Abstract
Background and Aims:
Medication errors occur at any point of the medication management process, and are a major cause of death and harm globally. The objective of this review was to compare the effectiveness of different interventions in reducing prescribing, dispensing and administration medication errors in acute medical and surgical settings.
Methods:
The protocol for this systematic review was registered in PROSPERO (CRD42019124587). The library databases, MEDLINE, CINAHL, EMBASE, PsycINFO, Cochrane Database of Systematic Reviews and the Cochrane Central Register of Controlled Trials were searched from inception to February 2019. Studies were included if they involved testing of an intervention aimed at reducing medication errors in adult, acute medical or surgical settings. Meta-analyses were performed to examine the effectiveness of intervention types.
Results:
A total of 34 articles were included with 12 intervention types identified. Meta-analysis showed that prescribing errors were reduced by pharmacist-led medication reconciliation, computerised medication reconciliation, pharmacist partnership, prescriber education, medication reconciliation by trained mentors and computerised physician order entry (CPOE) as single interventions. Medication administration errors were reduced by CPOE and the use of an automated drug distribution system as single interventions. Combined interventions were also found to be effective in reducing prescribing or administration medication errors. No interventions were found to reduce dispensing error rates. Most studies were conducted at single-site hospitals, with chart review being the most common method for collecting medication error data. Clinical significance of interventions was examined in 21 studies. Since many studies were conducted in a pre–post format, future studies should include a concurrent control group.
Conclusion:
The systematic review identified a number of single and combined intervention types that were effective in reducing medication errors, which clinicians and policymakers could consider for implementation in medical and surgical settings. New directions for future research should examine interdisciplinary collaborative approaches comprising physicians, pharmacists and nurses.
Lay summary
Activities to reduce medication errors in adult medical and surgical hospital areas
Introduction:
Medication errors or mistakes may happen at any time in hospital, and they are a major reason for death and harm around the world.
Objective:
To compare the effectiveness of different activities in reducing medication errors occurring with prescribing, giving and supplying medications in adult medical and surgical settings in hospital.
Methods:
Six library databases were examined from the time they were developed to February 2019. Studies were included if they involved testing of an activity aimed at reducing medication errors in adult medical and surgical settings in hospital. Statistical analysis was used to look at the success of different types of activities.
Results:
A total of 34 studies were included with 12 activity types identified. Statistical analysis showed that prescribing errors were reduced by pharmacists matching medications, computers matching medications, partnerships with pharmacists, prescriber education, medication matching by trained physicians, and computerised physician order entry (CPOE). Medication-giving errors were reduced by the use of CPOE and an automated medication distribution system. The combination of different activity types were also shown to be successful in reducing prescribing or medication-giving errors. No activities were found to be successful in reducing errors relating to supplying medications. Most studies were conducted at one hospital with reviewing patient charts being the most common way for collecting information about medication errors. In 21 out of 34 articles, researchers examined the effect of activity types on patient harm caused by medication errors. Many studies did not involve the use of a control group that does not receive the activity.
Conclusion:
A number of activity types were shown to be successful in reducing prescribing and medication-giving errors. New directions for future research should examine activities comprising health professionals working together.
Keywords: hospitals, medication errors, medical order entry systems, medication reconciliation, medication therapy management, nurses, patient safety, pharmacists, physicians, systematic review
Introduction
Medication errors occur at any point of the medication management process involving prescribing, transcribing, dispensing, administering and monitoring,1,2 have been reported to account for approximately one-quarter of all healthcare errors.3 Medication errors are a major cause of death and harm globally.4 According to the World Health Organisation (WHO), medication errors cost an estimated US$42 billion annually worldwide, which is 0.7% of the total global health expenditure.5
Systematic reviews examining interventions aimed at reducing medication errors have largely focused on specialty settings, such as patients situated in adult and paediatric intensive care units, emergency departments, and neonatal intensive care and paediatric units.6–10 Previous relevant systematic reviews relating to testing interventions for reducing medication errors in general hospital settings have focused on administration errors only,11,12 have involved adult and paediatric settings or have tested interventions in specialty and general hospital settings with no differentiation in results.11–13 This systematic review aims to compare the effectiveness of different interventions in reducing prescribing, dispensing and administration medication errors in acute medical and surgical settings. Information obtained from this review can inform clinicians and policymakers about the types of interventions that have been shown to be effective, which can guide the development of comprehensive guidelines for clinical practice and policy directives.
Methods
In conducting this systematic review, the authors followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.14 The review protocol was registered with PROSPERO (CRD42019124587).
Search strategy
A search was conducted of the following library databases, MEDLINE, CINAHL, EMBASE, PsycINFO, Cochrane Database of Systematic Reviews and the Cochrane Central Register of Controlled Trials, from inception to February 2019.
A search strategy was devised following consultation with a university research librarian to yield relevant studies. Keywords used in the search comprised five categories: the setting, with keywords ‘hospital’, ‘acute’, ‘medical’, ‘surgical’; perspective, with keywords ‘medication management’, ‘medication process’, ‘medicines management’, ‘prescribing’, ‘dispensing’, ‘administration’, ‘monitoring’; population, with keyword ‘adult’; activity, with keywords ‘program’ and ‘intervention’; and phenomenon of interest, with keywords ‘medication errors’, ‘preventive adverse drug events’, and ‘medicine errors’. Keywords in each category were searched using the operator OR, and then combined between categories using the operator AND. Search histories for all databases are listed in Supplemental file S1. Key article cross-checking was performed using citation-linking databases, Scopus and Web of Science in an attempt to identify further articles. Reference lists of relevant articles were checked to identify additional papers. Previous systematic reviews on a similar topic were also examined to determine possible papers for inclusion.11–13
Eligibility criteria
Studies were included if they involved testing an intervention aimed at reducing medication errors in adult acute medical or surgical settings. Adults were defined as patients aged 18 years or over. If patients received the intervention during hospitalisation and the effect on medication errors was measured in the hospital setting, these studies were included. Medication errors comprised any preventable events that may cause or lead to inappropriate medication use or patient harm during prescribing, dispensing or administration.15 The prevalence of medication errors must have been identified as a primary or secondary outcome to be included. Papers were considered for inclusion if they were published before 2000, as this was the year when the landmark publication, To Err is Human: Building a Safer Health System was released by the Institute of Medicine.16 This publication drew attention of the need for health services to develop tools and systems to address problems in patient safety, such as medication errors.
Near misses were not included as medication errors. Only papers published in English were included. Case studies, commentaries, editorials, reviews, epidemiological studies and conference abstracts were excluded. If studies examined medication-related problems as an outcome, which often comprised a combination of medication errors, as well as problems with medication knowledge, medication adherence and other aspects of medication management, these studies were not included. If the effect of the intervention was measured outside the hospital setting, these studies were excluded. Specialty wards such as intensive care, emergency care, perioperative care, neurological and cancer care were excluded. Outpatient settings and subacute settings, such as rehabilitation wards and geriatric evaluation and management units were excluded.
Study selection
Rayyan (Qatar Computing Research Institute), an online platform, was used for independent screening of articles at the title and abstract level, and subsequently at the full text level.17 Two authors reviewed titles and abstracts independently. The third author assessed discrepancies at the title and abstract level. Any uncertainty or disagreement about articles meeting the inclusion criteria was resolved after discussion among all authors. Full texts of papers were then examined independently by two authors to determine if studies were eligible for inclusion in the review. Any discrepancies identified at the full-text level were examined by the third author. Previous systematic reviews on similar topics were also examined to determine possible papers for inclusion.
Quality assessment
Quality assessment was undertaken using the Equator reporting guidelines whereby randomised controlled trials were assessed using the CONSORT guidelines,18 non-randomised studies were assessed using the TREND guidelines,19 and quality improvement studies were assessed using the SQUIRE guidelines.20 No study was excluded on the basis of the score obtained for quality assessment. Risk of bias assessment was also undertaken using Review Manager, version 5.3 (RevMan) (Cochrane Collaboration) software.
Data extraction
Data were extracted from each paper to a standard form for study design, country and setting, number of patients, intervention type, type of medication error analysed and effect of the intervention (Table 1). If the studies provided information about the severity of medication errors using their approach for measuring severity, these data were also included in data extraction.
Table 1.
Overview of studies included in the systematic review (n = 34).
| Reference (country) | Study design | Setting | Number of patients | Intervention type | Type of medication error analysed (method of data collection for medication errors), effect of intervention on medication error rate |
|---|---|---|---|---|---|
| PL-MR | |||||
| Al-Hashar et al.21 (Oman) | Prospective randomised controlled study | Medical wards of a tertiary care academic hospital with a bed capacity of 500 | 286 (intervention), 301 (control) | Pharmacist-led medication reconciliation (PL-MR) | Total number of preventable ADEs (review of electronic health record and patient interview) Control: 59 preventable ADEs (0.20 preventable ADEs/patient) 16% Intervention: 27 preventable ADEs (0.09 preventable ADEs/patient) 9.1%, p = 0.008 Severity of preventable ADEs Serious Control: 22 serious preventable ADEs Intervention: 7 serious preventable ADEs, p = 0.009 Significant Control: 36 significant preventable ADEs Intervention: 20 significant preventable ADEs, p = 0.041 |
| Batra et al.22 (US) | Prospective medical record review | Inpatient wards of 627-bed teaching hospital | 186 admissions for 105 patients with HIV | Pharmacist-led medication reconciliation (PL-MR) | Number of patients with prescribing errors (chart review) Retrospective chart reviews: 289/416 total admissions (35.1%) Intervention: 31/186 total admissions (16.7%) No p-value reported |
| Beckett et al.23 (US) | Prospective randomised, non-blinded study | Patients admitted to one of two general medicine floors or one general surgery floor | 41 (intervention), 40 (control) | Pharmacist-led medication reconciliation (PL-MR) | Medication discrepancies identified (chart review, patient and family interview) Control: 45 Intervention: 71, p = 0.074 No denominator term identified |
