Abstract
Cancer stem cells (CSCs) have been found to play a decisive role in cancer recurrence, metastasis, and chemo-, radio- and immuno-resistance. Understanding the mechanism of CSC self-renewal and proliferation may help overcome the limitations of clinical treatment. The microenvironment of tumor growth consists of a lack of oxygen, and hypoxia has been confirmed to induce cancer cell invasion, metastasis and epithelial-mesenchymal transition, and is usually associated with poor prognosis and low survival rates. Hypoxia inducible factor-1 (HIF-1) can be stably expressed under hypoxia and act as an important molecule to regulate the development of CSCs, but the specific mechanism remains unclear. The present review attempted to explain the role of HIF-1 in the generation and maintenance of CSCs from the perspective of epigenetics, metabolic reprogramming, tumor immunity, CSC markers, non-coding RNA and signaling pathways associated with HIF-1, in order to provide novel targets with HIF-1 as the core for clinical treatment, and extend the life of patients.
Keywords: cancer stem cell, hypoxia, hypoxia inducible factor-1, hypoxia inducible factor-1α, epigenetic modification, post-translational modification, non-coding RNA, cancer stem cell marker, tumor immunity, metabolic reprogramming, signal pathway
1. Introduction
Cancer stem cells (CSCs), a population of cells with similar characteristics to those of stem cells, are associated with the occurrence, recurrence, metastasis and chemoradiation resistance of cancer (1,2). The existence of CSCs is a challenge for tumor treatment, but can also provide a novel direction for clinical treatment. To date, there have been various speculations about the occurrence of CSCs, but it remains unclear. Hypoxia in the tumor microenvironment is usually found in advanced cancer and is associated with low survival rates and poor prognosis (3,4). An increasing number of studies have found that hypoxia can induce cancer cell invasion, metastasis and epithelial-mesenchymal transition (EMT), which can promote stem-like characteristics in cancer cells (5,6). Hypoxia inducible factor-1 (HIF-1), as a pivotal molecule in the regulation of CSCs by hypoxia, participates in tumor growth, immune evasion, metabolic reprogramming and drug resistance by regulating the transcription of target genes (4,6,7). HIF-1 seems to play an important, or even core, role in the generation and maintenance of CSCs, but the explicit mechanism remains to be elucidated. This review attempted to summarize the role and mechanism of HIF-1 in CSCs, in order to provide more targets to solve the limitations of clinical tumor treatment.
2. Structural characteristics of HIF-1
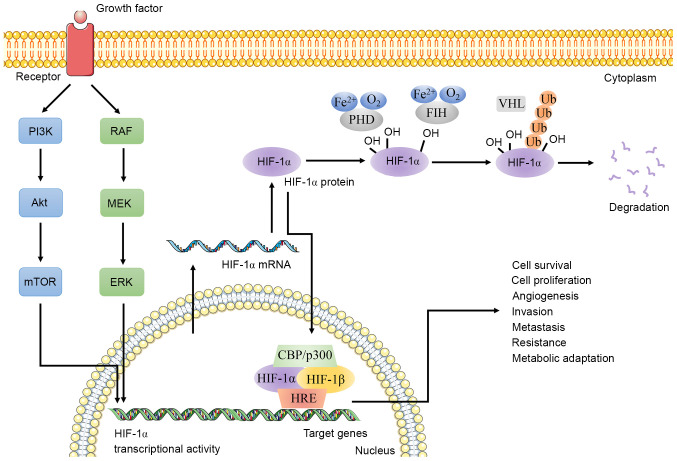
HIF-1 is a heterodimer composed of HIF-1α and HIF-1β (Fig. 1) (8,9). Under normoxic (21% O2) conditions, HIF-1 is degraded by intracellular oxygen-dependent ubiquitin protease degradation pathways, which are inhibited during hypoxia (8,10). HIF-1α is a hypoxia inducible transcription factor that contains two transactivation domains (C-TAD and N-TAD) (11,12). C-TAD can interact with co-activators such as CREB binding protein (CBP)/p300 to regulate the transcription of HIF-1α target genes. N-TAD participates in stabilizing HIF-1α (13,14). The core complex formed by HIF-1α and CBP/p300 mainly depends on the recognition of the two short-helical domains of C-TAD by CBP/p300 (15–17). In addition, these TADs are also regulatory targets for post-translational modifications, such as acetylation and phosphorylation (18). Under normoxic conditions, factor inhibiting HIF-1 (FIH), an asparaginyl hydroxylase, hydroxylates asparagine (N)803 residue within C-TAD in an oxygen-dependent manner to block the cooperative binding of CBP/p300 and C-TAD, thereby eliminating HIF-1α-mediated target gene transcription (18,19). However, the oxygen-dependent hydroxylation of N803 is inhibited in hypoxic conditions to promote this binding, leading to the transcriptional activation of target genes (13,20). Oxygen-dependent degradation domain, another special structure of HIF-1α, participates in mediating the degradation of HIF-1 (9). It is worth noting that HIF-1α must form a heterodimer with HIF-1β before exerting its biological function (8). HIF-1β is stably expressed in cells and maintains the stability of HIF-1 (21,22). The structures included in HIF-1α and HIF-1β are basic-helix-loop-helix and PER-ARNT-SIM domain, which promote DNA binding and dimerization (9,23). Increasing evidence has indicated that HIF-1α, as the active subunit of HIF-1, is involved in inducing and maintaining the phenotype of various CSCs (12,24,25). The present review focused more on the association between HIF-1α and CSCs than of HIF-1.
Figure 1.
Schematic diagram of HIF-1α and HIF-1β. Under hypoxic conditions, the PI3K/Akt/mTOR pathway and MAPK (RAF/MEK/ERK) pathway regulate HIF-1α transcriptional activity. The upregulated HIF-1α and HIF-1β form a heterodimer to regulate the expression of HIF-1α target genes with the participation of co-activators CBP/p300. Under normoxia, FIH hydroxylates asparagine (N803) residue within C-TAD to block the cooperative binding of CBP/p300 and C-TAD. PHD, whose activity depends on ferrous, dioxygen and 2-oxoglutarate, is involved in the hydroxylation of HIF-1α. Additionally, VHL, a tumor suppressor, regulates the expression of HIF-1α through ubiquitination and proteasome degradation. HIF-1, hypoxia inducible factor-1; CBP, CREB binding protein; FIH, factor inhibiting HIF-1; PHD, prolyl hydroxylase; VHL, Von Hippel-Lindau; Ub, ubiquitination; HRE, hypoxic response element; TAD, transactivation domain.
3. Role of epigenetic and post-translational modification of HIF-1 in CSCs
Epigenetic modifications are reversible, heritable changes in gene function that do not alter the DNA sequence (26,27). HIF-1α can be used as a key regulator of genomic methylation in hepatocellular carcinoma cells (28). The presence of HIF-1α binding sites on methionine adenosyltransferase 2A (MAT2A) can promote the transcription of MAT2A to maintain the genome of the demethylation state (28). Unfortunately, most currently available studies have focused on the genetic modification of HIF-1 downstream target genes or key enzymes (28). Research on the epigenetic modification of HIF-1 in CSCs is rare.
Post-translational modification is one of the most important regulatory mechanisms for dynamically and reversibly regulating proteins that have biological functions (29). A previous study has found that lysine methyltransferase G9a can mono- or di-methylate HIF-1α on lysine 674, which reduces the transcriptional activity of HIF-1α and expression of downstream genes by reducing the TAD activity of HIF-1α; meanwhile G9a is reduced in glioblastoma cells, maintaining the activity of HIF-1α and promoting HIF-1-dependent cell migration (30). As a small ubiquitin-like modifier (SUMO) protease, small ubiquitin-like modifier protease 1 (SENP1) forms a positive feedback loop with HIF-1α in hepatoma cells, which causes HIF-1α deSUMOylation and stable expression during hypoxia, and can promote the production of liver CSCs (31). In addition, the SENP1/HIF-1α positive feedback loop promotes hypoxia-induced cell EMT and invasion in osteosarcoma cells (32). Kinase ERK-mediated phosphorylation of HIF-1α increases its stability and accumulation in the nucleus to promote the transcriptional activation of target genes (25,33). Protein kinase A in HeLa cells can also phosphorylate threonine 63 and serine 692 of HIF-1α, inhibiting HIF-1α degradation and increasing HIF-1α transcription (34). The knockout of Y-box binding protein 1 in gliomas inhibits cell proliferation and blocks the cell cycle by downregulating the phosphorylation level of HIF-1, which may affect the proliferation, differentiation and metastasis of glioma cells (35,36). There is not enough information on the epigenetic and post-translational modification of HIF-1, thus further studies are needed to explore how the proliferation and growth of CSCs can be inhibited via the expression of HIF.
4. Role of HIF-1 in non-coding RNA associated with CSCs
Non-coding RNA (ncRNA) is a class of RNA that is transcribed from DNA but is not translated into a protein, types of ncRNA include small interfering RNA (siRNA), long ncRNA (lncRNA) and microRNA (miRNA) (37,38). A previous study demonstrated that the interaction between HIF-1 and ncRNA is significant in the self-renewal and proliferation of CSCs. miR-124 can reverse the resistance of breast CSCs to doxorubicin through the inhibition of the STAT3/HIF-1α signaling pathway (39). miR-200b can target and inhibit the anti-angiogenic Krüppel-like factor 2 gene during acute hypoxia, thereby stabilizing HIF-1 signaling to promote angiogenesis (40). miR-200c inhibits the metastasis and invasion of lung cancer cells by inhibiting HIF-1α expression (41). miR-18a targets HIF-1α and inhibits the distant metastasis of breast cancer through the HIF-1α-dependent hypoxic response; miR-18a-5p also increases the radiotherapy sensitivity of lung CSCs by inhibiting HIF-1α (42,43). The promoter of miR-302 responds to HIF-1α, which is beneficial for enhancing stem-like characteristics of hypoxic cancer cells (44). The expression of miR-210, an important regulator that inhibits DNA repair, is directly regulated by HIF-1α and promotes the degradation of normoxic gene mRNA (45,46). The knockout of miR-210 suppresses the self-renewal capacity and resistance of glioma stem-like cells induced by hypoxia (47). miR-21 and HIF-1α are positively correlated in multiple tumors, and miR-126 and HIF-1α are significantly negatively correlated in colon cancer, thus indicating that their expression could be used for the early diagnosis and screening of cancer (46,48,49). miR-21 and miR-130b activate EMT through the phosphatase and tensin homolog/Akt/HIF-1α pathway and promote hepatocellular carcinoma metastasis (50). HIF-1α binds to the miR-1275 promoter, which promotes miR-1275 expression and maintains the pluripotency of stem cells by activating the β-catenin and Notch signaling pathways in lung adenocarcinoma (51). miR-421 is upregulated by HIF-1α and promotes gastric cancer metastasis and chemotherapy resistance (52). miR-107 inhibits Ewing sarcoma cell proliferation, blocks the cell cycle and promotes apoptosis by targeting HIF-1β (53). miR-99a, which is reduced in breast CSCs, suppresses the stem-like phenotype of breast cancer by inhibiting the mTOR/HIF-1α signaling pathway (54). In addition, under normoxia, the upregulated miR-31 in head and neck squamous cell carcinoma can target the 3′ untranslated region of the FIH transcript to promote the transactivation activity of HIF, leading to increased tumorigenicity (55). Similar results were also found in oral squamous cell carcinoma and colorectal cancer (56,57). The upregulated lncRNA LOC554202 in non-small cell lung cancer is positively correlated with miR-31, thereby targeting FIH to promote the development of acquired gefitinib resistance (58). miR-31-5p, which is upregulated in lung cancer, induces glycolytic gene expression by regulating the FIH/HIF pathway, and ultimately promotes cell proliferation and tumor growth (59). miR-21 and miR-184, which are also upregulated in head and neck squamous cell carcinoma, have similar tumorigenic mechanisms to those of miR-31 (60).
Several hypoxia-related lncRNAs, such as HOTAIR, H19, lncRNA-NUTF2P3-001, lncRNA-UCA1, lncRNA-EFNA3, ANRIL, HINCUTs and GAPLINC, are directly regulated by HIF-1α, as their promoters have hypoxic response elements (HREs), which are required for HIF-1α-mediated transcriptional activation (61,62). The low expression of LINC00996 in colorectal cancer cells may participate in the occurrence and metastasis of colorectal cancer by regulating the HIF-1 signaling pathway (63). HIF-1α can promote the transcription of lincRNA-p21 (64). Conversely, hypoxia-related lncRNA-p21 can bind to HIF-1α, which can prevent the interaction of HIF-1α and Von Hippel-Lindau (VHL) and cause the accumulation of HIF-1α in cells (64). Beyond that, the knockout of lincRNA-p21 can also induce apoptosis through the HIF-1α/Akt/mTOR/P70S6 kinase 1 (S6K)-pathway and increase the sensitivity of radiotherapy (65). By forming a complex with HIF-1α, lncHIFCAR/MIR31HG promotes the binding of HIF-1α to the target promoters and increases the sphere-forming and metastatic ability of oral cancer cells (66). lncRNA PCGEM1 may be used as a scaffold to form a complex with HIF-1α and transcription factor Snail homolog 1 (SNAI1) and regulate the invasion and metastasis of gastric cancer cells (67). Hypoxia-induced lncRNA CRPAT4 is regulated by HIF-1α and plays a carcinogenic role by promoting the growth and migration of cancer cells (68). The presence of siRNA targeting HIF-1α may provide a novel direction for specific treatment against HIF-1α, and it could achieve molecular therapy by inducing apoptosis and increasing the sensitivity of radiotherapy (69,70).
