Abstract
Introduction:
Diabetes has emerged as an important risk factor for severe illness and death from COVID-19. There is a paucity of information on glycemic control among hospitalized COVID-19 patients with diabetes and acute hyperglycemia.
Methods:
This retrospective observational study of laboratory-confirmed COVID-19 adults evaluated glycemic and clinical outcomes in patients with and without diabetes and/or acutely uncontrolled hyperglycemia hospitalized March 1 to April 6, 2020. Diabetes was defined as A1C ≥6.5%. Uncontrolled hyperglycemia was defined as ≥2 blood glucoses (BGs) > 180 mg/dL within any 24-hour period. Data were abstracted from Glytec’s data warehouse.
Results:
Among 1122 patients in 88 U.S. hospitals, 451 patients with diabetes and/or uncontrolled hyperglycemia spent 37.8% of patient days having a mean BG > 180 mg/dL. Among 570 patients who died or were discharged, the mortality rate was 28.8% in 184 diabetes and/or uncontrolled hyperglycemia patients, compared with 6.2% of 386 patients without diabetes or hyperglycemia (P < .001). Among the 184 patients with diabetes and/or hyperglycemia who died or were discharged, 40 of 96 uncontrolled hyperglycemia patients (41.7%) died compared with 13 of 88 patients with diabetes (14.8%, P < .001). Among 493 discharged survivors, median length of stay (LOS) was longer in 184 patients with diabetes and/or uncontrolled hyperglycemia compared with 386 patients without diabetes or hyperglycemia (5.7 vs 4.3 days, P < .001).
Conclusion:
Among hospitalized patients with COVID-19, diabetes and/or uncontrolled hyperglycemia occurred frequently. These COVID-19 patients with diabetes and/or uncontrolled hyperglycemia had a longer LOS and markedly higher mortality than patients without diabetes or uncontrolled hyperglycemia. Patients with uncontrolled hyperglycemia had a particularly high mortality rate. We recommend health systems which ensure that inpatient hyperglycemia is safely and effectively treated.
Keywords: COVID-19, diabetes, glucose, Glytec, hospital, hyperglycemia, length of stay, mortality
Introduction
Diabetes has emerged as an important risk factor for severe illness and death from COVID-19.1-4 In a small retrospective study of 191 adult patients admitted to 2 hospitals in Wuhan, China, diabetes was present in 19% of patients, and nonsurvivors were significantly more likely to have diabetes than survivors (31% vs 14%, P = .0051).1 In a larger multicenter study assessing risk factors for complications of COVID-19 in 1099 patients hospitalized in China with COVID-19 illness, diabetes was present in 26.9% of patients achieving the primary endpoint of intensive care unit (ICU) admission, mechanical ventilation, or death, compared with 6.1% if none of these complications occurred.2
Preliminary reports have shown that diabetes is also common among patients hospitalized in the United States with COVID-19 illness. Among 7162 COVID-19 patients reported to the CDC February 12 to March 28, 2020 with accompanying information on underlying health conditions, diabetes was present in 24% of non-ICU and 32% of ICU patients.3 The Center for Disease Control’s (CDCs) COVID-19–Associated Hospitalization Surveillance Network (COVID-NET) identified the presence of COVID-19 in 28.3% of 178 hospitalized patients during March 2020.5 The New York Department of Health’s COVID-19 patient fatality dashboard reported that as of April 11, 2020, diabetes was present in 3490 of 9371 patients who died (37%).6
Despite a preponderance of evidence that diabetes is associated with poor COVID-19 outcomes, there is a paucity of information on inpatient glycemic control among patients with diabetes and acute hyperglycemia hospitalized with COVID-19. Direct correlation with clinical outcomes has not been established.
Glytec (Waltham, MA, United States), an insulin software titration company, maintains a large data warehouse of patient clinical and glycemic data including transmitted information from contracted hospitals for lab-confirmed COVID-19 patients, admission and discharge dates, and death notifications. Point-of-care blood glucose (BG) test results are transmitted and stored for all patients on contracted hospital units. In this study, we identified COVID-19 inpatients from the Glytec data warehouse treated during a 37-day period and analyzed all transmitted BGs during their hospital stay. We then characterized the COVID-19 patients from our contracted hospitals according to their (1) clinical characteristics at hospital presentation, (2) inpatient glycemic control, and (3) clinical outcomes.
Methods
Study Design
This retrospective observational study evaluated patients hospitalized with laboratory-confirmed COVID-19 illness between March 1 and April 6, 2020. We compared patients who had diabetes and/or uncontrolled hyperglycemia against each other and against contemporaneously hospitalized COVID-19 patients who did not have either diabetes or uncontrolled hyperglycemia. We evaluated (1) baseline demographic and clinical features, (2) inpatient glycemic control, and (3) clinical outcomes, including length of stay (LOS) and in-hospital death metrics. Institutional Review Board approval was not required for this observational, retrospective study of routinely transmitted patient information.
