Abstract
Despite substantial advances in anesthesia safety within the past decades, perioperative mortality remains a prevalent problem and can be considered among the top causes of death worldwide. Acute organ failure is a major risk factor of morbidity and mortality in surgical patients and develops primarily as a consequence of a dysregulated inflammatory response and insufficient tissue perfusion. Neurological dysfunction, myocardial ischemia, acute kidney injury, respiratory failure, intestinal dysfunction, and hepatic impairment are among the most serious complications impacting patient outcome and recovery. Pre-, intra-, and post-operative arrangements, such as enhanced recovery after surgery programs, can contribute to lowering the occurrence of organ dysfunction, and mortality rates have improved with the advent of specialized intensive care units and advances in procedures relating to extracorporeal organ support. However, no specific pharmacological therapies have proven effective in the prevention or reversal of perioperative organ injury. Therefore, understanding the underlying mechanisms of organ dysfunction is essential to identify novel treatment strategies to improve perioperative care and outcomes for surgical patients. This review focuses on recent knowledge of pathophysiological and molecular pathways leading to perioperative organ injury. Additionally, we highlight potential therapeutic targets relevant to the network of events that occur in clinical settings with organ failure.
Keywords: Perioperative Organ Injury, Hypoxia, Adenosine, Hypoxia-Inducible Factor, HIF, Adenosine, microRNAs, Acute Respiratory Distress Syndrome, Myocardial Ischemia, Acute Kidney Injury, Inflammation
Introduction
Anesthesia safety has improved steadily over the last century with the implementation of clinical practice guidelines and checklists, as well as with advances in training, medication, monitoring devices and equipment 1. The risk of death and major complications for the general surgical patient population is less than 1 % 2. However, recent estimates in Europe and the USA reveal that overall postoperative mortality remains higher than expected and can be considered the third leading cause of death after ischemic heart disease and cancer worldwide, if given its own category 3,4. It should be noted with this statistic that perioperative death is multifactorial and a clear discrimination between procedure-related death and mortality resulting from pre-existing disease can be difficult. More than 4.0 million patients die within 30 days of a procedure each year, accounting for 7.7% of all deaths globally 4,5.
The aging population, increasing comorbidities, and more complex surgical procedures negatively impact recovery from surgery and contribute to rising numbers of high-risk patients 6. Individualized integration of procedure- and patient-related risk factors into the pre-, intra-, and post-operative arrangements can contribute to improving patient outcomes, e.g. enhanced recovery after surgery programs 7. But, despite all preventive efforts, acute organ injury is a frequent complication and a major risk factor of morbidity and mortality for surgical patients 4,8. Common manifestations of perioperative organ injury include neurological complications 9, myocardial ischemia 10, acute kidney injury 11, respiratory failure 12, intestinal dysfunction 13, and hepatic impairment 14.
Inflammation and ischemia build the pathophysiological hallmarks of organ dysfunction. During the perioperative period, either the surgical insult or insufficient organ perfusion, initially induces a local inflammatory reaction resulting in a coordinated cytokine cascade to maintain substrate supply for essential organs and wound healing. However, dysregulated, excessive mediator release and oxidative stress can culminate in a prolonged systemic inflammatory reaction that can lead to considerable collateral damage of the host tissues 15. Increased oxidative metabolism and immunological activity of recruited leukocytes require high levels of oxygen, causing hypoxia in the inflamed lesion 16. Hypoxia and inflammation can exacerbate one another by bidirectional pathways, at the same time various key mediators in these signaling networks have the potential to break this loop and drive resolution and tissue repair 17. Despite promising results from preclinical studies, no biological response modifier has been conclusively confirmed as a pharmacological treatment for the prevention or reversal of organ failure in clinical trials, and current therapeutic approaches remain limited to organ supportive strategies. Further understanding of the underlying mechanisms of organ dysfunction is essential to improve perioperative care and to identify novel targets for therapeutic intervention.
Clinical presentation and interventions targeting acute organ dysfunction
Acute organ injury is characterized by the rapid functional decline of an organ system and subsequent failure to maintain physiologic homeostasis. The impact of injury on each organ can range from mild dysfunction to complete failure and is potentially reversible. Organ dysfunction after surgery primarily develops on the pathophysiological basis of an exacerbated inflammatory response towards local tissue injury, perioperative hemodynamic changes, sudden occlusive events, pre-existing organ susceptibility, predisposing comorbid conditions and/or procedure-related characteristics (Figure 1, Table 1). During and after the operation, cellular damage and immunological activity lead to the release of various molecules in a timely coordinated manner, which can be considered as diagnostic or predictive biomarkers for the development of organ dysfunction (Figure 2, Table 1). Initial impairment of one organ function is often followed by injury of other organs. The sequence of organ failure influences patient outcome, and mortality rates increase with the number of dysfunctional organs 18. In the following paragraphs we will present frequent manifestations of organ injury, discuss their impact on perioperative outcomes, and highlight some of the clinical practice approaches to prevent or improve individual organ dysfunctions.
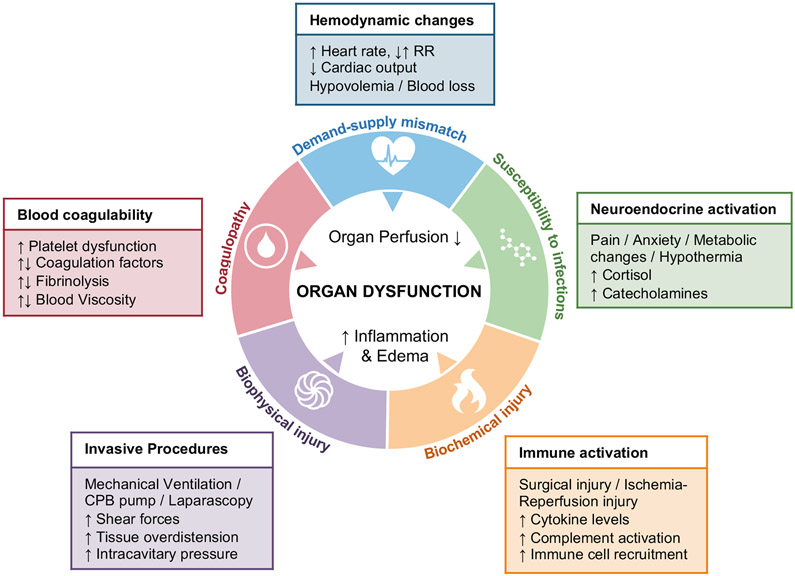
Figure 1: Pathophysiological mechanisms of perioperative organ injury.
Inflammation and ischemia are the pathophysiological hallmarks of perioperative single or multiple organ failure 155. During the perioperative period, organ perfusion can be significantly impacted by hemodynamic changes (blue) resulting from a demand-supply mismatch and/or hemostatic abnormalities (red) including either coagulopathic bleeding, or clotting. Neuroendocrine activation as part of the physiological stress response to the surgical insult can alter the immunological profile and contribute to increased susceptibility to infection (green). The surgical insult can trigger an uncontrolled inflammatory response with excessive release of inflammatory mediators and cytotoxic molecules, causing biochemical tissue damage, barrier dysfunction and edema (yellow). Concomitant activation of immune cells in a sterile environment can result in collateral tissue damage and organ dysfunction. Mechanical forces, such as mechanical ventilation, surgery on use of cardiopulmonary bypass pump or laparoscopy can cause tissue over distension and shear stress. Exposure to artificial surfaces and membrane oxygenators can contribute to immune cell activation and amplify collateral tissue damage (purple).
Table 1:
Overview of risk factors and biomarkers related to perioperative organ dysfunction.
| Organ Dysfunction | Top Surgeries associated with Perioperative Organ Injury |
Major Risk Factors for Organ Dysfunction (general surgical population) | Markers of Organ Injury (selection) | |||||
|---|---|---|---|---|---|---|---|---|
| (Ref.) | Preoperative | Intraoperative | Postoperative | Biomarker | Time to peak | Cellular Source | ||
| Stroke | Aortic/ Cardiac surgery Carotid endarterectomy Resection of head & neck tumors |
(19-22) | Carotid stenosis History of stroke or TIA Abrupt discontinuation of anticoagulation Coronary artery disease Arrhythmias |
Hyper-/ Hypotension Arrhythmias, CHF, MI Manipulation of aortic athero-sclerotic lesions Surgical complexity & duration Bypass time |
Arrhythmias, CHF, MI Hypercoagubility Dehydration Hemorrhage |
Copeptin GFAP* NSE S100B UCHL-1* |
rapid rapid early early rapid |
Hypothalamus Astrocytes Neurons Astrocytes, Glia Neurons |
| Delirium | Hip fracture repair Aortic/ Cardiac surgery Vascular surgery |
(23-25) | Advancing Age Multiple comorbidities Cognitive impairment (dementia) Substance abuse Premedication with Benzodiazepines |
Hypotension Deep anesthetic levels Anticholinergics Surgical complexity & duration Bypass time |
Insufficient pain control Opioid-based analgesic regimens Benzodiazepine sedation Prolonged mechanical ventilation Infection, Sepsis |
N/A | ||
| Myocardial infarction (Noncardiac surgery) | Vascular surgery Intraperitoneal surgery Orthopedic surgery |
(26-28) | Unstable or severe angina Recent MI Decompensated CHF Significant Arrhythmias Peripheral artery disease Renal insufficiency |
Tachyarrhythmia Hypo-/ Hypertension Blood loss, transfusion Hypercoagubility Surgical complexity & duration |
Hypo-/ Hypertension Arrhythmias Hypercoagubility Insufficient pain control Hypothermia |
CK-MB GPBB hFABP Myoglobin Troponin* |
early rapid rapid rapid early |
Myocard, Muscle Cardiomyocytes, Brain Cardiomyocytes, Kidney Myocard, Muscle Cardiomyocytes |
| Pulmonary complications | Aortic/ Cardiac surgery Thoracic surgery Upper abdominal surgery |
(29-31) | Chronic lung disease Major Trauma/ Severe Burns Aspiration Pneumectomy Obesity |
Mechanical Ventilation Massive transfusion Fluid overload Surgical complexity & duration Cardiopulmonary Bypass |
Pneumonia, Sepsis Prolonged mechanical ventilation Chest tubes Aspiration Nasogastric tubes |
Ang-1/2 sICAM sRAGE SP-D vWF |
early early early early early |
Endothelial cells Endothelial cells Alveolar Type I cells Alveolar Type II cells Endothelial cells |
| Acute kidney injury | Aortic-/ Cardiac surgery Major abdominal surgery Trauma surgery |
(32-34) | Hypertension Chronic kidney disease Coronary artery disease Peripheral artery disease Chronic lung disease |
Hypovolemia/ Fluid overload Anemia, transfusion Intraabdominal pressure Surgical complexity & duration Cross-clamp/ Bypass time Hypotension |
Nephrotoxic substances Infection, Sepsis Rhabdomyolysis Hypovolemia/ Fluid overload Hypotension |
Cystatin-C KIM-1* L-FABP NGAL TIMP2xIGFBP7* |
functional early rapid rapid early |
GFR Marker Proximal tubule Tubular epithelium Tubular epithelium, PMN Various |
| Liver Dysfunction | Hepatectomy | (14,35) | Cirrhosis Steatosis Cholestasis Arrhythmias, CHF, MI Renal insufficiency |
Blood loss, transfusion Fluid overload Major liver volume resection Intraabdominal pressure Arrhythmias, CHF, MI |
Intraabdominal Infection, Sepsis Biliary obstruction Portal vein thrombosis Hemorrhage Ascites |
ALT* CK-18 α-GST miR-122* |
early N/A early N/A |
Hepatocytes Hepatocytes Hepato-/ Enterocytes Hepatocytes |
| Bowel injury | Aortic-/ Cardiac surgery | (36,37) | Peripheral artery disease Arrhythmias, CHF, MI Chronic lung disease Coronary artery disease |
Blood loss, transfusion Cross-clamp/ Bypass time Hypotension, Hypovolemia |
Prolonged mechanical ventilation Vasopressors Infection, Sepsis Hypercoagubility Renal failure |
Citrulline* I-FABP α-GST |
N/A early early |
Enterocytes Enterocytes Hepato-/ Enterocytes |
indicates high specificity of biomarker, grey background indicates prognostic potential of biomarker.
(CHF, Congestive heart failure; MI, myocardial infarction; Ang1/2, Angiopoetin 1,2; ALT, Alanine aminotransferase; CK-18, Cytokeratin 18; CK-MB, Creatine kinase myocardial band; GFAP, Glial fibrillary acidic protein; GPBB, Glycogen phosphorylase isoenzyme BB; α-GST, α-Glutathione S-transferase; hFABP, Heart-type fatty acid binding protein; sICAM, soluble Intercellular adhesion molecule-1; IGFBP7, Insulin Like Growth Factor Binding Protein 7; I-FABP, Intestinal-type fatty acid-binding protein; KIM-1, Kidney Injury Molecule-1; L-FABP, liver-type fatty acid-binding protein; NSE, Neuron-specific enolase; NGAL, Neutrophil gelatinase-associated lipocalin; sRAGE, soluble Receptor for advanced glycation endproducts; SP-D, Surfactant protein D; vWF, von Willebrand factor; TIMP2, Tissue inhibitor of metalloproteinases 2; UCHL-1, Ubiquitin carboxy-terminal hydrolase L1)
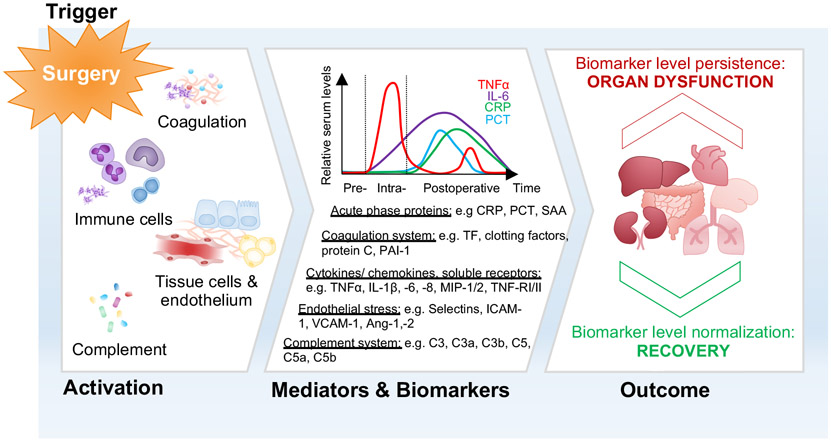
Figure 2: Simplified overview of the cellular sources and time-course of biomarker release after surgery.
