Abstract
Reduced cardiac sodium (Na+) channel current (INa) resulting from the loss-of-function of Na+ channel is a major cause of lethal arrhythmias in Brugada syndrome (BrS). Inspired by previous experimental studies which showed that in heart diseases INa was reduced along with expression changes in Na+ channel within myocytes, we hypothesized that the local decrease in INa caused by the alteration in Na+ channel expression in myocytes leads to the occurrence of phase-2 reentry, the major triggering mechanism of lethal arrhythmias in BrS. We constructed in silico human ventricular myocardial strand and ring models, and examined whether the Na+ channel expression changes in each myocyte cause the phase-2 reentry in BrS. Reducing Na+ channel expression in the lateral membrane of each myocyte caused not only the notch-and-dome but also loss-of-dome type action potentials and slowed conduction, both of which are typically observed in BrS patients. Furthermore, the selective reduction in Na+ channels on the lateral membrane of each myocyte together with spatial tissue heterogeneity of Na+ channel expression caused the phase-2 reentry and phase-2 reentry-mediated reentrant arrhythmias. Our data suggest that the BrS phenotype is strongly influenced by expression abnormalities as well as genetic abnormalities of Na+ channels.
Subject terms: Arrhythmias, Computational models, Predictive medicine, Computer modelling
Introduction
Cardiac Na+ channel (Nav1.5) encoded by SCN5A that controls the excitability of cardiomyocytes mainly contributes to the action potential (AP) initiation and its propagation. Na+ channel dysfunction in congenital or acquired heart diseases leads to a decrease in Na+ channel current (INa), which is known to be associated with slowed or blocked conduction, resulting in a potentially proarrhythmic substrate. Genetic abnormalities causing Na+ channel dysfunction have been linked to many arrhythmogenic diseases1, including sick sinus syndrome, progressive cardiac conduction disorders, and Brugada syndrome (BrS)2. A recent clinical study3 highlighted the correlation between the INa decrease and BrS phenotype in patients with SCN5A variants, which is one of the important disease genes for BrS4.
BrS is characterized by the electrocardiograms with both a right bundle-branch block pattern and ST-segment elevation in the right precordial leads (V1–V3) and by the higher incidence of sudden cardiac death due to ventricular tachycardia/fibrillation (VT/VF)5,6. It is commonly believed that VT/VF is elicited by the closely coupled premature ventricular contractions via the phase-2 reentry (P2R) mechanism6. This mechanism is that APs with phase-2 dome in some sites of the tissue re-excite neighboring repolarized sites via electrotonic interaction to evoke an additional conductive AP (triggered activity) (Fig. 1A). Although at first, it has been predicted that a global difference in AP duration (APD) causing a transmural voltage gradient in the right ventricle (RV) is a cause of P2R development7, several experimental studies using an optical mapping system showed that the epicardial local APD difference was involved in the occurrence of P2R8,9. Thus, the P2R mechanism in BrS is still controversial.
Figure 1.
Myocardial strand and ring models. (A) Schematic diagram of phase-2 reentry concept. (B) Schematic representation of a myocardial strand model comprising cylindrical 300 cells (a), of intercellular junction between myocytes (b), of each ventricular myocyte model (c), and of the equivalent circuit of the myocardial strand model (d). The cell membrane in each myocyte is divided into post-junctional (post-JM), lateral (LM), and pre-junctional membrane (pre-JM) segments, and each membrane segment comprises a modified O’Hara-Rudy dynamic (mORd) ventricular myocyte model10–12 and membrane capacitance, Cm,l, for l = post-JM, LM, and pre-JM (c). In (d), represents extracellular cleft potential just after the kth myocyte. , and , for l = post-JM, LM, and pre-JM, indicate the intracellular and extracellular potentials, respectively, of lth segment of the kth myocyte. denotes the transmembrane potential, i.e., . (C) Schematic representations of the myocardial ring model comprising 600 cells. Cell #1 (B) and #300 (C) were electrically stimulated at 1 Hz. Gg, gap junction conductance; Gj, radial conductance of intercellular cleft; Gd, axial conductance of intercellular cleft.
We previously showed that subcellular alterations in Na+ channel expression induced by myocardial ischemia, specifically reduced number of Na+ channels in the lateral membrane (LM) of cardiomyocytes, was strongly associated with both the decrease in local INa and slowed conduction13. Furthermore, the local decreasing of INa in cardiomyocytes led to the development of P2R13. Therefore, we hypothesized that the developments of P2R and VT/VF in BrS are brought about by local alterations in subcellular Na+ channel expression. To validate this hypothesis, we performed simulations of AP propagation in the in silico models and investigated the relationship between the subcellular alterations in Na+ channel expression and the occurrence of P2R, and whether the P2R leads to reentrant arrhythmia onsets in BrS.
Results
Reducing Na+ channel conductance in the LM causes AP morphological changes
Experiments on knock-in mice lacking the Serine-Isoleucine-Valine domain (∆SIV) of the Nav1.5 PDZ domain-binding motif have demonstrated that both INa and Na+ channel expression on the LM, but not on the intercalated discs (IDs), of cardiomyocytes were decreased by the lack of ∆SIV in Na+ channels14,15. Therefore, we first examined effects of selective decreasing of Na+ channel expression on the LM of cardiomyocytes on AP propagation by using a homogeneous myocardial strand model consisting of 300 cells (see “Methods” and Supplementary Methods; Fig. 1B). Throughout the article, we express Na+ channel conductances of the junctional membrane (JM), i.e. ID, and LM as percentages of the control conductance (see “Methods”), i.e., %gNa,JM and %gNa,LM, respectively.
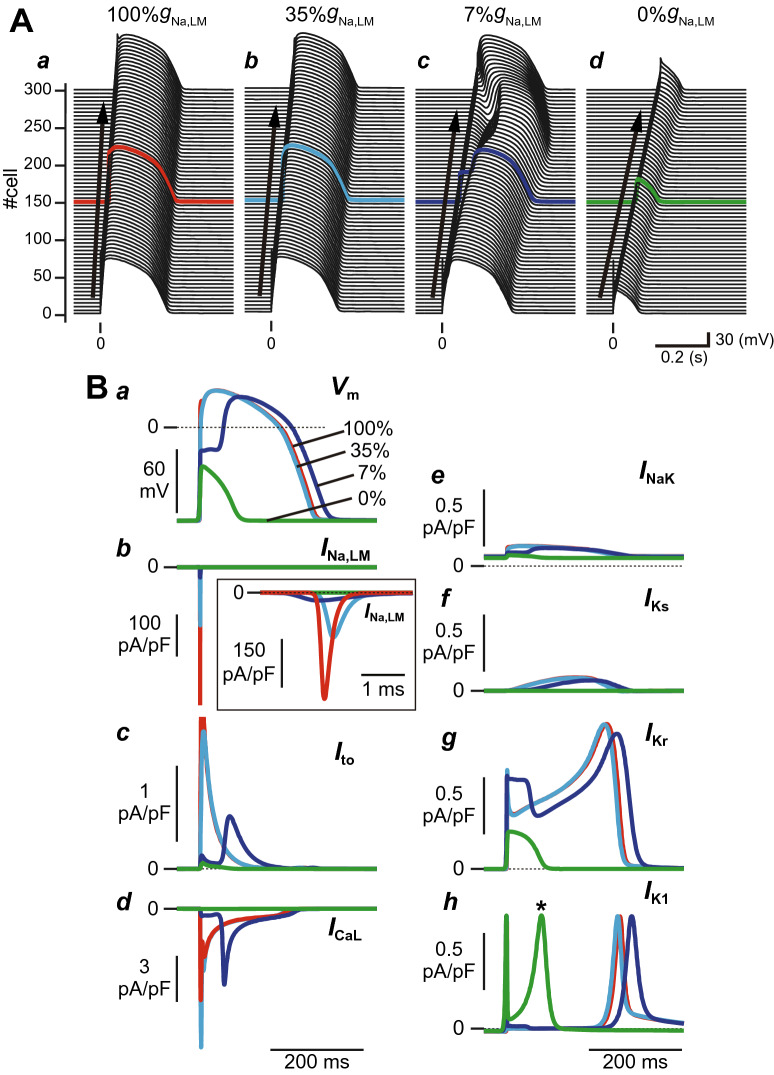
When the Na+ channel conductance in each myocyte of the myocardial fiber was set to the control condition (100%gNa,JM and 100%gNa,LM), the APs propagated through the myocardial strand (Fig. 2Aa) and exhibited a typical human ventricular AP morphology (Fig. 2Aa,Ba, red line). Reducing the %gNa,LM by 65%, corresponding to 51.3% of the total Na+ channel conductance (%gNa,tot) per myocyte, resulted in the decrease in the INa,LM peak in the 150th cell by 57.9% (Fig. 2Bb, cyan line, and Table 1) and slightly shortened the APD; APD at 90% repolarization (APD90) was 236.9 ms in 35%gNa,LM versus 241.8 ms in 100%gNa,LM (Fig. 2Ab,Ba, and Table 1). These results were similar to those experimentally determined by Shy et al.15 (see Supplementary Table S1). As the %gNa,LM further decreased step-by-step (7%gNa,LM; 26.6%gNa,tot), AP phase-0 amplitude decreased, and a larger phase-1 dip in the AP was produced (Fig. 2Ac,Ba, blue lines). As a result, the peak of the phase-2 dome was markedly delayed. APD90 with delayed phase-2 dome peak was 26.9 ms longer than that of the control condition (100%gNa,LM, see Table 1). Moreover, the loss of Na+ channels in the LM of each myocyte (0%gNa,LM; 21.0%gNa,tot) caused a further decrease in the AP phase-0 amplitude (Fig. 2Ba, green line), resulting in the failure of the transient outward K+ channel current (Ito) and L-type Ca2+ channel current (ICaL) to activate normally (Figs. 2Bc,d, green lines). The transient activation of the rapid component of delayed rectifier K+ channel current (IKr) and the Na+-K+ pump current (INaK), and subsequent activation of the inward rectifier K+ channel current (IK1), the late peak of which appeared earlier, allowed for earlier repolarization (Figs. 2Be,g,h). The slow component of delayed rectifier K+ channel current (IKs) hardly contributed to the repolarization (Fig. 2Bf, green line). This, in turn, led to AP propagation with a shortened APD (Fig. 2Ad).
