Abstract
Introduction:
The diagnosis of Ewing sarcoma family of tumours (ESFT) is challenging, especially in adults and in extra-skeletal or visceral location. Several morphologic mimics with varied treatment options and prognosis confer diagnostic dilemmas. Application of ancillary diagnostic modalities in surgical pathology in clinical routine has enabled accurate diagnosis of ESFT in bone, soft tissues, and viscera.
Aim:
The study aims to assess the clinicopathological features including molecular test results of ESFT with emphasis on sex, age, and location, especially extra-skeletal soft tissue and visceral location.
Material and Methods:
Data of clinicopathological, molecular tests (wherever performed), diagnosis rendered in 302 ESFT over a decade from our centre were reviewed. Statistical comparison of skeletal and extra-skeletal tumours with reference to age and sex was done using SPSS package. The P value of <.05 was considered significant.
Results:
The cohort included 302 ESFTs with 49% skeletal and 51% extra-skeletal tumours. Thigh was most common site among skeletal tumours; chest wall, paraspinal location, and retroperitoneum among soft tissues (39.4%); and kidney, ovary, and cervix among visceral tumours (11.3%). Fluorescence in situ hybridisation for EWSR1 gene rearrangement was positive in 54 patients and reverse-transcriptase polymerase chain reaction in 19 patients. Predominance of male sex, younger age and location in extremities among skeletal tumours and lack of gender predilection, higher age and axial location in extra-skeletal tumours were noted, which were statistically significant. Molecular tests were performed more frequently in extra-skeletal tumours, especially in visceral tumours to establish the diagnosis.
Conclusions:
The study showed statistically significant differences in the age, sex, and location between skeletal and extra-skeletal ESFT. The increased percentage of extra-skeletal tumours especially in viscera was attributed to the increased awareness and availability of ancillary techniques.
Keywords: Ewing sarcoma family of tumours, skeletal, extra-skeletal, visceral, fluorescence in situ hybridisation, reverse-transcriptase polymerase chain reaction
Introduction
The Ewing sarcoma family of tumours (ESFT) encompass all the previous terminologies such as Ewing sarcoma of bone, extra-skeletal Ewing sarcoma, peripheral primitive neuroectodermal tumour, Askin tumour of thoraco-pulmonary region, and atypical Ewing sarcoma based on the presence of a unifying pathognomonic chromosomal translocation.1-9 Tumours involve bones, soft tissues, and viscera and typically appear as solid sheets of uniform, small blue round cells with minimal cytoplasm and little extracellular matrix. The disease arises from some mesenchymal or neural crest–derived stem or progenitor cell and hence may show variable neuronal, mesenchymal, and epithelial differentiation.2,6,10 The potential mimics of ESFT with reference to age, site, and morphology are lymphoblastic lymphoma, rhabdomyosarcoma, neuroblastoma, poorly differentiated synovial sarcoma, desmoplastic small round cell tumour, and others.2-7,9,11The treatment and prognosis of these various tumours are very different, necessitating accurate diagnosis. The diagnosis is challenging, especially in adults and in extra-skeletal or visceral location.
Availability of ancillary techniques in diagnostic surgical pathology enables accurate diagnosis of more tumours in the extra-skeletal location.12-14 The clinicopathological features and prognosis were reported to be different in skeletal and extra-skeletal tumours.12-21 This article aims to study the clinicopathological features and the role of ancillary techniques in the diagnosis of ESFT with particular emphasis on extra-skeletal soft tissue and visceral location.
Materials and Methods
A total of 346 patients suspected clinically or on radiology as ESFT and tumours with small round cell morphology on core biopsies or resected specimens, with a possibility of ESFT during the period September 2009 to December 2019, were included in the study. The study was approved by the institutional ethics committee. The demographic, clinical, and radiology findings with respect to site of tumour were noted from the medical records. The biopsies and resected specimens were fixed in 10% neutral buffered formalin and processed for paraffin sections and stained with hematoxylin and eosin (H&E). The architectural details, cellularity, atypia, mitoses, areas of necrosis, and differentiation, if any, were noted.
Depending on the morphological differential diagnosis considered, immunohistochemistry (IHC) was performed on formalin-fixed paraffin embedded (FFPE) tissues as per the protocols on automated immunostainer Roche Ventana Benchmark XT. Antibodies used were cluster of differentiation 99 (CD99) (12E7 Dako; ready to use [RTU]), Friend leukaemia integration (FLI1) (MRQ1; Cell Marque, Rocklin, CA USA, 1:50), leukocyte common antigen (LCA) (PD7126/162B11; Biocare, CA USA, 1:100), vimentin (V9; Biocare, RTU), Bcl2 (124; Dako, Glostrup, Denmark, RTU), pan cytokeratin (pan CK) (AE1/AE3, RTU), synaptophysin, chromogranin, CD56, transducin-like enhancer of split 1 (TLE1), Wilms tumour gene 1 (WT1). Weak/moderate/strong staining was considered as positive staining and equivocal staining as negative staining; diffuse membranous staining was considered positive for CD99 and nuclear positivity for FLI1; focal staining in the tumour cells was reported as focal positivity, no staining as negative; endothelial cells and lymphocytes were used as internal control.
Fluorescence in situ hybridisation (FISH) test results of EWSR1 gene rearrangement status in FFPE tissues were reviewed. Locus-specific Vysis dual colour break-apart probe for EWSRI gene located at 22q12 (Vysis, Inc., Downer s Grove, IL, USA) was used. Hybridisation signals in 50 nonoverlapping nuclei were reported. Break in EWSR1 gene was considered to be positive for EWSR1 rearrangement. The cut-off established was 15%.
Reports of reverse-transcriptase polymerase chain reaction (RT-PCR) test, which was done in-house or out-sourced, were reviewed. The procedure involved extraction of total RNA from FFPE tissue, using Recover All Total Nucleic Acid Isolation kit (Invitrogen by Thermo Scientific, Baltics UAB, Lithuana). RNA, 1 µg, was reverse transcribed to cDNA using TRUPCR High Retro Transcriptase Starter kit (3B BlackBiotech India Ltd, Bhopal, India) as per manufacturer’s protocol. Amplicons were checked on 2% agarose gel. EWS-FLI1 was subtyped as Type 1, EWS (exon 7) with FLI1 (exon6) of 330 bp (base pairs) and Type II, EWS (exon7) and FLI1 (exon5) of 390 bp. The PCR products were sent to Bio-serve for sequencing on 3500Dx Genetic Analyzer. Bio-edit software programme (http://www.mbio.ncsu.edu/Bioedit/bioedit.html) was used to identify sequences.
