Abstract
Anterior pituitary somatotropes are important metabolic sensors responding to leptin by secreting growth hormone (GH). However, reduced leptin signals caused by fasting have not always correlated with reduced serum GH. Reports show that fasting may stimulate or reduce GH secretion, depending on the species. Mechanisms underlying these distinct somatotrope responses to fasting remain unknown. To define the somatotrope response to decreased leptin signaling we examined markers of somatotrope function over different time periods of fasting. Male and mice were fasted for 24 and 48 h, with female mice fasted for 24 h compared to fed ad libitum controls. Body weight and serum glucose were reduced in both males and females, but, unexpectedly, serum leptin was reduced only in males. Furthermore, in males serum GH levels showed a biphasic response with significant reductions at 24 h followed by a significant rise at 48 h, which coincided with the rise in serum ghrelin levels. In contrast, females showed an increase in serum GH at 24. We then explored mechanisms underlying the differential somatotrope responses seen in males and observed that pituitary levels of Gh mRNA increased, with no distinction between acute and prolonged fasting. By contrast, the Ghrhr mRNA (encoding GH releasing hormone receptor) and the Ghsr mRNA (encoding the ghrelin receptor) were both greatly increased at prolonged fasting times coincident with increased serum GH. These findings show sex differences in the somatotrope and adipocyte responses to fasting and support an adaptive role for somatotropes in males in response to multiple metabolic signals.
Keywords: Pituitary, growth hormone, somatotrope, leptin, ghrelin
INTRODUCTION
Malnutrition is a completely preventable global health crisis that is responsible for the most cases of ill health. The World Health Organization defines malnutrition as a result of either deficiency, excess, or imbalance in an individual’s energy and/or nutrient intake (World Health Organization, 2018). Malnutrition, therefore, can take on multiple forms, ranging from an over nourished-imbalanced diet to an undernourished, low nutrient diet. Undernutrition, as also seen with prolonged fasting, can result in stunted growth in children under five, anemia in women of reproductive age, and adults who are underweight (Fanzo et al., 2018).
Leptin and ghrelin are two major metabolic signals of energy homeostasis. Leptin is secreted by adipose tissue and is proportional to adiposity, with leptin receptors ubiquitously expressed and having major signaling targets in the brain (Lloyd et al., 2001, Bouret, 2010, Wada et al., 2014, Wauman et al., 2017). Hypothalamic leptin signaling regulates food intake, promoting an anorexigenic response, by signaling nutrient adequacy and fat storage levels (Ahima et al., 1996, Tao, 2010). In the fasted state, serum leptin levels are reduced (Chan et al., 2003, Longo and Mattson, 2014). In the pituitary gland, leptin receptors are predominantly expressed in somatotropes, where leptin stimulates the synthesis of the lipolytic hormone, growth hormone (GH) (Childs et al., 2011).
Ghrelin, serves to signal the opposite nutritional status from leptin. Ghrelin is secreted by the gastrointestinal tract, primarily from the stomach, and in the hypothalamus, ghrelin promotes an orexigenic response, signaling for increased nutrient intake (Wang et al., 2002, Muller et al., 2015b). Fasting or malnutrition cause a rise in serum ghrelin levels (Muller et al., 2002). In a broader sense, ghrelin is a growth hormone secretagogue and binds to growth hormone secretagogue receptors (GHSR) that are expressed in the hypothalamus, and in somatotropes of the anterior pituitary. Thus, in their role as metabolic sensors, somatotropes receive signals from both leptin and ghrelin to control synthesis and secretion of GH (Childs et al., 2011, Syed et al., 2013). However, the mechanisms by which complex metabolic signals are interpreted by somatotropes is only beginning to be understood.
Our studies have shown that loss of leptin signaling to somatotropes, through ablation of leptin receptors (somatotrope Cre GH Lepr-null), results in a deficiency in GH and growth hormone releasing hormone receptor (GHRHR) in the anterior pituitary and as adults, these mice are obese and metabolically dysfunctional (Allensworth-James et al., 2015, Akhter et al., 2012). Despite the function of ghrelin in promoting growth hormone synthesis and secretion, the Lepr-null somatotropes respond only partially to ghrelin by secreting or storing GH (Syed et al., 2013, Allensworth-James et al., 2020), suggesting that the somatotrope response to ghrelin is compromised when leptin mediated GHRHR expression is low.
To further define the link between leptin signaling and somatotrope function we utilized our food deprivation model (fasting) in this study, to reduce leptin signals (Crane et al., 2007). In this previous study of male rats, we showed that a 24 h fast reduced the number of cells storing GH and binding GHRHR, suggesting that somatotropes were responding to the lower leptin signals. However, in humans and in mouse models, fasting has historically been reported to result in an elevation of serum GH levels (Luque et al., 2007, Ho et al., 1988) suggesting that somatotrope function is not affected by the decline in leptin signals. In contrast, more recent findings have shown fasting to reduce serum GH levels (Steyn et al., 2011) in male C57BL/6 mice. These reported differences in somatotrope response to fasting have been suggested to be due to protocol differences in blood collection, animal handling and/or treatment times (Bartke et al., 2013), however, the mechanisms underlying these distinct observations remain unclear. Furthermore, there are no studies of somatotrope responses to fasting in female mice. To directly address this knowledge gap, we initiated studies of fasting effects upon somatotrope synthesis and secretion of GH, comparing males and females. These studies are the first to show a distinct sex difference in adipocyte and somatotrope responses to fasting. Unlike males, females do not show reduced serum leptin with 24 h fasting. They do show a rise in serum GH, however, which might be expected based on orexigenic responses. In contrast, these studies are the first to show that males show reduced serum leptin and exhibit a biphasic GH secretory response to fasting, which reflects this reduction and the gradual rise in ghrelin with the prolonged fast (48h).
MATERIALS AND METHODS
Animals
All animal care protocols were approved by the University of Arkansas for Medical Sciences Institutional Animal Care and Use Committee. At least 5 major groups (2–3 groups per fasting time, with 3–4 mice per condition) of 8–9 week old male or female FVB.129P2 mice (bred in house) were used in these studies. Mice were weaned at 21 days of age and housed with no more than 5 animals/cage at 27°C on a 14-hour light (06:00–20:00), 10-hour dark cycle. Mice were fed a diet of 22% crude protein, 5.5% crude fat, and 3.9% crude fiber (Teklad 8640, Harlan). We chose the FVB.129P2 (FVB) strain (Errijgers et al., 2007) because it is used for our transgenic models. We wanted to perform these experiments in the same strain in order to be able to compare results in ongoing studies with results with our transgenics. In addition, these mice were the hybrid strain of FVB, which do not lose sight at puberty.
Fasting protocol and tissue harvesting
The experimental approach consisted of a 24-hour singly housed acclimation period, followed by a fasting period, and then euthanasia. Mice were weighed at the start of the acclimation period, averaging 26g. The 6-hour and 12-hour fasting periods were done only on males. These periods were conducted during scotophase (starting at 20:00) because this was the time when they began their active eating period. The 24- and 48-hour fast were initiated at 09:00. The experimental conditions consisted of: 1) ad libitum fed (control), 2) fasted with water, and 3) fasted with 10% glucose in the drinking water (fasting+glucose), which was used as a control only for the 24-hr and 48-hr studies. At the conclusion of the fast, mice weights were measured. Mice were anesthetized under isoflurane (Piramal Critical Care). Blood glucose was collected via tail snip and measured in duplicate by an AlphaTRAK 2 glucometer. Mice were re-anesthetized under isoflurane and decapitated by guillotine. Trunk blood was collected on ice for serum analyses. Immediately after collection, serum was incubated on ice for 1 hour. Serum was centrifuged at 3200 x g for 20 minutes at 4°C. Supernatant was collected on ice and stored at −20°C until use. Pituitaries were collected in 150 μL ice-cold radioimmunoprecipitation assay (RIPA) buffer (Sigma Aldrich, R0278) containing 10 μL/mL protease inhibitor cocktail (Sigma-Aldrich, P8340). For each experimental time point for both male and female mice, 2–4 cohorts of mice were used. For each cohort, 3–4 mice were used per condition. This generated a total n of 5–9 mice per condition that was combined for analyses. In mRNA analysis of 48 h fasted male mice, only 4 mice per condition were used. Each mouse data was graphed as an individual data point.
Pituitary protein and mRNA extraction
Pituitaries collected in RIPA buffer cocktail were homogenized on ice with a pellet pestle for approximately 20 sec. A 20–30 μL aliquot was collected for RNA extraction and the remaining homogenate was incubated overnight at 4°C. The next morning, the homogenate was centrifuged at 19283 x g for 20 minutes at 4°C. Supernatant was collected and stored at −20°C for later use. From the homogenate aliquot, RNA was extracted by Maxwell 16 LEV simplyRNA Tissue Kit (Promega, AS1280) or using RNAzol RT (Sigma-Aldrich, R4533), and stored at −80°C. RNA quality was determined by using the Thermo Fisher NanoDrop™ 2000c spectrophotometer with an A260/A280 ratio of 1.4–2.0 as acceptable for use.
