Abstract
In Nepal, rapid urbanization and rural-to-urban migration especially due to internal civil conflict have catalyzed the development of temporary settlements, often along rivers on undeveloped land. This study conducted surveillance for viruses in small mammals and assessed potential risks for virus transmission to people in urban settlements along rivers in Kathmandu, Nepal. We collected samples from 411 small mammals (100 rodents and 311 shrews) at four riverside settlement sites and detected six viruses from four virus families including Thottapalayam virus; a strain of murine coronavirus; two new paramyxoviruses; and two new rhabdoviruses. Additionally, we conducted surveys of 264 residents to characterize animal–human contact. Forty-eight percent of individuals reported contact with wildlife, primarily with rodents and shrews (91%). Our findings confirm that rodents and shrews should be considered a health threat for residents of temporary settlements, and that assessment of disease transmission risk coupled with targeted surveillance for emerging pathogens could lead to improved disease control and health security for urban populations. Additionally, interventions focused on disease prevention should consider the unique urban ecology and social dynamics in temporary settlements, along with the importance of community engagement for identifying solutions that address specific multi-dimensional challenges that life on the urban river margins presents.
Electronic supplementary material
The online version of this article (10.1007/s10393-020-01499-4) contains supplementary material, which is available to authorized users.
Keywords: Zoonoses, Emerging infectious diseases, Interface, Urban, Spillover, Vulnerability, PREDICT, Nepal
Introduction
In 2009, more than 50% of the world’s population resided in cities (United Nations 2014), and rates of urbanization and growth of cities are projected to increase substantially, especially in low- and middle-income countries (LMICs) (Cohen 2004, 2006; Dye 2008). Increased urban population growth has profound impacts on health in LMICs, and effectively managing rapid urbanization without substantial capacity and resources (Alirol et al. 2011) is challenging where there is high population density in areas that lack infrastructure, conditions that facilitate disease transmission (Eckert and Kohler 2014; Neiderud 2015) and enable large-scale outbreaks (Gubler 2011). As evidenced by the recent Ebola virus disease epidemic in West Africa (Gatherer 2014), outbreaks in dense underserved communities are inherently difficult to control. In addition, demographic shifts in cities are not restricted to humans, as large influxes of domesticated animals often accompany people during rural-to-urban migrations. Rural-to-urban migration is a dynamic process, and customs and livelihoods, such as crop and animal production, are often imported into urban areas where the distinction between urban and rural systems becomes blurred. Consequently, human and animal health systems can become increasingly stressed as they cope with burgeoning populations.
Animal-origin pathogens have contributed the majority of emerging infectious diseases identified in people over the past 70 years (Taylor et al. 2001; Wolfe et al. 2007) and still pose an ongoing threat to global health (Hugh-Jones et al. 2008; Snowden 2008). Transmission of zoonotic pathogens from animals to people is dependent on a multitude of factors from the molecular to landscape level, including human–animal contact (Karesh et al. 2012). Other drivers of disease emergence include anthropogenic and environmental factors such as changes in land use, human population growth, agricultural intensification, international travel and trade, changes in human behavior and social structure, and breakdown of public health infrastructure (Lederberg et al. 2003). Such drivers of emergence are present and intensifying in established urban and peri-urban areas, which due to their role in commerce and trade are increasingly interconnected with the global community (Songsore 2004; Redman and Jones 2005; Alirol et al. 2011; Rydin et al. 2012).
Characterization of animal–human interactions that have facilitated zoonotic disease transmission from wildlife includes direct and indirect contact with wildlife in and around human dwellings and in agricultural settings (Johnson et al. 2015). However, the impact of urbanization on pathogen spillover from wildlife is complex. Urbanization can reduce the abundance of some wildlife parasites, though transmission has been shown to increase among urban-adapted hosts with detrimental effects on other rare wildlife species within or near a city’s edge (Bradley and Altizer 2007). In temporary settlements and urban slums that are common in LMICs (Marx et al. 2013), rapid and unplanned growth may influence adaptable synanthropic wildlife species such as rodents (Taylor et al. 2008) that can thrive in these urban settlements, attracted by easy access to food and housing for people and their animals (Mackenstedt et al. 2015). In these settlements, wildlife–human interactions are common through either shared food resources, surfaces contaminated with animal urine and feces, or via direct contact due to animal bites or scratches.
Globally among mammalian orders, the order Rodentia is known to have the highest number of species harboring previously detected zoonoses (Han et al. 2016), and rodent hosts were most likely to be implicated in transmission of zoonotic diseases to humans through contact in and around human dwellings and in agricultural fields (Taylor et al. 2008; Johnson et al. 2015). Important bacterial zoonotic diseases from rodents, such as plague and leptospirosis, can cause large epidemics in urban and semi-urban environments (Gupta and Sharma 2007; Vijayachari et al. 2008; Dikid et al. 2013). Rodents are also reservoirs for pathogens, such as Giardia and Leishmania (Meerburg et al. 2009), and act as reservoirs for zoonotic viruses of public health importance in South Asia, such as Crimean–Congo hemorrhagic fever virus (Tatera indica, Meriones hurrianae, Rattus rattus) (Darwish et al. 1983b), Kyasanur forest virus (Rattus rattus, Madromysblanfordi) (Mehla et al. 2009), and Hantavirus (Rattus nitidus, Bandicota indica, Mus caroli) (Blasdell et al. 2011). Antibodies against zoonotic flaviviruses have also been detected in rodent species commonly found in South Asian cities, including Zika virus (Bandicota bengalensis, Meriones hurrianae, Tatera indica) (Darwish et al. 1983a) and West Nile virus (Tatera indica, Rattus rattus, Millardia meltada) (Root 2013), although antibody detection for these specific pathogens could be due to cross-reactivity to other flaviviruses.
With a population of 2.5 million people, the Kathmandu Valley in Nepal is growing at 4% per year and is one of the fastest growing metropolitan cities in South Asia (Muzzini and Aparicio 2013). Internal economic migration is the largest contributor to this urban growth (Muzzini and Aparicio 2013), with net rural–urban migration rates estimated at 29%. In Kathmandu, rapid urbanization and rural-to-urban migration have led to an increase in the number of temporary settlements from 17 to 40 (Lumanti 2008). As in many cities around the world, migrants to urban areas in Kathmandu settle on undeveloped land, some defined as indigenous settlements (Lumanti 2008). These communities sometimes called “sukumbasis” (landless squatters) are concentrated on the banks of urban and peri-urban rivers, and residents can be subject to health and sanitation challenges due to poor quality housing and inadequate sewage, drainage, and drinking water facilities (Jassat et al. 2013). These communities are in close contact with synanthropic wildlife and at risk for exposure to zoonotic infections, as their homes and gardens are located along the banks of urban riparian corridors, providing habitat and forage for numerous wildlife species such as rodents. Small holdings in these settlements often feature dense animal and human communities, including pigs, cattle, goats, chickens, and ducks, often in close quarters with human dwellings, conditions well suited for transmission of zoonotic diseases.
These marginalized communities lack access to basic infrastructure and health services, as well as information for strengthening biosecurity for their small gardens and livestock holdings, and these communities can be overlooked by public and veterinary health surveillance systems. In LMICs, diseases are rarely recognized in pre-epidemic conditions due to limited surveillance and diagnostic capacity (Murdoch et al. 2004; Baker et al. 2010), despite widespread illness in people. Lower respiratory infections, diarrheal diseases, and encephalitic-type illnesses, often undiagnosed to a specific etiology, comprise the leading causes of life-years lost in Nepal (Karkey et al. 2008; Devleesschauwer et al. 2013, 2014). In addition, dense and inadequate housing infrastructure along with poor sanitation and hygiene can propagate transmission of viral diseases in these settlements and could be the source of disease outbreaks.
