Colonoscopy is performed routinely for colorectal cancer (CRC) screening, follow-up of other abnormal screening tests, workup of signs and symptoms of gastrointestinal disease, and surveillance after CRC and polyp removal. Post procedure, colonoscopists are expected to provide follow-up recommendations to patients and referring physicians. Recommendations for follow-up after normal colonoscopy among individuals age-eligible for screening, and post-polypectomy among all individuals with polyps are among the most common clinical scenarios requiring guidance.1
Risk of metachronous advanced neoplasia is associated with findings on prior colonoscopy. After high-quality colonoscopy, patients with no neoplasia detected are at the lowest risk, and those with polyps are risk-stratified based on the histology, number, location, and size of polyps detected. Since the release of the last US Multi-Society Task Force (Task Force) recommendations for post-colonoscopy follow-up and polyp surveillance in 2012,2 a number of articles have been published on risk of CRC based on colonoscopy findings and patient characteristics, as well as the potential impact of screening and surveillance colonoscopy on outcomes, such as incident CRC and polyps. Further, recent studies increasingly reflect the modern era of colonoscopy with more awareness of the importance of quality factors (eg, adequate bowel preparation, cecal intubation, adequate adenoma detection, and complete polyp resection), and utilization of state of the art technologies (eg, high-definition colonoscopes). Higher-quality colonoscopy could impact the importance of previously identified risk factors. Our aim was to review newly available evidence and update recommendations for follow-up after colonoscopy with or without polypectomy.
Methods
Evidence Review and Recommendation Development
To identify issues of greatest importance for the current revision, we developed PICO (patient, intervention, comparison, and outcome) questions (Supplementary Appendix A [SG and DL, with input from TK]). In consultation with a certified medical librarian (KH), literature searches were performed in PubMed, Embase, and CINAHL with a combination of controlled vocabulary and keyword terms for colonoscopy, polyps, and polypectomy surveillance (see Supplementary Appendix B for search terms). English-language articles since January 1, 2012 were retrieved. Searches were run on March 30, 2017, and identified a total of 1904 unique articles (see Supplementary Appendix C for article selection flow).
Criteria used for inclusion/exclusion of titles, abstracts, and articles are outlined in Table 1. All titles were reviewed by a single author (SG) and potentially relevant titles were selected for abstract review. All abstracts were reviewed by 2 authors (SG and DL) and potentially relevant abstracts were selected for full article review. Included articles were reviewed in detail by the same 2 authors. The final list of articles selected for review was supplemented by repeating the literature search through September 2018 to identify articles published since the time of the literature search, as well as through opportunistic identification of additional relevant articles. References directly relevant to final recommendations were identified through joint consensus (SG and DL). Based on prior findings and the current literature review, post-colonoscopy management recommendations were developed by 2 authors (SG and DL) and refined through consensus discussion with all authors after circulating both draft recommendations and a table summarizing key findings of articles that were included for article review. For each recommendation, the quality of evidence (Table 2) and strength of recommendation were rated using our previously described approach.3 Strong recommendations mean that most informed patients would choose the recommended management and that clinicians can structure their interactions with patients accordingly. Weak recommendations mean that patients’ choices will vary according to their values and preferences, and clinicians must ensure that patients’ care is in keeping with their values and preferences.
Table 1.
Criteria for Inclusion/Exclusion of Titles, Abstracts, and Articles
| Review phase (reviewer) | Inclusion/exclusion criteria |
|---|---|
| Title (SG) | Goal: Identify article(s) that might examine the relationship between baseline colonoscopy examination and subsequent neoplasia on follow-up |
Exclusion criteria
| |
| Abstract (SG and DL) | Goal: Identify article(s) that might examine relationship between the baseline colonoscopy examination and subsequent neoplasia on follow-up |
Exclusion Criteria
| |
| Article (SG and DL) | Goal: Identify article(s) that might examine relationship between baseline colonoscopy examination and subsequent neoplasia on follow-up, relevant to PICO questions |
Inclusion criteria
| |
Exclusion criteriaa
|
PICO, patient, intervention, comparison, and outcome.
Some articles excluded from main summary are included in Discussion as references.
Table 2.
Grading of Recommendations Assessment, Development, and Evaluation Ratings of Evidence
| Rating of evidence | Definition |
|---|---|
| A: High quality | Further research is very unlikely to change our confidence in the estimate of effect |
| B: Moderate quality | Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate |
| C: Low quality | Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate |
| D: Very low quality | Any estimate of effect is very uncertain |
This article does not include recommendations for follow-up for individuals with hereditary CRC syndromes (eg, Lynch syndrome and familial adenomatous polyposis), inflammatory bowel disease, a personal history of CRC (including malignant polyps), family history of CRC or colorectal neoplasia, or serrated polyposis syndrome. As such, our recommendations for follow-up after colonoscopy and polypectomy do not apply to these groups except in cases where polyp findings would result in a shorter colonoscopy interval than indicated based on the status of these clinical conditions. Further, recommendations for polypectomy technique were outside the scope of this article. Notably, the Task Force has recently issued recommendations for follow-up colonoscopy for individuals with Lynch syndrome4 and a personal history of CRC.3,5,6 Recommendations for follow-up of serrated polyposis syndrome, management of patients with a malignant polyp, as well as optimal polypectomy technique will be covered in subsequent Task Force recommendations.
Report Format
The primary goals of colonoscopy screening and post-polypectomy surveillance are to reduce CRC incidence and mortality. We provide a review of the available evidence on the impact of surveillance on these outcomes. Next, we provide recommendations for follow-up strategies, with a summary of new evidence, including an overall assessment of the quality of evidence and strength of recommendations. This is followed by a summary of key limitations of existing evidence, future research opportunities, and best practices for research in the field. Given the large amount of data on post-colonoscopy follow-up, we focus primarily on new publications since the Task Force recommendations in 2012.
Terms, Definitions, and Colonoscopy Quality Assumptions
Polyp terms and definitions.
The polyp surveillance literature varies in terms used for predictors and outcomes and associated definitions (Table 3). In this report, normal colonoscopy refers to a colonoscopy where no adenoma, sessile serrated adenoma/polyp or sessile serrated polyp (SSP), hyperplastic polyp (HP) ≥10 mm, traditional serrated adenoma (TSA), or CRC was found. We consider individuals with only HP <10 mm as having had normal colonoscopy. To summarize prior evidence, “low-risk adenoma” refers to having 1–2 tubular adenomas with low-grade dysplasia, each <10 mm in size. There are 2 higher-risk categories commonly described in the published literature, one based on size and histology (advanced neoplasia), and the other based on number of adenomas (multiple adenomas). Advanced neoplasia is defined as an adenoma ≥10 mm, adenoma with tubulovillous or villous histology, adenoma with high-grade dysplasia, or presence of invasive cancer. An adenoma with size ≥10 mm, with tubulovillous or villous histology, or with high-grade dysplasia in the absence of invasive CRC is commonly referred to as an advanced adenoma. As part of the definition of villous or tubulovillous histology, we do not quantify the proportion of adenoma with villous features, as this is rarely reported in clinical practice. Also, criteria used to define villous histology are often not reported in studies and, when reported, are often variable. Patients with 3 or more adenomas (often discussed as “multiple adenomas”) have been reported previously to be at an increased risk of metachronous advanced neoplasia and, in many studies, considered as belonging to a high-risk predictor or outcome group. As such, to summarize prior evidence in this report, “high-risk adenoma” refers to patients with advanced neoplasia or ≥3 adenomas. We recognize variability across studies in the use of the term high-risk adenoma, with some using this term as a synonym for advanced neoplasia (Table 3). However, when possible, we will make a distinction between advanced neoplasia and high-risk adenoma because implications of having any advanced neoplasia vs any high-risk adenoma (defined by advanced neoplasia and/or multiple adenomas) on risk for metachronous neoplasia may vary. We recognize that evidence on risks for metachronous neoplasia associated with SSPs and large HPs is evolving. For example, uncertainty exists as to whether HPs ≥10 mm in size represent lesions associated with increased risk. Because evidence of the risk of metachronous neoplasia associated with serrated lesions is evolving, whenever possible we have chosen not to include SSPs and HPs in our definitions of low-risk adenoma, high-risk adenoma, and advanced neoplasia, and will refer to these lesions separately.
Table 3.
Terms and Definitionsa
| Term | Definition |
|---|---|
| Average risk for CRC | Absence of inflammatory bowel disease, family history of CRC, hereditary syndrome associated with increased risk, serrated polyposis syndrome, personal history of CRC |
| Normal colonoscopy | A colonoscopy where no adenoma, SSP, TSA, HP ≥10 mm, or CRC is found |
| Low-risk adenoma | 1–2 nonadvanced adenomas <10 mm in size |
| Advanced adenoma | 1 or more of the following findings:
|
| Advanced neoplasia | 1 or more of the following findings:
|
| High-risk adenoma | 1 or more of the following findings:
|
| Adequate ADR | ADR ≥30% in men and ≥20% in women |
| Adequate bowel preparation | Bowel preparation adequate for visualization of polyps >5 mm in size |
| Complete examination | Complete colonoscopy to cecum, with photo documentation of cecal landmarks, such as the appendiceal orifice, terminal ileum, or ileocecal valve |
| High-quality examination | Examination complete to cecum with adequate bowel preparation performed by colonoscopist with adequate adenoma detection rate and attention to complete polyp excision |
We propose moving forward that rather than using categories such as “high-risk adenoma” or “low-risk adenoma,” that research articles specify the individual criteria being captured by the category (eg, use 1–2 adenomas <10 mm instead of the term low-risk adenoma) because evidence supporting level of risk for various criteria are constantly evolving.
We utilize specific findings (eg, 1–2 adenomas <10 mm) rather than summary categories (low-risk adenoma) to be as precise as possible in our updated scenario-specific recommendations because evidence supporting level of risk for various criteria are constantly evolving, and because prior terminology may be confusing (eg, use of high-risk adenoma to refer to both advanced neoplasia and/or having 3 or more adenomas) and limit precise risk stratification. All recommendations assume the colonoscopist has performed a high-quality examination (Table 3).
Colonoscopy quality assumptions.
For the purposes of this review, we have defined high quality based on colonoscopist performance, such as adequate adenoma detection rate (ADR), and examination-specific characteristics, such as examination complete to cecum, attention to complete polypectomy, and adequate bowel preparation to reliably detect lesions >5 mm. Benchmarks for ADR (ADR >30% in men; >20% in women), proportion of examinations with adequate preparation (>85%), and proportion of examinations complete to cecum (>95%) should be universally and routinely monitored as colonoscopy quality metrics in practice.7 Colonoscopists who are measuring quality metrics, but not meeting them, need to take steps to improve their examination quality and document this improvement. Polyp size is a major factor in our scenario-specific recommendations. Given the importance of polyp size for informing surveillance intervals, documentation of a polyp ≥10 mm within a report should be accompanied by an endoscopic photo of the polyp with comparison to an open snare or open biopsy forceps. Such documentation is important for lesions such as HPs, where small size (<10 mm) is associated with well documented low risk for subsequent advanced neoplasia, but size ≥10 mm may be associated with elevated risk. We define complete polypectomy or complete removal as removal of all visually detected polypoid tissue (regardless of morphology).
Results
Risk for Incident and Fatal Colorectal Cancer After Normal Colonoscopy and After Polyp Removal
Normal colonoscopy is associated with sustained reduced risk for incident and fatal CRC.
(High quality of evidence)
A cohort study of 304,774 individuals with normal colonoscopy vs 980,154 individuals with no lower endoscopy showed a reduced risk for incident CRC on long-term follow-up (hazard ratio [HR], 0.44; 95% confidence interval [CI], 0.38–0.52). The risk was persistently decreased across a range of years since last normal colonoscopy, ranging from an HR of 0.35 for ≤3 years to 0.65 at ≥15 years. Normal colonoscopy was also associated with reduced risk for fatal CRC (HR, 0.32; 95% CI, 0.24–0.45) over 300,000 person-years of follow-up.8 A cohort study comparing 131,349 individuals who had normal colonoscopy to the general population in Utah showed the standardized incidence ratio (SIR) for CRC was 0.26 (95% CI, 0.19–0.32) through 5 years and 0.60 (95% CI, 0.44–0.76) for 7–10 years of follow-up.9 A 70% relative risk (RR) reduction was observed through the 10-year follow-up period (SIR, 0.28; 95% CI, 0.24–0.33). Most recently, a cohort study of 1,251,318 adults at average risk for CRC served by a large health plan in the United States reported a 46% relative reduced risk for incident and a 88% relative reduced risk for fatal CRC among 99,166 who had a normal screening colonoscopy through the traditionally recommended 10-year follow-up period for these individuals (HR, 0.54; 95% CI, 0.31–0.94 for incident and HR, 0.12; 95% CI, 0.02–0.82 for fatal CRC).10 Notably, reduced risk was noted even up to 12 years post-normal screening colonoscopy. A strength of this study was the use of a validated approach to identifying screening colonoscopy procedures. A potential limitation was unmeasured differences between plan members who elected screening colonoscopy vs stool-based testing or sigmoidoscopy, including a potential healthy user bias. A modeling study, informed by age-specific rates of adenoma, advanced adenoma, and CRC observed among 4.3 million individuals who underwent screening colonoscopy, suggested that a normal colonoscopy was associated with a <0.5% 10-year risk of subsequent CRC.11 Since the 2012 review, we could identify no new data on risk of advanced neoplasia associated with small rectosigmoid HPs. Earlier literature has suggested that such patients have a risk of metachronous advanced neoplasia similar to that of patients with a normal examination, and recommendations for 10-year repeat examination remain unchanged.2
Incremental effectiveness of repeat colonoscopy after baseline normal colonoscopy for further reducing CRC incidence and mortality is uncertain.
(Insufficient evidence)
While we found no direct evidence to support the incremental effectiveness of repeat colonoscopy after 10 years, prior modeling studies have suggested that repeat colonoscopy in those with a baseline normal examination does confer additional benefit.12–14 Knudsen et al14 estimated that rescreening after initial normal colonoscopy resulted in a reduction from 31.3 lifetime CRC cases per 1000 persons with no further screening to as low as 7.7 cases per 1000 persons with repeat screening. Based on current available evidence, our recommendation for repeat colonoscopy 10 years after a normal colonoscopy remains unchanged.
Risk for incident and fatal CRC after baseline adenoma removal is uncertain.