Analysis of cytokines and anterior pituitary hormones
Serum leptin levels were measured at a 1:10 dilution using the Mouse/Rat Leptin Quantikine ELISA Kit (RNDSystems, MOB00). Total serum ghrelin levels were measured at a 1:4 dilution using the ghrelin (Rat, Mouse) EIA Kit (Phoenix Pharmaceuticals, EK-031–31). Anterior pituitary hormones were quantified at a 1:2.5 dilution for serum and a 1:300 dilution for pituitary content using the Luminex LX200 (Luminex Corp) xPONENT 3.1 with the Millipore MAP Multiplex kits (Millipore Corp).
Quantitative real time polymerase chain reaction (qRT-PCR)
Complementary DNA was synthesized using the iScript cDNA Synthesis Kit (Bio-Rad, 170–8890). qRT-PCR was performed as previously published (Odle et al., 2018). Primers (Table 1) and complementary DNA were added to Power SYBR Green PCR Master Mix (Applied Biosystems, 4367659). Reactions were performed with the QuantStudio 12K Flex system (Applied Biosystems, Life Technologies), under the following conditions: 1) incubation/denaturation stage: 50°C for 2 minutes and 95°C for 10 minutes; 2) polymerase chain reaction (PCR) amplification stage (40 cycles): 95°C for 15 seconds, 55°C for 15 seconds, and 72°C for 1 minute; and 3) melt curve stage: 95°C for 15 seconds (ramp rate = 1.6°/s), 60°C for 1 second (ramp rate = 1.6°/s), and 95°C for 15 seconds (ramp rate = 0.5°/s). For each sample, RQ values per target were normalized to peptidylprolyl isomerase A (Ppia, gene for cyclophilin A) expression using the QuantStudio 12K Flex software.
Table 1:
Primers used for quantitative real time PCR.
| Gene | Accession Number | Forward Sequence | Reverse Sequence |
|---|---|---|---|
| PPIA (cyclophilin A) | NM_008907.2 | TGG TCT TTG GGA AGG TGA AAG | TGT CCA CAG TCG GAA ATG GT |
| GH | NM_008117.3 | CCT CAG CAG GAT TTT CAC CA | CTT GAG GAT CTG CCC AAC AC |
| GHRHR | NM_001003685.3 | ACC CGT ATC CTC TGC TTG CT | AGG TGT TGT TGG TCC CCT CT |
| GHSR | NM_177330.4 | TCA GGG ACC AGA ACC ACA AA | CCA GCA GAG GAT GAA AGC AA |
| LHβ | NM_008497.2 | TGT CCT AGC ATG GTC CGA GT | AGG AAA GGA GAC TAT GGG GTC TA |
| MSI1 | NM_008629.1 | GCC ATG CTG ATG TTC GAC AA | CTA CGA TGT CCT CGC TCT CAA |
| MSI2 (isoforms 1,3,4) |
NM_054043.3 NM_001363195.1 NM_001363194.1 |
GCG ATG CTG ATG TTC GAC AA | TCT CCA CAA CGT CTT CAT TCT CA |
| POU1F1/PIT1 | NM_001362468.3 | AGC TGA GCA GGT CGG AGC TTT GT | GGA AGG CTT GCT GTG CTC CCC |
| PRL | NM_001163530.1 | GGC CAT CTT GGA GAA GTG TG | ACA GAT TGG CAG AGG CTG AA |
Statistics
Statistical analyses of mRNA and pituitary hormone levels were done on extracts from individual fractions from at least four mice. We used an ANOVA followed by Newman-Keul’s and Bonferroni’s post hoc tests (P <0.05 was considered significant). In some cases (indicated in the figure legends), a Student’s t test with Welch’s correction was used to compare values among two conditions (P < 0.05 was considered significant).
RESULTS
Changes in metabolic parameters, serum leptin, GH and ghrelin in 24 hour fasted mice
In humans and in mouse models, fasting normally results in reduced serum leptin and increased acylated ghrelin (Chan et al., 2003, Longo and Mattson, 2014, Muller et al., 2002, Muller et al., 2015b). We, therefore, initially tested male and female mice for 24 h. As a positive control, a fasted group was administered 10% glucose in the drinking water since glucose was shown to prevent the fasting-induced reduction in serum leptin (Crane et al., 2007). In comparison to ad libitum fed mice, both sexes had significant loses in body weight (males = 10.77% (p<0.0001) and females = 8.34% (p = 0.0004)) (Figure 1A) and blood glucose (males = 41.4% (p<0.0001) and females = 20.8% (p = 0.0133)) (Figure 2B). Glucose in the drinking water of 24 h fasted male mice prevented weight loss by 5.44% (p < 0.0001) but did not have a significant effect in female mice. As expected (Crane et al., 2007), the controls with 10% glucose in the drinking water prevented the fasting-induced decrease in blood glucose in males (Figure 1B). Mice fasted with 10% glucose in the drinking water consumed more water than the fed and fasted groups: the 24 h mice consumed 52.7% (p = 0.005) more compared to the fed mice and 53.5% (p = 0.0003) more compared to mice fasted alone (Supplemental Figure 1).
Figure 1: Fasted male FVB:P129 mice showed decreased body weight, blood glucose and leptin with 24 (red) and 48 (blue) hour fast. Serum GH in males had a biphasic response to the 24 and 48 h fast, and increased ghrelin with the 48 hr fast. Female fasted FVB:P129 mice had decreased body weight, blood glucose and leptin, while having increased serum GH with the 24 h (purple) fast.
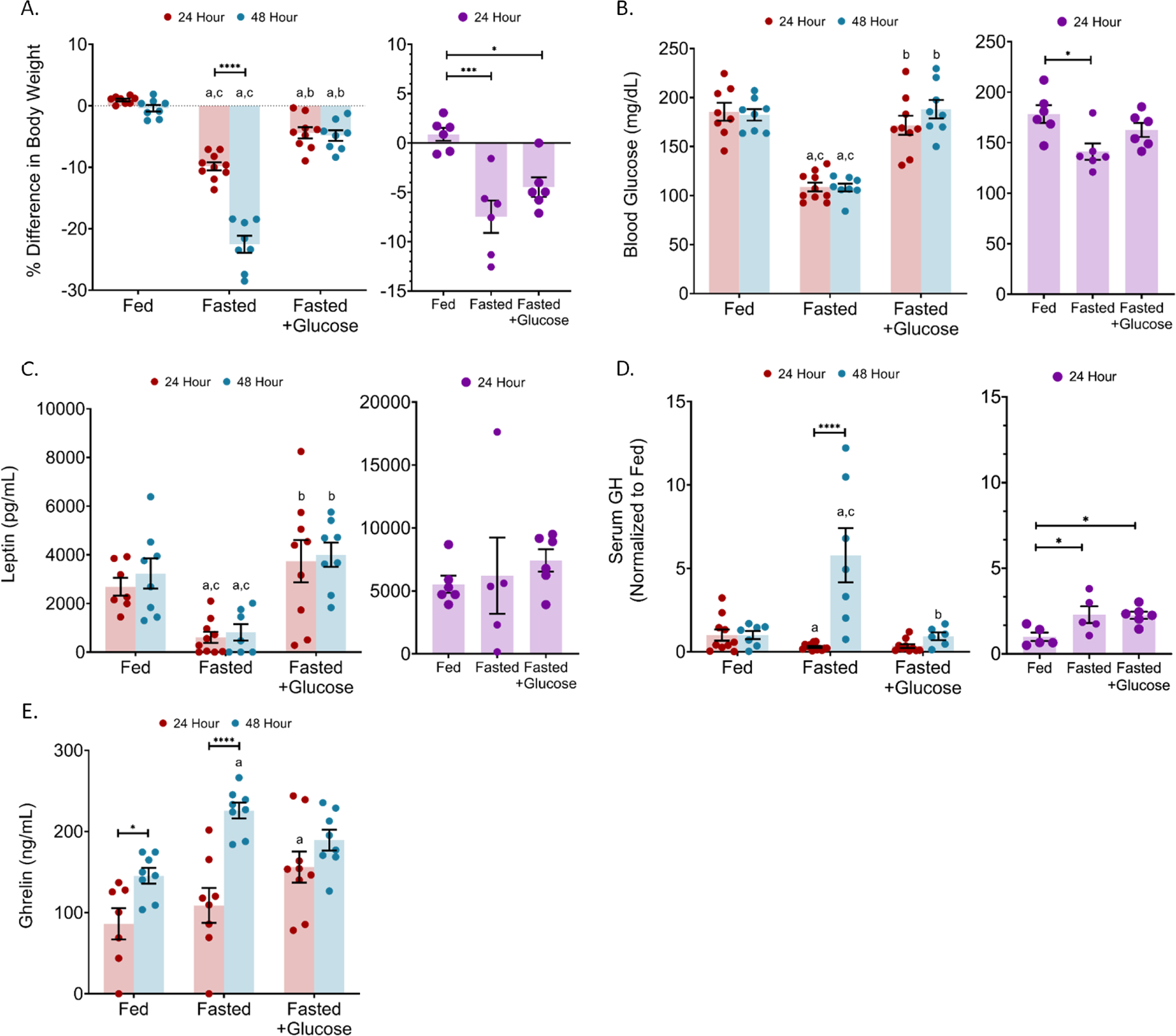
(a) the difference in body weight (b) blood glucose, (c) serum leptin, (d) serum GH and (e) serum ghrelin were measured relative to ad libitum fed controls (Fed). Addition of 10% glucose to drinking water resulted in partial reversal of fasting effects with 24 and 48 h (Fasted +Glucose). All sets; n = 5–8 per condition; error bars are SEM. Values that differ significantly among time points within a treatment condition: *P<0.05; **P<0.01, ***P<0.001, ****P<0.0001. Values that differ significantly (P < 0.05) with the same time point among different treatment conditions: “a” significant with fed group, “b” significant with fasted group, and “c” significant with fasted+glucose group. Two-way ANOVA (males) and one-way ANOVA (females).
