Abstract
Complement 3a (C3a) and complement 5a (C5a), small cleavage fragments generated by complement activation, has been previously shown to be obviously up-regulated in highly metastatic hepatocellular carcinoma (HCC) cells. However, their functional roles in HCC cells remains unclear. Here, we investigated the biological function of G protein-coupled receptor C3aR/C5aR using small interference RNA in HCC cells. Our data showed that C3aR and C5aR knockdown significantly inhibited the proliferation, migration and invasion of HCC cells using CCK-8, colony formation and transwell assays. Flow cytometry assay showed C3aR and C5aR knockdown induced cell cycle G0/G1 phase arrest and apoptosis in HCC cells. Moreover, we found down-regulation of C3aR/C5aR obviously down-regulated the expression of PCNA, Ki-67 and suppressed the epithelial-mesenchymal transition (EMT) markers (E-cadherin, N-cadherin and vimentin) in HCC cells. Collectively, our data demonstrated that targeting C3aR/C5aR may hold promise for the treatment of HCC.
Keywords: hepatocellular carcinoma, C3aR/C5aR, epithelial-mesenchymal transition
Introduction
Hepatocellular carcinoma (HCC), accounting for approximately 90% liver cancer, has been considered as the most common leading cause of cancer-related mortality in the world.1,2 It has been well established that some major factors, including viral hepatitis, non-alcoholic steatohepatitis or aflatoxin contribute to HCC progression.3,4 Despite feasible treatment strategies, including surgical resection, liver transplantation, and radiofrequency have been approved for HCC, the overall prognosis of patients still remains unsatisfactory.5,6 Therefore, exploring the detailed mechanism underlying HCC progression has become the key step to further improve the survival rate of HCC.
The occurrence and development of tumor is a complex process with unlimited cell proliferation, continuous formation of new blood vessels, apoptosis avoidance, tissue invasion and metastasis.7,8 In recent years, researchers have found the important role of complement in tumor immune surveillance.9,10 The complement system, which consists of numerous plasma proteins, membrane-bound and circulating complement regulators, and complement receptors, is part of the innate immune system of the body.11 Complement 3a (C3a) and complement 5a (C5a) are derived from the cleavage of complement C3/C5 involved in anaphylatoxic reactions and inflammatory responses via direct damage to the cell membrane or by indirectly binding to the G protein-coupled receptor C3aR/C5aR on the cell surface.12 Blockade of the C3aR/C5aR signaling in nTreg cells led to more functional Treg cells, causing decreased severity of autoimmune colitis and prolonged the survival time of allogeneic skin graft.13,14 Interestingly, Wang et al reported that C3aR and C5aR could be targeted for cancer immunotherapy by exerting as a new class of immune checkpoint receptors.15 Moreover, the complement components and their receptors C3aR/C5aR are found to be expressed in lung cancer.16,17 However, whether C3aR/C5aR participates in HCC progression has not been reported until now.
In this study, we analyzed the level of C3aR/C5aR in HCC tissues and cell lines. By performing loss-of-functionally assays, we investigated the functional role of C3aR/C5aR in HCC cells the underlying molecular mechanisms. Our findings might provide insights into potential treatment strategies for HCC.
Materials and Methods
Patients and Specimens
Paraffin-embedded tumor samples with matched paracarcinoma tissues were collected from 21 HCC patients after histologically and clinically diagnosis at General Hospital of Ningxia Medical University between March 2016 and October 2017. None of these patients (age of mean 58.35 years, range 32–72 years; male/female: 15/6) had received radiotherapy or chemotherapy. This study was approved by the Ethics Committee of General Hospital of Ningxia Medical University.
Immunohistochemistry
Paraffin-embedded specimens were made into 4-µm sections, deparaffinized in xylene, and rehydrated through a series of graded ethanol. Subsequently, the antigen was restored with EDTA antigenic retrieval buffer (pH 8.0) in a microwave oven. The tissue sections were blocked with 0.3% H2O2 for 15 min and then incubated with primary antibodies against C3aR and C5aR (Abcam, Cambridge, UK) overnight at 4°C, followed by incubated with horseradish peroxidase-conjugated secondary antibody for 1 h at room temperature. After incubated with streptavidinhorse-radish peroxidase, reaction product was developed with the DAB Kit (Boster Biological Technology, Ltd., Wuhan, China).