Definitions
A diagnosis of COVID-19 illness was defined as a positive Severe Adult Respiratory Syndrome (SARS)-CoV-2 lab result or the presence of ICD-10 code U07.1, which indicates acute illness from SARS-CoV-2. Hospital admission was defined as patient presence in the hospital for greater than 24 hours. Diabetes was defined as A1C ≥6.5%. Uncontrolled hyperglycemia was diagnosed when two or more BGs > 180 mg/dL occurred within any 24-hour period with an A1C < 6.5% or no A1C testing during hospitalization. Renal dysfunction was defined as estimated glomerular filtration rate (eGFR) < 60 mL/min. Admission lab tests were defined as having been time stamped within 24 hours of hospital presentation. An inactive patient was defined as a patient with a time stamped discharge or death notification. Active admissions were defined as patients without a time stamped discharge or death notification.
Data Collection
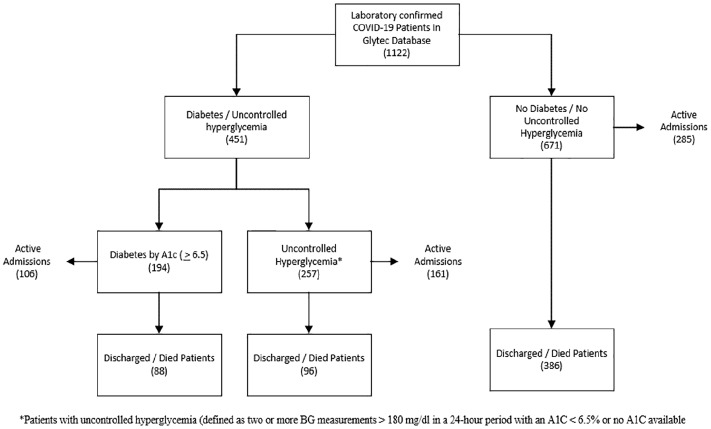
A query of the Glytec data warehouse for any transmitted SARS-CoV-2 lab result or the presence of ICD-10 code U07.1 is shown in Figure 1. For all COVID-19 patients identified in the Glytec data warehouse, we abstracted information on age, gender, and body mass index (BMI). Glucose, creatinine, eGFR, and anion gap were collected from admission labs. If A1C testing was performed during hospitalization, values transmitted to the Glytec data warehouse were abstracted. All transmitted BGs were abstracted, as well as time stamped admission, discharge, and death notifications.
Figure 1.
Study schematic of patient identification and classification.
Outcome Measures
Data for patients meeting criteria for either diabetes and/or uncontrolled hyperglycemia were combined and compared with data from patients without evidence of diabetes or uncontrolled hyperglycemia to assess differences in (1) baseline clinical and demographic characteristics, (2) inpatient glycemic control for all patients, (3) LOS for inactive patients, and (4) mortality for inactive patients.
A within-group subset analysis of inactive patients was performed comparing clinical characteristics and outcomes between patients meeting criteria for diabetes and patients with uncontrolled hyperglycemia against those with no diabetes designation.
Statistical Analysis
Differences in age and LOS were analyzed nonparametrically by median and Mann-Whitney tests because of nonnormal distributions. Other continuous variables were analyzed parametrically by means, standard deviations, and t-tests. Differences in categorical variables were calculated with the χ2 method and Fisher’s exact test. Analyses were performed with the use of Minitab 19.
Results
Patient Characteristics
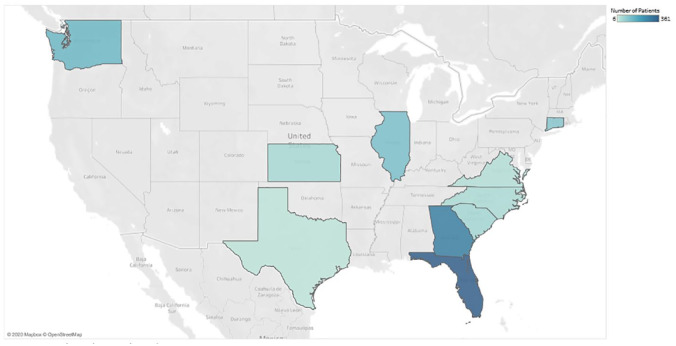
We identified 1122 patients with COVID-19 from 88 U.S. hospitals distributed across 10 states (Figure 2). A1C data were available for 282 patients. We identified 194 patients (17.3% of the total population) with diabetes (defined as having an A1C value ≥ 6.5%). We identified an additional 257 patients with uncontrolled hyperglycemia (defined as being present when two or more BGs > 180 mg/dL occurred within any 24-hour period with an A1C < 6.5% or no A1C testing during hospitalization) (Table 1).
Figure 2.
U.S. States with COVID-19 study patients, March 1 to April 6, 2020.
Table 1.