Figure 2: Increased levels of pituitary Gh, Prl and Lh mRNA in male mice fasted for 24 and 48 hour fasting. Fsh mRNA was increased in female mice with 24 hour fasting and decreased in male mice with 48 hour fasting.
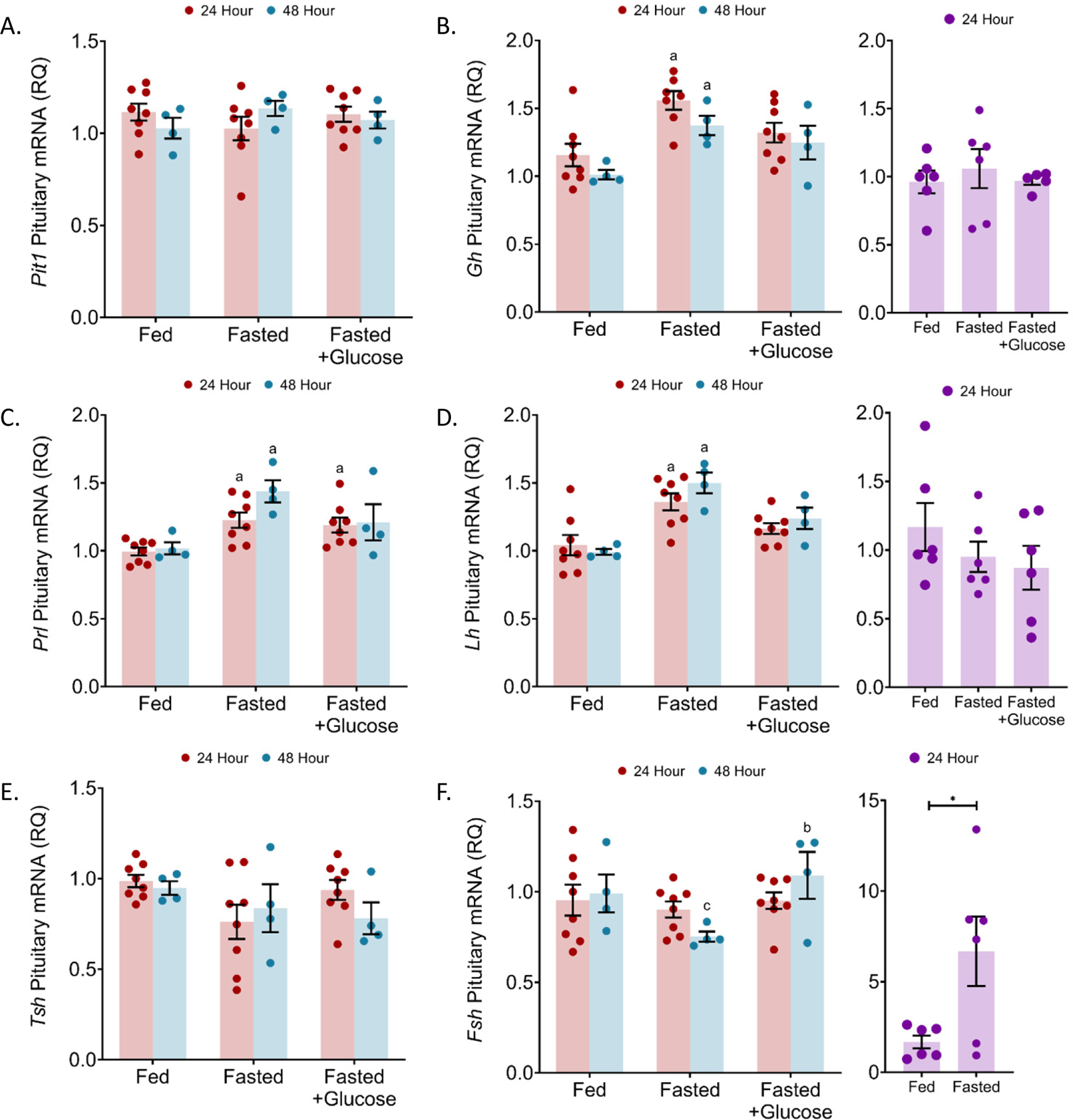
In the pituitary of 24 and 48 h fasted mice, mRNA levels were quantified by qRT-PCR for (a) pituitary-specific positive transcription factor 1 (Pit1), (b) growth hormone (Gh), (c) prolactin (Prl), (d) luteinizing hormone (Lh), (e) thyroid stimulating hormone, and (f) follicle-stimulating hormone (Fsh). n = 4–8 per condition; error bars are SEM. Values that differ significantly (P < 0.05) with the same time point among different treatment conditions: “a” significant with fed group and “b” significant with fasted group. Student’s t-test.
We next measured serum leptin, GH and ghrelin. Strikingly, female mice fasted for 24 h did not have a reduction in serum leptin (Figure 1C) while males had a 77.4% (p = 0.0256) significant reduction compared to ad libitum fed mice. Since, an objective of this study was to correlate the fasting-induced reduction in leptin with potential changes in somatotrope GH secretion we did not continue with additional fasting times in the females.
In addition, we detected sex differences in somatotrope responses. In the 24 h fasted males, serum GH was reduced by 71% (5512 pg/mL decrease (p = 0.0324); whereas with the females, serum GH was elevated by 129% (p = 0.0427) that was unaltered by 10% glucose in the water (Figure 1D). Serum ghrelin was unchanged in 24 h fasted male mice, but was increased in the presence of glucose by 81% (p = 0.0111) when compared to the fed mice (Figure 1E) (Alamri et al., 2016).
Changes in metabolic parameters, serum leptin, GH and ghrelin in 48 hour fasted mice
The observed decreases in serum leptin and GH with 24 h fasted male mice correlated with our studies where we showed leptin dependency in somatotropes of male mice. Furthermore, it was clear that serum ghrelin was not sufficiently high to rescue the somatotropes. Thus, we continued the studies of males, increasing the fasting time to 48 h. Male mice fasted for 48 h showed a 22.13% (p<0.0001) reduction in body weight, a 40.7% (p<0.0001) reduction in blood glucose and a 74.9% (p = 0.0095) reduction in serum leptin (Figure 1A–C). As with the 24 h fasted mice, mice fasted for 48 h mice consumed 32.8% (p<0.0001) more water with 10% glucose than the fed mice and 61.3% (p<0.0001) more than mice fasted alone (Supplemental Figure 1). Providing 10% glucose to males during the 48 h fast normalized glucose levels, prevented weight loss by 17.69% (Figure 1A), and blunted the reduction in serum leptin (Figure 1A–C). In spite of the reduction in serum leptin, serum GH was elevated 4.8 times (p<0.0001) compared to fed mice (Figure 1D), although providing 10% glucose maintained serum GH levels to those of the fed controls (Figure 1D).
The rise in serum GH with 48 h fasting correlated well with serum ghrelin levels, which were increased by 55.3% (p = 0.0029) over levels seen in ad libitum fed mice (Figure 1E). However, providing 10% glucose prevented this stark rise in ghrelin, which also correlates with the lack of change in serum GH. Thus, the 48 h fasted males provided a fasting model where serum leptin was reduced, while serum GH was elevated along with serum ghrelin.
Changes in transcripts of Pit1 and anterior pituitary hormones in 24 hour fasted male and female mice, and 48 hour male mice
To determine the effect of fasting upon anterior pituitary cell function, we continued studies of male pituitary responses, assessing the Pit1/Pou1f1 (pituitary-specific positive transcription factor 1) mRNA. Pit1/Pou1f1 is a progenitor cell marker and a transcription factor for induction of Gh, Prl, and Tsh gene expression (Ellsworth and Stallings, 2018, Davis et al., 2016). We have shown that synthesis of PIT1/POU1F1 protein is dependent upon leptin signaling (Odle et al., 2016). Pit1/Pou1f1 is also stimulated by ghrelin (Garcia et al., 2001, Kineman and Luque, 2007). Pit1/Pou1f1 mRNA levels, normalized to Ppia (cyclophilin A), were unchanged in 24 and 48 h fasted male mice (Figure 2A). Fasting with 10% glucose had no effect on Pit1/Pou1f1 mRNA expression in these mice.