(Low quality of evidence)
Four recent studies have shown that individuals with adenoma, despite adenoma removal, may have increased risk for CRC compared to the general population. An Irish cohort study of 6972 patients with adenomas identified between 2000 and 2005 found a 2.9-fold increased risk for incident CRC compared to the general population (SIR, 2.85; 95% CI, 2.61–3.25).15 Annual reported risk of CRC was 0.43% per year, and cumulative rate of CRC was <5% for men, and <3.5% for women with up to 10 years follow-up. This study was limited by lack of information on polyp size in the registry, limited information on type of follow-up patients received, and incomplete colonoscopy at baseline in some individuals. A French cohort study of 5779 patients diagnosed with any adenoma 1990–1999 followed through 2003 found risk of CRC increased 1.3-fold after first adenoma removal compared to the general population (SIR, 1.26; 95% CI, 1.01–1.56).16 Stratifying based on adenoma risk category (advanced adenoma and nonadvanced adenoma) showed baseline advanced adenoma was associated with a 2.2-fold increased CRC risk compared to the general population (SIR, 2.23; 95% CI, 1.67–2.92), while baseline nonadvanced adenoma was associated with reduced CRC risk (SIR, 0.68; 95% CI, 0.44–0.99). The 10-year cumulative probability of CRC in patients with advanced adenomas was 2.05% (95% CI, 1.14%–3.64%) with and 6.22% (95% CI, 4.26%–9.02%) without exposure to subsequent surveillance colonoscopy. A Norwegian cohort study of 40,826 patients with adenomas removed during years 1993–2007 and followed through 2011 found risk for fatal CRC was similar compared to the general population.17 Risk was decreased by 25% for those with low-risk adenoma (defined by single adenoma without advanced histology; standardized mortality ratio, 0.75; 95% CI, 0.63–0.88], but increased 1.2-fold for those with high-risk adenoma (defined by ≥2 adenomas, villous histology, or high-grade dysplasia; standardized mortality ratio, 1.16; 95% CI, 1.02–1.31). A limitation of this analysis was the inability to account for polyp size in the definition of high-risk adenoma. Among 15,935 participants in a US trial of sigmoidoscopy screening who completed subsequent colonoscopy, compared to those with no adenoma, the risk for incident and fatal CRC was increased among participants with advanced adenoma (RR, 2.7; 95% CI, 1.9–3.7 for incident; RR, 2.6; 95% CI, 1.2–5.7 for fatal), but similar among participants with nonadvanced adenoma (RR, 1.2; 95% CI, 0.8–1.7 for incident CRC and RR, 1.2; 95% CI, 0.5–2.7 for fatal CRC).18 Notably, 11.3% of the nonadvanced adenoma group had 3 or more adenomas, while 88.7% had 1–2 adenomas; none had villous features or high-grade dysplasia, and all were <10 mm. At median of 12.9 years follow-up, cumulative CRC incidence was 2.9% for the advanced adenoma group, 1.4% for the nonadvanced adenoma group, and 1.2% in the no adenoma group. Caution is warranted in interpreting the incident CRC outcomes for the nonadvanced vs no adenoma groups, as the nonadvanced group had greater exposure to subsequent colonoscopy follow-up, perhaps introducing detection bias; cumulative colonoscopy exposure after baseline examination was 53.0% vs 36.9% at 5 years and 78.1% vs 69.9% at 9 years follow-up for the nonadvanced vs no adenoma groups, respectively.
Surveillance colonoscopy after baseline removal of adenoma with high-risk features (eg, size ≥10 mm) may reduce risk for incident CRC, but impact on fatal CRC is uncertain.
(Low quality of evidence)
Incremental impact of surveillance colonoscopy after baseline removal of adenoma with low-risk features (such as 1–2 adenomas <10 mm) on risk for incident and fatal CRC is uncertain.
(Low quality of evidence)
Little prior research has examined the incremental benefit of surveillance (compared to no surveillance) colonoscopy on CRC risk after baseline polypectomy. Since the last review, 2 studies provided some evidence that surveillance may reduce CRC risk. A cohort study of 11,944 patients with intermediate-risk adenoma compared risk for incident CRC among patients exposed vs unexposed to surveillance colonoscopy, as well as for the entire group compared to the general UK population.19 Intermediate risk was based on UK polyp risk stratification guidelines, defined as having 1–2 adenomas ≥10 mm or 3–4 adenomas <10 mm in size; both of these groups would have been classified as high risk per 2012 Task Force guidelines. At median of 7.9 years follow-up, 42% did not receive surveillance colonoscopy. Exposure to 1 or 2 surveillance examinations was associated with a 43%–48% relative reduction in incident CRC risk (adjusted HR, 0.57 for 1 examination; 95% CI, 0.40–0.80 and HR, 0.52 for 2 examinations; 95% CI, 0.31–0.84). Risk for incident CRC was independently associated with increasing age, adenoma ≥20 mm in size, adenoma with high-grade dysplasia, proximal adenoma, incomplete baseline examination, and poor bowel preparation. The absolute risk for incident CRC was 2.3% with vs 2.7% without 1 surveillance examination. In a higher-risk group defined by having incomplete colonoscopy, poor preparation, high-grade dysplasia, proximal adenoma, or adenoma ≥20 mm, the absolute rate of incident CRC was 2.8% with vs 3.3% without a surveillance examination, corresponding to a statistically significant reduced CRC risk for exposure to surveillance for this higher-risk group (HR, 0.52; 95% CI, 0.36–0.75). Among individuals not meeting the criteria for the higher-risk group, the absolute rate of incident CRC among individuals exposed vs unexposed to at least 1 surveillance examination was 0.7% vs 1.1%, and associated with a nonstatistically significant reduced CRC risk (HR, 0.54; 95% CI, 0.20–1.43). Limitations of this study are that only patients with intermediate-risk adenomas were included, and that mortality was not assessed. In summary, this study demonstrates that surveillance colonoscopy, within a group of patients with 1–2 adenomas ≥10 mm or 3–4 adenomas <10 mm in size may reduce risk for incident CRC, particularly among those with baseline incomplete colonoscopy, poor preparation, high-grade dysplasia, adenoma ≥20 mm, and/or proximal adenoma. In patients without these findings, exposure to surveillance afforded no statistically significant observed reduction in risk for incident CRC. The previously mentioned French cohort study of 5779 patients with adenoma also reported on the impact of exposure to surveillance. Exposure to follow-up colonoscopy had a marked effect on risk of CRC, especially in patients with an advanced adenoma. The risk fell to that found within the general population if patients with an advanced adenoma had at least 1 follow-up colonoscopy (SIR, 1.10; 95% CI, 0.62–1.82), while this risk was more than 4 times higher in patients without follow-up colonoscopy (SIR, 4.26; 95% CI, 2.89–6.04).16
Taken together, new evidence suggests that adenoma-bearing patients with identifiable high-risk characteristics remain at increased risk for CRC in the absence of surveillance,17 and that exposure to surveillance is associated with reduced risk for some high-risk groups defined by baseline low quality of examination or polyp characteristics. Further, new evidence suggests that most adenoma patients (such as those with 1–2 small adenomas) are at lower than average risk for subsequent CRC than the general population after baseline polypectomy. The incremental benefit of subsequent surveillance is uncertain for all patients with polyps, but benefit among patients with higher-risk features (size ≥20 mm) is suggested by 2 studies. These studies highlight the importance of additional research to identify patients most likely to benefit from surveillance, and careful clinical management pending further clarification of which patients are at highest risk, and which strategies will be most effective for reducing risk. Limitations of prior studies include retrospective nature and subsequent inability to control for confounding factors that could be associated with CRC risk and likelihood of participation in surveillance, such as proclivity toward healthy behaviors and following medical recommendations for follow-up.
Risk for incident and fatal CRC among individuals with baseline SSP is uncertain.
(Very low quality of evidence)
In a Danish case-control study of 2045 CRC cases compared to 8105 CRC-free controls nested within a cohort of individuals who received colonoscopy between 1977 and 2009, having an SSP was associated with 3-fold increased odds for CRC (odds ratio [OR], 3.07; 95% CI, 2.30–4.10), while having SSP with dysplasia was associated with a nearly 5-fold increased odds for CRC (OR, 4.76; 95% CI, 2.59–8.73) compared to having no polyp.20 A limitation of this study is that it is unclear whether baseline polyps were excised or only biopsied because all SSP patients were identified based on pathology records, but colonoscopy records were not reviewed. A cohort study of patients included in a sigmoidoscopy screening trial compared CRC risk among 81 patients with ≥10 mm serrated lesions (including an SSP, TSA, HP, or unclassi fied serrated lesions) to risk among patients who had a nonadvanced adenoma, normal sigmoidoscopy, or no screening.21 Compared to the group with no screening, a 2.5-fold nonstatistically significant increased risk for incident CRC was observed in individuals with large serrated polyps (HR, 2.5; 95% CI, 0.8–7.8). Compared to the normal sigmoidoscopy group, a 4-fold increased risk for incident CRC was observed in individuals with large serrated polyps (HR, 4.2; 95% CI, 1.3–13.3). Risk for incident CRC for individuals with advanced adenoma at baseline compared to those with no screening was increased 2-fold (HR, 2.0; 95% CI, 1.3–2.9). On multivariable analyses adjusted for histology, size, and number of concomitant adenomas, having a large serrated polyp was associated with a 3.3-fold increased risk for incident CRC (OR, 3.3; 95% CI, 1.3–8.6). Interestingly, very little progression (including no progression to cancer) was observed in 23 large serrated polyps left in situ for a median 11 years of follow-up, suggesting that some serrated polyps may be a general biomarker of risk rather than an intermediate high-risk lesion. This study is limited by the small sample size, and uncertainty regarding whether a group of patients ascertained as a result of a sigmoidoscopy trial are representative of patients routinely encountered with SSP at colonoscopy. Despite data suggesting that patients with SSP have increased risk for CRC, the magnitude and significance of risk associated with SSPs is uncertain, given limitations of available studies.
Summary of risk for incident and fatal CRC after normal colonoscopy and after polyp removal.
Studies published since our last recommendations suggest the evidence to support a low risk for incident and fatal CRC after normal screening colonoscopy is stronger. There continues to be little evidence on the incremental effectiveness of a repeat screening colonoscopy at 10 years after normal colonoscopy, but modeling studies suggest benefit. Recent studies vary in estimates of risk for incident and fatal CRC after baseline adenoma removal, with some showing increased risk, and others showing decreased risk. New evidence suggests that exposure to surveillance colonoscopy after baseline adenoma removal may reduce CRC risk, but the magnitude of benefit associated with exposure to surveillance colonoscopy is unclear. Generally, individuals with more advanced findings at baseline (or colonoscopy with poor baseline quality) have higher risk for subsequent cancer relative to those with low-risk findings (eg, 1–2 small adenomas) and benefit of repeat surveillance colonoscopy is more demonstrable in the higher-risk groups. Further, determining which groups are most likely to benefit, and whether surveillance reduces CRC mortality, remains a challenge. Recent studies suggest patients with SSPs may have an increased risk for incident CRC, but magnitude and consistency of risk remains uncertain. Overall, more evidence is needed to understand which patients are at lowest and highest risk for incident and fatal CRC after initial colonoscopy, and whether surveillance can consistently improve outcomes. Nonetheless, pending generation of new evidence, we provide colonoscopy surveillance recommendations to guide patient care, given the prevailing conventional wisdom and available observational evidence suggesting that some patients remain at risk for CRC despite baseline polypectomy.
Recommended Post-Colonoscopy Surveillance Strategies for Reducing Colorectal Cancer Risk
For patients with normal, high-quality colonoscopy, repeat CRC screening in 10 years.
(Strong recommendation, high quality of evidence)
New observational and modeling studies of colonoscopy confirm and strengthen the evidence base to support the conclusion that individuals with normal colonoscopy are at lower than average risk for CRC, as mentioned previously.8–11 Based on this reduced risk, we recommend CRC screening in average-risk individuals be repeated 10 years after a normal examination complete to the cecum with bowel preparation adequate to detect polyps >5 mm in size. Future studies may clarify whether lengthening the interval beyond 10 years may be possible. A 10-year follow-up after normal colonoscopy is recommended regardless of indication for the colonoscopy, except for individuals at increased risk for CRC, such as those with history of a hereditary CRC syndrome, personal history of inflammatory bowel disease, personal history of hereditary cancer syndrome, serrated polyposis syndrome, malignant polyp, personal history of CRC, or family history of CRC (Tables 4 and 5; Figure 1).
For patients with 1–2 tubular adenomas <10 mm in size completely removed at a high-quality examination, repeat colonoscopy in 7–10 years.
(Strong recommendation, moderate quality of evidence)
Table 4.
US Multi-Society Task Force Recommendations for Post-Colonoscopy Follow-Up in Average-Risk Adults With Normal Colonoscopy or Adenomasa
| Baseline colonoscopy finding | Recommended interval for surveillance colonoscopy | Strength of recommendation | Quality of evidence |
|---|---|---|---|
| Normal | 10 yb | Strong | High |
| 1–2 tubular adenomas <10 mm | 7–10 yc | Strong | Moderate |
| 3–4 tubular adenomas <10 mm | 3–5 y | Weak | Very low |
| 5–10 tubular adenomas <10 mm | 3 y | Strong | Moderate |
| Adenoma ≥10 mm | 3 y | Strong | High |
| Adenoma with tubulovillous or villous histology | 3 yd | Strong | Moderate |
| Adenoma with high-grade dysplasia | 3 yd | Strong | Moderate |
| >10 adenomas on single examinatione | 1 y | Weak | Very low |
| Piecemeal resection of adenoma ≥20 mm | 6 mo | Strong | Moderatef |
All recommendations assume examination complete to cecum with bowel preparation adequate to detect lesions >5 mm in size; recommendations do not apply to individuals with a hereditary CRC syndrome, personal history of inflammatory bowel disease, personal history of hereditary cancer syndrome, serrated polyposis syndrome, malignant polyp, personal history of CRC, or family history of CRC, and must be judiciously applied to such individuals, favoring the shortest indicated interval based on either history or polyp findings.
Follow-up may be with colonoscopy or other screening modality for average-risk individuals.
Patients with recommendations issued before 2020 for shorter than 7- to 10-year follow-up after diagnosis of 1–2 tubular adenomas may follow original recommendations. If feasible, physicians may re-evaluate patients previously recommended an interval shorter than 10 y and reasonably choose to provide an updated recommendation for 7- to 10-year follow-up, taking into account factors such as quality of baseline examination, polyp history, and patient preferences.
Assumes high confidence of complete resection.
Patients with >10 adenomas or lifetime >10 cumulative adenomas may need to be considered for genetic testing based on absolute/cumulative adenoma number, patient age, and other factors such as family history of CRC (see text).
See US Multi-Society Task Force recommendations for endoscopic removal of colorectal lesions.69
Table 5.
US Multi-Society Task Force Recommendations for Post-Colonoscopy Follow-Up in Average-Risk Adults With Serrated Polypsa
| Baseline colonoscopy finding | Recommended interval for surveillance colonoscopy | Strength of recommendation | Quality of evidence |
|---|---|---|---|
| ≤20 HPs in rectum or sigmoid colon <10 mmf | 10 yb | Strong | Moderate |
| ≤20 HPs proximal to sigmoid colon <10 mmf | 10 y | Weak | Very low |
| 1–2 SSPs <10 mm | 5–10 y | Weak | Very low |
| 3–4 SSPs <10 mm | 3–5 y | Weak | Very low |
| 5–10 SSPs <10 mm | 3 y | Weak | Very low |
| SSP ≥10 mm | 3 y | Weak | Very low |
| SSP with dysplasiae | 3 y | Weak | Very low |
| HP ≥10 mm | 3–5 yc | Weak | Very low |
| TSA | 3 y | Weak | Very low |
| Piecemeal resection of SSP ≥20 mm | 6 mo | Strong | Moderated |
All recommendations assume examination complete to cecum with bowel preparation adequate to detect lesions >5 mm in size; recommendations do not apply to individuals with a hereditary CRC syndrome, personal history of inflammatory bowel disease, personal history of hereditary cancer syndrome, serrated polyposis syndrome, or malignant polyp, personal history of CRC, or family history of CRC, and must be judiciously applied to individuals with a personal or family history of CRC, favoring the shortest indicated interval based on either history or polyp findings.
