Abstract
In this study, we evaluated the antiproliferative and antimetastatic effects of the Pleurotus highking mushroom on the human triple-negative breast cancer cell lines MDA-MB-231 and HCC-1937 and attempted to elucidate the underlying molecular mechanisms. The antiproliferative effects of P. highking purified fraction-III (PEF-III) were investigated using colony formation and MTS assays. The antimigratory effects of PEF-III were determined by wound healing, transwell migration, and matrigel cell invasion assays. The protein expression levels were evaluated using Western blot analysis. The effect of PEF-III on tumor-sphere formation was examined in a 3D sphere-forming medium, and the mRNA expressions of proliferation- and migration-related genes in the cells from the tumor spheres were determined using RT-qPCR. PEF-III treatment caused a potent and concentration-dependent decrease in the numbers of colonies and viable cells. It also remarkably suppressed the migratory ability of the cells. Mechanistically, PEF-III treatment reduced the expression of pAkt, matrix metallopeptidase-9 (MMP-9), and vimentin. Furthermore, PEF-III reduced the number and size of the tumor spheres in the 3D culture system. It also significantly reduced the mRNA expression of Ki-67, MMP-9, and vimentin in the PEF-III-treated tumor-sphere cells. PEF-III exerted promising antiproliferative and antimigratory effects in triple-negative breast cancer cell lines by suppressing Akt signaling. Therefore, P. highking mushrooms may be considered a potential source for the development of potent anticancer drug(s) for the treatment of breast cancer.
Keywords: Akt signaling, antiproliferation, cellular migration, MMP-9, tumor sphere, vimentin
Introduction
Cancer is one of the most devastating diseases worldwide, despite recent medical advancements.1 Surgery, radiation, and immunotherapy are commonly used strategies to eradicate cancers, while chemotherapy is the choice of treatment in the initial stage.2 Vincristine, vinblastine, bleomycin, paclitaxel, and camptothecin are the best-known chemotherapeutic agents against cancer, although their low success rate, lack of selectivity, risk of toxicity, and high cost are drawbacks.3 Therefore, discovering safer, more selective, and cost-effective drugs from natural sources is critically needed to treat cancer and other chronic diseases. One such natural source is mushrooms, a plant-derived source and the focus of the current study.
Pleurotus highking (family Pleurotaceae), a type of oyster mushroom, is one of many edible and commercially cultivated varieties that are used worldwide for herbal and dietary purposes.4 Multiple anticancer compounds such as lentinan, hispolon, theanine, illudin-S, psilocybin, ganoderic acid, cordycepin, grifolin, and antroquinonol have been isolated from the different species of oyster mushrooms.5 Although several studies have assessed the antifungal and antibacterial,6 antitumour,7 anti-HIV,8 antigenotoxic,9 antilipidemic,10 anti-inflammatory,11 and immunomodulatory12 activities of different mushroom species, little is known about the antiproliferative and antimetastatic properties of P. highking against triple-negative breast cancer (TNBC) cells.
The results of our recent study suggested that P. highking can induce apoptosis by shifting the balance of apoptosis-related genes.13 Other studies have confirmed that the Pleurotus genus is a good source of antioxidants14 and anti-inflammatory agents.11 Therefore, we hypothesized that P. highking might inhibit cancer cell proliferation and metastasis by providing such potent metabolites. The current study focused on the antiproliferative and antimigratory roles of P. highking purified fraction-III (PEF-III) against the aggressive human TNBC cell lines MDA-MB-231 and HCC-1937 and on determining the molecular mechanisms underlying that activity.
Materials and Methods
Sample Collection and Crude Extract Production
Pleurotus highking is commercially cultivated in Bangladesh. It was obtained from the National Mushroom Development and Extension Centre (NMDEC), located in Dhaka, Bangladesh. The mushrooms were cleaned thoroughly, dried in the shade for more than 1 week until fully dried, and stored in an airtight container. Detailed authentication and deposition and extract production protocols were reported in our previous study.13
Fractionation and purification
The crude mushroom extract was fractionated using silica (60-230 mesh size) gel column chromatography using increasing concentrations of methanol in chloroform up to 100%. Elutes were combined for thin-layer chromatography (TLC), from which 5 isolated fractions were obtained and dried by rotary evaporation. The stock solution and serial dilution of the fractions were prepared in dimethyl sulphoxide (DMSO). The solutions were further diluted in Dulbecco’s modified Eagle’s medium (DMEM) and added to the cell cultures to attain the desired concentrations. The same concentration of DMSO was used in both the control medium and the test medium. Each of the fractions was screened for their anticancer activity against breast cancer cells and fraction-III (PEF-III) was selected for further study owing its potent breast cancer cell regrowth inhibitory capability (Supplementary Figure S1).