We also assessed levels of hormone mRNAs. In male mice fasted for 24 h without 10% glucose, growth hormone (Gh), prolactin (Prl), and luteinizing hormone-beta (Lhβ) mRNA expression were increased by 34.8% (p = 0.0014), 23.2% (p = 0.0143), and 30.4% (p = 0.0011), respectively (Figure 2B–D). In 24 h fasted female mice, Gh and Lh transcript expression were unchanged (Figure 2B, D). Male mice fasted for 48 h had a 33.4% (p = 0.0386) increase in growth hormone, a 41.3% (p = 0.0016) increase in prolactin, and a 51.4% (p = 0.0003) increase in luteinizing hormone-beta (Lhβ) mRNA expression (Figure 2B–D). The 10% glucose control with the 24 h fast resulted in Gh and Lh mRNA expression similar to those in ad libitum fed animals. Prl mRNA levels were increased by 20.1% (p = 0.0428) in 24 h fasted males receiving the 10% glucose water when compared to ad libitum fed males (Figure 2B–D). The expression of Tsh and Fsh mRNA was unaltered with 24 fasting in male mice, while Fsh mRNA levels were reduced with 48 h fasting (Figure 1E–F). Female mice fasted for 24 h had significantly elevated Fsh mRNA expression (p = 0.0281) (Figure 1F). The increased Gh mRNA in 48 h fasted male mice coincided with a small, but significant, increase in pituitary GH protein by 19.8% (p = 0.0408) (Figure 3A). The other increases in hormone mRNA levels did not result in changes in content of the pituitary hormones in males (24 or 48 h) or females (24 h) (Figure 3).
Figure 3: Protein content of anterior pituitary hormones and progenitor transcription factor, PIT1.
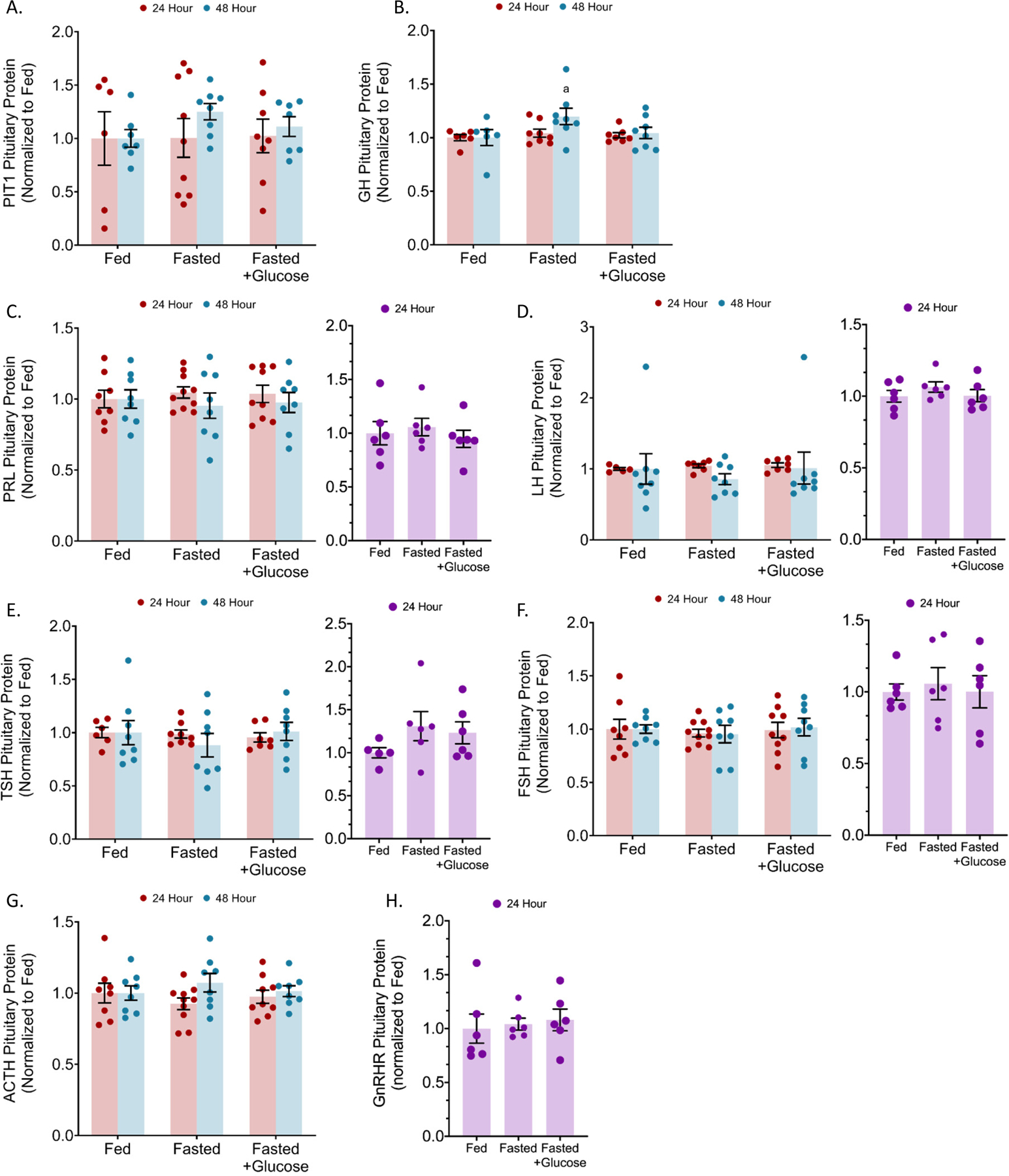
In the pituitary of 24 and 48 h fasted mice, pituitary content was quantified by multiplex enzyme immunoassay for (a) pituitary-specific positive transcription factor 1 (PIT1), (b) growth hormone (GH), (c) prolactin (PRL), (d) luteinizing hormone (LH), (e) thyroid-stimulating hormone (TSH), (f) follicular stimulating hormone (FSH), (g) adrenocorticotropic hormone (ACTH), and (h) gonadotropin releasing hormone receptor (GnRHR). n (males) = 8 and n (females) = 6 per condition; error bars are SEM. Two-way ANOVA.
For males fasted for 24 and 48 h, serum PRL (Figure 4A), LH (Figure 4C), and ACTH (Figure 4E) were unchanged. Serum TSH levels were significantly elevated by 37.3% (p = 0.0303) at the 48 h fast compared to the ad libitum fed group (Figure 4B). Serum FSH levels showed the opposite response and were significantly reduced by 47.5% (p = 0.0001) with the 48 h fast compared to the ad libitum fed group (Figure 4D). The serum FSH and TSH levels in the 10% glucose control were similar to that of the ad libitum fed group (Figure 4B,D). In 24 h female fasted mice, serum expression for anterior pituitary hormones were unchanged (Figure 4A–E).
Figure 4: Serum levels of anterior pituitary hormones in 24 and 48 hour fasted mice.
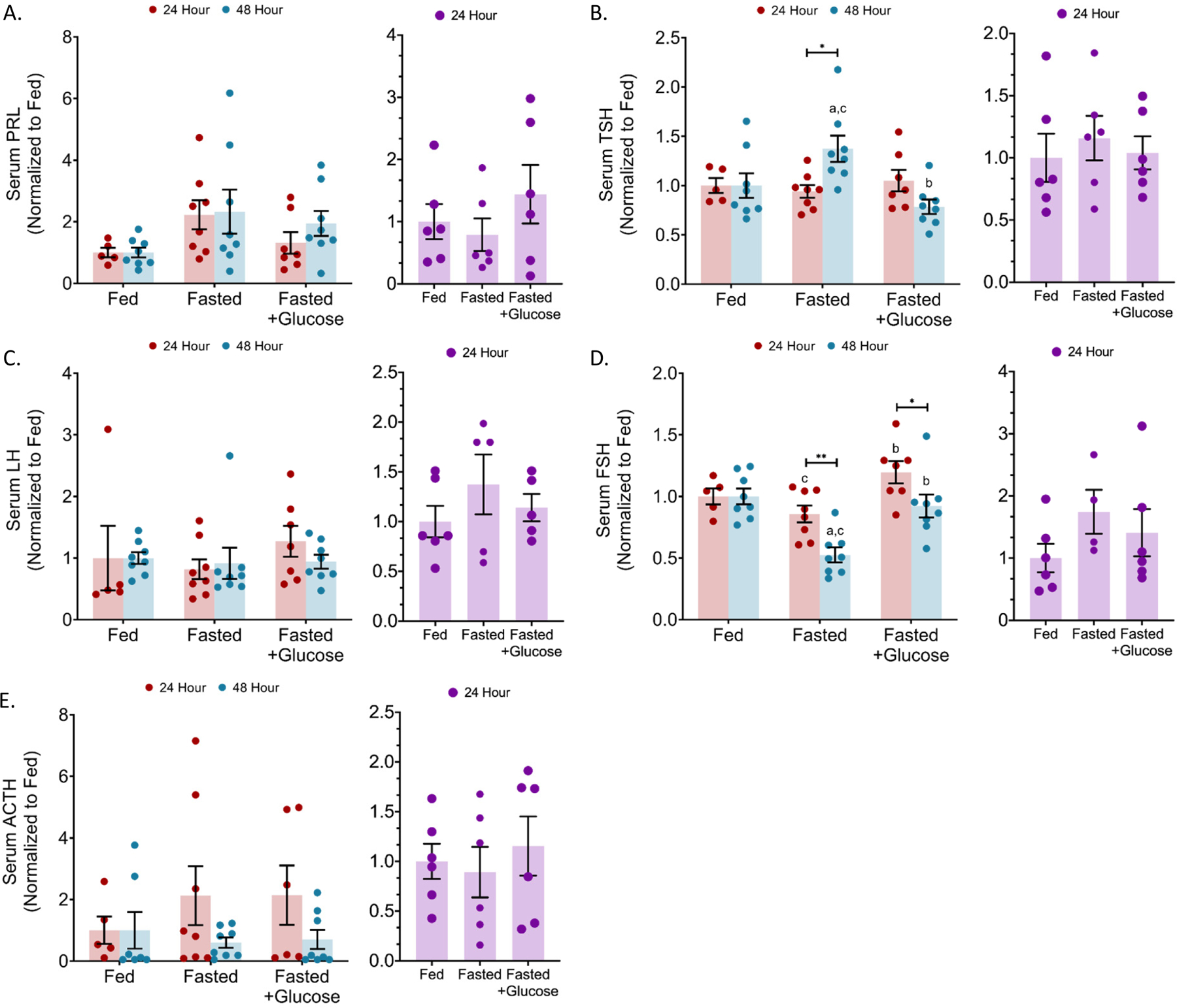
In the serum of 24 and 48 h fasted mice, anterior pituitary hormone levels were quantified by multiplex enzyme immunoassay for (a) prolactin (PRL), (b) thyroid-stimulating hormone (TSH), c) luteinizing hormone (LH), (d) follicular stimulating hormone (FSH), and (e) adrenocorticotropic hormone (ACTH). n (males) = 8 and n (females) = 6 per condition; error bars are SEM. Values that differ significantly: *P<0.05; **P<0.01, ***P<0.001. Two-way ANOVA.
