ABSTRACT
Auxin is an endogenous small molecule with an incredibly large impact on growth and development in plants. Movement of auxin between cells, due to its negative charge at most physiological pHs, strongly relies on families of active transporters. These proteins import auxin from the extracellular space or export it into the same. Mutations in these components have profound impacts on biological processes. Another transport route available to auxin, once the substance is inside the cell, are plasmodesmata connections. These small channels connect the cytoplasms of neighbouring plant cells and enable flow between them. Interestingly, the biological significance of this latter mode of transport is only recently starting to emerge with examples from roots, hypocotyls and leaves. The existence of two transport systems provides opportunities for reciprocal cross-regulation. Indeed, auxin levels influence proteins controlling plasmodesmata permeability, while cell–cell communication affects auxin biosynthesis and transport. In an evolutionary context, transporter driven cell–cell auxin movement and plasmodesmata seem to have evolved around the same time in the green lineage. This highlights a co-existence from early on and a likely functional specificity of the systems. Exploring more situations where auxin movement via plasmodesmata has relevance for plant growth and development, and clarifying the regulation of such transport, will be key aspects in coming years.
This article has an associated Future Leader to Watch interview with the author of the paper.
KEY WORDS: Plants, Plasmodesmata, Auxin, Cell–cell transport
Summary: The review discusses the occurrence and biological relevance of auxin transport via plasmodesmata in plants. Physical modes of transport of this hormone, feedbacks on its movement and evolutionary aspects are included.
INTRODUCTION
“In front of me there were two roads
I chose the less travelled road
And it made all the difference.”
Paulo Coelho – The Witch of Portobello (2006)
Plant growth and development is exquisitely responsive to a range of small, chemically different, endogenous molecules that have received the collective name of plant hormones. These substances can operate as information carriers, act both in proximity and distally from their initial site of biosynthesis and ultimately trigger specific biological responses via signalling or direct action (reviewed in Santner et al., 2009). One of these substances, auxin, is remarkable in the breadth of processes and the range of scales of biological form it has been found to be involved in: from organogenesis (Benková et al., 2003; Reinhardt et al., 2003), overall root (reviewed in Lavenus et al., 2013) and shoot architecture (reviewed in Domagalska and Leyser, 2011) via more local differential growth for tropisms (reviewed in Gilroy, 2008) to fine balances between maintenance of undifferentiated cells (Ding and Friml, 2010), cell division and differentiation (Di Mambro et al., 2017). Auxin is also important for adaptations to abiotic stresses and for interactions with other organisms (reviewed in Kazan, 2013). A comprehensive list would be incredibly long and is one of the reasons why so many plant scientists have had to deal – willingly or not – with this substance, being equally fascinated and challenged by the complexity of its biosynthesis, movement, action and interactions with other regulators. This review will mainly discuss some aspects of the cell–cell movement of auxin. I will employ the word auxin to indicate the main endogenous form of this family of chemicals, indole-3-acetic acid (IAA). However, it is important to remember that other native, biologically active forms also exist and differ in their properties (reviewed in Simon and Petrášek, 2011).
In chemical terms, auxin is a weak organic acid, so at the mildly acidic pHs of the extracellular space some of the molecules would remain protonated, while others would become negatively charged (Rubery and Sheldrake, 1974; Raven, 1975). While the formers can freely cross the membrane bilayer, active transporters of the AUXIN1/LIKE AUX1 (AUX/LAX) family enable cellular influx of the latter species (Bennett et al., 1996; Yang et al., 2006; Péret et al., 2012). Once inside, at cytosolic pH, auxin would be almost entirely in the deprotonated form (Rubery and Sheldrake, 1974; Raven, 1975). Efflux from the cells therefore has to be mediated by members of the PIN-FORMED (PIN) family (Gälweiler et al., 1998; Petrášek et al., 2006; Bennett et al., 2014a) or the ATP binding cassette B (ABCB) family (Noh et al., 2001; Terasaka et al., 2005) of active transporters. Polar localisation of the transporters on the membrane provides directionality to the auxin fluxes within tissues (Wiśniewska et al., 2006). This is especially the case for PINs while AUX/LAX and ABCBs display this to lower and more cell-type-dependent degrees, being in general more uniformly distributed in the membranes of cells (Gälweiler et al., 1998; Swarup et al., 2001; Geisler et al., 2005) (Fig. 1).
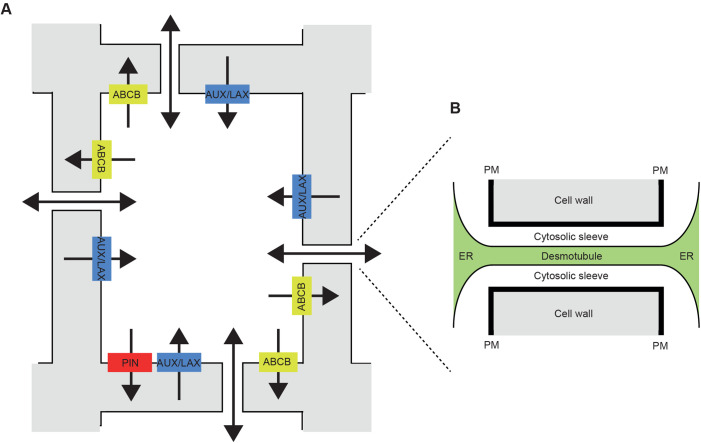
Fig. 1.
Auxin transport between plant cells. (A) Simplified schematic of a plant cell and its immediate neighbours. The cytosol is rendered as white space delimited by a dark line, representing the plasma membrane (PM). The extracellular space (cell wall) is coloured in grey. Transporters of the AUX/LAX family (auxin importers) and those of the PIN and ABCB families (auxin exporters) are depicted on the membrane of the central cell as coloured rectangles. The directionality of auxin transport provided by these proteins is shown as a black arrow. Fluxes across PD are shown as bidirectional arrows as different types of transport could be occurring (see Fig. 2). (B) Zoomed sketch of a PD also showing the endoplasmic reticulum (ER) transversing the channel, the desmotubule, and the cytosolic sleeves.
Other signalling substances and metabolites move between cells without the need for transporters and without having to leave the cellular symplastic space. Plasmodesmata (PD), membrane lined channels spanning the walls of neighbouring cells, enable this transport (reviewed in Li et al., 2020) (Fig. 1). Movement is largely governed by the size and shape of the substance, which has to be compatible with the aperture of the channel (Terry and Robards, 1987). The auxin molecule falls within such a category (Han et al., 2014; Rutschow et al., 2011) so transport is, and has always been, more likely to occur than not. Some authors did indeed consider this transport component very early in auxin research (Mitchison, 1980; Arisz, 1969, for example).
The argument around auxin movement via PD is whether this transport has biological significance. Namely, if it appreciably influences concentrations and distributions of the hormone in tissues and if it contributes to the developmental processes depending on such parameters. The alternative vision, perhaps implied until the recent developments discussed in this review, regards auxin PD movement as some form of non-functional leakage that actually creates issues for active auxin transport (Rutschow et al., 2011). I will show here that there is sufficient evidence to discard this second hypothesis and more attention should be given to auxin symplastic transport.
It is also important to consider that mutations in auxin transporters, while leading to phenotypes at times even severe (see for instance pin1 in Gälweiler et al., 1998), are not lethal (sextuple pin mutants in Verna et al., 2019). The lines without such proteins were instead instrumental in generating a breadth of literature on the relevance of active auxin transport. Mutations removing PD, conversely, have never been identified, and this will most likely continue to be the case. Even mutations altering PD permeability can be embryo lethal (Kim et al., 2002). A slight bias in considering the biological significance of auxin transport mechanisms might therefore have arisen from that.
This is not to challenge the centrality of transporter-driven auxin movement: this route remains an essential one especially in processes requiring transport up concentration gradients to generate auxin maxima or those involving sharp auxin gradients (Heisler et al., 2005; Benková et al., 2003, as examples). The transporters also provide an extremely refined and tuneable directionality system at the cellular scale (Zhang et al., 2020; Huang et al., 2010, as examples). PD transport could instead integrate into this picture and explain situations where transporters alone do not seem sufficient to explain the biology of the system (Mellor et al., 2020; Verna et al., 2019; Guenot et al., 2012, as potential examples).
In this review, I will highlight the various types of movement auxin could experience across PD. I will then link those to biological situations where auxin PD transport has been shown to, or could, contribute to developmental processes. I will then raise points on potential integration of transporter and PD-driven transport mechanisms, including feedbacks that have been observed between the two. I will conclude with a brief consideration on the evolutionary history of PD and the PIN family of auxin transporters.
TYPES OF MOVEMENT AUXIN MIGHT EXPERIENCE ACROSS PD AND THEIR BIOLOGICAL RELEVANCE
Diffusion
Diffusion down concentration gradients existing between cells is perhaps the simplest type of movement that can occur across PD (Schönknecht et al., 2008) (Fig. 2A). Diffusion of soluble molecules would primarily occur in the cytosolic sleeve of PD (also called cytoplasmic sleeve in the literature) (Fig. 1B). In the context of auxin, this was recently highlighted in Mellor et al. (2020). The authors, focusing on the root tip of Arabidopsis, compared the observed signal intensity of a DII-VENUS reporter (where fluorescence negatively correlates with auxin concentration) (Brunoud et al., 2012) with that predicted by a modified computational model of them (Band et al., 2014). In this version both auxin active transport and some local biosynthesis were included. Nonetheless, much sharper differences between high and low auxin areas were predicted than those that were observed. This held true even when extensive parameter space was surveyed and in several genetic backgrounds, namely mutants for active transporters. Transport via PD was incorporated into the model and the authors then tested whether such a route contributed to the functional auxin distribution within root tips. Symplastic auxin movement indeed reduced the sharpness of concentration differences and provided better agreement with those observed experimentally. Specifically, this form of movement was essential to facilitate reflux from the high auxin areas in the outer tissues, where active transporters direct flow shoot-ward, to the inner central tissues where flow is root-ward and the auxin concentration is lower (Mellor et al., 2020). This reflux component was theorised in Grieneisen et al., 2007 and is necessary to retain high auxin concentrations in the root tip, something necessary for many biological processes.