Cell lines and cell culture
The human TNBC cell lines MDA-MB-321 and HCC-1937 were obtained from the American Type Culture Collection and maintained in DMEM and RPMI-1640 medium, respectively, supplemented with fetal bovine serum (FBS) (10% v/v) and penicillin G/streptomycin 1% (v/v) at 37°C under 5% CO2. The absence of culture contamination by Mycoplasma was confirmed before the experiments.
Colony formation assay
The effect of PEF-III on breast cancer cell regrowth was assessed using a colony formation assay. Approximately 400 viable MDA-MB-231 and HCC-1937 cells were seeded in a 10-cm culture dish and incubated for 24 hours for attachment. After incubation, the cells were treated with different concentrations (20 and 30 μg/mL) of PEF-III (or DMSO for the control) and incubated again for 2 weeks. The medium was then discarded, and the colonies were washed with phosphate-buffered saline (PBS) twice. Next, the colonies were fixed with 4% paraformaldehyde and stained with crystal violet solution. Colonies consisting of more than 20 individual cells were counted.
Cell viability assay
The effect of PEF-III on breast cancer cell proliferation was determined using the CellTiter 96® AQueous One Solution Cell Proliferation Assay kit (Promega, USA). Briefly, 5 × 103 cells were seeded in each well of a 96-well plate for 1 day, and growth medium containing various concentrations (20 and 30 μg/mL) of PEF-III was added. Twenty microliters of CellTiter 96® AQueous One Solution kit was added to each well, and the cells were incubated for 2 hours. After incubation, the absorbance was measured at 490 nm with an enzyme-linked immunosorbent assay microplate reader (BioTek Instruments, USA). The absorbance value is directly proportional to the number of living cells.
Wound healing assay
To assess the metastatic ability of cancer cells, a wound-healing assay was performed according to a previously reported protocol.15 Briefly, approximately 5 × 105 MDA-MB-231 cells were seeded in a 6-well plate and incubated until confluence was reached then the culture scratched using a sterile P200 pipette tip. The cells were then washed with PBS, and fresh culture medium containing PEF-III (DMSO as the control) was added. Images were taken from 0 to 24 hours using a phase-contrast microscope. The wound closure gap was determined by dividing the area by the length of the scratch and then comparing it with the DMSO-treated group.
Transwell cell migration and invasion assay
The migratory and invasive abilities of the cancer cells were assayed in transwell chambers according to a published protocol,16 with some modifications. Briefly, the cells were treated with PEF-III for 24 hours, trypsinized, and washed twice with serum-free medium. Approximately 1 × 105 pretreated cells suspended in 100 μL of the serum-free medium were seeded to the upper chamber. The upper chambers coated with matrigel were used for the invasion assay, and uncoated chambers were used for the migration assay. The lower chambers were filled with approximately 500 μL of culture medium with 10% FBS and incubated for 24 hours. After incubation, the cells from the upper surface were wiped off with cotton swabs. The migrated or invaded cells on the opposite side of the transwell were washed twice with PBS, fixed with 4% paraformaldehyde, and stained with crystal violet solution. After 2 washes with Milli Q, 3 microscopic fields were taken randomly, and the migrated and invaded cells were counted using ImageJ software.
Lysate preparation and Western blot analysis
Lysates from both the treated and the control cells were prepared using TNE buffer, and the protein concentration was quantified using a protein DC quantification kit. Equal amounts of protein were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then transferred to polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA), and the membranes were blocked in 4% skim milk for 40 minutes. After blocking, the membranes were incubated with primary antibodies (antibody information provided in Supplementary Table 1) at 4°C overnight and washed with tris-buffered saline with Tween (TBST). Horseradish peroxidase (HRP)-conjugated secondary antibodies were added and incubated at 37°C for 40 minutes, the membranes were then washed with TBST, and the protein band was detected by use of the chemiluminescence kit Immuno Star Zeta (Wako, Japan) and EZ capture MG (ATTO Corporation, Japan). The normalized protein expression was calculated to that of the reference protein β-actin by use of ImageJ software.