Metabolic changes with 6 and 12 hour fasting
We continued our studies by investigating earlier fasting time points in the male mouse to determine how early loss of leptin, without changes in ghrelin (Figure 1E), would affect pituitary function. Therefore, additional groups of male mice were fasted for 6 and 12 h during scotophase at the start of nocturnal activity, which would detect changes at a time of active feeding (note, the 24 and 48 h fast began and ended during photophase). In comparison to ad libitum fed mice, fasted mice had a significant loss in body weight even at the earliest measured times [6 h: 5.296% (p = 0.0003) and 12 h: 8.796% (p<0.0001)] (Figure 5A). Fasting resulted in a significant drop in blood glucose at 6 h: 27.9% (p = 0.0087) and 12 h: 37.6% (p<0.0001) (Figure 5B) and in serum leptin at 6 h: 81.3% (p<0.0001) and 12 h : 88.1% (p = 0.0069) when compared to ad libitum fed mice (Figure 5C).
Figure 5: Fasted male FVB:P129 mice showed decreased body weight, blood glucose and leptin with 6 and 12 hour fasting.
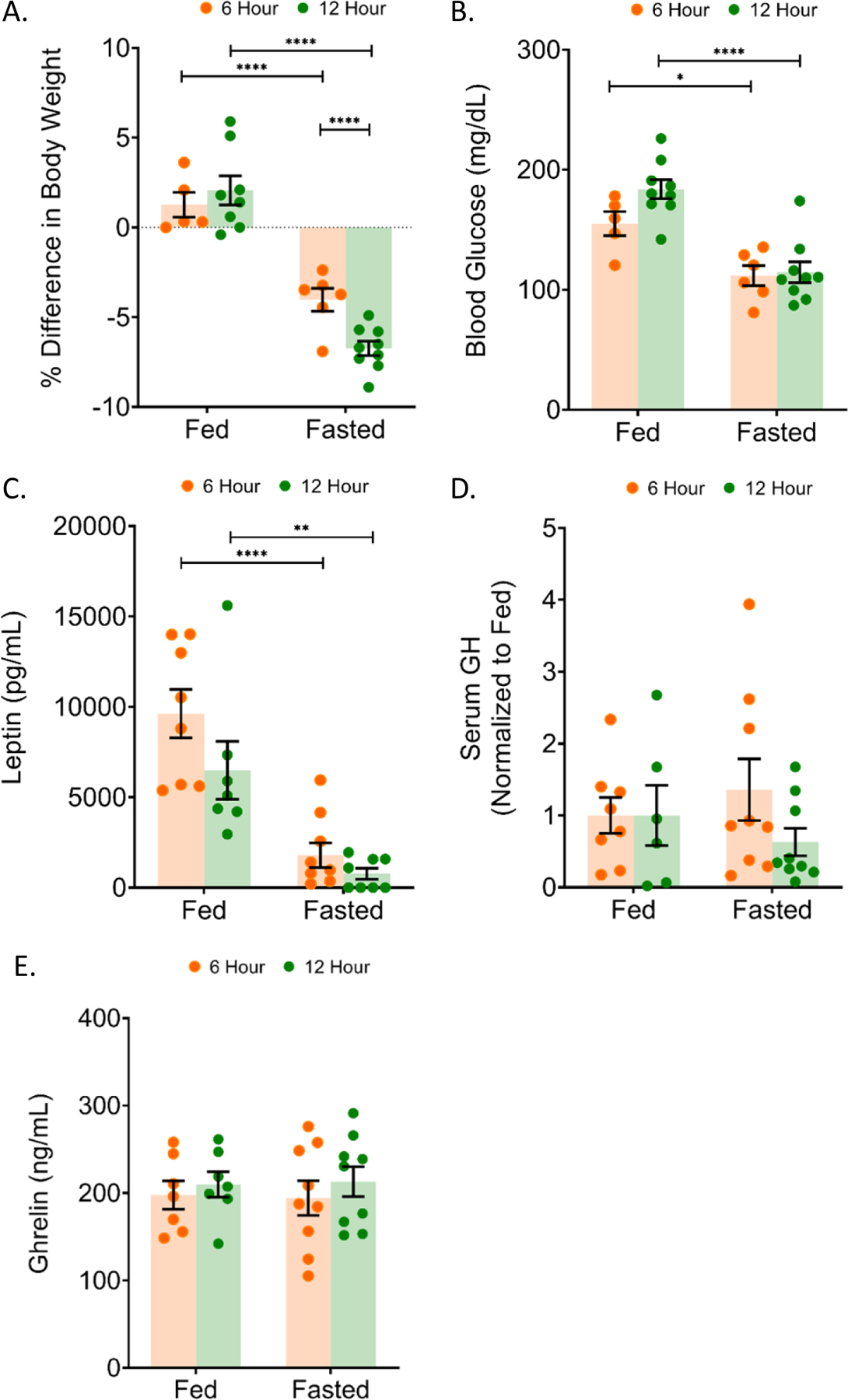
(a) the difference in body weight (b) blood glucose, (c) serum leptin, (d) serum GH, and (e) serum ghrelin were measured relative to ad libitum fed controls (Fed). Addition of 10% glucose to drinking water resulted in partial reversal of fasting effects with 24 and 48 h (Fasted +Glucose). All sets; n = 5–8 per condition; error bars are SEM. Values that differ significantly among time points within a treatment condition: *P<0.05; **P<0.01, ***P<0.001, ****P<0.0001. Values that differ significantly (P < 0.05) with the same time point among different treatment conditions: “a” significant with fed group, “b” significant with fasted group, and “c” significant with fasted+glucose group. Two-way ANOVA (males) and one-way ANOVA (females).
As expected based on the timing of the collection (20:00 h, scotophase), serum leptin levels in the control fed male mice were significantly higher at 6 h fasting (p <0.0001) compared to other groups of fed mice (Figure 5C). These higher levels reflect the normal nocturnal rise in leptin in response to feeding and activity (Jensen et al., 2019, Jensen et al., 2013). Similarly, ghrelin levels were unchanged comparing fed and fasted animals at 6 and 12 h, likely because this was at the start of their nocturnal activity and eating (Jensen et al., 2019, Jensen et al., 2013) (Figure 5E).
Pit1 transcript decreased with 6 hour fasting
We chose to measure transcripts in the 6 and 12 h fasted mice that were observed to be changed with the 24 and 48 h fasted male mice. In 6 h fasted male mice, Pit1/Pou1f1 mRNA was reduced significantly by 18.7% (p = 0.0206) compared to ad libitum fed mice (Figure 6A). For 12 h male mice, Pit1 was unchanged. Transcripts for Gh, Prl, and Lh were unchanged with 6 and 12 h fasting when compared to ad libitum fed mice (Figure 6B–D).
Figure 6: Pituitary Gh, Prl and Lh mRNA was unchanged with 6 and 12 hour fasting in male mice.