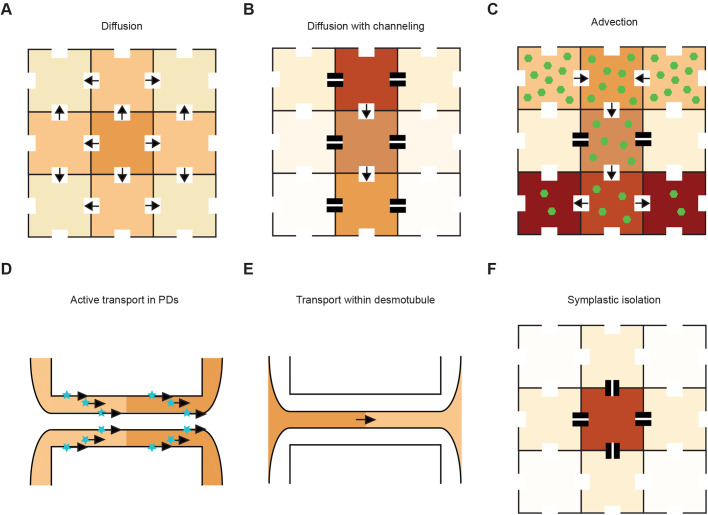
Fig. 2.
Potential modes of auxin transport across PD. (A–F) various proven, or potential, auxin transport mechanisms across PD. Cells are depicted as neighbouring tiles with dark contours. The colour of the squares is proportional to the intracellular auxin concentration, darker colours signifying higher amounts. PD are rendered as white gaps in the edges of the cells. Arrows show symplastic auxin fluxes between cells. Callose accumulation is depicted as two black rectangles. Metabolites (or other substances generating a drive for bulk transport) are shown as green hexagons. The number of hexagons is proportional to the intracellular concentration of the hypothetical metabolite. Zoomed views of PD depict a central desmotubule, surrounded by the cytosolic sleeve and delimited (above and below) by the plasma membrane of the cell. Potential factors interacting with auxin for its active transport (cytosolic or embedded in membranes) are shown as light blue stars. These factors, the metabolites and callose are not shown to scale. Transporter driven auxin fluxes would also be present in the cells represented (and could alter the patterns displayed) but are not considered for simplicity of representation.
Increasing PD permeabilities influenced auxin dependent DII-VENUS signals in general agreement with model predictions (Mellor et al., 2020). Changes were achieved both via chemical treatments (Rutschow et al., 2011) or using inducible antisense lines against a callose synthase gene (GSL8), whose polysaccharide product lines PD and regulates their aperture (Han et al., 2014). Differentials between observed and predicted values were, however, larger here, likely due to necessary approximations in the predicted effects of these manipulations on PD permeability.
When considering auxin movement across PD, attention should also be given to the negatively charged nature of this substance at cytosolic pHs (Rubery and Sheldrake, 1974; Raven, 1975). Electrostatic interactions with PD constituents could be occurring. The lipids on the inner leaflet of the plasma membranes (PM) lining PD are strongly negatively charged (Simon et al., 2016). Research regarding the importance of electrostatic charges of molecules crossing PD is limited (Terry and Robards, 1987, as an example). One paper did not find robust support for the idea that charge contributed significantly to the ease of movement of green fluorescent protein variants (Dashevskaya et al., 2008).
Diffusion with channelling effects
Passive – as opposed to energised – transport is not necessarily synonymous with unregulated transport. Movement across PD is indeed tightly and finely controlled (reviewed in Li et al., 2020). While still obeying movement down concentration gradients, the specific available space for passive transport could be restricted. As a result, transport would be granted a form of directionality (Fig. 2B).
An example of this, in the context of auxin, was recently provided in Gao et al. (2020). The authors quantified cell–cell coupling in leaf tissues using a photoactivatable dye (Martens et al., 2004). Movement, from the activated cell, was asymmetrical and biased in the longitudinal direction in midrib and petiole epidermal cells. The same was also observed, albeit to a lower extent, in deeper underlying tissues but not in leaf pavement cells to the sides of the midrib. As higher levels of callose were detected on the transverse walls, channelling of transport in the longitudinal direction might be achieved by altering the permeability of PD themselves. While the authors did not clarify how such selective callose deposition could be enforced, they showed that permeability differences were abolished in a gls8-callose synthase mutant. Using a radiolabeled version of auxin, applied to the leaf tip, more auxin arrived (sooner) in the petiole of wild-type plants compared to that of the mutant. Channelling might aid movement, possibly by reducing dissipation (Gao et al., 2020).
The paper also provides functional relevance for this symplastic auxin flow: the callose mutant displays reduced leaf hyponasty (leaf petiole bending) upon auxin application to the leaf tip (Gao et al., 2020). Hyponasty depends – among other processes – on auxin flow from the tip of the leaf into the petiole (van Zanten et al., 2009; Michaud et al., 2017; Pantazopoulou et al., 2017). The auxin active transporters involved in hyponasty did not display altered transcription in the callose mutant (Gao et al., 2020), so the effect might be largely attributable to PD transport. PIN proteins experience extensive post-translational modifications (reviewed in Löfke et al., 2013) so a contribution of that can't be ruled out, albeit how that could be connected to callose levels seems hard to envisage. The effect is not absolute, as some upward bending still occurs, nor does it obviously affect the fitness of the mutant allele employed, from what visible in the picture. However, testing the genotype in a crowded setting, upon flooding or thermal challenges would be relevant as the hyponastic responses displayed in those conditions (Millenaar et al., 2005) could be sharply relevant for plant fitness (reviewed in van Zanten et al., 2010).
Interestingly, the different PD densities across the various cell types of the root tip (Zhu et al., 1998) seemed determinant for the symplastic auxin fluxes described in Mellor et al. (2020). Modelled uniform densities were unable to recapitulate DII-VENUS patterns. PD distributions might therefore provide a quantitative form of directionality even in diffusive processes.
Channelling effects via callose deposition might not be restricted to leaves or the epidermal layer. For instance, PD in proto sieve elements higher up than those performing phloem unloading appear occluded by callose (Ross-Elliott et al., 2017) and the meta sieve element-companion cell complex is largely symplastically isolated from surrounding tissues in the root (Oparka et al., 1994; Knoblauch et al., 2015). These features could support long distance basipetal channelling of auxin within the phloem. Transport of the hormone in this tissue, in relation to bulk flow mechanisms, is described in the next section.
Advection
Another form of passive motion is advection, which occurs when the bulk flow of another fluid carries a substance along. Such movement would carry an overall directionality and, in addition, it could achieve movement of auxin up concentration gradients (Fig. 2C). Please note that while advective transport would be passive, an active mechanism might be required to generate the drive for bulk motion. Long distance transport in the phloem tissue is most likely based on bulk flow: osmotically generated pressure pushes the fluid towards areas of lower solute concentration (Knoblauch et al., 2016).
All steps of phloem function; loading, translocation and release of transported substances are dependent on PD, or modified forms of the same, connecting the relevant vascular cells (Ross-Elliott et al., 2017; Dettmer et al., 2014; Rennie and Turgeon, 2009). Advection is therefore an extremely relevant form of transport across PD.
Presence of radiolabelled auxin in the phloem, when the tracer is specifically applied to mature leaves, has been reported in a range of species and shown to be unaffected by inhibitors for active auxin transport (Morris and Thomas, 1978; Morris et al., 1973). Significant amounts of endogenous IAA have also been detected in phloem sap (Allen and Baker, 1980). Symplastic movement within phloem was clearly shown in Bishopp et al., 2011, when root-detected radiolabelled auxin was diminished upon phloem connectivity impairment. Phloem PD, and possibly sieve pores, were specifically occluded via induced callose deposition (Vatén et al., 2011). Potential feedback on active transport in response to blocked symplastic trafficking were not extensively assessed, but PIN7 displayed a slight reduction in its domain of expression in the root (Bishopp et al., 2011).
Active and passive transport of auxin in relation to the phloem might cooperate. While an active transport inhibitor did not affect the phloem translocation of the hormone, it did cause a reduction of its uptake in leaf veins (Goldsmith et al., 1974). In a mutant for AUX1, a reduction in an auxin reporter was observed in leaf vasculature (Marchant et al., 2002) and roots (Swarup et al., 2001). The authors speculated that the transporter might help load/unload auxin into/from the phloem. The transporter was expressed in protophloem of the root (Swarup et al., 2001) but was not specifically restricted to leaf vascular tissues (Marchant et al., 2002). The ABCB19 transporter has been suggested, conversely, to retain auxin in the phloem system along the transport pathway (Blakeslee et al., 2007). Shoot to root IAA transport was strongly reduced in the mutant (Noh et al., 2001) and the transporter displayed expression in the pericycle and endodermis, although present more broadly (Blakeslee et al., 2007).
However, especially in the context of advection, the presence of auxin does not immediately equate to functional relevance. In Bishopp et al. (2011) the impairment of symplastic phloem connectivity, and the associated reduced auxin transport, did not lead to large changes in root auxin responses. The changes observed were actually attributable to delivery of another hormone, cytokinins. The phloem is therefore unlikely to be a biologically necessary route, at least for the general signalling processes tested in that paper. Alternatively, compensation mechanisms might buffer reductions in this symplastic transport. Along similar lines, shoot-derived auxin (travelling via any potential route) was insufficient to maintain root meristem identity when local biosynthesis was compromised (Brumos et al., 2018).
This does not altogether rule out a significance for phloem auxin. Temporal differences might exist: lateral root emergence, when seedlings are young, was shown to depend on shoot-derived auxin (Bhalerao et al., 2002). Environmental ones are also possible, since increased lateral root development under high humidity conditions was attributed to higher auxin phloem transport (Chhun et al., 2007). Transport of auxin in the phloem would have a clear speed advantage, around one order of magnitude, to that via active transporters (Sabnis and Watson, 1982; Tsurumi and Wada, 1980; Kramer et al., 2011). It might therefore carry functional relevance in specific situations where fast communication is required or advantageous.
