Abstract
Introduction:
Breast cancer is the most prominent form of cancer and the second leading cause of death in women behind lung cancer. The primary modes of treatment today include surgical excision (lumpectomy, mastectomy), radiation, chemoablation, anti-HER2/neu therapy, and/or hormone therapy. The severe side effects associated with these therapies suggest a minimally invasive therapy with fewer quality of life issues would be advantageous for treatment of this pervasive disease. Cryoablation has been used in the treatment of other cancers, including prostate, skin, and cervical, for decades and has been shown to be a successful minimally invasive therapeutic option. To this end, the use of cryotherapy for the treatment of breast cancer has increased over the last several years. Although successful, one of the challenges in cryoablation is management of cancer destruction in the periphery of the ice ball as the tissue within this outer margin may not experience ablative temperatures. In breast cancer, this is of concern due to the lobular nature of the tumors. As such, in this study, we investigated the level of cell death at various temperatures associated with the margin of a cryogenic lesion as well as the impact of repetitive freezing and thawing methods on overall efficacy.
Methods:
Human breast cancer cells, MCF-7, were exposed to temperatures of −5°C, −10°C, −15°C, −20°C, or −25°C for 5-minute freeze intervals in a single or repeat freeze-thaw cycle. Samples were thawed with either passive or active warming for 5 or 10 minutes. Samples were assessed at 1, 2, and 3 days post-freeze to assess cell survival and recovery. In addition, the modes of cell death associated with freezing were assessed over the initial 24-hour post-thaw recovery period.
Results:
Exposure of MCF-7 cells to −5°C and −10°C resulted in minimal cell death regardless of the freeze/thaw conditions. Freezing to a temperature of −25°C resulted in complete cell death 1 day post-thaw with no cell recovery in all freeze/thaw scenarios evaluated. Exposure to a single freeze event resulted in a gradual increase in cell death at −15°C and −20°C. Application of a repeat freeze-thaw cycle (dual 5-minute freeze) resulted in an increase in cell death with complete destruction at −20°C and near complete death at −15°C (day 1 survival: single −15°C freeze/thaw = 20%; repeated −15°C freeze/thaw = 4%). Analysis of thaw interval time (5 vs 10 minute) demonstrated that the shorter 5-minute thaw interval between freezes resulted in increased cell destruction. Furthermore, investigation of thaw rate (active vs passive thawing) demonstrated that active thawing resulted in increased cell survival thereby less effective ablation compared with passive thawing (eg, −15°C 5/10/5 procedure survival, passive thaw: 4% vs active thaw: 29%).
Conclusions:
In summary, these in vitro findings suggest that freezing to temperatures of 25°C results in a high degree of breast cancer cell destruction. Furthermore, the data demonstrate that the application of a repeat freeze procedure with a passive 5-minute or 10-minute thaw interval between freeze cycles increases the minimal lethal temperature to the −15°C to −20°C range. The data also demonstrate that the use of an active thawing procedure between freezes reduces ablation efficacy at temperatures associated with the iceball periphery. These findings may be important to improving future clinical applications of cryoablation for the treatment of breast cancer.
Keywords: Cryosurgery, thermal dose, apoptosis, double freeze, active/passive thaw
Introduction
Breast cancer is a major cause of death in women. The World Health Organization estimates that by 2040, diagnosis and deaths from breast cancer will increase to ~3 million and ~1 million, respectively, globally.1,2 In the United States alone, billions of dollars are spent annually to treat this disease. Currently, the gold standard treatment for in situ and small invasive breast cancers is breast conservation surgery (known as a lumpectomy) followed by radiation therapy and systemic therapy.3,4 There are numerous adverse effects associated with these procedures. With the use of radiation therapy, there is always a risk of local skin reactions, swelling, and dryness. A study of a Dutch population who underwent radiation therapy as part of its breast cancer treatment plan showed that they experienced a significant excess risk of developing secondary non-breast cancers.5 While these treatment strategies have proven effective, there remains a need for the development of alternative minimally invasive targeted therapeutic options for the treatment of breast cancer.
With a continued rise in diagnosis and advances in biomarkers, the use of thermal ablation for pre- and metastatic breast cancer have experienced an increase in use and efficacy in the past 10 years.6-15 Ablative techniques such as cryotherapy have been used for the treatment of solid tumors for over 100 years.16,17 Thermal therapies include radiofrequency ablation (RFA), high-intensity focused ablation (HiFu), and cryoablation. Radiofrequency ablation and HiFu heat tissue to lethal temperatures (70°C to 90°C) and kill cells primarily by direct heat damage and necrosis; whereas cryoablation freezes tissue and kills cells through freeze rupture, necrosis, and apoptosis. Ablation therapy has been used for over 10 years to treat breast cancer.11-15 For instance, Kinoshita reported that localized tumors with a maximum diameter of 2 cm, preoperatively diagnosed by imaging and histopathology and treated with RFA yielded a 90% complete ablation rate based on histopathologic analysis.8 Ito et al9 conducted a retrospective analysis of 386 patients with breast cancer treated with RFA at 10 institutions and concluded “RFA in breast cancer is a safe and promising minimally invasive treatment for tumors ⩽2 cm in diameter.” Cazzato et al6 reported on the successful application of cryoablation for primary breast cancer in a patient cohort unsuitable for surgical excision. Simmons et al7 published the results of a Phase II clinical trial (ACOSOG Z1072) on cryoablation of early stage breast tumors ⩽2.0 cm in diameter reporting an overall success rate of 75.9%, and when patients with multifocal disease were excluded, a 92% success rate. More recently, Pediconi et al10 published a review of the use of ablation procedures (RFA, HiFu, Laser, and Cryo) using an magnetic resonance imaging (MRI) image guided approach and reported that “no serious complications were reported with these techniques and that these non-surgical approaches offer promise as a replacement to radical surgery when possible.” They also concluded that “further research utilizing these techniques should focus on the development of an even less invasive approach to breast neoplasms.” Finally, Pusceddu et al18 recently reviewed the breast cancer cryoablation literature and concluded that “. . . cryoablation as a alternative to surgery in patients with early stage disease is the most interesting aspect because it represents a conceptual shift towards a minimally invasive therapy.”