Figure 2.
Effects of changes in the subcellular Na+ channel expression on action potential (AP) morphology and conduction velocity (CV). (A) AP propagation observed in the myocardial strand model with a spatially-homogeneous reduction in Na+ channel conductance in the lateral membrane (LM) of 100%gNa,LM (a), 35%gNa,LM (b), 7%gNa,LM (c), and 0%gNa,LM (d). (B) AP morphological changes (a), INa at the LM, INa,LM (b), Ito (c), ICaL (d), INaK (e), IKs (f), IKr (g), and IK1 (h) at the LM. (C) Schematic diagram of a intercellular junction part in the myocardial strand model (a), the post-junctional (Im,post-JM) and pre-junctional transmembrane currents (Im,pre-JM) (b), the transmembrane current (Im,LM) and INa in the LM segments (c), the INa,JM of the pre-junctional membrane (pre-JM) in the 150th myocyte and of the post-junctional membrane (post-JM) in the 151st myocyte (d), the extracellular cleft potential (Vj) between the 150th and 151st myocytes (e), the intracellular potential (Vi) of the LM and pre-JM in 150th myocyte and of the post-JM in 151st myocyte (f), and the gap junctional current (Ig) between the 150th and 151st myocytes (g). The APs and ion currents were recorded in a myocyte located at the middle of the myocardial strand (cell #150). Each AP morphology corresponds to the waveforms in panel (A) indicated by red, cyan, blue, and green lines. CVs at 100%gNa,LM, 35%gNa,LM, 7%gNa,LM and 0%gNa,LM were 71.4, 53.6, 33.3, and 25.0 cm/s, respectively (see Table 1).
Table 1.
Effects of changes in Na+ channel density of the lateral membrane on epicardial action potential parameters, the regional Na+ channel current, and conduction velocity.
| 100%gNa,LM | 35%gNa,LM | 7%gNa,LM | 0%gNa,LM | |
|---|---|---|---|---|
| APD90 (ms) | 241.8 | 236.9 | 268.7 | 80.9 |
| (mV/ms) | 238.6 | 96.0 | 39.0 | 26.5 |
| RMP (mV) | − 87.7 | − 87.7 | − 87.7 | − 87.8 |
| AAP (mV) | 122.6 | 122.4 | 116.5 | 51.2 |
| Peak INa,LM (pA/pF) | 329.0 | 138.5 | 24.6 | 0.0 |
| Peak post-INa,JM (pA/pF) | 951.7 | 1064.9 | 1143.9 | 1162.4 |
| Peak pre-INa,JM (pA/pF) | 1035.0 | 1166.5 | 1222.8 | 1211.8 |
| CV (cm/s) | 71.4 | 53.6 | 33.3 | 25.0 |
Each parameter is determined for the LM segment of the 150th cell in the myocardial strand model comprised of 300 epicardial ventricular myocytes.
%gNa,LM a percent gNa in the lateral membrane (LM) segment of each cell of the strand model; APD90 action potential duration measured at 90% repolarization; , maximum upstroke velocity of action potential; RMP, resting membrane potential; AAP, amplitude of action potential; Peak INa,LM, post-INa,JM , and pre-INa,JM, the respective peak values of INa across the LM, post-JM, and pre-JM segments of the 150th cell in the myocardial strand model; CV, conduction velocity.
Furthermore, we examined effects of selective decreasing of Na+ channel expression on the JMs of cardiomyocytes (Supplementary Fig. S1), and of uniform decreasing of Na+ channel expression on both JMs and LM on AP propagation (Supplementary Fig. S2). As shown in Supplementary Fig. S1, the selective decreasing of Na+ channels on the JMs did not affect AP morphology. On the other hand, the uniform decreasing of the Na+ channels on both JMs and LM to 10% or less resulted in the AP morphology changes characterized by the loss-of-dome (see Supplementary Fig. S2). These results suggest that changes in Na+ channel expression on the LM have a greater effect on AP morphology than those on the JMs.
These alterations in subcellular Na+ channel distribution also modified the conduction velocity (CV). The pre-JM current (Im,pre-JM; Fig. 2Ca,b, orange lines) is the difference between the post-JM current (Im,post-JM; Fig. 2Ca,b, purple lines) and the transmembrane current in the LM (see Fig. 2Cc, gray lines), i.e., Im,pre-JM = Im,post-JM − Im,LM, and is determined by Gi and Vi,LM − Vi,pre-JM, where Vi,LM and Vi,pre-JM are the intracellular potentials in the LM and pre-JM compartments, respectively (see Fig. 2Ca,f). The decrease in Na+ channel expression on the LM (%gNa,LM from 100 to 0%; see Fig. 2Cc) led to not only a decrease in the INa,LM but also a slight increase in the INa,JM due to excessive localization of Na+ channels to the JMs (see Fig. 2Cd), consequently resulting in a large negative cleft potential (Vj) at each intercellular junctions, corresponding to augmentation of the ephaptic interaction (see Fig. 2Ce). This decrease in INa,LM also caused a decrease in the maximum upstroke velocity of Vi,LM , i.e., (see Fig. 2Cf, gray lines and see Table 1). Because the decrease in led to a decrease in the difference between the Vi,LM and Vi,pre-JM (Fig. 2Cf, gray and orange lines), the decrease in INa,LM also reduced Im,pre-JM (compare orange lines in left to right panels of Fig. 2Cb), resulting in the decrease in maximum upstroke velocity of Vi,pre-JM, , (compare orange lines in left to right panels of Fig. 2Cf). Thus, the decrease in led to a decrease in the difference between the Vi,pre-JM and the intracellular potential of the post-JM compartment in the neighbor myocyte (Vi,post-JM; purple lines in Fig. 2Cf). Subsequently, this caused both a marked decrease in the gap junctional current (Ig) defined by the equation for 150th myocyte (compare colored lines in left to right panels of Fig. 2Cg) and a decrease in CV (see Table 1).
