Abstract
In recent years, the study of single nucleotide polymorphisms (SNPs) has gained increasing importance in biomedical research, as they can either be at the molecular origin of a determined disorder or directly affect the efficiency of a given treatment. In this regard, sequence variations in genes involved in pro-survival cellular pathways are commonly associated with pathologies, as the alteration of these routes compromises cellular homeostasis. This is the case of autophagy, an evolutionarily conserved pathway that counteracts extracellular and intracellular stressors by mediating the turnover of cytosolic components through lysosomal degradation. Accordingly, autophagy dysregulation has been extensively described in a wide range of human pathologies, including cancer, neurodegeneration, or inflammatory alterations. Thus, it is not surprising that pathogenic gene variants in genes encoding crucial effectors of the autophagosome/lysosome axis are increasingly being identified. In this review, we present a comprehensive list of clinically relevant SNPs in autophagy-related genes, highlighting the scope and relevance of autophagy alterations in human disease.
Keywords: autophagy, pathology, SNPs, variants, polymorphisms, ATGs, autophagic receptors, lysosomes
1. Introduction
Sequence variations are the basis of genetic heterogeneity, which is essential for species to improve their fitness in the environment. Among these genetic changes, alterations with a minor allele frequency of at least 1% in a given population are called polymorphisms, and variants that affect only one base of the sequence (including exchanges, deletions or insertions) are termed single nucleotide polymorphisms (SNPs). From a clinical perspective, these variants are important because they alter the activity of the affected gene products. In other words, SNPs can be the underlying origin of different types of diseases or even explain the differential effect of some treatments in determined individuals. SNPs are mainly identified and analyzed by genome-wide association studies (GWASs), unveiling alleles that determine the susceptibility of their carriers to a given condition. It is not surprising that variants that negatively impact the function of genes involved in essential processes, such as DNA repair or autophagy, have been widely associated with different pathologies.
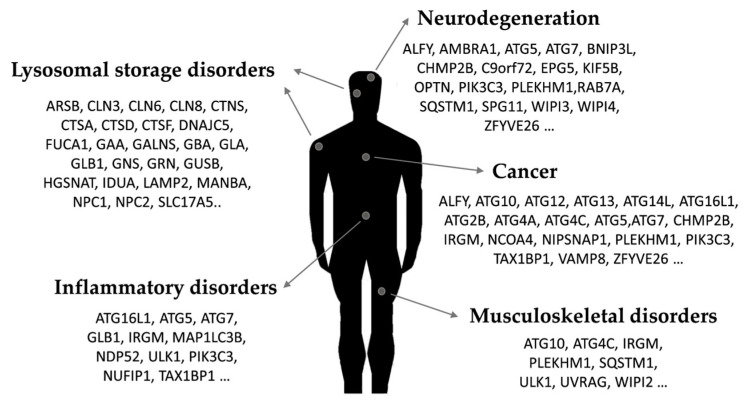
Tightly integrated into the cellular network of the stress response, autophagy is an essential mechanism for the maintenance of cellular homeostasis [1]. This catabolic pathway, present in all nucleated cells, can be defined as any mechanism which mediates the degradation of cellular components, including entire organelles, by the action of lysosomal hydrolases (in fact, “autophagy” derives from Greek, meaning “self-eating”) [2]. According to the way the cargo is transferred to the lysosome, there are three main autophagic pathways, namely microautophagy, chaperone-mediated autophagy (CMA) and macroautophagy [3]. During microautophagy, for example, substrates are directly engulfed by lysosomal protrusions or invaginations [4], while CMA requires the activity of chaperone Hsc70 (HSPA8) and LAMP2A to selectively recognize and internalize proteins showing the KFERQ motif [5]. In contrast, macroautophagy (which is the focus of this review, and will be hereafter referred to as “autophagy”) is based on the sequestration of cytoplasmic content by double-membrane vesicles, termed “autophagosomes” [6]. Once fully formed, autophagosomes eventually fuse their outer membrane with membranes of acidic lysosomes to become autolysosomes. Autolysosomes have hydrolytic activity, degrade their cargo, and recycle essential biomolecules to the cytoplasm [7]. Autophagy is active in all eukaryotic cells at basal rates, allowing the periodic renovation of cytosolic components or cytoplasmic organelles, acting as a housekeeping mechanism to preserve homeostasis. However, in response to a variety of cellular stresses, including nutrient deprivation, hypoxia, the accumulation of damaged organelles, protein aggregates or the presence of intracellular pathogens, the rate of autophagic degradation increases. This allows cells to eliminate damaged or harmful components through catabolism while supplying nutrients and energy to preserve cell viability. Given its fundamental roles in cell physiology, it was hypothesized early on that autophagy dysregulation could contribute to the pathogenesis of different diseases. Accordingly, a growing number of studies have linked autophagy alterations to a wide range of human pathologies, from immunological disorders to neurodegeneration or cancer [8] (Figure 1).
Figure 1.
Representative links between autophagy-related proteins and human pathology.
In this review, we collect and go through a comprehensive list of SNPs in autophagy-related genes that have been associated with human diseases. This approach highlights the relevance of specific genes and variants in human pathology, while giving us new insights into the real scope of autophagy-related SNPs in disease. We include variants in genes whose products are part of the main autophagy core machinery and also those in genes codifying lysosomal proteins, as its disruption leads to the alteration of the autophagosome/lysosome axis.
2. Autophagy Dysregulation in Disease
As it has been previously reviewed, several human disorders show alterations in autophagy [8,9]. Pathological dysregulation of autophagy is not restricted to a specific type of disease. In fact, a wide range of disorders that may not share a common etiology and affect different tissues or organs have been connected to autophagy dysregulation [10] (Figure 1). However, how autophagy alteration contributes to the pathogenesis of specific pathologies is far from being completely understood, as important questions remain unanswered [11].
One of the clinical fields in which autophagy’s role has been more extensively studied is oncology. Although it is still a long-standing subject of debate in the field, it is becoming increasingly clear that autophagy plays a dual role in this context, either favoring or fighting against the development of cancer [12]. On the one hand, the protective activity of this pathway prevents the initial malignant transformation of cancer cells. On the other hand, once the tumor is formed, autophagy would help the cancerous cells survive. All in all, the beneficial or detrimental effect of autophagy in cancer is likely to be tumor- and stage-dependent. Unsurprisingly, pathogenic and protective SNPs have been reported in autophagy-related genes, and a growing number of studies have been profiling the autophagy-related gene prognostic signature of different types of cancer [13,14,15,16,17,18,19,20,21,22,23,24]. The pivotal role of antitumor immunity against cancer progression adds even more complexity to the autophagy–cancer relation, as autophagy is also important for immunological processes.
In fact, autophagy plays an essential role in the correct functioning of the immune system, acting at different levels [25]. It has been shown that autophagy is implicated in the development of different immune cell populations [26], in antigen presentation [27] or in adaptive immunity [28]. Additionally, autophagy contributes to the control of innate immune signaling, participating in the finely tuned balance between activated and repressed immune responses [29]. In fact, autophagy dysregulation can alter this equilibrium, leading to chronic inflammatory diseases [30]. This could explain why mutations of autophagy-related genes have been associated with several autoimmune disorders, including systemic lupus erythematosus (SLE), different types of sclerosis or rheumatoid arthritis [31]. Well-studied examples of autophagy dysregulation in uncontrolled inflammatory responses are inflammatory bowel diseases (IBDs), particularly Crohn’s disease [32]. Autophagy is also an important defense barrier against infection, as it contributes to the degradation of intracellular pathogens (i.e., virus or bacteria) [33]. However, some of these infective agents have acquired molecular mechanisms to evade and use the autophagic machinery for their benefit, which aggravates infection in some cases [34].
Undoubtedly, one of the clinical contexts in which the link between deficient autophagy and pathology is more firmly-established is neurodegeneration [35]. In this regard, the inability to clear the accumulation of aggregation-prone misfolded proteins (including β-amyloid, huntingtin or α-synuclein) hampers cell viability, leading to the progressive loss of central nervous system function. This is the case of disorders such as Alzheimer’s, Huntington’s, or Parkinson’s diseases, with some of them also showing problems in mitophagy, the selective autophagic degradation of mitochondria [36]. Development of amyotrophic lateral sclerosis (ALS) has also been associated with the accumulation of different protein aggregates or defective mitochondrial clearance, and pathogenic variants in components of the autophagic pathway have been described in patients [37]. Additional neurological disorders that have been linked to autophagy failure are spastic paraplegias, beta-propeller protein-associated neurodegeneration or Charcot–Marie–Tooth diseases, where autophagosome maturation, transport and/or its fusion with the lysosome are blocked [38].
The musculoskeletal system also requires autophagy to maintain its homeostasis, and autophagy dysregulation has also been associated with musculoskeletal pathologies. For example, different myopathies have been termed autophagic myopathies, as most of them show blocked autophagy flux and accumulation of autophagic vacuoles [39]. In bone tissue, autophagy plays an important role in controlling the balance between bone resorption and formation. Consistently, alterations in the autophagic pathway have been found in different diseases caused by the perturbation of bone physiology, such as Paget’s disease, osteopetrosis or osteoporosis [40]. Moreover, chondrocytes of the cartilage are also more susceptible to cell death when autophagy is disrupted, leading to osteoarthritis [41].
Remarkably, both neurological and musculoskeletal alterations are common features observed in lysosomal storage diseases (LSDs). LSDs are rare metabolic disorders caused by alterations in genes that are required for lysosomal-mediated degradation [42]. These pathogenic variants often result in the accumulation of specific undegraded substrates in the lumen of this organelle, hindering lysosomal function [43]. The identity of the unprocessed molecule (sphingolipids, glycogen, glycosaminoglycans, etc.) is the base of the classification of LSDs. For example, mucopolysaccharidoses (MPSs) are mainly caused by mutations in specific lysosomal genes that contribute to the degradation of glycosaminoglycans (GAGs), while Danon disease and Pompe disease are characterized by the intralysosomal accumulation of glycogen. Deficiency in the catabolism of glucocerebrosides causes Gaucher disease and failure to degrade globotriaosylceramide results in Fabry disease. Niemann–Pick type C disease is caused by the accumulation of unesterified cholesterol in several organs, while cystinosis is characterized by the accumulation of the amino acid cystine. Other important LSDs are galactosialidosis (with sialyloligosaccharide accumulation), fucosidosis (witch lysosomal aggregation of different molecules containing fucose moieties), mannosidosis (characterized by a deficiency in the degradation of mannose-rich oligosaccharides) and sialic acid storage diseases. Given the intricate connection between autophagy and lysosomal activity, it is not surprising that defects on any of them have an impact on the other one [44,45].
3. Relevant Variants on Autophagy-Related Genes
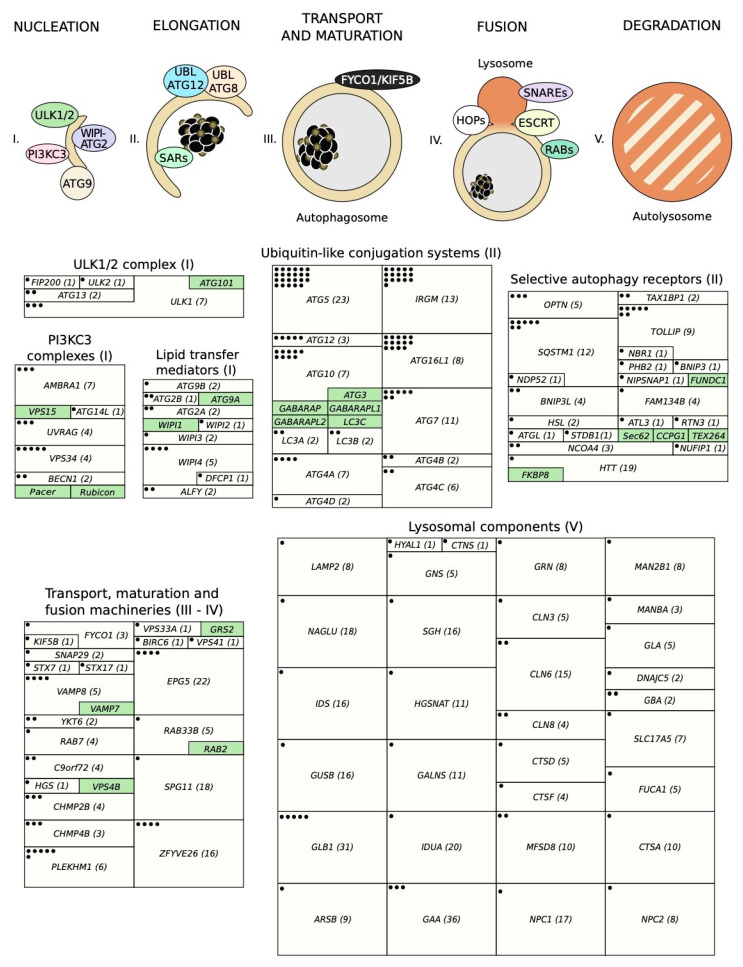
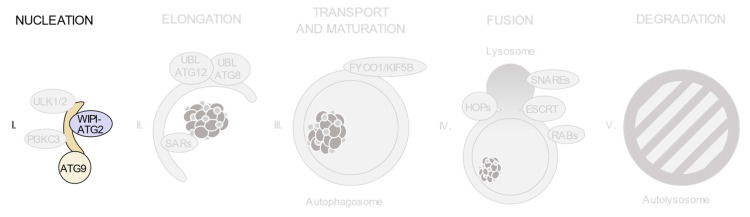
As shown in Figure 2, autophagic degradation involves different sequential stages, which operate from the regulation of autophagosome biogenesis to the last steps of autophagosome cargo degradation and recycling: (1) autophagy initiation, (2) membrane nucleation, (3) pre-autophagosomal membrane expansion, (4) autophagosome fusion with lysosomes and (5) degradation and efflux of basic components [46,47,48]. Each of these steps requires the coordinated temporal and spatial activation of several molecular components, namely the ULK1/2 kinase protein complex; the class III phosphatidylinositol 3-kinase (PI3KC3) protein complexes; phosphatidylinositol 3-phosphate (PI(3)P)-binding proteins and the ATG9-containing membranes; the ATG12 and ATG8 UBL conjugation systems; the selective autophagy receptors and the factors involved in autophagosome-lysosome fusion. Interestingly, pathogenic gene variants have already been described for all these different groups of effectors.
Figure 2.
Schematic view of the incidence of clinically relevant single nucleotide polymorphisms (SNPs) in genes throughout the autophagosome/lysosome axis. Autophagy can be divided in different stages: (I) initiation and membrane nucleation, (II) membrane expansion, (III) autophagosome maturation and transport, (IV) autophagosome-lysosome fusion and (V) lysosomal degradation. Pathological variants have been found in genes involved in all of the steps. Boxes show the genes whose products participate in each of these stages. The size of the boxes is proportional to the number of pathological SNPs found for each of the genes depicted. The total number of pathological SNPs found in a given gene is depicted between brackets. Each different disease linked to a determined gene is represented by a dot. Green boxes contain genes without any clinically relevant SNPs identified to date. PI3KC3, class III phosphatidylinositol 3-kinase protein complexes; UBL, ubiquitin-like conjugation system; SARs, selective autophagy receptors; HOPS, homotypic fusion and protein sorting tethering complex; ESCRT, endosomal sorting complexes required for transport.
3.1. The ULK1/2 Kinase Complex
The members of the ULK (Unc-51-like kinase) family of proteins are the orthologues of the yeast Atg1, a serine/threonine protein kinase essential for autophagy initiation (Figure 3). In human cells, there are five ULK proteins (ULK1, ULK2, ULK3, ULK4 and STK36) although among them, only ULK1 and ULK2 are involved in autophagy [48]. In living cells, ULK1 or ULK2 are part of a protein complex with at least ATG13, ATG101 and FIP200 (family-interacting protein of 200kDa, also known as RB1CC1). This complex is responsible for driving autophagy initiation upon autophagy-inducing stimuli [49]. When active, the ULK1/2 complex translocates to autophagosome formation sites and regulates the recruitment and activation of the class III phosphatidylinositol 3-kinase (PI3KC3) complex, which in turn will generate phosphatidylinositol 3-phosphate (PI(3)P), a signaling molecule that recruits other downstream factors involved in autophagosome biogenesis. Moreover, the ULK1/2 protein complex carries other different autophagy-related functions, such as ATG9-vesicle recruitment or regulation of ATG4B activity, and contributes to regulate mitophagy and degradation of protein aggregates [50]. Due to its importance in autophagy initiation, this protein complex is regulated by a variety of post-translational modifications, such as acetylation, ubiquitin conjugation or phosphorylation by protein kinases. Among these, adenosine monophosphate-activated protein kinase (AMPK) and mechanistic/mammalian target of rapamycin (mTOR) are the most relevant, connecting ULK1/2 complex activity to the nutritional and energetic status of the cell [51,52].
Figure 3.
The ULK1/2 kinase complex participates in the nucleation of the pre-autophagosomal membrane.
Several pathogenic variants of the ULK1/2 kinase complex have been identified (Table 1 and Table S1). For example, SNPs in ULK1 have been associated with Crohn’s disease susceptibility and clinical outcomes in different populations [53,54], supporting previous evidence of autophagy alterations in inflammatory bowel diseases. Additional variants of ULK1 have shown strong associations with tuberculosis [55,56] and also with a specific type of rheumatoid arthritis termed ankylosing spondylitis [57], further linking ULK1 activity with the immune system. SNPs in ULK2 have only been linked to asparaginase-associated pancreatitis to date [58]. Also related with the immune system is an ATG13 variant that may be associated with selective immunoglobulin A deficiency (IgAD), although it is not clear if this polymorphism affects ATG13 or AMBRA1 (which is another gene involved in autophagy) [59]. Additionally, altered ATG13 activity may also be involved in chemotherapy-induced cardiotoxicity in triple-negative breast cancer patients [60]. Regarding FIP200 only one pathogenic SNP has been documented, which predicts hypertension after metastatic colorectal cancer treatment [61]. In synthesis, polymorphisms in members of the ULK1/2 kinase complex have been associated with a variety of pathologies, some of them related to immune system dysfunction. It is also remarkable that seven different pathogenic SNPs have already been identified in the ULK1 gene (Figure 2). It is also noteworthy that different variants of this gene have been linked to a determined pathology (such as Crohn’s disease or tuberculosis).
Table 1.
Clinically relevant SNPs in ULK1/2 complex.
| Gene | Disease | dbSNP rsID |
|---|---|---|
| ATG13 | Selective immunoglobulin A deficiency | rs4565870 |
| ATG13 | Breast cancer | rs10838611 |
| FIP200 | Hypertension | rs1129660 |
| ULK1 | Crohn’s disease | rs12303764; rs10902469; rs7488085 |
| ULK1 | Tuberculosis | rs12297124; rs7138581; rs9481 |
| ULK1 | Ankylosing spondylitis | rs9652059 |
| ULK2 | Asparaginase-associated pancreatitis | rs281366 |
3.2. The Class III Phosphatidylinositol 3-Kinase (PI3KC3) Complexes
After being activated at the assembling site, the ULK1/2 kinase complex acts as a scaffold for the PI3KC3 complex, whose activity is essential for the nucleation of the pre-autophagosomal membranes by generating PI(3)P, an essential signal for autophagosome formation, which will in turn recruit additional downstream factors involved in autophagosome biogenesis [49] (Figure 4). This complex is formed by Beclin 1, VPS34/PIK3C3, VPS15/p150/PIK3R4 and Barkor/ATG14L [62]. The most important event for PI3KC3 regulation is the sequestration of Beclin 1 by BCL-2, which limits its ability to bind PI3KC3 complexes, resulting in autophagy inhibition [63]. Conversely, AMBRA1 can also bind to Beclin 1 (and other autophagy-related proteins), increasing PI3KC3 complex activity and thus supporting autophagosome formation [64]. Interestingly, there is an additional version of the PI3KC3 complex (often called PI3KC3-C2) in human cells, which is also involved in autophagy regulation. PI3KC3-C2 contains UVRAG instead of ATG14 and is involved in regulating the fusion of autophagosomes to lysosomes [65]. The activity of this complex can be repressed by binding of the negative regulator Rubicon/KIAA0226, which results in the inhibition of autophagosome/lysosome fusion [66,67]. Conversely, its activity can be enhanced by binding of the protein associated with UVRAG as autophagy enhancer (Pacer), which increases autophagic degradation [68].
Figure 4.
The phosphatidylinositol 3-kinase (PI3KC3) complexes participate in the nucleation of the pre-autophagosomal membrane.
Despite the relevance of Beclin 1 in autophagy, only two SNPs found in the BECN1 gene have so far been associated with diabetes [69] and Machado–Joseph disease [70], a neurodegenerative disorder characterized by progressive cerebellar ataxia (Table 2 and Table S1). On the other hand, polymorphisms on VPS34/PIK3C3 do correlate with increased cancer risk, specifically in pancreatic adenocarcinoma [71] and esophageal squamous cell carcinoma [72,73]. A third variant has been linked to gastric cardia adenocarcinoma, although it seems to be related to non-autophagical functions of VPS34 in the control of telomere length [74]. Another single nucleotide change on the promoter of VPS34 has been associated with both systemic lupus erythematosus [75] and with bipolar disorder and schizophrenia [76]. Interestingly, several variants of AMBRA1 have also been linked to schizophrenia [77], as well as to diverse forms of autism [78]. A polymorphism in ATG14 has been associated with testicular germ cell tumors [79], while different UVRAG alleles have been linked to a less efficient treatment response in multiple sclerosis [80], susceptibility to rheumatoid arthritis [81] and non-segmental vitiligo [82]. Altogether, these findings show that genetic variants in components of the PI3KC3 complexes are linked to a variety of pathologies, including cancer, autoimmune or neurological disorders. Among all of them, VPS34 and UVRAG seem to be more sensitive to nucleotide changes, with AMBRA1 (often associated with PI3KC3 complexes) also accumulating several pathogenic polymorphisms (Figure 2).
Table 2.
Clinically relevant SNPs in PI3KC3 complexes.
| Gene | Disease | dbSNP rsID |
|---|---|---|
| AMBRA1 | Schizophrenia | rs11819869; rs12574668; rs61882743; rs7112229; rs7130141 |
| AMBRA1 | Autism | rs3802890 |
| AMBRA1 | Selective immunoglobulin A deficiency | rs4565870 |
| ATG14L | Testicular germ cell tumor | rs1009647 |
| BECN1 | Machado–Joseph disease | rs60221525 |
| BECN1 | Diabetes | rs10512488 |
| UVRAG | Multiple sclerosis treatment | rs80191572 |
| UVRAG | Rheumatoid arthritis | rs7111334 |
| UVRAG | Non-segmental vitiligo | rs1458836; rs7933235 |
| VPS34 | Pancreatic cancer | rs76692125 |
| VPS34 | Esophageal squamous cell carcinoma | rs52911 |
| VPS34 | Gastric cancer | rs2162440 |
| VPS34 | Schizophrenia | rs3813065 |
| VPS34 | Systemic lupus erythematosus | rs3813065 |
3.3. PI(3)P-Binding Proteins and the ATG9-Containing Membranes
Production of PI(3)P by the PI3KC3 complex acts as a signal for the recruitment of autophagy-related proteins able to bind this phospholipid. Among these, the most characterized are DFCP1, WIPI proteins and ALFY [83]. DFCP1 recruitment to autophagosome formation sites occurs shortly after PI(3)P generation by PI3KC3. There, it labels the omegasome, a platform for the formation of autophagosomes that originate from the ER-associated membranes. DFCP1 colocalizes with LC3 and other essential proteins for autophagosome biogenesis. However, despite DFCP1 being often used as a marker for the omegasome, its depletion has no major effect on autophagy [84]. WIPI proteins are also recruited by the presence of PI(3)P at the pre-autophagosomal membranes, where they contribute to autophagosome formation by recruiting other autophagy essential proteins for this process [85] (Figure 5). In human cells, there are four WIPI proteins (WIPI 1-4). WIPI1 and WIPI2 proteins participate in the expansion of the autophagosomal membrane, with WIPI2 being responsible for recruiting the ATG5–ATG12/ATG16 complex to nascent autophagosomes [86]. In contrast, WIPI3 and WIPI4 interact with upstream regulators, such as the ULK1/2 complex and AMPK-activated TSC complex, coupling PI(3)P generation to the activity of upstream regulatory signaling [87]. Moreover, WIPI4 plays an important role in controlling the growth and size of autophagosomes through its interaction with ATG2 proteins. In yeast, Atg2 acts as a lipid transfer protein that supplies phospholipids to autophagosomal membranes [88]. In mammals, there are two Atg2 orthologues, ATG2A and ATG2B, that can translocate lipids from other membranes to nascent autophagosomes, forming a complex with WIPI4 that acts as a membrane tether with lipid transfer activity [89]. ALFY (autophagy-FYVE-linked protein) is also a PI(3)P-binding protein, which is recruited to nascent autophagosomal membranes close to protein aggregates. Although ALFY is not essential for autophagosome biogenesis during starvation-induced autophagy, it is required for protein aggregate autophagic degradation [90]. The only transmembrane proteins directly involved in autophagosome biogenesis are those from the ATG9 family, composed by ATG9A and ATG9B. ATG9 proteins assist with lipid transport and transferring to nascent autophagosomal membranes [49,91] (Figure 5). Concurrently with PI3KC3 complex activation, the ULK1 kinase complex also mediates the recruitment of vesicles containing either ATG9A or ATG9B (depending on the tissue or cell type). These transmembrane proteins drive the aforementioned vesicles to the assembling site so they can be used as membrane sources, contributing to the elongation of the autophagosomal membrane [83]. WIPI2 is also involved in the regulation of ATG9 traffic, as WIPI2 depletion hampers ATG9 dynamics and results in the accumulation of ATG9 pre-autophagosomal structures due to the inhibition of ATG9 retrieval to Golgi complex membranes [92].