| Boockvar et al.24 (US) | Cluster-randomised controlled trial | An inpatient unit of an urban veteran affair hospital with responsible specialties of medicine, surgery or psychiatry | 186 (intervention), 195 (control) | Pharmacist-led medication reconciliation (PL-MR) | Medication discrepancies (prescription coverage plan review, patients, family members, providers interviews) Control: 3.0 mean number of medication discrepancies/195 total number of patients Mean 3.0, SD 2.4 Intervention: 3.2 mean number of medication discrepancies/186 total number of patients Mean 3.2, SD 2.6, p = 0.452 ADEs: 37 patients (9.7%) had 41 ADEs with temporary symptoms. No differences between groups (OR 1.0, 95% CI, 0.49–2.1, p = 0.964) |
| Cadman et al.25 (UK) | Pilot randomised controlled trial | Five adult medical wards of a hospital | 96 (intervention), 102 (control) | Pharmacist-led medication reconciliation (PL-MR) | UDs (chart review, general practitioner and patient notes) Admission: Control: 3.0 per patient 309 UDs in 102 patients Intervention: 2.80 per patient 255 UDs in 96 patients (however one patient did not receive the intervention) Remained at discharge Control: 2.71 per patient 268 UDs in 99 patients Intervention: 0.02 per patient 2 UDs in 91 patients No p-value reported Unplanned readmission at 3 months Control 37 (36.6%) patients Intervention 30 (31.6%) patients Length of hospital stay Control: 109.3 h (95% CI 87.0 to 137.3) Intervention 99.6 (95% CI 76.59 to 129.63) |
| Tong et al.26 (Australia) | Unblinded, cluster randomised, controlled study | General medical unit of an adult major referral hospital | 431 (control), 401 (intervention) | Pharmacist-led medication reconciliation (PL-MR) | Patients’ discharge summaries with at least one medication error (prescribing errors) (discharge summary) Control: 265/431 patients (61.5%) Intervention: 60/401 patients (15%), p < 0.01 Severity of errors Control: insignificant 50 (18.9%), low 86 (32.5%), moderate 81 (30.6%) high 36 (13.6%), extreme 12 (4.5%) Intervention: insignificant 20 (33%), low 22 (37%), moderate 12 (20%), high 5 (8%), extreme 1 (2%), p < 0.01 |
| IT-MR | |||||
| Allison et al.27 (US) | Retrospective medical chart review of pre-post intervention | Medical settings of a tertiary hospital | 100 (pre-intervention), 100 (post-intervention) | Electronic discharge medication reconciliation tool (IT-MR) | Patients with at least one discharge antibiotic medication error (prescribing error) (chart review) Pre-intervention: 23/100 patients Post-intervention: 11/100 patients No p-value reported Total number of discharge medication errors Pre-intervention: 30/45 total number of errors Post-intervention: 15/45 total number of errors No p-value reported |
| Smith et al.28 (US) | Pre–post quasi-experimental study | General medicine, geriatrics, cardiology inpatients | 317 (pre-intervention), 243 (post-intervention) | IT-MR | Discharge medication errors (prescribing errors) (electronic medical record and chart review) Pre-intervention: 645 errors/3490 medication variance Post-intervention: 359 errors/2823 medication variance, p < 0.001 Clinically important medication errors (with potential for serious or life-threatening harm) Pre-intervention: 9/645 errors (1.4%) Post-intervention: 11/359 errors (3.1%), p = 0.10 |
| Medication reconciliation by trained mentors | |||||
| Schnipper et al.29 (US) | Quality improvement study | Medical or surgical units across 5 hospitals, no control units at hospital sites 4 and 5, no intervention units at hospital site 1 | 857 (control), 791 (intervention) | Local implementation of medication reconciliation best practices | Potentially harmful discrepancies in admission and discharge orders per patient (chart review) Results reported as mean number of errors per patient Site 1: did not implement the intervention. Site 2: Control units Pre-implementation: 0.98 Post-implementation: 1.32 Intervention units Pre-implementation: 1.00 Post-implementation: 0.88 Site 3 Control units Pre-implementation: 0.17 Post-implementation: 0.23 Intervention units Pre-implementation: 0.30 Post-implementation: 0.18 Site 4 and site 5: did not have control units at baseline. No p-value reported |
| CDSS | |||||
| Hernandez et al.30 (France) | Before and after observational study | 66-bed orthopaedic surgery unit of a 700-bed teaching hospital | 111 (pre-CPOE), 86 patients (post-CPOE) | CPOE with alerts for drug-allergy checking, therapeutic duplications, dose-range and age-based checking, and drug–drug interactions. No mention of CDSS | Prescribing errors (direct disguised observation) Pre-intervention: 479/1593 prescribed drugs (30.1%) Post-intervention: 33/1388 prescribed drugs (2.4%), p < 0.0001 Dispensing errors Pre-intervention: 430/1219 opportunities (35.3%) Post-intervention: 449/1407 opportunities (31.9%), p = 0.07 Administration errors Pre-intervention: 209/1222 opportunities (17.1%) Post-intervention: 200/1413 opportunities (14.2%), p < 0.05 |
| Milani et al.31 (US) | Prospective intervention | Patients with chronic kidney disease admitted with acute coronary syndrome to medical ward | 33 (intervention), 47 (control) | CPOE with alerts and CDSS for choice of medication, drug dosing based on clinical risk, patient weight, calculated creatinine clearance and consensus guidelines | Adverse drug events (Chart review) Contraindicated medications Control: 8/47 patients (17%) Intervention: 0/33 patients (0%), p = 0.01 In-hospital bleeding Control: 10/47 patients Intervention: 3/33 patients, p = 0.12 90-day mortality Control 7 (15%), Intervention 4 (12%), p = 0.50 Length of stay Control mean 9.1, SD 10.2 Intervention mean 4.8, SD 4.0, p = 0.01 |
| Pettit et al.32 (US) | Retrospective single centre, pre-post intervention study | Patients admitted to a 811-bed academic medical centre who continued on antiretroviral therapy | 167 (pre-intervention), 131 (post-intervention) | CPOE with alerts to drug-interactions and information on medication guidelines. No mention of CDSS | Prescribing errors (chart review) Pre-intervention: 84/167 patients (50.2%) Post-intervention: 37/131 patients (28.2%), p < 0.01 |
| Shawahna et al.33 (Pakistan) | Prospective review study | Various wards of hospital, three medical wards in one teaching hospital | Not available | Paper based versus electronic prescribing with no alerts or CDSS such as checks on drug interactions or allergies | Prescribing errors (chart review) (no numerator or denominator provided for medical wards) Prescription errors in medical ward 1 Control: 19.6% (95% CI 11.0–29.3) Intervention: 8.3% (95% CI 7.2–8.9), p < 0.05 Prescription errors in medical ward 2 Control: 19.6% (95% CI 13.2–24.5) Intervention: 6.3% (95% CI 5.2–7.1), p < 0.05 Prescription errors in medical ward 3 Control: 25.0% (95% CI 17.0–29.7) Intervention: 6.0% (95% CI 2.0–8.0), p < 0.05 Severity of prescription errors – no breakdown according to medical wards Prescription errors made but no clinical consequences Control: 510/3008 total inpatient prescription errors (17.0%) Intervention: 315/1147 total inpatient prescription errors (27.5%), p < 0.01 Prescription errors made that cause patient harm Control: 415/3008 total inpatient prescription errors (13.8%) Intervention: 215/1147 total inpatient prescription errors (18.7%), p < 0.01 Prescription errors made that could potentially result patient death Control: 230/3008 total inpatient prescription errors (7.6%) Intervention: 170/1147 total inpatient prescription errors (14.8%), p < 0.05 |
| van Doormaal et al.34 (The Netherlands) | Interrupted time-series design | Two medical wards of a university hospital and two medical wards of a teaching hospital | 592 (baseline) 603 (post-intervention) |
CPOE with alerts for drug interactions, overdoses and allergies, no CDSS | Prescribing errors (chart review) Baseline: 5724/9039 prescriptions (63.3%) Intervention: 1355/7210 prescriptions (18.8%), p < 0.05 Severe adverse drug events: Baseline: 102/9039 (1.1%) Intervention 54/7210 (0.7%), p < 0.05 |
| PP | |||||
| Garcia-Molina Saez et al.35 (Spain) | Quasi-experimental interrupted time-series study | Cardio-pneumology unit of general hospital | 3 phases: total 321 patients Pre-interventional: 119 Interventional: 105 Post-interventional: 97 |
PP | Reconciliation errors (structured interview with patients or family) Pre-intervention (period 1): 518/1087 total reconciliation errors (47.7%) Intervention (period 2): 188/1087 total reconciliation errors (17.3%) Post-intervention (period 3): 381/1087 total reconciliation errors (35.1%) p < 0.001 between period 1–2 and 2–3 p = 0.288 between period 1–3 Severity Error occurred but did not reach patient Pre-intervention: 273/518 errors (52.7%) Post-intervention: 201/381 errors (52.8%) Error occurred but did not cause patient harm Pre-intervention: 67/518 errors (12.9%) Post-intervention: 39/381 errors (10.2%) Error occurred and required monitoring Pre-intervention: 120/518 errors (23.2%) Post-intervention: 118/381 errors (31.0%) Error required intervention Pre-intervention: 55/518 errors (10.6%) Post-intervention: 19/381 errors (5.0%) Error required hospitalisation Pre-intervention:3/518 errors (0.6%) Post-intervention: 4/381 errors (1.0%) No p-value reported |
| Hassan et al.36 (Malaysia) | Pre intervention and post intervention study | 35-bed nephrology unit | 300 (intervention), 300 (control) | PP | Suspected ADEs (chart review and ward round participation) Control: 73 events, 64 patients/300 patients Intervention: 49 events, 48 patients/300 patients, p < 0.05 Inappropriate medication Control: 322/2814 total prescriptions (11.4%) Intervention: 176/2981 total prescriptions (5.9%), p < 0.001 Severity of ADEs Serious Control: 20 events/300 patients Intervention: 5 events/300 patients Significant Control: 42 events/300 patients Intervention: 36 events/300 patients Insignificant Control: 11 events/300 patients Intervention: 8 events/300 patients No p-value reported |
| Liedtke et al.37 (US) | Retrospective observational study | HIV-seropositive patients admitted to a large teaching hospital | Total 330 patient admissions: Pre-intervention: 153 patient admissions (96 patients) Intervention: 177 patient admissions (114 patients) |
PP | Total number of prescribing errors (sum of the above numbers) (chart review) Pre-intervention: 85 errors/330 admissions Intervention: 24 errors/330 admissions, p < 0.001 |
| PE | |||||
| Gursanscky et al.38 (Australia) | Cluster randomised trial involving prescribers | Four general medical units of a tertiary hospital | Intervention on doctors: 12 interns, 4 registrars | PE Education: Prescribing feedback and targeted education; e-learning on safe-prescribing |
Prescribing errors (chart review) Control: Baseline: 1171 total errors/2389 total medication orders Post-intervention: 1630 total errors/2771 total medication orders, p < 0.001 Pharmacist education: Pre-intervention: 621 total errors/1074 total medication orders (57.8%) Post-intervention: 493 total errors/1333 total medication orders (37.0%), p < 0.001 E-learning: Pre-intervention: 406 total errors/697 total medication orders Post-intervention: 882 total errors/1393 total medication orders, p = 0.025 Rates of clinically significant prescribing errors (potentially lethal, serious, significant errors) Control: Baseline: 104 errors/2389 total medication orders Post-intervention: 166 errors/2771 total medication orders, p < 0.01 Pharmacist education: Baseline: 70 errors/1074 total medication orders (6.52%) Post-intervention: 64 errors/1333 total medication orders (4.80%), p = 0.068 E-learning: Baseline: 42 errors/697 total medication orders Post-intervention: 83 errors/1393 total medication orders, p = 0.951 |
| PTE | |||||
| Weingart et al.39 (US) | Prospective randomised, controlled pilot trial | 40-bed general medicine unit of a Boston teaching hospital | 107 (intervention), 102 (control) | PTE | ADEs (chart review) Control: 3/102 total number of patients (2.9%) Intervention: 8/107 total number of patients (7.5%), p = 0.22 Severity Life threatening Control: 0 events/102 patients Intervention: 2 events/107 patients Serious Control: 0 events/102 patients Intervention: 3 events/107 patients Significant Control: 3 events/102 patients Intervention: 3 events/107 patients Little or none Control: 0 events/102 patients Intervention: 0 events/107 patients, p = 0.09 |
| TME | |||||
| Baqir et al.40 (UK) | Quasi-experimental study | One acute surgical and one acute medical ward at a district general hospital | 181 (intervention), 230 (intra-ward control), 369 (inter-ward control) | TME | Patient with at least one unacceptable omitted dose (administration errors) (chart review) Inter-ward control: 68/369 patients (18.5%) Intervention: 2/181 patients (1.1%), p < 0.0001 Critical unacceptable omitted dose Inter-ward control: 51/369 (13.8%) Intervention: 2/181 (1.1%), p = 0.03 |
