Abstract
Diabetes and non-coding RNAs are receiving increasing attention in contemporary medical research. The present study aimed to explore the role of the long non-coding RNA uc.48+ in the pathological changes of type 2 diabetes mellitus (T2DM) by observing the effects of uc.48+ small interfering RNA (siRNA) on the abdominal cells of a mouse model of T2DM. Mice with T2DM (DM group) were established by feeding with a high-sugar and -fat diet combined with intraperitoneal injections of low-dose streptozotocin. An intraperitoneal injection of uc.48+ siRNA was administered to the diabetic mice, and the serum levels of cytokines together with other clinical parameters, namely blood pressure, heart rate, mechanical withdrawal threshold (MWT) and thermal withdrawal latency (TWL) were examined. Following the collection and identification of abdominal cells from the mice, the mRNA levels of uc.48+, mRNA and protein levels of the P2X7 receptor, and phosphorylation levels of ERK1/2 were evaluated by reverse transcription-PCR and western blotting, respectively. The MWT and TWL were significantly decreased in the DM group compared with the non-diabetic control group. However, the reductions in MWT and TWL were significantly attenuated following uc.48+ siRNA injection. The systolic and diastolic blood pressure, as well as the serum levels of tumor necrosis factor α and interleukin 1β of mice in the DM group were significantly increased compared with those in the control group, whereas these changes were significantly attenuated following the injection of uc.48+ siRNA. In addition, the expression levels of P2X7 receptor mRNA and protein, and the degree of phosphorylation of ERK1/2 in the abdominal cells were significantly increased in the DM group compared with the control group. These changes were also significantly attenuated following transfection with uc.48+ siRNA in vivo. In conclusion, these data suggest that uc.48+ may play an important role in the pathological changes of blood pressure, neurology and abdominal cell function in T2DM via interaction with the P2X7 receptor.
Keywords: lncRNA uc.48+, abdominal cells, immune responses, inflammatory responses, P2X7 receptor, type 2 diabetes mellitus
Introduction
The global prevalence of diabetes has increased markedly. Due to the aging population and continued increase in obesity rates, the prevalence is expected to rise to 592 million by 2035(1). Concomitantly, the incidence of type 2 diabetes mellitus (T2DM) has increased rapidly, and patients with diabetes often experience a wide range of complications. T2DM is associated with low-grade inflammation, and this immune inflammatory response can be influenced and regulated by monocytes and macrophages via the secretion of cytokines and antigen-presenting cells (2-4). Previous studies have shown that the tissue macrophage status regulates the development and progression of T2DM (4,5). In addition, other studies have shown that peritoneal macrophages modulate the immune response by regulating cytokines and nitric oxide production in diabetic rats (6,7). Our previous study demonstrated that the P2X purinoceptor 7 (P2X7) receptor of the mononuclear phagocyte system is involved in the pathology of diabetes (8). As expression of the P2X7 receptor may be associated with inflammation (9), it would be interesting to investigate whether the P2X7 receptor is able to ameliorate the chronic inflammatory state of T2DM.
Long non-coding RNAs (lncRNAs) are generally RNA transcripts >200 nucleotides in length that lack the ability to encode proteins (10). Previous studies have shown that lncRNAs may contribute to the regulation of cell apoptosis, proliferation and differentiation at the RNA epigenetic level via the induction of changes in gene transcription and post-transcriptional modifications (11). uc.48+ is an lncRNA that has been observed to be expressed at increased levels, along with the P2X7 receptor, in the superior cervical ganglia of a rat model of T2DM, and is associated with cardiac autonomic dysfunction (12). In our previous study, uc.48+ small interfering RNA (siRNA) was found to influence immune and inflammatory responses in a diabetic monophagocyte system through the P2X7 receptor in vitro (13). A previous study revealed that macrophages may exist in large numbers in the peritoneum of mice (14). Therefore, the aim of the present study was to investigate whether uc.48+ exerts an effect on mouse abdominal cells through P2X7 receptors in vivo. The ERK signaling pathway is the key pathway by which signals are transmitted from surface receptors to the nucleus (15). Decreased ERK phosphorylation appears to be associated with decreased P2X7 receptor expression in RAW264.7 macrophages (13). In addition, uc.48+ siRNA has been shown to ameliorate diabetic sympathetic neuropathy in a rat model of T2DM (12). However, the exact mechanism by which uc.48+ affects the abdominal cells and neuropathological changes in mouse models of T2DM requires elucidation.
The present study aimed to investigate if uc.48+ siRNA has a beneficial effect on the function of abdominal cells, and whether it ameliorates the neuropathological changes associated with T2DM through the P2X7 receptor and ERK signaling pathway. Therefore, the role of uc.48+ and the mechanism underlying its effects on the pathological changes in T2DM were evaluated. This was achieved by monitoring and assessing the effects of uc.48+ siRNA on mice with T2DM and their abdominal cells in which P2X7 receptor expression was upregulated.
Materials and methods
Animals and animal groups
A total of 24 male Kunming mice of clean grade (32-42 g) were purchased from the Center of Laboratory Animal Science of Nanchang University at 8 weeks of age. They were acclimatized for 2 weeks at room temperature with 40-60% relative humidity and 12-h light/dark cycles and given standard feed with free access to drinking water, and randomly divided into four groups (n=6 in each group), comprising the control group, the DM group, DM treated with uc.48+ siRNA group (DM + uc.48+ si) and DM treated with scrambled siRNA group (DM + NCsi). All procedures involving animals were approved by the Animal Care and Use Committee of the Medical College of Nanchang University (approval no. 2018 16).
