Abstract
BACKGROUND
Diabetic vitreous hemorrhage (DVH) is a common complication of diabetes. While the diagnostic methods nowadays only concentrate on the eye injury in DVH patients, whether DVH leads to abnormalities of other visual systems, including the eye, the visual cortex, and other brain regions, remains unknown.
AIM
To explore the potential changes of brain activity in DVH using regional homogeneity (ReHo) and their relationships with clinical features.
METHODS
Thirty-one DVH patients and 31 matched healthy controls (HCs) were recruited. All subjects were examined by resting-state functional magnetic resonance imaging. The neural homogeneity in the brain region was estimated by ReHo method. Pearson correlation analysis was used to evaluate the relationships between average ReHo values and clinical manifestations in DVH patients.
RESULTS
Compared with HCs, the ReHo values in the bilateral cerebellar posterior lobes, right superior (RS)/middle occipital gyrus (MOG), and bilateral superior frontal gyrus were significantly increased. In contrast, in the right insula, bilateral medial frontal gyri, and right middle frontal gyrus, the ReHo values were significantly decreased. Furthermore, we found that best-corrected visual acuity of the contralateral eye in patients with DVH presented a positive correlation with the mean ReHo value of the RS/MOG. We also found that depression score of the DVH group presented a negative correlation with the mean ReHo values of the right insula, bilateral medial frontal gyrus, and right middle frontal gyrus.
CONCLUSION
We found that DVH may cause dysfunction in multiple brain areas, which may benefit the exploration of pathologic mechanisms in DVH patients.
Keywords: Diabetic vitreous hemorrhage, Diabetes mellitus, Regional homogeneity, Functional magnetic resonance imaging, Resting state, Regional homogeneity
Core Tip: We found that patients with diabetic vitreous hemorrhage (DVH) had abnormal spontaneous activity in many brain regions. The abnormalities of brain neural homogeneity may be associated with the altered best-corrected visual acuity of the contralateral eye and depression which occurred in DVH patients. These findings may provide important information for the understanding of neural changes in DVH patients and lay a foundation for further study.
INTRODUCTION
Diabetic vitreous hemorrhage (DVH) is a common complication of diabetes in which blood accumulates in or around the vitreous body. Vitreous hemorrhage (VH) can be associated with multiple symptoms, including visual losses, blurry vision, floaters, and photopsia[1]. The incidence of spontaneous VH is about 7 in 100000 cases[2]. Diabetes, proliferative diabetic retinopathy (DR), vitreous detachment, retinal vein occlusion (RVO), trauma, and retinal arterial aneurysms are the most common factors that lead to VH[3]. According to a survey in England, apart from diabetes mellitus, other risk factors are also present, such as hypertension and dyslipidemia[4,5]. The complications of persistent VHs are glaucoma and hemosiderosis bulbi. A mild VH may be re-absorbed spontaneously. However, in severe cases, laser photocoagulation, cryo-coagulation, and vitrectomy are the main treatments that are available[3].
Currently, computed tomography (CT) and B-scan ultrasonography are effective noninvasive technologies used to detect VH. Although information that is useful for diagnosis of VH can be provided by CT, it can only detect the integrity of the bulbus oculi, which means that it does not have a role in providing guidance for clinical treatment[4]. B-scan ultrasonography can not only be used to diagnose VH, but also identify retinal tears and retinal detachment in patients, which is of significance in planning therapeutic strategies for individuals with VH[2]. In addition, B-scan ultrasonography can be used to estimate the prognosis of VH. The diagnostic methods mentioned above only concentrate on the eye injury in VH patients. Visual systems include the visual cortex and the connecting pathways. Whether VH leads to alternations of other visual systems, including the eye and the connecting pathways through the visual cortex and other brain regions, remains unknown.
Functional magnetic resonance imaging (fMRI), a functional brain imaging examination technique, is thought to be a widely used measurement. The regional homogeneity (ReHo) method, which can be used to assess the patterns of coherent fluctuations of the blood oxygen level dependent signal surrounding adjacent voxels of the entire brain during rest, is also able to detect spontaneous hemodynamic responses of fMRI sensitively and reliably. The ReHo method is used effectively to study the local synchronization of spontaneous fMRI signals and it is developed to estimate regional resting-state brain activity. The ReHo method has been successfully applied in our previous studies to estimate neurological conditions in certain ocular diseases such as comitant strabismus, late monocular blindness, and acute eye pain[6-8].
The present study is the first to assess the alternations in ReHo, which might help to evaluate the synchronous neural activity changes in the brains of DVH patients. We hypothesized that DVH might cause altered activity of the visual cortex.
MATERIALS AND METHODS
Participants
DVH patients (16 men and 15 women) were enlisted, among whom 15 (8 men and 7 women) had VH caused by DR, and 16 (8 men and 8 women) had VH caused by RVO. The inclusion criteria of the study were: (1) Diabetes; (2) Normal vision in the affected eye before suffering sudden visual loss; (3) VH resulting from DR or RVO diagnosed by fundus camera (FC) and fluorescence fundus angiography (FFA) (in Figure 1, FC and FFA showed DR patients with scattered retinal hemorrhage and exudation around macula, and RVO patients with retinal hemorrhage, distorted retinal veins, and edema on optic disc and retina); and (4) Bilateral eyes without any ocular diseases (strabismus, cataracts, retinal degeneration, amblyopia, glaucoma, optic neuritis, and so on).
Figure 1.
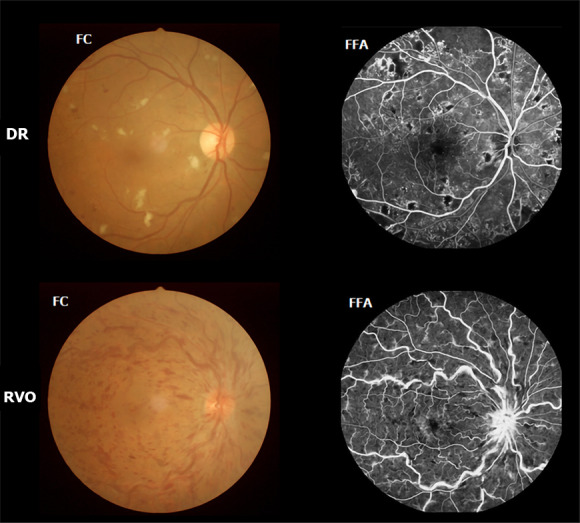
Example of diabetic vitreous hemorrhage caused by diabetic retinopathy and retinal vein occlusion seen on fundus camera and fluorescence fundus angiography. DR: Diabetic retinopathy; RVO: Retinal vein occlusion; FC: Fundus camera; FFA: Fluorescence fundus angiography.
