Abstract
The blood–brain barrier is playing a critical role in controlling the influx and efflux of biological substances essential for the brain’s metabolic activity as well as neuronal function. Thus, the functional and structural integrity of the BBB is pivotal to maintain the homeostasis of the brain microenvironment. The different cells and structures contributing to developing this barrier are summarized along with the different functions that BBB plays at the brain–blood interface. We also explained the role of shear stress in maintaining BBB integrity. Furthermore, we elaborated on the clinical aspects that correlate between BBB disruption and different neurological and pathological conditions. Finally, we discussed several biomarkers that can help to assess the BBB permeability and integrity in-vitro or in-vivo and briefly explain their advantages and disadvantages.
Keywords: Blood–brain barrier, Tight junctions, Transcytosis, Disruption, Permeability, Markers, CNS, Neuroinflammation, Degenerative, TEER, Integrity
Background
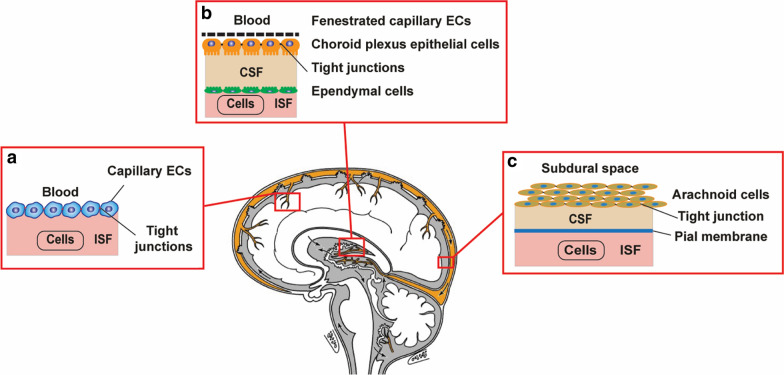
A highly controlled microenvironment is required to promote the normal functioning of the central nervous system (CNS). The existence of a biological barrier at the blood to brain interface effectively separating the brain from the rest of the body was established after the finding of Paul Ehrlich when he noticed that a peripherally infused dye did not stain the brain tissue. His finding was further supported by later observation from his associate Goldmann as he applied the same dye to the cerebrospinal fluid. It did stain only the brain tissue without extravasating in the periphery [1]. These biological barriers are established by different cells at three key interfaces: the blood–brain barrier (BBB), blood–CSF barrier (BCB), and the arachnoid barrier [2] (see Fig. 1).
Fig. 1.
Biological barriers are protecting the brain. a Blood–brain barrier; b blood–CSF barrier; c the arachnoid barrier
The BBB is formed by microvascular endothelial cells lining the cerebral capillaries penetrating the brain and spinal cord of most mammals and other organisms with a well-developed CNS [3]. It is considered the largest interface for blood–brain exchange as the combined surface area per average adult is assumed to fall between 12 and 18 m2 based on an average microvessels surface area of 150 and 200 cm2 per gram tissue [4]. The BBB is playing a critical role in protecting the brain parenchyma from blood-borne agents and providing a significant obstacle to the entry of drugs and other exogenous compounds into the central nervous system.
The second barrier is the blood–cerebrospinal fluid barrier (BCSFB), which is formed by the epithelial cells of the choroid plexus. The CSF secretion into the ventricular brain system is controlled by the choroid plexus epithelial cells [5]. In contrast, the interstitial fluid (ISF), which is consisting of the rest of the brain extracellular fluid, is derived at least partially by secretion across the capillary endothelium of the BBB [6–9]. ISF communicates freely with CSF over several locations, and its contribution to CSF is estimated between 10 and 60% [2].
Underlying the dura mater, we find the avascular arachnoid epithelium, which is considered the third barrier. The dura mater covers the CNS and completes the seal between the extracellular fluids of the central nervous system and the rest of the body [10]. Its contribution to the exchange between blood and brain is insignificant due to its avascular nature and the relatively small surface area compared to other barriers [11].
In this review, we will focus more on BBB as it is the main barrier contributing to CNS protection and maintaining the brain homeostasis. Unlike other vascular endothelial cells lining peripheral blood vessels, brain microvascular endothelial cells present distinctive morphological, structural, and functional characteristics that set them apart from other vascular endothelia. These include the following: (1) the expression of tight junctions (TJs), sealing the paracellular pathways between adjacent endothelial cells, thus preventing the unregulated passage of polar (water-soluble) molecules between the blood and the brain; (2) the absence of fenestrations; (3) the lack of pinocytic activity and the expression of active transport mechanisms to regulate the passage of essential molecule (including nutrients and essential amino acids) while blocking the passage of potentially undesired substance (both endogenous and xenobiotics) [12]. The transport barrier comprehends various efflux transporters, such as P-glycoprotein (P-gp) [13], breast cancer resistance protein (BCRP) [14], organic anion transporting polypeptide (OATP) [15]. These efflux systems may also share overlapping substrate affinities (such as P-gp and BCRP—[16]) actively pump compounds (including xenobiotics) out of the endothelial cells back into the blood circulation [17], resulting in reduced CNS exposure. Lastly, the presence of drug-metabolizing enzymes within the brain endothelial cells, such as CYP 450 enzymes (CYP1B1 and CYP2U1, CYP-3AF), results in the formation of the metabolic barrier [18–21]. CYP mRNAs have been found in several areas of the human brain which plays a role in neurodegenerative disease by metabolizing drugs and fatty acids [20]. For instance, CYP2U1 metabolizes fatty acids like arachidonic CYP1B1 has a role in the metabolism of endogenous compounds in the CNS [19]. CYP3A4 also showed to be functionally expressed at drug resistance of epileptic BBB and oxidizes a large group of xenobiotics, including antiepileptic drugs [22].
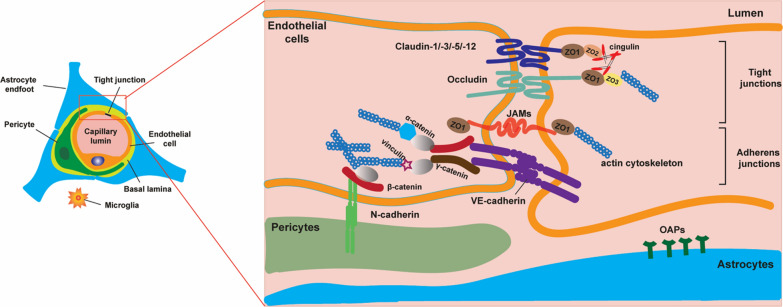
These integrated barrier systems, while protecting the brain from potentially harmful compounds, are also the major obstacle for delivering drugs into the CNS [23]. Overall, the BBB function as a dynamic interface regulating the brain homeostasis and protecting the CNS, which can respond to different physiological and pathological conditions [10]. The Induction and maintenance of the barrier function fall primarily on the interaction between the microvascular endothelium, the astrocytic foot processes (which invest close to 99% of the abluminal surface area of the brain capillary), and pericytes [24] (see also Fig. 2).
Fig. 2.
Cells association at- and molecular organization of the neurovascular unit (NVU)
In addition to microvascular endothelial cells, the capillary basement membrane (BM), astrocytes, pericytes (PCs) embedded within the BM, microglial and neuronal cells complete the structure of what we now refer to as the neurovascular unit (NVU) [25]. Below we provide a brief description of these cells and their role in maintaining the integrity of the BBB.
Different cells of the NVU
Endothelial cells
The BBB endothelial cells (ECs) in the mature mammalian brain are characterized by different features, which make them phenotypically different from other ECs located at different parts of the body. ECs are characterized by a flattened appearance, the expression of inter-endothelial tight junctions, the presence of very few caveolae at the luminal surface, and a high number of mitochondria [26] when compared to ECs from other vascular districts. During embryonic angiogenesis, the differentiation of the endothelium into a functioning barrier layer begins and is maintained during adulthood by a close inductive association with other cell types within the NVU, as we mentioned early [2].
The paracellular flux of hydrophilic molecules across the BBB endothelium is hindered by the tight junctions sealing the paracellular pathways between adjacent endothelial cells. The TJs also provide a fence around the cell, separating its luminal portion from the basolateral region [1]. Across the endothelium, there is rapid free diffusion of oxygen from the blood to the brain and carbon dioxide diffusion in the opposite direction, which is essential for normal brain metabolism and regulation of pH in the brain ISF, neurons, and other NVU cells. Besides, small lipophilic molecules, with a molecular weight (MW) < 400 Da forming < 8 hydrogen bonds, can cross the BBB [27]. Glucose, amino acids, and other nutrients enter the brain via carrier-mediated transporters. In contrast, the uptake of larger molecules such as insulin, leptin, and iron transferrin are facilitated via receptor-mediated endocytosis [28, 29].
Pericytes
Pericytes are essential constituents of the brain capillary with different frequencies in different vascular beds. They are most abundant in the CNS, particularly in the retina [1]. They share a basement membrane with endothelial cells and form direct synaptic-like peg-socket focal contacts with endothelium through N-cadherin and connexins [30] (see also Fig. 2). They can be visualized immunohistochemically by using antibodies against smooth muscle actin, desmin, platelet-derived growth factor b-receptor (PDGFR-b), aminopeptidase N, or the regulator of G-protein signalling-5 (RGS5) [31–33].
Their close association with ECs allows the exchange of ions, metabolites, second messengers, and ribonucleic acids between the two cell types [30]. Pericytes also play essential roles in maintaining BBB integrity, aiding in angiogenesis, and microvascular stability [30, 34]. Pericytes, which also feature contractile characteristics similar to smooth muscle cells, can regulate (to some extent) the capillary diameter and the cerebral blood flow (CBF) [35, 36]. Furthermore, pericytes may display phagocyting functions helping with the removal of toxic metabolites [37]. They have also been reported to have multipotent stem cell capabilities [38]. Studies have shown that PCs express receptors for vascular mediators, such as catecholamines [39], angiotensin I [40], vasoactive intestinal peptides [41], endothelin-1 [42], and vasopressin [43]. These data strongly suggest that PCs play an essential role in cerebral autoregulation.
Besides, in vitro studies [44] have shown that ECs associated with PCs are more resistant to apoptosis than isolated endothelial cells, further supporting the role of PCs in supporting the structural integrity and genesis of the BBB. The loss of PCs and the formation of microaneurysm in PDGF-B deficient mice suggest that PCs play an essential role in the maintenance of vascular wall stability [45]. These data are noteworthy from a clinical standpoint since the degeneration and injury of PCs have been reported in many neurological diseases, including Alzheimer’s disease (AD) [46–49], mild dementia [50], amyotrophic lateral sclerosis (ALS) [34], and stroke [35].
Astrocytes
ACs represent the most abundant cells in CNS and are involved in different physiological and biochemical tasks. These latter include (1) compartmentalization of the neural parenchyma; (2) maintenance of the ionic homeostasis of the extracellular space; (3) pH regulation; (4) neurotransmitter uptake and processing by providing energy-rich substrates to the neurons; (5) mediation of signals from neurons to the vasculature [51].
In particular, ACs are believed to play a decisive role in maintaining the barrier function of the brain microcapillary ECs and in controlling the CBF [2]. The astrocytic foot processes establish the direct interface between the vascular compartment and the neuroglial in the NVU. Recent literature stressed the role of astrocytic endfeet as key checkpoints of brain metabolism [52]. At the contact interface between astroglial endfeet and the superficial or perivascular basal lamina, there is a great density of intramembranous organic anion transporters (OAPs). OAPs’ density decreases where the glial cell membrane loses contact with the basal lamina [53]. This polar heterogeneity of astrocytic membrane domains represents one of the most impressive morphological features of mammalian and avian ACs, which seems to be correlated with the BBB maturation during development [1].
The contribution of different NVU cells in developing and maintaining the BBB have supported by several grafting and cell culture studies. When avascular tissue brain graft from a 3-day old quail was transplanted into the coelomic cavity of chick embryos, the endothelial cells vascularizing the graft formed a competent BBB [54]. In contrast, transplanting avascular embryonic quail coelomic graft into embryonic chick brain resulted in leaky capillaries and venules within the mesenchymal tissue of the graft. When cultured astrocytes were introduced into areas with normal leaky vessels, they induced tightening of the endothelium [55]. Another study showed that the optimal generation of BBB necessitates direct contact between endothelial cells and astrocytes [56].
The induction of BBB characteristics in ECs may be attributed not only to the NVU cells, but their cell-derived soluble factors as human or bovine endothelial cell monolayers showed a higher transendothelial resistance when being cultured in astrocyte-conditioned media [57]. Astrocytes participate in the dynamic regulations of the neural system and play a vital role in CNS inflammation in neurodegenerative diseases [58].
Tight junctions
Tight and adherens junction (AJs) constitutes the junction complex of the BBB. The TJs are present at the sites of fusion involving the outer surface of the plasma membrane of adjacent endothelial cells or the same cell (see Fig. 2). Adherens junctions are composed of a cadherin–catenin complex and its associated proteins. The TJs consists of three integral membrane proteins, namely, claudin, occludin, and junction adhesion molecules (JAMS), and several cytoplasmic accessory proteins including Zonula occludens-1, -2, -3 (ZO-1, ZO-2, ZO-3), cingulin, and others (see Fig. 2). The cytoplasmic proteins link membrane proteins to actin, which is the primary cytoskeleton protein for the maintenance of structural and functional integrity of the endothelium.
After early detection of the cytoplasmic accessory proteins, intramembrane junctional proteins were found to be directly contributing to the restricted interendothelial permeability. Occludin, a protein with four transmembrane domains, was the first discovered protein, which seemed to play a less important role in permeability restriction as occludin-deficient mouse mutant is viable and has intact biological barriers. The discovery of claudins, another four transmembrane domains protein with no homology to occludin, was of fundamental importance for TJs research. Also, JAMs and the endothelial selective adhesion molecule (ESAM), which are members of the immunoglobulin superfamily, are considered TJs components, which probably fulfill regulatory functions of the barrier [59].
Tight junction formation appears to be a very early feature of BBB development, and a barrier to the free movement of proteins and macromolecules is formed at the primary stages of brain development [2].
Claudins
Claudins-1 and -2 are 22 kDa phosphoproteins with four transmembrane domains. These claudins were identified as an integral component of TJs strands [60]. Claudins are the major components of TJs, where they bind homotypically to claudins on adjacent endothelial cells to form the primary seal of the TJ [61]. Claudins are linked to cytoplasmic proteins, including ZO-1, ZO-2, and ZO-3, through their carboxy-terminal [61]. In the brain, claudins-1 and -5, besides occludin, are the major components of endothelial TJs forming the BBB [62, 63]. In vivo and in vitro studies showed the loss of claudin-1 from cerebral vessels and ECs under pathologic conditions such as cancer, stroke, inflammation [62, 64, 65]. Also, the selective loss of claudin-3 or occludin was observed in experimental allergic encephalomyelitis (EAE) and glioblastoma multiform (GBM) resulting in a loss in BBB integrity along with some functional barrier loss [66]. The disappearance of claudin-5 from the tight junctional complexes can result in a compromised BBB as mice genetically altered to lack claudin-5 have shown severely compromised and leaky BBB and die shortly after birth [67], although their death is probably not solely related to the BBB defects [2]. Double knockdown of claudin-5 with occludin also increased the permeation of tracers between 3 and 10 kDa [68]. Furthermore, knock-down of claudin-5 was found to change the mRNA expression of other TJ proteins such as claudin-1 [69]. Previous studies have been shown that claudin-5, not only regulates paracellular ionic selectivity but also played a role in the regulation of the endothelial permeability in several pathological processes containing inflammation, trauma, toxic damage, and tumor cell motility [70, 71]. For instance, Persidsky et al. [72] showed that the downregulation of TJ proteins, claudin-5, and occludin, resulting in decreased barrier tightness and enhanced monocyte migration across the BBB during human immunodeficiency virus-1 (HIV-1) encephalitis.
Recently, the claudin-11 gene and protein were identified in brain endothelial cells and capillaries. It was originally recognized as oligodendrocyte-specific protein [73] and later named to the claudin protein family [69]. The partial overlap of Claudin-11 with claudin-5 was observed in the capillaries of the human brain section. It has been shown that claudin-11 plays a role in paracellular tightness by homophilic oligomerization [69]. Moreover, Uchida et al. [74] showed that claudin -11 was found to be significantly decreased in the brain and spinal cords capillaries of patients with MS and experimental autoimmune encephalomyelitis (EAE) mice.
Occludin
Occludin, a 65-kDa phosphoprotein, was first discovered by immunogold freeze-fracture microscopy in chickens [75] and then in mammals [76]. Also, the expression of occludin has been reported in rodents [77] and adult human brain [78] but not in the human newborn and fetal brain. Occludin is significantly larger than claudins and shows no amino acid sequence similarity with them. It has four transmembrane domains-as claudins, a long COOH-terminal cytoplasmic domain, and a short NH2-terminal cytoplasmic domain. The two extracellular loops of occludin and claudin originating from neighboring cells form the paracellular barrier of TJs while the cytoplasmic domain of occludin is directly associated with ZO proteins. Occludin is highly expressed in brain ECs compared to nonneural tissues and seems to be a regulatory protein that has a crucial role in paracellular permeability [77].
Occludins and claudins assemble into heteropolymers to form intramembranous strands, which have been proposed to contain fluctuating channels allowing the selective diffusion of ions and hydrophilic molecules [79]. Breakdown of the BBB in the tissue surrounding brain tumors occurs with concomitant loss of a 55-kDa occludin expression [78]. Claudins and occludins form the extracellular component of TJs and are both essential for the formation of the BBB [80].
Junctional adhesion molecules
Junctional adhesion molecules are recently identified 40 kDa proteins that belong to the immunoglobulin superfamily [81]. They have a single transmembrane domain, and their extracellular portion presents two immunoglobulins like loops that are formed by disulfide bonds. Studies in rodent brain sections showed that JAM-1 and JAM-3 are expressed in the brain blood vessels but not JAM-2 [82]. Studies also showed their role in cell-to-cell adhesion and monocyte transmigration across BBB [82, 83]. However, more investigations are required to unveil its function in the BBB.