Combination of the decreases in Na+ channel expression on the LM in myocytes and the spatially-heterogeneous Na+ channel distribution in tissue led to P2R development
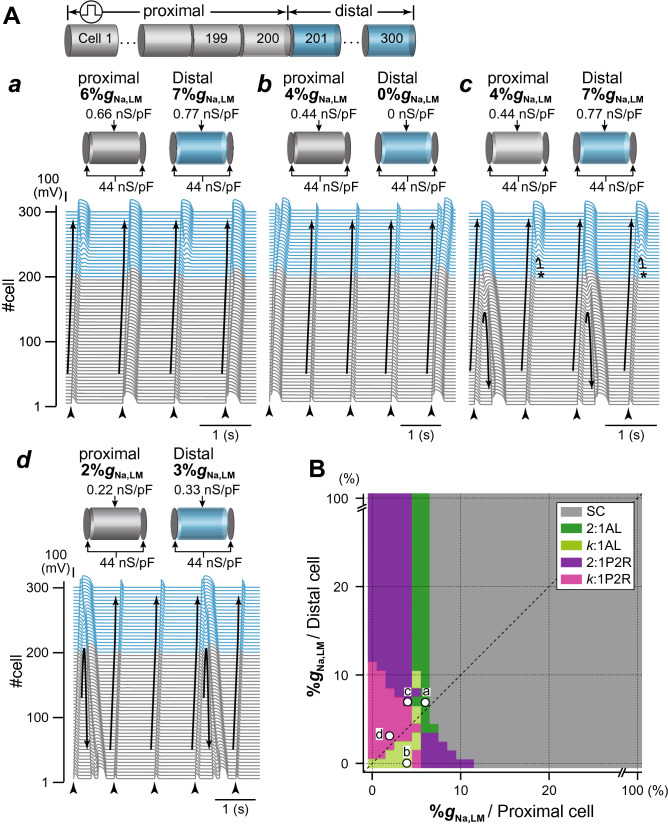
To test our hypothesis that alterations in the subcellular Na+ channel distribution lead to the development of P2R, we investigated the combined effects of both the regional INa decrease on the LM in myocytes and the spatial heterogeneity of Na+ channels in the myocardial tissue on AP propagation. The spatial heterogeneity of Na+ channels in the myocardial strand was achieved by changing the %gNa,LM in the proximal part of the myocardial strand (200 myocytes), while maintaining the 7%gNa,LM (26.6%gNa,tot) in the distal part of the myocardial strand (100 myocytes). The AP conduction exhibited AP alternans (Fig. 3Aa) when the %gNa,LM of the proximal myocytes was set to 6%gNa,LM (25.8%gNa,tot), as the slight spatial heterogeneity of Na+ channels in the myocardial strand. The ionic mechanism underlying AP alternans is shown in Supplementary Fig. S3. When the APD was prolonged due to reduced phase-0 amplitude and delayed phase-2 dome formation (e.g., green trace in Supplementary Fig. S3A), the recovery from the inactivation state of Na+ channels in the LM slightly delayed compared to that in the case of loss-of-dome (compare the blue and green traces in Supplementary Fig. S3C), slightly decreasing the peak INa,LM to cause the loss-of-dome at the next excitation (see Supplementary Fig. S3B–D). Consequently, the AP phase-0 amplitude slightly decreased (blue trace in Supplementary Fig. S3A), and the ICaL failed to activate (blue trace in Supplementary Fig. S3E). The failure of ICaL activation led to shortening of APD and prolongation of the diastolic interval (DI). The longer DI augmented the recovery from the inactivation state of the Na+ channel in the LM segment, causing the increase in the peak INa,LM (see green trace in Supplementary Fig. S3D) followed by the full AP accompanied by the delayed phase-2 dome (see green trace in Supplementary Fig. S3A). In addition, depending on the spatial heterogeneity of Na+ channels in the myocardial tissue, the AP propagation exhibited complex alternans patterns consisting of k − 1 times abbreviated AP conduction and a typical AP conduction (k:1 alternans) or a retrograde P2R (k:1 P2R). Figure 3Ab–d show examples of 4:1 AP alternans, 2:1 P2R, and 3:1 P2R, respectively.
Figure 3.
Simulated phase-2 reentry (P2R) development in the strand model. (A) Examples of typical patterns of AP propagation observed in the myocardial strand model with a spatially-heterogeneous Na+ channel distribution. The Na+ channel conductances on the LM in the proximal (cell #1–200) and the distal (cell #201–300) were set to 6%gNa,LM and 7%gNa,LM, respectively, to cause 2:1 AP alternans (a), 4%gNa,LM and 0%gNa,LM, respectively, to cause 4:1 AP alternans (b), 4%gNa,LM and 7%gNa,LM, respectively, to cause 2:1 retrograde P2R (c), and 2%gNa,LM and 3%gNa,LM, respectively, to cause 3:1 P2R consisting of a retrograde P2R and twice conductions with abbreviated AP (d). Arrows and black short bars indicate the direction of AP propagation and blockage, respectively. (B) A phase diagram of AP propagation patterns for the %gNa,LM in proximal myocytes vs. %gNa,LM in distal myocytes. Open circles labeled as a–d correspond to the parameter sets at which the AP propagation patterns shown in panels Aa–d. SC, stable conduction consisting of typical AP morphology of human ventricular myocytes; 2:1 AL, 2:1 AP alternans pattern; k:1 AL, AP alternans pattern including a typical AP conduction and k − 1 times conduction with abbreviated AP; 2:1 P2R, 2:1 alternans pattern including a retrograde phase-2 reentry and an abbreviated AP conduction; k:1 P2R, k:1 alternans pattern including a retrograde phase-2 reentry and k − 1 times conduction with abbreviated AP.
To understand relationships among Na+ channel distributions within myocytes, spatial heterogeneous Na+ channel distribution in tissue, and development of P2R, we systematically performed simulations of AP propagations while changing the subcellular Na+ channel distribution in the proximal and distal parts of the myocardial fiber every 1%gNa,LM. Figure 3B shows a phase diagram constructed by mapping the AP propagation patterns obtained from each simulation on the parameter plane of the %gNa,LM in proximal and distal myocytes. We can see that the 2:1 retrograde P2R as shown in Fig. 3Ab occurred when the %gNa,LM in the proximal myocyte was reduced to < 5% regardless of the %gNa,LM in the distal myocytes (Fig. 3B, purple region), and that the region where P2R occurs was largely the left upper region of the diagonal line which represents conditions of spatially-homogeneous Na+ channel distribution in myocardial strand. This implies that P2R occurs when a spatially-heterogeneous Na+ channel distribution exists on the myocardial fiber. These results suggest that selective decrease in Na+ channels on the LM of myocytes and the spatially-heterogeneous Na+ channel expression on the myocardial tissue cooperatively facilitate the occurrence of P2R.
In addition, we examined the effect of cleft width (cw) changes on the P2R development because AP propagation with both gap-junctional and ephaptic interacted mechanisms (see “Methods”) is greatly sensitive to the cw16,17. When the cw was in the range of 7 to 17 nm, the occurrence of P2R was confirmed (Supplementary Fig. S4). On the other hand, with cw < 7 nm, the ephaptic interaction, i.e., the cleft potential (Vj) at intercellular junctions, was significantly augmented because of an increase in the cleft resistance (see Fig. 1B, “Methods”) due to the decrease in cw. The Vj amplitude increase speeded up the AP depolarization in the JMs and reduced the potential differences between the pre- and post-JMs, leading to the gap-junctional current Ig. The Ig decrease caused AP propagation to fail. On the contrary, as the cw increases to more than 17 nm, the diminishing Vj (compare each panel c in Supplementary Fig. S4A–D, left to right) led to the relative increases in the local current consisting of the Ig and INa,JM (see panels d-h in Supplementary Fig. S4A–D). Thereby, the loss-of-dome AP observed in the proximal myocytes with smaller cw changed to a typical ventricular AP morphology (compare each panel b in Supplementary Fig. S4B,C). Furthermore, in the case of the gap-junctional mechanism alone corresponding to the loss of the Vj (no ephaptic coupling), the P2R did not occur, causing the normal AP conduction (see Supplementary Fig. S4D). These results suggest that ephaptic interactions are essential to the occurrences of P2R in patients with BrS.