Tumor-sphere formation assay
Three-dimensional, or tumor-sphere, culture is a recently introduced technique that is simple, inexpensive, and effective and provides a physiological environment that closely resembles that of cultured cells.17 This technique is now widely used for the screening of the antitumoural activity of drugs. An in vitro tumor-sphere formation assay was performed according to a reported protocol.18 Briefly, approximately 3 × 103 cells were resuspended in a poly-2-hydroxyethyl methacrylate- (Sigma, Aldrich) coated 6-well plate containing a sphere-forming medium containing either DMSO or PEF-III that had been incubated for 1 week. The number of tumor spheres was counted, and the diameter of each tumor sphere was measured.
RNA isolation and RT-qPCR
The total RNA from the PEF-II-treated tumor-sphere cells was isolated using TRIzol reagent (Invitrogen), and the concentration was measured using a NanoDrop spectrophotometer. The RT-qPCR was performed according to a published report,19 and the reactions were performed twice and repeated 3 times. All the primers used in this experiment are provided in Supplementary Table 2.
Statistical analysis
All the quantitative analytic data are represented as mean ± standard deviations (SDs) of 3 independent experiments. Differences between the control and test groups were determined using one-way analysis of variance (ANOVA). An unpaired t-test was used to compare the control and test groups. Probability values less than .05 were considered significant.
Results
Effects of PEF-III on breast cancer cell proliferation
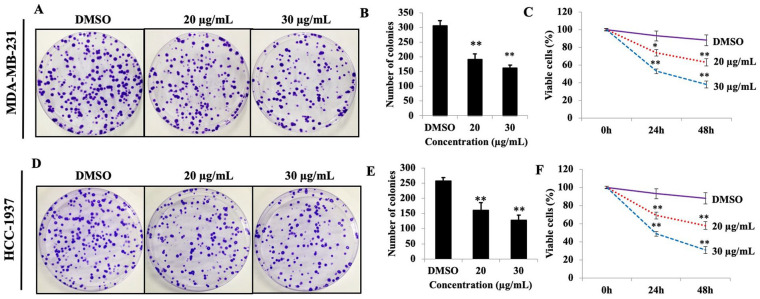
To explore the antiproliferative activity of PEF-III in the breast cancer cell lines MDA-MB-231 and HCC-1937, colony formation and MTS assays were used. PEF-III caused a significant dose-dependent reduction in the number of colonies compared to that of the control (DMSO-treated) cells (Figure 1A and D). A similar pattern was found in the MTS assay. When the breast cancer cells were challenged with 2 different concentrations of PEF-III (20 and 30 μg/mL), the number of living cells sharply decreased in a time- and concentration-dependent manner (Figure 1C and F). These data revealed that PEF-III has potent antiproliferative effects against breast cancer cells.
Figure 1.
PEF-III reduces TNBC cells regrowth and viability. Cells were treated with different concentrations of PEF-IIII (20 and 30 μg/mL) and incubated for 2 weeks. After incubation, the number of colonies was calculated and compared with that of the control group (A and D). Figure B and E depict the quantification of the colony numbers of 2 TNBC cell lines. After treatment of the cells with PEF-III (20 and 30 μg/mL) for 24 and 48 hours, the percentage of viable cells was determined using an MTS assay (C and F).
The results are presented as the means ± SDs of 3 independent experiments. Bars with asterisks indicate significant differences from the control at P ≤ .05 (*) or P ≤ .01 (**).
Effects of PEF-III on scratch wound closure and cell migration
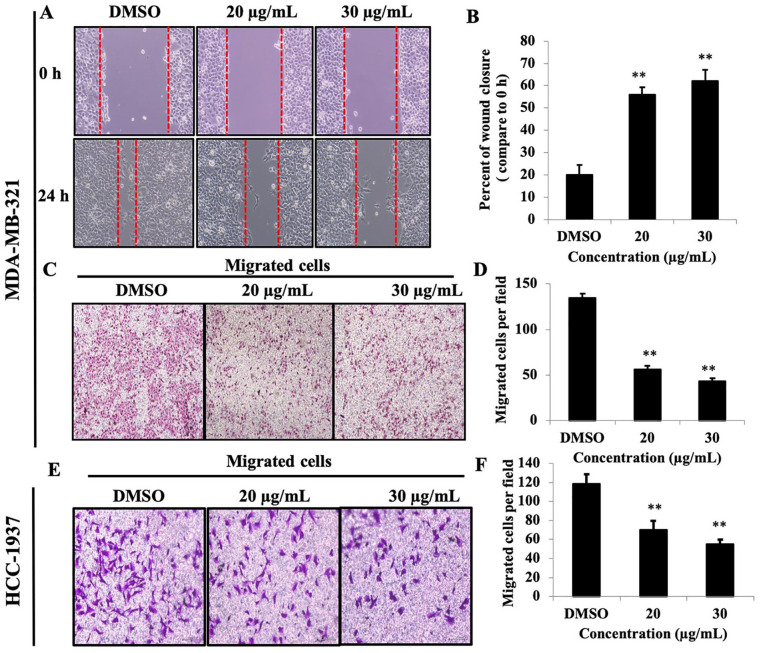
Migration and invasion of cancer cells are critical determinant steps of tumor metastasis. To evaluate the antimetastatic effect of PEF-III on breast cancer cells, cell migration, invasion, and wound healing assays were performed. As shown in Figure 2A, the ability of the cells exposed to PEF-III treatment to recover the wound gap was significantly less than the nontreated cells (P < .01). PEF-III treatment also significantly attenuated the migration of the breast cancer cells in a concentration-dependent manner (Figure 2C and E) compared to control cells (P < .01).