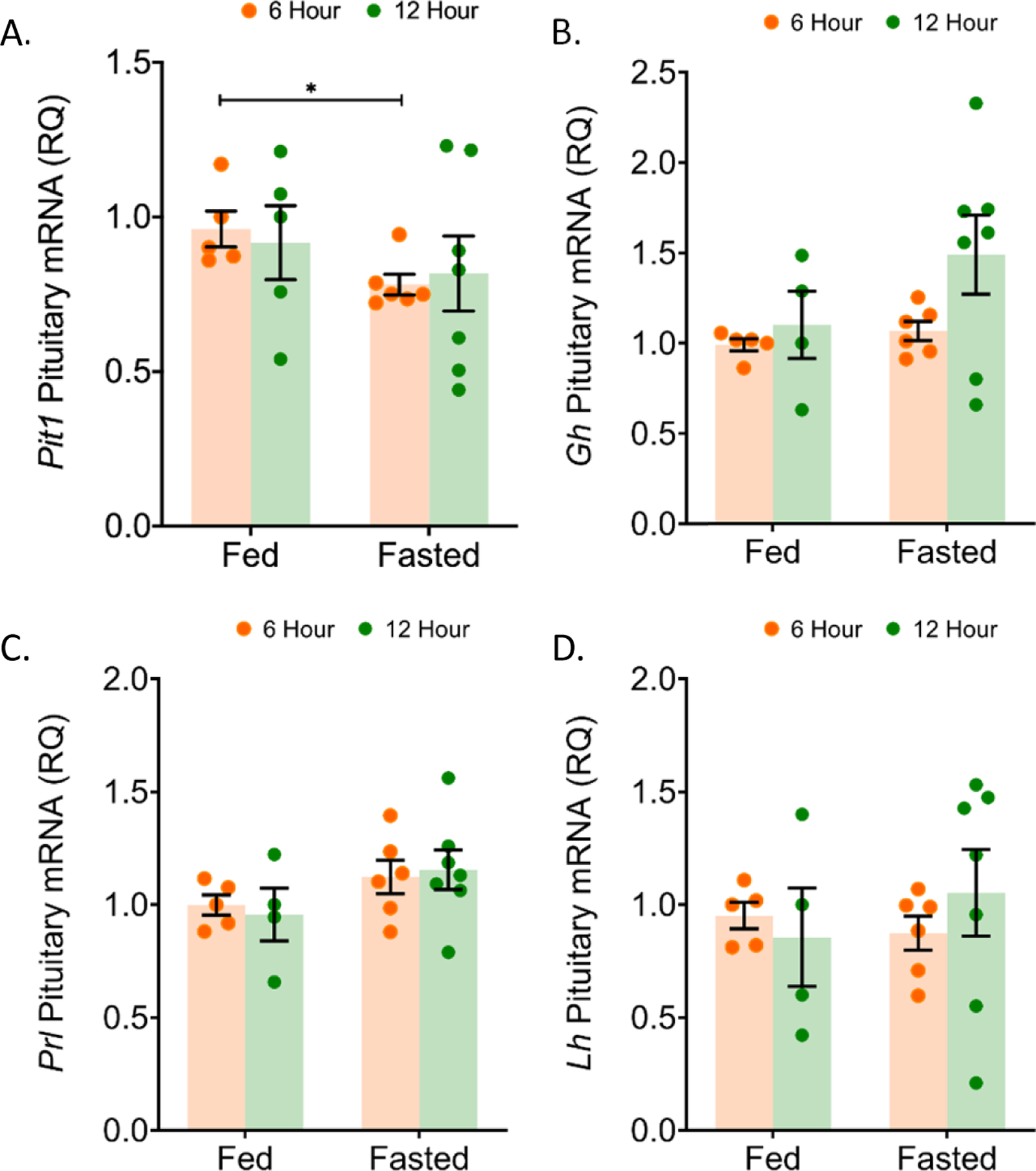
In the pituitary of 6 and 12 h fasted mice, mRNA levels were quantified by qRT-PCR for (a) pituitary-specific positive transcription factor 1 (Pit1), (b) growth hormone (Gh), (c) prolactin (Prl), and (d) luteinizing hormone (Lh). n = 5–8 per condition; error bars are SEM. Values that differ significantly among time points within a treatment condition: *P<0.05. Student’s t-test.
Changes in Ghrhr and Ghsr mRNA expression following fasting
We next wanted to determine potential mechanisms behind the differential serum GH levels that we observed in the acute and prolonged fasting. Therefore, we measured transcript expression of Ghrhr and Ghsr, which are both involved in GH synthesis and secretion. Ghrhr mRNA levels were increased at both the 24 h fast (87.38%, p = 0.0007) and at the 48 h fast (169.8%, p = 0.0002) compared to ad libitum fed group (Figure 7A). Water containing 10% glucose during the 48 h fast, blunted the increase in Ghrhr mRNA (94.63%, p = 0.0129) that was observed with fasting alone (Figure 7A). Similarly, the levels of Ghsr mRNA significantly increased with 48 h fasting when compared to the ad libitum fed group (82.1%, p = 0.0422) (Figure 7B). The 6 and 12 h fast had no significant effect on Ghrhr and Ghsr expression in male mice when compared to the ad libitum fed controls (Figure 7C–D).
Figure 7: Increased levels of pituitary Ghrhr and Ghsr mRNA with 24 and 48 hour fasting.
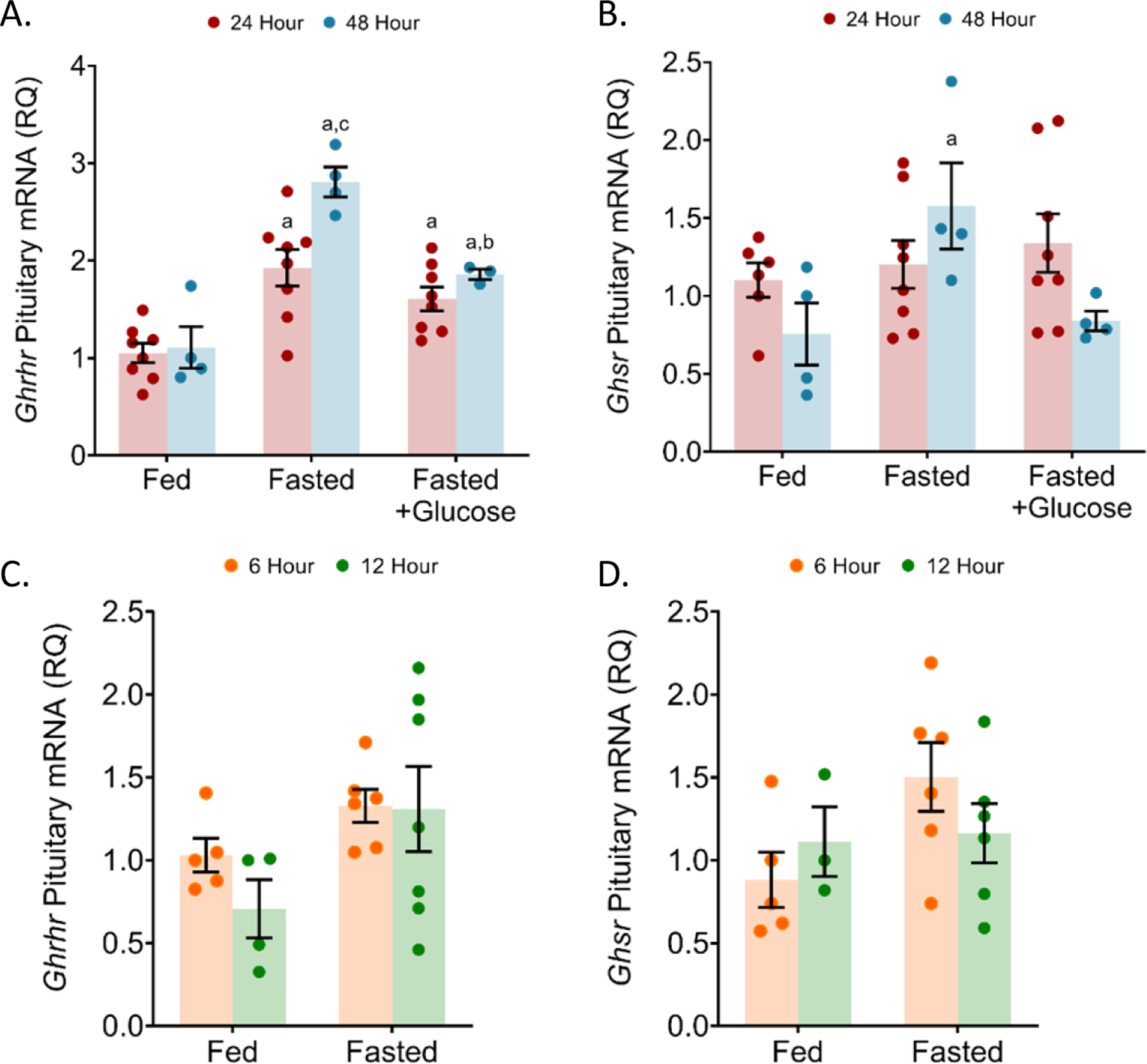
Quantified by qRT-PCR, Ghrhr (a) and Ghsr (b) mRNA levels were measured in male mice fasted for 24 and 48 h. In male mice fasted for 6 and 12 h, Ghrhr (c) and Ghsr (d) mRNA levels was also quantified by qRT-PCR. n = 4–8 per condition; error bars are SEM. Values that differ significantly (P < 0.05) with the same time point among different treatment conditions: “a” significant with fed group, “b” significant with fasted group, and “c” significant with fasted+glucose group. Student’s t-test.