DVH patients with the following conditions were excluded: (1) A history of scleral buckle or vitreous surgery, uveitis, trauma, complicated cataracts, or glaucoma surgery; (2) VH due to ocular trauma; (3) Systemic diseases, including heart disease and hypertension; (4) Additions (drug, cigarette, alcohol, and so on); and (5) Other disorders that would affect the ReHo measurement.
Healthy controls (HCs) were recruited, included 16 men and 15 women matched for age, gender, and socioeconomic status with the subjects in the DVH group. All the HCs met the following criteria: (1) Diabetes without VH; (2) No abnormalities in brain parenchyma identified by the head magnetic resonance imaging (MRI); (3) No ocular diseases with best-corrected visual acuity (BCVA) >1.0; (4) No psychiatric disease; and (5) Ability to be examined by MRI (no cardiac pacemaker, metallic false teeth, or implanted metal devices).
This study was approved by the Medical Ethics Committee of the First Affiliated Hospital of Nanchang University (ethical approval number: 2014022). All methods used followed the tenets of Declaration of Helsinki and complied with the principles of medical ethics. All volunteers participated in the study voluntarily, understood the purpose, methods, and potential risks, and provided written consent for the study.
Depression score assessment
Depression score (DS) assessment of all participants was performed with a questionnaire. The questionnaire used in this study was the Chinese translation of HADS which was designed by Zigmond AS and Snaith RP in 1983[9]. A higher DS means a higher level of depression.
MRI parameters
MRI scanning was carried out on a 3T MR scanner (Trio, Siemens, Munich, Germany). Whole-brain T1-weights were obtained with magnetization-prepared gradient echo images, using the following parameters: Repetition time = 1900 ms, echo time = 2.26 ms, thickness = 1.0 mm, gap = 0.5 mm, acquisition matrix = 256 × 256, field of view = 250 mm × 250 mm, and flip angle = 9°. Functional images with the parameters (repetition time = 2000 ms, echo time = 30 ms, thickness = 4.0 mm, gap = 1.2 mm, acquisition matrix = 64 × 64, flip angle = 90°, field of view = 220 mm × 220 mm, 29 axial) were corrected.
Processing of fMRI data
The functional diagrams were analyzed as described above[6]. Briefly, the data were filtered by MRIcro (Nottingham University, Nottingham, United Kingdom), then the rest of the images were preprocessed using Statistical Parametric Mapping SPM8 (The MathWorks, Inc., Natick, MA, United States) and Data Processing Assistant for rs-fMRI DPARSFA (Institute of Psychology, CAS., Beijing, China) software. Since there was time for signals to reach equilibrium, the first ten volumes of each subject were discarded. Then, the data were processed by head motion correction and spatial smoothing. For the effects of low-frequency drift and physiological high-frequency respiratory and cardiac noise which should be lessened, the fMRI images were de-trended and band-pass filtered (0.01 – 0.08 Hz). Each voxel in the brain was calculated in a voxel-wise manner by applying a cluster size of 26 voxels based on Kendall’s coefficient of concordance (KCC). The global signal was not regressed out according to the protocol from a previous study[10]. Finally, the remaining data were flattened with a Gaussian kernel of 6 mm × 6 mm × 6 mm full-width at half-maximum.
ReHo statistical analysis
ReHo computation was carried out with REST software (Institute of Psychology, CAS., Beijing, China). To estimate the consistency and comparability for individuals, ReHo analysis was carried out by counting the KCC of voxel time series for a given time series and its adjacent voxels in a voxel-wise analysis, assuming that a voxel in time is similar to its neighbors.
Statistical analysis
The cumulative clinical variables between patients and normal controls were analyzed using SPSS version 16.0 (SPSS Inc, Chicago, IL, United States) by an independent-sample t test. A P value < 0.05 was utilized as the cut-off for statistical significance.
A general linear model was used for statistical analysis with the SPM8 toolkit. ReHo maps were transformed to z-score maps. Areas with significant changes of frequency or overall ReHo were selected as regions of interest (ROIs). The two-sample t tests were used to examine the differences in ReHo at different frequency bands in these ROIs between the DVH groups and the HCs (P < 0.01) for multiple comparisons using Gaussian random field (GRF) theory (z > 2.3, P < 0.01, cluster > 40 voxels, AlphaSim corrected). Receiver operating characteristic (ROC) curves were used to analyze the average ReHo values in the various brain areas between the two groups.
Brian-behavior correlation analysis
Different brain areas between groups were classified as areas of interest using REST software according to the findings of ReHo. By calculating the average ReHo value of all voxels, the mean ReHo value for each area of interest was obtained. Pearson correlation was analyzed with GraphPad prism 7 (Graph Pad Software Inc., San Diego, CA, United States) to assess the correlations between the mean ReHo values in multiple brain areas in the DVH group and the associated behavioral performances (P < 0.05 indicates significant differences).
RESULTS
Demographics and visual measurements
There were no significant differences in age (P = 0.967) or weight (P = 0.906), but the differences in best-corrected VA-right (P < 0.001) and best-corrected VA-left (P < 0.001) between patients with DVH and HCs were significant. Additionally, the average value of the duration of DVH was 24.15 ± 20.97 d (Table 1).
Table 1.
Demographics and clinical measurements by group
|
Condition
|
DVH
|
HC
|
t
|
P
valuea
|
| Male/female | 16/15 | 16/15 | N/A | > 0.99 |
| Age (yr) | 56.03 ± 4.61 | 56.48 ± 4.29 | 0.041 | 0.967 |
| Weight (kg) | 60.48 ± 3.83 | 61.10 ± 3.49 | 0.118 | 0.906 |
| Handedness | 31 R | 31 R | N/A | > 0.99 |
| Duration of DVH (d) | 24.15 ± 20.97 | N/A | N/A | N/A |
| Best-corrected VA-right eye | 0.64 ± 0.05 | 1.07 ± 0.03 | -7.771 | < 0.001 |
| Best-corrected VA-left eye | 0.66 ± 0.06 | 1.06 ± 0.02 | -6.231 | < 0.001 |
| Duration of DM (yr) | 27.23 ± 19.89 | 25.85 ± 18.28 | 0.085 | 0.915 |
| HgbA1c (%) | 5.3 ± 0.4 | 4.8 ± 0.5 | 0.051 | > 0.99 |
P < 0.05, independent t-tests. Data shown are the mean ± SD. DVH: Diabetic vitreous hemorrhage; HC: Healthy control; N/A: Not applicable; R: Right; VA: Visual acuity; DM: Diabetes mellitus.