Mice in the DM group were fed with a high-sugar and -fat diet (consisting of 22% fat, 48% carbohydrate and 20% protein with a total calorific value of 44.3 kJ/kg) for 4 weeks and subsequently injected intraperitoneally (i.p.) with streptozotocin (STZ; 80 mg/kg). Control mice were fed with normal diet (consisting of 5% fat, 53% carbohydrate and 23% protein, with a total calorific value of 25 kJ/kg) for 4 weeks and subsequently injected i.p. with the same concentration of saline. On the third day after STZ injection (the last day of week 6), the blood glucose levels of the mice were measured. The successful establishment of the DM model was confirmed by fasting plasma glucose levels of >11.1 mmol/l and postprandial plasma glucose levels of >16.7 mmol/l (16,17).
uc.48+ siRNA treatment
The siRNA sequences specific for uc.48+ were purchased from Invitrogen (Thermo Fisher Scientific, Inc.). The following target sequence was used: sense, 5'-GGCACUACUACUUGCAGAATT-3' and anti-sense, 5'-UUCUGCAAGUAGUAGUGCCTT-3'. The uc.48+ siRNA was injected i.p. into DM model mice at the end of week 7 along with Entranster™-in vivo Transfection Reagent (Engreen Biosystem Co., Ltd.) to establish the DM + uc.48+ si group. Similarly, DM model mice were injected with scrambled siRNA: sense, 5'-UUCUCCGAACGUGUCACGUTT-3' and anti-sense, 5'-ACGUGACACGUUCGGAGAATT-3'; Invitrogen; Thermo Fisher Scientific, Inc.) and transfection reagent to establish the DM + NCsi group. According to the transfection reagent manufacturer's protocol, a mixture of 1 µg uc.48+ or scrambled siRNA in diluent (100 µl) and 0.5 µl transfection reagent in diluent (100 µl) was injected i.p. The control and DM groups were injected with the same volume of saline.
At the end of week 8, the animals were sacrificed with CO2 using a chamber displacement rate of 10-30%/min. Subsequently, 0.6-1.0 ml blood was collected by cardiac puncture and abdominal cells were harvested from the mice by intra-abdominal lavage.
Nociceptive behavior assays
Since uc.48+ siRNA has been shown to ameliorate diabetic sympathetic neuropathy in type 2 diabetic rats (8), the ability of uc.48+ siRNA to alleviate neuropathological changes in diabetic mice was investigated through behavioral assays in the present study. The behavioral assays comprised the assessment of mechanical withdrawal threshold (MWT) and thermal withdrawal latency (TWL) after 6 weeks (prior to STZ injection), 7 weeks (prior to siRNA injection) and 8 weeks (following siRNA injection), respectively.
Measurement of MWT
Noxious-pressure stimulation was used to evaluate mechanical hyperalgesia. The experimental protocol of Liu et al (18) was used.
Measurement of TWL
Noxious heat stimulation was applied using the Thermal Paw Stimulation System (BME-410C; Boerni Science and Technology Co., Ldt.) and hyperalgesia was assessed using thermal stimulation by Hargreaves' test. The experimental protocol published by Liu et al (18) was followed, except the difference in animal strains used.
Measurement of heart rate (HR) and blood pressure
HR and blood pressure are indicators that reflect the health of the cardiovascular system. Each mouse was assessed by measuring its HR and blood pressure, including systolic blood pressure (SBP) and diastolic blood pressure (DBP), after 6 weeks (prior to STZ injection), 7 weeks (prior to siRNA injection) and 8 weeks (following siRNA injection). HR and blood pressure were assessed through an indirect tailcuff method (Softron BP-98A; Softron Co., Ltd.). A tailcuff 1.5 cm in diameter and 3.2 cm in length was used. Systolic pulsation was detected using an electrosphygmograph coupler (ZHHX-Z, MD3000; Anhui Zhenghua Biologic Apparatus Facilities Co., Ltd.). In brief, the experimental protocol published by Wu et al (12) was followed, except the difference in animal strains used. The blood pressure of the mice was measured five times in the morning at each time point by one person.
Cytokine assays
The inflammatory state of the body can be evaluated by the determination of cytokines. The cytokine concentrations of each mouse were detected in the serum at the end of the 8-week period following animal sacrifice. The cytokines assessed comprised tumor necrosis factor (TNF)-α, interleukin (IL)-1β and IL-10. The concentration levels of IL-10 (cat. no. EK0417), IL-1β (cat. no. EK0394) and TNF-α (cat. no. EK0527) were determined using ELISA kits (Wuhan Boster Biological Technology, Ltd.) according to the manufacturer's protocol.
Isolation of abdominal cells
The effect of uc.48+ siRNA on P2X7 receptors in a diabetic mononuclear phagocyte system has previously been demonstrated in vitro (13). Therefore, the present study aimed to determine whether uc.48+ affects mouse abdominal cells through P2X7 receptors in vivo. Abdominal cells were harvested from the mice at the end of the 8-week period by intra-abdominal lavage, cultured overnight in RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.) containing 10% FBS (Hyclone; GE Healthcare Life Sciences), 100 U/ml penicillin and 100 mg/ml streptomycin sulphate at 37˚C in a humidified atmosphere containing 5% CO2, and enriched for abdominal cells by washing away non-adherent peritoneal cells with lukewarm serum-free culture medium. Wright-Giemsa dye was used for staining at room temperature for 5 min and samples were observed using SZ61 Olympus microscope. The cell seeding density was 5x105/ml for the Wright-Giemsa and trypan blue staining. The viability of the purified adherent abdominal cells was determined by trypan blue exclusion assay. A total of 0.1 ml trypan blue stock solution was added to 1 ml of cells at room temperature for 5 min. The number of stained and total cells was counted. Healthy log-phase cultures exhibit cell viability of ≥95% (19). The following equation was used in the present study: % Viable cells=[1.00-(number of blue-stained cells/number of all cells)] x100%.