In the regulation of HIF-1 expression, the cooperation of miRNA and related lncRNA is equally important. The significantly upregulated lncRNA TUG1 in osteosarcoma protects the HIF-1α mRNA 3′ untranslated region from miR-143-5p, thereby promoting osteosarcoma metastasis (71). lncRNA MIR31HG is the host gene of miR-31, which is located in the first intron of MIR31HG and has consistent transcriptional regulation (66,72). Studies have found that MIR31HG is a hypoxia-inducible lncRNA and acts as a HIF-1α co-activator to regulate the HIF-1 transcriptional network (66). Mechanistically, MIR31HG directly interacts with HIF-1α to promote the recruitment of HIF-1α and p300 to target gene promoters (66). It is worth noting that, although the expression of MIR31HG is positively correlated with miR-31 in certain types of cancer, the knockout of MIR31HG has no effect on miR-31, indicating that the tumor regulatory effect of MIR31HG may be independent of miR-31 (73). A variety of lncRNAs serve as competing endogenous RNAs (ceRNAs) to inhibit the interaction of miRNAs with their targets, thereby forming a lncRNA-miRNA-mRNA ceRNA network, which can regulate multiple signaling pathways, including the HIF-1α pathway (74,75). In addition, the HIF-1α-mediated hypoxia-induced upregulation of lncRNA-NEAT1 in hepatocellular carcinoma regulates the expression of the uridine-cytidine kinase 2 gene, which is associated with low survival rates of patients with liver cancer, by suppressing miR-199a-3p, and ultimately promotes tumor growth (76).
Although there are only a small number of studies on HIF-1-related ncRNAs, the existing independent studies are sufficient to illustrate HIF-1 as an important regulator or participant in CSC-related ncRNA (Table I), which may eradicate CSCs and prolong the life of patients by targeting ncRNA or HIF-1.
Table I.
Association between HIF-1 and non-coding RNA.
| Non-coding RNA | Category | Relationship with HIF-1 | Cancer type | (Refs.) |
|---|---|---|---|---|
| miR-124 | miRNA | Inhibits STAT3/HIF-1α pathway | Breast cancer | (39) |
| miR-200b | miRNA | Inhibits KLF2 gene and stabilizes HIF-1α signal | Human endothelial cells | (40) |
| miR-200c | miRNA | Inhibits HIF-1α expression | Lung cancer | (41) |
| miR-18a | miRNA | Inhibits HIF-1α expression | Breast cancer | (43) |
| miR-18a-5p | miRNA | Inhibits HIF-1α expression | Lung cancer | (42) |
| miR-302 | miRNA | Regulated by HIF-1α | HeLa cells | (44) |
| miR-210 | miRNA | Regulated by HIF-1α | Glioma; pancreatic cancer; colorectal cancer | (45–47) |
| miR-21 | miRNA | Activates PTEN/Akt/HIF-1α pathway; positive correlation with HIF-1α; targets FIH | Hepatocellular carcinoma; breast cancer; colon cancer; glioma | (46,48–50) |
| miR-126 | miRNA | Negative correlation with HIF-1α | Colon cancer | (46) |
| miR-130b | miRNA | Activates PTEN/Akt/HIF-1α pathway | Hepatocellular carcinoma | (50) |
| miR-1275 | miRNA | Regulated by HIF-1α and activates Notch and β-catenin pathway | Lung adenocarcinoma | (51) |
| miR-421 | miRNA | Upregulated by HIF-1α | Gastric cancer | (52) |
| miR-107 | miRNA | Targets HIF-1β | Ewing sarcoma | (53) |
| miR99a | miRNA | Inhibits mTOR/HIF-1α signal pathway | Breast cancer | (54) |
| miR-31 | miRNA | Targets the 3′ untranslated region of FIH transcript | Head and neck squamous cell carcinoma; oral squamous cell carcinoma; colorectal cancer; lung cancer | (55–58) |
| miR-31-5p | miRNA | Targets the 3′ untranslated region of FIH transcript | Lung cancer | (59) |
| miR-184 | miRNA | Targets FIH | head and neck squamous cell carcinoma | (60) |
| LOC554202 | lncRNA | Positively correlated with miR-31 | Lung cancer | (58) |
| LINC00996 | lncRNA | Regulates HIF-1α signal | Colorectal cancer | (63) |
| LincRNA-p21 | lncRNA | Regulated by HIF-1α and induces HIF-1α accumulation | HeLa cells | (64,65) |
| LncHIFCAR/ MIR31HG | lncRNA | Promotes the binding of HIF-1α to target genes | Oral cancer | (66) |
| PCGEM1 | lncRNA | Forms a complex with HIF-1α and SNAI1 | Gastric cancer | (67) |
| CRPAT4 | lncRNA | Regulated by HIF-1α | Clear cell renal cell carcinoma | (68) |
| TUG1 | lncRNA | Protects HIF-1α mRNA 3′ untranslated region from miR-143-5p | Osteosarcoma | (71) |
| miR31HG | lncRNA | Serves as a HIF-1α co-activator | Oral cancer | (66,72,73) |
| NEAT1 | lncRNA | Induced by HIF-1α and regulates the expression of UCK2 gene through suppressing miR-199a-3p | Hepatocellular carcinoma | (76) |
HIF-1, hypoxia inducible factor-1; miR/miRNA, microRNA; lncRNA, long non-coding RNA; KLF2, Krüppel-like factor 2; PTEN, phosphatase and tensin homolog; SNAI1, Snail homolog 1; FIH, factor inhibiting HIF-1; UCK2, uridine-cytidine kinase 2.
5. Role of HIF-1 in CSC markers
Markers of CSCs induce the pluripotency of cancer cells and are used to distinguish CSC subpopulations, some of which have been found to be associated with metastasis (77,78). Studies have found that HIF-1 is associated with the generation of CSC markers. The data has indicated that HIF-1α can induce the production of multiple stem cell markers, such as OCT4, SOX2, NANOG and Krüppel-like factor 4 (KLF4) (44,79,80). In addition, the silencing of HIF-1α can hinder the progression of cancer by inhibiting the expression of stem cell markers (81). HIF-1 was found to bind directly to the CD47 promoter to facilitate gene transcription, which helps to escape phagocytosis of macrophages and maintain the stem phenotype of breast CSCs (7,82). Endogenous HIF-1α is recruited to the promoter of CD24, which promotes CD24 expression, as well as tumor formation and metastasis (83). HIF-1α appears to bind to the CD133 promoter and promote the production of CD133+ glioblastoma, and colon and pancreatic CSCs via OCT4 and SOX2 (81,84–87). In addition, a correlation has been found between HIF-1α and CD133 in the cytoplasm, rather than other parts of the cell, such as the gland cavity (88). In turn, CD133 promotes HIF-1α expression and its translocation to the nucleus under hypoxia (89). However, there is a different opinion that hypoxia-induced HIF-1α expression leads to a decrease in CD133 expression in gastrointestinal cancer cells that overexpress CD133. Under normoxic conditions, the inhibition of mTOR signaling in CD133-overexpressing gastrointestinal cancer cells suppresses HIF-1α expression and promotes that of CD133 (90). In breast CSCs, HIF-1 increases the stability of NANOG mRNA through the transactivation of RNA demethylase ALKBH5, which is involved in encoding N6-methyladenosine demethylase (7). In prostate cancer samples, the co-localization of HIF-1α, OCT4 and NANOG suggests that HIF-1α may regulate the production of CSCs by regulating stem factors (44). Surprisingly, OCT4 isoform OCT4B in cervical cancer cells promotes neovascularization by upregulating HIF-1α production (91). The subtype of aldehyde dehydrogenase, 4-trimethylaminobutyraldehyde dehydrogenase (ALDH1A1), which is associated with the self-renewal, metastasis and resistance of cancer cells, is regulated by HIF-1α in breast cancer (81). In turn, ALDH1A1 promotes HIF-1α expression via retinoic acid signaling (81,92). In addition to promoting the production of mesenchymal or EMT marker proteins, HIF-1α also inhibits the expression of epithelial marker proteins, which can be confirmed by the use of HIF-1α inhibitors (93–95). HIF-1α can be used as a malignant marker of chondrosarcoma, due to its association with neovascularization (96). In conclusion, CSC markers can be used to isolate CSC subpopulations, and have been demonstrated to be involved in the self-renewal of CSCs, as well as cancer invasion and metastasis. Under hypoxic conditions, HIF-1α, as a direct or indirect upstream regulator of the marker protein, may become a novel target for the elimination of CSCs.
6. Role of HIF-1 in tumor immunity of CSCs
Hypoxia not only regulates the production of CSCs, but also participates in regulating the immune system. Hypoxia promotes the B cell differentiation potential of lymphoid-primed multipotent progenitors through HIF-1α, resulting in the production of B cells (97). Under hypoxic conditions, HIF-1α also regulates innate immune responses, induces regulatory T cells (Tregs), and mediates immune escape from cytotoxic T lymphocytes and other complex immune responses (98–100). In previous years, HIF-1α-mediated tumor immunity has been proposed as a direction to solve the problems associated with tumor therapy (101–103), so the immune response of CSCs mediated by HIF-1α is also worth exploring when investigating antitumor therapies.
During EMT, in addition to the induction of cancer stemness, immunosuppression is also observed, which can lead to increased malignancy of the tumor, drug resistance and metastasis (79). Studies have found that hypoxia further increases the production of immunosuppressive factors, inhibition of monocyte phagocytosis, inhibition of T cell proliferation, and activation and induction of Tregs in glioblastoma multiforme-related CSCs, which may be achieved via the phoshphorylated STAT3/HIF-1α/vascular endothelial growth factor (VEGF) pathway (104–106). During HIF-1α-induced EMT of liver cancer cells, the upregulated cytokine CCL20 promotes the expression of indoleamine 2,3-dioxygenase in monocyte-derived macrophages, which inhibits the activation and proliferation of T cells and induces Tregs by increasing the degradation of tryptophan (107). Immune escape and immunosuppression are equally important for the existence of CSCs (108). One of the breast CSC marker proteins, CD47, can bind to the signal regulatory protein α on the surface of macrophages to escape macrophage phagocytosis, and the induction of CD47 depends on the direct regulation of HIF-1α (82). There are two types of tumor-associated macrophages, M1 and M2, which inhibit or promote tumor growth, respectively. The M2 type is more common in the tumor microenvironment and promotes tumor invasion (108,109). Hypoxia induces nuclear factor-κB (NF-κB) and HIF-1α successively, leading to the infiltration of M2 macrophages in the tumor microenvironment and tumorigenesis (110). As compared with normal cells, triple-negative breast cancer cells have more HIF-1α-specific IgG, which indicates that HIF-1α is immunogenic (111). Treatment using a HIF-1α vaccine recruits type I T cells to the tumor tissue and effectively inhibits basal-like breast CSCs, which can inhibit tumor metastasis (111). In order to adapt to chronic hypoxia, CD8+ T cells increase the expression of active HIF-1α and increase their own effector functions (112). The production of NANOG in hypoxic tumor cells depends on the expression of HIF-1α, and upregulated NANOG reduces the sensitivity of hypoxic tumor cells to the lysis of cytotoxic T lymphocytes; however, this process is not caused by the increased phenotype of CSCs, but NANOG increased the viability of the tumor cells (113). At the same time, some studies have proposed that hypoxia-induced NANOG, which also depends on HIF-1α, promotes the self-renewal of melanoma cells and induces Tregs and macrophages by directly regulating TGF-β1, but whether this immunosuppression is associated with CSCs has not been reported (114). In summary, HIF-1α-mediated immunosuppression and immune escape are crucial in CSCs. This common regulatory mechanism in the tumor microenvironment gives CSCs a new definition and provides novel ideas for tumor immunotherapy.
7. Role of HIF-1 in metabolic reprogramming of CSCs
It is commonly known that cancer cells can metabolize and reprogram under hypoxic conditions to complete the conversion of oxidative phosphorylation (OXPHOS) to glycolysis to meet their own energy needs (7,115). However, a study recently proposed the concept of metabolic plasticity, that is, even within the same cancer cell population, cancer cells can complete the conversion of glycolysis to OXPHOS, while maintaining metabolic reprogramming and energy requirements (116). Indeed, hypoxic cells do not metabolize all glucose to lactic acid, and non-hypoxic cells do not metabolize all glucose to carbon dioxide and water, which makes metabolic balance particularly important (117). Among them, HIF-1 plays a crucial role as a regulator in metabolic reprogramming, and the role of HIF-1 in CSC metabolism is worthy of attention.