DISCUSSION
To define the somatotrope response to the fasting state, this study utilized a model of acute and prolonged fasting times in male and female FVB mice. The original objective was to determine if somatotropes were sensitive to a reduction in serum leptin and a rise in Ghrelin (Chan et al., 2003, Longo and Mattson, 2014, Muller et al., 2002, Muller et al., 2015b). In assessing the initial fasting responses of male and female mice, this study is the first report of two new findings. First, we discovered distinct sex differences in both somatotrope and adipocyte responses to fasting. Females showed no significant reduction in serum leptin with 24 h fasting, whereas males responded to acute fasting by a reduction in serum leptin levels. Further studies of males showed that this reduction could be seen as early as 6 h, even if fasting occurred during the scotophase. Second, we discovered that in females, fasting resulted in a significant increase in serum GH levels, whereas in males, somatotropes respond to fasting in a biphasic manner, with a decline in GH at 12–24 h of fasting followed by a rise at 48 h. These results indicate that somatotropes in male mice are more sensitive to the acute reduction in serum leptin caused by fasting than females, which confirms our studies of mice lacking LEPR in somatotropes showing that the absence of leptin signaling had a more profound effect on males. (Allensworth-James et al., 2015) Because our experimental model depended on a reduced serum leptin environment, we did not continue further studies of females. Subsequent studies in males were designed to determine the impact of different fasting times on somatotropes.
We established that in male mice our fasting model recapitulated metabolic parameters of fasted models in the literature (Ahima et al., 1996, Jensen et al., 2013, Luque et al., 2007, Park et al., 2004). We observed that with each fasting time, male mice had a significant progressive decrease in weight while glucose and leptin levels were reduced initially with the 6 h fast and the level of reduction was maintained with subsequent fasting times. In our FVB male mice, a rise in serum ghrelin was not detected until the prolonged 48 h fasting time. Ghrelin has been shown to be the main driving force of GH secretion during fasting in humans, in which ghrelin has a diurnal secretion pattern. (Muller et al., 2002). In humans, fasting is achieved by consuming little to no food or caloric beverages for 12 h to 3 weeks (Longo and Mattson, 2014) and in healthy human subjects, fasted for 72 h, body weight and fat mass composition was not reduced with fasting (Chan et al., 2003). Therefore a fasting time in mice is not a direct equivalent to human fasting time, consistent with the mouse accelerated lifespan (Dutta and Sengupta, 2016). With our male FVB mice, a 48 h fast resulted in a 22% reduction in body weight that is similar to what is seen in humans partially fasted (~50% calorie restricted diet) for 6 months, while the loss in weight seen by our male FVB mice at the earliest tested time of 6 h of fasting would be more consistent with an ~ 1 month partial fast in healthy male humans (Chan et al., 2003, Muller et al., 2015a, Kalm, 2005).
Response of Anterior Pituitary to Acute and Prolonged Fasting Times
We identified the responses of the anterior pituitary to acute and prolonged fasting and observed a rise in Gh, Prl, and Lh mRNA levels, a finding that differs from previous reports where C57BL/6J mice fasted for 12, 24 and 48 h had unchanged Gh pituitary mRNA levels (Luque et al., 2007). Notably, the severe reduction in leptin was not generally correlated with a loss of anterior pituitary transcript expression, and in fact, most gene expression changes indicated an increase in transcripts, correlating with the observed rise in serum ghrelin. However, the increased gene expression was not generally correlated with increased protein levels, suggesting that mRNA translation and protein synthesis may be regulated by leptin signaling (Akhter et al., 2014, Allensworth-James et al., 2015, Odle et al., 2017, Odle et al., 2016, Odle et al., 2018). Similarly, the mechanisms behind the decrease in serum FSH, and increase in serum TSH, with prolonged fasting are unknown at this point and may reflect distinct sensitivities to fasting.
Interestingly, the rise in Gh mRNA did not directly correlate with GH secretory events. Despite the increased Gh mRNA levels at both 24 h and 48 h, the 24 h fast resulted in a reduction in serum GH levels, whereas the 48 h fast resulted in elevated GH secretion in our male FVB mice. The pattern of our findings is similar to two studies with male C57BL/6J mice, where serum GH was reduced with an 18 h fast (Steyn et al., 2011) and elevated with 24 h fasting (Luque et al., 2007). Thus, a biphasic response by somatotropes is observed in both fasting models, with the timing of the secretory events in our FVB mice occurring later than what was reported in C57BL/6J mice.
We have reported previously that in male and female CreGH-Leprexon1 FVB mice, serum GH is reduced. (Allensworth-James et al., 2015) The reduced GH secretion, that we have previously observed in response to genetic ablation of leptin signaling, may be analogous to the reduced GH secretion that we observe in our 24 hour fasted mice, in which leptin signaling is naturally reduced by fasting. Leptin signaling has been shown to utilize the Jak2/STAT3 and STAT5, MAPK, PI3K-Akt-Foxo1, and AMPK pathways, in a tissue specific manner (Wauman et al., 2017). We have observed that inhibition of STAT3 activation prevents the leptin mediated increase in anterior pituitary GH stores, which suggests that leptin may use the JAK/STAT pathway to maintain GH stores (Allensworth-James et al., 2020). It will be interesting to determine if the JAK/STAT pathway plays a similar role in mediating GH responses in the prolonged fasted state.
Proposed Mechanisms for Biphasic GH Regulation with Fasting
To identify the mechanisms underlying the distinct effects of acute and prolonged fasting upon GH secretion we measured the expression of the Ghrhr mRNA, encoding the GHRH receptor, that stimulates GH synthesis and secretion, and also the Ghsr mRNA, encoding the ghrelin receptor, that also stimulates GH secretion. The expression of Ghsr mRNA increased with the 48 h fast while the Ghrhr mRNA steadily increased over all fasting times, such that both genes showed maximal expression at the prolonged 48 h fast time. Thus, the early rise in Ghrhr mRNA at 24 and continued expression at 48 h, coincides with the early rise in Gh mRNA and continued expression with the 24 and 48 h fast. These observations support a model in which the response by somatotropes to the loss of leptin signaling promotes an increased sensitivity to GHRH, by elevating GHRH receptor mRNA expression, resulting in an increased synthesis of Gh mRNA (Ho et al., 2020).
In the prolonged 48 h fasted FVB mice, we also observed increased Ghsr mRNA expression, indicating an enhanced sensitivity to ghrelin signaling. This observation is consistent with previous reports in which rats fasted for 48 h also showed increased pituitary Ghsr mRNA expression (Kim et al., 2003) and fasted C57BL/6J mice showed elevated Ghsr mRNA expression (Luque et al., 2007). Similarly, Zucker rats, lacking fully functional leptin receptors, had elevated Ghsr mRNA expression in the arcuate nucleus. In control, wild type rats fasted for 48 h, exogenous leptin administration reduced Ghsr mRNA expression and ghrelin administration further increased Ghsr mRNA expression in the arcuate nucleus indicating that both leptin and ghrelin have a significant roles in modulating Ghsr mRNA synthesis in the hypothalamus (Nogueiras et al., 2004). Similarly, our data in the pituitary support a model in which the rise in Ghsr mRNA is mediated through both a fasting induced drop in serum leptin and an increase in ghrelin signaling.
Conclusions
Findings from our male mouse fasting model indicate that anterior pituitary somatotropes respond in a dual phased manner to acute and prolonged fasting. Based upon findings from our previous studies in a genetic loss of leptin signaling model, we propose the biphasic response by male mouse somatotropes may be mediated through the initial loss of leptin signaling that is prompted by early fasting times and the enhanced ghrelin stimulation of the classic GH secretory response with prolonged fasting. These data shed further light onto the complex mechanistic relationship between metabolic signals and somatotrope functions. They also elucidate sex differences in responses to fasting, suggesting mechanisms whereby females resist the fasting-induced reduction in leptin and growth hormone.
Supplementary Material
ACKNOWLEDGEMENTS
The UAMS Division of Laboratory Animal Medicine (DLAM) administration and staff.
FUNDING
These studies were funded by National Institutes of Health R01HD059056 (GVC), 1R01DK113776-01 and R01HD093461 (GVC, AM, and MM) and R01HD087057 (GVC and AM); NIGMS P20 GM103425 and P30GM11070 (Dr. Edgar Garcia-Rill).
