 (0.05/8 items = 0.006; 8 items: diabetes, hypertension, smoking, physical activity, education, alcohol intake, APOE genotype, and GWAS risk alleles) were marked with an *. Kruskal–Wallis one-way analysis of variance or Pearson’s χ2 test were used to evaluate continuous and categorical variables by genotype. Missing data on covariates were imputed from age, sex, and population by multiple imputation.16 Missing values were <1% for modifiable risk factors. When performing a sensitivity analysis only including individuals with complete data, results were similar to those reported. Combining GWAS identified genotypes,9 excluding the APOE genotype, we generated a genetic score by summing the number of dementia increasing risk alleles in each individual. Subsequently, all individuals were categorized into four genetic score groups of approximately equal size. The score was generated in the total cohort.
Cox proportional hazards regression models with age as time scale (=age adjustment) and left truncation at study examination (delayed entry) were used to estimate hazard ratios for all-cause dementia and Alzheimer’s disease as a function of cardiovascular risk factors adjusted for APOE genotype and number of GWAS risk alleles. Death or emigration was taken into account as competing events by censoring on death, emigration, and end of follow-up. For Cox regression models, proportionality of hazards over time was assessed by plotting -ln(-ln[survival]) vs. ln(analysis time). There was no suspicion of non-proportionality. In combination with Cox regression models, Least Absolute Shrinkage and Selection Operator (LASSO) regression17 was further used to select the cardiovascular risk factors for stratification in 10-year absolute risk charts for all-cause dementia. Least Absolute Shrinkage and Selection Operator is helpful in parsing down to the most important terms, while testing all possible interactions of covariates, and thus identifies the most contributory factors in the dataset. Selection criteria were P-values < 0.05 from Cox regressions and/or delta Extended Bayesian Information Criteria (EBIC) values ≥10 from LASSO regressions for all but one covariate (smoking in men where we allowed a delta EBIC = 6).
Ten-year absolute risks of all-cause dementia were calculated using competing risk regression based on Fine and Gray proportional sub-hazards model,18 to account for the possibility of death or emigration as competing events. The Fine and Gray proportional sub-hazards model was chosen because a competing event prevents the event of interest, which is highly relevant with diseases of late life, while censoring merely obstructs the observation of the event of interest. When stratifying on midlife hypertension, we restricted the analyses to individuals aged 40–60 years at baseline. Because our focus for this study was dementias in late life, 10-year absolute risks of all-cause dementia were shown for individuals at or above 50 years. Twenty-year absolute risks of all-cause dementia were calculated when stratifying on midlife hypertension. Discriminative accuracy of 10- and 20-year absolute risk models stratified on modifiable risk factors was tested using Gray’s test.
Results
Table 1 shows baseline characteristics of the 61 664 individuals enrolled in the study by number of risk alleles for all-cause dementia and Alzheimer’s disease from GWAS.
Table 1.
Characteristics of study individuals by sex and number of GWAS risk alleles
| Women | Men | |||||||
|---|---|---|---|---|---|---|---|---|
| No. of risk alleles | 8–17 | 18–19 | 20–21 | 22–31 | 8–17 | 18–19 | 20–21 | 22–31 |
| No. of individuals | 9393 | 9685 | 8605 | 6260 | 7741 | 7927 | 7004 | 5049 |
| Age (years) | 57 (47–67) | 58 (47–67) | 57 (47–67) | 58 (47–67) | 58 (48–68) | 58 (48–67) | 58 (48–67) | 58 (47–67) |
| Diabetes (%) | 3.2 | 3.2 | 3.1 | 2.9 | 5.2 | 5.6 | 4.8 | 5.2 |
| Hypertension (%) | 52.9 | 52.6 | 52.6 | 51.9 | 64.9 | 65.2 | 65.0 | 64.5 |
| Smoking (%) | 59.2 | 59.1 | 60.1 | 60.9 | 65.9 | 67.1 | 67.0 | 66.6 |
| Physical inactivity (%) | 57.8 | 57.3 | 56.5 | 57.0 | 47.4 | 48.4 | 47.1 | 47.4 |
| Low education (%) | 13.8 | 13.9 | 13.3 | 14.9† | 14.7 | 14.3 | 15.1 | 15.0 |
| High alcohol intake (%) | 15.3 | 14.9 | 14.3 | 14.6 | 21.9 | 21.3 | 21.7 | 21.6 |
| APOE genotype | ||||||||
| ɛ22 (%) | 0.7 | 0.7 | 0.7 | 0.7 | 0.7 | 0.8 | 0.7 | 0.5 |
| ɛ32 (%) | 12.6 | 12.1 | 12.4 | 12.4 | 12.4 | 12.9 | 12.4 | 12.1 |
| ɛ33 (%) | 55.3 | 56.0 | 54.7 | 55.6 | 56.5 | 55.9 | 55.8 | 57.3 |
| ɛ42 (%) | 2.9 | 2.6 | 3.2 | 3.0 | 2.8 | 3.1 | 2.7 | 2.8 |
| ɛ43 (%) | 25.6 | 25.7 | 26.1 | 25.2 | 24.8 | 24.6 | 26.0 | 24.9 |
| ɛ44 (%) | 2.9 | 3.0 | 2.9 | 3.2 | 2.8 | 2.7 | 2.5 | 2.5 |
Values are numbers, median (interquartile range) or per cent, and are from the day of enrolment (2003 and onwards for the CGPS and 1991–94 or 2001–03 for the CCHS). P for trend was calculated using Kruskal–Wallis one-way analysis of variance with ties or Pearson’s χ2-test. † P < 0.05.
Cardiovascular risk factors at all ages and at midlife, and risk of dementia
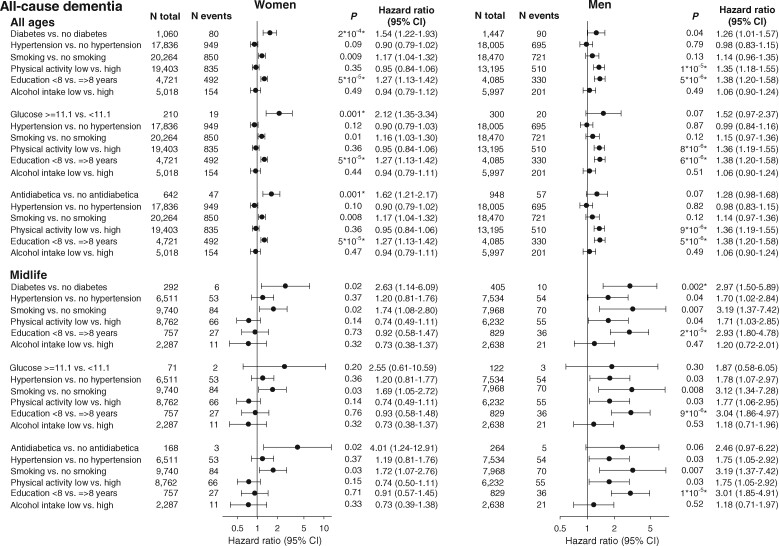
Multifactorially adjusted hazard ratios for all-cause dementia for diabetes by three definitions, hypertension, smoking, low physical activity, low education, and high alcohol intake for all ages (top three panels) and midlife (bottom three panels) are shown in Figure 1.
Figure 1.