Final histopathologic diagnosis rendered was based on correlation of clinical, morphologic, IHC (positivity for CD99 and FLI1), and/or positivity for EWSR1 rearrangement by FISH and/or EWS-FLI1 by RT-PCR. Tumours other than ESFT, confirmed on IHC, FISH, or RT-PCR, were excluded from the study.
Statistics
Analysis was done using SPSS statistical software version 17.0. Age and sex in skeletal versus extra-skeletal sites were correlated. The P value, from which statistical significance was assumed, was considered as significant when P < .05.
Results
Of the total of 346 suspected ESFT, 44 were excluded due to lack of clinical details in 17 and diagnosis of sarcomas other than ESFT in 27 patients. The cohort included 302 patients with 149 (49.34%) having tumours originating in skeletal system, 119 (77.7%) in soft tissues, and 34 (11.26%) in viscera. Final diagnosis was made correlating clinical features, imaging, morphology, and IHC. Molecular confirmation by FISH for EWSR1 was present in 54 patients and RT-PCR in 19 patients (including both in one).
Skeletal ESFT (n = 149): This group included 101 (67.8%) men and 48 (32.2%) women including 64 (42.9%) children (<15 years). Age ranged from 3 to 61 (median 16) years. The sites included long tubular bones in 74 (49.7%) with bones of upper limb (humerus, radius, and ulna) in 24 (32.4%) and bones of lower limb (femur, tibia, fibula, and calcaneum) in 50 (67.57%). Tumours involved flat bones in 75 (50.3%) that included cranial bones (8), mandible (4), maxilla (2), scapula (7), clavicle (6), ribs (20), pelvis (13), and vertebrae (4). Immunohistochemistry for both CD99 and FLI1 was positive in 109 (20 confirmed by FISH for EWSR1 rearrangement), CD99 and EWSR1 positivity in 6, only EWSR1 positivity in 5, only CD99 in 28, and only FLI1 in 1. Pan CK, desmin, and Bcl2 were variably positive and LCA was negative. Neuroendocrine markers (synaptophysin, chromogranin, CD56 and NSE) were present in 5. Fluorescence in situ hybridisation for EWSR1 gene rearrangement was positive in 31 patients.
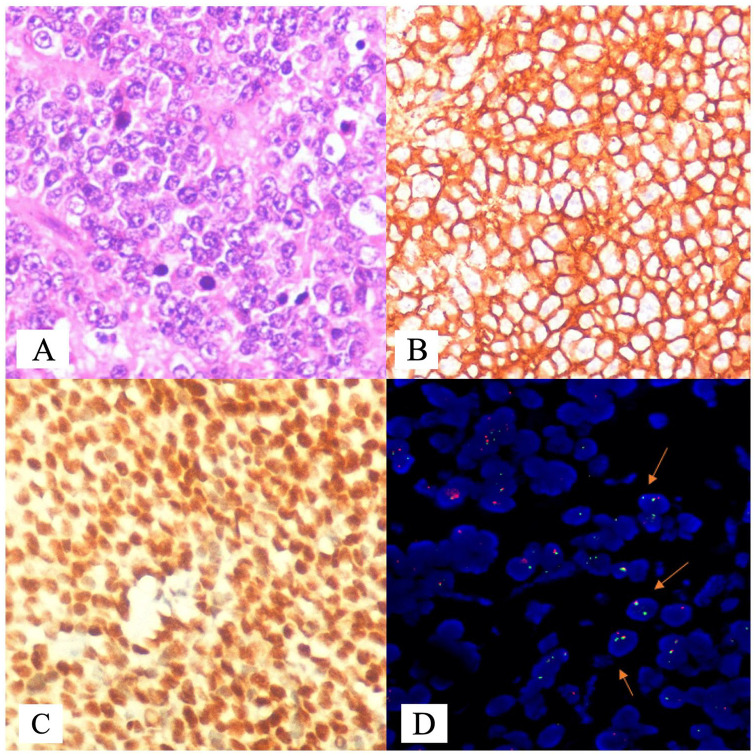
Extra-skeletal ESFT (n = 153): There were 79 (51.63%) men and 74 (48.37%) women, including 41 (26.80%) children (<15 years). Age ranged from 7 months to 65 (median 25) years. Soft tissue tumours were noted in 119 (77.7%) patients, which included arm (3), forearm (1), thigh (20), leg (4), popliteal fossa (2), gluteal region (5), cheek (2), orbit (2), nose and nasopharynx (2), scalp (1), neck (7), parapharyngeal (2), chest wall (29), mediastinum and thorax (5), paraspinal (20), abdomen and retroperitoneum (14). Visceral tumours were noted in 34 (22.22%) patients and the sites included ovary (4), cervix (3), vagina (1), kidney (7), bladder (2), prostate (1), lung (4), breast (5), lymph node (3), intestine (2), and brain (2). Immunohistochemistry for both CD99 and FLI1 was positive in 127 (16 confirmed by FISH for EWSR1 rearrangement and 19 for EWS-FLI1 by RT-PCR), CD99 and EWSR1 rearrangement by FISH in 4, FLI1 and EWSR1 rearrangement by FISH in 1, EWSR1 rearrangement by FISH in 2, only CD99 in 17, and only FLI1 in 2. Pan CK, desmin, and Bcl2 were variably positive and LCA was negative. Neuroendocrine markers (synaptophysin, chromogranin, CD56, and NSE) were present in 7. Fluorescence in situ hybridisation for EWSR1 rearrangement was positive in 23 and EWS-FLI1 by RT-PCR in 19 (including both in one) (Figure 1).
Figure 1.
Photomicrographs of ESFT: (A) diffuse sheets of round cells with vesicular nuclei, marginated nucleoli and moderate amounts of cytoplasm (hematoxylin-eosin, original magnification ×100, (B) immunohistochemistry (IHC) for CD 99 demonstrating membrane positivity in tumour cells (CD99×400), (C) IHC for FLI1 showing nuclear positivity (FLI1×400), and (D) representative flourescence in situ hybridisation (FISH) image showing EWSR1 rearrangement in 72% of cells. Predominant clone (34%) showed, standard break-apart signal pattern of one fusion and separated 3′ orange (centromeric) and 5′ green (telomeric) signal pattern (indicated by arrow). Two additional clones showed atypical break-apart pattern with one clone (24%) showing break and deletion of 3′ centromeric orange signal (1 fusion and one 5′ green) (indicated by arrow), and another clone 14% with break followed by deletion of 5′ (telomeric) green signal. Additional clones with atypical signal pattern demonstrate submicroscopic deletion of centromeric and telomeric regions of the EWSR1 gene, respectively. The same is represented as per International System for Human Cytogenetic Nomenclature (2016). nuc ish (EWSR1 ×2) (5′ EWSR1 sep 3′ EWSR1 ×1) [17/50]/nuc ish (5′ EWSR1 ×2, 3′ EWSR1 ×1) (5′ EWSR 1 con3′ EWSR1 ×1) [12/50]/nuc ish (5′ EWSR1 ×1, 3′ EWSR1 ×2) (5′ EWSR 1 con 3′ EWSR1 ×1) [7/50].