Cytoplasmic accessory proteins
Zonula occludens proteins (ZO-1, ZO-2, and ZO-3), cingulin, and several other proteins are essential for TJs functioning as they form the cytoplasmic bridge connecting the TJs to the cell cytoskeleton. ZO-1 (220 kDa), ZO-2 (160 kDa), and ZO-3 (130 kDa) have sequence similarity with each other and belong to the family of proteins known as membrane-associated guanylate kinase-like protein (MAGUKs). They contain three PDZ domains (PDZ1, PDZ2, and PDZ3), one SH3 domain, and one guanyl kinase-like (GUK) domain. These domains function as protein-binding molecules and thus play a role in organizing proteins at the plasma membrane. The PDZ1 domain of ZO-1, ZO-2, and ZO-3 has been reported to bind directly to the COOH-terminal of claudins [84]. For Occludin, the GUK domain is the site of interaction with ZO-1 [85]. Recently, studies show the ability of JAM to bind directly to ZO-1 and other PDZ-containing proteins [86]. Importantly, actin, the primary cytoskeleton protein, binds to the COOH-terminal of ZO-1 and ZO-2, which stabilizes transmembrane elements and provides structural support to the endothelial cells [87].
Adherens junctions
Cadherin is the main component of these junctional membrane proteins, which joins the actin cytoskeleton through intermediary proteins, catenins, to form adhesive contacts between cells. AJs assemble via homophilic interactions between the extracellular domains of calcium-dependent cadherin on the surface of adjacent cells. The submembrane proteins β- or γ-catenin connects the cytoplasmic domains of cadherins to the actin cytoskeleton via α-catenin (see Fig. 2). AJs components, including cadherin, alpha-actinin, and vinculin (α-catenin analog), have been demonstrated in intact microvessels of the BBB in rats. TJs and AJs components are known to interact, particularly ZO-1 and catenins, and influence TJs assembly [79].
The intracellular scaffold proteins ZO-1, ZO-2, and ZO-3, which link the junctional molecules claudin and occludin via cingulin to intracellular actin and the cytoskeleton appear to play a crucial role in the efficiency of the TJs [52, 59, 88]. Studies show that changes in calcium concentration both intracellular and extracellular can have a great impact on tight junction assembly and efficiency as a barrier and can alter the electrical resistance across the cell layer [10, 89]. As mentioned early, soluble factors released by many of the cell types associated with brain microvessels (including microglia and astrocytes) as well as nerve terminals adjacent to the endothelial extracellular matrix/basal lamina (such as vasoactive agents and cytokines) can modify tight junction assembly and barrier permeability [10, 90].
Hemodynamic modulatory functions in BBB physiology: role of shear stress
Mammalian endothelial cells are known to undergo dynamic changes under different stimuli. Cellular, molecular, and physical stimuli are the essential players that help ECs to acquire specialized functions. For most cell types, chemical signaling seems to play a pivotal role in cell physiology. Physical stimulation, such as shear stress, is one of the most important but underestimated physiological stimuli, which contributes to vascular ECs differentiation and maturation besides other cellular and molecular signaling. Akin to responses to inflammatory cytokines, shear stress has been shown to cause dramatic changes in ECs morphology [91], gene expression [92], and function [93]. Shear stress showed to induce the production of reactive oxygen species (ROS) and nitric oxide (NO). NO is known to cause vasodilation, autocrine signaling, and also increase the production of free radicals. A study showed that ECs might scavenge free radicals by increasing levels of GAPDH and other intracellular enzymes [94]. Another study showed that NO might have a protective role in BBB during reperfusion after the transient loss of flow in a condition mimicking ischemic stroke [95].
Shear stress was shown to induce a significant upregulation of tight and adherens junction proteins and genes in human brain microvascular endothelial cells (HBMECs). The study reported a 5.91- and 2.13-fold increase in claudin-5 and cadherin-5 gene expression, respectively. HBMECs showed higher maturation and differentiation under shear stress, as indicated by the increase in the trans-endothelial electrical resistance (TEER) from 100 to 700 Ω cm2. Also, SS induced the endothelial expression of different drug transporters and metabolic properties that allow the BBB to protect the CNS from harmful substances [96]. In contrast, a study on iPSCs-HBMECs d didn't show any significant changes in tight junctions, TEER, or cell morphology. These discrepancies highlight the importance of further studies to optimize culture models based on non-primary BBB ECs to better reflect the physiological responses of the BBB in vivo [97].
Akin to cytokines effect, shear stress has been shown to play a critical role in maintaining blood vessels’ homeostasis and a protective role. A study in HBMECs showed that shear stress was a much more potent stimulus for thrombomodulin (TM) release, yielding media TM levels of 1000 pg/105 cells when compared to 175 and 210 pg/105 cells for, tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), respectively. Thrombomodulin is an integral membrane receptor constitutively expressed on the luminal surface of vascular ECs and an essential determinant of blood vessel homeostasis. The study also showed that shear-conditioned media was able to completely block thrombin-induced permeabilization of HBMECs, which confirm the protective role of shear stress [98]. Overall, BBB studies must consider the effect of shear stress as it is playing an essential role in BBB maturation and homeostasis. In this respect, our group has summarized the advances in in-vitro BBB models [99, 100].
Physiological functions of the BBB at the blood–brain interface
Maintain ionic homeostasis and brain nutrition
The BBB provides a controlled microenvironment via a combination of specific ion channels and transporters, which keep the ionic composition optimal for neural and synaptic signaling functions. For instance, the levels of potassium in CSF and ISF is maintained at ~ 2.5–2.9 mM. In comparison, plasma concentration is approximately 4.5 mM, despite fluctuations that can occur in potassium plasma levels following exercise or a meal, imposed experimentally, or resulting from pathology [101, 102]. Other ions such as calcium and magnesium and pH are also actively regulated at the BBB and BCSFB [103, 104]. Calcium and potassium homeostasis controls neuronal excitability but is also essential for the transmigration of macrophage across the BBB [105]. Furthermore, Ca2+ is involved in the modulation of BBB integrity and endothelial morphology [106].
Specific ion channels and transporters at BBB provides the optimal preservation environment for synaptic and neural activity. As an example, the abluminal sodium pump (the Na+, K+-ATPase) maintains a high concentration of Na+ and low levels of K+ in brain ISF via transporting Na + into the brain and K + out of the brain. Or the luminal Na+, K+, Cl− cotransporter facilitate the transfer of Na+, K+, 2Cl− from blood to the endothelium. Calcium transporters (the Na+–Ca2+ exchanger) and voltage-gated K+ channel also regulate the ion transport across the BBB [107, 108].
For essential water-soluble nutrients and metabolites required by nervous tissue, the BBB allows for low passive permeability. In contrast, for other nutrients that cannot pass, there are specific transport systems expressed in the BBB to ensure an adequate supply of these substances. The selective and region-specific (luminal and abluminal surfaces of the ECs) expression of these transporters confers the normal polarity of the BBB endothelium [10, 52]. The differentiation of the endothelium into a barrier layer begins during embryonic angiogenesis and in the adult is primarily maintained by a close inductive association with several cell types, especially the endfeet of astrocytic glial cells.
Regulate levels of neurotransmitters
The central and peripheral nervous systems share many of the same neurotransmitters, so the BBB helps to keep the central and peripheral transmitter pools separate, minimizing ‘crosstalk’ and protecting the brain from unexpected changes in their plasma levels. For example, blood plasma contains high levels of the neuroexcitatory amino acid glutamate, which fluctuate significantly after the ingestion of food. High levels of glutamate in the brain ISF will have harmful effects on neuronal tissues. An example is a case of glutamate secretion from hypoxic neurons during ischemic stroke, which results in considerable and permanent neurotoxic/neuroexcitatory damage to neural tissue [10, 109].
The transfer of neurotransmitters from the brain to blood primarily dependent on Na+-coupled and Na+-independent amino acid transporters. The BBB limits the influx of some amino acids including the neurotransmitters glutamate and glycine, while it effluxes many other essential amino acids. Hladky and Barrand reviewed comprehensively the transport of amino acids across the BBB with different transport systems based on the type of amino acids [108, 110].
Limit plasma macromolecules leak into the brain
The production of CSF from plasma, under normal condition, passed through an efficient filtration process in the choroid plexus to remove unneeded plasma proteins. This process helps in controlling the protein content of CSF and results in minimal quantities of proteins in CSF compared to the plasma protein levels [2].
Under physiologic conditions, the BBB prevents many macromolecules from entering the brain through normal paracellular or diffusion routes. The leakage of these large molecular weight serum proteins into the brain across a damaged BBB can have severe pathological consequences. For example, the leakage of plasma proteins such as albumin, prothrombin, and plasminogen has a detrimental effect on nervous tissue, causing cellular activation, which can lead to apoptosis [111, 112]. There is a wide distribution of different activators for these proteins within the CNS. These include factor Xa, which converts prothrombin to thrombin, or tissue plasminogen activator, which converts plasminogen to plasmin. The resulting proteins, thrombin or plasmin, can bind to their receptors in brain tissue and initiate cascades resulting in seizures, glial activation, glial cell division and scarring, and cell death [113]. Thus, the BBB works as a “gatekeeper,” allowing the entry of only the beneficial materials.
Protect the brain against neurotoxins
Many potential neurotoxins are circulating in our blood, including those from endogenous sources such as metabolites or proteins, or exogenous ones such as xenobiotics ingested in the diet or otherwise acquired from the environment. The BBB function is to regulate the entry of different circulating substances based on CNS needs. The transport barrier represented by multiple ABC energy-dependent efflux transporters (ATP-binding cassette transporters) occupies the BBB luminal surface. It actively pumps many of these agents out of the brain [2]. The adult CNS has a limited regenerative capacity if damaged, and fully differentiated neurons have a minimal ability to divide and replace themselves under normal circumstances. There is a continuous steady rate of neuronal cell death from birth throughout life in the healthy human brain, with relatively low levels of neurogenesis [114]. That is why any factor promoting an acceleration of the natural rate of cell death (e.g., increased access of neurotoxins into the brain) would become prematurely debilitating.
Transport across the BBB
Passive diffusion
In general, a wide range of lipid-soluble molecules can diffuse passively through the BBB and enter the brain [115]. The lipid solubility of a drug is determined by calculating its logD (octanol/water) partition coefficient at pH 7.4. There is a general correlation between the rate at which a solute enters the CNS and its lipid solubility [116]. In contrast with logP, which only considers the partitioning of unionized species, logD includes unionized and ionized species present in solution [116]. Molecular weight is another crucial factor in determining the free diffusion of small molecules across the BBB. Once the MW is > 400 Da, the BBB permeability of the drug does not increase in proportion to lipid solubility [117]. Lipid soluble small molecular compounds are believed to cross the BBB via transitory formation of pores within the phospholipid bilayer that is created as the free fatty acyl side-chains kink in the process of normal molecular motion within the phospholipid bilayer [118, 119]. As the pores are of finite size, they restrict the movement of small molecules that have a spherical volume larger than the pore volume. An increase of the surface area of a drug from 52 A2 (e.g., a drug with a MW of 200 Da) to 105 A2 (e.g., a drug with a MW of 450 Da) dramatically decrease its BBB permeation [117].
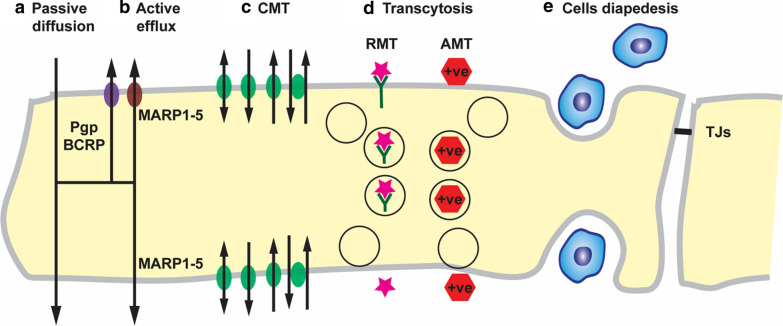
Also, a high polar surface area (PSA) greater than 80 Å2, and a tendency to form more than six hydrogen bonds are considered a limiting factor for the entry of compounds into the CNS. As a general rule, the BBB permeability of a drug decreases 1 log order in magnitude for each pair of H-bonds added to the molecule in the form of polar functional groups [120]. The number of H-bonds that drug forms with water can be calculated by inspecting the chemical structure [121]. Once the number of H-bonds is greater than eight, it is unlikely that the drug crosses the BBB via lipid-mediated free diffusion in therapeutically relevant amounts. Studies showed that the negative effect of H bonds on drug permeability might be attributed to the significant increase in the free energy required for moving the drug from an aqueous phase into the lipid of the cell membrane [116, 122]. The presence of rotatable bonds in the molecule and a high affinity of binding to plasma proteins with a low off-rate can also significantly reduce CNS penetration. It is no assured that drug meeting all the above criteria will be able to cross the BBB and give its action as it may increase the likelihood of becoming a substrate for active efflux transporter [123, 124]. A common misconception is that small molecules readily cross the BBB. However, > 98% of all small molecules do not cross the BBB either. There are > 7000 drugs in the Comprehensive Medicinal Chemistry (CMC) database, and only 5% of these drugs treat the CNS [125]. Figure 3 represents the different transport mechanisms across the BBB.
Fig. 3.
Different methods of transport across the BBB. CMT carrier mediated transport, RMT receptor-mediated transport, AMT adsorptive mediated transport
Active efflux
Several ATP-binding cassette (ABC) proteins are expressed on the luminal, blood-facing endothelial plasma membrane of the BBB. They are ATP-driven efflux pumps for xenobiotics and endogenous metabolites, which limit the permeability of multiple toxins, including therapeutic agents [126]. The pharmacoresistant characteristics of the CNS are attributed to their high expression. Decreased expression and/or functional activity of ABC BBB transporters were reported in different pathological conditions such as in patients with Alzheimer’s disease (AD) and Parkinson’s disease (PD) [25]. In an animal model of AD, ABC transporters are affected, which leads to the accumulation of amyloid β-peptide (Aβ) in the brain [127].
The primary efflux transporters at the BBB are the P-glycoprotein (Pgp—Multidrug Resistance Protein ABCB1), the Multidrug Resistance-associated Proteins (MRPs, ABCC1, 2, 4, 5 and possibly 3 and 6), and Breast Cancer Resistance Protein (BRCP, ABCG2). Pgp and BCRP are highly expressed in the luminal membrane of the BBB and are responsible for transporting substrate from endothelium to blood; recent studies report some cooperativity of action [127] and substrate overlap [16]. On the other hand, MRP isoforms appear to be expressed in either the luminal or the abluminal membrane [128]. As they favor water-soluble conjugates as substrates, a bi-directional efflux from the endothelium may be predicted, as conjugation by drug transforming enzymes will render them less cytotoxic.
While the brain ECs are considered the primary barrier interface, the transport activity of both pericytes [129] and perivascular astrocytic endfeet [52] may contribute to the barrier function, and may act as a ‘second line of defense’ if the primary barrier is breached or dysfunctional.
Carrier-mediated transport (CMT)
The BBB isolates the brain and limits the diffusion of many essential polar nutrients, including glucose and amino acids, which are essential for metabolism. Therefore, other routes for the essential nutrients to reach the brain are necessary. CMTs are encoded genes within the Solute Carrier (SLC) Transporter Gene Family. This includes more than 300 transporter genes encoding membrane-bound proteins that facilitate the transport of a wide array of substrates across biological membranes [130]. The SLC transporters facilitate the transcellular movement of a variety of molecules. These include amino acids, carbohydrates, monocarboxylic acids, fatty acids, hormones, nucleotides, organic anions, amines, choline, and vitamins.
Preferential distribution of these transporters over both sides of BBB confer the characteristic polarized behavior of BBB as some of these transport proteins are expressed on either the luminal or abluminal membrane only. In contrast, others are inserted into both membranes of the ECs [128, 131–133]. The orientation of these transporters may, therefore, result in preferential transport of substrates into or across the endothelial cell, and the direction of the transport may be from blood to brain or vice versa.
The tight junctions preserve the polarity of the BBB as they segregate transport proteins and lipid rafts to either the luminal or abluminal membrane domain and prevent their free movement from one side of the endothelium to the other [2].
Receptor-mediated transport (RMT)
The presence of peptide bonds limits the larger peptides and proteins from using the amino acid CMT systems to cross the BBB [134]. However, specific neuroactive peptides [135], regulatory proteins, hormones, and growth factors get the use of RMT systems to cross the BBB [136]. These Large molecular weight solutes can enter the CNS intact via endocytotic mechanisms in a process named transcytosis. Although most large blood-borne molecules are physically prevented from entering the brain by the presence of the BBB and TJs, specific and some non-specific transcytotic mechanisms exist to transport a variety of large molecules and complexes across the BBB.
There are two types of vesicular transport systems; one is based on receptor-mediated transcytosis (RMT) and the other on adsorptive-mediated transcytosis (AMT). In RMT, macromolecular binds to ligands specific receptors on the cell surface, which triggers an endocytotic event. Both receptors and their bound ligand cluster together, and a caveola are formed, which pinches off into a vesicle. Both ligand and receptors are internalized into the ECs and directed across the cytoplasm to be exocytosed at the opposite side of the cell [2]. Finally, the ligand and receptor dissociate during cellular transit or the exocytotic event (see also Fig. 3). While in AMT, positively charged large molecules interact with specific cell surface binding sites that induce endocytosis and subsequent transcytosis [137].
The lysosomal compartment within the cell needs to be avoided to achieve transcytosis of an intact protein or peptide. This is achieved by directing the primary sorting endosome and its contents away from this degradative lysosomal compartment. Lysosome escaping mechanisms appear to be a specific feature of the BBB endothelium not observed in many peripheral ECs [4]. Another specific feature of BBB endothelium is the presence of relatively few endocytotic vesicles in the cytoplasm of these cells compared to other endothelia, as observed in most electron microscopic studies [138]. However, there is a weak correlation between the protein permeability of a microvessel and the observable endocytotic activity [139]. Brain capillary endothelial cells are very thin, with the luminal and abluminal membranes being only separated by ~ 500 nm (5000 Å) or less. Caveolae are 50–80 nm in diameter, and thus the events of transcytosis may be difficult to capture within the cell using conventional electron microscopical techniques [2].
The RMT receptors may mediate functionally different processes, including (1) transcytosis of the ligand from blood to brain as in the case of insulin and transferrin [140, 141]; (2) reverse transcytosis from the brain to blood such as the transcytosis of IgG via Fc receptor (FcR) [142]; or (3) only endocytosis into the brain capillary endothelium without net transport across the endothelial cells. This latter includes scavenger proteins that facilitate the uptake of acetyl Low-Density Lipoprotein (LDL) from the blood into the BBB endothelium [143] and might be the reason why proteins targeting LDL related receptor type 1 are taken up by the brain at reduced rates compared with proteins that target other transcytosis receptor systems. Studies show that the brain uptake of melanotransferrin (p97) or angiopep-2, ligands that target lipoprotein receptor-related protein 1 (LRP1), is only 0.2% to 0.3% of injected dose (ID)/g in the mouse [144]. This is tenfold lower than the brain uptake of a monoclonal antibody (MAb) that targets the BBB transferrin receptor in the mouse [145].
