Abstract
Increased gut permeability has been suggested in patients with celiac disease (CD). We aimed to compare gut permeability in children using the lactulose/rhamnose permeability test. We prospectively recruited 55 children into 3 groups; 27 in group 1 (children with newly diagnosed celiac disease, 12 in group 2 (siblings of children with celiac disease) and 16 in group 3 (control group). The median age of participants was 11 years 2 months in group 1, 9 years 5 months in group 2 and 10 years 3 months in group 3. Standardized median delta rhamnose was lower in CD group as compared to control group (147.5 vs 3153.1, P = 0.040). The low median rhamnose absorption in children with celiac disease as compared to other groups suggests that this test can differentiate between damaged and healthy mucosa, hence can it potentially can be used as a noninvasive test of mucosal healing in children with celiac disease.
Keywords: Gut permeability, celiac disease, lactulose rhamnose test, children
Introduction
The prevalence of autoimmune disorders including celiac disease (CD) seems to be increasing globally.1-4 Interplay of genetic predisposition and environmental triggers are thought to be the main reasons for the increased prevalence. CD is distinct among the immune-mediated disorders since both the genetic predisposition (carrying HLA DQ2 or DQ8 genotype) and the environmental trigger (consuming gluten containing grains) are known.5-7 On the other hand, it is not clear why gluten triggers the autoimmune response that results in small bowel damage in some individual who carry the permissive HLA genotype for CD. Initial studies suggested that factors like early childhood infections, breast feeding and a delayed gluten exposure can increase the risk of CD.8-10 However, these differences in exposures are not supported as significant associations in recent literature.11-14 The potential role of other environmental factors like gut microbiome in the pathogenesis of CD is also being studied, although results are not conclusive.15 However, carrying CD permissive genotype HLA DQ2/DQ8 is still considered as the key predictor factor for developing CD.16-18 The observed high prevalence of CD in first degree relatives of patients with CD may also explain the genetic predisposition.19
The variability in gut permeability to luminal antigens, like gluten in CD, may explain why only subsets of people with genetic predisposition go on to develop autoimmune disease.20 The intestinal mucosal surface represents a large interface surface with the environment and functions as the first barrier of defense against pathogens and antigen triggers. Healthy individual are presumed to have normal gut barrier function that can be compromised in the settings of illness or damage. Patients with active CD have small bowel damage as a result of their autoimmune dysregulation.21,22
It is known that small bowel damage in children with CD results in increased gut permeability and the goal of treatment with gluten free diet (GFD) is to achieve mucosal healing.23 As there is no reliable noninvasive test available, many providers still rely on repeat endoscopy and small bowel biopsy (SBB) to assess for mucosal healing. On the other hand, the current ESPGHAN diagnostic guidelines suggest that CD diagnosis can be done without SBB.24 For that reason, a good noninvasive test that can assess permeability and mucosal damage in the presence of villous atrophy in CD and thus can differentiate from healthy and/or healed mucosa is of pivotal importance. Various noninvasive tests to assess gut permeability have been used in children. We believe that lactulose/rhamnose (L/R) permeability test is more promising as it lacks the limitations of traditional lactulose/mannitol (L/M) test (predominantly the “contamination” of the test by background intake of mannitol that is present in many foods, medications and skin products) and the results of this test have been recently validated by Faubion et al in their study to detect enteric dysfunction in children less than 5 years of age with poor hygiene and sanitary conditions.25 The children with CD are known to have small bowel damage leading to increased permeability while the controls and siblings do not have such mucosal damage. So if we can develop a noninvasive test to detect a permeability difference between these groups using the L/R permeability test, this can help differentiate mucosal damage in children with CD from controls. We carried out this cross-sectional study using the L/R permeability test with the aim to compare the difference in permeability among children with confirmed CD with villous atrophy, high risk group (siblings of children with CD) and controls and to see whether L/R test can be used as a reliable noninvasive test to identify increased permeability in the presence of small bowel damage.