Cell Culture and Transfection
High metastatic potential MHCC-97H and SK-HEP-1, and low metastatic potential Huh7, as well as an immortalized hepatocyte cell line, LO2 were provided by American Type Culture Collection (ATCC, Manassas, VA). All the above cells were cultured in DMEM medium (Gibco, Grand Island, NY, USA) with 10% fetal bovine serum (FBS) at 37°C in 5% CO2 humidified atmosphere. The small interring RNA targeting C3aR (si-C3aR), C5aR (si-C5aR) and negative control (si-NC) were chemically synthesized by GenePharma (Shanghai, China). For cell transfection, MHCC-97H cells were transfected with si-C3aR, while SK-HEP-1 cells were transfected with si-C5aR for 48 h by using Lipofectamine 2000 (Thermo Fisher Scientific, Waltham, MA, USA) following the manufacturer’s protocol.
Cell Counting Kit-8 (CCK-8) Assay
Cells were seeded onto 96-well plates at a density of 3 × 10 3 cells per well and cultured in DMEM medium for 24, 48, and 72 h, respectively. At each time point, cells were incubated with 10 µl CCK-8 (Dojindo, Kumamoto, Japan) at 37°C for 2 h. Next, the optical density (OD) value of each well was measured by a microplate reader at a wavelength of 450 nm.
Colony Formation Assay
Cells were plated onto 6-well plates at an initial density of 600 cells per well. After cultured for consecutive 2 weeks, formed colonies were washed with ice-cold PBS and fixed with 4% paraformaldehyde for 30 min. According to the instruction, colonies were stained with 0.1% crystal violet and counted under a light microscope.
Cell Cycle Analysis
Cells were seeded into 6 cm dishes at a density of 5 × 104 cells per well and incubated for 48 h until reaching 80% cell confluence. After trypsinization, cells were harvested and fixed with pre-cold 75% ethanol for 24 h at 4°C. Next day, cells were re-suspended in 100 µL RNaseA amd stained with propidium iodide (PI) at 4°C for 30 min. The percentage of cells at G0/G1, S and G2/M phase was analyzed using a flow cytometer (BD Bioscience, San Jose, CA, USA).
Cell Apoptosis Analysis
Cells were seeded into 6 cm dishes at a density of 5 × 104 cells per well. After 48 h, cells were collected, washed with PBS and subjected to Annexin V/PI double staining according to the instruction of Apoptosis Assays Kit (BD Bioscience, USA). The apoptosis of cells was determined by a flow cytometer (BD Bioscience, USA).
Transwell Assays
Cell migration and invasion were assessed using a 24-well transwell chamber (8 µm size, Corning, Beijing, China) pre-coated without and with 50% Matrigel (BD Bioscience, USA). In brief, transfected cells in serum-free medium were incubated in the upper chamber, while 500 µL of 10% FBS was added to the lower chamber. After 24 h incubation, migrated or invasive cells to the lower chamber were fixed with 4% paraformaldehyde, stained with 0.5% crystal violet and counted in 5 random fields under a microscope.
Western Blotting Assays
Protein samples were prepared from cells using RIPA buffer (Beyotime, Shanghai, China) according to the manufacturer’s instructions. Then, 30 µg protein per well was separated on 10% SDS-PAGE gels and transferred onto PVDF membrane (Millipore, Bedford, MA, USA). The membranes were blocked with 5% nonfat milk and incubated with primary antibodies against C3aR, C5aR, PCNA, Ki-67, E-cadherin, N-cadherin, Vimentin and GAPDH at 4°C overnight. After washed with PBS 3 times, the membranes were incubated with HRP-labeled secondary at temperature for 2 h and protein bands were visualized using ECL kit (Beyotime) with GAPDH as an internal control.