Figure 2.
PEF-III attenuates wound closure and cell migration of TNBC cells. Cell monolayers were scraped with a sterile p200 pipette tip, and the cells were treated with the indicated concentrations of PEF-III for 24 hours. The wound closure distances were quantified by measuring the distance between the edges (A and B). PEF-III also inhibited cell motility (C and E), as determined by a transwell cell migration assay. The migrated cells were stained with crystal violet, and photographs were taken for the inhibition calculation (D and F).*
The results are presented as the means ± SDs of 3 independent experiments. Bars with asterisks indicate significant differences from the control at P ≤ .05 (*) or P ≤ .01 (**).
Effect of PEF-III on the regulation of Akt signaling in breast cancer cells
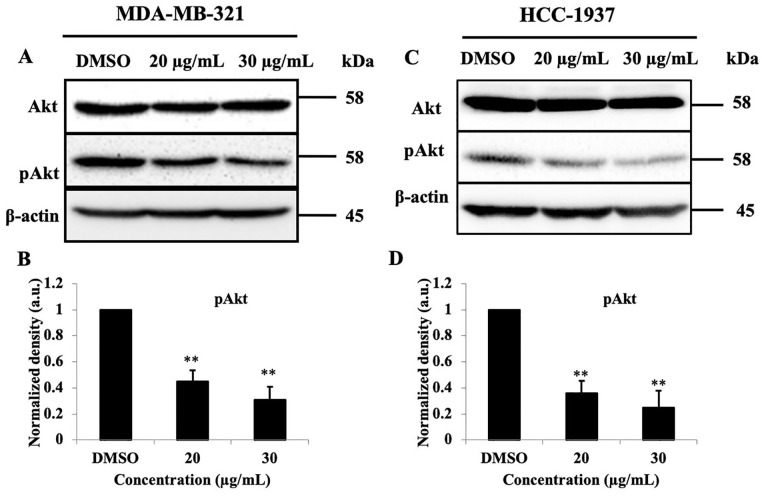
To determine the molecular mechanisms underlying PEF-III activities, we investigated the effect of PEF-III on Akt signaling in TNBC cells. Western blot analysis indicated that PEF-II treatment significantly (P < .01) reduced the amount of pAkt without altering the total Akt level (Figure 3A and C).
Figure 3.
PEF-III suppresses pAkt without changing the total Akt amount. PEF-III treatment in breast cancer cells decreased the expression of pAk without changing the total Akt (A and C) amount. The normalized band densities of pAkt relative to β-actin are shown in B and D for the MDA-AM-231 and HCC-1937 TNBC cell lines, respectively.*
The results are presented as the means ± SDs of 3 independent experiments. Bars with asterisks indicate significant differences from the control at P ≤ .05 (*) or P ≤ .01 (**).
Effect of PEF-III on MMP-9 and vimentin expression levels
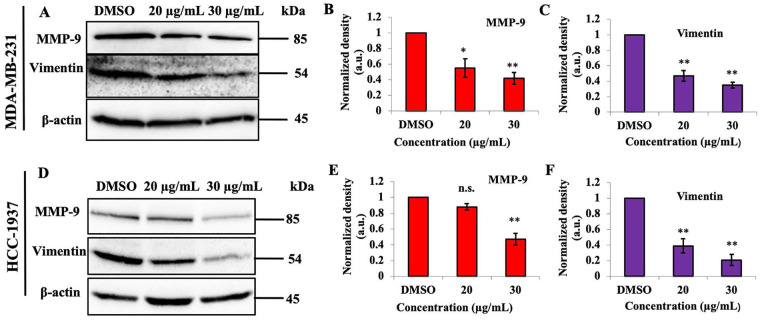
The protein levels of matrix metallopeptidase-9 (MMP-9) and vimentin in the breast cancer cell line challenged with different concentrations of PEF-III were determined. PEF-III decreased the amount of MMP-9 and vimentin proteins significantly (P < .01; Figure 4A and C).
