Abstract
The cytokinesis-block micronucleus cytome (CBMN) assay, introduced by Fenech, was used to demonstrate different types of DNA damage in MOLT-3 human lymphoblastoid cells exposed to 10 μM zidovudine (AZT). In addition, we explored the cytoprotective potential of two antioxidants, WR-1065 and Tempol, to decrease AZT-induced genotoxicity. Binucleated cells, arrested by Cytochalasin B (Cyt B), were evaluated for micronuclei (MN), caused by DNA damage or chromosomal loss, and chromatin nucleoplasmic bridges (NPBs), caused by telomere attrition. Additionally, nuclear buds (NBUDs), caused by amplified DNA, and apoptotic and necrotic (A/N) cells were scored. We hypothesized that AZT exposure would increase the frequency of genotoxic end points, and that the antioxidants Tempol and WR-1065 would protect against AZT-induced genotoxicity. MOLT-3 cells were exposed to 0 or 10 μM AZT for a total of 76 hr. After the first 24 hr, 0 or 5 μM WR-1065 and/or 0 or 200 μM Tempol were added for the remainder of the experiment. For the last 28 hr (of 76 hr), Cyt B was added to arrest replication after one cell division, leaving a predominance of binucleated cells. The nuclear division index (NDI) was similar for all treatment groups, indicating that the exposures did not alter cell viability. MOLT-3 cells exposed to AZT alone had significant (P < 0.05) increases in MN and NBs, compared to unexposed cells. Both Tempol and WR-1065 protected against AZT-induced MN formation (P < 0.003 for both), and WR-1065, but not Tempol, reduced the levels of A/N (P = 0.041). In cells exposed to AZT/Tempol there were significantly reduced levels of NBUDs, compared to cells exposed to AZT alone (P = 0.015). Cells exposed to AZT/WR-1065 showed reduced levels of NPBs, compared to cells exposed to AZT alone (P = 0.037). Thus WR-1065 and Tempol protected MOLT-3 cells against specific types of AZT-induced DNA damage.
Keywords: cytome assay, micronuclei, chromatin neoplasmic bridges, nuclear buds, apoptosis, necrosis, CBMN assay, MOLT-3 cells
INTRODUCTION
The cytokinesis-block micronucleus cytome assay (CBMN) [Fenech, 2007], constitutes a useful tool to study a variety of genotoxic endpoints. Based on the addition of Cytochalasin B (Cyt B) to prevent cytokinesis, the assay allows scoring of cells that have undergone one cell division, providing more information than the traditional micronucleus (MN) assay. The CBMN assay measures micronuclei (MN), caused by DNA damage or chromosomal loss, and chromatin nucleoplasmic bridges (NPBs), biomarkers of dicentric chromosomes resulting from telomeric end-fusions or DNA misrepair. Additionally, nuclear buds (NBUDs), caused by amplified DNA, and apoptotic and necrotic (A/N) cells were identified and scored using this assay [Fenech, 2007]. Overall, the CBMN assay has evolved into a comprehensive method for measuring chromosome breakage, DNA misrepair, chromosome loss, chromosomal non-disjunction, necrosis, apoptosis, and cytostasis [Fenech, 2007].
AZT is a nucleoside reverse transcriptase inhibitor (NRTI), a component of the Highly Active Antiretroviral Therapy mixtures used so successfully in patients infected with human immunodeficiency virus-1 (HIV-1). In a variety of in vitro and vivo models AZT and other NRTIs have been shown to induce host genotoxicity by DNA incorporation, and termination of DNA chain elongation during replication [IARC, 2000; Poirier et al., 2004; Olivero, 2007]. Other sequelae of NRTI exposure, which may or may not be directly related to NRTI-DNA incorporation, include mutagenesis [Meng et al., 2000; Bigbee et al., 2001; O’Neill et al., 2001], telomere shortening [Strahl and Blackburn, 1996; Gomez et al., 1998], tubulin polymerization [Olivero et al., 2006], and centrosomal amplification [Borojerdi et al., 2009; Yu et al., 2009a]. AZT is a moderately strong transplacental carcinogen in mice, and although most children born to HIV-1-infected mothers who received antiretroviral drugs during pregnancy have no evident adverse clinical effects, potential long-term genotoxic consequences of fetal exposure have been demonstrated in a transplacental non-human primate model [Olivero et al., 2013].
It has been previously shown that the prodrug amifostine, which acts as a cellular radioprotectant, is converted to the active thiol, WR-1065, by alkaline phosphatase. Use of the metabolite WR-1065 has been shown to attenuate cellular damage induced by radiation and chemotherapy [Grdina et al., 1985; Grdina et al., 1995; Grdina et al., 2000]. This attenuation occurs by a variety of mechanisms including inhibition of Topoisomerase II [Snyder and Grdina, 2000], induction of the mitochondrial Mn superoxide dismutase (SOD-2) [Murley et al., 2008], scavenging of free radicals [Koukourakis, 2002], and the enhancement of DNA repair mechanisms [Murley and Grdina, 1995]. In addition, WR-1065 has been shown to protect against NRTI-induced mutagenesis [Walker et al., 2009], and to have antiviral activity [Poirier et al., 2009].