The fascinating ability of RMT to transport intact large molecules peptides and proteins excite researchers to get the advantage of these receptors in drug delivery in what is called Trojan horses. Molecular Trojan horses are genetically engineered proteins that cross the blood–brain barrier (BBB) via endogenous RMT processes. They allow for the non-invasive delivery of large molecule therapeutics to the human brain [146]. Certain peptidomimetic monoclonal antibodies undergo RMT across the BBB in vivo. The receptor-specific mAb binds to the endogenous BBB peptide receptor at a site that is spatially distant from the endogenous ligand-binding site and carried across the BBB on the endogenous peptide RMT system. One of the most potent BBB molecular Trojan horses is a mAb for the human insulin receptor (HIR), which is active at both the BBB of humans and Old-World primates such as Rhesus monkeys [147]. Besides, molecular Trojan horses targeting the transferrin receptors (TRF) in rats and mice, which showed excellent efficiency. However, these receptor-specific mAbs are species-specific, which suggests that molecular Trojan horses that are used in preclinical research may require further development and optimization to be successfully used as human therapeutics. More efforts need to be directed toward the development of BBB drug targeting technology using molecular Trojan horses as it seems to be a promising technique [148].
Major facilitator superfamily
Essential omega-3 fatty acids, such as docosahexaenoic acid (DHA), are transported into the brain by the endothelial facilitator superfamily domain-containing protein 2a (MFSD2a) [149]. This family of transporters may have dual function as recent studies showed its importance in maintaining BBB integrity as mice lacking MFSD2a show brain DHA deficits and compromised BBB [150, 151].
Immune cell movement across the BBB
The CNS is considered as an immune-privileged site as a result of the low infiltration of neutrophils into the brain compared to other tissues and the strictly regulated immune cell-BBB interaction. Under normal physiological conditions, mononuclear cells enter the brain during embryonic development and become resident immunologically competent microglia [152]. They penetrate by process of diapedesis directly through the cytoplasm of the endothelial cells and not via a paracellular route involving re-arrangement and opening of the tight junctional complexes as had been previously suggested [153]. However, in inflammatory pathological conditions, the TJs between endothelial cells may be disrupted. This is the result of cytokines and other pro-inflammatory agents. Furthermore, mononuclear leukocytes, monocytes, and macrophages can enter the CNS via transcellular and paracellular routes and play roles complementary to those of the resident microglia [154, 155]. In some cases, these immune cells may transform into a microglial phenotype [2].
The transmission of immune cells across the BBB is a dynamic process carried out through a sequence of steps including tethering, rolling, crawling, arrest, and diapedesis across the ECs [156]. Multiple studies showed that during inflammation, ECs upregulate expression of vascular cell adhesion molecule 1 (VCAM-1) and intercellular adhesion molecule 1 (ICAM1) which resulted in arresting of CD4+ T cells onto the inflamed CNS vessels or primary brain EC monolayers through the interaction between αLβ2 [lymphocyte function-associated antigen 1 (LFA-1)], and α4β1 [very late antigen 4 (VLA4)] integrin of CD4+ T cells with ICAM-1 and VCAM-1 [156]. Moreover, it has been shown that the polarization and crawling of CD4+ T cells onto the inflamed vessel happened through the interactions of LFA-1-ICAM-1 [156].
Other studies indicated that the transmigration of CD4+ and CD8+ T cells across the CNS autoimmunity could be controlled through additional cell adhesion molecules such as melanoma cell adhesion molecule (MCAM) and activated leukocyte cell adhesion molecule (ALCAM) [157, 158].
Furthermore, it was demonstrated that tight junction and adhesion molecules including claudin-5, VE-Cadherin, JAM, PECAM-1, and CD99 played important roles in the paracellular migration of cell across the BBB [159]. Winger et al. [160] indicated that blocking CD99 in ameliorated EAE mice, diminished the accumulation of CNS inflammatory infiltrates, including dendritic cells, B-cells, and CD4+ and CD8+ T-cells. Administration of anti-CD99 was effective at the initiation of the disease symptoms and also blocked relapse when administered therapeutically after the recurrence of the symptoms [160].
BBB disruption in different pathological conditions
BBB dysfunction is reported in many CNS pathological conditions including multiple sclerosis [161]; hypoxic and ischemic insult [162]; Parkinson’s and Alzheimer’s disease [163]; epilepsy [164]; brain tumors [165]; glaucoma [166], and lysosomal storage diseases [167]. The observed barrier dysfunction can range from mild and transient changes in BBB permeability, resulting from tight junction opening, to chronic barrier breakdown, and changes in transport systems and enzymes can also occur. This process can also be associated with the degradation of the basement membrane [168]. Microglial activation and infiltration of different plasma components and immune cells into the brain parenchyma result in disturbance of CNS homeostasis and variable damage to the surrounding brain. In most cases, it is not possible to determine whether barrier compromise is causal in disease onset or a result of neurological disease progression. Still, barrier disturbance can often be seen to contribute to and exacerbate developing pathology [169].
As discussed earlier, under normal conditions, the BBB is relatively impermeable. In pathologic conditions, several vasoactive agents, cytokines, and chemical mediators are released that increase BBB permeability. Several in vitro and in vivo studies showed the opening effect on BBB of several mediators include glutamate, aspartate, taurine, ATP, endothelin-1, NO, TNF-α, and macrophage-inflammatory protein 2 (MIP2) which are produced by astrocytes [132, 170, 171]. Other humoral agents reported to increase BBB permeability are bradykinin, 5HT, histamine, thrombin, UTP, UMP, substance P, quinolinic acid, platelet-activating factor, and free radicals [132, 172, 173]. They are variable in the source as some of these agents are released by endothelium and have an autocrine effect on endothelium itself. For example, endothelin (ET-1) acts on Endothelin A (ETA) receptors in ECs. In physiologic conditions, chemical mediators released by nerve terminals of neurons associated with blood vessels such as histamine, substance P, and glutamate, influence and regulate BBB permeability. Table 1 summarizes different pathological conditions that are affecting BBB integrity.
Table 1.
Different CNS pathological conditions involving BBB disruption
| CNS pathology | BBB dysfunction |
|---|---|
| Stroke |
Astrocytes secrete transforming growth factor-β (TGFβ), which downregulates brain capillary endothelial Expression of fibrinolytic enzyme tissue Plasminogen activator (tPA) and anticoagulant thrombomodulin (TM) Proteolysis of vascular basement membrane/matrix Induction of aquaporin 4 (AQP4) mRNA and protein at BBB disruption |
| Trauma | Bradykinin (an inflammatory mediator) stimulates the production and the release of interleukin-6 (IL-6) from astrocytes, leading to the opening of the BBB |
| Infectious or inflammatory processes |
e.g., bacterial infections, meningitis, encephalitis, and sepsis The bacterial protein lipopolysaccharide (LPS) affects the permeability of BBB tight junctions. This is mediated by the production of free radicals, IL-6, and IL-1 β Interferon-β prevents BBB disruption Alterations in P-glycoprotein expression and activity in the BBB Increased pinocytosis in brain microvessel endothelium and swelling of astrocytes end-feet |
| Multiple sclerosis |
Breakdown of the BBB Tight junction abnormalities Downregulation of laminin in the basement membrane Selective loss of claudin3 in experimental autoimmune encephalomyelitis |
| HIV |
BBB tight junction disruption Cytokines secretion by activated macrophages and astrocytes, e.g., TNF-α, NO, platelet-activating factor, and quinolinic acid |
| Alzheimer’s disease |
Decreased glucose transport, downregulation of glucose transporter GLUT1, altered agrin levels, upregulation of AQP4 expression Accumulation of amyloid-β, a key neuropathological feature of Alzheimer’s disease, by decreased levels of P-glycoprotein transporter expression Altered cellular relations at the BBB, and changes in the basal lamina and amyloid-β clearance |
| Parkinson’s disease | Dysfunction of the BBB by reduced efficacy of P-glycoprotein |
| Epilepsy | Transient BBB opening in epileptogenic foci, and upregulated expression of P-glycoprotein and other drug efflux transporters in astrocytes and endothelium |
| Brain tumors |
Breakdown of the BBB Downregulation of tight junction protein claudin 1, 3, and occludin; redistribution of astrocyte AQP4 and Kir4.1 (inwardly rectifying K+ channel) |
| Pain | Inflammatory pain alters BBB tight junction protein expression and BBB permeability |
| Glaucoma | Opening of the BBB, possibly through the diffusion of endothelin-1 and matrix-metalloproteinase-9 into peri-capillary tissue |
| Lysosomal storage diseases (LSD) | May show changes in BBB permeability, and/or transport, depending on specific LSD |
Ischemic stroke
There are extensive studies showed the effect of hypoxia–ischemia on the BBB, which suggested the disruption of the TJs and the increase in BBB permeability. These events seem to be mediated by released soluble factors, including cytokines, vascular endothelial growth factor (VEGF), and NO. Elevated levels of proinflammatory cytokines, IL-1β, and TNF-α have been reported in animal brains after focal and global ischemia [174] and in CSF of stroke patients [175]. An in vitro study on the BBB model consisting of human cerebrovascular endothelial cells and astrocytes reported that simulated ischemia induces IL-8 and monocyte chemoattractant protein-1 (MCP-1) secretion from endothelial cells and astrocytes [176]. A different study by the same group of investigators observed that human astrocytes under in vitro hypoxic conditions release inflammatory mediators that are capable of up-regulating genes of IL-8, ICAM-1, E-selectin, IL-1 β, TNF-α, and MCP-1 in human cerebrovascular endothelial cells [177]. High levels of cytokines result in the up-regulation of endothelial and neutrophil adhesion molecules. This phenomenon subsequently leads to the transmigration of leukocytes across the endothelium and the BBB. The reported increase in phosphotyrosine staining, loss of TJs molecules (e.g., occludin and ZO), along with the apparent redistribution of AJs protein (such as vinculin), suggest that leukocyte recruitment may trigger signal transduction cascades that result in disorganization of TJs and BBB breakdown [178].
Another study using 14C sucrose, a marker of paracellular permeability, showed a 2.6-fold increase in sucrose permeability upon exposing primary bovine brain microvessel endothelial cells to hypoxic conditions. They also reported an increased expression of actin, and changes in occludin, ZO-1, and ZO-2 protein distribution [179]. In summary, these investigations suggest that TJs disruption and increased BBB permeability triggered by hypoxia–ischemia involves a cascade of events in which cytokines, VEGF, and NO are the leading players and astrocytes appear to play a protective role. Further in vivo studies are needed to validate these results as most of these experiments are performed on in vitro models.
Brain tumors
An increased vascular permeability has been reported in brain tumors due to the poorly developed and compromised BBB [180]. Studies have shown that there is a disruption of interendothelial TJs in human gliomas and metastatic adenocarcinoma [181]. The claudin-1 expression is lost in the microvessels of glioblastoma multiform, whereas claudin-5 and occludin are significantly down-regulated, and ZO-1 expression is unaffected [62]. On the other hand, the reported opening of endothelial TJs in astrocytoma and metastatic adenocarcinoma may be attributed to the loss of the 55 kDa occludin expression in the brain microvessels [78].
There is no clear explanation for the loss of TJs molecules in brain tumor microvessels. However, VEGF and cytokines [182], secreted by astrocytoma and other brain tumors, maybe the key players involved in down-regulating TJs molecules, increased vascular permeability, and cerebral edema. Neoplastic astrocytes are poorly differentiated, which may not be able to release factors necessary for BBB function.
Water channel molecule, aquaporin-4 (AQP4), has been suggested to play a role in BBB disruption in tumors as cerebral edema is an important sign of brain tumor. Multiple investigations have shown that AQP4 is extensively up-regulated in astrocytoma and metastatic adenocarcinoma, which correlates with the observed BBB opening visualized by contrast-enhanced computed tomograms [183]. Animal studies also highlight the AQP4 role in brain edema as Mice deficient in AQP4 have much better survival than wild-type mice in a model of brain edema caused by acute water intoxication. Besides the reported up-regulation of AQP4 in rat models of ischemia [184] and brain injury [185]. Thus, it seems that BBB disruption associated with brain tumors and other forms of brain insults up-regulate the expression of AQP4. Still, the exact mechanism behind the increased expression of AQP4 in different clinical situations is not known.
30 percent of tumors in the brain are metastatic lesions produced by cancers such as lung, breast, and melanoma [186]. The phenomenon surprisingly happens even though the brain is highly impermeable to cancerous cells and prevents their entry into the CNS. Partial disruption of the BBB could be an explanation for this and colonization of the tumor cells in the brain. Besides, this can be explained by transendothelial migration of tumor cells which principally resembles transendothelial migration of leukocytes i.e. rolling, adhesion, and diapedesis.
Certain chemotherapeutic agents can inhibit the growth of tumor cells outside the CNS while they are incapable of affecting the cells inside the brain as an example, trastuzumab in HER2-positive breast cancer [187]. This can be explained by the limited permeability of the agents through the BBB, which leads to sub-therapeutic concentrations in the brain [188]. Lockman et al. [189] showed that the BBB remains partially intact in experimental brain metastases and thus impair drug delivery, requiring a need for brain permeable molecular therapeutics. Similarly, Osswald et al. [190] showed that only the brain permeable compounds inhibit the growth of impermeable lesions in the brain compared to brain impermeable compounds.
Septic encephalopathy
The CNS pathophysiology in septic encephalopathy represented in decreased cerebral blood flow and oxygen extraction by the brain cells, cerebral edema, and BBB disruption may be related to several reasons including the effect of inflammatory mediators on the cerebrovascular microvessels, abnormal neurotransmitter composition of the reticular activating system, impaired astrocyte function, and neuronal degeneration [191]. Permeability studies in rodents using colloidal iron oxide [192], 14C amino acid [193], and 125I-albumin [194], showed their ability to enter the brain parenchyma under septic encephalopathy condition which suggests the breakdown of the BBB. Also, elevated CSF protein content has been observed. Several cellular pathologies underlying this BBB disruption have been reported in animal studies, including increased pinocytosis in the brain microvessel endothelium and swelling of astrocytes end-feet, detachment from microvessel walls, and dark, shrunken neurons [191, 192]. Studies also suggested the implication of the adrenergic system in the inflammatory response to sepsis where β2 adrenoreceptor stimulation seems to be suppressed, and α1 adrenoreceptor stimulation appears to trigger an inflammatory response and hence influence BBB permeability [195].
HIV encephalitis
CNS infection with human immunodeficiency virus (HIV) is associated with immune activation of astrocytes and macrophages. Activated macrophages and astrocytes release cytokines, chemokines, reactive oxygen species, and several neurotoxins, which impair cellular functioning, alter transmitter action, and result in leukoencephalopathy and neuronal dysfunction [196]. The severe neurologic pathologies seem to be attributed to TNF-α along with other neurotoxins such as arachidonic acid, NO, platelet-activating factor, and quinolinic acid. HIV-infected macrophages are released mainly TNF-α, which particularly affects oligodendrocytes [197]. It is not fully understood how the virus enters the CNS, but once there, it compromised BBB integrity, which facilitates viral entry to the brain and exaggerates the neuronal injury. Studies have shown serum protein leakage in the brains of HIV-associated dementia patients besides accumulation in subcortical neurons and glia [198]. Structural proteins of TJs, such as ZO-1 and occludin were absent or fragmented in brains of patients died from HIV-1 encephalitis where such changes was not observed in patients without encephalitis [199].
The gp120, an envelope glycoprotein, expression in HIV-1 gp120 transgenic mice cause extravasation of albumin and induce the expression of cellular and vascular adhesion molecules such as ICAM-1 and VCAM-1. Also, circulating gp120 has been shown to affect BBB integrity in the transgenic mice [200, 201]. Different studies reported that gp120 is cytotoxic to HUVECs and other ECs from the brain and lungs, which may be attributed to different factors including induced gelatinolytic activity, higher expression of metalloproteinases, and/or induced oxidative stress [202, 203].
Alzheimer’s disease (AD)
Amyloid beta (Aβ), a 36–43 amino acid peptides, is one of the main constituents of the amyloid plaques found in the brain of people with Alzheimer’s disease. The high levels of accumulated β-amyloid protein and related oligopeptides in the brain of diseased people activate microglia and astrocytes, which lead to a cascade of events producing toxic molecules, neuronal damage, and synaptic dysfunction [204]. Macrophages or microglia, associated with β-amyloid plaque, get activated and interact with astrocyte resulting in the release of different cytokines including interleukin-1 β, TNF-α, transforming growth factor-β, neurotrophic factors such as NGF and bNGF, and reactive oxygen species [205]. Besides, studies showed that β-amyloid stimulates NF-κB that induces the transcription of TNF-α, IL-1, IL-6, monocyte chemoattractant protein-1, and nitric oxide synthetase [206, 207]. Immune cells activation and migration, and the released cytokines affect the BBB integrity.
Amyloid-β is reversely transported from the brain to the blood by binding to LRP1 at the abluminal surface of BBB, which results in its rapid internalization and clearance from the brain. Phosphatidylinositol binding clathrin assembly protein (PICALM) is an essential factor in the internalization and transcytosis of the Aβ-LRP1 complex [208]. Different studies have shown that any mutation to the PICALM gene resulting in decreased expression in ECs may affect disease progression and is considered as a genetic risk factor for Alzheimer’s disease [209]. Furthermore, apolipoprotein E (APOE4), a protein responsible for Aβ clearance, the mutation is considered as the most substantial genetic risk factor for the late onset of AD. Unlike other forms, APOE4 showed to cause BBB disruption and increase fibrinogen and iron in the brain of AD patients. Studies in APOE4 transgenic mice suggested that vascular changes may occur early and proceeded by the neuronal behavioral changes [210].
Multiple sclerosis (MS)
Multiple sclerosis is an autoimmune disease in which reactive T cells interact with the antigen presented by macrophages- or microglia-expressing HLA-DR2a and HLADR2b, which lead to destroying myelin sheath and the underlying axons [211]. Nitric oxide and cytokines, including interferon-γ, TNF-α, and IL-3, are released by activated macrophages, which damage oligodendrocytes, thus causing interference with myelination and myelin gene expression [212, 213]. Disruption of the BBB is one of the initial critical steps in multiple sclerosis, which follows massive infiltration of T cells and the formation of demyelinated foci. Also, higher levels of reactive oxygen species have been observed in MS lesions, which result in brain damage and contribute to several mechanisms underlying the pathogenesis of MS lesions [214]. Lipid peroxidation products and nitric oxide metabolites are reported to be elevated in the serum of patients with MS [215].