Figure 4.
PEF-III treatment suppresses the amount of MMP-9 and vimentin proteins in TNBC cells. Breast cancer cells were treated with different concentrations of PEF-III (20 and 30 μg/mL) and then subjected to Western blot analysis to detect MMP-9 and vimentin levels (A and D). The relative band intensities of MMP-9 (B and E) and vimentin (C and F) compared with that of the control in 2 different TNBC cell lines are shown in bar charts.
The results are presented as the means ± SDs of 3 independent experiments. Bars with asterisks indicate significant differences from the control at P ≤ .05 (*) or P ≤ .01 (**).
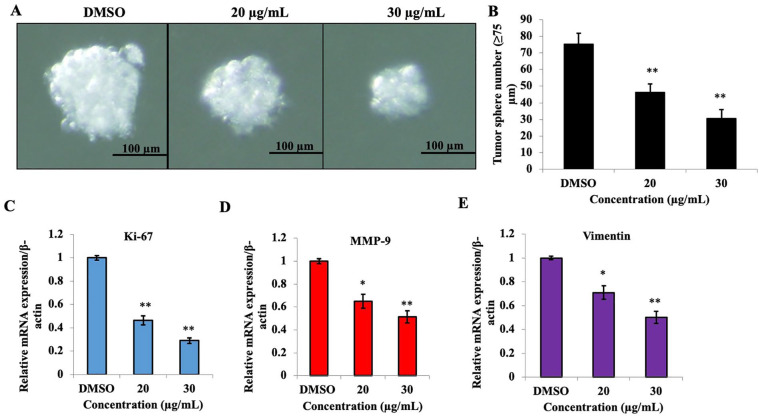
Effect of PEF-III on tumor-sphere formation and mRNA expression of cell proliferation-and migration-related genes in tumor-sphere cells
The effect of PEF-III on tumor-sphere formation was examined using tumor-sphere forming medium, and we found that PEF-III significantly reduced the number and size of the tumor-spheres (Figure 5A and B). The mRNA expression of the proliferation marker (Figure 5C) and the metastasis regulatory genes MMP-9 (Figure 5D) and vimentin (Figure 5E) were also significantly decreased in PEF-III-treated tumor-sphere cells compared to the control.
Figure 5.
PEF-III treatment inhibits tumor-sphere formation and suppresses cell proliferation- and cell migration-related gene expression. The cells were cultured in 3D sphere-forming medium with or without PEF-III and incubated for 5 days. After incubation, the size (A) and number (B) of the tumor spheres were determined. Total RNA from the tumor-sphere cells was collected, and the relative mRNA of Ki-67 (C), MMP-9 (D), and vimentin (E) were quantified by RT-qPCR. Data are presented as the means ± SDs of 3 independent experiments.
The bars with asterisks indicate significant differences from the control at P ≤ .05 (*) or P ≤ .01 (**).
Discussion
In this study, the possible mechanisms by which PEF-III reduces cell proliferation, as well as cell migration and invasion, were investigated using 2 human breast cancer cell lines. The findings demonstrate that PFE-III induced breast cancer cell death and inhibited cell migration and invasion by suppressing Akt signaling and metastasis regulatory molecules (MMP-9 and vimentin). Moreover, it also reduced the number and size of in vitro tumor-spheres in a 3D culture system. These findings may represent a novel mechanism for breast cancer cell sensitization and killing. Therefore, a compound(s) from PEF-III may be used for the chemoprevention and treatment of metastatic breast cancer.
Cancer cells have an uncontrolled growth rate, accelerated angiogenesis, and stimulated metastatic ability.20 Different external and internal factors may cause gene mutation and lead to cancer by accelerating various signaling pathways in the living system.20 Chemotherapeutic compounds interrupt these signaling pathways and control accelerated proliferation and metastasis, hence inducing cancer cell death.21 Medicinal herbs and mushrooms are commonly considered a starting point in the search for new anticancer compounds.5 In the current study, we found that PEF-III potently suppressed breast cancer cell proliferation and viability. The outcome was consistent with data from previous studies in which oyster mushrooms inhibited cell growth in various cancer cell lines.22,23 Concerning the cytotoxic effect of PEF-III to normal breast cells (MCF-10A), our data demonstrated that PEF-III has no or very little cytotoxicity to normal breast cells (Supplementary Figure S2).