ESFT indicates Ewing sarcoma family of tumours; FLI1, Friend leukaemia integration.
Comparison of skeletal and extra-skeletal ESFT: The skeletal ESFT showed predominance (67.79%) in men, whereas extra-skeletal ESFT had no gender predilection; this difference in sex distribution was not statistically significant (P = .0691). The median age was 16 in skeletal and 25 in extra-skeletal location, and this was statistically significant (P < .001). Number of children in skeletal location were 64 (42.95%) and 41 (26.80%) in extra-skeletal location and this was statistically significant (P = .0032). Location in extremities was 74 (49.66%) in skeletal compared with 35 (29.41%) in soft tissue ESFT; this was statistically significant (P = .019) (Table 1). Molecular tests were more frequently performed (27.45%) for the diagnosis of extra-skeletal ESFT, particularly more for visceral (47.06%), when compared with skeletal ESFT (20.85%). The FISH technique was performed more frequently (73.97%) than RT-PCR (26.03%).
Table 1.
Comparison of demographic features and sites of involvement in skeletal and extra-skeletal ESFT (n = 302).
| Parameter | Skeletal ESFT n = 149 |
Extra-skeletal ESFT n = 153 |
P value |
|---|---|---|---|
| Male: Female | 101: 48 | 79:74 | .0691 |
| Age (median), y | 3-61 (16) | 7 months-65 (25) | <.001 |
| Children <15 y | 64 | 41 | .0032 |
| Site | Upper limb: 24 Lower limb:50 |
Upper limb: 04 Lower limb: 31 |
.019 |
| Diagnosis CD99+FLI1 (FISH for EWSR1 and EWS-FLI1 by RT-PCR) CD99/FLI1+FISH for EWSR1/only FISH/only IHC |
109 (20 + 0) 39 |
127 (16 + 19)a
26 |
.048 |
| Molecular tests performed for diagnosis | 31(20.81%) 31 (FISH) |
42 (27.45%) 23 (FISH) + 19 (RT-PCR) Visceral (47.06%) |
Abbreviations: CD99, cluster of differentiation 99; ESFT, Ewing sarcoma family of tumours; FISH, flourescence in situ hybridisation; FLI1, Friend leukaemia integration; IHC, immunohistochemistry; RT-PCR, reverse-transcriptase polymerase chain reaction.
Includes one which was positive for both EWSR1 by FISH and EWS-FLI1 by RT-PCR.
Ewing sarcoma family of tumour in viscera (n = 34): The most common organ involved was kidney, followed by ovary and others. Fluorescence in situ hybridisation was more frequently (47.06%) performed for the diagnosis. The demographic details, system involved, details of IHC, and EWSR1 rearrangement by FISH are presented in Table 2.
Table 2.
ESFT of viscera: demographic details, site, immunohistochemical features, and FISH for EWSRI status (n = 34).
| S. no. | Organ | Gender/age in years | Immunohistochemistry | FISH for EWSR1 break |
|---|---|---|---|---|
| 1 | Ovary | F/18 | CD99:P; FLI1:P; LCA: N; pan CK: N; WT1: N | P |
| 2 | Ovary (post chemotherapy) | F/40 | CD99:P; FLI1:P; Vimentin:P; EMA: N; Calretinin, S100, SMA: N | P |
| 3 | Ovary | F/19 | CD99:P; FLI1: P | ND |
| 4 | Ovary | F/20 | CD99:P; FLI1:P; LCA: N; Tdt: N; Desmin: P; Bcl2: P; SMA: N | ND |
| 5 | Cervix | F/34 | CD99: P; FLI1:P; pan CK: weak | P |
| 6 | Cervix | F/5 | CD99: P; FLI1:P; Desmin: N; Bcl2: P | P |
| 7 | Cervix | F/21 | CD99: P; FLI1: FP; Desmin: N; Chromogranin, CD 56: FP; Synaptophysin: N; CD 34: N | P |
| 8 | Vagina | F/20 | CD99: P; FLI1:P; LCA: N; pan CK: N; Desmin: N; Bcl2: N; CD 10: N | ND |
| 9 | Kidney | F/17 | CD99: P; FLI1:P | P |
| 10 | Kidney | F/31 | CD99: P; FLI1:P; LCA: N; Desmin: N; Bcl2: P; WT1: P; Vimentin: P | P |
| 11 | Kidney | M/25 | CD99: P; FLI1:P; LCA: N; pan CK: N; WT1: N; Desmin: P; Bcl2: N; Vimentin: P | P |
| 12 | Kidney | F/30 | CD99: P; FLI:1P; pan CK: N; WT1: N | P |
| 13 | Kidney | M/40 | CD99: P; FLI1:P; LCA: N; pan CK: N; WT1: N; CD 10: N | ND |
| 14 | Kidney | M/30 | CD99: P; Vimentin: P; CK7: N; PAX8: N | ND |
| 15 | Kidney | F/26 | CD99: P; FLI1:P | ND |
| 16 | Bladder | F/4 | CD99: P; FLI1:P; LCA: N; Desmin: N; Myogenin: N; MELAN A: N | P |
| 17 | Bladder | M/22 | CD99: P; FLI1: FP; LCA: N; Bcl2: N | ND |
| 18 | Prostate | M/33 | CD99: P; FLI1: FP; TLE1: N; Inhibin: N; SALL4: N; CD 34: N | ND |
| 19 | Breast | F/50 | CD99: P; pan CK: N; Vimentin: P | P |
| 20 | Breast | F/31 | CD99: P; FLI1: FP; LCA: N; pan CK: N; Vimentin: P | ND |
| 21 | Breast | F/16 | CD99: P; FLI1: P; LCA: N; pan CK: N; Bcl2: N; S100: N | ND |
| 22 | Breast | F/20 | CD99: P; FLI1: FP; LCA: N; pan CK: N; Vimentin: P | ND |
| 23 | Breast | M/50 | CD99: N; FLI1: N; pan CK: N | P |
| 24 | Lung | M/33 | CD99: P; FLI1: P; ERG: N | P |
| 25 | Lung | F/32 | CD99: P; FLI1: P; TLE1: N; CD34: N | ND |
| 26 | Lung | M/34 | CD99: P; FLI1: FP; CD56: N | ND |
| 27 | Lung | M/41 | CD99: P; FLI1: P; Bcl2:P; TLE1: N; CD56: N | ND |
| 28 | Axillary lymph node | M/44 | CD99: P; FLI1: FP; LCA: N; pan CK: N | ND |
| 29 | Cervical lymph node | M/61 | CD99: ND; FLI1: P | P |
| 30 | Inguinal lymph node | M/21 | CD99: P; FLI1: FP; LCA: N; Tdt: N | ND |
| 31 | Rectosigmoid colon | M/37 | CD99: P; FLI1: P | ND |
| 32 | Intestine | M/11 | CD99: P; FLI1: N; Chromogranin: N; CK7: N; LCA: N; CK20: N | ND |
| 33 | Brain | M/28 | CD99: P; FLI1: FP; WT1: N | P |
| 34 | Brain | F/40 | CD99: P; FLI1: FP; LCA: N; TLE1: N; CK7: N; CK20: N; TTF1: N; Synaptophysin: N | Failed/ inconclusive |
Abbreviations: CD99, cluster of differentiation 99; CK, cytokeratin; FISH, flourescence-in-situ hybridisation; FLI1, Friend leukaemia integration; LCA, leukocyte common antigen; N, negative; ND, not done; P, positive; TLE1, transducin-like enhancer of split 1; WT, Wilms tumour gene 1.