HIF-1, as the main regulator of several glycolytic transporters and enzymes, including glucose transporter, monocarboxylate transporter, hexokinase and lactate dehydrogenase, regulates glycolytic transformation (95,118). HIF-1α also promotes the production of carbonic anhydrases, which interact with extracellular acidification to change the pH of the intracellular and extracellular environment of the cell, thereby affecting the absorption of anticancer drugs and producing drug resistance (119,120). Colon CSC clones with liver receptor homolog-1-overexpression, a target of GATA binding protein 6, were found to have increased intracellular hypoxia, HIF-1α and reactive oxygen species (ROS); it was further proven that glycolysis and OXPHOS co-exist in the clones but mainly mitochondrial respiration (121). The knockout of CSC marker protein CD44 induces glycolysis to OXPHOS via a complex signaling pathway involving HIF-1α (115). The loss of ATP synthase, especially the D subunit ATP5H, leads to the accumulation of ROS in cells and the stabilization of normoxic HIF-1α and activation of the HIF-1α pathway, affecting mitochondrial metabolic reprogramming, the production of stem-like ability, and therapeutic resistance (122). The α-KG analogue dimethyl 2-ketoglutarate allows excess succinate/fumarate to be transferred from the mitochondria to the cytoplasm, where it can impair prolyl hydroxylase and thus stabilize and activate HIF-1α, eventually leading to increased glycolysis and the acquisition of the stem-like characteristics of breast cancer cells (118). In addition, the upregulation of HIF-1α, under hypoxia, inhibits the expression of mitochondrial phosphoenolpyruvate carboxykinase, which leads to a weakened tricarboxylic acid cycle and the enrichment of fumarate, ultimately leading to increased ROS levels and breast CSC growth (123). In conclusion, HIF-1 appears to mainly act as an intermediate participant regulating the complex conversion mechanism of glycolysis and OXPHOS and the generation and maintenance of stemness in CSCs.
8. Role of HIF-1 in signaling pathways associated with CSCs
HIF-1 is regulated by multiple signaling pathways in CSCs and also participates in regulating the characteristics of CSCs through signaling pathways, such as the Notch, MAPK/ERK and Wnt signaling pathways (81). Studies have found that the PI3K/Akt/mTOR pathway maintains the transcription, translation and biological activity of HIF-1α (54,124). Hypoxia also induces tuftelin 1 in a HIF-1α-dependent manner; subsequently, tuftelin1 activates the Ca2+/PI3K/Akt pathway to induce EMT and metastasis in hepatocellular carcinoma (50). HIF-1α promotes the survival of prostate CSCs by inhibiting mTOR and activating Akt phosphorylation, which may be accomplished by the feedback regulation of PI3K via P70-S6K-mediated insulin receptor substrate 1 phosphorylation (125). The mitochondrial autophagy regulator NIX interacts with Ras homolog enriched in the brain to activate mTOR/Akt/HIF signaling, and subsequently increase the self-renewal capacity of glioma stem cells (126). The Wnt/β-catenin signaling pathway activates the transcription of HIF-1α, inhibits the apoptosis of hepatocellular carcinoma and promotes the occurrence of EMT, and then triggers the metastasis of hepatocellular carcinoma (50). Conversely, HIF-1α maintains the stemness of esophageal squamous cell carcinoma by activating the Wnt/β-catenin signaling pathway (127). HIF-1α induces the generation of breast CSCs and the enhancement of self-renewal capacity by upregulating the expression of yes-associated protein (YAP) and tafazzin (TAZ) in the Hippo pathway (7,86). HIF-1α, which can also function as a bidirectional co-activator of TAZ, is recruited by TAZ to the promoter of encoding connective tissue growth factor (CTGF) to activate the transcription of CTGF, which is involved in promoting the onset of EMT and maintaining the stem-like phenotype of breast CSCs (6,128). In addition, high-mobility group box 1 (HMGB1) released from injured or dying cells following X-ray radiation induces the dedifferentiation of CD133- pancreatic cancer cells, and promotes pancreatic CSC production and pancreatic cancer metastasis via the HMGB1/toll-like receptor 2 (TLR2)/YAP/HIF-1α axis, in which HMGB1-TLR2 promotes HIF-1α and YAP nuclear localization and HIF-1α DNA binding ability (129). The ROS-mediated transition of breast CSCs from a quiescent mesenchymal state to a proliferative epithelial state is promoted by the activation of the Notch pathway and AMP-activated protein kinase/HIF-1α axis (130,131).
HIF-1α maintains the stemness of leukemia and glioblastoma stem cells through the Notch signaling pathway (81,86). Studies have also suggested that the hypoxia/Notch1/SOX2 axis is essential for the development of ovarian CSCs (81). The combination of HIF-1α and notch intracellular domain activates Notch-responsive promoters and increases the expression of Notch downstream genes, such as Hes1 and Hey2, and Hes1 is important in the stemness maintenance and self-renewal of leukemia stem cells (50,81). In addition, studies have suggested that there is negative feedback regulation of the Hes1 expression, which is completed by the combination of Hes1 and N-boxes on the Hes1 promoter (132). Furthermore, HIF-1α may enhance the Notch pathway-induced Hes1 expression by inhibiting the negative feedback regulation of the Hes1 gene, and ultimately promote the maintenance of the stemness of mouse cholangiocarcinoma CSCs (132,133). STAT3 induced by HIF-1α through the JAK or adenylate receptor 2B pathway can upregulate interleukin-6 and Nanog, which can maintain the CSC phenotype (81). Under hypoxic conditions, the increased HIF-1α in glioma stem-like cells activates the JAK1/2-STAT3 signaling pathway by promoting the production of VEGF, a HIF-1α target, and ultimately enhances the self-renewal ability of glioma stem-like cells (134). HIF-1α inhibits the expression of E-cadherin and promotes EMT in hepatocellular carcinoma via the SNAI1 signaling pathway, in which HIF-1α binds to two HREs on the SNAI1 promoter to upregulate SNAI1, a transcriptional inhibitor of E-cadherin (50,135). At the same time, researchers found that HIF-1α promotes EMT in gastric CSCs by activating the Snail signaling pathway, and the same result was found in ovarian cancer (136,137). Dual-specificity phosphatases (DUSPs) negatively regulate MAPK pathway activity (138). Chemotherapy induces an increase in DUSP9 expression and a decrease in that of DUSP16 in a HIF-1-dependent manner; it then upregulates NANOG and KLF4 through the reduction of ERK activity and the increase of P38 activity, respectively, finally promoting the development of breast CSCs (138). Studies have found that hypoxia activates the Sonic Hedgehog signaling pathway to induce the production of CSC markers in cholangiocarcinoma cells in a HIF-1α-dependent manner, which can be blocked by HIF-1α inhibition (139). The expression level of glioma-associated oncogene homolog 1 (Gli1) in the Hedgehog pathway in prostate cancer was higher in the HIF-1α+ group, indicating that hypoxia promotes the expression of Gli1 and that the increased Gli1 expression is significantly associated with EMT of prostate cancer cells (140). Under hypoxia, upregulated HIF-1α promotes the expression of its downstream target gene, sirtuin type 1 (SIRT1), by activating the NF-κB signaling pathway, and increased SIRT1 promotes the maintenance of stem-like characteristics of ovarian cancer cells (141). Of note, studies have suggested that hypoxia-related factors switch HIF-1α to HIF-2α by activating the NF-κB pathway to increase the malignancy of bladder cancer and maintain the expression of stem cell markers (142).
In addition, some signaling pathways also regulate the HIF-1α-related complex. NF-κB-mediated inflammatory signaling can prevent the HIF-1α transcription network by directly competing for the binding of p300 to the promoter of HRE-encoding genes (143). Raf Kinase Trapping to Golgi in clear-cell renal cell carcinoma prevents the formation of the HIF-1α/p300 complex and transactivation of HIF-1α by inhibiting the MAPK pathway (144). Since PI3K promotes the induction of HIF-1α levels and there is a protein kinase B (PKB) phosphorylation site on p300, the PI3K/PKB pathway can boost the binding of HIF-1α/phosphorylated p300 to glucokinase gene (GK)-HRE, thereby promoting insulin-mediated GK gene expression (145). LB-1, a triptolide derivative, prevents the formation of the HIF-1α/p300/p-STAT3 complex by downregulating the PI3K/Akt/mTOR pathway that regulates HIF-1α at the translation level, and ultimately exerts anti-tumor properties (146). In turn, the increase in the Wnt signaling pathway mediated by CBP plays an important role in hypoxia-induced leukemia stem cells (147,148). In fact, multiple factors in the HIF-1α signaling pathway can also interfere with the formation of the HIF-1α/p300 complex. CBP/p300-interacting transactivator with an ED-rich tail 2 in hypoxia signaling was also found to prevent p300 from recruiting to N-TAD and C-TAD, thereby inactivating HIF-1α (149). The ferritin heavy chain in the hypoxia signaling pathway has also been demonstrated to interfere with p300 recruitment to HIF-1α by promoting FIH-mediated hydroxylation of N803 (150). In prostate cancer, N-myc downstream-regulated gene 3 regulates Akt-dependent HIF-1α hypoxia signaling through dissociating the p300 from HIF-1α (151).
In conclusion, HIF-1α promotes self-expression by interacting with multiple signaling pathways and participates in the maintenance of stem-like characteristics of CSCs (Table II).
Table II.
Association between HIF-1 and signaling pathways.
| Signaling pathway | Relationship with HIF-1 | Cancer type | (Refs.) |
|---|---|---|---|
| PI3K/Akt/mTOR | Maintains the transcription, translation and biological activity of HIF-1α; activated by HIF-1α-dependent tuftelin1; regulated by HIF-1α; involved in regulating the formation of HIF-1α/p300 complex | Breast cancer; hepatocellular carcinoma; prostate cancer; pancreatic cancer | (50,54,124,125,146) |
| Wnt/β-catenin | Activates the transcription of HIF-1α; also activated by HIF-1α | Hepatocellular carcinoma; esophageal squamous cell carcinoma | (50,127) |
| Hippo | Regulated by HIF-1α; TAZ recruits HIF-1α to promote CTGF expression | Breast cancer | (6,7,86,128) |
| Notch | Regulated by HIF-1α | Leukemia; glioblastoma; ovarian cancer | (50,81,86) |
| JAK/STAT | Activated by HIF-1α | Glioma | (81,134) |
| SNAI1 | Regulated by HIF-1α | Hepatocellular carcinoma | (50,135) |
| Snail | Activated by HIF-1α | Gastric cancer; ovarian cancer | (136,137) |
| MAPK | Regulated by DUSPs in a HIF-1-dependent manner under chemotherapy conditions; involved in regulating the formation of HIF-1α/p300 complex | Breast cancer; clear-cell renal cell carcinoma | (138,144) |
| Sonic Hedgehog | Activated by hypoxia in a HIF-1-dependent way | Cholangiocarcinoma; prostate cancer | (149,140) |
| NF-κB | Activated by HIF-1α; also activated by hypoxia-related factors to switch HIF-1α to HIF-2α; competes for the binding of p300 to the promoter of HRE-encoding genes | Bladder cancer; ovarian cancer | (141–143) |
| PI3K/PKB pathway | Promotes the binding of HIF-1α/phosphorylated p300 to GK-HRE | Hepatocytes | (145) |
| pSTAT3/HIF-1α/VEGF pathway | Promotes the occurrence of. immunosuppression | Glioblastoma multiforme | (104–106) |
HIF-1, hypoxia inducible factor-1; TAZ, tafazzin; CTGF, connective tissue growth factor; DUSPs, dual-specificity phosphatases; VEGF, vascular endothelial growth factor; SNAI1, Snail homolog 1; GK, glucokinase; HRE, hypoxic response element; p, phosphorylated.