Risk of all-cause dementia as a function of cardiovascular risk factors at all ages and during midlife. Hazard ratios were multifactorially adjusted for age (as time scale), APOE genotype, number of GWAS risk alleles, and the listed modifiable risk factors. Analyses in the lower three panels were restricted to individuals aged 40–60 years at baseline. P from Cox regression. P-values fulfilling a Bonferroni corrected criteria of 0.006 are marked with an *. For APOE genotype and number of GWAS risk alleles P was for trend. APOE, apolipoprotein E gene; APOE genotype, ε2/ε3/ε4 APOE genotype; CI, confidence interval; GWAS, genome-wide association study.
For women, the modifiable risk factors with the highest hazard ratios for all ages were diabetes, smoking, and low education with hazard ratios of 1.54 (95% CI 1.22–1.93) for diabetes vs. no diabetes, 1.17 (1.04–1.32) for smoking vs. no smoking, and 1.27 (1.13–1.42) for low vs. high education (Figure 1, top panel, left column). Two other definitions of diabetes are given in Figure 1, 2nd and 3rd panels, left column, for all ages and for midlife.
For men, the modifiable risk factors with the highest hazard ratios for all ages were diabetes, low physical activity, and low education with hazard ratios of 1.26 (1.01–1.57) for diabetes vs. no diabetes, 1.35 (1.18–1.55) for low vs. high physical activity, and 1.38 (1.20–1.58) for low vs. high education (Figure 1, top panel, right column). Two other definitions of diabetes are given in Figure 1, 2nd and 3rd panels, right column, for all ages and for midlife.
For women in midlife, the modifiable risk factors with the highest hazard ratios were diabetes and smoking with hazard ratios of 2.63 (1.14–6.09) for diabetes vs. no diabetes and 1.74 (1.08–2.80) for smoking vs. no smoking (Figure 1, 4th panel, left column). For men in midlife, the modifiable risk factors with the highest hazard ratios were diabetes, smoking, and low education with hazard ratios of 2.97 (1.50–5.89) for diabetes vs. no diabetes, 3.19 (1.37–7.42) for smoking vs. no smoking, and 2.93 (1.80–4.78) for low vs. high education (Figure 1, 4th panel, right column). In midlife men, hypertension and low physical activity also contributed to risk with hazard rations of 1.70 (1.02–2.84) for hypertension vs. no hypertension and 1.71 (1.03–2.85) for low physical activity vs. high physical activity.
Corresponding multifactorially adjusted hazard ratios for all-cause dementia for cardiovascular risk factors with hypertension by three different definitions showed similar patterns (Supplementary material online, Figure S1). Multifactorially adjusted hazard ratios for Alzheimer’s disease are shown in Supplementary material online, Figures S2 and S3.
Selection of cardiovascular risk factors for 10-year absolute risk charts for all-cause dementia
A combined strategy for selecting cardiovascular risk factors for 10-year absolute dementia risk was based on results from multifactorially adjusted Cox models and LASSO regressions. A priori we chose three main stratifications: sex, age, and diabetes, as these are well-established strong independent predictors of all-cause dementia. We then further stratified on education, APOE genotype and GWAS risk alleles, because these were both strong independent predictors in Cox models, as well as contributory factors in LASSO regressions. Finally, we stratified on smoking, because smoking was an independent predictor of dementia in midlife for both women and men and a contributory factor in LASSO regressions. No interaction terms from LASSO regressions between covariates appeared to contribute. In sensitivity analyses, we additionally stratified on alcohol intake and physical activity, because these two covariates either contributed to the Cox or the LASSO model in one sex or in both.
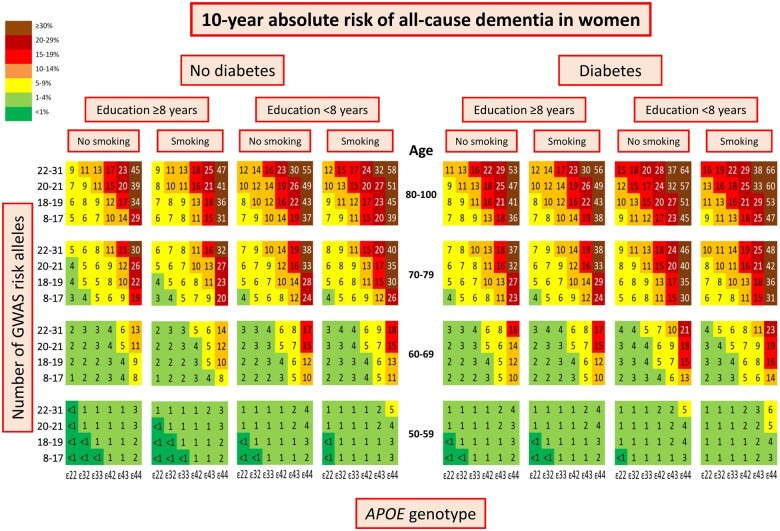
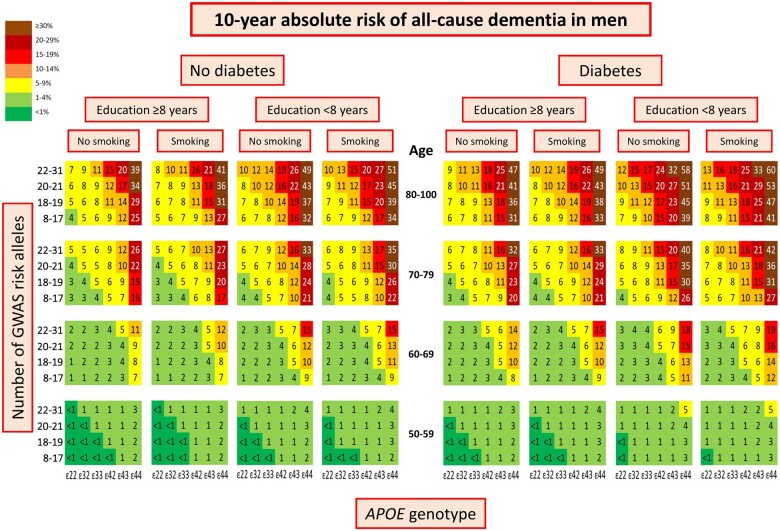
Ten-year absolute risk of dementia by genetics and cardiovascular risk factors
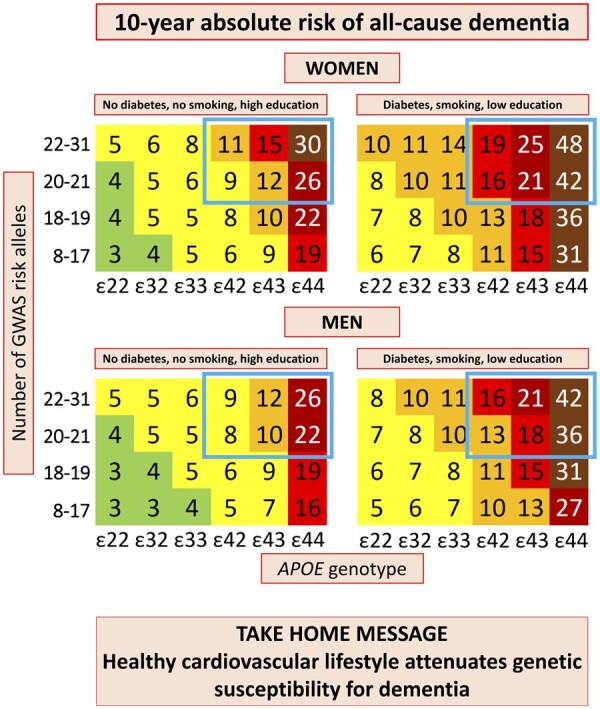
In women 10-year absolute risk of all-cause dementia stratified on diabetes status, education, and smoking status increased with increasing age, number of APOE ɛ4 alleles and number of GWAS risk alleles (Figure 2). The highest 10-year absolute risk of 66% was seen in women aged 80–100, with APOE ɛ44 genotype, 22–31 GWAS risk alleles, diabetes (P = 3*10−17* for diabetes status as discriminating factor), low education (P = 6*10−98* for educational level as discriminating factor), and smoking (P = 0.02 for smoking status as discriminating factor) (Figure 2, first right column). Corresponding 10-year absolute risk in men was 60% (P = 3*10−18* for diabetes status as discriminating factor, P = 4*10−74* for educational level as discriminating factor, and P = 1*10−17* for smoking status as discriminating factor) (Figure 3, first right column). Stratifications on alcohol intake and physical activity instead of smoking are shown in Supplementary material online, Figures S4–S7. Alcohol intake did not discriminate 10-year absolute risk of all-cause dementia (P = 0.10 for women, P = 0.34 for men), whereas physical activity did (P = 3*10−5* for women, P = 2*10−8* for men).