Spatial aspects might similarly apply. For instance, one can envisage a relevance for phloem movement of auxin within the transport pathway itself rather than at the terminal release point. Auxin is well known to be involved in vascular development (see Sachs, 1981, for a classic example). It is interesting that a callose mutant in maize, tie-died2, impaired in loading of molecules from companion cells to sieve elements, also presents vascular developmental defects (Slewinski et al., 2012). Movement of protein regulators (as speculated in Baker et al., 2013) but also perhaps auxin might be affected. PD auxin movement was recently speculated, in the context of leaf vein patterning, in Ravichandran et al., 2020.
The movement in the phloem of auxin-signalling components influencing responses to this hormone at sites of unloading has also been reported (Notaguchi et al., 2012; Spiegelman et al., 2015), but is not the specific focus of this review.
Combinations of types of transport are also possible, for instance phloem unloading in Arabidopsis is convective, combining both diffusion and advection (Ross-Elliott et al., 2017).
Active symplastic transport
Auxin transport across PD does not need to be (or always be) passive in nature. Energy-dependent movement could be relevant and could even enable transport against concentration gradients. While non-targeted transport across PD largely relies on structural properties of the substance, targeted movement involves the modification of PD permeability by the substance to be transported (or associated partners) to facilitate its own movement (Crawford and Zambryski, 2000) (Fig. 2D).
Directional yet non-targeted transport across PD in moss protonema (Kitagawa and Fujita, 2013) and in trichomes (Christensen et al., 2009) was also shown to rely on energy in some capacity, as it was abolished upon metabolic inhibition. The fact that directionality was not absolute (Kitagawa and Fujita, 2013; Christensen et al., 2009; Howell et al., 2020) and that the phenomenon was observed in both non-secreting and secreting trichomes (Christensen et al., 2009) makes the process less likely to be due to bulk flow. Overall, the lack of precise data (also in relation to auxin) would warrant further research into this type of directional transport via PD.
Diffusive transport within the desmotubule
So far, I have considered (auxin) movement across PD only within the cytosolic sleeve. This is the space between the plasma membrane lining these channels and a constricted form of the endoplasmic reticulum (ER) – the desmotubule – that also runs through PD (reviewed in Li et al. (2020)) (Fig. 1B). While the sleeve is likely the main route for transport of hydrophilic substances, diffusive movement within the constricted ER lumen has been shown for small dyes (Barton et al., 2011; Cantrill et al., 1999). Auxin size could be compatible with such transport (Fig. 2E).
In this regard, it is interesting that active auxin transporters are localised to the ER and are involved in import of the hormone into this cellular domain. These proteins include some PINs with specific structural features (Mravec et al., 2009; Ding et al., 2012) and the family of PIN-likes (PILS) proteins (Barbez et al., 2012). While ER import is regarded as a sequestration mechanism to determine cellular sensitivity to auxin (reviewed in Barbez and Kleine-Vehn, 2013), loading of auxin into the ER for cell-cell transport can't be ruled out.
Restricted symplastic transport
The presence of PD could be problematic for biological processes where auxin maxima are locally generated or gradients are enforced via active transport. Open PD could partially dissipate such auxin levels by leakage into surrounding cell layers. Preventing symplastic trafficking of auxin might be convenient in such scenarios and can be envisaged – with some flexibility on the term transport – as a form of highly restricted and regulated movement (Fig. 2F).
Auxin plays a central role in all stages of lateral root development: priming the future branching site, influencing division patterns and helping root emergence through overlying tissues. These processes are achieved via combinations of auxin maxima, gradients and overall signalling (reviewed in Lavenus et al., 2013). Symplastic connectivity of the lateral root primordium (LRP) and surrounding tissues also plays key roles in lateral root development (Benitez-Alfonso et al., 2013; Sager et al., 2020). Specifically, LRPs become fully isolated via callose accumulation around stages IV–V (Benitez-Alfonso et al., 2013). It is fascinating to speculate that this might be important to preserve the sharp auxin gradient (decreasing from the tip of primordium to its basal sides) observed using a DR5 auxin-signalling reporter at those stages (Benková et al., 2003). The gradient was dependent on active transport as more diffuse signals were observed upon treatments with transporter inhibitors or auxin analogues that are poorly taken up by transporters (Benková et al., 2003). It is less likely that LRP isolation has the goal to overall retain auxin, as much stronger DR5 signals are observed at earlier stages (Benková et al., 2003) when symplastic connectivity is still present (Benitez-Alfonso et al., 2013).
Active accumulation of auxin is also necessary for tropic responses of the hypocotyl. High auxin accumulates on the side that will go on to display increased growth and bending (Friml et al., 2002 as an example). However, GSL8-dependent accumulation of callose on that side was also observed during photo/gravitropisms (Han et al., 2014). Interestingly, in an inducible knockdown of GSL8 or upon treatments with callose synthesis inhibitors, the curvature responses were abolished. Higher transport of radiolabelled auxin and broader signals for auxin reporters were observed in the hypocotyls in the knockdown line. The normal auxin gradient across the hypocotyl was also specifically affected. Inhibitors of active transport did not modulate these aspects, suggesting that the phenotypes were most likely the result of increased auxin movement via PD (Han et al., 2014). PIN3 localisation was not affected (Han et al., 2014) but PIN4 and PIN7, equally important, were not checked. Callose accumulation might therefore be necessary to restrict high auxin concentrations to the side of the hypocotyl that will display tropic bending.
It is important to consider that symplastic isolation might have trade offs with delivery of metabolites to cells. Fine regulation of these processes is therefore likely required.
RECIPROCAL FEEDBACKS BETWEEN AUXIN AND PLASMODESMATA
Auxin modulating PD permeability
In a less specific way compared to targeted transport, auxin could generally regulate PD permeability and increase or decrease the movement of many substances (among which itself). For instance, in the context of the hypocotyl phototropic and gravitropic responses I described, auxin signalling directly upregulates transcription of the callose synthase GSL8, shown to be important for those processes. A positive feedback loop is generated: auxin promotes its own accumulation on one side of hypocotyl by blocking symplastic communication and diffusion (Han et al., 2014).
Additional examples come from the study of lateral root development. Expression of Plasmodesmata callose binding (PDCB) protein 1, involved in callose stabilisation and possibly deposition (Simpson et al., 2009), was upregulated by auxin signalling (Parizot et al., 2010; Maule et al., 2013). Reporters for the gene showed signal in LRP from stage III to VI (Maule et al., 2013). It is therefore likely that PDCB1 contributes to the LRP symplastic isolation at stages IV–VI. In this regard, it would be attractive to study phenotypes in loss-of-function mutants and see the impact on auxin fluxes.
However, overexpression of PDCB1 resulted in higher density of LRPs (Benitez-Alfonso et al., 2013), something not immediately relatable to their symplastic isolation at later stages. As the use of a constitutive promoter would cause ectopic callose accumulation early in the process, priming of future LRPs might have been affected. Clusters of high DR5 signal in xylem pole pericycle cells (the future branching sites) were indeed observed and later resulted in closely positioned, rather than orderly spaced, roots. The same phenotype was observed when callose was induced from a specific xylem pole promoter (Benitez-Alfonso et al., 2013). Cell–cell isolation likely disrupts the initial symplastic phase of LRPs. A speculative interpretation is that, at those stages, an initial active transport-driven auxin influx needs to be quickly dissipated for the signalling to be limited to a few founder cells (Casimiro et al., 2001). Sustained levels of auxin, because of impaired drainage, could lead to wider areas of priming. Movement of inhibitory regulators out of primed cells into surrounding ones is equally possible.
The LRP spacing phenotype also appeared in mutants for PD beta glucanases (PdBG) 1 and 2 (Benitez-Alfonso et al., 2013), which are involved in callose degradation (Levy et al., 2007; Iglesias and Meins, 2000). PdBGs are expressed in xylem pole pericycle cells and are also similarly induced by auxin signalling (Parizot et al., 2010; Benitez-Alfonso et al., 2013). However, as their strongest expression was at stage III, just before symplastic isolation, a specific function then is harder to envisage. Draining of the primordium of auxin or other signals might be necessary before isolation.
Another form of auxin regulation of PD occurs during the emergence of lateral roots. It was shown that ectopic induction of PDCB1 resulted in reduced emergence, likely because of callose induction in tissues overlying LRPs (Maule et al., 2013). This was confirmed in Sager et al. (2020) by overexpressing another protein, PD localised protein (PDLP) 5. When the native domain of expression of PDLP5 was studied, the authors observed that signal occurred in cell layers above LRPs and accompanied lateral root emergence. PDLP5 was also induced by auxin signalling. Connections with the movement of auxin itself were suggested: in the pdlp5 mutant (likely presenting more permeable PD), a higher number of DR5- and LAX3-expressing cells were observed in the layers above LRPs. LAX3 is normally involved in auxin influx from LRP into those overlying cells (Swarup et al., 2008). Higher auxin levels might therefore be present in those cells in the mutant. Considering the positive role of auxin in altering cell wall components to facilitate passage of the new root across the tissues of the primary one (reviewed in Péret et al., 2009), this well agrees with the observed increased lateral root emergence in the mutant (Sager et al., 2020).
Auxin can increase the activity of pectin methyl transferase proteins and modify the pectin component of the cell wall (Bryan and Newcomb, 1954; Laskowski et al., 2006; Braybrook and Peaucelle, 2013). Interestingly, these polysaccharides are enriched at PD (visible in Faulkner et al., 2008) and members of the pectin methyl transferase protein family have been localised to PD (Morvan et al., 1998). One member was also shown to interact with viral components and facilitate their spread, potentially by altering PD (Chen et al., 2000). Auxin might, therefore, not only facilitate separation of cells overlying lateral root primordia but also reinforce its own movement to achieve such effect.
However, the exact role played by PDLP5 in normal conditions remains puzzling: what selective advantage is provided by the induction of a protein negatively regulating lateral roots emergence, when the inducer itself favours it? Would this provide some control over lateral root numbers or would this once again serve to give full control to active auxin transport mechanisms in those cell layers? It will be interesting to see a future coherent framework integrating auxin processes and symplastic connectivity in lateral root development.