Markers to assess the blood–brain barrier integrity in vitro or in vivo
The raised interest in exploring BBB—after many studies showed its critical role in a wide range of neurological disorders—mandate the use of accurate and representative markers to demonstrate the integrity of the barriers between the blood, the brain, and the CSF [216]. The drug used should be metabolically inert, non-toxic at the applied doses, not bound to other molecules such as proteins in plasma or tissues, be available in a range of molecular sizes, can be visualized in the range from the naked eye to the electron microscopical level, and be reliably quantifiable.
To date, there is no single available marker that fulfills all the criteria mentioned above. Dextran, available in a wide range of molecular sizes labeled with either biotin or a fluorescent tag, has been extensively used in permeability studies, but it is tedious to quantify. Radiolabeled markers, which are also available in a wide range of molecular sizes, can be easily quantified. Still, they cannot be visualized with enough resolution and have many other limitations, which will be mentioned below. Recently 13C sucrose has been introduced as a small molecular weight marker, which can be precisely quantified but also cannot be visualized.
For the quantification of subtle BBB impairment, markers with small molecular weight, such as sucrose should be used, because even a minor degree of barrier damage is expected to have a noticeable effect on their permeability which cannot be quantified with larger molecular weight markers [217]. In general, physicochemical properties, such as size and polarity should be considered for measuring the BBB permeability. When the BBB is compromised and loses its integrity, the chance of large molecular weight markers such as dextran to enter the brain increases, and this could be considered as an option to evaluate the integrity and permeability of the BBB however, for accurately examining small changes in BBB permeability that occur in many neurological conditions, small molecular weight markers (with MW of less than 400 Da) have superiority. Thus, a combination of different markers is currently the most reliable approach to sufficiently assess barrier integrity in the developing or pathological brain. We will try to briefly explore the most used markers and elaborate on their advantages and disadvantages, hoping that will help researchers pick the best marker which can fit their application. A summary of different markers used for BBB permeability studies is represented in Table 2.
Table 2.
Summary of different markers that can be used for BBB permeability studies
| Marker | Size (Da) | Binding | Advantage | Disadvantage | |
|---|---|---|---|---|---|
| Protein | Tissue | ||||
| Radiolabeled-mannitol | 182 | No | No |
No interaction with proteins Metabolically stable Uncharged No interaction with BBB transporters Suitable for small molecules permeability prediction |
Contains lipophilic impurities Requires a radioactive license High cost It cannot be visualized |
| Biotin ethylenediamine | 286 | No | No |
It can be measured quantitatively with HPLC Visual qualification is feasible |
It has a low binding to plasma proteins |
| Radiolabeled-sucrose | 342 | No | No |
No interaction with proteins Metabolically stable Uncharged No interactions with BBB transporters Suitable for small molecules permeability prediction |
Contains lipophilic impurities Over-time degradation Requires a radioactive license High cost It cannot be visualized |
| 13C12 sucrose | 354 | No | No |
Non-radioactive A sensitive and specific method of detection No interaction with BBB transporters Metabolically stable No interaction with protein Suitable for small molecules permeability prediction |
Requires LC–MS/MS device for detection High cost It cannot be visualized |
| Sodium fluorescein | 376 | Weak | NR |
Easy detection method Freely diffusible Detectable in very low concentrations Inexpensive Non-radioactive Nontoxic It can be visually assessed Suitable for small molecules permeability prediction |
Interaction with BBB transporters Weekly binds to plasma proteins |
| Evans blue | 961 | Yes | Yes |
Visual investigation and qualification are feasible using different microscope techniques Allow assessment of vascular permeability to macromolecules due to binding to its extensive binding to albumin |
Quantification is unreliable It strongly binds to serum albumin in vivo and in vitro and, thereby, becomes a high molecular weight protein tracer (69 kDa) Potential in vivo toxicity Not suitable for small molecules permeability prediction |
| Trypan blue | 961 | Yes | Yes | Visual evaluation is feasible |
Quantification is unreliable Binds to plasma proteins Not suitable for small molecules permeability prediction |
| Radio-inulin | 7000 | No | No |
Quantification is feasible No protein binding |
Contains lipophilic impurities Requires a radioactive license High cost It cannot be visualized |
| Horseradish peroxidase | 44,000 | NR | NR | Can be easily visualized under light and electron microscopy |
The possibility of diffusion artifacts resulted in a distribution of the reaction product that may not reflect actual protein Found to be toxic in large doses Not suitable for small molecules permeability prediction Quantification is unreliable Species-specific degranulation of mast cells and histamine release |
| Albumin | 69,000 | No | No |
It is widely used in the radiolabeled or fluorescently labeled form The fluorescent-labeled version could be used for morphological studies The radiolabeled version allows for accurate quantification |
Requires radioactive license Not suitable for small molecules permeability prediction |
| Dextrans | 1500 to 70,000 | No | No |
It can be used for a broad range of molecular weights Can be visualized with both light microscopic and electron microscopic level due to conjugation with biotin of FITC |
Not suitable for small molecules permeability prediction Stability issue May be toxic at high concentrations |
NR not reported
Evans blue
Evans blue (T-1824) dye is one of the oldest dyes used in animal and human studies. It was used for a long time for plasma volume measurements. The first use in the assessment of BBB was reported in 1966 by Rössner and Temple [218]. Nowadays, Evans blue is widely used as a high-molecular-weight permeability marker to study capillary and cellular membrane permeability. Evans blue extensively binds to serum albumin as soon as it gets to the vascular system [219]. In the case of BBB disruption, the dye leaks through and stains the brain lesion where the BBB is impaired. The advantage of using Evans blue tracer is that it is easy to visualize the zone of altered permeability [220]. That is very useful in animal studies in which lesions with BBB breakdown in specific brain regions are investigated. For many years, the Evans blue method has been used to assess vascular protein leakage macroscopically [221].
With the advanced detection techniques and the wide availability of other accurate markers, researchers start to favor the use of other markers than Evans blue. Studies over the long period of Evans blue application, unveil many drawbacks which limit its use. These limitations include (1) a substantial amount of free dye being present in an animal following the amounts injected, (2) extensive binding to albumin and species-specific binding to other plasma proteins (researcher mainly used Evans blue to estimate albumin penetration through disrupted BBB), (3) unstable in saline and other salt solution, (4) different studies showed it binds to tissues, (5) inaccurate quantitative assessment of BBB damage due to spectroscopic limitations, Evans blue show spectral shifts in protein-containing solutions, (6) in vivo potential lethal toxicity [216].
Horseradish peroxidase
Horseradish peroxidase (HRP) has been used for years as a vascular permeability in morphological studies [222]. It is available commercially in several types such as types II, IV, and VI). The importance of the introduction of HRP is that the reaction product of this peroxidase forms electron-dense that can be visualized under electron microscopy. The use of horseradish peroxidase helps to expose the nature and location of the BBB and the critical contribution of the brain endothelium to its formation [223]. The main barrier for this tracer to enter the brain lies within the intercellular tight junction between adjacent endothelial cells and the scarcity of pinocytic vesicles in cerebral microvessels ECs. Also, the tight junctions between the epithelial cells of the choroid plexus showed to restrict the movement of HRP across the blood–CSF interface. Under several experimental and pathological conditions, HRP can cross the BBB, which provides morphological evidence for a barrier opening [224, 225].
There are a few limitations that need to be addressed when using this tracer. HRP can cause degranulation of mast cells leading to the release of histamine and serotonin, which subsequently affect vascular permeability [226]. This phenomenon seems to be species-specific as it was observed in certain strains of rats but not in the others [227]. The problem can be avoided by (1) using different strains such as Wistar rats, which did not show mast cell degranulation upon HRP injection [228]. Another solution is to concurrently treat the animal with antihistaminic and anti-serotonergic agents that will mask the degranulation effect. (2) Altogether, caution must be taken while interpreting the results of experiments using HRP, mainly when large doses were applied, and there was no pretreatment with antihistamines.
Sodium fluorescein
Sodium fluorescein, a 376 Da molecule, was the first visualizable small molecular-sized marker to be introduced into the BBB field [229, 230]. The required dose for injection into mice for barrier permeability experiments (50 mg/kg body weight) is very low considered to its LD 50 in mice, which was estimated as 4738 ± 1.23 mg/kg body weight [230]. Also, an injection of a single dose of 500 mg/kg in pregnant mice did not show to have any embryotoxic or teratogenic effects [231]. It was suggested that sodium fluorescein could be assayed spectrophotofluorometrically (excitation at 440 nm and emission at 525 nm), which will facilitate its detection in BBB permeability studies [220]. Thus, sodium fluorescein seems to be considerably less toxic than Evans blue or HRP. Unlike the Evans blue dye, it shows only weak binding to plasma proteins, which favor its use as a small molecular size marker for blood–brain barrier integrity.
Sucrose
The use of radiolabeled [14C] sucrose for BBB permeability studies was introduced by Dixon Woodbury, Hugh Davson, and Bill Oldendorf, three famous scientists in the BBB field [232–234]. The importance of using this marker is that it allows a quantitative determination of blood–brain or blood–CSF permeability. Experiments applying sucrose need to be designed carefully by ensuring that steady-state plasma levels of the maker are achieved, blood contamination of brain samples by the marker is estimated and secured isotopic labeling of the marker.
Recently, the disaccharide sucrose is considered the most widely accepted standard for the precise measurement of paracellular BBB permeability [217, 235, 236]. Sucrose is substantially better than other markers as being uncharged, not subjected to protein binding, metabolically stable after parenteral administration, falling within the molecular weight range of most small molecule drugs, and not a substrate for active or facilitative transporters in vertebrates animals [237]. However, radiotracer use is associated with special handling and licensing requirements. Impurities in the dosing solution might significantly impact the outcome of experiments [238, 239]. To overcome the drawbacks of radiolabeled tracer and avoid the non-specificity of total radioactivity measurement, [13C12] sucrose has been introduced as a superior non-radioactive marker, which can be precisely quantified by a sensitive and highly specific LC–MS/MS technique [240].
For an accurate estimation of the marker in the brain tissue, the method relies on transcardiac perfusion with buffer solution before tissue sampling to remove the marker from the brain vasculature. However, it is difficult to judge the full vascular washout in an individual animal. Besides, there are multiple variations in technical details on how the perfusions are performed, such as concerning total volume, duration, flow rate, temperature, and composition of a perfusion fluid, which may add to the experimental variability [241]. Furthermore, the accuracy of this method is questionable, particularly in experiments involving brain trauma, where part of the cerebral circulation is obstructed by post-trauma blood coagulation within vessels [216].
As an alternative, a second marker can be injected just before the terminal sampling time. This marker must be present in the circulation long enough to mix appropriately but not to penetrate the brain to any measurable extent. A second stable isotope-labeled sucrose variant [13C6] sucrose, which contains 6 of the carbons in the fructose moiety labeled with 13C isotope, can serve as a vascular marker. The method allows the simultaneous measurement of both analytes in the same sample in a single run [241]. For radiolabeled tracers, the use of radiolabeled markers such as 113mIndium has been reported [242]. Indium binds to transferrin and has the advantage of a very short half-life, but other radiolabeled markers such as albumin or inulin would also be suitable. Poduslo’s laboratory corrected the brain uptake of macromolecules by radioiodinated the same protein with either 125I or 131I, and one labeled species was used as a vascular marker [243].
Providing these factors are taken into consideration, the use of sucrose is a valuable way of obtaining an accurate quantitative estimate of any brain barrier dysfunction, particularly with its molecular weight falling within the molecular weight range of most small-molecule drugs. Their disadvantage is that it cannot be visualized in tissue sections, so the morphological nature of the disruption cannot be ascertained.
Dextrans
Dextrans are complex, branched polysaccharides consisting of many glucose molecules. They are commercially available labeled either with a fluorophore or biotin with their chain length varying from 3 to 2000 kDa. Ethylenediamine, a 286 Da biotin-labeled molecule, is also available, which is smaller than sucrose (342 Da), which is a commonly used marker for paracellular permeability in BBB studies [216].
The currently available biotin and fluorophore-labeled dextrans are highly purified, and only small amounts are required because of the sensitivity of the techniques utilized to visualize them. Biotin-labeled molecules can be visualized both under light and electron microscopy. Its permeability across the blood–CSF barrier is comparable to the permeability of more traditional permeability radiolabeled markers, sucrose, and inulin [244]. The use of fluorophore and biotin-labeled dextrans help in clarifying that the route of entry from the blood to the CSF is an intracellular path via the plexus epithelial cells, [245], rather than intercellularly via the tight junctions as generally believed [2]. In general, labeled dextrans are valuable markers of BBB integrity, which can be used safely in small concentrations. The biotin-labeled form is particularly valuable as it can be visualized under both light and electron microscopy.
Peripheral markers
Brain-derived proteins may work as markers of BBB integrity as they have several possible mechanisms across the BBB. Under physiologic conditions, the production of CSF from plasma involves an efficient filtration process in the choroid plexus that results in minimal quantities of proteins in the CSF [246]. However, many neurological disorders are associated with elevated CSF protein levels. Proteins in CSF can be detected by directly sampling the CSF (lumbar puncture) or intraoperative sampling from ventricles or the subarachnoid space. BBB integrity can also be assessed by contrast-enhanced computed tomography or MRI [247].
Accurate non-invasive techniques would be preferable, mainly to analyze multiple longitudinal samples. A few species of proteins are found exclusively or almost exclusively in the cerebrospinal fluid. Any dysfunction in BBB may allow protein leakage in both directions. Thus, measuring serum levels of CSF proteins represents a non-invasive approach for evaluating BBB integrity and may be of diagnostic value [248].
Currently, only invasive and expensive techniques such as contrast-enhanced magnetic resonance imaging, CT-scan, and lumbar puncture are available to assess BBB integrity clinically. Detection of alterations in blood composition has been proposed as an alternative way to predict BBB disruption [248]. Distinguishing between BBB defects and neuronal damage has immense clinical significance. In ischemic stroke, the delay between insult and the irreversible neuronal cell death offers a window of therapeutic opportunity. If BBB openings develop early after the initial arterial occlusion [249, 250], clinicians would have a unique opportunity to administer drugs that are usually BBB impermeant (e.g., nerve growth factors) before neurons were damaged. The opening time may be unpredictable, so a peripheral, non-invasive, easily repeatable test would be instrumental.
Because of the high interest in neuronal damage, many of the previous studies on biochemical markers have focused on targets that measure neuronal damage. However, most neurologic diseases are associated with increased BBB permeability, and thus the markers thought to indicate neuronal damage may indicate BBB dysfunction. Marker proteins under investigation have included neuron-specific enolase (NSE), glial fibrillary acidic protein (GFAP), and S100β (see Fig. 4) [251].
Fig. 4.
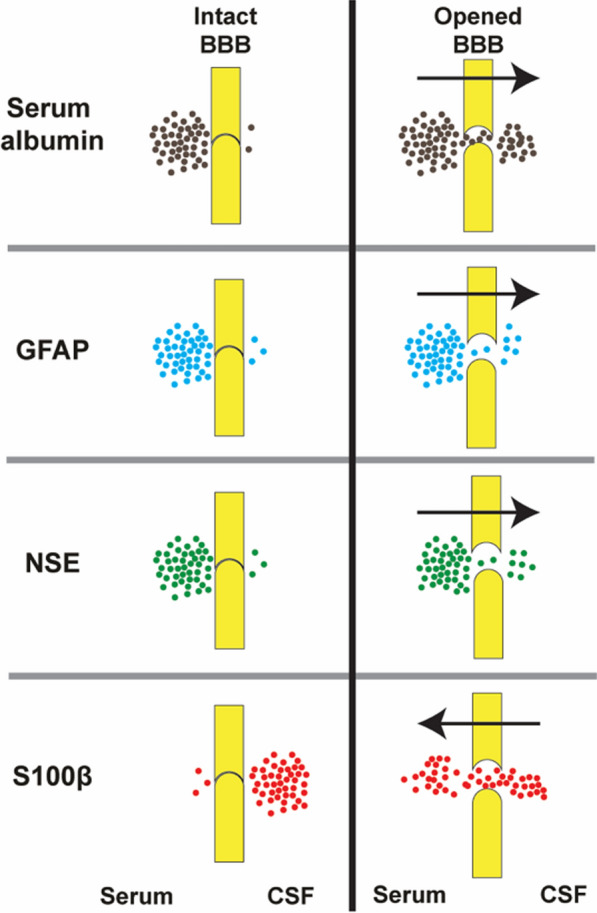
Protein biomarkers protein and their side selectivity. GFAP glial fibrillary acidic protein, NSE neuron specific enolase
S100β seems to be promising as its level is more correlated with BBB integrity rather than with neuronal damage, unlike monomeric transthyretin (TTR), a neuronal protein, that may be considered a potential marker of opening to the blood–CSF barrier [252]. In normal subjects, NSE is more concentrated in plasma, and S100β is primarily present in central nervous system fluids. Thus, opening the blood–brain barrier in the absence of neuronal damage would be expected to increase plasma S100β levels while leaving NSE levels markedly unchanged.
S100β is primarily synthesized in the brain by the end feet process of the astrocytes where it accumulates. When the BBB is disrupted, S100β is quickly released in the blood circulation [253, 254]. S100β has also been found in other tissues but at lower concentrations [255, 256]. While S100β appearance in plasma correlated well with BBB openings, S100β has been shown to increase in plasma, the CSF, or both as a consequence of other pathologies not limited to the brain. According to these authors, low levels of S100β are ordinarily present at the blood-to-brain interface and in the CSF. At the same time, disruption of the BBB will result in the sudden appearance of cerebral S100β in serum [257].
Conclusions
The BBB is a fundamental component of the CNS. Its functional and structural integrity is vital in maintaining the homeostasis of the brain microenvironment. Deterioration in BBB function may play a significant role in the pathogenesis of disease since the BBB dynamically responds to many events associated with flow disturbances, free radical release, and cytokine generation. Furthermore, many neurological disorders and lesions are associated with increased BBB permeability such as neoplasia, hypertension, dementia, epilepsy, infection, multiple sclerosis, and trauma. Any disorder which affects BBB function will cause secondary effects on cerebral blood flow and vascular tone, further influencing transport across the BBB. In this review, we covered several critical aspects of the BBB encompassing the role and functions of its cellular components as well as the contribution of physical stimuli such as shear stress. We also examined several neurological disorders related to the impairment of the BBB. Finally, we provided a comprehensive list of methods currently available to assess the viability of the BBB in vivo and in vitro and discuss the pros and cons of each method.