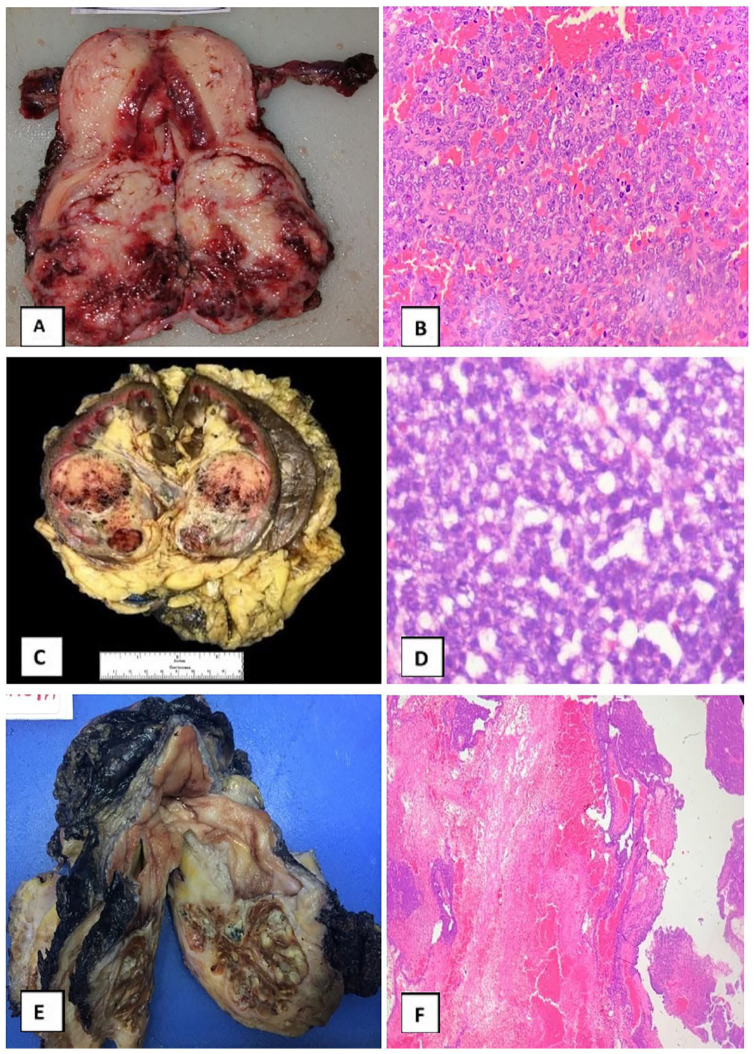
Ewing sarcoma family of tumour in female genital tract (n = 8): Most common site was the ovary in 4 (50%), cervix (3/8), and vagina (1/8). CD99 and FLI1 was positive in all 8 and FISH for EWSR1 was positive in 5 (2/4 ovary and 3/3 cervix) (Figure 2A and B).
Figure 2.
(A) Gross specimen of a fleshy to firm mass in cervix and lower uterine segment, (B) photomicrograph showing diffuse sheets of small round cells (hematoxylin-eosin, original magnification ×100), (C) gross specimen of kidney with a circumscribed firm lesion extending from middle region to lower pole with haemorrhage and cystic areas, (D) photomicrograph showing diffuse nests and sheets of oval cells with smoky chromatin and moderate amounts of cytoplasm (hematoxylin-eosin, original magnification ×400), (E) gross specimen showing polypoid mass arising from mucosal surface of urinary bladder, and (F) photomicrograph showing urothelium with submucosal sheets of round cells interspersed by areas of fibrosis (hematoxylin-eosin, original magnification ×100).
Ewing sarcoma family of tumour in genitourinary tract (n = 10): Tumours were more frequent in the kidney (7/10), whereas bladder (2/10) and prostate (1/10) were rare. The median age of patients with renal involvement was 30 (range: 17-40) years. CD99 and FLI1 expression was noted in all 10 and FISH for EWSR1 was positive in 5 (4/7 kidney and 1/2 bladder) (Figure 2C to F).
Ewing sarcoma family of tumour in breast (n = 5): The median age of patients with breast involvement was 31 (range: 16-50) years. Immunohistochemistry for CD99 and FLI1 was positive in 4 and FISH for EWSR1 was positive in 2. The one sample that was negative for both CD99 and FLI 1 was positive for EWSR1 gene rearrangement by FISH.
Ewing sarcoma family of tumour in lung (n = 4): The median age of patients with involvement of lung was 33.5 (range: 32-41) years. Immunohistochemistry for CD99 and FLI1 was positive in all 4 and FISH for EWSR1 was positive in 1. All were primary ESFT.
Ewing sarcoma family of tumour in lymph node (n = 3): The median age of patients with lymph node involvement was 44 (range: 21-61) years. Immunohistochemistry for CD99 and FLI1 was positive in 2 and one had the EWSR1 rearrangement. In one sample, where CD99 was not done, FLI1 was positive, and EWSR1 was positive for the rearrangement. All were primary ESFT.
Ewing sarcoma family of tumour in intestine (n = 2): The patients were aged 11 and 37 years. CD99 was positive in both but FLI1 was positive in 1. Fluorescence in situ hybridisation was not done in both.
Ewing sarcoma family of tumour in brain (n = 2): Two patients aged 28 and 40 years had ESFT of brain. CD99 was positive in both and FLI1 was focal positive in both. One was a EWSR1 rearranged tumour and FISH for EWSR1 was inconclusive in one.