ReHo differences
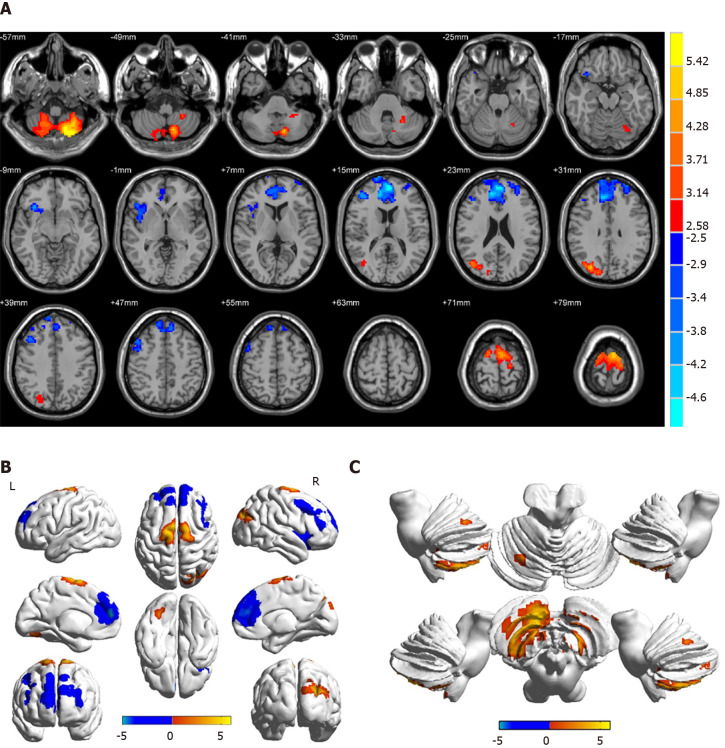
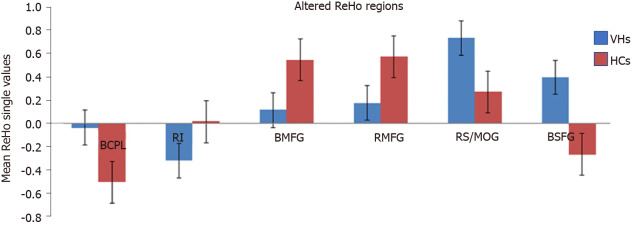
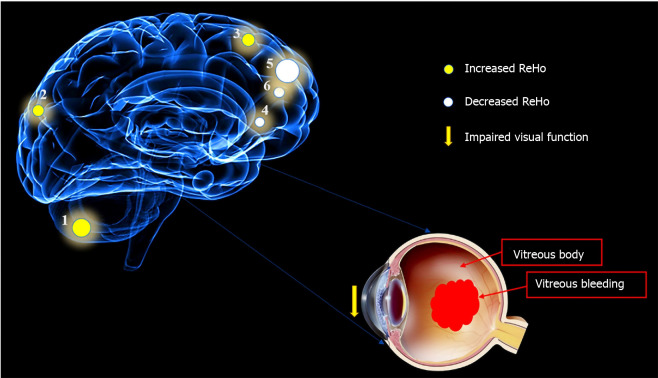
Compared with HCs, patients with DVH had significantly higher ReHo values bilaterally in the posterior lobe of the cerebellum (CPL), in the right superior (RS)/middle occipital gyrus (MOG), and bilateral superior frontal gyrus (SFG) as shown in Figure 2A-C (red) and Table 2. Meanwhile, the ReHo values of the right insula, bilateral medial frontal gyrus (MFG), and right middle frontal gyrus in the DVH patient group were obviously lower than those in HCs as shown in Figure 2A and B (blue) and Table 2 (P < 0.01 for multiple comparisons using the GRF theory, z > 2.3, P < 0.01, cluster > 40 voxels, AlphaSim corrected). The means of the altered ReHo values between the two groups are presented in Figure 3.
Figure 2.
Spontaneous brain activity in patients with diabetic vitreous hemorrhage vs healthy controls. Significant activity differences were observed in the bilateral cerebellum posterior lobe, right superior/middle occipital gyrus, bilateral superior frontal gyrus, right insula, bilateral medial frontal gyrus, and right middle frontal gyrus. Red denotes higher regional homogeneity values and blue denotes lower regional homogeneity values. P < 0.001 for multiple comparisons using Gaussian random field theory (z > 2.3, P < 0.001, cluster > 40 voxels, AlphaSim corrected). R: Right; L: Left.
Table 2.
Brain areas with significantly different regional homogeneity values between groups
| Brain region |
MNI coordinate |
BA |
Peak voxels |
t value |
||
| X | Y | Z | ||||
| DVH > HCs | ||||||
| BCPL | -21 | -75 | -57 | / | 914 | 5.9833 |
| RS/MOG | 30 | -78 | 30 | 19 | 262 | 4.8595 |
| BSFG | -6 | -6 | 78 | 6 | 345 | 5.0841 |
| DVH < HCs | ||||||
| RI | 33 | 21 | -12 | 47 | 247 | -4.1671 |
| BMFG | -3 | 54 | 15 | 10 | 1321 | -5.0857 |
| RMFG | 42 | 42 | 15 | 9 | 259 | -4.2382 |
The statistical threshold was set at the voxel level with P < 0.05 for multiple comparisons using Gaussian random field theory (z > 2.3, P < 0.01, cluster > 40 voxels, AlphaSim corrected). MNI: Montreal Neurological Institute; BA: Brodmann area; DVH: Diabetic vitreous hemorrhage; HCs: Healthy controls; BCPL: Bilateral cerebellum posterior lobe; RS: Right superior; MOG: Middle occipital gyrus; BSFG: Bilateral superior frontal gyrus; RI: Right insula; BMFG: Bilateral medial frontal gyrus; RMFG: Right middle frontal gyrus.
Figure 3.
Mean regional homogeneity values between the diabetic vitreous hemorrhage and healthy control groups. ReHo: Regional homogeneity; VH: Vitreous hemorrhage; HC: Healthy control; BCPL: Bilateral cerebellum posterior lobe; RI: Right insula; BMFG: Bilateral medial frontal gyrus; RMFG: Right middle frontal gyrus; RS: Right superior; MOG: Middle occipital gyrus; BSFG: Bilateral superior frontal gyrus.
Correlation analysis
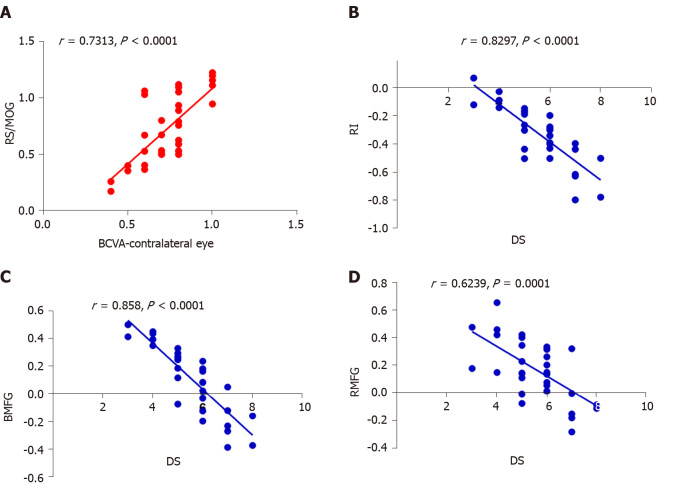
In the DVH group, BCVA of the contralateral eye showed a positive correlation with the mean ReHo signal value of the RS/MOG (r = 0.7313, P < 0.0001) (Figure 4A), while DS in the DVH group showed a negative correlation with the mean ReHo signal values of the right insula (r = -0.8297, P < 0.0001) (Figure 4B), bilateral MFG (r = -0.858, P < 0.0001) (Figure 4C), and right middle frontal gyrus (r = -0.6239, P = 0.0001) (Figure 4D).