Follow-up may be with colonoscopy or other screening modality for average risk individuals.
A 3-year follow-up interval is favored if concern about consistency in distinction between SSP and HP locally, bowel preparation, or complete excision, whereas a 5-year interval is favored if low concerns for consistency in distinction between SSP and HP locally, adequate bowel preparation, and confident complete excision.
See US Multi-Society Task Force recommendations for endoscopic removal of colorectal lesions.69
Assumes high confidence of complete resection.
Patients with cumulative >20 hyperplastic polyps distributed throughout the colon, with at least 5 being proximal to the rectum, as well as those with 5 serrated polyps proximal to the rectum > 5mm, with at least two ≥ 10 mm meet criteria for serrated polyposis syndrome and may require specialized management.112
Figure 1.
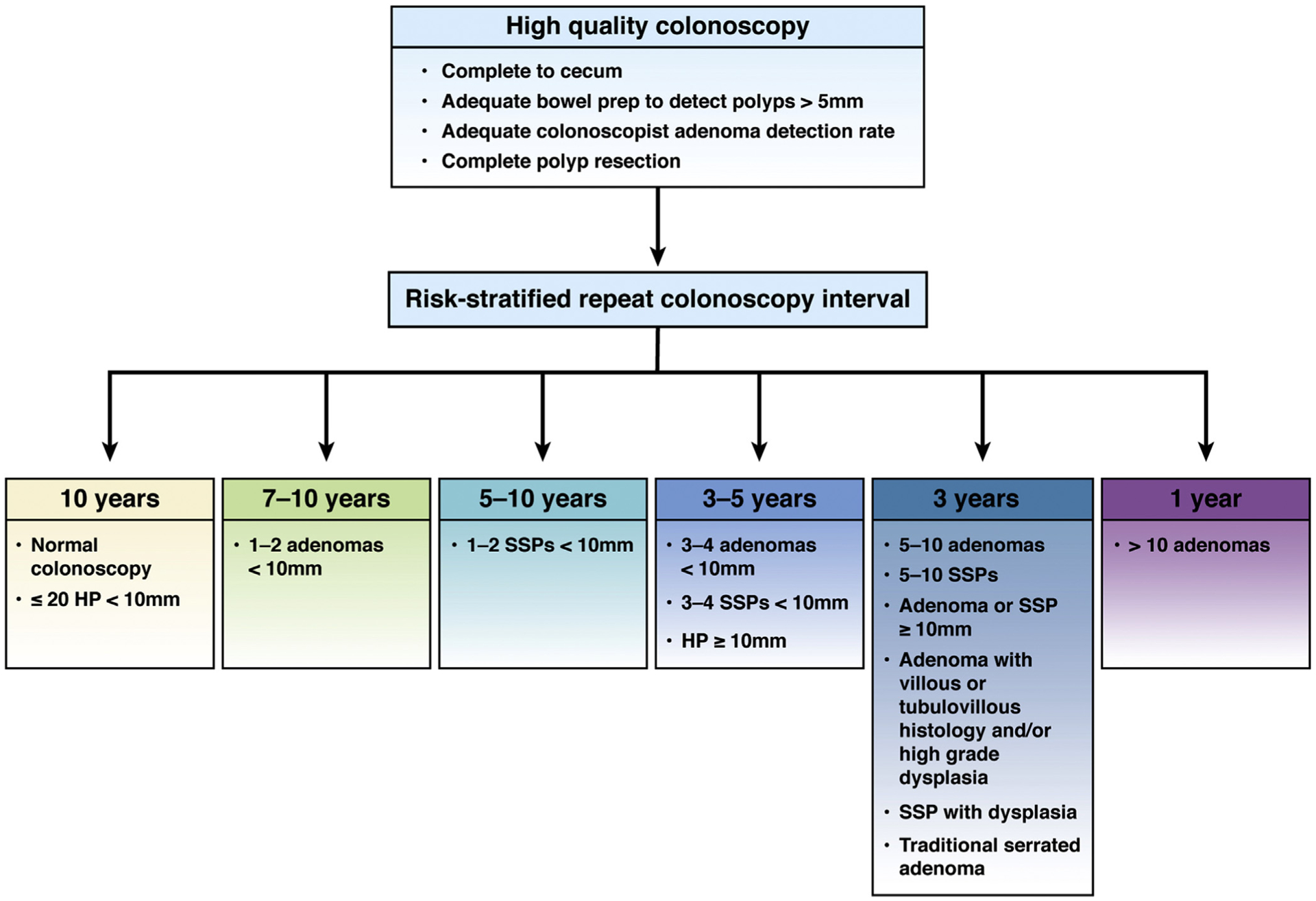
Recommendations for follow-up after colonoscopy and polypectomy. Recommendations for post-colonoscopy follow-up in average risk adults are depicted. After high-quality colonoscopy defined by examination complete to cecum adequate to detect polyps >5 mm, performed by a colonoscopist with adequate ADR with complete polyp resection, risk-stratified repeat colonoscopy intervals are provided. SSP, sessile serrated polyp/sessile serrated adenoma/sessile serrated lesion.
The Task Force previously recommended repeat colonoscopy within a range of 5–10 years for individuals with 1–2 small tubular adenomas. The shift in recommendation to a longer interval is based on new studies that confirm and extend prior evidence to suggest that individuals with low-risk adenomas have reduced risk for advanced neoplasia, as well as incident CRC on follow-up. Since our last review, 2 meta-analyses examining risk for metachronous advanced neoplasia among patients with low-risk adenomas have been published. The first pooled data from 11,387 individuals across 7 studies reported between 1992 and 2013 with 2–5 years follow-up after baseline colonoscopy. The pooled rate of metachronous advanced neoplasia was 3.6% for individuals with baseline low-risk adenoma and 1.6% for those with normal colonoscopy (RR, 1.8; 95% CI, 1.3–2.6).22 The most recent meta-analysis pooled data from 10,139 individuals across 8 studies reported between 2006 and 2015 with 3–10 years of follow-up after baseline colonoscopy (Figure 2).23 Five-year cumulative incidence of metachronous advanced adenoma on follow-up was 4.9% for the low-risk adenoma group (95% CI, 3.18%–6.97%) and 3.3% for the no adenoma group (95% CI, 1.85%–5.10%; RR, 1.55; 95% CI, 1.24–1.94). In contrast, the same meta-analysis reported the 5-year cumulative incidence of metachronous advanced adenoma on follow-up was 17.1% (95% CI, 11.97%–23.0%) for individuals with advanced adenoma. Limitations of both of these meta-analyses include short duration of follow-up, as well as inclusion of many patients from randomized trials of interventions to reduce polyp recurrence. Nonetheless, both meta-analyses suggest that the rate of metachronous advanced neoplasia is low among individuals with 1–2 adenomas <10 mm, and only marginally higher (no more than 2%) than the rate observed in people with normal colonoscopy at baseline. These studies are complemented by the aforementioned Norwegian cohort study, which found that the long-term risk of fatal CRC for 36,296 patients with a single adenoma without advanced histology (not taking into account size) was 25% lower than the general population (standardized mortality ratio, 0.75; 95% CI, 0.63–0.88)17 and the previously cited French cohort study, which reported baseline nonadvanced adenoma was associated with reduced CRC risk compared to the general population (SIR, 0.68; 95% CI, 0.44–0.99).16 The French cohort study also noted no statistically significant difference in risk for incident cancer compared to the general population among patients exposed to surveillance colonoscopy after removal of 1–2 adenomas <10 mm (SIR, 0.60; 95% CI, 0.30–1.07), although the point estimate for risk was higher among patients unexposed to surveillance (SIR, 0.82; 95% CI, 0.41–1.47).16 The previously mentioned US cohort study found cumulative CRC incidence at up to 15 years follow-up was 1.4% for individuals with nonadvanced adenoma vs 1.2% for individuals with no adenoma, and reported no difference in the rate of fatal CRC.18 A limitation of this study was inability to account for impact of exposure to surveillance colonoscopy, which occurred among 78.7% of nonadvanced adenoma and 69.9% of no adenoma patients at up to 9 years follow-up in the subset of 3492 individuals from whom follow-up colonoscopy data were collected and presented. Thus, it is possible that exposure to surveillance colonoscopy contributed to the lack of difference in incident CRC observed between the nonadvanced adenoma and colonoscopy groups.
Figure 2.
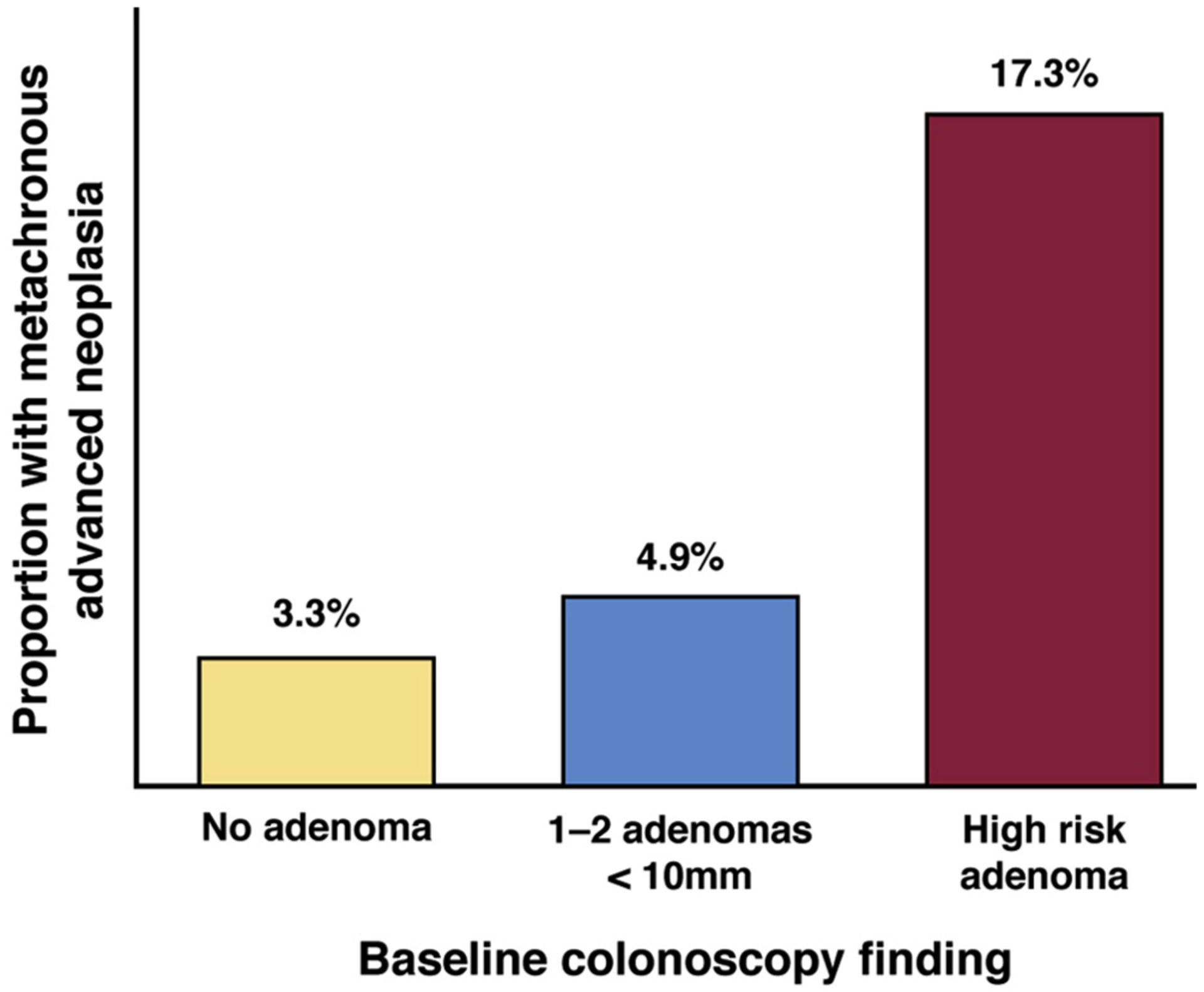
Risk for metachronous advanced neoplasia among individuals with normal colonoscopy, 1–2 adenomas <10 mm in size, or high-risk adenoma (adenoma >10 mm in size, adenoma with tubulovillous/villous histology, adenoma with high-grade dysplasia or ≥3 adenomas <10 mm) based on a meta-analysis of 10,139 across 8 surveillance studies is depicted.23 Risk for metachronous adenoma among individuals with no adenoma or 1–2 small adenomas is similar, and much lower than risk among individuals with baseline high-risk adenoma. In studies that defined high risk as advanced adenoma alone (n = 4 studies), cumulative advanced adenoma risk was 16% (95% CI, 9%–25%), and in studies that defined high risk as advanced adenoma or ≥3 adenomas <10 mm (n = 4 studies), cumulative advanced adenoma risk was 19% (95% CI, 10%–30%; C Dube, personal communication, September 18, 2018).
We specifically searched for articles evaluating factors that might increase risk among individuals with 1–2 adenomas <10 mm. In a pooled analysis of individuals with 1–2 small adenomas in 7 prospective polyp surveillance studies, an increased risk for metachronous advanced neoplasia was found for those with a history of polyps (absolute risk, 11.5%) or concurrent distal and proximal small adenomas (absolute risk, 11.0%).24 However, most studies contributing to this pooled analysis were randomized trials of strategies to reduce polyp recurrence, and were performed before the era of modern colonoscopy, impacting relevance to current practice in which baseline adenoma detection may have improved due to focus on optimizing bowel preparation and ADRs. In a separate study that included an analysis of 4496 patients with 1–2 nonadvanced adenomas, risk for incident CRC was similar among those with proximal only vs distal only adenomas (RR, 1.5; 95% CI, 0.7–2.8).18 More research is needed to determine whether subsets of individuals with low-risk adenoma, such as those with advanced age, youngonset adenoma, proximal adenoma, male sex, or other factors might benefit from shorter duration of follow-up.
We considered a recommendation of 10 years alone rather than a range of 7- to 10-year follow-up after removal of 1–2 adenomas <10 mm in size, given that evidence supports that these patients are at lower than average risk for CRC. The 7- to 10-year range was chosen because of ongoing uncertainty regarding whether the observed lower than average risk for CRC could be reduced further by exposure to surveillance,17 and also because we cannot rule out the possibility that exposure to surveillance colonoscopy in some studies contributed to the low risk of CRC observed in these patients.16,18 We anticipate that ongoing work may clarify whether surveillance colonoscopy can improve outcomes in patients with 1–2 small adenomas, and also whether characteristics (such as size <6 mm) may help guide the choice between recommending a shorter 7-year vs a longer 10-year surveillance interval.
The Task Force recognizes that many patients with 1–2 nonadvanced adenomas <10 mm will have had a prior documented recommendation for a 5-year examination or other interval shorter than 7–10 years, consistent with 2012 recommendations. Patients with recommendations before this publication for shorter than 7- to 10-year follow-up after diagnosis of 1–2 tubular adenomas <10 years can reasonably follow original recommendations. Based on the new evidence presented and our current recommendation for 7- to 10-year follow-up, if feasible, we suggest that physicians may re-evaluate patients previously recommended an interval shorter than 7–10 years and reasonably choose to provide an updated recommendation for follow-up between 7 and 10 years after the prior examination that diagnosed 1–2 adenomas <10 mm, taking into account factors such as quality of baseline examination, polyp history, and patient preferences.