| Greengold et al.41 (US) | Randomised, direct observation study | Medical and surgical units of an academic community hospital and a university teaching hospital | Total number of nurses: Medication nurses: 10 General nurses: 18 |
TME | Medication administration errors (observation) Control: 545/3661 opportunities for error (14.9%) Intervention: 912/5792 opportunities for error (15.7%), p < 0.84 No known patient harm or death during study periods (data were not shown) |
| Nguyen et al.42 (US) | Process improvement study | One medical-surgical ward in academic teaching hospital | Total number of nurses: 45 | TME - Teaching nurses to undertake medication pass time out |
Medication administration errors (observation) Pre-intervention: 2 errors/100 administered medications Post-intervention: 0 errors/100 administered medications, p value not stated |
| Schneider et al.43 (US) | Randomised, controlled, non-blinded study | Medical or medical-surgical units of three university hospitals | Total number of nurses: 30, assigned to either control or intervention group. | TME – CD-ROM to nurses | Medication administration error rate (incorrect time, dose preparation and technique) (observation): Control (pre): 29/266 (10.9%) Control (post): 25/284 (8.8%) Intervention (pre): 16/301 (5.3%) Intervention (post): 41/285 (14.4%) Odds ratio: 1.92 (95%CI 0.81–4.58), p = 0.14 |
| MD | |||||
| Dean and Barber44 (UK) | Prospective observational, before and after study | Medical ward and surgical ward of teaching hospital | 23 patients (surgical ward) 21 patients (medical ward) |
Patients bringing in own medications versus traditional pharmacy supply | Administration errors (observation): Surgical ward: Traditional: 66 errors/1510 opportunities (4.4%) Intervention: 64 errors/1279 opportunities (5.0%) Medical ward: Traditional: 86 errors/2066 opportunities (4.2%) Intervention: 41 errors/1212 opportunities (3.4%) Overall administration errors: Traditional: 152 errors/3576 opportunities 4.3% Intervention: 105 errors/2491 opportunities 4.2%, p = 0.99 Severity score (0–10, <3 minor, 3–7 moderate, >7 severe) Surgical wards Control: Mean 1.8 (SD 1.1) Intervention: Mean 1.8 (SD 1.1), Medical wards Control: Mean 1.9 (SD 1.1) Intervention: Mean 1.9 (1.0), p = 0.41 |
| Schimmel et al.45 (The Netherlands) | Prospective observational before and after study | 30-bed orthopaedic ward in a university medical centre | 45 (pre-intervention), 46 (post-intervention) | MD | Medication administration errors (observation) Pre-intervention: 114 errors/589 total observed medication administration (19.4%) Post-intervention: 170 errors/740 total observed medication administration (23.0%) Odds ratio: 1.24 (95%CI 0.95–1.62), p > 0.05 |
| DD ± eMAR | |||||
| Cousein et al.46 (France) | Before–after observational study | 40-bed short stay geriatric unit within a 1800 bed general hospital | 148 (pre-intervention), 166 (post intervention) Baseline: ward stock system |
DD Linking with or without eMAR |
Administration error rates (observation) Pre-intervention: 74/615 opportunities of errors (10.6%) Post-intervention: 41/783 opportunities of errors (5.0%) Without eMAR: 25/378 opportunities of errors (5.8%) p = 0.02 With eMAR: 16/405 opportunities of errors (4.1%) p = 0.001 Severity of errors No harm Control: 21.1% Intervention: 23.2% Minimum harm Control: 31.7% Intervention: 32.7% Monitoring Control: 35.0% Intervention: 33.3% Need for intervention Control: 12.2% Intervention: 10.7%, p < 0.01 |
| Combination of two types of interventions | |||||
| Cann et al.47 (Australia) | Pre–post test design | 29-bed acute surgical ward at tertiary-level regional hospital | 1115 (pre-intervention), 1069 (post-intervention) | PE, PP | Medication errors (did not specify which type) (online clinical incident reporting) Pre-intervention: 12.0 errors/100,000 patient hours Post-intervention: 10.9 errors/100,000 patient hours, p = 0.835 Patient falls Pre-intervention: 13.9/100,000 patient hours Post-intervention: 10.9/100,000 patient hours, p = 0.50 |
| Daniels et al.48 (US) | Prospective intervention | HIV-infected patients admitted to a 803-bed academic medical centre | 78 (intervention), 68 (control) | PE, CPOE | Types of errors (inpatient pharmacy medication system) Prescribing errors: Control: 62/119 total errors (52%) Intervention: 12/17 total errors (70%) Dispensing errors: Control: 39/119 total errors (33%) Intervention: 4/17 total errors (24%) No p-value reported Potential to cause moderate or severe discomfort or clinical deterioration Control: Initial regimen 38/68 (56%), During hospitalisation 44/68 (65%) Intervention: Initial regimen 12/78 (15%), During hospitalisation 17/78 (22%), p < 0.0001 |
| Gimenez-Manzorro et al.49 (Spain) | Pre–post intervention study with no equivalent control group | General surgery department | 107 (pre-intervention), 84 (post-intervention) | CPOE, IT-MR | Unintended discrepancies (prescribing errors) (patient interview) Pre-intervention: 102 unintended discrepancies/887 total number of discrepancies (10.6%) Post-intervention: 65 unintended discrepancies/791 total number of discrepancies (6.6%), p = 0.002 |
| Grimes et al.50 (Ireland) | Uncontrolled before–after study | Four acute medical care wards | 112 intervention group, 121 standard | PL-MR, PP | Errors on admission (prescribing errors) (pre-admission medication list, chart review, discharge medication list) Standard: 49 patients/121 total number of patients (40.5%) Intervention: 10 patients/112 total number of patients (9%), p < 0.0001 Errors at discharge (prescribing errors) Standard: 66 patients/101 total number of patients (65.3%) Intervention 15 patients/108 total number of patients (13.9%), p < 0.0001 No harm Standard: 35 (34.7%) Intervention: 93 (86.1%) Minor harm Standard: 6 (5.9%) Intervention: 2 (1.9%) Moderate harm Standard: 54 (53.5%) Intervention: 13 (12.0%) Severe harm Standard: 6 (5.9%) Intervention: 0 (0%), p < 0.001 |
| Jheeta et al.54 (UK) | Interrupted time series, pre–post intervention study | A 14-bed elderly medicine inpatient ward in a large teaching hospital | 86 (pre-intervention), 86 (post-intervention) | CPOE + electronic admin system (CA) | Medication administration errors (observation) Pre-intervention: 18/428 opportunities for error (4.2%) Post-intervention: 18/528 opportunities for error (3.4%), p = 0.64 Documentation discrepancies Pre-intervention: 5/460 observed documentations (1.1%) Post-intervention: 18/557 observed documentations (3.2%), p = 0.04 |
| Moura et al.51 (Brazil) | Quasi-experimental study | A 172-bed public institution providing primary and tertiary care | 1852 (pre-intervention), 295 (intervention) | CPOE, PP | Incidence rate of all drug-drug interactions (chart review) Pre-intervention: 27.5/1000 patient days Intervention: 13.2/1000 patient days, relative risk = 0.48 (0.44–0.52) Incidence rate of high-severity drug-drug interactions: Pre-intervention: 8.20/1000 patient days Intervention: 1.36/1000 patient days, relative risk = 0.17 (0.13–0.21) |
| Shea et al.52 (US) | Retrospective comparative cohort study | HIV-infected patients admitted to a 244-bed urban academic medical centre | Total 234 patient admissions Pre-intervention: 126 Post-intervention: 108 |
PE, PL-MR | Patient admissions with prescribing errors (chart review) Sum of incorrect/incomplete medication regimen, incorrect dosage regimen, incorrect renal dose adjustment, major drug interaction Pre-intervention: 73/126 total admission Post-intervention: 10/108 total admission Patient admissions with medication error types Incorrect/incomplete medication regimen Pre-intervention: 15/126 total admission (11.9%) Post-intervention: 0/108 total admission, p < 0.001 Incorrect dosage regimen Pre-intervention: 13/126 total admission (10.3%) Post-intervention: 0/108 total admission, p < 0.001 Incorrect renal dose adjustment Pre-intervention: 9/126 total admission (7.1%) Post-intervention: 0/108 total admission, p = 0.01 Incorrect administration Pre-intervention: 56/126 total admission (44.4%) Post-intervention: 3/108 total admission (2.8%), p < 0.001 Major drug interaction Pre-intervention: 36/126 total admission (28.6%) Post-intervention: 10/108 total admission (9.3%), p = 0.001 |
| Combination of three types of interventions | |||||
| Sanders et al.53 (US) | Retrospective before–after study | HIV infected patients admitted to a large academic medical centre | 162 (pre-intervention), 110 (post-intervention) | CPOE, PE, IC Antimicrobial stewardship program: updates of electronic medication records within CPOE, education, collaborative stewardship effort with infectious diseases department |
Prescribing errors (chart review) Pre-intervention: 124 total errors Post-intervention: 43 total errors No denominator given No p-value reported Number of admissions with a medication error Pre-intervention: 81/162 admissions (50%) Post-intervention: 37/110 admissions (34%), p < 0.001 |
ADE, adverse drug event; CA, CPOE + electronic administration system; CDSS, CPOE with or without clinical decision support system; CPOE, computerised physician order entry; DD, automated drug distribution system; eMAR, electronic medication administration record; HIV, human immunodeficiency virus; IC, interdisciplinary collaboration; IT-MR, computerised medication reconciliation; MD, medication dispensing; PE, prescriber education; PL-MR, pharmacist-led medication reconciliation; PP, pharmacist partnership; PTE, patient education; TME, trained medication experts; UD, unintentional discrepancies; UK, United Kingdom; US, United States.
Data synthesis
Data synthesis was undertaken qualitatively, which involved grouping results into meaningful clusters. These meaningful clusters comprised categorising results in terms of dispensing errors, prescribing errors, and administration errors, as well as examining the types of interventions used. Patterns of medication errors were examined across and between studies.
For the calculation of meta-analysis, data were entered into RevMan software according to intervention types. The risk ratio was calculated for categorical outcomes relating to dispensing, prescribing and administration medication errors. For medication error types expressed as continuous outcomes, the standard mean difference was calculated. Studies with incomplete data for RevMan entry were excluded from the meta-analysis. Statistical heterogeneity was calculated and reported in forest plots.
Results
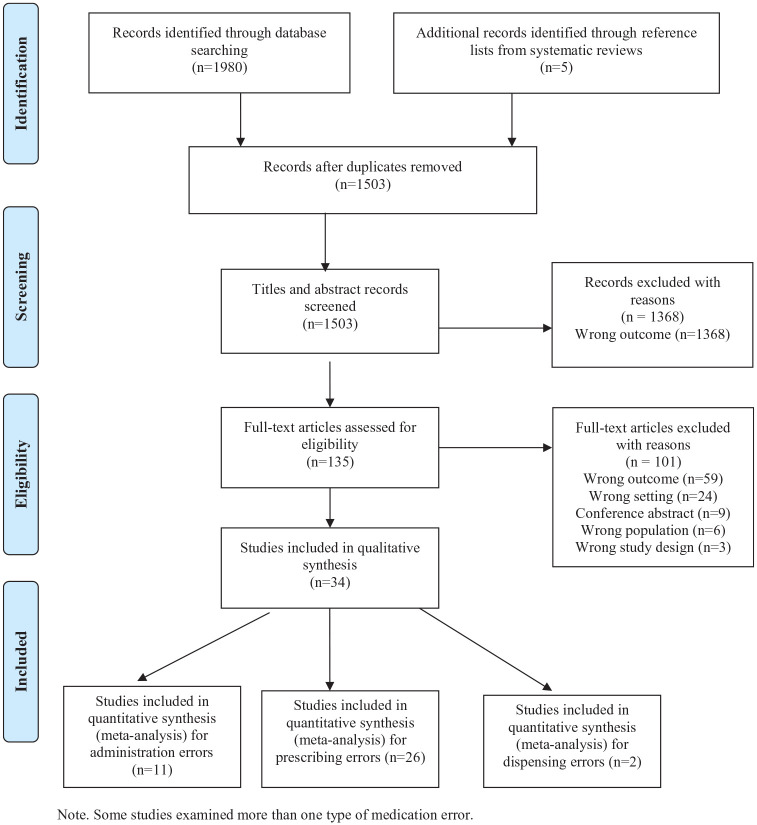
The initial search identified 1980 studies. No additional articles were identified after performing key article cross-checking on Web of Science and Scopus. There were 135 articles selected for full-text screening, of which 34 articles were included for data extraction. A PRISMA flow diagram is included in Figure 1. A total of 26 studies reported on prescribing errors, 11 studies on administration errors and 2 studies on dispensing errors (Table 1).
Figure 1.
PRISMA flow diagram. Some studies examined more than one type of medication error.
PRISMA, preferred reporting items for systematic reviews and meta-analyses.
Study and patient characteristics
The sample size ranged from 33 to 1115 patients in the intervention arm,31,47 and from 40 to 1852 patients in the control arm.23,51 The most common study design was a pre–post intervention design, used in 20 studies.27,28,30–32,35–37,40,44–54 Nine studies were randomised controlled trials (RCTs.21,23–26,38,39,41,43 There were two quality improvement studies,42,29 one study involved a prospective chart review with a historical control,22 one study involved an interrupted time series design34 and one study comprised a prospective observational design.33
A total of 9 studies involved implementation of interventions in both medical and surgical units; 21 studies were conducted in medical units while 4 studies were conducted in surgical units. Chart review was the most common data collection method used to obtain information about medication errors (n = 19), followed by observations (n = 8) and patient and family interviews (n = 5). Other methods used included review of discharge summaries (n = 2), electronic medical record review (n = 2), participation in ward rounds (n = 1), clinical incident reports (n = 1), the inpatient pharmacy system (n = 1), prescription coverage plan (n = 1), health provider interviews (n = 1), patient notes (n = 1) and the preadmission medication list (n = 1). In six studies, more than one method was used to collect data on medication errors. Out of the 34 included studies, 21 contained details about the clinical significance of the medication errors. This information was mainly in the form of severity of the medication errors in causing harm. Other studies provided details about clinical significance in relation to the medication errors prolonging length of hospital stay (n = 2), or contributing to hospital readmission (n = 1), patient mortality (n = 2) and falls (n = 1) (Table 1).