Figure 5.
The phosphatidylinositol 3-phosphate (PI(3)P)-binding proteins (WIPI or ATG2 proteins) and ATG9-containing vesicles participate in the nucleation of the pre-autophagosomal membrane.
To date, only one pathogenic SNP on DFCP1 has been found, which is linked to tuberculosis resistance [93]. In contrast, nucleotide changes on ALFY were identified in patients with microcephaly [94], as well as in oropharynx cancer [95]. WIPI4 pathogenic SNPs have been shown to cause neurological disorders, such as neurodegeneration with brain iron accumulation (NBIA) and Rett syndrome [96,97,98], while polymorphisms on WIPI2 and WIPI3 have been associated with osteoporosis [99] and a neurodevelopmental syndrome [100]. Two variants of ATG2A have been linked with both granuloma formation in Crohn’s disease and hyperuricemia [101,102]. Interestingly, the equivalent SNP in ATG2B increases susceptibility to neck squamous cell carcinoma in pharyngeal cancer [103] and correlates with both progression and recurrence of bladder cancer after treatment with bacillus Calmette–Guérin intravesical instillation [104]. Finally, a polymorphism associated with coronary artery disease has been identified on ATG9B [105] (Table 3 and Table S1). In summary, pathogenic SNPs that have been described on lipid transfer mediators are linked to pathologies of different origins. In contrast, pathogenic SNPs on genes coding for PI(3)P-binding proteins are mostly associated with neurological disorders. It is remarkable that distinct pathogenic polymorphisms related to very different diseases have been described for WIPI4 (Figure 2).
Table 3.
Clinically relevant SNPs in PI(3)P-binding proteins and ATG9 orthologues.
| Gene | Disease | dbSNP rsID |
|---|---|---|
| ATG2A | Granuloma formation in Crohn’s disease | rs17146441 |
| ATG2A | Hyperuricemia | rs188780113 |
| ATG2B | Non-muscle invasive bladder cancer | rs3759601 |
| ATG2B | Head and neck squamous cell carcinoma | rs3759601 |
| ATG9B | Coronary artery disease | rs2373929; rs7830 |
| ALFY | Microcephaly | rs1553924800 |
| ALFY | Malignant neoplasm of oropharynx | rs6847067 |
| DFCP1 | Tuberculosis | rs2333021 |
| WIPI2 | Osteoporosis | rs4720530 |
| WIPI3 | Neurodevelopmental disorder | rs786205510; rs1555647262 |
| WIPI4 | Rett syndrome | rs886041382; rs886041693 |
| WIPI4 | Neurodegeneration with brain iron accumulation | rs886041382 |
| WIPI4 | Early-onset epileptic encephalopathy | rs1064793294 |
| WIPI4 | β-propeller protein-associated neurodegeneration (BPAN) | rs387907330 |
3.4. The ATG12 and ATG8 Conjugation Systems
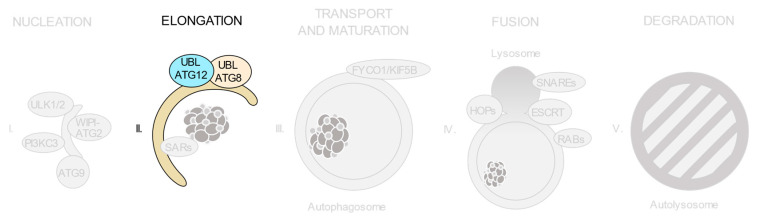
Two ubiquitin-like (UBL) conjugation systems are essential for autophagosome formation and autophagic cargo degradation (Figure 6). These UBL systems cooperate to drive the expansion of the nascent autophagosomal membrane, with the final product of the first system being the responsible enzyme for the last reaction in the second one [106]. The first UBL system, the ATG12 system, mediates the activation, transfer, and covalent conjugation of ATG12 to ATG5, a process that requires the sequential activities of the E1-like enzyme ATG7 and the E2-like enzyme ATG10. Once formed, two molecules of the ATG12–ATG5 conjugate interact with an ATG16 dimer, resulting in the final ATG16 complex. This complex is recruited to nascent autophagosomes by the actions of WIPI1 and WIPI2 proteins [86]. After being recruited, ATG16 keeps the ATG5–ATG12 conjugate at the pre-autophagosomal membrane, where it acts as an E3-ligase for the ATG8 UBL system, catalyzing ATG8 covalent conjugation [107]. It is interesting that although there are two ATG16 homologues (ATG16L1-2) in human cells, only ATG16L1 seems to play a prominent role in autophagy [108,109].
Figure 6.
The ubiquitin-like (UBL) conjugation systems of ATG12 and ATG8 participate in the elongation of the pre-autophagosomal membrane.
The second UBL system, the ATG8 system, mediates the conjugation of ATG8 molecules to phosphatidyl-ethanolamine (PE) phospholipids at the pre-autophagosomal membrane (Figure 6). In humans, there are six ATG8 proteins, grouped into two subfamilies: the MAP1-LC3 subfamily (including MAP1-LC3A, MAP1-LC3B and MAP1-LC3C) and the GABARAP subfamily (including GABARAP, GABARAPL1/ATG8L and GABARAPL2/GATE-16). All these proteins can be found either free or in their lipidated (PE-conjugated) form in human cells [110]. In normal conditions, the unconjugated ATG8 forms are mostly cytosolic. In contrast, ATG8 PE-conjugated forms are mainly associated with the inner and outer membranes of the autophagosome. ATG8 conjugation first requires the activity of ATG4 proteases (ATG4A-D, also called “autophagins” in humans) [111]. ATG4s cleave and activate ATG8 proteins, leaving a glycine residue at the carboxyl terminus, which is essential for ATG8 lipidation. ATG7 (E1-like enzyme), ATG3 (E2-like enzyme) and the ATG12-ATG5–ATG16L1 multimeric complex (E3-like enzyme) are necessary for ATG8 conjugation to PE. This conjugation is, in turn, essential for the expansion of the pre-autophagosomal membrane, autophagosome maturation and degradation of autophagic cargo [106]. Once ATG8s’ presence at the autophagosomal/autolysosomal membranes is no longer required, they can be deconjugated from PE by the action of ATG4s. In yeast, Atg8 deconjugation is important to keep an available pool of ready-to-use Atg8 in the cytosol and required for efficient autophagosome biogenesis and maturation [112,113].
Interestingly, most of the pathogenic polymorphisms that have been already identified on autophagy-related genes are located on the genomic sequence of members of the ATG12 conjugation system (Table 4 and Table S1). In fact, variants affecting different genes from this system are associated with the same pathology. For example, pathogenic alleles of ATG5, ATG10, ATG12 and ATG16L1 have been all linked to changes in susceptibility or treatment efficiency in neck squamous cell cancer [103,114,115,116], hepatocellular carcinoma [117] and lung adenocarcinoma [118] and others have been found to also affect development of other types of cancer, such as melanoma [119], brain metastases in patients with non-small lung cancer [120] or breast cancer [121,122,123,124]. Moreover, there is also an association between SNPs in ATG5 and ATG7 with clear cell renal cell carcinoma [125] and variants of ATG5 and ATG10 have been linked to non-small cell lung cancer [126,127]. Additional connections between changes on these genes and cancer are those of ATG5 in multiple myeloma [128] or non-medullary thyroid cancer [129]. Different ATG16L1 SNPs have been linked to cell-derived thyroid carcinoma [130], colorectal cancer [131], gastric cancer [132] or prostate cancer [133]. Moreover, several polymorphisms on the ATG12 conjugation system have also been associated with other pathologies besides cancer. In fact, SNPs in ATG5 and ATG7 are linked to the development of neurological disorders such as cerebral palsy (both ATG5 and ATG7) [134,135], Huntington’s disease (ATG7) [136,137], and Parkinson’s disease or spinocerebellar ataxia (ATG5) [138,139]. Additionally, nucleotide polymorphisms on ATG5, ATG10 and ATG12 have been related to pneumoconiosis in a population of coal workers [140], whereas other SNPs in ATG7 have been associated with ischemic stroke [141].
Table 4.
Clinically relevant SNPs in the ubiquitin-like conjugation systems ATG12 and ATG8.
| Gene | Disease | dbSNP rsID |
|---|---|---|
| ATG10 | Breast cancer | rs10514231; rs1864182; rs7707921 |
| ATG10 | Paget’s disease of the bone | rs1864183 |
| ATG10 | Vogt–Koyanagi–Harada syndrome | rs4703863 |
| ATG10 | Lung cancer | rs10514231; rs1864182; rs1864183; rs10036653 |
| ATG10 | Melanoma | rs1864182 |
| ATG10 | Brain metastasis | rs10036653 |
| ATG10 | Head and neck squamous cell carcinoma | rs10514231; rs1864183; rs4703533 |
| ATG10 | Pneumoconiosis | rs1864182 |
| ATG10 | Hepatocellular carcinoma | rs10514231; rs1864183 |
| ATG12 | Brain metastasis | rs26532 |
| ATG12 | Pneumoconiosis | rs26538 |
| ATG12 | Lung cancer | rs26538 |
| ATG12 | Head and neck squamous cell carcinoma | rs26537 |
| ATG12 | Hepatocellular carcinoma | rs26537 |
| ATG16L1 | Crohn’s disease | rs2241880 |
| ATG16L1 | Palmoplantar pustulosis | rs2241879; rs2241880; rs7587633 |
| ATG16L1 | Psoriasis vulgaris | rs10210302; rs12994971; rs13005285; rs2241879; rs2241880 |
| ATG16L1 | Cell-derived thyroid carcinoma | rs2241880 |
| ATG16L1 | Colorectal cancer | rs2241880 |
| ATG16L1 | Paget’s disease of the bone | rs2241880 |
| ATG16L1 | Prostate cancer | rs78835907 |
| ATG16L1 | Gastric cancer | rs2241880 |
| ATG16L1 | Melanoma | rs2241880 |
| ATG16L1 | Brain metastasis | rs2241880 |
| ATG16L1 | Head and neck squamous cell carcinoma | rs2241880; rs4663402 |
| ATG16L1 | Lung cancer | rs2241880 |
| ATG16L1 | Hepatocellular carcinoma | rs4663402 |
| ATG16L1 | Helicobacter pylori infection | rs2241880 |
| ATG4A | Cervical Cancer | rs5973822; rs4036579; rs807181; rs807182; rs807183 |
| ATG4A | Lung cancer | rs807185 |
| ATG4A | Granuloma formation in Crohn’s disease | rs5973822 |
| ATG4A | Clear cell renal cell carcinoma | rs7880351 |
| ATG4B | Obesity | rs7601000 |
| ATG4B | Atherosclerosis | rs139302128 |
| ATG4C | Clear cell renal cell carcinoma | rs6670694; rs6683832 |
| ATG4C | Kashin–Beck disease | rs11208030; rs4409690; rs12097658; rs6587988 |
| ATG4D | Granuloma formation in Crohn’s disease | rs7248036; rs2304165 |
| ATG5 | Systemic lupus erythematosus | rs6937876; rs3827644; rs573775; rs548234 |
| ATG5 | Asthma | rs12212740; rs11751513; rs12201458; rs2299863; rs510432 |
| ATG5 | Parkinson’s disease | rs510432 |
| ATG5 | Systemic sclerosis | rs3827644; rs9373839 |
| ATG5 | Non-medullary thyroid cancer | rs2245214 |
| ATG5 | Neuromyelitis optica | rs548234; rs6937876 |
| ATG5 | Paget’s disease of the bone | rs2245214 |
| ATG5 | Behçet’s disease | rs573775 |
| ATG5 | Spinocerebellar ataxia | rs1131692265 |
| ATG5 | Crohn’s disease | rs510432; rs9373839 |
| ATG5 | Multiple myeloma | rs9372120 |
| ATG5 | Melanoma | rs2245214; rs510432 |
| ATG5 | Sepsis | rs506027; rs510432 |
| ATG5 | Pneumoconiosis | rs510432 |
| ATG5 | Esophageal squamous cell carcinoma | rs1322178; rs3804329; rs671116 |
| ATG5 | Lung cancer | rs510432; rs688810; rs2245214 |
| ATG5 | Cerebral palsy | rs6568431 |
| ATG5 | Breast cancer | rs473543 |
| ATG5 | Clear cell renal cell carcinoma | rs490010 |
| ATG5 | Chronic Q fever | rs2245214 |
| ATG5 | Aplastic anaemia | rs473543; rs510432; rs573775; rs803360 |
| ATG5 | HBV infection | rs510432; rs6568431; rs548234 |
| ATG5 | Hepatocellular carcinoma | rs17067724 |
| ATG7 | Systemic lupus erythematosus | rs11706903; rs2736340 |
| ATG7 | Breast cancer | rs8154 |
| ATG7 | Ischemic stroke | rs2594966; rs2594973; rs4684776 |
| ATG7 | Lung cancer | rs8154 |
| ATG7 | Clear cell renal cell carcinoma | rs2606736; rs6442260 |
| ATG7 | Cerebral palsy | rs1470612; rs2594972 |
| ATG7 | Huntington’s disease | rs36117895 |
| IRGM | Crohn’s disease | rs10065172; rs1000113; rs10065172; rs11747270; rs11749391; rs180802994; rs4958843; rs4958847; rs72553867; rs7714584, rs9637876 |
| IRGM | Systemic lupus erythematosus | rs10065172; rs13361189 |
| IRGM | Ulcerative colitis | rs1000113; rs11747270; rs11749391; rs180802994; rs4958847 |
| IRGM | Tuberculosis | rs10051924; rs12654043; rs4958843; rs72553867 |
| IRGM | Celiac disease | rs10065172 |
| IRGM | Inflammatory bowel diseases | rs10065172; rs4958847 |
| IRGM | Ankylosing spondylitis | rs10065172; rs11749391 |
| IRGM | Arthritis | rs11747270; rs4958847 |
| IRGM | Chronic periodontitis | rs11747270 |
| IRGM | Asthma | rs11747270 |
| IRGM | Multiple sclerosis | rs11747270 |
| IRGM | Cholangitis | rs11749391 |
| IRGM | Psoriasis vulgaris | rs11749391 |
| IRGM | Pathologic fistula | rs4958847 |
| IRGM | Malignant neoplasm of stomach | rs4958847 |
| IRGM | Non-alcoholic fatty liver disease | rs4958847 |
| MAP1LC3A | Chronic Q fever | rs1040747 |
| MAP1LC3A | Coronary artery disease | rs2424994 |
| MAP1LC3B | Myopia | rs1054521 |
| MAP1LC3B | Systemic lupus erythematosus | rs933717 |
Perhaps the most studied SNP on an autophagy-related gene is rs2241880 on ATG16L1, resulting in a threonine-to-alanine substitution at amino acid position 300 (T300A). Its link to inflammatory bowel disease, first described by Hampe and collaborators [142], has been extensively confirmed in different populations by an immeasurable list of studies, impossible to entirely cite in this review. Polymorphisms on other proteins with autophagy-related functions have also been associated with inflammatory bowel diseases. For example, variants of IRGM, an important effector that links the autophagy molecular core to innate immunity receptors [143], are well-known risk alleles in pathologies such as Crohn’s disease [144]. Interestingly, two SNPs in ATG5 correlate to a positive response to Crohn’s disease therapy [145]. Moreover, variants of ATG16L1 and other effectors of the ATG12 conjugation system have also been linked to other inflammatory disorders, autoimmune diseases or other complications related to the immune system. In this regard, polymorphisms on ATG5, ATG10 and ATG16L1 have been associated with Paget’s disease of the bone [146], and SNPs in ATG5 and ATG7 have been extensively studied in the context of systemic lupus erythematosus [147,148,149,150,151,152,153]. In addition, several polymorphisms on ATG5 may play a role in the pathogenesis of other autoimmune disorders such as Behçet’s disease [154], neuromyelitis optica [155] and systemic sclerosis [156,157]. Additional inflammatory alterations linked to variants of ATG5 are aplastic anemia [158] or asthma [159,160] as well as complications related to infections, including chronic Q fever [161], sepsis [162] or Hepatitis B [163,164]. Similarly, SNPs in ATG16L1 are also associated with Helicobacter pylori infection and related gastric cancer [165,166,167] or skin conditions such as palmoplantar pustulosis [168] and psoriasis [169]. Finally, a variant of ATG10 has been associated with Vogt–Koyanagi–Harada disease, which is characterized by an autoimmune response against melanin-producing cells [154].
Fewer clinically relevant variants have been identified in genes encoding members of the ATG4 and ATG8 protein families (Table 4 and Table S1), perhaps because of their marked redundancy. Different SNPs in ATG4A have been associated with kidney, cervical and lung cancer [125,170,171]. Meanwhile, variants of ATG4B may be present in patients with obesity [172] or atherosclerosis [173]. Polymorphisms on ATG4C have also been linked to clear cell renal cell carcinoma [125], as well as an increase in susceptibility to Kashin–Beck disease, a osteochondropathy characterized by chondrocyte death and altered autophagy in growth plate and articular cartilage [174]. Nucleotide changes on ATG4A and ATG4D genes can also lead to the formation of granulomas during Crohn’s disease [101]. As for their substrates, the ATG8 proteins, only three gene variants have been associated with a pathology so far: two polymorphisms on MAP1LC3A that may contribute to progression of chronic Q fever [161] or coronary artery disease [175], and two SNPs that alter MAP1LC3B expression and correlate to myopia [176] and increased susceptibility to systemic lupus erythematosus [177]. To date, no pathogenic SNPs have been identified either in MAP1LC3C, GABARAP, GABARAPL1, GABARAPL2 nor in the gene encoding the E2-like enzyme ATG3.
In summary, alterations in autophagy UBL systems have been extensively linked to disease. This is shown not only by the high number of SNPs found in genes of these systems, but also by the total number of diseases to which they are associated (Figure 2). Specifically, genes such as ATG16L1, ATG5, ATG10 or ATG7, accumulate multiple SNPs linked to pathologies that include cancer, neurological disorders, or inflammatory bowel diseases.
3.5. Selective Autophagy Receptors
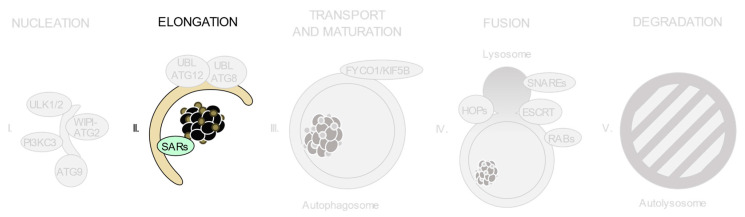
Autophagic degradation can be either bulk or selective. The last one requires the action of the so-called selective autophagy receptors (SARs), which mediate the recognition and engulfment of specific cargo in autophagosomes (Figure 7). Specifically, these adapters can simultaneously bind both to the target molecules and to the ATG8 proteins conjugated on the concave side of the autophagosomal membrane [178]. The identification and study of these SARs is likely one of the most exciting, fast-paced fields of autophagy research, as autophagy adapters show specificity to a wide variety of substrates. The most characterized mammalian SARs are those that bind ubiquitin molecules [179]. These molecules label a great variety of autophagic substrates, from protein aggregates to damaged mitochondria or intracellular pathogens. The most studied ubiquitin-binding SAR is p62/SQSTM1 [180], a multifunctional protein important for protein aggregate degradation (aggrephagy), mitophagy and the engulfment of intracellular pathogens by autophagosomes (xenophagy). Similarly, NBR1 is also involved in aggrephagy and acts synergically with p62/SQSTM1 for the degradation of ubiquitin-decorated protein aggregates [181]. OPTN is not only involved in aggrephagy [182] but also in mitophagy [183] and xenophagy [184]. Similarly, NDP52 is involved both in mitophagy [185] and xenophagy [186], as TAX1BP1 is [183,187]. TOLLIP has also been involved in mutant huntingtin-selective degradation, although it is less studied than other ubiquitin-binding SARs [188]. Apart from this group of SARs, many other proteins, each of them localized in a specific cellular structure/organelle, interact with ATG8s and act as specific SARs for their contained subcellular structures [189]. This is the case for BNIP3, BNIP3L/NIX, FUNDC1, FKBP8, PHB2 or NIPSNAP1/2, which are specific mitophagy SARs [190,191,192], or that of FAM134B, Sec62, RTN3, CCPG1, ATL3 and TEX264, which are specific SARs for ER-selective autophagy [193]. Apart from these, multiple other specific autophagy receptors for other selective autophagic processes are being constantly identified. This is the case of ATGL and HSL for lipophagy [194], STBD1 for glycophagy [195], NUFIP1 for ribophagy [196], or NCOA4 for ferritin degradation [197,198]. Certainly, new specific receptors for orphan-selective autophagic processes, such as zymophagy or granulophagy will be identified in the future.
Figure 7.
The autophagy receptors (SARs) participate in selective cytoplasmic cargo recognition (i.e., protein aggregates as depicted in the figure) during pre-autophagosomal membrane elongation.
Several studies have described important pathogenic SNPs in the genes of selective autophagy receptors (Table 5 and Table S1). Nucleotide changes on SQSTM1, for example, may contribute to the origin of neurological alterations [199], as well as being implicated in the pathogenesis of amyotrophic lateral sclerosis [200,201], dementia [202], apraxia of speech [203], myopathy [204] and Paget’s disease of the bone [205,206]. Additionally, NDP52 and NBR1 variants determine susceptibility to Crohn’s disease [207] and Brooke–Spiegler syndrome [208], a rare condition where tumors form from skin structures. Meanwhile, polymorphisms on OPTN also show clinical relevance, as they are associated with amyotrophic lateral sclerosis [209], Paget’s disease of the bone [210,211] and primary open-angle glaucoma [212]. Interestingly, SNPs in TOLLIP have been frequently linked to interstitial lung diseases [213] and different types of infections, including leishmaniasis [214], leprosy [215,216], malaria [217], tuberculosis [218] and septicemia [219]. Pathogenic alleles of TAX1BP1 have been identified in unrelated alterations such as oral cavity cancer [220] and hypospadias [221]. Variants of specific mitophagy adapters have been identified in patients with depression (in the case of BNIP3) [222], schizophrenia or impaired cognition (BNIP3L) [223], bisphosphonate-associated osteonecrosis of the jaw (PHB2) [224], and breast cancer (NIPSNAP1) [123]. Regarding reticulophagy receptors, patients with hereditary sensory autonomic neuropathy have SNPs in either FAM134B [225,226] or ATL3 [227,228], and a single nucleotide change on RTN3 increases the susceptibility to complications after malaria [229,230]. Changes on genes encoding lipophagy receptors ATGL and HSL are unsurprisingly associated with neutral lipid storage disease with myopathy [231] and familial partial lipodystrophy [232,233]. Additional links between polymorphisms on SARs genes and pathologies are those in STBD1 for Parkinson’s disease [234,235], NUFIP1 for asthma [236], and NCOA4 for cancer [237,238]. Finally, a high number of pathogenic variants in the gene encoding huntingtin protein (HTT) are the cause for Huntington’s disease [239]. Although it might not be considered a bona fide selective autophagy receptor, HTT can act as a scaffold for selective autophagy and interacts with ULK1, GABARAP and p62/SQSTM1 [193,240,241]. Taken together, these publications show that dysregulation of autophagy receptor function plays a role in the pathogenesis of a wide variety of diseases, with most pathogenic variants being identified in SQSTM1, TOLLIP and HTT (Figure 2).
Table 5.