For patients with 3–4 tubular adenomas <10 mm in size completely removed at a high-quality examination, repeat colonoscopy in 3–5 years.
(Weak recommendation, very low quality of evidence)
For patients with 5–10 tubular adenomas <10 mm in size completely removed at a high-quality examination, repeat colonoscopy in 3 years.
(Strong recommendation, moderate quality of evidence)
Since the 2012 recommendations, a number of studies have been published that included evaluation of risk among patients with 3–10 adenomas. These studies are consistent in demonstrating that individuals with 3–10 adenomas are at increased risk for advanced neoplasia25–30 and even CRC alone26,31 on follow-up. However, we were specifically interested in whether there was sufficient evidence to support longer surveillance intervals for patients with 3–4 small (<10 mm) adenomas. Our rationale for seeking such data is based on a postulate that the number of small adenomas found per patient may be increasing over time with greater attention to colonoscopy quality and use of high-definition colonoscopes.32 Several relevant studies were identified. In interpreting these studies, we considered the observation from the previously mentioned meta-analysis, which found 5-year cumulative risk of metachronous neoplasia was 3.3% for the no adenoma and 4.9% for the 1–2 <10-mm adenoma group.23 A cohort study of 561 individuals with 3–4 adenomas <10 mm suggested that the risk for metachronous advanced neoplasia among individuals with 3–4 adenomas was <5%.33 This study was limited by the absence of a comparison group with only 1–2 nonadvanced adenomas. In a cohort study of 443 individuals with 1–9 adenomas <10 mm, no group with <10-mm polyps (including those with between 5 and 9 adenomas) had a rate of metachronous advanced neoplasia >10% on follow-up that extended up to 32 months.34 A limitation of this study was small sample size, particularly for subgroup analyses by number and size of polyps, and that data on the subgroup of patients with 3–4 adenomas were not reported. A single-center retrospective study of 1414 patients cared for at a large academic gastroenterology practice between 2002 and 2012 with high awareness of colonoscopy quality strategies found 5% of patients with 5 or more adenomas <10 mm at baseline had metachronous advanced neoplasia on follow-up colonoscopy more than 200 days after baseline.35 Metachronous advanced neoplasia was found in just 1.8% of patients with 3–4 small adenomas at baseline, and 1.4% of those with 1–2 small adenomas. In comparison, the rate of metachronous advanced neoplasia was 16.3% for individuals with 5 or more adenomas with 1 ≥10 mm, and 8.6% for those with 3–4 adenomas with 1 ≥10 mm in size. As such, this study suggests that individuals with 1–2 low-risk adenomas, as well as those with 3–4 <10-mm adenomas, at baseline might have a similar very low risk for metachronous advanced neoplasia in settings that include high attention to colonoscopy quality. In a cohort study that compared 572 patients with 3 or more nonadvanced adenomas to 4496 patients with 1–2 nonadvanced adenomas, no difference in risk for incident CRC was observed (RR, 1.01; 95% CI, 0.4–2.4), and the cumulative rate of advanced adenoma removal through up to 9 years of follow-up was similar: 10.7% for individuals with 3 or more nonadvanced adenomas vs 7.1% for individuals with 1–2 nonadvanced adenomas.18 Outcomes stratified by exact number of adenomas in the 3 or more nonadvanced adenoma group were not reported.
Based on these studies, the Task Force suggests 3- to 5-year repeat colonoscopy for individuals with 3–4 adenomas <10 mm in size, and favors a 5-year interval based on current evidence. However, the Task Force recognizes very low quality of evidence to support the 3- to 5-year follow-up recommendation. More research is needed to determine if, in the modern era of colonoscopy, the risk for metachronous advanced neoplasia in individuals with 3–4 tubular adenomas <10 mm is low enough to permit a firm 5-year or even longer than 5-year interval to surveillance colonoscopy. Given limited available data to assess risk, the Task Force recommends 3-year repeat colonoscopy for individuals with 5–10 adenomas <10 mm in size. Future research may elucidate whether some individuals within this group (particularly those with 5–10 diminutive adenomas <6 mm in size) may have low risk also warranting longer follow-up intervals. The Task Force recommends that the number of small adenomas at a given examination should be considered in context of the cumulative number of lifetime adenomas, as differential management may be warranted based on having >10 adenomas, as is highlighted below.
For patients with 1 or more adenomas ≥10 mm in size completely removed at high-quality examination, repeat colonoscopy in 3 years.
(Strong recommendation, high quality of evidence)
Since the 2012 recommendations, additional studies have confirmed and extended the evidence supporting identification of 1 or more adenomas ≥10 mm size as a high-risk feature.25–27,30,31 A study of 2990 patients from the Netherlands diagnosed with adenoma 1988–2002 and followed through 2008 found size ≥10 mm was independently associated with 1.7-fold increased risk for metachronous advanced neoplasia (OR, 1.7; 95% CI, 1.2–2.3).30 A cohort study of 3300 patients diagnosed with adenomas at a large integrated US health care system found that size ≥10 mm was independently associated with 3.6-fold increased risk for advanced adenoma (OR, 3.6; 95% CI, 2.8–4.5) and 5.2-fold increased risk for CRC on follow-up (OR, 5.2; 95% CI, 1.8–15.1).26 An Australian cohort study of 5141 patients found having advanced neoplasia (defined as villous histology, size >9 mm, serrated histology, high-grade dysplasia, or >2 adenomas) was associated with increased risk for advanced neoplasia on follow-up, but risk associated with size >9 mm, villous histology, or high-grade dysplasia alone was not specifically examined. An additional limitation of this study was that half of the enrolled patients had a family history of CRC.27 As mentioned previously, a US cohort study found individuals with advanced adenoma had an increased risk for incident and fatal CRC compared to those with no adenoma, and the cumulative rate of advanced adenoma removal at up to 9 years follow-up was 13.0%.18 Although the study did not specifically report outcomes for individuals with adenoma ≥10 mm or larger, adenoma with high-grade dysplasia, or villous histology, the majority of individuals followed in the advanced adenoma group met the increased size criteria. As such, this study also supports closer follow-up for individuals with adenoma ≥10 mm. The Task Force acknowledges the importance of accurate polyp size estimation for this recommendation and suggests photodocumentation verifying polyp size ≥10 mm relative to an open forceps or open snare of known size.
For patients with adenoma containing villous histology completely removed at high-quality examination, repeat colonoscopy in 3 years.
(Strong recommendation, moderate quality of evidence)
Studies published since the 2012 recommendations continue to support villous histology as a potential risk factor for advanced neoplasia on follow-up. These studies include the aforementioned 2 large cohort studies from a large US health care system and the Netherlands.26,27,30
For patients with adenoma containing high-grade dysplasia completely removed at high-quality examination, repeat colonoscopy in 3 years.
(Strong recommendation, moderate quality of evidence)
The previously cited cohort study from the United States, as well as 1 additional cohort study, have confirmed and extended evidence to support high-grade dysplasia as a risk factor for metachronous advanced neoplasia26,27,36 and CRC.26 However, the Netherlands cohort of 2990 patients did not find baseline high-grade dysplasia to be an independent predictor of risk.30 Studying high-grade dysplasia as a risk factor is a major challenge because this finding is rare at baseline, perhaps accounting for some of the variability in risk observed across studies. The 3-year recommendation assumes that there was complete resection of neoplasia, including high-grade dysplasia at the baseline examination.
For patients with >10 adenomas completely removed at high-quality examination, repeat colonoscopy in 1 year.
(Weak recommendation, very low quality of evidence)
Since 2012, we found a single cohort study of 214 Korean patients with >10 adenomas in which risk for metachronous advanced adenoma was evaluated. At a median 4.3 years of follow-up, 26.6% had metachronous advanced adenoma.37 Patients with >10 adenomas may be at increased risk for having a hereditary polyposis syndrome, such as familial adenomatous polyposis or MYH-associated polyposis,38 and multiple groups have recommended patients with >10 cumulative lifetime adenomas be considered for genetic testing.39,40 Decision to perform genetic testing may be based on absolute or cumulative adenoma number, patient age, as well as other factors, such as family history of CRC and/or personal history of features associated with polyposis, such as desmoid tumor, hepatoblastoma, cribriform morular variant of papillary thyroid cancer, or multifocal/bilateral congenital hypertrophy of the retinal pigment epithelium.40
For patients with ≤ 20 HPs <10 mm in size in the rectum or sigmoid colon removed at a high-quality examination, repeat CRC screening in 10 years.
(Strong recommendation, moderate quality of evidence)
For patients with ≤ 20 HPs <10 mm in size proximal to the sigmoid colon removed at a high-quality examination, repeat colonoscopy in 10 years.
(Weak recommendation, very low quality of evidence)
Since the 2012 review, we could identify no new data on risk of advanced neoplasia associated with small rectosigmoid HPs. Prior literature has suggested that such patients have a similar risk of metachronous advanced neoplasia as patients with a normal examination, and recommendations for 10-year repeat examination remain unchanged,2 although previous studies have been limited by either small sample size or evaluating patients who had both conventional adenoma and distal HPs at baseline. We specifically searched for data to guide recommendations for patients with HPs <10 mm proximal to the sigmoid colon. We found no published studies on the risk for metachronous advanced neoplasia or large serrated polyps among patients with isolated HPs <10 mm proximal to the sigmoid colon without synchronous conventional adenoma. We do note that in a cohort study of patients with serrated polyps, among 698 patients with HPs and no concurrent conventional adenomas, the proportion with high-risk adenoma at follow-up was 3.7% (26 of 698), and large serrated polyp (defined as HP or SSP ≥10 mm) was 1.6% (11 of 698), supporting the concept that most individuals with isolated HPs are a low-risk group; data on outcomes stratified by size and location of baseline HPs were not provided.41 We do recognize concerns that in usual practice some SSPs may be misdiagnosed as HPs.42–47 If concerns regarding the ability of the local pathologist to distinguish between SSP and HPs exist, some clinicians may choose to follow the recommendations for patients with SSPs provided below for patients identified with isolated proximal HPs <10 mm.
For patients with 1–2 SSPs <10 mm in size completely removed at high-quality examination, repeat colonoscopy in 5–10 years.
(Weak recommendation, very low quality evidence)
We found 4 studies that evaluated outcomes among patients with 1–2 SSPs <10 mm. There are several challenges to interpreting and comparing these studies, including varying definitions of the baseline serrated polyp group and the outcome evaluated. For baseline serrated polyp group characterization, some studies restrict the group to SSPs, and others include SSPs plus TSA and large HP. For follow-up outcomes at surveillance, some used a definition of high-risk neoplasia that included conventional advanced adenoma (Table 3), while others used a definition that included conventional advanced adenoma, 3 or more conventional adenomas and/or SSPs, and SSPs or serrated polyp ≥10 mm. The varied ways studies of serrated polyp outcomes have characterized baseline findings and follow-up outcomes make the literature a major challenge to interpret.
Studies reviewed included a multiple cohort study that identified patients with serrated polyps vs those with conventional adenomas, who all had follow-up colonoscopy (n = 255).48 In this study, the serrated polyp group was defined by having SSP, TSA, or HP ≥10 mm. Primary outcomes were advanced adenoma (defined as adenoma ≥10 mm or with villous component or high-grade dysplasia) and advanced serrated polyp (defined as HP or SSP ≥10 mm, SSP with dysplasia, or TSA). Rate of metachronous advanced neoplasia was 20.7% (6 of 29) in patients with baseline conventional advanced neoplasia, and 6.3% (7 of 111) in the isolated serrated polyp group.48 Metachronous advanced serrated polyps (defined as HP or SSP ≥10 mm, SSP with dysplasia, or TSA of any size) were noted in 10% (3 of 30) and 12.5% (2 of 16) of patients with baseline serrated polyps and nonadvanced adenomas or advanced adenomas, respectively, and 5.4% (6 of 111) with isolated serrated polyps. Another multiple cohort study identified 4 baseline groups of patients who received surveillance colonoscopy: 1) low-risk conventional adenoma; 2) low-risk SSP (defined as 1–2 polyps <10 mm) ± conventional adenoma; 3) high-risk conventional adenoma and/or ≥3 conventional adenomas; and 4) low-risk SSP plus high-risk conventional adenoma or ≥3 conventional adenomas ± SSPs.49 SSP was defined by having histologically confirmed SSP. The primary outcome was advanced neoplasia, defined as adenoma or serrated polyp ≥10 mm or villous histology, or high-grade dysplasia, or CRC. Stratified by baseline group, the rate of advanced neoplasia (including large serrated polyp) was 18.2% with low-risk adenoma plus any SSP, 7.8% for low-risk adenoma without SSP, 17.9% for 1–2 SSP <10 mm, 15.9% for high-risk adenoma and/or ≥3 conventional adenomas without SSP.49 This suggests that having both conventional advanced neoplasia and SSP of any size could be associated with increased risk for having metachronous advanced neoplasia, defined as adenoma or serrated polyp ≥10 mm or adenoma with villous histology, or adenoma with high-grade dysplasia, or CRC. A very small study of 75 patients with histologically confirmed SSP at baseline suggested that those with synchronous high-risk adenoma (multiple adenomas or advanced adenoma), but not those with low-risk adenoma or absence of synchronous neoplasia, had increased risk for advanced neoplasia on follow-up, compared to samples of individuals with conventional high-risk adenoma, conventional low-risk adenoma, or normal colonoscopy at baseline.50
The largest study to date has been a cohort study of 5433 individuals with baseline colonoscopy and at least 1 surveillance colonoscopy ≥1 years after initial examination. Baseline categories included presence of normal colonoscopy, low-risk adenoma, high-risk adenoma, and/or SSP (defined as histologic SSP or TSA).41 Primary outcomes assessed on follow-up included risk for metachronous conventional high-risk adenoma, as well as large serrated polyp (HP, SSP, or TSA) ≥10 mm. Findings are summarized in Table 6. Rate of high-risk adenoma among patients with SSP but no synchronous high-risk adenoma was just 2.9%, much lower than the observed rate for individuals with isolated high-risk adenoma at baseline of 18.2%. Rate of high-risk adenoma was markedly higher in patients with both SSP and high-risk adenoma at baseline, estimated at 46.4%. Rate of serrated polyp ≥10 mm (HP, SSP, or TSA) at follow-up was substantially higher among patients with isolated SSP vs high-risk adenoma at baseline (9.6% vs 1.0%). Among patients with low-risk adenoma plus SSP at baseline, the rate of metachronous high-risk adenoma was 18.4% (9 of 49) and metachronous SSP ≥10 mm was 8.2% (4 of 49; Anderson JC, Butterly LF, Robinson CM, personal communication, March 14, 2018). These findings suggest that patients with isolated SSP have low rates of metachronous conventional high-risk adenoma unless they have synchronous conventional adenomas at baseline. However, patients with SSP at baseline appear to be at increased risk for metachronous large serrated polyps ≥10 mm (HP, SSP, or TSA), irrespective of whether concurrent conventional adenomas are present. While this is the largest study to date of metachronous findings among patients with and without SSPs, a limitation is that the risk estimates remain imprecise, owing to the relatively small number of patients with SSP at baseline available for evaluation in the various risk strata. In contrast to the aforementioned even smaller studies, however, it is interesting to note that patients with isolated SSP of any size as well as HPs ≥10 mm were not found to have increased risk for conventional high-risk adenoma on follow-up.
