Abstract
Background:
Conflicting evidence is available on the efficacy and safety of early intravenous beta-blockers before primary percutaneous coronary intervention for ST-segment elevation myocardial infarction. We performed a patient-pooled meta-analysis of trials comparing early intravenous beta-blockers with placebo or routine care in ST-segment elevation myocardial infarction patients undergoing primary percutaneous coronary intervention.
Aim:
The aim of this study was to evaluate the clinical and safety outcomes of intravenous beta-blockers in ST-segment elevation myocardial infarction patients undergoing primary percutaneous coronary intervention.
Methods:
Four randomized trials with a total of 1150 patients were included. The main outcome was one-year death or myocardial infarction. Secondary outcomes included biomarker-based infarct size, left ventricular ejection fraction during follow-up, ventricular tachycardia, and a composite safety outcome (cardiogenic shock, symptomatic bradycardia, or hypotension) during hospitalization.
Results:
One-year death or myocardial infarction was similar among beta-blocker (4.2%) and control patients (4.4%) (hazard ratio: 0.96 (95% confidence interval: 0.53–1.75, p=0.90, I2=0%). No difference was observed in biomarker-based infarct size. One-month left ventricular ejection fraction was similar, but left ventricular ejection fraction at six months was significantly higher in patients treated with early intravenous beta-blockade (52.8% versus 50.0% in the control group, p=0.03). No difference was observed in the composite safety outcome or ventricular tachycardia during hospitalization.
Conclusion:
In ST-segment elevation myocardial infarction patients undergoing primary percutaneous coronary intervention, the administration of early intravenous beta-blockers was safe. However, there was no difference in the main outcome of one-year death or myocardial infarction with early intravenous beta-blockers. A larger clinical trial is warranted to confirm the definitive efficacy of early intravenous beta-blockers.
Keywords: ST-segment elevation myocardial infarction, primary percutaneous coronary intervention, intravenous beta-blockers, beta-blockers, outcomes
Introduction
Primary percutaneous coronary intervention (PCI) is the preferred treatment in patients with ST-segment elevation myocardial infarction (STEMI). Although there have been large improvements in the outcomes for these patients, subgroups at high risk for mortality remain.1 The extent of myocardial necrosis/infarct size after STEMI is a well-known predictor for adverse outcomes including mortality.2 An early experimental study in dogs demonstrated that treatment with propranolol before coronary ligation was associated with a reduced infarct size.3 In patients, multiple randomized controlled trials have been performed evaluating the efficacy and safety of intravenous beta-blockers before primary PCI for STEMI, with inconsistent short-term results.4 This could possibly be explained by differences in time to treatment and heart rate before and after treatment. The objective of this patient-pooled meta-analysis was to the evaluate clinical and safety outcomes of intravenous beta-blockers in STEMI patients undergoing primary PCI.
Methods
Objective
The main objective of the current patient-pooled meta-analysis was to assess the effect of early beta-blockers on one-year death or myocardial infarction (MI) in patients undergoing primary PCI for STEMI. Secondary objectives included the evaluation of other clinical outcomes, secondary laboratory and imaging outcomes, and safety outcomes.
Search strategy
We searched electronic databases for randomized trials that compared early beta-blocker use with routine care or placebo in patients with STEMI. We performed a systematic review of the Medline, Web of Science, and Cochrane Register of Controlled Trials databases up to October 2016 with no language restriction using the following key words: “beta-blocker”, “adrenergic beta-antagonists”, “infarction” and “STEMI”. In order to avoid missing relevant studies, references of the identified studies were scrutinized. Studies that were conducted in the pre-PCI era or in non-ST elevation acute coronary syndrome patients were excluded.
Two reviewers screened title and abstract for eligibility (by NPGH and PD). Any uncertainties after screening of title and abstract were discussed until consensus was reached (by NPGH and PD). Relevant full-text articles were further reviewed for eligibility. We included four clinical trials from the PCI era and approached the principal investigators to participate in a collaborative patient-pooled meta-analysis.
Included trials and data collection
The principal investigators of the BEAT-AMI (BEtA-Blocker Therapy in Acute Myocardial Infarction), EARLY-BAMI (Early Beta-blocker Administration before primary PCI in patients with ST-elevation Myocardial Infarction), Hanada et al., and METOCARD-CNIC (Effect of Metoprolol in Cardioprotection During an Acute Myocardial Infarction) agreed to this collaborative meta-analysis. The study design and main results of the included trials have been described previously.5–8 A protocol was written summarizing the main pre-specified analyses and a common set of baseline and outcome variables. Individual patient data was provided to form a pooled-patient database. The database included core variables on demographics, clinical history, risk factors for coronary artery disease, STEMI timelines, primary PCI characteristics, laboratory and imaging data, and clinical outcomes. Data from each trial were sent to the coordinating center in Amsterdam. The merged database was checked for completeness and consistency by the participating investigators. The corresponding author had full access to all of the data in the study and had final responsibility for the decision to submit for publication.