Material and Methods
We prospectively recruited 3 groups of study participants including children with CD (group 1), siblings of children with CD (group 2) and a control group of children age matched to the CD cases (group 3). In group 1, we recruited the patients who presented with clinical signs and symptoms suggestive of CD and met the diagnostic criteria for CD based on NASPGHAN guidelines.26 Asymptomatic siblings of patients diagnosed with CD were subsequently tested for genotype. The siblings who were found to have positive permissive genotype were recruited in group 2. On the other hand, the siblings of CD patients who tested negative for the permissive genotype were offered to be part of the control group (group 3). Although asymptomatic, the subjects in both the sibling group and the control group were also screened for CD using serological markers before recruitment into respective groups (because of the high prevalence of CD in general population and the fact that some subjects can be asymptomatic in the early phase of CD). The subjects in these groups with positive celiac serology were excluded from the study and managed as clinically indicated. Subjects with any coexisting inflammatory condition that can alter the gut barrier function (like autoimmune enteropathy, inflammatory bowel disease, infectious gastroenteritis or other enteropathies) were excluded from the study. Informed consent/assent was obtained from patients/families. This study was approved by Mayo Clinic IRB (15-006018).
We performed lactulose/rhamnose gut permeability test on the participants in all 3 groups. All participants ingested monosaccharide (L-rhamnose 200 mg) and disaccharide (lactulose 1000 mg) in 10 ml of sterile water. The test was done early morning in fasting state. Urine samples were collected pre-dose and then 60 to 90 minutes post-ingestion to reflect small bowel permeability. Urine volume over the collection period was measured and an aliquot was analyzed by HPLC methods at the Immunochemistry Core Laboratory at Mayo Clinic. The rhamnose test had a lower detection limit of 0.4 ug /mL while lactulose had a lower detection limit of 0.3 ug/mL. Any values below these thresholds were set to the threshold. Pre, post, and delta lactulose and rhamnose concentrations were calculated for each subject. A pre, post, and delta measurement was also calculated for the lactulose-to-rhamnose ratio (LRR). In order to standardize the concentration values in urine, these values were calculated by multiplying with the urine volume to get mass of excreted sugar (i.e., ug), and then calculating the ratio by dividing the masses of sugars excreted during the standardized collection period.
Median (IQR) and counts (%) were used to summarize demographic and permeability results within each group. Because relatives of CD cases were recruited to the sibling and control groups, there exists an underlying correlation of the data across the groups. Due to this correlation and to the skewness of permeability results, associations between study groups and log-transformed permeability results were assessed using logistic regression using generalized estimating equations (GEE). CD cases and siblings were compared to controls in separate models. Study group assignment (CD case or sibling group) was the dependent variable in all GEE models. The control group and the sibling group were reference levels in separate models predicting CD case status. The control group was the reference level in models predicting sibling group status. The log-transformed permeability results were treated as the independent variables with an indicator for male sex included as an adjustment variable in all models. Analyzes were performed using SAS v9.4M5 (Cary, NC).
Results
A total of 55 participants were prospectively recruited into 3 groups. Out of these, 27 recently diagnosed children (within 8 weeks of diagnosis) were recruited in the CD group who met the diagnostic criteria for CD; 12 subjects were included in the siblings of children with CD group who had positive permissive gene for CD but negative celiac screening and 16 subjects were included in the control group who had negative serology. The median age of participants was 11 years 2 months in group 1, 9 years 5 months in group 2 and 10 years 3 months in group 3 (Table 1). The male to female ratio was 1:2 in CD cases but 2:1 in the other groups (Table 1). In the control group, 7/16 (43.8%) were found to carry the CD permissive gene (DQ2 and/or DQ8) while 1/16 (6.2%) had equivocal HLA type. The others 8/16 (50%) tested negative for CD permissive gene. The median volume of urine collected pre sugar administration was 80.0 ml in CD group, 93.7 ml in CD siblings group and 69.5 ml in control group. In post sugar dose, the median urine volume was 50.0 ml in CD group, 131.0 ml in the sibling group and 65.0 ml in the control group (Table 1).