ICaL plays an important role in the P2R development
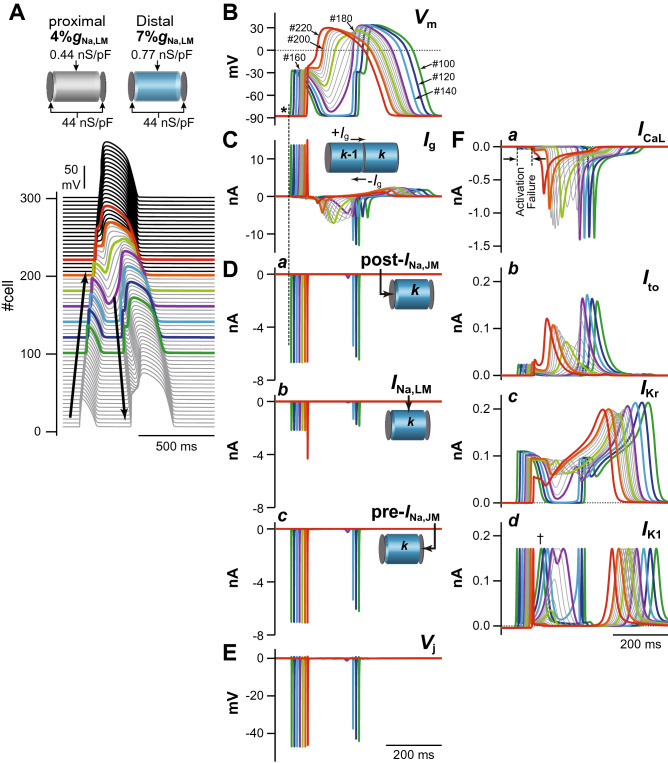
Based on the results shown in Fig. 3, we focused on how AP morphology is affected by changes in the ion channel currents. Figure 4 shows the AP profiles and main ion channel currents in several myocytes near the border between the proximal and distal parts of the myocardial fiber during the development of the 2:1 P2R shown in Fig. 3Ab. The initial depolarization of the regional membrane potential (Vm) in the LM segment of each myocyte, i.e., that is equal to Vi,LM, within the proximal part of the myocardial strand (#100, 120 and 140 in Fig. 4B, asterisk) was formed by the local current flows consisting of Ig (Fig. 4C) and post-INa,JM (Fig. 4Da). These current flows entered the myocyte through the post-JM, and depolarized the LM and pre-JM, resulting in the INa,LM (Fig. 4Db) and pre-INa,JM (Fig. 4Dc). Furthermore, these post- and pre-INa,JM led to the large negative cleft potential (Vj) at each intercellular junction (Fig. 4E). However, the local current was not able to depolarize the LM sufficiently (Fig. 4B), and both ICaL and Ito failed to activate (Fig. 4Fa,b). Moreover, the relatively large transient IKr activation (Fig. 4Fc) and faster onset of the late peak of IK1 in the proximal myocyte (Fig. 4Fd, dagger) accelerated the AP repolarization, leading to the loss of AP dome (Fig. 4A,B). Subsequently, the difference in Vm at the border between proximal and distal parts was increased, and consequently this elicited the retrograde Ig (the inward currents in Fig. 4C) from the distal to the proximal part. Unlike the anterograde Ig elicited by the initial depolarization differences in neighbor myocytes within the proximal part, the retrograde Ig continued to flow for a relatively long time because the notch-and-dome type prolonged AP in distal myocytes caused a long-lasting potential difference from the proximal loss-of-dome abbreviated AP. Continuous depolarizing loads of the proximal myocytes by the long-lasting retrograde Ig allow the depolarization of the Vm that activates ICaL of the LM in proximal myocytes, leading to re-excitation of the myocytes in the proximal part, i.e., retrograde P2R (Fig. 4A).
Figure 4.
Mechanism of phase-2 reentry (P2R). (A) The simulated AP propagation in the myocardial strand model during the development of 2:1 P2R shown in Fig. 3Ab. (B) Simulated behaviors of the membrane potential (Vm) in several myocytes (#100–220) during the 2:1 P2R development. (C) The gap junctional currents, Ig, flowing from the cell #100 to #220. (D) INa in the post-junctional membrane (INa,post-JM) (a), the lateral membrane (INa,LM) (b), and the pre-junctional membrane (INa,pre-JM) (c). (E) The extracellular cleft potential, Vj, in the cell #100 to #220. F, ICaL (a), Ito (b), IKr (c), and IK1 (d) during the P2R development.
This theoretical model for the P2R mechanism can also be used to investigate the mechanism of P2R inhibition. We conducted an additional, identical simulation under a β-AS condition18,19. The marked increase in ICaL due to the β-AS effect kept the AP dome of each myocyte even under the smaller %gNa,LM condition that caused loss-of-dome type abbreviated AP by the marked decrease in INa,LM in the proximal part of the myocardial fiber without β-AS (see Fig. 5). This resulted in an increase in Vm amplitude and APD prolongation in the proximal and distal parts of the myofiber (compare respective panels in Figs. 4 and 5). On the other hand, as shown in Supplementary Fig. S5, the P2R development could also be suppressed by reducing ICaL. Thus, not only β-AS that causes an increase in ICaL but also β-blocker may be effective for preventing P2R.
Figure 5.
Mechanisms for the prevention of phase-2 reentry (P2R) under β-AS condition. (A,B) The simulated AP propagation (A) and the membrane potential (Vm) change (B) of several myocytes in the myocardial strand model under the same condition as for Fig. 3Ab but during β-AS. (C–F) The gap junctional current, Ig (C), INa in the post-junctional membrane (JM), INa,post-JM (Da), INa in the lateral membrane, INa,LM (Db), and INa in the pre-JM, INa,pre-JM (Dc), The extracellular cleft potentials, Vj (E), ICaL (Fa), Ito (Fb), IKr (Fc), and IK1 (Fd) during β-AS.