It is important to remember, overall, that since auxin treatment did not increase cell–cell movement of fluorescent dyes in the root (Rutschow et al., 2011), tissue or temporal differences must also exist in these types of feedbacks.
Symplastic connectivity altering auxin biosynthesis and active transport
Feedbacks on auxin processes can also occur in response to altered cell–cell communication. An example of this was observed when cell-type-specific accumulation of callose was induced within the quiescent centre (Liu et al., 2017). This cellular domain acts as an organiser centre for the root meristem (Sarkar et al., 2007). In addition to loss of stem cell maintenance, the symplastic isolation also resulted in auxin reduction in the proximity of the quiescent centre. The auxin gradient normally present along the root cap was also disrupted (Liu et al., 2017). Auxin levels are fundamental for stem cell maintenance in the root (Ding and Friml, 2010). Interestingly, several auxin biosynthesis genes (but not active transporter genes) displayed reduced expression in the treated roots. PIN protein localisation was also largely unaltered, further suggesting that the observed auxin changes were largely due to biosynthesis (Liu et al., 2017). Movement of unknown regulators via PD might therefore instruct the formation of auxin gradients within the root tip. A symplastic auxin component diffusing from the QC might also contribute.
The callose inducible system (Vatén et al., 2011) was also employed to block symplastic communication to and from the endodermis. Aberrant periclinal cell divisions and altered endodermal identity in the resulting supernumerary cell files were observed. PIN2, for instance, which is not normally expressed in this cell type, appeared, and it additionally displayed an apolar localisation (Wu et al., 2016). This was consistent with signals moving cell-to-cell being required for correct patterning of this tissue (see, for example, Nakajima et al., 2001). However, in association with these phenotypes, the induced seedlings no longer properly responded to gravitropism. The DR5 signal in the root was altered and auxin did not seem to redistribute correctly upon the trophic stimulus (Wu et al., 2016), a process required for the differential growth and bending (Band et al., 2012). Perturbed active auxin transport (possibly due to the PIN2 appearance) is the likely cause, but a contribution from passive PD mechanisms can not be ruled out.
Evolutionary perspective on auxin movement
Some authors entertained the idea that, in the context of auxin movement, a passive PD transport system might have functionally pre-dated an active one (for example Benítez et al., 2018). Albeit the hypothesis is possible, it might erroneously rely on assumptions of lower ‘complexity’ of the former system.
Phylogenetic data, when focusing on multicellular families in the green lineage, do not particularly support this notion either. Active, PIN-driven, auxin transport emerged early in the evolution of streptophytes (the clade of land plants) and a PIN homolog was described in the filamentous algae Klebsormidium flaccidum (order Klebsormidiales). In heterologous systems, the protein performed true auxin transport. However, its localisation in algal cells was not polar and auxin seemed to be released in the environment rather than moving cell-to-cell (Skokan et al., 2019). True polar cell–cell auxin movement seems to appear in the order Charales (Boot et al., 2012). Overall, based on sequence data, PIN proteins are present in all strephophytes (reviewed in Bennett, 2015) but not in more distant chlorophytes (De Smet et al., 2011) (Fig. 3).
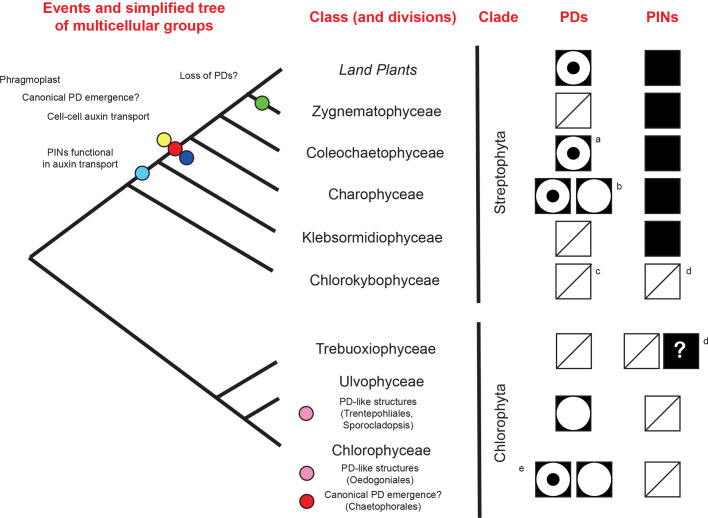
Fig. 3.
Evolution of Plasmodesmata and PIN proteins in multicellular groups of the green lineage. Only classes with multicellular species are depicted in the phylogenetic tree. Branch length does not reflect true evolutionary time. The phylogeny is based on One Thousand Plant Transcriptomes Initiative (2019) and the reviews Fang et al. (2017); Leliaert et al. (2012). The positions of events of interest are suggested on the tree as coloured dots. Presence or absence of PD and PINs in the phylogenetic classes is based on reviews from Nicolas et al. (2018); Raven (2018); Bennett (2015); the paper from De Smet et al. (2011) plus the additional notes. Presence of PD with desmotubules is depicted as a black square with a inscribed white circle and a second internal black circle. PD/PD-like structures without desmotubules are shown as a black squares with empty white inscribed circles. Presence of PINs is shown as a black square. Absence of either structure/protein is shown as a white square with a diagonal line. Notes: (A) no obvious clear image seems available in the literature but the morphology is inferred from Marchant and Pickett-Heaps (1973); (B) controversy regarding the presence (Kwiatkowska and Maszewski, 1986) or absence (Franceschi et al., 1994) of the desmotubule. It is present in at least some contexts (Cook et al., 1997); (C) absence of PD is based on Lokhorst et al. (1988) and Rogers et al. (1980); (D) absence of PINs in Chlorokybophyceae according to Wang et al. (2020), in this study the sequence for a PIN protein was potentially found in Chlorophyta, unlike what concluded in De Smet et al. (2011); (E) desmotubule is present in at least one species based on the figures in Stewart et al. (1973).
The evolution of PD is more complicated and less studied. For instance, it has not been revisited since the sister group to land plants was changed from the order Charales to that of Zygnematales (One Thousand Plant Transcriptomes Initiative, 2019 for the current phylogeny and Zhong et al., 2015 for a review of the changes). Canonical PD forms, with a desmotubule, are restricted to land plants, the order Charales, most likely the order Coleochetales and a specific order in the Chlorophyceae class (Marchant and Pickett-Heaps, 1973 and reviewed in Nicolas et al., 2018; Raven, 2018). PD forms without ER are reported in other orders of the Chlorophyceae and Ulvophyceae classes (reviewed in Nicolas et al., 2018; Raven, 2018). Therefore, in the green lineage, PD and PD-like connections likely appeared multiple times, at least once in streptophytes and multiple times in the Chlorophytes. The development of analogous structures across independent lineages likely bears testimony to the effectiveness of cytosolic continuity as a strategy (among others) for cell–cell communication. Interestingly, PD seem absent in Klabsormidales and Zygnematales (reviewed in Raven, 2018). The former might be a case of functional loss if we assume that PD containing ER evolved after the appearance of the phragmoplast in the green lineage (reviewed in Graham et al., 2000) (Fig. 3). This structure is involved in cell plate formation and ER strands remain embedded inside it (Hepler, 1982). Presence of the desmotubules in some members of the Chlorophyceae class (Stewart et al., 1973) is surprising because those cells do not display a phragmoplast.
In summary, in some charophytes, PD-like structures have been present in absence of PINs but in streptophytes canonical PD likely appeared after PIN emergence and around the same time as these proteins might have started to perform cell–cell auxin movement. Functional specificity between passive and active transport is therefore a more likely explanation for the presence of both systems.
The moss Physcomitrella patens (Bryophytes) provides an example of this. While active auxin transport is necessary for apex maintenance, leaf development in the gametophyte phase and for branching regulation in the sporophyte generation of this species (Viaene et al., 2014; Bennett et al., 2014b; Fujita et al., 2008), it is not involved in the control of gametophyte branching (Coudert et al., 2015). Chemical inhibition of PINs or ABCBs transporters did not cause phenotypes. Bidirectional auxin transport, required in the authors’ computational model to reproduce branching patterns, might be instead provided by PD. Treatment with a callose synthesis inhibitor indeed reduced branching in the moss, consistent with higher auxin fluxes in the model. Homologues of callose synthases and glucanases (and presence of callose itself) occur in bryophytes and even basal streptophytes (Del Bem and Vincentz, 2010; Zaveska-Drabkova and Honys, 2017; Gaudioso-Pedraza and Benitez-Alfonso, 2014; Scherp et al., 2001). However, PD regulation by callose has only been shown in vascular plants.
CONCLUSION
A growing body of publications is now highlighting the functional contribution of auxin moving via PD and the influence on auxin processes of other signals also moving via PD. It is therefore an exciting time for researchers in both fields. Knowledge exchange and synergies between the two communities will be beneficial to push forward some of these aspects. For instance, it will be highly interesting to re-evaluate known processes where auxin levels play key roles and try to investigate if fluxes via PD meaningfully contribute. A few of those cases have been mentioned in this review. Further dissecting the feedbacks and reciprocal impacts of transporter and PD fluxes will also be highly valuable. Manipulating PD aperture in active transport mutants (or manipulating transporters in inducible lines with altered PD aperture) would be challenging yet attractive possibilities. Ultimately, I envisage that comprehensive and quantitative models incorporating the various routes and regulators of auxin movement will be produced for various tissues. These will provide more holistic visions of cells and their robust mechanisms for growth and development. As a community of (molecular) scientific explorers, in coming years, we shall map the symplastic route of auxin movement. While hidden in plain sight, knowledge treasures might await us there.
Acknowledgements
I want to thank Emmanuelle Bayer (Laboratory of Membrane Biogenesis – Bordeaux), Yvon Jallais (ENS – Lyon) and Calum Ashcroft (University of Cambridge – Cambridge) for critical reading of the manuscript and Ottoline Leyser (Sainsbury Laboratory – University of Cambridge) for her encouragement in submitting the proposal for this review.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Funding
This work was supported by the Gatsby Foundation [GAT3395/PR3 awarded to Yka Helariutta and supporting A.P.]