Table 1.
Patient Characteristics and Sugar Data among 3 Groups.
| Sugar | Controls N = 16 | Siblings of CD N = 12 | CD cases N = 27 |
|---|---|---|---|
| Age (years), Median (Q1, Q3) | 10.3 (8.2, 15.0) | 9.4 (7.9, 11.7) | 11.2 (8.75, 13.7) |
| Female Sex, N (%) | 6 (37.5%) | 4 (33.3%) | 17 (63.0%) |
| Pre-Urine Volume (mL), Median (Q1, Q3) | 69.5 (62.0, 443.0) | 93.7 (64.2, 161.2) | 80.0 (70.0, 150.0) |
| Post-Urine Volume (mL), Median (Q1, Q3) | 65.0 (40.0, 258.1) | 131.0 (48.9, 274.9) | 50.0 (25.0, 75.0) |
| Pre-Rhamnose (µg), Median (Q1, Q3) | 192.1 (98.6, 299.7) | 114.7 (37.5, 271.4) | 60.0 (28.8, 120.0) |
| Post-Rhamnose (µg), Median (Q1, Q3) | 5846.4 (2739.0, 7085.0) | 6895.0 (3824.1, 8025.2) | 327.5 (33.0, 3234.4) |
| Delta Rhamnose (µg), Median (Q1, Q3) | 3153.1 (1511.6, 6354.2) | 6622.9 (3525.5, 7996.0) | 147.5 (−27.0, 3122.0) |
| Pre-Lactulose (µg), Median (Q1, Q3) | 54.3 (19.5, 132.9) | 29.9 (19.3, 59.6) | 29.9 (21.6, 45.0) |
| Post-Lactulose (µg), Median (Q1, Q3) | 522.9 (326.4, 694.6) | 524.6 (365.4, 617.8) | 506.0 (200.0, 989.9) |
| Delta Lactulose (µg), Median (Q1, Q3) | 405.0 (223.2, 551.8) | 444.5 (291.5, 580.8) | 446.0 (125.5, 976.0) |
| Pre-LRR, Median (Q1, Q3) | 0.3 (0.2, 0.8) | 0.3 (0.2, 0.8) | 0.8 (0.5, 0.8) |
| Post-LRR, Median (Q1, Q3) | 0.1 (0.1, 0.2) | 0.1 (0.1, 01) | 0.7 (0.2, 6.6) |
| Delta-LRR, Median (Q1, Q3) | 0.1 (0.1, 0.1) | 0.1 (0.1, 0.1) | 0.2 (0.1, 4.1) |
Abbreviations: CD, celiac disease; LRR, lactulose-rhamnose ratio.
The CD group had the lowest pre- (60.0 µg), post- (327.5 µg), and delta (147.5 µg) median standardized rhamnose values compared to the sibling and control groups (Table 1). The control group had the highest median standardized pre-lactulose value (54.3 µg) compared to CD cases and siblings (29.9 µg in both groups). The post- and delta-median standardized lactulose values were similar across all 3 groups (Table 1). Due to the lower rhamnose value in the CD group, this group had a higher median delta lactulose/rhamnose ratio (LRR) value when compared to controls (0.2 vs 0.1) and the sibling group (0.2 vs 0.1).