9. Potential targets for CSC therapy
It is worth noting that the regulatory effect of HIF-1 is also different in specific types of cancer (Table III). As HIF-1/HIF-1α is a key factor regulating CSCs, its inhibitor may be used for adjuvant therapy. Acriflavine, a HIF-1 inhibitor, prevents the dimerization of HIF-1α and HIF-1β, leading to undimerized HIF-1α degradation and inhibition of hypoxia-induced gene expression (82). Acriflavine can effectively inhibit the stemness and growth of chronic myeloid leukemia cells (81). Digoxin, also a HIF-1 inhibitor, or acriflavine can prevent glioblastoma stem cells from responding to hypoxia by reducing the expression of receptor for advanced glycation end products, which is a membrane receptor that senses the necrotic stimulation of cells that die due to hypoxia (152). The targeted inhibition of HIF-1 by siRNA can increase the radiotherapy sensitivity of malignant gliomas, but the limited delivery efficiency of siRNA still needs to be resolved (153,154). The HIF-1α vaccine is proposed to inhibit breast cancer metastasis from the perspective of tumor immunity (111). Not only that, the combination of HIF-1α and co-activators can also become a potential therapeutic target. Even under hypoxic conditions, 3-(5-hydroxymethyl-2-furyl)-1-benzyl indazole can stimulate FIH to bind to C-TAD and reduce the recruitment of p300 to inhibit HIF-1α in a N803 hydroxylation-independent manner (155). Bortezomib, which is used for clinical testing of multiple tumors, has similar FIH-mediated anticancer effects (156). Osmium metal complex 1 can be used as an inhibitor to directly interfere with the interaction between HIF-1α and p300 to stop the HIF-1α expression and inhibit cell proliferation (157). Chetomin, discorhabdin L (2) and melatonin play a similar role (153,158–160). The combination of enzalutamide, an androgen receptor antagonist, and chetomin effectively inhibits the growth of castration-resistant prostate cancer cells (158). TEL03, a perylene derivative, acts as a dual targeted inhibitor of STAT3 and HIF-1α, interrupts the phosphorylation of STAT3 and inhibits its transcription; it also inhibits the combination of HIF-1α and CBP/p300 to induce HIF-1α degradation, thereby inducing apoptosis and suppressing tumor growth (161). LB-1 also prevents the formation of the HIF-1α/p300/p-STAT3 complex by targeting both the HIF-1α and STAT3 pathways to inhibit the growth of prostate cancer cells (146). Minnelide, a pro-drug of triptolide, has shown clinical prospects in a recent phase I trial of advanced gastrointestinal malignancies, with the phase II trial in preparation (162). In addition, the inhibition of key enzymes and signaling pathways (Table II), key gene knockout, as well as CSC-related immune and metabolic regulation, all comprise potential targets (Table IV) for CSC therapy.
Table III.
Potential effects of HIF-1 modulation in specific types of cancer.
| Cancer type | Potential effect of HIF-1 | Refs. |
|---|---|---|
| Brain tumor | Promotes CD133+ glioblastoma production and maintains self-renewal; regulates tumor immune microenvironment; also regulates cell proliferation and metastasis | (30,35,36,47,81,86,87,104–106,128,134,152–154) |
| Liver cancer | Promotes CSC production and EMT occurrence; regulates genomic methylation | (28,31,50,76) |
| Osteosarcoma | SENP1/HIF-1α positive feedback loop promotes EMT occurrence and cell invasion | (32,71) |
| Breast cancer | Participates in the regulation of breast CSC chemotherapy resistance, self-renewal, and breast cancer metastasis; promotes immune escape | (6,7,39,43,54,81,82,86,92,111,118,123,128,130,138) |
| Lung cancer | Participates in the regulation of lung CSC radiotherapy resistance and lung cancer invasion and metastasis; also maintains stemness | (41–43,51,58,59) |
| Gastric cancer | Promotes gastric cancer metastasis and chemotherapy resistance; promotes EMT occurrence | (52,67,136) |
| Ewing sarcoma | Participates in the regulation of cell proliferation and survival | (53) |
| Colorectal cancer | Participates in colorectal cancer occurrence and metastasis; promotes CSC production | (46,48,49,57,63,81,84,121) |
| Oral cancer | Regulates sphere formation, metabolism and metastasis | (56,66) |
| Pancreatic cancer | Promotes CSC production | (85,129) |
| Prostate cancer | Regulates CSC production and survival; also related to EMT occurrence | (44,125,140,151,142,146) |
| Cervical cancer | Participates in regulating neovascularization | (91) |
| Melanoma | Promotes melanoma cell self-renewal and regulates tumor immune microenvironment | (114) |
| Esophageal cancer | Maintains stemness | (127) |
| Leukemia | Maintains stemness and self-renewal | (50,81,86,147,148) |
| Ovarian cancer | Maintains stemness and promotes EMT occurrence | (81,136,137,141) |
| Cholangiocarcinoma | Participates in regulating CSC stemness | (132,133,139) |
| Bladder cancer | The switch of HIF-1α to HIF-2α is related to the malignancy and stemness maintenance of bladder cancer | (142) |
HIF-1, hypoxia inducible factor-1; CSC, cancer stem cell; EMT, epithelial-mesenchymal transition; SENP1, small ubiquitin-like modifier proteases 1.
Table IV.
Potential therapeutic targets associated with HIF-1.
| Potential targets | Possible mechanism | (Refs.) |
|---|---|---|
| MAT2A | Maintains the demethylation status of genes | (28) |
| SENP1 | Serves as a SUMO protease and forms a positive feedback loop with HIF-1α | (31,32) |
| ERK | Phosphorylates HIF-1α and increases its expression and stability | (25,33) |
| PKA | Prevents HIF-1α degradation | (34) |
| YB-1 | Participates in regulating HIF-1 phosphorylation | (35,36) |
| Indoleamine 2,3-dioxygenase | Breaks down tryptophan and regulates tumor immunity | (107) |
| Carbonic anhydrase | Regulates intracellular PH | (119,120) |
HIF-1, hypoxia inducible factor-1; MAT2A, methionine adenosyltransferase 2A; SENP1, small ubiquitin-like modifier proteases 1; PKA, protein kinase A; YB-1, Y-box binding protein 1; SUMO, small ubiquitin-like modifier.
10. Conclusions and perspectives
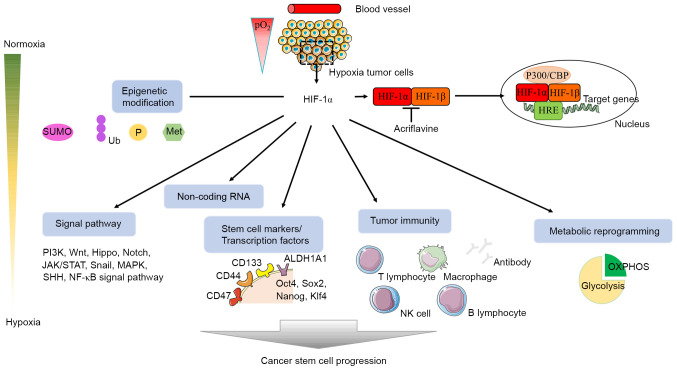
CSCs are a population with the potential for differentiation and self-renewal, and participate in tumor metastasis, recurrence and treatment resistance (81,163). Understanding the mechanisms through which CSCs produce and maintain stemness may help overcome the limitations of clinical cancer treatment. Hypoxia regulates angiogenesis, tumorigenesis, immune response, cancer recurrence and metastasis, and participates in EMT progression and CSC production (81,82,164). HIF-1 stably expressed under hypoxic conditions binds to HRE on the promoter of key genes and regulates glycolysis, angiogenesis, cell apoptosis, tissue invasion and PH regulation (165,166). HIF-1α, as the active subunit of HIF-1, is a primary transcription regulator in hypoxic adaptive responses (81,82). Therefore, the present review focused more on the role of HIF-1α in CSCs instead of HIF-1, in order to propose novel methods for the eradication of CSCs from various perspectives, including epigenetic modification, immune response, metabolic reprogramming, stem cell marker, and ncRNA and signaling pathways associated with CSCs (Fig. 2).
Figure 2.
Hypoxia regulates CSCs with HIF-1 as the core. HIF-1α and HIF-1β form a heterodimer and bind to HRE on the target genes to activate transcription, which can be suppressed by acriflavine, a HIF-1α inhibitor. This chart attempts to show the role and regulation of HIF-1 in CSCs from multiple directions, including epigenetic modification, signaling pathway, non-coding RNA, stem cell marker, immunity and metabolic reprogramming. CSCs, cancer stem cells; HIF-1, hypoxia inducible factor-1; SUMO, small ubiquitin-like modifier; Ub, ubiquitination; P, phosphorylation; Met, methylation; HRE, hypoxic response element; OXPHOS, oxidative phosphorylation; CBP, CREB binding protein; NK, natural killer; Klf4, Krüppel-like factor 4; ALDH1A1, 4-trimethylaminobutyraldehyde dehydrogenase; SHH, Sonic Hedgehog.
The current tumor treatment methods, combined with adjuvant therapy with HIF-1/HIF-1α as the core, may prevent the recurrence and metastasis of cancer cells, and ultimately improve the cure rate and prolong the life of patients.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- CSC
cancer stem cell
- EMT
epithelial-mesenchymal transition
- HIF-1
hypoxia-inducible factor-1
- TAD
transactivation domain
- FIH
factor inhibiting HIF-1
- MAT2A
methionine adenosyltransferase 2A
- SENP1
small ubiquitin-like modifier proteases 1
- ncRNA
non-coding RNA
- siRNA
small interfering RNA
- lncRNA
long ncRNA
- miRNA
microRNA
- ceRNA
competing endogenous RNA
- HRE
hypoxic response element
- VHL
Von Hippel-Lindau
- SNAI1
Snail homolog 1
- Treg
regulatory T cell
- OXPHOS
oxidative phosphorylation
- YAP
yes-associated protein
- TAZ
tafazzin
- CTGF
connective tissue growth factor
- HMGB1
high-mobility group box 1
- VEGF
vascular endothelial growth factor
- DUSPs
dual-specificity phosphatases
- Gli1
glioma-associated oncogene homolog 1
- SIRT1
sirtuin type 1
Funding
This review was supported by the National Natural Science Foundation of China (grant nos. 81372835 and 81670143), and National Key Research and Development Program of China (grant no. 2018YFA0106902).
Availability of data and materials
Not applicable.
