Abstract
Mondor’s disease (MD), or superficial thrombophlebitis of the anterolateral thoracoabdominal wall, is a rare disease that presents with a palpable cord-like induration beneath the skin. It is a benign, self-limiting condition with probably underestimated significance due to the fact it may be a rare manifestation of an underlying breast carcinoma. It can also resemble breast malignancy and, if physician is not familiar with clinical features of MD, it may lead to unnecessary biopsy. The diagnosis is straightforward in most cases and it may be based on a thorough history and physical examination and it can be ultrasonographically confirmed. Raising awareness of this condition may facilitate recognition and diagnosing MD and eventually limit unnecessary diagnostic procedures.
Keywords: Mondor’s disease, breast, thrombophlebitis
Introduction
Mondor’s disease (MD) is a rare entity characterized by sclerosing thrombophlebitis of the superficial veins of thoracoabdominal wall.1 The disease is approximately 3 times more prevalent in women than in men and it mostly presents in middle aged patients.1,2 The etiology of MD remains largely unknown—most commonly it is idiopathic.1 It is characterized with sudden onset of soreness and subcutaneous cord-like lesion, which is red and tender on presentation, but subsequently turns into a nonpainful fibrous band that disappears after recanalization of the affected vein.3 MD is a benign, self-limiting condition which usually spontaneously resolves over 2 to 8 weeks and leaves no permanent sequela.1,2
Case Report
A previously healthy 37-year-old female presented with pain and skin retraction of the right breast of 10-days duration. Physical examination revealed a palpable subcutaneous cord-like fibrous lesion and linear retraction of the breast skin, which extended from the upper lateral quadrant to the lower lateral quadrant, next to the areola of the right breast (Figure 1). The rest of the clinical examination was unremarkable and all vital signs were within normal range.
Figure 1.
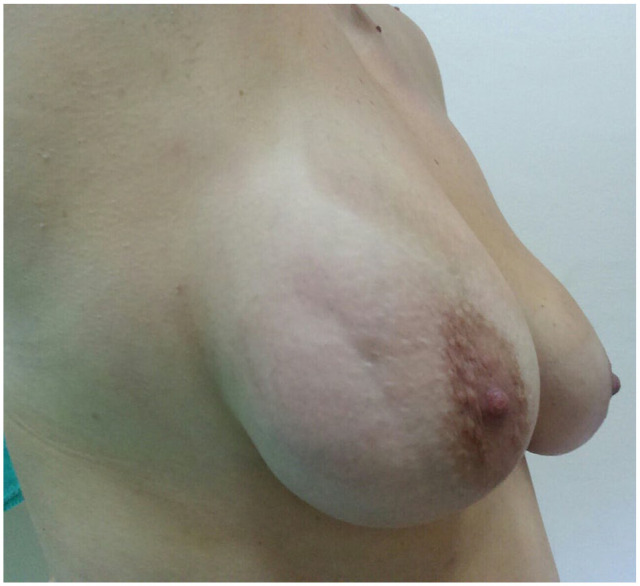
Physical examination: groove-like retraction of the breast skin.
Ultrasonography of the right breast showed a long, superficial, beaded, tubular, anechogenic structure. It measured 7mm in its widest diameter. There was no blood flow on Doppler imaging, except in surrounding fibroglandular tissue in the proximal part of the lesion (Figure 2).
Figure 2.
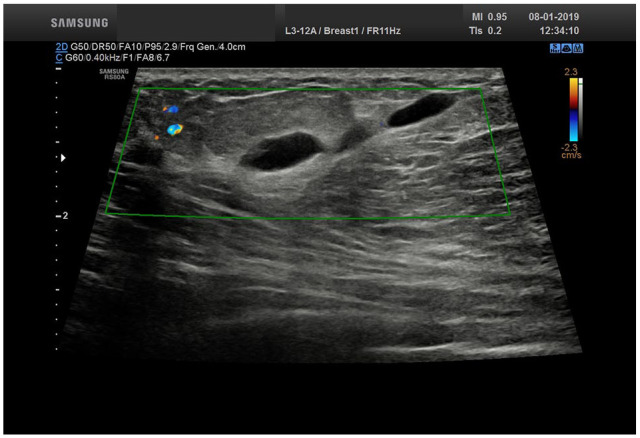
Ultrasonography: subcutaneous, tubular, anechogenic structure with no blood flow.
The patient was diagnosed with superficial thrombophlebitis of the lateral thoracic vein—Mondor’s disease.
The cord-like lesion and tenderness completely resolved in 4 weeks after conservative treatment with NSAIDs (ibuprofen).