Table 6.
Risk for High-Risk Adenoma and Large Serrated Polyps Stratified by Baseline Colonoscopy Findings in the New Hampshire Colonoscopy Registry
| Surveillance colonoscopy finding | ||
|---|---|---|
| Baseline finding | HRA,a % (n) | SPb ≥10 mm, % (n) |
| No adenoma | 4.8 (116/2396) | 0.7 (18/2396) |
| LRAc | 9.7 (96/991) | 0.5 (5/991) |
| HRA | 18.2 (11/603) | 1.0 (6/603) |
| LRA + SSP | 18.4 (9/49) | 8.2 (4/49) |
| HRA + SSP | 46.4 (13/28) | 3.6 (1/28) |
| SSA/Pd | 2.9 (3/104) | 9.6 (10/104) |
| SP ≥10 mm | 3.1 (2/65) | 12.3 (8/65) |
NOTE. From Anderson et al,41 adapted with permission. Previously unpublished data provided through personal communication with JC Anderson, LF Butterly, CM Robinson, March 14, 2018, with permission.
HRA, high-risk adenoma; LRA, low-risk adenoma; SSA/P, sessile serrated adenoma/polyp.
HRA includes advanced neoplasia or >2 adenomas.
SP includes HP, SP, or TSA.
LRA includes 1–2 adenomas <10 mm in size.
Included TSA in SSA/P group.
Taken together, very low quality of evidence exists to support recommendations for surveillance after removal of 1–2 SSPs <10 mm. Specifically, subgroups describing outcomes in those with serrated lesions are small and there are very limited data on subsequent risk for the most important outcomes (ie, CRC). The largest traditional cohort study suggests patients with isolated SSPs have low risk for traditionally defined high-risk adenomas, those with synchronous SSPs and conventional adenoma may have high risk for traditionally defined high-risk adenomas, and that all patients with SSPs are at elevated risk for large serrated polyps on follow-up. Smaller studies at higher risk of bias that used disparate definitions of predictors and outcomes are variably consistent with these observations. Taking into account the absence of consistent, higher-quality evidence, uncertainty regarding implications of having a large serrated polyp at follow-up on CRC risk, and the known challenges of adequate detection51 and complete resection of SSPs,52 the Task Force recommends patients with 1–2 SSPs <10 mm receive repeat colonoscopy in 5–10 years until new evidence can clarify risk for this group. The recommendation for 5- to 10-year follow-up of patients with 1–2 SSPs <10 mm is more aggressive than the recommendation for 7- to 10-year follow-up of patients with 1–2 isolated conventional adenomas because the evidence base to support longer follow-up for 1–2 isolated conventional adenomas is strong, whereas the evidence base to support follow-up recommendations for individuals with 1–2 SSPs <10 mm is weak.
For patients with TSA completely removed at a high-quality examination, repeat colonoscopy in 3 years.
(Weak recommendation, very low quality of evidence)
We found little new evidence to guide the follow-up recommendation for patients with TSA. A cross-sectional study compared risk for advanced neoplasia and/or 3 adenomas at surveillance colonoscopy for patients with prior isolated TSA (n = 186) vs a group of age-/sex-matched patients with prior conventional adenoma (n = 372). Proportion with metachronous high-risk adenoma was higher in the TSA vs conventional adenoma group (47.3% vs 32.0%), and associated with higher risk on adjusted analyses (high-risk adenoma OR, 2.37; 95% CI, 1.55–3.63),53 supporting our recommendation for repeat colonoscopy in 3 years after TSA diagnosis.
For patients with 3–4 SSPs <10 mm at high-quality examination, repeat colonoscopy in 3–5 years.
(Weak recommendation, very low quality of evidence)
For patients with any combination of 5–10 SSPs <10 mm at high-quality examination, repeat colonoscopy in 3 years.
(Weak recommendation, very low quality of evidence)
We were unable to identify published articles that specifically examined risk for metachronous neoplasia in patients with 3–10 SSPs, or any combination of 3–10 SSPs and conventional adenomas. The previously mentioned unpublished data on 49 patients with a combination of low-risk adenoma and SSP at baseline with unknown total number suggests increased risk for metachronous advanced neoplasia and for large SSP. In the absence of additional data, we have chosen to recommend 3- to 5-year repeat colonoscopy for individuals with 3–4 SSPs <10 mm, and 3- year repeat colonoscopy for individuals with 5–10 SSPs <10 mm. These are the same recommendations provided for individuals in the groups with 3–4 and 5–10 isolated conventional adenomas, respectively. Future research may clarify whether patients with a combination of <10-mm SSPs and conventional adenomas have a distinct risk that should merit different management.
For patients with SSP ≥10 mm at a high-quality examination, repeat colonoscopy in 3 years.
(Weak recommendation, very low quality of evidence)
For patients with HP ≥10 mm, repeat colonoscopy in 3–5 years. A 3-year follow-up interval is favored if concern about pathologist consistency in distinguishing SSPs from HPs, quality of bowel preparation, or complete polyp excision, whereas a 5-year interval is favored if low concerns for consistency in distinguishing between SSP and HP by the pathologist, adequate bowel preparation, and confident complete polyp excision.
(Weak recommendations, very low quality of evidence)
We found little new evidence to guide management of patients with SSP ≥10 mm or HP ≥10 mm. In the previously cited New Hampshire Colonoscopy registry study, among 65 patients with large serrated polyps (HP, SSP, or TSA), 3.1% had high-risk adenoma on follow-up compared to 4.8% among 2396 patients with no adenoma at index colonoscopy.41 However, having any serrated polyp ≥10 mm in size was associated with increased risk for large serrated polyp (≥10 mm SSP, TSA, or HP), ranging from an absolute risk of 12.3% (8 of 65) for no concurrent conventional adenoma to 11.2% (2 of 18) for concurrent high-risk adenoma, compared to an absolute risk of 0.7% (18 of 2396) for those without adenoma or any serrated polyp. Thus, based on this new evidence, the implications of having a large serrated polyp on risk for subsequent conventional high-risk adenoma are uncertain. However, having a large serrated polyp at baseline does appear to be associated with risk for subsequent large serrated polyps. A challenge in interpreting available literature is a lack of data separating outcomes for those with ≥10 mm SSP, TSA, and HP. Because of variation in consistent distinction by pathologists between SSPs and HPs in usual care,42–47 a conservative approach might be to assume all HPs ≥10 mm are SSPs. However, this may subject some patients (especially if consultant pathology expertise in distinguishing SSPs from HPs is high) to over-diagnosis and more aggressive surveillance than necessary if rates of advanced neoplasia or large serrated polyp on follow-up among individuals with large SSPs vs large HPs differ. An added problem in making recommendations for large serrated polyps is the potential challenge of resection of SSPs ≥10 mm. For example, Pohl et al52 reported 47% of SSPs 10–20 mm had evidence of incomplete resection. Given uncertainties regarding implications of having serrated polyp ≥10 mm and whether outcomes differ for those with SSP vs HP ≥10 mm, as well as observed variation in ability of pathologists to distinguish SSPs from HPs, and the known challenge of resection of ≥10 mm SSPs, the Task Force recommends 3-year follow-up for individuals with SSP ≥10 mm in size, and 3- to 5-year follow-up for individuals with HP ≥10 mm. For HP ≥10 mm, a 3-year follow-up interval is favored if concern about consistency in distinction by the consult pathologist between SSP and HP, adequacy of bowel preparation, or complete excision, whereas a 5-year interval is favored if there are limited concerns about consult pathologist ability to distinguish SSP from HP, adequacy of bowel preparation, or complete polyp excision. The Task Force acknowledges the importance of accurate polyp size estimation for this recommendation and recommends photo documentation verifying polyp size relative to an open forceps or open snare of known size.
For patients with SSP containing dysplasia at a high-quality examination, repeat colonoscopy in 3 years.
(Weak recommendation, very low quality of evidence)
No new evidence regarding outcomes of surveillance in individuals with isolated SSP containing dysplasia was identified. SSP with dysplasia is rare; in one series of 179,111 patients with polyps submitted for histologic examination, of 2139 SSPs identified, 302 contained low- or high-grade dysplasia.54 Dysplastic SSPs have more features consistent with CRC than SSPs without dysplasia. In absence of additional data on whether metachronous neoplasia risk differs for individuals with SSP and dysplasia compared to SSP without dysplasia, the Task Force recommends repeat colonoscopy in 3 years after SSP with dysplasia diagnosis, as long as a high-confidence complete resection of the lesion was performed.
For patients with history of baseline adenoma removal and 1 subsequent colonoscopy, recommendations for subsequent surveillance should take into account findings at baseline and first surveillance (Table 7).
(Weak recommendation, low quality of evidence)
Table 7.
Recommendations for Second Surveillance Stratified by Adenoma Findings at Baseline and First Surveillance
| Baseline finding | Recommended interval for first surveillance | Finding at first surveillance | Recommended interval for next surveillance |
|---|---|---|---|
| 1–2 tubular adenomas <10 mm | 7–10 y | Normal colonoscopya | 10 y |
| 1–2 tubular adenomas <10 mm | 7–10 y | ||
| 3–4 tubular adenomas <10 mm | 3–5 y | ||
| Adenoma ≥10 mm in size; or adenoma with tubulovillous/villous histology; or adenoma with high grade dysplasia; or 5–10 adenomas <10 mm | 3 y | ||
| 3–4 tubular adenomas <10 mm | 3–5 y | Normal colonoscopya | 10 y |
| 1–2 tubular adenomas <10 mm | 7–10 y | ||
| 3–4 tubular adenomas <10 mm | 3–5 y | ||
| Adenoma ≥10 mm in size; or adenoma with tubulovillous/villous histology; or adenoma with high grade dysplasia; or 5–10 adenomas <10 mm | 3 y | ||
| Adenoma ≥10 mm in size; or adenoma with tubulovillous/villous histology; or adenoma with high-grade dysplasia; or 5–10 adenomas <10 mm | 3 y | Normal colonoscopya | 5 y |
| 1–2 tubular adenomas <10 mm | 5 y | ||
| 3–4 tubular adenomas <10 mm | 3–5 y | ||
| Adenoma ≥10 mm in size; or adenoma with tubulovillous/villous histology; or adenoma with high grade dysplasia; or 5–10 adenomas <10 mm | 3 y |
Normal colonoscopy is defined as colonoscopy where no adenoma, SSP, or CRC is found.
We identified several studies on serial surveillance published since 2012.30,55–59 Findings from the largest of these studies,30,55,56 as well as those considered as part of the 2012 recommendations, are summarized in Table 8. Across all studies, individuals with low-risk adenoma at baseline and no adenoma at first surveillance had low rates of high-risk adenoma on follow-up, ranging from 1% to 6.6%. Similarly, across all but one of the studies reviewed, individuals with high-risk adenoma at both baseline and subsequent surveillance examination have >18% rate of metachronous high-risk adenoma on follow-up, supporting our recommendation for follow-up colonoscopy in 3 years. However, the outcomes at second surveillance for other clinical scenarios of baseline and first surveillance findings are more variable across studies. Our recommendations for second surveillance colonoscopy based on findings at baseline and first surveillance are summarized in Table 7. More evidence is needed to clarify the best intervals for surveillance in patients who have had baseline and repeat colonoscopy, particularly for those with low-risk adenoma at baseline and follow-up. Also, new evidence is required to guide serial surveillance of individuals with SSPs and large HPs.
There is insufficient evidence to recommend use of currently published prediction models for polyp surveillance recommendations.
(Weak recommendation, very low quality of evidence)
Table 8.
Risk for Neoplasia at Second Surveillance Stratified by Findings at Baseline and First Surveillance HRA, high-risk adenoma; advanced adenoma or ≥3 adenomas; LRA, low-risk adenoma; 1–2 nonadvanced adenomas.
| HRA at second surveillance, % | |||||||
|---|---|---|---|---|---|---|---|
| Baseline finding | First surveillance finding | Morelli et al, 201355 (n = 965) | Park et al, 201556 (n = 2087) | van Heijningen, et al, 201330 (n = 1482)a | Pinsky et al, 2009109 (n = 1032)a | Laiyemo et al, 2009110 (n = 1297) | Robertson et al, 2009111 (n = 564) |
| LRA | No adenoma | 6.6 | 6.0 | 1.0 | 3.9 | 2.8 | 4.9 |
| LRA | 13.8 | 10.6 | 1.0 | 5.7 | 4.7 | 9.5 | |
| HRA | 18.0 | 16.4 | 0.0 | 15.6 | 6.9 | 20.0 | |
| HRA | No adenoma | 9.6 | 6.7 | 4.0 | 5.9 | 4.8 | 12.3 |
| LRA | 14.0 | 24.3 | 3.0 | 6.7 | 8.9 | 13.6 | |
| HRA | 22.0 | 38.2 | 4.0 | 19.3 | 30.6 | 18.2 | |
Risk and outcome characterized based on nonadvanced and advanced adenoma.
Multiple models have been developed to stratify the risk of metachronous neoplasia and guide surveillance.27,30,58,60–64 Results are promising, but incremental value over current risk-stratification recommendations informed by number, size, and histology of polyps is unclear. For example, a comprehensive model including polyp size, villous histology, proximal location, and number of adenomas had a superior C-statistic compared with the 2012 Task Force guidelines, but the magnitude of improvement was small (0.71 for the model vs 0.66 for 2012 guidelines).30 An important limitation of current published work is that many of these studies have not included a test and independent validation set, raising concerns about generalizability.27,30,60,61 Additionally, the range of variables utilized as part of models varies considerably. Notably, models reviewed here suggest the best predictors of future risk for advanced neoplasia remain baseline colonoscopy polyp findings.
Evidence is insufficient to recommend differential management for patients with proximal adenoma.
(Weak recommendation, very low quality of evidence)
Among patients with 1–2 adenomas <10 mm in size, having at least 1 proximal adenoma was associated with increased risk for metachronous advanced neoplasia in a pooled analysis of 7 prospective studies.24 In another study, among patients with any adenoma, having at least one proximal adenoma was associated with 1.17-fold increased risk for any metachronous adenoma, but no increased risk for metachronous advanced neoplasia.65 A cohort study in the Netherlands of 2990 patients diagnosed with adenoma from 1988 to 2002 and followed through 2008 with medical record review found proximal location was associated with a 1.6-fold increased risk for advanced adenoma at follow-up.30 As mentioned previously, a study of intermediate risk (1–2 >10 mm adenomas or 3–4 adenomas any size) found that proximal adenoma was associated with increased risk for incident CRC,19 but another study found similar risk for incident CRC among individuals with 1–2 proximal only vs distal only adenomas <10 mm in size.18 Taken together, given these varying results, more research is needed to determine whether proximal adenoma location should be considered as a specific factor for modifying surveillance recommendations.
For patients with piecemeal resection of adenoma or SSP >20 mm, repeat colonoscopy in 6 months.