Demographic and Clinical Characteristics of 1122 Hospitalized COVID-19 Patients, Comparing Presence (n = 451) vs Absence (n = 671) of Diabetes and/or Uncontrolled Hyperglycemia.
| Variable | + Diabetes and/or uncontrolled hyperglycemia (n = 451) |
– Diabetes or uncontrolled hyperglycemia (n = 671) |
P-value |
|---|---|---|---|
| Patients, n (%) | 451 (40.2) | 671 (59.8) | |
| Male gender, n (%) | 268 (59.4) | 356 (53.1) | .035 |
| Median age in years (range) | 65 (24-95) | 61 (18-101) | .005 |
| Mean BMI, kg/m2 (SD) | 31.8 (±13) | 30.8 (±15) | .053 |
| Mean A1C, % (SD)a | 8.7 (±2.4) | 5.8 (±0.4) | <.001 |
| Diabetes by A1C criteria, n (%) | 194 (17.3) | 0 (0.0) |
Abbreviations: BMI, body mass index; SD, standard deviation.
A1C was available in 221 of 451 diabetes and/or uncontrolled hyperglycemia patients (49.0%) and in 61 of 671 patients without diabetes or uncontrolled hyperglycemia (9.1%).
The combined group of 451 patients with diabetes by A1C criteria and/or uncontrolled hyperglycemia (194 + 257), compared with 671 patients who did not meet diabetes or uncontrolled hyperglycemia criteria, contained a significantly higher percentage of males (59% vs 53%, P = .035). Patients with diabetes and/or uncontrolled hyperglycemia, compared with patients without diabetes or hyperglycemia, were significantly older (median age of 65 vs 61 years, P = .005). In the combined group of patients with diabetes and/or uncontrolled hyperglycemia, mean A1C was significantly higher at 8.7% compared with patients without diabetes or hyperglycemia (mean A1C: 5.8%, P < .001)
Admission Lab
The 451 patients in the combined diabetes and/or uncontrolled hyperglycemia group, compared with 671 patients without evidence of diabetes or ensuing uncontrolled hyperglycemia, demonstrated higher rates of hyperglycemia at admission (mean BG: 202 vs 114 mg/dL, P < .001) and renal dysfunction (40.6% vs 23.5% with eGFR < 60, P < .001), as well as a higher mean anion gap (15.8 vs 13.4 mEq/dL, P < .001; Table 2).
Table 2.
Admission Lab Characteristics of 1122 Patients, Comparing Presence (n = 451) vs Absence (n = 671) of Diabetes and/or Uncontrolled Hyperglycemia.
| Variable | + Diabetes and/or uncontrolled hyperglycemia (n = 451) | – Diabetes or uncontrolled hyperglycemia (n = 671) | P-value |
|---|---|---|---|
| Mean blood glucose mg/dL (SD) | 202.4 (±117.7) | 114.5 (±20.0) | <.001 |
| Mean anion gap mEq/dL (SD)a | 15.8 (±6.3) | 13.4 (±4.9) | <.001 |
| Mean serum creatinine mg/dL (SD)b | 1.6 (±1.9) | 1.3 (±1.6) | .004 |
| Mean eGFR, mL/min (SD)c | 63.7 (±30.9) | 73.5 (±30.1) | <.001 |
| eGFR categories, mL/min | |||
| eGFR < 15, n (%) | 21 (4.8) | 28 (4.3) | .069 |
| eGFR 15-29, n (%) | 44 (10.0) | 18 (2.8) | <.001 |
| eGFR 30-59, n (%) | 113 (25.8) | 107 (16.4) | <.001 |
| eGFR ≥ 60, n (%) | 260 (59.4) | 499 (76.5) | <.001 |
Abbreviations: eGFR, estimated glomerular filtration rate; SD, standard deviation.
Anion gap was available in 431 of 451 patients with diabetes and/or uncontrolled hyperglycemia (95.6%) and in 651 of 671 patients without diabetes or uncontrolled hyperglycemia (97.5%).
Creatinine was available in 432 of 451 patients with diabetes and/or uncontrolled hyperglycemia (95.8%) and in 651 of 671 patients without diabetes or uncontrolled hyperglycemia (97.5%).
eGFR was available in 438 of 451 patients with diabetes and/or uncontrolled hyperglycemia patients (97.1%) and in 652 of 671 patients without diabetes or uncontrolled hyperglycemia (97.2%).
Glycemic Outcomes
The combined diabetes and/or hyperglycemia group spent 3885 patient days in the hospital and the patients without diabetes or hyperglycemia accounted for 3793 patient days (Table 3). Out of the 3885 patient days of the combined diabetes and/or hyperglycemia group, 1470 patient days (37.8%) were spent with a mean BG > 180 mg/dL and 1004 patient days (25.8%) included at least one BG > 250 mg/dL. A hypoglycemic glucose concentration (BG < 70 mg/dL) was more common in patients with diabetes and/or hyperglycemia, occurring in 137 out of 3885 patient days (3.5%) compared with 39 out of 3793 patient days in patients without diabetes or hyperglycemia (1.0%, P < .001). Likewise, severe hypoglycemia (BG < 40 mg/dL) was significantly more frequent in the diabetes and/or hyperglycemia group, occurring in 25 patient days (0.6%) compared with 3 patient days in those without diabetes or uncontrolled hyperglycemia (0.1%, P < .001).