Total RNA isolation and reverse transcription-PCR (RT-PCR) analysis
Total RNA was isolated from the abdominal cells using TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. The quality assessment of the RNA and synthesized cDNA was performed according to our previously published method (13). The PCR amplification of the P2X7 receptor and β-actin (internal standard for quantification) genes was performed according to our previously published method (20). The amplification system included 2 µl cDNA, 12.5 µl PCR mixture (Tiangen Biotech Co., Ltd.), 2 µl primers (1 µl each of sense and antisense primers) and 8.5 µl nuclease-free water. The sequences of the primers used for RT-PCR analysis were as follows: P2X7 receptor (171 bp): sense, 5'-GCACGAATTATGGCACCGTC-3' and antisense, 5'-CCCCACCCTCTGTGACATTC-3'; uc.48+ (231 bp): sense, 5'-GCAAACTGGATGAGGAT-3' and antisense, 5'-GTAGTGCCACAAGGAGA-3'; β-actin (240 bp): sense, 5'-TAAAGACCTCTATGCCAACACAGT-3' and antisense, 5'-CACGATGGAGGGGCCGGACTCATC-3'. The PCR conditions used to detect these genes and analyze the PCR products were as described in the study by Wu et al (13).
Western blot analysis
Total protein was extracted using RIPA buffer containing a protease/phosphatase inhibitor mixture (diluted 1:100; Vazyme Biotech Co., Ltd.). The total protein concentration in the supernatant was measured using a bicinchoninic acid assay (Beyotime Institute of Biotechnology). The steps of the western blot analysis for P2X7 receptor and (p-)ERK1/2 were performed as described previously in the study of Wu et al (13).
Statistical analysis
All experiments were carried out in triplicate to confirm the accuracy of the results and data are presented as the mean ± standard deviation. The data of the abdominal cell experiments were normalized to those of the control group. Statistical analyses were carried out using SPSS 11.5 software (SPSS, Inc.). Statistical significance was determined by one-way analysis of variance. The Tukey's test was used for comparison between groups. P<0.05 was considered to indicate a statistically significant difference.
Results
Effects of uc.48+ siRNA on the nociceptive behavior of DM mice
The MWTs and TWLs of the mice were measured. Prior to the STZ injection (at the end of week 6), no significant differences in MWT were detected among the four groups, while the TWLs of the mice in the DM groups were lower than those of the control group. Following STZ injection (at the end of week 7), the MWTs and TWLs in the DM groups were lower than those of the control group. Following siRNA injection (at the end of week 8), the MWTs and TWLs (Fig. 1) in the DM + uc.48+ si group were higher than those of the DM group.
Figure 1.
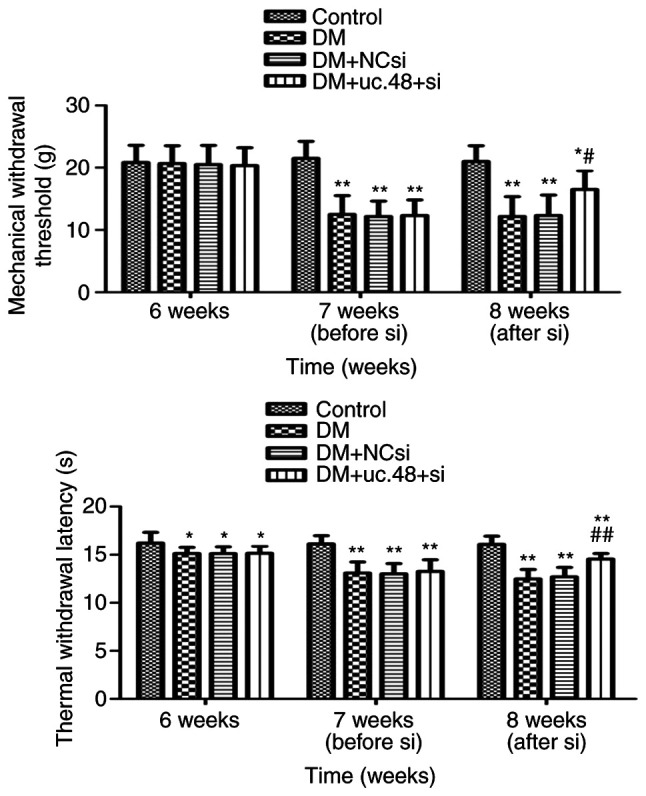
Effects of uc.48+ siRNA on the nociceptive behavior of type 2 diabetic mice. The behavior of the mice was assessed using MWT (upper panel) and TWL (lower panel) assays. At 6W, no significant differences in MWT were detected among the four groups, while the TWLs of the DM, DM + NCsi and DM + uc.48+ si groups were lower than that of the control group. At 7W, the MWTs and TWLs of the DM, DM + NCsi and DM + uc.48+ si groups were lower compared with those of the control group. At 8W, the MWT and TWL of the DM + uc.48+ si group were higher than those of the DM and DM + NCsi groups. n=6/group. *P<0.05, **P<0.01 vs. the control group; #P<0.05, ##P<0.01 vs. the DM group. siRNA, small interfering RNA; uc.48+ si, uc.48+ siRNA; NCsi, scrambled control siRNA; MWT, mechanical withdrawal threshold; TWL, thermal withdrawal latency; 6W, 6 weeks (prior to STZ injection); 7W, 7 weeks (following STZ injection); 8W, 8 weeks (after siRNA injection); DM, diabetes mellitus; STZ, streptozotocin.