Patients
Patients included were presenting with STEMI within six hours of symptom onset to reperfusion (within 12 h in EARLY-BAMI) and a Killip class I or II. Exclusion criteria generally included a low systolic blood pressure (<120 mm Hg in METOCARD-CNIC, <100 mm Hg in EARLY-BAMI, mean arterial pressure <65 mm Hg in BEAT-AMI), a heart rate <60 beats/min, and atrioventricular block type II or III.
Study intervention
The METOCARD-CNIC and EARLY-BAMI trials evaluated pre-reperfusion intravenous beta-blocker use, the BEAT-AMI evaluated early post-reperfusion intravenous beta-blocker use. In the METOCARD-CNIC trial, patients randomized to intravenous beta-blockers received up to three boluses of 5 mg metoprolol tartrate two minutes apart in the ambulance during transfer to the PCI center. Patients randomized to intravenous beta-blockers in the EARLY-BAMI trial received 5 mg of intravenous metoprolol in the ambulance, followed by 5 mg of intravenous metoprolol at the catheterization laboratory pre-PCI. In BEAT-AMI, active therapy consisted of weight-adapted continuous infusion of esmolol plus an additional bolus of esmolol targeting a heart rate of 60 beats per min. It was initiated immediately after transfer from the catheterization laboratory to the intensive care unit. Patients randomized to the control arm received a placebo. In the study by Hanada et al, patients were randomized post-PCI, the landiolol group received intravenous infusion of beta-blocker started at 3 μg per kg per min without loading and continued for 24 h. Patients were concomitantly treated according to clinical guidelines in all studies. Generally clinical guidelines recommend the initiation of oral beta-blockers within 12 h of hospitalization, unless contra-indicated.
Outcomes
Infarct size was estimated by biomarker-based infarct size (peak value of creatinine-kinase (CK), CK isoenzyme muscle/brain (CK-MB), and troponin T and I). Left ventricular ejection fraction (LVEF) was assessed by imaging methods (cardiac magnetic resonance in METOCARD-CNIC and EARLY-BAMI, echocardiography in BEAT-AMI, and left ventricular angiography in Hanada et al.). The main outcome of the patient-pooled meta-analysis was one-year death or MI. Secondary clinical outcomes included death or MI, (cardiac) death, and nonfatal MI at seven days after randomization, in addition to one-year (cardiac) death and one-year nonfatal MI. Safety outcomes included ventricular tachycardia and the composite of cardiogenic shock, symptomatic bradycardia, or hypotension during hospitalization.
Statistical analysis
We analyzed data by the intention-to-treat principle. Baseline and procedural characteristics, and outcomes, were tabulated by treatment group. Normally distributed continuous variables were compared with the Student’s t test. Continous variables with a skewed distribution were compared uisng the Wilcoxon rank-sum test. Categorical variables were compared with the Chi-square test. Hazard ratios (HRs) were pooled using the generic inverse variance method and a DerSimonian and Laird random effects model. Whereas for odds ratios we used the inverse variance method. Heterogeneity was assessed using I2 and τ2. We weighted studies with infinite HRs due to zero or low event rates with zero. In addition, the Kaplan-Meier method was used to calculate one-year clinical outcome estimates. HR, 95% confidence interval (CI), and p-values of the univariable association between randomized treatment and outcomes were derived from Cox regression models. Pre-specified subgroup analyses included infarct-related artery, time to reperfusion, and heart rate before study treatment. No adjustments for multiple comparisons were performed. All analyses were performed using SPSS (version 25.0) and R (version 3.5.1) using the meta (version 4.9.2) and survival (version 2.43.3).