Figure 2.
Ten-year absolute risk of all-cause dementia in women. Ten-year absolute risk is read by identifying age group, diabetes status, educational level, smoking status, APOE genotype, and number of GWAS risk alleles. Diabetes status, educational level, and smoking status had the highest hazard ratios by Cox regressions and/or were the best discriminating factors in LASSO regressions. APOE, apolipoprotein E gene; APOE genotype, ε2/ε3/ε4 APOE genotype; GWAS, genome-wide association study.
Figure 3.
Ten-year absolute risk of all-cause dementia in men. Ten-year absolute risk is read by identifying age group, diabetes status, educational level, smoking status, APOE genotype, and number of GWAS risk alleles. Diabetes status, educational level, and smoking status had the highest hazard ratios by Cox regressions and/or were the best discriminating factors in LASSO regressions. APOE, apolipoprotein E gene; APOE genotype, ε2/ε3/ε4 APOE genotype; GWAS, genome-wide association study.
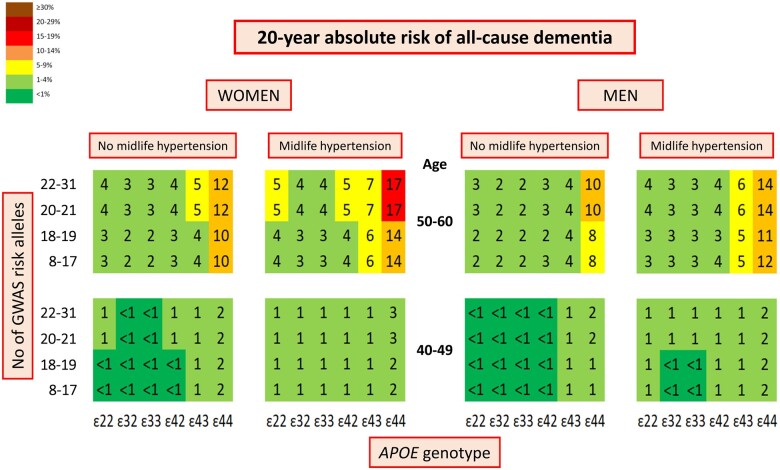
Twenty-year absolute risk of dementia by midlife hypertension status
When addressing midlife hypertension, by limiting the analysis to individuals aged 40–60 years at baseline, 20-year absolute risk of all-cause dementia stratified on midlife hypertension increased with increasing age, number of APOE ɛ4 alleles and number of GWAS risk alleles, to a maximum of 17% for women aged 50–60, with the APOE ɛ44 genotype, 22–31 GWAS risk alleles, and midlife hypertension (P = 0.006* for midlife hypertension as discriminating factor) (Figure 4, second left column). The corresponding value in men was 14% (P = 0.002*) (Figure 4, first right column).
Figure 4.
Twenty-year absolute risk of all-cause dementia stratified by midlife hypertension. Twenty-year absolute risk is read by identifying the age group and sex, midlife hypertension status, APOE genotype, and number of GWAS risk alleles. APOE, apolipoprotein E gene; APOE genotype, ε2/ε3/ε4 APOE genotype; GWAS, genome-wide association study.
Ten-year absolute risk of dementia separately in APOE ε44 homozygotes, ε43 heterozygotes, and ε33 homozygotes
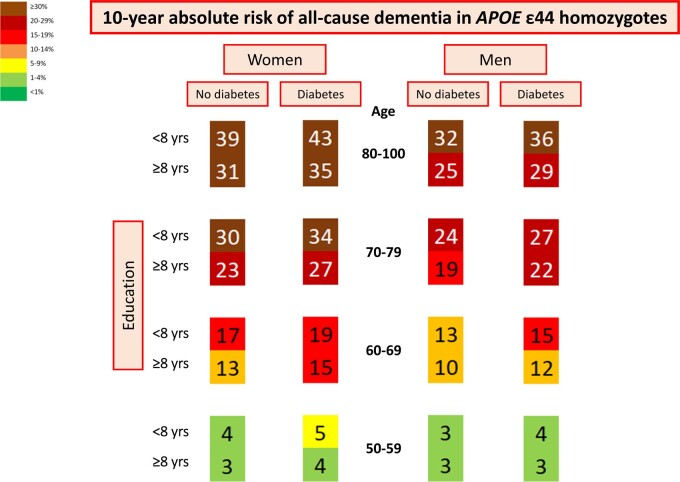
In APOE ε44 homozygotes, 10-year absolute risk varied from 48% to 19% in women aged 70–79, depending on the burden of GWAS risk alleles and modifiable risk factors (women aged 70–79 with APOE ε44, diabetes, low education, smoking, and 22–31 GWAS risk alleles vs. women aged 70–79 with APOE ε44, no diabetes, high education, no smoking, and 8–17 GWAS risk alleles) (Figure 2). Corresponding 10-year absolute risk in men varied from 42% to 16% (Figure 3).
In LASSO regressions only including ε44 homozygotes, diabetes and education were the best discriminating modifiable risk factors. In women, 10-year absolute risk of all-cause dementia stratified on diabetes status and education increased with increasing age. The highest 10-year absolute risk of 43% was seen in women aged 80–100 with diabetes (P = 0.006* for diabetes status as discriminating factor) and low education (P = 1*10−7* for educational level as discriminating factor) (Figure 5, 2nd left column). Corresponding 10-year absolute risk in men was 36% (P = 0.96 for diabetes status as discriminating factor, P = 2*10−7* for educational level as discriminating factor) (Figure 5, first right column).
Figure 5.
Ten-year absolute risk of all-cause dementia in APOE ε44 homozygotes. Ten-year absolute risk is read by identifying age group and sex, diabetes status, and educational level. Diabetes status and educational level had the highest hazard ratios by Cox regressions and/or were the best discriminating factors in LASSO regressions in ε44 homozygotes. APOE, apolipoprotein E gene.
In APOE ε43 heterozygotes and APOE ε33 homozygotes, 10-year absolute risk of all-cause dementia stratified on the best discriminating risk factors from LASSO regressions are shown in Supplementary material online, Figures S8 and S9. In ɛ43 heterozygotes, diabetes and education remained as discriminating factors in women, whereas education remained in men (diabetes: P = 1*10−4*, education: P = 1*10−62* in women; diabetes: P = 0.14, education: P = 4*10−24* in men). For ɛ33 homozygotes, diabetes and education remained as discriminating factors in women, whereas diabetes, education and physical activity remained in men (diabetes: P = 3*10−8*, education: P = 8*10−49* in women; diabetes: P = 6*10−15*, education: P = 4*10−41*, physical activity: P = 2*10−6* in men).