The P2R triggered reentrant arrhythmias
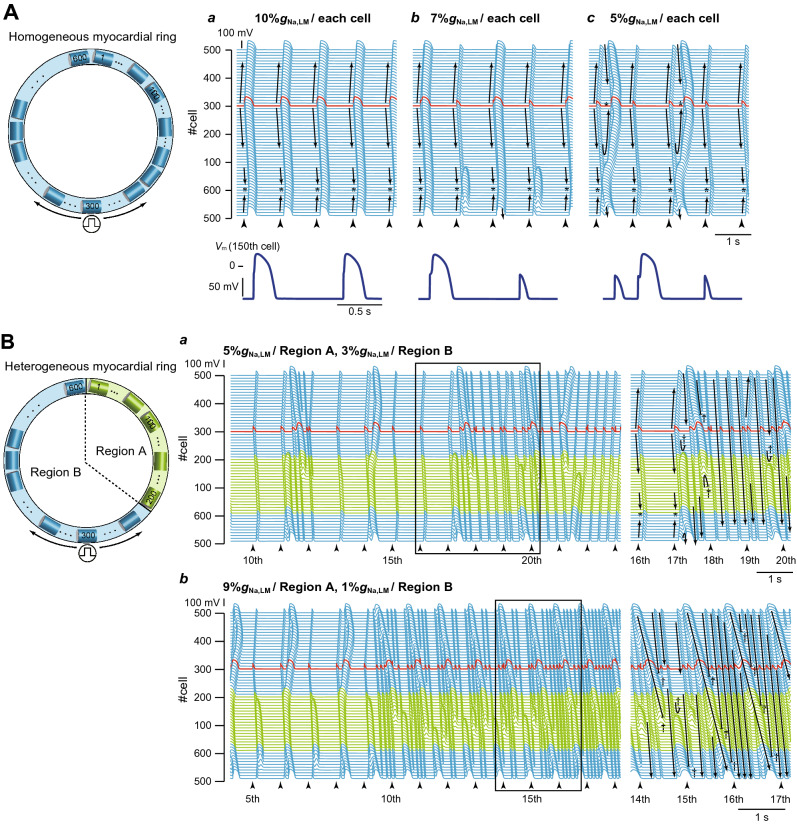
Whether or not the P2R triggers reentrant arrhythmias was investigated using a myocardial ring model (Fig. 1C). In the myocardial ring with spatially-homogeneous Na+ channel distribution (Fig. 6A), the AP exhibited bidirectional conduction from the stimulus site (300th myocyte), resulting in the collision of excitation wavefronts at the opposite side of the stimulus site (Fig. 6Aa, asterisk). When the %gNa,LM of each myocyte in the myocardial ring was set to 7%, we could observe the bidirectional conduction with 2:1 alternans pattern such that the loss-of-dome AP and the AP with decreased AP phase-0 amplitude and delayed phase-2 dome formation appear alternately (see Fig. 6Ab). When the %gNa,LM in the LM of each myocyte in the myocardial ring was homogeneously reduced by 95%, the bidirectional conduction of shortened AP from the stimulus site was observed (Fig. 6Ac); the bidirectional excitation wavefronts collided, causing a bidirectional P2R in the myocardial ring. However, each excitation wavefront originating from the bidirectional P2R collided and disappeared at around stimulus site (Fig. 6Ac); no proarrhythmic changes were observed in the myocardial ring model with spatially-homogeneous Na+ channel distribution even under the condition of the markedly decreased %gNa,LM. However, in the same myocardial ring model but with the spatially-heterogeneous Na+ channel distribution (Fig. 6B), AP conduction properties obviously changed (see Fig. 6Ba,b). With setting the %gNa,LM of 200 cells (cells #1–200; region A in Fig. 6B) to 5% (0.55 nS/pF), the %gNa,LM in each 400 cells (cells #201–600; region B in Fig. 6B) was uniformly reduced to 3% (0.33 nS/pF), thereby producing the spatial heterogeneity of Na+ channel distribution within the tissue. The bidirectional excitation wavefronts elicited at the stimulus site (cell #300) collided at the border between the regions A and B (asterisk in the right panel of Fig. 6Ba) and were subsequently followed by bidirectional P2R occurrence. However, the clockwise-rotating excitation wave of the P2R exhibited decremental conduction and block (dagger in the right panel of Fig. 6Ba); in contrast, the counterclockwise-rotating excitation wave continued to conduct in the myocardial ring, causing the P2R-mediated reentrant tachyarrhythmia. During 1-Hz pacing, the occurrence of reentrant arrhythmias was intermittent and irregular (see Fig. 6Ba, left). The further augmentation of the spatial heterogeneity of Na+ channel distribution in the tissue (9%gNa,LM in region A and 1%gNa,LM in region B on the myocardial ring) induced P2R-mediated persistent reentry (Fig. 6Bb). Notably, a reentrant wave with a large amplitude slowly rotated on the myocardial ring, while continuously emitting fast but low-amplitude waves. This low-amplitude fast wave circled around and collided with the tail of the slow wave (Fig. 6Bb). To know what degree of the spatial heterogeneity in %gNa,LM for the two regions in the myocardial ring is needed to cause reentrant arrhythmias, we constructed a phase diagram of the AP conduction properties (see Fig. 6C). This result indicates that the P2R-mediated reentry occurred mostly when the %gNa,LM in the region B was reduced to < 5% and the %gNa,LM in the region A was in the 5 ~ 10% range (Fig. 6C, magenta region).
Figure 6.

Rotatory reentry induction in the myocardial ring model. (A) Overall views (top) of simulated AP propagations in the myocardial ring with spatially-homogeneous Na+ channel distribution with 10%gNa,LM (a), 7%gNa,LM (b), and 5%gNa,LM (c) in the lateral membrane (LM) segment of each myocyte and enlarged views (bottom) of the time-dependent behaviours of membrane potential (Vm) of the 150th myocyte. (B) Overall (left) and enlarged views (right) of simulated AP propagations in the same model but with a spatially-heterogeneous Na+ channel distribution with gNa,LM in the LM of the 1st to 200th myocytes (Region A) and of the 201st to 600th myocytes (Region B): 5%gNa,LM in Region A and 3%gNa,LM in Region B (a) and 9%gNa,LM in Region A and 1%gNa,LM in Region B (b). Arrows and black short bars indicate the direction of AP propagation and blockage, respectively. Asterisks and dagger symbols represent the collision of excitation wavefronts, and the blockade of AP propagation, respectively. The red trace represents the AP behavior at the stimulus site (cell #300). (C) A phase diagram of AP propagation patterns for the %gNa,LM of each myocyte within Region A vs. %gNa,LM of each myocyte within Region B. Open circles labeled as a–c correspond to the parameter sets at which the AP propagation patterns shown in Aa–c occurred. Open circles labeled as (d) and (e) show the parameter sets for (Ba,b).
Discussion
Although it has so far believed that in addition to the loss-of-function mutation of Na+ channels, impaired Nav1.5 trafficking20–23 or Na+ channel expression abnormality14,15 to the cell surface membrane is one of the major causes of fatal arrhythmias in patients with BrS, the link between the loss-of-function of Na+ channels and arrhythmogenesis was not clear. In the present study, we found that selective decreases in INa,LM of each ventricular myocyte without changing INa,JM (Na+ channel located at junctional membranes) caused both morphological changes in AP and the conduction slowing that are typically observed in BrS patients24,25. These findings suggest that both the BrS mechanisms, previously represented as repolarization and depolarization abnormalities26, can at least in part be explained by this decrease in INa,LM. Furthermore, we showed that the marked decrease in INa,LM together with an existence of the spatial heterogeneity at tissue-level in the Na+ channel expression is essential for the P2R development, as well as the reentry initiation triggered by the P2R. To the best of our knowledge, this is the first report demonstrating mechanistic links between the INa decrease as the subcellular Na+ channel expression changes and P2R in BrS.
Selectively reducing Na+ channels from the LM led to the notch-and-dome AP morphology (Fig. 2Ac,B, blue lines). The underdeveloped AP phase-0 amplitude resulted in a marked decrease in Ito during AP phase-1 and the delay of ICaL activation because both Ito and ICaL activate as Vm exceeds − 30 mV10. The decrease in Ito and delay in ICaL activation caused a large phase-1 dip and the delay of the AP phase-2 dome peak, respectively. This AP shape is greatly similar to those reported in our previous studies13,16, and in Wei et al.17, which has shown the occurrence of AP with the delayed phase-2 dome by changing the cleft width at the intercellular junction. There is a common mechanism for APs generated in those models, and the success or failure of L-type Ca2+ channel activation determines whether an AP exhibits the delayed phase-2 dome or the loss-of-dome. This morphology resembled that of epicardial monophasic AP recordings from the RV outflow tract (RVOT) in patients with BrS who were undergoing open chest surgery24. This implies that the characteristic monophasic AP morphology recorded in a patient with BrS might be attributed to the selective decrease of INa,LM in epicardial myocytes located in the RVOT.
In epicardial myocytes in the RVOT, the loss-of-dome AP followed by marked shortening of APD, i.e., repolarization abnormality7,26, is considered to be one of the mechanisms of coved-type ST-segment elevation (Brugada-type ECG)2,6. One of the factors causing the loss-of-dome is thought to be the ventricular gradient of Ito through the RV wall, as well as endogenously heterogeneous Ito in the RV epicardial layers7,26. We showed that even without changing Ito, the loss-of-dome type abbreviated AP was also caused by extreme decrease and/or loss of Na+ channels from the LM, but not JMs of each myocyte (Fig. 2Ad,B; green lines). Indeed, the loss of INa,LM further decreased the AP phase-0 amplitude, therefore preventing the Vm depolarization at AP phase-0 from reaching a threshold potential for ICaL activation; this thus failed to activate ICaL, resulting in the loss-of-dome type abbreviated AP. These results suggest that the Na+ channel abnormality, which is commonly believed to be the cause of the depolarization abnormality in BrS26, may also be responsible for the repolarization abnormality of BrS.