This study was part of the US Agency for International Development’s Emerging Pandemic Threats PREDICT project, a global project aimed at strengthening capacity for surveillance and detection of virus threats in wildlife and targeting viral families with a high proportion of zoonotic viruses, including several high consequence pathogens and select agents, as well as viruses that have caused previous pandemics. We designed this study to address a critical knowledge gap on risks for viral transmission at the urban wildlife–human interface in vulnerable urban settlements of Nepal. In this study, we characterized the potential risk for human exposure to viruses from rodents and small mammals at four informal urban settlement sites along the rivers in the capital of Nepal, Kathmandu. Specifically, we collected samples from wild rodents and shrews and tested them for potentially zoonotic viruses, and we conducted observational research and surveyed residents in these communities to better understand human activities and social factors that could be associated with zoonotic disease transmission risk.
Materials and Methods
Study Location and Site Selection
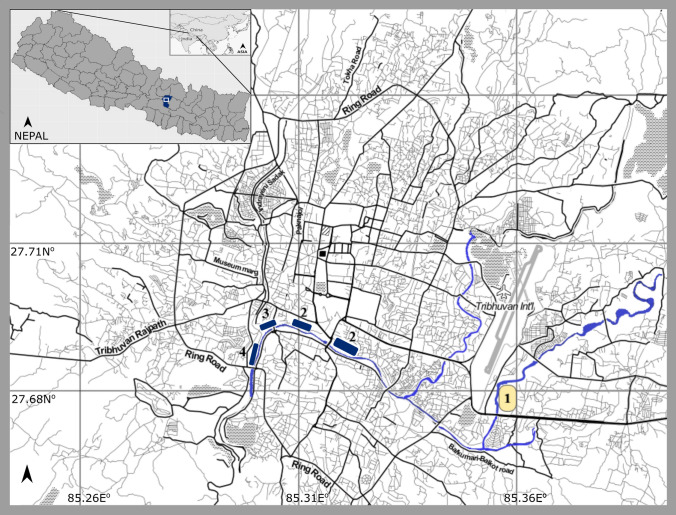
The study conducted in Kathmandu, Nepal, was designed to specifically address virus threat risk from wildlife in urban and peri-urban areas, defined here as areas with a mix of urban and rural development processes, economies, and livelihoods on the periphery of cities (Rohilla 2005). The study targeted unplanned informal settlements along rivers in urban and peri-urban areas as these sites were hypothesized to be especially at risk for a number of threats that impact human health (e.g., low-income communities exposed to weather due to types of dwellings, a lack of access to improved sanitation and safe water sources, and location of homes in areas near wildlife habitat, especially rodents and small mammals). Sites that fit the definition of temporary settlements based on the Kathmandu municipality’s categorization were verified by our site visits to meet our hypothesized criteria above and further confirmed through local community member reports regarding frequent observation and contact with rodents and small mammals. Four sites were selected for our study (Table 1). At each of the sites, we identified key features for risk of wildlife contact and potential for zoonotic virus transmission and spread (Supplementary Table S1). Each of the four sites (1 to 4) was selected for wildlife sampling, and behavioral risk surveys were conducted from 2013–2014 (Fig. 1). Of these four, three were defined as urban settlements in areas predominantly developed and built-up along the holy river, centrally located within Kathmandu municipal city limits and surrounded by residential and commercial areas; one was defined as a peri-urban settlement situated outside Kathmandu city limits east of the international airport on the banks of the river in an area surrounded by open fields used for agricultural production and rice cultivation and scattered forest. Land use and cover classifications for the Kathmandu Valley were consulted to confirm the urban vs. peri-urban distinction (Ishtiaque et al. 2017). Except for Site-3, the smallest and most densely populated urban site, each site was delineated with animal trapping and sampling lines designed to capture animals across all available habitat from the river edge to within and around dwellings.
Table 1.
Settlement Sites, Their Attributes, Research Team Observations, and Reported Local Community Insights
| Observations and insights | Unplanned settlement sitesa | |||
|---|---|---|---|---|
| Site-1 (peri-urban) |
Site-2 (urban) |
Site-3 (urban) |
Site-4 (urban) |
|
| Within Kathmandu municipal boundaries | No | Yes | Yes | Yes |
| Along a river | Yes | Yes | Yes | Yes |
| Animal production on site | Yes (Cattle, pigs, ducks, cats, chickens, dogs, goats) | Yes (Cattle, pigs, ducks, cats, chickens, dogs, goats) | Yes (Cattle, pigs, ducks, cats, sheep, chickens, dogs, goats, geese) | Yes (Cattle, pigs, cats, rats, chickens, dogs, goats) |
| Agriculture production on site | Yes (gardens and crops in peri-urban fields) | Yes (small household gardens) | Yes (household gardens and large vegetable and fruit market) | Yes (small household gardens) |
| Temple area (cremation and religious ceremonies) | No | Yes | Yes | No |
| Landfill or municipal dump on site | No | No | Yes | No |
|
Direct contact between people and rodents at this site (e.g., aggressive interactions, bites, scratches, harassing rodents, etc.) |
Yes | Yes | Yes | Yes |
| Rodent interaction with livestock or domestic animals at this site | Yes | Yes | Yes | Yes |
|
Signs that rodents have been near or in homes, living areas (within 10 meters) |
Yes | Yes | Yes | Yes |
| Signs that rodents have been feeding on crops used for food | Yes | Yes | Yes | Yes |
| No. of people interviewed | 40 | 96 | 48 | 80 |
aBased on municipality categorization
Figure 1.
Study sites in the Kathmandu, Nepal. In yellow is the peri-urban settlement (Site-1) along the river just east of Kathmandu municipal boundaries. In blue are the urban settlements (Site-2–4) along the river. Shown in the inset is the Kathmandu Valley of Nepal
Animal Trapping and Sampling
Rodents and shrews were captured and sampled under protocols approved by the UC Davis Institutional Animal Care and Use Committee (IACUC #17504) and through permission granted by the Department of National Parks and Wildlife Conservation of Nepal. Trapping was conducted from February 2013 to February 2014 during both the monsoon and dry seasons targeting a minimum of 50 animals per site per season. Seasons were classified using precipitation data from nearby Tribhuvan International Airport in Kathmandu with the dry season ranging from mid-May to late October. Animals were live-captured using Sherman live trap (22.9 × 8.9 × 7.6 cm3) and small locally produced wire-mesh traps. Traps were set at night along trap lines corresponding to the range of habitat observed at each site: near the river edge, along the river banks in riparian shrubs or gardens irrigated by river water by settlement residents, and with resident’s permission, around and within settlement structures such as dwellings and outbuildings.
Animals were anesthetized with the ball-drop method using isoflurane (Nagate et al. 2007). Oral and rectal samples were collected in duplicate using sterile polyester swabs, placed into viral transport media (VTM) (Thermo Scientific™ MicroTest™ # R12557) and lysis buffer, and stored in liquid nitrogen until transferring to an ultra-low freezer (− 80 °C). Blood samples were also collected from these animals by retro-orbital bleeding, preserved in VTM, and stored at − 80 °F freezer until DNA extraction for barcoding. All samples were collected from apparently healthy animals, and individuals were released following sampling.
Rodent and Shrew Species Identification
While anesthetized, morphometric measurements and photographs were taken of each animal for species identification using a field guide (Baral and Shah 2008). When identification from morphometric measurements was uncertain, especially for Rattus species, identification was completed using DNA barcoding targeting the Cytochrome-b mitochondrial gene by polymerase chain reaction (PCR) and sequence confirmation (Schmidt and Gold 1993). In brief, DNA was extracted from the blood samples of 224 animals using the Qiagen DNeasy Blood and Tissue Kit according to manufacturer’s instructions (Qiagen 2006) followed by PCR. Sample code, Blast match, and identity percentage for each sequence were tabulated, and species were then evaluated for consistency with habitat and range maps provided by the International Union for Conservation of Nature (IUCN) (www.iucn.org).