Surgery (‘Trigger’) causes localized organ injury and triggers the release of danger signals, thereby activating the coagulation and complement system, and the immune response including stimulation of inflammatory and tissue cells (‘Activation’). During and after the operation, cellular damage and immunological activity lead to the release of various mediators in a timely coordinated manner, which relate to the course of the response to the surgical insult (‘Mediators and Biomarker’). These molecules are considered as biomarkers and have been suggested to have predictive values before tissue injury for specific organs becomes irreversible. The normalization of biomarker levels over time indicates recovery from tissue damage, whereas biomarker persistence points toward a significant and potentially permanent impact on organ function (‘Outcome’).
(Ang, Angiopoetin, C3 and C5, complement component 3 and 5; CRP, C-reactive protein; ICAM, Intercellular adhesion molecule 1; IL, Interleukin; MIP, Macrophage inflammatory protein; PCT, Procalcitonin; PAI-1, Plasminogen activator inhibitor 1; SAA, Serum Amyloid; TF, Tissue Factor; TNFα, Tumor-necrosis factor α; TNF-R, Tumor necrosis factor receptor; VCAM, vascular cell adhesion molecule-1)
a). Cardiovascular dysfunction
Perioperative myocardial ischemia (PMI) and infarction continue to be major causes of morbidity and mortality in noncardiac surgical patients 10. Cardiac injury is defined as myocardial cell death, reflected by elevated serum cardiac troponin levels within 30 days of non-cardiac surgery. The peak post-operative troponin (≥ 0.3 ng/ml) during the first three days after surgery has prognostic value and an absolute change of 5 ng/ml predicts 30-day mortality 38. High-sensitivity troponin assays could increase the detection of myocardial injury 39, while post-operative troponin kinetics were not useful for further mortality risk assessment 40. PMI peaks during the early post-operative period (24-48 hours) and is significantly associated with myocardial infarction (MI) and cardiac complications 10. In more than half of the cases, myocardial infarction is silent and is preceded by ST-depression type ischemia rather than ST elevations 41. Despite improved pre-operative risk stratification and advances in intra-operative care fluid resuscitation, PMI occurs in up to 6.2% of the surgeries with fatal outcomes in 2-25% of the cases 27,42.
PMI is primarily considered the result of imbalances in myocardial oxygen supply and demand (oxygen supply-demand mismatch) from tachycardia, hypotension, hypoxia, or anemia 43. Thus, abrupt changes in heart rate, blood pressure, and intravascular volume during surgery can culminate in cardio-myocyte necrosis and subsequent infarction in susceptible patients. A strong correlation of hypotension and myocardial injury is widely accepted. However, the vulnerable threshold and critical duration of intraoperative hypotension is not consistently defined 44,45. Prolonged exposure to either an absolute mean arterial pressure (MAP) below 65 mmHg or relative thresholds of 20% from the pre-induction MAP 45, and even short periods of an intraoperative MAP less than 55 mmHg could relate to the occurrence of myocardial injury 44.
Besides demand-supply mismatch associated cardiac ischemia, patients with preexisting coronary artery disease are at a high risk of suffering from occlusive events during surgery. Increased coronary artery sheer stress can precipitate destabilization and rupture of vulnerable plaques, low coronary flow and blood hypercoagulability, combined with stress-induced vasoconstriction, can favor thrombotic occlusion of the coronaries 43. To protect the heart from sustained ischemia, myocardial preconditioning using volatile anesthetics has been suggested based on the observations in animal studies 46. However, cardio-protective effects of volatile over intravenous anesthetics have not been convincingly demonstrated in several clinical trials and meta-analyses 47,48, suggesting that myocardial ischemia cannot be prevented by anesthetic agents ‘preconditioning’ the heart, but depends on the anesthesiologist’s skills to use the available tools to control the hemodynamic homeostasis of the patient 49.
b). Neurological complication
Cerebral dysfunction occurs frequently after surgery and can manifest as stroke or confusional states, such as delirium. Perioperative stroke, defined as either hemorrhagic or ischemic brain infarction within 30 days of the procedure, is one of the most devastating complications with significant impact on patient outcome and recovery 50. Mortality rates are as high as 24% and a large proportion of survivors face the challenges of long-term neurological disability 9. In particular, patients undergoing cardiovascular and carotid artery surgery (1.9 - 9.7 %) are affected 19,21. In the general surgical patient population cerebrovascular insult is considered rare (0.1 - 1.9%) 9,51, but due to increasing patient age and comorbidities, a larger population at risk of perioperative stroke is expected in the future 51. Diagnosis is often delayed because neurological symptoms may present mild and be incorrectly mistaken as post-operative confusional states 52. Embolic events are considered the major etiology of strokes after surgery. Early strokes are commonly associated to direct manipulation of the heart, the aorta, or the carotid artery 19,21. Delayed cerebrovascular accidents from the second post-operative day onward are frequently attributable to cardiogenic embolism on the basis of post-operative atrial fibrillation, myocardial infarction, or a hypercoagulable state 19,51,53. Hypoperfusion as a cause of stroke is considered rare 54. However, recent studies identify beta-blockade as a risk factor for perioperative stroke in the general surgical population and there is rejuvenated interest in linking intraoperative hypotension to strokes 55,56. The cerebral perfusion is critically dependent on the mean arterial pressure, and a non-selective beta-blockade may cause malperfusion by impairing cerebral vasodilation and reducing cardiac output. Consistently, Bijker and colleagues observed an association of post-operative ischemic strokes and a MAP reduction by more than 30% from baseline blood pressure in general surgical patients 57.
Post-operative delirium, characterized by acute fluctuations in awareness, cognition and consciousness, is a more frequent neurological complication with high incidence rates reported from 5-50% and seems to affect elderly patients in particular 58. Among those admitted to critical care units, the occurrence of delirious patients is even higher, with 60–80% of mechanically ventilated patients and 20–50% of non-mechanically ventilated patients 59. The wide variation of reported incidence rates may reflect the fact that delirium is not a clinical diagnosis but rather a variably operationalized concept defined by decline in postoperative cognitive performance assessed by a mental testing algorithms 60. Although often considered less serious than other types of organ injury after surgery, there is increasing evidence that delirium significantly predicts and relates to adverse surgical outcomes 58,61. Recent studies demonstrated an association of delirium with patients’ functional decline, longer duration of mechanical ventilation and intensive care unit (ICU) stay, increased length of overall hospital stay, and post-discharge mortality 62,63. Notably, post-operative delirium negatively impacts patient outcome and recovery independent of other risk factors such as comorbidities and illness severity 61,64. Given the significant impact on patient outcome and the lack of evidence for efficient pharmacological treatments, early recognition and prevention of delirium is of high importance. Opioid-based analgesic regimens on the one hand, and untreated pain on the other hand, are both potential risk factors of delirium 65. Thus, effective and opioid-sparing pain treatment is considered by many experts as an important goal to prevent acute confusional states after surgery. Until then, reducing opioids by appropriately-timed administration of dexmedetomidine can be considered a promising approach to lower the occurrence of cognitive dysfunction 66,67, whereas adjunctive treatment with Gabapentin 68 or a single subanesthetic dose of ketamine 69 did not indicate beneficial effects, but has been associated with an increased incidence of confusion and nightmares in elderly patients. For mechanically ventilated ICU patients, dexmedetomidine compared to standard sedatives was shown to increase delirium-free and coma-free days 70,71, but did not affect 90-day mortality and was more frequently associated with bradycardia and hypotension 72.
The underlying pathophysiology of delirium is considered to be multifactorial and involves acute central cholinergic deficiency, decreased GABAergic activity and abnormalities in serotonergic and dopaminergic pathways combined with cerebral inflammation 65. Anesthetic agents were hypothesized to differentially have brain protective effects by modifying these pathways, but recent clinical trials and retrospective analysis for Xenon, Sevofluran, or Propofol-based anesthetics failed to demonstrate evidence 48,73,74. Thus, it is more likely that the overall perioperative management, not just the anesthetic agent per se, impacts delirium risk.
c). Respiratory dysfunction
Patients are at risk for several types of respiratory dysfunctions in the perioperative period, including pneumonia, atelectasis, pneumothorax, and acute respiratory distress syndrome (ARDS) 12. Nowadays, mechanical ventilation is an indispensable tool in general anesthesia and intensive care medicine to provide sufficient oxygenation. Though being necessary to preserve life, invasive ventilation can cause injurious forces that can precipitate or exacerbate most of the above lung damage, referred to as ventilator-induced lung injury (VILI) 75. These mechanisms include exposure to high inflation transpulmonary pressures (barotrauma), alveolar overdistention (volutrauma), and/or high shear forces from repetitive opening and closing of atelectatic alveoli (atelectrauma), collectively leading to structural damage of the alveolar epithelial-endothelial unit and subsequent inflammation (biotrauma) 75,76. In 2000, a landmark trial by the ARDSnet translated the advances in understanding VILI into clinical success. The implementation of lung-protective ventilation strategies (≤6 vs. 12 ml/kg predicted body weight ventilation, optimal FiO2/PEEP titration, limited plateau pressure to ≤30 vs 50 cm H2O) has proven great implications for ICU patients and remains the cornerstone to prevent lung injury and improve outcomes 77. The knowledge from these studies may as well provide a pathway toward what would be the best use of lung protective strategies in the OR to reduce post-operative pulmonary complications. Although few clinical studies focus on the general surgical patient population, increasing evidence suggests that low tidal ventilation 78,79 and low driving pressures 80 could potentially prevent the occurrence of major respiratory dysfunctions. A few years ago, the original definition of VILI was extended to include dynamic work during ventilation, such as respiratory rate and flow, which distributes energy to the lung causing injurious strain 81,82. Cressoni and colleagues introduced the concept of mechanical power, which combines the lung damaging static (transpulmonary driving pressure, tidal volume) and dynamic (respiratory rate, flow) components into one variable, and is defined as energy per breath times respiratory rate (J/min) 81. These studies were the first to indicate that a power threshold, rather than focusing on individual parameter limits, should be taken into account to minimize VILI in healthy and diseased lungs 81. Although the concept of mechanical force is promising, the current mathematical equation has limitations and lacks an appropriate representation of the PEEP and aerated lung tissue 83,84. Improved modeling will be critical for large multicenter trials relating mechanical power to the risk of VILI to define a ‘safe’ mechanical power threshold for clinical practice and ventilator settings 84-86.
ARDS is one of the most serious pulmonary complications characterized by hypoxemia, non-cardiogenic pulmonary edema, and excessive lung inflammation with high mortality rates ranging from 27-46% 87,88. According to the Berlin definition proposed in 2012, ARDS is categorized in mild (PaO2/FiO2 ≤ 300 mmHg), moderate (PaO2/FiO2 ≤ 200 mmHg), or severe ARDS (PaO2/FiO2 ≤100 mmHg) with a significant mortality increase across the severity categories 88,89. The incidence in the general surgical population is low (0.2%), however, several factors such as pneumonia, extrapulmonary sepsis, aspiration, high risk surgeries, imbalanced ventilator and fluid management, significantly propagate the risk to develop lung failure after surgery 90. Excessive inflammatory activation and degradation of the alveolar-capillary barrier resulting in pulmonary edema formation, are considered central processes in the pathogenesis of acute lung injury 75,91. Improvements in outcomes of ARDS patients are primarily attributable to advances in supportive care on specialized ICUs (e.g. limiting fluid overload 92, early prone positioning93, extracorporeal membrane oxygenation? 94) and evolving lung-protective mechanical ventilation concepts (low tidal volume and plateau pressure 77, ‘open lung’ approach (controversial) 95,96, low driving pressures 97, and low mechanical power? 81). However, despite intense research over four decades and more than 20 large multicenter clinical trials, no specific pharmacological therapies have proven effective in the treatment of ARDS 98. Therefore, the paradigm has shifted to earlier interventions to prevent ARDS. The Lung Injury Prediction Score (LIPS) and the Checklist for Lung Injury Prevention (CLIP) have been suggested to standardize early recognition and initiate good practices for ARDS patients 99,100. Knowledge of ARDS risk factors provides the rationale for Phase III clinical trials from the Prevention & Early Treatment of Acute Lung Injury (PETAL) Network and the Lung Injury Prevention Study (LIPS) Group. Unfortunately, most of the trials published until now remain negative and did not provide any substantial breakthroughs for ARDS prevention and therapy. Ongoing study efforts continue to focus on basic physiological concepts and attenuation of the immune response. Adding to the list of negative studies last year, PETAL investigators reevaluated the benefits of early neuromuscular blockade to reduce patient–ventilator dyssynchrony and the work of breathing for patients with moderate-severe ARDS 101. Equally disappointing were the results obtained from studies addressing the potential of antioxidants and immune-modulators, such as statins 102, Vitamin C 103, and Vitamin D 104. Linking platelet immune functions to ARDS is increasingly recognized as a potential therapeutic intervention. However, despite repeated demonstration that the inhibition of platelet signaling attenuates lung inflammation in preclinical studies 105, the use of aspirin compared with placebo was not successful to reduce the risk of ARDS at 7 days in a multicenter trial randomizing 390 patients (LIPS-A trial) 106.