Acknowledgements
Not applicable.
Abbreviations
- BBB
Blood–brain barrier
- CNS
Central nervous system
- CSF
Cerebrospinal fluid
- BCB
Blood–CSF barrier
- ISF
Interstitial fluid
- TJs
Tight junctions
- P-gp
P-glycoprotein
- BCRP
Breast cancer resistance protein
- OATP
Organic anion transporting polypeptide
- CYP 450
Cytochrome P450
- BM
Basement membrane
- PCs
Pericytes
- NVU
Neurovascular unit
- ECs
Endothelial cells
- PDGFR-b
Platelet-derived growth factor b-receptor
- RGS5
Regulator of G-protein signalling-5 (RGS5)
- Ajs
Adherens junctions
- JAMs
Junction adhesion molecules
- ZO
Zonula occludens
- EAE
Encephalomyelitis
- GBM
Glioblastoma multiforme
- MAGUKs
Membrane-associated guanylate kinase-like protein
- GUK
Guanyl kinase-like
- ROS
Reactive oxygen species
- NO
Nitric oxide
- HBMECs
Human brain microvascular endothelial cells
- TEER
Trans-endothelial electrical resistance
- TM
Thrombomodulin
- TNF-α
Tumor necrosis factor-α
- Il-6
Interleukin-6
- MW
Molecular weight
- PSA
Polar surface area
- MRP
Multidrug resistance-associated proteins
- CMT
Carrier-mediated transport
- RMT
Receptor mediated transport
- SLC
Solute carrier
- AMT
Adsorptive-mediated transcytosis
- LDL
Low density lipoprotein
- LRP1
Lipoprotein receptor-related protein 1
- MAb
Monoclonal antibody
- TRF
Transferrin receptors
- DHA
Docosahexaenoic acid
- MFSD2a
Major facilitator superfamily domain-containing protein 2a
- ICAM1
Intercellular adhesion molecule 1
- VCAM-1
Vascular cell adhesion molecule 1
- LFA-1
Lymphocyte function-associated antigen 1
- VLA4
Very late antigen 4
- VEGF
Vascular endothelial growth factor
- MCP-1
Monocyte chemoattractant protein-1
- AQP4
Aquaporin-4
- HIV
Human immunodeficiency virus
- PICALM
Phosphatidylinositol binding clathrin assembly protein
- APOR4
Apolipoprotein E
- MS
Multiple sclerosis
- HRP
Horseradish peroxidase
- NSE
Neuron-specific enolase
- GFAP
Glial fibrillary acidic protein
- TTR
Monomeric transthyretin
Authors’ contributions
All authors conceived the study and prepared the drafting of the manuscript. LC assisted with the drafting of the manuscript and preparation of the figures. LC also oversaw all the studies and provided funding. All authors reviewed the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the National Institutes of Health/National Institute on Drug Abuse 2R01-DA029121-01A1, 1R01DA049737-01, and 1R01NS117906-01 to Dr. Luca Cucullo.
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Liebner S, Czupalla CJ, Wolburg H. Current concepts of blood–brain barrier development. Int J Dev Biol. 2011;55(4–5):467–476. doi: 10.1387/ijdb.103224sl. [DOI] [PubMed] [Google Scholar]
- 2.Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood–brain barrier. Neurobiol Dis. 2010;37(1):13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 3.Abbott NJ. Dynamics of CNS barriers: evolution, differentiation, and modulation. Cell Mol Neurobiol. 2005;25(1):5–23. doi: 10.1007/s10571-004-1374-y. [DOI] [PubMed] [Google Scholar]
- 4.Nag S, Begley D. Pathology and genetics cerebrovascular diseases. Basel: ISN Neuropath Press; 2005. Blood–brain barrier, exchange of metabolites and gases; pp. 22–29. [Google Scholar]
- 5.Brown P, Davies S, Speake T, Millar I. Molecular mechanisms of cerebrospinal fluid production. Neuroscience. 2004;129(4):955–968. doi: 10.1016/j.neuroscience.2004.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abbott NJ. Evidence for bulk flow of brain interstitial fluid: significance for physiology and pathology. Neurochem Int. 2004;45(4):545–552. doi: 10.1016/j.neuint.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 7.Cserr HF, Cooper DN, Suri PK, Patlak CS. Efflux of radiolabeled polyethylene glycols and albumin from rat brain. Am J Physiol. 1981;240(4):F319–F328. doi: 10.1152/ajprenal.1981.240.4.F319. [DOI] [PubMed] [Google Scholar]
- 8.Cserr H, Patlak C. Physiology and pharmacology of the blood–brain barrier. New York: Springer; 1992. Secretion and bulk flow of interstitial fluid; pp. 245–261. [Google Scholar]
- 9.Dolman D, Drndarski S, Abbott NJ, Rattray M. Induction of aquaporin 1 but not aquaporin 4 messenger RNA in rat primary brain microvessel endothelial cells in culture. J Neurochem. 2005;93(4):825–833. doi: 10.1111/j.1471-4159.2005.03111.x. [DOI] [PubMed] [Google Scholar]
- 10.Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood–brain barrier. Nat Rev Neurosci. 2006;7(1):41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 11.Kandel ER, Schwartz JH, Jessell TM, Biochemistry Do. Jessell MBT, Siegelbaum S, et al. Principles of neural science. New York: McGraw-hill; 2000. [Google Scholar]
- 12.Ballabh P, Braun A, Nedergaard M. The blood–brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004;16(1):1–13. doi: 10.1016/j.nbd.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 13.Cordon-Cardo C, O'Brien JP, Casals D, Rittman-Grauer L, Biedler JL, Melamed MR, et al. Multidrug-resistance gene (P-glycoprotein) is expressed by endothelial cells at blood–brain barrier sites. Proc Natl Acad Sci USA. 1989;86(2):695–698. doi: 10.1073/pnas.86.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eisenblatter T, Galla HJ. A new multidrug resistance protein at the blood–brain barrier. Biochem Biophys Res Commun. 2002;293(4):1273–1278. doi: 10.1016/S0006-291X(02)00376-5. [DOI] [PubMed] [Google Scholar]
- 15.Gao B, Hagenbuch B, Kullak-Ublick GA, Benke D, Aguzzi A, Meier PJ. Organic anion-transporting polypeptides mediate transport of opioid peptides across blood–brain barrier. J Pharmacol Exp Ther. 2000;294(1):73–79. [PubMed] [Google Scholar]
- 16.Agarwal S, Hartz AM, Elmquist WF, Bauer B. Breast cancer resistance protein and P-glycoprotein in brain cancer: two gatekeepers team up. Curr Pharm Des. 2011;17(26):2793–2802. doi: 10.2174/138161211797440186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Lange EC. Potential role of ABC transporters as a detoxification system at the blood–CSF barrier. Adv Drug Deliv Rev. 2004;56(12):1793–1809. doi: 10.1016/j.addr.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 18.Ghersi-Egea JF, Leininger-Muller B, Cecchelli R, Fenstermacher JD. Blood–brain interfaces: relevance to cerebral drug metabolism. Toxicol Lett. 1995;82–83:645–653. doi: 10.1016/0378-4274(95)03510-9. [DOI] [PubMed] [Google Scholar]
- 19.Shawahna R, Uchida Y, Decleves X, Ohtsuki S, Yousif S, Dauchy S, et al. Transcriptomic and quantitative proteomic analysis of transporters and drug metabolizing enzymes in freshly isolated human brain microvessels. Mol Pharm. 2011;8(4):1332–1341. doi: 10.1021/mp200129p. [DOI] [PubMed] [Google Scholar]
- 20.Ghosh C, Puvenna V, Gonzalez-Martinez J, Janigro D, Marchi N. Blood–brain barrier P450 enzymes and multidrug transporters in drug resistance: a synergistic role in neurological diseases. Curr Drug Metab. 2011;12(8):742–749. doi: 10.2174/138920011798357051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dauchy S, Dutheil F, Weaver RJ, Chassoux F, Daumas-Duport C, Couraud PO, et al. ABC transporters, cytochromes P450 and their main transcription factors: expression at the human blood–brain barrier. J Neurochem. 2008;107(6):1518–1528. doi: 10.1111/j.1471-4159.2008.05720.x. [DOI] [PubMed] [Google Scholar]
- 22.Ghosh C, Gonzalez-Martinez J, Hossain M, Cucullo L, Fazio V, Janigro D, et al. Pattern of P450 expression at the human blood–brain barrier: roles of epileptic condition and laminar flow. Epilepsia. 2010;51(8):1408–1417. doi: 10.1111/j.1528-1167.2009.02428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pardridge WM. The blood–brain barrier: bottleneck in brain drug development. NeuroRx. 2005;2(1):3–14. doi: 10.1602/neurorx.2.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pardridge WM. Blood–brain barrier biology and methodology. J Neurovirol. 1999;5(6):556–569. doi: 10.3109/13550289909021285. [DOI] [PubMed] [Google Scholar]
- 25.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci. 2011;12(12):723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liebner S, Engelhardt B. The blood brain barrier and its microenvironment: basic physiology to neurological disease. New York: Taylor and Francis; 2005. Development of the blood–brain barrier; pp. 1–25. [Google Scholar]
- 27.Pardridge WM. Blood–brain barrier endogenous transporters as therapeutic targets: a new model for small molecule CNS drug discovery. Expert Opin Ther Targets. 2015;19(8):1059–1072. doi: 10.1517/14728222.2015.1042364. [DOI] [PubMed] [Google Scholar]
- 28.Pardridge WM, Eisenberg J, Yang J. Human blood–brain barrier insulin receptor. J Neurochem. 1985;44(6):1771–1778. doi: 10.1111/j.1471-4159.1985.tb07167.x. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y, Pardridge WM. Rapid transferrin efflux from brain to blood across the blood–brain barrier. J Neurochem. 2001;76(5):1597–1600. doi: 10.1046/j.1471-4159.2001.00222.x. [DOI] [PubMed] [Google Scholar]
- 30.Armulik A, Genove G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21(2):193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 31.Smyth LCD, Rustenhoven J, Scotter EL, Schweder P, Faull RLM, Park TIH, et al. Markers for human brain pericytes and smooth muscle cells. J Chem Neuroanat. 2018;92:48–60. doi: 10.1016/j.jchemneu.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 32.Alarcon-Martinez L, Yilmaz-Ozcan S, Yemisci M, Schallek J, Kilic K, Can A, et al. Capillary pericytes express alpha-smooth muscle actin, which requires prevention of filamentous-actin depolymerization for detection. Elife. 2018;7:e34861. doi: 10.7554/eLife.34861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R, et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68(3):409–427. doi: 10.1016/j.neuron.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Winkler EA, Bell RD, Zlokovic BV. Central nervous system pericytes in health and disease. Nat Neurosci. 2011;14(11):1398–1405. doi: 10.1038/nn.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hall CN, Reynell C, Gesslein B, Hamilton NB, Mishra A, Sutherland BA, et al. Capillary pericytes regulate cerebral blood flow in health and disease. Nature. 2014;508(7494):55–60. doi: 10.1038/nature13165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peppiatt CM, Howarth C, Mobbs P, Attwell D. Bidirectional control of CNS capillary diameter by pericytes. Nature. 2006;443(7112):700–704. doi: 10.1038/nature05193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sagare AP, Bell RD, Zhao Z, Ma Q, Winkler EA, Ramanathan A, et al. Pericyte loss influences Alzheimer-like neurodegeneration in mice. Nat Commun. 2013;4:2932. doi: 10.1038/ncomms3932. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Nakagomi T, Kubo S, Nakano-Doi A, Sakuma R, Lu S, Narita A, et al. Brain vascular pericytes following ischemia have multipotential stem cell activity to differentiate into neural and vascular lineage cells. Stem Cells. 2015;33(6):1962–1974. doi: 10.1002/stem.1977. [DOI] [PubMed] [Google Scholar]
- 39.Elfont RM, Sundaresan PR, Sladek CD. Adrenergic receptors on cerebral microvessels: pericyte contribution. Am J Physiol. 1989;256(1 Pt 2):R224–R230. doi: 10.1152/ajpregu.1989.256.1.R224. [DOI] [PubMed] [Google Scholar]
- 40.Healy DP, Wilk S. Localization of immunoreactive glutamyl aminopeptidase in rat brain. II. Distribution and correlation with angiotensin II. Brain Res. 1993;606(2):295–303. doi: 10.1016/0006-8993(93)90997-2. [DOI] [PubMed] [Google Scholar]
- 41.Benagiano V, Virgintino D, Maiorano E, Rizzi A, Palombo S, Roncali L, et al. VIP-like immunoreactivity within neurons and perivascular neuronal processes of the human cerebral cortex. Eur J Histochem. 1996;40(1):53–56. [PubMed] [Google Scholar]
- 42.Dehouck MP, Vigne P, Torpier G, Breittmayer JP, Cecchelli R, Frelin C. Endothelin-1 as a mediator of endothelial cell-pericyte interactions in bovine brain capillaries. J Cereb Blood Flow Metab. 1997;17(4):464–469. doi: 10.1097/00004647-199704000-00012. [DOI] [PubMed] [Google Scholar]
- 43.van Zwieten EJ, Ravid R, Swaab DF, Van de Woude T. Immunocytochemically stained vasopressin binding sites on blood vessels in the rat brain. Brain Res. 1988;474(2):369–373. doi: 10.1016/0006-8993(88)90453-2. [DOI] [PubMed] [Google Scholar]
- 44.Ramsauer M, Krause D, Dermietzel R. Angiogenesis of the blood–brain barrier in vitro and the function of cerebral pericytes. FASEB J. 2002;16(10):1274–1276. doi: 10.1096/fj.01-0814fje. [DOI] [PubMed] [Google Scholar]
- 45.Lindahl P, Johansson BR, Leveen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277(5323):242–245. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- 46.Baloyannis SJ, Baloyannis IS. The vascular factor in Alzheimer’s disease: a study in Golgi technique and electron microscopy. J Neurol Sci. 2012;322(1–2):117–121. doi: 10.1016/j.jns.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 47.Farkas E, Luiten PG. Cerebral microvascular pathology in aging and Alzheimer’s disease. Prog Neurobiol. 2001;64(6):575–611. doi: 10.1016/S0301-0082(00)00068-X. [DOI] [PubMed] [Google Scholar]
- 48.Halliday MR, Pomara N, Sagare AP, Mack WJ, Frangione B, Zlokovic BV. Relationship between cyclophilin a levels and matrix metalloproteinase 9 activity in cerebrospinal fluid of cognitively normal apolipoprotein e4 carriers and blood–brain barrier breakdown. JAMA Neurol. 2013;70(9):1198–1200. doi: 10.1001/jamaneurol.2013.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sengillo JD, Winkler EA, Walker CT, Sullivan JS, Johnson M, Zlokovic BV. Deficiency in mural vascular cells coincides with blood–brain barrier disruption in Alzheimer’s disease. Brain Pathol. 2013;23(3):303–310. doi: 10.1111/bpa.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Montagne A, Barnes SR, Sweeney MD, Halliday MR, Sagare AP, Zhao Z, et al. Blood–brain barrier breakdown in the aging human hippocampus. Neuron. 2015;85(2):296–302. doi: 10.1016/j.neuron.2014.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119(1):7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wolburg H, Noell S, Wolburg-Buchholz K, Mack A, Fallier-Becker P. Agrin, aquaporin-4, and astrocyte polarity as an important feature of the blood–brain barrier. Neuroscientist. 2009;15(2):180–193. doi: 10.1177/1073858408329509. [DOI] [PubMed] [Google Scholar]
- 53.Wolburg H, Wolburg-Buchholz K, Fallier-Becker P, Noell S, Mack AF. Structure and functions of aquaporin-4-based orthogonal arrays of particles. Int Rev Cell Mol Biol. 2011;287:1–41. doi: 10.1016/B978-0-12-386043-9.00001-3. [DOI] [PubMed] [Google Scholar]
- 54.Stewart PA, Wiley MJ. Developing nervous tissue induces formation of blood–brain barrier characteristics in invading endothelial cells: a study using quail–chick transplantation chimeras. Dev Biol. 1981;84(1):183–192. doi: 10.1016/0012-1606(81)90382-1. [DOI] [PubMed] [Google Scholar]
- 55.Janzer RC, Raff MC. Astrocytes induce blood–brain barrier properties in endothelial cells. Nature. 1987;325(6101):253–257. doi: 10.1038/325253a0. [DOI] [PubMed] [Google Scholar]
- 56.Rubin LL, Barbu K, Bard F, Cannon C, Hall DE, Horner H, et al. Differentiation of brain endothelial cells in cell culture. Ann N Y Acad Sci. 1991;633:420–425. doi: 10.1111/j.1749-6632.1991.tb15631.x. [DOI] [PubMed] [Google Scholar]
- 57.Neuhaus J, Risau W, Wolburg H. Induction of blood–brain barrier characteristics in bovine brain endothelial cells by rat astroglial cells in transfilter coculture. Ann N Y Acad Sci. 1991;633:578–580. doi: 10.1111/j.1749-6632.1991.tb15667.x. [DOI] [PubMed] [Google Scholar]
- 58.Clarke LE, Barres BA. Emerging roles of astrocytes in neural circuit development. Nat Rev Neurosci. 2013;14(5):311–321. doi: 10.1038/nrn3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wolburg H, Lippoldt A. Tight junctions of the blood–brain barrier: development, composition and regulation. Vasc Pharmacol. 2002;38(6):323–337. doi: 10.1016/S1537-1891(02)00200-8. [DOI] [PubMed] [Google Scholar]
- 60.Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S. Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol. 1998;141(7):1539–1550. doi: 10.1083/jcb.141.7.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Furuse M, Sasaki H, Tsukita S. Manner of interaction of heterogeneous claudin species within and between tight junction strands. J Cell Biol. 1999;147(4):891–903. doi: 10.1083/jcb.147.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liebner S, Fischmann A, Rascher G, Duffner F, Grote EH, Kalbacher H, et al. Claudin-1 and claudin-5 expression and tight junction morphology are altered in blood vessels of human glioblastoma multiforme. Acta Neuropathol. 2000;100(3):323–331. doi: 10.1007/s004010000180. [DOI] [PubMed] [Google Scholar]