These results reveal that the MWTs and TWLs in the DM group were significantly decreased compared with those of the control group. However, such effects were significantly attenuated following the injection of uc.48+ siRNA. Moreover, the TWL appeared to be significantly affected by a high-sugar and high-fat diet while the MTL was not.
Effects of uc.48+ siRNA on the HR and blood pressure of DM mice
The effects of uc.48+ siRNA treatment on HR and blood pressure are shown in Table I. The HR of the DM group was significantly increased compared with that of the control group. However, no significant differences were noted in HR between the DM, DM + NCsi and DM + uc.48+ siRNA groups following uc.48+ siRNA injection. The SBP and DBP in the DM group were significantly increased compared with those of the control group. However, these changes were significantly diminished following uc.48+ siRNA injection (Table I).
Table I.
Effects of uc.48+ siRNA on the heart rate, SBP and DBP of DM model mice.
| Group | ||||
|---|---|---|---|---|
| Parameter | Control | DM | DM + NCsi | DM + uc.48+ si |
| Heart rate | ||||
| 6 weeks | 579.80±44.68 | 617.43±37.08 | 617.83±58.58 | 620.15±40.65 |
| 7 weeks | 572.63±46.50 | 636.37±44.46a | 637.62±57.09a | 635.03±52.45a |
| 8 weeks | 568.08±52.03 | 636.93±47.73a | 634.65±58.10a | 601.62±59.16 |
| SBP | ||||
| 6 weeks | 112.15±14.53 | 140.55±16.17b | 140.50±20.08b | 141.50±16.21b |
| 7 weeks | 115.42±11.91 | 146.45±16.83b | 147.75±15.91b | 146.83±14.73b |
| 8 weeks | 115.33±12.89 | 141.20±16.21b | 142.83±11.63b | 122.83±11.63c |
| DBP | ||||
| 6 weeks | 75.57±5.86 | 85.27±11.07 | 86.50±8.04 | 85.67±9.69 |
| 7 weeks | 76.37±8.78 | 90.43±8.47a | 90.33±7.84a | 89.50±8.83a |
| 8 weeks | 75.03±6.80 | 89.73±6.37b | 89.27±8.98b | 80.00±7.44c |
Values are presented as the mean ± standard deviation (n=6/group). aP<0.05, bP<0.01 vs. control group; cP<0.05 vs. the DM group. siRNA, small interfering RNA; SBP, systolic blood pressure; DBP, diastolic blood pressure; DM, diabetes mellitus; 6 weeks, prior to STZ injection; 7 weeks, prior to siRNA injection; 8 weeks, after siRNA injection.
Effects of uc.48+ siRNA on the cytokine levels of DM mice
The serum TNF-α and IL-1β concentrations of the DM group were significantly higher compared with those of the control group, and these increases were significantly attenuated following the injection of uc.48+ siRNA. The serum IL-10 concentration of the DM group was significantly decreased compared with the corresponding concentration in the control group, whereas uc.48+ siRNA injection restored the IL-10 concentration to its initial levels (Fig. 2).
Figure 2.
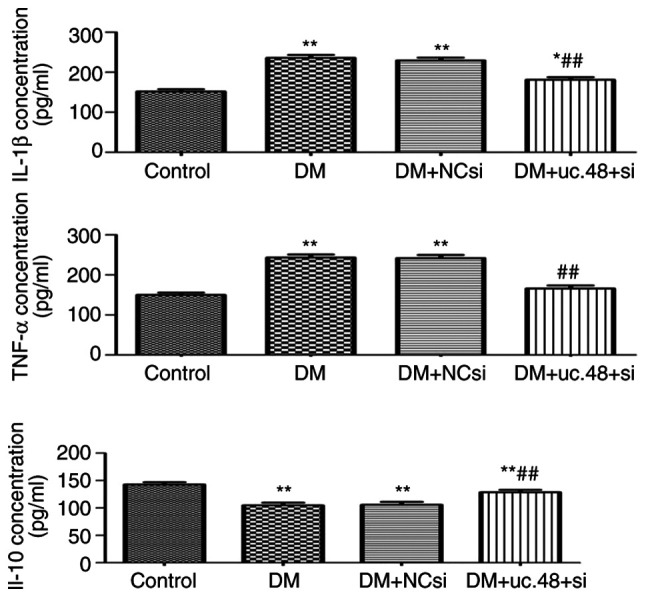
Effects of uc.48+ siRNA on the cytokine levels of type 2 diabetic mice. The serum expression levels of the cytokines IL-1β, TNF-α and IL-10 were evaluated by ELISA. IL-1β and TNF-α levels were increased significantly in the DM group (235.78±17.84 and 243.07±18.97 pg/ml, respectively) compared with those in the control group (151.52±14.51 and 149.52±13.84 pg/ml, respectively), but were significantly decreased by uc.48+ siRNA injection (to 181.30±15.38 and 165.75±19.44 pg/ml, respectively). The IL-10 expression levels demonstrated opposite trends compared with those observed for TNF-α and IL-1β. n=6/group. *P<0.05, **P<0.01 vs. the control group; ##P<0.01 vs. the DM group. siRNA, small interfering RNA; uc.48+ si, uc.48+ siRNA; NCsi, scrambled control siRNA; DM, diabetes mellitus; IL, interleukin; TNF, tumor necrosis factor.
Morphological identification and viability of abdominal cells
The scattered distribution, variable size, irregular shape and strong refractive index of the adherent abdominal cells were observed by microscopy (Fig. 3A). Following Wright-Giemsa staining, these cells exhibited the morphological features of macrophages. They were oval, round or irregularly shaped. They possessed abundant cytoplasm with irregular margins and pseudopodia and often exhibited single eccentric nuclei (Fig. 3B). No marked changes were noted with regard to the size, morphology and number of abdominal cells following transfection with uc.48+ siRNA.