(Strong recommendation, moderate quality of evidence)
Piecemeal polyp resection contributes to risk for metachronous neoplasia. A meta-analysis by Belderbos et al66 of 33 studies found risk for recurrent neoplasia was 20% for piecemeal vs just 3% for en bloc resection utilizing endoscopic mucosal resection (EMR) technique. In the subgroup with EMR of polyps 10–20 mm in size, piecemeal resection was associated with an 18% risk for recurrent neoplasia, similar to the 19% rate observed for polyps 20–30 mm and >30 mm in size. Pohl et al52 studied rate of incomplete resection using biopsy immediately after assumed complete resection of 5–20 mm polyps, including patients with and without EMR. Incomplete resection was more common with piecemeal (20%) vs en bloc resection (8.4%), but piecemeal resection was not an independent predictor of incomplete resection after adjusting for size and histology. For polyps ≥20 mm, additional articles67,68 since the Belderbos et al meta-analysis have reported high risk for recurrent neoplasia associated with piecemeal vs en block resection. These findings suggest that colonoscopists must emphasize complete polyp excision at baseline and, particularly for polyps ≥20 mm in size, consider strategies for verifying complete excision. The evidence base to support management of patients with polyps ≥20 mm in size resected piecemeal has been reviewed in detail in the recent Task Force recommendations on endoscopic removal of colorectal lesions.69 Based on the evidence reviewed, the Task Force recommended patients with polyps ≥20 mm resected piecemeal have first surveillance colonoscopy at approximately 6 months, second surveillance 1 year from first surveillance, and third surveillance 3 years from the second surveillance.
Other Risk Factors for Metachronous Neoplasia
Since the 2012 recommendations, a number of studies have reported on risk factors for metachronous neoplasia. Smoking may be associated with risk for recurrent conventional adenoma as well as serrated polyps.70,71 Environmental factors, such as rural vs urban residence, may contribute to risk for cancer after advanced adenoma removal.72 Metabolic syndrome71,73,74 (as well as components of this diagnosis, such as increased waist to hip ratio, increased hip circumference) and obesity73–75 have been reported by a number of studies to be associated with increased risk for recurrent neoplasia. Race does not appear to modify risk for recurrent adenoma and metachronous advanced neoplasia. A retrospective cohort study of 246 whites and 203 black patients who had an adenoma at baseline and at least 1 surveillance colonoscopy found similar rates of recurrent adenoma and advanced neoplasia.76 A cohort study of participants in the Polyp Prevention Trial compared risk for metachronous adenoma and advanced neoplasia among 1668 white and 153 black patients with adenoma at baseline, all of whom received surveillance colonoscopy, found no difference in rate of metachronous adenoma or advanced neoplasia.77 Thus, while there is evidence that black patients have a higher age-adjusted incidence and mortality from CRC and develop CRC at a younger age than other racial and ethnic groups in the United States, once screened, there is no robust evidence that black race modifies the risk for recurrent adenoma or advanced neoplasia. Having a flat adenoma may increase risk for recurrent neoplasia, but more data are needed to support differential management.78 Diet might modify risk, but new evidence to support its impact is limited. One study found no clear association between fruit and vegetable intake and risk for adenoma recurrence,79 and another pooled study of 1727 participants from 2 randomized trials did not identify a relationship between proinflammatory diet and risk for adenoma, advanced adenoma, or 3 or more adenomas on follow-up colonoscopy after initial polypectomy.80 Lifestyle factors, such as increased sedentary behavior, may increase risk for adenoma recurrence,81 but it is unclear whether specifically modifying behavior will reduce risk.
Since 2012, several studies have been published on chemopreventive strategies for reducing risk for recurrent neoplasia. A large, well-done randomized controlled trial found that supplementation with calcium or vitamin D (alone or in combination) was not associated with reduced risk for recurrent neoplasia,82 and a small study that included intervention with calcitriol, aspirin, and calcium also found no benefit on risk for recurrent neoplasia.83 A prospective cohort study reported that dietary supplement use was not associated with reduced risk of metachronous neoplasia.84 An observational study demonstrated that exposure to metformin was associated with reduced risk for finding adenoma at surveillance colonoscopy among diabetics,85 and a pilot randomized controlled trial of nondiabetic subjects found that low-dose metformin was associated with reduced risk for recurrent adenoma at 1 year,86 suggesting metformin may be a promising chemopreventive agent warranting further study.
Newly published work has confirmed that aspirin and exposure to nonsteroidal anti-inflammatory medications may reduce risk for adenoma recurrence, but optimal dose, mechanism of action, and characteristics of patients most likely to benefit have not been well established.87,88 While there is insufficient evidence to support routine recommendation of aspirin for cancer and adenoma prevention in patients with baseline adenoma, the overall impact of aspirin on cardiovascular disease (CVD) and CRC risk reduction might support recommending aspirin for some patients. Specifically, it should be noted that, for patients aged 50–59 years who have ≥10% risk for CVD and life expectancy of ≥10 years, without increased risk for bleeding, the US Preventive Services Task Force has recommended use of aspirin 81 mg per day for primary prevention of both CVD and CRC (grade B recommendation), and has recommended that aspirin could also be considered for patients aged 60–69 years based on shared decision making, taking into account potential harms and benefits (grade C recommendation).89 Thus, for patients who inquire about strategies to reduce future CRC risk after polypectomy, an opportunity exists to recommend estimation of cardiovascular risk and to consider aspirin for both CVD and CRC risk reduction if these criteria are met.
In summary, there is little evidence that lifestyle factors, such as diet, smoking, obesity, and sedentary behavior, increase the risk of metachronous neoplasia, or that modification of these behaviors reduces the risk. Likewise, there is little new evidence that chemoprevention impacts the risk of metachronous advanced neoplasia in patients with adenoma. At this time, there is insufficient evidence to recommend modification of surveillance intervals based on these factors. More work needs to be done to identify risk factors and chemopreventive strategies that can reduce risk for metachronous neoplasia and possibly allow for less frequent surveillance colonoscopy.
Discussion
Currently, the interval for screening and surveillance colonoscopy is based on stratification of risk for metachronous advanced neoplasia. Since the last recommendations by the Task Force in 2012, evidence to support low risk for incident and fatal cancer after normal colonoscopy has strengthened the recommendation to defer repeat screening for at least 10 years. Among patients with polyps, new data suggest that patients with 1–2 adenomas <10 mm are at lower than average risk for incident and fatal CRC and can undergo colonoscopy at longer intervals. Individuals with advanced neoplasia appear to remain at a greater than population risk for CRC after polypectomy. New data are emerging to support less frequent surveillance among individuals with 3–4 adenomas <10 mm in size. The literature on risk for subsequent neoplasia in those with serrated lesions is at an early stage (relative to those with conventional adenomas) and continues to evolve. Those with a combination of both serrated lesions and conventional adenomas appear to be a higher-risk group for subsequent advanced neoplasia. Encouragingly, 2 studies suggest that exposure to surveillance colonoscopy after baseline polypectomy (compared to no surveillance) may reduce risk for incident CRC among high-risk patients, but more data are needed to support the incremental benefit of post-polypectomy surveillance for reducing incidence and mortality from CRC.
Given that risk for metachronous advanced neoplasia has been accepted thus far as a surrogate for risk for incident CRC, and the plethora of studies that have examined risk for metachronous advanced neoplasia among individuals with baseline polyps, the Task Force has provided updated recommendations for surveillance based on the relationship of baseline findings to risk for metachronous advanced neoplasia. Key updates since the 2012 US Multi-Society Task Force recommendations are summarized in Table 9. Recommendations for patients with advanced adenoma, including those with adenoma ≥10 mm, or containing high-grade dysplasia and/or villous features are unchanged, with evidence to support close surveillance in 3 years strengthened. One year, rather than a more general recommendation for <3-year follow-up colonoscopy for individuals with >10 adenomas at a single examination, has been recommended to simplify follow-up, although the evidence base to support this strategy has not been markedly strengthened. Emerging evidence suggests that individuals with 3–4 adenomas <10 mm are at low risk for metachronous neoplasia, supporting our recommendation for a 3- to 5-year interval rather than a strict 3-year follow-up colonoscopy for this group of patients. Another significant change from prior guidance is our recommendation for surveillance colonoscopy in 7–10 years rather than 5–10 years for patients with 1–2 adenomas <10 mm, based on the growing body of evidence to support low risk for metachronous advanced neoplasia. In this population, the risk for metachronous advanced neoplasia is similar to that for individuals with no adenoma (Figure 2). Importantly, the observed risk for fatal CRC among individuals with 1–10 adenomas <10 mm is lower than average for the general population. The largest cohort study to date including patients with SSPs offers evidence to support follow-up in <10 years (5–10 years for 1–2 SSPs <10 mm, 3–5 years for 3–4 SSPs <10 mm, and 3 years for 5–10 SSPs, SSP ≥10 mm, or SSP with dysplasia), based on observed increased risk for metachronous large SSP.
Table 9.
Key Updates Since 2012 Recommendations Provided in the 2019 US Multi-Society Task Force Recommendations for Follow-Up After Colonoscopy and Polypectomy
|
Our review highlights several opportunities for research to clarify risk stratification and management of patients post polypectomy. In order to optimize risk-reduction strategies, the mechanisms driving metachronous advanced neoplasia after baseline polypectomy and their relative frequency need to be better understood through studies that include large numbers of patients with interval cancers and/or advanced neoplasia after baseline polypectomy. Mechanisms may include new/incident growth, incomplete baseline resection, and missed neoplasia; each of these potential causes may require different interventions for improvement.90 For example, if most interval cancers after polypectomy are attributable to missed neoplasia,91,92 redoubled focus on quality of baseline examination may be indicated. Indeed, quality factors, such as incomplete examination and poor bowel preparation, have been associated with risk for cancer after polypectomy.19,30,31 Further, it is plausible that the ADR of a colonoscopist, which has been tied closely with risk for interval cancer after normal screening colonoscopy,93,94 might have a similar correlation with risk for interval cancer after polypectomy. If incomplete resection is the major cause of metachronous neoplasia after polypectomy,65 focus on implementing strategies that improve polypectomy technique may be indicated. If the main driver is incident neoplasia, then strategies that optimize risk stratification and timing of colonoscopy (early for high risk and deferred for low risk) might be most impactful. Interestingly, one study has found that the attributable fraction of risk for CRC after baseline polypectomy is highest for incomplete polyp removal and not having “on time” follow-up colonoscopy, underscoring the importance of complete removal and appropriate follow-up intervals.31 More work is needed to identify the key drivers of metachronous advanced neoplasia, particularly CRC. Application of precision medicine, such as offering chemoprevention to individuals with genotypes associated with response to therapy, may improve effectiveness of chemoprevention, but requires further study.95 Biomarkers of adenoma recurrence also merit study.96–100 Widespread promotion of colonoscopist ADR as a quality metric is likely to increase the frequency of diagnosing patients with multiple small adenomas. Because finding multiple small adenomas may be a marker of careful colonoscopy, patients with multiple (eg, 1–4) small adenomas may be subject to a so-called “adenoma detector paradox,” in which they are currently recommended short-interval (eg, 3 years) colonoscopy despite potentially having very low risk for incident CRC secondary to having a very-high-quality examination. Although we have recommended 3- to 5-year follow-up for individuals with 3–4 small adenomas based on emerging evidence, understanding the implications of having multiple small adenomas should be a key focus of future research. We found few data to guide management of individuals with isolated HPs <10 mm. Future research should clarify whether these individuals are indeed a low-risk group, as uncertainties remain about frequency of misdiagnosis of small SSPs as HPs, and whether patients with small HPs proximal to the sigmoid colon or in the rectum or sigmoid colon have significantly increased risk for either large serrated polyps or advanced neoplasia on follow-up.
Beyond risk stratification, more fundamental research on the potential benefits of surveillance is needed. In particular, better evidence is needed to support whether exposure to surveillance colonoscopy, compared to no surveillance, reduces CRC incidence or mortality. Such evidence is needed given the increasing proportion of patients who are having adenomas detected as part of increased participation in CRC screening.
Several areas not covered by our current recommendations also warrant investigation. We do not provide recommendations for management of young patients (<50 years) with incidentally detected adenoma, although evidence to guide management is emerging.101,102 At the other end of the age spectrum, more research is needed to determine whether the potential cancer prevention and early detection benefits of surveillance outweigh immediate procedure-related risks for individuals older than age 75 years, or with multiple comorbidities. Cost-effectiveness of surveillance, as well as alternative strategies for surveillance (such as fecal immunochemical testing or multi-target fecal immunochemical testing-DNA) requires further study. Indeed, one modeling study has suggested that surveillance fecal immunochemical testing (rather than colonoscopy) might be effective post-polypectomy.103
As a result of our review, we have several suggestions for best practices to improve the quality and comparability of future research on post-polypectomy surveillance. Studies vary in their definitions of high-risk adenoma. Ideally, when considering both predictors and outcomes, we suggest as a best practice reporting presence of individual findings (eg, villous adenoma, SSP, and HP ≥10 mm) in addition to several potentially clinical relevant summary categories, including advanced neoplasia, advanced adenoma, and large serrated polyp (HP or SSP ≥10 mm). Because our understanding of the risks and outcomes among patients with SSPs is still limited, we suggest it is particularly important to separate SSPs from aggregate predictor or outcome categories, such as advanced neoplasia. Further, we suggest specifically reporting SSP, HPs, and TSAs separately as predictors and outcomes, and clearly defining any aggregate categories (such as serrated polyps ≥10 mm) precisely. Providing histology-specific data will allow for greater comparability across studies, and better assessment of whether outcomes differ by serrated polyp histology. For example, histology-specific outcome data could help elucidate whether individuals with HP ≥10 mm have outcomes similar to those of patients with SSP ≥10 mm. More studies are needed that include patients that are racially and ethnically diverse. Most surveillance studies provide limited data on the quality of baseline colonoscopy, which could help in interpreting results. Additionally, we recommend that both relative and absolute risks for outcomes, such as metachronous advanced neoplasia, be provided in surveillance studies. Absolute risks are key to providing perspective to patients and physicians on the true risk associated with a given polyp-finding scenario. Studies examining the potential benefit of exposure to surveillance vs no surveillance should seek to avoid several potential sources of bias. First, risk for cancer associated with adenoma is often compared to the general population, not to people who had normal colonoscopy. Comparing cancer risk among individuals with adenoma removal to a general population without ascertaining for presence of CRC or adenoma may bias towards underestimating risk reduction that can be gained by removing adenomas.8,16,17,104 Second, risk for cancer associated with surveillance is often compared to the general population, not to people who had polypectomy but no surveillance; this may bias towards an overestimation of the benefit of surveillance.8,15,16 Also, risk for cancer associated with surveillance often excludes cancers diagnosed within 1 year, which may bias towards overestimating benefit of surveillance because in usual practice, surveillance time frames are assigned based on initial results, not initial results plus clinical course within a year.16,105 Finally, some studies may compare outcomes among patients who did not receive surveillance to those who survived cancer free and received surveillance.19 This is analogous to a per-protocol analysis of a randomized trial, may overestimate the benefit of surveillance, and may be considered a form of immortal time bias. Additionally, we note that very few randomized trials of surveillance strategies have been done. In the United States, the National Polyp Study is the only randomized controlled trial of surveillance colonoscopy. This study was conducted in the 1980s before availability of modern technology (eg, high-definition colonoscopies) and widespread awareness of importance of quality on outcomes, employed a highly aggressive baseline polyp-clearing strategy, and compared a very short 1- vs 3-year follow-up interval among patients with baseline adenoma.106 The European Polyp Surveillance trial, which includes arms randomized to different surveillance intervals based on specific baseline polyp-finding strata, is well underway and will likely offer new insights to guide polyp surveillance.107 Lack of randomized trials in the area of surveillance is quite remarkable, given the frequency of surveillance colonoscopy in usual practice and in the context of the many trials that are available on CRC screening.