Figure 4.
Correlations between mean regional homogeneity signal values of different areas and behavior performances. A: In diabetic vitreous hemorrhage (DVH) group, best-corrected visual acuity of the contralateral eye showed a positive correlation with the mean regional homogeneity (ReHo) signal value of the right superior/middle occipital gyrus (r = 0.7313, P < 0.0001); B: Depression score in DVH group showed a negative correlation with the mean ReHo signal value of the right insula (r = -0.8297, P < 0.0001); C: Bilateral medial frontal gyrus (r = -0.858, P < 0.0001); D: Right middle frontal gyrus (r = -0.6239, P = 0.0001). RS: Right superior; MOG: Middle occipital gyrus; BCVA: Best-corrected visual acuity; RI: Right insula; DS: Depression score; BMFG: Bilateral medial frontal gyrus; RMFG: Right middle frontal gyrus.
ROC curve analysis
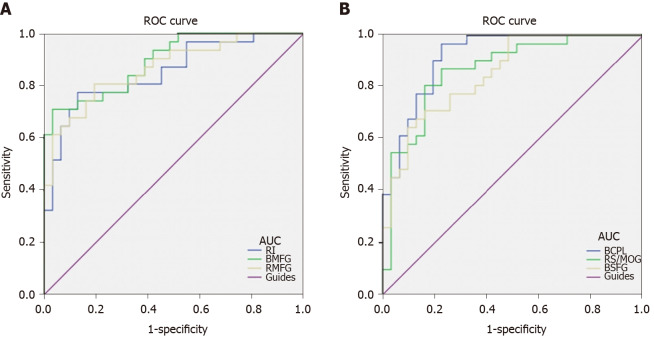
We hypothesized that the ReHo values of the altered brain regions in patients with DVH might be used as diagnostic markers for VH. The mean ReHo values in multiple brain regions were analyzed by ROC method. The area under the ROC curve stands for diagnostic rate. The areas under the ROC curves for ReHo values were as follows: Bilateral cerebellum posterior lobe 0.919; RS/MOG 0.868; bilateral SFG 0.851 (Figure 5A, DVHs > HCs); right insula 0.852; bilateral MFG 0.894; and right middle frontal gyrus 0.867 (Figure 5B, DVHs < HCs).
Figure 5.
Receiver operating characteristic curve analysis of mean regional homogeneity values for altered brain regions. A: The areas under the receiver operating characteristic (ROC) curves were 0.919 [P < 0.001; 95% confidence interval (CI): 0.852-0.986] for bilateral cerebellum posterior lobe, 0.868 (P < 0.001; 95%CI: 0.777-0.959) for the right superior/middle occipital gyrus, and 0.851 (P < 0.001; 95%CI: 0.759-0.943) for bilateral superior frontal gyrus; B: The areas under the ROC curves were 0.852 (P < 0.001; 95%CI: 0.757-0.947) for the right insula, 0.894 (P < 0.001; 95%CI: 0.818-0.970) for bilateral medial frontal gyrus, and 0.867 (P < 0.001; 95%CI: 0.778-0.955) for right middle frontal gyrus. ROC: Receiver operating characteristic; AUC: Area under the curve; RI: Right insula; BMFG: Bilateral medial frontal gyrus; RMFG: Right middle frontal gyrus; BCPL: Bilateral cerebellum posterior lobe; RS: Right superior; MOG: Middle occipital gyrus; BSFG: Bilateral superior frontal gyrus.
DISCUSSION
VH, as an important ocular disease, raises much concern on its underlying pathogenesis and its relationship with brain function activities. A previous study reported that intracerebral hemorrhage might be responsible for VH[8]. The intracerebral hemorrhage which means the rupture of blood vessels in the brain and bleeding can be caused by traumatic brain injury, hypertension, and some other risk factors, and may lead to VH. In addition, the blood accumulated in the vitreous body can result in impaired visual function which may be involved in depression[11]. Therefore, brain regions that are associated with emotion processing may present abnormal neural activities (Figure 6).
Figure 6.
Relationship between magnetic resonance imaging images and diabetic vitreous hemorrhage. Intracerebral hemorrhage may lead to vitreous hemorrhage, and blood accumulated in vitreous body can result in impaired visual function that may be involved in depression. Brain regions that are associated with emotion processing may present abnormal neural activities.
fMRI has become widely used in clinical medicine. Compared with traditional MRI, fMRI can not only provide new insights into the pathophysiology of diseases (including anatomical structure and basic physiology) but also produce dynamic brain activation maps with a relatively high resolution in a fast and non-invasive way[12]. ReHo is an analytical method of resting state fMRI that can determine the local synchronization of spontaneous fMRI signals, as well as detect and quantitate activation sites in the brain. This method has been successfully applied in several ophthalmological diseases and is predicted to have huge prospects for further development in medicine (Table 3).
Table 3.