Clinically relevant SNPs in selective autophagy receptors.
| Gene | Disease | dbSNP rsID |
|---|---|---|
| ATGL | Neutral lipid storage disease with myopathy | rs121918259 |
| ATL3 | Hereditary sensory autonomic neuropathy | rs587777108 |
| BNIP3 | Major depressive disorder | rs9419139 |
| BNIP3L | Schizophrenia | rs1042992; rs73219805; rs73219806 |
| BNIP3L | Cognitive decline | rs77609452 |
| HSL | Lipodystrophy | rs766817317; rs587777699 |
| HTT | Huntington’s disease | rs1210554604; rs10015979; rs110501; rs11731237; rs2071655; rs2269499; rs2285086; rs2298969; rs2471347; rs362272; rs363066; rs363092; rs363096; rs3856973; rs6855981; rs82333; rs916171; rs118005095; rs13102260 |
| NBR1 | Brooke–Spiegler syndrome | rs202122812 |
| NCOA4 | Prostate cancer | rs10740051; rs10761581 |
| NCOA4 | Papillary thyroid carcinoma | rs782237788 |
| NDP52 | Crohn’s disease | rs2303015 |
| NIPSNAP1 | Breast cancer | rs183421746 |
| NUFIP1 | Asthma | rs114280567 |
| OPTN | Amyotrophic lateral sclerosis | rs267606928; rs267606929 |
| OPTN | Primary open-angle glaucoma | rs28939688 |
| OPTN | Paget’s disease of the bone | rs1561570; rs2234968 |
| PHB2 | Bisphosphonate-associated osteonecrosis of the jaw | rs11064477 |
| FAM134B | Hereditary sensory autonomic neuropathy | rs137852737; rs137852738; rs137852739; rs886037748 |
| RTN3 | Malaria | rs542998 |
| SQSTM1 | Frontotemporal dementia | rs776749939; rs772889843; rs1355424687 |
| SQSTM1 | Paget’s disease of the bone | rs796051869; rs104893941 |
| SQSTM1 | Amyotrophic lateral sclerosis | rs796052214; rs796051870; rs796051870 |
| SQSTM1 | Neurodegeneration | rs886039780 |
| SQSTM1 | Parkinson’s disease | rs200396166 |
| SQSTM1 | Atypical apraxia of speech | rs796052214 |
| SQSTM1 | Sporadic inclusion body myositis | rs11548633 |
| STBD1 | Parkinson’s disease | rs6812193 |
| TAX1BP1 | Head and neck carcinoma | rs11540483 |
| TAX1BP1 | Hypospadias | rs10214930 |
| TOLLIP | Leishmaniasis | rs3750920; rs5743899 |
| TOLLIP | Leprosy | rs3793964; rs3750920 |
| TOLLIP | Malaria | rs3750920 |
| TOLLIP | Tuberculosis | rs3750920; rs5743867 |
| TOLLIP | Sepsis | rs5743867 |
| TOLLIP | Idiopathic pulmonary fibrosis | rs5743890; rs111521887; rs3750920 |
| TOLLIP | Fibrotic idiopathic interstitial pneumonia | rs3168046; rs3750920; rs3793964; rs3829223; rs5744034 |
3.6. Cellular Machineries Involved in Autophagosome-Lysosome Fusion
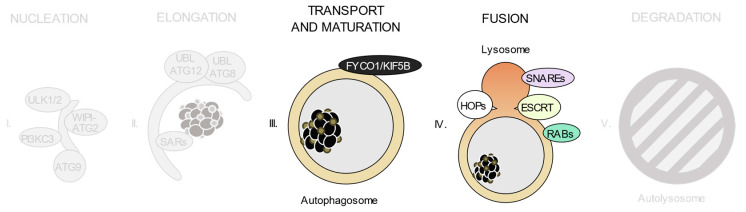
Once fully formed, autophagosomes move along microtubules depending on the actions of the minus-end-directed motor protein dynein and a plus-end-directed motor kinesin/FYCO1 [242]. This bidirectional transport leads to autophagosome clustering around the perinuclear area, where they eventually fuse with lysosomes [243]. In fact, disruption of either dynein or KIF5B, the heavy chain of kinesin-1, impairs autophagosome/lysosome fusion, blocking autophagic degradation [244,245]. Once mature autophagosomes and lysosomes encounter, lysosomal and outer autophagosomal membranes fuse forming a new organelle called an autolysosome, in which degradation of autophagic cargo occurs [246]. This fusion requires the coordination of SNAREs, small GTPases, tethering factors, and other proteins [247]. The SNARE proteins involved in autophagosome/lysosome fusion are the Q-SNAREs STX17 and SNAP29 and the R-SNARE YKT6, all present at the autophagosomal membrane. At the lysosomal membrane, R-SNAREs such as VAMP8 or VAMP7 and Q-SNARE STX7 have been reported to interact with autophagosomal SNAREs to mediate membrane fusion. Rab GTPases also play a major role in this process, recruiting other proteins that act coordinately to enhance the efficiency and specificity of fusion [248,249]. In this context, Rab7 is likely the most important Rab protein, as it has been reported to recruit tethering factors, including EPG5, PLEKHM1, and VPS33A and VPS41 from the HOPS complex [250], which all promote the assembly of trans-SNARE complexes for fusion [251,252]. A similar role for the Rab2 protein has also been proposed in autophagy [253,254] and Rab33b has been shown to recruit the ATG16L1 complex to pre-autophagosomal membranes [255]. Moreover, ATG14L has also been shown to act in this process stabilizing the STX17–SNAP29 complex to promote autophagosome/lysosome fusion [256]. Finally, other tethering factors, such as GRS2/GRASP55 [257] or BIRC6/BRUCE [258] have been reported to play a role in this process.
To become autolysosomes, autophagosomes may either fuse directly with lysosomes or fuse their external membrane with endosomes and become an organelle called amphisome, which will eventually fuse with lysosomes [259]. This event is sometimes required for efficient autolysosome formation and disruptions in autophagosome/endosome fusion often result in autophagosome accumulation and autophagic degradation blockage [260]. Members of the ESCRT families of proteins have been shown to be required for autophagosome/endosome fusion, and thus for adequate autolysosome formation. In this regard, the ESCRT-associated AAA-ATPase VPS4B/SKD1 has been shown to be required for efficient autophagosome clearance [261]. Depletion of ESCRT-0 HRS/HGS also leads to impairment in autophagosome maturation and fusion with lysosomes [262]. Similarly, depletion of CHMP4B/SNF7-2/VPS32-2 (ESCRT-III) or the expression of a mutant form of CHMP2B causes an accumulation of autophagosomes [263]. Other proteins that have been suggested to be involved in endocytic transport and autophagy are C9orf72 [264], ZFYVE26, SPG11 [265], and Rab33B [266].
Altogether, the coordinated activity of all these proteins results in autolysosome formation, which is the last step preceding autophagosome cargo degradation (Figure 8). Table 6 shows polymorphisms on the genes encoding these effectors that are connected to diseases (with additional references collected in Table S1). Regarding the genes encoding motor proteins, SNPs in FYCO1 and KIF5B have been associated with cataracts [267] and bipolar disorder [268], respectively. As for genes encoding SNARE proteins, several links with alterations have been established: STX7 and neuronal heterotopia [269], STX17 and alopecia [270], YKT6 and diabetes or birth weight [271,272], and SNAP29 and a neurocutaneous condition termed Cednik syndrome [273]. Variants of another SNARE, VAMP8, are present in patients with coronary artery disease [274], cerebrovascular accident [275], tuberculosis [276] or prostate cancer [277]. An SNP in Rab7 has been identified in patients with Charcot–Marie–Tooth disease type 2B [278,279,280], while SNPs in Rab33B are associated with Smith–McCort osteochondrodysplasia [281,282,283]. As for changes on genes from members of HOPS complex, a variant of VPS33A has been linked to a new type of mucopolysaccharidosis [284], with a specific allele of VPS41 being implicated in major depressive disorder [285]. Additionally, SNPs in PLEKHM1 have been involved in several diseases, including osteopetrosis [286], Parkinson’s disease [287], ovarian cancer [288,289], depression [290], and alopecia [291]. Some pathogenic variants on EPG5 are responsible for Vici syndrome, a congenital multisystem disorder [292], while others have also been linked to Alzheimer’s disease [293] or depression [294]. Only one polymorphism of BIRC6 has been described in a disease, specifically glaucoma [295]. Variants on components of the ESCRT-III complex component are associated with a wide range of disorders. For example, SNPs in CHMP2B are linked to neuroblastoma [296], frontotemporal dementia [297,298] or ALS [299] and those in CHMP4B have been associated with cataracts [300], dysphagia [301] or diabetes mellitus [302]. Polymorphisms in C9orf72 are also associated with frontotemporal dementia and ALS [264,303], while one SNP in HGS correlates with age-related macular degeneration [304]. Finally, variants of ZFYVE26 [305] and SPG11 [306] have been extensively analyzed in patients with spastic paraplegia, while SNPs in ZFYVE26 have been also linked to ALS [307] and breast cancer [308]. All in all, polymorphisms on genes encoding proteins involved in autophagosome-lysosome fusion often result in the development of different diseases, with dementia, ALS and paraplegia being the most frequent ones. It is remarkable that a large number of clinically relevant SNPs have been identified in the genes encoding EPG5, SPG11 and ZFYVE26 (Figure 2).
Figure 8.
Different cellular machineries (including the HOPs and ESCRT complexes) and effectors (like motor proteins, as well as members of the SNARE or Rab family proteins) are involved in autophagosome transport and maturation, as well as in their fusion with lysosomes.
Table 6.
Clinically relevant SNPs in cellular machineries involved in autophagosome-lysosome fusion.
| Gene | Disease | dbSNP rsID |
|---|---|---|
| BIRC6 | Glaucoma | rs2754511 |
| C9orf72 | Amyotrophic lateral sclerosis | rs3849943; rs774359; rs3849942 |
| C9orf72 | Familial frontotemporal dementia with amyotrophic lateral sclerosis | rs71492753 |
| CHMP2B | Neuroblastoma | rs63750355; rs63750653 |
| CHMP2B | Frontotemporal dementia | rs78268395 |
| CHMP2B | Amyotrophic lateral sclerosis | rs281864934 |
| CHMP4B | Bilateral cataracts | rs118203966 |
| CHMP4B | Diabetes mellitus, non-insulin-dependent | rs7274168 |
| CHMP4B | Dysphagia | rs2747539 |
| EPG5 | Alzheimer’s disease | rs9963463; rs11082498 |
| EPG5 | Depressive disorders | rs58682566 |
| EPG5 | Vici syndrome | rs1470797555; rs1555673917; rs1568107449; rs1568112516; rs1568112543; rs1568118775; rs1568133724; rs1568133760; rs201757275; rs587776940; rs587776941; rs587776942; rs762639913; rs767638289; rs780889226; rs863225064; rs866435487; rs961245497; rs863225064 |
| EPG5 | Cataract | rs201757275 |
| FYCO1 | Cataract | rs387906963; rs387906964; rs387906965 |
| HGS | Age-related macular degeneration | rs8070488 |
| KIF5B | Bipolar disorder | rs1775715 |
| PLEKHM1 | Osteopetrosis | rs786205055 |
| PLEKHM1 | Parkinson’s disease | rs11012 |
| PLEKHM1 | Alopecia | rs144733372 |
| PLEKHM1 | Unipolar depression | rs144733372 |
| PLEKHM1 | Major depressive disorder | rs144733372 |
| PLEKHM1 | Ovarian cancer | rs1879586; rs2077606; rs17631303 |
| RAB33B | Smith–McCort dysplasia | rs1085307129; rs886044716; rs1085307131; rs1085307128; rs587776958 |
| RAB7 | Charcot–Marie–Tooth disease type 2B | rs121909080; rs121909078; rs121909079; rs121909081 |
| SNAP29 | Cednik syndrome | rs387907363; rs869312906 |
| SPG11 | Spastic paraplegia | rs1085307097; rs118203963; rs140385286; rs1555447432; rs141848292; rs312262720; rs312262721; rs312262722; rs312262737; rs312262749; rs312262752; rs312262764; rs312262779; rs371334506; rs747220413; rs764647588; rs765477482; rs767798272 |
| STX17 | Alopecia | rs10760706 |
| STX7 | Neuronal heterotopia | rs864309676 |
| VAMP8 | Cerebrovascular accident | rs1010 |
| VAMP8 | Tuberculosis | rs1010 |
| VAMP8 | Coronary artery disease | rs1010 |
| VAMP8 | Prostate cancer | rs10187424; rs3731827 |
| VPS33A | MPS-like disorder | rs767748011 |
| VPS41 | Major depressive disorder | rs10274968 |
| YKT6 | Diabetes | rs2908282 |
| YKT6 | Birth weight and subsequent risk factors | rs138715366 |
| ZFYVE26 | Spastic paraplegia | rs1049504575; rs1057518016; rs1214483973; rs1555394376; rs200832994; rs558285072; rs767164213; rs768176054; rs769329153; rs774809466; rs941230062; rs981804211 |
| ZFYVE26 | Amyotrophic lateral sclerosis | rs12891047 |
| ZFYVE26 | Breast cancer | rs200595749 |
| ZFYVE26 | Movement disorders | rs752283089; rs869312914 |
Once the autolysosome is formed, the inner membrane and the internal content of the original autophagosome are degraded by the action of acidic hydrolases (Figure 9). After that, the resulting new biomolecules (nucleotides, lipids, amino acids, etc.) return to the cytoplasm by the action of permeases and other transporters. Therefore, the lysosome becomes an essential player in autophagy. Disruption of lysosomal activity impacts autophagic degradation, leading to the accumulation of autophagosomes and/or autolysosomes, which physically stresses the cell while undesired cytoplasmic components accumulate without being degraded. In fact, alterations in the balance of lysosomal lipids can hinder autophagy, either by blocking autophagosome-lysosome fusion (which is the case in multiple sulfatase deficiency (MSD) or in mucopolysaccharidosis type IIIA [309,310]), impeding autophagosomal closure (by, for example, the accumulation of sphingomyelin in Niemann–Pick type A and B [311]), impairing lysosomal proteolysis (shown in Niemann–Pick disease type C [312]) or resulting in lysosomal permeabilization (as it has been described in Niemann–Pick disease type A [313]).
Figure 9.
Lysosomal components are direct effectors of autophagosome cargo degradation.
Classic examples of human pathologies caused by alterations in lysosomal function are LSDs. These pathologies are originated by variants that alter the activity of either specific lysosomal proteins (including hydrolases, transferases, membrane proteins, activators or transporters) or non-lysosomal ones that are required for the lysosomal-mediated degradation of different molecules. Thus, glycosaminoglycans are accumulated in different types of mucopolysaccharidoses. In fact, a long list of diseases are originated by pathogenic SNPs in the genes involved in their degradation, such as ARSB (MPS VI or Maroteaux–Lamy syndrome) [314], GALNS (MPS IVA or Morchio A syndrome) [315], GLB1 (MPS IVB or Morchio B syndrome) [316,317,318], GNS (MPS IIID or Sanfilippo syndrome type D) [319] GUSB (MPS VII or Sly syndrome) [320], HGSNAT (MPS IIIC or Sanfilippo syndrome type C) [319], HYAL1 (MPS IX) [321], IDS (MPS II or Hunter syndrome) [322], IDUA (different subtypes of MPS I and II) [323], NAGLU (MPS IIIB or Sanfilippo syndrome type B) [319] or SGSH MPS IIIA or Sanfilippo syndrome type A) [319]. One of the most well-studied pathologies caused by lysosomal deficiency is Danon disease, which has been extensively associated with LAMP2 deficiency [324]. Another well-documented glycogen storage disorder, Pompe disease, is caused by mutations in GAA [325], which has also been implicated in Friedreich ataxia [326] and hypochondrogenesis [327]. Deficiency of the lysosomal hydrolase β-glucocerebrosidase (GCase), encoded by GBA, causes Gaucher disease [328], and a polymorphism on this gene has also been linked to Parkinson’s disease [329], whereas several pathogenic SNPs in GLA, the gene encoding the lysosomal enzyme α-galactosidase A (a-Gal A), are associated with Fabry disease [330]. Polymorphisms in NPC1 and NPC2 genes results in Niemann–Pick type C disease [331], while changes in CTNS have been associated with cystinosis [332]. Different types of neuronal ceroid lipofuscinoses can be caused by mutations on different genes, such as GRN, several CLNs, CTSD, CTSF, DNAJC5 or MFSD8 [333]. Finally, pathogenic SNPs on CTSA, FUCA1, SLC17A5, and MANBA or MAN2B1 lead to galactosialidosis [334], fucosidosis [335], sialic acid storage diseases [336] or mannosidosis [337,338], respectively. All these clinically relevant SNPs are shown in Table 7, with additional references collected in Table S1. It is remarkable that numerous SNPs on a given lysosomal gene are only associated with just one or two pathologies (GLB1 being the single exception) (Figure 2). This contrasts with the case of the genes encoding proteins involved in autophagosome biogenesis, in which fewer variants are linked to a wider range of diseases.
Table 7.
Clinically relevant SNPs in lysosomal components.
| Gene | Disease | dbSNP rsID |
|---|---|---|
| ARSB | Mucopolysaccharidosis type VI (Maroteaux–Lamy syndrome) | rs118203938; rs118203939; rs118203940; rs431905493; rs431905495; rs431905496; rs118203942; rs118203944; rs118203943 |
| CLN3 | Neuronal ceroid lipofuscinosis type 3 | rs121434286; rs267606737; rs386833720; rs786201028; rs121434286 |
| CLN6 | Neuronal ceroid lipofuscinosis type 6 | rs104894483; rs104894486; rs121908079; rs121908080; rs397515352; rs774543080; rs786205065; rs786205066; rs786205067; rs104894484 |
| CLN6 | Adult neuronal ceroid lipofuscinosis | rs154774633; rs154774634; rs154774635; rs154774636 |
| CLN8 | Neuronal ceroid lipofuscinosis type 8 | rs104894060; rs137852883; rs28940569 |
| CLN8 | Northern epilepsy syndrome | rs104894064 |
| CTNS | Cystinosis | rs375952052 |
| CTSA | Galactosialidosis | rs137854540; rs137854544; rs137854546; rs137854547; rs137854548; rs137854549; rs786200859; rs875989777; rs137854544; rs137854543 |
| CTSD | Neuronal ceroid lipofuscinosis type 10 | rs786205105; rs797045137; rs797045138; rs121912789; rs121912790 |
| CTSF | Neuronal ceroid lipofuscinosis type 13 | rs753084727; rs797045136; rs143889283; rs397514731 |
| DNAJC5 | Neuronal ceroid lipofuscinosis, Parry type, | rs587776892; rs387907043 |
| FUCA1 | Fucosidosis | rs118204450; rs80358195; rs80358196; rs80358197; rs80358198 |
| GAA | Glycogen storage disease type II (Pompe disease) | rs1057516581; rs12450199; rs140826989; rs121907940; rs121907941; rs1393386120; rs1414146587; rs121907942; rs1344266804; rs121907943; rs121907944; rs1221948995; rs1245412108; rs121907938; rs121907945; rs121907936; rs1800309; rs121907937; rs1800307; rs147804176; rs1555600061; rs1555601773; rs1800312; rs200856561; rs1555601773; rs1800312; rs200856561; rs369531647; rs1057516277; rs886043343; rs892129065; rs28940868;rs1057516215; rs1055945806; |
| GAA | Friedreich ataxia | rs1245992455 |
| GAA | Hypochondrogenesis | rs1289257741 |
| GALNS | Mucopolysaccharidosis IVA (Morchio A syndrome) | rs1028668536; rs118204438; rs118204449; rs786205899; rs118204435; rs118204441; rs118204442; rs118204446; rs118204447; rs118204448; rs267606838 |
| GBA | Parkinson’s disease | rs75548401 |
| GBA | Gaucher disease | rs421016 |
| GLA | Fabry disease | rs104894828; rs104894834; rs104894845; rs28935197; rs869312142 |
| GLB1 | GM1 gangliosidosis | rs192732174; rs376663785; rs587776524; rs794727165; rs794729217; rs781658798; rs778423653; rs778700089; rs879050821; rs72555361; rs72555364; rs72555368; rs72555370; rs72555390; rs72555393; rs794729217; rs72555392; rs72555362; rs1214295886; rs1553606128; rs1553610382; rs1553610553; rs1553612189; rs1559401428; rs192732174; rs189115557; |
| GLB1 | Mucopolysaccharidosis IVB (Morchio B syndrome) | rs72555363; rs1553606128; rs1553610382; rs1553610553; rs1553612220; rs189115557; rs192732174; rs794729217; rs794727165; rs778700089; rs778423653 |
| GLB1 | Neuraminidase 1 deficiency | rs1356418704 |
| GLB1 | Respiratory tract diseases | rs9828592 |
| GLB1 | Asthma | rs79337446 |
| GNS | Mucopolysaccharidosis type IIID (Sanfilippo syndrome) | rs119461974; rs119461975; rs483352898; rs483352899; rs483352900 |
| GRN | Presenile dementia | rs373885474 |
| GRN | Frontotemporal lobar degeneration | rs606231220; rs63749801; rs63750077; rs63751006; rs63750331; rs63751294; rs63751243 |
| GUSB | Mucopolysaccharidosis type VII (Sly syndrome) | rs121918179; rs121918181; rs121918185; rs377519272; rs786200863; rs121918180; rs121918173; rs121918174; rs121918175; rs121918176; rs121918177; rs121918178; rs121918182; rs121918183; rs121918184; rs121918172 |
| HGSNAT | Mucopolysaccharidosis type IIIC (Sanfilippo syndrome type C) | rs121908282; rs121908283; rs121908284; rs121908285; rs121908286; rs193066451; rs483352896; rs753355844; rs754875934; rs764206492; rs797045120 |
| HYAL1 | Mucopolysaccharidosis IX | rs104893743 |
| IDS | Mucopolysaccharidosis type II (Hunter syndrome) | rs113993946; rs113993947; rs199422230; rs483352904; rs483352905; rs797044671; rs869025304; rs869025305; rs869025306; rs869025307; rs869025308; rs104894856; rs104894861; rs199422228; rs199422229; rs199422231 |
| IDUA | Mucopolysaccharidosis type I (Hurler and Scheie syndrome) | rs121965025; rs121965033; rs199801029; rs387906504; rs398123258; rs762411583; rs786200915; rs869025584; rs121965021; rs121965026; rs121965027; rs121965031; rs121965023; rs121965019; rs121965021; rs121965030; rs764196171; rs121965019; rs121965033; rs121965024; |
| LAMP2 | Danon disease | rs104894857; rs104894858; rs1060502302; rs137852527; rs727503118; rs727503119; rs727503120; rs727504742 |
| MAN2B1 | Alpha-mannosidosis | rs121434331; rs121434332; rs775200333; rs80338677; rs80338678; rs80338679; rs80338680; rs80338681 |
| MANBA | Beta-mannosidosis | rs121434334; rs121434335; rs121434336 |
| MFSD8 | Neuronal ceroid lipofuscinosis type 7 | rs11820397; rs587778809; rs724159971; rs727502801; rs118203975; rs118203976; rs140948465; rs267607235; rs749704755 |
| MFSD8 | Late-infantile neuronal ceroid lipofuscinosis | rs200319160 |
| NAGLU | Mucopolysaccharidosis type IIIB (Sanfilippo syndrome type B) | rs104894591; rs104894592; rs104894597; rs104894598; rs118204025; rs746006696; rs886039894; rs886039895; rs118204024; rs104894590; rs104894593; rs104894594; rs104894595; rs104894597; rs104894598; rs753520553; rs796052122; rs104894601 |
| NPC1 | Niemann–Pick disease, type C | rs1055204017; rs1057518711; rs1474434210; rs753768576; rs139751448; rs143124972; rs28942104; rs756815030; rs758231839; rs886042270; rs80358257; rs80358254; rs80358259; rs150334966; rs1555634422; rs768999208; rs80358259 |
| NPC2 | Niemann–Pick disease type C | rs80358262; rs80358263; rs80358266; rs80358268; rs11694; rs80358261; rs80358264; rs104894458 |
| SGSH | Mucopolysaccharidosis type IIIA (Sanfilippo syndrome type A) | rs104894635; rs104894637; rs138504221; rs1057521801; rs374621913; rs770947426; rs777956287; rs778700037; rs104894638; rs104894640; rs104894642; rs104894643; rs104894636; rs104894641; rs138504221; rs104894635 |
| SLC17A5 | Sialic acid storage diseases (SASDs) | rs386833987; rs386833994; rs727504156; rs119491109; rs119491110; rs80338795; rs80338794 |
4. Concluding Remarks
As the number of studies on autophagy research grows, it becomes clearer how essential this catabolic pathway is for cellular homeostasis and health. What was first inferred from the characterization of animal models deficient in autophagy it is now directly described in human pathology, with an increasing record of papers showing that autophagy dysregulation drives or sustains a wide range of disorders. The extensive list of autophagy-related SNPs collected in this review further reflects the relevance of autophagy and its effectors in human pathology. In this regard, several variants present in different populations can decisively affect the origin, development or prognosis of different diseases, remarking the importance of defining autophagy-related gene signatures in these disorders. Thus, the existence of variants on autophagy-related genes allows us to better understand the role of this route in pathophysiology.