When performing solid tumor cryoablation, the temperature typically reached at the center of a cryogenic lesion ranges between −80°C and −180°C.19-22 Extending radially from the center of the cryolesion, temperatures increase until the edge of the frozen mass, where temperatures are 0°C, nominally. The corresponding isothermal gradient (temperature gradient profile) within the frozen mass varies depending on application time, freeze repetition, and cryogen used.19-24
Attainment of the minimal lethal temperature necessary to destroy a specific type of cancer plays an important role in procedural outcome. Numerous studies have shown that different cancer tissue types and even different molecular variants of cancer from the same tissue can have a differential response to mild freezing, thereby affecting the minimal lethal temperature.25-29 For instance, in prostate cancer, the loss of androgen receptor expression (shift from hormone responsive to unresponsive cancer) or increase in integrin expression can result in increased tolerance to freezing, shifting the minimal lethal temperature from −25°C to −40°C.26,30 As such, for prostate cancer, a minimal lethal temperature of −40°C is typically targeted to assure complete cancer ablation.16,19,27 In vitro studies have identified −20°C as the minimal lethal temperature for colorectal cancer31,32; −25°C for renal cancer,29,33 pancreatic cancer,34,35 and bladder cancer36,37; and −35° for liver cancer.24,28,38 Other studies have demonstrated that the application of a repeat or double freeze procedure can elevate the minimal lethal temperature for a given cancer type. For instance, Santucci et al36 reported a repeat freeze can elevate the lethal temperature in bladder cancer to −20°C. Baumann et al34 found a similar response in pancreatic cancer with a repeat freeze. Klossner et al26 demonstrated that repeat freezing of androgen insensitive prostate cancer cells yielded complete cell death at −40°C whereas a single freeze was not effective, and the surviving cells repopulated quickly. Clarke et al33 reported that application of a double freeze in a renal engineered tissue model resulted in an elevation of the lethal temperature from −25°C to ~−15°C. Furthermore, studies have shown that cancer cells in general have a higher tolerance to freezing compared with their non-cancerous counterparts.27,28,33 As such, the aim of this study was to characterize these factors in an effort to provide quantitative data to help refine the procedural application of cryosurgery in treatment of breast cancer. This dosing information could play an important guidance role in the use of cryoablation to treat breast cancer enabling expanded application, reducing variability in clinical outcomes and improving procedural efficacy.
Methods
Cell culture
The MCF-7 cells (ATCC, Manassas, Virginia) were maintained in Eagle’s Minimum Essential Medium (EMEM) media with 10% Foetal Bovine Serum (FBS), 1% Penicillin-Streptomycin, 0.05% bovine insulin at 37°C, 5% CO2. Cells were lifted using TrypLE Express (Invitrogen, Carlsbad, California) and seeded onto Costar 96-well strip plates (Corning Incorporated, Tewksbury, Massachusetts) and cultured for 3 days prior to experimentation to allow formation of a cell monolayer and complete attachment of cells.
Freezing protocol
Samples in Costar 8-well strips (75 µL medium/well) were exposed to freezing temperatures of −5°C, −10°C, −15°C, −20°C, or −25°C in a refrigerated circulating bath (Neslab/Thermo Scientific, Waltham, Massachusetts) for 5 minutes. Culture medium was aspirated and replaced with 75 µL per well of appropriate culture medium 30 minutes prior to freezing. Strips were placed into pre-cooled aluminum blocks within the freezing baths, containing a thin coating of ethanol to facilitate complete thermal contact with each well. Ice nucleation was initiated at −2°C using liquid nitrogen vapor to prevent supercooling. Sample temperature was recorded at 1-second intervals using a type T thermocouple (Omega HH806AU, Omega, Stamford, Connecticut). For single freeze conditions, samples were held for a total time of 5 minutes in the freezing blocks, passively (p = thawed under a laminar flow hood at room temperature) or actively thawed (a = thawed in a 37°C incubator) for 5 (p5, a5) or 10 (p10, a10) minutes and then placed at 37°C for recovery and assessment. For repeat (double) freeze conditions, samples were held for 5 minutes, passively or actively thawed for 5 or 10 minutes, and then frozen again for an additional 5 minutes (5/5/5 or 5/10/5 protocol). Following the second freeze interval, samples were again passively or actively thawed and then returned to the 37°C incubator for recovery and assessment.
Viability assessment
The metabolic activity indicator alamarBlue (Invitrogen) was used to assess cell viability. Stock alamarBlue was diluted 1:20 in Hank Balanced Salt Solution (HBSS, Corning/Mediatech, Tewksbury, Massachusetts) and applied to samples for 60 minutes (±1 minute) at 37°C. Raw fluorescent units were obtained using a Tecan SPECTRAFluorPlus plate reader (excitation 530 nm and emission 590 nm, Tecan Austria GmBH, Grodig, Austria) and analyzed using Microsoft Excel. Raw fluorescence units were converted to percentages based on pre-freeze control values (± SD). Assessments were conducted at 1, 2, and 3 days of recovery. A minimum of 3 experimental repeats with an intra-experimental repeat of 7 wells were performed for each condition (n ⩾ 21). Statistical significance was determined by single factor analysis of variance (ANOVA), where P < .005 was applied as the significance threshold.
Fluorescence microscopy
Samples were frozen as described and then fluorescence imaging was conducted at 1, 4, 8, and 24 hours post-freeze. Prior to imaging, samples were labeled with tri-stain fluorescence probes Hoechst 33258 (living cells, 0.06 µg/µL), propidium iodide (necrotic cells, 0.007 µg/µL), and YO-PRO-1 (apoptotic cells, 0.8 µM) (Molecular Probes, Invitrogen, Eugene, Oregon) for identification of living, necrotic, and apoptotic populations within each sample. Samples were incubated for 15 minutes in the dark before visualization and image acquisition using Zeiss Axiovert software (Carl Zeiss Microimaging, Thornwood, New York) at 20× magnification. Images were analyzed using ImageJ to quantitate the level of living, apoptotic, and necrotic cells at each time point for the various conditions.
Results
Single freeze exposure response of breast cancer cells
To identify the minimal lethal temperature for breast cancer, MCF-7 cells were exposed to a single 5-minute freeze at −5°C, −10°C, −15°C, −20°C, or −25°C, passively thawed, allowed to recover in culture and assessed for initial cell viability (24 hours) as well as recovery over a 3-day period. Analysis of MCF-7 samples revealed high survival (minimal death) following exposure to a −5°C or −10°C single freeze, whereas −15°C resulted in a significant decline in MCF-7 survival at day 1 post-freeze to 20.1% (±6.7) (Figure 1). Exposure to −20°C yielded a further reduction in MCF-7 survival to 2.9% (±1.5) 1 day post-freeze. When MCF-7 cells were treated with a single freeze at −25°C, complete cell death (no survival) was observed (day 1 survival = 0.5% [±0.25]). Assessment of cell recovery over the 3-day post-culture interval revealed a continued decrease in cell survival from day 1 to day 3 most notably in −15°C and −20°C samples (−15°C D1: 20.1% vs D3 4%, P < .01). While significant, due to the in vitro nature of this study, the continued decrease in survival may not reflect in vivo conditions. As such, we focused primarily on day 1 results as a means of assessing ablation efficacy.
Figure 1.