Logistic regression using generalized estimating equations (GEE) were used to assess whether the sugars were associated with CD group status. The results of these model fits are shown in Table 2. A lower pre-rhamnose (OR = 0.47, P = .034), post-rhamnose (OR = .32, P = .011), delta rhamnose (OR = .51, P = .040) while larger post-LRR (OR = 5.56, P = .003) and delta-LRR (OR = 4.62, P = .016) were statistically significantly associated with the CD group when compared against the controls. Only post-LRR (OR = 126.41, P = .004) and delta-LRR (OR = 71.72, P = .003) were statistically significantly associated with the CD group compared to the siblings. No permeability measures were found to be statistically significant associated with the siblings when compared against the controls. No differences were observed when further stratifying by CD permission gene status in either group, suggesting that only villous atrophy in the children with CD resulted in abnormal rhamnose absorption as shown by the lower level of rhamnose detected in the post-study urine sample.
Table 2.
Results from Logistic Regression Models Using Generalized Estimating Equations (GEE).
| Predicting CD caseversus control | Predicting CD case versussiblings of CD | Predicting sibling ofCD versus control | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Predictor1 | OR (95% CI) | P-value | QIC | OR (95% CI) | P-value | QIC | OR (95% CI) | P-value | QIC |
| Pre-Rhamnose | 0.47 (0.23, 0.94) | .034 | 47.5497 | 0.73 (0.40, 1.31) | .289 | 47.5360 | 0.48 (0.18, 1.24) | .127 | 38.2412 |
| Post-Rhamnose | 0.32 (0.13, 0.77) | .011 | 42.5406 | 0.13 (0.01, 1.24) | .076 | 33.6368 | 3.00 (0.60, 14.99) | .181 | 42.2691 |
| Delta Rhamnose | 0.51 (0.27, 0.97) | .040 | 40.5775 | 0.16 (0.02, 1.52) | .111 | 32.7561 | 4.68 (0.97, 22.61) | .055 | 36.0702 |
| Pre-Lactulose | 0.60 (0.27, 1.30) | .194 | 54.6145 | 0.88 (0.45, 1.75) | .721 | 48.1849 | 0.57 (0.23, 1.41) | .228 | 39.9885 |
| Post-Lactulose | 0.97 (0.50, 1.85) | .918 | 58.9741 | 0.96 (0.49, 1.89) | .905 | 51.4581 | 1.33 (0.18, 9.76) | .777 | 44.2914 |
| Delta Lactulose | 1.09 (0.60, 1.98) | .783 | 53.0134 | 0.94 (0.51, 1.76) | .853 | 47.8575 | 2.14 (0.40, 11.44) | .374 | 39.6058 |
| Pre-LRR | 1.62 (0.92, 2.84) | .092 | 57.4045 | 1.39 (0.67, 2.90) | .382 | 51.7015 | 1.25 (0.65, 2.41) | .502 | 45.0847 |
| Post-LRR | 5.56 (1.79, 17.30) | .003 | 39.2982 | 126.41 (4.81, 3320.89) | .004 | 23.1750 | 0.23 (0.05, 1.08) | .063 | 42.1659 |
| Delta-LRR | 4.62 (1.33, 16.05) | .016 | 37.8503 | 71.72 (4.31, 1194.03) | .003 | 23.2956 | 0.31 (0.05, 1.77) | .186 | 42.3924 |
Abbreviations: CD, celiac disease; LRR, lactulose-rhamnose ratio; OR, odds ratio; QIC, quasilikelihood under the independence model criterion statistic.
Predictors were log-transformed in all models.
The significant P-values are highlighted in bold.
Discussion
Mucosal injury in CD is associated with increased gut permeability and many non-invasive tests to evaluate intestinal barrier function in children by measuring urinary excretion of different sugars have been used. Other noninvasive methods like measurement of plasma intestinal-fatty acid binding protein have also been studied.27 The conventional lactulose and mannitol test to assess small bowel disease including CD was first described in 1982 by Pearson et al in an attempt to develop a non-invasive test.28 Despite being studied widely, the lactulose/mannitol (L/M) test has certain limitations. A recent systemic review by Deno et al. concluded that although L/M test is safe, it can’t be used as a stand-alone test for diagnostic or screening purposes because of limitations like heterogeneity in enzymatic methods used, timing of urine collection and lack of normal values for high risk children.29 Baseline mannitol levels can be affected by non-food items as well which is another disadvantage of L/M test.30 The monosaccharide L-rhamnose is similar to mannitol in terms of molecular weight and volume; however it has several advantages over the conventional L/M test including decreased sugar administration, decreased time of urine collection and decreased risk of environmental contamination as it is not commonly used in food materials. Mayo clinic investigators compared the results of healthy and sick infants’ permeability using this test in their ongoing work on environmental enteropathy and were able to validate results. It was found that this test is safe, well tolerated and more accurate than the -conventional lactulose/mannitol test.