Figure 3.
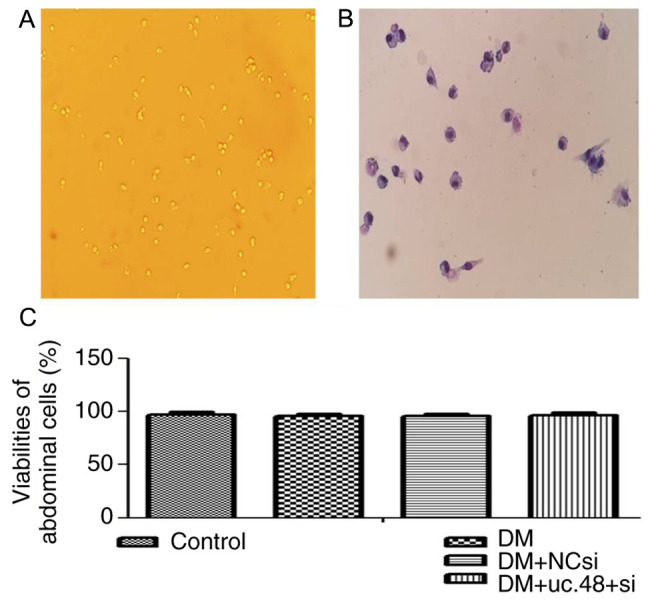
Morphological identification and viabilities of mouse abdominal cells. (A) Surviving abdominal cells (magnification, x10) and (B) abdominal cells following Wright-Giemsa staining (magnification, x20). (C) Viabilities of the adherent abdominal cells were 97.28±2.46, 95.77±2.29, 95.68±2.38 and 96.63±2.54 in the control, DM, DM + NC si and DM + NC si groups, respectively. DM, diabetes mellitus; NCsi, scrambled control siRNA; uc.48+ si, uc.48+ siRNA; siRNA, small interfering RNA.
The viabilities of the adherent abdominal cells in all four groups were >95% as determined by the trypan blue exclusion assay, and no significant difference was detected among the groups (Fig. 3C).
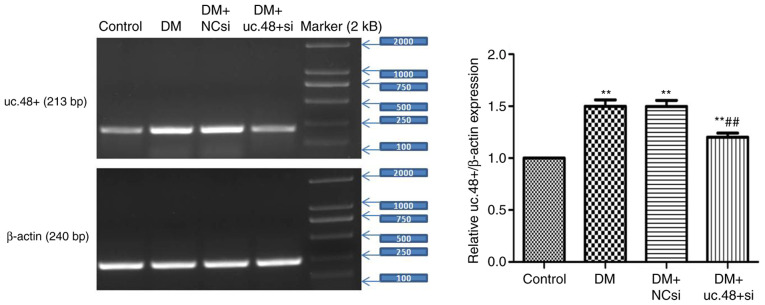
Changes in the uc.48+ expression levels in the abdominal cells of DM mice following uc.48+ siRNA treatment
The expression levels of uc.48+ in the abdominal cells were significantly reduced following treatment with uc.48+ siRNA (1.20±0.10) compared with the DM group, whereas no significant differences were noted between the DM (1.50±0.15) and the DM + NCsi (1.49±0.14) groups (Fig. 4). These results indicate that the targeting of uc.48+ with siRNA effectively suppressed the expression of uc.48+ in the abdominal cells of DM model mice.
Figure 4.
Change in the expression levels of uc.48+ in the abdominal cells of type 2 diabetic mice following uc.48+ siRNA treatment. uc.48+ expression was significantly increased in the DM group compared with the control group, and was significantly decreased following treatment with uc.48+ siRNA (1.20±0.10). However, no significant difference was detected between the DM (1.50±0.15) and DM + NCsi groups (1.49±0.14). n=6/group. **P<0.01 vs. the control; ##P<0.01 vs. the DM group. DM, diabetes mellitus; NCsi, scrambled control siRNA; uc.48+ si, uc.48+ siRNA; siRNA, small interfering RNA.
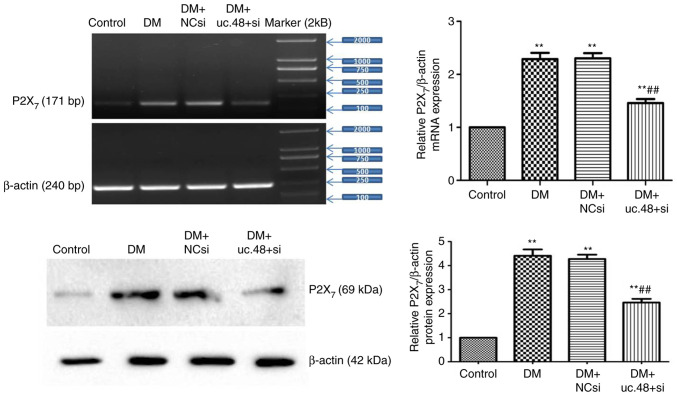
Changes in the expression levels of P2X7 receptor mRNA and protein in the abdominal cells of DM mice following uc.48+ siRNA treatment
As shown in Fig. 5, the upregulated mRNA and protein levels of the P2X7 receptor were significantly decreased following uc.48+ siRNA transfection in vivo (1.46±0.19 and 2.46±0.38, respectively) compared with those in the DM (2.29±0.29 and 4.41±0.63, respectively) group. No significant differences were detected between the DM and the DM + NCsi groups.
Figure 5.