One of the lessons of the many failed trials is that ARDS represents a heterogeneous syndrome, and it is unlikely that one therapeutic strategy is suitable for all patients. Thus, identifying subgroups of patients that could benefit from specific interventions may provide a more promising approach for ARDS prevention, treatment, and trial design 107,108.
d). Acute kidney injury
Acute kidney injury (AKI), defined by a rapid decline of kidney function within a few hours or days, is a morbid complication of the surgical patient and is associated with poor outcomes and increased mortality 11. Traditionally, the concept of acute renal failure focused on severe, and relatively rare total loss of kidney function, thereby overlooking mild and moderate stages of renal impairment that occur more frequently 109. However, all severity levels can be associated with adverse outcomes and in particular, the milder forms remain underdiagnosed 110,111. To streamline research and clinical practice, the consensus definition of AKI has been revised with the intention to standardize assessment of kidney injury, define different severity categories and predict patient prognosis [Risk, Injury, Failure, Loss, End Stage Renal Disease (RIFLE) criteria, 2004; Acute Kidney Injury Network (AKIN) criteria, 2007; Kidney Disease: Improving Global Outcomes (KDIGO) criteria, 2012]. Since the release of the KDIGO criteria, the incidence of documented AKI in hospitalized patients is progressively increasing 112 underscoring the clinical importance of renal injury. Yet, the epidemiological change is not only attributable to the new criteria, but also indicates a real rise of AKI cases most likely reflecting the aging population, increasing comorbidities, and more complex surgeries 113,114. The incidence of AKI is considered rare in the general surgical population 115, but recent studies identified particular patient groups at high risk of renal injury. To this point, an incidence of 8.5% after gastric bypass surgery 116 is reported, 26% for trauma patients 117, 7-39% for patients undergoing major abdominal surgery 34, 19-46% for cardiac surgical patients 32, 48% after orthotopic liver transplantation 118, and rates as high as 75% for patients undergoing ruptured abdominal aortic aneurysm repair 33.
Renal hypoperfusion and inflammation are considered major contributors to cellular damage and tubular cell dysfunction in the kidneys 11. Hemodynamic instability and hypovolemia often occur temporarily during the perioperative period, potentially altering both MAP and cardiac output, and subsequently impairing renal blood flow 119. Initially, compensatory mechanisms involving the sympathetic nervous system, hormones, and the renin–angiotensin axis control the renal blood flow by regulating the diameter of the renal vasculature to maintain glomerular filtration. However, persistent hypoperfusion can exceed the auto-regulatory capacity of the kidney, leading to cellular hypoxia, tubular necrosis, and the release of danger signals (damage-associated molecular patterns, DAMPs) 120. Combined with surgical injury, these ischemic episodes can trigger a systemic inflammatory response resulting in recruitment of immune cells, endothelial dysfunction and renal microcirculatory alterations, thereby causing further tubular damage 121. Nephrotoxic drugs, such as antimicrobial substances including aminoglycosides and amphotericin B, nonsteroidal anti-inflammatory medication, or iodinated contrast imaging agents, can further increase the susceptibility of the kidney to perioperative stressors 122.
With the limited understanding of the pathogenesis of AKI, therapeutic approaches and preventive efforts remain majorly based on physiological concepts. Hemodynamic optimization and avoidance of intravenous fluid over- or underload are well-established concepts in reno-protection 123-125. Early detection, precise risk stratification, and supportive strategies can improve patient outcomes, however, the number of clinical trials remains inadequate 126. Recent pharmacological approaches to attenuate inflammatory activity include perioperative aspirin and clonidine 127, rosuvastatin 128 or high-dose atorvastatin administration 129, short-term perioperative medication of oral spironolactone 130, or treatment with THR-184—a bone morphogenetic protein-7 agonist 131. Unfortunately, all of the investigations failed to demonstrate outcome improvements in clinical trials. While renal replacement therapy continues to be the cornerstone of treatment for patients with severe kidney injury, the question of when (‘early vs. late’) and at which AKI stage to initiate extracorporeal organ support, remains at the center of intensive research efforts 132,133.
e). Intestinal dysfunction
For more than 30 years, the gut has been considered a central modulator in the development of multiple organ failure and sepsis 134. Traditionally, distant organ dysfunction was attributed to direct translocation of indigenous bacteria and toxins through the intestinal wall into systemic circulation due to inflammatory mucosal hyperpermeability 13. Critically ill patients in intensive care units frequently experience splanchnic hypoperfusion and intestinal barrier dysfunction 135. During the perioperative period, the incidence of mesenteric ischemia is only well documented for cardiac (< 1%) 36 and aortic surgery patients (1.6-15.2%) 37. Either as transient mesenteric ischemia triggering a gut-derived systemic inflammation or as mesenteric ischemic necrosis, bowel injury can be caused by an acute embolic or thrombotic obstruction of the mesenteric vasculature, or as non-occlusive mesenteric ischemia due to low flow situations, such as acute heart failure, cardiac arrhythmia or during high-dose administration of vasopressors 136,137. Acute bowel ischemia is generally a devastating complication that requires early diagnosis and intervention to prevent bowel necrosis and patient death. Primarily, patients with several comorbidities and poor cardiovascular conditions are affected 36. It is now recognized that not only the leakiness of the gut, but also the composition of bowel microbiome, has a significant impact on patient outcome 138. Critical illness can cause acute changes of the microbiome, and vice versa, the gut bacteria impacts critical illness. Intestinal microbiota can directly influence the cytokine response to injury, and thus, has the potential to drive immune responses into either a protective or an injurious direction 139,140. Several stressors during critical disease states, such as antibiotics, proton pump inhibitors, vasopressors and tissue hypoxia, can cause alterations of the gut microbiome leading to a detrimental immune profile and disease-exacerbating neutrophil subsets 141, which can trigger the development of organ failure 139,142.
f). Liver dysfunction
Acute liver dysfunction is defined as a sudden onset of jaundice, hepatic encephalopathy, and coagulopathy. Patients undergoing hepatectomy or cases with pre-existing liver disease have a particular high risk to develop liver failure after surgery 14,35. In addition, liver injury can be precipitated by cardiogenic causes, such as myocardial infarction or sustained arrhythmia resulting in passive acute liver congestion, and congestive heart failure leading to ‘cardiac cirrhosis’ 143. Portal hypertension, arteriovenous shunting and reduced splanchnic inflow can result in decreased hepatic arterial and venous perfusion at baseline, thereby increasing the susceptibility of the liver to ischemic injury. Intraoperative hypotension, hemorrhage, and vasoactive drugs, as well as mechanical compression of the liver by positive-pressure ventilation or pneumoperitoneum during laparoscopic surgery, further contribute to reduce liver circulation 144,145. To a limited extent, the liver can increase oxygen extraction to compensate for the decrease in hepatic blood flow, however, impaired oxygen and substrate delivery to the remaining functional hepatocytes and liver sinusoidal endothelial cells is likely to precipitate acute hepatic decompensation. ATP depletion and mitochondrial dysfunction due to ischemia lead to the accumulation of toxic mediators, such as lactate and reactive oxygen species causing hepatic inflammation 146. On the cellular level, resident liver macrophages (Kupffer cells) are critical for promoting liver resistance toward ischemia and reperfusion injury, for example through reprogramming via hypoxia-inducible transcription factors 147,148. Importantly, HIF stabilization confers hepato-protection during acute liver damage and is essential for tissue recovery and repair 148. Practice approaches to prevent or minimize acute hepatic failure in patients undergoing liver surgery include treating comorbid conditions, limiting the extent of surgery and increasing the volume of the future remnant liver by portal vein embolization 149. Preclinical studies in rodents indicate that following hepatic injury platelets are recruited into the liver, and that increasing platelet counts improved liver regeneration and survival after hepatectomy 150,151. Evidence is increasing that the induction of thrombocytosis either by splenectomy or platelet transfusion may promote regenerative processes after liver resection or transplantation 152,153. To make use of the regenerative potential of platelets without increasing the risk of transfusion- or splenectomy-related side effects, further understanding of the underlying mechanisms by which platelets stimulate regenerative programs in hepatocytes and liver sinusoidal endothelial cells will be essential to develop targeted therapies for perioperative liver dysfunction 154.
Cellular mechanisms leading to organ dysfunction
Many pathways inducing cellular damage are similar for all organs systems and include a combination of ischemic tissue injury and a dysregulated inflammatory response. On the cellular level, tissue hypoxia and inflammation are closely linked: Inflammatory conditions are often characterized by hypoxia, and conditions of low oxygen levels are often characterized by the onset of tissue inflammation (Figure 3). Perioperative reduction of blood supply during episodes of low blood pressure or occlusive events limits substrate and oxygen availability, causing cell-type specific transcriptional reprogramming involving hypoxia-inducible factors (HIF). Injured cells release ‘danger signals’ and induce trafficking of leukocytes to the side of tissue damage, which is associated with a profound increase in local oxygen demand. Activated immune cells release inflammatory mediators and cytotoxic molecules to pre-empt impending infection, but at the same time potentially cause collateral tissue injury. Cellular destruction can induce an amplification loop, in which leukocyte recruitment is maintained through sustained release of tissue injury signals. (Figure 4). If the immunological exacerbation is triggered by pathogen invasion, the clinical syndrome is defined as sepsis and causes infectious organ failure, which will be discussed elsewhere. In the following section, we will exemplify molecular pathways leading to organ dysfunction, and present potential therapeutic strategies to target these mechanistic networks to improve clinical outcomes.
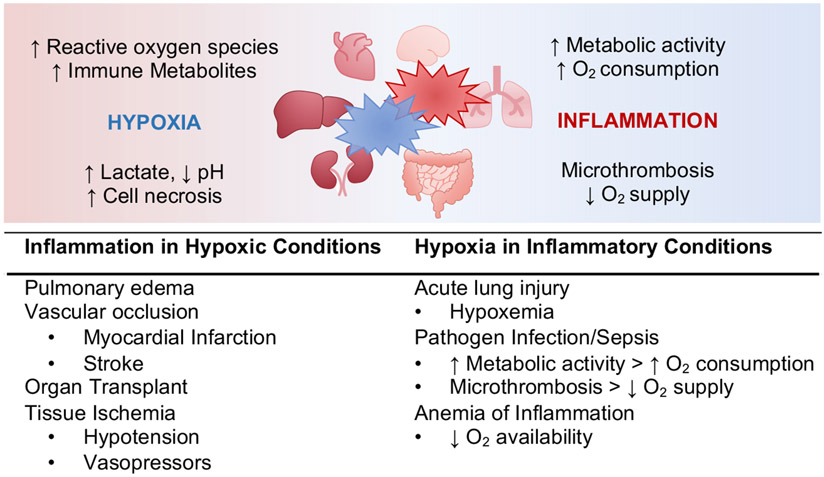
Figure 3: Links between Hypoxia and Inflammation.
Inflammed tissue (red) lesions are profoundly hypoxic, and hypoxia (blue) is a proinflammatory stimulus. Limited cellular oxygen availability results in the accumulation of cytotoxic metabolites, causing tissue damage and necrosis. Inflammation causes localized hypoxia by increased metabolic activity and oxygen (O2) consumption by immune and tissue cells. In addition, activated endothelial cells promote platelet aggregation and microthrombosis, thereby reducing oxygen supply. Examples for clinical condition primarily characterized by tissue hypoxia that causes inflammatory changes are summarized in the left panel, and perioperative inflammatory manifestations leading to tissue hypoxia on the right.
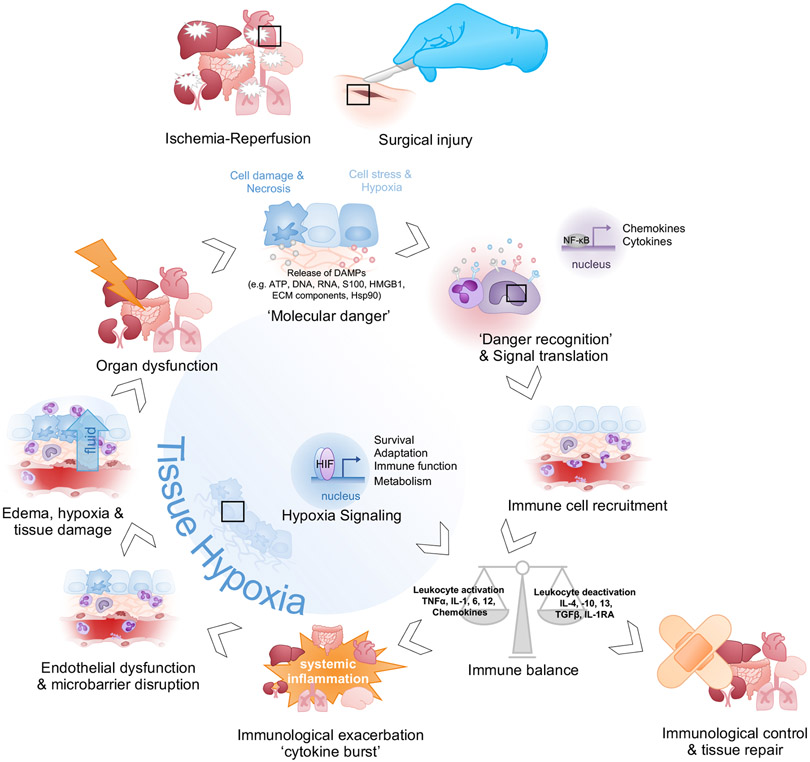
Figure 4: Cellular mechanisms leading to organ dysfunction.
Ischemia-reperfusion or surgical injury leads to local cellular damage, hypoxia and necrosis, and leads to the release of endogenous danger signals (DAMPs) from injured tissues (‘Molecular Danger’). DAMPs bind to pattern-recognition receptors (PRRs) on immune, endothelial, and epithelial cells and induce pro-inflammatory cytokine release and upregulation of adhesion molecules on the endothelium (‘Danger Recognition and Signal translation’). Activated leukocytes traffic to the site of injury and release cytokines, chemokines, and cytotoxic molecules to pre-empt impending infection (‘Immune cell recruitment’). The net inflammatory activity (‘Immune balance’) can either drive resolution and tissue repair (‘Immunological control’) or induce uncontrolled, systemic inflammation (‘Immunological exacerbation’). Cytotoxic molecules and reactive species from immune cells damage endothelial cells, leading to plasma leakage and subsequent tissue edema (‘Endothelial Dysfunction & Microbarrier disruption’). Tissue swelling and sustained inflammatory activity cause hypoxia and cellular damage (‘Edema, hypoxia & tissue damage’), leading to organ injury (‘Organ dysfunction’). Persistent cellular destruction can induce an amplification loop, in which leukocyte recruitment is maintained through sustained release of signals of tissue injury (‘Molecular Danger’). Nevertheless, although hypoxia can generate cytotoxic metabolites that induce proinflammatory responses and break down tissue barriers, there are many examples in which stabilization of HIFs induces tissue-protective responses (‘Hypoxia Signaling’).