References
- Allen J. R. F. and Baker D. A. (1980). Free tryptophan and indole-3-acetic acid levels in the leaves and vascular pathways of Ricinus communis L. Planta 148, 69-74. 10.1007/BF00385444 [DOI] [PubMed] [Google Scholar]
- Arisz W. H. (1969). Intercellular polar transport and the role of the plasmodesmata in coleoptiles and Vallisneria leaves. Acta Botánica Neerlandica 18, 14-38. 10.1111/j.1438-8677.1969.tb00567.x [DOI] [Google Scholar]
- Baker R. F., Slewinski T. L. and Braun D. M. (2013). The tie-dyed pathway promotes symplastic trafficking in the phloem. Plant Signaling and Behavior 8, e24540 10.4161/psb.24540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Band L. R., Wells D. M., Larrieu A., Sun J., Middleton A. M., French A. P., Brunoud G., Sato E. M., Wilson M. H., Péret B. et al. (2012). Root gravitropism is regulated by a transient lateral auxin gradient controlled by a tipping-point mechanism. Proceedings of the National Academy of Sciences 109, 4668-4673. 10.1073/pnas.1201498109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Band L. R., Wells D. M., Fozard J. A., Ghetiu T., French A. P., Pound M. P., Wilson M. H., Yu L., Li W., Hijazi H. I. et al. (2014). Systems analysis of auxin transport in the Arabidopsis root apex. The Plant Cell 26, 862-875. 10.1105/tpc.113.119495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbez E. and Kleine-Vehn J. (2013). Divide Et Impera—cellular auxin compartmentalization. Curr. Opin. Plant Biol. 16, 78-84. 10.1016/j.pbi.2012.10.005 [DOI] [PubMed] [Google Scholar]
- Barbez E., Kubeš M., Rolčík J., Béziat C., Pěnčík A., Wang B., Rosquete M. R., Zhu J., Dobrev P. I., Lee Y. et al. (2012). A novel putative auxin carrier family regulates intracellular auxin homeostasis in plants. Nature 485, 119-122. 10.1038/nature11001 [DOI] [PubMed] [Google Scholar]
- Barton D. A., Cole L., Collings D. A., Liu D. Y., Smith P. M., Day D. A. and Overall R. L. (2011). Cell–to–cell transport via the lumen of the endoplasmic reticulum. The Plant Journal 66, 806-817. 10.1111/j.1365-313X.2011.04545.x [DOI] [PubMed] [Google Scholar]
- Benitez-Alfonso Y., Faulkner C., Pendle A., Miyashima S., Helariutta Y. and Maule A. (2013). Symplastic intercellular connectivity regulates lateral root patterning. Dev. Cell 26, 136-147. 10.1016/j.devcel.2013.06.010 [DOI] [PubMed] [Google Scholar]
- Benítez M., Hernández-Hernández V., Newman S. A. and Niklas K. J. (2018). Dynamical patterning modules, biogeneric materials, and the evolution of multicellular plants. Frontiers in Plant Science 9, 871 10.3389/fpls.2018.00871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benková E., Michniewicz M., Sauer M., Teichmann T., Seifertová D., Jürgens G. and Friml J. (2003). Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115, 591-602. 10.1016/S0092-8674(03)00924-3 [DOI] [PubMed] [Google Scholar]
- Bennett T. (2015). PIN proteins and the evolution of plant development. Trends Plant Sci. 20, 498-507. 10.1016/j.tplants.2015.05.005 [DOI] [PubMed] [Google Scholar]
- Bennett M. J., Marchant A., Green H. G., May S. T., Ward S. P., Millner P. A., Walker A. R., Schulz B. and Feldmann K. A. (1996). Arabidopsis AUX1 gene: a permease-like regulator of root gravitropism. Science 273, 948-950. 10.1126/science.273.5277.948 [DOI] [PubMed] [Google Scholar]
- Bennett T., Brockington S. F., Rothfels C., Graham S. W., Stevenson D., Kutchan T., Rolf M., Thomas P., Wong G. K., Leyser O. et al. (2014a). Paralogous radiations of PIN proteins with multiple origins of noncanonical PIN structure. Mol. Biol. Evol. 31, 2042-2060. 10.1093/molbev/msu147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett T. A., Liu M. M., Aoyama T., Bierfreund N. M., Braun M., Coudert Y., Dennis R. J., O'Connor D., Wang X. Y., White C. D. et al. (2014b). Plasma membrane-targeted PIN proteins drive shoot development in a moss. Curr. Biol., 24, 2776-2785. 10.1016/j.cub.2014.09.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhalerao R. P., Eklöf J., Ljung K., Marchant A., Bennett M. and Sandberg G. (2002). Shoot–derived auxin is essential for early lateral root emergence in Arabidopsis seedlings. The Plant Journal 29, 325-332. 10.1046/j.0960-7412.2001.01217.x [DOI] [PubMed] [Google Scholar]
- Bishopp A., Lehesranta S., Vatén A., Help H., El-Showk S., Scheres B., Helariutta K., Mahonen A. P., Sakakibara H. and Helariutta Y. (2011). Phloem-transported cytokinin regulates polar auxin transport and maintains vascular pattern in the root meristem. Curr. Biol. 21, 927-932. 10.1016/j.cub.2011.04.049 [DOI] [PubMed] [Google Scholar]
- Blakeslee J. J., Bandyopadhyay A., Lee O. R., Mravec J., Titapiwatanakun B., Sauer M., Makam S. N., Cheng Y., Bouchard R., Adamec J. et al. (2007). Interactions among PIN-FORMED and P-glycoprotein auxin transporters in Arabidopsis. Plant Cell 19, 131-147. 10.1105/tpc.106.040782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boot K. J., Libbenga K. R., Hille S. C., Offringa R. and van Duijn B. (2012). Polar auxin transport: an early invention. J. Exp. Bot. 63, 4213-4218. 10.1093/jxb/ers106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braybrook S. A. and Peaucelle A. (2013). Mechano-chemical aspects of organ formation in Arabidopsis thaliana: the relationship between auxin and pectin. PLoS One 8, e57813 10.1371/journal.pone.0057813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumos J., Robles L. M., Yun J., Vu T. C., Jackson S., Alonso J. M. and Stepanova A. N. (2018). Local auxin biosynthesis is a key regulator of plant development. Dev. Cell 47, 306-318. 10.1016/j.devcel.2018.09.022 [DOI] [PubMed] [Google Scholar]
- Brunoud G., Wells D. M., Oliva M., Larrieu A., Mirabet V., Burrow A. H., Beeckman T., Kepinski S., Traas J., Bennett M. J. et al. (2012). A novel sensor to map auxin response and distribution at high spatio-temporal resolution. Nature 482, 103-106. 10.1038/nature10791 [DOI] [PubMed] [Google Scholar]
- Bryan W. H. and Newcomb K. H. (1954). Stimulation of pectin methylesterase activity of cultured tobacco pith by indoleacetic acid. Physiol. Plant 7, 290-297. 10.1111/j.1399-3054.1954.tb07578.x [DOI] [Google Scholar]
- Cantrill L. C., Overall R. L. and Goodwin P. B. (1999). Cell-to-cell communication via plant endomembranes. Cell Biol. Int. 23, 653-661. 10.1006/cbir.1999.0431 [DOI] [PubMed] [Google Scholar]
- Casimiro I., Marchant A., Bhalerao R. P., Beeckman T., Dhooge S., Swarup R., Graham N., Inzé D., Sandberg G., Casero P. J. et al. (2001). Auxin transport promotes Arabidopsis lateral root initiation. Plant Cell 13, 843-852. 10.1105/tpc.13.4.843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M.-H., Sheng J., Hind G., Handa A. K. and Citovsky V. (2000). Interaction between the tobacco mosaic virus movement protein and host cell pectin methylesterases is required for viral cell–to–cell movement. EMBO J. 19, 913-920. 10.1093/emboj/19.5.913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhun T., Uno Y., Taketa S., Azuma T., Ichii M., Okamoto T. and Tsurumi S. (2007). Saturated humidity accelerates lateral root development in rice (Oryza sativa L.) seedlings by increasing phloem-based auxin transport. J. Exp. Bot. 58, 1695-1704. 10.1093/jxb/erm026 [DOI] [PubMed] [Google Scholar]
- Christensen N. M., Faulkner C. and Oparka K. (2009). Evidence for unidirectional flow through plasmodesmata. Plant Physiol. 150, 96-104. 10.1104/pp.109.137083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook M. E., Graham L. E., Botha C. E. J. and Lavin C. A. (1997). Comparative ultrastructure of plasmodesmata of Chara and selected bryophytes: toward an elucidation of the evolutionary origin of plant plasmodesmata. Am. J. Bot. 84, 1169-1178. 10.2307/2446040 [DOI] [PubMed] [Google Scholar]
- Coudert Y., Palubicki W., Ljung K., Novak O., Leyser O. and Harrison C. J. (2015). Three ancient hormonal cues co-ordinate shoot branching in a moss. eLife 4, e06808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford K. M. and Zambryski P. C. (2000). Subcellular localization determines the availability of non-targeted proteins to plasmodesmatal transport. Curr. Biol. 10, 1032-1040. 10.1016/S0960-9822(00)00657-6 [DOI] [PubMed] [Google Scholar]
- Dashevskaya S., Kopito R. B., Friedman R., Elbaum M. and Epel B. L. (2008). Diffusion of anionic and neutral GFP derivatives through plasmodesmata in epidermal cells of Nicotiana benthamiana. Protoplasma 234, 13-23. 10.1007/s00709-008-0014-7 [DOI] [PubMed] [Google Scholar]
- De Smet I., Voß U., Lau S., Wilson M., Shao N., Timme R. E., Swarup R., Kerr I., Hodgman C., Bock R. et al. (2011). Unraveling the evolution of auxin signaling. Plant Physiol. 155, 209-221. 10.1104/pp.110.168161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Bem L. E. V. and Vincentz M. G. (2010). Evolution of xyloglucan-related genes in green plants. BMC Evol. Biol. 10, 1-17. 10.1186/1471-2148-10-341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettmer J., Ursache R., Campilho A., Miyashima S., Belevich I., O'Regan S., Mullendore D. L., Yadav S. R., Lanz C., Beverina L. et al. (2014). CHOLINE TRANSPORTER-LIKE1 is required for sieve plate development to mediate long-distance cell-to-cell communication. Nat. Commun. 5, 4276 10.1038/ncomms5276 [DOI] [PubMed] [Google Scholar]