Virus Detection and Discovery
Virus detection was performed using consensus PCR (cPCR) targeting virus families or genera of zoonotic interest prioritized by the PREDICT project to detect known potentially zoonotic viruses in wildlife as well as their genetic near neighbors. Specifically, samples were tested for alphaviruses (Sánchez-Seco et al. 2001, targeting 193 bp of NSP4 gene), mammarenaviruses (Lozano et al. 1997, targeting 460 bp of S-gene), the orthobunyavirus genus within the Peribunyaviridae family (Briese et al. 2007, targeting 930 bp, 898 bp and 586 bp of S-, M-, and L-segments, respectively), coronaviruses (Quan et al. 2010, targeting 328 bp of Rdrp gene; Watanabe et al. 2010, targeting 434 bp of Rdrp gene), flaviviruses (Sánchez-Seco et al. 2005 targeting 270 bp of NS5 gene; Moureau et al. 2007 targeting 141 bp of NS5 gene), Hantaviridae (Aitichou et al. 2005, targeting 236 bp of S-segment; Raboni et al. 2005, targeting 417 bp of S-segment), alphainfluenzaviruses (Anthony et al. 2012, targeting 243 bp of M gene and Anthony et al. 2015 targeting 236 bp of PB1 gene), paramyxoviruses within the Paramyxoviridae family (Tong et al. 2008, targeting 561 bp of pol gene), rhabdoviruses within the Rhabdoviridae family (Bourhy et al. 2005, targeting 260 bp of L-gene), and the seadornavirus genus within the Reoviridae family (Anthony et al. 2015, targeting 747 bp of genome segment 1). Oral and rectal swabs (or fresh feces when available) in lysis buffer were prioritized for RNA extraction and virus detection. RNA was extracted using QIAamp Virus Mini Kits (Qiagen 2006) and cDNA synthesis performed using SuperScript III first-strand synthesis supermix (Invitrogen). PCR was performed using Platinum Taq DNA polymerase (Invitrogen) with cycling conditions according to published protocols (Anthony et al. 2015). Synthetic DNA constructs were used as positive controls made from DNA plasmids containing short regions of overlapping virus sequences providing primer binding sites for the different PCR assays. Amplified products of the expected size were cloned (pCR4-TOPO vector; Invitrogen Corp.) and sequenced. The resulting sequences were edited manually in Geneious Pro (ver 9.1.3, Biomatters, Auckland, NZ) and compared with known sequences in the GenBank database.
Virus sequences were classified as belonging to virus taxa according to established cutoffs and methods (Anthony et al. 2017). Virus sequences with percent identity below the cutoff to a known sequence were labeled sequentially as PREDICT viruses (e.g., for paramyxoviruses: PREDICT_PMV-1, -2, -3, etc.), while groups sharing greater percent identity than the cutoff to a sequence already in the GenBank database were given the same name as the matching sequence. Based on these criteria, the sequences detected were assigned to discrete virus taxa.
Animal Contact and Human Behavioral Risk Investigations
A structured questionnaire was used to understand human behavior that could lead to disease exposure in the settlement areas by quantifying human and animal contact, identifying potential risk factors for virus spillover from animals to people, and exploring the awareness of zoonotic disease transmission in people. The questionnaire collected quantitative data on demographic categories, livelihoods, frequency and type of contact with wildlife and domesticated species over the past year, and other factors potentially associated with indirect contact with animals and viral transmission through fomites and environmental contamination (e.g., water, contaminated food, etc.). We have included the questionnaire in Supplementary Materials as a resource for others wishing to conduct similar or comparative investigations. This survey study was approved by the Nepal Health Research Council (#232/141/2014) and University of California Davis Institutional Review Board (#626851). The questionnaire was translated into Nepali, and interviews were conducted in Nepali by trained interviewers at all four settlements in September 2014. Systematic random sampling was performed to select the households at each site, with the first household selected at random and subsequent interviews continuing at every fifth household until reaching the targeted minimum sample size of at least 40 individuals per settlement and with children comprising no more than 40%.
Interview data were translated, coded, and analyzed in R (R Core Development Team). For analysis, all contacts with wildlife were counted irrespective of type. We calculated the total number of reported wildlife contacts by taxa for each individual over the entire 12-month timeline. We used a zero-inflated Poisson (ZIP) regression model to study the associations between putative risk factors and number of contacts with wildlife. Zero-inflated Poisson models are generally used to model count data with excessive zeros and contain two parts, a Poisson count model and the logit model for predicting an excess of zeros (Long and Freese 2006; Hardin et al. 2007). We focused on an individual’s demographic characteristics (age, gender, occupation, residency time in the settlement), and all categorical variables (except age) were evaluated statistically for the model. Reported occupation (business or trade, student, animal agriculture, unskilled labor, or unemployed) was also assessed to enable comparisons across groups. For the inclusion of a risk factor, a forward manual stepwise model building algorithm was implemented with a two-sided p value ≤0.10 in the count model. An interaction term of occupation and gender was also included. The ZIP regression model was run using “pscl” package in “R” (R Core Development Team).
Results
Site Characterization and Emerging Virus Spillover Vulnerability
Detailed site attributes for the four settlements included in the study are shown in Table 1 and Supplementary Table S1. Despite similar site attributes and characteristics, qualitative data recorded in all settlements demonstrated considerable differences, even within sites. For example, Site-3 is densely clustered with dwellings, adjacent to the municipal dump where some residents scavenge materials and raise pigs. Site-2 by contrast consists of an older established settlement with access to improved sanitation and piped water on the west end and a collection of tarp-roofed dwellings with limited sanitation and water access on the east end.
Rodent and Shrew Species at Settlement Sites
A total of 411 animals (100 rodents and 311 shrews) were captured and sampled at the four sites. The majority of these animals were captured during the dry months (n = 302); a total of 109 animals were captured during the monsoon (Table 2). Across all settlement sites, eight species from four genera and two taxonomic orders were captured (Fig. 1). Suncus murinus, the Asian house shrew, was the most common species captured at all sites (n = 313; 76.7%). In addition, we captured 79 animals (19.2%) from the genus Rattus (Rattus tanezumi, Rattus rattus, Rattus nitidus, and another unclassified Rattus sp.), seven Bandicota bengalensis (1.7%), and 10 rodents from Mus genus (2.4%). Of the four settlement sites, Site-2 had the highest species diversity with animals from all four genera followed by Site-4 site with species from three genera. The highest numbers of Rattus sp. were captured at the Site-4, which is next to a large urban market, while all Bandicota sp. were captured at Site-2, and all but one of these animals were captured during the monsoon season.
Table 2.
Species of Rodents and Shrews Sampled by Site and Season (n = 411 Animals)
| Species | Site and season | Grand Total |
|||||
|---|---|---|---|---|---|---|---|
| Site-1 (peri-urban) |
Site-2 (urban) |
Site-3 (urban) |
Site-4 (urban) |
||||
| Dry | Monsoona | Dry | Monsoona | Dry | Dry | ||
| Bandicota bengalensis | 0 | 0 | 1 | 6 | 0 | 0 | 7 |
| Bandicota indica | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| Bandicota spp. | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| Mus musculus | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Mus musculus castaneus | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| Mus spp. | 1 | 0 | 5 | 0 | 0 | 2 | 8 |
| Rattus nitidus | 0 | 0 | 0 | 1 | 1 | 0 | 2 |
| Rattus rattus | 3 | 0 | 1 | 2 | 10 | 19 | 35 |
| Rattus spp. | 0 | 2 | 0 | 1 | 1 | 32 | 36 |
| Rattus tanezumi | 2 | 0 | 0 | 0 | 2 | 2 | 6 |
| Suncus murinus | 54 | 47 | 80 | 48 | 37 | 47 | 313 |
| Grand total | 60 | 49 | 88 | 60 | 51 | 103 | 411 |
aThe monsoon season was classified as May 15, 2013, to October 25, 2013, using precipitation data from the Tribhuvan airport weather station in Kathmandu, Nepal
Virus Detections in Rodents at Settlement Sites
A total of 564 specimens from the 411 animals were tested by PCR for 10 virus families. Oral swabs were tested from 410 sampled animals from all sites, and rectal swabs or feces were tested from 154 animals from two sites (Site-1 & Site-3). Virus RNA was detected in seven oral and seven rectal swabs from 13 individuals. Six viruses from four virus families were confirmed by sequencing with at least one virus detected at each of the four sampling sites (Table 3). Virus sequences were deposited in GenBank, and accession numbers are provided in Table 3.