Results
Baseline characteristics
Our search identified 286 studies (Figure 1). A total of 275 studies were excluded. We evaluated 11 full-text articles in detail. Four trials with a total of 1150 patients were included in this study. Key features of the included trials are displayed in Table 1. There was an overall low risk of bias among the studies (Supplementary Material Table 1). Of 1150 patients, 572 were randomized to early intravenous beta-blockade and 528 to control. The median time to follow-up was 365 days (interquartile range 365–373). Table 2 shows the baseline characteristics of the study population. The mean age of the population was 61 years, and around three-quarters were male. Over 90% of the patients underwent primary PCI. There were no significant differences in baseline characteristics. Post-treatment heart rate was 72 beats/min in the intravenous beta-blocker group versus 78 beats/min in the control group (p<0.001). The baseline characteristics of the individual studies are presented in Supplementary Material Table 2.
Figure 1.
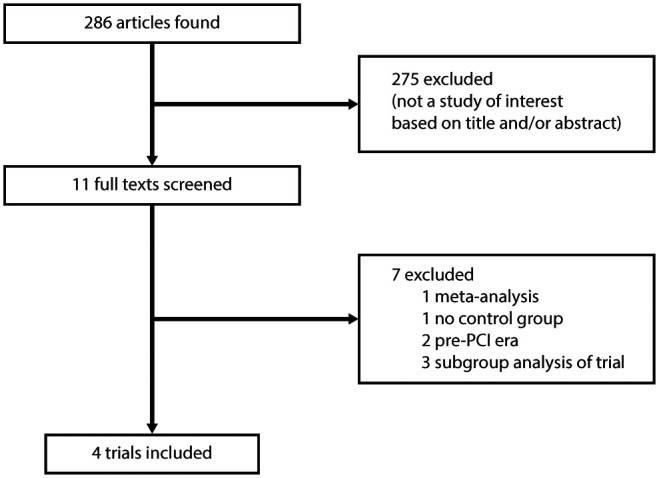
Flow diagram of trial selection. PCI: percutaneous coronary intervention.
Table 1.
Key features of included trials.
| Beta-blocker | Control | Design | Administration | Major inclusion criteria | Major exclusion criteria | |
|---|---|---|---|---|---|---|
| BEAT-AMI5 | Esmolol infusion for target HR of 60/min | Placebo | Single center 1:1 randomized, single-blind, placebo-controlled | 60 min after PCI | STEMI patients with successful PCI, symptom onset to PCI<6 h | Killip class III or IV, HR <60/min, mean arterial BP<65 mm Hg |
| EARLY-BAMI6 | Metoprolol 2 intravenous doses of 5 mg | Placebo | International multicenter 1:1 randomized, double-blind, placebo-controlled | First bolus in ambulance. Second bolus at immediately before PCI | STEMI patients eligible for primary PCI, symptoms >30 min to <12 h | Killip class III and IV, HR<60/min, systolic BP<100 mm Hg, type II or III AV-block |
| Hanada et al.7 | Landiolol 3 µg/kg/min infusion for 24 h, no loading dose | Routine care | Single-center: 1:1 randomized, non-blinded, open-label | Directly after PCI | STEMI patients undergoing primary PCI, symptoms for <12 h | Killip class III or IV, HR<50/min, systolic BP<90 mm Hg, type II or III AV-block |
| METOCARD-CINC8 | Metoprolol up to 3 intravenous doses of 5 mg | Routine care | Multicenter 1:1, randomized, single-blind | During transfer to PCI or at the emergency department | Anterior STEMI patients, symptom onset >30 min to <4.5 h | Killip class II or IV, HR<60/min, systolic BP<120 mm Hg, type II–III AV-block |
AV: atrioventricular; BEAT-AMI: BEtA-Blocker Therapy in Acute Myocardial Infarction; BP: blood pressure; EARLY-BMI: Early Beta-blocker Administration before primary PCI in patients with ST-elevation Myocardial Infarction; HR: heart rate; METOCARD-CNIC: Effect of Metoprolol in Cardioprotection During an Acute Myocardial Infarction; PCI: percutaneous coronary intervention; STEMI: ST-segment elevation myocardial infarction.
Table 2.
Baseline characteristics.