An additional antioxidant and stable free radical, the nitroxide Tempol, acts as a scavenger of superoxide anions in vitro [Chatterjee et al., 2000] and may be a genuine “SOD-mimetic” agent [Krishna et al., 1996]. Tempol reduces the formation of hydroxyl radicals either by scavenging superoxide anions or by reducing the intracellular concentrations of Fe2+ and, hence, reducing the formation of hydroxyl radicals that would occur via the Fenton or Haber–Weiss reactions [Mitchell et al., 1990].
The current study was designed to examine AZT-induced genotoxic damage in human MOLT-3 cells by the CBMN assay, and to evaluate the potential of WR-1065 and Tempol to attenuate AZT induced genotoxicity. The protective properties of these antioxidant agents were evaluated as single agents and in combination.
MATERIALS AND METHODS
Cell Culture Conditions and Treatments
The lymphoblastoid cell line MOLT-3 [American Type Culture Collection, (ATCC), Manassas, VA] was cultured in Roswell Park Memorial Institute (RPMI) media (ATCC), and supplemented with 10% fetal bovine serum (ATCC) with the addition of penicillin-streptomycin (100IU/ml each, Invitrogen, Carlsbad, CA) in a 5% CO2 controlled atmosphere with 95% humidified ambient air at 37°C. AZT (SIGMA-Aldrich, Saint Louis, MO) was dissolved in a stock phosphate buffered saline (PBS) solution and the final AZT concentration was established by spectrophotometer, using the peak at 266 nm and an extinction coefficient of 11,500 M−1 cm−1 RPMI media containing AZT was prepared, and a stock solution was stored at 4°C until used.
Cytome Assay, Cell Treatment, and Procedure
The protocol used here for the CBMN assay is shown in Figure 1. Cells with and without AZT, were grown for 76 hr in 12-well plates containing a total of 2 × 105 cells/well in 3 ml of media. The antioxidants, WR-1065 (NCI Drug Synthesis and Chemistry Branch) and Tempol (Gift from J.M.), were added for the last 52 hr of culture. Cyt B (SIGMA-Aldrich) was added for the last 28 hr before cells were harvested (76 hr total, Fig. 1). Two experiments were performed on separate occasions, and each experiment involved triplicate exposures. At harvest, cells were cytospun in a Shandon cytospin 2 (Shandon, Thermo Scientific, Kalamazoo, MI) at 400 rpm for 4 min onto glass slides, using double cytofunnel sample chambers (Shandon). Subsequently, spreads were allowed to air dry for no longer than 10 min (to preserve cell morphology), and immediately fixed with 90% cold methanol (SIGMA, Aldrich) for 5 min. Once slides were dry, they were stored until scoring, at which time they were stained with a freshly prepared solution of acridine orange, 12.5 μg/ml (N,N,N′,N′-tetramethylacridine-3,6-diamine), in 1× PBS for 1 min at room temperature. One thousand binucleated cells were analyzed, for each of three wells per treatment group per experiment. The frequency of MN, NBUDs, NPBs, and A/N [Fenech 2007] was calculated as an average of two separate exposures. Representative images of an unexposed MOLT-3 cell and MN, NPBs, and NBUDs are shown in Figure 2.
Fig. 1.
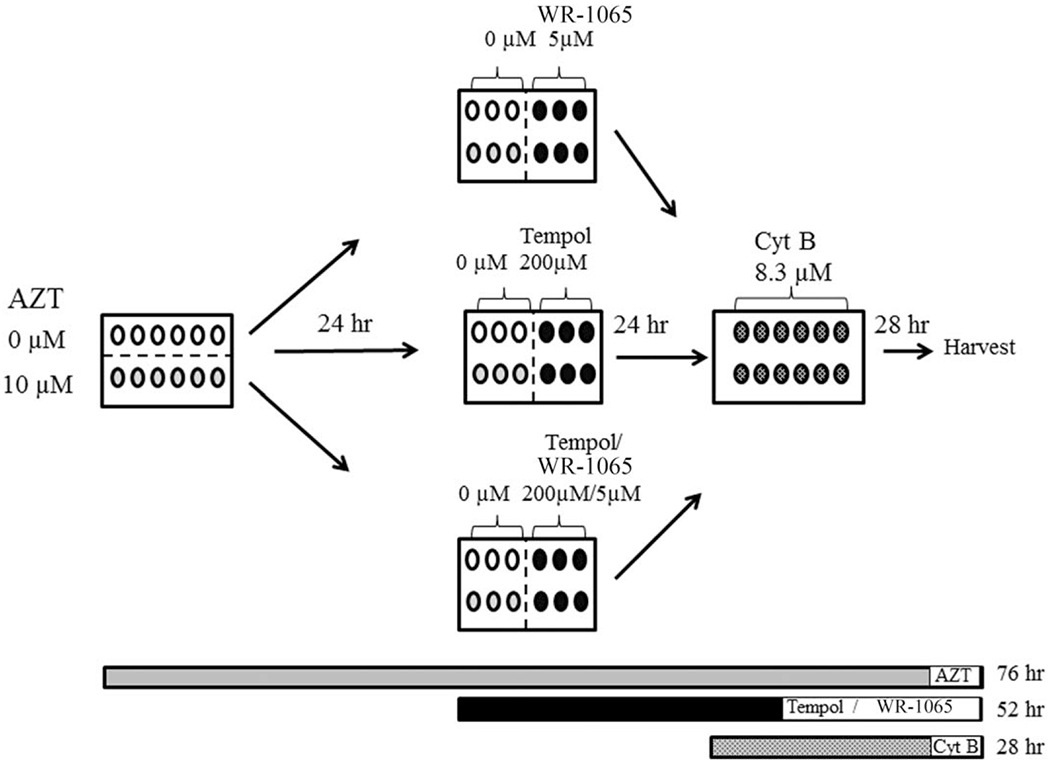
MOLT-3 cells, with and without AZT, were grown in 12-well plates containing 3 ml of media/well and a total of 2 × 105 cells/well for 76 hr. The center of the figure shows that WR-1065 (top), Tempol (middle), and Tempol plus WR-1065 (bottom) were added to half of the wells on the plate for 52 hr. For the last 28 hr, 8.3 μM Cyt B was added to all groups. The experiment was performed in triplicate on two separate occasions. Cells were exposed to AZT for a total of 76 hr, to antioxidants for 52 hr, and to Cyt B for 28 hr.