Quality of studies
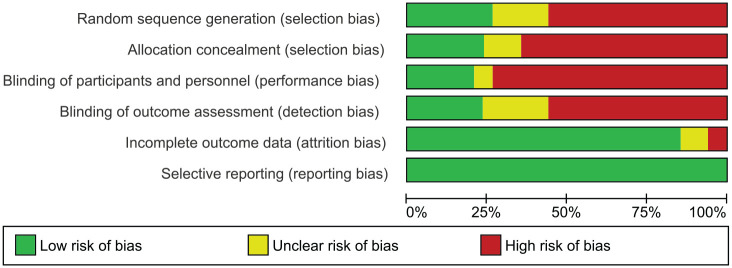
A total of 9 randomised controlled studies scored 49–70% using the CONSORT guideline (Table 2). The quality improvement studies scored 48–80% using the SQUIRE guideline (Table 3); 23 studies scored 36–73% according to the TREND guideline (Table 4). Figure 2 contains the risk of bias graph while Figure 3 shows the risk of bias summary.
Table 2.
Quality assessment for randomized controlled trials and cluster randomized controlled trials using the CONSORT guidelines (n = 9).
| Reference (Country) (intervention) | Study design | Title and Abst | Intro | Trial Desig | Part | Int | Outc | Sp Sz | Rand Seq Gen | Rand Alloc | Rand Impl | Blind | Stat. Meth | Part Flow | Recru | Bas. Data | Num Ana | Out & Est | Anc Anal | Harm | Lim. | Gen | Intp | Reg | Prot | Fund |
n/N
% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Al-Hashar et al.21 (Oman) (PL-MR) |
Prospective randomised controlled study | 1/2 | 2/2 | 1/2 | 2/2 | 1/1 | 1/2 | 1/2 | 2/2 | 1/1 | 1/1 | 0/2 | 1/2 | 2/2 | 1/2 | 1/1 | 1/1 | 1/2 | 1/1 | 0/1 | 1/1 | 1/1 | 1/1 | 1/1 | 0/1 | 1/1 | 26/37 70% |
| Beckett et al.23 (US) (PL-MR) |
Prospective randomised, non-blinded study | 1/2 | 2/2 | 1/2 | 2/2 | 1/1 | 1/2 | 0/2 | 1/2 | 0/1 | 1/1 | 0/2 | 1/2 | 1/2 | 1/2 | 1/1 | 1/1 | 1/2 | 0/1 | 0/1 | 1/1 | 1/1 | 1/1 | 0/1 | 0/1 | 1/1 | 20/37 54% |
| Boockvar et al.24 (US) (PL-MR) |
Cluster-randomised controlled trial | 2/2 | 2/2 | 2/2 | 2/2 | 1/1 | 1/2 | 1/2 | 0/2 | 0/1 | 0/3 | 1/2 | 2/2 | 2/2 | 1/2 | 1/1 | 1/1 | 1/2 | 1/1 | 0/1 | 1/1 | 1/1 | 1/1 | 1/1 | 1/1 | 1/1 | 27/39 69% |
| Cadman et al.25 (UK) (PL-MR) |
Pilot randomised controlled trial | 2/2 | 2/2 | 2/2 | 2/2 | 1/1 | 1/2 | 1/2 | 2/2 | 1/1 | 1/1 | 0/2 | 2/2 | 1/2 | 1/2 | 0/1 | 1/1 | 1/2 | 0/1 | 0/1 | 1/1 | 1/1 | 1/1 | 1/1 | 0/1 | 1/1 | 26/37 70% |
| Tong et al.26 (Australia) (PL-MR) |
Unblinded, cluster randomised, controlled study | 2/2 | 2/2 | 1/2 | 2/2 | 1/1 | 1/2 | 1/2 | 1/2 | 1/1 | 2/3 | 0/2 | 1/2 | 2/2 | 1/2 | 1/1 | 1/1 | 1/2 | 0/1 | 0/1 | 1/1 | 1/1 | 1/1 | 1/1 | 0/1 | 1/1 | 26/39 66% |
| Gursanscky et al.38 (Australia) (PE) |
Cluster randomised trial involving prescribers | 1/2 | 2/2 | 2/2 | 2/2 | 1/1 | 0/2 | 0/2 | 1/2 | 1/1 | 1/3 | 1/2 | 2/2 | 1/2 | 1/2 | 0/1 | 1/1 | 1/2 | 1/1 | 0/1 | 1/1 | 1/1 | 1/1 | 0/1 | 0/1 | 0/1 | 22/39 56% |
| Weingart et al.39 (US) (PTE) | Prospective randomised, controlled pilot trial | 1/2 | 2/2 | 1/2 | 2/2 | 1/1 | 0/2 | 0/2 | 1/2 | 1/1 | 1/1 | 1/2 | 2/2 | 2/2 | 1/2 | 1/1 | 1/1 | 1/2 | 1/1 | 0/1 | 1/1 | 1/1 | 1/1 | 0/1 | 1/1 | 1/1 | 25/37 68% |
| Greengold et al.41 (US) (TME) |
Randomised, direct observation study | 2/2 | 2/2 | 1/2 | 1/2 | 1/1 | 1/2 | 0/2 | 1/2 | 1/1 | 1/1 | 0/2 | 1/2 | 0/2 | 1/2 | 0/1 | 1/1 | 1/2 | 0/1 | 0/1 | 1/1 | 1/1 | 1/1 | 0/1 | 0/1 | 0/1 | 18/37 49% |
| Schneider et al.43 (US) (TME) | Randomised, controlled, non-blinded study | 1/2 | 1/2 | 2/2 | 2/2 | 1/2 | 1/2 | 1/2 | 2/2 | 0/1 | 0/1 | 0/2 | 1/2 | 1/2 | 0/2 | 2/2 | 2/2 | 2/2 | 1/1 | 0/1 | 0/1 | 0/1 | 1/1 | 0/1 | 0/1 | 1/1 | 22/37 59% |
Anc Anal, ancillary analyses; Bas Data, baseline data; Blind, blinding; Fund, funding; Gen, generalizability; Harm, harms, Int, interventions; Intp, interpretation; Intro, introduction; Lim, limitations; Num Ana, numbers analysed; Out & Est, outcomes and estimation; Outc, outcomes; Part, participants; Part Flow, participant flow; PE, prescriber education; PL-MR, pharmacist-led medication reconciliation; Prot, protocol; PTE, patient education; Rand Alloc, randomisation allocation; Rand Impl, randomisation implementation; Rand Seq Gen, randomisation sequence generation; Recru, recruitment; Reg, registration; Sp Sz, sample size; Stat Meth, statistical methods; Title & Abst, title and abstract; TME; trained medication experts; Trial Desig, trial design; US, United States.
Table 3.
Quality assessment for the quality improvement study using the SQUIRE guidelines (n = 2).
| Reference (Country) (intervention) | Title | Abst | Prob. desc | Avail know | Ration | Spec aims | Context | Interv | Study of the interv | Measu | Analy | Eth consid | Results | Summary | Interp | Limit | Conclu | Fund |
n/N
% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Schnipper et al.29 (US) (MR) | 1/1 | 2/2 | 1/1 | 1/1 | 1/1 | 1/1 | 1/1 | 1/2 | 2/2 | 1/3 | 2/2 | 0/1 | 5/6 | 2/2 | 5/5 | 3/3 | 2/5 | 1/1 | 32/40 80% |
| Nguyen et al.42 (US) (TME) | 1/1 | 1/1 | 1/1 | 1/1 | 0/1 | 0/1 | 0/1 | 1/2 | 1/2 | 1/3 | 0/2 | 1/1 | 3/6 | 1/2 | 2/5 | 2/3 | 2/5 | 1/1 | 19/40 48% |
Abst, abstract; Analy, analysis; Avail Know, available knowledge; Conclu, conclusions; Eth Consid, ethical consideration; Fund, funding; Interp, interpretation; Interv, intervention; Limit, limitations, Measu, measures; MR, medication reconciliation; Prob Desc, problem description; Ration, rationale; Spec Aims, specific aims; Study of the Interv, study of the intervention; TME, trained medication experts; US, United States.
Table 4.
Quality assessment for quasi-experimental studies using the TREND guidelines (n = 23).
| Reference (Country) (intervention) | Title and abst | Bgd | Partic | Int | Obj | Outc | Sp Sz | Assign. mtd | Bld | Unit of anal | Stat mtd | Part flow | Recru | Basel data | Basel equiv | No anal | Outc & estim | Anc anal | Adv ev | Inter | Gen | Ov evid |
n/N
% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Batra et al.22 (US) (PL-MR) |
2/3 | 1/2 | 4/4 | 3/9 | 1/1 | 1/3 | 0/1 | 0/3 | 0/1 | 1/2 | 3/4 | 2/7 | 1/1 | 1/4 | 0/1 | 1/2 | 1/3 | 0/1 | 0/1 | 2/4 | 0/1 | 1/1 | 25/59 42% |
| Allison et al.27 (US) (IT-MR) |
1/3 | 1/2 | 4/4 | 5/9 | 1/1 | 2/3 | 0/1 | 0/3 | 0/1 | 1/2 | 3/4 | 4/7 | 1/1 | 3/4 | 1/1 | 1/2 | 1/3 | 0/1 | 0/1 | 3/4 | 1/1 | 1/1 | 34/59 58% |
| Smith et al.28 (US) (IT-MR) |
2/3 | 1/2 | 4/4 | 4/9 | 1/1 | 2/3 | 1/1 | 0/3 | 0/1 | 1/2 | 2/4 | 1/7 | 1/1 | 3/4 | 1/1 | 1/2 | 3/3 | 1/1 | 0/1 | 3/4 | 0/1 | 1/1 | 33/59 56% |
| Hernandez et al.30 (France) (CPOE) |
2/3 | 1/2 | 4/4 | 4/9 | 1/1 | 1/3 | 0/1 | 0/3 | 1/1 | 1/2 | 2/4 | 1/7 | 1/1 | 3/4 | 0/1 | 1/2 | 2/3 | 1/1 | 0/1 | 3/4 | 0/1 | 1/1 | 30/59 51% |
| Milani et al.31 (US) (CPOE + CDSS) |
2/3 | 1/2 | 4/4 | 4/9 | 1/1 | 1/3 | 0/1 | 0/3 | 0/1 | 1/2 | 3/4 | 3/7 | 1/1 | 2/4 | 1/1 | 1/2 | 1/3 | 1/1 | 0/1 | 3/4 | 1/1 | 1/1 | 32/59 54% |
| Pettit et al.32 (US) (CPOE) |
2/3 | 1/2 | 4/4 | 4/9 | 1/1 | 1/3 | 0/1 | 0/3 | 0/1 | 1/2 | 2/4 | 1/7 | 1/1 | 0/4 | 0/1 | 1/2 | 1/3 | 1/1 | 0/1 | 3/4 | 0/1 | 1/1 | 25/59 42% |
| Shawahna et al.33 (US) (CPOE) | 3/3 | 1/2 | 2/4 | 5/9 | 1/1 | 2/3 | 0/1 | 1/3 | 1/1 | 0/2 | 2/4 | 0/7 | 0/1 | 0/4 | 0/1 | 1/2 | 2/3 | 1/1 | 1/1 | 2/4 | 0/1 | 1/1 | 26/59 44% |