We found that the conduction delay in RVOT under structurally normal hearts in patients with BrS27, i.e., depolarization disorder hypothesis26, may be attributable to the selective decrease in INa,LM causing decreases in Ig without the Gg change. The mechanism of INa,LM-mediated decreases in Ig proposed in the present theoretical study is as follows: (1) The marked decrease in INa,LM decreases the pre-JM current (compare left to right panels of Fig. 2Cc) and thus slows the depolarization of the pre-JM (compare left to right panels of Fig. 2Ce). (2) The slower and smaller depolarization of the pre-JM due to the reduced pre-JM current leads to decreases in the difference between and , for k = 1, 2,…, 299, resulting in the Ig decrease. This notion is also supported by previous experimental studies14,15, which showed that mutant mice hearts with a selective decrease in Na+ channel expression on the LM had slower CV than the wild type, without affecting the connexin 43 (Cx43; gap-junction channel-forming proteins) expression (see Supplementary Table S1).
The underlying mechanism of P2R has been explained by the electrotonic interaction between the region with normal AP morphology and the adjacent region with the loss of the AP dome. Such P2R mechanism were explored in numerous experimental28,29 and computational studies30–32. For the onset of P2R, excitable tissues such as RV and RVOT, in general, are required to be separated into two regions by the segment of reduced excitability and/or reduced electrical coupling. The existence of a local difference in AP waveform (or local APD difference) at the RVOT epicardium has been confirmed by several experimental studies9,33. Our results showed that an existence of the slight spatial heterogeneity at tissue-level in Na+ channel expressions accompanied by a marked decrease in INa,LM (reduced excitability) could induce P2R (Fig. 3). Although the causes that make the local differences of APs in the RVOT/RV are not clear, one possible cause is an endogenous heterogeneity in Na+ channel expression at the RVOT epicardial layer as in the transmural difference in Na+ channel expression in the RVOT and the RV free wall34. On the other hand, Auerbach and co-workers35 have shown by combined experimental and computational studies that a structural heterogeneity (a geometric expansion) can produce P2R. In addition to differences in electrophysiological properties due to differences in the developmental origin of the RVOT and RV36, a tissue structure of the RVOT with more aligned fiber orientations is different from that of the RV free wall which comprised of network-like structures of myocardial fibers; the structural heterogeneity between the RV free wall and RVOT might be also responsible for the P2R development in patients with BrS.
A previous clinical observation37 has demonstrated that BrS is associated with epicardial interstitial fibrosis and reduced Cx43 expression in the RVOT. Furthermore, previous experimental studies using the SCN5A heterozygous (Scn5a+/−) mice38,39 have shown that fibrosis, accompanied by downregulation and redistribution of Cx43, is increased with aging. The Ig decrease with Gg change leads to a decrease in the local current (Im,LM) consisting of Ig and Im,post-JM (Fig. 2Cb,g). Besides INa,LM reduction, decreases in Ig could result in the loss-of-dome abbreviated AP32. Therefore, we speculate that the decrease in gap junctions at the RVOT, where Na+ channel expression is impaired along with aging-associated fibrosis, may also cause the loss of the AP dome followed by P2R resulting in VT/VF.
Miyoshi et al.31 suggested that ICaL plays critical roles in the formation of local APD differences even during AP propagation and the following second excitation during P2R. Indeed, as shown in Figs. 2B and 4B, ICaL was the main determinant of AP dome formation in each myocyte of the myocardial strand and contributed to maintaining the second excitation wave propagation during P2R, consequently leading to reentrant excitation wave propagations (VT/VF) in a myocardial ring model (Fig. 6). Notably, the reentrant waves shown in Fig. 6Bb combined slow (ICaL based AP phase-2 dome propagation) and fast (INa and intercellular local current based loss-of-dome AP propagation) wavefronts, and are likely to share a similar mechanism with the bi-stable wave propagation reported by Chang et al.40 On the other hand, it is known that the sympathetic activation in patients with BrS decreases VT/VF initiation41, predicting that the greater ICaL enhancement contributes to preventing P2R. We demonstrated that β-AS by the administration of isoproterenol could reduce the local APD difference and could prevent P2R (Fig. 5). Based on our result as shown in Fig. 4, ICaL inhibition by Ca2+ channel blocker and/or β-blocker administration is likely to prevent the P2R development (Supplementary Fig. S5), consequently preventing VT/VF. However, the effects of β-AS and/or β-blockers on AP propagation in patient with BrS are not fully understood. Thus, more quantitative experimental verification and theoretical studies using more elaborate intracellular Ca2+-signaling models42 are required to understand the exact roles of ICaL modulation by β-AS and/or β-blocker in P2R and arrhythmia preventions.
Based on our simulation data (Supplemental Fig. S4), it is suggested that the ephaptic coupling mechanism is essential for the loss of AP dome followed by P2R development. Such ephaptic coupling might be involved in several heart diseases. Tolkacheva and co-workers have investigated the role of Na+ channel distribution on AP conduction during regional ischemia17,43. They observed small APs with a reduced amplitude that propagates and impacts conduction failure. In patients with long QT syndrome type 3, the distribution changes in Na+ channels with the gain-of-function mutation have significantly affected the propagation in APs with early afterdepolarizations44–46. Also Jaeger et al.47 have demonstrated a mechanism of conduction failure due to changes in Na+ channel distribution. As with these, we will need to perform additional simulations employing more sophisticated, three-dimensional, whole ventricle models to elucidate the development mechanism of severe arrhythmia disorders such as BrS.
Methods
Myocardial strand and ring models and subcellular Na+ channel distribution
To perform computer simulations examining effects of alterations in subcellular Na+ channel distribution on AP propagation behaviors, we constructed myocardial strand and ring models comprising 300 (Fig. 1Ba,d) and 600 ventricular myocytes (Fig. 1C), respectively. As shown in Fig. 1Bb, adjacent myocytes were electrically coupled with both gap junctions and an electric field mechanism (ephaptic coupling)48,49, the latter of which is an electrical interference effect caused at intercellular cleft space; electrical communication between myocytes through the electric field mechanism is mediated by large negative changes in the extracellular potential elicited within the narrow intercellular cleft space facing the IDs.
As in our previous study13,16, to achieve the inhomogeneous Na+ channel distribution within a myocyte found in humans and other mammals50,51, the whole cell membrane of each myocyte was divided into three segments (Fig. 1Bc), one segment for the LM and the other two segments for the pre- and post-junctional membranes (JMs), namely the IDs. Allocating Na+ channel conductances to each membrane segment and changing those individually, we altered the subcellular expression of Na+ channels. The electrophysiological property of each membrane segment was represented by a modified O’Hara-Rudy dynamic (mORd) model10,11, which is the most sophisticated human ventricular AP model to date that extensively validated against experimental data from more than 100 non-diseased human hearts. For better correspondence with the experimental CV of human RV epicardium52, the formula of fast INa in the O’Hara-Rudy dynamic model was replaced with that of the ten Tusscher-Panfilov (TP) model12. We set the control conductance of fast INa to 11 nS/pF for the LM (GNaF,LM) and 44 nS/pF for the JMs (GNaF,JM) so that INa amplitude obtained from the simulation matched the one recorded experimentally for basal condition15; the conductance of late INa was also set to 0.0045 nS/pF for the LM (GNaL,LM) and 0.018 nS/pF for the JMs (GNaL,JM) as the control condition. The fast and late Na+ channel conductances of the JM and LM were expressed as percentages of the GNaF,JM (GNaL,JM) and GNaF,LM (GNaL,LM), i.e., %gNa,JM and %gNa,LM, respectively. Specific parameters in the myocardial strand and ring models consisting of the mORd model can be found in the Supplementary Table S2.
To test whether the sympathetic activity inhibits the P2R development, we additionally performed simulations of AP propagation using the myocardial strand model under the condition of β-adrenergic stimulation (β-AS) mimicking sympathetic activation. Modifications of parameters for simulating the condition of β-AS18,19 were listed in the Supplementary Table S3.
Simulations
Numerical simulations of AP propagation were performed as described previously13,16 and details were provided in Supplementary Methods. Pacing stimuli consisting of 300 pA/pF, 1 ms current pulse with a basic cycle length of 1 s were applied to a myocyte located at the end of the myocardial strand (the 300th myocyte in the myocardial ring) and repeated 30 times to minimize transient responses in each simulation.
Preprint
A previous version of this manuscript was published as a preprint53.