Statistical Analysis
Statistical analysis was performed using SPSS 21.0 software. Data were expressed as mean ± SD from at least 3 independent experiments. Significant differences were evaluated using Student’s test or one-way ANOVA followed by Dunnett’s multiple comparison. All differences were deemed significant at p < 0.05.
Results
The Expression of C3aR and C5aR Was Up-Regulated in HCC
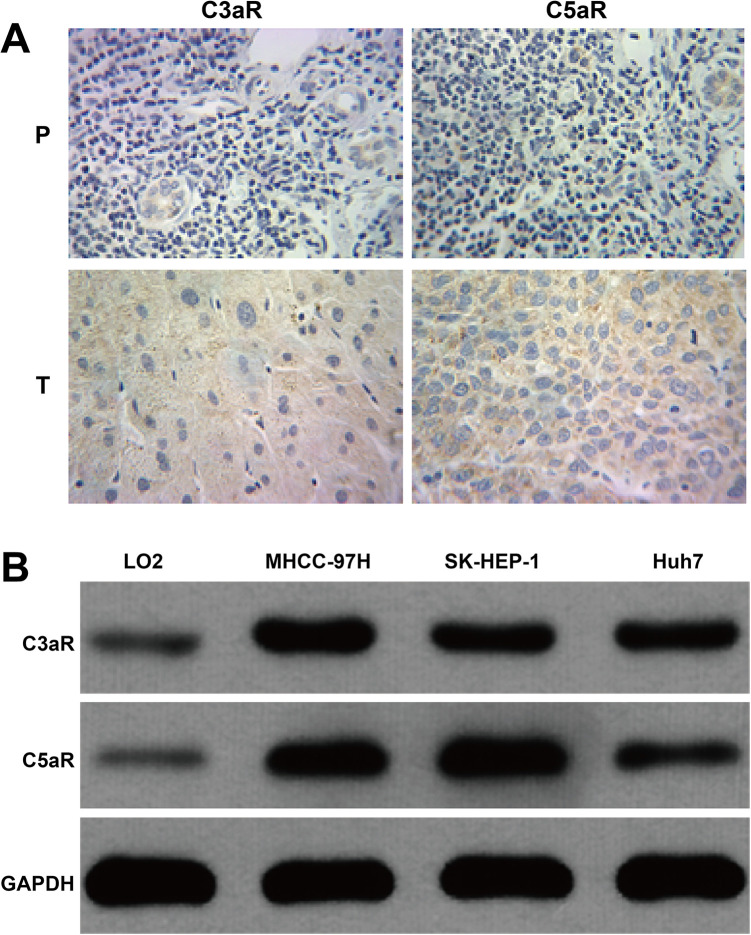
To investigate the correlation between C3aR/C5aR and HCC progression, immunohistochemistry analysis was performed on a TMA comprising 21 HCC and matched paracarcinoma tissues. As shown in Figure 1A, obviously positive immunohistochemical staining of C3aR/C5aR were observed in tumor tissues compared with paracarcinoma tissues, which were all yellow brown, with all proteins mainly in cytoplasm, and partially in the cell nucleus. In addition, we analyzed the expression of C3aR/C5aR in HCC cell lines. The results showed that C3aR/C5aR expression levels were obviously increased in 3 HCC cell lines compared with LO2 cells, of which MHCC-97H and SK-HEP-1 cells presented relative higher C3aR and C5aR protein expression, respectively (Figure 1B). These data support a key role for C3aR and C5aR in the pathogenesis of HCC.
Figure 1.
Detection of protein expression of C3aR/C5aR in HCC. A, Representative images of immunohistochemistry (n = 21) showing C3aR/C5aR expression in the tumor (T) sample and the adjacent paracarcinoma (P) tissues derived from HCC patients. B, The protein expression of C3aR/C5aR was measured using western blotting in HCC cell lines and LO2 cells.