Fig. 2.
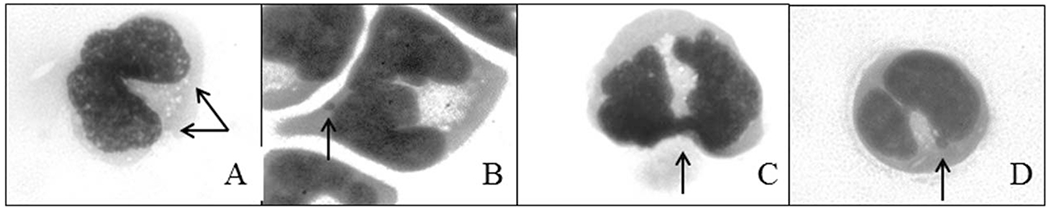
Representative images showing: (A) a binucleated MOLT-3 cell (arrows indicate both nuclei) arrested by Cyt B. Aberrations scored by the Cytome assay: (B) MN (arrow) in binucleated cell; (C) NPB (arrow), and (D) pedunculated NBUD (arrow).
Cell Proliferation Measured by Nuclear Division Index
The Nuclear Division Index (NDI), as previously described [Eastmond and Tucker 1989], is a measure of cytotoxicity. The assay involves scoring the frequency of cells that have undergone 1, 2, or more cell divisions during Cyt B blocking. The minimum index value is 1, which occurs when all the cells failed to divide during the cytokinesis-block period and hence are all mononucleated. If all viable cells completed one nuclear division, and are all binucleated, the NDI value is 2.0. An NDI value can only be greater than 2.0 if a substantial proportion of viable cells have completed more than one nuclear division during the cytokinesis-block phase and therefore contains more than two nuclei [Eastmond and Tucker 1989; Fenech 2007]. In these experiments, approximately 1,000 cells were scored for NDI after 76 hr of AZT treatment, with or without the addition of WR-1065 or Tempol for 52 hr, and addition of Cyt B for the last 28 hr of culture.
Statistics
Comparisons between exposed and unexposed groups (n = 2 experiments, each one performed by triplicate) and unexposed control cells with cells exposed to either antioxidant, individually and in combination were performed by a two-tailed unpaired Students’ t test, using Prism Graphpad statistic software. The Kruskal-Wallis One Way Analysis of Variance on Ranks was used to compare differences in the mean incidence values for MN between treatment groups. Additionally, we applied Holm-Sidak and Dunn’s methods for pairwise multiple comparisons, in order to identify a trend revealing that the anti-clastogenic effect of WR-1065 + Tempol versus either agent alone was significant. A two way ANOVA was performed to determine interactions between combined incidence of MN, NPBs, and NBUDs for control and treatment group comparisons.
RESULTS
Nuclear Division Index
The NDI is based on a comparison of the distribution of mononucleated and multinucleated cells present in cytokinesis-arrested Cyt B cells. In this experiment the exposure groups were: control; AZT; AZT/Tempol; AZT/WR-1065; and AZT/Tempol/WR-1065. Cells exposed to WR-1065, Tempol, and the combination WR-1065/Tempol were processed and scored for every endpoint (MN, NBUDs, NPBs, and A/N). Statistical analysis comparing unexposed controls and cells exposed to both anti-oxidant drugs show no difference in any of the endpoints analyzed (Supporting Information Table I), in consequence the data have been excluded from analysis for this contribution.
When a total of ~1,000 cells were scored for each treatment group, there were no major differences in ploidy among the five treatment groups and the results are shown in Table I. The binucleated cells were by far in the majority (80–90% of total cells examined), while the mononucleated cells were less than 6%. The overall NDI for each exposure group was essentially 2, indicating that none of the test compounds arrested survival. However, there were threefold more mononucleated cells in the AZT/WR-1065 and AZT/WR-1065/Tempol groups compared to the controls, and an increase in tetranucleated cells in the unexposed control group. Therefore, although the first cell division after the addition of Cyt B was not affected by AZT treatment, the second cell division, which produced the tetranucleated cells, was less frequent in the drug-exposed groups, possibly due to a delay in cell cycle.