Authors' contributions
QZ wrote the manuscript. ZH, YZ, JC and WL reviewed the manuscript critically. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Lagunas-Rangel FA. Circular RNAs and their participation in stemness of cancer. Med Oncol. 2020;37:42. doi: 10.1007/s12032-020-01373-x. [DOI] [PubMed] [Google Scholar]
- 2.Wang H, Cui G, Yu B, Sun M, Yang H. Cancer stem cell niche in colorectal cancer and targeted therapies. Curr Pharm Des. 2020;26:1979–1993. doi: 10.2174/1381612826666200408102305. [DOI] [PubMed] [Google Scholar]
- 3.Sureshbabu SK, Chaukar D, Chiplunkar SV. Hypoxia regulates the differentiation and anti-tumor effector functions of γδT cells in oral cancer. Clin Exp Immunol. 2020;201:40–57. doi: 10.1111/cei.13436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang C, Samanta D, Lu H, Bullen JW, Zhang H, Chen I, He X, Semenza GL. Hypoxia induces the breast cancer stem cell phenotype by HIF-dependent and ALKBH5-mediated m6A-demethylation of NANOG mRNA. Proc Natl Acad Sci USA. 2016;113:E2047–E2056. doi: 10.1073/pnas.1602883113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu QH, Xiao Y, Li XQ, Fan L, Zhou CC, Cheng L, Jiang ZD, Wang GH. Resveratrol Counteracts Hypoxia-Induced Gastric Cancer Invasion and EMT through Hedgehog Pathway Suppression. Anticancer Agents Med Chem. 2020;20:1105–1114. doi: 10.2174/1871520620666200402080034. [DOI] [PubMed] [Google Scholar]
- 6.Ajduković J. HIF-1 - a big chapter in the cancer tale. Exp Oncol. 2016;38:9–12. doi: 10.31768/2312-8852.2016.38(1):9-12. [DOI] [PubMed] [Google Scholar]
- 7.Schito L, Semenza GL. Hypoxia-inducible factors: Master regulators of cancer progression. Trends Cancer. 2016;2:758–770. doi: 10.1016/j.trecan.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 8.Lu Y, Wang L, Ding W, Wang D, Wang X, Luo Q, Zhu L. Ammonia mediates mitochondrial uncoupling and promotes glycolysis via HIF-1 activation in human breast cancer MDA-MB-231 cells. Biochem Biophys Res Commun. 2019;519:153–159. doi: 10.1016/j.bbrc.2019.08.152. [DOI] [PubMed] [Google Scholar]
- 9.Azimi I. The interplay between HIF-1 and calcium signalling in cancer. Int J Biochem Cell Biol. 2018;97:73–77. doi: 10.1016/j.biocel.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Hong M, Shi H, Wang N, Tan HY, Wang Q, Feng Y. Dual effects of Chinese herbal medicines on angiogenesis in cancer and ischemic stroke treatments: Role of HIF-1 network. Front Pharmacol. 2019;10:696. doi: 10.3389/fphar.2019.00696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Albadari N, Deng S, Li W. The transcriptional factors HIF-1 and HIF-2 and their novel inhibitors in cancer therapy. Expert Opin Drug Discov. 2019;14:667–682. doi: 10.1080/17460441.2019.1613370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garner KEL, Hull NJ, Sims AH, Lamb R, Clarke RB. The milk protein alpha-casein suppresses triple negative breast cancer stem cell activity via STAT and HIF-1alpha signalling pathways in breast cancer cells and fibroblasts. J Mammary Gland Biol Neoplasia. 2019;24:245–256. doi: 10.1007/s10911-019-09435-1. [DOI] [PubMed] [Google Scholar]
- 13.Masoud GN, Li W. HIF-1α pathway: Role, regulation and intervention for cancer therapy. Acta Pharm Sin B. 2015;5:378–389. doi: 10.1016/j.apsb.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan ST, Patel PR, Ransom TR, Henrich CJ, McKee TC, Goey AK, Cook KM, Figg WD, McMahon JB, Schnermann MJ, et al. Structural elucidation and synthesis of eudistidine A: An unusual polycyclic marine alkaloid that blocks interaction of the protein binding domains of p300 and HIF-1α. J Am Chem Soc. 2015;137:5569–5575. doi: 10.1021/jacs.5b02156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freedman SJ, Sun ZY, Kung AL, France DS, Wagner G, Eck MJ. Structural basis for negative regulation of hypoxia-inducible factor-1alpha by CITED2. Nat Struct Biol. 2003;10:504–512. doi: 10.1038/nsb936. [DOI] [PubMed] [Google Scholar]
- 16.Kasper LH, Boussouar F, Boyd K, Xu W, Biesen M, Rehg J, Baudino TA, Cleveland JL, Brindle PK. Two transactivation mechanisms cooperate for the bulk of HIF-1-responsive gene expression. EMBO J. 2005;24:3846–3858. doi: 10.1038/sj.emboj.7600846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henchey LK, Kushal S, Dubey R, Chapman RN, Olenyuk BZ, Arora PS. Inhibition of hypoxia inducible factor 1-transcription coactivator interaction by a hydrogen bond surrogate alpha-helix. J Am Chem Soc. 2010;132:941–943. doi: 10.1021/ja9082864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cai X, Huang Y, Zhang X, Wang S, Zou Z, Wang G, Wang Y, Zhang Z. Cloning, characterization, hypoxia and heat shock response of hypoxia inducible factor-1 (HIF-1) from the small abalone Haliotis diversicolor. Gene. 2014;534:256–264. doi: 10.1016/j.gene.2013.10.048. [DOI] [PubMed] [Google Scholar]
- 19.Hu CJ, Sataur A, Wang L, Chen H, Simon MC. The N-terminal transactivation domain confers target gene specificity of hypoxia-inducible factors HIF-1alpha and HIF-2alpha. Mol Biol Cell. 2007;18:4528–4542. doi: 10.1091/mbc.e06-05-0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soñanez-Organis JG, Peregrino-Uriarte AB, Gómez-Jiménez S, López-Zavala A, Forman HJ, Yepiz-Plascencia G. Molecular characterization of hypoxia inducible factor-1 (HIF-1) from the white shrimp Litopenaeus vannamei and tissue-specific expression under hypoxia. Comp Biochem Physiol C Toxicol Pharmacol. 2009;150:395–405. doi: 10.1016/j.cbpc.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 21.Soni S, Padwad YS. HIF-1 in cancer therapy: Two decade long story of a transcription factor. Acta Oncol. 2017;56:503–515. doi: 10.1080/0284186X.2017.1301680. [DOI] [PubMed] [Google Scholar]
- 22.Ju UI, Park JW, Park HS, Kim SJ, Chun YS. FBXO11 represses cellular response to hypoxia by destabilizing hypoxia-inducible factor-1α mRNA. Biochem Biophys Res Commun. 2015;464:1008–1015. doi: 10.1016/j.bbrc.2015.07.037. [DOI] [PubMed] [Google Scholar]
- 23.Mandl M, Depping R. Hypoxia-inducible aryl hydrocarbon receptor nuclear translocator (ARNT) (HIF-1β): Is it a rare exception? Mol Med. 2014;20:215–220. doi: 10.2119/molmed.2014.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qiu Y, Shan W, Yang Y, Jin M, Dai Y, Yang H, Jiao R, Xia Y, Liu Q, Ju L, et al. Reversal of sorafenib resistance in hepatocellular carcinoma: Epigenetically regulated disruption of 14-3-3η/hypoxia-inducible factor-1α. Cell Death Discov. 2019;5:120. doi: 10.1038/s41420-019-0200-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karagiota A, Kourti M, Simos G, Mylonis I. HIF-1α-derived cell-penetrating peptides inhibit ERK-dependent activation of HIF-1 and trigger apoptosis of cancer cells under hypoxia. Cell Mol Life Sci. 2019;76:809–825. doi: 10.1007/s00018-018-2985-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh A, Gupta S, Sachan M. Epigenetic biomarkers in the management of ovarian cancer: Current prospectives. Front Cell Dev Biol. 2019;7:182. doi: 10.3389/fcell.2019.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Urbano A, Smith J, Weeks RJ, Chatterjee A. Gene-specific targeting of DNA methylation in the mammalian genome. Cancers. 2019;11:1515. doi: 10.3390/cancers11101515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Q, Liu L, Zhao Y, Zhang J, Wang D, Chen J, He Y, Wu J, Zhang Z, Liu Z. Hypoxia induces genomic DNA demethylation through the activation of HIF-1α and transcriptional upregulation of MAT2A in hepatoma cells. Mol Cancer Ther. 2011;10:1113–1123. doi: 10.1158/1535-7163.MCT-10-1010. [DOI] [PubMed] [Google Scholar]
- 29.Han ZJ, Feng YH, Gu BH, Li YM, Chen H. The post-translational modification, SUMOylation, and cancer (Review) Int J Oncol. 2018;52:1081–1094. doi: 10.3892/ijo.2018.4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bao L, Chen Y, Lai HT, Wu SY, Wang JE, Hatanpaa KJ, Raisanen JM, Fontenot M, Lega B, Chiang CM, et al. Methylation of hypoxia-inducible factor (HIF)-1α by G9a/GLP inhibits HIF-1 transcriptional activity and cell migration. Nucleic Acids Res. 2018;46:6576–6591. doi: 10.1093/nar/gky449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cui CP, Wong CC, Kai AK, Ho DW, Lau EY, Tsui YM, Chan LK, Cheung TT, Chok KS, Chan AC, et al. SENP1 promotes hypoxia-induced cancer stemness by HIF-1α deSUMOylation and SENP1/HIF-1α positive feedback loop. Gut. 2017;66:2149–2159. doi: 10.1136/gutjnl-2016-313264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang X, Liang X, Liang H, Wang B. SENP1/HIF-1α feedback loop modulates hypoxia-induced cell proliferation, invasion, and EMT in human osteosarcoma cells. J Cell Biochem. 2018;119:1819–1826. doi: 10.1002/jcb.26342. [DOI] [PubMed] [Google Scholar]
- 33.Mylonis I, Kourti M, Samiotaki M, Panayotou G, Simos G. Mortalin-mediated and ERK-controlled targeting of HIF-1α to mitochondria confers resistance to apoptosis under hypoxia. J Cell Sci. 2017;130:466–479. doi: 10.1242/jcs.195339. [DOI] [PubMed] [Google Scholar]
- 34.Bullen JW, Tchernyshyov I, Holewinski RJ, DeVine L, Wu F, Venkatraman V, Kass DL, Cole RN, Van Eyk J, Semenza GL. Protein kinase A-dependent phosphorylation stimulates the transcriptional activity of hypoxia-inducible factor 1. Sci Signal. 2016;9:ra56. doi: 10.1126/scisignal.aaf0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gong H, Gao S, Yu C, Li M, Liu P, Zhang G, Song J, Zheng J. Effect and mechanism of YB-1 knockdown on glioma cell growth, migration, and apoptosis. Acta Biochim Biophys Sin (Shanghai) 2020;52:168–179. doi: 10.1093/abbs/gmz161. [DOI] [PubMed] [Google Scholar]
- 36.El-Naggar AM, Veinotte CJ, Cheng H, Grunewald TG, Negri GL, Somasekharan SP, Corkery DP, Tirode F, Mathers J, Khan D, et al. Translational activation of HIF1α by YB-1 promotes sarcoma metastasis. Cancer Cell. 2015;27:682–697. doi: 10.1016/j.ccell.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 37.Yu AM, Batra N, Tu MJ, Sweeney C. Novel approaches for efficient in vivo fermentation production of noncoding RNAs. Appl Microbiol Biotechnol. 2020;104:1927–1937. doi: 10.1007/s00253-020-10350-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Panir K, Schjenken JE, Robertson SA, Hull ML. Non-coding RNAs in endometriosis: A narrative review. Hum Reprod Update. 2018;24:497–515. doi: 10.1093/humupd/dmy014. [DOI] [PubMed] [Google Scholar]
- 39.Liu C, Xing H, Guo C, Yang Z, Wang Y, Wang Y. MiR-124 reversed the doxorubicin resistance of breast cancer stem cells through STAT3/HIF-1 signaling pathways. Cell Cycle. 2019;18:2215–2227. doi: 10.1080/15384101.2019.1638182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bartoszewski R, Serocki M, Janaszak-Jasiecka A, Bartoszewska S, Kochan-Jamrozy K, Piotrowski A, Króliczewski J, Collawn JF. miR-200b downregulates Krüppel Like Factor 2 (KLF2) during acute hypoxia in human endothelial cells. Eur J Cell Biol. 2017;96:758–766. doi: 10.1016/j.ejcb.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Byun Y, Choi YC, Jeong Y, Lee G, Yoon S, Jeong Y, Yoon J, Baek K. MiR-200c downregulates HIF-1α and inhibits migration of lung cancer cells. Cell Mol Biol Lett. 2019;24:28. doi: 10.1186/s11658-019-0152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen X, Wu L, Li D, Xu Y, Zhang L, Niu K, Kong R, Gu J, Xu Z, Chen Z, et al. Radiosensitizing effects of miR-18a-5p on lung cancer stem-like cells via downregulating both ATM and HIF-1α. Cancer Med. 2018;7:3834–3847. doi: 10.1002/cam4.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krutilina R, Sun W, Sethuraman A, Brown M, Seagroves TN, Pfeffer LM, Ignatova T, Fan M. MicroRNA-18a inhibits hypoxia-inducible factor 1α activity and lung metastasis in basal breast cancers. Breast Cancer Res. 2014;16:R78. doi: 10.1186/bcr3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mathieu J, Zhang Z, Zhou W, Wang AJ, Heddleston JM, Pinna CM, Hubaud A, Stadler B, Choi M, Bar M, et al. HIF induces human embryonic stem cell markers in cancer cells. Cancer Res. 2011;71:4640–4652. doi: 10.1158/0008-5472.CAN-10-3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ho AS, Huang X, Cao H, Christman-Skieller C, Bennewith K, Le QT, Koong AC. Circulating miR-210 as a novel hypoxia marker in pancreatic cancer. Transl Oncol. 2010;3:109–113. doi: 10.1593/tlo.09256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sabry D, El-Deek SE, Maher M, El-Baz MA, El-Bader HM, Amer E, Hassan EA, Fathy W, El-Deek HE. Role of miRNA-210, miRNA-21 and miRNA-126 as diagnostic biomarkers in colorectal carcinoma: Impact of HIF-1α-VEGF signaling pathway. Mol Cell Biochem. 2019;454:177–189. doi: 10.1007/s11010-018-3462-1. [DOI] [PubMed] [Google Scholar]