Down-Regulation of C3aR/C5aR Suppressed HCC Cell Proliferation, Induced Cell Cycle Arrest and Apoptosis
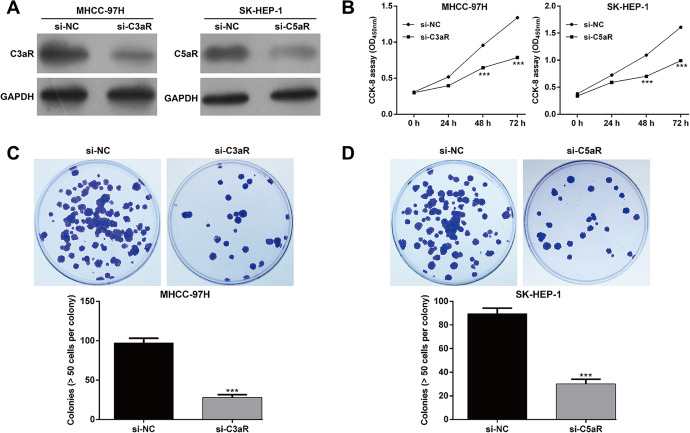
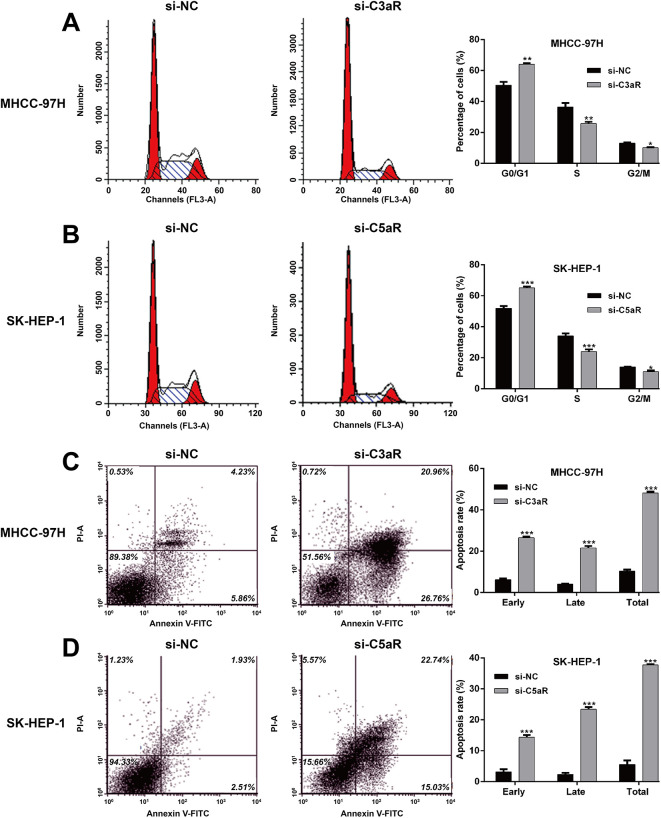
According to the expression of C3aR/C5aR in HCC cells, MHCC-97H and SK-HEP-1 cells were selected for performing loss-of-functional assays. As demonstrated by western blotting, si-C3aR transfection remarkably down-regulated C3aR protein expression in MHCC-97H cells and si-C5aR transfection remarkably down-regulated C5aR protein expression in SK-HEP-1 cells (Figure 2A). The results from CCK-8 assay indicated that knockdown of C3aR in MHCC-97H cells and C5aR in SK-HEP-1 cells significantly attenuated cell proliferation (Figure 2B). By performing colony formation assay, the number of colonies was notably decreased after si-C3aR transfection in MHCC-97H cells (Figure 2C) and also reduced after si-C5aR transfection in SK-HEP-1 cells (Figure 2D). Moreover, flow cytometry with PI staining demonstrated that the percentage of G0/G1 phase was significantly elevated, while S phase and G2/M phase were accordingly reduced in si-C3aR-transfected MHCC-97H cells (Figure 3A) and si-C5aR-transfected SK-HEP-1 cells (Figure 3B) compared with corresponding si-NC transfection, suggesting that C3aR/C5aR critically promotes the G1/S phase transition in HCC cells. Furthermore, either C3aR or C5aR knockdown dramatically enhanced early, late and total apoptotic rate in MHCC-97H (Figure 3C) and SK-HEP-1 cells (Figure 3D). These results collectively supported the contention that C3aR/C5aR facilitated HCC cell proliferation.