TABLE I.
Nuclear Division Index (NDI) and Frequency of Cells with 1, 2, 3, or 4 Nuclei/Total Cells, in MOLT-3 Cells Exposed to 0 or 10 μM AZT, With and Without 200 μM Tempol, or 5 μM WR-1065, or Both
| Control | AZT | AZT/Tempol | AZT/WR-1065 | AZT/WR-1065/Tempol | |
|---|---|---|---|---|---|
| Mononucleated | 14 | 27 | 41 | 55 | 56 |
| Binucleated | 785 | 881 | 919 | 860 | 910 |
| Trinucleated | 59 | 27 | 13 | 31 | 17 |
| Tetranucleated | 144 | 77 | 22 | 70 | 18 |
| Total cells scored | 1002 | 1012 | 968 | 1016 | 1001 |
| NDI | 2.33 | 2.15 | 1.94 | 2.11 | 1.99 |
The Cytome Assay in Cells Exposed to AZT for 24 hr Prior to Exposure to Protective Agents
Examples of cells with MN, NPBs, and NBUDs are shown in Figures 2B–2D, respectively. Table II (column 2) shows incidence of MN in MOLT-3 cells under different exposure conditions. MN were significantly elevated in cells exposed to AZT, with a value of 29.0 ± 1.5 MN/1,000 binucleated cells, compared to controls with a value of 21.0 ± 1.5 MN/1,000 binucleated cells (P = 0.003, Table II). The addition of WR-1065 along with the AZT exposure reduced the incidence of MN significantly from 29.0 ± 1.5 to 20.0 ± 1.3 (P = 0.003). A significant reduction from 29.0 ± 1.5 to 21.0 ± 0.6 (P = 0.0006) was observed when cells were exposed to the AZT/Tempol combination. The reduction was even more pronounced when both antioxidants were used in combination, with a significant reduction from 29.0 ± 1.5 to 17.0 ± 1.8 MN/1,000 binucleated cells (P = 0.0005).
TABLE II.
Number of Changes/1,000 cells (±SE, n = 2 Experiments) Grown in Unexposed MOLT-3 Cells or Cells Exposed to AZT Alone or With WR-1065 and/or Tempola
| Treatment | MNb | NPBc | NBUDsd | A/Ne |
|---|---|---|---|---|
| Control | 21.0 ± 1.5 | 5.0 ± 1.5 | 5.0 ± 0.8 | 11.0 ± 1.1 |
| AZT | 29.0 ± 1.5 (P = 0.003) | 8.0 ± 1.5 (P = 1.09) | 9.0 ± 0.7 (P = 0.005) | 13.0 ± 1.3 (P = 0.407) |
| AZT/WR-1065 | 20.0 ± 1.3 (P = 0.003) | 4.0 ± 0.9 (P = 0.037) | 8.0 ± 0.5 (P = 0.244) | 7.0 ± 1.9 (P = 0.041) |
| AZT/Tempol | 21.0 ± 0.6 (P = 0.006) | 7.0 ± 0.9 (P = 0.5) | 5.0 ± 1.1 (P = 0.015) | 12.0 ± 1.3 (P = 0.547) |
| AZT/WR-1065/Tempol | 17.0 ± 1.8 (P = 0.005) | 6.0 ± 0.5 (P = 0.138) | 7.0 ± 0.6 (P = 0.113) | 11.0 ± 1.4 (P = 0.361) |
Cells with and without AZT (0 or 10μM) were grown for 76 hr in 12 well plates containing 2 × 105 cells/well in 3 ml of RPMI medium, and the antioxidants WR-1065 (5μM) and/or Tempol (200 μM) were added for the last 52 hr of culture. Cytochalasin B was added for the last 28 hr before cells were harvested (see text and Figure 1 for details).
MN, micronuclei.
NPBs, chromatin nucleoplasmic bridges.
NBUDs, nuclear buds.
A/N, apoptotic/necrotic cells.
The profile for NPB formation was somewhat different than for MN, and is shown in Table II, column 3. In MOLT-3 cells grown without AZT there were 5.0 ± 1.2 NPBs/1,000 cells, while AZT induced 8.0 ± 1.5 NPBs/1,000 cells (P = 1.09, Table II, column 3). The incidence of NPBs induced by AZT alone was significantly reduced with the addition of WR-1065 from 8.0 ± 1.5 to 4.0 ± 0.9 (P = 0.037). However, no significant reduction was observed with either Tempol (P = 0.5) or Tempol/WR-1065 (P = 0.138).
The data for NBUDs is shown in Table II, column 4. In MOLT-3 cells growing without AZT there were 5.0 ± 0.8 NBUDs/1,000 binucleated cells, and in cells growing with AZT there were 9.0 ± 0.7 NBUDs/1,000 binucleated cells (P = 0.005). The addition of WR-1065 reduced the initial incidence of NBUDs to 8 ± 0.5 (P = 0.244), while in cells growing with AZT/Tempol NBUDs were significantly reduced from 9.0 ± 0.7 to 5.0 ± 1.1 (P = 0.015). Cells growing with AZT, Tempol, and WR-1065 exhibited an incidence of 7.0 ± 0.6 (P = 0.113) NBUDs/1,000 binucleated cells.