Discussion
Ewing sarcoma family of tumours are aggressive mesenchymal neoplasms and constitute 6% to 8% of primary bone tumours in children and young adults with a peak incidence in the second decade and male predominance.7,12 In this study of 302 ESFT, male predominance (59.6%) was noted in skeletal ESFT versus the extra-skeletal ESFT, and this difference was statistically significant. Further ESFT was noted in 105 (34.77%) tumours occurring in children less than 15 years. The median age was higher (25 years) in patients with extra-skeletal tumours compared with those with skeletal (16 years) tumours, which was statistically significant. Similar observations were made earlier.12,13,16
The reported frequency of extra-skeletal location of tumours in various series ranged from 21% to 31% in cohort ranging from 120 to 2202 patients.12,14,15 Reports from India described extra-skeletal tumours in small sample size of 51 to 58 patients and described the frequency to range from 23% to 29%.19-21 In this study of 302 patients, ESFT of extra-skeletal location was seen in 50.7% with diagnosis in visceral organs being more. The relatively high frequency was probably related to the referral bias, subspeciality reporting, and use of ancillary techniques in the diagnosis. The sites of involvement for skeletal ESFT are diaphysis or metaphyseo-diaphyseal portion of long bones such as femur, tibia or humerus, flat bones of pelvis, and rib and less commonly, bones of skull, vertebra, scapula, and short tubular bones of hands and feet.7,17 The number of tumours in the long bones of the extremities was 74 (49.66%), and those in axial skeleton involving flat bones was 75 (50.34%). Applebaum reported that axial and nonpelvic locations were more frequent in extra-skeletal tumours when compared with skeletal location, which were statistically significant.12,15 In this study, the extra-skeletal sites constituted 153 (50.66%) of ESFT including soft tissues in 119 (39.40%) and viscera in 34 (11.26%); however, the age and sex did not differ significantly between soft tissue and visceral location. The common sites of soft tissues involved were chest wall, extremities, paraspinal, abdomen, and others as reported in literature.7,12,18-22 Furthermore, involvement of the extremities between skeletal and extra-skeletal tumours was observed to be statistically significant in our study. Salah et al14 reported soft tissue location in 23 (79%). Visceral ESFTs have been described in studies with small sample size, ranging from 29 to 58 with percentage of such cases varying from 6% to 21% (Table 3).14,19-21
Table 3.
Demographic features in skeletal and extra-skeletal ESFT in different series compared with present series.
| Author(s), year, (reference); number, duration | Skeletal | Extra-skeletal | |
|---|---|---|---|
| Soft tissues | Visceral | ||
| Applebaum et al12; n = 2202 1973-2007 |
1519 (69%) M:F 63.3%:36.7% Mean age: 16.3 (0-39) y |
683 (31%) M:F 53.4%:46.6% Mean age: 19.5 (0-39) y |
NA |
| Salah et al14; n = 120 January, 2006 to June, 2018 |
91 (76%) | 23 (19.17%) | 6 (5%) |
| Cash et al15; n = 1029 | 816 (79.30%) | 213 (20.70%) | NA |
| Rekhi et al19; n = 58 M: F: 65.52%: 34.48% Median age: 16 (1-65) y |
Not specified | Not specified | 12 (20.69%) Ovary:5 Kidney:5 Prostate:1 Vagina:1 |
| Priya et al20; n = 51 August 2009-April 2014 M: F: 53%:47% Mean age: 10 (2-14) y |
36 (71%) | 12 (23%) | 3 (6%) Kidney:2 Adrenal:1 |
| Mittal et al21; n = 58 January 2014-May 2016 M:F: 63%:37% Mean age: 20 (3-65) y |
35 (61%) | 17 (29%) | 6 (10%) Spinal: 2 Brain: 2 Nose and nasopharynx: 1 Urinary bladder: 1 |
| Present study n = 302 September 2009 to December 2019 |
149 (49.34%) Median age: 16 (3-61) y |
119 (39.40%) Median age: 25 (7 months-65) y |
34 (11.26%) |
Abbreviation: ESFT, Ewing sarcoma family of tumours.
The significant number of ESFTs in visceral location in this study may probably be due to heightened awareness and application of IHC, FISH, and RT-PCR as diagnostic methods. Molecular tests were more frequently (47.06%) performed to establish the diagnosis in visceral ESFT, when compared with skeletal or soft tissue location, highlighting the importance of ancillary techniques for accurate diagnosis.
The morphology of ESFT may be varied; however, skeletal and extra-skeletal ESFTs are indistinguishable and are defined by the same molecular alteration. However, depending on the age, site, morphology, and differentiation, especially in extra-skeletal location, the differential diagnosis varies, necessitating a panel of IHC markers. The diagnosis of round cell sarcoma as ESFT is routinely established by diffuse membrane positivity of CD99 along with nuclear positivity of FLI1 and negative LCA.19 NKX2.2 has been established as a highly sensitive but only moderately specific marker for ES.23 In this study, positivity of both CD99 and FLI1 along with other IHC markers was considered for diagnosing a tumour as ESFT when diagnosis was based on IHC. NKX2-2 is a new marker and was not done during that study period. FISH positivity for EWSR1 rearrangement confirmed the diagnosis of ESFT in 31 skeletal and 23 in extra-skeletal location and in 15/34 (44.12%) tumours in the visceral location. The RT-PCR for EWS-FLI1 fusion was positive in 19 of the extra-skeletal ESFT including one in visceral location.
Primary ESFT of the female genital tract is very rare with the most common site being the ovary, followed by the uterine corpus and rarely cervix, vagina, and vulva.24-32 Chao et al25 reported 4 cases involving the ovary and reviewed 15 published cases in the period 1980 to 2017. In the last decade of review of our cohort of 8 cases, 4 involved the ovary, 3 cervix, and 1 vagina. Diagnosis was established by morphology and IHC in all with confirmation by FISH studies in 5. The use of IHC and FISH in the diagnosis of ESFT in the female genital tract was stressed earlier.24,25,29
Primary ESFT of the kidney is rarely reported.33-41 However, in the present series, involvement of kidney was seen in 7 (median age of presentation: 30 years) patients. Further ESFTs in bladder and prostate noted in this cohort were rarely reported.42-47
Metastatic disease involving lung, lymph nodes, and several organs has been described12,48,49; however, primary ESFTs of breast,50-52 lung,53-55 intestine,56,57 and brain22,58-62 were rarely reported.