Figure 2.
Down-regulation of C3aR/C5aR suppressed HCC cell proliferation. MHCC-97H and SK-HEP-1 cells were transfected with si-C3aR and si-C5aR, respectively. A, The protein expression of C3aR/C5aR was detected in MHCC-97H and SK-HEP-1 cells using western blotting. B, CCK-8 assay was conducted to determine cell proliferation in MHCC-97H and SK-HEP-1 cells. C-D, The colony formation was assessed in si-C3aR-transfected MHCC-97H cells and si-C5aR-transfected SK-HEP-1 cells. Data were expressed as mean ± SD. ***p < 0.001, compared with si-NC.
Figure 3.
Down-regulation of C3aR/C5aR induced cell cycle arrest and apoptosis in HCC cells. MHCC-97H and SK-HEP-1 cells were transfected with si-C3aR and si-C5aR, respectively. A-B, The percentage of cells at G0/G1, S and G2/M phase was determined in MHCC-97H and SK-HEP-1 cells using flow cytometry with PI staining. C-D, Apoptotic rate was determined in MHCC-97H and SK-HEP-1 cells by flow cytometry with Annexin V/PI double staining. Data were expressed as mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001, compared with si-NC.
Down-Regulation of C3aR/C5aR Impaired Cell Migration and Invasion Ability in HCC
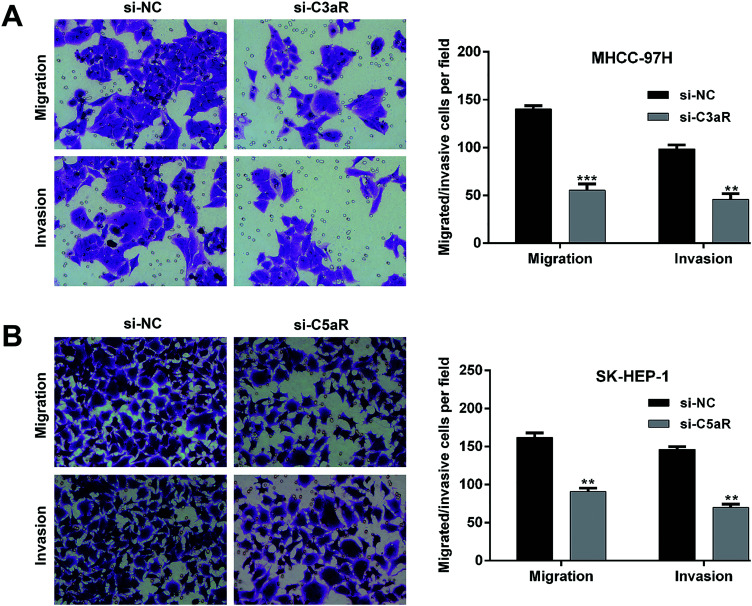
In the transwell assay, the number of migrated (55.3 ± 6.8 vs. 140.3 ± 3.5) and invasive cells (45.7 ± 6.0 vs. 98.7 ± 4.0) was significantly decreased in MHCC-97H cells after C3aR knockdown compared with control groups (Figure 4A). Similarly, C5aR knockdown dramatically inhibited cell invasion in SK-HEP-1 cells, as reflected by decreased invasive cells in si-C5aR group (90.7 ± 4.7) compared with si-NC group (162.0 ± 6.0). Thus, knockdown of C3aR/C5aR suppressed tumor cell migration and invasion in HCC cells (Figure 4B).
Figure 4.
Down-regulation of C3aR/C5aR impaired cell migration and invasion ability in HCC. MHCC-97H and SK-HEP-1 cells were transfected with si-C3aR and si-C5aR, respectively. A, The migrated and invasive cells were determined in si-C3aR-transfected MHCC-97H cells. B, The migrated and invasive cells were determined in si-C5aR-transfected SK-HEP-1 cells. Data were expressed as mean ± SD. **p < 0.01, ***p < 0.001, compared with si-NC.