The incidence of A/N, shown in Table II, column 5, was 11.0 ± 1.1/1,000 cells in untreated MOLT-3 cells and 13.0 ± 1.3 in AZT treated cells (P = 0.407). Reduction of cells with A/N was observed in cultures treated with AZT/WR-1065, which had a value of 7.0 ± 1.9, compared to 13.0 ± 1.3 in cells exposed only to AZT (P = 0.041). In cells exposed to AZT/Tempol, or the combination AZT/Tempol/WR-1065, there was no reduction in A/N compared to cells exposed only to AZT.
DISCUSSION
The CBMN assay, as used here, demonstrated enhanced formation of MN and NBUDs in human lymphoblastoid MOLT-3 cells exposed for 76 hr to AZT at plasma levels (10 μM), compared to unexposed controls. Additionally, the CBMN assay was used to explore the hypothesis that WR-1065 and/or Tempol might protect against AZT-induced MN, NPB, NBUD, or A/N formation. Our experiments indicate that the addition of WR-1065, to cells treated with AZT, reduced the incidence of MN, NPBs, and cells with A/N. Tempol reduced the incidence of MN and NBUDs occurring in AZT-exposed cells. The combination of WR-1065 and Tempol further reduced the number of MN, compared to either agent alone, but had no effect on NPBs, NBUDs, or number of cells with A/N. The data suggest that the most successful protection occurs against MN. A subset of MN induced by AZT exposure contains whole chromosomes, and may predispose to cancer. Therefore, presumably any agent that could reduce MN formation and protect against loss of a whole chromosome, would protect against AZT-induced aneuploidy and chromosomal instability.
Chromosome instability, measured as aneuploidy or an abnormal number of chromosomes, is associated with the carcinogenic process [Wilkens et al., 2004]. Chromosome instability and centrosomal amplification have been well-documented to occur in cells exposed to AZT [Borojerdi et al., 2009] and other NRTIs [Yu et al., 2009]. In addition, AZT increased the frequency of erythrocyte MN [Bishop et al., 2004; Witt et al., 2004; Dobrovolsky et al., 2005], and the formation of MN containing whole chromosomes [Borojerdi et al., 2009]. The persistence of NRTI-induced aneuploidy was documented in a non-human primate model revealing the presence of MN in cultured bone marrow cells taken from 3 year old monkeys exposed to NRTIs in utero and during the first 6 weeks of life [Olivero et al., 2013]. Taken together these events suggest the value in potential attenuation of NRTI-induced genotoxicity, with agents like WR-1065 and Tempol.
Introduced by Fenech [2007], and based on Cyt B cytokinesis arrest, the CBMN assay provides a convenient tool to evaluate cellular damage induced as a response to xenobiotic exposure [Fenech, 2007]. The use of Cyt B facilitates the scoring of MN exclusively in cells that have undergone one cell division under the effect of the blocking, and are easily identifiable as binucleated. Additionally, the presence of chromatin bridges connecting the nuclei of binucleated cells allows speculation about chromosomal rearrangements favored by telomeric attrition. The scoring of NPBs and NBUDs adds a new dimension to the classical MN scoring, and complements the understanding of the complex process of DNA damage and response that cells experience when exposed to a genotoxic agent. The origin of NBUDs is still under study, and some authors believe that NBUDs are aberrations formed to eliminate amplified DNA [Shimizu et al., 1998, 2000; Serrano-Garcia and Montero-Montoya, 2001]. Using fluorescent in situ hybridization probes in bone marrow stem cells of DNA repair deficient mice, the chromosomal content of AZT-induced NBUDs was found to be random, with no preference for any specific chromosome [Dutra et al., 2010].
AZT becomes incorporated into virus and host DNA in place of thymidine, inducing DNA replication chain termination, DNA fragmentation, mutagenesis, and the formation of MN and other types of genotoxic damage [Gonzalez and Larripa, 1994; Stern et al., 1994; Dertinger et al., 1996; Poirier et al., 2004; Olivero, 2007]. There is evidence that AZT is preferentially incorporated into the telomeric regions of chromosomes, presumably because telomerase has an affinity for the drug, and this results in telomere shortening [Gomez et al., 1995, 1998; Olivero et al., 1997]. It has been proposed that NPBs may be surrogate markers for telomeric shortening [Rudolph et al., 2001].
The antioxidant and cytoprotective agent WR-1065 has been used in the clinic to prevent the side effects of radiotherapy and chemotherapy [Grdina et al., 1985, 1995, 2000]. The use of WR-1065 to induce SOD-2-mediated radioprotection has been reported in human colon carcinoma cells, where the authors showed that WR-1065 reduced the incidence of ionizing radiation-induced MN and increased levels of SOD-2 [Murley et al., 2011]. An analog of WR-1065, WR-151326, was proven to have anti-mutagenic activity in HepG2 cells when added either concomitantly or following exposure of cells to a 18.7 mM concentration of AZT. The authors report that the agent decreased the level of HPRT (hypoxanthine phosphoribosyltransferase) mutations twofold and concluded that this analog has anti-mutagenic properties [Grdina et al., 1992]. In addition, protection against the induction of NRTI-induced centrosomal amplification was observed in cells exposed to NRTIs in the presence of WR-1065 [Yu, 2009]. The selective protection exerted by WR-1065 on formation of MN and/or NPBs may reveal mechanisms underlying the protective capacity of this agent. Some of the bridges observed may be due to telomeric attrition and unprotected “sticky ends,” but translocations due to DNA breakage may also play a role.