| Beta-blocker | Control | p-Value | |
|---|---|---|---|
| (n=572) (%) | (n=578) (%) | ||
| Baseline characteristics | |||
| Age, year, mean±SD | 61.2±12.4 | 61.4±12.3 | 0.79 |
| Male | 451/572 (78.8) | 449/578 (77.7) | 0.63 |
| BMI, mean±SD | 27.0±4.2 | 27.2±4.1 | 0.59 |
| Hypertension | 249/566 (44.0) | 248/572 (43.4) | 0.83 |
| Diabetes | 105/565 (18.6) | 113/575 (19.7) | 0.65 |
| Smoking | 260/547 (47.5) | 256/548 (46.7) | 0.79 |
| Hyperlipidemia | 197/559 (35.2) | 187/565 (33.1) | 0.45 |
| Previous MI | 15/429 (3.5) | 17/445 (3.8) | 0.80 |
| Previous PCI | 30/430 (7.0) | 24/446 (5.4) | 0.33 |
| Previous CABG | 4/430 (0.9) | 6/446 (1.3) | 0.56 |
| Prior beta-blocker use | 54/329 (16.4) | 60/343 (17.5) | 0.71 |
| Clinical presentation | |||
| Killip class I | 445/472 (94.3) | 447/485 (92.2) | 0.19 |
| Killip class II | 24/472 (5.1) | 31/485 (6.4) | 0.39 |
| Baseline SBP, mm Hg, mean±SD | 143.8±23.3 | 143.5±23.3 | 0.86 |
| Baseline DBP, mm Hg, mean±SD | 87.8±15.8 | 87.8±16.7 | 0.98 |
| Pretreatment HR, beat/min, mean±SD | 79.6±14.9 | 80.5±14.9 | 0.30 |
| SBP after intervention, mm Hg, mean±SD | 131.0±22.2 | 134.1±25.5 | 0.04 |
| DBP after intervention, mm Hg, mean±SD | 79.4±15.1 | 79.8±16.0 | 0.74 |
| HR after intervention, beat/min, mean±SD | 72.1±13.1 | 77.9±15.0 | <0.001 |
| Hospitalization | |||
| Reperfusion time, min, median (IQR) | 182 (137–258) | 183 (130–259) | 0.58 |
| Infarct-related artery | |||
| LAD | 316/535 (59.1) | 337/540 (62.4) | 0.26 |
| LCX | 61/535 (11.4) | 56/540 (10.4) | 0.59 |
| RCA | 155/535 (29.0) | 142/540 (26.3) | 0.33 |
| No. diseased coronary vessels | |||
| 1 | 225/427 (52.7) | 254/439 (57.9) | 0.13 |
| 2 | 124/427 (29.0) | 91/439 (20.7) | 0.005 |
| 3 | 62/427 (14.5) | 73/439 (16.6) | 0.39 |
| Primary PCI during admission | 529/561 (94.3) | 528/569 (92.8) | 0.31 |
| CABG during admission | 12/572 (2.1) | 22/578 (3.8) | 0.09 |
| Beta-blocker at discharge | 420/515 (81.6) | 413/519 (79.6) | 0.42 |
| Admission length, days, median (IQR) | 3.0 (1.0–5.8) | 3.0 (1.0–6.0) | 0.87 |
BMI: body mass index, CABG: coronary artery bypass grafting, DBP: diastolic blood pressure, IQR: interquartile range, LAD: anterior descending artery, LCX: left circumflex artery, MI: myocardial infarction, PCI: percutaneous coronary intervention, RCA: right coronary artery, SBP: systolic blood pressure, SD: standard deviation.
Biomarker-based infarct size
Biomarker-based infarct size is displayed in Table 3. No significant difference was observed in peak CK, CK-MB, and troponin T or I. Biomarker-based infarct size of the individual studies are presented in Supplementary Material Table 3.
Table 3.
Biomarker-based infarct size.
| Beta-blocker |
Control |
p-Value | |
|---|---|---|---|
| (n=572) | (n=578) | ||
| CK peak, U/l, median (IQR) | 1427 (471–3232) | 1650 (513–3589) | 0.29 |
| (n=558) | (n=561) | ||
| CK-MB, U/l, median (IQR) | 141 (59–316) | 160 (60–299) | 0.52 |
| (n=50) | (n=50) | ||
| CK-MB, µg/l, median (IQR) | 155 (65–440) | 114 (41–324) | 0.25 |
| (n=63) | (n=70) | ||
| CK-MB AUC, (U*h/l), median (IQR) | 2610 (926–5885) | 2561 (929–5498) | 0.45 |
| (n=204) | (n=202) | ||
| Troponin T peak, ng/l, median (IQR) | 1620 (299–5455) | 2000 (267–5800) | 0.73 |
| (n=341) | (n=354) | ||
| Troponin I peak, µg/l, median (IQR) | 45 (11–211) | 63 (20–133) | 0.82 |
| (n=29) | (n=34) | ||
| Troponin T AUC, (ng*h/l), median (IQR) | 51230 (8653–153,825) | 52826 (13,580–133,547) | 0.93 |
| (n=226) | (n=228) |
AUC: area under the curve; CK: creatine-kinase; CK-MB: creatine-kinase isoenzyme brain/muscle; IQR: interquartile range.