Several limitations may be considered in interpreting and applying our recommendations to practice. Our recommendations for surveillance intervals depend on the performance of a high-quality examination (as evidenced by examination complete to the cecum with adequate bowel preparation and complete polyp resection) by a high-quality colonoscopist (based on adequate ADR). This requires that colonoscopists continuously strive to improve quality, but also use caution in applying surveillance recommendations when concerns about quality exist. We focused on updating our recommendations based on a literature review of articles published since the prior recommendations were issued in 2012, and did not perform pooled or meta-analyses. A more comprehensive literature review of all articles published relevant to surveillance over a longer time period, as well as meta-analyses, were beyond the scope of this work. In many cases, our recommendations are based on very-low- or low-quality evidence. Even where evidence was judged to be of moderate or high quality, few studies were randomized trials. Thus, future research has a high likelihood of producing evidence that may change recommendations, particularly those based on lower-quality evidence. We recognize the challenge of applying new recommendations in practice, such as a 7- to 10-year, rather than a 5- to 10-year follow-up recommendation for patients with 1–2 adenomas <10 mm. Patients, primary care physicians, and colonoscopists may have concerns about lengthening a previously recommended interval, and will need to engage in shared decision making regarding whether to lengthen the follow-up interval based upon the guidance here or utilize the recommendation made at the time of the prior colonoscopy.
Conclusions
CRC incidence and mortality are decreasing secondary to improvements in risk factor exposures, screening, treatment, and perhaps exposure to surveillance among patients with polyps.108 Given that some patients with polyps appear to have persistently increased risk for CRC, and many have increased risk for advanced neoplasia on follow-up, surveillance colonoscopy to attempt to reduce CRC risk is clinically rational and recommended. Evidence to support best practices for surveillance colonoscopy has strengthened and has helped to support close follow-up for some groups, as well as less intense follow-up for others. More work is needed to fully understand which patient populations are most likely to benefit from surveillance, and the ideal surveillance interventions to apply for optimizing CRC prevention and early detection.
Supplementary Material
Acknowledgments
The views expressed in this article are those of the author(s) and do not necessarily represent the views of the Department of Veterans Affairs.
The authors thank Karen Heskett, medical librarian, UC San Diego, for her assistance with conduct of our literature review.
Funding
This work was supported in part by the National Cancer Institute (NCI) of the National Institutes of Health (NIH) under Award Number R37 CA222866 and the Department of Veterans Affairs under Award Number I01 HX001574, as well as NIH awards P30CA06516 and UO1 CA864000.
Abbreviations used in this paper:
- ADR
adenoma detection rate
- CI
confidence interval
- CRC
colorectal cancer
- CVD
cardiovascular disease
- EMR
endoscopic mucosal resection
- HR
hazard ratio
- SIR
standardized incidence ratio
- SSP
sessile serrated polyp
- HP
hyperplastic polyp
- OR
odds ratio
- RR
relative risk
- TSA
traditional serrated adenoma
Footnotes
Supplementary Material
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at https://doi.org/10.1053/j.gastro.2019.10.026.
Conflicts of interest
The authors disclose no conflicts of interest relative to the current work since 2016. Industry relationships for authors (consulting, research, reimbursement) without conflict of interest relevant to the current work since 2016: Douglas K. Rex (Olympus, Boston Scientific, Covidien, Lumendi, Salix, Aries, Cook Medical, ERBE, Bausch Health Inc, Novo Nordisk, Endochoice, Braintree Laboratories, Norgine, Endokey, EndoAid, Medivators, Satisfai Health); Sapna Syngal (Chirhoclin, Cook, Myriad Genetics, Inc, DC Health, Inc); David Lieberman (Covidien, Freenome Holdings, Inc, Ironwood, Check-Cap, CEGX); Douglas Robertson (Covidien, Freenome Holdings, Inc, Amadix); Tonya Kaltenbach (Aries Pharmaceuticals, Micro-Tech Endoscopy, Olympus, Boston Scientific, Medtronic); Aasma Shaukat (None); Samir Gupta (Freenome Holdings, Inc, Guardant Health, Inc, Mallinckrodt Pharmaceuticals); Carol Burke (Salix Pharmaceuticals, Ferring Pharmaceuticals, Aries Pharmaceuticals, Intuitive Surgical, Pfizer, Covidien, Boston Scientific, US Endoscopy, Abbvie Cancer Prevention Pharmaceuticals, Janssen Pharmaceuticals; SLA Pharma AG; Freenome Holdings, Inc). Jason Dominitz (None); Joseph C. Anderson (None).
References
- 1.Heitman SJ, Ronksley PE, Hilsden RJ, et al. Prevalence of adenomas and colorectal cancer in average risk individuals: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2009;7:1272–1278. [DOI] [PubMed] [Google Scholar]
- 2.Lieberman DA, Rex DK, Winawer SJ, et al. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2012;143:844–857. [DOI] [PubMed] [Google Scholar]
- 3.Kahi CJ, Boland CR, Dominitz JA, et al. Colonoscopy surveillance after colorectal cancer resection: recommendations of the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2016;150:758–768.e11. [DOI] [PubMed] [Google Scholar]
- 4.Giardiello FM, Allen JI, Axilbund JE, et al. Guidelines on genetic evaluation and management of Lynch syndrome: a consensus statement by the US Multi-society Task Force on colorectal cancer. Am J Gastroenterol 2014; 109:1159–1179. [DOI] [PubMed] [Google Scholar]
- 5.Kahi CJ, Boland CR, Dominitz JA, et al. Colonoscopy surveillance after colorectal cancer resection: recommendations of the US Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol 2016;111:337–346; quiz 347. [DOI] [PubMed] [Google Scholar]
- 6.Kahi CJ, Boland CR, Dominitz JA, et al. Colonoscopy surveillance after colorectal cancer resection: recommendations of the US Multi-Society Task Force on colorectal cancer. Gastrointest Endosc 2016;83:489–498.e10. [DOI] [PubMed] [Google Scholar]
- 7.Rex DK, Schoenfeld PS, Cohen J, et al. Quality indicators for colonoscopy. Gastrointest Endosc 2015; 81:31–53. [DOI] [PubMed] [Google Scholar]
- 8.Nishihara R, Wu K, Lochhead P, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med 2013;369:1095–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Samadder NJ, Pappas L, Boucherr KM, et al. Long-term colorectal cancer incidence after negative colonoscopy in the state of Utah: the effect of family history. Am J Gastroenterol 2017;112:1439–1447. [DOI] [PubMed] [Google Scholar]
- 10.Lee JK, Jensen CD, Levin TR, et al. Long-term risk of colorectal cancer and related deaths after a colonoscopy with normal findings. JAMA Intern Med 2019;179:153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brenner H, Altenhofen L, Stock C, et al. Incidence of colorectal adenomas: birth cohort analysis among 4.3 million participants of screening colonoscopy. Cancer Epidemiol Biomarkers Prev 2014;23:1920–1927. [DOI] [PubMed] [Google Scholar]
- 12.Sonnenberg A, Delco F. Cost-effectiveness of a single colonoscopy in screening for colorectal cancer. Arch Intern Med 2002;162:163–168. [DOI] [PubMed] [Google Scholar]
- 13.Knudsen AB, Zauber AG, Rutter CM, et al. Estimation of benefits, burden, and harms of colorectal cancer screening strategies: modeling study for the US Preventive Services Task Force. JAMA 2016;315:2595–2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knudsen AB, Hur C, Gazelle GS, et al. Rescreening of persons with a negative colonoscopy result: results from a microsimulation model. Ann Intern Med 2012;157:611–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coleman HG, Loughrey MB, Murray LJ, et al. Colorectal cancer risk following adenoma removal: a large prospective population-based cohort study. Cancer Epidemiol Biomarkers Prev 2015;24:1373–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cottet V, Jooste V, Fournel I, et al. Long-term risk of colorectal cancer after adenoma removal: a population-based cohort study. Gut 2012;61:1180–1186. [DOI] [PubMed] [Google Scholar]
- 17.Løberg M, Kalager M, Holme Ø, et al. Long-term colorectal-cancer mortality after adenoma removal. N Engl J Med 2014;371:799–807. [DOI] [PubMed] [Google Scholar]
- 18.Click B, Pinsky PF, Hickey T, et al. Association of colonoscopy adenoma findings with long-term colorectal cancer Laiyemo Lincidence. JAMA 2018;319:2021–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Atkin W, Wooldrage K, Brenner A, et al. Adenoma surveillance and colorectal cancer incidence: a retrospective, multicentre, cohort study. Lancet Oncol 2017; 18:823–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Erichsen R, Baron JA, Hamilton-Dutoit SJ, et al. Increased risk of colorectal cancer development among patients with serrated polyps. Gastroenterology 2016; 150:895–902.e5. [DOI] [PubMed] [Google Scholar]
- 21.Holme O, Bretthauer M, Eide TJ, et al. Long-term risk of colorectal cancer in individuals with serrated polyps. Gut 2015;64:929–936. [DOI] [PubMed] [Google Scholar]
- 22.Hassan C, Gimeno-Garcia A, Kalager M, et al. Systematic review with meta-analysis: the incidence of advanced neoplasia after polypectomy in patients with and without low-risk adenomas. Aliment Pharmacol Ther 2014;39:905–912. [DOI] [PubMed] [Google Scholar]
- 23.Dube C, Yakubu M, McCurdy BR, et al. Risk of advanced adenoma, colorectal cancer, and colorectal cancer mortality in people with low-risk adenomas at baseline colonoscopy: a systematic review and meta-analysis. Am J Gastroenterol 2017;112:1790–1801. [DOI] [PubMed] [Google Scholar]
- 24.Gupta S, Jacobs ET, Baron JA, et al. Risk stratification of individuals with low-risk colorectal adenomas using clinical characteristics: a pooled analysis. Gut 2017; 66:446–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bjerrum A, Milter MC, Andersen O, et al. Risk stratification and detection of new colorectal neoplasms after colorectal cancer screening with faecal occult blood test: experiences from a Danish screening cohort. Eur J Gastroenterol Hepatol 2015;27:143–147. [DOI] [PubMed] [Google Scholar]
- 26.Fairley KJ, Li J, Komar M, et al. Predicting the risk of recurrent adenoma and incident colorectal cancer based on findings of the baseline colonoscopy. Clin Transl Gastroenterol 2014;5:e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Good NM, Macrae FA, Young GP, et al. Ideal colonoscopic surveillance intervals to reduce incidence of advanced adenoma and colorectal cancer. J Gastroenterol Hepatol 2015;30:1147–1154. [DOI] [PubMed] [Google Scholar]
- 28.Jang HW, Park SJ, Hong SP, et al. Risk factors for recurrent high-risk polyps after the removal of high-risk polyps at initial colonoscopy. Yonsei Med J 2015; 56:1559–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park SK, Song YS, Jung YS, et al. Do surveillance intervals in patients with more than five adenomas at index colonoscopy be shorter than those in patients with three to four adenomas? A Korean Association for the Study of Intestinal Disease study. J Gastroenterol Hepatol 2017;32:1026–1031. [DOI] [PubMed] [Google Scholar]
- 30.van Heijningen EM, Lansdorp-Vogelaar I, Kuipers EJ, et al. Features of adenoma and colonoscopy associated with recurrent colorectal neoplasia based on a large community-based study. Gastroenterology 2013; 144:1410–1418. [DOI] [PubMed] [Google Scholar]
- 31.Brenner H, Chang-Claude J, Jansen L, et al. Role of colonoscopy and polyp characteristics in colorectal cancer after colonoscopic polyp detection: a population-based case-control study. Ann Intern Med 2012; 157:225–232. [DOI] [PubMed] [Google Scholar]
- 32.Buchner AM, Shahid MW, Heckman MG, et al. High-definition colonoscopy detects colorectal polyps at a higher rate than standard white-light colonoscopy. Clin Gastroenterol Hepatol 2010;8:364–370. [DOI] [PubMed] [Google Scholar]
- 33.Pérez-Cuadrado-Robles E, Torrella-Cortés E, Bebia-Conesa P, et al. Intermediate-risk patients with three to four small adenomas should be considered low risk for colorectal cancer screening. Dig Endosc 2016;28:450–455. [DOI] [PubMed] [Google Scholar]
- 34.Sneh Arbib O, Zemser V, Leibovici Weissman Y, et al. Risk of advanced lesions at the first follow-up colonoscopy after polypectomy of diminutive versus small adenomatous polyps of low-grade dysplasia. Gastrointest Endosc 2017;86:713–721.e2. [DOI] [PubMed] [Google Scholar]
- 35.Vemulapalli KC, Rex DK. Risk of advanced lesions at first follow-up colonoscopy in high-risk groups as defined by the United Kingdom post-polypectomy surveillance guideline: data from a single U.S. center. Gastrointest Endosc 2014;80:299–306. [DOI] [PubMed] [Google Scholar]
- 36.van Enckevort CC, de Graaf AP, Hollema H, et al. Predictors of colorectal neoplasia after polypectomy: based on initial and consecutive findings. Neth J Med 2014; 72:139–145. [PubMed] [Google Scholar]
- 37.Park SK, Hwang SW, Kim KO, et al. Risk of advanced colorectal neoplasm in patients with more than 10 adenomas on index colonoscopy: A Korean Association for the Study of Intestinal Diseases (KASID) study. J Gastroenterol Hepatol 2017;32:803–808. [DOI] [PubMed] [Google Scholar]
- 38.Grover S, Kastrinos F, Steyerberg EW, et al. Prevalence and phenotypes of APC and MUTYH mutations in patients with multiple colorectal adenomas. JAMA 2012; 308:485–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Syngal S, Brand RE, Church JM, et al. ACG clinical guideline: genetic testing and management of hereditary gastrointestinal cancer syndromes. Am J Gastroenterol 2015;110:223–262; quiz 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Provenzale D, Gupta S, Ahnen D, et al. NCCN Clinical Practice Guidelines in Oncology: Genetic/Familial High-Risk Assessment: Colorectal Version 1.2018. J Natl Compr Canc Netw 2018;16:939–949. [DOI] [PubMed] [Google Scholar]
- 41.Anderson JC, Butterly LF, Robinson CM, et al. Risk of metachronous high-risk adenomas and large serrated polyps in individuals with serrated polyps on index colonoscopy: data from the New Hampshire Colonoscopy Registry. Gastroenterology 2018;154:117–127 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Payne SR, Church TR, Wandell M, et al. Endoscopic detection of proximal serrated lesions and pathologic identification of sessile serrated adenomas/polyps vary on the basis of center. Clin Gastroenterol Hepatol 2014; 12:1119–1126. [DOI] [PubMed] [Google Scholar]
- 43.Ensari A, Bilezikci B, Carneiro F, et al. Serrated polyps of the colon: how reproducible is their classification? Virchows Arch 2012;461:495–504. [DOI] [PubMed] [Google Scholar]