Supplementary information
Acknowledgements
This work was supported by grants from the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Numbers 24790214 and 16KT0194, Takeda Science Foundation (to K.T.), Hiroshi and Aya Irisawa Memorial Promotion Award for Young Physiologists from the Physiological Society of Japan (to K.T.), and Grant for Promoted Research (S2019-2) from Kanazawa Medical University (to K.T.), and the Japanese Ministry of Education, Culture, Sports, Science and Technology (MEXT) KAKENHI Grant Number 22136002 (to Yo.K.).
Author contributions
K.T., T.A., A.A., Ya.K., and Yo.K. conceived and designed the experiments; K.T., N.N., and T.S. conducted the simulations, performed numerical calculations and prepared figures; K.T., T.A. N.N., T.S., A.A., Ya.K., and Yo.K. analyzed and interpreted results; K.T., T.A., Ya.K., A.A., and Yo.K. drafted and edited the manuscript. All authors reviewed and approved the final version of manuscript.
Data availability
All data generated or analyzed during this study are included in this published article (and its Supplementary Information files). Furthermore, these codes used to simulate the myocardial strand and ring models are available in the repository: https://github.com/92tsumoto/BrS-P2R-strand-ORd2011model-withTNNP_INa-FT_IKr, and https://github.com/92tsumoto/BrS-P2R-ring-ORd2011model-withTNNP_INa-FT_IKr.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-76681-3.
References
- 1.Abriel H. Cardiac sodium channel Na(v)1.5 and interacting proteins: Physiology and pathophysiology. J. Mol. Cell. Cardiol. 2010;48(1):2–11. doi: 10.1016/j.yjmcc.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 2.Brugada P, Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical and electrocardiographic syndrome. A multicenter report. J. Am. Coll. Cardiol. 1992;20(6):1391–1396. doi: 10.1016/0735-1097(92)90253-J. [DOI] [PubMed] [Google Scholar]
- 3.Kroncke BM, Glazer AM, Smith DK, Blume JD, Roden DM. SCN5A (NaV1.5) variant functional perturbation and clinical presentation: variants of a certain significance. Circ. Genom. Precis Med. 2018;11(5):e002095. doi: 10.1161/CIRCGEN.118.002095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Q, et al. Genetic basis and molecular mechanism for idiopathic ventricular fibrillation. Nature. 1998;392(6673):293–296. doi: 10.1038/32675. [DOI] [PubMed] [Google Scholar]
- 5.Brugada R, et al. Sodium channel blockers identify risk for sudden death in patients with ST-segment elevation and right bundle branch block but structurally normal hearts. Circulation. 2000;101(5):510–515. doi: 10.1161/01.CIR.101.5.510. [DOI] [PubMed] [Google Scholar]
- 6.Sendfeld F, et al. Experimental models of Brugada syndrome. Int. J. Mol. Sci. 2019;20(9):E2123. doi: 10.3390/ijms20092123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antzelevitch C. The Brugada syndrome: ionic basis and arrhythmia mechanisms. J. Cardiovasc. Electrophysiol. 2001;12(2):268–272. doi: 10.1046/j.1540-8167.2001.00268.x. [DOI] [PubMed] [Google Scholar]
- 8.Aiba T, et al. Cellular basis for trigger and maintenance of ventricular fibrillation in the Brugada syndrome model: high-resolution optical mapping study. J. Am. Coll. Cardiol. 2006;47(10):2074–2085. doi: 10.1016/j.jacc.2005.12.064. [DOI] [PubMed] [Google Scholar]
- 9.Morita H, et al. Repolarization heterogeneity in the right ventricular outflow tract: correlation with ventricular arrhythmias in Brugada patients and in an in vitro canine Brugada model. Heart Rhythm. 2008;5(5):725–733. doi: 10.1016/j.hrthm.2008.02.028. [DOI] [PubMed] [Google Scholar]
- 10.O'Hara T, Virág L, Varró A, Rudy Y. Simulation of the undiseased human cardiac ventricular action potential: model formulation and experimental validation. PLoS Comput. Biol. 2011;7(5):e1002061. doi: 10.1371/journal.pcbi.1002061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furutani K, et al. Facilitation of IKr current by some hERG channel blockers suppresses early afterdepolarizations. J. Gen. Physiol. 2019;151(2):214–230. doi: 10.1085/jgp.201812192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.ten Tusscher KH, Panfilov AV. Alternans and spiral breakup in a human ventricular tissue model. Am. J. Physiol. Heart. Circ. Physiol. 2006;291(3):H1088–H1100. doi: 10.1152/ajpheart.00109.2006. [DOI] [PubMed] [Google Scholar]
- 13.Tsumoto K, Ashihara T, Haraguchi R, Nakazawa K, Kurachi Y. Ischemia-related subcellular redistribution of sodium channels enhances the proarrhythmic effect of class I antiarrhythmic drugs: a simulation study. PLoS ONE. 2014;9(10):e109271. doi: 10.1371/journal.pone.0109271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petitprez S, et al. SAP97 and dystrophin macromolecular complexes determine two pools of cardiac sodium channels Nav15 in cardiomyocytes. Circ. Res. 2011;108(3):294–304. doi: 10.1161/CIRCRESAHA.110.228312. [DOI] [PubMed] [Google Scholar]
- 15.Shy D, et al. PDZ domain-binding motif regulates cardiomyocyte compartment-specific NaV1.5 channel expression and function. Circulation. 2014;130(2):147–160. doi: 10.1161/CIRCULATIONAHA.113.007852. [DOI] [PubMed] [Google Scholar]
- 16.Tsumoto K, Ashihara T, Haraguchi R, Nakazawa K, Kurachi Y. Roles of subcellular Na+ channel distributions in the mechanism of cardiac conduction. Biophys. J. 2011;100(3):554–563. doi: 10.1016/j.bpj.2010.12.3716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wei N, Mori Y, Tolkacheva EG. The dual effect of ephaptic coupling on cardiac conduction with heterogeneous expression of connexin 43. J Theor Biol. 2016;397:103–114. doi: 10.1016/j.jtbi.2016.02.029. [DOI] [PubMed] [Google Scholar]
- 18.Zeng J, Rudy Y. Early afterdepolarizations in cardiac myocytes: mechanism and rate dependence. Biophys. J. 1995;68(3):949–964. doi: 10.1016/S0006-3495(95)80271-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kurata Y, et al. Dynamical mechanisms of phase-2 early afterdepolarizations in human ventricular myocytes: insights from bifurcation analyses of two mathematical models. Am. J. Physiol. Heart. Circ. Physiol. 2017;312(1):H106–H127. doi: 10.1152/ajpheart.00115.2016. [DOI] [PubMed] [Google Scholar]
- 20.Baroudi G, Acharfi S, Larouche C, Chahine M. Expression and intracellular localization of an SCN5A double mutant R1232W/T1620M implicated in Brugada syndrome. Circ. Res. 2002;90(1):E11–E16. doi: 10.1161/hh0102.102977. [DOI] [PubMed] [Google Scholar]
- 21.Mohler PJ, et al. Nav1.5E1053K mutation causing Brugada syndrome blocks binding to ankyrin-G and expression of Nav15 on the surface of cardiomyocytes. Proc. Natl. Acad. Sci. USA. 2004;101(50):17533–17538. doi: 10.1073/pnas.0403711101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.London B, et al. Mutation in glycerol-3-phosphate dehydrogenase 1 like gene (GPD1-L) decreases cardiac Na+ current and causes inherited arrhythmias. Circulation. 2007;116(20):2260–2268. doi: 10.1161/CIRCULATIONAHA.107.703330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ishikawa T, et al. A novel disease gene for Brugada syndrome: sarcolemmal membrane-associated protein gene mutations impair intracellular trafficking of hNav1.5. Circ. Arrhythm Electrophysiol. 2012;5(6):1098–1107. doi: 10.1161/CIRCEP.111.969972. [DOI] [PubMed] [Google Scholar]
- 24.Kurita T, et al. The electrophysiologic mechanism of ST-segment elevation in Brugada syndrome. J. Am. Coll. Cardiol. 2002;40(2):330–334. doi: 10.1016/S0735-1097(02)01964-2. [DOI] [PubMed] [Google Scholar]