LVEF
There was no significant difference in LVEF at one month between the two groups (beta-blockers 51.1% versus control 49.8%, p=0.15), as is displayed in Table 4. LVEF at six months after STEMI was higher in patients treated with early intravenous beta-blockade (52.8% versus 50.0% in the control group, p=0.03). At both one and six months follow-up there was a lower number of patients with severely depressed LVEF (<35%) (one month beta-blocker: 8.9% versus control: 14.2%, p=0.035 and six months beta-blocker: 7.4% versus control: 14.7%, p=0.035).
Table 4.
Clinical outcomes.
| Beta-blocker | Control | p-Value | |
|---|---|---|---|
| (n=572) (%) | (n=578) (%) | ||
| Left ventricular ejection fraction | |||
| One month, mean±SD | 51.1±11.4 | 49.8±11.7 | 0.15 |
| (n=316) | (n=332) | ||
| Six months, mean±SD | 52.8±11.2 | 50.0±12.4 | 0.03 |
| (n=189) | (n=191) | ||
| Seven days clinical outcomes | |||
| Death or myocardial infarction | 10/521 (2.0%) | 9/525 (1.7%) | 0.81 |
| All-cause mortality | 6/571 (1.1%) | 7/576 (1.2%) | 0.79 |
| Cardiac death | 5/571 (0.9%) | 7/576 (1.2%) | 0.57 |
| Myocardial infarction | 4/521 (0.8%) | 2/525 (0.4%) | 0.41 |
| One-year clinical outcomes | |||
| Death or myocardial infarction | 21/521 (4.2) | 22/525 (4.4) | 0.90 |
| All-cause mortality | 16/571 (3.0) | 19/576 (3.6) | 0.62 |
| Cardiac death | 11/571 (2.0) | 14/576 (2.6) | 0.56 |
| Myocardial infarction | 5/521 (1.0) | 4/525 (0.8) | 0.73 |
| Safety outcomes during hospitalization | |||
| Composite safety outcome | 22/520 (4.2) | 19/525 (3.6) | 0.61 |
| Ventricular tachycardia | 36/522 (6.9) | 32/527 (6.1) | 0.59 |
SD: standard deviation.
Safety outcome is the composite of cardiogenic shock, symptomatic bradycardia or hypotension. One-year efficacy outcomes are Kaplan-Meier estimates, compared with a log-rank test. Safety outcome and ventricular tachycardia are percentages, compared with a Chi-square test.
Clinical outcomes
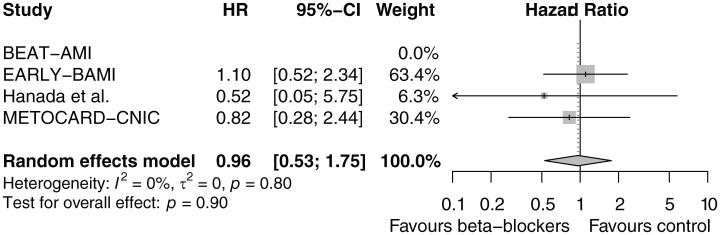
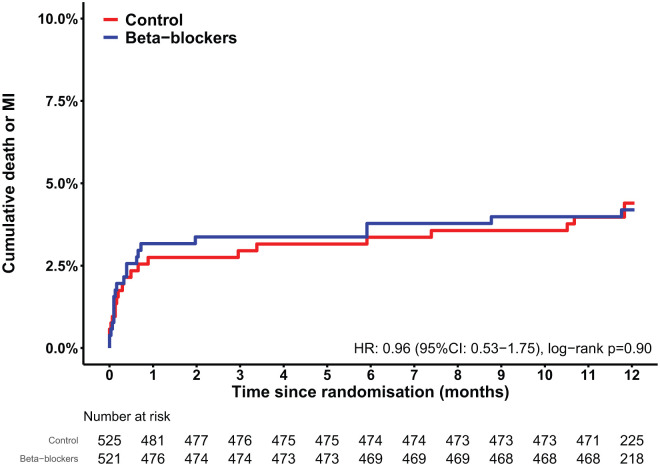
There was no significant effect of early intravenous beta-blocker administration on the main outcome of one-year death or MI (HR: 0.96, CI 95% CI: 0.53–1.75, p=0.90, I2=0%), as is displayed in the forest plot in Figure 2. Death or MI occurred in 4.2% of the beta-blocker group and 4.4% of the control group (Table 4). The Kaplan-Meier curves are shown in Figure 3. No difference was observed in the individual outcomes death, cardiac death, or MI. Forest plots of the individual outcomes are presented in Supplementary Material Figures 1 and 2. Seven-days clinical outcomes were similar among the two groups. There were comparable rates of the composite safety outcome of cardiogenic shock, symptomatic bradycardia or hypotension. Forest plots of the safety outcomes are presented in Supplementary Material Figures 3 and 4. Clinical outcomes of the individual studies are presented in Supplementary Material Table 4.