Our study showed that permeability to rhamnose is decreased and lactulose is unchanged when comparing CD cases to controls. The delta lactulose/rhamnose (LRR) value was higher in CD group when compared to controls (P = 0.016) and siblings (P = 0.003), indicating that L/R test can detect altered gut permeability that results from mucosal damage (Figure 1). Similar results were first described by Stenhammer et al in 1988 when they found increased gut permeability in patients with CD as compared to various GI disorders.31 Recently, Faubion et al. also used L/R test in malnourished children with enteric dysfunction and demonstrated increased gut permeability to lactulose leading to high lactulose rhamnose ratio (LRR) in these children as compared to healthy controls.25 The difference in permeability between the groups is driven mainly by differences in rhamnose absorption rather than lactulose. The precise physiological reason for this difference is not known, but can be a result of the mucosal damage associated with CD that results in loss of absorptive surface in the celiac group, whereas the lamina is largely intact and able to excrete lactulose. Sex difference can also have an impact over the gut permeability. Edogawa et al have demonstrated that women have lower permeability as compared to men.32 So it is reasonable to expect that rhamnose would behave the same way. This may partly explain why the CD group in our study, which has a female to male ratio of 2:1, shows lower uptake of rhamnose than control group in which female to male ratio is 1:2.
Figure 1.
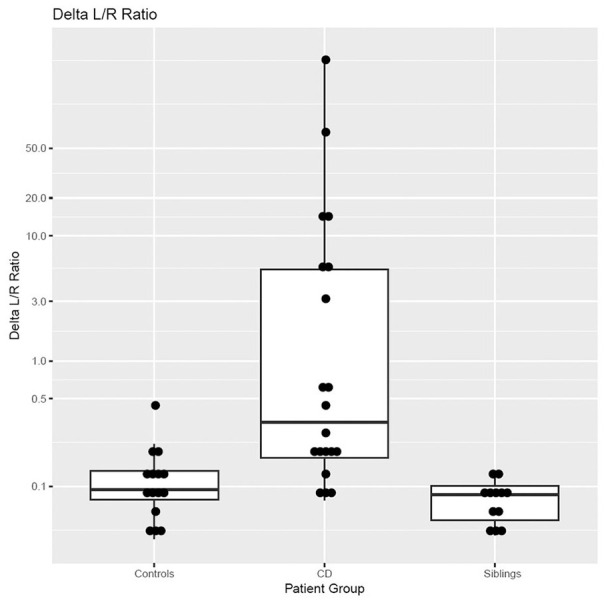
Comparison of the delta lactulose/rhamnose value among 3 study groups.
Previous studies have shown that carrying the CD permissive genotype (HLA DQ2/DQ8) may also be associated with increased gut permeability, even in the absence of CD. Vazquez et al. showed that patients with IBS-D who carried the HLA DQ2/DQ8 genotype had significantly high small bowel permeability as compared to healthy controls.33 Our study did not show any difference in permeability between children carrying the permissive gene for celiac disease (HLA DQ2/DQ8) and healthy controls. Increased permeability to rhamnose was seen only in children with CD, suggesting that that L/R test can identify mucosal damage in children with CD.