(ATP, adenosine triphosphate; ECM, extracellular matrix; IL, interleukin; IL1-RA, interleukin-1 receptor antagonist; NF-κB, nuclear factor kappa B; HMGB-1, high-mobility group protein box 1; Hsp90, heat-shock protein 90; PRR, pattern-recognition receptor; S100, S100 protein; TGFβ, transforming growth factor β; TNFα, tumor necrosis factor α;)
a). Immunological activity and acute organ injury
Neutrophil recruitment to the site of infection is an essential early step in initiating innate immunity and effective bacterial clearance, yet excessive or inappropriate inflammation is associated with considerable collateral damage of the host tissues. Several complications that can be present during the perioperative period, such as acute myocardial infarction, stroke, reversal of cardiac arrest or organ transplant, are characterized by an initial tissue hypoperfusion followed by a sudden restoration of blood flow (ischemia-reperfusion) 155. This sequence has been shown to cause an inflammatory activation sharing parallels with neutrophilic inflammation directed towards microorganisms 156. Besides microbe-derived pathogen-associated molecular patterns (PAMPs), the host response is activated by damage-associated molecular patterns (DAMPs) released from injured or hypoxic cells 157. Whereas PAMP-signaling has a clear role in pathogen defense, the purpose of neutrophil recruitment into damaged tissues without infection is less understood. Recently, Huang and Niethammer used an elegant approach in zebrafish to uncouple tissue damage- and microbe-induced signaling during bacterial infection. Interestingly, they showed that neutrophils ignore bacteria in the absence of DAMPs, providing evidence for the indispensability of danger signals for microbial defense 158.
Given the adverse clinical effects of excessive inflammation, Kang et al. suggest a blood-cleansing device for patients with sepsis, which may also be of interest for perioperative patients with a systemic inflammatory response. In that approach, DAMPs and PAMPS are continuously removed from the blood using magnetic beads coupled to mannose-binding ligand, and the cleansed blood is returned back to the individual (‘biospleen device’) 159. However, this approach has so far only been studied in animal models. More advanced and of clinical use in Japan and parts of Western Europe, is direct hemoperfusion to remove circulating endotoxins via high affinity binding to polymyxin B immobilized to polystyrene-derived fibers. Despite evidence for improvements of hemodynamics, and oxygenation as well as renal function in pilot trials, polymyxin B hemoperfusion failed to improve survival at 28 days for patients with septic shock in a North American multicenter, randomized controlled trial in a cohort of 450 patients with septic shock and high endotoxin activity levels (Evaluating the Use of Polymyxin B Hemoperfusion in a Randomized Controlled trial of Adults Treated for Endotoxemia and Septic Shock, EUPHRATES trial) 160. Results from these studies indicate that filter-based approaches need to be designed more selectively in the future to specifically eliminate pro- over anti-inflammatory mediators.
Although it was established more than 25 years ago that dysregulated inflammatory activity and oxidative stress can result in damage of cellular structures and cell death 161, to date, no biological response modifier has proven effective for the treatment of acute organ injury. Clinical trials investigating broad-spectrum immuno-modulatory agents to block the excessive inflammatory response of multiple cell types, such as corticosteroids, keep yielding conflicting data about their benefit. Recent medium to large randomized-controlled trials in patients with severe acquired pneumonia, sepsis, and kidney injury only added to this controversy, and still lack a clear rationale for the use of corticosteroids either to prevent or treat organ failure 162-164. Equally disappointing were the results from two multicenter trials attempting to take advantage of the anti-inflammatory and antioxidant properties of vitamin C or statins in patients with sepsis-associated lung failure 102,103. One of the lessons of many failed trials of immunomodulatory treatment for organ dysfunction is that ubiquitous immune modulators are not for every patient, suggesting that an individualized and context-dependent fine-tuning of the inflammatory response is more likely to improve outcomes.
Currently, selective inhibition of particular drivers of inflammation is coming into the focus of clinical investigations. Upon exposure to DAMPs and PAMPs, cytokines and chemokines (including TNFα, IL-6, IL-1β and IL-8) as well as complement peptides are released, which activate the endothelium and stimulate leukocyte adhesion and transmigration 15. Numerous in vitro and genetic mouse experiments contributed to the current understanding of pro- and anti-inflammatory signaling. Although neutralizing compounds, such as inhibitory antibodies, were shown to reduce inflammatory markers and improve outcomes in experimental animal models, only a few small trials exist indicating potential benefits of such approaches as a therapy for organ injury. For example, specific inhibition of TNF-Receptor 1 signaling could dampen inflammation in ARDS patients, as suggested by a pilot study in healthy volunteers 165. In small patient cohorts, the inhibition of the IL-6 receptor by Tocilizumab 166,167, and targeting the interleukin-1β innate immunity pathway with canakinumab 168, were shown to have protective effects on myocardial injury. In a cohort of 80 stroke patients in a single-center, randomized placebo-controlled phase 2 trial, subcutaneous IL-1 Receptor antagonist reduced plasma inflammatory markers in patients with ischemic stroke 169.
Although neutrophils are generally considered to exacerbate tissue injury through the release of proteases and oxidants, recent work has implicated that neutrophils may also exhibit anti-inflammatory and reparative characteristics 170,171. They can actively contribute to host protection, for example neutrophils have been shown to shuttle micro-vesicles containing immuno-modulating microRNAs to damaged epithelial cells in models of acute lung 172. Currently, evidence is increasing for a role of neutrophils in terminating and resolving inflammation. Cessation of leukocyte influx, apoptosis, and subsequent efferocytosis are fundamental events in all organs during the resolution of inflammation 173. Neutrophil-derived proteases, such as matrix-metalloprotease 9, can proteolytically degrade DAMPs including HMGB1 and Hsp90 and thus, could dampen danger signaling and recruitment of additional immune cells by clearing immunogenic molecules 174. In a model of acute liver injury, it has just recently been demonstrated that reactive oxygen species can induce a reparative phenotype of macrophages that drives liver repair, suggesting that neutrophils can coordinate the surrounding cells and initiate resolution programs 175. Accordingly, neutrophils orchestrate post-myocardial infarction healing by inducing macrophage efferocytosis via neutrophil gelatinase-associated lipocalin (NGAL) 176.
Given that the net output of inflammatory activity can either resolve inflammation, or drive a detrimental amplification loop that can result in excessive, systemic inflammation and collateral tissue damage (Figure 4), balanced fine tuning of leukocytes and particular immune-modulators at different phases of inflammation might provide future strategies for organ protection.
b). Linking hypoxia signaling to organ protection
Only recently, William Kaelin, Peter Ratcliffe, and Gregg Semenza were awarded the Nobel Prize in Physiology or Medicine 2019 for their exceptional discoveries of fundamental pathways by which cells respond to hypoxia. Perioperative hemodynamic changes, hypovolemia, and hemostatic abnormalities can result in insufficient organ perfusion and limit cellular oxygen availability. The adaptive response to reduced oxygen levels is primarily mediated by hypoxia-inducible transcription factors (HIFs), which orchestrate transcriptional programs that regulate tissue metabolism and maintain homeostasis in conditions of low oxygen 177,178.
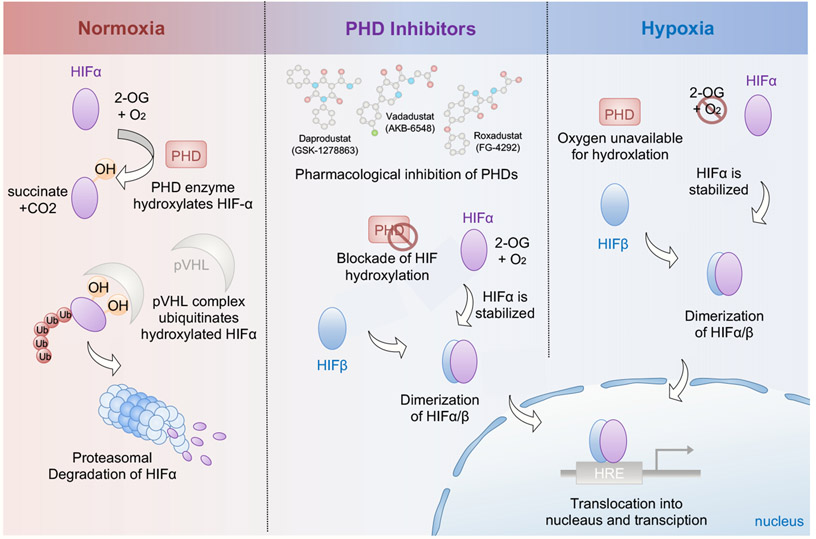
HIFs are basic helix-loop-helix transcription factors composed of two subunits, HIF-α and HIF-β. The stability of HIFs is controlled by oxygen-dependent prolyl hydroxylases (PHDs) and von Hippel–Lindau (VHL), an E3 ubiquitin ligase 178. Under normoxic conditions, HIF-α subunits are hydroxylated by PHDs at two conserved proline residues, inducing their polyubiquitination by VHL and subsequent degradation at the proteasome 179. Factor-inhibiting HIF (FIH), an oxygen-dependent asparaginyl hydroxylase, also regulates hypoxia signaling through hydroxylation of asparaginyl residues of HIF-α subunit, which prevents the association of HIFs with transcription coactivators 180. Under hypoxic conditions, PHDs and FIHs are inactive due to the low availability of oxygen as one of their essential substrates. Consequently, the degradation of the HIF-α subunit is blocked and combines with HIF-β, resulting in a functional heterodimeric transcription factor. Following dimerization, HIF translocates into the nucleus and binds to hypoxia-responsive elements (HREs) in target gene promotors (Figure 5). Several lines of evidence suggest that besides the traditional oxygen-dependent HIF activation, pro-inflammatory factors such as bacterial products or citric acid cycle intermediates, can stabilize HIFs directly 181,182. In models of acute sepsis, lipopolysaccharide (LPS) has been shown to increase HIF-1α stability and decrease PHDs via toll-like receptor 4 under normoxia 181. Other studies show, that hypoxia increases the activity of pro-inflammatory transcription factor NFκB (nuclear-factor-κ-light-chain enhancer of activated B cells) through PHD-dependent hydroxylation of its negative regulator IκB kinase-β 183.
Figure 5: Regulation of Hypoxia-inducible factor (HIF) during normoxia and hypoxia.
Insufficient organ perfusion, respiratory system failure, and anemia can lead to cellular hypoxia. In normoxic conditions, the proline residues of HIFα subunits are constantly hydroxylated by oxygen-dependent prolyl-4-hydroxylases (PHDs). Von Hippel–Lindau protein (pVHL), an E3 ubiquitin ligase, recognizes hydroxylated HIFα and targets it for proteasomal degradation (left panel, light red). When oxygen levels drop (right panel, light blue), molecular O2 as an essential co-substrate for PHDs is unavailable, thereby inhibiting hydroxylase activity. Small PHD-inhibitors, such as roxadustat, vadadustat and daprodustat, block the function of PHDs and can mimic cellular hypoxia (middle). Subsequently, HIFα escapes the PHD-dependent hydroxylation under hypoxic conditions, dimerizes with the HIFβ subunit and translocates into the nucleus. Binding of the HIF-α:HIF-β transcription factor complex to the hypoxia-responsive elements (HREs) in the promoter regions activates target gene expression.
(HIF, hypoxia-inducible factor; 2-OG, 2-oxoglutarate; O2, oxygen, CO2, carbon dioxide, OH, hydroxyl group; PHD, prolyl hydroxylase domain protein; pVHL, von Hippel–Lindau tumor suppressor; Ub, ubiquitin; HRE, hypoxia-responsive element; p300/CBP, p300/CREB-binding protein)
Although hypoxia can generate cytotoxic metabolites that induce pro-inflammatory responses and break down tissue barriers, there are many examples in which stabilization of HIFs induces tissue-protective responses 184. HIF-elicited transcriptional programs have been shown to dampen organ injury in a variety of inflammatory disease models through the production of anti-inflammatory mediators, such as adenosine 185-188. Activated immune cells and damaged epithelia release adenosine triphosphate (ATP) or adenosine diphosphate (ADP) from the intracellular compartment. Hypoxia can induce the expression of a cascade of nucleotide converting enzymes, including ectonucleoside triphosphate diphosphohydrolase 1 (CD39) and ecto-5’ nucleotidase (CD73), both in a HIF-dependent and independent manner 188. The signaling molecule adenosine is the end product of the hydrolysis from adenosine monophosphate (AMP) to adenosine and phosphate by CD73 189. The increase of extracellular adenosine activates protective down-stream signaling via purinergic receptors such as A2A and A2B adenosine receptors (AR), which themselves are known HIF targets 190,191. For example, HIF-dependent adenosine signaling contributes to cardio-protection during myocardial ischemia-reperfusion injury as demonstrated by larger infarct sizes in mice with a genetic deletion of CD39 and CD73, which could be reversed by the administration of AMP or apyrase to CD39-deficient mice or 5’-nucleotidase application to CD73 knockout mice, respectively 192-194. In line with these findings, a protective role of extracellular adenosine during acute lung injury has been suggested. HIF-1α transcriptionally upregulates the immunosuppressive A2B adenosine receptor, and thereby attenuates pulmonary edema, inflammation, and gas exchange 195. Finally, extracellular adenosine levels are regulated by equilibrative nucleoside transporters (ENTs), which terminate purinergic signaling by adenosine re-uptake 190,196. In hypoxic conditions, HIF-dependent transcriptional ENT repression is an innate mechanism to increase extracellular adenosine. Accordingly, pharmacological blockade of ENTs using dipyridamole or nitrobenzylthioinosine, can attenuate ventilator-induced lung injury as well as bacterial gram-negative lung inflammation 190,197.
The understanding that inflammatory lesions are profoundly hypoxic and that the enhancement of adenosine signaling by HIFs is an essential endogenous organprotective response, provides a strong rationale for using pharmacological HIF activators to attenuate organ injury. Remote ischemic preconditioning (RIPC), defined as repeated, short periods of ischemia and reperfusion applied to an extremity (e.g. the arm or the leg) to protect remote tissues during and after prolonged ischemia, was one of the first approaches to make use of this favorable, adaptive response 198. Several preclinical and clinical studies support the concept of RIPC for tissue protection in various target organs, including the heart, kidney, lung, and the brain 199-202. Despite promising results from a multicenter, randomized double-blind trial enrolling 240 patients at high risk for acute kidney injury by Zarbock and colleagues 200, the evidence for RIPC is still inconclusive as demonstrated by two other large clinical studies by Meybohm et al., and Hausenloy et al. 203,204. These conflicting results may reflect the challenges to choose the optimum type, duration, and timing of the ischemic intervention according to patient-related factors such as skeletal muscle mass, hepatic function, and comorbidities. A propofol-based anesthetic technique may as well make a contribution to these findings in that RIPC effectivity has been shown to be reduced by propofol in a preclinical study 205.