Tempol, a catalytic antioxidant, has been used as a radiation protector providing protection against oxidative- and radiation-induced damage [Soule et al., 2007]. Tempol protects against side effects resulting from cisplatin-based chemoradiation in mice without interfering with tumor response [Krishna et al., 1994]. Administered to mice in their diet Tempol can decrease cancer, and extend survival when administered immediately after non-lethal total body radiation [Mitchell et al., 2012]. Our data suggest that Tempol is able to protect MOLT-3 cells against some of the genotoxicity induced by AZT, mainly MN and NBUDs.
In summary, we have shown statistically significant formation of MN, in human lymphoblastoid cells exposed to plasma-levels of AZT for 76 hr (24 hr alone and additional 52 hr in combination with antioxidants). The incidence of MN, NPBs, and A/N was decreased when the antioxidant WR-1065 was added to the cultures during the last 52 hr of a total of 76 hr exposure to AZT. Tempol, on the other hand, showed protective effects in decreasing the incidence of MN and NBUDs. Additive protection was observed when Tempol was added to cultures with AZT and WR-1065. However, a statistical pairwise comparison revealed that addition of WR-1065 to cultures in the presence of AZT and Tempol did not produce any extra protection (Supporting Information Table II). Since some MN also contains whole chromosomes, treatment with WR-1065 and/or Tempol may protect against aneuploidy occurring during NRTI-induced neoplastic transformation.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the NCI Drug Synthesis and Chemistry Branch for providing WR-1065.
Grant sponsor: Intramural Research Program of the Center for Cancer Research, National Cancer Institute, NIH, Bethesda, MD.
Abbreviations:
- AIDS
acquired immunodeficiency syndrome
- AZT
3′-azido-3′-deoxythymidine, zidovudine, Retrovir®
- Cyt B
cytochalasin B
- CBMN
cytokinesis-block micronucleus cytome assay
- MN
micronucleus
- NBUD
nuclear bud
- NPB
nucleoplasmic bridge
- PBS
phosphate buffer saline
- RPMI
Roswell Park Memorial Institute
- WR-1065
2-ethanethiol-dihydrochloride
Footnotes
Additional Supporting Information may be found in the online version of this article.
REFERENCES
- Bigbee WL, Ness RB, Bass DC, Welsh MJ, Walker VE. 2001. Glycophorin A (GPA) locus somatic mutations in umbilical cord blood erythroid cells of infants exposed in utero to NRTIS. Environ Mol Mutagen 37 (Suppl 32):22. [Google Scholar]
- Bishop JB, Witt KL, Tice RR, Wolfe GW. 2004. Genetic damage detected in CD-1 mouse pups exposed perinatally to 3′-azido-3′-deoxythymidine and dideoxyinosine via maternal dosing, nursing, and direct gavage. Environ Mol Mutagen 43:3–9. [DOI] [PubMed] [Google Scholar]
- Borojerdi JP, Ming J, Cooch C, Ward Y, Semino-Mora C, Yu M, Braun HM, Taylor BJ, Poirier MC, Olivero OA. 2009. Centrosomal amplification and aneuploidy induced by the antiretroviral drug AZT in hamster and human cells. Mutat Res 665:67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee PK, Cuzzocrea S, Brown PA, Zacharowski K, Stewart KN, Mota-Filipe H, Thiemermann C. 2000. Tempol, a membrane-permeable radical scavenger, reduces oxidant stress-mediated renal dysfunction and injury in the rat. Kidney Int 58:658–673. [DOI] [PubMed] [Google Scholar]
- Dertinger SD, Torous DK, Tometsko KR. 1996. Induction of micronuclei by low doses of azidothymidine (AZT). Mutat Res 368: 301–307. [DOI] [PubMed] [Google Scholar]
- Dobrovolsky VN, McGarrity LJ, VonTungeln LS, Mittelstaedt RA, Morris SM, Beland FA, Heflich RH. 2005. Micronucleated erythrocyte frequency in control and azidothymidine-treated Tk+/+, Tk+/− and Tk−/− mice. Mutat Res 570:227–235. [DOI] [PubMed] [Google Scholar]
- Dutra A, Pak E, Wincovitch S, John K, Poirier MC, Olivero OA. 2010. Nuclear bud formation: A novel manifestation of Zidovudine genotoxicity. Cytogenet Genome Res 128:105–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastmond DA, Tucker JD. 1989. Kinetochore localization in micro-nucleated cytokinesis-blocked Chinese hamster ovary cells: A new and rapid assay for identifying aneuploidy-inducing agents. Mutat Res 224:517–525. [DOI] [PubMed] [Google Scholar]
- Fenech M 2007. Cytokinesis-block micronucleus cytome assay. Nat Protoc 2:1084–1104. [DOI] [PubMed] [Google Scholar]