- 44.Khalid O, Radaideh S, Cummings OW, et al. Reinterpretation of histology of proximal colon polyps called hyperplastic in 2001. World J Gastroenterol 2009; 15:3767–3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wong NA, Hunt LP, Novelli MR, et al. Observer agreement in the diagnosis of serrated polyps of the large bowel. Histopathology 2009;55:63–66. [DOI] [PubMed] [Google Scholar]
- 46.Bustamante-Balen M, Bernet L, Cano R, et al. Assessing the reproducibility of the microscopic diagnosis of sessile serrated adenoma of the colon. Rev Esp Enferm Dig 2009;101:258–264. [PubMed] [Google Scholar]
- 47.Farris AB, Misdraji J, Srivastava A, et al. Sessile serrated adenoma: challenging discrimination from other serrated colonic polyps. Am J Surg Pathol 2008; 32:30–35. [DOI] [PubMed] [Google Scholar]
- 48.Macaron C, Vu HT, Lopez R, et al. Risk of metachronous polyps in individuals with serrated polyps. Dis Colon Rectum 2015;58:762–768. [DOI] [PubMed] [Google Scholar]
- 49.Melson J, Ma K, Arshad S, et al. Presence of small sessile serrated polyps increases rate of advanced neoplasia upon surveillance compared with isolated low-risk tubular adenomas. Gastrointest Endosc 2016; 84:307–314. [DOI] [PubMed] [Google Scholar]
- 50.Pereyra L, Zamora R, Gomez EJ, et al. Risk of metachronous advanced neoplastic lesions in patients with sporadic sessile serrated adenomas undergoing colonoscopic surveillance. Am J Gastroenterol 2016; 111:871–878. [DOI] [PubMed] [Google Scholar]
- 51.Anderson JC, Butterly LF, Weiss JE, et al. Providing data for serrated polyp detection rate benchmarks: an analysis of the New Hampshire Colonoscopy Registry. Gastrointest Endosc 2017;85:1188–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pohl H, Srivastava A, Bensen SP, et al. Incomplete polyp resection during colonoscopy-results of the complete adenoma resection (CARE) study. Gastroenterology 2013;144:74–80.e1. [DOI] [PubMed] [Google Scholar]
- 53.Yoon JY, Kim HT, Hong SP, et al. High-risk metachronous polyps are more frequent in patients with traditional serrated adenomas than in patients with conventional adenomas: a multicenter prospective study. Gastrointest Endosc 2015;82:1087–1093.e3. [DOI] [PubMed] [Google Scholar]
- 54.Lash RH, Genta RM, Schuler CM. Sessile serrated adenomas: prevalence of dysplasia and carcinoma in 2139 patients. J Clin Pathol 2010;63:681–686. [DOI] [PubMed] [Google Scholar]
- 55.Morelli MS, Glowinski EA, Juluri R, et al. Yield of the second surveillance colonoscopy based on the results of the index and first surveillance colonoscopies. Endoscopy 2013;45:821–826. [DOI] [PubMed] [Google Scholar]
- 56.Park HW, Han S, Lee JY, et al. Probability of high-risk colorectal neoplasm recurrence based on the results of two previous colonoscopies. Dig Dis Sci 2015;60:226–233. [DOI] [PubMed] [Google Scholar]
- 57.Chung SH, Park SJ, Cheon JH, et al. Factors predictive of high-risk adenomas at the third colonoscopy after initial adenoma removal. J Korean Med Sci 2013; 28:1345–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Imperiale TF, Juluri R, Sherer EA, et al. A risk index for advanced neoplasia on the second surveillance colonoscopy in patients with previous adenomatous polyps. Gastrointest Endosc 2014;80:471–478. [DOI] [PubMed] [Google Scholar]
- 59.Suh KH, Koo JS, Hyun JJ, et al. Risk of adenomas with high-risk characteristics based on two previous colonoscopy. J Gastroenterol Hepatol 2014;29:1985–1990. [DOI] [PubMed] [Google Scholar]
- 60.Botteri E, Crosta C, Bagnardi V, et al. Predictors of advanced colorectal neoplasia at initial and surveillance colonoscopy after positive screening immunochemical faecal occult blood test. Dig Liver Dis 2016;48:321–326. [DOI] [PubMed] [Google Scholar]
- 61.Facciorusso A, Di Maso M, Serviddio G, et al. Development and validation of a risk score for advanced colorectal adenoma recurrence after endoscopic resection. World J Gastroenterol 2016;22:6049–6056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Facciorusso A, Di Maso M, Serviddio G, et al. Factors associated with recurrence of advanced colorectal adenoma after endoscopic resection. Clin Gastroenterol Hepatol 2016;14:1148–1154.e4. [DOI] [PubMed] [Google Scholar]
- 63.Lee JY, Park HW, Kim MJ, et al. Prediction of the risk of a metachronous advanced colorectal neoplasm using a novel scoring system. Dig Dis Sci 2016;61:3016–3025. [DOI] [PubMed] [Google Scholar]
- 64.Liu L, Messer K, Baron JA, et al. A prognostic model for advanced colorectal neoplasia recurrence. Cancer Causes Control 2016;27:1175–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pohl H, Robertson DJ, Mott LA, et al. Association between adenoma location and risk of recurrence. Gastrointest Endosc 2016;84:709–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Belderbos TD, Leenders M, Moons LM, et al. Local recurrence after endoscopic mucosal resection of non-pedunculated colorectal lesions: systematic review and meta-analysis. Endoscopy 2014;46:388–402. [DOI] [PubMed] [Google Scholar]
- 67.Pellise M, Burgess NG, Tutticci N, et al. Endoscopic mucosal resection for large serrated lesions in comparison with adenomas: a prospective multicentre study of 2000 lesions. Gut 2017;66:644–653. [DOI] [PubMed] [Google Scholar]
- 68.Rex KD, Vemulapalli KC, Rex DK. Recurrence rates after EMR of large sessile serrated polyps. Gastrointest Endosc 2015;82:538–541. [DOI] [PubMed] [Google Scholar]
- 69.Kaltenbach TA, Anderson JC, Burke CA, et al. Endoscopic removal of colorectal lesions—recommendations by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2020;158 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 70.Figueiredo JC, Crockett SD, Snover DC, et al. Smoking-associated risks of conventional adenomas and serrated polyps in the colorectum. Cancer Causes Control 2015; 26:377–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim MC, Jung SW, Kim CS, et al. Metabolic syndrome is associated with increased risk of recurrent colorectal adenomas in Korean men. Int J Obes (Lond) 2012; 36:1007–1011. [DOI] [PubMed] [Google Scholar]
- 72.Fournel I, Cottet V, Binquet C, et al. Rural-urban differences in the long-term risk of colorectal cancer after adenoma removal: a population-based study. Dig Liver Dis 2014;46:376–382. [DOI] [PubMed] [Google Scholar]
- 73.Kim NH, Park JH, Park DI, et al. Metabolic syndrome is a risk factor for adenoma occurrence at surveillance colonoscopy: a single-center experience in Korea. Medicine (Baltimore) 2016;95:e4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim TJ, Kim JE, Choi YH, et al. Obesity-related parameters and colorectal adenoma development. J Gastroenterol 2017;52:1221–1229. [DOI] [PubMed] [Google Scholar]
- 75.Kitahara CM, Berndt SI, de Gonzalez AB, et al. Prospective investigation of body mass index, colorectal adenoma, and colorectal cancer in the prostate, lung, colorectal, and ovarian cancer screening trial. J Clin Oncol 2013;31:2450–2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kwah J, Schroy PC 3rd, Jacobson BC, et al. Whites and blacks have similar risk of metachronous advanced colorectal neoplasia. Dig Dis Sci 2014; 59:2264–2271. [DOI] [PubMed] [Google Scholar]
- 77.Laiyemo AO, Doubeni C, Brim H, et al. Short- and long-term risk of colorectal adenoma recurrence among whites and blacks. Gastrointest Endosc 2013;77:447–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McGill SK, Soetikno R, Rouse RV, et al. Patients with nonpolypoid (flat and depressed) colorectal neoplasms at increased risk for advanced neoplasias, compared with patients with polypoid neoplasms. Clin Gastroenterol Hepatol 2017;15:249–256.e1. [DOI] [PubMed] [Google Scholar]
- 79.Kunzmann AT, Coleman HG, Huang WY, et al. Fruit and vegetable intakes and risk of colorectal cancer and incident and recurrent adenomas in the PLCO cancer screening trial. Int J Cancer 2016;138:1851–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sardo Molmenti CL, Steck SE, Thomson CA, et al. Dietary inflammatory index and risk of colorectal adenoma recurrence: a pooled analysis. Nutr Cancer 2017; 69:238–247. [DOI] [PubMed] [Google Scholar]
- 81.Molmenti CL, Hibler EA, Ashbeck EL, et al. Sedentary behavior is associated with colorectal adenoma recurrence in men. Cancer Causes Control 2014;25:1387–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Baron JA, Barry EL, Mott LA, et al. A trial of calcium and vitamin D for the prevention of colorectal adenomas. N Engl J Med 2015;373:1519–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pommergaard HC, Burcharth J, Rosenberg J, et al. Aspirin, calcitriol, and calcium do not prevent adenoma recurrence in a randomized controlled trial. Gastroenterology 2016;150:114–122.e4. [DOI] [PubMed] [Google Scholar]
- 84.Heine-Broring RC, Winkels RM, Botma A, et al. Dietary supplement use is not associated with recurrence of colorectal adenomas: a prospective cohort study. Int J Cancer 2013;132:666–675. [DOI] [PubMed] [Google Scholar]
- 85.Han MS, Lee HJ, Park SJ, et al. The effect of metformin on the recurrence of colorectal adenoma in diabetic patients with previous colorectal adenoma. Int J Colorectal Dis 2017;32:1223–1226. [DOI] [PubMed] [Google Scholar]
- 86.Higurashi T, Hosono K, Takahashi H, et al. Metformin for chemoprevention of metachronous colorectal adenoma or polyps in post-polypectomy patients without diabetes: a multicentre double-blind, placebo-controlled, randomised phase 3 trial. Lancet Oncol 2016;17:475–483. [DOI] [PubMed] [Google Scholar]
- 87.Fedirko V, Bradshaw PT, Figueiredo JC, et al. Urinary metabolites of prostanoids and risk of recurrent colorectal adenomas in the Aspirin/Folate Polyp Prevention Study (AFPPS). Cancer Prev Res (Phila) 2015;8:1061–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dulai PS, Singh S, Marquez E, et al. Chemoprevention of colorectal cancer in individuals with previous colorectal neoplasia: systematic review and network meta-analysis. BMJ 2016;355:i6188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bibbins-Domingo K. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med 2016;164:836–845. [DOI] [PubMed] [Google Scholar]
- 90.Huang Y, Gong W, Su B, et al. Risk and cause of interval colorectal cancer after colonoscopic polypectomy. Digestion 2012;86:148–154. [DOI] [PubMed] [Google Scholar]
- 91.Pohl H, Robertson DJ. Colorectal cancers detected after colonoscopy frequently result from missed lesions. Clin Gastroenterol Hepatol 2010;8:858–864. [DOI] [PubMed] [Google Scholar]
- 92.Robertson DJ, Lieberman DA, Winawer SJ, et al. Colorectal cancers soon after colonoscopy: a pooled multi-cohort analysis. Gut 2014;63:949–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Corley DA, Jensen CD, Marks AR, et al. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med 2014;370:1298–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kaminski MF, Wieszczy P, Rupinski M, et al. Increased rate of adenoma detection associates with reduced risk of colorectal cancer and death. Gastroenterology 2017; 153:98–105. [DOI] [PubMed] [Google Scholar]
- 95.Barry EL, Peacock JL, Rees JR, et al. Vitamin D receptor genotype, vitamin D3 supplementation, and risk of colorectal adenomas: a randomized clinical trial. JAMA Oncol 2017;3:628–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fedirko V, McKeown-Eyssen G, Serhan CN, et al. Plasma lipoxin A4 and resolvin D1 are not associated with reduced adenoma risk in a randomized trial of aspirin to prevent colon adenomas. Mol Carcinog 2017;56:1977–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kang M, Peery AF, Locklear C, et al. Plasma insulin, glucose, IGF-I, IGF-II, and IGFBP-3 and risk of recurrent colorectal adenomas. J Gastroenterol Hepatol Res 2013; 2:531–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kim NH, Suh JY, Park JH, et al. Parameters of glucose and lipid metabolism affect the occurrence of colorectal adenomas detected by surveillance colonoscopies. Yonsei Med J 2017;58:347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Macaron C, Lopez R, Pai RK, et al. Expression of annexin A10 in serrated polyps predicts the development of metachronous serrated polyps. Clin Transl Gastroenterol 2016;7:e205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Uchiyama T, Takahashi H, Endo H, et al. Number of aberrant crypt foci in the rectum is a useful surrogate marker of colorectal adenoma recurrence. Dig Endosc 2012;24:353–357. [DOI] [PubMed] [Google Scholar]
- 101.Kim HG, Cho YS, Cha JM, et al. Risk of metachronous neoplasia on surveillance colonoscopy in young patients with colorectal neoplasia. Gastrointest Endosc 2018; 87:666–673. [DOI] [PubMed] [Google Scholar]
- 102.Nagpal SJS, Mukhija D, Sanaka M, et al. Metachronous colon polyps in younger versus older adults: a case-control study. Gastrointest Endosc 2018;87:657–665. [DOI] [PubMed] [Google Scholar]
- 103.Greuter MJE, de Klerk CM, Meijer GA, et al. Screening for colorectal cancer with fecal immunochemical testing with and without postpolypectomy surveillance colonoscopy: a cost-effectiveness analysis. Ann Intern Med 2017;167:544–554. [DOI] [PubMed] [Google Scholar]
- 104.Zauber AG, Winawer SJ, O’Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med 2012;366:687–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Goodman M, Fletcher RH, Doria-Rose VP, et al. Observational methods to assess the effectiveness of screening colonoscopy in reducing right colon cancer mortality risk: SCOLAR. J Comp Eff Res 2015;4:541–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Winawer SJ, Zauber AG, O’Brien MJ, et al. Randomized comparison of surveillance intervals after colonoscopic removal of newly diagnosed adenomatous polyps. The National Polyp Study Workgroup. N Engl J Med 1993; 328:901–906. [DOI] [PubMed] [Google Scholar]
- 107.Jover R, Bretthauer M, Dekker E, et al. Rationale and design of the European Polyp Surveillance (EPoS) trials. Endoscopy 2016;48:571–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Welch HG, Robertson DJ. Colorectal cancer on the decline—why screening can’t explain it all. N Engl J Med 2016;374:1605–1607. [DOI] [PubMed] [Google Scholar]
- 109.Pinsky PF, Schoen RE, Weissfeld JL, et al. The yield of surveillance colonoscopy by adenoma history and time to examination. Clin Gastroenterol Hepatol 2009; 7:86–92. [DOI] [PubMed] [Google Scholar]
- 110.Laiyemo AO, Pinsky PF, Marcus PM, et al. Utilization and yield of surveillance colonoscopy in the continued follow-up study of the polyp prevention trial. Clin Gastroenterol Hepatol 2009;7:562–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Robertson DJ, Burke CA, Welch G, et al. Using the results of a baseline and a surveillance colonoscopy to predict recurrent adenomas with high-risk characteristics. Ann Intern Med 2009;151:103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.World Health Organization. Classification of tumours of the digestive tract. IARC Press: Lyon; 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