Regional homogeneity method applied in ophthalmological diseases
|
Author
|
Year
|
Disease
|
Brain areas
|
|
|
UDs > HCs
|
UDs < HCs
|
|||
| Song et al[11] | 2014 | Glaucoma | RDACC; BMFG; RCAL | BC; BPG; BP/PG; LIPL; LCPL |
| Cui et al[12] | 2014 | Diabetic retinopathy | PLC; ACC; FL | OL; PG |
| Shao et al[6] | 2015 | Optic neuritis | LFG; RIPL | LCPL; LMTG; RI; RSTG; LMFG;BACC; BMFG; L/RSFG; RPG |
| Huang et al[13]; Huang et al[14] | 2016; 2016 | Comitant Strabismus; Open-globe injury | RITC/FG/CAL; RLG; BCG; RCPL/LG; LSTG/IFG; LIFG; LPCC/PCUN; LPrCO | |
| Huang, et al[15] | 2017 | Retinal detachment | ROL; RSTG; BC; LMFG | |
| Tang et al[33] | 2018 | Acute eye pain | LSFG; RIPL; LP | LP/PG; RP/PG; LMFG |
| Shao et al[34] | 2019 | Strabismus and amblyopia | LLG; RMOG/P; BAC; LMOG; BPG | LIFG |
| Wen et al[35] | 2019 | Retinal vein occlusion | RCPL; RFG; RITG | RC |
| Xu et al[36] | 2019 | Corneal ulcer | RCPL; LCPL; LITG; RLG; LMFG; LAG; LCG; RAG; BSFG | LPG; RAC |
HCs: Healthy controls; RDACC: Right dorsal anterior cingulated cortex; BMFG: Bilateral medial frontal gyrus; RCAL: Right cerebellar anterior lobe; BC: Bilateral calcarine; BPG: Bilateral precuneus gyrus; BP/PG: Bilateral pre/postcentral gyrus; LIPL: Left inferior parietal lobule; LCPL: Left cerebellum posterior lobe; PLC: Posterior lobe of cerebellum; ACC: Anterior cingulate cortex; FL: Frontal lobe; OL: Occipital lobe; LFG: Left fusiform gyrus; RIPL: Right inferior parietal lobule; LMTG: Left middle temporal gyrus; RI: Right insula; RSTG: Right superior temporal gyrus; LMFG: Left middle frontal gyrus; BACC: Bilateral anterior cingulate cortex; L/RSFG: Left/right superior frontal gyrus; RPG: Right precentral gyrus; RITC/FG/CAL: Right inferior temporal cortex/fusiform gyrus/cerebellum anterior lobe; RLG: Right lingual gyrus; BCG: Bilateral cingulate gyrus; RCPL/LG: Right cerebellum posterior lobe/lingual gyrus; LSTG/IFG: Left superior temporal gyrus/inferior frontal gyrus; LIFG: Left inferior frontal gyrus; LPCC/PCUN: Left posterior cingulate cortex/precuneus; LPrCO: Left precentral operculum; ROL: Right occipital lobe; LSFG: Left superior frontal gyrus; LP: Left precuneus; RP: Right precentral; LLG: Left lingual gyrus; RMOG/P: Right Middle occipital gyrus/precuneus; BAC: Bilateral anterior cingulate; LMOG: Left middle occipital gyrus; RFG: Right fusiform gyrus; RITG: Right inferior temporal gyrus; RC: Right cuneus; LAG: Left angular gyrus; LCG: Left cingulate gyrus; RAG: Right angular gyrus; BSFG: Bilateral superior frontal gyrus; LPG: Left precentral gyrus; RAC: Right anterior cingulate. LITG: Left inferior temporal gyrus.
To our knowledge, this is the first study in which the ReHo technique was used to evaluate regional spontaneous neural activity changes in patients with DVH. Compared to the HCs, patients with DVH had significantly higher ReHo values in the bilateral posterior CPL, RS/MOG, and bilateral SFG. DVH patients also had lower ReHo values in the right insula, right middle frontal gyrus, and bilaterally in the MFG (Figure 7). Moreover, we found that in the DVH group, BCVA of the contralateral eye presented a positive correlation with the mean ReHo signal value of the RS/MOG (r = 0.7313, P < 0.0001). We also observed that DS of the DVH group presented a negative correlation with the mean ReHo signal values of the right insula (r = -0.8297, P < 0.0001), bilateral MFG (r = -0.858, P < 0.0001), and right middle frontal gyrus (r = -0.6239, P = 0.0001). And a higher DS means a higher level of depression. Thus, the depression level of DVH patients presented a negative correlation with the mean ReHo signal values of the three brain areas above.
Figure 7.
Mean regional homogeneity values of altered brain regions in the diabetic vitreous hemorrhage group. Compared with the healthy controls, the regional homogeneity values of the following regions were increased to various extents: 1- cerebellum posterior lobe, bilateral (-, t = 5.9833); 2- superior/middle occipital gyrus, right (Brodmann area [BA] 19, t = 4.8595); and 3- superior frontal gyrus, bilateral (BA6, t = 5.0841), whereas the ReHo values of the following regions were decreased: 4- insula, right (BA47, t = -4.1671); 5- medial frontal gyrus, bilateral (BA10, t = -5.0857); and 6- middle frontal gyrus, right (BA9, t = -4.2382). The sizes of the spots denote the degree of quantitative changes. ReHo: Regional homogeneity.
The cerebellum plays a key role in various central nervous system functions including sensorimotor control and language, spatial, and executive functions[12]. Previous studies have shown that the cerebellum may be involved in cognition, and that injuries in this region may cause cognitive dysfunction. The posterior lobe of the cerebellum is a part of the cerebellum beneath the primary fissure. Baumann et al[13] reported that inputs from the brainstem and cerebral cortex are received by the posterior CPL, and this activation is linked to happiness. Casual et al[14] found that the CPL is associated with motor control. Moreover, dysfunction of the CPL occurs in many diseases, such as comitant strabismus, primary angle closure glaucoma, and optic neuritis[6,15,16]. In our study, we found that patients with DVH showed increased ReHo values bilaterally in the CPL, which indicates dysfunctional brain activity in this structure.
Apart from the frontal eye field (FEF), the supplementary eye field (SEF) is another important brain region that is related to the oculomotor pathway of saccadic eye movements. The SEF lies in the superior area of the medial wall, the same area as the SFG. The SEF regulates saccades and the coordination of eye and hands in an indirect way. Min et al[17] demonstrated that strabismus patients with amblyopia had higher ALFF value in the right SFG. In our study, higher ReHo values were detected bilaterally in the SFG of DVH patients. We hypothesized that the hyperactivated neurons in the SFG might elicit SEF hyperfunction to compensate for the FEF hypofunction that occurs in DVH patients.
The occipital lobe is the anatomical region of the visual cortex that is closely related to visual processing. The occipital lobe contains the primary visual cortex (V1), and four other visual areas that are referred to as V2, V3, V4, and V5[18]. The primary visual cortex is a vital region that receives visual signals from the lateral geniculate nucleus. Reislev et al[19] performed diffusion tensor imaging and found that blindness is associated with decreased fractional anisotropy in the primary visual cortex. Hence, dysfunction of the occipital lobe may cause disorders of visual processing. The MOG is part of the dorsal visual stream (DVS) in the visual center which is located at the area V2[20]. The DVS is associated with spatial information analyses including motion, location, direction, and, action plans. Renier et al[21] demonstrated that the right MOG provides spatial processing of both auditory and tactile stimuli, and the activity of the MOG is associated with the performance of sound localization in humans. Thus, lesions in the MOG may affect the processing of spatial information. The superior occipital gyrus (SOG) is another region in the occipital lobe. Zhang et al[22] reported that compared with controls, female migraineurs without aura exhibited altered network properties, and that the nodal centralities in the SOG of migraine patients were significantly lower. Song et al[23] found that pituitary adenoma patients with visual impairment had significantly increased ReHo values in the left SOG. In our study, the ReHo value of the RS/MOG in patients with DVH was increased, reflecting abnormal synchronization of brain activity in the RS/MOG. In addition, we also observed that BCVA of the contralateral eye in DVH patients showed a positive correlation with the mean ReHo signal value of the RS/MOG (r = 0.7313, P < 0.0001). Thus, we inferred that DVH patients had hyperfunction of the brain involved in activities in the RS/MOG, which was related to the better BCVA of the contralateral eye. It might be explained by visual compensation of the contralateral eye after visual impairment in the eye with DVH.