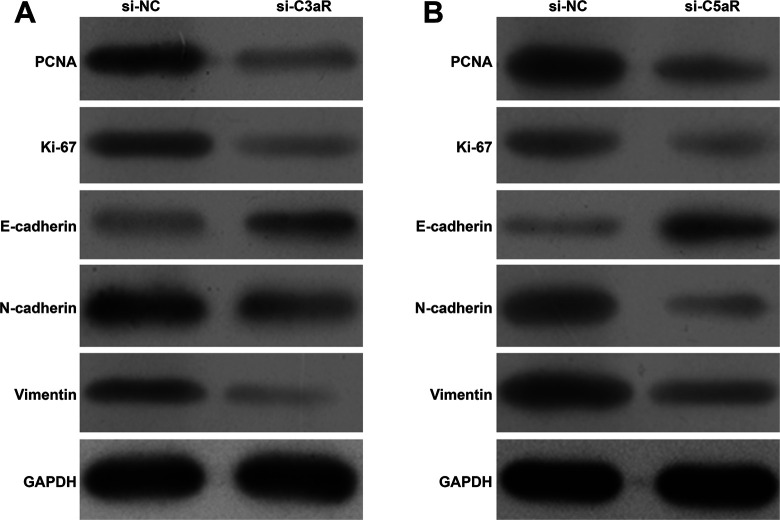
Down-Regulation of C3aR/C5aR Altered the Molecular Expression Associated With Proliferation and EMT
To explore the mechanism underlying C3aR/C5aR regulating HCC proliferation, migration and invasion, we analyzed related gene expression levels using western blotting. As shown in Figure 5A-B, the protein expression of PCNA and Ki-67, associated with cell proliferation was obviously down-regulated after C3aR/C5aR knockdown in MHCC-97H and SK-HEP-1 cells. The expression of the EMT markers E-cadherin, N-cadherin and Vimentin was examined by western blotting. In accordance with decreased migration and invasion, the expression of E-cadherin (epithelial marker) was up-regulated and the expression of N-cadherin and Vimentin (mesenchymal markers) was down-regulated when C3aR/C5aR expression was decreased in MHCC-97H (Figure 5A) and SK-HEP-1 cells (Figure 5B).
Figure 5.
Down-regulation of C3aR/C5aR altered the molecular expression associated with proliferation and EMT. MHCC-97H and SK-HEP-1 cells were transfected with si-C3aR and si-C5aR, respectively. The protein expression of PCNA, Ki-67 and EMT markers (E-cadherin, N-cadherin, and Vimentin) was examined by western blotting in MHCC-97H (A) and SK-HEP-1 (B) cells.
Discussion
In this study, the expression of C3aR/C5aR was observed to be significantly up-regulated in HCC tissues and cell lines. We further demonstrated that knockdown of C3aR/C5aR remarkably suppressed cell proliferation, migration and invasion in HCC cells. In lines with our data, Nabizadeh et al18 reported that therapeutic inhibition of C3aR may therefore be an effective means to trigger an antitumor response in melanoma and other cancers. C5a contributes to the immunosuppressive microenvironment required for tumor growth.19 In addition, blockade of C5a could restore antitumor immune responses, inhibit tumor cell growth, and improve outcomes of patients with lung cancer.20 In squamous carcinogenesis, therapeutic inhibition of C5aR1 via the peptide antagonist PMX-53 improved efficacy of paclitaxel chemotherapy associated with increased presence.21
The role of complement and its receptors in antitumor immunity has been recently been reported by Wang et al.15 Related studies indicated that tumor complement levels are not only positively associated with tumor size, but also correlated with poor outcome in different tumors.10,22 The complement could inhibit the antitumor immunity through activation of myeloid-derived suppressor cells (MDSCs) recruitment into the tumor microenvironment (TME)23,24 or inhibition of dendritic cells (DCs)/NK cell activation.25 There results suggested that C3aR/C5aR might be an oncogene in HCC progression.