Although the molecular mechanisms involved in RIPC remain incompletely understood, there is increasing evidence that HIFs are activated in RIPC leading to the secretion of HIF-dependent cytoprotective molecules in peripheral tissues to protect remote organs, such as IL-10 release from the ischemic limb musculature for cardioprotection 194,199. By using pharmacological HIF activators, HIF-dependent gene expression programs that mimic protective endogenous pathways could be turned on, which may exhibit a more reliable mode of action and allow for dosage titration to overcome the variability of RIPC effects. Normoxic HIF stabilization can be elicited pharmacologically by several classes of compounds, which are mainly PHD inhibitors 206. Blocking the catalytic activity of PHDs efficiently stabilizes HIF1 by preventing its proteasomal degradation (Figure 5). Both, transgenic PHD knockout models and administration of PHD inhibitors, indicated a protective role for HIF activation in various disease models, including acute lung injury, stroke, myocardial ischemia/reperfusion injury, acute kidney dysfunction, liver damage and organ transplantation 194,207-211. Several companies have developed orally available small-molecule inhibitors [roxadustat (FG-4592), vadadustat (AKB-6548), daprodustat (GSK1278863), desidustat (ZYAN1) and molidustat (BAY 85-3934)], which have been established in numerous phase-2 clinical trials as a treatment of anemia and chronic kidney disease (CKD) by increasing endogenous erythropoietin and improving iron metabolism 212-215. Vadadustat, daprodustat, and roxadustat advanced to phase III clinical trials. Meanwhile, roxadustat is the first small-molecule PHD inhibitor approved by China for the treatment of anemia in patients with dialysis-dependent CKD 216. Until now, no major side effects have been reported in these trials. However, given the complexity of HIF target genes, unknown effects of different inhibitor affinities to PHD isoforms, and a potential to interfere with signaling pathways that involve proline-hydroxylation of non-HIF signaling molecules—in particular long-term effects during chronic application—remains an area of investigation 217. Nevertheless, current results from basic research on hypoxia pathways together with clinical trials, provide a strong rationale for implementing HIF activators in the context of perioperative organ injury and encourage novel clinical investigations, particularly as these compounds would be used for short-term organ protection, rather than for long-term use.
Conclusions and future directions
Perioperative organ injury is primarily caused by a dysregulated inflammatory response or insufficient tissue perfusion, and represents a major risk factor for morbidity and mortality in surgical patients. Progressive barrier dysfunction, edema, and subsequent bacterial translocation can transition single-organ injury to multi-organ failure and sepsis.
Patient-centered, multidisciplinary care protocols can contribute to achieve early recovery after surgical procedures by maintaining pre-operative organ function and reducing surgical stress responses, while supportive care on specialized ICUs and extracorporeal organ replacement strategies remain the cornerstone of treating organ failure. However, only few other therapeutic approaches have proven effective in the management of organ dysfunction.
Understanding the molecular basis of inflammatory and oxygen sensing pathways has proven great implications for the field of perioperative medicine. It is now appreciated that inflammatory activation and the initiation of resolution programs overlay in a manner that will determine whether organ injury is repaired or progresses to loss of organ function. Inflamed lesions are often profoundly hypoxic and it is important to emphasize that although hypoxia is a proinflammatory stimulus, stabilization of HIF transcription factors can promote an anti-inflammatory and tissue-protective response. A significant challenge that remains is the movement of this knowledge into clinical practice. The protective effects of remote ischemic preconditioning in various organs has been shown to require the stabilization of HIFs. Recent positive trials of the HIF activator roxadustat 215,218 and vadadustat 219 for renal anemia hopefully give rise to clinical investigations of these compounds for perioperative kidney, cardiac or lung protection in surgical patients soon. Indeed, prophylactic pre-conditioning using HIF activators could represent an innovative therapeutic approach for organ protection. Unfortunately, from our experience it is currently challenging to receive support from pharmaceutical companies for trials in the field of perioperative medicine, as these enterprises prioritize to introduce HIF activators for the treatment of renal anemia. Due to the fact that potential side-effects reported in trials for organ protection could jeopardize marketing of these drugs, the pharmaceutical partners remain hesitant in providing orally available HIF activators for other clinical applications. We hope that such barriers will be overcome in the near future once these compounds have received FDA approval in the USA, to advance these pathways closer to being preventive approaches or treatments for perioperative organ injury.
Acknowledgment
Funding: Deutsche Forschungsgemeinschaft Grant CO 2096/1-1 to C.C. and National Institute of Health Grants R01 DK097075, POI-HL114457, R01-HL109233, R01-DK109574, R01-HL119837 and R01-HL133900 to H.K.E.
Abbreviations
- ADP
Adenosine Diphosphate
- AKI
Acute Kidney Injury
- AKIN
Acute Kidney Injury Network
- ALT
Alanine aminotransferase
- AMP
Adenosine Monophosphate
- Ang-2
Angiopoetin-2
- AR
Adenosine Receptor
- ARDS
Acute Respiratory Distress Syndrome
- ATP
Adenosine Triphosphate
- CC-16
Club cell protein 16
- CK-18
Cytokeratin 18
- CKD
Chronic Kidney Disease
- CK-MB
Creatine kinase myocardial band
- CLIP
Checklist for Lung Injury Prevention
- DAMPs
damage-associated molecular patterns
- ENT
Equilibrative Nucleoside Transporter
- EUPHRATES
Evaluating the Use of Polymyxin B Hemoperfusion in a Randomized Controlled trial of Adults Treated for Endotoxemia and Septic Shock
- FIH
Factor-inhibiting HIF
- GABA
γ-aminobutyric acid
- GFAP
Glial fibrillary acidic protein
- α-GST
α-Glutathione S-transferase
- GPBB
Glycogen phosphorylase isoenzyme BB
- hFABP
Heart-type fatty acid binding protein
- HIF
Hypoxia-inducible factor
- HRE
Hypoxia-responsive elements
- ICAM
Intercellular adhesion molecule-1
- ICU
Intensive Care Unit
- I-FABP
Intestinal fatty acid-binding protein
- KDIGO
Kidney Disease: Improving Global Outcomes
- KIM-1
Kidney Injury Molecule-1
- L-FABP
liver-type fatty acid-binding protein
- LIPS
Lung Injury Prevention Score
- LIPS-A
Lung Injury Prevention Score with Aspirin
- LPS
Lipopolysaccharide
- MAP
Mean Arterial Pressure
- MI
Myocardial Infarction
- NFκB
Nuclear-factor-κ-light-chain enhancer of activated B cells
- NGAL
neutrophil gelatinase-associated lipocalin
- NSE
Neuron specific enolase
- PAMPs
pathogen-associated molecular patterns
- PEEP
Positive End Expiratory Pressure
- PETAL
Prevention & Early Treatment of Acute Lung Injury
- PHD
Prolyl Hydroxylase
- PMI
Perioperative Myocardial Ischemia
- PPR
Pattern Recognition Receptor
- RAGE
Receptor for advanced glycation endproducts
- RIFLE
Risk, Injury, Failure, Loss, End Stage
- RIPC
Remote Ischemic Preconditioning
- UCHL-1
Ubiquitin carboxy-terminal hydrolase L1
- VHL
von Hippel–Lindau
- VILI
Ventilator-induced lung injury
- vWF
von Willebrand factor
Footnotes
Conflicts of Interests/Financial Disclosures: none
References
- 1.Haynes AB, Weiser TG, Berry WR, et al. A surgical safety checklist to reduce morbidity and mortality in a global population. N Engl J Med. 2009;360(5):491–499. [DOI] [PubMed] [Google Scholar]
- 2.Glance LG, Lustik SJ, Hannan EL, et al. The Surgical Mortality Probability Model: derivation and validation of a simple risk prediction rule for noncardiac surgery. Ann Surg. 2012;255(4):696–702. [DOI] [PubMed] [Google Scholar]
- 3.Pearse RM, Moreno RP, Bauer P, et al. Mortality after surgery in Europe: a 7 day cohort study. Lancet. 2012;380(9847):1059–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nepogodiev D, Martin J, Biccard B, Makupe A, Bhangu A, Surgery NIfHRGHRUoG. Global burden of postoperative death. Lancet. 2019;393(10170):401. [DOI] [PubMed] [Google Scholar]
- 5.Rose J, Weiser TG, Hider P, Wilson L, Gruen RL, Bickler SW. Estimated need for surgery worldwide based on prevalence of diseases: a modelling strategy for the WHO Global Health Estimate. Lancet Glob Health. 2015;3 Suppl 2:S13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whitlock EL, Feiner JR, Chen LL. Perioperative Mortality, 2010 to 2014: A Retrospective Cohort Study Using the National Anesthesia Clinical Outcomes Registry. Anesthesiology. 2015;123(6):1312–1321. [DOI] [PubMed] [Google Scholar]
- 7.Ljungqvist O, Scott M, Fearon KC. Enhanced Recovery After Surgery: A Review. JAMA Surg. 2017;152(3):292–298. [DOI] [PubMed] [Google Scholar]
- 8.Bainbridge D, Martin J, Arango M, Cheng D, Group E-bP-oCORE. Perioperative and anaesthetic-related mortality in developed and developing countries: a systematic review and meta-analysis. Lancet. 2012;380(9847):1075–1081. [DOI] [PubMed] [Google Scholar]
- 9.Mashour GA, Shanks AM, Kheterpal S. Perioperative stroke and associated mortality after noncardiac, nonneurologic surgery. Anesthesiology. 2011;114(6):1289–1296. [DOI] [PubMed] [Google Scholar]
- 10.Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology. 2014;120(3):564–578. [DOI] [PubMed] [Google Scholar]
- 11.Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet. 2012;380(9843):756–766. [DOI] [PubMed] [Google Scholar]
- 12.Slinger P Perioperative lung injury. Best Pract Res Clin Anaesthesiol. 2008;22(1):177–191. [DOI] [PubMed] [Google Scholar]
- 13.Klingensmith NJ, Coopersmith CM. The Gut as the Motor of Multiple Organ Dysfunction in Critical Illness. Crit Care Clin. 2016;32(2):203–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mayo SC, Shore AD, Nathan H, et al. Refining the definition of perioperative mortality following hepatectomy using death within 90 days as the standard criterion. HPB (Oxford). 2011;13(7):473–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huber-Lang M, Lambris JD, Ward PA. Innate immune responses to trauma. Nat Immunol. 2018;19(4):327–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campbell EL, Bruyninckx WJ, Kelly CJ, et al. Transmigrating neutrophils shape the mucosal microenvironment through localized oxygen depletion to influence resolution of inflammation. Immunity. 2014;40(1):66–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koeppen M, Eckle T, Eltzschig HK. The hypoxia-inflammation link and potential drug targets. Curr Opin Anaesthesiol. 2011;24(4):363–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakr Y, Lobo SM, Moreno RP, et al. Patterns and early evolution of organ failure in the intensive care unit and their relation to outcome. Crit Care. 2012;16(6):R222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bucerius J, Gummert JF, Borger MA, et al. Stroke after cardiac surgery: a risk factor analysis of 16,184 consecutive adult patients. Ann Thorac Surg. 2003;75(2):472–478. [DOI] [PubMed] [Google Scholar]
- 20.McKhann GM, Grega MA, Borowicz LM, Baumgartner WA, Selnes OA. Stroke and encephalopathy after cardiac surgery: an update. Stroke. 2006;37(2):562–571. [DOI] [PubMed] [Google Scholar]
- 21.Bonati LH, Dobson J, Featherstone RL, et al. Long-term outcomes after stenting versus endarterectomy for treatment of symptomatic carotid stenosis: the International Carotid Stenting Study (ICSS) randomised trial. Lancet. 2015;385(9967):529–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nosan DK, Gomez CR, Maves MD. Perioperative stroke in patients undergoing head and neck surgery. Ann Otol Rhinol Laryngol. 1993;102(9):717–723. [DOI] [PubMed] [Google Scholar]
- 23.Gustafson Y, Berggren D, Brännström B, et al. Acute confusional states in elderly patients treated for femoral neck fracture. J Am Geriatr Soc. 1988;36(6):525–530. [DOI] [PubMed] [Google Scholar]
- 24.Böhner H, Hummel TC, Habel U, et al. Predicting delirium after vascular surgery: a model based on pre- and intraoperative data. Ann Surg. 2003;238(1):149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benoit AG, Campbell BI, Tanner JR, et al. Risk factors and prevalence of perioperative cognitive dysfunction in abdominal aneurysm patients. J Vasc Surg. 2005;42(5):884–890. [DOI] [PubMed] [Google Scholar]
- 26.Devereaux PJ, Xavier D, Pogue J, et al. Characteristics and short-term prognosis of perioperative myocardial infarction in patients undergoing noncardiac surgery: a cohort study. Ann Intern Med. 2011;154(8):523–528. [DOI] [PubMed] [Google Scholar]
- 27.Parashar A, Agarwal S, Krishnaswamy A, et al. Percutaneous Intervention for Myocardial Infarction After Noncardiac Surgery: Patient Characteristics and Outcomes. J Am Coll Cardiol. 2016;68(4):329–338. [DOI] [PubMed] [Google Scholar]