Changes in the expression levels of P2X7 receptor mRNA and protein in the abdominal cells of type 2 diabetic mice following uc.48+ siRNA treatment. The mRNA and protein levels (top panel and bottom panel, respectively) of the P2X7 receptor were downregulated in the uc.48+ siRNA group (1.46±0.19 and 2.46±0.38, respectively) as determined by RT-PCR and western blotting, respectively, following transfection of uc.48+ siRNA in vivo, while no significant differences were detected between the DM (2.29±0.29 and 4.41±0.63, respectively) and the DM + NCsi groups (2.30±0.24 and 4.27±0.44, respectively). n=6/group. **P<0.01 vs. the control group; ##P<0.01 vs. the DM group. P2X7, P2X purinoceptor 7; DM, diabetes mellitus; NCsi, scrambled control siRNA; uc.48+ si, uc.48+ siRNA; siRNA, small interfering RNA; RT-PCR, reverse transcription PCR.
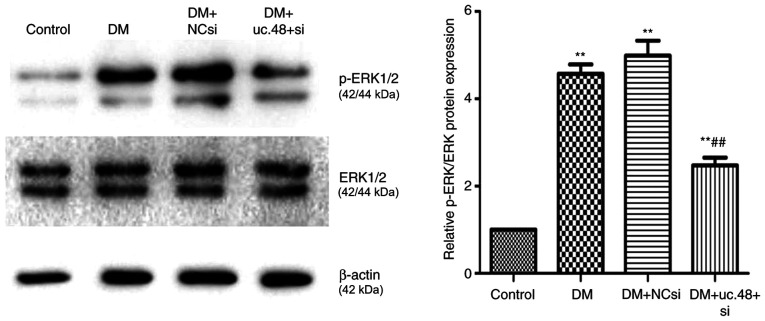
Changes in phosphorylated (p-)ERK1/2 levels in the abdominal cells of DM mice following uc.48+ siRNA treatment
The levels of p-ERK1/2 were normalized to the total ERK1/2 protein levels. The normalized p-ERK1/2 levels were significantly increased in the DM group (4.57±0.52) compared with the control group (1.00±0.00; Fig. 6). The knockdown of uc.48+ with uc.48+ siRNA (2.47±0.44) significantly decreased the ratio of p-ERK1/2 to total ERK1/2 compared with the DM group, while scrambled siRNA (4.99±0.85) exhibited no significant effects on p-ERK levels (Fig. 6).
Figure 6.
Changes of p-ERK1/2 levels in the abdominal cells of type 2 diabetic mice following uc.48+ siRNA treatment. The ratio of p-ERK1/2 to total ERK1/2 (4.57±0.52) was increased in the DM group compared with the control group, and uc.48+ siRNA (2.47±0.44) significantly decreased the proportion of p-ERK, while scrambled siRNA (4.99±0.85) did not significantly affect it. n=6/group. **P<0.01 vs. the control group; ##P<0.01 vs. the DM group. DM, diabetes mellitus; NCsi, scrambled control siRNA; uc.48+ si, uc.48+ siRNA; siRNA, small interfering RNA; p, phosphorylated.
Discussion
Diabetes is a considerable global health problem and has been classified as a major disease that requires prevention and control by the World Health Organization (21). The etiology of T2DM has not been fully clarified. Previous studies have shown that diabetic autonomic neuropathy, a complication of T2DM, can lead to cardiac dysfunction (22), while a chronic low-grade inflammatory response serves an important role in the occurrence and development of T2DM (2). In the present study, it was observed that the MWT and TWL of mice in the DM group were significantly lower than those in the control group, indicating that DM damages autonomic nerves and causes autonomic dysfunction, resulting in significant increases in blood pressure and HR. Concomitantly, the DM group displayed significantly higher serum levels of the anti-inflammatory cytokines TNF-α and IL-1β in comparison with those in the control group. The results presented in the current study indicate that inflammatory reactions occurred in the T2DM model mice, which exhibited effects on multiple systems, such as the central nervous and cardiovascular systems, and resulted in physiological dysfunction.
In vitro studies have shown that lncRNAs exhibit important cellular functions (23-25). Experiments using knockout animal models have confirmed that multiple lncRNAs serve roles in disease pathogenesis (26,27). A number of specific lncRNAs have been shown to participate in pathological processes of the endocrine system, including DM (28,29). The RT-PCR results of the present study indicate that the expression levels of uc.48+ in the abdominal cells of mice were significantly higher in the DM group than in the control group. The results further revealed that the MWT and TWL of mice with DM were significantly reduced following uc.48+ siRNA injection, suggesting that uc.48+ siRNA treatment may relieve diabetic neuropathic pain. Concomitantly, the blood pressure and expression levels of the anti-inflammatory cytokines IL-1β and TNF-α were also significantly decreased in DM model mice following uc.48+ siRNA injection, demonstrating that the downregulation of uc.48+ is associated with changes that relieve the diabetic inflammatory state and cardiovascular disease.
Monocytes/macrophages play an important role in the occurrence and development of T2DM, which is regarded as a type of low-grade inflammation (4,5,30,31). The in vitro results of our previous study using RAW264.7 macrophages revealed that uc.48+ siRNA is able to regulate immune and inflammatory responses, thus influencing the course and outcome of these effects, which are mediated by the P2X7 receptor (13). In the present study, the role of uc.48+ in the pathological changes of T2DM were investigated by monitoring the effects of uc.48+ siRNA on the abdominal cells of a mouse model of T2DM. The results indicated that the mRNA and protein expression levels of the P2X7 receptor and p-ERK1/2 level in the abdominal cells were significantly increased in DM model mice compared with the control group. However, these changes were significantly attenuated following transfection with uc.48+ siRNA in vivo. The experimental results indicate that uc.48+ serves an important role in the pathological changes of T2DM via regulation of the function of abdominal cells, while uc.48+ siRNA treatment may influence the ERK signaling pathway via the P2X7 receptor. However, it is unclear whether uc.48+ regulates the expression of cytokines through the ERK signaling pathway, or the expression of cytokines activates the ERK signaling pathway. Therefore, the specific mechanism requires further study. In particular, more experiments to verify that the effects of uc.48+ siRNA therapy are mediated by the P2X7 receptor and ERK signaling are necessary. In future studies, rescue experiments in which P2X7 receptor functions or ERK activity are blocked will be conducted to elucidate the mechanism of uc.48+ siRNA treatment.