Interestingly, in some cases, the very same SNPs have been described to be associated with different disorders either with a pathogenic or a protective effect. This is the case, for example, of rs2241880, the threonine-to-alanine substitution at amino acid position 300 of ATG16L1 (T300A) that has been intensively analyzed in the context of inflammatory bowel diseases. This variant has additionally been linked to different types of cancer or to Paget’s disease of the bone, and the same allele may be protective or pathogenic depending on the disorder and the population. This redundancy is also true for other genes, such as ATG5 (rs510432, rs573775 or rs2245214) or ATG10 (rs10514231, rs1864182 or rs1864183). Although identification of the same variant in different pathologies could be explained by the fact that already-studied SNPs are more likely to be analyzed again in the context of additional diseases, it nevertheless shows that alteration in the activity of autophagy proteins definitely contributes to the progression or repression of a given disorder. This bias could also explain why most of the autophagy-related polymorphisms that we have collected are located on genes of effectors mediating ATG12 conjugation (Table 4), as many researchers first focus on these proteins when addressing autophagy in a pathological context. Similarly, the analysis of SNPs in lysosomal genes has been favored by the direct link between mutations on these genes and lysosomal storage disorders (Table 7), entailing a fast, straightforward approach to find pathogenic variants in diseases.
Finally, and although this review is focused on SNPs, it is worth mentioning that other less-frequent pathogenic variants of autophagy-related genes have also been described in the literature. For example, the monoallelic deletion of the 17q21 region, affecting the BECN1 gene, is associated with breast, ovarian and prostate cancer [339,340]. Two variants of WIPI4 (c.439 + 1G > T and c.1033_1034dupAA) were identified in patients with beta-propeller protein-associated neurodegeneration [98], while a third one is specifically linked to developmental and epileptic encephalopathy in patients with the same neurological disorder [341]. Sequence variants affecting the expression levels of ATG12 (115842507G>T, 115842394C>T and 115841817_18del) [342] and ATG7 (11313449G>A, 11313811T>C, 1313913G>A and 11314041G>A) [343] are present in patients with sporadic Parkinson’s disease. Other examples of less-common risk alleles are those of SQSTM1 associated with frontotemporal dementia (c.1142C>T, K341V and K344E) [202,344] or muscle disorders such as sporadic inclusion body myositis (G194R) [204] and distal myopathies with rimmed vacuoles (p.G351_P388del and p.Glu389delinsAspLysTer) [345]. Although these and other minority variants have only been identified in few patients, their real allelic frequency in populations could be much higher, increasing their relevance and consolidating the clinical importance of pathogenic autophagy variants.
As a conclusion, it has to be considered that medicine is firmly progressing toward a personalized approach, given that patients respond differently to the same treatments. Although other factors are surely involved, the presence of SNPs plays a pivotal role in the specific response of a patient to a determined treatment. In this regard, autophagy-related polymorphisms are not only involved in the development of pathologies, but also may influence the effectiveness of a determined treatment. This additional clinical relevance of variants on autophagy-related genes has already been described in cancer [61,104,114,118,121], inflammatory bowel diseases [145,346,347] and others [80]. For this reason, the identification of new clinically significant SNPs is not only important in terms of disease prevention, but also to design new therapeutic approaches aimed at modulating autophagy for clinically relevant purposes. This review should be of great help in advancing to design new therapeutic strategies.
Abbreviations
| ALFY | Autophagy-linked FYVE protein |
| AMBRA1 | Activating molecule in BECN1-regulated autophagy protein 1 |
| AMPK | Adenosine monophosphate-activated protein kinase |
| ARSB | Arylsulfatase B |
| ATG10 | Autophagy-related protein 10 |
| ATG101 | Autophagy-related protein 101 |
| ATG12 | Autophagy-related protein 12 |
| ATG13 | Autophagy-related protein 13 |
| ATG14 | Autophagy-related protein 14 |
| ATG16L1 | Autophagy-related protein 16 like 1 |
| ATG2A | Autophagy-related protein 2 homolog A |
| ATG2B | Autophagy-related protein 2 homolog B |
| ATG4A | Autophagy related 4A cysteine peptidase |
| ATG4B | Autophagy related 4B cysteine peptidase |
| ATG4C | Autophagy related 4C cysteine peptidase |
| ATG4D | Autophagy related 4D cysteine peptidase |
| ATG5 | Autophagy-related protein 5 |
| ATG7 | Autophagy-related protein 7 |
| ATG9A | Autophagy-related protein 9A |
| ATG9B | Autophagy-related protein 9B |
| ATGL | Adipose triglyceride lipase |
| ATL3 | Atlastin-3 |
| Barkor | Beclin 1-associated autophagy-related key regulator |
| BCL-2 | Apoptosis regulator Bcl-2 |
| BECN1 | Beclin-1 |
| BIRC6 | Baculoviral IAP repeat-containing protein 6 |
| BNIP3 | BCL2/adenovirus E1B 19 kDa protein-interacting protein 3 |
| BNIP3L | BCL2/adenovirus E1B 19 kDa protein-interacting protein 3-like |
| CCPG1 | Cell cycle progression protein 1 |
| CHMP2B | Charged multivesicular body protein 2b |
| CHMP4B | Charged multivesicular body protein 4b |
| CTNS | Cystinosin |
| CTSD | Cathepsin D |
| CTSF | Cathepsin F |
| DFCP1 | Double FYVE-containing protein 1 |
| DNAJC5 | DnaJ homolog subfamily C member 5 |
| EPG5 | Ectopic P granules protein 5 homolog |
| FAM134B | Family With Sequence Similarity 134, Member B |
| FKBP8 | Peptidyl-prolyl cis-trans isomerase FKBP8 |
| FUCA1 | Tissue alpha-L-fucosidase |
| FUNDC1 | FUN14 domain-containing protein 1 |
| FYCO1 | FYVE and coiled-coil domain-containing protein 1 |
| GAA | Lysosomal alpha-glucosidase |
| GABARAP | Gamma-aminobutyric acid receptor-associated protein |
| GABARAPL1 | Gamma-aminobutyric acid receptor-associated protein-like 1 |
| GALNS | N-acetylgalactosamine-6-sulfatase |
| GARARAPL2 | Gamma-aminobutyric acid receptor-associated protein-like 2 |
| GLB1 | Beta-galactosidase |
| GNS | N-acetylglucosamine-6-sulfatase |
| GRASP55 | Golgi reassembly-stacking protein of 55 kDa |
| GRN | Progranulin |
| GUSB | Beta-glucuronidase |
| HGS | Hepatocyte growth factor-regulated tyrosine kinase substrate |
| HGSNAT | Heparan-alpha-glucosaminide N-acetyltransferase |
| HOPS | Hepatocyte odd Ppotein shuttling protein |
| HSC70 | Heat shock cognate 71 kDa protein |
| HSL | Hormone-sensitive lipase |
| HTT | Huntingtin |
| HYAL1 | Hyaluronidase-1 |
| IDS | Iduronate 2-sulfatase |
| IDUA | Alpha-L-iduronidase |
| KIF5B | Kinesin-1 heavy chain |
| LAMP2 | Lysosome-associated membrane protein 2 |
| MAN2B1 | Lysosomal alpha-mannosidase |
| MANBA | Beta-mannosidase |
| MAP1-LC3A | Microtubule-associated proteins 1A/1B light chain 3A |
| MAP1-LC3B | Microtubule-associated proteins 1A/1B light chain 3B |
| MAP1-LC3C | Microtubule-associated proteins 1A/1B light chain 3C |
| MFSD8 | Major facilitator superfamily domain-containing protein 8 |
| mTOR | Mechanistic/mammalian target of rapamycin |
| NAGLU | Alpha-N-acetylglucosaminidase |
| NBR1 | Next to BRCA1 gene 1 protein |
| NCOA4 | Nuclear receptor coactivator 4 |
| NDP52 | Nuclear domain 10 protein 52 |
| NPC1 | NPC intracellular cholesterol transporter 1 |
| NPC2 | NPC intracellular cholesterol transporter 2 |
| NUFIP1 | Nuclear fragile X mental retardation-interacting protein 1 |
| OPTN | Optineurin |
| PI3KC3 | Phosphatidylinositol 3-kinase catalytic subunit type 3 |
| PIK3R4 | Phosphoinositide 3-kinase regulatory subunit 4 |
| PLEKHM1 | Pleckstrin homology domain-containing family M member 1 |
| RB1CC1 | RB1-inducible coiled-coil protein 1 |
| RTN3 | Reticulon-3 |
| SEC62 | Translocation protein SEC62 |
| SGSH | N-sulphoglucosamine sulphohydrolase |
| SLC17A5 | Sialin |
| SNAP29 | Synaptosomal-associated protein 29 |
| SQSTM1 | Sequestosome-1 |
| STK36 | Serine/threonine-protein kinase 36 |
| STX17 | Syntaxin-17 |
| TAX1BP1 | Tax1-binding protein 1 |
| TEX264 | Testis-expressed protein 264 |
| TOLLIP | Toll-interacting protein |
| ULK1 | Unc-51 like autophagy activating kinase |
| UVRAG | UV radiation resistance-associated gene protein |
| VAMP7 | Vesicle-associated membrane protein 7 |
| VAMP8 | Vesicle-associated membrane protein 8 |
| VPS4B | Vacuolar protein sorting-associated protein 4B |
| WIPI1 | WD repeat domain phosphoinositide-interacting protein 1 |
| WIPI2 | WD repeat domain phosphoinositide-interacting protein 2 |
| WIPI3 | WD repeat domain phosphoinositide-interacting protein 3 |
| WIPI4 | WD repeat domain phosphoinositide-interacting protein 4 |
| YKT6 | Synaptobrevin homolog YKT6 |
Supplementary Materials
Supplementary Materials can be found at https://www.mdpi.com/1422-0067/21/21/8196/s1.
Funding
This work was funded by Principality of Asturias Government (IDI/2018/000159) and Instituto de Salud Carlos III (RTICC-Spain).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kroemer G., Mariño G., Levine B. Autophagy and the Integrated Stress Response. Mol. Cell. 2010;40:280–293. doi: 10.1016/j.molcel.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galluzzi L., Baehrecke E.H., Ballabio A., Boya P., Bravo-San Pedro J.M., Cecconi F., Choi A.M., Chu C.T., Codogno P., Colombo M.I., et al. Molecular definitions of autophagy and related processes. Embo J. 2017;36:1811–1836. doi: 10.15252/embj.201796697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parzych K.R., Klionsky D.J. An overview of autophagy: Morphology, mechanism, and regulation. Antioxid. Redox Signal. 2014;20:460–473. doi: 10.1089/ars.2013.5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mijaljica D., Prescott M., Devenish R.J. Microautophagy in Mammalian Cells: Revisiting a 40-Year-Old Conundrum. Autophagy. 2011;7:673–682. doi: 10.4161/auto.7.7.14733. [DOI] [PubMed] [Google Scholar]
- 5.Kaushik S., Cuervo A.M. The coming of age of chaperone-mediated autophagy. Nat. Rev. Mol. Cell Biol. 2018;19:365–381. doi: 10.1038/s41580-018-0001-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawabata T., Yoshimori T. Autophagosome Biogenesis and Human Health. Cell Discov. 2020;6:33. doi: 10.1038/s41421-020-0166-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh R., Cuervo A.M. Autophagy in the cellular energetic balance. Cell Metab. 2020;6:33. doi: 10.1016/j.cmet.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levine B., Kroemer G. Biological Functions of Autophagy Genes: A Disease Perspective. Cell. 2019;176:11–42. doi: 10.1016/j.cell.2018.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao Y.G., Zhang H. Core autophagy genes and human diseases. Curr. Opin. Cell Biol. 2019;61:117–125. doi: 10.1016/j.ceb.2019.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Choi A.M.K., Ryter S.W., Levine B. Autophagy in Human Health and Disease. N. Engl. J. Med. 2013;368:651–662. doi: 10.1056/NEJMra1205406. [DOI] [PubMed] [Google Scholar]
- 11.Yang Y., Klionsky D.J. Autophagy and disease: Unanswered questions. Cell Death Differ. 2020;27:858–871. doi: 10.1038/s41418-019-0480-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galluzzi L., Pietrocola F., Bravo-San Pedro J.M., Amaravadi R.K., Baehrecke E.H., Cecconi F., Codogno P., Debnath J., Gewirtz D.A., Karantza V., et al. Autophagy in malignant transformation and cancer progression. Embo J. 2015;34:856–880. doi: 10.15252/embj.201490784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lai J., Chen B., Mok H., Zhang G., Ren C., Liao N. Comprehensive analysis of autophagy-related prognostic genes in breast cancer. J. Cell. Mol. Med. 2020;24:9145–9153. doi: 10.1111/jcmm.15551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu Y., Wang R., Chen W., Chen Q., Zhou J. Construction of a prognosis-predicting model based on autophagy-related genes for hepatocellular carcinoma (HCC) patients. Aging. 2020;12:14582. doi: 10.18632/aging.103507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng H., Zhong L., Yang X., Wan Q., Pei X., Wang J. Development and validation of prognostic index based on autophagy-related genes in patient with head and neck squamous cell carcinoma. Cell Death Discov. 2020;6:1–8. doi: 10.1038/s41420-020-00294-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu D., Jiang L., Luo S., Zhao X., Hu H., Zhao G., Tang W. Development of an autophagy-related gene expression signature for prognosis prediction in prostate cancer patients. J. Transl. Med. 2020;18:1–12. doi: 10.1186/s12967-020-02323-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun Z., Jing C., Xiao C., Li T. An autophagy-related long non-coding RNA prognostic signature accurately predicts survival outcomes in bladder urothelial carcinoma patients. Aging. 2020;12:15624–15637. doi: 10.18632/aging.103718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen M., Zhang S., Nie Z., Wen X., Gao Y. Identification of an Autophagy-Related Prognostic Signature for Clear Cell Renal Cell Carcinoma. Front. Oncol. 2020;10:873. doi: 10.3389/fonc.2020.00873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen C., Chen S., Cao H., Wang J., Wen T., Hu X., Li H. Prognostic significance of autophagy-related genes within esophageal carcinoma. BMC Cancer. 2020;20:1–11. doi: 10.1186/s12885-020-07303-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xing Q., Ji C., Zhu B., Cong R., Wang Y. Identification of small molecule drugs and development of a novel autophagy-related prognostic signature for kidney renal clear cell carcinoma. Cancer Med. 2020;9:7034–7051. doi: 10.1002/cam4.3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huo X., Qi J., Huang K., Bu S., Yao W., Chen Y., Nie J. Identification of an autophagy-related gene signature that can improve prognosis of hepatocellular carcinoma patients. BMC Cancer. 2020;20:771. doi: 10.1186/s12885-020-07277-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Du J.-X., Chen C., Luo Y.-H., Cai J.-L., Cai C.-Z., Xu J., Ni X.-J., Zhu W. Establishment and validation of a novel autophagy-related gene signature for patients with breast cancer. Gene. 2020;762:144974. doi: 10.1016/j.gene.2020.144974. [DOI] [PubMed] [Google Scholar]
- 23.Wang H., Ma X., Liu J., Wan Y., Jiang Y., Xia Y., Cheng W. Prognostic value of an autophagy-related gene expression signature for endometrial cancer patients. Cancer Cell Int. 2020;20:306. doi: 10.1186/s12935-020-01413-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang H., Han M., Li H. Construction and Validation of an Autophagy-Related Prognostic Risk Signature for Survival Predicting in Clear Cell Renal Cell Carcinoma Patients. Front. Oncol. 2020;10:707. doi: 10.3389/fonc.2020.00707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shibutani S.T., Saitoh T., Nowag H., Münz C., Yoshimori T. Autophagy and Autophagy-Related Proteins in the Immune System. Nat. Immunol. 2015;16:1014–1024. doi: 10.1038/ni.3273. [DOI] [PubMed] [Google Scholar]
- 26.Clarke A.J., Simon A.K. Autophagy in the Renewal, Differentiation and Homeostasis of Immune Cells. Nat. Rev. Immunol. 2019;19:170–183. doi: 10.1038/s41577-018-0095-2. [DOI] [PubMed] [Google Scholar]
- 27.Münz C. Autophagy proteins in antigen processing for presentation on MHC molecules. Immunol. Rev. 2016;272:17–27. doi: 10.1111/imr.12422. [DOI] [PubMed] [Google Scholar]
- 28.Cui B., Lin H., Yu J., Yu J., Hu Z. Autophagy and the Immune Response. Adv. Exp. Med. Biol. 2019;1206:595–634. doi: 10.1007/978-981-15-0602-4_27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deretic V., Levine B. Autophagy Balances Inflammation in Innate Immunity. Autophagy. 2018;14:243–251. doi: 10.1080/15548627.2017.1402992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cadwell K. Crosstalk between Autophagy and Inflammatory Signalling Pathways: Balancing Defence and Homeostasis. Nat. Rev. Immunol. 2016;16:661–675. doi: 10.1038/nri.2016.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yin H., Wu H., Chen Y., Zhang J., Zheng M., Chen G., Li L., Lu Q. The Therapeutic and Pathogenic Role of Autophagy in Autoimmune Diseases. Front. Immunol. 2018;9:1512. doi: 10.3389/fimmu.2018.01512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim S., Eun H., Jo E.-K. Roles of Autophagy-Related Genes in the Pathogenesis of Inflammatory Bowel Disease. Cells. 2019;8:77. doi: 10.3390/cells8010077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levine B., Mizushima N., Virgin H.W. Autophagy in Immunity and Inflammation. Nature. 2011;469:323–335. doi: 10.1038/nature09782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choi Y., Bowman J.W., Jung J.U. Autophagy during Viral infection A Double-Edged Sword. Nat. Rev. Microbiol. 2018;16:341–354. doi: 10.1038/s41579-018-0003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Menzies F.M., Fleming A., Caricasole A., Bento C.F., Andrews S.P., Ashkenazi A., Füllgrabe J., Jackson A., Jimenez Sanchez M., Karabiyik C., et al. Autophagy and Neurodegeneration: Pathogenic Mechanisms and Therapeutic Opportunities. Neuron. 2017;93:1015–1034. doi: 10.1016/j.neuron.2017.01.022. [DOI] [PubMed] [Google Scholar]
- 36.Park H., Kang J.H., Lee S. Autophagy in Neurodegenerative Diseases: A Hunter for Aggregates. Int. J. Mol. Sci. 2020;21:3369. doi: 10.3390/ijms21093369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vicencio E., Beltrán S., Labrador L., Manque P., Nassif M., Woehlbier U. Implications of Selective Autophagy Dysfunction for ALS Pathology. Cells. 2020;9:381. doi: 10.3390/cells9020381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu Y., Runwal G., Obrocki P., Rubinsztein D.C. Autophagy in childhood neurological disorders. Dev. Med. Child Neurol. 2019;61:639–645. doi: 10.1111/dmcn.14092. [DOI] [PubMed] [Google Scholar]
- 39.Margeta M. Autophagy Defects in Skeletal Myopathies. Annu. Rev. Pathol. Mech. Dis. 2020;15:261–285. doi: 10.1146/annurev-pathmechdis-012419-032618. [DOI] [PubMed] [Google Scholar]
- 40.Pierrefite-Carle V., Santucci-Darmanin S., Breuil V., Camuzard O., Carle G.F. Autophagy in bone: Self-eating to stay in balance. Ageing Res. Rev. 2015;24:206–217. doi: 10.1016/j.arr.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 41.Vinatier C., Domínguez E., Guicheux J., Caramés B. Role of the Inflammation-Autophagy-Senescence Integrative Network in Osteoarthritis. Front. Physiol. 2018;9:706. doi: 10.3389/fphys.2018.00706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ballabio A., Bonifacino J.S. Lysosomes as dynamic regulators of cell and organismal homeostasis. Nat. Rev. Mol. Cell Biol. 2020;21:101–118. doi: 10.1038/s41580-019-0185-4. [DOI] [PubMed] [Google Scholar]
- 43.Sun A. Lysosomal storage disease overview. Ann. Transl. Med. 2018;6:476. doi: 10.21037/atm.2018.11.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Palhegyi A.M., Seranova E., Dimova S., Hoque S., Sarkar S. Biomedical Implications of Autophagy in Macromolecule Storage Disorders. Front. Cell Dev. Biol. 2019;7:179. doi: 10.3389/fcell.2019.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parenti G., Andria G., Ballabio A. Lysosomal storage diseases: From pathophysiology to therapy. Annu. Rev. Med. 2015;66:471–486. doi: 10.1146/annurev-med-122313-085916. [DOI] [PubMed] [Google Scholar]
- 46.Ariosa A.R., Klionsky D.J. Autophagy core machinery: Overcoming spatial barriers in neurons. J. Mol. Med. 2016;94:1217–1227. doi: 10.1007/s00109-016-1461-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koyama-Honda I., Itakura E., Fujiwara T.K., Mizushima N. Temporal analysis of recruitment of mammalian ATG proteins to the autophagosome formation site. Autophagy. 2013;9:1491–1499. doi: 10.4161/auto.25529. [DOI] [PubMed] [Google Scholar]
- 48.Wesselborg S., Stork B. Autophagy Signal Transduction by ATG Proteins: From Hierarchies to Networks. Cell Mol Life Sci. 2015;72:4721–4757. doi: 10.1007/s00018-015-2034-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakatogawa H. Mechanisms governing autophagosome biogenesis. Nat. Rev. Mol. Cell Biol. 2020;21:439–458. doi: 10.1038/s41580-020-0241-0. [DOI] [PubMed] [Google Scholar]
- 50.Zachari M., Ganley I.G. The Mammalian ULK1 Complex and Autophagy Initiation. Esssays Biochem. 2017;61:585–596. doi: 10.1042/EBC20170021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tamargo-Gómez I., Mariño G. AMPK: Regulation of Metabolic Dynamics in the Context of Autophagy. Int. J. Mol. Sci. 2018;19:3812. doi: 10.3390/ijms19123812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Y., Zhang H. Regulation of Autophagy by mTOR Signaling Pathway. Volume 1206. Springer; Berlin/Heidelberg, Germany: 2019. pp. 67–83. [DOI] [PubMed] [Google Scholar]
- 53.Morgan A.R., Lam W.J., Han D.Y., Fraser A.G., Ferguson L.R. Association analysis of ULK1 with Crohn‘s disease in a New Zealand population. Gastroenterol. Res. Pract. 2012 doi: 10.1155/2012/715309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Henckaerts L., Cleynen I., Brinar M., John J.M., Van Steen K., Rutgeerts P., Vermeire S. Genetic variation in the autophagy gene ULK1 and risk of Crohn’s disease. Inflamm. Bowel Dis. 2011;17:1392–1397. doi: 10.1002/ibd.21486. [DOI] [PubMed] [Google Scholar]
- 55.Zhang R.R., Liang L., Chen W.W., Wen C., Wan B.S., Luo L.L., Zhao Y.L., Chen J., Yue J. ULK1 polymorphisms confer susceptibility to pulmonary tuberculosis in a Chinese population. Int. J. Tuberc. Lung Dis. 2019;23:265–271. doi: 10.5588/ijtld.18.0174. [DOI] [PubMed] [Google Scholar]
- 56.Horne D.J., Graustein A.D., Shah J.A., Peterson G., Savlov M., Steele S., Narita M., Hawn T.R. Human ULK1 Variation and Susceptibility to Mycobacterium tuberculosis Infection. J. Infect. Dis. 2016;214:1260–1267. doi: 10.1093/infdis/jiw347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang X., Han R., Wang M., Li X., Yang X., Xia Q., Liu R., Yuan Y., Hu X., Chen M., et al. Association between the autophagy-related gene ULK1 and ankylosing spondylitis susceptibility in the Chinese Han population: A case-control study. Postgrad. Med. J. 2017;93:752–757. doi: 10.1136/postgradmedj-2017-134964. [DOI] [PubMed] [Google Scholar]