Discussion
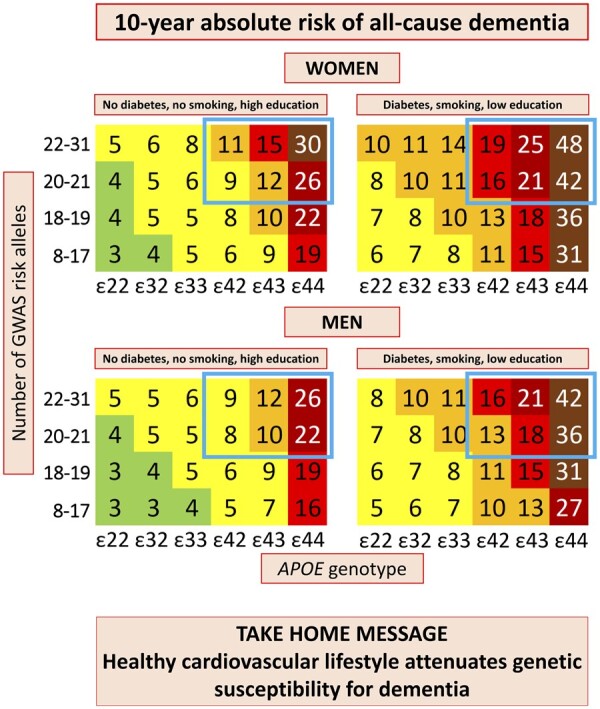
The principal findings of this study are that 10-year absolute risk of all-cause dementia increase with increasing age, number of APOE ɛ4 alleles, GWAS risk alleles, and with diabetes, low education, and smoking. Physical inactivity also contributed, especially in men. These findings are timely and facilitate identification of high-risk individuals, those that are anticipated to benefit the most from targeted prevention (Take home figure).
Take home figure.

Among individuals 70–79 years of age with high genetic risk (blue framing), 10-year absolute risk increased 60–80% depending on the burden of cardiovascular risk factors. Diabetes status, smoking status, and educational level were the best discriminating factors by Cox regressions and/or LASSO regressions. Colour code: dark green <1%; light green 1–4%; yellow 5–9%; orange 10–14%; red 15–19%; dark red 20–29%; brown ≥30%. APOE, apolipoprotein E gene; APOE genotype, ε2/ε3/ε4 APOE genotype; GWAS, genome-wide association study.
To the best of our knowledge, this is the first study to assess 10-year absolute risk of dementia by age, APOE genotype, GWAS risk alleles, and cardiovascular risk factors taking risk of death as a competing event into account in large prospective cohorts of the general population. Because we found diabetes, smoking, low education, physical activity, and alcohol intake to be the strongest modifiable risk factors for all-cause dementia in Cox and LASSO regressions, we stratified the 10-year absolute risk on these risk factors. The Cox model has the advantage of estimating the independent contribution of each covariate, whereas LASSO regression has the advantage of parsing down to the most important terms, while testing all possible interactions of covariates, and thus helps identifying the most contributory factors in the dataset. Diabetes, low education, and smoking eventually turned out to be the discriminatory modifiable risk factors in Fine and Gray analyses of 10-year absolute risk, and diabetes and low education when ɛ44 homozygotes, ɛ43 heterozygotes, and ɛ33 homozygotes were assessed separately. Furthermore, we observed that both untreated and treated diabetes were predictors of all-cause dementia in all ages and midlife, whereas hypertension in accordance with the litterature1 was only a predictor in midlife, when assessed as the combined covariate or as measured blood pressure. The present findings for the contribution of age and genetic variants are in accordance with a recent report from the Rotterdam study,19 summarizing that common genetic variants with small individual effects jointly modify the risk and age at onset of all-cause dementia and Alzheimer’s disease, particularly in APOE ɛ4-carriers (homozygotes and heterozygotes combined). To facilitate the usability of risk scores, we now provide stratification by age, sex, cardiovascular risk factors, GWAS risk alleles, and by exact APOE genotype representing specific individuals. Since 2006 several risk prediction models for dementia, mainly focusing on cardiovascular risk factors, blood biomarkers, cognitive testing, and brain imaging, have been published.1 , 8 , 20 , 21 These risk scores are based on scoring systems summing up points for the different test results, cardiovascular risk factors, age, sex, and in some cases APOE genotype. In contrast, the present risk score is similar to the widely used SCORE22 for risk of cardiovascular disease and takes risk of death as a competing event into account, which is crucial for diseases of late life.
The mechanisms behind how cardiovascular risk factors affect dementia are not well-established; however, suggestive evidence exists that will be reviewed in the following. Diabetes is strongly associated with increased risk of dementia.7 One potential mechanism may be that peripheral insulin anomalies cause a decrease in brain insulin production, which can impair amyloid clearance.1 Also, inflammation and high blood glucose concentrations are suggested as potential mechanisms by which diabetes impairs cognition.1 Low educational level, equivalent to a maximum of finalized primary school, as a potential modifiable risk factor has shown the most consistent association with risk of dementia.23 , 24 Satizabal et al. 25 examined dementia incidences in the Framingham Heart Study and found a declining incidence over three decades, but only among people who had at least a high school diploma. It is a widespread opinion that this is due to higher cognitive reserve in individuals with higher level of education. Individuals with higher educational level tolerate more severe brain pathology without developing clinical dementia compared with individuals with lower educational level.1 , 26 However, it is also likely that those with low education have a less favourable lifestyle than those with high education, potentially explaining a part of the high risk of dementia in individuals with low vs. high education. Smoking associates with increased risk of dementia,24 , 27 and is likely mediated by cardiovascular pathology and the content of neurotoxins in cigarette smoke.1 , 28 Midlife hypertension is consistently associated with increased risk of dementia1 , 5 , 29—an association we can confirm at least for men in the present study—and is suggested to be through increased risk of cerebrovascular disease and the metabolic syndrome.1 Physical activity is inversely associated with risk of dementia,1 , 5 , 30 an association mainly explained by cardiovascular risk factors.1 , 30 Finally, the current evidence for the association between alcohol intake and risk of dementia is unclear.1 , 7
Intervention trials to prevent dementia has shown that decreasing the risk of dementia requires extensive multidomain interventions.2 , 4 , 31 The preDIVA trial ‘provided modestly enhanced care to non-selected or non-targeted patients already connected to medical practice’ to identify and try to reduce vascular risk of dementia, however, without success.4 In contrast, in the FINGER trial—a proof-of-concept randomized controlled trial with intensive, multidomain intervention on nutritional guidance, exercise, cognitive training, and management of metabolic and cardiovascular risk factors applied to at-risk elderly people from the general population—the participants in the intervention group showed improved or maintained cognitive function after 2 years of intervention independent of baseline characteristics.2 , 3 This intensive multidomain intervention will, however, most likely be an unrealistic economic burden for many societies to implement in all individuals at risk of dementia. Therefore, the implementation of a combined genetic- and risk factor score identifying high-risk individuals who are likely to benefit the most from a targeted preventive intervention is in high demand. Based on the present data, we suggest that focus must be on raising the educational level for children, adolescents, and young adults, and that a targeted preventive intervention on cardiovascular risk factors starts at age 60 at the latest. Furthermore, a general aggressive healthy lifestyle should be reinforced throughout the life course.