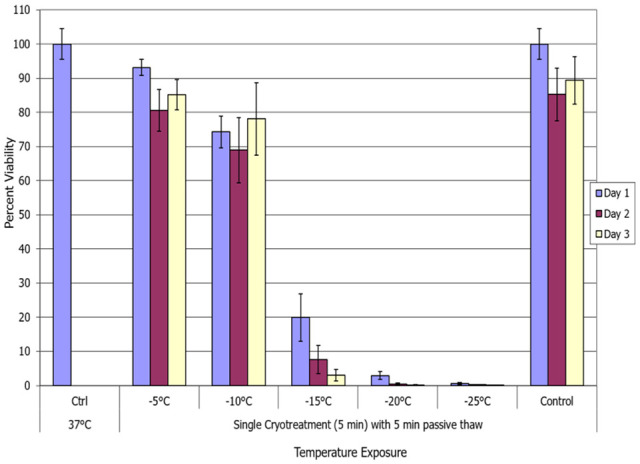
Assessment of breast cancer cell viability following a single freeze/passive thaw event. The MCF-7 cells were subjected to a 5-minute freeze at −10°C, −15°C, −20°C, or −25°C followed by a 5-minute passive thaw, and survival was assessed. Data suggest that complete cell death with no recovery is attained following exposure to −25°C whereas −15°C and −20°C exposure results in a substantial level of cell death.
Repeat (double) freeze exposure response of breast cancer cells
Repeat freezing increased cell death
With the identification of −25°C as completely lethal for MCF-7 cells and a significant cell loss at −15°C and −20°C, studies were conducted to assess the impact of a repeat (double) freeze exposure on cell survival and recovery. Samples were exposed to repeat freezing at −5°C, −10°C, −15°C, −20°C, and −25°C and allowed to passively thaw for 5 minutes at room temperature between and following freezes (5/p5/5 protocol). Repeat freeze exposure (double 5-minute freezes) to −5°C yielded minimal cell death (Figure 2). Repeat freezing at −10°C resulted in a significant decrease in cell survival compared with a single freeze exposure, repeat versus single = 36.9% (±7.1) versus 74.2% (±4.5), P < .005. Furthermore, the repeat −10°C sample survival was found to decline over the 3-day recovery interval. Repeat freezing at −15°C resulted in a further decrease in cell survival in MCF-7 samples at day 1 post-freeze compared with a single freeze exposure, repeat versus single = 3.8% (±0.9) versus 20.1% (±6.7), P < .005, with no recovery over the 3-day assessment interval. Repeat freezing at −20°C resulted in near complete cell death (minimal survival) at day 1 which was a small, yet significant, improvement compared with single freeze samples, 1.1% (±0.3) versus 2.9% (±1.5); P < .005. As with single −25°C samples, repeat freeze −25°C exposure yielded no survival. The results from the double 5-minute freeze with 5-minute passive thaw (5/p5/5) experiments suggested that the repeat exposure results in an elevation of the minimal lethal temperature to near −20°C.
Figure 2.
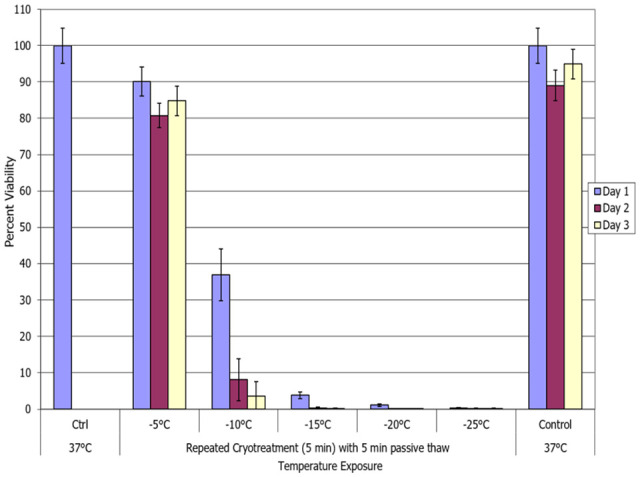
Impact of repeat freeze/passive thaw on breast cancer cell viability. The MCF-7 cells were subjected to a double 5-minute freeze at −5°C, −10°C, −15°C, −20°C, or −25°C with a 5-minute passive thaw (5/p5/5) and survival was assessed. Data suggest that a double freeze at −20°C results in complete breast cancer cell death with no recovery. Double freeze to −15°C resulted in a significant decrease in cell survival. However, a low level of surviving cells was noted over the assessment interval.
Impact of increasing thaw interval during a repeat (double) freeze protocol
Extending the thaw interval decreases overall ablation efficacy at warmer temperatures
With the identification of the increased level of cell death following a repeat freeze, we then explored the impact of increasing the thaw time between freezes from 5 to 10 minutes. A 10-minute thaw interval was tested given that this is the standard used clinically in prostate and other cancer cryoablation.7,11-13,39-45 To this end, a 5-minute freeze/10-minute passive thaw/5-minute freeze protocol (5/p10/5) was employed, followed by a final 10-minute passive thaw and return to culture at normothermic temperatures. As with the other conditions, minimal cell death was observed following exposure to −5°C (Figure 3). Repeat freezing at −10°C resulted in a decrease in cell survival at day 1 post-freeze compared with a single freeze exposure, repeat p10 versus single = 52.8% (±8.8) versus 74.2% (±4.5), P < .005. Interestingly, when comparing the repeat 10-minute and 5-minute thaw conditions, the increase in the time of passive thawing resulted in a significant increase in breast cancer cell survival following repeat freezing at −10°C, D1: 5/p10/5 = 52.8% (±8.8) versus 5/p5/5 = 36.9% (±7.1), respectively; P < .005. Repeat −15°C freezing/10-minute passive thaw (5/p10/5) samples yielded 3.3% (±1.2) survival with no recovery over the 3-day assessment interval, which was not significantly different from −15°C 5/p5/5 samples (P = .35). A similar response was noted in −20°C and −25°C 10-minute thaw samples compared with the 5-minute thaw samples, where near complete cell destruction (⩽1% survival) was noted in all 4 conditions. The results from the double 5-minute freeze/10-minute passive thaw (5/p10/5) experiments suggested that extension of the thaw interval yielded a negative impact following the repeat exposure to −10°C. However, at temperatures of −15°C or colder, no significant impact was noted. As with the 5-minute passive thaw protocol (5/p5/5), the 10-minute passive thaw protocol (5/p10/5) yielded an elevation in the minimal lethal temperature to around −20°C versus −25°C for a single 5-minute freeze protocol.
Figure 3.