Table 3.
Summary of Virus Detected by Species, Specimen Type, and Settlement Site
| Virus family, virus (# of animal species, season sampled) |
No. of positive / No. of tested specimens | Settlement sites (urban or peri-urban) |
||
|---|---|---|---|---|
| No. of oral swabs tested |
No. of rectal swabs tested |
Total specimens testeda | ||
| Hantavirus | ||||
|
Thottapalayam virusc (1 Suncus murinus, dry season) |
0.24% (1/410) | 0% (0/154) | 0.18% (1/560) | Site-1 (peri-urban)b |
| Coronavirus | ||||
|
Murine coronavirusd (2 Mus musculus, dry season) |
0.49% (2/410) | 0% (0/154) | 0.36% (2/560) |
Site-4 (urban) & Site-1 (peri-urban)b |
| Paramyxovirus | ||||
|
PREDICT_PMV-54e (6 Suncus murinus, dry & monsoon season) |
0.24% (1/410) | 3.90% (6/154) | 1.25% (7/560) | Site-1 (peri-urban)b & Site-3 (urban) sitesb |
|
PREDICT_PMV-55f (1 Rattus tanezumi, dry season) |
0.24% (1/410) | 0.00% (0/154) | 0.18% (1/560) | Site-1 (peri-urban)b |
| Rhabdovirus | ||||
|
PREDICT_RbdV-5 g (2 Suncus murinus, monsoon season) |
0.24% (1/410) | 0.65% (1/154) | 0.36% (2/560) | Site-1 (peri-urban)b |
|
PREDICT_RbdV-6 h (1 Suncus murinus, dry season) |
0.24% (1/410) | 0.00% (0/154) | 0.18% (1/560) | Site-2 (urban) |
| Total | 1.46% (6/410) | 4.55% (7/154) | 2.32% (13/560) | |
aAll virus findings were from adult animals (n = 13) and no animals tested positive for more than one virus
bRectal swabs or feces were tested from these sites only
cGenBank accession number KX442774
dGenBank accession number KX285432 & KX285433
eGenBank accession number KP963851-57; PREDICT_PMV-54 virus was detected in both oral and rectal swabs from one animal (Suncus murinus)
fGenBank accession KP963858
gGenBank accession MG956834 & MG956836
hGenBank accession MG956835
We did not detect any known zoonotic viruses in rodents at these four sites using the PCR protocols designed for the PREDICT project. Following methods of Anthony et al. 2017, strains of murine coronavirus (≥ 90% nucleotide similarity; GenBank accession numbers: KX285432 and KX285433) were detected in oral swabs from two Mus musculus. We detected a hantavirus in an oral swab collected from a Suncus murinus, and this sequence had > 99% nucleotide similarity to other sequences in GenBank for Thottapalayam virus (GenBank accession number: KX442774).
In this study, we detected two new paramyxoviruses (PREDICT_PMV-54 & -55) with sequences that were < 85% nucleotide similarity to known paramyxovirus sequences. The PREDICT_PMV-54 (GenBank accession numbers: KP963851-57) was identified in oral and rectal swabs of six Suncus murinus, whereas the PREDICT_PMV-55 was identified in an oral swab of Rattus tanezumi, (GenBank accession number: KP963858). Similarly, we also detected two new rhabdoviruses, PREDICT_Rbdv-5 (GenBank accession numbers: MG956834 and MG956836) and PREDICT_Rbdv_6 (GenBank accession number: MG956835), distantly related to a group of insect-transmitted rhabdoviruses. Both rhabdovirus sequences were < 80% nucleotide similarity to known rhabdovirus sequences and were detected in oral and rectal swabs from three Suncus murinus. All virus findings were from adult animals (n = 13) and no animals tested positive for more than one virus.
Human–Wildlife Contact in Temporary Settlements
Interviews were conducted with 264 people across all four settlements sites: 152 were female (57.9%), and 112 were male (42.4%) with a mean age of 31.6 years (range 6–81 years, median 29.5, standard deviation 17.0). Ethnic groups represented in the study included disadvantaged Janajati (indigenous peoples: 40.5%), upper caste individuals (31.8%), relatively advantaged Janajati (14%), Dalit (lower caste: 11.4%), and Muslims (1.9%). Religious groups included Hindus (65.5%), Christians (18.9%), Buddhists (14.0%), and Muslims (1.52%). Residency times (ranging from less than 1 year, one to 10 years, and greater than 10 years) at the settlements varied by location and ethnicity. A broad range of occupations was reported, and multiple occupations and livelihood strategies were frequently reported by a single individual. For analysis, occupation was grouped into five main categories: unemployed (20.5%), unskilled laborers (21.3%), students (25.5%), business or traders (29.3%), and workers engaged in animal agriculture, livestock production, and related practices (3.4%).
One hundred twenty-six (47.9%) individuals reported contact with wildlife within the past year, and contacts with rodents and shrews represented the vast majority (91.1%). Based on multivariable zero-inflated Poisson model results, males (RR = 2.37, CI = 1.47–3.78, p <0.005) and individuals working with animals in agricultural occupations, such as raising pigs, poultry, or small ruminants (RR = 4.38, CI = 1.95–9.83, p <0.005), had a significantly higher likelihood of contact with wildlife. Similarly, residing in one of the settlements for more than one year increased the individual’s risk for reported contact (RR = 4.22, CI = 2.23–7.97, p <0.005 with a residency time between 1 and 10 years, and RR=4.82, CI = 2.54–9.32, p <0.005 with a residency time greater than 10 years). Individuals from Site-3 showed a reduced risk (RR = 0.46, CI = 0.29–0.72, p <0.005) of contact with wildlife compared to other sites. The logit part of the model described an interaction term between males with white-collar occupations resulting in reduced odds for contact with wildlife (OR = 0.14, CI = 0.02–0.81, p =0.027). None of the other covariates, including ethnic and religious groups, were found to be significantly associated with wildlife contact. To explore awareness of zoonotic disease transmission, people were asked whether they believed that people could become sick from contact with animals. The vast majority (97.4%; 257/264) responded “never”, while 2.27% (6/264) responded “sometimes”, and one individual understood that contact with animals could result in illness.
Discussion
Our study is one of the first to evaluate the potential for zoonotic disease risks in informal settlement communities in urban Nepal and the first study to describe contact between small mammals and people at this unique animal–human interface. Our findings demonstrate that urban and peri-urban human inhabitants interact intimately with diverse wild and domestic animal communities sharing this riparian landscape and are routinely exposed to animals that could act as reservoir hosts for potentially infectious zoonotic pathogens. Small mammals, such as rodents and shrews, are habituated to human dwellings and contaminate food and grain storage areas, water supplies, and gardens and should be considered as sources for exposure to zoonotic viruses and other microbial pathogens. Though outside of the scope for testing in this study, bacteria that cause the rodent-borne diseases leptospirosis, rat-bite fever, plague, and tularemia are all recognized as threats for residents of urban slums worldwide (Corburn and Riley 2016). Thus, our finding that approximately half of survey respondents reported contact with rodents and shrews illustrates the potential public health risk that contact with wildlife may pose to communities in these urban and peri-urban settlements.