EMT frequently occurs in the initiation of tumor invasion, which is a crucial first step in the development of tumor metastasis.26 Here, we further demonstrated that the expression of epithelial marker E-cadherin was increased, while the expression of mesenchymal markers (N-cadherin and Vimentin) was decreased under C3aR/C5aR knockdown. Consistently, the exosomes from high-metastatic MHCC97H cells could promote HCC cell migration via stimulating the EMT process.27 In addition, the interaction of C5a/C5aR1 plays a positive role in the migratory ability of muscle-derived satellite cells (MDSCs) into tumor cells and the suppressive ability of tumor-associated MDSCs.23 These facts indicated that C3aR/C5aR promoted HCC cell migration and invasion by regulating EMT markers.
In summary, our present study showed inhibition of C3aR/C5aR could attenuate cell proliferation, invasion and EMT in HCC cells. Therefore, C3aR/C5aR might be a potential target in overcoming therapy for HCC in the future.
Footnotes
Authors’ Note: Bendong Chen, MD, Wenyan Zhou, MM, and Chaofeng Tang, MM, contributed equally to this work. The datasets used during the current study are available from the corresponding author on reasonable request. CBD and ZWY were the main designers for the research. ZWY and TCF performed the experiments and collected the data. WGW and YP were responsible for the data analysis. ZY, BSC and MJL reviewed the literature and the data examining. MJL and LJZ were responsible for the figure format. All authors read and approved the final manuscript. Patient samples were collected with written informed consent in accordance to the Declaration of Helsinki and under the Ethical Committee of General Hospital of Ningxia Medical University (Approval number: NM20171326A).
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study received the grants from the Nature Science Foundation of Ningxia (grants no. NGY2016122, 2018AAC03155).
ORCID iD: Bendong Chen  https://orcid.org/0000-0003-3908-8428
https://orcid.org/0000-0003-3908-8428
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 2. Bertuccio P, Turati F, Carioli G, et al. Global trends and predictions in hepatocellular carcinoma mortality. J Hepatol. 2017;67(2):302–309. [DOI] [PubMed] [Google Scholar]
- 3. Della Corte C, Aghemo A, Colombo M. Individualized hepatocellular carcinoma risk: the challenges for designing successful chemoprevention strategies. World J Gastroenterol. 2013;19(9):1359–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. El-Serag HB. Hepatocellular carcinoma: an epidemiologic view. J Clin Gastroenterol. 2002;35(5 Suppl 2): S72–S78. [DOI] [PubMed] [Google Scholar]
- 5. Zhu MX, Wei CY, Zhang PF, et al. Elevated TRIP13 drives the AKT/mTOR pathway to induce the progression of hepatocellular carcinoma via interacting with ACTN4. J Exp Clin Cancer Res. 2019;38(1):409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gu X, Mao Y, Shi C, et al. MAGEC2 correlates with unfavorable prognosis and promotes tumor development in HCC via epithelial-mesenchymal transition. Onco Targets Ther. 2019;12(2):7843–7855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. He M, Rosen J, Mangiameli D, Libutti SK. Cancer development and progression. Adv Exp Med Biol. 2007;593:117–133. [DOI] [PubMed] [Google Scholar]
- 8. Freeman SM, Abboud CN, Whartenby KA, et al. The “bystander effect”: tumor regression when a fraction of the tumor mass is genetically modified. Cancer Res. 1993;53(21):5274. [PubMed] [Google Scholar]