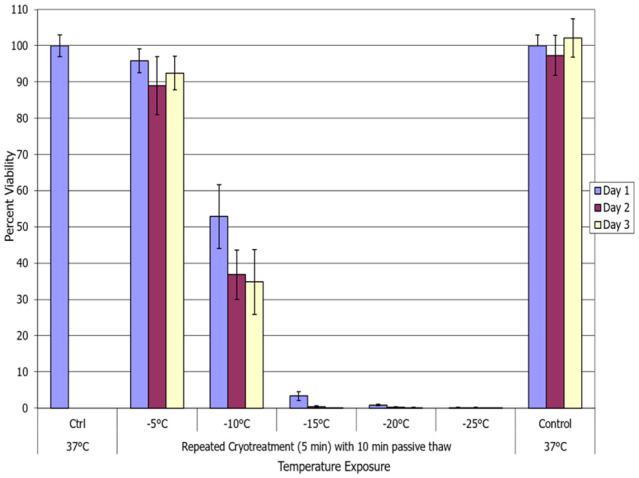
Assessment of the impact of elongating the thaw interval during a repeat freeze. The MCF-7 cells were subjected to a repeat (double) 5-minute freeze with a 10-minute passive thaw (5/p10/5) at −5°C, −10°C, −15°C, −20°C, or −25°C and survival was assessed. Data suggest that lengthening the thaw interval between freezes to 10 minutes resulted in a decrease in cell destruction (increased survival) following freezing to −10°C. However, at −15°C or colder, no significant effect was observed.
Assessment of the impact of active versus passive thawing techniques
Slower thawing rate increases cell death following freezing
In addition to temperature, number of freeze cycles and thaw interval time, the manner (rate) of thaw between freezing cycles was evaluated to determine if a faster, active thaw affects survival.30,46,47 This was conducted as an active thaw is often desired as it allows for shortened procedural times. Comparison of 5-minute and 10-minute active and passive thaw intervals was conducted with MCF-7 samples frozen to −5°C, −10°C, −15°C, −20°C, or −25°C using both a single (data not shown) and repeat freeze technique (Figure 4). Samples exposed to a repeat freeze at −10°C with an active 5-minute thaw (5/a5/5) yielded a significant increase in survival to 68.4% (±5.3) compared with 5-minute passive thaw samples (5/p5/5) which yielded 36.9% (±7.1) (P < .005) viable cells. Similar results were attained with a 10-minute active thaw protocol (5/a10/5) wherein day 1 post-thaw survival was found to increase to 84.6% (±3.9) compared with 52.8% (±3.9) following the 10-minute passive thaw protocol (5/p10/5) (P < .005). Examining the −15°C condition, it was observed that implementation of an active thaw also resulted in increased survival compared with the passive thaw protocol. Specifically, samples frozen to −15°C with a 10-minute active thaw protocol yielded 29.1% (±9.4) day 1 survival compared with 3.3% (±1.2) survival following a 10-minute passive thaw protocol (P < .005). A similar pattern of increased survival was observed in the −15°C freeze/5-minute active versus passive thaw samples, 7% (±2.2) versus 3.8% (±0.9), respectively; P = .01. In the −20°C repeat freeze samples, it was found that while an active thaw protocol resulted in an increase in survival compared with a passive thaw protocol, overall day 1 survival was below 2% and no statistical difference was observed across each of the 4 conditions when compared with one another (P > .05 for all conditions). Furthermore, analysis over the 3-day assessment interval revealed complete cell destruction (<0.25% [±0.2] survival for all 4 conditions). Overall, the data suggest that passive thawing between freeze cycles provides more effective breast cancer cell destruction than active thawing. In addition, using the shorter 5-minute duration thaw interval, compared with the longer 10-minute interval between freezes, increased cell death.
Figure 4.
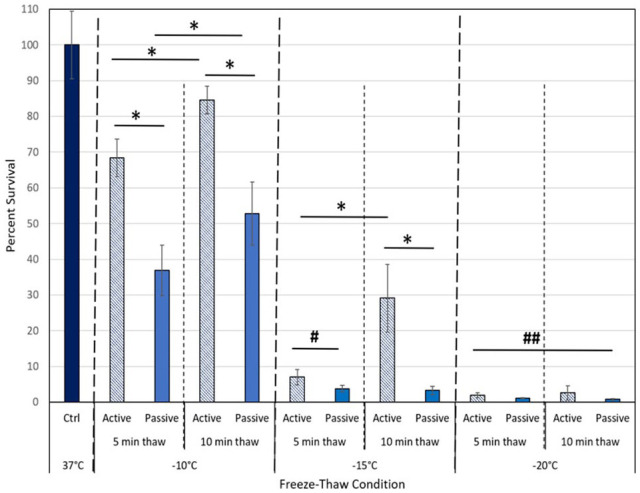
Comparison of active versus passive thaw protocol during a repeat freeze. The MCF-7 cells were subjected to a double 5-minute freeze to −10°C, −15°C, or −20°C with either a 5-minute or 10-minute slow, passive (solid bars), or a fast, active thaw (striped bars) between and following the freeze intervals. Data illustrate that the usage of a rapid, active thaw process resulted in a significant increase in cell survival following freezing to −10°C or −15°C. This decrease in ablation efficacy was found with either a 5-minute or 10-minute thaw interval. Active thawing of −20°C samples also yielded a small, but insignificant, increase in post-thaw survival compared with passively thawed samples. The data suggest that a 5-minute passive thaw protocol results in the greatest cell destruction.
Assessment of modes of cell death following freezing
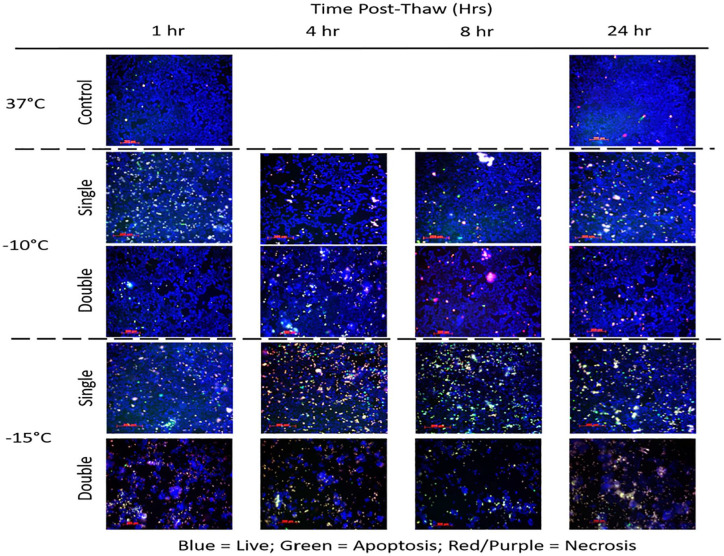
With the identification of increased MCF-7 cell death following a double freeze with passive thawing at −10°C and −15°C, analysis of the modes (apoptosis and necrosis) and timing of cell death over the initial 24 hours post-freeze was assessed via fluorescence image analysis. To this end, samples were frozen to −10°C or −15°C using a single or repeat freeze with a passive 10-minute thaw and analyzed at 1, 4, 8, and 24 hours with the YoPro-PI Cell Death Assay (Figure 5). Image analysis revealed a low level of necrosis and minimal apoptosis in untreated 37°C control populations (Figure 5). Analysis of −10°C images following a single freeze revealed a low level of apoptosis (green) and necrosis (red) and a high level of living (blue) cells across all time points. Analysis of the cell population at 24 hours post-freeze revealed 83% overall survival when compared with 24-hour controls. Analysis of the repeat freeze −10°C samples revealed increased cell loss compared with −10°C single samples with an overall survival of 33% at 24 hours compared with controls.