Cancer metastasis is a multistep process and a significant cause of cancer-associated mortalities.24 Various cascades of events such as cell adhesion, cell motility and invasion, cell movement, and degradation of the cellular matrix are associated with metastasis pathways.25 During the metastatic stage, cancer cells escape from the primary tumor site and travel to a distant area, where they establish a secondary tumor.26 Inhibition of cancer cell migration is a novel strategy for the treatment of metastatic cancers. Notably, our data showed that PEF-III effectively suppressed breast cancer cell migration and invasion (Supplementary Figure S3). These findings are consistent with those of reports27,28 in which oyster mushrooms inhibited cell migration and invasion in various types of cancer cells.
In a study conducted to elucidate the mechanism underlying cell growth inhibition, we found that P. highking alters the balance of Bax and Bcl-2,13 which are closely associated with Akt signaling.29 Furthermore, dysregulation of Akt signaling is one common cause of uncontrolled cell growth.30 Our current study revealed that treatment with P. highking markedly attenuated Akt phosphorylation without changing the total Akt level in TNBC cells. These data corroborate the findings of previous studies performed with different oyster mushrooms and using different cancer cells.29,31
MMP-9, one of the crucial determinants of cancer cell metastasis, is responsible for the degradation of the extracellular matrix of blood or lymph vessels and facilitates cancer cell migration and invasion.32 Vimentin, an intermediate filament protein and one of the essential elements of the cytoskeleton, also regulates cell migration and invasion.33 Many studies suggest that MMP-9 and vimentin expression are upregulated in aggressive and metastatic breast cancer cell lines, especially basal-like TNBC.34 Hence, therapies targeting MMP-9 and vimentin may provide a potential additional option for the treatment of aggressive and metastatic breast cancer. Our investigation demonstrates that PEF-III treatment significantly reduced these metastasis regulatory genes in breast cancer cells at both the transcriptional and translational level. As far as we know, this is the first report of suppression of these metastatic regulatory genes by P. highking treatment.
In vitro tumor-sphere formation is a new and inexpensive method considered a potential alternative for in vivo experiments and is now frequently used for the screening of anticancer drugs.17 In the present study, the number and size of tumor spheres were sharply reduced by PEF-III treatment in a concentration-dependent manner. The mRNA profile of PEF-III-treated tumor-sphere cells showed that PEF-III treatment clearly suppressed the proliferation gene and expression of metastasis-modulating genes. This suppression may be due to the inhibition of breast cancer cell proliferation and attenuation of phosphorylation of Akt signaling by PEF-III.
Conclusion
Our study has revealed potential antiproliferative and antimetastatic effects of P. highking against human breast cancer cells by suppression of Akt signaling phosphorylation and metastasis regulatory mediators (MMP-9 and vimentin). Our data firmly suggest that P. highking may have a beneficial therapeutic application for the treatment of aggressive and metastatic breast cancer. This study contributes to the growing body of evidence supporting the importance of edible mushrooms in the treatment of metastatic cancers, and will subsequently lead to the purification and isolation of the responsible compound(s). An essential next step is conducting in vivo experiments for further understanding of the relevant mechanisms.
Supplemental Material
Supplemental material, 3._Supplementary_Figures_and_Tables_R2_EDITS for Pleurotus highking mushrooms potentiate antiproliferative and antimigratory activity against triple-negative breast cancer cells by suppressing Akt signaling by Md. Anwarul Haque, A. S. M. Ali Reza, Mst. Samima Nasrin and Md. Atiar Rahman in Integrative Cancer Therapies
Acknowledgments
We would like to express our thankful appreciation to F. Miyamasu (Medical English Communications Center, Faculty of Medicine, University of Tsukuba) for critical English editing of the manuscript.