| van Doormaal et al.34 (The Netherlands) (CPOE) | 2/3 | 1/2 | 2/4 | 7/9 | 1/1 | 3/3 | 1/1 | 2/3 | 0/1 | 1/2 | 2/4 | 5/7 | 1/1 | 3/4 | 1/1 | 2/2 | 2/3 | 1/1 | 1/1 | 3/4 | 1/1 | 1/1 | 43/59 73% |
| Garcia-Molina Saez et al.35 (Spain) (PP) |
2/3 | 1/2 | 4/4 | 5/9 | 1/1 | 1/3 | 0/1 | 0/3 | 0/1 | 1/2 | 3/4 | 2/7 | 1/1 | 3/4 | 1/1 | 1/2 | 1/3 | 1/1 | 0/1 | 3/4 | 1/1 | 1/1 | 33/59 56% |
| Hassan et al.36 (Malaysia) (PP) |
2/3 | 1/2 | 4/4 | 4/9 | 1/1 | 1/3 | 0/1 | 0/3 | 0/1 | 1/2 | 3/4 | 0/7 | 1/1 | 1/4 | 1/1 | 1/2 | 1/3 | 0/1 | 0/1 | 3/4 | 1/1 | 1/1 | 27/59 46% |
| Liedtke et al.37 (US) (PP) |
3/3 | 1/2 | 4/4 | 4/9 | 1/1 | 2/3 | 0/1 | 0/3 | 1/1 | 1/2 | 3/4 | 2/7 | 1/1 | 3/4 | 0/1 | 1/2 | 1/3 | 0/1 | 0/1 | 3/4 | 1/1 | 1/1 | 33/59 56% |
| Dean and Barber44 (UK) (MD) | 1/3 | 1/2 | 2/4 | 4/9 | 1/1 | 3/3 | 1/1 | 2/3 | 0/1 | 2/2 | 3/4 | 2/7 | 0/1 | 0/4 | 0/1 | 1/2 | 3/3 | 1/1 | 1/1 | 4/4 | 1/1 | 1/1 | 34/59 58% |
| Schimmel et al.45 (Netherland) (MD) |
2/3 | 1/2 | 3/4 | 4/9 | 1/1 | 2/3 | 1/1 | 0/3 | 0/1 | 1/2 | 3/4 | 2/7 | 1/1 | 2/4 | 1/1 | 1/2 | 2/3 | 0/1 | 0/1 | 3/4 | 0/1 | 1/1 | 31/59 53% |
| Baqir et al.40 (UK) | 2/3 | 1/2 | 4/4 | 5/9 | 1/1 | 2/3 | 0/1 | 0/3 | 0/1 | 1/2 | 3/4 | 3/7 | 1/1 | 0/4 | 0/1 | 1/2 | 1/3 | 0/1 | 0/1 | 4/4 | 1/1 | 1/1 | 31/59 53% |
| Cann et al.47 (Australia) (PP, PE) |
2/3 | 1/2 | 3/4 | 5/9 | 1/1 | 2/3 | 0/1 | 0/3 | 0/1 | 1/2 | 2/4 | 1/7 | 1/1 | 0/4 | 0/1 | 1/2 | 2/3 | 0/1 | 0/1 | 3/4 | 1/1 | 1/1 | 27/59 46% |
| Daniels et al.48 (US) (PE, CPOE) |
2/3 | 1/2 | 4/4 | 5/9 | 1/1 | 2/3 | 0/1 | 0/3 | 0/1 | 1/2 | 0/4 | 0/7 | 1/1 | 0/4 | 0/1 | 1/2 | 1/3 | 0/1 | 0/1 | 3/4 | 1/1 | 1/1 | 24/59 41% |
| Gimenez-Manzorro et al.49 (Spain) (CPOE, IT-MR) |
2/3 | 1/2 | 4/4 | 4/9 | 1/1 | 2/3 | 1/1 | 1/3 | 0/1 | 1/2 | 3/4 | 5/7 | 1/1 | 3/4 | 1/1 | 1/2 | 1/3 | 0/1 | 0/1 | 4/4 | 1/1 | 1/1 | 38/59 64% |
| Grimes et al.50 (Ireland) (PL-MR, PP) |
2/3 | 1/2 | 4/4 | 5/9 | 1/1 | 2/3 | 0/1 | 1/3 | 0/1 | 1/2 | 3/4 | 1/7 | 1/1 | 3/4 | 1/1 | 1/2 | 3/3 | 1/1 | 0/1 | 3/4 | 1/1 | 1/1 | 36/59 61% |
| Moura et al.51 (Brazil) (CPOE, PP) |
2/3 | 1/2 | 4/4 | 4/9 | 1/1 | 2/3 | 0/1 | 0/3 | 0/1 | 1/2 | 3/4 | 0/7 | 1/1 | 1/4 | 0/1 | 1/2 | 1/3 | 0/1 | 0/1 | 2/4 | 0/1 | 1/1 | 25/59 42% |
| Shea et al.52 (US) (PE, PL-MR) |
2/3 | 1/2 | 4/4 | 4/9 | 1/1 | 2/3 | 0/1 | 0/3 | 0/1 | 1/2 | 3/4 | 3/7 | 1/1 | 0/4 | 0/1 | 1/2 | 1/3 | 0/1 | 0/1 | 3/4 | 1/1 | 1/1 | 29/59 49% |
| Sanders et al.53 (US) (COPE, PE, IC) |
2/3 | 1/2 | 4/4 | 5/9 | 1/1 | 1/3 | 0/1 | 0/3 | 0/1 | 1/2 | 3/4 | 1/7 | 1/1 | 3/4 | 1/1 | 1/2 | 3/3 | 1/1 | 0/1 | 3/4 | 0/1 | 0/1 | 32/59 54% |
| Cousein et al.46 (France) (DD) |
2/3 | 1/2 | 3/4 | 4/9 | 1/1 | 1/3 | 0/1 | 0/3 | 1/1 | 1/2 | 3/4 | 0/7 | 1/1 | 3/4 | 1/1 | 1/2 | 1/3 | 0/1 | 0/1 | 3/4 | 0/1 | 1/1 | 28/59 47% |
| Jheeta et al.54 (UK) (CPOE, CA) |
2/3 | 1/2 | 1/4 | 4/9 | 1/1 | 1/3 | 0/1 | 0/3 | 0/1 | 1/2 | 1/4 | 2/7 | 1/1 | 0/4 | 0/1 | 1/2 | 1/3 | 0/1 | 0/1 | 3/4 | 0/1 | 1/1 | 21/59 36% |
Adv Ev, adverse events; Anc Anal, ancillary analyses; Assign Mtd, assignment method; Basel Data, baseline data; Basel Equiv, baseline equivalence; Bgd, background; Bld, blinding; CA, electronic administration system, CDSS, clinical decision support system; CPOE, computerized physician order entry; DD, automated drug distribution system, Gen, generalizability; Int, interventions; Inter, interpretation; IT-MR, computerized medication reconciliation; MD, medication dispensing; No Anal, numbers analysed; Obj, objectives; Out, outcomes; Outc & Estim, outcomes and estimation; Ov Evid, overall evidence; Part Flow, participant flow; Partic, participants; PE, prescriber education; PL-MR, pharmacist-Led medication reconciliation; PP, pharmacist partnership; Recru, recruitment; Sp Sz, sample size; Stat Mtd, statistical methods; Title & Abst, title and abstract; TME, trained medication experts; Unit of Anal, unit of analysis; UK, United Kingdom; US, United States.
Figure 2.
Risk of bias graph.
Figure 3.
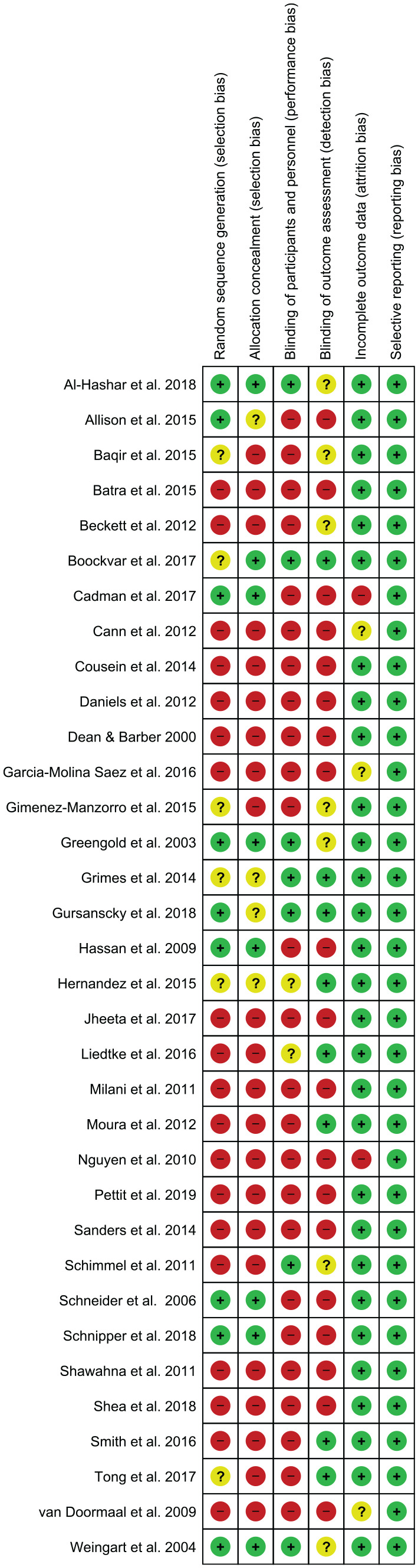
Risk of bias summary.
Identified interventions
The 12 intervention types identified were: pharmacist-led medication reconciliation, computerised medication reconciliation, medication reconciliation by trained mentors, computerised physician order entry (CPOE) with or without a clinical decision support system, pharmacist partnership, prescriber education, patient education, trained medication experts, medication dispensing, use of an automated drug distribution system with or without electronic medication administration record, interdisciplinary collaboration and electronic administration system (Table 5). Various combinations of interventions were also identified.
Table 5.
Types of interventions.
| PL-MR | Pharmacists identify the most accurate list of medications and provide patients with the correct medications in hospital. This is usually conducted at admission and/or discharge. |
| IT-MR | Electronic systems are used to identify the most accurate list of medications and provide patients with the correct medications in hospital. This is usually conducted at admission and/or discharge. |
| CPOE with or without CDSS | Electronic systems designed to automates the medication order process with the use of standardized and complete order. Sometimes this is complemented with the availability of CDSS, providing information on medication dose, route, and frequency. |
| PP | Pharmacists involved as part of the team. This can include ward rounds, providing monitoring service and/or prescription reviews. |
| PE | Educating the prescribers through online modules or pharmacist-led sessions. |
| PTE | Patient education especially on the medical terms on how to take the medication. This is usually conducted by pharmacists. |
| IC | Collaboration with various health care discipline groups for better medication management. |
| TME | Experts who were trained in medication administration. |
| CA | Electronic systems designed to facilitate medication administration. |
| DD | Electronic systems designed to facilitate medication administration via automating drug distribution. |
| eMAR | Electronic records that comprise tools for medication prescription and administration. |
| MD | Different methods of medication cart filling methods to facilitate administration, for example, medications arranged by round time or by their names. |
CA, CPOE + electronic administration system; CDSS, CPOE with or without clinical decision support system; CPOE, computerised physician order entry; DD, automated drug distribution system; IC, interdisciplinary collaboration; IT-MR, computerised medication reconciliation; MD, medication dispensing; PE, prescriber education; PL-MR, pharmacist-led medication reconciliation; PP, pharmacist partnership; PTE, patient education; TME, trained medication experts.