In conclusion, the present study demonstrated that the uc.48+ expression levels of abdominal cells were significantly increased in a mouse model of DM compared with those in non-diabetic controls. Treatment of the mice with uc.48+ siRNA ameliorated the blood pressure and neuropathological changes associated with T2DM, and also downregulated the expression levels of the P2X7 receptor. In addition, uc.48+ siRNA regulated the inflammatory response and ERK signaling pathway in the abdominal cells of the diabetic mice. These effects may be mediated by the P2X7 receptor in T2DM. It is suggested that uc.48+ may serve an important role in T2DM via regulation of the P2X7 receptor, and thereby exert an effect on pathological changes in blood pressure, neuropathological changes and abdominal cells function.
Acknowledgements
The authors would like to thank Professor Shangdong Liang [Department of Physiology, Medical College of Nanchang University (Nanchang, China)] for guidance us during the present study.
Funding
The present study was supported by a grant from the National Natural Science Foundation of China (grant no. 81660144).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
YN and HW were responsible for the conception and design of the study. YN, HW, FW, MJ and QL acquired the data. HW drafted the manuscript and YN revised the manuscript for important intellectual content. All authors read and approved the final manuscript.
Ethics approval and consent to participate
All procedures involving animals were approved by the Animal Care and Use Committee of the Medical College of Nanchang University (approval no. 2018 16).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Harris-Hayes M, Schootman M, Schootman JC, Hastings MK. The role of physical therapists in fighting the type 2 diabetes epidemic. J Orthop Sports Phys Ther. 2020;50:5–16. doi: 10.2519/jospt.2020.9154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prattichizzo F, De Nigris V, Spiga R, Mancuso E, La Sala L, Antonicelli R, Testa R, Procopio AD, Olivieri F, Ceriello A. Inflammageing and metaflammation: The yin and yang of type 2 diabetes. Ageing Res Rev. 2018;41:1–17. doi: 10.1016/j.arr.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Bonnet F, Scheen AJ. Effects of SGLT2 inhibitors on systemic and tissue low-grade inflammation: The potential contribution to diabetes complications and cardiovascular disease. Diabetes Metab. 2018;44:457–464. doi: 10.1016/j.diabet.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 4.Ward MG, Li G, Hao M. Apoptotic β-cells induce macrophage reprogramming under diabetic conditions. J Biol Chem. 2018;293:16160–16173. doi: 10.1074/jbc.RA118.004565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saika F, Kiguchi N, Matsuzaki S, Kobayashi D, Kishioka S. Inflammatory macrophages in the sciatic nerves facilitate neuropathic pain associated with type 2 diabetes mellitus. J Pharmacol Exp Ther. 2019;368:535–544. doi: 10.1124/jpet.118.252668. [DOI] [PubMed] [Google Scholar]
- 6.Maciel FR, Punaro GR, Rodrigues AM, Bogsan CS, Rogero MM, Oliveira MN, Mouro MG, Higa EM. Immunomodulation and nitric oxide restoration by a probiotic and its activity in gut and peritoneal macrophages in diabetic rats. Clin Nutr. 2016;35:1066–1072. doi: 10.1016/j.clnu.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 7.Breuillard C, Bonhomme S, Couderc R, Cynober L, De Bandt JP. In vitro anti-inflammatory effects of citrulline on peritoneal macrophages in Zucker diabetic fatty rats. Br J Nutr. 2015;113:120–124. doi: 10.1017/S0007114514002086. [DOI] [PubMed] [Google Scholar]
- 8.Wu H, Nie Y, Xiong H, Liu S, Li G, Huang A, Guo L, Wang S, Xue Y, Wu B, et al. P2X7 receptor expression in peripheral blood monocytes is correlated with plasma C-reactive protein and cytokine levels in patients with type 2 diabetes mellitus: A preliminary report. Inflammation. 2015;38:2076–2081. doi: 10.1007/s10753-015-0189-y. [DOI] [PubMed] [Google Scholar]
- 9.Adinolfi E, Giuliani AL, De Marchi E, Pegoraro A, Orioli E, Di Virgilio F. The P2X7 receptor: A main player in inflammation. Biochem Pharmacol. 2018;151:234–244. doi: 10.1016/j.bcp.2017.12.021. [DOI] [PubMed] [Google Scholar]
- 10.Bhat SA, Ahmad SM, Mumtaz PT, Malik AA, Dar MA, Urwat U, Shah RA, Ganai NA. Long non-coding RNAs: Mechanism of action and functional utility. Noncoding RNA Res. 2016;1:43–50. doi: 10.1016/j.ncrna.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dinescu S, Ignat S, Lazar AD, Constantin C, Neagu M, Costache M. Epitranscriptomic signatures in lncRNAs and their possible roles in cancer. Genes (Basel) 2019;10(52) doi: 10.3390/genes10010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu B, Zhang C, Zou L, Ma Y, Huang K, Lv Q, Zhang X, Wang S, Xue Y, Yi Z, et al. LncRNA uc.48+ siRNA improved diabetic sympathetic neuropathy in type 2 diabetic rats mediated by P2X7 receptor in SCG. Auton Neurosci. 2016;197:14–18. doi: 10.1016/j.autneu.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 13.Wu H, Wen F, Jiang M, Liu Q, Nie Y. LncRNA uc.48+ is involved in the diabetic immune and inflammatory responses mediated by P2X7 receptor in RAW264.7 macrophages. Int J Mol Med. 2018;42:1152–1160. doi: 10.3892/ijmm.2018.3661. [DOI] [PubMed] [Google Scholar]