- 63.Morita K, Sasaki H, Furuse M, Tsukita S. Endothelial claudin: claudin-5/TMVCF constitutes tight junction strands in endothelial cells. J Cell Biol. 1999;147(1):185–194. doi: 10.1083/jcb.147.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liebner S, Kniesel U, Kalbacher H, Wolburg H. Correlation of tight junction morphology with the expression of tight junction proteins in blood–brain barrier endothelial cells. Eur J Cell Biol. 2000;79(10):707–717. doi: 10.1078/0171-9335-00101. [DOI] [PubMed] [Google Scholar]
- 65.Lippoldt A, Kniesel U, Liebner S, Kalbacher H, Kirsch T, Wolburg H, et al. Structural alterations of tight junctions are associated with loss of polarity in stroke-prone spontaneously hypertensive rat blood–brain barrier endothelial cells. Brain Res. 2000;885(2):251–261. doi: 10.1016/S0006-8993(00)02954-1. [DOI] [PubMed] [Google Scholar]
- 66.Wolburg H, Wolburg-Buchholz K, Kraus J, Rascher-Eggstein G, Liebner S, Hamm S, et al. Localization of claudin-3 in tight junctions of the blood–brain barrier is selectively lost during experimental autoimmune encephalomyelitis and human glioblastoma multiforme. Acta Neuropathol. 2003;105(6):586–592. doi: 10.1007/s00401-003-0688-z. [DOI] [PubMed] [Google Scholar]
- 67.Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, et al. Size-selective loosening of the blood–brain barrier in claudin-5-deficient mice. J Cell Biol. 2003;161(3):653–660. doi: 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hashimoto Y, Shirakura K, Okada Y, Takeda H, Endo K, Tamura M, et al. Claudin-5-binders enhance permeation of solutes across the blood–brain barrier in a mammalian model. J Pharmacol Exp Ther. 2017;363(2):275–283. doi: 10.1124/jpet.117.243014. [DOI] [PubMed] [Google Scholar]
- 69.Berndt P, Winkler L, Cording J, Breitkreuz-Korff O, Rex A, Dithmer S, et al. Tight junction proteins at the blood–brain barrier: far more than claudin-5. Cell Mol Life Sci. 2019;76(10):1987–2002. doi: 10.1007/s00018-019-03030-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Greene C, Hanley N, Campbell M. Claudin-5: gatekeeper of neurological function. Fluids Barriers CNS. 2019;16(1):3. doi: 10.1186/s12987-019-0123-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jia W, Lu R, Martin TA, Jiang WG. The role of claudin-5 in blood–brain barrier (BBB) and brain metastases (review) Mol Med Rep. 2014;9(3):779–785. doi: 10.3892/mmr.2013.1875. [DOI] [PubMed] [Google Scholar]
- 72.Persidsky Y, Heilman D, Haorah J, Zelivyanskaya M, Persidsky R, Weber GA, et al. Rho-mediated regulation of tight junctions during monocyte migration across the blood–brain barrier in HIV-1 encephalitis (HIVE) Blood. 2006;107(12):4770–4780. doi: 10.1182/blood-2005-11-4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bronstein JM, Popper P, Micevych PE, Farber DB. Isolation and characterization of a novel oligodendrocyte-specific protein. Neurology. 1996;47(3):772–778. doi: 10.1212/WNL.47.3.772. [DOI] [PubMed] [Google Scholar]
- 74.Uchida Y, Sumiya T, Tachikawa M, Yamakawa T, Murata S, Yagi Y, et al. Involvement of claudin-11 in disruption of blood–brain, -spinal cord, and -arachnoid barriers in multiple sclerosis. Mol Neurobiol. 2019;56(3):2039–2056. doi: 10.1007/s12035-018-1207-5. [DOI] [PubMed] [Google Scholar]
- 75.Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S, et al. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123(6 Pt 2):1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ando-Akatsuka Y, Saitou M, Hirase T, Kishi M, Sakakibara A, Itoh M, et al. Interspecies diversity of the occludin sequence: cDNA cloning of human, mouse, dog, and rat-kangaroo homologues. J Cell Biol. 1996;133(1):43–47. doi: 10.1083/jcb.133.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hirase T, Staddon JM, Saitou M, Ando-Akatsuka Y, Itoh M, Furuse M, et al. Occludin as a possible determinant of tight junction permeability in endothelial cells. J Cell Sci. 1997;110(Pt 14):1603–1613. doi: 10.1242/jcs.110.14.1603. [DOI] [PubMed] [Google Scholar]
- 78.Papadopoulos MC, Saadoun S, Woodrow CJ, Davies DC, Costa-Martins P, Moss RF, et al. Occludin expression in microvessels of neoplastic and non-neoplastic human brain. Neuropathol Appl Neurobiol. 2001;27(5):384–395. doi: 10.1046/j.0305-1846.2001.00341.x. [DOI] [PubMed] [Google Scholar]
- 79.Matter K, Balda MS. Signalling to and from tight junctions. Nat Rev Mol Cell Biol. 2003;4(3):225–236. doi: 10.1038/nrm1055. [DOI] [PubMed] [Google Scholar]
- 80.Sonoda N, Furuse M, Sasaki H, Yonemura S, Katahira J, Horiguchi Y, et al. Clostridium perfringens enterotoxin fragment removes specific claudins from tight junction strands: evidence for direct involvement of claudins in tight junction barrier. J Cell Biol. 1999;147(1):195–204. doi: 10.1083/jcb.147.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Martin-Padura I, Lostaglio S, Schneemann M, Williams L, Romano M, Fruscella P, et al. Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J Cell Biol. 1998;142(1):117–127. doi: 10.1083/jcb.142.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Aurrand-Lions M, Johnson-Leger C, Wong C, Du Pasquier L, Imhof BA. Heterogeneity of endothelial junctions is reflected by differential expression and specific subcellular localization of the three JAM family members. Blood. 2001;98(13):3699–3707. doi: 10.1182/blood.V98.13.3699. [DOI] [PubMed] [Google Scholar]
- 83.Bazzoni G, Martinez-Estrada OM, Mueller F, Nelboeck P, Schmid G, Bartfai T, et al. Homophilic interaction of junctional adhesion molecule. J Biol Chem. 2000;275(40):30970–30976. doi: 10.1074/jbc.M003946200. [DOI] [PubMed] [Google Scholar]
- 84.Itoh M, Furuse M, Morita K, Kubota K, Saitou M, Tsukita S. Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J Cell Biol. 1999;147(6):1351–1363. doi: 10.1083/jcb.147.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mitic LL, Van Itallie CM, Anderson JM. Molecular physiology and pathophysiology of tight junctions I. Tight junction structure and function: lessons from mutant animals and proteins. Am J Physiol Gastrointest Liver Physiol. 2000;279(2):G250–G254. doi: 10.1152/ajpgi.2000.279.2.G250. [DOI] [PubMed] [Google Scholar]
- 86.Ebnet K, Schulz CU, Meyer Zu Brickwedde MK, Pendl GG, Vestweber D. Junctional adhesion molecule interacts with the PDZ domain-containing proteins AF-6 and ZO-1. J Biol Chem. 2000;275(36):27979–27988. doi: 10.1074/jbc.M002363200. [DOI] [PubMed] [Google Scholar]
- 87.Haskins J, Gu L, Wittchen ES, Hibbard J, Stevenson BR. ZO-3, a novel member of the MAGUK protein family found at the tight junction, interacts with ZO-1 and occludin. J Cell Biol. 1998;141(1):199–208. doi: 10.1083/jcb.141.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bauer H-C, Traweger A, Bauer H. Blood–spinal cord and brain barriers in health and disease. Amsterdam: Elsevier; 2004. Proteins of the tight junction in the blood–brain barrier; pp. 1–10. [Google Scholar]
- 89.Balda MS, Gonzalez-Mariscal L, Contreras RG, Macias-Silva M, Torres-Marquez ME, Garcia-Sainz JA, et al. Assembly and sealing of tight junctions: possible participation of G-proteins, phospholipase C, protein kinase C and calmodulin. J Membr Biol. 1991;122(3):193–202. doi: 10.1007/BF01871420. [DOI] [PubMed] [Google Scholar]
- 90.Rennels ML, Gregory TF, Fujimoto K. Innervation of capillaries by local neurons in the cat hypothalamus: a light microscopic study with horseradish peroxidase. J Cereb Blood Flow Metab. 1983;3(4):535–542. doi: 10.1038/jcbfm.1983.82. [DOI] [PubMed] [Google Scholar]
- 91.Ott MJ, Olson JL, Ballermann BJ. Chronic in vitro flow promotes ultrastructural differentiation of endothelial cells. Endothelium. 1995;3(1):21–30. doi: 10.3109/10623329509024655. [DOI] [Google Scholar]
- 92.Akimoto S, Mitsumata M, Sasaguri T, Yoshida Y. Laminar shear stress inhibits vascular endothelial cell proliferation by inducing cyclin-dependent kinase inhibitor p21(Sdi1/Cip1/Waf1) Circ Res. 2000;86(2):185–190. doi: 10.1161/01.RES.86.2.185. [DOI] [PubMed] [Google Scholar]
- 93.Ngai AC, Winn HR. Modulation of cerebral arteriolar diameter by intraluminal flow and pressure. Circ Res. 1995;77(4):832–840. doi: 10.1161/01.RES.77.4.832. [DOI] [PubMed] [Google Scholar]
- 94.Desai SY, Marroni M, Cucullo L, Krizanac-Bengez L, Mayberg MR, Hossain MT, et al. Mechanisms of endothelial survival under shear stress. Endothelium. 2002;9(2):89–102. doi: 10.1080/10623320212004. [DOI] [PubMed] [Google Scholar]
- 95.Krizanac-Bengez L, Kapural M, Parkinson F, Cucullo L, Hossain M, Mayberg MR, et al. Effects of transient loss of shear stress on blood–brain barrier endothelium: role of nitric oxide and IL-6. Brain Res. 2003;977(2):239–246. doi: 10.1016/S0006-8993(03)02689-1. [DOI] [PubMed] [Google Scholar]
- 96.Cucullo L, Hossain M, Puvenna V, Marchi N, Janigro D. The role of shear stress in blood–brain barrier endothelial physiology. BMC Neurosci. 2011;12:40. doi: 10.1186/1471-2202-12-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.DeStefano JG, Xu ZS, Williams AJ, Yimam N, Searson PC. Effect of shear stress on iPSC-derived human brain microvascular endothelial cells (dhBMECs) Fluids Barriers CNS. 2017;14(1):20. doi: 10.1186/s12987-017-0068-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rochfort KD, Cummins PM. Thrombomodulin regulation in human brain microvascular endothelial cells in vitro: role of cytokines and shear stress. Microvasc Res. 2015;97:1–5. doi: 10.1016/j.mvr.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 99.Bhalerao A, Sivandzade F, Archie SR, Chowdhury EA, Noorani B, Cucullo L. In vitro modeling of the neurovascular unit: advances in the field. Fluids Barriers CNS. 2020;17(1):22. doi: 10.1186/s12987-020-00183-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sivandzade F, Cucullo L. In-vitro blood–brain barrier modeling: A review of modern and fast-advancing technologies. J Cereb Blood Flow Metab. 2018;38(10):1667–1681. doi: 10.1177/0271678X18788769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bradbury MW, Stubbs J, Hughes IE, Parker P. The distribution of potassium, sodium, chloride and urea between lumbar cerebrospinal fluid and blood serum in human subjects. Clin Sci. 1963;25:97–105. [PubMed] [Google Scholar]
- 102.Hansen AJ. Effect of anoxia on ion distribution in the brain. Physiol Rev. 1985;65(1):101–148. doi: 10.1152/physrev.1985.65.1.101. [DOI] [PubMed] [Google Scholar]
- 103.Jeong SM, Hahm KD, Shin JW, Leem JG, Lee C, Han SM. Changes in magnesium concentration in the serum and cerebrospinal fluid of neuropathic rats. Acta Anaesthesiol Scand. 2006;50(2):211–216. doi: 10.1111/j.1399-6576.2006.00925.x. [DOI] [PubMed] [Google Scholar]
- 104.Nischwitz V, Berthele A, Michalke B. Speciation analysis of selected metals and determination of their total contents in paired serum and cerebrospinal fluid samples: an approach to investigate the permeability of the human blood–cerebrospinal fluid-barrier. Anal Chim Acta. 2008;627(2):258–269. doi: 10.1016/j.aca.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 105.Gendelman HE, Ding S, Gong N, Liu J, Ramirez SH, Persidsky Y, et al. Monocyte chemotactic protein-1 regulates voltage-gated K+ channels and macrophage transmigration. J Neuroimmune Pharmacol. 2009;4(1):47–59. doi: 10.1007/s11481-008-9135-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wilhelm I, Farkas AE, Nagyoszi P, Varo G, Balint Z, Vegh GA, et al. Regulation of cerebral endothelial cell morphology by extracellular calcium. Phys Med Biol. 2007;52(20):6261–6274. doi: 10.1088/0031-9155/52/20/012. [DOI] [PubMed] [Google Scholar]
- 107.Sweeney MD, Zhao Z, Montagne A, Nelson AR, Zlokovic BV. Blood–brain barrier: from physiology to disease and back. Physiol Rev. 2019;99(1):21–78. doi: 10.1152/physrev.00050.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hladky SB, Barrand MA. Fluid and ion transfer across the blood–brain and blood–cerebrospinal fluid barriers; a comparative account of mechanisms and roles. Fluids Barriers CNS. 2016;13(1):19. doi: 10.1186/s12987-016-0040-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bernacki J, Dobrowolska A, Nierwinska K, Malecki A. Physiology and pharmacological role of the blood–brain barrier. Pharmacol Rep. 2008;60(5):600–622. [PubMed] [Google Scholar]
- 110.Hladky SB, Barrand MA. Elimination of substances from the brain parenchyma: efflux via perivascular pathways and via the blood–brain barrier. Fluids Barriers CNS. 2018;15(1):30. doi: 10.1186/s12987-018-0113-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nadal A, Fuentes E, Pastor J, McNaughton PA. Plasma albumin is a potent trigger of calcium signals and DNA synthesis in astrocytes. Proc Natl Acad Sci USA. 1995;92(5):1426–1430. doi: 10.1073/pnas.92.5.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gingrich MB, Traynelis SF. Serine proteases and brain damage—is there a link? Trends Neurosci. 2000;23(9):399–407. doi: 10.1016/S0166-2236(00)01617-9. [DOI] [PubMed] [Google Scholar]
- 113.Lewey L. Force-feeding-a clinical or administrative decision? Can Med Assoc J. 1977;116(4):416–419. [PMC free article] [PubMed] [Google Scholar]
- 114.Lim DA, Huang YC, Alvarez-Buylla A. The adult neural stem cell niche: lessons for future neural cell replacement strategies. Neurosurg Clin N Am. 2007;18(1):81–92, ix. [DOI] [PubMed]
- 115.Liu X, Tu M, Kelly RS, Chen C, Smith BJ. Development of a computational approach to predict blood–brain barrier permeability. Drug Metab Dispos. 2004;32(1):132–139. doi: 10.1124/dmd.32.1.132. [DOI] [PubMed] [Google Scholar]
- 116.Clark DE. In silico prediction of blood–brain barrier permeation. Drug Discov Today. 2003;8(20):927–933. doi: 10.1016/S1359-6446(03)02827-7. [DOI] [PubMed] [Google Scholar]
- 117.Fischer H, Gottschlich R, Seelig A. Blood–brain barrier permeation: molecular parameters governing passive diffusion. J Membr Biol. 1998;165(3):201–211. doi: 10.1007/s002329900434. [DOI] [PubMed] [Google Scholar]
- 118.Trauble H. The movement of molecules across lipid membranes: a molecular theory. J Membr Biol. 1971;4(1):193–208. doi: 10.1007/BF02431971. [DOI] [PubMed] [Google Scholar]
- 119.Marrink SJ, Jahnig F, Berendsen HJ. Proton transport across transient single-file water pores in a lipid membrane studied by molecular dynamics simulations. Biophys J. 1996;71(2):632–647. doi: 10.1016/S0006-3495(96)79264-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pardridge WM, Mietus LJ. Transport of steroid hormones through the rat blood–brain barrier. Primary role of albumin-bound hormone. J Clin Invest. 1979;64(1):145–154. doi: 10.1172/JCI109433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Diamond JM, Wright EM. Molecular forces governing non-electrolyte permeation through cell membranes. Proc R Soc Lond B Biol Sci. 1969;171(1028):273–316. doi: 10.1098/rspb.1969.0022. [DOI] [PubMed] [Google Scholar]
- 122.Gleeson MP. Generation of a set of simple, interpretable ADMET rules of thumb. J Med Chem. 2008;51(4):817–834. doi: 10.1021/jm701122q. [DOI] [PubMed] [Google Scholar]
- 123.Eilers M, Roy U, Mondal D. MRP (ABCC) transporters-mediated efflux of anti-HIV drugs, saquinavir and zidovudine, from human endothelial cells. Exp Biol Med (Maywood) 2008;233(9):1149–1160. doi: 10.3181/0802-RM-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Forster F, Volz A, Fricker G. Compound profiling for ABCC2 (MRP2) using a fluorescent microplate assay system. Eur J Pharm Biopharm. 2008;69(1):396–403. doi: 10.1016/j.ejpb.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 125.Ghose AK, Viswanadhan VN, Wendoloski JJ. A knowledge-based approach in designing combinatorial or medicinal chemistry libraries for drug discovery. 1. A qualitative and quantitative characterization of known drug databases. J Comb Chem. 1999;1(1):55–68. doi: 10.1021/cc9800071. [DOI] [PubMed] [Google Scholar]