Figure 5.
Analysis of the modes of cell death following freezing using a single or repeat freeze protocol. The MCF-7 cells were exposed to a single or repeat freeze to −10°C or −15°C followed by passive thawing and then assessed for apoptotic, necrotic, and living populations using YoPro-1, Propidium Iodide, and Hoechst 33258 (green, red, blue, respectively) at 1, 4, 8, and 24 hours post-thaw. Image analysis of −10°C samples revealed a significant increase in necrosis and a low level of apoptosis during the post-thaw interval following both a single and double freeze. Increased levels were noted in the double samples resulting in increased cell death. Analysis of −15°C samples revealed a marked increase of apoptosis and necrosis compared with −10°C samples, most notably at 4 and 8 hours post-thaw. Repeat −15°C freeze samples revealed increased cell loss and necrosis characterized by reduced apoptosis over the entire time course.
Analysis of −15°C images following a single freeze revealed an increase and shift in the levels of cell death compared with −10°C samples. This was most notable at the 4-hour and 8-hour time points. Specifically, in the 4 hour −15°C single freeze samples, 32% of the population was necrotic and 8% apoptotic. At 8 hours, necrosis decreased to 24% whereas apoptosis increased to 15%. By 24 hours, necrotic and apoptotic levels were below 10% and overall cell survival was 30% compared with controls. Image analysis of −15°C double freeze samples revealed a marked reduction in total cell number at all time points compared with single freeze and controls. At 4 hours post-thaw, 43% of the remaining population was necrotic and 5% apoptotic. At 8 hours, 46% was necrotic and 3% apoptotic. By 24 hours, the levels of necrosis and apoptosis were 65% and 3%, respectively. Overall survival at 24 hours post-thaw in the double −15°C samples was found to be 2% when compared with controls. Overall, these findings suggested that both necrosis and apoptosis play a role in cell loss following mild freezing. Furthermore, assessment of overall survival via image analysis at 24 hours correlated well with the day 1 viability assessment findings.
Discussion
While the use of cryoablation continues to grow for the treatment of breast cancer,6,7,11-15,45,48 there remains a void in the literature surrounding the basic response of breast cancer cells to freezing. Although the exact clinical techniques needed for the complete cryoablation in vivo are difficult to characterize, it is now known that successful cryoablation is dependent on several factors including the cancer phenotype, the number of freeze-thaw cycles, the rate of cooling and thawing, the final tissue temperature, and the duration of the freezing episode.16,19,24,26-28,46,48
To this end, this study investigated the survival response of a breast cancer cell line following a freezing insult in an effort to identify the minimal lethal temperature (dose) necessary for complete cell destruction. In addition, investigation into the impact of repeat freezing (single vs repeat freeze), thaw protocol (active vs passive), thaw time (5 vs 10 minutes), and modes of cell death were conducted. These studies were conducted as cryoablation is often applied in a repeat (double) freeze procedure for the treatment of many cancers including breast, prostate, renal, and liver.6,7,11,25,27,32,42-45,49 Furthermore, thaw protocol (time and type) is often discussed as there is a continual push to reduce overall procedure times.
Our studies focused on the warmer sub-freezing temperatures associated with the periphery (outer edge) of the freeze lesion, as it is generally accepted that temperatures below −40°C result in complete cancer cell lysis through physical ice rupture.23,24,29,35,50 The thermal range of 0°C to −40°C is characterized by a region of heterogeneous cell responses which includes cell lysis, activation of necrotic and apoptotic pathways, as well as some cell survival.19,27,40,51,52 Numerous reports by our group and others have demonstrated the transition from complete cell death to complete cell survival lies within this region.23,24,27,29,41,53,54 Given these facts, it is important to identify the critical lethal temperature for breast cancer cells as well as understand the mechanisms of cell death to develop more effective treatment paths. The data revealed the minimal lethal temperature for MCF-7 cells following a single freeze event was −25°C in vitro whereas a low, yet significant, level of survival was noted following freezing to −20°C and −15°C (Figure 1). These samples were found to contain a heterogeneous mix of necrotic, apoptotic, and surviving cell populations (Figure 4). Application of a repeat freeze/passive 5-minute thaw protocol (5/p5/5) resulted in an increase of the minimal lethal temperature to −20°C which was significant compared with a single freeze at −15°C, 3.8% (±0.9) versus 20.1% (±6.7), respectively, P < .005 (Figure 2). The repeat freeze was also found to decrease survival following exposure to −10°C compared with a single freeze, 36.9% (±7.1) versus 74.2% (±4.5), respectively, P < .005, although complete destruction was not attained (Figure 2). Elongation of the thaw interval between freeze cycles to 10 minutes (5/p10/5) had no significant impact on cell survival following freezing to −15°C or −20°C (Figure 3). Interestingly, at −10°C, extending the thaw interval to 10 minutes resulted in a significant increase in cell survival versus a 5-minute thaw (52.8% vs 36.9%, respectively. P < .005) (Figure 3). From these studies, it was found that a repeat freeze resulted in an elevation of the minimal lethal temperature from −25°C to ~−20°C. Furthermore, it was found that a 5-minute thaw interval was effective and that extension to 10 minutes had a potentially negative impact, depending on the nadir temperature attained.
Another factor influencing cryosurgical outcome is the thawing rate. In clinical practice, there is a desire to reduce operating room time and as such the practice of using active heating of the probes between the freeze cycles is commonplace.42 In examining the impact of thaw rate, compared with passive thawing, it was found that faster thaw rates associated with active thawing decreased the level of cell death (increased survival) at all temperatures examined (Figure 4). The most significant impact was observed at −15°C and −10°C wherein application of either a 5-minute or 10-minute active thaw resulted in a >50% increase in survival compared with matched passive thaw samples (P < .005). In the case of −20°C samples, active thawing also resulted in increased survival compared with passive thawing following either 5 or 10 minutes of thawing. However, this was not significant (P > .01). Passive thawing between freezing cycles allows tissues to remain at hypothermic temperatures and in osmotic imbalance for a greater duration, yielding increased cell destruction due in part to elongated intervals of oxidative stress.19 A similar process occurs in vivo when ischemia is prolonged during the extended hypothermic temperatures and reperfusion injury occurs, exacerbating post-thaw cell death.16,24,29,33,51 We hypothesize that the increased cell death noted following the shorter 5-minute passive thaw interval was a result of a combination of these factors along with a reduced interval for the activation of cell stress recovery signaling.