Significant differences were reported in clinical presentation, treatment strategy, and outcomes for extra-skeletal ESFT compared with skeletal tumours.12 Although the key tumour genomic features are same, the differences may arise due to subtle biologic differences in the tumour microenvironment between skeletal and extra-skeletal location.12,15 Extra-skeletal ESFT is an important subtype of Ewing sarcoma that may require different treatment strategies, and the prognosis is more favourable compared with skeletal tumours, independent of age, race, and primary site.15
Accurate diagnosis of ESFT is challenging when patients present with unusual clinical features, disease onset in older age, visceral location, rare sites, inconclusive, or aberrant expression of IHC markers. Confirmation of diagnosis by molecular studies may involve demonstration of gene rearrangement by FISH or chimeric fusion transcript by RT-PCR. Interphase FISH is a simple, robust, sensitive, and reliable ancillary technique, with a targeted approach, to detect the presence or absence of EWSR1 gene rearrangement, in the FFPE specimen as 82% to 92% ESFT show EWSR1 rearrangement.5,11 The RT-PCR has better yield on fresh tissue and has the advantage of identifying the fusion partner.11 However, both the techniques can be applied to FFPE tissues, are complementary to each other, and a combination of the techniques was recommended to enhance the accuracy of the diagnosis.5-8,11 Both FISH and RT-PCR were applied in this study to establish diagnosis, especially for tumours in visceral and unusual locations. However, molecular techniques may not be available or are too expensive for routine use. Therefore, not all patients were subjected to molecular testing in this study. In addition, molecular techniques also have several limitations in technique and interpretation and in more than 10% of cases may not yield good-quality DNA or RNA.63 A targeted enrichment strategy for library preparation for targeted next-generation sequencing (NGS) of soft tissue sarcomas has been reported to have higher sensitivity and specificity in soft tissue sarcomas.64
The use of multiple technologies and knowhow of applying these in the right context is an intellectual and time-driven exercise for the pathologists. Accurate diagnosis and timeliness of the report are essential for appropriate management of the disease. The utilisation of these techniques in an algorithmic manner (assuming a turnaround time for histopathology and IHC to be 4 days, FISH additional 5 days, and RT-PCR where necessary 5-7 days) may add up to the time for diagnosis significantly. This may be shortened with a planned molecular profiling by NGS which would take about 10 to 15 days but with more information and specificity. Therefore, all the techniques (IHC, FISH, RT-PCR) complement each other especially in settings where access to more sensitive technologies like anchored multiplex PCR–based NGS testing is not available.64 Next-generation sequencing testing incorporating other fusion partners (CIC-DUX4 and BCOR) may help in picking up the rare subset of tumours as well.
Conclusions
This study of a large cohort of 302 ESFT demonstrated statistically significant differences between skeletal and extra-skeletal tumours. Male sex predominance, younger age, and extremity location were noted in skeletal tumours. However, no gender predilection, older age, and axial location were seen in extra-skeletal tumours. Molecular tests were more frequently performed and contributed to the diagnosis of ESFT more frequently in extra-skeletal sites including soft tissues and viscera. High index of suspicion and appropriate use of molecular techniques help in the diagnosis of ESFT even in uncommon locations.
Acknowledgments
The authors are thankful to personnel from Departments of Pathology, Radiology, and Surgical Oncology with particular reference to Dr Manjula, Dr Sahithi, Dr Rajasekhar, Dr Shankari, Mr U Ravinder, Mr A Hussain, Mrs M Padma, Mrs Swarnalatha, Ms D Vijaya, Mr Sampath Kumar, Mr Sudhakar, Mr Vijay, Mr Sambasiva Reddy, Mr Ramachander Reddy, and Mr Praveen.
Footnotes
Author Contributions: Concept and design: SSM, SC, TSR.
Provision of study material or patients: SJR, KVVNR, TSR, RS, KMM, VCK.
Collection and assembly of data: SSM, SC, SDG.
Data analysis and interpretation: SSM, DF, BVR, SDG, SK, FA, MM, LN.
Manuscript writing: SSM, SC, SDG.
Final approval of manuscript: All authors.
Declaration of Conflicting Interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Sudha S Murthy  https://orcid.org/0000-0001-6406-7659
https://orcid.org/0000-0001-6406-7659
L Nambaru  https://orcid.org/0000-0002-1628-3258
https://orcid.org/0000-0002-1628-3258
References
- 1. Delattre O, Zucman J, Melot T, et al. The Ewing family of tumors – a subgroup of small-round-cell tumors defined by specific chimeric transcripts. N Engl J Med. 1994;331:294-299. [DOI] [PubMed] [Google Scholar]
- 2. Folpe AL, Hill CE, Parham DM, O’Shea PA, Weiss SW. Immunohistochemical detection of FLI-1 protein expression: a study of 132 round cell tumors with emphasis on CD99-positive mimics of Ewing’s sarcoma/primitive neuroectodermal tumor. Am J Surg Pathol. 2000;24:1657-1662. [DOI] [PubMed] [Google Scholar]
- 3. O’Sullivan MJ, Perlman EJ, Furman J, Humphrey PA, Dehner LP, Pfeifer JD. Visceral primitive peripheral neuroectodermal tumors: a clinicopathologic and morphologic study. Hum Pathol. 2001;32:1109-1115. [DOI] [PubMed] [Google Scholar]
- 4. Khoury JD. Ewing sarcoma family of tumors. Adv Anatpathol. 2005;12:212-220. [DOI] [PubMed] [Google Scholar]
- 5. Gamberi G, Cocchi S, Benini S, et al. Molecular diagnosis in Ewing family tumors: the Rizzoli experience – 222 consecutive cases in four years. J Mol Diagn. 2011;13:313-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pinto A, Dickman P, Parham D. Pathobiologic markers of the Ewing sarcoma family of tumors: state of the art and prediction of behaviour. Sarcoma. 2011;2011:856190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. de Alava E, Lessnick SL, Sorensen PH. Ewing sarcoma. In: Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F, eds. WHO classification of tumours of soft tissue and bone. Lyon, France: WHO Press; 2013:305-309. [Google Scholar]