- 126.Miller DS. Regulation of ABC transporters blood–brain barrier: the good, the bad, and the ugly. Adv Cancer Res. 2015;125:43–70. doi: 10.1016/bs.acr.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 127.Cirrito JR, Deane R, Fagan AM, Spinner ML, Parsadanian M, Finn MB, et al. P-glycoprotein deficiency at the blood–brain barrier increases amyloid-beta deposition in an Alzheimer disease mouse model. J Clin Invest. 2005;115(11):3285–3290. doi: 10.1172/JCI25247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Roberts LM, Black DS, Raman C, Woodford K, Zhou M, Haggerty JE, et al. Subcellular localization of transporters along the rat blood–brain barrier and blood–cerebral–spinal fluid barrier by in vivo biotinylation. Neuroscience. 2008;155(2):423–438. doi: 10.1016/j.neuroscience.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 129.Shimizu F, Sano Y, Maeda T, Abe MA, Nakayama H, Takahashi R, et al. Peripheral nerve pericytes originating from the blood–nerve barrier expresses tight junctional molecules and transporters as barrier-forming cells. J Cell Physiol. 2008;217(2):388–399. doi: 10.1002/jcp.21508. [DOI] [PubMed] [Google Scholar]
- 130.Lin L, Yee SW, Kim RB, Giacomini KM. SLC transporters as therapeutic targets: emerging opportunities. Nat Rev Drug Discov. 2015;14(8):543–560. doi: 10.1038/nrd4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ohtsuki S, Terasaki T. Contribution of carrier-mediated transport systems to the blood–brain barrier as a supporting and protecting interface for the brain; importance for CNS drug discovery and development. Pharm Res. 2007;24(9):1745–1758. doi: 10.1007/s11095-007-9374-5. [DOI] [PubMed] [Google Scholar]
- 132.Abbott NJ. Astrocyte-endothelial interactions and blood–brain barrier permeability. J Anat. 2002;200(6):629–638. doi: 10.1046/j.1469-7580.2002.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Begley DJ, Brightman MW. Structural and functional aspects of the blood–brain barrier. Prog Drug Res. 2003;61:39–78. doi: 10.1007/978-3-0348-8049-7_2. [DOI] [PubMed] [Google Scholar]
- 134.Zlokovic BV, Begley DJ, Chain-Eliash DG. Blood–brain barrier permeability to leucine-enkephalin, d-alanine2-d-leucine5-enkephalin and their N-terminal amino acid (tyrosine) Brain Res. 1985;336(1):125–132. doi: 10.1016/0006-8993(85)90423-8. [DOI] [PubMed] [Google Scholar]
- 135.Zlokovic BV. Cerebrovascular permeability to peptides: manipulations of transport systems at the blood–brain barrier. Pharm Res. 1995;12(10):1395–1406. doi: 10.1023/A:1016254514167. [DOI] [PubMed] [Google Scholar]
- 136.Zlokovic BV. The blood–brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57(2):178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 137.Sauer I, Dunay IR, Weisgraber K, Bienert M, Dathe M. An apolipoprotein E-derived peptide mediates uptake of sterically stabilized liposomes into brain capillary endothelial cells. Biochemistry. 2005;44(6):2021–2029. doi: 10.1021/bi048080x. [DOI] [PubMed] [Google Scholar]
- 138.Claudio L, Kress Y, Norton WT, Brosnan CF. Increased vesicular transport and decreased mitochondrial content in blood–brain barrier endothelial cells during experimental autoimmune encephalomyelitis. Am J Pathol. 1989;135(6):1157–1168. [PMC free article] [PubMed] [Google Scholar]
- 139.Stewart PA. Endothelial vesicles in the blood–brain barrier: are they related to permeability? Cell Mol Neurobiol. 2000;20(2):149–163. doi: 10.1023/A:1007026504843. [DOI] [PubMed] [Google Scholar]
- 140.Duffy KR, Pardridge WM. Blood–brain barrier transcytosis of insulin in developing rabbits. Brain Res. 1987;420(1):32–38. doi: 10.1016/0006-8993(87)90236-8. [DOI] [PubMed] [Google Scholar]
- 141.Skarlatos S, Yoshikawa T, Pardridge WM. Transport of [125I]transferrin through the rat blood–brain barrier. Brain Res. 1995;683(2):164–171. doi: 10.1016/0006-8993(95)00363-U. [DOI] [PubMed] [Google Scholar]
- 142.Zhang Y, Pardridge WM. Mediated efflux of IgG molecules from brain to blood across the blood–brain barrier. J Neuroimmunol. 2001;114(1–2):168–172. doi: 10.1016/S0165-5728(01)00242-9. [DOI] [PubMed] [Google Scholar]
- 143.Triguero D, Buciak J, Pardridge WM. Capillary depletion method for quantification of blood–brain barrier transport of circulating peptides and plasma proteins. J Neurochem. 1990;54(6):1882–1888. doi: 10.1111/j.1471-4159.1990.tb04886.x. [DOI] [PubMed] [Google Scholar]
- 144.Che C, Yang G, Thiot C, Lacoste MC, Currie JC, Demeule M, et al. New angiopep-modified doxorubicin (ANG1007) and etoposide (ANG1009) chemotherapeutics with increased brain penetration. J Med Chem. 2010;53(7):2814–2824. doi: 10.1021/jm9016637. [DOI] [PubMed] [Google Scholar]
- 145.Zhou QH, Boado RJ, Lu JZ, Hui EK, Pardridge WM. Monoclonal antibody-glial-derived neurotrophic factor fusion protein penetrates the blood–brain barrier in the mouse. Drug Metab Dispos. 2010;38(4):566–572. doi: 10.1124/dmd.109.031534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Pardridge WM. Molecular Trojan horses for blood–brain barrier drug delivery. Discov Med. 2006;6(34):139–143. [PubMed] [Google Scholar]
- 147.Pardridge WM. Blood–brain barrier drug targeting: the future of brain drug development. Mol Interv. 2003;3(2):90–105, 51. [DOI] [PubMed]
- 148.Pardridge WM. Blood–brain barrier delivery. Drug Discov Today. 2007;12(1–2):54–61. doi: 10.1016/j.drudis.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 149.Nguyen LN, Ma D, Shui G, Wong P, Cazenave-Gassiot A, Zhang X, et al. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature. 2014;509(7501):503–506. doi: 10.1038/nature13241. [DOI] [PubMed] [Google Scholar]
- 150.Ben-Zvi A, Lacoste B, Kur E, Andreone BJ, Mayshar Y, Yan H, et al. Mfsd2a is critical for the formation and function of the blood–brain barrier. Nature. 2014;509(7501):507–511. doi: 10.1038/nature13324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhao Z, Zlokovic BV. Blood–brain barrier: a dual life of MFSD2A? Neuron. 2014;82(4):728–730. doi: 10.1016/j.neuron.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Glezer I, Simard AR, Rivest S. Neuroprotective role of the innate immune system by microglia. Neuroscience. 2007;147(4):867–883. doi: 10.1016/j.neuroscience.2007.02.055. [DOI] [PubMed] [Google Scholar]
- 153.Engelhardt B, Wolburg H. Mini-review: Transendothelial migration of leukocytes: through the front door or around the side of the house? Eur J Immunol. 2004;34(11):2955–2963. doi: 10.1002/eji.200425327. [DOI] [PubMed] [Google Scholar]
- 154.Davoust N, Vuaillat C, Androdias G, Nataf S. From bone marrow to microglia: barriers and avenues. Trends Immunol. 2008;29(5):227–234. doi: 10.1016/j.it.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 155.Anthony DC, Bolton SJ, Fearn S, Perry VH. Age-related effects of interleukin-1 beta on polymorphonuclear neutrophil-dependent increases in blood–brain barrier permeability in rats. Brain. 1997;120(Pt 3):435–444. doi: 10.1093/brain/120.3.435. [DOI] [PubMed] [Google Scholar]
- 156.Carman CV, Martinelli R. T lymphocyte-endothelial interactions: emerging understanding of trafficking and antigen-specific immunity. Front Immunol. 2015;6:603. doi: 10.3389/fimmu.2015.00603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Larochelle C, Cayrol R, Kebir H, Alvarez JI, Lecuyer MA, Ifergan I, et al. Melanoma cell adhesion molecule identifies encephalitogenic T lymphocytes and promotes their recruitment to the central nervous system. Brain. 2012;135(Pt 10):2906–2924. doi: 10.1093/brain/aws212. [DOI] [PubMed] [Google Scholar]
- 158.Cayrol R, Wosik K, Berard JL, Dodelet-Devillers A, Ifergan I, Kebir H, et al. Activated leukocyte cell adhesion molecule promotes leukocyte trafficking into the central nervous system. Nat Immunol. 2008;9(2):137–145. doi: 10.1038/ni1551. [DOI] [PubMed] [Google Scholar]
- 159.Liebner S, Dijkhuizen RM, Reiss Y, Plate KH, Agalliu D, Constantin G. Functional morphology of the blood–brain barrier in health and disease. Acta Neuropathol. 2018;135(3):311–336. doi: 10.1007/s00401-018-1815-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Winger RC, Harp CT, Chiang MY, Sullivan DP, Watson RL, Weber EW, et al. Cutting edge: CD99 is a novel therapeutic target for control of T cell-mediated central nervous system autoimmune disease. J Immunol. 2016;196(4):1443–1448. doi: 10.4049/jimmunol.1501634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Correale J, Villa A. The blood–brain-barrier in multiple sclerosis: functional roles and therapeutic targeting. Autoimmunity. 2007;40(2):148–160. doi: 10.1080/08916930601183522. [DOI] [PubMed] [Google Scholar]
- 162.Kaur C, Ling EA. Blood brain barrier in hypoxic-ischemic conditions. Curr Neurovasc Res. 2008;5(1):71–81. doi: 10.2174/156720208783565645. [DOI] [PubMed] [Google Scholar]
- 163.Desai BS, Monahan AJ, Carvey PM, Hendey B. Blood–brain barrier pathology in Alzheimer’s and Parkinson’s disease: implications for drug therapy. Cell Transplant. 2007;16(3):285–299. doi: 10.3727/000000007783464731. [DOI] [PubMed] [Google Scholar]
- 164.Remy S, Beck H. Molecular and cellular mechanisms of pharmacoresistance in epilepsy. Brain. 2006;129(Pt 1):18–35. doi: 10.1093/brain/awh682. [DOI] [PubMed] [Google Scholar]
- 165.Bronger H, Konig J, Kopplow K, Steiner HH, Ahmadi R, Herold-Mende C, et al. ABCC drug efflux pumps and organic anion uptake transporters in human gliomas and the blood–tumor barrier. Cancer Res. 2005;65(24):11419–11428. doi: 10.1158/0008-5472.CAN-05-1271. [DOI] [PubMed] [Google Scholar]
- 166.Grieshaber MC, Flammer J. Does the blood–brain barrier play a role in glaucoma? Surv Ophthalmol. 2007;52(Suppl 2):S115–S121. doi: 10.1016/j.survophthal.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 167.Begley DJ, Pontikis CC, Scarpa M. Lysosomal storage diseases and the blood–brain barrier. Curr Pharm Des. 2008;14(16):1566–1580. doi: 10.2174/138161208784705504. [DOI] [PubMed] [Google Scholar]
- 168.Moya ML, Triplett M, Simon M, Alvarado J, Booth R, Osburn J, et al. A reconfigurable in vitro model for studying the blood–brain barrier. Ann Biomed Eng. 2020;48(2):780–793. doi: 10.1007/s10439-019-02405-y. [DOI] [PubMed] [Google Scholar]
- 169.Persidsky Y, Ramirez SH, Haorah J, Kanmogne GD. Blood–brain barrier: structural components and function under physiologic and pathologic conditions. J Neuroimmune Pharmacol. 2006;1(3):223–236. doi: 10.1007/s11481-006-9025-3. [DOI] [PubMed] [Google Scholar]
- 170.Chen Y, McCarron RM, Azzam N, Bembry J, Reutzler C, Lenz FA, et al. Endothelin-1 and nitric oxide affect human cerebromicrovascular endothelial responses and signal transduction. Acta Neurochir Suppl. 2000;76:131–135. doi: 10.1007/978-3-7091-6346-7_27. [DOI] [PubMed] [Google Scholar]
- 171.Kustova Y, Grinberg A, Basile AS. Increased blood–brain barrier permeability in LP-BM5 infected mice is mediated by neuroexcitatory mechanisms. Brain Res. 1999;839(1):153–163. doi: 10.1016/S0006-8993(99)01734-5. [DOI] [PubMed] [Google Scholar]
- 172.Annunziata P, Cioni C, Toneatto S, Paccagnini E. HIV-1 gp120 increases the permeability of rat brain endothelium cultures by a mechanism involving substance P. AIDS. 1998;12(18):2377–2385. doi: 10.1097/00002030-199818000-00006. [DOI] [PubMed] [Google Scholar]
- 173.St'astny F, Skultetyova I, Pliss L, Jezova D. Quinolinic acid enhances permeability of rat brain microvessels to plasma albumin. Brain Res Bull. 2000;53(4):415–420. doi: 10.1016/S0361-9230(00)00368-3. [DOI] [PubMed] [Google Scholar]
- 174.Feuerstein GZ, Liu T, Barone FC. Cytokines, inflammation, and brain injury: role of tumor necrosis factor-alpha. Cerebrovasc Brain Metab Rev. 1994;6(4):341–360. [PubMed] [Google Scholar]
- 175.Tarkowski E, Rosengren L, Blomstrand C, Wikkelso C, Jensen C, Ekholm S, et al. Intrathecal release of pro- and anti-inflammatory cytokines during stroke. Clin Exp Immunol. 1997;110(3):492–499. doi: 10.1046/j.1365-2249.1997.4621483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zhang W, Smith C, Shapiro A, Monette R, Hutchison J, Stanimirovic D. Increased expression of bioactive chemokines in human cerebromicrovascular endothelial cells and astrocytes subjected to simulated ischemia in vitro. J Neuroimmunol. 1999;101(2):148–160. doi: 10.1016/S0165-5728(99)00137-X. [DOI] [PubMed] [Google Scholar]
- 177.Zhang W, Smith C, Howlett C, Stanimirovic D. Inflammatory activation of human brain endothelial cells by hypoxic astrocytes in vitro is mediated by IL-1beta. J Cereb Blood Flow Metab. 2000;20(6):967–978. doi: 10.1097/00004647-200006000-00009. [DOI] [PubMed] [Google Scholar]
- 178.Bolton SJ, Anthony DC, Perry VH. Loss of the tight junction proteins occludin and zonula occludens-1 from cerebral vascular endothelium during neutrophil-induced blood–brain barrier breakdown in vivo. Neuroscience. 1998;86(4):1245–1257. doi: 10.1016/S0306-4522(98)00058-X. [DOI] [PubMed] [Google Scholar]
- 179.Mark KS, Davis TP. Cerebral microvascular changes in permeability and tight junctions induced by hypoxia-reoxygenation. Am J Physiol Heart Circ Physiol. 2002;282(4):H1485–H1494. doi: 10.1152/ajpheart.00645.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Groothuis DR, Vriesendorp FJ, Kupfer B, Warnke PC, Lapin GD, Kuruvilla A, et al. Quantitative measurements of capillary transport in human brain tumors by computed tomography. Ann Neurol. 1991;30(4):581–588. doi: 10.1002/ana.410300411. [DOI] [PubMed] [Google Scholar]
- 181.Long DM. Capillary ultrastructure and the blood–brain barrier in human malignant brain tumors. J Neurosurg. 1970;32(2):127–144. doi: 10.3171/jns.1970.32.2.0127. [DOI] [PubMed] [Google Scholar]
- 182.de Vries HE, Blom-Roosemalen MC, van Oosten M, de Boer AG, van Berkel TJ, Breimer DD, et al. The influence of cytokines on the integrity of the blood–brain barrier in vitro. J Neuroimmunol. 1996;64(1):37–43. doi: 10.1016/0165-5728(95)00148-4. [DOI] [PubMed] [Google Scholar]
- 183.Saadoun S, Papadopoulos MC, Davies DC, Krishna S, Bell BA. Aquaporin-4 expression is increased in oedematous human brain tumours. J Neurol Neurosurg Psychiatry. 2002;72(2):262–265. doi: 10.1136/jnnp.72.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Taniguchi M, Yamashita T, Kumura E, Tamatani M, Kobayashi A, Yokawa T, et al. Induction of aquaporin-4 water channel mRNA after focal cerebral ischemia in rat. Brain Res Mol Brain Res. 2000;78(1–2):131–137. doi: 10.1016/S0169-328X(00)00084-X. [DOI] [PubMed] [Google Scholar]
- 185.Vizuete ML, Venero JL, Vargas C, Ilundain AA, Echevarria M, Machado A, et al. Differential upregulation of aquaporin-4 mRNA expression in reactive astrocytes after brain injury: potential role in brain edema. Neurobiol Dis. 1999;6(4):245–258. doi: 10.1006/nbdi.1999.0246. [DOI] [PubMed] [Google Scholar]
- 186.Norden AD, Wen PY, Kesari S. Brain metastases. Curr Opin Neurol. 2005;18(6):654–661. doi: 10.1097/01.wco.0000191514.37498.2b. [DOI] [PubMed] [Google Scholar]
- 187.Steeg PS, Camphausen KA, Smith QR. Brain metastases as preventive and therapeutic targets. Nat Rev Cancer. 2011;11(5):352–363. doi: 10.1038/nrc3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Arvanitis CD, Ferraro GB, Jain RK. The blood–brain barrier and blood–tumour barrier in brain tumours and metastases. Nat Rev Cancer. 2020;20(1):26–41. doi: 10.1038/s41568-019-0205-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lockman PR, Mittapalli RK, Taskar KS, Rudraraju V, Gril B, Bohn KA, et al. Heterogeneous blood–tumor barrier permeability determines drug efficacy in experimental brain metastases of breast cancer. Clin Cancer Res. 2010;16(23):5664–5678. doi: 10.1158/1078-0432.CCR-10-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Osswald M, Blaes J, Liao Y, Solecki G, Gommel M, Berghoff AS, et al. Impact of blood–brain barrier integrity on tumor growth and therapy response in brain metastases. Clin Cancer Res. 2016;22(24):6078–6087. doi: 10.1158/1078-0432.CCR-16-1327. [DOI] [PubMed] [Google Scholar]
- 191.Papadopoulos MC, Davies DC, Moss RF, Tighe D, Bennett ED. Pathophysiology of septic encephalopathy: a review. Crit Care Med. 2000;28(8):3019–3024. doi: 10.1097/00003246-200008000-00057. [DOI] [PubMed] [Google Scholar]
- 192.Clawson CC, Hartmann JF, Vernier RL. Electron microscopy of the effect of gram-negative endotoxin on the blood–brain barrier. J Comp Neurol. 1966;127(2):183–198. doi: 10.1002/cne.901270204. [DOI] [PubMed] [Google Scholar]
- 193.Jeppsson B, Freund HR, Gimmon Z, James JH, von Meyenfeldt MF, Fischer JE. Blood–brain barrier derangement in sepsis: cause of septic encephalopathy? Am J Surg. 1981;141(1):136–142. doi: 10.1016/0002-9610(81)90026-X. [DOI] [PubMed] [Google Scholar]