- 47.Yang W, Wei J, Guo T, Shen Y, Liu F. Knockdown of miR-210 decreases hypoxic glioma stem cells stemness and radioresistance. Exp Cell Res. 2014;326:22–35. doi: 10.1016/j.yexcr.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 48.Han M, Wang Y, Liu M, Bi X, Bao J, Zeng N, Zhu Z, Mo Z, Wu C, Chen X. MiR-21 regulates epithelial-mesenchymal transition phenotype and hypoxia-inducible factor-1α expression in third-sphere forming breast cancer stem cell-like cells. Cancer Sci. 2012;103:1058–1064. doi: 10.1111/j.1349-7006.2012.02281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hermansen SK, Nielsen BS, Aaberg-Jessen C, Kristensen BW. miR-21 is linked to glioma angiogenesis: A co-localization study. J Histochem Cytochem. 2016;64:138–148. doi: 10.1369/0022155415623515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guo Y, Xiao Z, Yang L, Gao Y, Zhu Q, Hu L, Huang D, Xu Q. Hypoxia inducible factors in hepatocellular carcinoma (Review) Oncol Rep. 2020;43:3–15. doi: 10.3892/or.2019.7397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jiang N, Zou C, Zhu Y, Luo Y, Chen L, Lei Y, Tang K, Sun Y, Zhang W, Li S, et al. HIF-1α-regulated miR-1275 maintains stem cell-like phenotypes and promotes the progression of LUAD by simultaneously activating Wnt/β-catenin and Notch signaling. Theranostics. 2020;10:2553–2570. doi: 10.7150/thno.41120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ge X, Liu X, Lin F, Li P, Liu K, Geng R, Dai C, Lin Y, Tang W, Wu Z, et al. MicroRNA-421 regulated by HIF-1α promotes metastasis, inhibits apoptosis, and induces cisplatin resistance by targeting E-cadherin and caspase-3 in gastric cancer. Oncotarget. 2016;7:24466–24482. doi: 10.18632/oncotarget.8228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen J, Zhou X, Xiao Q, Wang T, Shao G, Li Y, Zhang Z. MiR-107 suppresses cell proliferation and tube formation of Ewing sarcoma cells partly by targeting HIF-1β. Hum Cell. 2018;31:42–49. doi: 10.1007/s13577-017-0183-9. [DOI] [PubMed] [Google Scholar]
- 54.Yang Z, Han Y, Cheng K, Zhang G, Wang X. miR-99a directly targets the mTOR signalling pathway in breast cancer side population cells. Cell Prolif. 2014;47:587–595. doi: 10.1111/cpr.12146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu CJ, Tsai MM, Hung PS, Kao SY, Liu TY, Wu KJ, Chiou SH, Lin SC, Chang KW. miR-31 ablates expression of the HIF regulatory factor FIH to activate the HIF pathway in head and neck carcinoma. Cancer Res. 2010;70:1635–1644. doi: 10.1158/0008-5472.CAN-09-2291. [DOI] [PubMed] [Google Scholar]
- 56.Hung PS, Tu HF, Kao SY, Yang CC, Liu CJ, Huang TY, Chang KW, Lin SC. miR-31 is upregulated in oral premalignant epithelium and contributes to the immortalization of normal oral keratinocytes. Carcinogenesis. 2014;35:1162–1171. doi: 10.1093/carcin/bgu024. [DOI] [PubMed] [Google Scholar]
- 57.Chen T, Yao LQ, Shi Q, Ren Z, Ye LC, Xu JM, Zhou PH, Zhong YS. MicroRNA-31 contributes to colorectal cancer development by targeting factor inhibiting HIF-1α (FIH-1) Cancer Biol Ther. 2014;15:516–523. doi: 10.4161/cbt.28017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.He J, Jin S, Zhang W, Wu D, Li J, Xu J, Gao W. Long non-coding RNA LOC554202 promotes acquired gefitinib resistance in non-small cell lung cancer through upregulating miR-31 expression. J Cancer. 2019;10:6003–6013. doi: 10.7150/jca.35097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhu B, Cao X, Zhang W, Pan G, Yi Q, Zhong W, Yan D. MicroRNA-31-5p enhances the Warburg effect via targeting FIH. FASEB J. 2019;33:545–556. doi: 10.1096/fj.201800803R. [DOI] [PubMed] [Google Scholar]
- 60.Kao SY, Tsai MM, Wu CH, Chen JJ, Tseng SH, Lin SC, Chang KW. Co-targeting of multiple microRNAs on factor-Inhibiting hypoxia-Inducible factor gene for the pathogenesis of head and neck carcinomas. Head Neck. 2016;38:522–528. doi: 10.1002/hed.23912. [DOI] [PubMed] [Google Scholar]
- 61.Shih JW, Kung HJ. Long non-coding RNA and tumor hypoxia: New players ushered toward an old arena. J Biomed Sci. 2017;24:53. doi: 10.1186/s12929-017-0358-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dong J, Xu J, Wang X, Jin B. Influence of the interaction between long noncoding RNAs and hypoxia on tumorigenesis. Tumour Biol. 2016;37:1379–1385. doi: 10.1007/s13277-015-4457-0. [DOI] [PubMed] [Google Scholar]
- 63.Ge H, Yan Y, Wu D, Huang Y, Tian F. Potential role of LINC00996 in colorectal cancer: A study based on data mining and bioinformatics. OncoTargets Ther. 2018;11:4845–4855. doi: 10.2147/OTT.S173225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang F, Zhang H, Mei Y, Wu M. Reciprocal regulation of HIF-1α and lincRNA-p21 modulates the Warburg effect. Mol Cell. 2014;53:88–100. doi: 10.1016/j.molcel.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 65.Shen Y, Liu Y, Sun T, Yang W. LincRNA-p21 knockdown enhances radiosensitivity of hypoxic tumor cells by reducing autophagy through HIF-1/Akt/mTOR/P70S6K pathway. Exp Cell Res. 2017;358:188–198. doi: 10.1016/j.yexcr.2017.06.016. [DOI] [PubMed] [Google Scholar]
- 66.Shih JW, Chiang WF, Wu AT, Wu MH, Wang LY, Yu YL, Hung YW, Wang WC, Chu CY, Hung CL, et al. Long noncoding RNA LncHIFCAR/MIR31HG is a HIF-1α co-activator driving oral cancer progression. Nat Commun. 2017;8:15874. doi: 10.1038/ncomms15874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang J, Jin HY, Wu Y, Zheng ZC, Guo S, Wang Y, Yang D, Meng XY, Xu X, Zhao Y. Hypoxia-induced LncRNA PCGEM1 promotes invasion and metastasis of gastric cancer through regulating SNAI1. Clin Transl Oncol. 2019;21:1142–1151. doi: 10.1007/s12094-019-02035-9. [DOI] [PubMed] [Google Scholar]
- 68.Zhang W, Wang J, Chai R, Zhong G, Zhang C, Cao W, Yan L, Zhang X, Xu Z. Hypoxia-regulated lncRNA CRPAT4 promotes cell migration via regulating AVL9 in clear cell renal cell carcinomas. OncoTargets Ther. 2018;11:4537–4545. doi: 10.2147/OTT.S169155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang W, Sun T, Cao J, Fan S. Hypoxia-inducible factor-1α downregulation by small interfering RNA inhibits proliferation, induces apoptosis, and enhances radiosensitivity in chemical hypoxic human hepatoma SMMC-7721 cells. Cancer Biother Radiopharm. 2011;26:565–571. doi: 10.1089/cbr.2011.0955. [DOI] [PubMed] [Google Scholar]
- 70.Staab A, Fleischer M, Loeffler J, Said HM, Katzer A, Plathow C, Einsele H, Flentje M, Vordermark D. Small interfering RNA targeting HIF-1alpha reduces hypoxia-dependent transcription and radiosensitizes hypoxic HT 1080 human fibrosarcoma cells in vitro. Strahlenther Onkol. 2011;187:252–259. doi: 10.1007/s00066-011-2167-0. [DOI] [PubMed] [Google Scholar]
- 71.Yu X, Hu L, Li S, Shen J, Wang D, Xu R, Yang H. Long non-coding RNA Taurine upregulated gene 1 promotes osteosarcoma cell metastasis by mediating HIF-1α via miR-143-5p. Cell Death Dis. 2019;10:280. doi: 10.1038/s41419-019-1509-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Augoff K, McCue B, Plow EF, Sossey-Alaoui K. miR-31 and its host gene lncRNA LOC554202 are regulated by promoter hypermethylation in triple-negative breast cancer. Mol Cancer. 2012;11:5. doi: 10.1186/1476-4598-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sun Y, Jia X, Wang M, Deng Y. Long noncoding RNA MIR31HG abrogates the availability of tumor suppressor microRNA-361 for the growth of osteosarcoma. Cancer Manag Res. 2019;11:8055–8064. doi: 10.2147/CMAR.S214569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu Y, Zhu J, Ma X, Han S, Xiao D, Jia Y, Wang Y. ceRNA network construction and comparison of gastric cancer with or without Helicobacter pylori infection. J Cell Physiol. 2019;234:7128–7140. doi: 10.1002/jcp.27467. [DOI] [PubMed] [Google Scholar]
- 75.Sun J, Yan J, Yuan X, Yang R, Dan T, Wang X, Kong G, Gao S. A computationally constructed ceRNA interaction network based on a comparison of the SHEE and SHEEC cell lines. Cell Mol Biol Lett. 2016;21:21. doi: 10.1186/s11658-016-0022-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang Q, Cheng Q, Xia M, Huang X, He X, Liao J. Hypoxia-induced lncRNA-NEAT1 sustains the growth of hepatocellular carcinoma via regulation of miR-199a-3p/UCK2. Front Oncol. 2020;10:998. doi: 10.3389/fonc.2020.00998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.van Schaijik B, Davis PF, Wickremesekera AC, Tan ST, Itinteang T. Subcellular localisation of the stem cell markers OCT4, SOX2, NANOG, KLF4 and c-MYC in cancer: A review. J Clin Pathol. 2018;71:88–91. doi: 10.1136/jclinpath-2017-204815. [DOI] [PubMed] [Google Scholar]
- 78.Wang Z, Zöller M. Exosomes, metastases, and the miracle of cancer stem cell markers. Cancer Metastasis Rev. 2019;38:259–295. doi: 10.1007/s10555-019-09793-6. [DOI] [PubMed] [Google Scholar]
- 79.Lin YT, Wu KJ. Epigenetic regulation of epithelial-mesenchymal transition: Focusing on hypoxia and TGF-β signaling. J Biomed Sci. 2020;27:39. doi: 10.1186/s12929-020-00632-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rankin EB, Nam JM, Giaccia AJ. Hypoxia: Signaling the metastatic cascade. Trends Cancer. 2016;2:295–304. doi: 10.1016/j.trecan.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hajizadeh F, Okoye I, Esmaily M, Ghasemi Chaleshtari M, Masjedi A, Azizi G, Irandoust M, Ghalamfarsa G, Jadidi-Niaragh F. Hypoxia inducible factors in the tumor microenvironment as therapeutic targets of cancer stem cells. Life Sci. 2019;237:116952. doi: 10.1016/j.lfs.2019.116952. [DOI] [PubMed] [Google Scholar]
- 82.Zhang H, Lu H, Xiang L, Bullen JW, Zhang C, Samanta D, Gilkes DM, He J, Semenza GL. HIF-1 regulates CD47 expression in breast cancer cells to promote evasion of phagocytosis and maintenance of cancer stem cells. Proc Natl Acad Sci USA. 2015;112:E6215–E6223. doi: 10.1073/pnas.1520032112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Thomas S, Harding MA, Smith SC, Overdevest JB, Nitz MD, Frierson HF, Tomlins SA, Kristiansen G, Theodorescu D. CD24 is an effector of HIF-1-driven primary tumor growth and metastasis. Cancer Res. 2012;72:5600–5612. doi: 10.1158/0008-5472.CAN-11-3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ohnishi S, Maehara O, Nakagawa K, Kameya A, Otaki K, Fujita H, Higashi R, Takagi K, Asaka M, Sakamoto N, et al. hypoxia-inducible factors activate CD133 promoter through ETS family transcription factors. PLoS One. 2013;8:e66255. doi: 10.1371/journal.pone.0066255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hashimoto O, Shimizu K, Semba S, Chiba S, Ku Y, Yokozaki H, Hori Y. Hypoxia induces tumor aggressiveness and the expansion of CD133-positive cells in a hypoxia-inducible factor-1alpha-dependent manner in pancreatic cancer cells. Pathobiology. 2011;78:181–192. doi: 10.1159/000325538. [DOI] [PubMed] [Google Scholar]