- 25.Coronel R, et al. Right ventricular fibrosis and conduction delay in a patient with clinical signs of Brugada syndrome: a combined electrophysiological, genetic, histopathologic, and computational study. Circulation. 2005;112(18):2769–2777. doi: 10.1161/CIRCULATIONAHA.105.532614. [DOI] [PubMed] [Google Scholar]
- 26.Meregalli PG, Wilde AA, Tan HL. Pathophysiological mechanisms of Brugada syndrome: depolarization disorder, repolarization disorder, or more? Cardiovasc. Res. 2005;67(3):367–378. doi: 10.1016/j.cardiores.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 27.Nagase S, et al. Epicardial electrogram of the right ventricular outflow tract in patients with the Brugada syndrome: using the epicardial lead. J. Am. Coll. Cardiol. 2002;39(12):1992–1995. doi: 10.1016/S0735-1097(02)01888-0. [DOI] [PubMed] [Google Scholar]
- 28.Davidenko J, Antzelevitch C. The effects of milrinone on action potential characteristics, conduction, automaticity, and reflected reentry in isolated myocardial fibers. J. Cardiovasc. Pharmacol. 1985;7(2):341–349. doi: 10.1097/00005344-198503000-00021. [DOI] [PubMed] [Google Scholar]
- 29.Lukas A, Antzelevitch C. Phase 2 reentry as a mechanism of initiation of circus movement reentry in canine epicardium exposed to simulated ischemia. Cardiovasc. Res. 1996;32(3):593–603. doi: 10.1016/S0008-6363(96)00115-0. [DOI] [PubMed] [Google Scholar]
- 30.Cabo C, Barr RC. Reflection after delayed excitation in a computer model of a single fiber. Circ. Res. 1992;71(2):260–270. doi: 10.1161/01.RES.71.2.260. [DOI] [PubMed] [Google Scholar]
- 31.Miyoshi S, et al. A mathematical model of phase 2 reentry: role of L-type Ca current. Am. J. Physiol. Heart. Circ. Physiol. 2003;284(4):H1285–H1294. doi: 10.1152/ajpheart.00849.2002. [DOI] [PubMed] [Google Scholar]
- 32.Bueno-Orovio A, Cherry EM, Evans SJ, Fenton FH. Basis for the induction of tissue-level phase-2 reentry as a repolarization disorder in the Brugada syndrome. Biomed. Res. Int. 2015;2015:197586. doi: 10.1155/2015/197586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kimura M, et al. Mechanism of ST elevation and ventricular arrhythmias in an experimental Brugada syndrome model. Circulation. 2004;109(1):125–131. doi: 10.1161/01.CIR.0000105762.94855.46. [DOI] [PubMed] [Google Scholar]
- 34.Szabó G, et al. Asymmetrical distribution of ion channels in canine and human left-ventricular wall: epicardium versus midmyocardium. Pflugers Arch. 2005;450(5):307–316. doi: 10.1007/s00424-005-1445-z. [DOI] [PubMed] [Google Scholar]
- 35.Auerbach DS, et al. Structural heterogeneity promotes triggered activity, reflection and arrhythmogenesis in cardiomyocyte monolayers. J. Physiol. 2011;589(Pt 9):2363–2381. doi: 10.1113/jphysiol.2010.200576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boukens BJ, Christoffels VM, Coronel R, Moorman AF. Developmental basis for electrophysiological heterogeneity in the ventricular and outflow tract myocardium as a substrate for life-threatening ventricular arrhythmias. Circ. Res. 2009;104(1):19–31. doi: 10.1161/CIRCRESAHA.108.188698. [DOI] [PubMed] [Google Scholar]
- 37.Nademanee K, et al. Fibrosis, connexin-43, and conduction abnormalities in the Brugada syndrome. J. Am. Coll. Cardiol. 2015;66(18):1976–1986. doi: 10.1016/j.jacc.2015.08.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Veen TA, et al. Impaired impulse propagation in Scn5a-knockout mice: combined contribution of excitability, connexin expression, and tissue architecture in relation to aging. Circulation. 2005;112(13):1927–1935. doi: 10.1161/CIRCULATIONAHA.105.539072. [DOI] [PubMed] [Google Scholar]
- 39.Jansen JA, et al. Reduced heterogeneous expression of Cx43 results in decreased Nav1.5 expression and reduced sodium current that accounts for arrhythmia vulnerability in conditional Cx43 knockout mice. Heart Rhythm. 2012;9(4):600–607. doi: 10.1016/j.hrthm.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chang MG, Sato D, de Lange E, et al. Bi-stable wave propagation and early afterdepolarization-mediated cardiac arrhythmias. Heart Rhythm. 2012;9(1):115–122. doi: 10.1016/j.hrthm.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Watanabe A, et al. Low-dose isoproterenol for repetitive ventricular arrhythmia in patients with Brugada syndrome. Eur. Heart J. 2006;27(13):1579–1583. doi: 10.1093/eurheartj/ehl060. [DOI] [PubMed] [Google Scholar]
- 42.Heijman J, Volders PG, Westra RL, Rudy Y. Local control of β-adrenergic stimulation: effects on ventricular myocyte electrophysiology and Ca2+-transient. J. Mol. Cell. Cardiol. 2011;50(5):863–871. doi: 10.1016/j.yjmcc.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wei N, Tolkacheva EG. Interplay between ephaptic coupling and complex geometry of border zone during acute myocardial ischemia: effect on arrhythmogeneity. Chaos. 2020;30(3):033111. doi: 10.1063/1.5134447. [DOI] [PubMed] [Google Scholar]
- 44.Weinberg SH. Ephaptic coupling rescues conduction failure in weakly coupled cardiac tissue with voltage-gated gap junctions. Chaos. 2017;27(9):093908. doi: 10.1063/1.4999602. [DOI] [PubMed] [Google Scholar]
- 45.Greer-Short A, George SA, Poelzing S, Weinberg SH. Revealing the concealed nature of long-QT type 3 syndrome. Circ. Arrhythm Electrophysiol. 2017;10(2):e004400. doi: 10.1161/CIRCEP.116.004400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nowak MB, et al. Intercellular sodium regulates repolarization in cardiac tissue with sodium channel gain of function. Biophys. J. 2020;118(11):2829–2843. doi: 10.1016/j.bpj.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jæger KH, Edwards AG, McCulloch A, Tveito A. Properties of cardiac conduction in a cell-based computational model. PLoS Comput Biol. 2019;15(5):e1007042. doi: 10.1371/journal.pcbi.1007042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kucera JP, Rohr S, Rudy Y. Localization of sodium channels in intercalated disks modulates cardiac conduction. Circ. Res. 2002;91(12):1176–1182. doi: 10.1161/01.RES.0000046237.54156.0A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mori Y, Fishman GI, Peskin CS. Ephaptic conduction in a cardiac strand model with 3D electrodiffusion. Proc. Natl. Acad. Sci. USA. 2008;105(17):6463–6468. doi: 10.1073/pnas.0801089105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cohen SA. Immunocytochemical localization of rH1 sodium channel in adult rat heart atria and ventricle Presence in terminal intercalated disks. Circulation. 1996;94(12):3083–3086. doi: 10.1161/01.CIR.94.12.3083. [DOI] [PubMed] [Google Scholar]
- 51.Maier SK, et al. An unexpected role for brain-type sodium channels in coupling of cell surface depolarization to contraction in the heart. Proc. Natl. Acad. Sci. USA. 2002;99(6):4073–4078. doi: 10.1073/pnas.261705699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taggart P, et al. Inhomogeneous transmural conduction during early ischaemia in patients with coronary artery disease. J. Mol. Cell. Cardiol. 2000;32(4):621–630. doi: 10.1006/jmcc.2000.1105. [DOI] [PubMed] [Google Scholar]
- 53.Tsumoto, K. et al. Specific decreasing of Na+ channel expression on the lateral membrane of cardiomyocytes causes fatal arrhythmias in Brugada syndrome. bioRxiv. 10.1101/805986 (2019). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article (and its Supplementary Information files). Furthermore, these codes used to simulate the myocardial strand and ring models are available in the repository: https://github.com/92tsumoto/BrS-P2R-strand-ORd2011model-withTNNP_INa-FT_IKr, and https://github.com/92tsumoto/BrS-P2R-ring-ORd2011model-withTNNP_INa-FT_IKr.