For cardiovascular disease, implementation of genetic risk scores for primary prevention is anticipated to reach clinical practice in the near future.32 The genetic contribution to common forms of age-related dementia is much stronger than for cardiovascular disease, exemplified very clearly by the well-known APOE effect but also by the additive effect of other common risk alleles.10 Since the genetic contribution to dementia is stronger than the contribution from modifiable risk factors—in sharp contrast to cardiovascular disease—polygenic information is key for a robust dementia risk score. The selection of risk alleles for the score was based on a conservative judgement, where the strongest genetic hits that were validated in both testing and replication samples were chosen.9 For future updates of the score, additional risk alleles from recent GWAS may be worthwhile to include,10 , 33 although these probably will have minor contributions. Reassuringly, the use of genetic information for risk prediction and targeted intervention is anticipated to be received well, as a previous study revealed that knowing your APOE genotype in relation to risk of Alzheimer’s disease does not result in emotional distress, even with no available treatment.34 Importantly, we now show that among APOE ε44 homozygotes 10-year absolute risk can vary substantially depending on the burden of additional genetic risk variants and cardiovascular risk factors.
The present study has potential limitations and strengths that need to be addressed. As events are based on ICD registry codes from hospitals and death certificates, only individuals with a dementia diagnosis as a cause or contributing cause of death, or those referred to hospitals, are included in the present study, in contrast to research studies examining all participants using study physicians and standardized diagnostic methods.35 The use of hospital record data, however, reduces attrition bias.30 Even though the national Danish registries are regarded among the best of its kind,36 , 37 and the quality of the Danish registry-based dementia diagnoses previously has been validated,38 the use of registry-based diagnoses suffer from potential underdiagnosis. We ensured, by scrutinizing department codes for all events, that 91–93% of all-cause dementia diagnoses were given from departments of internal medicine, dementia clinics, other neurological outpatient clinics, departments of neurology and neurosurgery, or in- and outpatient clinics at departments of geriatrics.15 This information together with the fact that APOE ε44 vs. ε33 homozygotes have 8- to 10-fold risk of Alzheimer’s disease in the present populations13 , 39 provide confidence that the present Alzheimer’s disease diagnosis is acceptable to use. Strengths of our study are the prospective design and the large, well-characterized, ethnically homogeneous cohorts of the general population with no losses to follow-up, and the use of competing risk regression. Ethnically homogeneity may, however, lead to limited applicability for individuals of other ethnicities, supporting the need for more extensive studies in diverse populations.
In conclusion, in combined and easily applicable 10-year absolute risk scores of all-cause dementia, we find that risk increases with increasing age, number of APOE ε4 alleles, GWAS risk alleles, and with diabetes, low education, and smoking. These findings are timely and may serve as tools for comprehensive risk stratification to facilitate efficient preventive and curative trials in high-risk groups of individuals, those that likely will benefit the most from interventions.
Supplementary Material
Acknowledgements
We are indebted to staff and participants of the Copenhagen General Population Study and the Copenhagen City Heart Study for their important contributions to our study.
Funding
This work was supported by the Danish Medical Research Council (grant no 10-081618), by the Research Council at Rigshospitalet (PhD fellowship to I.J.R.), by the Danish Alzheimer Research Foundation, by the Lundbeck Foundation (grant no R278-2018-804), and by M.L. Jørgensen & Gunnar Hansen’s Fund. The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Conflict of interest: none declared.
Contributor Information
Ida Juul Rasmussen, Department of Clinical Biochemistry, Rigshospitalet, Copenhagen University Hospital, Blegdamsvej 9, DK-2100 Copenhagen, Denmark; The Copenhagen General Population Study, Herlev and Gentofte Hospital, Copenhagen University Hospital, Borgmester Ib Juuls Vej 1, DK-2730 Herlev, Denmark; Department of Clinical Medicine, Faculty of Health and Medical Sciences, University of Copenhagen, Blegdamsvej 3B, DK-2200 Copenhagen, Denmark.
Katrine Laura Rasmussen, Department of Clinical Biochemistry, Rigshospitalet, Copenhagen University Hospital, Blegdamsvej 9, DK-2100 Copenhagen, Denmark; The Copenhagen General Population Study, Herlev and Gentofte Hospital, Copenhagen University Hospital, Borgmester Ib Juuls Vej 1, DK-2730 Herlev, Denmark.
Børge G Nordestgaard, The Copenhagen General Population Study, Herlev and Gentofte Hospital, Copenhagen University Hospital, Borgmester Ib Juuls Vej 1, DK-2730 Herlev, Denmark; Department of Clinical Medicine, Faculty of Health and Medical Sciences, University of Copenhagen, Blegdamsvej 3B, DK-2200 Copenhagen, Denmark; Department of Clinical Biochemistry, Herlev and Gentofte Hospital, Copenhagen University Hospital, Borgmester Ib Juuls Vej 1, DK-2730 Herlev, Denmark; The Copenhagen City Heart Study, Bispebjerg and Frederiksberg Hospital, Copenhagen University Hospital, Nordre Fasanvej 57, DK-2000 Frederiksberg, Denmark.
Anne Tybjærg-Hansen, Department of Clinical Biochemistry, Rigshospitalet, Copenhagen University Hospital, Blegdamsvej 9, DK-2100 Copenhagen, Denmark; The Copenhagen General Population Study, Herlev and Gentofte Hospital, Copenhagen University Hospital, Borgmester Ib Juuls Vej 1, DK-2730 Herlev, Denmark; Department of Clinical Medicine, Faculty of Health and Medical Sciences, University of Copenhagen, Blegdamsvej 3B, DK-2200 Copenhagen, Denmark; The Copenhagen City Heart Study, Bispebjerg and Frederiksberg Hospital, Copenhagen University Hospital, Nordre Fasanvej 57, DK-2000 Frederiksberg, Denmark.
Ruth Frikke-Schmidt, Department of Clinical Biochemistry, Rigshospitalet, Copenhagen University Hospital, Blegdamsvej 9, DK-2100 Copenhagen, Denmark; The Copenhagen General Population Study, Herlev and Gentofte Hospital, Copenhagen University Hospital, Borgmester Ib Juuls Vej 1, DK-2730 Herlev, Denmark; Department of Clinical Medicine, Faculty of Health and Medical Sciences, University of Copenhagen, Blegdamsvej 3B, DK-2200 Copenhagen, Denmark.