We identified a range of non-zoonotic and potentially zoonotic RNA viruses for the first time in urban small mammal populations in the Kathmandu Valley, including new viruses within Paramyxoviridae and Rhabdoviridae families. Viruses within the Paramyxoviridae family have been detected in a wide variety of hosts, including humans and other mammals, rodents, reptiles, birds, and aquatic animals (MacLachlan and Dubovi 2011). Some of these viruses have caused devastating diseases with high morbidity and mortality in animals (e.g., rinderpest, canine distemper, Newcastle disease, etc.) and humans (e.g., measles, mumps, etc.) (MacLachlan and Dubovi 2011). Viruses within this family such as morbilliviruses and rinderpest are also known for cross-species transmission, and others such as Nipah virus and Hendra virus are important zoonotic threats to both people and domestic animals (Zeltina et al. 2016; Thibault et al. 2017). In rodents, Sendai virus (murine parainfluenza 1) is recognized as a highly contagious virus and is closely related to human parainfluenza virus 1 (MacLachlan and Dubovi 2011). Members of Rhabdoviridae family have been isolated from a broad range of vertebrates, plants, and arthropods. Zoonotic rhabdoviruses such as Lyssavirus can cause fatal encephalitis (rabies) in mammals including rodents. Additionally, vesicular stomatitis, an insect-borne rhabdovirus, infects livestock and can cause debilitating disease and loss of productivity (MacLachlan and Dubovi 2017).
We also identified a known member of the Hantaviridae family. Hantaviruses have been isolated from insectivores such as shrews and moles, bats, and even reptiles (Laenen et al. 2019) though they are perhaps best known as rodent-borne zoonotic pathogens causing acute hantavirus pulmonary syndrome or hemorrhagic fever with renal syndrome (Burrell et al. 2017a, b). The hantavirus identified in this study, Thottapalayam virus (TPMV), is a rodent-borne hantavirus found previously in insectivorous Asian house shrews (Suncus murinus) (Carey et al. 1971; Kang et al. 2011). Though TPMV is not known to cause disease in humans, Asian house shrews are peri-domestic and frequently share living space with humans providing ample opportunities for close human contact and disease transmission (Song et al. 2007). Asian shrews are considered to be the natural reservoir species of many members of the hantavirus family and should be a considered a potential spillover host for emerging viral threats in animals and people (Kang et al. 2011; Burrell et al. 2017a; Laenen et al. 2019).
Coronaviruses (CoVs) are a large family of RNA viruses, and α- and β-CoVs are adept at infecting various mammalian species worldwide (Burrell et al. 2017b; Chang et al. 2020). While the majority of currently known CoVs cause self-limited respiratory illnesses in humans and infected livestock, recently emerged zoonotic CoVs such as severe acute respiratory syndrome coronavirus (SARS-CoV) in 2002-2003 and Middle Eastern respiratory syndrome coronavirus (MERS-CoV) in 2012 have caused severe illness in humans (Fehr and Perlman 2015). The most recent coronavirus to emerge, SARS-CoV-2, was first recognized in 2019 and is the cause of an ongoing pandemic with sustained human-to-human transmission. The emergence of these new CoVs, widely thought to have originated in bats (Li et al. 2005; Hu et al. 2015; Zhou et al. 2020), provides evidence that CoVs, especially those circulating in understudied wildlife populations, warrant further investigation and evaluation as zoonotic threats. Two coronaviruses that circulate in humans causing mild disease, HCoV-OC43 and HCoV-HKU1, likely emerged from rodents (Corman et al. 2018). Murine coronaviruses, including mouse hepatitis virus (MVH) detected in this study, are known to cause hepatitis with some strains associated with respiratory, enteric, or neurologic disease in mice but not in humans (Weiss and Navas-Martin 2005). While MVH is a known CoV that does not cause disease in people, the detection of this virus along with the other viruses detected in our study could be indicators of viral risk, especially within the context of these vulnerable settlements where there is direct evidence of frequent rodent–human contact. In these urban settings, there is a need to continue to advance an understanding of the public health implications of potential rodent-borne viral threats, especially given the heavy burden of febrile illnesses in Kathmandu, many of which remain poorly characterized due to limited diagnostic capabilities (Murdoch et al. 2004).
Few studies of rodent-borne pathogens have been conducted in Nepal to date, and the majority of these studies have focused on bacteria or parasites, though other studies have also identified zoonotic viruses belonging to Hantaviridae or coronaviruses (Kang et al. 2011, retracted paper; Blasdell et al. 2011; Bordes et al. 2013; Van Cuong et al. 2015; Blasdell et al. 2015, 2016). These studies were based on either serology or RT-PCR and used tissue samples to detect or characterize viral shedding and diversity. For the PREDICT project, we relied on oral and rectal swabs and conventional consensus-based PCR for virus detection, which might be less sensitive for detecting viruses circulating in these populations.
In general, urbanization has been linked to declines in synanthropic species density due to landscape change and habitat degradation, though a few studies report capturing more diverse rodents species in urban areas compared to peri-urban or rural areas (Davis et al. 2005; Bordes et al. 2013; Morand et al. 2019). While this study was not designed to estimate or compare small mammal diversity across sites, we did experience greater species diversity among the four sampled genera represented in our study (Bandicota, Mus, Rattus, and Suncus) at the urban sites (Sites-2 & -4) compared to the peri-urban site on the city’s periphery. Bandicota sp. were only captured at the Site-2, where sampling occurred during both wet and dry seasons compared to only the dry season at the other urban sites. It is possible that Bandicota species are more abundant or easier to capture during the rainy (monsoon) season, which according to local community members corresponds to greater forage and food availability, or that the animals are more likely to move into sheltered areas at these sites during the monsoon. Despite capturing more small mammal species at the urban sites, five out of six viruses were detected at the single peri-urban site of this study, indicating that viral shedding and subsequent diversity in these small mammal populations might be linked to land use or degree of fragmentation of habitat or urbanization (Morand et al. 2019). Our peri-urban site was located at the city’s periphery with considerably more open space, gardens, and crop lands, and a wide range of domestic species (cattle, sheep, goats, chickens, ducks) raised by residents, so it is possible that the diversity of viruses detected here may be related to habitat and ecology though further investigation of this trend along with implications for public and veterinary health is recommended.
This study, while grounded in the One Health approach, did not investigate spillover or exposure to zoonotic viruses in the at-risk human community at the time of publication. To more comprehensively characterize zoonotic disease risks at this unique human–animal interface, we have initiated follow-up investigations that assess the presence of viruses in domestic animals, evaluate people for virus presence or exposure, and better determine whether any of the viruses detected in rodents or shrews have spilled over into domestic species or people. To understand the real potential for viral sharing and transmission at this interface, including the new viruses we detected, longitudinal and concurrent surveillance of people, wildlife, and domestic animals will be important. In addition, while emphasizing the importance of surveillance for emerging viral threats, future studies should also seek to incorporate screening for well-recognized zoonotic bacteria and parasites in order to provide a more comprehensive zoonotic risk assessment and inform risk mitigation and prevention strategies specific to these communities.
While we engaged these communities and promoted health education and disease awareness throughout the duration of the study, significant investments are required to address the unique health security challenges that these urban and peri-urban interfaces present. Moving One Health beyond the ecological and biological domain to include contributions from urban planners and the social sciences is perhaps the best path to address the considerable challenges in managing rapid and unorganized growth while promoting health in these riverside settlement communities. Our data suggest that there is considerable lack of awareness among community members that diseases can be transmitted from animals, and our surveys revealed a clear correlation between occupation and wildlife contact and, therefore, potential for exposure to zoonoses. Our study identified conditions that place informal settlements at risk for transmission of zoonotic diseases. This specific human–wildlife interface should be addressed by planners and the health community, and we recommend the development of policies and interventions that carefully consider the social structures within these settlements, especially among the most at-risk groups (women, children, and those with high-risk occupations). In addition, we encourage the engagement of community members together with government officials to develop and implement effective solutions that adequately address the multitude of environmental, ecological, and social challenges that life on the river margins presents.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This study was made possible by the generous support of the American people through the United States Agency for International Development (USAID) Emerging Pandemic Threats PREDICT project (cooperative agreement number GHN-A-OO-09-00010-00). Additionally, we would like to thank the Government of Nepal’s Department of National Parks and Wildlife Conservation (Ministry of Forest and Soil Conservation), Department of Livestock Services (Ministry of Livestock Development), Epidemiology and Disease Control Division (Ministry of Health & Population), and Nepal Health Research Council for giving us valuable guidance and permissions to conduct this study. We would like to thank the field team from the Center for Molecular Dynamics Nepal, the laboratory team at UC Davis, and to express our deep appreciation for the cooperation and participation of the informal settlement communities of the Kathmandu Valley.