Table 3.
Glycemic Outcomes in 1122 Patients With Diabetes and/or Uncontrolled Hyperglycemia (n = 451) Compared With Patients Without Diabetes or Hyperglycemia (n = 671).
| Variable | + Diabetes and/or uncontrolled hyperglycemia (n = 451) | – Diabetes or uncontrolled hyperglycemia (n = 671) | P-value |
|---|---|---|---|
| BG events, n (%) | 19 168 (100) | 6532 (100) | |
| Mean glucose, mg/dL (SD) | 178.5 (±71.0) | 116.6 (±25.9) | <.001 |
| BGs > 250 mg/dL, n (%) | 2795 (14.6) | 6 (0.1) | <.001 |
| BGs > 180 mg/dL, n (%) | 7499 (39.1) | 91 (1.4) | <.001 |
| BGs 70-180 mg/dL, n (%) | 11 473 (59.9) | 6389 (97.8) | <.001 |
| BGs < 70 mg/dL, n (%) | 196 (1.0) | 52 (0.8) | .106 |
| BGs < 54 mg/dL, n (%) | 69 (0.4) | 10 (0.2) | .009 |
| BGs < 40 mg/dL, n (%) | 31 (0.2) | 4 (0.1) | .057 |
| Patient days, n (%) | 3885 (50.6) | 3793 (49.4) | |
| Patient days with mean BG > 180 mg/dL, n (%) | 1470 (37.8) | 46 (1.2) | <.001 |
| Patient days with at least 1 BG > 250 mg/dL, n (%) | 1004 (25.8) | 6 (0.2) | <.001 |
| Patient days with at least 1 BG > 180 mg/dL, n (%) | 2252 (58.0) | 91 (2.4) | <.001 |
| Patient days with at least 1 BG < 70 mg/dL, n (%) | 137 (3.5) | 39 (1.0) | <.001 |
| Patient days with at least 1 BG < 54 mg/dL, n (%) | 63 (1.6) | 16 (0.4) | <.001 |
| Patient days with at least 1 BG < 40 mg/dL, n (%) | 25 (0.6) | 3 (0.1) | <.001 |
Abbreviations: BG, blood glucose; SD, standard deviation.
Clinical Outcomes
A total of 552 patients were still hospitalized at the time of analysis. Of the 570 inactive patients, 77 patients died for a mortality rate of 13.5% within these inactive patients. Of these 77 patients who died, 53 were among the 184 patients in the combined diabetes and/or uncontrolled hyperglycemia group (28.8%) compared with 24 who were among the 386 patients in the comparison unaffected group (6.2%, P < .001) (Table 4; Figure 3).
Table 4.
Glycemic and Clinical Outcomes Among 570 Patients Who Were Discharged or Died Comparing Diabetes and/or Uncontrolled Hyperglycemia (n = 184) With Patients Without Diabetes or Hyperglycemia (n = 386).
| Variable | + Diabetes and/or uncontrolled hyperglycemia (n = 184) | – Diabetes or uncontrolled hyperglycemia (n = 386) | P-value |
|---|---|---|---|
| Patients, n (%) | 184 (32.3) | 386 (67.7) | |
| Admission mean glucose mg/dL, (SD) | 206.0 (±115.6) | 113.6 (±19.6) | <.001 |
| Mean A1C (SD)a | 8.5 (±2.3) | 5.9 (±0.51) | <.001 |
| BG events, n (%) | 6076 (100) | 2744 (100) | |
| Mean glucose, mg/dL (SD) | 178.2 (±72.9) | 110.9 (±22.6) | |
| BGs > 250 mg/dL, n (%) | 822 (13.5) | 1 (0.0) | <.001 |
| BGs > 180 mg/dL, n (%) | 2389 (39.3) | 24 (0.9) | <.001 |
| BGs 70-180 mg/dL, n (%) | 3628 (59.7) | 2702 (98.5) | <.001 |
| BGs < 70 mg/dL, n (%) | 59 (1.0) | 18 (0.7) | .169 |
| BGs < 54 mg/dL, n (%) | 27 (0.4) | 2 (0.1) | .018 |
| BGs < 40 mg/dL, n (%) | 13 (0.2) | 2 (0.1) | .290 |
| Patient days, n (%) | 1407 (43.8) | 1804 (56.2) | |
| Patient days with mean BG > 180 mg/dL, n (%) | 520 (37.0) | 12 (0.7) | <.001 |
| Patient days with at least 1 BG > 250 mg/dL, n (%) | 337 (24.0) | 1 (0.1) | <.001 |
| Patient days with at least 1 BG > 180 mg/dL, n (%) | 803 (57.1) | 24 (1.3) | <.001 |
| Patient days with at least 1 BG < 70 mg/dL, n (%) | 43 (3.1) | 15 (0.8) | <.001 |
| Patient days with at least 1 BG < 54 mg/dL, n (%) | 26 (1.8) | 4 (0.2) | <.001 |
| Patient days with at least 1 BG < 40 mg/dL, n (%) | 10 (0.7) | 1 (0.1) | .005 |
| Died in hospital, n (%) | 53 (28.8) | 24 (6.2) | <.001 |
| Mean duration in days from admission to death (SD) | 8.7 (±4.6) | 7.9 (±4.4) | .494 |
| Median duration in days from admission to death (IQR) | 7.5 (2.0-20.1) | 6.9 (1.3-20.4) | .560 |
| Discharged alive from hospital, n (%) | 131 (71.2) | 362 (93.8) | |
| Mean LOS in days (SD) | 6.2 (±3.7) | 5.0 (±3.3) | <.001 |
| Median LOS in days (IQR) | 5.7 (1.1-24.6) | 4.3 (1.0-21.2) | <.001 |
Abbreviations: BG, blood glucose; IQR, interquartile range; LOS, length of stay; SD, standard deviation.