The insula lies in the lateral sulcus, and anatomically connects to the temporal, frontal, and parietal lobes[24]. Dysfunction of the insula has been found to be involved in negative emotional experiences and anxiety[25]. Moreover, abnormalities of the insula are also related to Alzheimer’s disease and epilepsy[26]. In our study, we found that DVH patients exhibited significantly lower ReHo values in the right insula, indicating that the insula was dysfunctional. Furthermore, we also observed that DS showed a negative correlation with the mean ReHo signal value of the right insula (r = -0.8297, P < 0.0001). It can be concluded that DVH patients with impaired insula function that was indicated by lower ReHo values also had a higher level of depression. Hence, we can speculate that DVH may lead to patients suffering depression and the insula that is involved in negative emotion regulation may present abnormal brain function activity. Thus, DVH severity may has a close relationship with insula function.
The MFG, including the FEF, lies in the middle of the frontal gyrus and is closely related to the empathy[27]. Additionally, the MFG is associated with negative emotion[28]. The dysfunction of the MFG is seen in plenty of psychiatric disorders. Myung et al[29] reported that reduced gray matter volume in the right MFG was observed in patients with adjustment disorder. Additionally, Gao et al[30] demonstrated that in patients with schizophrenia, ReHo of the left medial SFG was increased, which might indicate the altered local synchronization of spontaneous brain activity. In this study, we detected that DVH subjects had decreased ReHo values in the bilateral MFG, which reflected the impaired local synchronization of brain activities. Furthermore, we found that DS showed a negative correlation with the mean ReHo signal value of bilateral MFG (r = -0.858, P < 0.0001), indicating that DVH patients with lower ReHo values in the bilateral MFG may also suffer mental diseases, which is consistent with the findings of Myung et al[29]. DVH might therefore lead to depression and be associated with dysfunction of bilateral MFG.
The middle frontal gyrus, which makes up about one-third of the frontal lobe, is located between the superior and inferior frontal sulci. It plays a vital role in cognitive function and attention[31]. Quinn et al[32] found that dysfunction of the middle frontal gyrus was associated with depression. Our previous studies reported that the middle frontal gyrus is involved in monocular blindness and acute eye pain[7,33]. In this study, we found that DVH was associated with significantly lower ReHo value in the right middle frontal gyrus, and thus can speculate that DVH may give rise to the dysfunction of the middle frontal gyrus. Moreover, we found that DS showed a negative correlation with the mean ReHo signal value of the right middle frontal gyrus (r = -0.6239, P = 0.0001), which was consistent with the findings of Quinn et al[32]. Therefore, our study suggested that DVH has a harmful influence on the right middle frontal gyrus, which might be related to depression.
ARTICLE HIGHLIGHTS
Research background
Diabetic vitreous hemorrhage (DVH) is a common complication of diabetes. However, there are few studies that have focused on synchronous neural activities in DVH patients. Our study is the first to use the ReHo method to evaluate the synchronous neural activity changes in the brain of DVH patients.
Research motivation
The diagnostic methods nowadays only concentrate on the eye injury in vitreous hemorrhage (VH) patients, and whether VH leads to abnormalities of other visual systems, including the eye, the visual cortex, and other brain regions, remains unknown. In this study, we investigated the underlying regional homogeneity (ReHo) of brain-activity abnormalities in patients with DVH and their relationship with clinical features. Our study may contribute to understanding altered neural mechanisms present in patients with DVH.
Research objectives
To investigate the underlying ReHo of brain-activity abnormalities in patients with DVH and their relationship with behavioral performance. Our findings might provide useful information for exploration of neural mechanisms in DVH patients.
Research methods
Thirty-one DVH patients and 31 matched healthy controls were recruited. All subjects were examined by resting-state functional magnetic resonance imaging. The neural homogeneity in the brain region was estimated by ReHo method. Pearson correlation analysis was used to evaluate the relationships between average ReHo values and clinical manifestations in DVH patients.
Research results
We found that DVH may cause dysfunction in multiple brain areas like bilateral cerebellar posterior lobes, right superior/middle occipital gyrus, bilateral superior frontal gyrus, right insula, bilateral medial frontal gyri, and right middle frontal gyrus. And DVH may be associated with depression that occurred in patients. There are some limitations to our study, such as the relatively small sample size. Moreover, for some subjects, the scan time was too long, so body movement could have affected the ReHo findings.
Research conclusions
We hypothesized that DVH might cause altered activity of visual cortex. Our results revealed that spontaneous activity in multiple regions of the brain changed in patients with DVH. The abnormalities of brain neural homogeneity may be associated with the altered best-corrected visual acuity of the contralateral eye and depression that occurred in DVH patients. These findings may point to altered neural mechanisms present in patients with DVH and lay a foundation for further study.
Research perspectives
We found that DVH may cause dysfunction in multiple brain areas, which may benefit the exploration of pathologic mechanisms in DVH patients. We included both right eye and left eye injury patients, which might affect the ReHo findings. Future studies should distinguish the difference and measure brain function activity changes more accurately.
Footnotes
Institutional review board statement: The study methods and protocols were approved by the Medical Ethics Committee of the First Affiliated Hospital of Nanchang University (Ethical approval number: 2014022), and followed the principles of the Declaration of Helsinki. All subjects were notified of the objectives and content of the study and latent risks, and then provided written informed consent to participate.
Informed consent statement: All study participants, or their legal guardian, provided informed written consent prior to study enrollment.
Conflict-of-interest statement: This is not an industry supported study. The authors report no conflicts of interest in this work.
CONSORT 2010 statement: The manuscript was prepared and revised according to the CONSORT 2010 statement.
Manuscript source: Unsolicited manuscript
Peer-review started: February 21, 2020
First decision: April 22, 2020
Article in press: September 27, 2020
Specialty type: Ophthalmology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Essawy BE S-Editor: Liu M L-Editor: Wang TQ P-Editor: Ma YJ
Contributor Information
Yu-Qing Zhang, Department of Ophthalmology, The First Affiliated Hospital of Nanchang University, Nanchang 330006, Jiangxi Province, China; Department of Ophthalmology, Jiangxi Province Ocular Disease Clinical Research Center, Nanchang 330006, Jiangxi Province, China.
Fei-Yin Zhu, Department of Ophthalmology, The First Affiliated Hospital of Nanchang University, Nanchang 330006, Jiangxi Province, China; Department of Ophthalmology, Jiangxi Province Ocular Disease Clinical Research Center, Nanchang 330006, Jiangxi Province, China.