References
- Aitichou M, Saleh SS, McElroy AK, Schmaljohn C, Ibrahim MS. Identification of Dobrava, Hantaan, Seoul, and Puumala viruses by one-step real-time RT-PCR. Journal of Virological Methods. 2005;124:21–26. doi: 10.1016/j.jviromet.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Alirol E, Getaz L, Stoll B, Chappuis F, Loutan L. Urbanisation and infectious diseases in a globalised world. The Lancet infectious diseases. 2011;11:131–141. doi: 10.1016/S1473-3099(10)70223-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony S, Leger JS, Pugliares K, Ip H, Chan J, Carpenter Z, Navarrete-Macias I, Sanchez-Leon M, Saliki J, Pedersen J. Emergence of fatal avian influenza in New England harbor seals. MBio. 2012;3:e00112–e00166. doi: 10.1128/mBio.00166-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony SJ, Islam A, Johnson C, Navarrete-Macias I, Liang E, Jain K, Hitchens PL, Che X, Soloyvov A, Hicks AL, Ojeda-Flores R, Zambrana-Torrelio C, Ulrich W, Rostal MK, Petrosov A, Garcia J, Haider N, Wolfe N, Goldstein T, Morse SS, et al. Non-random patterns in viral diversity. Nature communications. 2015;6:8147. doi: 10.1038/ncomms9147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony SJ, Johnson CK, Greig DJ, Kramer S, Che X, Wells H, Hicks AL, Joly DO, Wolfe ND, Daszak P, Karesh W, Lipkin WI, Morse SS, PREDICT Consortium, Mazet JAK, Goldstein T Global patterns in coronavirus diversity. Virus Evol. 2017;3:1–15. doi: 10.1093/ve/vex012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker S, Favorov M, Dougan G. Searching for the elusive typhoid diagnostic. BMC infectious diseases. 2010;10:45. doi: 10.1186/1471-2334-10-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baral HS, Shah K. Wild mammals of Nepal. Nepal: Himalayan Nature; 2008. [Google Scholar]
- Blasdell K, Cosson JF, Chaval Y, Herbreteau V, Douangboupha B, Jittapalapong S, Lundqvist A, Hugot J-P, Morand S, Buchy P. Rodent-Borne Hantaviruses in Cambodia, Lao PDR, and Thailand. EcoHealth. 2011;8:432–443. doi: 10.1007/s10393-011-0725-7. [DOI] [PubMed] [Google Scholar]
- Blasdell K, Bordes F, Chaisiri K, Chaval Y, Claude J, Cosson J-F, Latinne A, Michaux J, Morand S, Pagès M, Tran A. Progress on research on rodents and rodent-borne zoonoses in South-east Asia. Wildl Res. 2015;42:98–107. doi: 10.1071/WR14201. [DOI] [Google Scholar]
- Blasdell K, Morand S, Henttonen H, Tran A, Buchy P. Hantavirus seropositivity in rodents in relation to habitat heterogeneity in human-shaped landscapes of Southeast Asia. Spat Spatio-Temporal Epidemiol. 2016;17:27–35. doi: 10.1016/j.sste.2016.04.002. [DOI] [PubMed] [Google Scholar]
- Bordes F, Herbreteau V, Dupuy S, Chaval Y, Tran A, Morand S (2013) The diversity of microparasites of rodents: a comparative analysis that helps in identifying rodent-borne rich habitats in Southeast Asia. Infect Ecol Epidemiol 3 [DOI] [PMC free article] [PubMed]
- Bourhy H, Cowley J, Larrous F, Holmes E, Walker P. Phylogenetic relationships among rhabdoviruses inferred using the L polymerase gene. Journal of General Virology. 2005;86:2849–2858. doi: 10.1099/vir.0.81128-0. [DOI] [PubMed] [Google Scholar]
- Bradley CA, Altizer S. Urbanization and the ecology of wildlife diseases. Trends in Ecology & Evolution. 2007;22:95–102. doi: 10.1016/j.tree.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briese T, Kapoor V, Lipkin W. Natural M-segment reassortment in Potosi and Main Drain viruses: implications for the evolution of orthobunyaviruses. Archives of virology. 2007;152:2237–2247. doi: 10.1007/s00705-007-1069-z. [DOI] [PubMed] [Google Scholar]
- Burrell CJ, Howard CR, Murphy FA. Chapter 29 - Bunyaviruses. In: Burrell CJ, Howard CR, Murphy FA, editors. Fenner and White’s Medical Virology. 5. London: Academic Press; 2017. pp. 407–424. [Google Scholar]
- Burrell CJ, Howard CR, Murphy FA. Chapter 31 - Coronaviruses. In: Burrell CJ, Howard CR, Murphy FA, editors. Fenner and White’s Medical Virology. 5. London: Academic Press; 2017. pp. 437–446. [Google Scholar]
- Carey DE, Reuben R, Panicker KN, Shope RE, Myers RM. Thottapalayam virus: a presumptive arbovirus isolated from a shrew in India. Indian J Med Res. 1971;59:1758–1760. [PubMed] [Google Scholar]
- Chang L, Yan Y, Wang L (2020) Coronavirus Disease 2019: Coronaviruses and Blood Safety. Transfus Med Rev [DOI] [PMC free article] [PubMed]
- Cohen B. Urban growth in developing countries: a review of current trends and a caution regarding existing forecasts. World development. 2004;32:23–51. [Google Scholar]
- Cohen B. Urbanization in developing countries: Current trends, future projections, and key challenges for sustainability. Technology in society. 2006;28:63–80. [Google Scholar]
- Corburn J, Riley L. Slum health: from the cell to the street. Oakland, California: Univ of California Press; 2016. [Google Scholar]
- Corman VM, Muth D, Niemeyer D, Drosten C (2018) Chapter Eight - Hosts and Sources of Endemic Human Coronaviruses. In: Kielian M, Mettenleiter TC, Roossinck MJ (eds) Advances in Virus Research. Academic Press, pp 163–188 [DOI] [PMC free article] [PubMed]
- Darwish MA, Hoogstraal H, Roberts TJ, Ahmed IP, Omar F. A sero-epidemiological survey for certain arboviruses (Togaviridae) in Pakistan. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1983;77:442–445. doi: 10.1016/0035-9203(83)90106-2. [DOI] [PubMed] [Google Scholar]
- Darwish MA, Hoogstraal H, Roberts TJ, Ghazi R, Amer T. A sero-epidemiological survey for Bunyaviridae and certain other arboviruses in Pakistan. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1983;77:446–450. doi: 10.1016/0035-9203(83)90108-6. [DOI] [PubMed] [Google Scholar]
- Davis S, Calvet E, Leirs H. Fluctuating Rodent Populations and Risk to Humans from Rodent-Borne Zoonoses. Vector-Borne Zoonotic Dis. 2005;5:305–314. doi: 10.1089/vbz.2005.5.305. [DOI] [PubMed] [Google Scholar]