- 194.Deng X, Wang X, Andersson R. Endothelial barrier resistance in multiple organs after septic and nonseptic challenges in the rat. J Appl Physiol. 1995;78(6):2052–2061. doi: 10.1152/jappl.1995.78.6.2052. [DOI] [PubMed] [Google Scholar]
- 195.Tighe D, Moss R, Bennett D. Cell surface adrenergic receptor stimulation modifies the endothelial response to SIRS. Systemic inflammatory response syndrome. New Horiz. 1996;4(4):426–442. [PubMed] [Google Scholar]
- 196.Sharer LR. Pathology of HIV-1 infection of the central nervous system: a review. J Neuropathol Exp Neurol. 1992;51(1):3–11. doi: 10.1097/00005072-199201000-00002. [DOI] [PubMed] [Google Scholar]
- 197.Johnson RT, McArthur JC, Narayan O. The neurobiology of human immunodeficiency virus infections. FASEB J. 1988;2(14):2970–2981. doi: 10.1096/fasebj.2.14.2846395. [DOI] [PubMed] [Google Scholar]
- 198.Petito CK, Cash KS. Blood–brain barrier abnormalities in acquired immunodeficiency syndrome: immunohistochemical localization of serum proteins in postmortem brain. Ann Neurol. 1992;32(5):658–666. doi: 10.1002/ana.410320509. [DOI] [PubMed] [Google Scholar]
- 199.Dallasta LM, Pisarov LA, Esplen JE, Werley JV, Moses AV, Nelson JA, et al. Blood–brain barrier tight junction disruption in human immunodeficiency virus-1 encephalitis. Am J Pathol. 1999;155(6):1915–1927. doi: 10.1016/S0002-9440(10)65511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Toneatto S, Finco O, van der Putten H, Abrignani S, Annunziata P. Evidence of blood–brain barrier alteration and activation in HIV-1 gp120 transgenic mice. AIDS. 1999;13(17):2343–2348. doi: 10.1097/00002030-199912030-00005. [DOI] [PubMed] [Google Scholar]
- 201.Cioni C, Annunziata P. Circulating gp120 alters the blood–brain barrier permeability in HIV-1 gp120 transgenic mice. Neurosci Lett. 2002;330(3):299–301. doi: 10.1016/S0304-3940(02)00814-5. [DOI] [PubMed] [Google Scholar]
- 202.Huang MB, Hunter M, Bond VC. Effect of extracellular human immunodeficiency virus type 1 glycoprotein 120 on primary human vascular endothelial cell cultures. AIDS Res Hum Retrovir. 1999;15(14):1265–1277. doi: 10.1089/088922299310160. [DOI] [PubMed] [Google Scholar]
- 203.Louboutin JP, Strayer DS. Blood–brain barrier abnormalities caused by HIV-1 gp120: mechanistic and therapeutic implications. Sci World J. 2012;2012:482575. doi: 10.1100/2012/482575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Giulian D, Haverkamp LJ, Li J, Karshin WL, Yu J, Tom D, et al. Senile plaques stimulate microglia to release a neurotoxin found in Alzheimer brain. Neurochem Int. 1995;27(1):119–137. doi: 10.1016/0197-0186(95)00067-I. [DOI] [PubMed] [Google Scholar]
- 205.Klegeris A, McGeer PL. beta-amyloid protein enhances macrophage production of oxygen free radicals and glutamate. J Neurosci Res. 1997;49(2):229–235. doi: 10.1002/(SICI)1097-4547(19970715)49:2<229::AID-JNR11>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 206.Akama KT, Albanese C, Pestell RG, Van Eldik LJ. Amyloid beta-peptide stimulates nitric oxide production in astrocytes through an NFkappaB-dependent mechanism. Proc Natl Acad Sci USA. 1998;95(10):5795–5800. doi: 10.1073/pnas.95.10.5795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Sivandzade F, Prasad S, Bhalerao A, Cucullo L. NRF2 and NF-B interplay in cerebrovascular and neurodegenerative disorders: molecular mechanisms and possible therapeutic approaches. Redox Biol. 2019;21:101059. doi: 10.1016/j.redox.2018.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Zhao Z, Sagare AP, Ma Q, Halliday MR, Kong P, Kisler K, et al. Central role for PICALM in amyloid-beta blood–brain barrier transcytosis and clearance. Nat Neurosci. 2015;18(7):978–987. doi: 10.1038/nn.4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Lambert JC, Heath S, Even G, Campion D, Sleegers K, Hiltunen M, et al. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer’s disease. Nat Genet. 2009;41(10):1094–1099. doi: 10.1038/ng.439. [DOI] [PubMed] [Google Scholar]
- 210.Bell RD, Winkler EA, Singh I, Sagare AP, Deane R, Wu Z, et al. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature. 2012;485(7399):512–516. doi: 10.1038/nature11087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Wekerle H, Hohlfeld R. Molecular mimicry in multiple sclerosis. N Engl J Med. 2003;349(2):185–186. doi: 10.1056/NEJMcibr035136. [DOI] [PubMed] [Google Scholar]
- 212.Chao CC, Hu S, Sheng WS, Peterson PK. Tumor necrosis factor-alpha production by human fetal microglial cells: regulation by other cytokines. Dev Neurosci. 1995;17(2):97–105. doi: 10.1159/000111278. [DOI] [PubMed] [Google Scholar]
- 213.Merrill JE, Ignarro LJ, Sherman MP, Melinek J, Lane TE. Microglial cell cytotoxicity of oligodendrocytes is mediated through nitric oxide. J Immunol. 1993;151(4):2132–2141. [PubMed] [Google Scholar]
- 214.Bonda DJ, Wang X, Perry G, Nunomura A, Tabaton M, Zhu X, et al. Oxidative stress in Alzheimer disease: a possibility for prevention. Neuropharmacology. 2010;59(4–5):290–294. doi: 10.1016/j.neuropharm.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 215.Ortiz GG, Macias-Islas MA, Pacheco-Moises FP, Cruz-Ramos JA, Sustersik S, Barba EA, et al. Oxidative stress is increased in serum from Mexican patients with relapsing-remitting multiple sclerosis. Dis Markers. 2009;26(1):35–39. doi: 10.1155/2009/325847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Saunders NR, Dziegielewska KM, Mollgard K, Habgood MD. Markers for blood–brain barrier integrity: how appropriate is Evans blue in the twenty-first century and what are the alternatives? Front Neurosci. 2015;9:385. doi: 10.3389/fnins.2015.00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Noorani B, Chowdhury EA, Alqahtani F, Ahn Y, Patel D, Al-Ahmad A, et al. LC–MS/MS-based in vitro and in vivo investigation of blood–brain barrier integrity by simultaneous quantitation of mannitol and sucrose. Fluids Barriers CNS. 2020;17(1):61. doi: 10.1186/s12987-020-00224-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Rossner W, Tempel K. Quantitative determination of the permeability of the so-called blood–brain barrier of Evans blue (T 1824) Med Pharmacol Exp Int J Exp Med. 1966;14(2):169–182. [PubMed] [Google Scholar]
- 219.Wolman M, Klatzo I, Chui E, Wilmes F, Nishimoto K, Fujiwara K, et al. Evaluation of the dye-protein tracers in pathophysiology of the blood–brain barrier. Acta Neuropathol. 1981;54(1):55–61. doi: 10.1007/BF00691332. [DOI] [PubMed] [Google Scholar]
- 220.Kaya M, Ahishali B. Assessment of permeability in barrier type of endothelium in brain using tracers: Evans blue, sodium fluorescein, and horseradish peroxidase. Methods Mol Biol. 2011;763:369–382. doi: 10.1007/978-1-61779-191-8_25. [DOI] [PubMed] [Google Scholar]
- 221.Clasen RA, Pandolfi S, Hass GM. Vital staining, serum albumin and the blood–brain barrier. J Neuropathol Exp Neurol. 1970;29(2):266–284. doi: 10.1097/00005072-197004000-00008. [DOI] [PubMed] [Google Scholar]
- 222.Reese TS, Karnovsky MJ. Fine structural localization of a blood–brain barrier to exogenous peroxidase. J Cell Biol. 1967;34(1):207–217. doi: 10.1083/jcb.34.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Brightman MW, Reese TS. Junctions between intimately apposed cell membranes in the vertebrate brain. J Cell Biol. 1969;40(3):648–677. doi: 10.1083/jcb.40.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Reyners H, de Reyners EG, Jadin JM, Maisin JR. An ultrastructural quantitative method for the evaluation of the permeability to horseradish peroxidase of cerebral cortex endothelial cells of the rat. Cell Tissue Res. 1975;157(1):93–99. doi: 10.1007/BF00223232. [DOI] [PubMed] [Google Scholar]
- 225.Broadwell RD, Charlton HM, Balin BJ, Salcman M. Angioarchitecture of the CNS, pituitary gland, and intracerebral grafts revealed with peroxidase cytochemistry. J Comp Neurol. 1987;260(1):47–62. doi: 10.1002/cne.902600105. [DOI] [PubMed] [Google Scholar]
- 226.Majno G, Palade GE, Schoefl GI. Studies on inflammation. II. The site of action of histamine and serotonin along the vascular tree: a topographic study. J Biophys Biochem Cytol. 1961;11:607–626. doi: 10.1083/jcb.11.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Cotran RS, Karnovsky MJ. Ultrastructural studies on the permeability of the mesothelium to horseradish peroxidase. J Cell Biol. 1968;37(1):123–137. doi: 10.1083/jcb.37.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Cotran RS, Karnovsky MJ, Goth A. Resistance of Wistar-Furth rats to the mast cell-damaging effect of horseradish peroxidase. J Histochem Cytochem. 1968;16(5):382–383. doi: 10.1177/16.5.382. [DOI] [PubMed] [Google Scholar]
- 229.Hoffman HJ, Olszewski J. Spread of sodium fluorescein in normal brain tissue. A study of the mechanism of the blood–brain barrier. Neurology. 1961;11:1081–1085. doi: 10.1212/WNL.11.12.1081. [DOI] [PubMed] [Google Scholar]
- 230.Malmgren LT, Olsson Y. Differences between the peripheral and the central nervous system in permeability to sodium fluorescein. J Comp Neurol. 1980;191(1):103–107. doi: 10.1002/cne.901910106. [DOI] [PubMed] [Google Scholar]
- 231.Salem H, Loux JJ, Smith S, Nichols CW. Evaluation of the toxicologic and teratogenic potentials of sodium fluorescein in the rat. Toxicology. 1979;12(2):143–150. doi: 10.1016/0300-483X(79)90040-4. [DOI] [PubMed] [Google Scholar]
- 232.Reed DJ, Woodbury DM. Kinetics of movement of iodide, sucrose, inulin and radio-iodinated serum albumin in the central nervous system and cerebrospinal fluid of the rat. J Physiol. 1963;169:816–850. doi: 10.1113/jphysiol.1963.sp007298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Davson H, Segal MB. Effect of cerebrospinal fluid on volume of distribution of extracellular markers. Brain. 1969;92(1):131–136. doi: 10.1093/brain/92.1.131. [DOI] [PubMed] [Google Scholar]
- 234.Oldendorf WH, Davson H. Brain extracellular space and the sink action of cerebrospinal fluid. Measurement of rabbit brain extracellular space using sucrose labeled with carbon 14. Arch Neurol. 1967;17(2):196–205. doi: 10.1001/archneur.1967.00470260086010. [DOI] [PubMed] [Google Scholar]
- 235.Ziylan YZ, Robinson PJ, Rapoport SI. Blood–brain barrier permeability to sucrose and dextran after osmotic opening. Am J Physiol. 1984;247(4 Pt 2):R634–R638. doi: 10.1152/ajpregu.1984.247.4.R634. [DOI] [PubMed] [Google Scholar]
- 236.Preston E, Webster J. Differential passage of [14C]sucrose and [3H]inulin across rat blood–brain barrier after cerebral ischemia. Acta Neuropathol. 2002;103(3):237–242. doi: 10.1007/s004010100458. [DOI] [PubMed] [Google Scholar]
- 237.Miah MK, Chowdhury EA, Bickel U, Mehvar R. Evaluation of [(14)C] and [(13)C]sucrose as blood–brain barrier permeability markers. J Pharm Sci. 2017;106(6):1659–1669. doi: 10.1016/j.xphs.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 238.Preston E, Foster DO, Mills PA. Effects of radiochemical impurities on measurements of transfer constants for [14C]sucrose permeation of normal and injured blood–brain barrier of rats. Brain Res Bull. 1998;45(1):111–116. doi: 10.1016/S0361-9230(97)00278-5. [DOI] [PubMed] [Google Scholar]
- 239.Alqahtani F, Chowdhury EA, Bhattacharya R, Noorani B, Mehvar R, Bickel U. Brain uptake of [13C] and [14C]sucrose quantified by microdialysis and whole tissue analysis in mice. Drug Metab Dispos. 2018;46(11):1514–1518. doi: 10.1124/dmd.118.082909. [DOI] [PubMed] [Google Scholar]
- 240.Miah MK, Bickel U, Mehvar R. Development and validation of a sensitive UPLC-MS/MS method for the quantitation of [(13)C]sucrose in rat plasma, blood, and brain: Its application to the measurement of blood–brain barrier permeability. J Chromatogr B Anal Technol Biomed Life Sci. 2016;1015–1016:105–110. doi: 10.1016/j.jchromb.2016.02.017. [DOI] [PubMed] [Google Scholar]
- 241.Chowdhury EA, Alqahtani F, Bhattacharya R, Mehvar R, Bickel U. Simultaneous UPLC-MS/MS analysis of two stable isotope labeled versions of sucrose in mouse plasma and brain samples as markers of blood–brain barrier permeability and brain vascular space. J Chromatogr B Anal Technol Biomed Life Sci. 2018;1073:19–26. doi: 10.1016/j.jchromb.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 242.Evans CA, Reynolds JM, Reynolds ML, Saunders NR, Segal MB. The development of a blood–brain barrier mechanism in foetal sheep. J Physiol. 1974;238(2):371–386. doi: 10.1113/jphysiol.1974.sp010530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Poduslo JF, Curran GL, Berg CT. Macromolecular permeability across the blood–nerve and blood–brain barriers. Proc Natl Acad Sci USA. 1994;91(12):5705–5709. doi: 10.1073/pnas.91.12.5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Ek CJ, Dziegielewska KM, Stolp H, Saunders NR. Functional effectiveness of the blood–brain barrier to small water-soluble molecules in developing and adult opossum (Monodelphis domestica) J Comp Neurol. 2006;496(1):13–26. doi: 10.1002/cne.20885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Liddelow SA, Dziegielewska KM, Ek CJ, Johansson PA, Potter AM, Saunders NR. Cellular transfer of macromolecules across the developing choroid plexus of Monodelphis domestica. Eur J Neurosci. 2009;29(2):253–266. doi: 10.1111/j.1460-9568.2008.06571.x. [DOI] [PubMed] [Google Scholar]
- 246.Neuwelt EA, Abbott NJ, Drewes L, Smith QR, Couraud PO, Chiocca EA, et al. Cerebrovascular biology and the various neural barriers: challenges and future directions. Neurosurgery. 1999;44(3):604–608. doi: 10.1097/00006123-199903000-00095. [DOI] [PubMed] [Google Scholar]
- 247.Marchi N, Cavaglia M, Fazio V, Bhudia S, Hallene K, Janigro D. Peripheral markers of blood–brain barrier damage. Clin Chim Acta. 2004;342(1–2):1–12. doi: 10.1016/j.cccn.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 248.Kapural M, Krizanac-Bengez L, Barnett G, Perl J, Masaryk T, Apollo D, et al. Serum S-100beta as a possible marker of blood–brain barrier disruption. Brain Res. 2002;940(1–2):102–104. doi: 10.1016/S0006-8993(02)02586-6. [DOI] [PubMed] [Google Scholar]
- 249.Mossakowski MJ, Lossinsky AS, Pluta R, Wisniewski HM. Abnormalities of the blood–brain barrier in global cerebral ischemia in rats due to experimental cardiac arrest. Acta Neurochir Suppl (Wien) 1994;60:274–276. doi: 10.1007/978-3-7091-9334-1_73. [DOI] [PubMed] [Google Scholar]
- 250.Pluta R, Lossinsky AS, Wisniewski HM, Mossakowski MJ. Early blood–brain barrier changes in the rat following transient complete cerebral ischemia induced by cardiac arrest. Brain Res. 1994;633(1–2):41–52. doi: 10.1016/0006-8993(94)91520-2. [DOI] [PubMed] [Google Scholar]
- 251.Reiber H. Cerebrospinal fluid—physiology, analysis and interpretation of protein patterns for diagnosis of neurological diseases. Mult Scler J. 1998;4(3):99–107. doi: 10.1177/135245859800400302. [DOI] [PubMed] [Google Scholar]
- 252.Marchi N, Fazio V, Cucullo L, Kight K, Masaryk T, Barnett G, et al. Serum transthyretin monomer as a possible marker of blood-to-CSF barrier disruption. J Neurosci. 2003;23(5):1949–1955. doi: 10.1523/JNEUROSCI.23-05-01949.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Dyck RH, Van Eldik LJ, Cynader MS. Immunohistochemical localization of the S-100 beta protein in postnatal cat visual cortex: spatial and temporal patterns of expression in cortical and subcortical glia. Brain Res Dev Brain Res. 1993;72(2):181–192. doi: 10.1016/0165-3806(93)90183-B. [DOI] [PubMed] [Google Scholar]
- 254.Mercier F, Hatton GI. Immunocytochemical basis for a meningeo-glial network. J Comp Neurol. 2000;420(4):445–465. doi: 10.1002/(SICI)1096-9861(20000515)420:4<445::AID-CNE4>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 255.Jonsson H, Johnsson P, Alling C, Backstrom M, Bergh C, Blomquist S. S100beta after coronary artery surgery: release pattern, source of contamination, and relation to neuropsychological outcome. Ann Thorac Surg. 1999;68(6):2202–2208. doi: 10.1016/S0003-4975(99)00851-6. [DOI] [PubMed] [Google Scholar]
- 256.Brochez L, Naeyaert JM. Serological markers for melanoma. Br J Dermatol. 2000;143(2):256–268. doi: 10.1046/j.1365-2133.2000.03649.x. [DOI] [PubMed] [Google Scholar]
- 257.Marchi N, Rasmussen P, Kapural M, Fazio V, Kight K, Mayberg MR, et al. Peripheral markers of brain damage and blood–brain barrier dysfunction. Restor Neurol Neurosci. 2003;21(3–4):109–121. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.