Examination of the modes of cell death post-freeze over the initial 24-hour recovery interval in −10°C and −15°C samples following a single or double freeze protocol allowed for qualitative visualization of alterations in cell death and cell survival between the 2 freeze protocols (Figure 5). Image analysis revealed that following a single freeze, necrosis (red/purple cells) was the predominant form of cell death. An increase in the necrotic population was found in the −15°C samples compared with −10°C samples at all time points examined. In addition to necrosis, a low level of apoptosis (green cells) was also noted in the single freeze samples. This was most notable at 4 and 8 hours post-freeze in −15°C samples (Figure 5, −15°C single). A high level of surviving (blue) cells was noted at 24 hours post-thaw following a single freeze event at −10°C or −15°C. Application of a repeat, double freeze/passive thaw protocol increased the levels of necrosis compared with single freeze at all time points examined. In the −15°C repeat freeze samples, the increased necrosis was accompanied with a decrease in the level of apoptosis as well as surviving cells (blue cells). This was attributed to increased cell stress levels experienced following a repeat freeze. These findings correlated with the increased cell death found in the viability studies when comparing single and double −15°C samples. While promising, given the in vitro nature of the study, it is difficult to determine if the increase in necrosis at 4, 8, and 24 hours post-thaw was due completely to new necrotic activity or partially a result of the progression of apoptotic cells into secondary necrosis due to the absence of macrophages in the in vitro model. Regardless, importantly, these analyses demonstrated that the increased cell death following exposure to −15°C (single or repeat) was not due to freeze rupture alone, but a result of delayed (4-8 hours) activation of molecular-based cell death (delayed necrosis and apoptosis). If the predominant form of cell death at these temperatures was freeze rupture, the cell loss would be noted immediately (within 1 hour post-freeze). These findings are consistent with several reports on the activation of molecular-based cell death, as well as an immunologic, in response to freezing injury in both normal and cancerous cells and in vivo studies.25,30,54-58
Several reports detailing the successful application of cryoablation to treat breast cancer have appeared in the literature.6,7,11-15,59 In a pilot 27-patient study, Sabel et al59 reported successful ablation of localized breast tumors <1.5 cm in diameter in 100% of the patients. More recently, Simmons et al extended this work in a Phase II clinical study (ACOSOG Z1072) targeting unifocal invasive ductal carcinoma ⩽2.0 cm and reported 100% ablation of tumor <1.0 cm in diameter and 77.4% in tumor ⩾ 1.0 cm. In these studies, a double freeze 6/10/6 (<1.0 cm tumors) or 8/10/8 (>1.0 cm tumors) freeze protocol was employed. Similar protocols are being employed in ongoing clinical trials (FROST and ICE-3 Trials60,61) which are focused on the cryoablation of unifocal primary invasive breast carcinoma ⩽1.5 cm in diameter.
When comparing our in vitro findings with the reported clinical outcomes, the data suggest in the larger tumor cohort that a minimal temperature of −25°C may not have been attained throughout the tumors thereby resulting in the ~25% increase in failure rate reported when cryoablation was applied to >1.0 cm tumors. Correlating the reported isotherm profile created in room temperature ultrasound gel models by today’s cryosystems62,63 using a 6-minute freeze protocol with the reported significant impact that the in vivo heat load (37°C) experienced clinically has on reducing isotherm distribution (20%-40%, cryogen and isotherm dependent),20-23 the data suggest a 1.2 to 1.6 cm diameter frozen mass below −25°C. This correlates well with our current findings which suggested attaining −20°C to −25°C or colder (protocol dependent) was necessary to achieve complete breast cancer cell destruction.
Conclusions
The data from this study suggest that cryoablation is an effective means of thermally ablating breast cancer tumors. In vitro investigations into cryosurgical techniques may eventually lead to clinical improvements in cryosurgical outcome. This study investigated several variables common to cryosurgical procedures in an in vitro model. The findings provide a baseline characterization of the cellular and molecular responses of a human breast cancer cell line to freezing and identifies −25°C as the critical temperature for complete breast cancer cell destruction following a single freeze. Usage of a double freeze protocol elevated the minimal lethal temperature to around −20°C. Application of a slower, passive thaw between freezes was found to be more effective than faster, active thaw procedures. The combination of repeat freezing and passive thawing was found to increase the level of necrosis post-thaw thereby increasing the level of cell death. When considering our in vitro findings in combination with the Phase II clinical results,7 the data suggest a minimal lethal temperature of −25°C or colder should be targeted. Given the reported decrease in success rate in tumors >1.0 cm coupled with the current focus of clinical trials on tumors ⩽1.5 cm,60,61 active monitoring of the temperature at the tumor edge, using thermocouples, during a procedure may be of benefit.40,42,64 Furthermore, as different cryogens, devices, probes, and protocols yield different thermal profiles under heat load,20-22,65 temperature monitoring clinically could help to further establish the critical dosing parameters (time and temperature) to attain complete tumor destruction thereby further positioning cryoablation as a first-line treatment option for breast cancer.
In conclusion, the data presented in this study suggest that by employing repetitive freeze-thaw cycles, attaining temperatures of −25°C and passive thawing, cryosurgical efficacy may be increased. The collective optimization of these various parameters, both in vitro and in vivo, should improve the efficacy and clinical outcome of breast cancer cryoablation.
Acknowledgments
The authors would like to thank Kristin Andrascik and Dr Daniel Arsenault for their contributions to data acquisition for this manuscript.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported in part by funding from the National Institutes of Health, National Cancer Institute Grant No. 1R43CA224431-01A1 awarded to CPSI Biotech.
Declaration of conflicting interests:The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: J.M.B., K.K.S., and R.G.V.B. are employees of CPSI Biotech. J.G.B. has no competing interests.
Availability of Data and Material: The data that support the findings of this study are available from CPSI Biotech but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors on reasonable request and with permission of CPSI Biotech.
Author Contributions: KKS and JMB performed all experimental design, experimentation, and data analysis for this study. RGVB and JGB conducted data and experimental design review and assisted in data interpretation. JMB prepared the draft manuscript. JMB, KKS, RGVB, and JGB provided review and revision input for the manuscript. All authors read and approved the final manuscript.
ORCID iD: John M Baust  https://orcid.org/0000-0003-0867-8062
https://orcid.org/0000-0003-0867-8062
References
- 1. Cancer facts and figures. American Cancer Society. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2019.html. Updated 2019.
- 2. Cancer Tomorrow. World Health Organization International Agency for Research on Cancer. http://gco.iarc.fr/tomorrow/home. Updated 2019.