- 9. Wang Y, Sun SN, Liu Q, et al. Autocrine complement inhibits IL10-dependent T-cell-mediated antitumor immunity to promote tumor progression. Cancer Discov. 2016;6(9):1022–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pio R, Corrales L, Lambris JD. The role of complement in tumor growth. Adv Exp Med Biol. 2014;772:229–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schreiber A, Xiao H, Jennette JC, Schneider W, Luft FC, Kettritz R. C5a receptor mediates neutrophil activation and ANCA-induced glomerulonephritis. J Am Soc Nephrol. 2009;20(2):289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li L, Chen L, Zang J, et al. C3a and C5a receptor antagonists ameliorate endothelial-myofibroblast transition via the Wnt/beta-catenin signaling pathway in diabetic kidney disease. Metabolism. 2015;64(5):597–610. [DOI] [PubMed] [Google Scholar]
- 13. Strainic MG, Shevach EM, An F, Lin F, Medof ME. Absence of signaling into CD4(+) cells via C3aR and C5aR enables autoinductive TGF-beta1 signaling and induction of Foxp3(+) regulatory T cells. Nat Immunol. 2013;14(2):162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kwan WH, van der Touw W, Paz-Artal E, Li MO, Heeger PS. Signaling through C5a receptor and C3a receptor diminishes function of murine natural regulatory T cells. J Exp Med. 2013;210(2):257–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang Y, Zhang H, He YW. The complement receptors C3aR and C5aR are a new class of immune checkpoint receptor in cancer immunotherapy. Front Immunol. 2019;10:1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vadrevu SK, Chintala NK, Sharma SK, et al. Complement c5a receptor facilitates cancer metastasis by altering T-cell responses in the metastatic niche. Cancer Research. 2014;74(13):3454–3465. [DOI] [PubMed] [Google Scholar]
- 17. Kwak JW, Laskowski J, Li HY, et al. Complement activation via a C3a receptor pathway alters CD4+ T lymphocytes and mediates lung cancer progression. Cancer Res. 2018;78(1):143-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nabizadeh JA, Manthey HD, Steyn FJ, et al. The complement C3a receptor contributes to melanoma tumorigenesis by inhibiting neutrophil and CD4+ T cell responses. J Immunol. 2016;196(11):4783–4792. [DOI] [PubMed] [Google Scholar]
- 19. Corrales L, Ajona D, Rafail S, et al. Anaphylatoxin C5a creates a favorable microenvironment for lung cancer progression. J Immunol. 2012;189(9):4674–4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ajona D, Ortiz-Espinosa S, Moreno H, et al. A combined PD-1/C5a blockade synergistically protects against lung cancer growth and metastasis. Cancer Discov. 2017;7(7):694–703. [DOI] [PubMed] [Google Scholar]
- 21. Medler TR, Murugan D, Horton W, et al. Complement C5a fosters squamous carcinogenesis and limits T cell response to chemotherapy. Cancer Cell. 2018;34(4):561–578 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu XY, Wang XY, Li RY, et al. Recent progress in the understanding of complement activation and its role in tumor growth and anti-tumor therapy. Biomed Pharmacother. 2017;91:446–456. [DOI] [PubMed] [Google Scholar]
- 23. Markiewski MM, DeAngelis RA, Benencia F, et al. Modulation of the antitumor immune response by complement. Nat Immunol. 2008;9(11):1225–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Qing X, Koo GC, Salmon JE. Complement regulates conventional DC-mediated NK-cell activation by inducing TGF-β1 in Gr-1+ myeloid cells. Eur J Immunol. 2012;42(7):1723–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Janelle V, Marie-Pierre L, Tarrab E, Lapierre P, Poliquin L, Lamarre A. Transient complement inhibition promotes a tumor-specific immune response through the implication of natural killer cells. Cancer Immunol Res. 2014;2(3):200–206. [DOI] [PubMed] [Google Scholar]
- 26. Qu Z, Feng J, Pan H, Jiang Y, Duan Y, Fa Z. Exosomes derived from HCC cells with different invasion characteristics mediated EMT through TGF-beta/Smad signaling pathway. Onco Targets Ther. 2019;12:6897–6905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen L, Guo P, He Y, Chen Z, Guo H. HCC-derived exosomes elicit HCC progression and recurrence by epithelial-mesenchymal transition through MAPK/ERK signalling pathway. Cell Death Dis. 2018;9(5):513. [DOI] [PMC free article] [PubMed] [Google Scholar]