- Devleesschauwer B, Ale A, Torgerson P, Praet N, de Noordhout CM, Pandey BD, Pun SB, Lake R, Vercruysse J, Joshi DD. The burden of parasitic zoonoses in Nepal: a systematic review. PLoS Negl Trop Dis. 2014;8:e2634. doi: 10.1371/journal.pntd.0002634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devleesschauwer B, Pruvot M, Joshi DD, De Craeye S, Jennes M, Ale A, Welinski A, Lama S, Aryal A, Victor B. Seroprevalence of zoonotic parasites in pigs slaughtered in the Kathmandu Valley of Nepal. Vector-Borne and Zoonotic Diseases. 2013;13:872–876. doi: 10.1089/vbz.2013.1313. [DOI] [PubMed] [Google Scholar]
- Dikid T, Jain S, Sharma A, Kumar A, Narain J. Emerging & re-emerging infections in India: an overview. The Indian journal of medical research. 2013;138:19. [PMC free article] [PubMed] [Google Scholar]
- Dye C. Health and urban living. Science. 2008;319:766–769. doi: 10.1126/science.1150198. [DOI] [PubMed] [Google Scholar]
- Eckert S, Kohler S. Urbanization and health in developing countries: a systematic review. World Health Popul. 2014;15:7–20. doi: 10.12927/whp.2014.23722. [DOI] [PubMed] [Google Scholar]
- Fehr AR, Perlman S (2015) Coronaviruses: an overview of their replication and pathogenesis. In: Coronaviruses. Springer, pp 1–23 [DOI] [PMC free article] [PubMed]
- Gatherer D. The 2014 Ebola virus disease outbreak in West Africa. J Gen Virol. 2014;95:1619–1624. doi: 10.1099/vir.0.067199-0. [DOI] [PubMed] [Google Scholar]
- Gubler DJ. Dengue, urbanization and globalization: the unholy trinity of the 21st century. Trop. Med. Health. 2011;39:S3–S11. doi: 10.2149/tmh.2011-S05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta ML, Sharma A. Pneumonic plague, northern India, 2002. Emerging infectious diseases. 2007;13:664. doi: 10.3201/eid1304.051105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han BA, Kramer AM, Drake JM. Global patterns of zoonotic disease in mammals. Trends in Parasitology. 2016;32:565–577. doi: 10.1016/j.pt.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin JW, Hardin JW, Hilbe JM, Hilbe J (2007) Generalized linear models and extensions. Stata press
- Hu B, Ge X, Wang L-F, Shi Z. Bat origin of human coronaviruses. Virololgy Journal. 2015;12:221. doi: 10.1186/s12985-015-0422-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugh-Jones ME, Hubbert WT, Hagstad HV (2008) Zoonoses: recognition, control, and prevention. John Wiley & Sons,
- Johnson CK, Hitchens PL, Evans TS, Goldstein T, Thomas K, Clements A, Joly DO, Wolfe ND, Daszak P, Karesh WB. Spillover and pandemic properties of zoonotic viruses with high host plasticity. Scientific reports. 2015;5:14830. doi: 10.1038/srep14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jassat W, Naicker N, Naidoo S, Mathee A. Rodent control in urban communities in Johannesburg, South Africa: From research to action. Int J Environ Health Res. 2013;23:474–483. doi: 10.1080/09603123.2012.755156. [DOI] [PubMed] [Google Scholar]
- Ishtiaque A, Shrestha M, Chhetri N. Rapid Urban Growth in the Kathmandu Valley, Nepal: Monitoring Land Use Land Cover Dynamics of a Himalayan City with Landsat Imageries. Environments. 2017;4:72. [Google Scholar]
- Kang HJ, Kosoy MY, Shrestha SK, Shrestha MP, Pavlin JA, Gibbons RV, Yanagihara R. Genetic Diversity of Thottapalayam Virus, a Hantavirus Harbored by the Asian House Shrew (Suncus murinus) in Nepal. Am J Trop Med Hyg. 2011;85:540–545. doi: 10.4269/ajtmh.2011.11-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karesh WB, Dobson A, Lloyd-Smith JO, Lubroth J, Dixon MA, Bennett M, Aldrich S, Harrington T, Formenty P, Loh EH. Ecology of zoonoses: natural and unnatural histories. The Lancet. 2012;380:1936–1945. doi: 10.1016/S0140-6736(12)61678-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkey A, Aryjal A, Basnyat B, Baker S. Kathmandu, Nepal: still an enteric fever capital of the world. The Journal of Infection in Developing Countries. 2008;2:461–465. doi: 10.3855/jidc.162. [DOI] [PubMed] [Google Scholar]
- Laenen L, Vergote V, Calisher CH, Klempa B, Klingström J, Kuhn JH, Maes P (2019) Hantaviridae: Current Classification and Future Perspectives. Viruses 11 [DOI] [PMC free article] [PubMed]
- Lederberg J, Hamburg MA, Smolinski MS (2003) Microbial threats to health: emergence, detection, and response. National Academies Press [PubMed]
- Li W, Shi Z, Yu M, Ren W, Smith C, Epstein JH, Wang H, Crameri G, Hu Z, Zhang H, Zhang J, McEachern J, Field H, Daszak P, Eaton BT, Zhang S, Wang L-F. Bats Are Natural Reservoirs of SARS-Like Coronaviruses. Science. 2005;310:676. doi: 10.1126/science.1118391. [DOI] [PubMed] [Google Scholar]
- Long SJ, Freese J (2006) Regression models for categorical dependent variables using Stata. Stata press
- Lozano M, Posik D, Albarino C, Schujman G, Ghiringhelli P, Calderon G, Sabattini M, Romanowski V. Characterization of arenaviruses using a family-specific primer set for RT-PCR amplification and RFLP analysis: its potential use for detection of uncharacterized arenaviruses. Virus research. 1997;49:79–89. doi: 10.1016/s0168-1702(97)01458-5. [DOI] [PubMed] [Google Scholar]
- Lumanti (2008) Status of squatter communities along the Bagmati River and its tributaries in the Kathmandu Valley. Kathmandu: (LSGS) LSGfS
- Mackenstedt U, Jenkins D, Romig T. The role of wildlife in the transmission of parasitic zoonoses in peri-urban and urban areas. International Journal for Parasitology: Parasites and Wildlife. 2015;4:71–79. doi: 10.1016/j.ijppaw.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLachlan NJ, Dubovi EJ (eds) (2011) Chapter 17—Paramyxoviridae. In: Fenner’s Veterinary Virology (Fourth Edition). Academic Press, San Diego, pp 299–325
- MacLachlan, N.J., Dubovi, E.J. (Eds.), 2017. Chapter 18 - Rhabdoviridae, in: Fenner’s Veterinary Virology (Fifth Edition). Academic Press, Boston, pp. 357–372.