- 58.Wolthers B.O., Frandsen T.L., Abrahamsson J., Albertsen B.K., Helt L.R., Heyman M., Jónsson G., Kõrgvee L.T., Lund B., Raja R.A., et al. Asparaginase-associated pancreatitis: A study on phenotype and genotype in the NOPHO ALL2008 protocol. Leukemia. 2017;31:325–332. doi: 10.1038/leu.2016.203. [DOI] [PubMed] [Google Scholar]
- 59.Bronson P.G., Chang D., Bhangale T., Seldin M.F., Ortmann W., Ferreira R.C., Urcelay E., Pereira L.F., Martin J., Plebani A., et al. Common variants at PVT1, ATG13-AMBRA1, AHI1 and CLEC16A are associated with selective IgA deficiency. Nat. Genet. 2016;48:1425–1429. doi: 10.1038/ng.3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu B., An T., Li M., Yi Z., Li C., Sun X., Guan X., Li L., Wang Y., Zhang Y., et al. The association between early-onset cardiac events caused by neoadjuvant or adjuvant chemotherapy in triple-negative breast cancer patients and some novel autophagy-related polymorphisms in their genomic DNA: A real-world study. Cancer Commun. 2018;38:71. doi: 10.1186/s40880-018-0343-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Berger M.D., Yamauchi S., Cao S., Hanna D.L., Sunakawa Y., Schirripa M., Matsusaka S., Yang D., Groshen S., Zhang W., et al. Autophagy-related polymorphisms predict hypertension in patients with metastatic colorectal cancer treated with FOLFIRI and bevacizumab: Results from TRIBE and FIRE-3 trials. Eur. J. Cancer. 2017;77:13–20. doi: 10.1016/j.ejca.2017.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hurley J.H., Young L.N. Mechanisms of autophagy initiation. Annu. Rev. Biochem. 2017;86:225–244. doi: 10.1146/annurev-biochem-061516-044820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kang R., Zeh H.J., Lotze M.T., Tang D. The Beclin 1 Network Regulates Autophagy and Apoptosis. Cell Death Differ. 2011;18:571–580. doi: 10.1038/cdd.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cianfanelli V., De Zio D., Di Bartolomeo S., Nazio F., Strappazzon F., Cecconi F. Ambra1 at a glance. J. Cell Sci. 2015;128:2003–2008. doi: 10.1242/jcs.168153. [DOI] [PubMed] [Google Scholar]
- 65.Kim Y.M., Jung C.H., Seo M., Kim E.K., Park J.M., Bae S.S., Kim D.H. MTORC1 phosphorylates UVRAG to negatively regulate autophagosome and endosome maturation. Mol. Cell. 2015;57:207–218. doi: 10.1016/j.molcel.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhong Y., Wang Q.J., Li X., Yan Y., Backer J.M., Chait B.T., Heintz N., Yue Z. Distinct regulation of autophagic activity by Atg14L and Rubicon associated with Beclin 1-phosphatidylinositol-3-kinase complex. Nat. Cell Biol. 2009;11:468–476. doi: 10.1038/ncb1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Matsunaga K., Saitoh T., Tabata K., Omori H., Satoh T., Kurotori N., Maejima I., Shirahama-Noda K., Ichimura T., Isobe T., et al. Two Beclin 1-binding proteins, Atg14L and Rubicon, reciprocally regulate autophagy at different stages. Nat. Cell Biol. 2009;11:385–396. doi: 10.1038/ncb1846. [DOI] [PubMed] [Google Scholar]
- 68.Cheng X., Ma X., Ding X., Li L., Jiang X., Shen Z., Chen S., Liu W., Gong W., Sun Q. Pacer Mediates the Function of Class III PI3K and HOPS Complexes in Autophagosome Maturation by Engaging Stx17. Mol. Cell. 2017;65:1029–1043.e5. doi: 10.1016/j.molcel.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 69.Hamet P., Haloui M., Harvey F., Marois-Blanchet F.-C., Sylvestre M.-P., Tahir M.-R., Simon P.H.G., Kanzki B.S., Raelson J., Long C., et al. PROX1 gene CC genotype as a major determinant of early onset of type 2 diabetes in slavic study participants from Action in Diabetes and Vascular Disease. J. Hypertens. 2017;35:S24–S32. doi: 10.1097/HJH.0000000000001241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kazachkova N., Raposo M., Ramos A., Montiel R., Lima M. Promoter Variant Alters Expression of the Autophagic BECN1 Gene: Implications for Clinical Manifestations of Machado-Joseph Disease. Cerebellum. 2017;16:957–963. doi: 10.1007/s12311-017-0875-4. [DOI] [PubMed] [Google Scholar]
- 71.Zhao L.-L., Liu H.-L., Luo S., Walsh K.M., Li W., Wei Q. Associations of novel variants in PIK3C3, INSR and MAP3K4 of the ATM pathway genes with pancreatic cancer risk. Am. J. Cancer Res. 2020;10:2128–2144. [PMC free article] [PubMed] [Google Scholar]
- 72.Ng D., Hu N., Hu Y., Wang C., Giffen C., Tang Z.-Z., Han X.-Y., Yang H.H., Lee M.P., Goldstein A.M., et al. Replication of a genome-wide case-control study of esophageal squamous cell carcinoma. Int. J. Cancer. 2008;123:1610–1615. doi: 10.1002/ijc.23682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hu N., Wang C., Hu Y., Yang H.H., Giffen C., Tang Z.Z., Han X.Y., Goldstein A.M., Emmert-Buck M.R., Buetow K.H., et al. Genome-wide association study in esophageal cancer using GeneChip mapping 10K array. Cancer Res. 2005;65:2542–2546. doi: 10.1158/0008-5472.CAN-04-3247. [DOI] [PubMed] [Google Scholar]
- 74.Zhang N., Zheng Y., Liu J., Lei T., Xu Y., Yang M. Genetic variations associated with telomere length confer risk of gastric cardia adenocarcinoma. Gastric Cancer. 2019;22:1089–1099. doi: 10.1007/s10120-019-00954-8. [DOI] [PubMed] [Google Scholar]
- 75.Niewold T.B., Kariuki S.N., Franek B.S., Mikolaitis R.A., Utset T.O., Jolly M., Skol A.D. Promoter variant of PIK3C3 is associated with autoimmunity against Ro and Sm epitopes in African-American lupus patients. J. Biomed. Biotechnol. 2010:2010. doi: 10.1155/2010/826434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stopkova P., Saito T., Papolos D.F., Vevera J., Paclt I., Zukov I., Bersson Y.B., Margolis B.A., Strous R.D., Lachman H.M. Identification of PIK3C3 promoter variant associated with bipolar disorder and schizophrenia. Biol. Psychiatry. 2004;55:981–988. doi: 10.1016/j.biopsych.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 77.Rietschel M., Mattheisen M., Degenhardt F., Mühleisen T.W., Kirsch P., Esslinger C., Herms S., Demontis D., Steffens M., Strohmaier J., et al. Association between genetic variation in a region on chromosome 11 and schizophrenia in large samples from Europe. Mol. Psychiatry. 2012;17:906–917. doi: 10.1038/mp.2011.80. [DOI] [PubMed] [Google Scholar]
- 78.Mitjans M., Begemann M., Ju A., Dere E., Wüstefeld L., Hofer S., Hassouna I., Balkenhol J., Oliveira B., van der Auwera S., et al. Sexual dimorphism of AMBRA1-related autistic features in human and mouse. Transl. Psychiatry. 2017;7:e1247. doi: 10.1038/tp.2017.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Litchfield K., Levy M., Orlando G., Loveday C., Law P.J., Migliorini G., Holroyd A., Broderick P., Karlsson R., Haugen T.B., et al. Identification of 19 new risk loci and potential regulatory mechanisms influencing susceptibility to testicular germ cell tumor. Nat. Genet. 2017;49:1133–1140. doi: 10.1038/ng.3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ross C.J., Towfic F., Shankar J., Laifenfeld D., Thoma M., Davis M., Weiner B., Kusko R., Zeskind B., Knappertz V., et al. A pharmacogenetic signature of high response to Copaxone in late-phase clinical-trial cohorts of multiple sclerosis. Genome Med. 2017;9:50. doi: 10.1186/s13073-017-0436-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim H.-K., Lee W.-Y., Kwon J.-T., Sohn D.-R., Hong S.-J., Kim H.-J. Association of ultraviolet radiation resistance-associated gene polymorphisms with rheumatoid arthritis. Biomed. Rep. 2014;2:117–121. doi: 10.3892/br.2013.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jeong T.-J., Shin M.-K., Uhm Y.-K., Kim H.-J., Chung J.-H., Lee M.-H. Association of UVRAG polymorphisms with susceptibility to non-segmental vitiligo in a Korean sample. Exp. Dermatol. 2010;19:e323–e325. doi: 10.1111/j.1600-0625.2009.01039.x. [DOI] [PubMed] [Google Scholar]
- 83.Mercer T.J., Gubas A., Tooze S.A. A Molecular Perspective of Mammalian Autophagosome Biogenesis. Biol. Chem. 2018;293:5386–5395. doi: 10.1074/jbc.R117.810366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Axe E.L., Walker S.A., Manifava M., Chandra P., Roderick H.L., Habermann A., Griffiths G., Ktistakis N.T. Autophagosome formation from membrane compartments enriched in phosphatidylinositol 3-phosphate and dynamically connected to the endoplasmic reticulum. J. Cell Biol. 2008;182:685–701. doi: 10.1083/jcb.200803137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Proikas-Cezanne T., Takacs Z., Dönnes P., Kohlbacher O. WIPI Proteins: Essential PtdIns3P Effectors at the Nascent Autophagosome. J. Cell Sci. 2015;128:207–217. doi: 10.1242/jcs.146258. [DOI] [PubMed] [Google Scholar]
- 86.Dooley H.C., Razi M., Polson H.E.J., Girardin S.E., Wilson M.I., Tooze S.A. WIPI2 Links LC3 Conjugation with PI3P, Autophagosome Formation, and Pathogen Clearance by Recruiting Atg12-5-16L1. Mol. Cell. 2014;55:238–252. doi: 10.1016/j.molcel.2014.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bakula D., Müller A.J., Zuleger T., Takacs Z., Franz-Wachtel M., Thost A.K., Brigger D., Tschan M.P., Frickey T., Robenek H., et al. WIPI3 and WIPI4 β-propellers are scaffolds for LKB1-AMPK-TSC signalling circuits in the control of autophagy. Nat. Commun. 2017;8:1–18. doi: 10.1038/ncomms15637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Osawa T., Kotani T., Kawaoka T., Hirata E., Suzuki K., Nakatogawa H., Ohsumi Y., Noda N.N. Atg2 mediates direct lipid transfer between membranes for autophagosome formation. Nat. Struct. Mol. Biol. 2019;26:281–288. doi: 10.1038/s41594-019-0203-4. [DOI] [PubMed] [Google Scholar]
- 89.Osawa T., Noda N.N. Atg2: A novel phospholipid transfer protein that mediates de novo autophagosome biogenesis. Protein Sci. A Publ. Protein Soc. 2019;28:1005–1012. doi: 10.1002/pro.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Filimonenko M., Isakson P., Finley K.D., Anderson M., Jeong H., Melia T.J., Bartlett B.J., Myers K.M., Birkeland H.C.G., Lamark T., et al. The Selective Macroautophagic Degradation of Aggregated Proteins Requires the PI3P-Binding Protein Alfy. Mol. Cell. 2010;38:265–279. doi: 10.1016/j.molcel.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Noda T. Autophagy in the Context of the Cellular Membrane-Trafficking System: The Enigma of Atg9 Vesicles. Biochem. Soc. Trans. 2017;45:1323–1331. doi: 10.1042/BST20170128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Orsi A., Razi M., Dooley H.C., Robinson D., Weston A.E., Collinson L.M., Tooze S.A. Dynamic and transient interactions of Atg9 with autophagosomes, but not membrane integration, are required for autophagy. Mol. Biol. Cell. 2012;23:1860–1873. doi: 10.1091/mbc.e11-09-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sobota R.S., Stein C.M., Kodaman N., Maro I., Wieland-Alter W., Igo R.P., Magohe A., Malone L.L., Chervenak K., Hall N.B., et al. A chromosome 5q31.1 locus associates with tuberculin skin test reactivity in HIV-positive individuals from tuberculosis hyper-endemic regions in east Africa. PLoS Genet. 2017;13:e1006710. doi: 10.1371/journal.pgen.1006710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kadir R., Harel T., Markus B., Perez Y., Bakhrat A., Cohen I., Volodarsky M., Feintsein-Linial M., Chervinski E., Zlotogora J., et al. ALFY-Controlled DVL3 Autophagy Regulates Wnt Signaling, Determining Human Brain Size. PLoS Genet. 2016;12:e1005919. doi: 10.1371/journal.pgen.1005919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lesseur C., Diergaarde B., Olshan A.F., Wünsch-Filho V., Ness A.R., Liu G., Lacko M., Eluf-Neto J., Franceschi S., Lagiou P., et al. Genome-wide association analyses identify new susceptibility loci for oral cavity and pharyngeal cancer. Nat. Genet. 2016;48:1544–1550. doi: 10.1038/ng.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ohba C., Nabatame S., Iijima Y., Nishiyama K., Tsurusaki Y., Nakashima M., Miyake N., Tanaka F., Ozono K., Saitsu H., et al. De novo WDR45 mutation in a patient showing clinically Rett syndrome with childhood iron deposition in brain. J. Hum. Genet. 2014;59:292–295. doi: 10.1038/jhg.2014.18. [DOI] [PubMed] [Google Scholar]
- 97.Tschentscher A., Dekomien G., Ross S., Cremer K., Kukuk G.M., Epplen J.T., Hoffjan S. Analysis of the C19orf12 and WDR45 genes in patients with neurodegeneration with brain iron accumulation. J. Neurol. Sci. 2015;349:105–109. doi: 10.1016/j.jns.2014.12.036. [DOI] [PubMed] [Google Scholar]
- 98.Saitsu H., Nishimura T., Muramatsu K., Kodera H., Kumada S., Sugai K., Kasai-Yoshida E., Sawaura N., Nishida H., Hoshino A., et al. De novo mutations in the autophagy gene WDR45 cause static encephalopathy of childhood with neurodegeneration in adulthood. Nat. Genet. 2013;45:445–449. doi: 10.1038/ng.2562. [DOI] [PubMed] [Google Scholar]
- 99.Lee H.-S., Park T. Nuclear receptor and VEGF pathways for gene-blood lead interactions, on bone mineral density, in Korean smokers. PLoS ONE. 2018;13:e0193323. doi: 10.1371/journal.pone.0193323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Suleiman J., Allingham-Hawkins D., Hashem M., Shamseldin H.E., Alkuraya F.S., El-Hattab A.W. WDR45B-related intellectual disability, spastic quadriplegia, epilepsy, and cerebral hypoplasia: A consistent neurodevelopmental syndrome. Clin. Genet. 2018;93:360–364. doi: 10.1111/cge.13054. [DOI] [PubMed] [Google Scholar]
- 101.Brinar M., Vermeire S., Cleynen I., Lemmens B., Sagaert X., Henckaerts L., Van Assche G., Geboes K., Rutgeerts P., De Hertogh G. Genetic variants in autophagy-related genes and granuloma formation in a cohort of surgically treated Crohn’s disease patients. J. Crohn’s Colitis. 2012;6:43–50. doi: 10.1016/j.crohns.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 102.Yamada Y., Sakuma J., Takeuchi I., Yasukochi Y., Kato K., Oguri M., Fujimaki T., Horibe H., Muramatsu M., Sawabe M., et al. Identification of C21orf59 and ATG2A as novel determinants of renal function-related traits in Japanese by exome-wide association studies. Oncotarget. 2017;8:45259–45273. doi: 10.18632/oncotarget.16696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fernández-Mateos J., Seijas-Tamayo R., Klain J.C.A., Borgonõn M.P., Pérez-Ruiz E., Mesiá R., Del Barco E., Coloma C.S., Dominguez A.R., Daroqui J.C., et al. Analysis of autophagy gene polymorphisms in Spanish patients with head and neck squamous cell carcinoma. Sci. Rep. 2017;7:1–8. doi: 10.1038/s41598-017-07270-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Buffen K., Oosting M., Quintin J., Ng A., Kleinnijenhuis J., Kumar V., van de Vosse E., Wijmenga C., van Crevel R., Oosterwijk E., et al. Autophagy Controls BCG-Induced Trained Immunity and the Response to Intravesical BCG Therapy for Bladder Cancer. PLoS Pathog. 2014;10:e1004485. doi: 10.1371/journal.ppat.1004485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mehrabi Pour M., Nasiri M., Kamfiroozie H., Zibaeenezhad M.J. Association of the ATG9B gene polymorphisms with coronary artery disease susceptibility: A case-control study. J. Cardiovasc. Thorac. Res. 2019;11:109–115. doi: 10.15171/jcvtr.2019.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mizushima N. The ATG conjugation systems in autophagy. Curr. Opin. Cell Biol. 2020;63:1–10. doi: 10.1016/j.ceb.2019.12.001. [DOI] [PubMed] [Google Scholar]
- 107.Hanada T., Noda N.N., Satomi Y., Ichimura Y., Fujioka Y., Takao T., Inagaki F., Ohsumi Y. The Atg12-Atg5 conjugate has a novel E3-like activity for protein lipidation in autophagy. J. Biol. Chem. 2007;282:37298–37302. doi: 10.1074/jbc.C700195200. [DOI] [PubMed] [Google Scholar]
- 108.Khor B., Conway K.L., Omar A.S., Biton M., Haber A.L., Rogel N., Baxt L.A., Begun J., Kuballa P., Gagnon J.D., et al. Distinct Tissue-Specific Roles for the Disease-Associated Autophagy Genes ATG16L2 and ATG16L1. J. Immunol. 2019;203:1820–1829. doi: 10.4049/jimmunol.1800419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ishibashi K., Fujita N., Kanno E., Omori H., Yoshimori T., Itoh T., Fukuda M. Atg16L2, a novel isoform of mammalian Atg16L that is not essential for canonical autophagy despite forming an Atg12-5-16L2 complex. Autophagy. 2011;7:1500–1513. doi: 10.4161/auto.7.12.18025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wesch N., Kirkin V., Rogov V.V. Atg8-Family Proteins-Structural Features and Molecular Interactions in Autophagy and Beyond. Cells. 2020;9:2008. doi: 10.3390/cells9092008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fernandez A.F., Lopez-Otin C. The functional and pathologic relevance of autophagy proteases. J. Clin. Investig. 2015;125:33–41. doi: 10.1172/JCI73940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nair U., Yen W.L., Mari M., Cao Y., Xie Z., Baba M., Reggiori F., Klionsky D.J. A role for Atg8-PE deconjugation in autophagosome biogenesis. Autophagy. 2012;8:780–793. doi: 10.4161/auto.19385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yu Z.Q., Ni T., Hong B., Wang H.Y., Jiang F.J., Zou S., Chen Y., Zheng X.L., Klionsky D.J., Liang Y., et al. Dual roles of Atg8 - PE deconjugation by Atg4 in autophagy. Autophagy. 2012;8:883–892. doi: 10.4161/auto.19652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yang Z., Liu Z. Potentially functional variants of autophagy-related genes are associated with the efficacy and toxicity of radiotherapy in patients with nasopharyngeal carcinoma. Mol. Genet. Genom. Med. 2019;7:1–8. doi: 10.1002/mgg3.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Song X., Yuan Z., Yuan H., Wang L., Ji P., Jin G., Dai J., Ma H. ATG12 expression quantitative trait loci associated with head and neck squamous cell carcinoma risk in a Chinese Han population. Mol. Carcinog. 2018;57:1030–1037. doi: 10.1002/mc.22823. [DOI] [PubMed] [Google Scholar]
- 116.Yang P.W., Hsieh M.S., Chang Y.H., Huang P.M., Lee J.M. Genetic polymorphisms of ATG5 predict survival and recurrence in patients with early-stage esophageal squamous cell carcinoma. Oncotarget. 2017;8:91494–91504. doi: 10.18632/oncotarget.20793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shen M., Lin L. Functional variants of autophagy-related genes are associated with the development of hepatocellular carcinoma. Life Sci. 2019;235:116675. doi: 10.1016/j.lfs.2019.116675. [DOI] [PubMed] [Google Scholar]
- 118.Yuan J., Zhang N., Yin L., Zhu H., Zhang L., Zhou L., Yang M. Clinical Implications of the Autophagy Core Gene Variations in Advanced Lung Adenocarcinoma Treated with Gefitinib. Sci. Rep. 2017;7:1–10. doi: 10.1038/s41598-017-18165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.White K.A.M., Luo L., Thompson T.A., Torres S., Hu C.A.A., Thomas N.E., Lilyquist J., Anton-Culver H., Gruber S.B., From L., et al. Variants in autophagy-related genes and clinical characteristics in melanoma: A population-based study. Cancer Med. 2016;5:3336–3345. doi: 10.1002/cam4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li Q.X., Zhou X., Huang T.T., Tang Y., Liu B., Peng P., Sun L., Wang Y.H., Yuan X.L. The Thr300Ala variant of ATG16L1 is associated with decreased risk of brain metastasis in patients with non-small cell lung cancer. Autophagy. 2017;13:1053–1063. doi: 10.1080/15548627.2017.1308997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li M., Ma F., Wang J., Li Q., Zhang P., Yuan P., Luo Y., Cai R., Fan Y., Chen S., et al. Genetic polymorphisms of autophagy-related gene 5 (ATG5) rs473543 predict different disease-free survivals of triple-negative breast cancer patients receiving anthracycline- and/or taxane-based adjuvant chemotherapy. Chin. J. Cancer. 2018;37:4. doi: 10.1186/s40880-018-0268-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhou J., Hang D., Jiang Y., Chen J., Han J., Zhou W., Jin G., Ma H., Dai J. Evaluation of genetic variants in autophagy pathway genes as prognostic biomarkers for breast cancer. Gene. 2017;627:549–555. doi: 10.1016/j.gene.2017.06.053. [DOI] [PubMed] [Google Scholar]
- 123.Michailidou K., Beesley J., Lindstrom S., Canisius S., Dennis J., Lush M.J., Maranian M.J., Bolla M.K., Wang Q., Shah M., et al. Genome-wide association analysis of more than 120,000 individuals identifies 15 new susceptibility loci for breast cancer. Nat. Genet. 2015;47:373–380. doi: 10.1038/ng.3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Qin Z., Xue J., He Y., Ma H., Jin G., Chen J., Hu Z., Liu X.a., Shen H. Potentially functional polymorphisms in ATG10 are associated with risk of breast cancer in a Chinese population. Gene. 2013;527:491–495. doi: 10.1016/j.gene.2013.06.067. [DOI] [PubMed] [Google Scholar]
- 125.Santoni M., Piva F., De Giorgi U., Mosca A., Basso U., Santini D., Buti S., Lolli C., Terrone C., Maruzzo M., et al. Autophagic gene polymorphisms in liquid biopsies and outcome of patients with metastatic clear cell renal cell carcinoma. Anticancer Res. 2018;38:5773–5782. doi: 10.21873/anticanres.12916. [DOI] [PubMed] [Google Scholar]
- 126.Nikseresht M., Shahverdi M., Dehghani M., Abidi H., Mahmoudi R., Ghalamfarsa G., Manzouri L., Ghavami S. Association of single nucleotide autophagy-related protein 5 gene polymorphism rs2245214 with susceptibility to non–small cell lung cancer. J. Cell. Biochem. 2019;120:1924–1931. doi: 10.1002/jcb.27467. [DOI] [PubMed] [Google Scholar]
- 127.Xie K., Liang C., Li Q., Yan C., Wang C., Gu Y., Zhu M., Du F., Wang H., Dai J., et al. Role of ATG10 expression quantitative trait loci in non-small cell lung cancer survival. Int. J. Cancer. 2016;139:1564–1573. doi: 10.1002/ijc.30205. [DOI] [PubMed] [Google Scholar]