- 86.Chiu DK, Zhang MS, Tse AP, Wong CC. Assessment of stabilization and activity of the HIFs important for hypoxia-induced signalling in cancer cells. Methods Mol Biol. 2019;1928:77–99. doi: 10.1007/978-1-4939-9027-6_6. [DOI] [PubMed] [Google Scholar]
- 87.Soeda A, Park M, Lee D, Mintz A, Androutsellis-Theotokis A, McKay RD, Engh J, Iwama T, Kunisada T, Kassam AB, et al. Hypoxia promotes expansion of the CD133-positive glioma stem cells through activation of HIF-1alpha. Oncogene. 2009;28:3949–3959. doi: 10.1038/onc.2009.252. [DOI] [PubMed] [Google Scholar]
- 88.Hashimoto K, Aoyagi K, Isobe T, Kouhuji K, Shirouzu K. Expression of CD133 in the cytoplasm is associated with cancer progression and poor prognosis in gastric cancer. Gastric Cancer. 2014;17:97–106. doi: 10.1007/s10120-013-0255-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Maeda K, Ding Q, Yoshimitsu M, Kuwahata T, Miyazaki Y, Tsukasa K, Hayashi T, Shinchi H, Natsugoe S, Takao S. CD133 modulate HIF-1alpha expression under hypoxia in EMT phenotype pancreatic cancer stem-like cells. Int J Mol Sci. 2016;17:1025. doi: 10.3390/ijms17071025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Matsumoto K, Arao T, Tanaka K, Kaneda H, Kudo K, Fujita Y, Tamura D, Aomatsu K, Tamura T, Yamada Y, et al. mTOR signal and hypoxia-inducible factor-1 alpha regulate CD133 expression in cancer cells. Cancer Res. 2009;69:7160–7164. doi: 10.1158/0008-5472.CAN-09-1289. [DOI] [PubMed] [Google Scholar]
- 91.Li SW, Wu XL, Dong CL, Xie XY, Wu JF, Zhang X. The differential expression of OCT4 isoforms in cervical carcinoma. PLoS One. 2015;10:e0118033. doi: 10.1371/journal.pone.0118033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ciccone V, Terzuoli E, Donnini S, Giachetti A, Morbidelli L, Ziche M. Stemness marker ALDH1A1 promotes tumor angiogenesis via retinoic acid/HIF-1α/VEGF signalling in MCF-7 breast cancer cells. J Exp Clin Cancer Res. 2018;37:311. doi: 10.1186/s13046-018-0975-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen Y, Xu H, Shi Q, Gu M, Wan X, Chen Q, Wang Z. Hypoxia-inducible factor 1α (HIF-1α) mediates the epithelial-mesenchymal transition in benign prostatic hyperplasia. Int J Clin Exp Pathol. 2019;12:295–304. [PMC free article] [PubMed] [Google Scholar]
- 94.Abouhashem NS, Ibrahim DA, Mohamed AM. Prognostic implications of epithelial to mesenchymal transition related proteins (E-cadherin, Snail) and hypoxia inducible factor 1α in endometrioid endometrial carcinoma. Ann Diagn Pathol. 2016;22:1–11. doi: 10.1016/j.anndiagpath.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 95.Lee SY, Ju MK, Jeon HM, Lee YJ, Kim CH, Park HG, Han SI, Kang HS. Oncogenic metabolism acts as a prerequisite step for induction of cancer metastasis and cancer stem cell phenotype. Oxid Med Cell Longev. 2018;2018:1027453. doi: 10.1155/2018/1027453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kouvaras E, Christoni Z, Siasios I, Malizos K, Koukoulis GK, Ioannou M. Hypoxia-inducible factor 1-alpha and vascular endothelial growth factor in cartilage tumors. Biotech Histochem. 2019;94:283–289. doi: 10.1080/10520295.2018.1556806. [DOI] [PubMed] [Google Scholar]
- 97.Chabi S, Uzan B, Naguibneva I, Rucci J, Fahy L, Calvo J, Arcangeli ML, Mazurier F, Pflumio F, Haddad R. Hypoxia regulates lymphoid development of human hematopoietic progenitors. Cell Rep. 2019;29:2307–2320.e6. doi: 10.1016/j.celrep.2019.10.050. [DOI] [PubMed] [Google Scholar]
- 98.Barsoum IB, Smallwood CA, Siemens DR, Graham CH. A mechanism of hypoxia-mediated escape from adaptive immunity in cancer cells. Cancer Res. 2014;74:665–674. doi: 10.1158/0008-5472.CAN-14-2598. [DOI] [PubMed] [Google Scholar]
- 99.Polke M, Seiler F, Lepper PM, Kamyschnikow A, Langer F, Monz D, Herr C, Bals R, Beisswenger C. Hypoxia and the hypoxia-regulated transcription factor HIF-1α suppress the host defence of airway epithelial cells. Innate Immun. 2017;23:373–380. doi: 10.1177/1753425917698032. [DOI] [PubMed] [Google Scholar]
- 100.Flück K, Breves G, Fandrey J, Winning S. Hypoxia-inducible factor 1 in dendritic cells is crucial for the activation of protective regulatory T cells in murine colitis. Mucosal Immunol. 2016;9:379–390. doi: 10.1038/mi.2015.67. [DOI] [PubMed] [Google Scholar]
- 101.Sun X, Kanwar JR, Leung E, Lehnert K, Wang D, Krissansen GW. Gene transfer of antisense hypoxia inducible factor-1 alpha enhances the therapeutic efficacy of cancer immunotherapy. Gene Ther. 2001;8:638–645. doi: 10.1038/sj.gt.3301388. [DOI] [PubMed] [Google Scholar]
- 102.Liu F, Wang P, Jiang X, Tan G, Qiao H, Jiang H, Krissansen GW, Sun X. Antisense hypoxia-inducible factor 1alpha gene therapy enhances the therapeutic efficacy of doxorubicin to combat hepatocellular carcinoma. Cancer Sci. 2008;99:2055–2061. doi: 10.1111/j.1349-7006.2008.00905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tartour E, Pere H, Maillere B, Terme M, Merillon N, Taieb J, Sandoval F, Quintin-Colonna F, Lacerda K, Karadimou A, et al. Angiogenesis and immunity: A bidirectional link potentially relevant for the monitoring of antiangiogenic therapy and the development of novel therapeutic combination with immunotherapy. Cancer Metastasis Rev. 2011;30:83–95. doi: 10.1007/s10555-011-9281-4. [DOI] [PubMed] [Google Scholar]
- 104.Wei J, Wu A, Kong LY, Wang Y, Fuller G, Fokt I, Melillo G, Priebe W, Heimberger AB. Hypoxia potentiates glioma-mediated immunosuppression. PLoS One. 2011;6:e16195. doi: 10.1371/journal.pone.0016195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wu A, Wei J, Kong LY, Wang Y, Priebe W, Qiao W, Sawaya R, Heimberger AB. Glioma cancer stem cells induce immunosuppressive macrophages/microglia. Neuro Oncol. 2010;12:1113–1125. doi: 10.1093/neuonc/noq082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wei J, Barr J, Kong LY, Wang Y, Wu A, Sharma AK, Gumin J, Henry V, Colman H, Priebe W, et al. Glioblastoma cancer-initiating cells inhibit T-cell proliferation and effector responses by the signal transducers and activators of transcription 3 pathway. Mol Cancer Ther. 2010;9:67–78. doi: 10.1158/1535-7163.MCT-09-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ye LY, Chen W, Bai XL, Xu XY, Zhang Q, Xia XF, Sun X, Li GG, Hu QD, Fu QH, et al. Hypoxia-induced epithelial-to-Mesenchymal transition in hepatocellular carcinoma induces an immunosuppressive tumor microenvironment to promote metastasis. Cancer Res. 2016;76:818–830. doi: 10.1158/0008-5472.CAN-15-0977. [DOI] [PubMed] [Google Scholar]
- 108.Noman MZ, Hasmim M, Messai Y, Terry S, Kieda C, Janji B, Chouaib S. Hypoxia: A key player in antitumor immune response. A review in the theme: Cellular responses to hypoxia. Am J Physiol Cell Physiol. 2015;309:C569–C579. doi: 10.1152/ajpcell.00207.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Guo X, Xue H, Shao Q, Wang J, Guo X, Chen X, Zhang J, Xu S, Li T, Zhang P, et al. Hypoxia promotes glioma-associated macrophage infiltration via periostin and subsequent M2 polarization by upregulating TGF-beta and M-CSFR. Oncotarget. 2016;7:80521–80542. doi: 10.18632/oncotarget.11825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yu X, Li Z, Zhang Y, Xu M, Che Y, Tian X, Wang R, Zou K, Zou L. β-elemene inhibits radiation and hypoxia-induced macrophages infiltration via Prx-1/NF-κB/HIF-1α signaling pathway. OncoTargets Ther. 2019;12:4203–4211. doi: 10.2147/OTT.S196910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cecil DL, Slota M, O'Meara MM, Curtis BC, Gad E, Dang Y, Herendeen D, Rastetter L, Disis ML. Immunization against HIF-1α inhibits the growth of basal mammary tumors and targets mammary stem cells in vivo. Clin Cancer Res. 2017;23:3396–3404. doi: 10.1158/1078-0432.CCR-16-1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.de Almeida PE, Mak J, Hernandez G, Jesudason R, Herault A, Javinal V, Borneo J, Kim JM, Walsh KB. Anti-VEGF treatment enhances CD8+ T-cell antitumor activity by amplifying hypoxia. Cancer Immunol Res. 2020;8:806–818. doi: 10.1158/2326-6066.CIR-19-0360. [DOI] [PubMed] [Google Scholar]
- 113.Hasmim M, Noman MZ, Lauriol J, Benlalam H, Mallavialle A, Rosselli F, Mami-Chouaib F, Alcaide-Loridan C, Chouaib S. Hypoxia-dependent inhibition of tumor cell susceptibility to CTL-mediated lysis involves NANOG induction in target cells. J Immunol. 2011;187:4031–4039. doi: 10.4049/jimmunol.1101011. [DOI] [PubMed] [Google Scholar]
- 114.Hasmim M, Noman MZ, Messai Y, Bordereaux D, Gros G, Baud V, Chouaib S. Cutting edge: Hypoxia-induced Nanog favors the intratumoral infiltration of regulatory T cells and macrophages via direct regulation of TGF-β1. J Immunol. 2013;191:5802–5806. doi: 10.4049/jimmunol.1302140. [DOI] [PubMed] [Google Scholar]
- 115.Nam K, Oh S, Shin I. Ablation of CD44 induces glycolysis-to-oxidative phosphorylation transition via modulation of the c-Src-Akt-LKB1-AMPKα pathway. Biochem J. 2016;473:3013–3030. doi: 10.1042/BCJ20160613. [DOI] [PubMed] [Google Scholar]
- 116.Moldogazieva NT, Mokhosoev IM, Terentiev AA. Metabolic heterogeneity of cancer cells: An interplay between HIF-1, GLUTs, and AMPK. Cancers (Basel) 2020;12:862. doi: 10.3390/cancers12040862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Semenza GL. Hypoxia-inducible factors: Coupling glucose metabolism and redox regulation with induction of the breast cancer stem cell phenotype. EMBO J. 2017;36:252–259. doi: 10.15252/embj.201695204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kuo CY, Cheng CT, Hou P, Lin YP, Ma H, Chung Y, Chi K, Chen Y, Li W, Kung HJ, et al. HIF-1-alpha links mitochondrial perturbation to the dynamic acquisition of breast cancer tumorigenicity. Oncotarget. 2016;7:34052–34069. doi: 10.18632/oncotarget.8570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Deshmukh A, Deshpande K, Arfuso F, Newsholme P, Dharmarajan A. Cancer stem cell metabolism: A potential target for cancer therapy. Mol Cancer. 2016;15:69. doi: 10.1186/s12943-016-0555-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shen YA, Pan SC, Chu I, Lai RY, Wei YH. Targeting cancer stem cells from a metabolic perspective. Exp Biol Med (Maywood) 2020;245:465–476. doi: 10.1177/1535370220909309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lai HT, Chiang CT, Tseng WK, Chao TC, Su Y. GATA6 enhances the stemness of human colon cancer cells by creating a metabolic symbiosis through upregulating LRH-1 expression. Mol Oncol. 2020;14:1327–1347. doi: 10.1002/1878-0261.12647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Song KH, Kim JH, Lee YH, Bae HC, Lee HJ, Woo SR, Oh SJ, Lee KM, Yee C, Kim BW, et al. Mitochondrial reprogramming via ATP5H loss promotes multimodal cancer therapy resistance. J Clin Invest. 2018;128:4098–4114. doi: 10.1172/JCI96804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tang K, Yu Y, Zhu L, Xu P, Chen J, Ma J, Zhang H, Fang H, Sun W, Zhou L, et al. Hypoxia-reprogrammed tricarboxylic acid cycle promotes the growth of human breast tumorigenic cells. Oncogene. 2019;38:6970–6984. doi: 10.1038/s41388-019-0932-1. [DOI] [PubMed] [Google Scholar]
- 124.Patra K, Jana S, Sarkar A, Mandal DP, Bhattacharjee S. The inhibition of hypoxia-induced angiogenesis and metastasis by cinnamaldehyde is mediated by decreasing HIF-1α protein synthesis via PI3K/Akt pathway. Biofactors. 2019;45:401–415. doi: 10.1002/biof.1499. [DOI] [PubMed] [Google Scholar]
- 125.Marhold M, Tomasich E, El-Gazzar A, Heller G, Spittler A, Horvat R, Krainer M, Horak P. HIF1α regulates mTOR signaling and viability of prostate cancer stem cells. Mol Cancer Res. 2015;13:556–564. doi: 10.1158/1541-7786.MCR-14-0153-T. [DOI] [PubMed] [Google Scholar]