- Marx B, Stoker T, Suri T. The economics of slums in the developing world. Journal of Economic Perspectives. 2013;27:187–210. [Google Scholar]
- Meerburg BG, Singleton GR, Kijlstra A. Rodent-borne diseases and their risks for public health. Critical Reviews in Microbiology. 2009;35:221–270. doi: 10.1080/10408410902989837. [DOI] [PubMed] [Google Scholar]
- Mehla R, Kumar SR, Yadav P, Barde PV, Yergolkar PN, Erickson BR, Carroll SA, Mishra AC, Nichol ST, Mourya DT. Recent ancestry of Kyasanur Forest disease virus. Emerging infectious diseases. 2009;15:1431. doi: 10.3201/eid1509.080759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morand S, Blasdell K, Bordes F, Buchy P, Carcy B, Chaisiri K, Chaval Y, Claude J, Cosson J-F, Desquesnes M, Jittapalapong S, Jiyipong T, Karnchanabanthoen A, Pornpan P, Rolain J-M, Tran A. Changing landscapes of Southeast Asia and rodent-borne diseases: decreased diversity but increased transmission risks. Ecol Appl. 2019;29:e01886. doi: 10.1002/eap.1886. [DOI] [PubMed] [Google Scholar]
- Moureau G, Temmam S, Gonzalez J, Charrel R, Grard G, De Lamballerie X. A real-time RT-PCR method for the universal detection and identification of flaviviruses. Vector-Borne and Zoonotic Diseases. 2007;7:467–478. doi: 10.1089/vbz.2007.0206. [DOI] [PubMed] [Google Scholar]
- Murdoch DR, Woods CW, Zimmerman MD, Dull PM, Belbase RH, Keenan AJ, Scott RM, Basnyat B, Archibald LK, Reller LB. The etiology of febrile illness in adults presenting to Patan hospital in Kathmandu. Nepal. The American journal of tropical medicine and hygiene. 2004;70:670–675. [PubMed] [Google Scholar]
- Muzzini E, Aparicio G. Urban Growth and Spatial Transition in Nepal: An Initial Assessment. Washington, DC: World Bank; 2013. [Google Scholar]
- Nagate T, Chino T, Nishiyama C, Okuhara D, Tahara T, Maruyama Y, Kasahara H, Takashima K, Kobayashi S, Motokawa Y. Diluted isoflurane as a suitable alternative for diethyl ether for rat anaesthesia in regular toxicology studies. Journal of Veterinary Medical Science. 2007;69:1137–1143. doi: 10.1292/jvms.69.1137. [DOI] [PubMed] [Google Scholar]
- Neiderud C-J (2015) How urbanization affects the epidemiology of emerging infectious diseases. Infection ecology & epidemiology 5 [DOI] [PMC free article] [PubMed]
- Qiagen (2006) DNeasy® Blood & Tissue Handbook. In: Qiagen (ed) Qiagen.com
- Quan P-L, Firth C, Street C, Henriquez JA, Petrosov A, Tashmukhamedova A, Hutchison SK, Egholm M, Osinubi MO, Niezgoda M. Identification of a severe acute respiratory syndrome coronavirus-like virus in a leaf-nosed bat in Nigeria. MBio. 2010;1:e00208–e00210. doi: 10.1128/mBio.00208-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raboni SM, Rubio G, De Borba L, Zeferino A, Skraba I, Goldenberg S, Dos Santos CND. Clinical survey of hantavirus in southern Brazil and the development of specific molecular diagnosis tools. The American journal of tropical medicine and hygiene. 2005;72:800–804. [PubMed] [Google Scholar]
- Redman CL, Jones NS. The environmental, social, and health dimensions of urban expansion. Population and environment. 2005;26:505–520. [Google Scholar]
- Rohilla SK. Defining Peri-Urban: A Review. In: Dupont V, editor. Peri-Urban Dynamics: Population, Habitat, and Environment on the Peripheries of Large Indian Metropolises. Centre de Sciences Humaines: A Review Concepts and General Issues, New Delhi; 2005. [Google Scholar]
- Root JJ. West Nile virus associations in wild mammals: a synthesis. Archives of virology. 2013;158:735–752. doi: 10.1007/s00705-012-1516-3. [DOI] [PubMed] [Google Scholar]
- Rydin Y, Bleahu A, Davies M, Dávila JD, Friel S, De Grandis G, Groce N, Hallal PC, Hamilton I, Howden-Chapman P. Shaping cities for health: complexity and the planning of urban environments in the 21st century. Lancet. 2012;379:2079. doi: 10.1016/S0140-6736(12)60435-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Seco M, Rosario D, Domingo C, Hernández L, Valdés K, Guzmán M, Tenorio A. Generic RT-nested-PCR for detection of flaviviruses using degenerated primers and internal control followed by sequencing for specific identification. Journal of Virological Methods. 2005;126:101–109. doi: 10.1016/j.jviromet.2005.01.025. [DOI] [PubMed] [Google Scholar]
- Sánchez-Seco MP, Rosario D, Quiroz E, Guzmán G, Tenorio A. A generic nested-RT-PCR followed by sequencing for detection and identification of members of the alphavirus genus. Journal of Virological Methods. 2001;95:153–161. doi: 10.1016/s0166-0934(01)00306-8. [DOI] [PubMed] [Google Scholar]
- Schmidt TR, Gold JR. Complete sequence of the mitochondrial cytochrome b gene in the cherryfin shiner, Lythrurus roseipinnis (Teleostei: Cyprinidae) Copeia. 1993;1993:880–883. [Google Scholar]
- Snowden FM. Emerging and reemerging diseases: a historical perspective. Immunological reviews. 2008;225:9–26. doi: 10.1111/j.1600-065X.2008.00677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J-W, Baek LJ, Schmaljohn CS, Yanagihara R. Thottapalayam virus, a prototype shrewborne hantavirus. Emerging infectious diseases. 2007;13:980. doi: 10.3201/eid1307.070031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songsore J (2004) Urbanization and health in Africa: exploring the interconnections between poverty, inequality, and the burden of disease. Ghana Universities Press Accra
- Taylor LH, Latham SM, Mark E. Risk factors for human disease emergence. Philosophical Transactions of the Royal Society of London B: Biological Sciences. 2001;356:983–989. doi: 10.1098/rstb.2001.0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor PJ, Arntzen L, Hayter M, Iles M, Frean J, Belmain S. Understanding and managing sanitary risks due to rodent zoonoses in an African city: beyond the Boston Model. Integrative Zoology. 2008;3:38–50. doi: 10.1111/j.1749-4877.2008.00072.x. [DOI] [PubMed] [Google Scholar]
- Thibault PA, Watkinson RE, Moreira-Soto A, Drexler JF, Lee B. Zoonotic Potential of Emerging Paramyxoviruses: Knowns and Unknowns. Advances in Virus Research. 2017;98:1–55. doi: 10.1016/bs.aivir.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong S, Chern S-WW, Li Y, Pallansch MA, Anderson LJ. Sensitive and broadly reactive reverse transcription-PCR assays to detect novel paramyxoviruses. Journal of Clinical Microbiology. 2008;46:2652–2658. doi: 10.1128/JCM.00192-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations (2014) More than half of world’s population now living in urban areas, United Nation survey finds. UN News. Available online at: http://www.un.org/apps/news/story.asp?NewsID=48240#.WP3KUzolHDc. [Accessed April 21, 2017]
- Van Cuong N, Carrique-Mas J, Vo Be H, An NN, Tue NT, Anh NL, Anh PH, Phuc NT, Baker S, Voutilainen L, Jääskeläinen A, Huhtamo E, Utriainen M, Sironen T, Vaheri A, Henttonen H, Vapalahti O, Chaval Y, Morand S, Bryant JE. Rodents and Risk in the Mekong Delta of Vietnam: Seroprevalence of Selected Zoonotic Viruses in Rodents and Humans. Vector Borne Zoonotic Dis. 2015;15:65–72. doi: 10.1089/vbz.2014.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayachari P, Sugunan A, Shriram A. Leptospirosis: an emerging global public health problem. Journal of Biosciences. 2008;33:557–569. doi: 10.1007/s12038-008-0074-z. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Masangkay JS, Nagata N, Morikawa S, Mizutani T, Fukushi S, Alviola P, Omatsu T, Ueda N, Iha K. Bat coronaviruses and experimental infection of bats, the Philippines. Emerging infectious diseases. 2010;16:1217. doi: 10.3201/eid1608.100208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss SR, Navas-Martin S. Coronavirus pathogenesis and the emerging pathogen severe acute respiratory syndrome coronavirus. Microbiol Mol Biol Rev. 2005;69:635–664. doi: 10.1128/MMBR.69.4.635-664.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe ND, Dunavan CP, Diamond J. Origins of major human infectious diseases. Nature. 2007;447:279. doi: 10.1038/nature05775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeltina A, Bowden TA, Lee B. Emerging Paramyxoviruses: Receptor Tropism and Zoonotic Potential. PLOS Pathogens. 2016;12(2):e1005390. doi: 10.1371/journal.ppat.1005390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P, Yang X-L, Wang X-G, Hu B, Zhang L, Zhang W, Si H-R, Zhu Y, Li B, Huang C-L, Chen H-D, Chen J, Luo Y, Guo H, Jiang R-D, Liu M-Q, Chen Y, Shen X-R, Wang X, Zheng X-S, Zhao K, Chen Q-J, Deng F, Liu L-L, Yan B, Zhan F-X, Wang Y-Y, Xiao G-F, Shi Z-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.