A1C was available for 125 of 184 patients with diabetes (67.9%) and 54 of 386 patients with uncontrolled hyperglycemia (14.0%).
Figure 3.
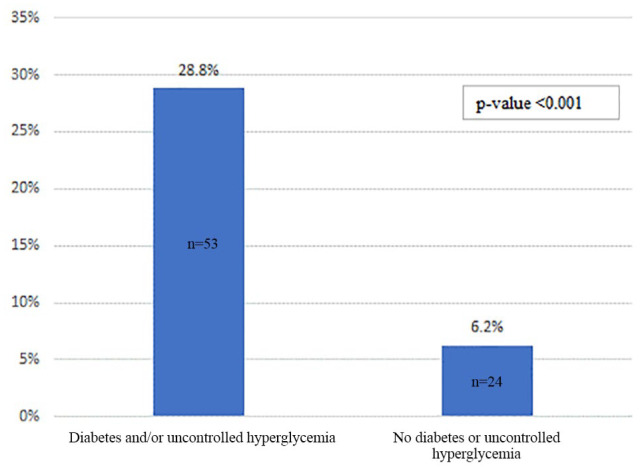
Mortality rates among patients who were discharged or died comparing diabetes and/or uncontrolled hyperglycemia (n = 184) with patients without diabetes or hyperglycemia (n = 386).
Of 493 inactive patients who were discharged alive, the combined diabetes and/or uncontrolled hyperglycemia patient group (131 patients) experienced a significantly longer median LOS at 5.7 days compared with patients without diabetes or hyperglycemia at 4.3 days (362 patients, P < .001).
Subset Analysis
In a within-group subset analysis of 184 inactive patients with diabetes and/or uncontrolled hyperglycemia, 88 patients met criteria for diabetes (47.8%) and 96 met criteria for uncontrolled hyperglycemia (52.2%) (Table 5). Patients with diabetes had a higher admission mean BG at 238.3 mg/dL compared with uncontrolled hyperglycemia patients at 175.3 mg/dL (P < .001) and a higher mean A1C (9.1% vs 5.9%, P < .001).
Table 5.
Glycemic and Clinical Outcomes Among 184 Patients With Diabetes Status (n = 88) or Uncontrolled Hyperglycemia Status (n = 96) Who Were Discharged or Died.