Li-Ying Tang, Department of Ophthalmology, The First Affiliated Hospital of Nanchang University, Nanchang 330006, Jiangxi Province, China; Department of Ophthalmology, Jiangxi Province Ocular Disease Clinical Research Center, Nanchang 330006, Jiangxi Province, China; Department of Ophthalmology, Xiang’an Hospital of Xiamen University, Xiamen 361101, Fujian Province, China; Fujian Provincial Key Laboratory of Ophthalmology and Visual Science, Xiamen 361102, Fujian Province, China; Department of Ophthalmology, Eye Institute of Xiamen University, Xiamen 361102, Fujian Province, China.
Biao Li, Department of Ophthalmology, The First Affiliated Hospital of Nanchang University, Nanchang 330006, Jiangxi Province, China; Department of Ophthalmology, Jiangxi Province Ocular Disease Clinical Research Center, Nanchang 330006, Jiangxi Province, China.
Pei-Wen Zhu, Department of Ophthalmology, The First Affiliated Hospital of Nanchang University, Nanchang 330006, Jiangxi Province, China; Department of Ophthalmology, Jiangxi Province Ocular Disease Clinical Research Center, Nanchang 330006, Jiangxi Province, China.
Wen-Qing Shi, Department of Ophthalmology, The First Affiliated Hospital of Nanchang University, Nanchang 330006, Jiangxi Province, China; Department of Ophthalmology, Jiangxi Province Ocular Disease Clinical Research Center, Nanchang 330006, Jiangxi Province, China.
Qi Lin, Department of Ophthalmology, The First Affiliated Hospital of Nanchang University, Nanchang 330006, Jiangxi Province, China; Department of Ophthalmology, Jiangxi Province Ocular Disease Clinical Research Center, Nanchang 330006, Jiangxi Province, China.
You-Lan Min, Department of Ophthalmology, The First Affiliated Hospital of Nanchang University, Nanchang 330006, Jiangxi Province, China; Department of Ophthalmology, Jiangxi Province Ocular Disease Clinical Research Center, Nanchang 330006, Jiangxi Province, China.
Yi Shao, Department of Ophthalmology, The First Affiliated Hospital of Nanchang University, Nanchang 330006, Jiangxi Province, China; Department of Ophthalmology, Jiangxi Province Ocular Disease Clinical Research Center, Nanchang 330006, Jiangxi Province, China.
Qiong Zhou, Department of Ophthalmology, The First Affiliated Hospital of Nanchang University, Nanchang 330006, Jiangxi Province, China; Department of Ophthalmology, Jiangxi Province Ocular Disease Clinical Research Center, Nanchang 330006, Jiangxi Province, China. qiongz-ms@126.com.
Data sharing statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
References
- 1.Yoonessi R, Hussain A, Jang TB. Bedside ocular ultrasound for the detection of retinal detachment in the emergency department. Acad Emerg Med. 2010;17:913–917. doi: 10.1111/j.1553-2712.2010.00809.x. [DOI] [PubMed] [Google Scholar]
- 2.Sandinha MT, Kotagiri AK, Owen RI, Geenen C, Steel DH. Accuracy of B-scan ultrasonography in acute fundus obscuring vitreous hemorrhage using a standardized scanning protocol and a dedicated ophthalmic ultrasonographer. Clin Ophthalmol. 2017;11:1365–1370. doi: 10.2147/OPTH.S133938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cuevas P, Outeiriño LA, Azanza C, Angulo J, Giménez-Gallego G. Dramatic resolution of vitreous hemorrhage after an intravitreal injection of dobesilate. Mil Med Res. 2015;2:23. doi: 10.1186/s40779-015-0050-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frasure SE, Saul T, Lewiss RE. Bedside ultrasound diagnosis of vitreous hemorrhage and traumatic lens dislocation. Am J Emerg Med 2013; 31: 1002.e1-1002. :e2. doi: 10.1016/j.ajem.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Banerjee S, Denniston AK, Gibson JM, Dodson PM. Does cardiovascular therapy affect the onset and recurrence of preretinal and vitreous haemorrhage in diabetic eye disease? Eye (Lond) 2004;18:821–825. doi: 10.1038/sj.eye.6701338. [DOI] [PubMed] [Google Scholar]
- 6.Shao Y, Cai FQ, Zhong YL, Huang X, Zhang Y, Hu PH, Pei CG, Zhou FQ, Zeng XJ. Altered intrinsic regional spontaneous brain activity in patients with optic neuritis: a resting-state functional magnetic resonance imaging study. Neuropsychiatr Dis Treat. 2015;11:3065–3073. doi: 10.2147/NDT.S92968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang X, Ye CL, Zhong YL, Ye L, Yang QC, Li HJ, Jiang N, Peng DC, Shao Y. Altered regional homogeneity in patients with late monocular blindness: a resting-state functional MRI study. Neuroreport. 2017;28:1085–1091. doi: 10.1097/WNR.0000000000000855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Czorlich P, Skevas C, Knospe V, Vettorazzi E, Richard G, Wagenfeld L, Westphal M, Regelsberger J. Terson syndrome in subarachnoid hemorrhage, intracerebral hemorrhage, and traumatic brain injury. Neurosurg Rev. 2015;38:129–36; discussion 136. doi: 10.1007/s10143-014-0564-4. [DOI] [PubMed] [Google Scholar]
- 9.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 10.Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. 2009;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song Y, Mu K, Wang J, Lin F, Chen Z, Yan X, Hao Y, Zhu W, Zhang H. Altered spontaneous brain activity in primary open angle glaucoma: a resting-state functional magnetic resonance imaging study. PLoS One. 2014;9:e89493. doi: 10.1371/journal.pone.0089493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cui Y, Jiao Y, Chen YC, Wang K, Gao B, Wen S, Ju S, Teng GJ. Altered spontaneous brain activity in type 2 diabetes: a resting-state functional MRI study. Diabetes. 2014;63:749–760. doi: 10.2337/db13-0519. [DOI] [PubMed] [Google Scholar]
- 13.Huang X, Li SH, Zhou FQ, Zhang Y, Zhong YL, Cai FQ, Shao Y, Zeng XJ. Altered intrinsic regional brain spontaneous activity in patients with comitant strabismus: a resting-state functional MRI study. Neuropsychiatr Dis Treat. 2016;12:1303–1308. doi: 10.2147/NDT.S105478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang X, Li HJ, Ye L, Zhang Y, Wei R, Zhong YL, Peng DC, Shao Y. Altered regional homogeneity in patients with unilateral acute open-globe injury: a resting-state functional MRI study. Neuropsychiatr Dis Treat. 2016;12:1901–1906. doi: 10.2147/NDT.S110541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang X, Li HJ, Zhang Y, Peng DC, Hu PH, Zhong YL, Zhou FQ, Shao Y. Microstructural changes of the whole brain in patients with comitant strabismus: evidence from a diffusion tensor imaging study. Neuropsychiatr Dis Treat. 2016;12:2007–2014. doi: 10.2147/NDT.S108834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malikovic A, Vucetic B, Milisavljevic M, Tosevski J, Sazdanovic P, Milojevic B, Malobabic S. Occipital sulci of the human brain: variability and morphometry. Anat Sci Int. 2012;87:61–70. doi: 10.1007/s12565-011-0118-6. [DOI] [PubMed] [Google Scholar]