- Di Mambro R., De Ruvo M., Pacifici E., Salvi E., Sozzani R., Benfey P. N., Busch W., Novak O., Ljung K., Di Paola L. et al. (2017). Auxin minimum triggers the developmental switch from cell division to cell differentiation in the Arabidopsis root. Proc. Natl Acad. Sci. USA 114, E7641-E7649. 10.1073/pnas.1705833114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Z. and Friml J. (2010). Auxin regulates distal stem cell differentiation in Arabidopsis roots. Proc. Natl Acad. Sci. USA 107, 12046-12051. 10.1073/pnas.1000672107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Z., Wang B., Moreno I., Dupláková N., Simon S., Carraro N., Reemmer J., Pěnčík A., Chen X., Tejos R. et al. (2012). ER-localized auxin transporter PIN8 regulates auxin homeostasis and male gametophyte development in Arabidopsis. Nat. Commun. 3, 1-11. 10.1038/ncomms1941 [DOI] [PubMed] [Google Scholar]
- Domagalska M. A. and Leyser O. (2011). Signal integration in the control of shoot branching. Nat. Rev. Mol. Cell Biol. 12, 211-221. 10.1038/nrm3088 [DOI] [PubMed] [Google Scholar]
- Fang L., Leliaert F., Zhang Z.-H., Penny D. and Zhong B.-J. (2017). Evolution of the Chlorophyta: insights from chloroplast phylogenomic analyses. Journal of Systematics and Evolution 55, 322-332. 10.1111/jse.12248 [DOI] [Google Scholar]
- Faulkner C., Akman O. E., Bell K., Jeffree C. and Oparka K. (2008). Peeking into pit fields: a multiple twinning model of secondary plasmodesmata formation in tobacco. Plant Cell 20, 1504-1518. 10.1105/tpc.107.056903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi V. R., Ding B. and Lucas W. J. (1994). Mechanism of plasmodesmata formation in characean algae in relation to evolution of intercellular communication in higher plants. Planta 192, 347-358. 10.1007/BF00198570 [DOI] [Google Scholar]
- Friml J., Wiśniewska J., Benková E., Mendgen K. and Palme K. (2002). Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature 415, 806-809. 10.1038/415806a [DOI] [PubMed] [Google Scholar]
- Fujita T., Sakaguchi H., Hiwatashi Y., Wagstaff S. J., Ito M., Deguchi H., Sato T. and Hasebe M. (2008). Convergent evolution of shoots in land plants: lack of auxin polar transport in moss shoots. Evol. Dev. 10, 176-186. 10.1111/j.1525-142X.2008.00225.x [DOI] [PubMed] [Google Scholar]
- Gälweiler L., Guan C., Müller A., Wisman E., Mendgen K., Yephremov A. and Palme K. (1998). Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science 282, 2226-2230. 10.1126/science.282.5397.2226 [DOI] [PubMed] [Google Scholar]
- Gao C., Liu X., De Storme N., Jensen K. H., Xu Q., Yang J., Liu X., Chen S., Martens H. J., Schulz A. et al. (2020). Directionality of plasmodesmata-mediated transport in Arabidopsis leaves supports auxin channeling. Curr. Biol. 30, 1970-1977. 10.1016/j.cub.2020.03.014 [DOI] [PubMed] [Google Scholar]
- Gaudioso-Pedraza R. and Benitez-Alfonso Y. (2014). A phylogenetic approach to study the origin and evolution of plasmodesmata-localized glycosyl hydrolases family 17. Frontiers in Plant Science 5, 212 10.3389/fpls.2014.00212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler M., Blakeslee J. J., Bouchard R., Lee O. R., Vincenzetti V., Bandyopadhyay A., Titapiwatanakun B., Peer W. A., Bailly A., Richards E. L. et al. (2005). Cellular efflux of auxin catalyzed by the Arabidopsis MDR/PGP transporter AtPGP1. The Plant Journal 44, 179-194. 10.1111/j.1365-313X.2005.02519.x [DOI] [PubMed] [Google Scholar]
- Gilroy S. (2008). Plant tropisms. Curr. Biol. 18, R275-R277. 10.1016/j.cub.2008.02.033 [DOI] [PubMed] [Google Scholar]
- Goldsmith M. H. M., Cataldo D. A., Karn J., Brenneman T. and Trip P. (1974). The rapid non-polar transport of auxin in the phloem of intact Coleus plants. Planta 116, 301-317. 10.1007/BF00390855 [DOI] [PubMed] [Google Scholar]
- Graham L. E., Cook M. E. and Busse J. S. (2000). The origin of plants: body plan changes contributing to a major evolutionary radiation. Proceedings of the National Academy of Sciences 97, 4535-4540. 10.1073/pnas.97.9.4535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieneisen V. A., Xu J., Marée A. F., Hogeweg P. and Scheres B. (2007). Auxin transport is sufficient to generate a maximum and gradient guiding root growth. Nature 449, 1008-1013. 10.1038/nature06215 [DOI] [PubMed] [Google Scholar]
- Guenot B., Bayer E., Kierzkowski D., Smith R. S., Mandel T., Žádníková P., Benková E. and Kuhlemeier C. (2012). PIN1-independent leaf initiation in Arabidopsis. Plant Physiol. 159, 1501-1510. 10.1104/pp.112.200402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X., Hyun T. K., Zhang M., Kumar R., Koh E. J., Kang B. H., Lucas W. J. and Kim J. Y. (2014). Auxin-callose-mediated plasmodesmal gating is essential for tropic auxin gradient formation and signaling. Dev. Cell 28, 132-146. 10.1016/j.devcel.2013.12.008 [DOI] [PubMed] [Google Scholar]
- Heisler M. G., Ohno C., Das P., Sieber P., Reddy G. V., Long J. A. and Meyerowitz E. M. (2005). Patterns of auxin transport and gene expression during primordium development revealed by live imaging of the Arabidopsis inflorescence meristem. Curr. Biol. 15, 1899-1911. 10.1016/j.cub.2005.09.052 [DOI] [PubMed] [Google Scholar]
- Hepler P. K. (1982). Endoplasmic reticulum in the formation of the cell plate and plasmodesmata. Protoplasma 111, 121-133. 10.1007/BF01282070 [DOI] [Google Scholar]
- Howell A. H., Peters W. S. and Knoblauch M. (2020). The diffusive injection micropipette (DIMP). J. Plant Physiol. 244, 153060 10.1016/j.jplph.2019.153060 [DOI] [PubMed] [Google Scholar]
- Huang F., Zago M. K., Abas L., van Marion A., Galván-Ampudia C. S. and Offringa R. (2010). Phosphorylation of conserved PIN motifs directs Arabidopsis PIN1 polarity and auxin transport. Plant Cell 22, 1129-1142. 10.1105/tpc.109.072678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias V. A. and Meins F. Jr. (2000). Movement of plant viruses is delayed in a β-1, 3-glucanase-deficient mutant showing a reduced plasmodesmatal size exclusion limit and enhanced callose deposition. The Plant Journal 21, 157-166. 10.1046/j.1365-313x.2000.00658.x [DOI] [PubMed] [Google Scholar]
- Kazan K. (2013). Auxin and the integration of environmental signals into plant root development. Annals of Botany 112, 1655-1665. 10.1093/aob/mct229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I., Hempel F. D., Sha K., Pfluger J. and Zambryski P. C. (2002). Identification of a developmental transition in plasmodesmatal function during embryogenesis in Arabidopsis thaliana. Development 129, 1261-1272. [DOI] [PubMed] [Google Scholar]
- Kitagawa M. and Fujita T. (2013). Quantitative imaging of directional transport through plasmodesmata in moss protonemata via single-cell photoconversion of Dendra2. J. Plant Res. 126, 577-585. 10.1007/s10265-013-0547-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoblauch M., Vendrell M., De Leau E., Paterlini A., Knox K., Ross-Elliot T., Reinders A., Brockman S. A., Ward J. and Oparka K. (2015). Multispectral phloem-mobile probes: properties and applications. Plant Physiol. 167, 1211-1220. 10.1104/pp.114.255414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoblauch M., Knoblauch J., Mullendore D. L., Savage J. A., Babst B. A., Beecher S. D., Dodgen A. C., Jensen K. H. and Holbrook N. M. (2016). Testing the Münch hypothesis of long distance phloem transport in plants. eLife 5, e15341 10.7554/eLife.15341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer E. M., Rutschow H. L. and Mabie S. S. (2011). AuxV: a database of auxin transport velocities. Trends Plant Sci. 16, 461-463. 10.1016/j.tplants.2011.05.003 [DOI] [PubMed] [Google Scholar]
- Kwiatkowska M. and Maszewski J. (1986). Changes in the occurrence and ultrastructure of plasmodesmata in antheridia of Chara vulgaris L. during different stages of spermatogenesis. Protoplasma 132, 179-188. 10.1007/BF01276998 [DOI] [Google Scholar]
- Laskowski M., Biller S., Stanley K., Kajstura T. and Prusty R. (2006). Expression profiling of auxin-treated Arabidopsis roots: toward a molecular analysis of lateral root emergence. Plant Cell Physiol. 47, 788-792. 10.1093/pcp/pcj043 [DOI] [PubMed] [Google Scholar]
- Lavenus J., Goh T., Roberts I., Guyomarc'h S., Lucas M., De Smet I., Fukaki H., Beeckman T., Bennett M. and Laplaze L. (2013). Lateral root development in Arabidopsis: fifty shades of auxin. Trends Plant Sci. 18, 450-458. 10.1016/j.tplants.2013.04.006 [DOI] [PubMed] [Google Scholar]
- Leliaert F., Smith D. R., Moreau H., Herron M. D., Verbruggen H., Delwiche C. F. and De Clerck O. (2012). Phylogeny and molecular evolution of the green algae. Crit. Rev. Plant Sci. 31, 1-46. 10.1080/07352689.2011.615705 [DOI] [Google Scholar]