Discussion
Superficial thrombophlebitis is a common, benign, self-limited disease representing an inflammatory process of superficial veins associated with thrombus formation. Although this term is most commonly related to leg veins, this condition can also affect the superficial veins of the other parts of the body.4
Mondor’s disease (MD) is a rare entity characterized by sclerosing thrombophlebitis of the thoracoepigastic, lateral thoracic, or the superior epigastric vein.1 In our patient, the lateral thoracic vein was involved.
Etiology of MD is still unclear and most of the cases are considered idiopathic.1,2 Identified predisposing factors include recent surgical resection or breast augmentation, breast biopsy, trauma, vigorous physical activity (extensive stretching and relaxing of the vein), hormone therapy, infection (abscess or mastitis), large pendulous breasts and tight clothing (pressure on the vein with consequential stagnation of blood).1,5-10 Based on the history and physical examination, no risk factor was identified in our patient.
MD is characterized by sudden appearance of a linear, cordlike, partially or completely occluded, thrombosed vein which is sore and erythematous at first. The lesion subsequently, while the thrombus organizes and recanalizes, turns into a painless, fibrous band which may persist up to several weeks.11 Elevation of the breast or abduction of the ipsilateral arm may accentuate the cord, which may also present as retracted breast skin.8,12 In cases of breast MD, it is imperative for clinicians to correctly differentiate this condition from a breast cancer, which may be challenging—due to palpable finding and skin retraction, MD can resemble breast cancer on clinical exam, but, according to a recent systematic review by Amano and Shimizu,1 5% of MD cases are actually associated with underlying occult breast cancer.
Ultrasonography is considered the first line in the imaging evaluation of MD. On ultrasound examination, our patient had typical presentation with subcutaneous anechogenic, uncompressible tubular structure with multiple areas of narrowing, giving a beaded appearance. Presence of blood flow signals depends on the status of thrombosis or recanalization and may be useful in follow-up.13-15
There is no need for additional diagnostic evaluation if physician is familiar with MD and if the clinical features are typical. If there is doubt, mammography should be performed to exclude eventual presence of underlying malignancy. Some authors suggest that mammography should be performed in all patients with unidentified underlying cause of MD, but the others underline that it may be inconclusive, or it may show a tubular density, which may be mistaken for a dilated duct and lead to unnecessary biopsy.16-18
MD on the chest wall is considered as original MD, but similar conditions recognized as variants of MD may rarely arise on other sites, such as penis (penile Mondor’s disease—PMD) and axilla (axillary web syndrome—AWS).19,20 There is uncertainty in the literature whether it is correct to define AWS as a form of MD. AWS usually appears in the early postoperative period following axillary lymph node dissection or sentinel lymph node biopsy and clinical presentation is similar to MD.20 However, ultrasonographic examination doesn’t show any reproducibly identifiable structure and the diagnosis is based on clinical finding of subcutaneous cording.21 Also, there are different views on the pathophysiology of AWS—some authors suggest that it affects lymphatic or veinous system or both and others believe that AWS cords represent abnormal fascial tissue.20,22 Physical therapy and exercising can reduce pain and improve range of motion.22
Differential diagnoses of Mondor’s disease include inflammatory breast cancer, mastitis, abscess and dilated duct (Table 1).9,18,23
Table 1.
Mondor’s disease and differential diagnoses.
| Symptoms | Skin changes | Palpatory finding | US finding | |
|---|---|---|---|---|
| MD | Mild, aching pain may be present initially; soreness. | Often erythematous; may be raised or retracted. | Linear, cord-like lesion adherent to the skin. | Tubular anechogenic, beaded structure; thrombus distending the vein may be visible. |
| Inflammatory breast cancer | Nonpainful in spite of the alarming appearance on examination; progressive worsening. | Possible skin retraction; Peau d’orange, erythema, crusting, blistering, or retraction of the nipple. | There may or may not be an underlying palpable mass, which may become adherent to the skin; fixed palpable ipsilateral axillary lymph nodes may be present. | Hypoechogenic shadowing mass; skin thickening; pectoral muscle invasion; axillary involvement. |