- 17.Min YL, Su T, Shu YQ, Liu WF, Chen LL, Shi WQ, Jiang N, Zhu PW, Yuan Q, Xu XW, Ye L, Shao Y. Altered spontaneous brain activity patterns in strabismus with amblyopia patients using amplitude of low-frequency fluctuation: a resting-state fMRI study. Neuropsychiatr Dis Treat. 2018;14:2351–2359. doi: 10.2147/NDT.S171462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang F, Zeng FF, Yu C, Ye YQ, Zeng XJ. Altered whole-brain gray matter volume in primary angle closure glaucoma patients: a voxel-based morphometry study. Neuroreport. 2018;29:1405–1412. doi: 10.1097/WNR.0000000000001126. [DOI] [PubMed] [Google Scholar]
- 19.Reislev NL, Dyrby TB, Siebner HR, Kupers R, Ptito M. Simultaneous Assessment of White Matter Changes in Microstructure and Connectedness in the Blind Brain. Neural Plast. 2016;2016:6029241. doi: 10.1155/2016/6029241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wandell BA, Dumoulin SO, Brewer AA. Visual field maps in human cortex. Neuron. 2007;56:366–383. doi: 10.1016/j.neuron.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 21.Renier LA, Anurova I, De Volder AG, Carlson S, VanMeter J, Rauschecker JP. Preserved functional specialization for spatial processing in the middle occipital gyrus of the early blind. Neuron. 2010;68:138–148. doi: 10.1016/j.neuron.2010.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang J, Su J, Wang M, Zhao Y, Zhang QT, Yao Q, Lu H, Zhang H, Li GF, Wu YL, Liu YS, Liu FD, Zhuang MT, Shi YH, Hou TY, Zhao R, Qiao Y, Li J, Liu JR, Du X. The Posterior Insula Shows Disrupted Brain Functional Connectivity in Female Migraineurs Without Aura Based on Brainnetome Atlas. Sci Rep. 2017;7:16868. doi: 10.1038/s41598-017-17069-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song G, Qiu J, Li C, Li J, Gui S, Zhu H, Zhang Y. Alterations of regional homogeneity and functional connectivity in pituitary adenoma patients with visual impairment. Sci Rep. 2017;7:13074. doi: 10.1038/s41598-017-13214-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uddin LQ, Nomi JS, Hébert-Seropian B, Ghaziri J, Boucher O. Structure and Function of the Human Insula. J Clin Neurophysiol. 2017;34:300–306. doi: 10.1097/WNP.0000000000000377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stein MB, Simmons AN, Feinstein JS, Paulus MP. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. Am J Psychiatry. 2007;164:318–327. doi: 10.1176/ajp.2007.164.2.318. [DOI] [PubMed] [Google Scholar]
- 26.Dylgjeri S, Taussig D, Chipaux M, Lebas A, Fohlen M, Bulteau C, Ternier J, Ferrand-Sorbets S, Delalande O, Isnard J, Dorfmüller G. Insular and insulo-opercular epilepsy in childhood: an SEEG study. Seizure. 2014;23:300–308. doi: 10.1016/j.seizure.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 27.Seitz RJ, Nickel J, Azari NP. Functional modularity of the medial prefrontal cortex: involvement in human empathy. Neuropsychology. 2006;20:743–751. doi: 10.1037/0894-4105.20.6.743. [DOI] [PubMed] [Google Scholar]
- 28.Harenski CL, Hamann S. Neural correlates of regulating negative emotions related to moral violations. Neuroimage. 2006;30:313–324. doi: 10.1016/j.neuroimage.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 29.Myung W, Na KS, Ham BJ, Oh SJ, Ahn HW, Jung HY. Decreased medial frontal gyrus in patients with adjustment disorder. J Affect Disord. 2016;191:36–40. doi: 10.1016/j.jad.2015.11.028. [DOI] [PubMed] [Google Scholar]
- 30.Gao B, Wang Y, Liu W, Chen Z, Zhou H, Yang J, Cohen Z, Zhu Y, Zang Y. Spontaneous Activity Associated with Delusions of Schizophrenia in the Left Medial Superior Frontal Gyrus: A Resting-State fMRI Study. PLoS One. 2015;10:e0133766. doi: 10.1371/journal.pone.0133766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tops M, Boksem MA. A potential role of the inferior frontal gyrus and anterior insula in cognitive control, brain rhythms, and event-related potentials. Front Psychol. 2011;2:330. doi: 10.3389/fpsyg.2011.00330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quinn ME, Stange JP, Jenkins LM, Corwin S, DelDonno SR, Bessette KL, Welsh RC, Langenecker SA. Cognitive control and network disruption in remitted depression: a correlate of childhood adversity. Soc Cogn Affect Neurosci. 2018;13:1081–1090. doi: 10.1093/scan/nsy077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang LY, Li HJ, Huang X, Bao J, Sethi Z, Ye L, Yuan Q, Zhu PW, Jiang N, Gao GP, Shao Y. Assessment of synchronous neural activities revealed by regional homogeneity in individuals with acute eye pain: a resting-state functional magnetic resonance imaging study. J Pain Res. 2018;11:843–850. doi: 10.2147/JPR.S156634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shao Y, Li QH, Li B, Lin Q, Su T, Shi WQ, Zhu PW, Yuan Q, Shu YQ, He Y, Liu WF, Ye L. Altered brain activity in patients with strabismus and amblyopia detected by analysis of regional homogeneity: A restingstate functional magnetic resonance imaging study. Mol Med Rep. 2019;19:4832–4840. doi: 10.3892/mmr.2019.10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wen SM, Min YL, Yuan Q, Li B, Lin Q, Zhu PW, Shi WQ, Shu YQ, Shao Y, Zhou Q. Altered spontaneous brain activity in retinal vein occlusion as determined by regional homogeneity: a resting-state fMRI study. Acta Radiol. 2019;60:1695–1702. doi: 10.1177/0284185119845089. [DOI] [PubMed] [Google Scholar]
- 36.Xu MW, Liu HM, Tan G, Su T, Xiang CQ, Wu W, Li B, Lin Q, Xu XW, Min YL, Liu WF, Gao GP, Shao Y. Altered Regional Homogeneity in Patients With Corneal Ulcer: A Resting-State Functional MRI Study. Front Neurosci. 2019;13:743. doi: 10.3389/fnins.2019.00743. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.