- 28.Beaulieu RJ, Sutzko DC, Albright J, Jeruzal E, Osborne NH, Henke PK. Association of High Mortality With Postoperative Myocardial Infarction After Major Vascular Surgery Despite Use of Evidence-Based Therapies. JAMA Surg. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arozullah AM, Daley J, Henderson WG, Khuri SF. Multifactorial risk index for predicting postoperative respiratory failure in men after major noncardiac surgery. The National Veterans Administration Surgical Quality Improvement Program. Ann Surg. 2000;232(2):242–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Canet J, Gallart L, Gomar C, et al. Prediction of postoperative pulmonary complications in a population-based surgical cohort. Anesthesiology. 2010;113(6):1338–1350. [DOI] [PubMed] [Google Scholar]
- 31.Miskovic A, Lumb AB. Postoperative pulmonary complications. Br J Anaesth. 2017;118(3):317–334. [DOI] [PubMed] [Google Scholar]
- 32.Hoste EA, Kellum JA, Katz NM, Rosner MH, Haase M, Ronco C. Epidemiology of acute kidney injury. Contrib Nephrol. 2010;165:1–8. [DOI] [PubMed] [Google Scholar]
- 33.van Beek SC, Legemate DA, Vahl A, et al. Acute kidney injury defined according to the 'Risk,' 'Injury,' 'Failure,' 'Loss,' and 'End-stage' (RIFLE) criteria after repair for a ruptured abdominal aortic aneurysm. J Vasc Surg. 2014;60(5):1159–1167.e1151. [DOI] [PubMed] [Google Scholar]
- 34.Gameiro J, Fonseca JA, Neves M, Jorge S, Lopes JA. Acute kidney injury in major abdominal surgery: incidence, risk factors, pathogenesis and outcomes. Ann Intensive Care. 2018;8(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Northup PG, Friedman LS, Kamath PS. AGA Clinical Practice Update on Surgical Risk Assessment and Perioperative Management in Cirrhosis: Expert Review. Clin Gastroenterol Hepatol. 2019;17(4):595–606. [DOI] [PubMed] [Google Scholar]
- 36.Lorusso R, Mariscalco G, Vizzardi E, Bonadei I, Renzulli A, Gelsomino S. Acute bowel ischemia after heart operations. Ann Thorac Surg. 2014;97(6):2219–2227. [DOI] [PubMed] [Google Scholar]
- 37.Ultee KH, Zettervall SL, Soden PA, et al. Incidence of and risk factors for bowel ischemia after abdominal aortic aneurysm repair. J Vasc Surg. 2016;64(5):1384–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Devereaux PJ, Biccard BM, Sigamani A, et al. Association of Postoperative High-Sensitivity Troponin Levels With Myocardial Injury and 30-Day Mortality Among Patients Undergoing Noncardiac Surgery. JAMA. 2017;317(16):1642–1651. [DOI] [PubMed] [Google Scholar]
- 39.Brown JC, Samaha E, Rao S, et al. High-Sensitivity Cardiac Troponin T Improves the Diagnosis of Perioperative MI. Anesth Analg. 2017;125(5):1455–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Waes JA, Peelen LM, Kemperman H, Grobben RB, Nathoe HM, van Klei WA. Kinetics of troponin I in patients with myocardial injury after noncardiac surgery. Clin Chem Lab Med. 2017;55(4):586–594. [DOI] [PubMed] [Google Scholar]
- 41.Landesberg G, Luria MH, Cotev S, et al. Importance of long-duration postoperative ST-segment depression in cardiac morbidity after vascular surgery. Lancet. 1993;341(8847):715–719. [DOI] [PubMed] [Google Scholar]
- 42.Badner NH, Knill RL, Brown JE, Novick TV, Gelb AW. Myocardial infarction after noncardiac surgery. Anesthesiology. 1998;88(3):572–578. [DOI] [PubMed] [Google Scholar]
- 43.Landesberg G The pathophysiology of perioperative myocardial infarction: facts and perspectives. J Cardiothorac Vasc Anesth. 2003;17(1):90–100. [DOI] [PubMed] [Google Scholar]
- 44.Walsh M, Devereaux PJ, Garg AX, et al. Relationship between intraoperative mean arterial pressure and clinical outcomes after noncardiac surgery: toward an empirical definition of hypotension. Anesthesiology. 2013;119(3):507–515. [DOI] [PubMed] [Google Scholar]
- 45.Salmasi V, Maheshwari K, Yang D, et al. Relationship between Intraoperative Hypotension, Defined by Either Reduction from Baseline or Absolute Thresholds, and Acute Kidney and Myocardial Injury after Noncardiac Surgery: A Retrospective Cohort Analysis. Anesthesiology. 2017;126(1):47–65. [DOI] [PubMed] [Google Scholar]
- 46.Pagel PS, Crystal GJ. The Discovery of Myocardial Preconditioning Using Volatile Anesthetics: A History and Contemporary Clinical Perspective. J Cardiothorac Vasc Anesth. 2018;32(3):1112–1134. [DOI] [PubMed] [Google Scholar]
- 47.Lurati Buse GA, Schumacher P, Seeberger E, et al. Randomized comparison of sevoflurane versus propofol to reduce perioperative myocardial ischemia in patients undergoing noncardiac surgery. Circulation. 2012;126(23):2696–2704. [DOI] [PubMed] [Google Scholar]
- 48.Landoni G, Lomivorotov VV, Nigro Neto C, et al. Volatile Anesthetics versus Total Intravenous Anesthesia for Cardiac Surgery. N Engl J Med. 2019;380(13):1214–1225. [DOI] [PubMed] [Google Scholar]
- 49.De Hert S, Moerman A. Anesthetic Preconditioning: Have We Found the Holy Grail of Perioperative Cardioprotection? J Cardiothorac Vasc Anesth. 2018;32(3):1135–1136. [DOI] [PubMed] [Google Scholar]
- 50.Mashour GA, Moore LE, Lele AV, Robicsek SA, Gelb AW. Perioperative care of patients at high risk for stroke during or after non-cardiac, non-neurologic surgery: consensus statement from the Society for Neuroscience in Anesthesiology and Critical Care*. J Neurosurg Anesthesiol. 2014;26(4):273–285. [DOI] [PubMed] [Google Scholar]
- 51.Bateman BT, Schumacher HC, Wang S, Shaefi S, Berman MF. Perioperative acute ischemic stroke in noncardiac and nonvascular surgery: incidence, risk factors, and outcomes. Anesthesiology. 2009;110(2):231–238. [DOI] [PubMed] [Google Scholar]
- 52.Saltman AP, Silver FL, Fang J, Stamplecoski M, Kapral MK. Care and Outcomes of Patients With In-Hospital Stroke. JAMA Neurol. 2015;72(7):749–755. [DOI] [PubMed] [Google Scholar]
- 53.Kaatz S, Douketis JD, Zhou H, Gage BF, White RH. Risk of stroke after surgery in patients with and without chronic atrial fibrillation. J Thromb Haemost. 2010;8(5):884–890. [DOI] [PubMed] [Google Scholar]
- 54.Likosky DS, Marrin CA, Caplan LR, et al. Determination of etiologic mechanisms of strokes secondary to coronary artery bypass graft surgery. Stroke. 2003;34(12):2830–2834. [DOI] [PubMed] [Google Scholar]
- 55.Devereaux PJ, Yang H, Yusuf S, et al. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet. 2008;371(9627):1839–1847. [DOI] [PubMed] [Google Scholar]
- 56.Ashes C, Judelman S, Wijeysundera DN, et al. Selective β1-antagonism with bisoprolol is associated with fewer postoperative strokes than atenolol or metoprolol: a single-center cohort study of 44,092 consecutive patients. Anesthesiology. 2013;119(4):777–787. [DOI] [PubMed] [Google Scholar]
- 57.Bijker JB, Persoon S, Peelen LM, et al. Intraoperative hypotension and perioperative ischemic stroke after general surgery: a nested case-control study. Anesthesiology. 2012;116(3):658–664. [DOI] [PubMed] [Google Scholar]
- 58.Gleason LJ, Schmitt EM, Kosar CM, et al. Effect of Delirium and Other Major Complications on Outcomes After Elective Surgery in Older Adults. JAMA Surg. 2015;150(12):1134–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA. 2001;286(21):2703–2710. [DOI] [PubMed] [Google Scholar]
- 60.Daiello LA, Racine AM, Yun Gou R, et al. Postoperative Delirium and Postoperative Cognitive Dysfunction: Overlap and Divergence. Anesthesiology. 2019;131(3):477–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim SW, Han HS, Jung HW, et al. Multidimensional frailty score for the prediction of postoperative mortality risk. JAMA Surg. 2014;149(7):633–640. [DOI] [PubMed] [Google Scholar]
- 62.Rudolph JL, Inouye SK, Jones RN, et al. Delirium: an independent predictor of functional decline after cardiac surgery. J Am Geriatr Soc. 2010;58(4):643–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pisani MA, Kong SY, Kasl SV, Murphy TE, Araujo KL, Van Ness PH. Days of delirium are associated with 1-year mortality in an older intensive care unit population. Am J Respir Crit Care Med. 2009;180(11):1092–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Witlox J, Eurelings LS, de Jonghe JF, Kalisvaart KJ, Eikelenboom P, van Gool WA. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA. 2010;304(4):443–451. [DOI] [PubMed] [Google Scholar]
- 65.Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Su X, Meng ZT, Wu XH, et al. Dexmedetomidine for prevention of delirium in elderly patients after non-cardiac surgery: a randomised, double-blind, placebo-controlled trial. Lancet. 2016;388(10054):1893–1902. [DOI] [PubMed] [Google Scholar]
- 67.Subramaniam B, Shankar P, Shaefi S, et al. Effect of Intravenous Acetaminophen vs Placebo Combined With Propofol or Dexmedetomidine on Postoperative Delirium Among Older Patients Following Cardiac Surgery: The DEXACET Randomized Clinical Trial. JAMA. 2019;321(7):686–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Leung JM, Sands LP, Chen N, et al. Perioperative Gabapentin Does Not Reduce Postoperative Delirium in Older Surgical Patients: A Randomized Clinical Trial. Anesthesiology. 2017;127(4):633–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Avidan MS, Maybrier HR, Abdallah AB, et al. Intraoperative ketamine for prevention of postoperative delirium or pain after major surgery in older adults: an international, multicentre, double-blind, randomised clinical trial. Lancet. 2017;390(10091):267–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pandharipande PP, Pun BT, Herr DL, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. 2007;298(22):2644–2653. [DOI] [PubMed] [Google Scholar]
- 71.Riker RR, Shehabi Y, Bokesch PM, et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. 2009;301(5):489–499. [DOI] [PubMed] [Google Scholar]
- 72.Shehabi Y, Howe BD, Bellomo R, et al. Early Sedation with Dexmedetomidine in Critically Ill Patients. N Engl J Med. 2019;380(26):2506–2517. [DOI] [PubMed] [Google Scholar]
- 73.Coburn M, Sanders RD, Maze M, et al. The hip fracture surgery in elderly patients (HIPELD) study to evaluate xenon anaesthesia for the prevention of postoperative delirium: a multicentre, randomized clinical trial. Br J Anaesth. 2018;120(1):127–137. [DOI] [PubMed] [Google Scholar]
- 74.Oh CS, Park S, Wan Hong S, Kang WS, Yoon TG, Kim SH. Postoperative Delirium in Patients Undergoing Off-Pump Coronary Artery Bypass Grafting According to the Anesthetic Agent: A Retrospective Study. J Cardiothorac Vasc Anesth. 2017;31(6):1988–1995. [DOI] [PubMed] [Google Scholar]
- 75.Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med. 2014;370(10):980. [DOI] [PubMed] [Google Scholar]
- 76.Fan E, Villar J, Slutsky AS. Novel approaches to minimize ventilator-induced lung injury. BMC Med. 2013;11:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Laffey JG, Kavanagh BP. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury. N Engl J Med. 2000;343(11):812; author reply 813-814. [DOI] [PubMed] [Google Scholar]
- 78.Futier E, Constantin JM, Paugam-Burtz C, et al. A trial of intraoperative low-tidal-volume ventilation in abdominal surgery. N Engl J Med. 2013;369(5):428–437. [DOI] [PubMed] [Google Scholar]
- 79.Güldner A, Kiss T, Serpa Neto A, et al. Intraoperative protective mechanical ventilation for prevention of postoperative pulmonary complications: a comprehensive review of the role of tidal volume, positive end-expiratory pressure, and lung recruitment maneuvers. Anesthesiology. 2015;123(3):692–713. [DOI] [PubMed] [Google Scholar]
- 80.Neto AS, Hemmes SN, Barbas CS, et al. Association between driving pressure and development of postoperative pulmonary complications in patients undergoing mechanical ventilation for general anaesthesia: a meta-analysis of individual patient data. Lancet Respir Med. 2016;4(4):272–280. [DOI] [PubMed] [Google Scholar]
- 81.Cressoni M, Gotti M, Chiurazzi C, et al. Mechanical Power and Development of Ventilator-induced Lung Injury. Anesthesiology. 2016;124(5):1100–1108. [DOI] [PubMed] [Google Scholar]
- 82.Marini JJ, Gattinoni L. Energetics and the Root Mechanical Cause for Ventilator-induced Lung Injury. Anesthesiology. 2018;128(6):1062–1064. [DOI] [PubMed] [Google Scholar]
- 83.Collino F, Rapetti F, Vasques F, et al. Positive End-expiratory Pressure and Mechanical Power. Anesthesiology. 2019;130(1):119–130. [DOI] [PubMed] [Google Scholar]
- 84.Huhle R, Serpa Neto A, Schultz MJ, Gama de Abreu M. Is mechanical power the final word on ventilator-induced lung injury?-no. Ann Transl Med. 2018;6(19):394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gattinoni L, Tonetti T, Quintel M. Intensive care medicine in 2050: ventilator-induced lung injury. Intensive Care Med. 2018;44(1):76–78. [DOI] [PubMed] [Google Scholar]
- 86.Marini JJ, Rocco PRM, Gattinoni L. Static and Dynamic Contributors to Ventilator-induced Lung Injury in Clinical Practice. Pressure, Energy, and Power. Am J Respir Crit Care Med. 2020;201(7):767–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Blum JM, Stentz MJ, Dechert R, et al. Preoperative and intraoperative predictors of postoperative acute respiratory distress syndrome in a general surgical population. Anesthesiology. 2013;118(1):19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bellani G, Laffey JG, Pham T, et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA. 2016;315(8):788–800. [DOI] [PubMed] [Google Scholar]
- 89.Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–2533. [DOI] [PubMed] [Google Scholar]