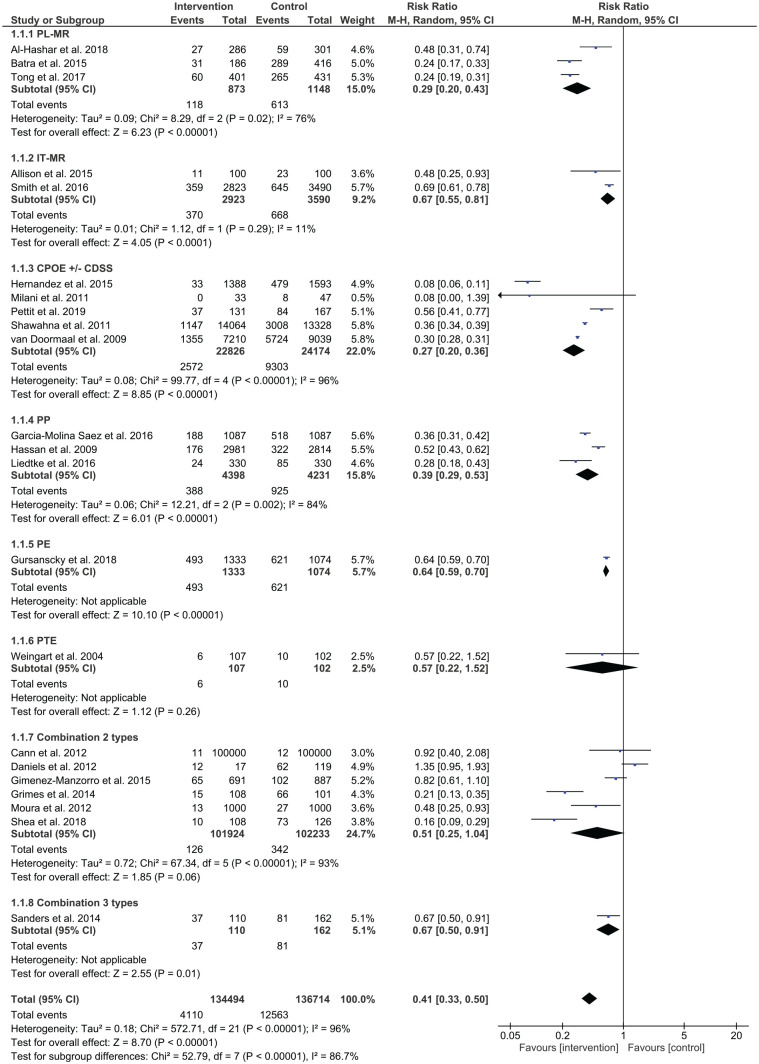
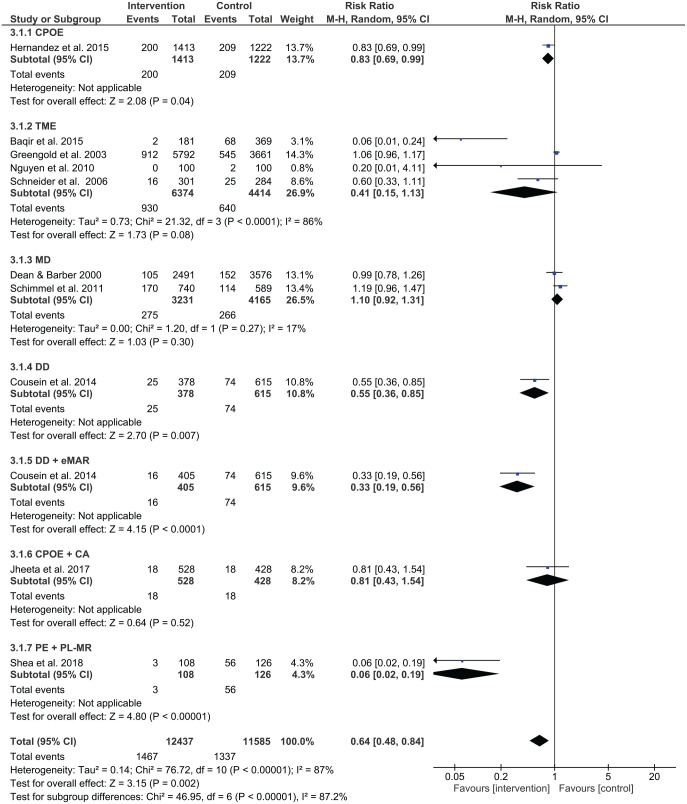
Prescribing error rates were reduced in 14 out of 26 studies, while administration error rates were reduced in 4 out of 11 studies. Out of two studies using interventions for dispensing, no studies reported a significant reduction in dispensing errors. Figure 4 shows a summary of risk ratios for studies that reported on prescribing errors as categorical variables. Figure 5 shows the mean differences for studies reporting on prescribing errors as continuous variables, whereas Figures 6 and 7 present the risk ratio summaries for administration and dispensing errors respectively.
Figure 4.
Risk ratio summary for prescription errors.
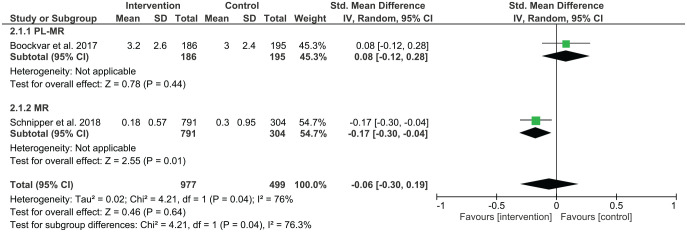
Figure 5.
Standard mean difference summary for prescribing errors.
Figure 6.
Risk ratio summary for administration errors.
Figure 7.
Risk ratio summary for dispensing errors.
Pharmacist-led medication reconciliation
Six studies investigated the effect of pharmacist-led medication reconciliation on prescribing errors, with two out of the six studies reporting a reduction in prescribing error rates. Al-Hashar et al. showed a reduction of preventable adverse drug events (ADEs) from 16% to 9.1% (p = 0.008).21 The percentage of patients with prescribing errors reduced from 35.1% to 16.7% in the work of Batra et al.22 A pilot randomised controlled trial reported a reduction of unintentional discrepancies (UDs) from 2.71 errors per patient in the intervention group (268 UDs in 99 patients) to 0.02 errors per patient in the control group (2 UDs in 91 patients).25 There was no significant difference in prescribed medication discrepancies in the study by Beckett et al., with 45 medication discrepancies in the control group and 71 in the intervention group (p = 0.074).23 Boockvar et al. reported no difference in mean medication discrepancy (3.0 versus 3.2, p = 0.452).24
Computerised medication reconciliation
Two studies employed computerised medication reconciliation to reduce medication errors at discharge and only one showed a significant reduction in errors. In a medication antimicrobial reconciliation program at discharge, Allison et al. undertook a retrospective examination of patients’ medical records to investigate the presence of intravenous antibiotics in their discharge orders before and after the implementation of the intervention.27 Patients with at least one discharge medication error decreased from 23/100 in the pre-intervention period to 11/100 in the post-intervention period (p-value was not reported). Smith et al. conducted a quasi-experimental study and reported a significant reduction (p < 0.001) in discharge medication errors from 645 errors/3490 medication variance in the pre-intervention period to 359 errors/2823 medication variance in the post-intervention period.28 The study also found no change in medication errors having the potential to produce serious or life-threatening harm with 1.4% (9/645 errors) at pre-intervention and 3.1% (11/359 errors at post-intervention, p = 0.10).
Medication reconciliation by trained mentors
One study specified that trained mentors comprising physicians with medication safety experience carried out medication reconciliation.29 Three hospitals were intervention sites and two hospitals were concurrent controls. The outcome was reported as potentially harmful discrepancies in admission and discharge orders per patient. Only two sites (sites 2 and 3) out of five had results for both control units and intervention units. In site 2, the mean number of errors per patient decreased from 1.00 to 0.88. A similar result was found in site 3 where the mean number of errors per patient decreased from 0.30 to 0.18.
CPOE with or without a clinical decision support system
Five studies examined the use of CPOE and reported significant improvements in reduction of medication errors. Hernandez et al. conducted a before-and-after observational study in an orthopaedic surgery unit using CPOE without a clinical decision support system.30 Prescribing errors decreased from 30.1% (479 errors/1593 prescribed medications) in the pre-intervention period to 2.4% (33 errors/1388 prescribed medications) in the post-intervention period (p < 0.0001). The study also found a significant reduction in administration errors (p < 0.05) but showed no significant change in dispensing errors. CPOE with a clinical decision support system was employed by Milani et al. for patients with chronic kidney disease who were admitted with acute coronary syndrome.31 The number of patients with contraindicated medications decreased from 8/47 in the control group to 0/33 in the intervention group (p = 0.01). Pettit et al. found a significant reduction in the number of patients with prescribing errors from 84/167 (50.2%) in the pre-intervention period to 37/131 (28.2%) in the post-intervention period (p < 0.01).32 Shawahna et al. compared paper based with electronic prescribing in Pakistan in a prospective chart review.33 They found prescribing errors differed significantly between control and intervention wards with a mean prescription errors of 21.4% and 6.9% errors respectively. van Doormaal et al. undertook an interrupted time series design and found following CPOE, prescribing errors reduced from 63.3% at baseline to 18.8% following the intervention.34
Pharmacist partnership
Three studies examined the effect of pharmacist partnership and showed significant reductions in prescribing errors. Garcia-Molina Saez et al. involved pharmacists who participated on the medical team who entered patients’ pre-admission medications in a computerised tool, which were then integrated into their clinical history.35 Results showed a significant decrease in prescription reconciliation errors from 47.7% (518/1087) in the pre-intervention period to 17.3% (188/1087) following the intervention period (p < 0.001). Pharmacists were involved in ward rounds in the study conducted by Hassan et al., where the number of inappropriate medications were lower in the intervention group (11.4%, 322/2, 814 total prescriptions) compared with the control group (5.9%, 176/2, 981; p < 0.001).36 Liedtke et al. assessed the effect of a pharmacist monitoring service on admitted human immunodeficiency virus (HIV)-seropositive patients. Prescribing errors reduced following the intervention comprising 24 errors/330 admissions compared with 85 errors/330 admissions at pre-intervention (p < 0.001).37
Prescriber education
One study using a cluster randomised trial examined prescriber education in general medicine units.38 Three groups, each consisting of junior doctors, were assigned to either a control group, a feedback and targeted education by pharmacist group, or an e-learning group. Detailed discussions regarding recently observed prescribing errors were provided by pharmacists during three 10-min sessions per week over the 4-week intervention period. The e-learning group completed an online course with modules on safe and correct prescribing practices. Both the control group and the e-learning group showed a significant increase in prescribing errors from their baselines, with the control group moving from 1171/2389 (49.0%) at baseline to 1630/2771 (58.8%) at post-intervention (p < 0.001), and the e-learning group moving from 406/697 (58.2%) at baseline to 882/1393 (63.3%) at post-intervention (p = 0.025). The pharmacist education group showed decreased prescribing errors from 57.8% (621 errors/1074 total medication orders) to 37.0% (493 errors/1333 total medication orders), p < 0.001. This study also reported on the rate of clinically significant prescribing errors, including potentially lethal, serious, and significant errors, which showed no change in the pharmacist education group between baseline and intervention groups (p = 0.068).
Patient education
One study involved examination of patient education. Weingart et al. conducted a randomised controlled pilot trial, in which patients received a copy of their current medication list with a glossary, explaining common medical terms upon discharge.39 The percentage of patients with potential ADEs did not differ between the control and intervention groups (10 potential ADEs/102 total number of patients versus 6/107 respectively, p = 0.30). This study also found no change in actual ADEs, comprising 2.9% (3 ADEs/102 total number of patients) in the control group and 7.5% (8 ADEs/107 total number of patients) in the intervention group (p = 0.22).
Trained medication experts
Four studies examined the effect of trained medication experts on administration errors and one showed a significant improvement. Baqir et al. investigated the effect of having dedicated trained pharmacy assistants participate in clinical settings and found that the administration error rate in the intervention group (2/181 patients) was less than the rate in the control ward group (68/369 patients), p < 0.0001.40 However, Greengold et al. found no significant change in the administration error rate (p = 0.84) in their study involved the use of dedicated medication nurses in the intervention group.41 The resulting administration error rate was 14.9% (545 errors/3661 opportunities for error) in the control group, while the administration error rate in the intervention group was 15.7% (912 errors/5792 opportunities for error). In the process improvement study undertaken by Nguyen et al., the goal was to teach nurses to focus on reconciling medication orders, on administering medications, on checking medication labels, and on charting medication administration, while at the same time reducing interruptions.42 It was difficult to determine the impact of the intervention as the administration error rate reduced from 2 errors/100 medication administrations to 0 errors/100 medication administrations. Schneider et al. completed a randomised non-blinded controlled study in providing nurse training in medication administration.43 They found no difference in the medication administration error rate (odds ratio = 1.92, 95% CI 0.81–4.58, p = 0.14).
Medication dispensing
Two studies examined the effects of medication dispensing. Using a prospective, observational, before-and-after study, Dean and Barber assessed the effects of patients using their own medications in hospital compared with pharmacists bringing in their supply to the clinical setting.44 Overall, there was no difference in administration errors between the traditional pharmacy supply approach (152 errors/3576 opportunities for error, 4.3%) and patients bringing in their medications (105 errors/2491 opportunities for error, 4.2%, p = 0.99). Using a prospective before-and-after study, Schimmel et al. implemented a medication dispensing intervention in an orthopaedic ward involving medication cart filling by arranging medications by names, compared with usual care of arranging medications by what medications had to be delivered for a particular medication round.45 After the intervention, there was no change in medication administration error rates (19.4% at pre-intervention and 23.0% at post-intervention, odds ratio = 1.24, 95% CI 0.95–1.62).