- 128.Mitchell J.S., Li N., Weinhold N., Försti A., Ali M., Van Duin M., Thorleifsson G., Johnson D.C., Chen B., Halvarsson B.M., et al. Genome-wide association study identifies multiple susceptibility loci for multiple myeloma. Nat. Commun. 2016;7:22. doi: 10.1038/ncomms12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Plantinga T.S., van de Vosse E., Huijbers A., Netea M.G., Joosten L.A.B., Smit J.W.A., Netea-Maier R.T. Role of Genetic Variants of Autophagy Genes in Susceptibility for Non-Medullary Thyroid Cancer and Patients Outcome. PLoS ONE. 2014;9:e94086. doi: 10.1371/journal.pone.0094086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Huijbers A., Plantinga T.S., Joosten L.A.B., Aben K.K.H., Gudmundsson J., den Heijer M., Kiemeney L.A.L.M., Netea M.G., Hermus A.R.M.M., Netea-Maier R.T. The effect of the ATG16L1 Thr300Ala Polymorphism on Susceptibility and Outcome of Patients with Epithelial Cell-Derived Thyroid Carcinoma. Endocr. Relat. Cancer. 2012;19:L15–L18. doi: 10.1530/ERC-11-0302. [DOI] [PubMed] [Google Scholar]
- 131.Grimm W.A., Messer J.S., Murphy S.F., Nero T., Lodolce J.P., Weber C.R., Logsdon M.F., Bartulis S., Sylvester B.E., Springer A., et al. The Thr300Ala variant in ATG16L1 is associated with improved survival in human colorectal cancer and enhanced production of type I interferon. Gut. 2016;65:456–464. doi: 10.1136/gutjnl-2014-308735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Burada F., Ciurea M.E., Nicoli R., Streata I., Vilcea I.D., Rogoveanu I., Ioana M. ATG16L1 T300A Polymorphism is Correlated with Gastric Cancer Susceptibility. Pathol. Oncol. Res. 2016;22:317–322. doi: 10.1007/s12253-015-0006-9. [DOI] [PubMed] [Google Scholar]
- 133.Huang C.Y., Huang S.P., Lin V.C., Yu C.C., Chang T.Y., Lu T.L., Chiang H.C., Bao B.Y. Genetic variants of the autophagy pathway as prognostic indicators for prostate cancer. Sci. Rep. 2015;5:14045. doi: 10.1038/srep14045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Xia L., Xu J., Song J., Xu Y., Zhang B., Gao C., Zhu D., Zhou C., Bi D., Wang Y., et al. Autophagy-Related Gene 7 Polymorphisms and Cerebral Palsy in Chinese Infants. Front. Cell. Neurosci. 2019;13:494. doi: 10.3389/fncel.2019.00494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Xu J., Xia L., Shang Q., Du J., Zhu D., Wang Y., Bi D., Song J., Ma C., Gao C., et al. A Variant of the Autophagy-Related 5 Gene Is Associated with Child Cerebral Palsy. Cell. Neurosci. 2017;11:407. doi: 10.3389/fncel.2017.00407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Metzger S., Saukko M., Van Che H., Tong L., Puder Y., Riess O., Nguyen H.P. Age at onset in Huntington’s disease is modified by the autophagy pathway: Implication of the V471A polymorphism in Atg7. Hum. Genet. 2010;128:453–459. doi: 10.1007/s00439-010-0873-9. [DOI] [PubMed] [Google Scholar]
- 137.Metzger S., Walter C., Riess O., Roos R.A.C., Nielsen J.E., Craufurd D., Nguyen H.P. The V471A Polymorphism in Autophagy-Related Gene ATG7 Modifies Age at Onset Specifically in Italian Huntington Disease Patients. PLoS ONE. 2013;8:e68951. doi: 10.1371/journal.pone.0068951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kim M., Sandford E., Gatica D., Qiu Y., Liu X., Zheng Y., Schulman B.A., Xu J., Semple I., Ro S.H., et al. Mutation in ATG5 reduces autophagy and leads to ataxia with developmental delay. eLife. 2016;5:e12245. doi: 10.7554/eLife.12245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chen D., Zhu C., Wang X., Feng X., Pang S., Huang W., Hawley R.G., Yan B. A novel and functional variant within the ATG5 gene promoter in sporadic Parkinson’s disease. Neurosci. Lett. 2013;538:49–53. doi: 10.1016/j.neulet.2013.01.044. [DOI] [PubMed] [Google Scholar]
- 140.Yuan J., Han R., Esther A., Wu Q., Yang J., Yan W., Ji X., Liu Y., Li Y., Yao W., et al. Polymorphisms in autophagy related genes and the coal workers’ pneumoconiosis in a Chinese population. Gene. 2017;632:36–42. doi: 10.1016/j.gene.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 141.Lee T.H., Ko T.M., Chen C.H., Chang Y.J., Lu L.S., Chang C.H., Huang K.L., Chang T.Y., Lee J.D., Chang K.C., et al. A genome-wide association study links small-vessel ischemic stroke to autophagy. Sci. Rep. 2017;7:1–7. doi: 10.1038/s41598-017-14355-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hampe J., Franke A., Rosenstiel P., Till A., Teuber M., Huse K., Albrecht M., Mayr G., De La Vega F.M., Briggs J., et al. A genome-wide association scan of nonsynonymous SNPs identifies a susceptibility variant for Crohn disease in ATG16L1. Nat. Genet. 2007;39:207–211. doi: 10.1038/ng1954. [DOI] [PubMed] [Google Scholar]
- 143.Chauhan S., Mandell M.A., Deretic V. IRGM governs the core autophagy machinery to conduct antimicrobial defense. Mol. Cell. 2015;58:507–521. doi: 10.1016/j.molcel.2015.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bekpen C., Xavier R.J., Eichler E.E. Human IRGM gene “to be or not to be”. Semin. Immunopathol. 2010;32:437–444. doi: 10.1007/s00281-010-0224-x. [DOI] [PubMed] [Google Scholar]
- 145.Dezelak M., Repnik K., Koder S., Ferkolj I., Potočnik U. A Prospective Pharmacogenomic Study of Crohn’s Disease Patients during Routine Therapy with Anti-TNF-α Drug Adalimumab: Contribution of ATG5, NFKB1, and CRP Genes to Pharmacodynamic Variability. Omics A J. Integr. Biol. 2016;20:296–309. doi: 10.1089/omi.2016.0005. [DOI] [PubMed] [Google Scholar]
- 146.Usategui-Martín R., García-Aparicio J., Corral-Gudino L., Calero-Paniagua I., Del Pino-Montes J., González Sarmiento R. Polymorphisms in Autophagy Genes Are Associated with Paget Disease of Bone. PLoS ONE. 2015;10:e0128984. doi: 10.1371/journal.pone.0128984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kamel A.M., Badary M.S., Mohamed W.A., Ahmed G.H., El-Feky M.A. Evaluation of autophagy-related genes in Egyptian systemic lupus erythematosus patients. Int. J. Rheum. Dis. 2020;5:1–7. doi: 10.1111/1756-185X.13910. [DOI] [PubMed] [Google Scholar]
- 148.Ciccacci C., Perricone C., Alessandri C., Latini A., Politi C., Delunardo F., Pierdominici M., Conti F., Novelli G., Ortona E., et al. Evaluation of ATG5 polymorphisms in Italian patients with systemic lupus erythematosus: Contribution to disease susceptibility and clinical phenotypes. Lupus. 2018;27:1464–1469. doi: 10.1177/0961203318776108. [DOI] [PubMed] [Google Scholar]
- 149.Dang J., Li J., Xin Q., Shan S., Bian X., Yuan Q., Liu N., Ma X., Li Y., Liu Q. Gene–gene interaction of ATG5, ATG7, BLK and BANK1 in systemic lupus erythematosus. Int. J. Rheum. Dis. 2016;19:1284–1293. doi: 10.1111/1756-185X.12768. [DOI] [PubMed] [Google Scholar]
- 150.López P., Alonso-Pérez E., Rodríguez-Carrio J., Suárez A. Influence of Atg5 Mutation in SLE Depends on Functional IL-10 Genotype. PLoS ONE. 2013;8:e78756. doi: 10.1371/journal.pone.0078756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Alonso-Perez E., Suarez-Gestal M., Calaza M., Ordi-Ros J., Balada E., Bijl M., Papasteriades C., Carreira P., Skopouli F.N., Witte T., et al. Further Evidence of Subphenotype Association with Systemic Lupus Erythematosus Susceptibility Loci: A European Cases Only Study. PLoS ONE. 2012;7:e45356. doi: 10.1371/journal.pone.0045356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhou X.J., Lu X.L., Lv J.C., Yang H.Z., Qin L.X., Zhao M.H., Su Y., Li Z.G., Zhang H. Genetic association of PRDM1-ATG5 intergenic region and autophagy with systemic lupus erythematosus in a Chinese population. Ann. Rheum. Dis. 2011;70:1330–1337. doi: 10.1136/ard.2010.140111. [DOI] [PubMed] [Google Scholar]
- 153.Harley J.B., Alarcón-Riquelme M.E., Criswell L.A., Jacob C.O., Kimberly R.P., Moser K.L., Tsao B.P., Vyse T.J., Langefeld C.D., Nath S.K., et al. Genome-wide association scan in women with systemic lupus erythematosus identifies susceptibility variants in ITGAM, PXK, KIAA1542 and other loci. Nat. Genet. 2008;40:204–210. doi: 10.1038/ng.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zheng M., Yu H., Zhang L., Li H., Liu Y., Kijlstra A., Yang P. Association of ATG5 gene polymorphisms with behçet’s disease and ATG10 gene polymorphisms with VKH syndrome in a chinese han population. Investig. Ophthalmol. Vis. Sci. 2015;56:8280–8287. doi: 10.1167/iovs.15-18035. [DOI] [PubMed] [Google Scholar]
- 155.Cai P.P., Wang H.X., Zhuang J.C., Liu Q.B., Zhao G.X., Li Z.X., Wu Z.Y. Variants of autophagy-related gene 5 are associated with neuromyelitis optica in the Southern Han Chinese population. Autoimmunity. 2014;47:563–566. doi: 10.3109/08916934.2014.929668. [DOI] [PubMed] [Google Scholar]
- 156.Mayes M.D., Bossini-Castillo L., Gorlova O., Martin J.E., Zhou X., Chen W.V., Assassi S., Ying J., Tan F.K., Arnett F.C., et al. Immunochip analysis identifies multiple susceptibility loci for systemic sclerosis. Am. J. Hum. Genet. 2014;94:47–61. doi: 10.1016/j.ajhg.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Martin J.E., Assassi S., Diaz-Gallo L.M., Broen J.C., Simeon C.P., Castellvi I., Vicente-Rabaneda E., Fonollosa V., Ortego-Centeno N., González-Gay M.A., et al. A systemic sclerosis and systemic lupus erythematosus pan-meta-GWAS reveals new shared susceptibility loci. Hum. Mol. Genet. 2013;22:4021–4029. doi: 10.1093/hmg/ddt248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.You Y., Huo J., Huang J., Wang M., Shao Y., Ge M., Li X., Huang Z., Zhang J., Nie N., et al. Contribution of autophagy-related gene 5 variants to acquired aplastic anemia in Han-Chinese population. J. Cell. Biochem. 2019;120:11409–11417. doi: 10.1002/jcb.28418. [DOI] [PubMed] [Google Scholar]
- 159.Martin L.J., Gupta J., Jyothula S.S.S.K., Butsch Kovacic M., Biagini Myers J.M., Patterson T.L., Ericksen M.B., He H., Gibson A.M., Baye T.M., et al. Functional Variant in the Autophagy-Related 5 Gene Promotor is Associated with Childhood Asthma. PLoS ONE. 2012;7:e33454. doi: 10.1371/journal.pone.0033454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Poon A.H., Chouiali F., Tse S.M., Litonjua A.A., Hussain S.N.A., Baglole C.J., Eidelman D.H., Olivenstein R., Martin J.G., Weiss S.T., et al. Genetic and histologic evidence for autophagy in asthma pathogenesis. J. Allergy Clin. Immunol. 2012;129:569–571. doi: 10.1016/j.jaci.2011.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Jansen A.F.M., Schoffelen T., Bleeker-Rovers C.P., Wever P.C., Jaeger M., Oosting M., Adriaans A., Joosten L.A.B., Netea M.G., van Deuren M., et al. Genetic variations in innate immunity genes affect response to Coxiella burnetii and are associated with susceptibility to chronic Q fever. Clin. Microbiol. Infect. 2019;25:e11–e631. doi: 10.1016/j.cmi.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 162.Shao Y., Chen F., Chen Y., Zhang W., Lin Y., Cai Y., Yin Z., Tao S., Liao Q., Zhao J., et al. Association between genetic polymorphisms in the autophagy-related 5 gene promoter and the risk of sepsis. Sci. Rep. 2017;7:1–14. doi: 10.1038/s41598-017-09978-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Li N., Fan X., Wang X., Zhang X., Zhang K., Han Q., Lv Y., Liu Z. Genetic association of polymorphisms at the intergenic region between PRDM1 and ATG5 with hepatitis B virus infection in Han Chinese patients. J. Med Virol. 2020;92:1198–1205. doi: 10.1002/jmv.25629. [DOI] [PubMed] [Google Scholar]
- 164.Li N., Fan X., Wang X., Deng H., Zhang K., Zhang X., Han Q., Lv Y., Liu Z. Autophagy-Related 5 Gene rs510432 Polymorphism Is Associated with Hepatocellular Carcinoma in Patients with Chronic Hepatitis B Virus Infection. Immunol. Investig. 2019;48:378–391. doi: 10.1080/08820139.2019.1567532. [DOI] [PubMed] [Google Scholar]
- 165.Tanaka S., Nagashima H., Uotani T., Graham D.Y., Yamaoka Y. Autophagy-related genes in Helicobacter pylori infection. Helicobacter. 2017;22:1–10. doi: 10.1111/hel.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Castaño-Rodríguez N., Kaakoush N.O., Goh K.L., Fock K.M., Mitchell H.M. Autophagy in Helicobacter pylori Infection and Related Gastric Cancer. Helicobacter. 2015;20:353–369. doi: 10.1111/hel.12211. [DOI] [PubMed] [Google Scholar]
- 167.Raju D., Hussey S., Ang M., Terebiznik M.R., Sibony M., Galindo-Mata E., Gupta V., Blanke S.R., Delgado A., Romero-Gallo J., et al. Vacuolating cytotoxin and variants in Atg16L1 that disrupt autophagy promote helicobacter pylori infection in humans. Gastroenterology. 2012;142:1160–1171. doi: 10.1053/j.gastro.2012.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Douroudis K., Kingo K., Traks T., Rätsep R., Silm H., Vasar E., Kõks S. ATG16L1 gene polymorphisms are associated with palmoplantar pustulosis. Hum. Immunol. 2011;72:613–615. doi: 10.1016/j.humimm.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 169.Douroudis K., Kingo K., Traks T., Reimann E., Raud K., Rätsep R., Mössner R., Silm H., Vasar E., Kõks S. Polymorphisms in the ATG16L1 Gene are Associated with Psoriasis Vulgaris. Acta Derm. Venereol. 2012;92:85–87. doi: 10.2340/00015555-1183. [DOI] [PubMed] [Google Scholar]
- 170.Mao J.J., Wu L.X., Wang W., Ye Y.Y., Yang J., Chen H., Yang Q.F., Zhang X.Y., Wang B., Chen W.X. Nucleotide variation in ATG4A and susceptibility to cervical cancer in southwestern chinese women. Oncol. Lett. 2018;15:2992–3000. doi: 10.3892/ol.2017.7663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.He Q., Lu Y., Hu S., Huang Q., Li S., Huang Y., Hu Q., Wu L., Chen W. An intron SNP rs807185 in ATG4A decreases the risk of lung cancer in a southwest Chinese population. Eur. J. Cancer Prev. 2016;25:255–258. doi: 10.1097/CEJ.0000000000000174. [DOI] [PubMed] [Google Scholar]
- 172.Turcot V., Lu Y., Highland H.M., Schurmann C., Justice A.E., Fine R.S., Bradfield J.P., Esko T., Giri A., Graff M., et al. Protein-altering variants associated with body mass index implicate pathways that control energy intake and expenditure in obesity. Nat. Genet. 2018;50:26–41. doi: 10.1038/s41588-017-0011-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Franceschini N., Giambartolomei C., de Vries P.S., Finan C., Bis J.C., Huntley R.P., Lovering R.C., Tajuddin S.M., Winkler T.W., Graff M., et al. GWAS and colocalization analyses implicate carotid intima-media thickness and carotid plaque loci in cardiovascular outcomes. Nat. Commun. 2018;9:1–14. doi: 10.1038/s41467-018-07340-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wu C., Wen Y., Guo X., Yang T., Shen H., Chen X., Tian Q., Tan L., Deng H.W., Zhang F. Genetic association, mRNA and protein expression analysis identify ATG4C as a susceptibility gene for Kashin–Beck disease. Osteoarthr. Cartil. 2017;25:281–286. doi: 10.1016/j.joca.2016.09.019. [DOI] [PubMed] [Google Scholar]
- 175.Portilla-Fernandez E., Ghanbari M., van Meurs J.B.J., Danser A.H.J., Franco O.H., Muka T., Roks A., Dehghan A. Dissecting the association of autophagy-related genes with cardiovascular diseases and intermediate vascular traits: A population-based approach. PLoS ONE. 2019;14:e0214137. doi: 10.1371/journal.pone.0214137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hysi P.G., Choquet H., Khawaja A.P., Wojciechowski R., Tedja M.S., Yin J., Simcoe M.J., Patasova K., Mahroo O.A., Thai K.K., et al. Meta-analysis of 542,934 subjects of European ancestry identifies new genes and mechanisms predisposing to refractive error and myopia. Nat. Genet. 2020;52:401–407. doi: 10.1038/s41588-020-0599-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Qi Y.Y., Zhou X.J., Nath S.K., Sun C., Wang Y.N., Hou P., Mu R., Li C., Guo J.P., Li Z.G., et al. A Rare Variant (rs933717) at FBXO31-MAP1LC3B in Chinese Is Associated With Systemic Lupus Erythematosus. Arthritis Rheumatol. 2018;70:287–297. doi: 10.1002/art.40353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Johansen T., Lamark T. Selective Autophagy: ATG8 Family Proteins, LIR Motifs and Cargo Receptors. J. Mol. Biol. 2020;432:80–103. doi: 10.1016/j.jmb.2019.07.016. [DOI] [PubMed] [Google Scholar]
- 179.Khaminets A., Behl C., Dikic I. Ubiquitin-Dependent and Independent Signals In Selective Autophagy. Trends Cell Biol. 2016;26:6–16. doi: 10.1016/j.tcb.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 180.Katsuragi Y., Ichimura Y., Komatsu M. P62/SQSTM1 Functions as a Signaling Hub and an Autophagy Adaptor. FEBS J. 2015;82:4672–4678. doi: 10.1111/febs.13540. [DOI] [PubMed] [Google Scholar]
- 181.Kirkin V., McEwan D.G., Novak I., Dikic I. A Role for Ubiquitin in Selective Autophagy. Volume 34. Elsevier; Amsterdam, The Netherlands: 2009. pp. 259–269. [DOI] [PubMed] [Google Scholar]
- 182.Korac J., Schaeffer V., Kovacevic I., Clement A.M., Jungblut B., Behl C., Terzic J., Dikic I. Ubiquitin-independent function of optineurin in autophagic clearance of protein aggregates. J. Cell Sci. 2013;126:580–592. doi: 10.1242/jcs.114926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lazarou M., Sliter D.A., Kane L.A., Sarraf S.A., Wang C., Burman J.L., Sideris D.P., Fogel A.I., Youle R.J. The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature. 2015;524:309–314. doi: 10.1038/nature14893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wild P., Farhan H., McEwan D.G., Wagner S., Rogov V.V., Brady N.R., Richter B., Korac J., Waidmann O., Choudhary C., et al. Phosphorylation of the autophagy receptor optineurin restricts Salmonella growth. Science. 2011;333:228–233. doi: 10.1126/science.1205405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Vargas J.N.S., Wang C., Bunker E., Hao L., Maric D., Schiavo G., Randow F., Youle R.J. Spatiotemporal Control of ULK1 Activation by NDP52 and TBK1 during Selective Autophagy. Mol. Cell. 2019;74:347–362.e6. doi: 10.1016/j.molcel.2019.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ravenhill B.J., Boyle K.B., von Muhlinen N., Ellison C.J., Masson G.R., Otten E.G., Foeglein A., Williams R., Randow F. The Cargo Receptor NDP52 Initiates Selective Autophagy by Recruiting the ULK Complex to Cytosol-Invading Bacteria. Mol. Cell. 2019;74:320–329.e6. doi: 10.1016/j.molcel.2019.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Tumbarello D.A., Manna P.T., Allen M., Bycroft M., Arden S.D., Kendrick-Jones J., Buss F. The Autophagy Receptor TAX1BP1 and the Molecular Motor Myosin VI Are Required for Clearance of Salmonella Typhimurium by Autophagy. PLoS Pathog. 2015;11:e1005174. doi: 10.1371/journal.ppat.1005174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lu K., Psakhye I., Jentsch S. Autophagic clearance of PolyQ proteins mediated by ubiquitin-Atg8 adaptors of the conserved CUET protein family. Cell. 2014;158:549–563. doi: 10.1016/j.cell.2014.05.048. [DOI] [PubMed] [Google Scholar]
- 189.Gatica D., Lahiri V., Klionsky D.J. Cargo recognition and degradation by selective autophagy. Nat. Cell Biol. 2018;20:233–242. doi: 10.1038/s41556-018-0037-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Yoo S.M., Jung Y.K. A Molecular Approach to Mitophagy and Mitochondrial Dynamics. Mol. Cells. 2018;41:18–26. doi: 10.14348/molcells.2018.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wei Y., Chiang W.C., Sumpter R., Mishra P., Levine B. Prohibitin 2 Is an Inner Mitochondrial Membrane Mitophagy Receptor. Cell. 2017;168:224–238.e10. doi: 10.1016/j.cell.2016.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Princely Abudu Y., Pankiv S., Mathai B.J., Håkon Lystad A., Bindesbøll C., Brenne H.B., Yoke Wui Ng M., Thiede B., Yamamoto A., Mutugi Nthiga T., et al. NIPSNAP1 and NIPSNAP2 Act as “Eat Me” Signals for Mitophagy. Dev. Cell. 2019;49:509–525.e12. doi: 10.1016/j.devcel.2019.03.013. [DOI] [PubMed] [Google Scholar]
- 193.Kirkin V., Rogov V.V. A Diversity of Selective Autophagy Receptors Determines the Specificity of the Autophagy Pathway. Mol. Cell. 2019;76:268–285. doi: 10.1016/j.molcel.2019.09.005. [DOI] [PubMed] [Google Scholar]
- 194.Martinez-Lopez N., Garcia-Macia M., Sahu S., Athonvarangkul D., Liebling E., Merlo P., Cecconi F., Schwartz G.J., Singh R. Autophagy in the CNS and Periphery Coordinate Lipophagy and Lipolysis in the Brown Adipose Tissue and Liver. Cell Metab. 2016;23:113–127. doi: 10.1016/j.cmet.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Jiang S., Wells C.D., Roach P.J. Starch-binding domain-containing protein 1 (Stbd1) and glycogen metabolism: Identification of the Atg8 family interacting motif (AIM) in Stbd1 required for interaction with GABARAPL1. Biochem. Biophys. Res. Commun. 2011;413:420–425. doi: 10.1016/j.bbrc.2011.08.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wyant G.A., Abu-Remaileh M., Frenkel E.M., Laqtom N.N., Dharamdasani V., Lewis C.A., Chan S.H., Heinze I., Ori A., Sabatini D.M. Nufip1 is a ribosome receptor for starvation-induced ribophagy. Science. 2018;360:751–758. doi: 10.1126/science.aar2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Dowdle W.E., Nyfeler B., Nagel J., Elling R.A., Liu S., Triantafellow E., Menon S., Wang Z., Honda A., Pardee G., et al. Selective VPS34 inhibitor blocks autophagy and uncovers a role for NCOA4 in ferritin degradation and iron homeostasis in vivo. Nat. Cell Biol. 2014;16:1069–1079. doi: 10.1038/ncb3053. [DOI] [PubMed] [Google Scholar]
- 198.Mancias J.D., Wang X., Gygi S.P., Harper J.W., Kimmelman A.C. Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature. 2014;508:105–109. doi: 10.1038/nature13148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Haack T.B., Ignatius E., Calvo-Garrido J., Iuso A., Isohanni P., Maffezzini C., Lönnqvist T., Suomalainen A., Gorza M., Kremer L.S., et al. Absence of the Autophagy Adaptor SQSTM1/p62 Causes Childhood-Onset Neurodegeneration with Ataxia, Dystonia, and Gaze Palsy. Am. J. Hum. Genet. 2016;99:735–743. doi: 10.1016/j.ajhg.2016.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Teyssou E., Takeda T., Lebon V., Boillée S., Doukouré B., Bataillon G., Sazdovitch V., Cazeneuve C., Meininger V., Leguern E., et al. Mutations in SQSTM1 encoding p62 in amyotrophic lateral sclerosis: Genetics and neuropathology. Acta Neuropathol. 2013;125:511–522. doi: 10.1007/s00401-013-1090-0. [DOI] [PubMed] [Google Scholar]
- 201.Fecto F., Yan J., Vemula S.P., Liu E., Yang Y., Chen W., Zheng J.G., Shi Y., Siddique N., Arrat H., et al. SQSTM1 mutations in familial and sporadic amyotrophic lateral sclerosis. Arch. Neurol. 2011;68:1440–1446. doi: 10.1001/archneurol.2011.250. [DOI] [PubMed] [Google Scholar]