References
- 1. Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, Ballard C, Banerjee S, Burns A, Cohen-Mansfield J, Cooper C, Fox N, Gitlin LN, Howard R, Kales HC, Larson EB, Ritchie K, Rockwood K, Sampson EL, Samus Q, Schneider LS, Selbæk G, Teri L, Mukadam N. Dementia prevention, intervention, and care. Lancet 2017;390:2673–2734. [DOI] [PubMed] [Google Scholar]
- 2. Ngandu T, Lehtisalo J, Solomon A, Levälahti E, Ahtiluoto S, Antikainen R, Bäckman L, Hänninen T, Jula A, Laatikainen T, Lindström J, Mangialasche F, Paajanen T, Pajala S, Peltonen M, Rauramaa R, Stigsdotter-Neely A, Strandberg T, Tuomilehto J, Soininen H, Kivipelto M. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet 2015;385:2255–2263. [DOI] [PubMed] [Google Scholar]
- 3. Rosenberg A, Ngandu T, Rusanen M, Antikainen R, Bäckman L, Havulinna S, Hänninen T, Laatikainen T, Lehtisalo J, Levälahti E, Lindström J, Paajanen T, Peltonen M, Soininen H, Stigsdotter-Neely A, Strandberg T, Tuomilehto J, Solomon A, Kivipelto M. Multidomain lifestyle intervention benefits a large elderly population at risk for cognitive decline and dementia regardless of baseline characteristics: the FINGER trial. Alzheimers Dement 2018;14:263–270. [DOI] [PubMed] [Google Scholar]
- 4. Charante EV, Richard E, Eurelings LS, Dalen JV, Ligthart SA, Bussel EV, Hoevenaar-Blom MP, Vermeulen M, Gool WV. Effectiveness of a 6-year multidomain vascular care intervention to prevent dementia (preDIVA): a cluster-randomised controlled trial. Lancet 2016;388:797–805. [DOI] [PubMed] [Google Scholar]
- 5. Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol 2014;13:788–794. [DOI] [PubMed] [Google Scholar]
- 6. Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer’s disease. Alzheimers Dement 2007;3:186–191. [DOI] [PubMed] [Google Scholar]
- 7. Baumgart M, Snyder HM, Carrillo MC, Fazio S, Kim H, Johns H. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: a population-based perspective. Alzheimers Dement 2015;11:718–726. [DOI] [PubMed] [Google Scholar]
- 8. Exalto LG, Quesenberry CP, Barnes D, Kivipelto M, Biessels GJ, Whitmer RA. Midlife risk score for the prediction of dementia four decades later. Alzheimers Dement 2014;10:562–570. [DOI] [PubMed] [Google Scholar]
- 9. Lambert J-C, Ibrahim-Verbaas CA, Harold D, Naj AC, Sims R, Bellenguez C, Jun G, DeStefano AL, Bis JC, Beecham GW, Grenier-Boley B, Russo G, Thornton-Wells TA, Jones N, Smith AV, Chouraki V, Thomas C, Ikram MA, Zelenika D, Vardarajan BN, Kamatani Y, Lin C-F, Gerrish A, Schmidt H, Kunkle B, Dunstan ML, Ruiz A, Bihoreau M-T, Choi S-H, Reitz C, Pasquier F, Hollingworth P, Ramirez A, Hanon O, Fitzpatrick AL, Buxbaum JD, Campion D, Crane PK, Baldwin C, Becker T, Gudnason V, Cruchaga C, Craig D, Amin N, Berr C, Lopez OL, De Jager PL, Deramecourt V, Johnston JA, Evans D, Lovestone S, Letenneur L, Morón FJ, Rubinsztein DC, Eiriksdottir G, Sleegers K, Goate AM, Fiévet N, Huentelman MJ, Gill M, Brown K, Kamboh MI, Keller L, Barberger-Gateau P, McGuinness B, Larson EB, Green R, Myers AJ, Dufouil C, Todd S, Wallon D, Love S, Rogaeva E, Gallacher J, St George-Hyslop P, Clarimon J, Lleo A, Bayer A, Tsuang DW, Yu L, Tsolaki M, Bossù P, Spalletta G, Proitsi P, Collinge J, Sorbi S, Sanchez-Garcia F, Fox NC, Hardy J, Naranjo MCD, Bosco P, Clarke R, Brayne C, Galimberti D, Mancuso M, Matthews F, Moebus S, Mecocci P, Del Zompo M, Maier W, Hampel H, Pilotto A, Bullido M, Panza F, Caffarra P, Nacmias B, Gilbert JR, Mayhaus M, Lannfelt L, Hakonarson H, Pichler S, Carrasquillo MM, Ingelsson M, Beekly D, Alvarez V, Zou F, Valladares O, Younkin SG, Coto E, Hamilton-Nelson KL, Gu W, Razquin C, Pastor P, Mateo I, Owen MJ, Faber KM, Jonsson PV, Combarros O, O'Donovan MC, Cantwell LB, Soininen H, Blacker D, Mead S, Mosley TH, Bennett DA, Harris TB, Fratiglioni L, Holmes C, de Bruijn RFAG, Passmore P, Montine TJ, Bettens K, Rotter JI, Brice A, Morgan K, Foroud TM, Kukull WA, Hannequin D, Powell JF, Nalls MA, Ritchie K, Lunetta KL, Kauwe JSK, Boerwinkle E, Riemenschneider M, Boada M, Hiltunen M, Martin ER, Schmidt R, Rujescu D, Wang L-S, Dartigues J-F, Mayeux R, Tzourio C, Hofman A, Nöthen MM, Graff C, Psaty BM, Jones L, Haines JL, Holmans PA, Lathrop M, Pericak-Vance MA, Launer LJ, Farrer LA, van Duijn CM, Van Broeckhoven C, Moskvina V, Seshadri S, Williams J, Schellenberg GD, Amouyel P, European Alzheimer's Disease Initiative (EADI). Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat Genet 2013;45:1452–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kunkle BW, Grenier-Boley B, Sims R, Bis JC, Damotte V, Naj AC, Boland A, Vronskaya M, van der Lee SJ, Amlie-Wolf A, Bellenguez C, Frizatti A, Chouraki V, Martin ER, Sleegers K, Badarinarayan N, Jakobsdottir J, Hamilton-Nelson KL, Moreno-Grau S, Olaso R, Raybould R, Chen Y, Kuzma AB, Hiltunen M, Morgan T, Ahmad S, Vardarajan BN, Epelbaum J, Hoffmann P, Boada M, Beecham GW, Garnier J-G, Harold D, Fitzpatrick AL, Valladares O, Moutet M-L, Gerrish A, Smith AV, Qu L, Bacq D, Denning N, Jian X, Zhao Y, Del Zompo M, Fox NC, Choi S-H, Mateo I, Hughes JT, Adams HH, Malamon J, Sanchez-Garcia F, Patel Y, Brody JA, Dombroski BA, Naranjo MCD, Daniilidou M, Eiriksdottir G, Mukherjee S, Wallon D, Uphill J, Aspelund T, Cantwell LB, Garzia F, Galimberti D, Hofer E, Butkiewicz M, Fin B, Scarpini E, Sarnowski C, Bush WS, Meslage S, Kornhuber J, White CC, Song Y, Barber RC, Engelborghs S, Sordon S, Voijnovic D, Adams PM, Vandenberghe R, Mayhaus M, Cupples LA, Albert MS, De Deyn PP, Gu W, Himali JJ, Beekly D, Squassina A, Hartmann AM, Orellana A, Blacker D, Rodriguez-Rodriguez E, Lovestone S, Garcia ME, Doody RS, Munoz-Fernadez C, Sussams R, Lin H, Fairchild TJ, Benito YA, Holmes C, Karamujić-Čomić H, Frosch MP, Thonberg H, Maier W, Roshchupkin G, Ghetti B, Giedraitis V, Kawalia A, Li S, Huebinger RM, Kilander L, Moebus S, Hernández I, Kamboh MI, Brundin R, Turton J, Yang Q, Katz MJ, Concari L, Lord J, Beiser AS, Keene CD, Helisalmi S, Kloszewska I, Kukull WA, Koivisto AM, Lynch A, Tarraga L, Larson EB, Haapasalo