- 3. Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233-1241. [DOI] [PubMed] [Google Scholar]
- 4. Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347:1227-1232. [DOI] [PubMed] [Google Scholar]
- 5. Schaapveld M, Visser O, Louwman MJ, et al. Risk of new primary nonbreast cancers after breast cancer treatment: a Dutch population-based study. J Clin Oncol. 2008;26:1239-1246. [DOI] [PubMed] [Google Scholar]
- 6. Cazzato RL, de Lara CT, Buy X, et al. Single-centre experience with percutaneous cryoablation of breast cancer in 23 consecutive non-surgical patients. Cardiovasc Intervent Radiol. 2015;38:1237-1243. [DOI] [PubMed] [Google Scholar]
- 7. Simmons RM, Ballman KV, Cox C, et al. A phase II trial exploring the success of cryoablation therapy in the treatment of invasive breast carcinoma: results from ACOSOG (Alliance) Z1072. Ann Surg Oncol. 2016;23:2438-2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kinoshita T. RFA experiences, indications and clinical outcomes. Int J Clin Oncol. 2019;24:603-607. [DOI] [PubMed] [Google Scholar]
- 9. Ito T, Oura S, Nagamine S, et al. Radiofrequency ablation of breast cancer: a retrospective study. Clin Breast Cancer. 2018;18:e495-e500. [DOI] [PubMed] [Google Scholar]
- 10. Pediconi F, Marzocca F, Cavallo Marincola B, Napoli A. MRI-guided treatment in the breast. J Magn Reson Imaging. 2018;48:1479-1488. [DOI] [PubMed] [Google Scholar]
- 11. Littrup PJ, Jallad B, Chandiwala-Mody P, D’Agostini M, Adam BA, Bouwman D. Cryotherapy for breast cancer: a feasibility study without excision. J Vasc Interv Radiol. 2009;20:1329-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ward RC, Lourenco AP, Mainiero MB. Ultrasound-guided breast cancer cryoablation. AJR: Am J Roentgenol. 2019;213:716-722. [DOI] [PubMed] [Google Scholar]
- 13. Swintelski C, Plaza M. Successful cryoablation of breast cancer. Breast J. 2018;24:704-706. [DOI] [PubMed] [Google Scholar]
- 14. Pusztaszeri M, Vlastos G, Kinkel K, Pelte MF. Histopathological study of breast cancer and normal breast tissue after magnetic resonance-guided cryotherapy ablation. Cryobiology. 2007;55:44-51. [DOI] [PubMed] [Google Scholar]
- 15. Takada M, Toi M. Cryosurgery for primary breast cancers, its biological impact, and clinical outcomes. Int J Clin Oncol. 2019;24:608-613. [DOI] [PubMed] [Google Scholar]
- 16. Gage AA, Baust JG. Cryosurgery for tumors: a clinical overview. Technol Cancer Res Treat. 2004;3:187-199. [DOI] [PubMed] [Google Scholar]
- 17. Huston TL, Simmons RM. Ablative therapies for the treatment of malignant diseases of the breast. Am J Surg. 2005;189:694-701. [DOI] [PubMed] [Google Scholar]
- 18. Pusceddu C, Paliogiannis P, Nigri G, Fancellu A. Cryoablation in the management of breast cancer: evidence to date. Breast Cancer (Dove Med Press). 2019;11:283-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Baust JG, Snyder KK, Santucci KL, Robilotto AT, Van Buskirk RG, Baust JM. Cryoablation: physical and molecular basis with putative immunological consequences. Int J Hyperthermia. 2019;36:10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Baust JM, Robilotto A, Snyder KK, et al. Assessment of cryosurgical device performance using a 3D tissue-engineered cancer model. Technol Cancer Res Treat. 2017;16:900-909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Littrup PJ, Jallad B, Vorugu V, et al. Lethal isotherms of cryoablation in a phantom study: effects of heat load, probe size, and number. J Vasc Interv Radiol. 2009;20:1343-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shah TT, Arbel U, Foss S, et al. Modeling cryotherapy ice ball dimensions and isotherms in a novel gel-based model to determine optimal cryo-needle configurations and settings for potential use in clinical practice. Urology. 2016;91:234-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Larson TR, Rrobertson Corica A, Bostwick DG. In vivo interstitial temperature mapping of the human prostate during cryosurgery with correlation to histopathologic outcomes. Urology. 2000;55:547-552. [DOI] [PubMed] [Google Scholar]
- 24. Seifert JK, Gerharz CD, Mattes F, et al. A pig model of hepatic cryotherapy. In vivo temperature distribution during freezing and histopathological changes. Cryobiology. 2003;47:214-226. [DOI] [PubMed] [Google Scholar]
- 25. Baust JG, Bischof JC, Jiang-Hughes S, et al. Re-purposing cryoablation: a combinatorial “therapy” for the destruction of tissue. Prostate Cancer Prostatic Dis. 2015;18:87-95. [DOI] [PubMed] [Google Scholar]
- 26. Klossner DP, Baust JM, VanBuskirk RG, Gage AA, Baust JG. Cryoablative response of prostate cancer cells is influenced by androgen receptor expression. BJU Int. 2008;101:1310-1316. [DOI] [PubMed] [Google Scholar]
- 27. Klossner DP, Robilotto AT, Clarke DM, et al. Cryosurgical technique: assessment of the fundamental variables using human prostate cancer model systems. Cryobiology. 2007;55:189-199. [DOI] [PubMed] [Google Scholar]
- 28. Bischof J, Christov K, Rubinsky B. A morphological study of cooling rate response in normal and neoplastic human liver tissue: cryosurgical implications. Cryobiology. 1993;30:482-492. [DOI] [PubMed] [Google Scholar]
- 29. Rupp CC, Hoffmann NE, Schmidlin FR, Swanlund DJ, Bischof JC, Coad JE. Cryosurgical changes in the porcine kidney: histologic analysis with thermal history correlation. Cryobiology. 2002;45:167-182. [DOI] [PubMed] [Google Scholar]
- 30. Baust JG, Klossner DP, Vanbuskirk RG, et al. Integrin involvement in freeze resistance of androgen-insensitive prostate cancer. Prostate Cancer Prostatic Dis. 2010;13:151-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bang HJ, Littrup PJ, Currier BP, et al. Percutaneous cryoablation of metastatic lesions from colorectal cancer: efficacy and feasibility with survival and cost-effectiveness observations. ISRN Minim Invasive Surg. 2012;2012:942364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Seifert JK, Zhao J, Ahkter J, Bolton E, Junginger T, Morris DL. Cryoablation of human colorectal cancer in vivo in a nude mouse xenograft model. Cryobiology. 1998;37:30-37. [DOI] [PubMed] [Google Scholar]
- 33. Clarke DM, Robilotto AT, Rhee E, et al. Cryoablation of renal cancer: variables involved in freezing-induced cell death. Technol Cancer Res Treat. 2007;6:69-79. [DOI] [PubMed] [Google Scholar]