| Mastitis | Exquisite tenderness; fever. | Erythematous skin; mild skin thickening. | Warm; indurated breast; nipple retraction may be present; nodal enlargement is common. | Ill-defined area with hyperechogenic, infiltrated and inflamed fat lobules, hypoechogenic areas in the glandular parenchyma; mild skin thickening; inflammatory axillary lymph nodes. |
| Abscess | Exquisite tenderness; fever. | Erythematous skin. | Warm; rounded lesion; indurated breast; palpable fluctuance; nodal enlargement is common. | Hypoechogenic multiloculated collection with an hyperechogenic, vascular rim; acoustic enhancement due to fluid content; no vascularity within the collection; inflammatory axillary lymph nodes. |
| Dilated duct | Often asymptomatic; pain or tenderness may be present. | Often asymptomatic; nipple retraction may be present; nipple discharge may be present. | Often asymptomatic; sometimes may present as a palpable mass. | Distended (>2mm) anechogenic branching or tubular structures. |
MD is generally considered self-limited and benign, but there is a potential link with serious underlying diseases—malignancy (breast cancer, vascular neoplasm, cutaneous metastasis of any type of cancer), hypercoagulable state or systemic vasculitis, so these patients should be carefully examined and followed-up.1,16,24,25
Today, the pandemic of COVID-19 is challenging healthcare systems all around the world. Available evidence suggest the presence of hypercoagulable state in COVID-19 patients and thrombophlebitis could be clinical manifestation due to tight interconnection between inflammation and hemostasis abnormalities. Clinicians should also be aware of rare forms of venous thrombosis such as Mondor’s disease.26
In most cases, a proper explanation of the condition, strong reassurance and symptomatic treatment with anti-inflammatory drugs and warm compresses are helpful and lesion resolves completely within 2-8 weeks.1,2,25
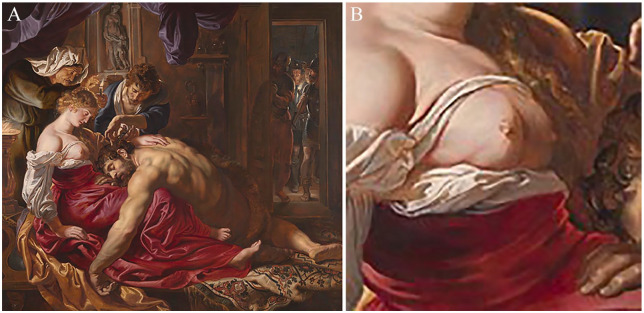
Cases with cord-like lesions on the chest wall were initially reported in the early 1850s, but this condition was acknowledged by and named after Henri Mondor, a French surgeon who reported a series of four cases and thoroughly described them in 1939.27 However, there is possible evidence that it was noticed much earlier by one of the Old Masters, Peter Paul Rubens, in his famous painting “Samson and Delilah” (1609-1610) (Figure 3A). If we look closer, we can see an interesting detail on Delilah’s right breast—in lower lateral quadrant, there is an oblique indentation that extends towards the nipple (Figure 3B). Did Rubens’ Delilah have Mondor’s disease?28 This medico-artistic diagnosis is controversial, but in accordance with Rubens’s pursuit of realism, it is likely correct.
Figure 3.
Rubens’s “Samson and Delilah” (A), and detail of Delilah (B), The National Gallery, London.
Story of Mondor’s disease is almost like a never-ending story—from Peter Paul Rubens to COVID-19 era. However, until this day, only approximately 500 cases have been described in the literature (Laroche, Galanaud and Labau, 2012). There is a possibility that it is more common than reported since the lesion is often nonpainful and self-resolving, so some patients do not seek medical attention and due to lack of needed awareness of physicians to recognize it. The correct diagnosis is very important for avoiding unnecessary invasive diagnostic procedures and providing optimal management and it can be straightforward if physicians are familiar with the condition.
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: Katarina Obradovic and Nina Adzic wrote the manuscript. Dragana Pavlovic Stankovic, Ivana Petkovic and Vladimir Urban examined the patient and helped in drafting the manuscript. Zorica Milosevic supervised this case report, revised the manuscript and approved the final version.
Patient’s Consent: Written informed consent was obtained from the patient for publication of this case report.
ORCID iDs: Katarina Obradovic  https://orcid.org/0000-0002-7544-1527
https://orcid.org/0000-0002-7544-1527
Nina Adzic  https://orcid.org/0000-0003-0540-0890
https://orcid.org/0000-0003-0540-0890
Zorica Milosevic  https://orcid.org/0000-0001-9602-2084
https://orcid.org/0000-0001-9602-2084
References
- 1. Amano M, Shimizu T. Mondor’s disease: a review of the literature. Intern Med. 2018;57:2607-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Laroche JP, Galanaud J, Labau D, et al. Mondor’s disease: what’s new since 1939? Thromb Res. 2012;130(suppl 1):S56-S58. [DOI] [PubMed] [Google Scholar]