Footnotes
Author’s Contribution: Md. Anwarul Haque formulated research ideas overarching research goals and aims. MAH with A. S. M. Ali Reza and Mst. Samima Nasrin designed methodology, created models and applied statistical or other formal techniques to analyze study data. ASMAR, AAH conducted bench work and accomplished the experimental work. MAH prepared the manuscript and polished with the help of ASMAR. Md Atiar Rahman supervised and administrated the research planning and execution, interpreted the data and oversighted the manuscript to make suitable for submission.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Md. Anwarul Haque  https://orcid.org/0000-0001-6785-4121
https://orcid.org/0000-0001-6785-4121
A. S. M. Ali Reza  https://orcid.org/0000-0002-1457-0245
https://orcid.org/0000-0002-1457-0245
Md. Atiar Rahman  https://orcid.org/0000-0002-4902-8923
https://orcid.org/0000-0002-4902-8923
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674. [DOI] [PubMed] [Google Scholar]
- 2. Yildizhan H, Barkan NP, Turan SK, et al. Treatment strategies in cancer from past to present. In: Grumezescu AM. ed. Drug Targeting and Stimuli Sensitive Drug Delivery systems. William Andrew; 2018: 1-37. [Google Scholar]
- 3. Salem ML, Shoukry NM, Teleb WK, Abdel-Daim MM, Rahman MAA. In vitro and in vivo antitumor effects of the Egyptian scorpion Androctonus amoreuxi venom in an Ehrlich ascites tumor model. Springerplus. 2016;5:570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pereira A, Maraschin M. Banana (Musa spp) from peel to pulp: ethnopharmacology, source of bioactive compounds and its relevance for human health. J Ethnopharmacol. 2015;160:49-163. [DOI] [PubMed] [Google Scholar]
- 5. Patel S, Arun G. Recent developments in mushrooms as anti-cancer therapeutics: a review. 3 Biotech. 2012;2:1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Synytsya A, Mickova K, Synytsya A, Jablonsky I, Spevacek J, Erban V. Glucans from fruit bodies of cultivated mushrooms Pleurotus ostreatus and Pleurotus eryngii: structure and potential prebiotic activity. Carbohydr Polym. 2009;76;548-556. [Google Scholar]
- 7. Ye M, Luo X, Li L, et al. Grifolin, apotential antitumor natural product from the mushroom Albatrellus confluens, induces cell-cycle arrest in G1 phase via the ERK1/2 pathway. Cancer Lett. 2007;258:199-207. [DOI] [PubMed] [Google Scholar]
- 8. Piraino F, Brandt CR. Isolation and partial characterization of an antiviral, RC-183, from the edible mushroom Rozites caperata. Antivir Res. 1999;3:67-78. [DOI] [PubMed] [Google Scholar]
- 9. Wang JC, Hu SH, Liang ZC, Lee MY. Antigenotoxicity of extracts from Pleurotus citrinopileatus. J Sci Food Agric. 2005;85:770-778. [Google Scholar]
- 10. Miyazawa N, Okazaki M, Ohga S. Antihypertensive effect of Pleurotus nebrodensis in spontaneously hypertensive rats. J Oleo Sci. 2008;57:675-681. [DOI] [PubMed] [Google Scholar]
- 11. Sripanidkulchai K, Teepsawang S, Sripanidkulchai B. Protective effect of Cratoxylum formosum extract against acid/alcohol-induced gastric mucosal damage in rats. J Med Food. 2010;13:1097-1103. [DOI] [PubMed] [Google Scholar]
- 12. Vaz JA, Barros L, Martins AC, Buelga CS, Vasconcelos MH, Ferreira ICFR. Chemical composition of wild edible mushrooms and antioxidant properties of their water soluble polysaccharidic and ethanolic fractions. Food Chem. 2011;126:610-616. [Google Scholar]
- 13. Haque MA, Islam MAU. Pleurotus highking mushroom induces apoptosis by altering the balance of proapoptotic and antiapoptotic genes in breast cancer cells and inhibits tumor sphere formation. Medicina. 2019;55:716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Roupas P, Keogh J, Noakes M, Margetts C, Taylor P. The role of edible mushrooms in health: evaluation of the evidence. J Funct Foods. 2012;4:687-709. [Google Scholar]
- 15. Liang C, Park A, Guan J. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007:2:329-333. [DOI] [PubMed] [Google Scholar]