Recently, there is a move toward no biopsy diagnosis of CD relying on the highly sensitive serologic markers. The ESPGHAN guidelines recommend that small bowel biopsy (SBB) for the diagnosis of CD can be skipped in children with highly positive (>10 upper limit normal) anti tissue transglutaminase antibodies.24,34 Some studies have shown that combined use of CD serological markers including anti-tissue transglutaminase immunoglobulin A and anti-deamidated gliadin peptide IgG antibodies can be used as marker of mucosal recovery.35 There is no reliable diagnostic test available to assess compliance with gluten free diet in patients with CD although utility of detection of gluten immunogenic peptides in feces and urine is being studied and seems promising.36 However, currently there is no reliable non-invasive test to confirm the presence of mucosal damage (villous atrophy) in children with CD. There is no correlation between tissue transglutaminase (TTG) titers, symptoms resolution or mucosal healing.37 Hence a noninvasive test that can confirm the presence of mucosal damage can be used not only to confirm the diagnosis but also to assess mucosal healing in response to GFD. Our study using L/R test showed that gut permeability is significantly altered in newly diagnosed children with CD as compared to controls. Our study did not show any significant permeability changes either in high risk group like siblings of CD or controls, differentiating between damaged and normal small bowel mucosa. The main limitation of our study was its small sample size and lack of power calculation for sample size. The other limitation is sex disparity between groups.
In conclusion, the lactulose rhamnose ratio did not show significant difference between siblings and controls regardless of HLA typing. However, the loss of absorptive surface due to mucosal damage in children with newly diagnosed CD resulted in low rhamnose absorption when compared to the high risk siblings and controls group in this cohort. L/R test is not specific for CD; however our study suggests that this test can be potentially employed as a noninvasive tests for CD diagnosis in patients who have suggestive signs/symptoms and highly positive serology potential. Also, the role of L/R test could be ancillary, when coupled with serology, dietary interview and other innovative non-invasive modalities such as urinary/fecal gluten immunogenic peptides (GIPs) to assess mucosal healing. Prospective studies with larger sample size are needed to validate these findings and assess the potential role of L/R test as a noninvasive tool to assess mucosal damage. In future, this test can also be potentially employed as a noninvasive confirmatory test of mucosal healing in children with CD.
Footnotes
Author Contributions: MRK: contributed to data acquisition, analysis, draft preparation/revision and final approval. WAF: contributed to study concept, design, draft revision and final approval. RD: contributed to data acquisition, draft revision and final approval. RS: contributed to data acquisition, draft revision and final approval. JJL: contributed to data analysis, draft revision and final approval. IA: contributed to study concept/design, data acquisition, draft preparation/revision and final approval.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Muhammad Rehan Khan  https://orcid.org/0000-0003-1288-6905
https://orcid.org/0000-0003-1288-6905
Joseph J. Larson  https://orcid.org/0000-0001-8372-0465
https://orcid.org/0000-0001-8372-0465
References
- 1. Fasano A, Berti I, Gerarduzzi T, et al. Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: a large multicenter study. Arch Intern Med. 2003;163:286-292. [DOI] [PubMed] [Google Scholar]
- 2. Cavell B, Stenhammar L, Ascher H, et al. Increasing incidence of childhood coeliac disease in Sweden. Results of a national study. Acta Paediatr. 1992;81:589-592. [DOI] [PubMed] [Google Scholar]