- 8. Sand LG, Szuhai K, Hogendoorn PC. Sequencing overview of Ewing sarcoma: a journey across genomic, epigenomic and transcriptomic landscapes. Int J Mol Sci. 2015;16:16176-16215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wei S, Henderson-Jackson E, Qian X, Bui MM. Soft tissue tumor immunohistochemistry update: illustrative examples of diagnostic pearls to avoid pitfalls. Arch Pathol Lab Med. 2017;141:1072-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kovar H, Amatruda J, Brunet E, et al. The second European interdisciplinary Ewing sarcoma research summit – a joint effort to deconstructing the multiple layers of a complex disease. Oncotarget. 2016;7:8613-8624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Noujaim J, Jones RL, Swansbury J, et al. The spectrum of EWSR1-rearranged neoplasms at a tertiary sarcoma centre; assessing 772 tumour specimens and the value of current ancillary molecular diagnostic modalities. Br J Cancer. 2017;116:669-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Applebaum MA, Worch J, Matthay KK, et al. Clinical features and outcomes in patients with extraskeletal Ewing sarcoma. Cancer. 2011;117:3027-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pradhan A, Grimer RJ, Spooner D, et al. Oncological outcomes of patients with Ewing’s sarcoma: is there a difference between skeletal and extra-skeletal Ewing’s sarcoma? J Bone Joint Surg Br. 2011;93:531-536. [DOI] [PubMed] [Google Scholar]
- 14. Salah S, Abuhijla FJ, Ismaeel T, et al. Outcomes of extra-skeletal versus skeletal Ewing sarcoma patients treated with standard chemotherapy protocol. J Clin Oncol. 2019;37:11027. [DOI] [PubMed] [Google Scholar]
- 15. Cash T, McIlvaine E, Krailo MD, et al. Comparison of clinical features and outcomes in patients with extraskeletal versus skeletal localized Ewing sarcoma: a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2016;63:1771-1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gurria JP, Dasgupta R. Rhabdomyosarcoma and extraosseous Ewing sarcoma. Children. 2018;5:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Casali PG, Blay JY, ESMO/CONTICANET/EUROBONET Consensus panel of experts. Soft tissue sarcomas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21:v198-v203. doi: 10.1093/annonc/mdq209. [DOI] [PubMed] [Google Scholar]
- 18. Galyfos G, Karantzikos GA, Kavouras N, Sianou A, Palogos K, Filis K. Extraosseous Ewing sarcoma: diagnosis, prognosis and optimal management. Indian J Surg. 2016;78:49-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rekhi B, Vogel U, Basak R, Desai SB, Jambhekar NA. Clinicopathological and molecular spectrum of Ewing sarcomas/PNETs, including validation of EWSR1 rearrangement by conventional and array FISH technique in certain cases. Pathol Oncol Res. 2014;20:503-516. [DOI] [PubMed] [Google Scholar]
- 20. Priya D, Kumar RV, Appaji L, Aruna Kumari BS, Padma M, Kumari P. Histological diversity and clinical characteristics of Ewing sarcoma family of tumors in children: a series from a tertiary care center in South India. Indian J Cancer. 2015;52:331-335. [DOI] [PubMed] [Google Scholar]
- 21. Mittal A, Mehta J, Mangal K, Jain A, Agarwal N, Solanki MK. Clinicopathological and immunophenotypic characteristics of Ewings sarcoma family of tumors: special emphasis on role of Friend Leukemia Integration - 1(Fli-1) antibody and occurrence of tumor on rare sites. Int J Cur Res Rev. 2016;8:10-16. [Google Scholar]
- 22. Bhattacharjee S, Venkata SR, Uppin MS. Skull and spinal Ewing’s sarcoma in children: an institutional study. J Pediatr Neurosci. 2018;13:392-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hung YP, Fletcher CD, Hornick JL. Evaluation of NKX2-2 expression in round cell sarcomas and other tumors with EWSR1 rearrangement: imperfect specificity for Ewing sarcoma. Mod Pathol. 2016;29:370-380. [DOI] [PubMed] [Google Scholar]
- 24. Chiang S, Snuderl M, Kojiro-Sanada S, et al. Primitive neuroectodermal tumors of the female genital tract: a morphologic, immunohisto-chemical, and molecular study of 19 cases. Am J Surg Pathol. 2017;41:761-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chao X, Bi Y, Li L. Ovarian primary primitive neuroectodermal tumor: a review of cases at PUMCH and in the published literature. Orphanet J Rare Dis. 2019;14:147. doi: 10.1186/s13023-019-1106-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ostwal V, Rekhi B, Noronha V, et al. Primitive neuroectodermal tumor of ovary in a young lady, confirmed with molecular and cytogenetic results – a rare case report with a diagnostic and therapeutic challenge. Pathol Oncol Res. 2012;18:1101-1106. [DOI] [PubMed] [Google Scholar]
- 27. Chu LH, Chang WC, Kuo KT, Sheu BC. Primary primitive neuroectodermal tumor of the ovary. Taiwan J Obstet Gynecol. 2014;53:409-412. [DOI] [PubMed] [Google Scholar]
- 28. Lateef R, Bali A. Ewing’s sarcoma of uterus – case report and review of literature. Gynecol Obstet (Sunnyvale). 2016;6:362. [Google Scholar]
- 29. Rekhi B, Agrawal R, Shetty O, et al. Five rare cases of Ewing sarcoma, including with epithelial differentiation, involving the female genital tract, displaying EWSR1 rearrangement: diagnostic challenge and treatment implications. Ann Diagn Pathol. 2019;41:1-7. [DOI] [PubMed] [Google Scholar]
- 30. Rekhi B, Qureshi S, Basak R, et al. Primary vaginal Ewing’s sarcoma or primitive neuroectodermal tumor in a 17-year-old woman: a case report. J Med Case Rep. 2010;4:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rekhi B, Chinnaswamy G, Vora T, Shah S, Rangarajan V. Primary Ewing sarcoma of vulva, confirmed with molecular cytogenetic analysis: a rare case report with diagnostic and treatment implications. Indian J Pathol Microbiol. 2015;58:341-344. [DOI] [PubMed] [Google Scholar]
- 32. Dutta S, Dasgupta C, Choudhury K. Paravaginal peripheral primitive neuroectodermal tumor: a rare tumor. Indian J Med Paediatr Oncol. 2011;32:171-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jimenez RE, Folpe AL, Lapham RL, et al. Primary Ewing’s sarcoma/primitive neuroecto-dermal tumor of the kidney: a clinicopathologic and immunohistochemical analysis of 11 cases. Am J Surg Pathol. 2002;26:320-327. [DOI] [PubMed] [Google Scholar]
- 34. Thyavihally YB, Tongaonkar HB, Gupta S, et al. Primitive neuroectodermal tumor of the kidney: a single institute series of 16 patients. Urology. 2008;71:292-296. [DOI] [PubMed] [Google Scholar]