- 3. Álvarez-Garrido H, Garrido-Ríos AA, Sanz-Muñoz C, et al. Mondor’s disease. Clin Exp Dermatol. 2009;34:753-756. [DOI] [PubMed] [Google Scholar]
- 4. Czysz A, Higbee SL. Superficial thrombophlebitis. In: StatPearls. StatPearls Publishing; 2020. [PubMed] [Google Scholar]
- 5. Khan UD. Incidence of mondor disease in breast augmentation: a retrospective study of 2052 breasts using inframammary incision. Plast Reconstr Surg. 2008;122:88e-89e. [DOI] [PubMed] [Google Scholar]
- 6. Salemis NS, Vasilara G, Lagoudianakis E. Mondor’s disease of the breast as a complication of ultrasound-guided core needle biopsy: management and review of the literature. Breast Dis. 2015;35:73-76. [DOI] [PubMed] [Google Scholar]
- 7. Tröbinger C, Wiedermann CJ. Bodybuilding-induced Mondor’s disease of the chest wall. Phys Ther Sport. 2017;23:133-135. [DOI] [PubMed] [Google Scholar]
- 8. Kadioglu H, Yildiz S, Ersoy YE, et al. An unusual case caused by a common reason: Mondor’s disease by oral contraceptives. Int J Surg Case Rep. 2013;4:855-857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boonjunwetvat D, Chakkabat P, Techanithisawat P, et al. Mondor’s disease secondary to abscess in the axillary region. Breast J. 2019;25:746-748. [DOI] [PubMed] [Google Scholar]
- 10. Poongodi V, Annal MA, Lavanya S. Mastitis with Mondor’s disease. Pon J Nurs. 2019;12:15-17. [Google Scholar]
- 11. Hogan G. Mondor’s disease. Arch Intern Med. 1964;113:881-885. [DOI] [PubMed] [Google Scholar]
- 12. Ichinose A, Fukunaga A, Terashi H, et al. Objective recognition of vascular lesions in Mondor’s disease by immunohistochemistry. J Eur Acad Dermatol Venereol. 2008;22:168-173. [DOI] [PubMed] [Google Scholar]
- 13. Johnson WC, Wallrich R, Helwig EB. Superficial thrombophlebitis of the chest wall. JAMA.1962;180:103-108. [Google Scholar]
- 14. Oldfield MC. Mondor’s disease: a superficial phlebitis of the breast. Lancet. 1962;1:994-996. [DOI] [PubMed] [Google Scholar]
- 15. Lin JC, Liu CL, Liu TP. The sonographic findings of Mondor’s disease in the breast. J Med Ultrasound. 2002;10:202-204. [Google Scholar]
- 16. Catania S, Zurrida S, Veronesi P, Galimberti V, Bono A, Pluchinotta A. Mondor’s disease and breast cancer. Cancer. 1992;69:2267-2270. [DOI] [PubMed] [Google Scholar]
- 17. Shetty M, Watson AB. Mondor’s disease of the breast: sonographic and mammographic findings. AJR Am J Roentgenol. 2001;177:893-896. [DOI] [PubMed] [Google Scholar]
- 18. Adeniji-Sofoluwe A, Afolabi O. Mondor’s disease: classical imaging findings in the breast. BMJ Case Rep. 2011;2011:bcr0720114521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mukendi AM, Mahlobo F. Penile Mondor’s disease: clinical and sonographic images. Clin Case Rep. 2019;7:2283-2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mayo RC, Leung JWT. Axillary web syndrome: what the radiologist needs to know. Breast J. 2019;25:153. [DOI] [PubMed] [Google Scholar]
- 21. Koehler LA, Hunter DW, Haddad TC, Blaes AH, Hirsch AT, Ludewig PM. Characterizing axillary web syndrome: ultrasonographic efficacy. Lymphology. 2014;47:156-163. [PMC free article] [PubMed] [Google Scholar]
- 22. de Sire A, Invernizzi M, Lippi L, Cisari C, Özçakar L, Franchignoni F. Blurred lines between axillary web syndrome and Mondor’s disease after breast cancer surgery: a case report. Ann Phys Rehabil Med. 2020;63:365-367. [DOI] [PubMed] [Google Scholar]
- 23. Menta A, Fouad TM, Lucci A, et al. Inflammatory breast cancer: what to know about this unique, aggressive breast cancer. Surg Clin North Am. 2018;98:787-800. [DOI] [PubMed] [Google Scholar]
- 24. de Godoy JM, Godoy MF, Batigália F, Braile DM. The association of Mondor’s disease with protein S deficiency: case report and review of literature. J Thromb Thrombolysis. 2002;13:187-189. [DOI] [PubMed] [Google Scholar]
- 25. Pasta V, D’Orazi V, Sottile D, Del Vecchio L, Panunzi A, Urciuoli P. Breast Mondor’s disease: diagnosis and management of six new cases of this underestimated pathology. Phlebology. 2015;30:564-568. [DOI] [PubMed] [Google Scholar]
- 26. Agus G. COVID-19 era: Mondor’s disease and rembrandt. What ties? Veins and Lymphatics. 2020;9:23-24. [Google Scholar]
- 27. Mondor H. Tronculite sous-cutané subaigue de la paroi thoracique antéro-latérale. Mem Acad Chir (Paris). 1939;65:1271-1278. [Google Scholar]
- 28. King B, Shortis A, King AJ. Did Rubens’ Delilah have Mondor’s disease? ANZ J Surg. 2013;83:146-148. [DOI] [PubMed] [Google Scholar]