- 202.Le Ber I., Camuzat A., Guerreiro R., Bouya-Ahmed K., Bras J., Nicolas G., Gabelle A., Didic M., De Septenville A., Millecamps S., et al. SQSTM1 Mutations in french patients with frontotemporal dementia or frontotemporal dementia with amyotrophic lateral sclerosis. JAMA Neurol. 2013;70:1403–1410. doi: 10.1001/jamaneurol.2013.3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Boutoleau-Bretonnière C., Camuzat A., Le Ber I., Bouya-Ahmed K., Guerreiro R., Deruet A.L., Evrard C., Bras J., Lamy E., Auffray-Calvier E., et al. A phenotype of atypical apraxia of speech in a family carrying SQSTM1 mutation. J. Alzheimer’s Dis. 2015;43:625–630. doi: 10.3233/JAD-141512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Gang Q., Bettencourt C., Machado P.M., Brady S., Holton J.L., Pittman A.M., Hughes D., Healy E., Parton M., Hilton-Jones D., et al. Rare variants in SQSTM1 and VCP genes and risk of sporadic inclusion body myositis. Neurobiol. Aging. 2016;47:e1–e218. doi: 10.1016/j.neurobiolaging.2016.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Rea S.L., Walsh J.P., Ward L., Yip K., Ward B.K., Kent G.N., Steer J.H., Xu J., Ratajczak T. A novel mutation (K378X) in the Sequestosome 1 gene associated with increased NF-κB signaling and Paget’s disease of bone with a severe phenotype. J. Bone Miner. Res. 2006;21:1136–1145. doi: 10.1359/jbmr.060405. [DOI] [PubMed] [Google Scholar]
- 206.Hocking L.J., Lucas G.J.A., Daroszewska A., Mangion J., Olavesen M., Cundy T., Nicholson G.C., Ward L., Bennett S.T., Wuyts W., et al. Domain-specific mutations in sequestosome 1 (SQSTM1) cause familial and sporadic Paget’s diease. Hum. Mol. Genet. 2002;11:2735–2739. doi: 10.1093/hmg/11.22.2735. [DOI] [PubMed] [Google Scholar]
- 207.Ellinghaus D., Zhang H., Zeissig S., Lipinski S., Till A., Jiang T., Stade B., Bromberg Y., Ellinghaus E., Keller A., et al. Association between variants of PRDM1 and NDP52 and crohn’s disease, based on exome sequencing and functional studies. Gastroenterology. 2013;145:339–347. doi: 10.1053/j.gastro.2013.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Pap É.M., Farkas K., Széll M., Németh G., Rajan N., Nagy N. Identification of putative phenotype-modifying genetic factors associated with phenotypic diversity in Brooke-Spiegler syndrome. Exp. Dermatol. 2020 doi: 10.1111/exd.14161. [DOI] [PubMed] [Google Scholar]
- 209.Maruyama H., Morino H., Ito H., Izumi Y., Kato H., Watanabe Y., Kinoshita Y., Kamada M., Nodera H., Suzuki H., et al. Mutations of optineurin in amyotrophic lateral sclerosis. Nature. 2010;465:223–226. doi: 10.1038/nature08971. [DOI] [PubMed] [Google Scholar]
- 210.Silva I.A.L., Conceição N., Gagnon É., Caiado H., Brown J.P., Gianfrancesco F., Michou L., Cancela M.L. Effect of genetic variants of OPTN in the pathophysiology of Paget’s disease of bone. Biochim. Et Biophys. Acta Mol. Basis Dis. 2018;1864:143–151. doi: 10.1016/j.bbadis.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 211.Albagha O.M.E., Visconti M.R., Alonso N., Langston A.L., Cundy T., Dargie R., Dunlop M.G., Fraser W.D., Hooper M.J., Isaia G., et al. Genome-wide association study identifies variants at CSF1, OPTN and TNFRSF11A as genetic risk factors for Paget’s disease of bone. Nat. Genet. 2010;42:520–524. doi: 10.1038/ng.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Rezaie T., Child A., Hitchings R., Brice G., Miller L., Coca-Prados M., Héon E., Krupin T., Ritch R., Kreutzer D., et al. Adult-onset primary open-angle glaucoma caused by mutations in optineurin. Sci. N.Y. 2002;295:1077–1079. doi: 10.1126/science.1066901. [DOI] [PubMed] [Google Scholar]
- 213.Newton C.A., Oldham J.M., Ley B., Anand V., Adegunsoye A., Liu G., Batra K., Torrealba J., Kozlitina J., Glazer C., et al. Telomere length and genetic variant associations with interstitial lung disease progression and survival. Eur. Respir. J. 2019;53:1–4. doi: 10.1183/13993003.01641-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Araujo F.J.d., Silva L.D.O.d., Mesquita T.G., Pinheiro S.K., Vital W.d.S., Chrusciak-Talhari A., Guerra J.A.d.O., Talhari S., Ramasawmy R. Polymorphisms in the TOLLIP Gene Influence Susceptibility to Cutaneous Leishmaniasis Caused by Leishmania guyanensis in the Amazonas State of Brazil. PLoS Negl. Trop. Dis. 2015;9:e0003875. doi: 10.1371/journal.pntd.0003875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Montoya-Buelna M., Fafutis-Morris M., Tovar-Cuevas A.J., Alvarado-Navarro A., Valle Y., Padilla-Gutierrez J.R., Muñoz-Valle J.F., Figuera-Villanueva L.E. Role of toll-interacting protein gene polymorphisms in leprosy Mexican patients. Biomed Res. Int. 2013;2013 doi: 10.1155/2013/459169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Shah J.A., Berrington W.R., Vary J.C., Wells R.D., Peterson G.J., Kunwar C.B., Khadge S., Hagge D.A., Hawn T.R. Genetic variation in toll-interacting protein is associated with leprosy susceptibility and cutaneous expression of interleukin 1 receptor antagonist. J. Infect. Dis. 2016;213:1189–1197. doi: 10.1093/infdis/jiv570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Brasil L.W., Barbosa L.R.A., De Araujo F.J., Da Costa A.G., Da Silva L.D.O., Pinheiro S.K., De Almeida A.C.G., Kuhn A., Vitor-Silva S., De Melo G.C., et al. TOLLIP gene variant is associated with Plasmodium vivax malaria in the Brazilian Amazon. Malar. J. 2017;16:116. doi: 10.1186/s12936-017-1754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Wu S., Huang W., Wang D., Wang Y., Wang M., Zhang M., He J.Q. Evaluation of TLR2, TLR4, and TOLLIP polymorphisms for their role in tuberculosis susceptibility. Apmis. 2018;126:501–508. doi: 10.1111/apm.12855. [DOI] [PubMed] [Google Scholar]
- 219.Song Z., Yin J., Yao C., Sun Z., Shao M., Zhang Y., Tao Z., Huang P., Tong C. Variants in the Toll-interacting protein gene are associated with susceptibility to sepsis in the Chinese Han population. Crit. Care. 2011;15:R12. doi: 10.1186/cc9413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Ruiz M.T., Balachi J.F., Fernandes R.A., Galbiatti A.L.S., Manigua J.V., Pavarino-Bertelli É.C., Goloni-Bertollo E.M. Analysis of the TAX1BP1 gene in head and neck cancer patients. Braz. J. Otorhinolaryngol. 2010;76:193–198. doi: 10.1590/S1808-86942010000200008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Geller F., Feenstra B., Carstensen L., Pers T.H., Van Rooij I.A.L.M., Körberg I.B., Choudhry S., Karjalainen J.M., Schnack T.H., Hollegaard M.V., et al. Genome-wide association analyses identify variants in developmental genes associated with hypospadias. Nat. Genet. 2014;46:957–963. doi: 10.1038/ng.3063. [DOI] [PubMed] [Google Scholar]
- 222.Lin E., Kuo P.-H., Liu Y.-L., Yu Y.W.Y., Yang A.C., Tsai S.-J. A Deep Learning Approach for Predicting Antidepressant Response in Major Depression Using Clinical and Genetic Biomarkers. Front. Psychiatry. 2018;9:290. doi: 10.3389/fpsyt.2018.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Ikeda M., Takahashi A., Kamatani Y., Momozawa Y., Saito T., Kondo K., Shimasaki A., Kawase K., Sakusabe T., Iwayama Y., et al. Genome-wide association study detected novel susceptibility genes for schizophrenia and shared trans-populations/diseases genetic effect. Schizophr. Bull. 2019;45:824–834. doi: 10.1093/schbul/sby140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Nicoletti P., Cartsos V.M., Palaska P.K., Shen Y., Floratos A., Zavras A.I. Genomewide Pharmacogenetics of Bisphosphonate-Induced Osteonecrosis of the Jaw: The Role of RBMS3. Oncologist. 2012;17:279–287. doi: 10.1634/theoncologist.2011-0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Ilgaz Aydinlar E., Rolfs A., Serteser M., Parman Y. Mutation in FAM134B causing hereditary sensory neuropathy with spasticity in a Turkish family. Muscle Nerve. 2014;49:774–775. doi: 10.1002/mus.24145. [DOI] [PubMed] [Google Scholar]
- 226.Murphy S.M., Davidson G.L., Brandner S., Houlden H., Reilly M.M. Mutation in FAM134B causing severe hereditary sensory neuropathy. J. Neurol. Neurosurg. Psychiatry. 2012;83:119–120. doi: 10.1136/jnnp.2010.228965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Fischer D., Schabhüttl M., Wieland T., Windhager R., Strom T.M., Auer-Grumbach M. A Novel Missense Mutation Confirms ATL3 as a Gene for Hereditary Sensory Neuropathy Type 1. Brain. 2014;137:286. doi: 10.1093/brain/awu091. [DOI] [PubMed] [Google Scholar]
- 228.Kornak U., Mademan I., Schinke M., Voigt M., Krawitz P., Hecht J., Barvencik F., Schinke T., Gießelmann S., Beil F.T., et al. Sensory neuropathy with bone destruction due to a mutation in the membrane-shaping atlastin GTPase 3. Brain. 2014;137:683–692. doi: 10.1093/brain/awt357. [DOI] [PubMed] [Google Scholar]
- 229.Manjurano A., Clark T.G., Nadjm B., Mtove G., Wangai H., Sepulveda N., Campino S.G., Maxwell C., Olomi R., Rockett K.R., et al. Candidate Human Genetic Polymorphisms and Severe Malaria in a Tanzanian Population. PLoS ONE. 2012;7:e47463. doi: 10.1371/journal.pone.0047463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Apinjoh T.O., Anchang-Kimbi J.K., Njua-Yafi C., Ngwai A.N., Mugri R.N., Clark T.G., Rockett K.A., Kwiatkowski D.P., Achidi E.A. Association of candidate gene polymorphisms and TGF-beta/IL-10 levels with malaria in three regions of Cameroon: A case-control study. Malar. J. 2014;13:236. doi: 10.1186/1475-2875-13-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Tavian D., Missaglia S., Redaelli C., Pennisi E.M., Invernici G., Wessalowski R., Maiwald R., Arca M., Coleman R.A. Contribution of novel ATGL missense mutations to the clinical phenotype of NLSD-M: A strikingly low amount of lipase activity may preserve cardiac function. Hum. Mol. Genet. 2012;21:5318–5328. doi: 10.1093/hmg/dds388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Zolotov S., Xing C., Mahamid R., Shalata A., Sheikh-Ahmad M., Garg A. Homozygous LIPE mutation in siblings with multiple symmetric lipomatosis, partial lipodystrophy, and myopathy. Am. J. Med Genet. Part A. 2017;173:190–194. doi: 10.1002/ajmg.a.37880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Farhan S.M.K., Robinson J.F., McIntyre A.D., Marrosu M.G., Ticca A.F., Loddo S., Carboni N., Brancati F., Hegele R.A. A Novel LIPE Nonsense Mutation Found Using Exome Sequencing in Siblings With Late-Onset Familial PartialLipodystrophy. Can. J. Cardiol. 2014;30:1649–1654. doi: 10.1016/j.cjca.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 234.Jansen I.E., Gibbs J.R., Nalls M.A., Price T.R., Lubbe S., van Rooij J., Uitterlinden A.G., Kraaij R., Williams N.M., Brice A., et al. Establishing the role of rare coding variants in known Parkinson’s disease risk loci. Neurobiol. Aging. 2017;59:e11–e220. doi: 10.1016/j.neurobiolaging.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.International Parkinson’s Disease Genomics C., Wellcome Trust Case Control C. A two-stage meta-analysis identifies several new loci for Parkinson’s disease. PLoS Genet. 2011;7:e1002142. doi: 10.1371/journal.pgen.1002142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Yucesoy B., Kaufman K.M., Lummus Z.L., Weirauch M.T., Zhang G., Cartier A., Boulet L.P., Sastre J., Quirce S., Tarlo S.M., et al. Genome-wide association study identifies novel loci associated with diisocyanate-induced occupational asthma. Toxicol. Sci. 2015;146:192–201. doi: 10.1093/toxsci/kfv084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Wang Y., Ray A.M., Johnson E.K., Zuhlke K.A., Cooney K.A., Lange E.M. Evidence for an association between prostate cancer and chromosome 8q24 and 10q11 genetic variants in African American men: The flint men’s health study. Prostate. 2011;71:225–231. doi: 10.1002/pros.21234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Sheu S.Y., Schwertheim S., Worm K., Grabellus F., Schmid K.W. Diffuse sclerosing variant of papillary thyroid carcinoma: Lack of BRAF mutation but occurrence of RET/PTC rearrangements. Mod. Pathol. 2007;20:779–787. doi: 10.1038/modpathol.3800797. [DOI] [PubMed] [Google Scholar]
- 239.Lee J.M., Gillis T., Mysore J.S., Ramos E.M., Myers R.H., Hayden M.R., Morrison P.J., Nance M., Ross C.A., Margolis R.L., et al. Common SNP-based haplotype analysis of the 4p16.3 Huntington disease gene region. Am. J. Hum. Genet. 2012;90:434–444. doi: 10.1016/j.ajhg.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Martinez-Vicente M., Talloczy Z., Wong E., Tang G., Koga H., Kaushik S., de Vries R., Arias E., Harris S., Sulzer D., et al. Cargo recognition failure is responsible for inefficient autophagy in Huntington’s disease. Nat. Neurosci. 2010;13:567–576. doi: 10.1038/nn.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Rui Y.N., Xu Z., Patel B., Chen Z., Chen D., Tito A., David G., Sun Y., Stimming E.F., Bellen H.J., et al. Huntingtin functions as a scaffold for selective macroautophagy. Nat. Cell Biol. 2015;17:262–275. doi: 10.1038/ncb3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Kast D.J., Dominguez R. The Cytoskeleton–Autophagy Connection. Curr Biol. 2017;27:318–326. doi: 10.1016/j.cub.2017.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Jahreiss L., Menzies F.M., Rubinsztein D.C. The itinerary of autophagosomes: From peripheral formation to kiss-and-run fusion with lysosomes. Traffic. 2008;9:574–587. doi: 10.1111/j.1600-0854.2008.00701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Cardoso C.M.P., Groth-Pedersen L., Høyer-Hansen M., Kirkegaard T., Corcelle E., Andersen J.S., Jäättelä M., Nylandsted J. Depletion of Kinesin 5B Affects Lysosomal Distribution and Stability and Induces Peri-Nuclear Accumulation of Autophagosomes in Cancer Cells. PLoS ONE. 2009;4:e4424. doi: 10.1371/journal.pone.0004424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Kimura S., Noda T., Yoshimori T. Dynein-dependent movement of autophagosomes mediates efficient encounters with lysosomes. Cell Struct. Funct. 2008;33:109–122. doi: 10.1247/csf.08005. [DOI] [PubMed] [Google Scholar]
- 246.Lőrincz P., Juhász G. Autophagosome-Lysosome Fusion. J Mol Biol. 2020;432:2462–2482. doi: 10.1016/j.jmb.2019.10.028. [DOI] [PubMed] [Google Scholar]
- 247.Zhao Y.G., Zhang H. Autophagosome Maturation: An Epic Journey from the ER to Lysosomes. J Cell Biol. 2019;218:757–770. doi: 10.1083/jcb.201810099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Takáts S., Boda A., Csizmadia T., Juhász G. Small GTPases controlling autophagy-related membrane traffic in yeast and metazoans. Small Gtpases. 2018;9:465–471. doi: 10.1080/21541248.2016.1258444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Szatmári Z., Sass M. The Autophagic Roles of Rab Small GTPases and Their Upstream Regulators. Autophagy. 2014;10:1154–1166. doi: 10.4161/auto.29395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Bröcker C., Kuhlee A., Gatsogiannis C., Kleine Balderhaar H.J., Hönscher C., Engelbrecht-Vandré S., Ungermann C., Raunser S. Molecular architecture of the multisubunit homotypic fusion and vacuole protein sorting (HOPS) tethering complex. Proc. Natl. Acad. Sci. USA. 2012;109:1991–1996. doi: 10.1073/pnas.1117797109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Wang Z., Miao G., Xue X., Guo X., Yuan C., Wang Z., Zhang G., Chen Y., Feng D., Hu J., et al. The Vici Syndrome Protein EPG5 Is a Rab7 Effector that Determines the Fusion Specificity of Autophagosomes with Late Endosomes/Lysosomes. Mol. Cell. 2016;63:781–795. doi: 10.1016/j.molcel.2016.08.021. [DOI] [PubMed] [Google Scholar]
- 252.McEwan D.G., Popovic D., Gubas A., Terawaki S., Suzuki H., Stadel D., Coxon F.P., MirandadeStegmann D., Bhogaraju S., Maddi K., et al. PLEKHM1 regulates autophagosome-lysosome fusion through HOPS complex and LC3/GABARAP proteins. Mol. Cell. 2015;57:39–54. doi: 10.1016/j.molcel.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 253.Ding X., Jiang X., Tian R., Zhao P., Li L., Wang X., Chen S., Zhu Y., Mei M., Bao S., et al. RAB2 regulates the formation of autophagosome and autolysosome in mammalian cells. Autophagy. 2019;15:1774–1786. doi: 10.1080/15548627.2019.1596478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Lörincz P., Tóth S., Benkö P., Lakatos Z., Boda A., Glatz G., Zobel M., Bisi S., Hegedüs K., Takáts S., et al. Rab2 promotes autophagic and endocytic lysosomal degradation. J. Cell Biol. 2017;216:1937–1947. doi: 10.1083/jcb.201611027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Pantoom S., Konstantinidis G., Voss S., Han H., Hofnagel O., Li Z., Wu Y.W. RAB33B recruits the ATG16L1 complex to the phagophore via a noncanonical RAB binding protein. Autophagy. 2020:1–15. doi: 10.1080/15548627.2020.1822629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Diao J., Liu R., Rong Y., Zhao M., Zhang J., Lai Y., Zhou Q., Wilz L.M., Li J., Vivona S., et al. ATG14 promotes membrane tethering and fusion of autophagosomes to endolysosomes. Nature. 2015;520:563–566. doi: 10.1038/nature14147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Zhang X., Wang L., Lak B., Li J., Jokitalo E., Wang Y. GRASP55 Senses Glucose Deprivation through O-GlcNAcylation to Promote Autophagosome-Lysosome Fusion. Dev. Cell. 2018;45:245–261.e6. doi: 10.1016/j.devcel.2018.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Ebner P., Poetsch I., Deszcz L., Hoffmann T., Zuber J., Ikeda F. The IAP family member BRUCE regulates autophagosome–lysosome fusion. Nat. Commun. 2018;9:1–15. doi: 10.1038/s41467-018-02823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Nakamura S., Yoshimori T. New Insights into Autophagosome-Lysosome Fusion. J Cell Sci. 2017;130:209–1216. doi: 10.1242/jcs.196352. [DOI] [PubMed] [Google Scholar]
- 260.Lefebvre C., Legouis R., Culetto E. ESCRT and autophagies: Endosomal functions and beyond. Semin. Cell Dev. Biol. 2018;74:21–28. doi: 10.1016/j.semcdb.2017.08.014. [DOI] [PubMed] [Google Scholar]
- 261.Nara A., Mizushima N., Yamamoto A., Kabeya Y., Ohsumi Y., Yoshimori T. SKD1 AAA ATPase-dependent endosomal transport is involved in autolysosome formation. Cell Struct. Funct. 2002;27:29–37. doi: 10.1247/csf.27.29. [DOI] [PubMed] [Google Scholar]
- 262.Tamai K., Tanaka N., Nara A., Yamamoto A., Nakagawa I., Yoshimori T., Ueno Y., Shimosegawa T., Sugamura K. Role of Hrs in maturation of autophagosomes in mammalian cells. Biochem. Biophys. Res. Commun. 2007;360:721–727. doi: 10.1016/j.bbrc.2007.06.105. [DOI] [PubMed] [Google Scholar]
- 263.Lee J.A., Beigneux A., Ahmad S.T., Young S.G., Gao F.B. ESCRT-III Dysfunction Causes Autophagosome Accumulation and Neurodegeneration. Curr. Biol. 2007;17:1561–1567. doi: 10.1016/j.cub.2007.07.029. [DOI] [PubMed] [Google Scholar]
- 264.Farg M.A., Sundaramoorthy V., Sultana J.M., Yang S., Atkinson R.A.K., Levina V., Halloran M.A., Gleeson P.A., Blair I.P., Soo K.Y., et al. C9ORF72, implicated in amytrophic lateral sclerosis and frontotemporal dementia, regulates endosomal trafficking. Hum. Mol. Genet. 2014;23:3579–3595. doi: 10.1093/hmg/ddu068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Vantaggiato C., Panzeri E., Castelli M., Citterio A., Arnoldi A., Santorelli F.M., Liguori R., Scarlato M., Musumeci O., Toscano A., et al. ZFYVE26/SPASTIZIN and SPG11/SPATACSIN mutations in hereditary spastic paraplegia types AR-SPG15 and AR-SPG11 have different effects on autophagy and endocytosis. Autophagy. 2019;15:34–57. doi: 10.1080/15548627.2018.1507438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Morgan N.E., Cutrona M.B., Simpson J.C. Multitasking Rab Proteins in Autophagy and Membrane Trafficking: A Focus on Rab33b. Int J Mol Sci. 2019;20:3916. doi: 10.3390/ijms20163916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Chen J., Ma Z., Jiao X., Fariss R., Kantorow W.L., Kantorow M., Pras E., Frydman M., Pras E., Riazuddin S., et al. Mutations in FYCO1 cause autosomal-recessive congenital cataracts. Am. J. Hum. Genet. 2011;88:827–838. doi: 10.1016/j.ajhg.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Goes F.S., Hamshere M.L., Seifuddin F., Pirooznia M., Belmonte-Mahon P., Breuer R., Schulze T., Nöthen M., Cichon S., Rietschel M., et al. Genome-wide association of mood-incongruent psychotic bipolar disorder. Transl. Psychiatry. 2012;2:e180. doi: 10.1038/tp.2012.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Zillhardt J.L., Poirier K., Broix L., Lebrun N., Elmorjani A., Martinovic J., Saillour Y., Muraca G., Nectoux J., Bessieres B., et al. Mosaic parental germline mutations causing recurrent forms of malformations of cortical development. Eur. J. Hum. Genet. 2016;24:611–614. doi: 10.1038/ejhg.2015.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Petukhova L., Duvic M., Hordinsky M., Norris D., Price V., Shimomura Y., Kim H., Singh P., Lee A., Chen W.V., et al. Genome-wide association study in alopecia areata implicates both innate and adaptive immunity. Nature. 2010;466:113–117. doi: 10.1038/nature09114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Xue A., Wu Y., Zhu Z., Zhang F., Kemper K.E., Zheng Z., Yengo L., Lloyd-Jones L.R., Sidorenko J., Wu Y., et al. Genome-wide association analyses identify 143 risk variants and putative regulatory mechanisms for type 2 diabetes. Nat. Commun. 2018;9:1–14. doi: 10.1038/s41467-018-04951-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Horikoshi M., Beaumont R.N., Day F.R., Warrington N.M., Kooijman M.N., Fernandez-Tajes J., Feenstra B., Van Zuydam N.R., Gaulton K.J., Grarup N., et al. Genome-wide associations for birth weight and correlations with adult disease. Nature. 2016;538:248–252. doi: 10.1038/nature19806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Fuchs-Telem D., Stewart H., Rapaport D., Nousbeck J., Gat A., Gini M., Lugassy Y., Emmert S., Eckl K., Hennies H.C.C., et al. CEDNIK syndrome results from loss-of-function mutations in SNAP29. Br. J. Dermatol. 2011;164:610–616. doi: 10.1111/j.1365-2133.2010.10133.x. [DOI] [PubMed] [Google Scholar]
- 274.Bare L.A., Morrison A.C., Rowland C.M., Shiffman D., Luke M.M., Iakoubova O.A., Kane J.P., Malloy M.J., Ellis S.G., Pankow J.S., et al. Five common gene variants identify elevated genetic risk for coronary heart disease. Genet. Med. 2007;9:682–689. doi: 10.1097/GIM.0b013e318156fb62. [DOI] [PubMed] [Google Scholar]