- Gomez DE, Kassim A, Olivero OA. 1995. Preferential incorporation of 3′-Azido-2′,3′-dideoxythymidine (AZT) in telomeric sequences of CHO cells. Int J Oncol 7:1057–1060. [DOI] [PubMed] [Google Scholar]
- Gomez DE, Tejera AM, Olivero OA. 1998. Irreversible telomere shortening by 3′-azido-2′, 3′-dideoxythymidine (AZT) treatment. Biochem Biophys Res Commun 246:107–110. [DOI] [PubMed] [Google Scholar]
- Gonzalez CM, Larripa I. 1994. Genotoxicity of azidothymidine (AZT) in in vitro systems. Mutat Res 321:113–118. [DOI] [PubMed] [Google Scholar]
- Grdina DJ, Nagy B, Hill CK, Wells RL, Peraino C. 1985. The radioprotector WR1065 reduces radiation-induced mutations at the hypoxanthine-guanine phosphoribosyl transferase locus in V79 cells. Carcinogenesis 6:929–931. [DOI] [PubMed] [Google Scholar]
- Grdina DJ, Dale P, Weichselbaum R. 1992. Protection against AZT-induced mutagenesis at the HGPRT locus in a human cell line by WR-151326. Int J Radiat Oncol Biol Phys 22: 813–815. [DOI] [PubMed] [Google Scholar]
- Grdina DJ, Shigematsu N, Dale P, Newton GL, Aguilera JA, Fahey RC. 1995. Thiol and disulfide metabolites of the radiation protector and potential chemopreventive agent WR-2721 are linked to both its anti-cytotoxic and anti-mutagenic mechanisms of action. Carcinogenesis 16:767–774. [DOI] [PubMed] [Google Scholar]
- Grdina DJ, Kataoka Y, Murley JS. 2000. Amifostine: Mechanisms of action underlying cytoprotection and chemoprevention. Drug Metabol Drug Interact 16:237–279. [DOI] [PubMed] [Google Scholar]
- IARC. 2000. Some Antiviral and Antineoplastic Drugs, and Other Pharmaceutical Agents. World Health Organization, International Agency for Research On Cancer, Lyon, France. [Google Scholar]
- Koukourakis MI. 2002. Hypofractionated and accelerated radiotherapy with amifostine cytoprotection (HypoARC): A new concept in radiotherapy and encouraging results in breast cancer. Semin Oncol 29(6 Suppl 19):42–46. [DOI] [PubMed] [Google Scholar]
- Krishna MC, Dewhirst MW, Friedman HS, Cook JA, DeGraff W, Samuni A, Russo A, Mitchell JB. 1994. Hyperthermic sensitization by the radical initiator 2,2′-azobis (2-amidinopropane) dihydrochloride (AAPH). I. In vitro studies. Int J Hyperthermia 10: 271–281. [DOI] [PubMed] [Google Scholar]
- Krishna MC, Russo A, Mitchell JB, Goldstein S, Dafni H, Samuni A. 1996. Do nitroxide antioxidants act as scavengers of O2− or as SOD mimics? J Biol Chem 271:26026–26031. [DOI] [PubMed] [Google Scholar]
- Meng Q, Su T, Olivero OA, Poirier MC, Shi X, Ding X, Walker VE. 2000. Relationships between DNA incorporation, mutant frequency, and loss of heterozygosity at the TK locus in human lymphoblastoid cells exposed to 3′-azido-3′-deoxythymidine. Toxicol Sci 54:322–329. [DOI] [PubMed] [Google Scholar]
- Mitchell JB, Samuni A, Krishna MC, DeGraff WG, Ahn MS, Samuni U, Russo A. 1990. Biologically active metal-independent superoxide dismutase mimics. Biochemistry 29:2802–2807. [DOI] [PubMed] [Google Scholar]
- Mitchell JB, Anver MR, Sowers AL, Rosenberg PS, Figueroa M, Thetford A, Krishna MC, Albert PS, Cook JA. 2012. The antioxidant tempol reduces carcinogenesis and enhances survival in mice when administered after nonlethal total body radiation. Cancer Res 72:4846–4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murley JS, Grdina DJ. 1995. The effects of cycloheximide and WR-1065 on radiation-induced repair processes: A mechanism for chemoprevention. Carcinogenesis 16:2699–2705. [DOI] [PubMed] [Google Scholar]
- Murley JS, Nantajit D, Baker KL, Kataoka Y, Li JJ, Grdina DJ. 2008. Maintenance of manganese superoxide dismutase (SOD2)-mediated delayed radioprotection induced by repeated administration of the free thiol form of amifostine. Radiat Res 169:495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murley JS, Kataoka Y, Miller RC, Li JJ, Woloschak G, Grdina DJ. 2011. SOD2-mediated effects induced by WR1065 and low-dose ionizing radiation on micronucleus formation in RKO human colon carcinoma cells. Radiat Res 175:57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill JP, Gundel RM, Trombley LM, Walker VE. 2001. HPRT mutant frequency and mutation spectrum in newborns with perinatal exposure to antiviral drugs. Environ Mol Mutagen 37 (Suppl 32): 58. [Google Scholar]
- Olivero OA. 2007. Mechanisms of genotoxicity of nucleoside reverse transcriptase inhibitors. Environ Mol Mutagen 48:215–223. [DOI] [PubMed] [Google Scholar]