- 3. Murray JA, Van Dyke C, Plevak MF, Dierkhising RA, Zinsmeister AR, Melton LJ. Trends in the identification and clinical features of celiac disease in a North American community, 1950-2001. Clin Gastroenterol H. 2003;1:19-27. [DOI] [PubMed] [Google Scholar]
- 4. Ludvigsson JF, Rubio-Tapia A, Van Dyke CT, et al. Increasing incidence of celiac disease in a North American population. Am J Gastroenterol. 2013;108:818-824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bardella MT, Fredella C, Prampolini L, Marino R, Conte D, Giunta AM. Gluten sensitivity in monozygous twins: a long-term follow-up of five pairs. Am J Gastroenterol. 2000;95:1503-1505. [DOI] [PubMed] [Google Scholar]
- 6. Van Belzen MJ, Koeleman BPC, Crusius JBA, et al. Defining the contribution of the HLA region to cis DQ2-positive coeliac disease patients. Genes Immun. 2004;5:215-220. [DOI] [PubMed] [Google Scholar]
- 7. Husby S, Koletzko S, Korponay-Szabo IR. European society for pediatric gastroenterology, hepatology, and nutrition guidelines for the diagnosis of coeliac disease (vol 54, pg 136, 2012). J Pediatr Gastr Nutr. 2012;54:572-573. [DOI] [PubMed] [Google Scholar]
- 8. Ivarsson A, Hernell O, Stenlund H, Persson LA. Breast-feeding protects against celiac disease. Am J Clin Nutr. 2002;75:914-921. [DOI] [PubMed] [Google Scholar]
- 9. Eidelman AI, Schanler RJ, Johnston M, et al. Breastfeeding and the use of human milk. Pediatrics. 2012;129:E827-E841. [DOI] [PubMed] [Google Scholar]
- 10. Agostoni C, Decsi T, Fewtrell M, et al. Complementary feeding: a commentary by the ESPGHAN committee on nutrition. J Pediatr Gastr Nutr. 2008;46:99-110. [DOI] [PubMed] [Google Scholar]
- 11. Szajewska H, Shamir R, Mearin L, et al. Gluten introduction and the risk of coeliac disease: a position paper by the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastr Nutr. 2016;62:507-513. [DOI] [PubMed] [Google Scholar]
- 12. Pinto-Sanchez MI, Verdu EF, Liu E, et al. Gluten introduction to infant feeding and risk of celiac disease: systematic review and meta-analysis. J Pediatr. 2016;168:132-U214. [DOI] [PubMed] [Google Scholar]
- 13. Marlid K, Ludvigsson J, Sanz Y, Ludvigsson JF. Antibiotic exposure in pregnancy and risk of coeliac disease in offspring: a cohort study. Bmc Gastroenterol. 2014;14:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kemppainen KM, Vehik K, Lynch KF, et al. Association between early-life antibiotic use and the risk of islet or celiac disease autoimmunity. Jama Pediatr. 2017;171:1217-1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Valitutti F, Cucchiara S, Fasano A. Celiac disease and the microbiome. Nutrients. 2019;11:2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vriezinga SL, Auricchio R, Bravi E, et al. Randomized feeding intervention in infants at high risk for celiac disease. N Engl J Med. 2014;371:1304-1315. [DOI] [PubMed] [Google Scholar]
- 17. Lionetti E, Castellaneta S, Francavilla R, et al. Introduction of gluten, HLA status, and the risk of celiac disease in children. N Engl J Med. 2014;371:1295-1303. [DOI] [PubMed] [Google Scholar]
- 18. Romanos J, Rosen A, Kumar V, et al. Improving coeliac disease risk prediction by testing non-HLA variants additional to HLA variants. Gut. 2014;63:415-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nellikkal SS, Hafed Y, Larson JJ, Murray JA, Absah I. High prevalence of celiac disease among screened first-degree relatives. Mayo Clin Proc. 2019;94:1807-1813. [DOI] [PubMed] [Google Scholar]
- 20. Elli L, Ferretti F, Orlando S, et al. Management of celiac disease in daily clinical practice. Eur J Intern Med. Epub ahead of print December 2018. doi: 10.1016/j.ejim.2018 [DOI] [PubMed] [Google Scholar]