- 34. Baumann KW, Baust JM, Snyder KK, Baust JG, Van Buskirk RG. Dual thermal ablation of pancreatic cancer cells as an improved combinatorial treatment strategy. Liver Pancreat Sci. 2017;2:1-10. [Google Scholar]
- 35. Korpan NN. Cryosurgery: ultrastructural changes in pancreas tissue after low temperature exposure. Technol Cancer Res Treat. 2007;6:59-67. [DOI] [PubMed] [Google Scholar]
- 36. Santucci KL, Baust JM, Snyder KK, et al. Investigation of bladder cancer cell response to cryoablation and adjunctive cisplatin based cryo/chemotherapy. Clin Res Open Access. 2020;6:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liang Z, Fei Y, Lizhi N, et al. Percutaneous cryotherapy for metastatic bladder cancer: experience with 23 patients. Cryobiology. 2014;68:79-83. [DOI] [PubMed] [Google Scholar]
- 38. El-Shakhs SA, Shimi SA, Cuschieri A. Effective hepatic cryoablation: does it enhance tumor dissemination? World J Surg. 1999;23:306-310. [DOI] [PubMed] [Google Scholar]
- 39. Klatte T, Kroeger N, Zimmermann U, Burchardt M, Belldegrun AS, Pantuck AJ. The contemporary role of ablative treatment approaches in the management of renal cell carcinoma (RCC): focus on radiofrequency ablation (RFA), high-intensity focused ultrasound (HIFU), and cryoablation. World J Urol. 2014;32:597-605. [DOI] [PubMed] [Google Scholar]
- 40. Baust JG, Gage AA, Klossner D, et al. Issues critical to the successful application of cryosurgical ablation of the prostate. Technol Cancer Res Treat. 2007;6:97-109. [DOI] [PubMed] [Google Scholar]
- 41. Gage AA, Baust JM, Baust JG. Experimental cryosurgery investigations in vivo. Cryobiology. 2009;59:229-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Babaian RJ, Donnelly B, Bahn D, et al. Best practice statement on cryosurgery for the treatment of localized prostate cancer. J Urol. 2008;180:1993-2004. [DOI] [PubMed] [Google Scholar]
- 43. Wagstaff P, Ingels A, Zondervan P, de la Rosette JJ, Laguna MP. Thermal ablation in renal cell carcinoma management: a comprehensive review. Curr Opin Urol. 2014;24:474-482. [DOI] [PubMed] [Google Scholar]
- 44. Hinshaw JL, Lee FT., Jr. Cryoablation for liver cancer. Tech Vasc Interv Radiol. 2007;10:47-57. [DOI] [PubMed] [Google Scholar]
- 45. Bland KL, Gass J, Klimberg VS. Radiofrequency, cryoablation, and other modalities for breast cancer ablation. Surg Clin North Am. 2007;87:539-550.xii. [DOI] [PubMed] [Google Scholar]
- 46. Gage AA, Guest K, Montes M, Caruana JA, Whalen DA., Jr. Effect of varying freezing and thawing rates in experimental cryosurgery. Cryobiology. 1985;22:175-182. [DOI] [PubMed] [Google Scholar]
- 47. Seifert JK, Junginger T, Morris DL. A collective review of the world literature on hepatic cryotherapy. J R Coll Surg Edinb. 1998;43:141-154. [PubMed] [Google Scholar]
- 48. Hanai A, Yang WL, Ravikumar TS. Induction of apoptosis in human colon carcinoma cells HT29 by sublethal cryo-injury: mediation by cytochrome c release. Int J Cancer. 2001;93:526-533. [DOI] [PubMed] [Google Scholar]
- 49. Sabel MS, Su G, Griffith KA, Chang AE. Rate of freeze alters the immunologic response after cryoablation of breast cancer. Ann Surg Oncol. 2010;17:1187-1193. [DOI] [PubMed] [Google Scholar]
- 50. Whittaker DK. Mechanisms of tissue destruction following cryosurgery. Ann R Coll Surg Engl. 1984;66:313-318. [PMC free article] [PubMed] [Google Scholar]
- 51. Hoffmann NE, Bischof JC. The cryobiology of cryosurgical injury. Urology. 2002;60:40-49. [DOI] [PubMed] [Google Scholar]
- 52. Baust JG, Gage AA. The molecular basis of cryosurgery. BJU Int. 2005;95:1187-1191. [DOI] [PubMed] [Google Scholar]
- 53. Hollister WR, Mathew AJ, Baust JG, et al. The effects of freezing on cell viability and mechanisms of cell death in an in vitro human prostate cancer cell line. Mol Urol. 1998;2:13-18. [Google Scholar]
- 54. Jiang J, Goel R, Iftekhar MA, et al. Tumor necrosis factor-alpha-induced accentuation in cryoinjury: mechanisms in vitro and in vivo. Mol Cancer Ther. 2008;7:2547-2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Robilotto AT, Clarke D, Baust JM, Van Buskirk RG, Gage AA, Baust JG. Development of a tissue engineered human prostate tumor equivalent for use in the evaluation of cryoablative techniques. Technol Cancer Res Treat. 2007;6:81-89. [DOI] [PubMed] [Google Scholar]
- 56. Robilotto AT, Baust JM, Van Buskirk RG, Gage AA, Baust JG. Temperature-dependent activation of differential apoptotic pathways during cryoablation in a human prostate cancer model. Prostate Cancer Prostatic Dis. 2013;16:41-49. [DOI] [PubMed] [Google Scholar]
- 57. Sabel MS, Nehs MA, Su G, Lowler KP, Ferrara JL, Chang AE. Immunologic response to cryoablation of breast cancer. Breast Cancer Res Treat. 2005;90:97-104. [DOI] [PubMed] [Google Scholar]
- 58. Yang WL, Addona T, Nair DG, Qi L, Ravikumar TS. Apoptosis induced by cryo-injury in human colorectal cancer cells is associated with mitochondrial dysfunction. Int J Cancer. 2003;103:360-369. [DOI] [PubMed] [Google Scholar]
- 59. Sabel MS, Kaufman CS, Whitworth P, et al. Cryoablation of early-stage breast cancer: work-in-progress report of a multi-institutional trial. Ann Surg Oncol. 2004;11:542-549. [DOI] [PubMed] [Google Scholar]
- 60. Cryoablation of small breast tumors in early stage breast cancer (FROST). https://clinicaltrials.gov/ct2/show/NCT01992250. Updated 2018.
- 61. Cryoablation of low risk small breast cancer—Ice3 trial. https://clinicaltrials.gov/ct2/show/NCT02200705. Updated 2020.
- 62. IceCure™ Medical Ltd. ProSense™ User Manual. DSR3200000 rev. G. https://icecure-medical.com/wp-content/uploads/2020/05/DSR3200000-rev.G-SW3.4.2e_ProSense-User-Manual-_Final.pdf.
- 63. Sanarus Medical. Product Specification, Visica II. PS-71-0001 Rev. E. https://dichthuatasean.com/wp-content/uploads/2016/07/Mau-CV-8.pdf.
- 64. Martin JW, Patel RM, Okhunov Z, Vyas A, Vajgrt D, Clayman RV. Multipoint thermal sensors associated with improved oncologic outcomes following cryoablation. J Endourol. 2017;31:355-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Baust JM, Robilotto AT, Gage AA, Baust JG. Enhanced cryoablative methodologies. In: Bischof J, Ca XY, ed. Multiscale Technologies for Cryomedicine. London, England: World Scientific Publishing Co; 2016:388. [Google Scholar]