- Levy A., Erlanger M., Rosenthal M. and Epel B. L. (2007). A plasmodesmata–associated β-1, 3–glucanase in Arabidopsis. The Plant Journal 49, 669-682. 10.1111/j.1365-313X.2006.02986.x [DOI] [PubMed] [Google Scholar]
- Li Z. P., Paterlini A., Glavier M. and Bayer E. M. (2020). Intercellular trafficking via plasmodesmata: molecular layers of complexity. Cell. Mol. Life Sci. 10.1007/s00018-020-03622-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Xu M., Liang N., Zheng Y., Yu Q. and Wu S. (2017). Symplastic communication spatially directs local auxin biosynthesis to maintain root stem cell niche in Arabidopsis. Proc. Natl Acad. Sci. USA 114, 4005-4010. 10.1073/pnas.1616387114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löfke C., Luschnig C. and Kleine-Vehn J. (2013). Posttranslational modification and trafficking of PIN auxin efflux carriers. Mech. Dev. 130, 82-94. 10.1016/j.mod.2012.02.003 [DOI] [PubMed] [Google Scholar]
- Lokhorst G. M., Sluiman H. J. and Star W. (1988). The ultrastructure of mitosis and cytokinesis in the sarcinoid Chlorokybus atmophyticus (Chlorophyta, Charophyceae) revealed by rapid freeze fixation and freeze substitution. J. Phycol. 24, 237-248. 10.1111/j.1529-8817.1988.tb04239.x [DOI] [Google Scholar]
- Marchant H. J. and Pickett-Heaps J. D. (1973). Mitosis and cytokinesis in coleochaete scutata. J. Phycol. 9, 461-471. 10.1111/j.1529-8817.1973.tb04122.x [DOI] [Google Scholar]
- Marchant A., Bhalerao R., Casimiro I., Eklöf J., Casero P. J., Bennett M. and Sandberg G. (2002). AUX1 promotes lateral root formation by facilitating indole-3-acetic acid distribution between sink and source tissues in the Arabidopsis seedling. Plant Cell 14, 589-597. 10.1105/tpc.010354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens H. J., Hansen M. and Schulz A. (2004). Caged probes: a novel tool in studying symplasmic transport in plant tissues. Protoplasma 223, 63-66. 10.1007/s00709-003-0029-z [DOI] [PubMed] [Google Scholar]
- Maule A. J., Gaudioso-Pedraza R. and Benitez-Alfonso Y. (2013). Callose deposition and symplastic connectivity are regulated prior to lateral root emergence. Communicative & Integrative Biology 6, e26531 10.4161/cib.26531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor N. L., Voß U., Janes G., Bennett M. J., Wells D. M. and Band L. R. (2020). Auxin fluxes through plasmodesmata modify root-tip auxin distribution. Development 147, 1-12. 10.1242/dev.181669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud O., Fiorucci A.-S., Xenarios I. and Fankhauser C. (2017). Local auxin production underlies a spatially restricted neighbor-detection response in Arabidopsis. Proc. Natl Acad. Sci. USA 114, 7444-7449. 10.1073/pnas.1702276114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millenaar F. F., Cox M. C., van Berkel Y. E. D. J., Welschen R. A., Pierik R., Voesenek L. A. and Peeters A. J. (2005). Ethylene-induced differential growth of petioles in Arabidopsis. Analyzing natural variation, response kinetics, and regulation. Plant Physiol. 137, 998-1008. 10.1104/pp.104.053967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison G. J. (1980). A model for vein formation in higher plants. Proceedings of the Royal Society of London. Series B. Biological Sciences 207, 79-109. 10.1098/rspb.1980.0015 [DOI] [Google Scholar]
- Morris D. A. and Thomas A. G. (1978). A microautoradiographic study of auxin transport in the stem of intact pea seedlings (Pisum sativum L.). J. Exp. Bot. 29, 147-157. 10.1093/jxb/29.1.147 [DOI] [Google Scholar]
- Morris D. A., Kadir G. O. and Barry A. J. (1973). Auxin transport in intact pea seedlings (Pisum sativum L.): The inhibition of transport by 2,3,5-triiodobenzoic acid. Planta 110: 173-182. 10.1007/BF00384840 [DOI] [PubMed] [Google Scholar]
- Morvan O., Quentin M., Jauneau A., Mareck A. and Morvan C. (1998). Immunogold localization of pectin methylesterases in the cortical tissues of flax hypocotyl. Protoplasma 202, 175-184. 10.1007/BF01282545 [DOI] [Google Scholar]
- Mravec J., Skůpa P., Bailly A., Hoyerová K., Křeček P., Bielach A., Petrášek J., Zhang J., Gaykova V., Stierhof Y. et al. (2009). Subcellular homeostasis of phytohormone auxin is mediated by the ER-localized PIN5 transporter. Nature 459, 1136-1140. 10.1038/nature08066 [DOI] [PubMed] [Google Scholar]
- Nakajima K., Sena G., Nawy T. and Benfey P. N. (2001). Intercellular movement of the putative transcription factor SHR in root patterning. Nature 413, 307-311. 10.1038/35095061 [DOI] [PubMed] [Google Scholar]
- Nicolas W. J., Grison M. S. and Bayer E. M. (2018). Shaping intercellular channels of plasmodesmata: the structure-to-function missing link. J. Exp. Bot. 69, 91-103. 10.1093/jxb/erx225 [DOI] [PubMed] [Google Scholar]
- Noh B., Murphy A. S. and Spalding E. P. (2001). Multidrug resistance–like genes of Arabidopsis required for auxin transport and auxin-mediated development. Plant Cell 13, 2441-2454. 10.1105/tpc.010350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notaguchi M., Wolf S. and Lucas W. J. (2012). Phloem–Mobile Aux/IAA Transcripts Target to the Root Tip and Modify Root Architecture F. Journal of Integrative Plant Biology 54, 760-772. 10.1111/j.1744-7909.2012.01155.x [DOI] [PubMed] [Google Scholar]
- One Thousand Plant Transcriptomes Initiative (2019). One thousand plant transcriptomes and the phylogenomics of green plants. Nature 574, 679 10.1038/s41586-019-1693-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oparka K. J., Duckett C. M., Prior D. A. M. and Fisher D. B. (1994). Real–time imaging of phloem unloading in the root tip of Arabidopsis. The Plant Journal 6, 759-766. 10.1046/j.1365-313X.1994.6050759.x [DOI] [Google Scholar]
- Pantazopoulou C. K., Bongers F. J., Küpers J. J., Reinen E., Das D., Evers J. B., Anten N. P. R. and Pierik R. (2017). Neighbor detection at the leaf tip adaptively regulates upward leaf movement through spatial auxin dynamics. Proc. Natl Acad. Sci. USA 114, 7450-7455. 10.1073/pnas.1702275114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parizot B., De Rybel B. and Beeckman T. (2010). VisuaLRTC: a new view on lateral root initiation by combining specific transcriptome data sets. Plant Physiol. 153, 34-40. 10.1104/pp.109.148676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Péret B., De Rybel B., Casimiro I., Benková E., Swarup R., Laplaze L., Beeckman T. and Bennett M. J. (2009). Arabidopsis lateral root development: an emerging story. Trends Plant Sci. 14, 399-408. 10.1016/j.tplants.2009.05.002 [DOI] [PubMed] [Google Scholar]
- Péret B., Swarup K., Ferguson A., Seth M., Yang Y., Dhondt S., James N., Casimiro I., Perry P., Syed A. et al. (2012). AUX/LAX genes encode a family of auxin influx transporters that perform distinct functions during Arabidopsis development. The Plant Cell 24, 2874-2885. 10.1105/tpc.112.097766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrášek J., Mravec J., Bouchard R., Blakeslee J. J., Abas M., Seifertová D., Wisniewska J., Tadele Z., Kubes M., Covanová M. et al. (2006). PIN proteins perform a rate-limiting function in cellular auxin efflux. Science 312, 914-918. 10.1126/science.1123542 [DOI] [PubMed] [Google Scholar]
- Raven J. A. (1975). Transport of indoleacetic acid in plant cells in relation to pH and electrical potential gradients, and its significance for polar IAA transport. New Phytol. 74, 163-172. 10.1111/j.1469-8137.1975.tb02602.x [DOI] [Google Scholar]
- Raven J. A. (2018). Evolution of plasmodesmata. Annual Plant Reviews online 18, 33-52. 10.1002/9781119312994.apr0174 [DOI] [Google Scholar]
- Ravichandran S. J., Linh N. M. and Scarpella E. (2020). The canalization hypothesis–challenges and alternatives. New Phytol. 1051-1059. 10.1111/nph.16605 [DOI] [PubMed] [Google Scholar]
- Reinhardt D., Pesce E.-R., Stieger P., Mandel T., Baltensperger K., Bennett M., Traas J., Friml J. and Kuhlemeier C. (2003). Regulation of phyllotaxis by polar auxin transport. Nature 426, 255-260. 10.1038/nature02081 [DOI] [PubMed] [Google Scholar]
- Rennie E. A. and Turgeon R. (2009). A comprehensive picture of phloem loading strategies. Proceedings of the National Academy of Science USA 106, 14162-14167. 10.1073/pnas.0902279106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers C. E., Mattox K. R. and Stewart K. D. (1980). The zoospore of Chlorokybus atmophyticus, a charophyte with sarcinoid growth habit. Am. J. Bot. 67, 774-783. 10.1002/j.1537-2197.1980.tb07706.x [DOI] [Google Scholar]
- Ross-Elliott T. J., Jensen K. H., Haaning K. S., Wager B. M., Knoblauch J., Howell A. H., Mullendore D. L., Monteith A. G., Paultre D., Yan D. et al. (2017). Phloem unloading in Arabidopsis roots is convective and regulated by the phloem-pole pericycle. eLife 6, e24125 10.7554/eLife.24125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubery P. H. and Sheldrake A. R. (1974). Carrier-mediated auxin transport. Planta 118, 101-121. 10.1007/BF00388387 [DOI] [PubMed] [Google Scholar]