A, Lawlor B, Mosley TH, Lipton RB, Solfrizzi V, Gill M, Longstreth WT, Montine TJ, Frisardi V, Diez-Fairen M, Rivadeneira F, Petersen RC, Deramecourt V, Alvarez I, Salani F, Ciaramella A, Boerwinkle E, Reiman EM, Fievet N, Rotter JI, Reisch JS, Hanon O, Cupidi C, Andre Uitterlinden AG, Royall DR, Dufouil C, Maletta RG, de Rojas I, Sano M, Brice A, Cecchetti R, George-Hyslop PS, Ritchie K, Tsolaki M, Tsuang DW, Dubois B, Craig D, Wu C-K, Soininen H, Avramidou D, Albin RL, Fratiglioni L, Germanou A, Apostolova LG, Keller L, Koutroumani M, Arnold SE, Panza F, Gkatzima O, Asthana S, Hannequin D, Whitehead P, Atwood CS, Caffarra P, Hampel H, Quintela I, Carracedo Á, Lannfelt L, Rubinsztein DC, Barnes LL, Pasquier F, Frölich L, Barral S, McGuinness B, Beach TG, Johnston JA, Becker JT, Passmore P, Bigio EH, Schott JM, Bird TD, Warren JD, Boeve BF, Lupton MK, Bowen JD, Proitsi P, Boxer A, Powell JF, Burke JR, Kauwe JSK, Burns JM, Mancuso M, Buxbaum JD, Bonuccelli U, Cairns NJ, McQuillin A, Cao C, Livingston G, Carlson CS, Bass NJ, Carlsson CM, Hardy J, Carney RM, Bras J, Carrasquillo MM, Guerreiro R, Allen M, Chui HC, Fisher E, Masullo C, Crocco EA, DeCarli C, Bisceglio G, Dick M, Ma L, Duara R, Graff-Radford NR, Evans DA, Hodges A, Faber KM, Scherer M, Fallon KB, Riemenschneider M, Fardo DW, Heun R, Farlow MR, Kölsch H, Ferris S, Leber M, Foroud TM, Heuser I, Galasko DR, Giegling I, Gearing M, Hüll M, Geschwind DH, Gilbert JR, Morris J, Green RC, Mayo K, Growdon JH, Feulner T, Hamilton RL, Harrell LE, Drichel D, Honig LS, Cushion TD, Huentelman MJ, Hollingworth P, Hulette CM, Hyman BT, Marshall R, Jarvik GP, Meggy A, Abner E, Menzies GE, Jin L-W, Leonenko G, Real LM, Jun GR, Baldwin CT, Grozeva D, Karydas A, Russo G, Kaye JA, Kim R, Jessen F, Kowall NW, Vellas B, Kramer JH, Vardy E, LaFerla FM, Jöckel K-H, Lah JJ, Dichgans M, Leverenz JB, Mann D, Levey AI, Pickering-Brown S, Lieberman AP, Klopp N, Lunetta KL, Wichmann H-E, Lyketsos CG, Morgan K, Marson DC, Brown K, Martiniuk F, Medway C, Mash DC, Nöthen MM, Masliah E, Hooper NM, McCormick WC, Daniele A, McCurry SM, Bayer A, McDavid AN, Gallacher J, McKee AC, van den Bussche H, Mesulam M, Brayne C, Miller BL, Riedel-Heller S, Miller CA, Miller JW, Al-Chalabi A, Morris JC, Shaw CE, Myers AJ, Wiltfang J, O’Bryant S, Olichney JM, Alvarez V, Parisi JE, Singleton AB, Paulson HL, Collinge J, Perry WR, Mead S, Peskind E, Cribbs DH, Rossor M, Pierce A, Ryan NS, Poon WW, Nacmias B, Potter H, Sorbi S, Quinn JF, Sacchinelli E, Raj A, Spalletta G, Raskind M, Caltagirone C, Bossù P, Orfei MD, Reisberg B, Clarke R, Reitz C, Smith AD, Ringman JM, Warden D, Roberson ED, Wilcock G, Rogaeva E, Bruni AC, Rosen HJ, Gallo M, Rosenberg RN, Ben-Shlomo Y, Sager MA, Mecocci P, Saykin AJ, Pastor P, Cuccaro ML, Vance JM, Schneider JA, Schneider LS, Slifer S, Seeley WW, Smith AG, Sonnen JA, Spina S, Stern RA, Swerdlow RH, Tang M, Tanzi RE, Trojanowski JQ, Troncoso JC, Van Deerlin VM, Van Eldik LJ, Vinters HV, Vonsattel JP, Weintraub S, Welsh-Bohmer KA, Wilhelmsen KC, Williamson J, Wingo TS, Woltjer RL, Wright CB, Yu C-E, Yu L, Saba Y, Pilotto A, Bullido MJ, Peters O, Crane PK, Bennett D, Bosco P, Coto E, Boccardi V, De Jager PL, Lleo A, Warner N, Lopez OL, Ingelsson M, Deloukas P, Cruchaga C, Graff C, Gwilliam R, Fornage M, Goate AM, Sanchez-Juan P, Kehoe PG, Amin N, Ertekin-Taner N, Berr C, Debette S, Love S, Launer LJ, Younkin SG, Dartigues J-F, Corcoran C, Ikram MA, Dickson DW, Nicolas G, Campion D, Tschanz JAnn, Schmidt H, Hakonarson H, Clarimon J, Munger R, Schmidt R, Farrer LA, Van Broeckhoven C, C. O’Donovan M, DeStefano AL, Jones L, Haines JL, Deleuze J-F, Owen MJ, Gudnason V, Mayeux R, Escott-Price V, Psaty BM, Ramirez A, Wang L-S, Ruiz A, van Duijn CM, Holmans PA, Seshadri S, Williams J, Amouyel P, Schellenberg GD, Lambert J-C, Pericak-Vance MA, Alzheimer Disease Genetics Consortium (ADGC), European Alzheimer’s Disease Initiative (EADI), Cohorts for Heart and Aging Research in Genomic Epidemiology Consortium (CHARGE), Genetic and Environmental Risk in AD/Defining Genetic, Polygenic and Environmental Risk for Alzheimer’s Disease Consortium (GERAD/PERADES). Genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Aβ, tau, immunity and lipid processing. Nat Genet 2019;51:414–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Frikke-Schmidt R, Nordestgaard BG, Stene MCA, Sethi AA, Remaley AT, Schnohr P, Grande P, Tybjærg-Hansen A. Association of loss-of-function mutations in the ABCA1 gene with high-density lipoprotein cholesterol levels and risk of ischemic heart disease. JAMA 2008;299:2524–2532. [DOI] [PubMed] [Google Scholar]
- 12. Jørgensen AB, Frikke-Schmidt R, Nordestgaard BG, Tybjærg-Hansen A. Loss-of-function mutations in APOC3 and risk of ischemic vascular disease. N Engl J Med 2014;371:32–41. [DOI] [PubMed] [Google Scholar]
- 13. Rasmussen KL, Tybjaerg-Hansen A, Nordestgaard BG, Frikke-Schmidt R. Plasma levels of Apolipoprotein E and risk of dementia in the general population. Ann Neurol 2015;77:301–311. [DOI] [PubMed] [Google Scholar]
- 14. Rasmussen KL, Tybjærg-Hansen A, Nordestgaard BG, Frikke-Schmidt R. Plasma levels of Apolipoprotein E, APOE genotype, and all-cause and cause-specific mortality in 105 949 individuals from a white general population cohort. Eur Heart J 2019;40:2813–2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rasmussen KL, Tybjaerg-Hansen A, Nordestgaard BG, Frikke-Schmidt R. Plasma Apolipoprotein E levels and risk of dementia: a Mendelian randomization study of 106,562 individuals. Alzheimers Dement 2018;14:71–80. [DOI] [PubMed] [Google Scholar]
- 16. White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377–399. [DOI] [PubMed] [Google Scholar]
- 17. Tibshirani R. Regression shrinkage and selection via the lasso. J R Stat Soc Ser B 1996;58:267–288. [Google Scholar]
- 18. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496–509. [Google Scholar]