- 16. Wu GS, Song YL, Yin ZQ, et al. Ganoderiol A-Enriched extract suppresses migration and adhesion of MDA-MB-231 cells by inhibiting FAK-SRC-Paxillin cascade pathway. PLoS One. 2013;8:e76620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhu ZW, Chen L, Liu JX, et al. A novel three-dimensional tumorsphere culture system for the efficient and low-cost enrichment of cancer stem cells with natural polymers. Exp Ther Med. 2018;15:85-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee CH, Yu CC, Wang BY, Chang WW. Tumorsphere as an effeective in vitro platform for screening anti-cancer stem cell drugs. Oncotarget. 2016;7:1215-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Singh R, Mandhani A, Agrawal V, Garg M. Positive correlation between matrix metalloproteinases and epithelial-to-mesenchymal transition and its association with clinical outcome in bladder cancer patients. Cancer Microenviron. 2018;11:23-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hassan M, Watari H, Abu Almaaty A, Ohba Y, Sakuragi N. Apoptosis and molecular targeting therapy in cancer. Biomed Res Int. 2014;2014:150845. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21. Schwartz GK, Shah MA. Targeting the cell cycle: a new approach to cancer therapy. J Clin Oncol. 2005;23:9408-9421. [DOI] [PubMed] [Google Scholar]
- 22. Adams LS, Phung S, Wu X, Ki L, Chen S. White button mushroom (Agaricus bisporus) exhibits antiproliferative and proapoptotic properties and inhibits prostate tumor growth in athymic mice. Nutr Cancer. 2008;60:744-756. [DOI] [PubMed] [Google Scholar]
- 23. Chan YY, Chang CS, Chien LH, Wu TF. Apoptotic effects of a high performance liquid chromatography (HPLC) fraction of Antrodia camphorata mycelia are mediated by down-regulation of the expressions of four tumor-related genes in human non small cell lung carcinoma A549 cell. J Ethnopharmacol. 2010;127:652-661. [DOI] [PubMed] [Google Scholar]
- 24. Dillekas H, Rogers MS, Straume O. Are 90% of deaths from cancer caused by metastases? Cancer Med. 2019;8:5574-5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Geiger TR, Peeper DS. Metastasis mechanisms. Biochim Biophys Acta. 2009;1796:293-308. [DOI] [PubMed] [Google Scholar]
- 26. Aznavoorian S, Murphy AN, Stetler-Stevenson WG, Liotta LA. Molecular aspects of tumor cell invasion and metastasis. Cancer. 1993;71:1368-1383. [DOI] [PubMed] [Google Scholar]
- 27. Hsu YL, Kuo PL, Cho CY, et al. Antrodia cinnamomea fruiting bodies extract suppresses the invasive potential of human liver cancer cell line PLC/PRF/5 through inhibition of nuclear factor kB pathway. Food Chem Toxicol. 2007;45:1249-1257. [DOI] [PubMed] [Google Scholar]
- 28. Song QTY, Lin HC, Yang NC, Hu ML. Antiproliferative and antimetastatic effects of the ethanolic extract of Phellinus igniarius (Linnearus: Fries). J Ethnopharmacol. 2008;115:50-56. [DOI] [PubMed] [Google Scholar]
- 29. Xu W, Huang JJH, Cheung PCK. Extract of Pleurotus pulmonarius suppresses liver cancer development and progression through inhibition of VEGF-Induced PI3K/AKT signaling pathway. PLoS One. 2012;7:e34406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vara JAF, Casado F, Castro J, Cejas P, Belda-Iniesta C, Gonzalez-Baron M. PI3K/Akt signalling pathway and cancer. Cancer Treat Rev. 2004;30:193-204. [DOI] [PubMed] [Google Scholar]
- 31. Yang HL, Kuo YH, Tsa CT, et al. HAnti-metastatic activities of Antrodia camphorata against human breast cancer cells mediated through suppression of the MAPK signaling pathway. Food Chem Toxicol. 2011;49:290-298. [DOI] [PubMed] [Google Scholar]
- 32. Jin UH, Suh SJ, Chang HW, et al. Tanshinone IIA from Salvia miltiorrhiza BUNGE inhibits human aortic smooth muscle cell migration and MMP-9 activity through AKT signaling pathway. J Cell Biochem. 2008;104:15-26. [DOI] [PubMed] [Google Scholar]
- 33. Nieminen M, Henttinen T, Merinen M, Marttila-Ichihara F, Eriksson JE, Jalkanen S. Vimentin function in lymphocyte adhesion and transcellular migration. Nat Cell Biol. 2006:8:156-62. [DOI] [PubMed] [Google Scholar]
- 34. Falconer RA, Loadman PM. Membrane-type matrix metalloproteinases: expression, roles in metastatic prostate cancer progression and opportunities for drug targeting. J Cancer Metastasis Treat. 2017;3:315-327. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, 3._Supplementary_Figures_and_Tables_R2_EDITS for Pleurotus highking mushrooms potentiate antiproliferative and antimigratory activity against triple-negative breast cancer cells by suppressing Akt signaling by Md. Anwarul Haque, A. S. M. Ali Reza, Mst. Samima Nasrin and Md. Atiar Rahman in Integrative Cancer Therapies