Figure 2.
Forest plot of one-year death or myocardial infarction (MI) after early intravenous beta-blockers versus control. BEAT-MI: BEtA-Blocker Therapy in Acute Myocardial Infarction; CI: confidence interval; EARLY-BMI: Early Beta-blocker Administration before primary PCI in patients with ST-elevation Myocardial Infarction; HR: hazard ratio; METOCARD-CNIC: Effect of Metoprolol in Cardioprotection During an Acute Myocardial Infarction.
Figure 3.
Kaplan-Meier estimates of the cumulative one-year death or myocardial infarction (MI). CI: confidence interval; HR: hazard ratio.
Relation between beta-blockers and outcomes in subgroups
The unadjusted HR for one-year death of MI was 0.96 (95% CI: 0.53–1.75, p=0.90). After adjustment for pre-specified heart-rate pre-treatment, infarct-related artery, and time to treatment, the adjusted HR was 0.91 (95% CI: 0.45–1.82, p=0.78). Furthermore, there was no interaction with these pre-specified variables.
Discussion
We report the outcomes from a patient-pooled meta-analysis comparing early intravenous beta-blockers with control in STEMI patients undergoing primary PCI. First, our results show administration of early intravenous beta-blockers was safe. Second, there was no difference in the main outcome of one-year death or MI. Third, biomarker-based infarct size was not different between the two groups. Fourth, we did not observe a difference in LVEF at one month. However, there was a significant but small absolute increase (2.8%) in LVEF at six months in the beta-blocker group. Finally, results were comparable when heart rate, time to treatment, and infarct-related artery were taken into account.
Included trials
There is a discrepancy in the efficacy results of the largest two trials. In METOCARD-CNIC, there was a reduction in cardiac magnetic resonance imaging (CMR) infarct size at one week and six months with early intravenous beta-blockade.5 No difference in infarct size was observed at one month in the EARLY-BAMI trial.7 This might be explained by the following factors. First, infarct size was smaller in the EARLY-BAMI trial, making it less likely to show a reduction with early beta-blockade. Second, the dose of metoprolol used in the METOCARD-CNIC was 15 mg, while 10 mg was used in the EARLY-BAMI trial. A third explanation might be related to timing of metoprolol administration. In a subanalysis of METOCARD-CNIC, longer exposure to intravenous beta-blockade was associated with smaller infarct size. In EARLY-BAMI, the second bolus of medication was administered just before PCI. In BEAT-AMI, esmolol treatment statistically significantly decreased cardiac troponin, CK, and CK-MB as surrogate markers for myocardial injury. In the trial by Hanada et al., early intravenous administration of landiolol was associated with an improvement in six-month LVEF. With regards to safety, all four trials show comparable results.
Pathophysiological effects of beta-blockers
A potential beneficial effect of beta-blockers might explained by the following mechanisms. In general, blockade of beta-1 receptors cause a reduction of heart rate, myocardial contractility, and lowered systemic blood pressure; resulting in a reduced myocardial workload and oxygen demand. This has been demonstrated in porcine models, in which intravenous metoprolol during coronary occlusion and before mechanical reperfusion was a highly effective cardioprotective agent, resulting in a 27% smaller MI than placebo, despite an initially equivalent amount of myocardium at risk.9 This cardioprotective effect was independent of its negative chronotropic effects. It has to be noted that the beneficial effect was independent of the heart rate. Furthermore, prolongation of the diastolic phase caused by the chronotropic effects of beta-blockers may increase myocardial perfusion.
Recently, a study has shown that neutrophil stunning by metoprolol might be an explanation for a reduced infarct size.10 It has been previously shown that infarct size is related to a pro-inflammatory response occurring during and post-MI.11 In both human and animal ischemia-reperfusion models, metoprolol inhibits the potential deleterious neutrophil inflammatory response.