- 126.Jung J, Zhang Y, Celiku O, Zhang W, Song H, Williams BJ, Giles AJ, Rich JN, Abounader R, Gilbert MR, et al. Mitochondrial NIX promotes tumor survival in the hypoxic niche of glioblastoma. Cancer Res. 2019;79:5218–5232. doi: 10.1158/0008-5472.CAN-19-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lv Z, Liu RD, Chen XQ, Wang B, Li LF, Guo YS, Chen XJ, Ren XQ. HIF 1α promotes the stemness of oesophageal squamous cell carcinoma by activating the Wnt/β catenin pathway. Oncol Rep. 2019;42:726–734. doi: 10.3892/or.2019.7203. [DOI] [PubMed] [Google Scholar]
- 128.Xiang L, Gilkes DM, Hu H, Luo W, Bullen JW, Liang H, Semenza GL. HIF-1α and TAZ serve as reciprocal co-activators in human breast cancer cells. Oncotarget. 2015;6:11768–11778. doi: 10.18632/oncotarget.4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhang L, Shi H, Chen H, Gong A, Liu Y, Song L, Xu X, You T, Fan X, Wang D, et al. Dedifferentiation process driven by radiotherapy-induced HMGB1/TLR2/YAP/HIF-1α signaling enhances pancreatic cancer stemness. Cell Death Dis. 2019;10:724. doi: 10.1038/s41419-019-1956-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Qian J, Rankin EB. Hypoxia-induced phenotypes that mediate tumor heterogeneity. Adv Exp Med Biol. 2019;1136:43–55. doi: 10.1007/978-3-030-12734-3_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Luo M, Shang L, Brooks MD, Jiagge E, Zhu Y, Buschhaus JM, Conley S, Fath MA, Davis A, Gheordunescu E, et al. Targeting breast cancer stem cell state equilibrium through modulation of redox signaling. Cell Metab. 2018;28:69–86.e6. doi: 10.1016/j.cmet.2018.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang Y, Liu Y, Malek SN, Zheng P, Liu Y. Targeting HIF1α eliminates cancer stem cells in hematological malignancies. Cell Stem Cell. 2011;8:399–411. doi: 10.1016/j.stem.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hirata H, Yoshiura S, Ohtsuka T, Bessho Y, Harada T, Yoshikawa K, Kageyama R. Oscillatory expression of the bHLH factor Hes1 regulated by a negative feedback loop. Science. 2002;298:840–843. doi: 10.1126/science.1074560. [DOI] [PubMed] [Google Scholar]
- 134.Almiron Bonnin DA, Havrda MC, Lee MC, Liu H, Zhang Z, Nguyen LN, Harrington LX, Hassanpour S, Cheng C, Israel MA. Secretion-mediated STAT3 activation promotes self-renewal of glioma stem-like cells during hypoxia. Oncogene. 2018;37:1107–1118. doi: 10.1038/onc.2017.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhang L, Huang G, Li X, Zhang Y, Jiang Y, Shen J, Liu J, Wang Q, Zhu J, Feng X, et al. Hypoxia induces epithelial-mesenchymal transition via activation of SNAI1 by hypoxia-inducible factor −1α in hepatocellular carcinoma. BMC Cancer. 2013;13:108. doi: 10.1186/1471-2407-13-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yang SW, Zhang ZG, Hao YX, Zhao YL, Qian F, Shi Y, Li PA, Liu CY, Yu PW. HIF-1α induces the epithelial-mesenchymal transition in gastric cancer stem cells through the Snail pathway. Oncotarget. 2017;8:9535–9545. doi: 10.18632/oncotarget.14484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Du J, Sun B, Zhao X, Gu Q, Dong X, Mo J, Sun T, Wang J, Sun R, Liu Y. Hypoxia promotes vasculogenic mimicry formation by inducing epithelial-mesenchymal transition in ovarian carcinoma. Gynecol Oncol. 2014;133:575–583. doi: 10.1016/j.ygyno.2014.02.034. [DOI] [PubMed] [Google Scholar]
- 138.Lu H, Tran L, Park Y, Chen I, Lan J, Xie Y, Semenza GL. Reciprocal regulation of DUSP9 and DUSP16 expression by HIF1 controls ERK and p38 MAP kinase activity and mediates chemotherapy-induced breast cancer stem cell enrichment. Cancer Res. 2018;78:4191–4202. doi: 10.1158/0008-5472.CAN-18-0270. [DOI] [PubMed] [Google Scholar]
- 139.Bhuria V, Xing J, Scholta T, Bui KC, Nguyen MLT, Malek NP, Bozko P, Plentz RR. Hypoxia induced Sonic Hedgehog signaling regulates cancer stemness, epithelial-to-mesenchymal transition and invasion in cholangiocarcinoma. Exp Cell Res. 2019;385:111671. doi: 10.1016/j.yexcr.2019.111671. [DOI] [PubMed] [Google Scholar]
- 140.Lv L, Yang Z, Ma T, Xuan Y. Gli1, a potential cancer stem cell marker, is strongly associated with prognosis in prostate cancer. Int J Clin Exp Pathol. 2018;11:4957–4966. [PMC free article] [PubMed] [Google Scholar]
- 141.Qin J, Liu Y, Lu Y, Liu M, Li M, Li J, Wu L. Hypoxia-inducible factor 1 alpha promotes cancer stem cells-like properties in human ovarian cancer cells by upregulating SIRT1 expression. Sci Rep. 2017;7:10592. doi: 10.1038/s41598-017-09244-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Guan Z, Ding C, Du Y, Zhang K, Zhu JN, Zhang T, He D, Xu S, Wang X, Fan J. HAF drives the switch of HIF-1α to HIF-2α by activating the NF-κB pathway, leading to malignant behavior of T24 bladder cancer cells. Int J Oncol. 2014;44:393–402. doi: 10.3892/ijo.2013.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mendonça DB, Mendonça G, Aragão FJ, Cooper LF. NF-κB suppresses HIF-1α response by competing for P300 binding. Biochem Biophys Res Commun. 2011;404:997–1003. doi: 10.1016/j.bbrc.2010.12.098. [DOI] [PubMed] [Google Scholar]
- 144.Zhang Y, Jiang X, Qin X, Ye D, Yi Z, Liu M, Bai O, Liu W, Xie X, Wang Z, et al. RKTG inhibits angiogenesis by suppressing MAPK-mediated autocrine VEGF signaling and is downregulated in clear-cell renal cell carcinoma. Oncogene. 2010;29:5404–5415. doi: 10.1038/onc.2010.270. [DOI] [PubMed] [Google Scholar]
- 145.Roth U, Curth K, Unterman TG, Kietzmann T. The transcription factors HIF-1 and HNF-4 and the coactivator p300 are involved in insulin-regulated glucokinase gene expression via the phosphatidylinositol 3-kinase/protein kinase B pathway. J Biol Chem. 2004;279:2623–2631. doi: 10.1074/jbc.M308391200. [DOI] [PubMed] [Google Scholar]
- 146.Niu F, Li Y, Lai FF, Ni L, Ji M, Jin J, Yang HZ, Wang C, Zhang DM, Chen XG. LB-1 exerts antitumor activity in pancreatic cancer by inhibiting HIF-1α and Stat3 signaling. J Cell Physiol. 2015;230:2212–2223. doi: 10.1002/jcp.24949. [DOI] [PubMed] [Google Scholar]
- 147.Kida A, Kahn M. Hypoxia selects for a quiescent, CML stem/leukemia initiating-like population dependent on CBP/catenin transcription. Curr Mol Pharmacol. 2013;6:204–210. doi: 10.2174/1874467207666140219121219. [DOI] [PubMed] [Google Scholar]
- 148.Bordonaro M, Lazarova DL. CREB-binding protein, p300, butyrate, and Wnt signaling in colorectal cancer. World J Gastroenterol. 2015;21:8238–8248. doi: 10.3748/wjg.v21.i27.8238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yoon H, Lim JH, Cho CH, Huang LE, Park JW. CITED2 controls the hypoxic signaling by snatching p300 from the two distinct activation domains of HIF-1α. Biochim Biophys Acta. 2011;1813:2008–2016. doi: 10.1016/j.bbamcr.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 150.Jin P, Kang J, Lee MK, Park JW. Ferritin heavy chain controls the HIF-driven hypoxic response by activating the asparaginyl hydroxylase FIH. Biochem Biophys Res Commun. 2018;499:475–481. doi: 10.1016/j.bbrc.2018.03.173. [DOI] [PubMed] [Google Scholar]
- 151.Lee GY, Shin SH, Shin HW, Chun YS, Park JW. NDRG3 lowers the metastatic potential in prostate cancer as a feedback controller of hypoxia-inducible factors. Exp Mol Med. 2018;50:1–13. doi: 10.1038/s12276-018-0083-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Papale M, Buccarelli M, Mollinari C, Russo MA, Pallini R, Ricci-Vitiani L, Tafani M. Hypoxia, inflammation and necrosis as determinants of glioblastoma cancer stem cells progression. Int J Mol Sci. 2020;21:2660. doi: 10.3390/ijms21082660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kessler J, Hahnel A, Wichmann H, Rot S, Kappler M, Bache M, Vordermark D. HIF-1α inhibition by siRNA or chetomin in human malignant glioma cells: Effects on hypoxic radioresistance and monitoring via CA9 expression. BMC Cancer. 2010;10:605. doi: 10.1186/1471-2407-10-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lo WL, Chien Y, Chiou GY, Tseng LM, Hsu HS, Chang YL, Lu KH, Chien CS, Wang ML, Chen YW, et al. Nuclear localization signal-enhanced RNA interference of EZH2 and Oct4 in the eradication of head and neck squamous cell carcinoma-derived cancer stem cells. Biomaterials. 2012;33:3693–3709. doi: 10.1016/j.biomaterials.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 155.Li SH, Shin DH, Chun YS, Lee MK, Kim MS, Park JW. A novel mode of action of YC-1 in HIF inhibition: Stimulation of FIH-dependent p300 dissociation from HIF-1{alpha} Mol Cancer Ther. 2008;7:3729–3738. doi: 10.1158/1535-7163.MCT-08-0074. [DOI] [PubMed] [Google Scholar]
- 156.Shin DH, Chun YS, Lee DS, Huang LE, Park JW. Bortezomib inhibits tumor adaptation to hypoxia by stimulating the FIH-mediated repression of hypoxia-inducible factor-1. Blood. 2008;111:3131–3136. doi: 10.1182/blood-2007-11-120576. [DOI] [PubMed] [Google Scholar]
- 157.Yang C, Wang W, Li GD, Zhong HJ, Dong ZZ, Wong CY, Kwong DW, Ma DL, Leung CH. Anticancer osmium complex inhibitors of the HIF-1α and p300 protein-protein interaction. Sci Rep. 2017;7:42860. doi: 10.1038/srep42860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Fernandez EV, Reece KM, Ley AM, Troutman SM, Sissung TM, Price DK, Chau CH, Figg WD. Dual targeting of the androgen receptor and hypoxia-inducible factor 1α pathways synergistically inhibits castration-resistant prostate cancer cells. Mol Pharmacol. 2015;87:1006–1012. doi: 10.1124/mol.114.097477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Goradel NH, Asghari MH, Moloudizargari M, Negahdari B, Haghi-Aminjan H, Abdollahi M. Melatonin as an angiogenesis inhibitor to combat cancer: Mechanistic evidence. Toxicol Appl Pharmacol. 2017;335:56–63. doi: 10.1016/j.taap.2017.09.022. [DOI] [PubMed] [Google Scholar]
- 160.Harris EM, Strope JD, Beedie SL, Huang PA, Goey AK, Cook KM, Schofield CJ, Chau CH, Cadelis MM, Copp BR, et al. Preclinical evaluation of discorhabdins in antiangiogenic and antitumor models. Mar Drugs. 2018;16:241. doi: 10.3390/md16070241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chen H, Guan Y, Yuan G, Zhang Q, Jing N. A perylene derivative regulates HIF-1α and Stat3 signaling pathways. Bioorg Med Chem. 2014;22:1496–1505. doi: 10.1016/j.bmc.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 162.McGinn O, Gupta VK, Dauer P, Arora N, Sharma N, Nomura A, Dudeja V, Saluja A, Banerjee S. Inhibition of hypoxic response decreases stemness and reduces tumorigenic signaling due to impaired assembly of HIF1 transcription complex in pancreatic cancer. Sci Rep. 2017;7:7872. doi: 10.1038/s41598-017-08447-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wang T, Shigdar S, Gantier MP, Hou Y, Wang L, Li Y, Shamaileh HA, Yin W, Zhou SF, Zhao X, et al. Cancer stem cell targeted therapy: Progress amid controversies. Oncotarget. 2015;6:44191–44206. doi: 10.18632/oncotarget.6176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Xiang L, Semenza GL. Hypoxia-inducible factors promote breast cancer stem cell specification and maintenance in response to hypoxia or cytotoxic chemotherapy. Adv Cancer Res. 2019;141:175–212. doi: 10.1016/bs.acr.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 165.Yeo CD, Kang N, Choi SY, Kim BN, Park CK, Kim JW, Kim YK, Kim SJ. The role of hypoxia on the acquisition of epithelial-mesenchymal transition and cancer stemness: A possible link to epigenetic regulation. Korean J Intern Med. 2017;32:589–599. doi: 10.3904/kjim.2016.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lee JH, Hur W, Hong SW, Kim JH, Kim SM, Lee EB, Yoon SK. ELK3 promotes the migration and invasion of liver cancer stem cells by targeting HIF-1α. Oncol Rep. 2017;37:813–822. doi: 10.3892/or.2016.5293. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.