- 35. Hakky TS, Gonzalvo AA, Lockhart JL, Rodriguez AR. Primary Ewing sarcoma of the kidney: a symptomatic presentation and review of the literature. Ther Adv Urol. 2013;5:153-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Risi E, Iacovelli R, Altavilla A, et al. Clinical and pathological features of Primary neuroectodermal tumor/Ewing sarcoma of the kidney. Urology. 2013;82:382-386. [DOI] [PubMed] [Google Scholar]
- 37. Almeida MFA, Patnana M, Korivi BR, Kalhor N, Marcal L. Ewing sarcoma of the kidney: a rare entity. Case Rep Radiol. 2014;2014:283902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Celli R, Cai G. Ewing sarcoma/primitive neuroectodermal tumor of the kidney. A rare and lethal entity. Arch Pathol Lab Med. 2016;140:281-285. [DOI] [PubMed] [Google Scholar]
- 39. Choubey S, Pipara G, Kumar A. Ewings sarcoma of the kidney: a rare entity. World J Nephrol Urol. 2017;6:18-20. [Google Scholar]
- 40. Doroudinia A, Ahmadi S, Mehrian P, Pourabdollah M. Primary Ewing sarcoma of the kidney. BMJ Case Rep CP. 2019;12:bcr-2018-227198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Alghamdi MHA, Alawad SA, Alharbi MG, Alabdulsalam AK, Almodhen F, Alasker A. A rare case of Ewing’s sarcoma of the kidney. Urol Case Rep. 2020;29:101094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mohsin R, Hashmi A, Mubarak M, et al. Primitive neuroectodermal tumor/Ewing’s sarcoma in adult uro-oncology: a case series from a developing country. Urol Ann. 2011;3:103-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Okada Y, Kamata S, Akashi T, Kurata M, Nakamura T, Kihara K. Primitive neuroectodermal tumor/Ewing’s sarcoma of the urinary bladder: a case report and its molecular diagnosis. Int J Clin Oncol. 2011;16:435-438. [DOI] [PubMed] [Google Scholar]
- 44. Vallonthaiel AG, Kaur K, Jain D, et al. Ewing sarcoma of urinary bladder showing EWSR1 rearrangement on FISH analysis and unique response to chemotherapy. Clin Genitourin Cancer. 2016;14:e183-e186. [DOI] [PubMed] [Google Scholar]
- 45. Tonyali S, Yazici S, Yesilirmak A, Ergen A. The Ewing’s sarcoma family of tumors of urinary bladder: a case report and review of the literature. Balkan Med J. 2016;33:462-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kumar V, Khurana N, Rathi AK, et al. Primitive neuroectodermal tumor of prostate. Indian J Pathol Microbiol. 2008;51:386-388. [DOI] [PubMed] [Google Scholar]
- 47. Liao C, Wu X, Wang X, Li H. Primitive neuroectodermal tumor of the prostate: case report from China. J Cancer Res Ther. 2015;11:664. [DOI] [PubMed] [Google Scholar]
- 48. Caballero Vázquez A, García Flores P, Herrera Chilla Á. Involvement of mediastinal lymph nodes by Ewing’s sarcoma. Arch Bronconeumol. 2017;53:215-216. [DOI] [PubMed] [Google Scholar]
- 49. Vias P, Thakur S, Seam RK, Fotedar V, Saklani A. Superficial extraskeletal Ewing’s sarcoma of forefoot with inguinal lymph node metastasis and lung metastasis: a rare case. Clin Cancer Investig J. 2019;8:73-75. [Google Scholar]
- 50. Vindal A, Kakar AK. Primary primitive neuroectodermal tumor of the breast. J Clin Oncol. 2010;28:e453-e455. [DOI] [PubMed] [Google Scholar]
- 51. Chuthapisith S, Prasert W, Warnnissorn M, Pradniwat K, Srimuninnimit V, Angsusinha T. Ewing’s sarcoma and primitive neuroectodermal tumour (ES/PNET) presenting as a breast mass. Oncol Lett. 2012;4:67-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Majid N, Amrani M, Ghissassi I, et al. Bilateral Ewing sarcoma/primitive neuroecto-dermal tumor of the breast: a very rare entity and review of the Literature. Case Rep Oncol Med. 2013;2013:964568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hwang SK, Kim DK, Park S-I, Kim Y-H, Hyeong Ryul Kim HR. Primary Ewing’s sarcoma of the lung. Korean J Thorac Cardiovasc Surg. 2014;47:47-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Purkayastha A, Pathak A, Sharma N, Viswanath S, Dutta V. Primitive neuroectodermal tumor of lungs in adults: a rare series of three cases treated with upfront chemo-radiation. Transl Lung Cancer Res. 2016;5:350-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sobh E, El-Sheshtawy WH, Anis SE. Primary pulmonary extraskeletal Ewing sarcoma/ primitive neuroectodermal tumor: two case reports. Egypt J Bronchol. 2017;11:161-164. [Google Scholar]
- 56. Graham DK, Stork LC, Wei Q, et al. Molecular genetic analysis of a small bowel primitive neuroectodermal tumor. Pediatr Dev Pathol. 2002;5:86-90. [DOI] [PubMed] [Google Scholar]
- 57. Cantu C, Bressler E, Dermawan J, Paral K. Extraskeletal Ewing sarcoma of the jejunum: a case report. Perm J. 2019;23:18-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kazmi SA, Perry A, Pressey JG, Wellons JC, Hammers Y, Palmer CA. Primary Ewing sarcoma of the brain: a case report and literature review. Diagn Mol Pathol. 2007;16:108-111. [DOI] [PubMed] [Google Scholar]
- 59. Choudhury KB, Sharma S, Kothari R, Majumder A. Primary extraosseous intracranial Ewing’s sarcoma: case report and literature review. Indian J Med Paediatr Oncol. 2011;32:118-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ibrahim GM, Fallah Shahideh M, Tabori U, Rutka JT. Primary Ewing’s sarcoma affecting the central nervous system: a review and proposed prognostic considerations. J Clin Neurosci. 2012;19:203-209. [DOI] [PubMed] [Google Scholar]
- 61. Ke CS, Duan QH, Yang H, et al. Meningeal Ewing sarcoma/peripheral PNET: clinicopathological, immunohistochemical and FISH study of four cases. Neuropathology. 2017;37:35-44. [DOI] [PubMed] [Google Scholar]
- 62. Chen J, Jiang Q, Zhang Y, et al. Clinical features and long-term outcome of primary intracranial Ewing sarcoma/peripheral primitive neuroectodermal tumors: 14 cases from a single institution. World Neurosurg. 2019;122:e1606-e1614. [DOI] [PubMed] [Google Scholar]
- 63. Machado I, Noguera R, Pellin A, et al. Molecular diagnosis of Ewing sarcoma family of tumors: a comparative analysis of 560 cases with FISH and RT-PCR. Diagn Mol Pathol. 2009;18:189-199. [DOI] [PubMed] [Google Scholar]
- 64. Lam SW, Cleton-Jansen AM, Cleven A, et al. Molecular analysis of gene fusions in bone and soft tissue tumors by anchored multiplex PCR-based targeted next-generation sequencing. J Mol Diagn. 2018;20:653-663. [DOI] [PubMed] [Google Scholar]