- 90.Rezoagli E, Fumagalli R, Bellani G. Definition and epidemiology of acute respiratory distress syndrome. Ann Transl Med. 2017;5(14):282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tremblay L, Valenza F, Ribeiro SP, Li J, Slutsky AS. Injurious ventilatory strategies increase cytokines and c-fos m-RNA expression in an isolated rat lung model. J Clin Invest. 1997;99(5):944–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354(24):2564–2575. [DOI] [PubMed] [Google Scholar]
- 93.Guérin C, Reignier J, Richard JC, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368(23):2159–2168. [DOI] [PubMed] [Google Scholar]
- 94.Combes A, Hajage D, Capellier G, et al. Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome. N Engl J Med. 2018;378(21):1965–1975. [DOI] [PubMed] [Google Scholar]
- 95.Brower RG, Lanken PN, MacIntyre N, et al. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351(4):327–336. [DOI] [PubMed] [Google Scholar]
- 96.Cavalcanti AB, Suzumura É, Laranjeira LN, et al. Effect of Lung Recruitment and Titrated Positive End-Expiratory Pressure (PEEP) vs Low PEEP on Mortality in Patients With Acute Respiratory Distress Syndrome: A Randomized Clinical Trial. JAMA. 2017;318(14):1335–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Amato MB, Meade MO, Slutsky AS, et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med. 2015;372(8):747–755. [DOI] [PubMed] [Google Scholar]
- 98.Matthay MA, McAuley DF, Ware LB. Clinical trials in acute respiratory distress syndrome: challenges and opportunities. Lancet Respir Med. 2017;5(6):524–534. [DOI] [PubMed] [Google Scholar]
- 99.Kor DJ, Talmor DS, Banner-Goodspeed VM, et al. Lung Injury Prevention with Aspirin (LIPS-A): a protocol for a multicentre randomised clinical trial in medical patients at high risk of acute lung injury. BMJ Open. 2012;2(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gajic O, Dabbagh O, Park PK, et al. Early identification of patients at risk of acute lung injury: evaluation of lung injury prediction score in a multicenter cohort study. Am J Respir Crit Care Med. 2011;183(4):462–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Moss M, Huang DT, Brower RG, et al. Early Neuromuscular Blockade in the Acute Respiratory Distress Syndrome. N Engl J Med. 2019;380(21):1997–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Truwit JD, Bernard GR, Steingrub J, et al. Rosuvastatin for sepsis-associated acute respiratory distress syndrome. N Engl J Med. 2014;370(23):2191–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fowler AA, Truwit JD, Hite RD, et al. Effect of Vitamin C Infusion on Organ Failure and Biomarkers of Inflammation and Vascular Injury in Patients With Sepsis and Severe Acute Respiratory Failure: The CITRIS-ALI Randomized Clinical Trial. JAMA. 2019;322(13):1261–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ginde AA, Brower RG, Caterino JM, et al. Early High-Dose Vitamin D. N Engl J Med. 2019;381(26):2529–2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Looney MR, Nguyen JX, Hu Y, Van Ziffle JA, Lowell CA, Matthay MA. Platelet depletion and aspirin treatment protect mice in a two-event model of transfusion-related acute lung injury. J Clin Invest. 2009;119(11):3450–3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kor DJ, Carter RE, Park PK, et al. Effect of Aspirin on Development of ARDS in At-Risk Patients Presenting to the Emergency Department: The LIPS-A Randomized Clinical Trial. JAMA. 2016;315(22):2406–2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Calfee CS, Delucchi K, Parsons PE, et al. Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med. 2014;2(8):611–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wilson JG, Calfee CS. ARDS Subphenotypes: Understanding a Heterogeneous Syndrome. Crit Care. 2020;24(1):102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ricci Z, Cruz DN, Ronco C. Classification and staging of acute kidney injury: beyond the RIFLE and AKIN criteria. Nat Rev Nephrol. 2011;7(4):201–208. [DOI] [PubMed] [Google Scholar]
- 110.Mehta RL, Pascual MT, Soroko S, et al. Spectrum of acute renal failure in the intensive care unit: the PICARD experience. Kidney Int. 2004;66(4):1613–1621. [DOI] [PubMed] [Google Scholar]
- 111.Kork F, Balzer F, Spies CD, et al. Minor Postoperative Increases of Creatinine Are Associated with Higher Mortality and Longer Hospital Length of Stay in Surgical Patients. Anesthesiology. 2015;123(6):1301–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang HE, Muntner P, Chertow GM, Warnock DG. Acute kidney injury and mortality in hospitalized patients. Am J Nephrol. 2012;35(4):349–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kolhe NV, Muirhead AW, Wilkes SR, Fluck RJ, Taal MW. The epidemiology of hospitalised acute kidney injury not requiring dialysis in England from 1998 to 2013: retrospective analysis of hospital episode statistics. Int J Clin Pract. 2016;70(4):330–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kolhe NV, Muirhead AW, Wilkes SR, Fluck RJ, Taal MW. National trends in acute kidney injury requiring dialysis in England between 1998 and 2013. Kidney Int. 2015;88(5):1161–1169. [DOI] [PubMed] [Google Scholar]
- 115.Kheterpal S, Tremper KK, Heung M, et al. Development and validation of an acute kidney injury risk index for patients undergoing general surgery: results from a national data set. Anesthesiology. 2009;110(3):505–515. [DOI] [PubMed] [Google Scholar]
- 116.Thakar CV, Kharat V, Blanck S, Leonard AC. Acute kidney injury after gastric bypass surgery. Clin J Am Soc Nephrol. 2007;2(3):426–430. [DOI] [PubMed] [Google Scholar]
- 117.Bihorac A, Delano MJ, Schold JD, et al. Incidence, clinical predictors, genomics, and outcome of acute kidney injury among trauma patients. Ann Surg. 2010;252(1):158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cabezuelo JB, Ramírez P, Ríos A, et al. Risk factors of acute renal failure after liver transplantation. Kidney Int. 2006;69(6):1073–1080. [DOI] [PubMed] [Google Scholar]
- 119.Rhee CJ, Kibler KK, Easley RB, et al. Renovascular reactivity measured by near-infrared spectroscopy. J Appl Physiol (1985). 2012;113(2):307–314. [DOI] [PubMed] [Google Scholar]
- 120.Meersch M, Schmidt C, Zarbock A. Perioperative Acute Kidney Injury: An Under-Recognized Problem. Anesth Analg. 2017;125(4):1223–1232. [DOI] [PubMed] [Google Scholar]
- 121.Victorino F, Sojka DK, Brodsky KS, et al. Tissue-Resident NK Cells Mediate Ischemic Kidney Injury and Are Not Depleted by Anti-Asialo-GM1 Antibody. J Immunol. 2015;195(10):4973–4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mehran R, Dangas GD, Weisbord SD. Contrast-Associated Acute Kidney Injury. N Engl J Med. 2019;380(22):2146–2155. [DOI] [PubMed] [Google Scholar]
- 123.Futier E, Lefrant JY, Guinot PG, et al. Effect of Individualized vs Standard Blood Pressure Management Strategies on Postoperative Organ Dysfunction Among High-Risk Patients Undergoing Major Surgery: A Randomized Clinical Trial. JAMA. 2017;318(14):1346–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sun LY, Wijeysundera DN, Tait GA, Beattie WS. Association of intraoperative hypotension with acute kidney injury after elective noncardiac surgery. Anesthesiology. 2015;123(3):515–523. [DOI] [PubMed] [Google Scholar]
- 125.Myles PS, Bellomo R, Corcoran T, et al. Restrictive versus Liberal Fluid Therapy for Major Abdominal Surgery. N Engl J Med. 2018;378(24):2263–2274. [DOI] [PubMed] [Google Scholar]
- 126.Kellum JA, Bellomo R, Ronco C. Progress in Prevention and Treatment of Acute Kidney Injury: Moving Beyond Kidney Attack. JAMA. 2018;320(5):437–438. [DOI] [PubMed] [Google Scholar]
- 127.Garg AX, Kurz A, Sessler DI, et al. Perioperative aspirin and clonidine and risk of acute kidney injury: a randomized clinical trial. JAMA. 2014;312(21):2254–2264. [DOI] [PubMed] [Google Scholar]
- 128.Zheng Z, Jayaram R, Jiang L, et al. Perioperative Rosuvastatin in Cardiac Surgery. N Engl J Med. 2016;374(18):1744–1753. [DOI] [PubMed] [Google Scholar]
- 129.Jaber S, Paugam C, Futier E, et al. Sodium bicarbonate therapy for patients with severe metabolic acidaemia in the intensive care unit (BICAR-ICU): a multicentre, open-label, randomised controlled, phase 3 trial. Lancet. 2018;392(10141):31–40. [DOI] [PubMed] [Google Scholar]
- 130.Barba-Navarro R, Tapia-Silva M, Garza-Garcia C, et al. The Effect of Spironolactone on Acute Kidney Injury After Cardiac Surgery: A Randomized, Placebo-Controlled Trial. Am J Kidney Dis. 2017;69(2):192–199. [DOI] [PubMed] [Google Scholar]
- 131.Himmelfarb J, Chertow GM, McCullough PA, et al. Perioperative THR-184 and AKI after Cardiac Surgery. J Am Soc Nephrol. 2018;29(2):670–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zarbock A, Mehta RL. Timing of Kidney Replacement Therapy in Acute Kidney Injury. Clin J Am Soc Nephrol. 2019;14(1):147–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gumbert SD, Kork F, Jackson ML, et al. Perioperative Acute Kidney Injury. Anesthesiology. 2020;132(1):180–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Carrico CJ, Meakins JL, Marshall JC, Fry D, Maier RV. Multiple-organ-failure syndrome. Arch Surg. 1986;121(2):196–208. [DOI] [PubMed] [Google Scholar]
- 135.Leone M, Bechis C, Baumstarck K, et al. Outcome of acute mesenteric ischemia in the intensive care unit: a retrospective, multicenter study of 780 cases. Intensive Care Med. 2015;41(4):667–676. [DOI] [PubMed] [Google Scholar]
- 136.Acosta S, Ogren M, Sternby NH, Bergqvist D, Björck M. Fatal nonocclusive mesenteric ischaemia: population-based incidence and risk factors. J Intern Med. 2006;259(3):305–313. [DOI] [PubMed] [Google Scholar]
- 137.Deitch EA. Gut-origin sepsis: evolution of a concept. Surgeon. 2012;10(6):350–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Haak BW, Wiersinga WJ. The role of the gut microbiota in sepsis. Lancet Gastroenterol Hepatol. 2017;2(2):135–143. [DOI] [PubMed] [Google Scholar]
- 139.Schuijt TJ, Lankelma JM, Scicluna BP, et al. The gut microbiota plays a protective role in the host defence against pneumococcal pneumonia. Gut. 2016;65(4):575–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Schirmer M, Smeekens SP, Vlamakis H, et al. Linking the Human Gut Microbiome to Inflammatory Cytokine Production Capacity. Cell. 2016;167(7):1897. [DOI] [PubMed] [Google Scholar]
- 141.Zhang D, Chen G, Manwani D, et al. Neutrophil ageing is regulated by the microbiome. Nature. 2015;525(7570):528–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cisalpino D, Fagundes CT, Brito CB, et al. Microbiota-Induced Antibodies Are Essential for Host Inflammatory Responsiveness to Sterile and Infectious Stimuli. J Immunol. 2017;198(10):4096–4106. [DOI] [PubMed] [Google Scholar]
- 143.Portincasa P The two congested failing giants: heart and liver. Intern Emerg Med. 2019;14(6):907–910. [DOI] [PubMed] [Google Scholar]
- 144.Sato K, Kawamura T, Wakusawa R. Hepatic blood flow and function in elderly patients undergoing laparoscopic cholecystectomy. Anesth Analg. 2000;90(5):1198–1202. [DOI] [PubMed] [Google Scholar]
- 145.Friedman LS. Surgery in the patient with liver disease. Trans Am Clin Climatol Assoc. 2010;121:192–204; discussion 205. [PMC free article] [PubMed] [Google Scholar]
- 146.Dar WA, Sullivan E, Bynon JS, Eltzschig H, Ju C. Ischaemia reperfusion injury in liver transplantation: Cellular and molecular mechanisms. Liver Int. 2019;39(5):788–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gao RY, Wang M, Liu Q, et al. Hypoxia-Inducible Factor-2α Reprograms Liver Macrophages to Protect Against Acute Liver Injury Through the Production of Interleukin-6. Hepatology. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ju C, Colgan SP, Eltzschig HK. Hypoxia-inducible factors as molecular targets for liver diseases. J Mol Med (Berl). 2016;94(6):613–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Farges O, Belghiti J, Kianmanesh R, et al. Portal vein embolization before right hepatectomy: prospective clinical trial. Ann Surg. 2003;237(2):208–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Murata S, Ohkohchi N, Matsuo R, Ikeda O, Myronovych A, Hoshi R. Platelets promote liver regeneration in early period after hepatectomy in mice. World J Surg. 2007;31(4):808–816. [DOI] [PubMed] [Google Scholar]
- 151.Matsuo R, Nakano Y, Ohkohchi N. Platelet administration via the portal vein promotes liver regeneration in rats after 70% hepatectomy. Ann Surg. 2011;253(4):759–763. [DOI] [PubMed] [Google Scholar]
- 152.Kim J, Yi NJ, Shin WY, Kim T, Lee KU, Suh KS. Platelet transfusion can be related to liver regeneration after living donor liver transplantation. World J Surg. 2010;34(5):1052–1058. [DOI] [PubMed] [Google Scholar]
- 153.Cescon M, Sugawara Y, Takayama T, et al. Role of splenectomy in living-donor liver transplantation for adults. Hepatogastroenterology. 2002;49(45):721–723. [PubMed] [Google Scholar]
- 154.Meyer J, Lejmi E, Fontana P, Morel P, Gonelle-Gispert C, Bühler L. A focus on the role of platelets in liver regeneration: Do platelet-endothelial cell interactions initiate the regenerative process? J Hepatol. 2015;63(5):1263–1271. [DOI] [PubMed] [Google Scholar]
- 155.Eltzschig HK, Eckle T. Ischemia and reperfusion—from mechanism to translation. Nature Medicine. 2011;17:1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.McDonald B, Pittman K, Menezes GB, et al. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science. 2010;330(6002):362–366. [DOI] [PubMed] [Google Scholar]