- 275.Luke M.M., Lalouschek W., Rowland C.M., Catanese J.J., Bolonick J.I., Bui N.D., Greisenegger S., Endler G., Devlin J.J., Mannhalter C. Polymorphisms Associated with Both Noncardioembolic Stroke and Coronary Heart Disease: Vienna Stroke Registry. Cerebrovasc. Dis. 2009;28:499–504. doi: 10.1159/000236914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Cheng S., Sun C., Lao W., Kang H. Association of VAMP8 rs1010 Polymorphism with Host Susceptibility to Pulmonary Tuberculosis in a Chinese Han Population. Genet. Test. Mol. Biomark. 2019;23:299–303. doi: 10.1089/gtmb.2018.0265. [DOI] [PubMed] [Google Scholar]
- 277.Hoffmann T.J., Van Den Eeden S.K., Sakoda L.C., Jorgenson E., Habel L.A., Graff R.E., Passarelli M.N., Cario C.L., Emami N.C., Chao C.R., et al. A large multiethnic genome-wide association study of prostate cancer identifies novel risk variants and substantial ethnic differences. Cancer Discov. 2015;5:878–891. doi: 10.1158/2159-8290.CD-15-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Houlden H., King R.H.M., Muddle J.R., Warner T.T., Reilly M.M., Orrell R.W., Ginsberg L. A novel RAB7 mutation associated with ulcero-mutilating neuropathy. Ann. Neurol. 2004;56:586–590. doi: 10.1002/ana.20281. [DOI] [PubMed] [Google Scholar]
- 279.Meggouh F., Bienfait H.M.E., Weterman M.A.J., De Visser M., Baas F. Charcot-Marie-tooth disease due to a de novo mutation of the RAB7 gene. Neurology. 2006;67:1476–1478. doi: 10.1212/01.wnl.0000240068.21499.f5. [DOI] [PubMed] [Google Scholar]
- 280.Verhoeven K., De Jonghe P., Coen K., Verpoorten N., Auer-Grumbach M., Kwon J.M., FitzPatrick D., Schmedding E., De Vriendt E., Jacobs A., et al. Mutations in the small GTP-ase late endosomal protein RAB7 cause Charcot-Marie-Tooth type 2B neuropathy. Am. J. Hum. Genet. 2003;72:722–727. doi: 10.1086/367847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Alshammari M.J., Al-Otaibi L., Alkuraya F.S. Mutation in RAB33B, which encodes a regulator of retrograde Golgi transport, defines a second Dyggve-Melchior-Clausen locus. J. Med Genet. 2012;49:455–461. doi: 10.1136/jmedgenet-2011-100666. [DOI] [PubMed] [Google Scholar]
- 282.Dupuis N., Lebon S., Kumar M., Drunat S., Graul-Neumann L.M., Gressens P., El Ghouzzi V. A Novel RAB33B Mutation in Smith-McCort Dysplasia. Hum. Mutat. 2013;34:283–286. doi: 10.1002/humu.22235. [DOI] [PubMed] [Google Scholar]
- 283.Salian S., Cho T.J., Phadke S.R., Gowrishankar K., Bhavani G.S.L., Shukla A., Jagadeesh S., Kim O.H., Nishimura G., Girisha K.M. Additional three patients with Smith-McCort dysplasia due to novel RAB33B mutations. Am. J. Med Genet. Part A. 2017;173:588–595. doi: 10.1002/ajmg.a.38064. [DOI] [PubMed] [Google Scholar]
- 284.Kondo H., Maksimova N., Otomo T., Kato H., Imai A., Asano Y., Kobayashi K., Nojima S., Nakaya A., Hamada Y., et al. Mutation in VPS33A affects metabolism of glycosaminoglycans: A new type of mucopolysaccharidosis with severe systemic symptoms. Hum. Mol. Genet. 2017;26:173–183. doi: 10.1093/hmg/ddw377. [DOI] [PubMed] [Google Scholar]
- 285.Nagel M., Jansen P.R., Stringer S., Watanabe K., De Leeuw C.A., Bryois J., Savage J.E., Hammerschlag A.R., Skene N.G., Muñoz-Manchado A.B., et al. Meta-analysis of genome-wide association studies for neuroticism in 449,484 individuals identifies novel genetic loci and pathways. Nat. Genet. 2018;50:920–927. doi: 10.1038/s41588-018-0151-7. [DOI] [PubMed] [Google Scholar]
- 286.Van Wesenbeeck L., Odgren P.R., Coxon F.P., Frattini A., Moens P., Perdu B., MacKay C.A., Van Hul E., Timmermans J.P., Vanhoenacker F., et al. Involvement of PLEKHM1 in osteoclastic vesicular transport and osteopetrosis in incisors absent rats and humans. J. Clin. Investig. 2007;117:919–930. doi: 10.1172/JCI30328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Edwards T.L., Scott W.K., Almonte C., Burt A., Powell E.H., Beecham G.W., Wang L., Züchner S., Konidari I., Wang G., et al. Genome-Wide association study confirms SNPs in SNCA and the MAPT region as common risk factors for parkinson disease. Ann. Hum. Genet. 2010;74:97–109. doi: 10.1111/j.1469-1809.2009.00560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Couch F.J., Wang X., McGuffog L., Lee A., Olswold C., Kuchenbaecker K.B., Soucy P., Fredericksen Z., Barrowdale D., Dennis J., et al. Genome-Wide Association Study in BRCA1 Mutation Carriers Identifies Novel Loci Associated with Breast and Ovarian Cancer Risk. PLoS Genet. 2013;9:e1003212. doi: 10.1371/journal.pgen.1003212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Phelan C.M., Kuchenbaecker K.B., Tyrer J.P., Kar S.P., Lawrenson K., Winham S.J., Dennis J., Pirie A., Riggan M.J., Chornokur G., et al. Identification of 12 new susceptibility loci for different histotypes of epithelial ovarian cancer. Nat. Genet. 2017;49:680–691. doi: 10.1038/ng.3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Amare A.T., Schubert K.O., Tekola-Ayele F., Hsu Y.H., Sangkuhl K., Jenkins G., Whaley R.M., Barman P., Batzler A., Altman R.B., et al. Association of the polygenic scores for personality traits and response to selective serotonin reuptake inhibitors in patients with major depressive disorder. Front. Psychiatry. 2018;9:16. doi: 10.3389/fpsyt.2018.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Hagenaars S.P., Hill W.D., Harris S.E., Ritchie S.J., Davies G., Liewald D.C., Gale C.R., Porteous D.J., Deary I.J., Marioni R.E. Genetic prediction of male pattern baldness. PLoS Genet. 2017;13:e1006594. doi: 10.1371/journal.pgen.1006594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Piano Mortari E., Folgiero V., Marcellini V., Romania P., Bellacchio E., D’Alicandro V., Bocci C., Carrozzo R., Martinelli D., Petrini S., et al. The Vici syndrome protein EPG5 regulates intracellular nucleic acid trafficking linking autophagy to innate and adaptive immunity. Autophagy. 2018;14:22–37. doi: 10.1080/15548627.2017.1389356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Wang K.S., Liu X., Xie C., Liu Y., Xu C. Non-parametric Survival Analysis of EPG5 Gene with Age at Onset of Alzheimer’s Disease. J. Mol. Neurosci. 2016;60:436–444. doi: 10.1007/s12031-016-0821-9. [DOI] [PubMed] [Google Scholar]
- 294.Mekli K., Phillips D.F., Arpawong T.E., Vanhoutte B., Tampubolon G., Nazroo J.Y., Lee J., Prescott C.A., Stevens A., Pendleton N. Genome-wide scan of depressive symptomatology in two representative cohorts in the United States and the United Kingdom. J. Psychiatr. Res. 2018;100:63–70. doi: 10.1016/j.jpsychires.2018.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Ayub H., Micheal S., Akhtar F., Khan M.I., Bashir S., Waheed N.K., Ali M., Schoenmaker-Koller F.E., Shafique S., Qamar R., et al. Association of a Polymorphism in the BIRC6 Gene with Pseudoexfoliative Glaucoma. PLoS ONE. 2014;9:e105023. doi: 10.1371/journal.pone.0105023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.van der Zee J., Urwin H., Engelborghs S., Bruyland M., Vandenberghe R., Dermaut B., De Pooter T., Peeters K., Santens P., De Deyn P.P., et al. CHMP2B C-truncating mutations in frontotemporal lobar degeneration are associated with an aberrant endosomal phenotype in vitro. Hum. Mol. Genet. 2008;17:313–322. doi: 10.1093/hmg/ddm309. [DOI] [PubMed] [Google Scholar]
- 297.Ferrari R., Kapogiannis D., Huey E.D., Grafman J., Hardy J., Momeni P. Novel missense mutation in charged multivesicular body protein 2B in a patient with frontotemporal dementia. Alzheimer Dis. Assoc. Disord. 2010;24:397–401. doi: 10.1097/WAD.0b013e3181df20c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Skibinski G., Parkinson N.J., Brown J.M., Chakrabarti L., Lloyd S.L., Hummerich H., Nielsen J.E., Hodges J.R., Spillantini M.G., Thusgaard T., et al. Mutations in the endosomal ESCRTIII-complex subunit CHMP2B in frontotemporal dementia. Nat. Genet. 2005;37:806–808. doi: 10.1038/ng1609. [DOI] [PubMed] [Google Scholar]
- 299.Cox L.E., Ferraiuolo L., Goodall E.F., Heath P.R., Higginbottom A., Mortiboys H., Hollinger H.C., Hartley J.A., Brockington A., Burness C.E., et al. Mutations in CHMP2B in Lower Motor Neuron Predominant Amyotrophic Lateral Sclerosis (ALS) PLoS ONE. 2010;5:e9872. doi: 10.1371/journal.pone.0009872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Shiels A., Bennett T.M., Knopf H.L.S., Yamada K., Yoshiura K.I., Niikawa N., Shim S., Hanson P.I. CHMP4B, a novel gene for autosomal dominant cataracts linked to chromosome 20q. Am. J. Hum. Genet. 2007;81:596–606. doi: 10.1086/519980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Raginis-Zborowska A., Mekli K., Payton A., Ollier W., Hamdy S., Pendleton N. Genetic determinants of swallowing impairments among community dwelling older population. Exp. Gerontol. 2015;69:196–201. doi: 10.1016/j.exger.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 302.Kichaev G., Bhatia G., Loh P.R., Gazal S., Burch K., Freund M.K., Schoech A., Pasaniuc B., Price A.L. Leveraging Polygenic Functional Enrichment to Improve GWAS Power. Am. J. Hum. Genet. 2019;104:65–75. doi: 10.1016/j.ajhg.2018.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Shamim U., Ambawat S., Singh J., Thomas A., Pradeep-Chandra-Reddy C., Suroliya V., Uppilli B., Parveen S., Sharma P., Chanchal S., et al. C9orf72 hexanucleotide repeat expansion in Indian patients with ALS: A common founder and its geographical predilection. Neurobiol. Aging. 2020;88:e1–e156. doi: 10.1016/j.neurobiolaging.2019.12.024. [DOI] [PubMed] [Google Scholar]
- 304.Logue M.W., Schu M., Vardarajan B.N., Farrell J., Lunetta K.L., Jun G., Baldwin C.T., DeAngelis M.M., Farrer L.A. A search for age-related macular degeneration risk variants in Alzheimer disease genes and pathways. Neurobiol. Aging. 2014;35:e7–e1510. doi: 10.1016/j.neurobiolaging.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Morais S., Raymond L., Mairey M., Coutinho P., Brandão E., Ribeiro P., Loureiro J.L., Sequeiros J., Brice A., Alonso I., et al. Massive sequencing of 70 genes reveals a myriad of missing genes or mechanisms to be uncovered in hereditary spastic paraplegias. Eur. J. Hum. Genet. 2017;25:1217–1228. doi: 10.1038/ejhg.2017.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Bowling K.M., Thompson M.L., Amaral M.D., Finnila C.R., Hiatt S.M., Engel K.L., Cochran J.N., Brothers K.B., East K.M., Gray D.E., et al. Genomic diagnosis for children with intellectual disability and/or developmental delay. Genome Med. 2017;9:1–11. doi: 10.1186/s13073-017-0433-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Xie T., Deng L., Mei P., Zhou Y., Wang B., Zhang J., Lin J., Wei Y., Zhang X., Xu R. A genome-wide association study combining pathway analysis for typical sporadic amyotrophic lateral sclerosis in Chinese Han populations. Neurobiol. Aging. 2014;35:e9–e1778. doi: 10.1016/j.neurobiolaging.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 308.Sagona A.P., Nezis I.P., Bache K.G., Haglund K., Bakken A.C., Skotheim R.I., Stenmark H. A tumor-associated mutation of FYVE-CENT prevents its interaction with Beclin 1 and interferes with cytokinesis. PLoS ONE. 2011;6:1–10. doi: 10.1371/journal.pone.0017086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Settembre C., Fraldi A., Jahreiss L., Spampanato C., Venturi C., Medina D., de Pablo R., Tacchetti C., Rubinsztein D.C., Ballabio A. A block of autophagy in lysosomal storage disorders. Hum Mol Genet. 2008;17:119–129. doi: 10.1093/hmg/ddm289. [DOI] [PubMed] [Google Scholar]
- 310.Fraldi A., Annunziata F., Lombardi A., Kaiser H.J., Medina D.L., Spampanato C., Fedele A.O., Polishchuk R., Sorrentino N.C., Simons K., et al. Lysosomal fusion and SNARE function are impaired by cholesterol accumulation in lysosomal storage disorders. Embo J. 2010;29:3607–3620. doi: 10.1038/emboj.2010.237. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 311.Corcelle-Termeau E., Vindelov S.D., Hamalisto S., Mograbi B., Keldsbo A., Brasen J.H., Favaro E., Adam D., Szyniarowski P., Hofman P., et al. Excess sphingomyelin disturbs ATG9A trafficking and autophagosome closure. Autophagy. 2016;12:833–849. doi: 10.1080/15548627.2016.1159378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Elrick M.J., Yu T., Chung C., Lieberman A.P. Impaired proteolysis underlies autophagic dysfunction in Niemann-Pick type C disease. Hum Mol Genet. 2012;21:4876–4887. doi: 10.1093/hmg/dds324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Gabande-Rodriguez E., Boya P., Labrador V., Dotti C.G., Ledesma M.D. High sphingomyelin levels induce lysosomal damage and autophagy dysfunction in Niemann Pick disease type A. Cell Death Differ. 2014;21:864–875. doi: 10.1038/cdd.2014.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Tomanin R., Karageorgos L., Zanetti A., Al-Sayed M., Bailey M., Miller N., Sakuraba H., Hopwood J.J. Mucopolysaccharidosis type VI (MPS VI) and molecular analysis: Review and classification of published variants in the ARSB gene. Hum. Mutat. 2018;39:1788–1802. doi: 10.1002/humu.23613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Morrone A., Caciotti A., Atwood R., Davidson K., Du C., Francis-Lyon P., Harmatz P., Mealiffe M., Mooney S., Oron T.R., et al. Morquio a syndrome-associated mutations: A review of alterations in the GALNS gene and a new locus-specific database. Hum. Mutat. 2014;35:1271–1279. doi: 10.1002/humu.22635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Brunetti-Pierri N., Scaglia F. GM1 gangliosidosis: Review of clinical, molecular, and therapeutic aspects. Mol. Genet. Metab. 2008;94:391–396. doi: 10.1016/j.ymgme.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 317.Zhu Z., Zhu X., Liu C.L., Shi H., Shen S., Yang Y., Hasegawa K., Camargo C.A., Jr., Liang L. Shared genetics of asthma and mental health disorders: A large-scale genome-wide cross-trait analysis. Eur. Respir. J. 2019;54:1901507. doi: 10.1183/13993003.01507-2019. [DOI] [PubMed] [Google Scholar]
- 318.Callahan J.W. Molecular basis of GM1 gangliosidosis and Morquio disease, type B. Structure-function studies of lysosomal β-galactosidase and the non-lysosomal β-galactosidase-like protein. Biochim. Et Biophys. Acta Mol. Basis Dis. 1999;1455:85–103. doi: 10.1016/S0925-4439(99)00075-7. [DOI] [PubMed] [Google Scholar]
- 319.Jakobkiewicz-Banecka J., Gabig-Ciminska M., Kloska A., Malinowska M., Piotrowska E., Banecka-Majkutewicz Z., Banecki B., Wegrzyn A., Wegrzyn G. Glycosaminoglycans and mucopolysaccharidosis type III. Front Biosci. 2016;1:1393–1409. doi: 10.2741/4463. [DOI] [PubMed] [Google Scholar]
- 320.Tomatsu S., Montano A.M., Dung V.C., Grubb J.H., Sly W.S. Mutations and polymorphisms in GUSB gene in mucopolysaccharidosis VII (sly syndrome) Hum. Mutat. 2009;30:511–519. doi: 10.1002/humu.20828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Triggs-Raine B. Biology of hyaluronan: Insights from genetic disorders of hyaluronan metabolism. World J. Biol. Chem. 2015;6:110. doi: 10.4331/wjbc.v6.i3.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Froissart R., Da Silva I.M., Maire I. Mucopolysaccharidosis type II: An update on mutation spectrum. Acta Paediatr. Int. J. Paediatr. 2007;96:71–77. doi: 10.1111/j.1651-2227.2007.00213.x. [DOI] [PubMed] [Google Scholar]
- 323.Poletto E., Pasqualim G., Giugliani R., Matte U., Baldo G. Worldwide distribution of common IDUA pathogenic variants. Clin. Genet. 2018;94:95–102. doi: 10.1111/cge.13224. [DOI] [PubMed] [Google Scholar]
- 324.Rowland T.J., Sweet M.E., Mestroni L., Taylor M.R.G. Danon disease dysregulation of autophagy in a multisystem disorder with cardiomyopathy. J. Cell Sci. 2016;129:2135–2143. doi: 10.1242/jcs.184770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Peruzzo P., Pavan E., Dardis A. Molecular genetics of Pompe disease: A comprehensive overview. Ann. Transl. Med. 2019;7:278. doi: 10.21037/atm.2019.04.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Potter N.T., Miller C.A., Anderson I.J. Mutation detection in an equivocal case of Friedreich’s ataxia. Pediatric Neurol. 2000;22:413–415. doi: 10.1016/S0887-8994(00)00136-3. [DOI] [PubMed] [Google Scholar]
- 327.Bogaert R., Tiller G.E., Weis M.A., Gruber H.E., Rimoin D.L., Cohn D.H., Eyre D.R. An amino acid substitution (Gly853 → Glu) in the collagen α1(II) chain produces hypochondrogenesis. J. Biol. Chem. 1992;267:22522–22526. [PubMed] [Google Scholar]
- 328.Kinghorn K.J., Asghari A.M., Castillo-Quan J.I. The emerging role of autophagic-lysosomal dysfunction in Gaucher disease and Parkinson’s disease. Neural Regen. Res. 2017;12:380–384. doi: 10.4103/1673-5374.202934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Benitez B.A., Davis A.A., Jin S.C., Ibanez L., Ortega-Cubero S., Pastor P., Choi J., Cooper B., Perlmutter J.S., Cruchaga C. Resequencing analysis of five Mendelian genes and the top genes from genome-wide association studies in Parkinson’s Disease. Mol. Neurodegener. 2016;11:1–12. doi: 10.1186/s13024-016-0097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Ortiz A., Germain D.P., Desnick R.J., Politei J., Mauer M., Burlina A., Eng C., Hopkin R.J., Laney D., Linhart A., et al. Fabry disease revisited: Management and treatment recommendations for adult patients. Mol. Genet. Metab. 2018;123:416–427. doi: 10.1016/j.ymgme.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 331.Bräuer A.U., Kuhla A., Holzmann C., Wree A., Witt M. Current challenges in understanding the cellular and molecular mechanisms in niemann-pick disease type C1. Int. J. Mol. Sci. 2019;20:4392. doi: 10.3390/ijms20184392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Wilmer M.J., Schoeber J.P., Van Den Heuvel L.P., Levtchenko E.N. Cystinosis: Practical tools for diagnosis and treatment. Pediatric Nephrol. 2011;26:205–215. doi: 10.1007/s00467-010-1627-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Mole S.E., Cotman S.L. Genetics of the neuronal ceroid lipofuscinoses (Batten disease) Biochim. Et Biophys. Acta Mol. Basis Dis. 2015;1852:2237–2241. doi: 10.1016/j.bbadis.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Caciotti A., Catarzi S., Tonin R., Lugli L., Perez C.R., Michelakakis H., Mavridou I., Donati M.A., Guerrini R., d’Azzo A., et al. Galactosialidosis: Review and analysis of CTSA gene mutations. Orphanet J. Rare Dis. 2013;8:114. doi: 10.1186/1750-1172-8-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Wali G., Wali G.M., Sue C.M., Kumar K.R. A Novel Homozygous Mutation in the FUCA1 Gene Highlighting Fucosidosis as a Cause of Dystonia: Case Report and Literature Review. Neuropediatrics. 2019;50:248–252. doi: 10.1055/s-0039-1684052. [DOI] [PubMed] [Google Scholar]
- 336.Aula N., Salomäki P., Timonen R., Verheijen F., Mancini G., Månsson J.E., Aula P., Peltonen L. The spectrum of SLC17A5-gene mutations resulting in free sialic acid-storage diseases indicates some genotype-phenotype correlation. Am. J. Hum. Genet. 2000;67:832–840. doi: 10.1086/303077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Malm D., Nilssen Ø. Alpha-mannosidosis. Orphanet J. Rare Dis. 2008;3:1–10. doi: 10.1186/1750-1172-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Molho-Pessach V., Bargal R., Abramowitz Y., Doviner V., Ingber A., Raas-Rothschild A., Ne’eman Z., Zeigler M., Zlotogorski A. Angiokeratoma corporis diffusum in human β-mannosidosis: Report of a new case and a novel mutation. J. Am. Acad. Dermatol. 2007;57:407–412. doi: 10.1016/j.jaad.2007.01.037. [DOI] [PubMed] [Google Scholar]
- 339.Saito H., Inazawa J., Saito S., Kasumi F., Koi S., Sagae S., Kudo R., Saito J., Noda K., Nakamura Y. Detailed Deletion Mapping of Chromosome 17q in Ovarian and Breast Cancers: 2-cM Region on 17q21.3 Often and Commonly Deleted in Tumors. Cancer Res. 1993;53:3382–3385. [PubMed] [Google Scholar]
- 340.Gao X., Zacharek A., Salkowski A., Grignon D.J., Sakr W., Porter A.T., Honn K.V. Loss of heterozygosity of the BRCA1 and other loci on chromosome 17q in human prostate cancer. Cancer Res. 1995;55:1002–1005. [PubMed] [Google Scholar]
- 341.Carvill G.L., Liu A., Mandelstam S., Schneider A., Lacroix A., Zemel M., McMahon J.M., Bello-Espinosa L., Mackay M., Wallace G., et al. Severe infantile onset developmental and epileptic encephalopathy caused by mutations in autophagy gene WDR45. Epilepsia. 2018;59:e5–e13. doi: 10.1111/epi.13957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Li Y., Huang J., Pang S., Wang H., Zhang A., Hawley R.G., Yan B. Novel and functional ATG12 gene variants in sporadic Parkinson’s disease. Neurosci. Lett. 2017;643:22–26. doi: 10.1016/j.neulet.2017.02.028. [DOI] [PubMed] [Google Scholar]
- 343.Chen D., Pang S., Feng X., Huang W., Hawley R.G., Yan B. Genetic analysis of the ATG7 gene promoter in sporadic Parkinson’s disease. Neurosci. Lett. 2013;534:193–198. doi: 10.1016/j.neulet.2012.12.039. [DOI] [PubMed] [Google Scholar]
- 344.Elisa R., Rainero I., Chiò A., Rogaeva E., Galimberti D., Fenoglio P., Grinberg Y., Isaia G., Calvo A., Gentile S., et al. SQSTM1 mutations in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Neurology. 2012;79:1556–1562. doi: 10.1212/WNL.0b013e31826e25df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Bucelli R.C., Arhzaouy K., Pestronk A., Pittman S.K., Rojas L., Sue C.M., Evilä A., Hackman P., Udd B., Harms M.B., et al. SQSTM1 splice site mutation in distal myopathy with rimmed vacuoles. Neurology. 2015;85:665–674. doi: 10.1212/WNL.0000000000001864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Nuij V.J.A.A., Peppelenbosch M.P., Woude C.J., Fuhler G.M. Genetic polymorphism in ATG16L1 gene is associated with adalimumab use in inflammatory bowel disease. J. Transl. Med. 2017;15:248. doi: 10.1186/s12967-017-1355-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Koder S., Repnik K., Ferkolj I., Pernat C., Skok P., Weersma R.K., Potočnik U. Genetic polymorphism in ATG16L1 gene influences the response to adalimumab in Crohn’s disease patients. Pharmacogenomics. 2015;16:191–204. doi: 10.2217/pgs.14.172. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.