- 21. Vilela EG, Torres HO, Ferrari ML, Lima AS, Cunha AS. Gut permeability to lactulose and mannitol differs in treated Crohn’s disease and celiac disease patients and healthy subjects. Braz J Med Biol Res. 2008;41:1105-1109. [DOI] [PubMed] [Google Scholar]
- 22. Visser J, Rozing J, Sapone A, Lammers K, Fasano A. Tight junctions, intestinal permeability, and autoimmunity: celiac disease and type 1 diabetes paradigms. Ann N Y Acad Sci. 2009;1165:195-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Valitutti F, Fasano A. Breaking down barriers: how understanding celiac disease pathogenesis informed the development of novel treatments. Dig Dis Sci. 2019;64:1748-1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Husby S, Koletzko S, Korponay-Szabo I, et al. European society paediatric gastroenterology, hepatology and nutrition guidelines for diagnosing coeliac disease 2020. J Pediatr Gastroenterol Nutr. 2020;70:141-156. [DOI] [PubMed] [Google Scholar]
- 25. Faubion WA, Camilleri M, Murray JA, et al. Improving the detection of environmental enteric dysfunction: a lactulose, rhamnose assay of intestinal permeability in children aged under 5 years exposed to poor sanitation and hygiene. BMJ Glob Health. 2016;1:e000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hill ID, Fasano A, Guandalini S, et al. NASPGHAN clinical report on the diagnosis and treatment of gluten-related disorders. J Pediatr Gastroenterol Nutr. 2016;63:156-165. [DOI] [PubMed] [Google Scholar]
- 27. Adriaanse MPM, Mubarak A, Riedl RG, et al. Progress towards non-invasive diagnosis and follow-up of celiac disease in children; a prospective multicentre study to the usefulness of plasma I-FABP. Sci Rep. 2017;7:8671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pearson AD, Eastham EJ, Laker MF, Craft AW, Nelson R. Intestinal permeability in children with Crohn’s disease and coeliac disease. Br Med J (Clin Res Ed). 1982;285:20-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Denno DM, VanBuskirk K, Nelson ZC, Musser CA, Hay Burgess DC, Tarr PI. Use of the lactulose to mannitol ratio to evaluate childhood environmental enteric dysfunction: a systematic review. Clin Infect Dis. 2014;59:S213-S219. [DOI] [PubMed] [Google Scholar]
- 30. Grover M, Camilleri M, Hines J, et al. (13) C mannitol as a novel biomarker for measurement of intestinal permeability. Neurogastroenterol Motil. 2016;28:1114-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stenhammar L, Stromberg S. Intestinal permeability to lactulose/L-rhamnose in children with celiac disease and other gastrointestinal disorders. J Pediatr Gastroenterol Nutr. 1988;7:304-306. [PubMed] [Google Scholar]
- 32. Edogawa S, Peters SA, Jenkins GD, et al. Sex differences in NSAID-induced perturbation of human intestinal barrier function and microbiota. FASEB J. 2018;32:6615-6625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vazquez-Roque MI, Camilleri M, Smyrk T, et al. Association of HLA-DQ gene with bowel transit, barrier function, and inflammation in irritable bowel syndrome with diarrhea. Am J Physiol Gastrointest Liver Physiol. 2012;303:G1262-G1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Husby S, Koletzko S, Korponay-Szabo IR, et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr. 2012;54:136-160. [DOI] [PubMed] [Google Scholar]
- 35. Bannister EG, Cameron DJ, Ng J, et al. Can celiac serology alone be used as a marker of duodenal mucosal recovery in children with celiac disease on a gluten-free diet? Am J Gastroenterol. 2014;109:1478-1483. [DOI] [PubMed] [Google Scholar]
- 36. Moreno ML, Rodriguez-Herrera A, Sousa C, Comino I. Biomarkers to monitor gluten-free diet compliance in celiac patients. Nutrients. 2017;9:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Leonard MM, Weir DC, DeGroote M, et al. Value of IgA tTG in predicting mucosal recovery in children with celiac disease on a gluten-free diet. J Pediatr Gastroenterol Nutr. 2017;64:286-291. [DOI] [PMC free article] [PubMed] [Google Scholar]