- 157.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140(6):805–820. [DOI] [PubMed] [Google Scholar]
- 158.Huang C, Niethammer P. Tissue Damage Signaling Is a Prerequisite for Protective Neutrophil Recruitment to Microbial Infection in Zebrafish. Immunity. 2018;48(5):1006–1013.e1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kang JH, Super M, Yung CW, et al. An extracorporeal blood-cleansing device for sepsis therapy. Nat Med. 2014;20(10):1211–1216. [DOI] [PubMed] [Google Scholar]
- 160.Dellinger RP, Bagshaw SM, Antonelli M, et al. Effect of Targeted Polymyxin B Hemoperfusion on 28-Day Mortality in Patients With Septic Shock and Elevated Endotoxin Level: The EUPHRATES Randomized Clinical Trial. JAMA. 2018;320(14):1455–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bone RC. Toward an epidemiology and natural history of SIRS (systemic inflammatory response syndrome). JAMA. 1992;268(24):3452–3455. [PubMed] [Google Scholar]
- 162.Blum CA, Nigro N, Briel M, et al. Adjunct prednisone therapy for patients with community-acquired pneumonia: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet. 2015;385(9977):1511–1518. [DOI] [PubMed] [Google Scholar]
- 163.Venkatesh B, Finfer S, Cohen J, et al. Adjunctive Glucocorticoid Therapy in Patients with Septic Shock. N Engl J Med. 2018;378(9):797–808. [DOI] [PubMed] [Google Scholar]
- 164.Annane D, Renault A, Brun-Buisson C, et al. Hydrocortisone plus Fludrocortisone for Adults with Septic Shock. N Engl J Med. 2018;378(9):809–818. [DOI] [PubMed] [Google Scholar]
- 165.Proudfoot A, Bayliffe A, O'Kane CM, et al. Novel anti-tumour necrosis factor receptor-1 (TNFR1) domain antibody prevents pulmonary inflammation in experimental acute lung injury. Thorax. 2018;73(8):723–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Orrem HL, Nilsson PH, Pischke SE, et al. IL-6 Receptor Inhibition by Tocilizumab Attenuated Expression of C5a Receptor 1 and 2 in Non-ST-Elevation Myocardial Infarction. Front Immunol. 2018;9:2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Holte E, Kleveland O, Ueland T, et al. Effect of interleukin-6 inhibition on coronary microvascular and endothelial function in myocardial infarction. Heart. 2017;103(19):1521–1527. [DOI] [PubMed] [Google Scholar]
- 168.Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med. 2017;377(12):1119–1131. [DOI] [PubMed] [Google Scholar]
- 169.Smith CJ, Hulme S, Vail A, et al. SCIL-STROKE (Subcutaneous Interleukin-1 Receptor Antagonist in Ischemic Stroke): A Randomized Controlled Phase 2 Trial. Stroke. 2018;49(5):1210–1216. [DOI] [PubMed] [Google Scholar]
- 170.Wang J, Hossain M, Thanabalasuriar A, Gunzer M, Meininger C, Kubes P. Visualizing the function and fate of neutrophils in sterile injury and repair. Science. 2017;358(6359):111–116. [DOI] [PubMed] [Google Scholar]
- 171.Peiseler M, Kubes P. More friend than foe: the emerging role of neutrophils in tissue repair. J Clin Invest. 2019;129(7):2629–2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Neudecker V, Brodsky KS, Clambey ET, et al. Neutrophil transfer of. Sci Transl Med. 2017;9(408). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6(12):1191–1197. [DOI] [PubMed] [Google Scholar]
- 174.Cauwe B, Martens E, Proost P, Opdenakker G. Multidimensional degradomics identifies systemic autoantigens and intracellular matrix proteins as novel gelatinase B/MMP-9 substrates. Integr Biol (Camb). 2009;1(5-6):404–426. [DOI] [PubMed] [Google Scholar]
- 175.Yang W, Tao Y, Wu Y, et al. Neutrophils promote the development of reparative macrophages mediated by ROS to orchestrate liver repair. Nat Commun. 2019;10(1):1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Horckmans M, Ring L, Duchene J, et al. Neutrophils orchestrate post-myocardial infarction healing by polarizing macrophages towards a reparative phenotype. Eur Heart J. 2017;38(3):187–197. [DOI] [PubMed] [Google Scholar]
- 177.Bowser JL, Lee JW, Yuan X, Eltzschig HK. The hypoxia-adenosine link during inflammation. J Appl Physiol (1985). 2017;123(5):1303–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lee P, Chandel NS, Simon MC. Cellular adaptation to hypoxia through hypoxia inducible factors and beyond. Nat Rev Mol Cell Biol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ivan M, Kondo K, Yang H, et al. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292(5516):464–468. [DOI] [PubMed] [Google Scholar]
- 180.Mahon PC, Hirota K, Semenza GL. FIH-1: a novel protein that interacts with HIF-1alpha and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev. 2001;15(20):2675–2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Peyssonnaux C, Cejudo-Martin P, Doedens A, Zinkernagel AS, Johnson RS, Nizet V. Cutting edge: Essential role of hypoxia inducible factor-1alpha in development of lipopolysaccharide-induced sepsis. J Immunol. 2007;178(12):7516–7519. [DOI] [PubMed] [Google Scholar]
- 182.Tannahill GM, Curtis AM, Adamik J, et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature. 2013;496(7444):238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Cummins EP, Berra E, Comerford KM, et al. Prolyl hydroxylase-1 negatively regulates IkappaB kinase-beta, giving insight into hypoxia-induced NFkappaB activity. Proc Natl Acad Sci U S A. 2006;103(48):18154–18159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Eltzschig HK, Carmeliet P. Hypoxia and inflammation. N Engl J Med. 2011;364(7):656–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kiers D, Wielockx B, Peters E, et al. Short-Term Hypoxia Dampens Inflammation in vivo via Enhanced Adenosine Release and Adenosine 2B Receptor Stimulation. EBioMedicine. 2018;33:144–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ahmad A, Ahmad S, Glover L, et al. Adenosine A2A receptor is a unique angiogenic target of HIF-2alpha in pulmonary endothelial cells. Proc Natl Acad Sci U S A. 2009;106(26):10684–10689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Eltzschig HK, Abdulla P, Hoffman E, et al. HIF-1-dependent repression of equilibrative nucleoside transporter (ENT) in hypoxia. J Exp Med. 2005;202(11):1493–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Idzko M, Ferrari D, Riegel AK, Eltzschig HK. Extracellular nucleotide and nucleoside signaling in vascular and blood disease. Blood. 2014;124(7):1029–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Eltzschig HK, Weissmuller T, Mager A, Eckle T. Nucleotide metabolism and cell-cell interactions. Methods Mol Biol. 2006;341:73–87. [DOI] [PubMed] [Google Scholar]
- 190.Eckle T, Hughes K, Ehrentraut H, et al. Crosstalk between the equilibrative nucleoside transporter ENT2 and alveolar Adora2b adenosine receptors dampens acute lung injury. FASEB J. 2013;27(8):3078–3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Eckle T, Kewley EM, Brodsky KS, et al. Identification of hypoxia-inducible factor HIF-1A as transcriptional regulator of the A2B adenosine receptor during acute lung injury. J Immunol. 2014;192(3):1249–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Eckle T, Krahn T, Grenz A, et al. Cardioprotection by ecto-5'-nucleotidase (CD73) and A2B adenosine receptors. Circulation. 2007;115(12):1581–1590. [DOI] [PubMed] [Google Scholar]
- 193.Kohler D, Eckle T, Faigle M, et al. CD39/ectonucleoside triphosphate diphosphohydrolase 1 provides myocardial protection during cardiac ischemia/reperfusion injury. Circulation. 2007;116(16):1784–1794. [DOI] [PubMed] [Google Scholar]
- 194.Eckle T, Köhler D, Lehmann R, El Kasmi K, Eltzschig HK. Hypoxia-inducible factor-1 is central to cardioprotection: a new paradigm for ischemic preconditioning. Circulation. 2008;118(2):166–175. [DOI] [PubMed] [Google Scholar]
- 195.Eckle T, Grenz A, Laucher S, Eltzschig HK. A2B adenosine receptor signaling attenuates acute lung injury by enhancing alveolar fluid clearance in mice. J Clin Invest. 2008;118(10):3301–3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Aherne CM, Collins CB, Rapp CR, et al. Coordination of ENT2-dependent adenosine transport and signaling dampens mucosal inflammation. JCI Insight. 2018;3(20). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Chambers ED, White A, Vang A, et al. Blockade of equilibrative nucleoside transporter 1/2 protects against Pseudomonas aeruginosa-induced acute lung injury and NLRP3 inflammasome activation. FASEB J. 2020;34(1):1516–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Hausenloy DJ, Yellon DM. The therapeutic potential of ischemic conditioning: an update. Nat Rev Cardiol. 2011;8(11):619–629. [DOI] [PubMed] [Google Scholar]
- 199.Cai Z, Luo W, Zhan H, Semenza GL. Hypoxia-inducible factor 1 is required for remote ischemic preconditioning of the heart. Proc Natl Acad Sci U S A. 2013;110(43):17462–17467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Zarbock A, Schmidt C, Van Aken H, et al. Effect of remote ischemic preconditioning on kidney injury among high-risk patients undergoing cardiac surgery: a randomized clinical trial. JAMA. 2015;313(21):2133–2141. [DOI] [PubMed] [Google Scholar]
- 201.Zhou X, Jiang R, Dong Y, Wang L. Remote ischemic preconditioning attenuates cardiopulmonary bypass-induced lung injury. PLoS One. 2017;12(12):e0189501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Jensen HA, Loukogeorgakis S, Yannopoulos F, et al. Remote ischemic preconditioning protects the brain against injury after hypothermic circulatory arrest. Circulation. 2011;123(7):714–721. [DOI] [PubMed] [Google Scholar]
- 203.Meybohm P, Bein B, Brosteanu O, et al. A Multicenter Trial of Remote Ischemic Preconditioning for Heart Surgery. N Engl J Med. 2015;373(15):1397–1407. [DOI] [PubMed] [Google Scholar]
- 204.Hausenloy DJ, Candilio L, Evans R, et al. Remote Ischemic Preconditioning and Outcomes of Cardiac Surgery. N Engl J Med. 2015;373(15):1408–1417. [DOI] [PubMed] [Google Scholar]
- 205.Behmenburg F, van Caster P, Bunte S, et al. Impact of Anesthetic Regimen on Remote Ischemic Preconditioning in the Rat Heart In Vivo. Anesth Analg. 2018;126(4):1377–1380. [DOI] [PubMed] [Google Scholar]
- 206.Eltzschig HK, Bratton DL, Colgan SP. Targeting hypoxia signalling for the treatment of ischaemic and inflammatory diseases. Nat Rev Drug Discov. 2014;13(11):852–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Zhang Y, Fan F, Zeng G, et al. Temporal analysis of blood-brain barrier disruption and cerebrospinal fluid matrix metalloproteinases in rhesus monkeys subjected to transient ischemic stroke. J Cereb Blood Flow Metab. 2016:271678X16680221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Bernhardt WM, Gottmann U, Doyon F, et al. Donor treatment with a PHD-inhibitor activating HIFs prevents graft injury and prolongs survival in an allogenic kidney transplant model. Proc Natl Acad Sci U S A. 2009;106(50):21276–21281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Schneider M, Van Geyte K, Fraisl P, et al. Loss or silencing of the PHD1 prolyl hydroxylase protects livers of mice against ischemia/reperfusion injury. Gastroenterology. 2010;138(3):1143–1154 e1141-1142. [DOI] [PubMed] [Google Scholar]
- 210.Rajendran G, Schonfeld MP, Tiwari R, et al. Inhibition of Endothelial PHD2 Suppresses Post-Ischemic Kidney Inflammation through Hypoxia-Inducible Factor-1. J Am Soc Nephrol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Nagamine Y, Tojo K, Yazawa T, et al. Inhibition of Prolyl Hydroxylase Attenuates Fas Ligand-Induced Apoptosis and Lung Injury in Mice. Am J Respir Cell Mol Biol. 2016;55(6):878–888. [DOI] [PubMed] [Google Scholar]
- 212.Pergola PE, Spinowitz BS, Hartman CS, Maroni BJ, Haase VH. Vadadustat, a novel oral HIF stabilizer, provides effective anemia treatment in nondialysis-dependent chronic kidney disease. Kidney Int. 2016;90(5):1115–1122. [DOI] [PubMed] [Google Scholar]
- 213.Besarab A, Provenzano R, Hertel J, et al. Randomized placebo-controlled dose-ranging and pharmacodynamics study of roxadustat (FG-4592) to treat anemia in nondialysis-dependent chronic kidney disease (NDD-CKD) patients. Nephrol Dial Transplant. 2015;30(10):1665–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Akizawa T, Tsubakihara Y, Nangaku M, et al. Effects of Daprodustat, a Novel Hypoxia-Inducible Factor Prolyl Hydroxylase Inhibitor on Anemia Management in Japanese Hemodialysis Subjects. Am J Nephrol. 2017;45(2):127–135. [DOI] [PubMed] [Google Scholar]
- 215.Chen N, Hao C, Liu BC, et al. Roxadustat Treatment for Anemia in Patients Undergoing Long-Term Dialysis. N Engl J Med. 2019;381(11):1011–1022. [DOI] [PubMed] [Google Scholar]
- 216.Dhillon S Roxadustat: First Global Approval. Drugs. 2019;79(5):563–572. [DOI] [PubMed] [Google Scholar]
- 217.Haase VH. Therapeutic targeting of the HIF oxygen-sensing pathway: Lessons learned from clinical studies. Exp Cell Res. 2017;356(2):160–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Chen N, Hao C, Peng X, et al. Roxadustat for Anemia in Patients with Kidney Disease Not Receiving Dialysis. N Engl J Med. 2019;381(11):1001–1010. [DOI] [PubMed] [Google Scholar]
- 219.Haase VH, Chertow GM, Block GA, et al. Effects of vadadustat on hemoglobin concentrations in patients receiving hemodialysis previously treated with erythropoiesis-stimulating agents. Nephrol Dial Transplant. 2019;34(1):90–99. [DOI] [PMC free article] [PubMed] [Google Scholar]