| Variable | Diabetes and/or uncontrolled hyperglycemia (n = 184) | P-value | |
|---|---|---|---|
| Diabetes by A1C criteria (n = 88) | Uncontrolled hyperglycemia by BG criteria (n = 96) | ||
| Patients, n (%) | 88 (47.8) | 96 (52.2) | |
| Admission mean glucose mg/dL (SD) | 238.3 (±121.6) | 175.3 (±107.2) | <.001 |
| Mean A1C (SD)a | 9.1 (±2.3) | 5.9 (±0.4) | <.001 |
| BG events, n (%) | 2664 (100) | 3412 (100) | |
| Mean glucose, mg/dL (SD) | 177.8 (±64.5) | 178.5 (±78.9) | .704 |
| BGs > 250 mg/dL, n (%) | 330 (12.4) | 492 (14.4) | .024 |
| BGs > 180 mg/dL, n (%) | 1060 (39.8) | 1329 (39.0) | .527 |
| BGs 70-180 mg/dL, n (%) | 1589 (59.6) | 2039 (59.8) | .875 |
| BGs < 70 mg/dL, n (%) | 15 (0.6) | 44 (1.3) | .006 |
| BGs < 54 mg/dL, n (%) | 8 (0.3) | 19 (0.6) | .089 |
| BGs < 40 mg/dL, n (%) | 4 (0.2) | 9 (0.3) | .444 |
| Patient days, n (%) | 603 (42.9) | 804 (57.1) | |
| Patient days with mean BG > 180 mg/dL, n (%) | 233 (38.6) | 287 (35.7) | .265 |
| Patient days with at least 1 BG > 250 mg/dL, n (%) | 138 (22.9) | 199 (24.8) | .409 |
| Patient days with at least 1 BG > 180 mg/dL, n (%) | 362 (60.0) | 441 (54.9) | .056 |
| Patient days with at least 1 BG < 70 mg/dL, n (%) | 12 (2.0) | 31 (3.9) | .042 |
| Patient days with at least 1 BG < 54 mg/dL, n (%) | 7 (1.2) | 19 (2.4) | .102 |
| Patient days with at least 1 BG < 40 mg/dL, n (%) | 4 (0.7) | 6 (0.7) | 1.000 |
| Died in the hospital, n (%) | 13 (14.8%) | 40 (41.7%) | <.001 |
| Mean duration from admission to death (SD) | 7.2 (±4.9) | 9.2 (±4.5) | <.001 |
| Median duration from admission to death (IQR) | 6.0 (2.0-18.9) | 8.4 (2.1-20.1) | <.001 |
| Discharged alive from hospital, n (%) | 75 (85.2) | 56 (58.3) | |
| Mean LOS in days (SD) | 5.8 (±3.5) | 6.8 (±3.8) | <.001 |
| Median LOS in days (IQR) | 5.0 (1.1-24.6) | 6.2 (1.1-20.7) | <.001 |
Abbreviations: BG, blood glucose; IQR, interquartile range; LOS, length of stay; SD, standard deviation.
A1C was available for 88 patients with diabetes (100% by study design) and 27 of 96 patients with uncontrolled hyperglycemia (28.1%).
Among these 184 inactive patients, death occurred in 40 of 96 uncontrolled hyperglycemia patients (41.7%) compared with death in 13 of 88 patients with diabetes (14.8%, P < .001) (Figure 4). In this 184-patient cohort, patients designated as having uncontrolled hyperglycemia, compared to patients with diabetes, had a longer median duration from admission to death (8.4 vs 6.0 days, P < .001). Among the 493 patients who were discharged alive, 131 patients had either diabetes or uncontrolled hyperglycemia. Among them were 56 patients with uncontrolled hyperglycemia (42.7%) and 75 patients with diabetes (57.3%). The former group, compared to the latter group, had a longer median hospital LOS (6.2 vs 5.0 days, P < .001).
Figure 4.
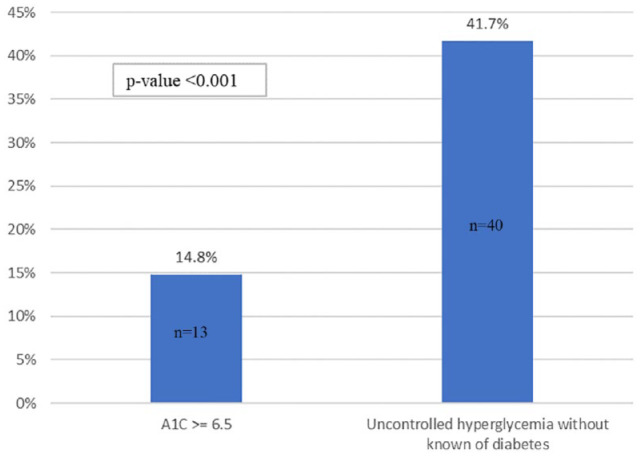
Mortality rates among patients who were discharged or died comparing patients with diabetes (n = 88) with hyperglycemia patients (n = 96).
Discussion
Among 1122 patients in 88 U.S. hospitals for COVID-19 treatment, 38.5% were found to have either diabetes by A1C criteria or uncontrolled hyperglycemia. As a combined group, patients with diabetes and/or uncontrolled hyperglycemia presented to the hospital at an older age, with a lower eGFR and with a higher anion gap than their comparison group without these diagnoses.
During admission, 37.8% of patient days in this group with diabetes and/or uncontrolled hyperglycemia were spent with a mean BG > 180 mg/dL, which is the American Diabetes Association (ADA) recommended upper limit target for most inpatients.7 This group also experienced higher rates of hypoglycemia, occurring in 3.5% of patient days, compared with 1.0% of patient days in the group without diabetes or uncontrolled hyperglycemia.
The overall mortality rate for inactive patients in our study population was 13.5%. In the combined diabetes and/or uncontrolled hyperglycemia group, 28.8% of patients did not survive hospitalization, representing a more than fourfold higher in-hospital mortality rate compared with the mortality rate for COVID-19 inpatients without evidence for diabetes or uncontrolled hyperglycemia (6.2%).