- Rutschow H. L., Baskin T. I. and Kramer E. M. (2011). Regulation of solute flux through plasmodesmata in the root meristem. Plant Physiol. 155, 1817-1826. 10.1104/pp.110.168187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabnis D. D. and Watson B. T. (1982). Transport of 14C-IAA in Cucurbita maxima: a comparative study of rapid, non-polar movement and slow, polar auxin transport. J. Exp. Bot. 33, 1279-1285. 10.1093/jxb/33.6.1279 [DOI] [Google Scholar]
- Sachs T. (1981). The control of the patterned differentiation of vascular tissues. In Advances in botanical research (vol. 9, pp. 151-262). Academic Press. [Google Scholar]
- Sager R., Wang X., Hill K., Yoo B. C., Caplan J., Nedo A., Tran T., Bennett M. J. and Lee J. Y. (2020). Auxin-dependent control of a plasmodesmal regulator creates a negative feedback loop modulating lateral root emergence. Nat. Commun. 11, 1-10. 10.1038/s41467-019-14226-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar A. K., Luijten M., Miyashima S., Lenhard M., Hashimoto T., Nakajima K., Scheres B., Heidstra R. and Laux T. (2007). Conserved factors regulate signalling in Arabidopsis thaliana shoot and root stem cell organizers. Nature 446, 811-814. 10.1038/nature05703 [DOI] [PubMed] [Google Scholar]
- Santner A., Calderon-Villalobos L. I. A. and Estelle M. (2009). Plant hormones are versatile chemical regulators of plant growth. Nat. Chem. Biol. 5, 301-307. 10.1038/nchembio.165 [DOI] [PubMed] [Google Scholar]
- Scherp P., Grotha R. and Kutschera U. (2001). Occurrence and phylogenetic significance of cytokinesis-related callose in green algae, bryophytes, ferns and seed plants. Plant Cell Rep. 20, 143-149. 10.1007/s002990000301 [DOI] [PubMed] [Google Scholar]
- Schönknecht G., Brown J. E. and Verchot-Lubicz J. (2008). Plasmodesmata transport of GFP alone or fused to potato virus X TGBp1 is diffusion driven. Protoplasma 232, 143 10.1007/s00709-008-0293-z [DOI] [PubMed] [Google Scholar]
- Simon S. and Petrášek J. (2011). Why plants need more than one type of auxin. Plant Science 180, 454-460. 10.1016/j.plantsci.2010.12.007 [DOI] [PubMed] [Google Scholar]
- Simon M. L. A., Platre M. P., Marquès-Bueno M. M., Armengot L., Stanislas T., Bayle V., Caillaud M. and Jaillais Y. (2016). A PtdIns (4) P-driven electrostatic field controls cell membrane identity and signalling in plants. Nature Plants 2, 1-10. 10.1038/nplants.2016.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson C., Thomas C., Findlay K., Bayer E. and Maule A. J. (2009). An Arabidopsis GPI-anchor plasmodesmal neck protein with callose binding activity and potential to regulate cell-to-cell trafficking. Plant Cell 21, 581-594. 10.1105/tpc.108.060145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skokan R., Medvecká E., Viaene T., Vosolsobě S., Zwiewka M., Müller K., Skůpa P., Karady M., Zhang Y., Janacek D. P. et al. (2019). PIN-driven auxin transport emerged early in streptophyte evolution. Nature Plants 5, 1114-1119. 10.1038/s41477-019-0542-5 [DOI] [PubMed] [Google Scholar]
- Slewinski T. L., Baker R. F., Stubert A. and Braun D. M. (2012). Tie-dyed2 encodes a callose synthase that functions in vein development and affects symplastic trafficking within the phloem of maize leaves. Plant Physiol. 160, 1540-1550. 10.1104/pp.112.202473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelman Z., Ham B.-K., Zhang Z., Toal T. W., Brady S. M., Zheng Y., Lucas W. J. and Wolf S. (2015). A tomato phloem–mobile protein regulates the shoot-to-root ratio by mediating the auxin response in distant organs. The Plant Journal 83, 853-863. 10.1111/tpj.12932 [DOI] [PubMed] [Google Scholar]
- Stewart K. D., Mattox K. R. and Floyd G. L. (1973). Mitosis, cytokinesis, the distribution of plasmodesmata, and other cytological characteristics in the Ulotrichales, Ulvales, and Chaetophorales: phylogenetic and taxonomic consideration. J. Phycol. 9, 128-141. 10.1111/j.1529-8817.1973.tb04068.x [DOI] [Google Scholar]
- Swarup R., Friml J., Marchant A., Ljung K., Sandberg G., Palme K. and Bennett M. (2001). Localization of the auxin permease AUX1 suggests two functionally distinct hormone transport pathways operate in the Arabidopsis root apex. Genes Dev. 15, 2648-2653. 10.1101/gad.210501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup K., Benková E., Swarup R., Casimiro I., Péret B., Yang Y., Parry G., Nielsen E., De Smet I., Vanneste S. et al. (2008). The auxin influx carrier LAX3 promotes lateral root emergence. Nat. Cell Biol. 10, 946-954. 10.1038/ncb1754 [DOI] [PubMed] [Google Scholar]
- Terasaka K., Blakeslee J. J., Titapiwatanakun B., Peer W. A., Bandyopadhyay A., Makam S. N., Ran O. R., Richards E. L., Murphy A. S., Sato F. et al. (2005). PGP4, an ATP binding cassette P-glycoprotein, catalyzes auxin transport in Arabidopsis thaliana roots. Plant Cell 17, 2922-2939. 10.1105/tpc.105.035816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry B. R. and Robards A. W. (1987). Hydrodynamic radius alone governs the mobility of molecules through plasmodesmata. Planta 171, 145-157. 10.1007/BF00391090 [DOI] [PubMed] [Google Scholar]
- Tsurumi S. and Wada S. (1980). Transport of shoot-and cotyledon-applied indole-3-acetic acid to Vicia faba root. Plant Cell Physiol. 21, 803-816. 10.1093/oxfordjournals.pcp.a076055 [DOI] [PubMed] [Google Scholar]
- van Zanten M., Voesenek L. A., Peeters A. J. and Millenaar F. F. (2009). Hormone-and light-mediated regulation of heat-induced differential petiole growth in Arabidopsis. Plant Physiol. 151, 1446-1458. 10.1104/pp.109.144386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zanten M., Pons T. L., Janssen J. A. M., Voesenek L. A. C. J. and Peeters A. J. M. (2010). On the relevance and control of leaf angle. Critical Reviews in Plant Science 29, 300-316. 10.1080/07352689.2010.502086 [DOI] [Google Scholar]
- Vatén A., Dettmer J., Wu S., Stierhof Y.-D., Miyashima S., Yadav S. R., Roberts C. J., Campilho A., Bulone V., Lichtenberger R. et al. (2011). Callose biosynthesis regulates symplastic trafficking during root development. Dev. Cell 21, 1144-1155. 10.1016/j.devcel.2011.10.006 [DOI] [PubMed] [Google Scholar]
- Verna C., Ravichandran S. J., Sawchuk M. G., Linh N. M. and Scarpella E. (2019). Coordination of tissue cell polarity by auxin transport and signaling. eLife 8, e51061 10.7554/eLife.51061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viaene T., Landberg K., Thelander M., Medvecka E., Pederson E., Feraru E., Cooper E. D., Karimi M., Delwiche C. F., Ljung K. et al. (2014). Directional auxin transport mechanisms in early diverging land plants. Curr. Biol. 24, 2786-2791. 10.1016/j.cub.2014.09.056 [DOI] [PubMed] [Google Scholar]
- Wang S., Li L., Li H., Sahu S. K., Wang H., Xu Y., Xian W., Song B., Liang H., Cheng S. et al. (2020). Genomes of early-diverging streptophyte algae shed light on plant terrestrialization. Nature Plants 6, 95-106. 10.1038/s41477-019-0560-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiśniewska J., Xu J., Seifertová D., Brewer P. B., Růžička K., Blilou I., Rouquie D., Benkova E., Scheres B. and Friml J. (2006). Polar PIN localization directs auxin flow in plants. Science 312, 883-883 10.1126/science.1121356 [DOI] [PubMed] [Google Scholar]
- Wu S., O'Lexy R., Xu M., Sang Y., Chen X., Yu Q. and Gallagher K. L. (2016). Symplastic signaling instructs cell division, cell expansion, and cell polarity in the ground tissue of Arabidopsis thaliana roots. Proc. Natl Acad. Sci. USA 113, 11621-11626. 10.1073/pnas.1610358113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Hammes U. Z., Taylor C. G., Schachtman D. P. and Nielsen E. (2006). High-affinity auxin transport by the AUX1 influx carrier protein. Curr. Biol. 16, 1123-1127. 10.1016/j.cub.2006.04.029 [DOI] [PubMed] [Google Scholar]
- Zaveska-Drabkova L. and Honys D. (2017). Evolutionary history of callose synthases in terrestrial plants with emphasis on proteins involved in male gametophyte development. PLoS One 12, e0187331 10.1371/journal.pone.0187331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Adamowski M., Marhava P., Tan S., Zhang Y., Rodriguez L., Zwiewka M., Pukyšová V., Sánchez A. S., Raxwal V. K. et al. (2020). Arabidopsis flippases cooperate with ARF GTPase exchange factors to regulate the trafficking and polarity of PIN auxin transporters. Plant Cell 32, 1644-1664. 10.1105/tpc.19.00869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong B., Sun L. and Penny D. (2015). The origin of land plants: a phylogenomic perspective. Evolutionary Bioinformatics, 11, EBO-S29089 10.4137/EBO.S29089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu T., Lucas W. J. and Rost T. L. (1998). Directional cell-to-cell communication in the Arabidopsis root apical meristem I. An ultrastructural and functional analysis. Protoplasma 203, 35-47. [Google Scholar]