- 14.Dos Anjos Cassado A. F4/80 as a major macrophage marker: The case of the peritoneum and spleen. Results Probl Cell Differ. 2017;62:161–179. doi: 10.1007/978-3-319-54090-0_7. [DOI] [PubMed] [Google Scholar]
- 15.Jain R, Watson U, Vasudevan L, Saini DK. ERK activation pathways downstream of GPCRs. Int Rev Cell Mol Biol. 2018;338:79–109. doi: 10.1016/bs.ircmb.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 16.Ma L, Zhang S, Du M. Cordycepin from Cordyceps militaris prevents hyperglycemia in alloxan-induced diabetic mice. Nutr Res. 2015;35:431–439. doi: 10.1016/j.nutres.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 17.Zhang J, Qiu H, Huang J, Ding S, Huang B, Wu Q, Jiang Q. Establishment of a diabetic myocardial hypertrophy model in Mus musculus castaneus mouse. Int J Exp Pathol. 2018;99:295–303. doi: 10.1111/iep.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu S, Zou L, Xie J, Xie W, Wen S, Xie Q, Gao Y, Li G, Zhang C, Xu C, et al. LncRNA NONRATT021972 siRNA regulates neuropathic pain behaviors in type 2 diabetic rats through the P2X7 receptor in dorsal root ganglia. Mol Brain. 2016;9(44) doi: 10.1186/s13041-016-0226-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adan A, Kiraz Y, Baran Y. Cell proliferation and cytotoxicity assays. Curr Pharm Biotechnol. 2016;17:1213–1221. doi: 10.2174/1389201017666160808160513. [DOI] [PubMed] [Google Scholar]
- 20.Wang S, Xu H, Zou L, Xie J, Wu H, Wu B, Yi Z, Lv Q, Zhang X, Ying M, et al. LncRNA uc.48+ is involved in diabetic neuropathic pain mediated by the P2X3 receptor in the dorsal root ganglia. Purinergic Signal. 2016;12:139–148. doi: 10.1007/s11302-015-9488-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Begum M, Lewison G, Sommariva S, Ciani O, Tarricone R, Sullivan R. European diabetes research and its funding, 2002-2013. Diabet Med. 2017;34:1354–1360. doi: 10.1111/dme.13411. [DOI] [PubMed] [Google Scholar]
- 22.Tarquini R, Lazzeri C, Pala L, Rotella CM, Gensini GF. The diabetic cardiomyopathy. Acta Diabetol. 2011;48:173–181. doi: 10.1007/s00592-010-0180-x. [DOI] [PubMed] [Google Scholar]
- 23.Andersen RE, Lim DA. Forging our understanding of lncRNAs in the brain. Cell Tissue Res. 2018;371:55–71. doi: 10.1007/s00441-017-2711-z. [DOI] [PubMed] [Google Scholar]
- 24.Dong Y, Yoshitomi T, Hu JF, Cui J. Long noncoding RNAs coordinate functions between mitochondria and the nucleus. Epigenetics Chromatin. 2017;10(41) doi: 10.1186/s13072-017-0149-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lennox KA, Behlke MA. Cellular localization of long non-coding RNAs affects silencing by RNAi more than by antisense oligonucleotides. Nucleic Acids Res. 2016;44:863–877. doi: 10.1093/nar/gkv1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen X, Yan CC, Zhang X, You ZH. Long non-coding RNAs and complex diseases: From experimental results to computational models. Brief Bioinform. 2017;18:558–576. doi: 10.1093/bib/bbw060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Magagula L, Gagliardi M, Naidoo J, Mhlanga M. Lnc-ing inflammation to disease. Biochem Soc Trans. 2017;45:953–962. doi: 10.1042/BST20160377. [DOI] [PubMed] [Google Scholar]
- 28.He X, Ou C, Xiao Y, Han Q, Li H, Zhou S. LncRNAs: Key players and novel insights into diabetes mellitus. Oncotarget. 2017;8:71325–71341. doi: 10.18632/oncotarget.19921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li X, Zhao Z, Gao C, Rao L, Hao P, Jian D, Li W, Tang H, Li M. The diagnostic value of whole blood lncRNA ENST00000550337.1 for pre-diabetes and type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes. 2017;125:377–383. doi: 10.1055/s-0043-100018. [DOI] [PubMed] [Google Scholar]
- 30.Alvarado-Vázquez PA, Grosick RL, Moracho-Vilrriales C, Ward E, Threatt T, Romero-Sandoval EA. Cytokine production capabilities of human primary monocyte-derived macrophages from patients with diabetes mellitus type 2 with and without diabetic peripheral neuropathy. J Pain Res. 2018;12:69–81. doi: 10.2147/JPR.S186372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dhananjayan K, Gunawardena D, Hearn N, Sonntag T, Moran C, Gyengesi E, Srikanth V, Münch G. Activation of macrophages and microglia by interferon-γ and lipopolysaccharide increases methylglyoxal production: A new mechanism in the development of vascular complications and cognitive decline in type 2 diabetes mellitus? J Alzheimers Dis. 2017;59:467–479. doi: 10.3233/JAD-161152. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.