Our study design combined patients meeting criteria for both diabetes and uncontrolled hyperglycemia to reduce the impact of potential misclassification bias from failing to include for analysis many patients with diabetes who did not have an A1C performed during hospitalization. In a subset analysis of outcomes within the diabetes and/or uncontrolled hyperglycemia group, we found that of the 53 deaths in this combined group, 75% occurred in patients designated as uncontrolled hyperglycemia (and 25% in patients designated as having diabetes). Patients designated as uncontrolled hyperglycemia presented to the hospital with a significantly lower mean BG and were hospitalized for a longer duration between admission and death than patients designated as having diabetes, raising the possibility that acute hyperglycemia is an independent risk factor for mortality from COVID-19.
Stress hyperglycemia in hospitalized patients, typically characterized as a transient elevation in blood glucose in the setting of acute illness or after surgery in a patient with an A1C < 6.5%, is associated with longer LOS, longer ventilator management time, and increased mortality in critically ill patients.8-11 A variety of immune system abnormalities have been postulated to explain the relationship between hyperglycemia and immune dysfunction, including impairment in polymorphonuclear and monocytic white blood cell chemotaxis and phagocytosis, complement function, and cytokine dysregulation.12-14 During the 2003 SARS epidemic, acute hyperglycemia in the previously healthy patients without known diabetes (as well as patients with known diabetes) was identified as a complication of SARS illness and a risk factor for respiratory failure and death.15,16 Furthermore, immunohistochemical stains of cadaveric pancreatic tissue revealed angiotensin converting enzyme 2 immunostaining in pancreatic islet cells similar to lung alveolar epithelium and myocardium.17 Angiotensin converting enzyme 2 is a recognized receptor protein for corona virus attachment and direct islet cell toxicity during infection appears plausible as a contributor to acute hyperglycemia.17
The findings in this retrospective, observational study are subject to several limitations. Our study protocol was designed for a descriptive analysis and it was beyond the scope of this assessment to quantify the magnitude of association between diabetes and/or uncontrolled hyperglycemia and clinical outcomes, or to assess the impact of insulin therapy. Our dataset did not include recognized comorbidities for death from COVID-19, such as hypertension or cardiovascular disease.1-4 Furthermore, it was beyond the scope of this study to capture other recognized predictors of clinical outcomes, such as health system size and glycemic care policies, patient demographics such as ethnicity or payer source, or clinically relevant information on ICU admission or ventilator use. Because of limited SARS-CoV-2 test availability and the newness of the ICD-10 code U07.1 for COVID-19 illness, our data warehouse query likely missed patients for whom either lab test results or diagnosis coding was not reported before discharge. Diagnosis codes for diabetes and A1C data, potentially available from previous admissions, were not abstracted for this analysis. The absence of this information likely led to misclassification bias toward uncontrolled hyperglycemia in patients with underlying diabetes.
To our knowledge, this is the first published report characterizing glycemic control in patients hospitalized with COVID-19 in the United States. Two important questions raised by these data are (1) whether the high rates of death from COVID-19 in this study are predominantly due to metabolic derangements with associated sequelae and (2) whether acute hyperglycemia plays a role which can be ameliorated through effective glycemic management. A longitudinal assessment of glycemic control was beyond the scope of this evaluation. To address study limitations and provide greater insights into the impact of glycemic control on clinical outcomes, additional studies will be forthcoming.
The finding that patients who would go on to have uncontrolled hyperglycemia had lower admission mean BG concentrations compared with patients with diabetes suggests an opportunity to manage uncontrolled hyperglycemia over the course of a hospitalization. Hospitals are already constrained in managing the care of COVID-19 positive patients because of the scarcity of personal protection equipment as well as fear of catching the disease by some hospital workers.