- Olivero OA, Anderson LM, Diwan BA, Haines DC, Harbaugh SW, Moskal TJ, Jones AB, Rice JM, Riggs CW, Logsdon D, et al. 1997. Transplacental effects of 3′-azido-2′,3′-dideoxythymidine (AZT): Tumorigenicity in mice and genotoxicity in mice and monkeys. J Natl Cancer Inst 89:1602–1608. [DOI] [PubMed] [Google Scholar]
- Olivero OA, Borojerdi JP, Semino-Mora C, Ward Y, Poirier MC. 2006. Genomic instability induced by AZT in cultured normal human mammary epithelial cells (NHMECs) generates aneuploidy. Retrovirology 3 (Suppl 1):48.16893449 [Google Scholar]
- Olivero OA, Torres LR, Gorjifard S, Momot D, Marrogi E, Divi RL, Liu Y, Woodward RA, Sowers MJ, Poirier MC. 2013. Perinatal exposure of patas monkeys to antiretroviral nucleoside reverse-transcriptase inhibitors induces genotoxicity persistent for up to 3 years of age. J Infect Dis 208:244–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier MC, Olivero OA, Walker DM, Walker VE. 2004. Perinatal genotoxicity and carcinogenicity of anti-retroviral nucleoside analog drugs. Toxicol Appl Pharmacol 199:151–161. [DOI] [PubMed] [Google Scholar]
- Poirier MC, Olivero OA, Hardy AW, Franchini G, Borojerdi JP, Walker VE, Walker DM, Shearer GM. 2009. Antiretroviral activity of the aminothiol WR1065 against Human Immunodeficiency virus (HIV-1) in vitro and Simian Immunodeficiency virus (SIV) ex vivo. AIDS Res Ther 6:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph KL, Millard M, Bosenberg MW, DePinho RA. 2001. Telomere dysfunction and evolution of intestinal carcinoma in mice and humans. Nat Genet 28:155–159. [DOI] [PubMed] [Google Scholar]
- Serrano-Garcia L, Montero-Montoya R. 2001. Micronuclei and chromatid buds are the result of related genotoxic events. Environ Mol Mutagen 38:38–45. [DOI] [PubMed] [Google Scholar]
- Shimizu N, Itoh N, Utiyama H, Wahl GM. 1998. Selective entrapment of extrachromosomally amplified DNA by nuclear budding and micronucleation during S phase. J Cell Biol 140:1307–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu N, Shimura T, Tanaka T. 2000. Selective elimination of acentric double minutes from cancer cells through the extrusion of micronuclei. Mutat Res 448:81–90. [DOI] [PubMed] [Google Scholar]
- Snyder RD, Grdina DJ. 2000. Further evidence that the radioprotective aminothiol, WR-1065, catalytically inactivates mammalian topoisomerase II. Cancer Res 60:1186–1188. [PubMed] [Google Scholar]
- Soule BP, Hyodo F, Matsumoto K, Simone NL, Cook JA, Krishna MC, Mitchell JB. 2007. The chemistry and biology of nitroxide compounds. Free Radic Biol Med 42:1632–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern M, Cid MG, Larripa I, Slavutsky I. 1994. AZT-induction of micronuclei in human lymphocyte subpopulations. Toxicol Lett 70: 235–242. [DOI] [PubMed] [Google Scholar]
- Strahl C, Blackburn EH. 1996. Effects of reverse transcriptase inhibitors on telomere length and telomerase activity in two immortalized human cell lines. Mol Cell Biol 16:53–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DM, Kajon AE, Torres SM, Carter MM, McCash CL, Swenberg JA, Upton PB, Hardy AW, Olivero OA, Shearer GM, et al. 2009. WR1065 mitigates AZT-ddI-induced mutagenesis and inhibits viral replication. Environ Mol Mutagen 50:460–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkens L, Flemming P, Gebel M, Bleck J, Terkamp C, Wingen L, Kreipe H, Schlegelberger B. 2004. Induction of aneuploidy by increasing chromosomal instability during dedifferentiation of heaptocellular carcinoma. Proc Natl Acad Sci USA 101:1309–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt KL, Tice RR, Wolfe GW, Bishop JB. 2004. Genetic damage detected in CD-1 mouse pups exposed perinatally to 3′-azido-3′-deoxythymidine or dideoxyinosine via maternal dosing, nursing, and direct gavage. II. Effects of the individual agents compared to combination treatment. Environ Mol Mutagen 44:321–328. [DOI] [PubMed] [Google Scholar]
- Yu M, Ward Y, Poirier MC, Olivero OA. 2009a. Centrosome amplification induced by the antiretroviral nucleoside reverse transcriptase inhibitors lamivudine, stavudine, and didanosine. Environ Mol Mutagen 50:718–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Ward Y, Davila K, Poirier MC, Olivero OA. 2009b. Centrosomal Amplification, A Genotoxic Mechanism Induced by Multiple Nucleoside Reverse Transcriptase Inhibitors is Inhibited by the Aminothiol WR-1065. Baltimore. p 195. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