- 19. Lee SVD, Wolters FJ, Ikram MK, Hofman A, Ikram MA, Amin N, Duijn CV. The effect of APOE and other common genetic variants on the onset of Alzheimer’s disease dementia: a community-based cohort study. Lancet Neurol 2018;17:434–444. [DOI] [PubMed] [Google Scholar]
- 20. Reitz C, Tang M-X, Schupf N, Manly JJ, Mayeux R, Luchsinger JA. A summary risk score for the prediction of Alzheimer disease in elderly persons. Arch Neurol 2010;67:835–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li J, Ogrodnik M, Devine S, Auerbach S, Wolf PA, Au R. Practical risk score for 5-, 10-, and 20-year prediction of dementia in elderly persons: Framingham Heart Study. Alzheimers Dement 2018;14:35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer GG, Delgado V, Ference BA, Graham IM, Halliday A, Landmesser U, Mihaylova B, Pedersen TR, Riccardi G, Richter DJ, Sabatine MS, Taskinen M-R, Tokgozoglu L, Wiklund O; ESC Scientific Document Group. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 2020;41:111–188. [DOI] [PubMed] [Google Scholar]
- 23. Caamaño-Isorna F, Corral M, Montes-Martínez A, Takkouche B. Education and dementia: a meta-analytic study. Neuroepidemiology 2006;26:226–232. [DOI] [PubMed] [Google Scholar]
- 24. Beydoun MA, Beydoun HA, Gamaldo AA, Teel A, Zonderman AB, Wang Y. Epidemiologic studies of modifiable factors associated with cognition and dementia: systematic review and meta-analysis. BMC Public Health 2014;14:643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Satizabal CL, Beiser AS, Chouraki V, Chêne G, Dufouil C, Seshadri S. Incidence of dementia over three decades in the Framingham Heart Study. N Engl J Med 2016;374:523–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vemuri P, Lesnick TG, Przybelski SA, Machulda M, Knopman DS, Mielke MM, Roberts RO, Geda YE, Rocca WA, Petersen RC, Jack CR. Association of lifetime intellectual enrichment with cognitive decline in the older population. JAMA Neurol 2014;71:1017–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhong G, Wang Y, Zhang Y, Guo JJ, Zhao Y. Smoking is associated with an increased risk of dementia: a meta-analysis of prospective cohort studies with investigation of potential effect modifiers. PLoS One 2015;10:e0118333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Swan GE, Lessov-Schlaggar CN. The effects of tobacco smoke and nicotine on cognition and the brain. Neuropsychol Rev 2007;17:259–273. [DOI] [PubMed] [Google Scholar]
- 29. Lennon MJ, Makkar SR, Crawford JD, Sachdev PS. Midlife hypertension and Alzheimer’s disease: a systematic review and meta-analysis. J Alzheimers Dis 2019;71:307–316. [DOI] [PubMed] [Google Scholar]
- 30. Kivimäki M, Singh-Manoux A, Pentti J, Sabia S, Nyberg ST, Alfredsson L, Goldberg M, Knutsson A, Koskenvuo M, Koskinen A, Kouvonen A, Nordin M, Oksanen T, Strandberg T, Suominen SB, Theorell T, Vahtera J, Väänänen A, Virtanen M, Westerholm P, Westerlund H, Zins M, Seshadri S, Batty GD, Sipilä PN, Shipley MJ, Lindbohm JV, Ferrie JE, Jokela M. IPD-Work consortium. Physical inactivity, cardiometabolic disease, and risk of dementia: an individual-participant meta-analysis. BMJ 2019;365:l1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schneider LS. Reduce vascular risk to prevent dementia? Lancet 2016;388:738–740. [DOI] [PubMed] [Google Scholar]
- 32. Khera AV, Chaffin M, Aragam KG, Haas ME, Roselli C, Choi SH, Natarajan P, Lander ES, Lubitz SA, Ellinor PT, Kathiresan S. Genome-wide polygenic scores for common diseases identify individuals with risk equivalent to monogenic mutations. Nat Genet 2018;50:1219–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rojas I. D, Moreno-Grau S, Tesi N, Grenier-Boley B, Andrade V, Jansen I, Pedersen NL, Stringa N, Zettergren A, Hernández I, Montrreal L, Antúnez C, Antonell A, Tankard RM, Bis JC, Sims R, Bellenguez C, Quintela I, González-Perez A, Calero M, Franco E, Macías J, Blesa R, Menéndez-González M, Frank-Garcia A, Royo JL, Moreno F, Huerto R, Baquero M, Diez-Fairen M, Lage C, Garcia-Madrona S, Garcia P, Alarcon-Martin E, Valero S, Sotolongo-Grau O, EADB, GR@ACE, DEGESCO, IGAP (ADGC, CHARGE, EADI, GERARD), PGC-ALZ, Garcia-Ribas G, Sanchez-Juan P, Pastor P, Perez-Tur J, Pinol-Ripoll G, de Munain AL, Garcia-Alberca JM, Bullido MJ, Alvarez V, Lleo A, Real LM, Mir P, Medina M, Scheltens P, Holstege H, Marquie M, Saez ME, Carracedo A, Amouyel P, Williams J, Seshadri S, van Duijn CM, Mather KA, Sanchez-Valle R, Serrano-Rios M, Orellana A, Tarraga L, Blennow K, Huisman M, Andreassen OA, Posthuma D, Clarimon J, Boada M, van der Flier WM, Ramirez A, Lambert JC, van der Lee SJ, Ruiz A. Common variants in Alzheimer’s disease: novel association of six genetic variants with AD and risk stratification by polygenic risk scores. medRxiv doi: 10.1101/19012021 (17 July 2020). [DOI] [Google Scholar]
- 34. Green RC, Roberts JS, Cupples LA, Relkin NR, Whitehouse PJ, Brown T, Eckert SL, Butson M, Sadovnick AD, Quaid KA, Chen C, Cook-Deegan R, Farrer LA. Disclosure of APOE genotype for risk of Alzheimer’s disease. N Engl J Med 2009;361:245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hofman A, Brusselle GGO, Murad SD, Duijn CV, Franco OH, Goedegebure A, Ikram MA, Klaver CCW, Nijsten TEC, Peeters RP, Stricker BHC, Tiemeier HW, Uitterlinden AG, Vernooij MW. The Rotterdam Study: 2016 objectives and design update. Eur J Epidemiol 2015;30:661–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scand J Public Health 2011;39:30–33. [DOI] [PubMed] [Google Scholar]
- 37. Helweg-Larsen K. The Danish Register of causes of death. Scand J Public Health 2011;39:26–29. [DOI] [PubMed] [Google Scholar]
- 38. Phung TKT, Andersen BB, Høgh P, Kessing LV, Mortensen PB, Waldemar G. Validity of dementia diagnoses in the Danish hospital registers. Dement Geriatr Cogn Disord 2007;24:220–228. [DOI] [PubMed] [Google Scholar]
- 39. Rasmussen KL, Tybjærg-Hansen A, Nordestgaard BG, Frikke-Schmidt R. Absolute 10-year risk of dementia by age, sex and APOE genotype: a population-based cohort study. Can Med Assoc J 2018;190:E1033–E1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.