Automated drug distribution system ± electronic medication administration record
One study assessed the effect of an automated drug distribution system with and without an electronic medication administration record, showing significant reductions in administration errors in both interventions.46 In the pre-intervention period, 74 errors were identified out of 615 opportunities for errors (10.6%). Without the electronic medication administration record, the administration error reduced to 5.8% (25/378 opportunities for errors, p = 0.02). The error rate reduced even further with the use of electronic medication administration record, where only 16 errors were identified out of 405 opportunities for errors (4.1%, p = 0.001).
Combining intervention types
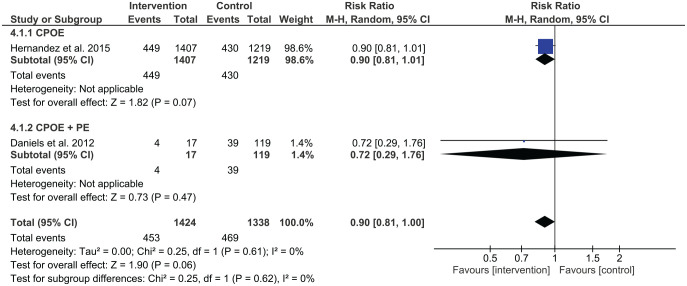
The effect of combining interventions was also investigated in studies. Prescriber education, pharmacist partnership and CPOE were the most frequent components of combinations for prescribing errors. In studies examining the combinations of two interventions to test the effects on prescribing errors, meta-analysis identified mixed results. Grimes et al. assessed the effectiveness of pharmacist-led medication reconciliation and pharmacist partnership in acute medical units, finding a lower prescribing error rate at discharge in the intervention group (13.9%) compared with the control group (65.3%, p < 0.0001).50 Shea et al. demonstrated that the combination of prescriber education and pharmacist-led medication reconciliation was effective in reducing prescribing errors.52 However, the combination of prescriber education and CPOE in the study by Daniels et al. did not reduce prescribing errors.48 Cann et al. applied prescriber education (PE) and pharmacist partnership in an acute surgical ward, with no significant change in medication errors with 12.0 errors at pre-intervention and 10.9 errors per 100,000 patient hours [relative risk (RR) 0.92, 95% confidence interval (CI) 0.40–2.08, p = 0.835].47 Gimenez-Manzorro et al. recruited patients from general surgical units and utilised computerised medication reconciliation integrated into the computerised physician order system.49 Unintended discrepancies decreased from 10.6% in the pre-intervention phase to 6.6% in the post-intervention phase (p = 0.002). The combination of three different types of interventions, CPOE, prescriber education and interdisciplinary collaboration for HIV-infected patients admitted to acute medical and surgical services decreased the rate of medication errors from 50% in the pre-intervention period to 34% in the post-intervention period (p < 0.001).53
Three studies assessed the combination of two different types of interventions involving administration errors. Shea et al. found that administration errors reduced with pharmacist-led medication reconciliation and prescriber education, p < 0.001.52 Jheeta et al. examined the effect of combining CPOE and electronic administration system, which showed no significant change in administration errors (p = 0.64).54 Cousein et al. found in examining an automated drug distribution system and an electronic medication administration record, administration errors significantly reduced following the combined intervention.46
The study by Daniels et al. was the only one that assessed dispensing errors using a combination of interventions.48 Dispensing error rates were 39/119 (33%) at pre-intervention and 4/17 (24%) at post-intervention with the implementation of CPOE and prescriber education. However, this change was insignificant (RR 0.72, 95% CI 0.29–1.76) (Figure 5).
Discussion
This systematic review investigated the effectiveness of various types of interventions in reducing medication errors in adult acute medical and surgical settings. Meta-analysis results showed that prescribing errors were reduced by pharmacist-led medication reconciliation, computerised medication reconciliation, pharmacist partnership, prescriber education, medication reconciliation by trained mentors, and CPOE as single interventions. Medication administration errors were reduced by CPOE and the use of an automated drug distribution system as single interventions. Furthermore, combined interventions that included CPOE, prescriber education and interdisciplinary collaboration were effective for prescribing errors while combined interventions that included automated drug distribution and use of the electronic medical record, or prescriber education and pharmacist-led medication reconciliation were found to be effective in reducing administration errors. No interventions were found to reduce dispensing error rates.
Pharmacist-led medication reconciliation showed mixed results in terms of effectiveness in reducing prescribing errors. Effectiveness of this intervention type was demonstrated in three studies, comprising implementation of HIV-specialised pharmacists reconciling prescribing errors within 24 h by liaising with the inpatient team,22 targeting discharge summary errors by having pharmacists complete discharge medication documentation,26 and examining medication reconciliation on admission and discharge, while undertaking bedside counselling.21 Results in two of these studies may be biased as the error-identifying assessor was not blinded as to who completed the discharge plans. Al-Hashar et al. found a lower effectiveness of pharmacist-led medication reconciliation compared with studies by Batra et al. and Tong et al. because patients were contacted 30 days after discharge and recall bias may have influenced the results.21,22,26 Beckett et al. found an increase in prescribing errors, which was explained by having a greater number of patients not being fully alert or oriented who were allocated to the intervention group under randomisation.23 These patients possibly required medications to manage their mental state in addition to the treatment for their admissions. Boockvar et al. reported no change in error rates.24 The authors found that charging for accessing prescription information led to blocked availability of medication details if a transactional payment was not affordable. This issue demonstrates the importance of changing context in determining the impact of effectiveness.
Computerised medication reconciliation was comparatively less effective than pharmacist-led medication reconciliation at reducing prescribing errors. Only two studies used computerised medication reconciliation, and neither of the studies included surgical patients.27,28 Further studies using this intervention could examine the effectiveness in surgical patients with a larger sample size.
The quality improvement study by Schnipper et al. achieved implementation of medication reconciliation by trained mentors across five different sites without providing additional resources to hospitals.29 The study was an example of a potentially cost-saving strategy of long-term implementation. The study was conducted in diverse settings, including academic medical centres, community hospitals and veteran affairs medical centres, thereby indicating that the results could be generalised to other similar hospitals.
Studies utilising CPOE showed beneficial results. The results from Hernandez et al. favoured the intervention.30 However, with prescriptions routinely checked by pharmacist post-intervention, it is difficult to assess the add-on effect from involving the pharmacist. The study Milani of et al. was the only one examining the effects of both CPOE and clinical decision support31; however, it involved small sample sizes (n = 33 and 47 in the intervention and control groups, respectively). Other studies with alerts showed significant reductions in prescribing errors.32,34 Interestingly, Shawahna et al.’s study, which comprised neither alerts nor clinical decision support, also demonstrated reduced prescription errors in intervention wards.33 However, the effect of the intervention was more pronounced on minor errors without clinical consequences compared with those that were likely to cause patient harm.
Prescriber education as a single intervention was examined in one study, showing a significant effect on prescribing errors.38 However, it is difficult to deduce the individual effect of prescriber education when combined with other interventions.47,48,52,53 One cluster randomised trial investigated the effect of e-learning tools in comparison to pharmacists’ targeted feedback and education.38 In this study, prescribing errors showed no change in medication errors after prescribers finished e-learning modules. This lack of change could have occurred due to difficulties in prescribers applying general knowledge of prescribing practice learnt from e-learning modules to clinical scenarios, in the absence of targeted feedback and education sessions. There appears to be limited benefit in the use of e-learning modules and future research could focus on examining this use of this type of intervention with application to clinical scenarios and targeted feedback.
A total of 11 studies examined the effect of interventions on administration errors. For all single and multifaceted interventions, generally a small number of studies were undertaken for each intervention type. Possible reasons for lack of impact of interventions for some studies included small patient samples and the short period for embedding the intervention before testing occurred.45,54 To understand the trends and impact of interventions, future work should encompass the conduct of well-designed studies with adequate sample sizes.
There were methodological concerns with included studies, which comprised lack of information about sample size calculations, how participants were recruited in studies and lack of blinding to the intervention. The quality improvement study conducted by Schnipper et al. scored the highest in the quality assessment. Most studies were conducted at a single site, relaying difficulties in generalising results to other hospitals and settings.29 Many studies were conducted in a pre–post format. Future studies should involve examining the effect of time on the intervention by including a concurrent control group. Out of the 34 studies, only 21 studies contained information about the clinical significance of medication errors. Where clinical significance of medication errors was not provided, it is difficult to understand the true impact of interventions. Such difficulties arise in medication reconciliation studies where relatively minor discrepancies may have been regarded as medication errors. It is important for intervention studies to have details provided about clinical significance of medication errors. The use of universal reporting standards, such as the one endorsed by the National Coordinating Council for Medication Error Reporting and Prevention,15 would enable consistent scoring and facilitate greater comprehension of the impact of interventions on patient harm. In addition, it is vital to use independent panels to assess the likely clinical significance of medication errors.
Several interventions have been identified as effective in reducing prescribing and administration errors, including medication reconciliation by trained mentors. While pharmacist-led medication reconciliation was time-consuming and costly, computerised medication reconciliation could be a suitable alternative, although a computerised system may not be able to replace a pharmacist taking the best possible medication history. With more hospitals adopting computerised systems, adding features to the system, such as computerised medication reconciliation and CPOE with or without clinical decision support system might cost proportionally less overall. The effectiveness of CPOE in reducing administration errors could also be an added benefit. Further research examining the effect of computerised medication reconciliation and CPOE should confirm whether this combination is still effective in reducing both prescribing and administration errors. As the systematic review did not identify improvements in dispensing errors with prescriber education and CPOE, the addition of pharmacist-led medication reconciliation or pharmacist partnership may help to facilitate a reduction in dispensing errors.
There are limitations of this systematic review. There may be unpublished studies that have demonstrated insignificant error results. Results reported in conference abstracts were not included. Similarly, studies not reported in English were also not included. Medication error calculations comprised a variety of formats, including the proportion of medication errors in relation to the opportunity for errors as well as the proportion of patients with medication errors. These error calculations were directly inserted into RevMan for meta-analysis. The variability of the units for medication errors probably contributed to the extensive heterogeneity of meta-analysis results. For the systematic review, the definition used for medication errors was broad, encompassing any preventable medication event that may cause inappropriate medication use or lead to patient harm. Subsequently, the systematic review included studies where the outcome variables comprised medication errors, as well as ADEs, which involve harm caused by medications as a result of medication errors, and unintended medication discrepancies where there were unexplained differences in medications prescribed across patient transfers. There was also variability in the calculation of medication error rates. Rates were variably expressed as the number of errors obtained as a proportion of the total opportunities of errors, the number of patients experiencing as least one error compared with the total number of patients involved, and the number of errors involved in relation to the total number of patients. The data collection method used to determine medication errors also varied between studies. These factors all contributed to the relatively high level of heterogeneity between studies.
Conclusion
This systematic review examined the efficacy of interventions in reducing medication errors within medical and surgical settings. The systematic review identified a number of single and combined intervention types that were effective in reducing medication errors that clinicians and policymakers could consider for implementation in medical and surgical settings. There were no effective interventions identified for reducing dispensing errors. More research is needed in the conduct of randomised intervention studies and well-constructed observational studies, with a greater focus on the clinical significance of the interventions. Interventions comprising interdisciplinary approaches including physicians, pharmacists and nurses are also warranted.
Supplemental Material
Supplemental material, TADS_Supplementary_file_1_20200527 for Interventions to reduce medication errors in adult medical and surgical settings: a systematic review by Elizabeth Manias, Snezana Kusljic and Angela Wu in Therapeutic Advances in Drug Safety
Acknowledgments
Many thanks to Jim Berryman for his help with developing the search terms.
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Elizabeth Manias  https://orcid.org/0000-0002-3747-0087
https://orcid.org/0000-0002-3747-0087
Snezana Kusljic  https://orcid.org/0000-0002-2465-9884
https://orcid.org/0000-0002-2465-9884
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Elizabeth Manias, School of Nursing and Midwifery, Centre for Quality and Patient Safety Research, Institute for Health Transformation, Deakin University, 221 Burwood Highway, Burwood, Victoria 3125, Australia; Melbourne School of Health Sciences, The University of Melbourne, Melbourne, Australia; Department of Medicine, Royal Melbourne Hospital.