The reduction in ventricular arrhythmias, observed in previous studies, might be related to the stabilizing effect on the myocardial cell membrane and the inhibition of the re-entrant pathways as a consequence of the infarcted myocardium.12 In the current analysis there was no difference in ventricular tachycardia during hospitalization. A possible explanation for this finding could be due to differences in timing (pre- or post-PCI) and dose of beta-blocker administration in the various studies.
Safety
The safety outcome of the current analysis was a composite of cardiogenic shock, symptomatic bradycardia, or hypotension. Physicians might be reluctant to use early intravenous beta-blockers because of the potential occurrence of these outcomes. However, during hospitalization, no significant increase was observed in the safety outcome in the intravenous beta-blocker group in more than 1000 randomized patients. Therefore we conclude that this therapy is safe in selected STEMI patients undergoing primary PCI.
LVEF during follow-up
We observed no difference in LVEF at one month and a small but significant increase in LVEF at six months. One possible explanation could be a reduction of total infarct size. However, this was not supported by cardiac necrosis markers. On the other hand, the observed benefit might be a spurious finding, with an LVEF difference of 2.8% in favor of the intravenous beta-blocker group. Although this difference was statistically significant, it might not be clinically relevant since there was no benefit of early intravenous beta-blockers with regard to clinical outcomes. Longer follow-up might be necessary to reveal differences in clinical outcomes.
Implications
Based on the current available evidence, the latest European Society of Cardiology guidelines for STEMI state that early administration of intravenous beta-blockers at the time of presentation followed by oral beta-blockers should be considered in hemodynamically stable patients undergoing primary PCI.13 This excludes patients with signs of acute heart failure or a systolic blood pressure lower than 120 mm Hg. Our current patient-pooled meta-analysis remains neutral towards this statement, since we did not observe an evident clinical benefit or harm of early beta-blocker administration.
Lessons learned from the two largest trial EARLY-BAMI and METOCARD-CNIC could help to conduct a novel large trial to assess the effect of intravenous metoprolol in STEMI patients. This study should involve double-blind early administration (preferably in the ambulance) of the high dose of 15 mg metoprolol (used in METOCARD-CNIC) or placebo in both anterior and non-anterior STEMI patients. An imaging-based primary endpoint using CMR based infarct size at six months would be preferred. However, LVEF should be assessed by echocardiography at one and six months as a secondary endpoint, to account for CMR dropout. Pre-specified analyses should include anterior vs non-anterior MI and small vs larger infarct size. Conducting such a novel could be challenging however, since it would be costly and EARLY-BAMI and METOCARD-CNIC faced some difficulties with patient enrolment and dropout from CMR.
Limitations
A number of limitations should be mentioned. First, a small but increase in six-month LVEF was observed. However, this finding should be interpreted with caution as it may no longer be statistically significant after correction for multiple comparison. Second, LVEF was assessed using different imaging modalities in the various trials. However, this is true for both treatment strategies. Third, different beta-blockers were used in the various trials, with metoprolol being in used in the largest two trials, and landiolol and esmolol in the smaller trials. Fourth, we did not have data not only on the use of beta-blockers, but also angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, after discharge which may have impacted the outcome of the trials.
Conclusion
In STEMI patients undergoing primary PCI, the use of early intravenous beta-blockers was safe. However, there was no difference in the main outcome of one-year death or MI with the administration of intravenous beta-blockers. Given the apparent safety of intravenous metoprolol when administered in STEMI patients, a novel larger trial comparing the effect of early (preferably in the ambulance) high dose of 15 mg metoprolol versus placebo on clinical outcomes is warranted. If successful, this could result in an inexpensive treatment for STEMI patients undergoing primary PCI in absence of cardiogenic shock at presentation.
Supplemental Material
Supplemental material, Supplemental_Material for Early intravenous beta-blockers in patients undergoing primary percutaneous coronary intervention for ST-segment elevation myocardial infarction: A patient-pooled meta-analysis of randomized clinical trials by Niels PG Hoedemaker, Vincent Roolvink, Robbert J de Winter, Niels van Royen, Valentin Fuster, José M García-Ruiz, Fikret Er, Natig Gassanov, Kenji Hanada, Ken Okumura, Borja Ibáñez, Arnoud W van ’t Hof and Peter Damman in European Heart Journal: Acute Cardiovascular Care
Acknowledgments
The following contributions were made: PD had the idea for and designed the study, did the systematic review, collected and analyzed data, and wrote the article. VR, VF, JMGR, FE, NG, KH, KO, BI, and AWvtH designed the study, extracted data, and revised the article. NPGH did the systematic review, analyzed data and wrote the article. RJdW and NvR revised the article.