As a result, the medical team might try to reduce caregiver-patient contact, with attendant risk of decreasing the frequency of BG assessments and avoiding IV insulin and basal-bolus insulin therapy. In our view, in the absence of evidence to the contrary, clinicians should interpret COVID-19–associated hyperglycemia as a potential indicator of pancreatic islet cell injury and a risk for poor outcome. Clinicians should treat hyperglycemia to achieve BG targets < 180 mg/dL for most patients. This equates to basal-bolus insulin therapy in most non-ICU patients and continuous insulin infusion in the critically ill as directed by national guidelines.7,18-19
Conclusion
Among hospitalized patients with COVID-19, diabetes and/or uncontrolled hyperglycemia occurred frequently. These COVID-19 patients with diabetes and/or uncontrolled hyperglycemia had a longer LOS and markedly higher mortality than patients without diabetes or uncontrolled hyperglycemia. Patients with uncontrolled hyperglycemia had a particularly high mortality rate. We recommend health systems which ensure that inpatient hyperglycemia is safely and effectively treated.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Bruce Bode is an advisory board member for Glytec and owns stock in Aseko/Glytec; his employer, Atlanta Diabetes Associates, receives grant support from DexCom, Insulet, Lilly, Medtronic, Novo Nordisk, and Sanofi. He is on the speaker bureau and consults with Lilly, Medtronic, Novo Nordisk, and Sanofi. Valerie Garrett, Jordan Messler, Raymie McFarland, Jennifer Crowe, and Robby Booth are employed by Glytec. David Klonoff is a consultant to Abbott, Ascensia, Dexcom, EOFlow, Fractyl, Lifecare, Novo, Roche, and Thirdwayv.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Raymie McFarland  https://orcid.org/0000-0003-0123-9286
https://orcid.org/0000-0003-0123-9286
References
- 1. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guan W-J, Ni Z-Y, Hu Y, et al. ; China Medical Treatment Expert Group. Clinical characteristics of coronavirus disease 2019 in China [published online ahead of print February 28, 2020]. N Engl J Med. doi: 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention. Preliminary Estimates of the Prevalence of Selected Underlying Health Conditions Among Patients with Coronavirus Disease 2019 - United States, February 12–March 28, 2020 [Internet]. Centers for Disease Control and Prevention; 2020. https://www.cdc.gov/mmwr/volumes/69/wr/mm6913e2.htm Accessed April 12, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 323(16):1574-1581. doi: 10.1001/jama.2020.5394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Centers for Disease Control and Prevention. Hospitalization Rates and Characteristics of Patients Hospitalized with Laboratory-Confirmed Coronavirus Disease 2019 - COVID-NET, 14 States, March 1–30, 2020 [Internet]. Centers for Disease Control and Prevention; 2020. https://www.cdc.gov/mmwr/volumes/69/wr/mm6915e3.htm. Accessed April 12, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. New York State Department of Health. Workbook: NYS-COVID19-Tracker [Internet]. COVID19; https://covid19tracker.health.ny.gov/views/NYS-COVID19-Tracker/NYSDOHCOVID-19Tracker-Fatalities?:embed=yes&:toolbar=no&:tabs=n. Accessed April 12, 2020. [Google Scholar]
- 7. American Diabetes Association. 15. Diabetes care in the hospital: standards of medical care in diabetes-2020. Diabetes Care. 2020;43(Suppl 1):S193-S202. https://care.diabetesjournals.org/content/43/Supplement_1/S193. Accessed April 12, 2020. [DOI] [PubMed] [Google Scholar]
- 8. Olariu E, Pooley N, Danel A, Miret M, Preiser J-C. A systematic scoping review on the consequences of stress-related hyperglycaemia. PLos One. 2018;13(4):e0194952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Valent F, Tonutti L, Grimaldi F. Does diabetes mellitus comorbidity affect in-hospital mortality and length of stay? Analysis of administrative data in an Italian Academic Hospital. Acta Diabetologica. 2017;54(12):1081-1090. [DOI] [PubMed] [Google Scholar]
- 10. Krinsley JS, Egi M, Kiss A, Devendra AN, Schuetz P, Maurer PM, et al. Diabetic status and the relation of the three domains of glycemic control to mortality in critically ill patients: an international multicenter cohort study. Critical care. 2013;17(2):R37 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3733432/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: An Independent Marker of In-Hospital Mortality in Patients with Undiagnosed Diabetes. The Journal of Clinical Endocrinology & Metabolism. 2002;87(3):978–82. [DOI] [PubMed] [Google Scholar]
- 12. Ilyas R, Wallis R, Soilleux EJ, et al. High glucose disrupts oligosaccharide recognition function via competitive inhibition: a potential mechanism for immune dysregulation in diabetes mellitus. Immunobiology. 2011;216(1-2):126-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Price CL, Hassi HOSA, English NR, Blakemore AIF, Stagg AJ, Knight SC. Methylglyoxal modulates immune responses: relevance to diabetes. J Cell Mol Med. 2009;14(68):1806-1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Geerlings SE, Hoepelman AI. Immune dysfunction in patients with diabetes mellitus (DM). FEMS Immunol Med Microbiol. 1999;26(3-4):259-265. [DOI] [PubMed] [Google Scholar]
- 15. Yang JK, Feng Y, Yuan MY, et al. Plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS. Diabet Med. 2006;23(6):623-628. [DOI] [PubMed] [Google Scholar]
- 16. Liao Z. The Clinical Features of SARS Patients with Diabetes or Secondary Hyperglycemia [Internet]. American Diabetes Association; 1970. https://professional.diabetes.org/abstract/clinical-features-sars-patients-diabetes-or-secondary-hyperglycemia. Accessed April 12, 2020. [Google Scholar]
- 17. Yang J-K, Lin S-S, Ji X-J, Guo L-M. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2009;47(3):193-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304-377. [DOI] [PubMed] [Google Scholar]
- 19. Jacobi J, Bircher N, Krinsley J, et al. Guidelines for the use of an insulin infusion for the management of hyperglycemia in critically ill patients. Crit Care Med. 2012;40(12):3251-3276. [DOI] [PubMed] [Google Scholar]