Snezana Kusljic, Department of Nursing, The University of Melbourne, Melbourne, Victoria, Australia; The Florey Institute of Neuroscience and Mental Health, Melbourne, Australia.
Angela Wu, Melbourne Medical School, The University of Melbourne, Melbourne, Victoria, Australia.
References
- 1. Barker KN, Mikeal RL, Pearson RE, et al. Medication errors in nursing homes and small hospitals. Am J Hosp Pharm 1982; 39: 987–991. [PubMed] [Google Scholar]
- 2. Fahimi F, Abbasi Nazari M, Abrishami R, et al. Transcription errors observed in a teaching hospital. Arch Iran Med 2009; 12: 173–175. [PubMed] [Google Scholar]
- 3. Patel I, Balkrishnan R. Medication error management around the globe: an overview. Indian J Pharm Sci 2010; 72: 539–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Makary MA, Daniel M. Medical error—the third leading cause of death in the US. BMJ 2016; 353: i2139. [DOI] [PubMed] [Google Scholar]
- 5. Donaldson LJ, Kelley ET, Dhingra-Kumar N, et al. Medication without harm: WHO’s third global patient safety challenge. Lancet 2017; 389: 1680–1681. [DOI] [PubMed] [Google Scholar]
- 6. Manias E, Kinney S, Cranswick N, et al. Interventions to reduce medication errors in pediatric intensive care. Ann Pharmacother 2014; 48: 1313–1331. [DOI] [PubMed] [Google Scholar]
- 7. Manias E, Williams A, Liew D. Interventions to reduce medication errors in adult intensive care: a systematic review. Br J Clin Pharmacol 2012; 74: 411–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nguyen M-NR, Mosel C, Grzeskowiak LE. Interventions to reduce medication errors in neonatal care: a systematic review. Ther Adv Drug Saf 2018; 9: 123–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rinke ML, Bundy DG, Velasquez CA, et al. Interventions to reduce pediatric medication errors: a systematic review. Pediatrics 2014; 134: 338–360. [DOI] [PubMed] [Google Scholar]
- 10. Weant KA, Bailey AM, Baker SN. Strategies for reducing medication errors in the emergency department. Open Access Emerg Med 2014; 6: 45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Berdot S, Roudot M, Schramm C, et al. Interventions to reduce nurses’ medication administration errors in inpatient settings: a systematic review and meta-analysis. Int J Nurs Stud 2016; 53: 342–350. [DOI] [PubMed] [Google Scholar]
- 12. Keers RN, Williams SD, Cooke J, et al. Impact of interventions designed to reduce medication administration errors in hospitals: a systematic review. Drug Saf 2014; 37: 317–332. [DOI] [PubMed] [Google Scholar]
- 13. Acheampong F, Anto BP, Koffuor GA. Medication safety strategies in hospitals–a systematic review. Int J Risk Saf Med 2014; 26: 117–131. [DOI] [PubMed] [Google Scholar]
- 14. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009; 151: 264–269. [DOI] [PubMed] [Google Scholar]
- 15. National Coordinating Council for Medication Error Reporting and Prevention. About medication errors, https://www.nccmerp.org/about-medication-errors (2020).
- 16. Institute of Medicine Committee (US) on Quality of Health Care in America. In: Kohn LT, Corrigan JM, Donaldson MS. (eds) To err is human: building a safer health system. Washington, DC: National Academies Press, 2000. [PubMed] [Google Scholar]
- 17. Ouzzani M, Hammady H, Fedorowicz Z, et al. Rayyan QCRI, the systematic reviews web app, https://rayyan.qcri.org/welcome (2016). [DOI] [PMC free article] [PubMed]
- 18. Schulz KF, Altman DG, Moher D; CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010; 340: c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Des Jarlais DC, Lyles C, Crepaz N. Improving the reporting quality of nonrandomized evaluations of behavioral and public health interventions: the TREND statement. Am J Public Health 2004; 94: 361–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ogrinc G, Davies L, Goodman D, et al. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf 2016; 25: 986–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Al-Hashar A, Al-Zakwani I, Eriksson T, et al. Impact of medication reconciliation and review and counselling, on adverse drug events and healthcare resource use. Int J Clin Pharm 2018; 40: 1154–1164. [DOI] [PubMed] [Google Scholar]
- 22. Batra R, Wolbach-Lowes J, Swindells S, et al. Impact of an electronic medical record on the incidence of antiretroviral prescription errors and HIV pharmacist reconciliation on error correction among hospitalized HIV-infected patients. Antivir Ther 2015; 20: 555–559. [DOI] [PubMed] [Google Scholar]
- 23. Beckett RD, Crank CW, Wehmeyer A. Effectiveness and feasibility of pharmacist-led admission medication reconciliation for geriatric patients. J Pharm Pract 2012; 25: 136–141. [DOI] [PubMed] [Google Scholar]
- 24. Boockvar KS, Ho W, Pruskowski J, et al. Effect of health information exchange on recognition of medication discrepancies is interrupted when data charges are introduced: results of a cluster-randomized controlled trial. J Am Med Inform Assoc 2017; 24: 1095–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cadman B, Wright D, Bale A, et al. Pharmacist provided medicines reconciliation within 24 hours of admission and on discharge: a randomised controlled pilot study. BMJ Open 2017; 7: e013647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tong EY, Roman CP, Mitra B, et al. Reducing medication errors in hospital discharge summaries: a randomised controlled trial. Med J Aust 2017; 206: 36–39. [DOI] [PubMed] [Google Scholar]
- 27. Allison GM, Weigel B, Holcroft C. Does electronic medication reconciliation at hospital discharge decrease prescription medication errors? Int J Health Care Qual Assur 2015; 28: 564–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Smith KJ, Handler SM, Kapoor WN, et al. Automated communication tools and computer-based medication reconciliation to decrease hospital discharge medication errors. Am J Med Qual 2016; 31: 315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schnipper JL, Mixon A, Stein J, et al. Effects of a multifaceted medication reconciliation quality improvement intervention on patient safety: final results of the MARQUIS study. BMJ Qual Saf 2018; 27: 954–964. [DOI] [PubMed] [Google Scholar]
- 30. Hernandez F, Majoul E, Montes-Palacios C, et al. An observational study of the impact of a computerized physician order entry system on the rate of medication errors in an orthopaedic surgery unit. PLoS One 2015; 10: e0134101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Milani RV, Oleck SA, Lavie CJ. Medication errors in patients with severe chronic kidney disease and acute coronary syndrome: the impact of computer-assisted decision support. Mayo Clin Proc 2011; 86: 1161–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pettit NN, Han Z, Choksi A, et al. Reducing medication errors involving antiretroviral therapy with targeted electronic medical record modifications. AIDS Care 2019; 31: 893–896. [DOI] [PubMed] [Google Scholar]
- 33. Shawahna R, Rahman NU, Ahmad M, et al. Electronic prescribing reduces prescribing error in public hospitals. J Clin Nurs 2011; 20: 3233–3245. [DOI] [PubMed] [Google Scholar]
- 34. van Doormaal JE, van den Bemt PM, Zaal RJ, et al. The influence that electronic prescribing has on medication errors and preventable adverse drug events: an interrupted time-series study. J Am Med Inform Assoc 2009; 16: 816–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. García-Molina Sáez C, Urbieta Sanz E, Madrigal de Torres M, et al. Computerized pharmaceutical intervention to reduce reconciliation errors at hospital discharge in Spain: an interrupted time-series study. J Clin Pharm Ther 2016; 41: 203–208. [DOI] [PubMed] [Google Scholar]
- 36. Hassan Y, Al-Ramahi RJ, Aziz NA, et al. Impact of a renal drug dosing service on dose adjustment in hospitalized patients with chronic kidney disease. Ann Pharmacother 2009; 43: 1598–1605. [DOI] [PubMed] [Google Scholar]
- 37. Liedtke MD, Tomlin CR, Skrepnek GH, et al. HIV pharmacist’s impact on inpatient antiretroviral errors. HIV Med 2016; 17: 717–723. [DOI] [PubMed] [Google Scholar]
- 38. Gursanscky J, Young J, Griffett K, et al. Benefit of targeted, pharmacist-led education for junior doctors in reducing prescription writing errors – a controlled trial. J Pharm Pract Res 2018; 48: 26–35. [Google Scholar]
- 39. Weingart SN, Toth M, Eneman J, et al. Lessons from a patient partnership intervention to prevent adverse drug events. Int J Qual Health Care 2004; 16: 499–507. [DOI] [PubMed] [Google Scholar]
- 40. Baqir W, Jones K, Horsley W, et al. Reducing unacceptable missed doses: pharmacy assistant-supported medicine administration. Int J Pharm Pract 2015; 23: 327–332. [DOI] [PubMed] [Google Scholar]
- 41. Greengold NL, Shane R, Schneider P, et al. The impact of dedicated medication nurses on the medication administration error rate: a randomized controlled trial. Arch Intern Med 2003; 163: 2359–2367. [DOI] [PubMed] [Google Scholar]
- 42. Nguyen EE, Connolly PM, Wong V. Medication safety initiative in reducing medication errors. J Nurs Care Qual 2010; 25: 224–230. [DOI] [PubMed] [Google Scholar]
- 43. Schneider PJ, Pedersen CA, Montanya KR, et al. Improving the safety of medication administration using an interactive CD-ROM program. Am J Health Syst Pharm 2006; 63: 59–64. [DOI] [PubMed] [Google Scholar]
- 44. Dean B, Barber N. The effects of a patient’s own drugs scheme on the incidence and severity of medication administration errors. Int J Pharm Pract 2000; 8: 209–216. [Google Scholar]
- 45. Schimmel AM, Becker ML, van den Bout T, et al. The impact of type of manual medication cart filling method on the frequency of medication administration errors: a prospective before and after study. Int J Nurs Stud 2011; 48: 791–797. [DOI] [PubMed] [Google Scholar]
- 46. Cousein E, Mareville J, Lerooy A, et al. Effect of automated drug distribution systems on medication error rates in a short-stay geriatric unit. J Eval Clin Pract 2014; 20: 678–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cann T, Gardner A. Change for the better: an innovative model of care delivering positive patient and workforce outcomes. Collegian 2012; 19: 107–113. [DOI] [PubMed] [Google Scholar]
- 48. Daniels LM, Raasch RH, Corbett AH. Implementation of targeted interventions to decrease antiretroviral-related errors in hospitalized patients. Am J Health Syst Pharm 2012; 69: 422–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gimenez-Manzorro A, Romero-Jimenez RM, Calleja-Hernandez MA, et al. Effectiveness of an electronic tool for medication reconciliation in a general surgery department. Int J Clin Pharm 2015; 37: 159–167. [DOI] [PubMed] [Google Scholar]
- 50. Grimes TC, Deasy E, Allen A, et al. Collaborative pharmaceutical care in an Irish hospital: uncontrolled before-after study. BMJ Qual Saf 2014; 23: 574–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Moura CS, Prado NM, Belo NO, et al. Evaluation of drug-drug interaction screening software combined with pharmacist intervention. Int J Clin Pharm 2012; 34: 547–552. [DOI] [PubMed] [Google Scholar]
- 52. Shea KM, Hobbs AL, Shumake JD, et al. Impact of an antiretroviral stewardship strategy on medication error rates. Am J Health Syst Pharm 2018; 75: 876–885. [DOI] [PubMed] [Google Scholar]
- 53. Sanders J, Pallotta A, Bauer S, et al. Antimicrobial stewardship program to reduce antiretroviral medication errors in hospitalized patients with human immunodeficiency virus infection. Infect Control Hosp Epidemiol 2014; 35: 272–277. [DOI] [PubMed] [Google Scholar]
- 54. Jheeta S, Franklin BD. The impact of a hospital electronic prescribing and medication administration system on medication administration safety: an observational study. BMC Health Serv Res 2017; 17: 547. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, TADS_Supplementary_file_1_20200527 for Interventions to reduce medication errors in adult medical and surgical settings: a systematic review by Elizabeth Manias, Snezana Kusljic and Angela Wu in Therapeutic Advances in Drug Safety