Footnotes
Conflict of interest: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1. Damman P, Beijk MA, Kuijt WJ, et al. Multiple biomarkers at admission significantly improve the prediction of mortality in patients undergoing primary percutaneous coronary intervention for acute ST-segment elevation myocardial infarction. J Am Coll Cardiol 2011; 57: 29–36. [DOI] [PubMed] [Google Scholar]
- 2. Stone GW, Selker HP, Thiele H, et al. Relationship between infarct size and outcomes following primary PCI: Patient-level analysis from 10 randomized trials. J Am Coll Cardiol 2016; 67: 1674–1683. [DOI] [PubMed] [Google Scholar]
- 3. Rasmussen MM, Reimer KA, Kloner RA, et al. Infarct size reduction by propranolol before and after coronary ligation in dogs. Circulation 1977; 56: 794–798. [DOI] [PubMed] [Google Scholar]
- 4. Elgendy IY, Elgendy AY, Mahmoud AN, et al. Intravenous beta-blockers for patients undergoing primary percutaneous coronary intervention: A meta-analysis of randomized trials. Int J Cardiol 2016; 223: 891–897. [DOI] [PubMed] [Google Scholar]
- 5. Er F, Dahlem KM, Nia AM, et al. Randomized control of sympathetic drive with continuous intravenous esmolol in patients with acute ST-segment elevation myocardial infarction: The BEtA-Blocker Therapy in Acute Myocardial Infarction (BEAT-AMI) Trial. JACC Cardiovasc Interv 2016; 9: 231–240. [DOI] [PubMed] [Google Scholar]
- 6. Roolvink V, Ibanez B, Ottervanger JP, et al. Early intravenous beta-blockers in patients with ST-segment elevation myocardial infarction before primary percutaneous coronary intervention. J Am Coll Cardiol 2016; 67: 2705–2715. [DOI] [PubMed] [Google Scholar]
- 7. Hanada K, Higuma T, Nishizaki F, et al. Randomized study on the efficacy and safety of landiolol, an ultra-short-acting beta1-adrenergic blocker, in patients with acute myocardial infarction undergoing primary percutaneous coronary intervention. Circ J 2012; 76: 439–445. [DOI] [PubMed] [Google Scholar]
- 8. Ibanez B, Macaya C, Sanchez-Brunete V, et al. Effect of early metoprolol on infarct size in ST-segment-elevation myocardial infarction patients undergoing primary percutaneous coronary intervention: The Effect of Metoprolol in Cardioprotection During an Acute Myocardial Infarction (METOCARD-CNIC) trial. Circulation 2013; 128: 1495–1503. [DOI] [PubMed] [Google Scholar]
- 9. Ibanez B, Prat-Gonzalez S, Speidl WS, et al. Early metoprolol administration before coronary reperfusion results in increased myocardial salvage: Analysis of ischemic myocardium at risk using cardiac magnetic resonance. Circulation 2007; 115: 2909–2916. [DOI] [PubMed] [Google Scholar]
- 10. Garcia-Prieto J, Villena-Gutierrez R, Gomez M, et al. Neutrophil stunning by metoprolol reduces infarct size. Nat Commun 2017; 8: 14780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van der Laan AM, Hirsch A, Robbers LF, et al. A proinflammatory monocyte response is associated with myocardial injury and impaired functional outcome in patients with ST-segment elevation myocardial infarction: Monocytes and myocardial infarction. Am Heart J 2012; 163: 57–65.e52. [DOI] [PubMed] [Google Scholar]
- 12. Norris RM, Barnaby PF, Brown MA, et al. Prevention of ventricular fibrillation during acute myocardial infarction by intravenous propranolol. Lancet 1984; 2: 883–886. [DOI] [PubMed] [Google Scholar]
- 13. Ibanez B, James S, Agewall S, et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the Management of Acute Myocardial Infarction in Patients Presenting with ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J 2018; 39: 119–177. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplemental_Material for Early intravenous beta-blockers in patients undergoing primary percutaneous coronary intervention for ST-segment elevation myocardial infarction: A patient-pooled meta-analysis of randomized clinical trials by Niels PG Hoedemaker, Vincent Roolvink, Robbert J de Winter, Niels van Royen, Valentin Fuster, José M García-Ruiz, Fikret Er, Natig Gassanov, Kenji Hanada, Ken Okumura, Borja Ibáñez, Arnoud W van ’t Hof and Peter Damman in European Heart Journal: Acute Cardiovascular Care