Footnotes
DECLARATION OF INTEREST
There is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
REFERENCES
- AHIMA RS, PRABAKARAN D, MANTZOROS C, QU D, LOWELL B, MARATOS-FLIER E & FLIER JS 1996. Role of leptin in the neuroendocrine response to fasting. Nature, 382, 250–2. [DOI] [PubMed] [Google Scholar]
- AKHTER N, CARLLEE T, SYED MM, ODLE AK, COZART MA, HANEY AC, ALLENSWORTH-JAMES ML, BENES H & CHILDS GV 2014. Selective deletion of leptin receptors in gonadotropes reveals activin and GnRH-binding sites as leptin targets in support of fertility. Endocrinology, 155, 4027–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AKHTER N, ODLE AK, ALLENSWORTH-JAMES ML, HANEY AC, SYED MM, COZART MA, CHUA S, KINEMAN R & CHILDS GV 2012. Ablation of leptin signaling to somatotropes: changes in metabolic factors that cause obesity. Endocrinology, 153, 4705–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALAMRI BN, SHIN K, CHAPPE V & ANINI Y 2016. The role of ghrelin in the regulation of glucose homeostasis. Horm Mol Biol Clin Investig, 26, 3–11. [DOI] [PubMed] [Google Scholar]
- ALLENSWORTH-JAMES M, ODLE AK, HANEY A & CHILDS GV 2015. Sex Differences in Somatotrope Dependency on Leptin Receptors in Young Mice: Ablation of LEPR Causes Severe Growth Hormone Deficiency and Abdominal Obesity in Males. Endocrinology, 156, 3253–3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALLENSWORTH-JAMES ML, ODLE AK, LIM J, LAGASSE AN, MILES TK, HARDY LL, HANEY AC, MACNICOL MC, MACNICOL AM & CHILDS GV 2020. Metabolic signalling to somatotrophs: Transcriptional and post-transcriptional mediators. J Neuroendocrinol, e12883. [DOI] [PMC free article] [PubMed]
- BARTKE A, SUN LY & LONGO V 2013. Somatotropic signaling: trade-offs between growth, reproductive development, and longevity. Physiol Rev, 93, 571–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOURET SG 2010. Neurodevelopmental actions of leptin. Brain Res, 1350, 2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAN JL, HEIST K, DEPAOLI AM, VELDHUIS JD & MANTZOROS CS 2003. The role of falling leptin levels in the neuroendocrine and metabolic adaptation to short-term starvation in healthy men. J Clin Invest, 111, 1409–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHILDS GV, AKHTER N, HANEY A, SYED M, ODLE A, COZART M, BRODRICK Z, GADDY D, SUVA LJ, AKEL N, et al. 2011. The somatotrope as a metabolic sensor: deletion of leptin receptors causes obesity. Endocrinology, 152, 69–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRANE C, AKHTER N, JOHNSON BW, IRUTHAYANATHAN M, SYED F, KUDO A, ZHOU YH & CHILDS GV 2007. Fasting and glucose effects on pituitary leptin expression: is leptin a local signal for nutrient status? J Histochem Cytochem, 55, 1059–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS SW, KEISLER JL, PEREZ-MILLAN MI, SCHADE V & CAMPER SA 2016. All Hormone-Producing Cell Types of the Pituitary Intermediate and Anterior Lobes Derive From Prop1-Expressing Progenitors. Endocrinology, 157, 1385–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUTTA S & SENGUPTA P 2016. Men and mice: Relating their ages. Life Sci, 152, 244–8. [DOI] [PubMed] [Google Scholar]
- ELLSWORTH BS & STALLINGS CE 2018. Molecular Mechanisms Governing Embryonic Differentiation of Pituitary Somatotropes. Trends Endocrinol Metab, 29, 510–523. [DOI] [PubMed] [Google Scholar]
- ERRIJGERS V, VAN DAM D, GANTOIS I, VAN GINNEKEN CJ, GROSSMAN AW, D’HOOGE R, DE DEYN PP & KOOY RF 2007. FVB.129P2-Pde6b(+) Tyr(c-ch)/Ant, a sighted variant of the FVB/N mouse strain suitable for behavioral analysis. Genes Brain Behav, 6, 552–7. [DOI] [PubMed] [Google Scholar]
- FANZO J, HAWKES C, UDOMKESMALEE E, AFSHIN A, ALLEMANDI L, ASSERY O, BAKER P, BATTERSBY J, BHUTTA Z & CHEN K 2018. 2018 Global Nutrition Report: Shining a light to spur action on nutrition.
- GARCIA A, ALVAREZ CV, SMITH RG & DIEGUEZ C 2001. Regulation of Pit-1 expression by ghrelin and GHRP-6 through the GH secretagogue receptor. Mol Endocrinol, 15, 1484–95. [DOI] [PubMed] [Google Scholar]
- HO KY, VELDHUIS JD, JOHNSON ML, FURLANETTO R, EVANS WS, ALBERTI KG & THORNER MO 1988. Fasting enhances growth hormone secretion and amplifies the complex rhythms of growth hormone secretion in man. J Clin Invest, 81, 968–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HO Y, HU P, PEEL MT, CHEN S, CAMARA PG, EPSTEIN DJ, WU H & LIEBHABER SA 2020. Single-cell transcriptomic analysis of adult mouse pituitary reveals sexual dimorphism and physiologic demand-induced cellular plasticity. Protein Cell. [DOI] [PMC free article] [PubMed]
- JENSEN TL, KIERSGAARD MK, MIKKELSEN LF & SORENSEN DB 2019. Fasting of male mice - Effects of time point of initiation and duration on clinical chemistry parameters and animal welfare. Lab Anim, 53, 587–597. [DOI] [PubMed] [Google Scholar]
- JENSEN TL, KIERSGAARD MK, SORENSEN DB & MIKKELSEN LF 2013. Fasting of mice: a review. Lab Anim, 47, 225–40. [DOI] [PubMed] [Google Scholar]
- Kalm LM, Semba RD. They starved so that others be better fed: remembering Ancel Keys and the Minnesota experiment. J Nutr 2005. June;135(6):1347–52. doi: 10.1093/jn/135.6.1347. [DOI] [PubMed] [Google Scholar]
- KIM MS, YOON CY, PARK KH, SHIN CS, PARK KS, KIM SY, CHO BY & LEE HK 2003. Changes in ghrelin and ghrelin receptor expression according to feeding status. Neuroreport, 14, 1317–20. [DOI] [PubMed] [Google Scholar]
- KINEMAN RD & LUQUE RM 2007. Evidence that Ghrelin Is as Potent as Growth Hormone (GH)-Releasing Hormone (GHRH) in Releasing GH from Primary Pituitary Cell Cultures of a Nonhuman Primate (Papio anubis), Acting through Intracellular Signaling Pathways Distinct from GHRH. Endocrinology, 148, 4440–4449. [DOI] [PubMed] [Google Scholar]
- LLOYD RV, JIN L, TSUMANUMA I, VIDAL S, KOVACS K, HORVATH E, SCHEITHAUER BW, COUCE ME & BURGUERA B 2001. Leptin and leptin receptor in anterior pituitary function. Pituitary, 4, 33–47. [DOI] [PubMed] [Google Scholar]
- LONGO VD & MATTSON MP 2014. Fasting: molecular mechanisms and clinical applications. Cell Metab, 19, 181–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUQUE RM, PARK S & KINEMAN RD 2007. Severity of the catabolic condition differentially modulates hypothalamic expression of growth hormone-releasing hormone in the fasted mouse: potential role of neuropeptide Y and corticotropin-releasing hormone. Endocrinology, 148, 300–9. [DOI] [PubMed] [Google Scholar]
- MULLER AF, LAMBERTS SW, JANSSEN JA, HOFLAND LJ, KOETSVELD PV, BIDLINGMAIER M, STRASBURGER CJ, GHIGO E & VAN DER LELY AJ 2002. Ghrelin drives GH secretion during fasting in man. Eur J Endocrinol, 146, 203–7. [DOI] [PubMed] [Google Scholar]
- MULLER MJ, ENDERLE J, POURHASSAN M, BRAUN W, EGGELING B, LAGERPUSCH M, GLUER CC, KEHAYIAS JJ, KIOSZ D & BOSY-WESTPHAL A 2015a. Metabolic adaptation to caloric restriction and subsequent refeeding: the Minnesota Starvation Experiment revisited. Am J Clin Nutr, 102, 807–19. [DOI] [PubMed] [Google Scholar]
- MULLER TD, NOGUEIRAS R, ANDERMANN ML, ANDREWS ZB, ANKER SD, ARGENTE J, BATTERHAM RL, BENOIT SC, BOWERS CY, BROGLIO F, et al. 2015b. Ghrelin. Mol Metab, 4, 437–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOGUEIRAS R, TOVAR S, MITCHELL SE, RAYNER DV, ARCHER ZA, DIEGUEZ C & WILLIAMS LM 2004. Regulation of growth hormone secretagogue receptor gene expression in the arcuate nuclei of the rat by leptin and ghrelin. Diabetes, 53, 2552–8. [DOI] [PubMed] [Google Scholar]
- ODLE AK, AKHTER N, SYED MM, ALLENSWORTH-JAMES ML, BENES H, MELGAR CASTILLO AI, MACNICOL MC, MACNICOL AM & CHILDS GV 2017. Leptin Regulation of Gonadotrope Gonadotropin-Releasing Hormone Receptors As a Metabolic Checkpoint and Gateway to Reproductive Competence. Front Endocrinol (Lausanne), 8, 367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ODLE AK, ALLENSWORTH-JAMES ML, AKHTER N, SYED M, HANEY AC, MACNICOL M, MACNICOL AM & CHILDS GV 2016. A Sex-Dependent, Tropic Role for Leptin in the Somatotrope as a Regulator of POU1F1 and POU1F1-Dependent Hormones. Endocrinology, 157, 3958–3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ODLE AK, BENES H, MELGAR CASTILLO A, AKHTER N, SYED M, HANEY A, ALLENSWORTH-JAMES M, HARDY L, WINTER B, MANOHARAN R, et al. 2018. Association of Gnrhr mRNA With the Stem Cell Determinant Musashi: A Mechanism for Leptin-Mediated Modulation of GnRHR Expression. Endocrinology, 159, 883–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARK S, SOHN S & KINEMAN RD 2004. Fasting-induced changes in the hypothalamic-pituitary-GH axis in the absence of GH expression: lessons from the spontaneous dwarf rat. J Endocrinol, 180, 369–78. [DOI] [PubMed] [Google Scholar]
- STEYN FJ, HUANG L, NGO ST, LEONG JW, TAN HY, XIE TY, PARLOW AF, VELDHUIS JD, WATERS MJ & CHEN C 2011. Development of a method for the determination of pulsatile growth hormone secretion in mice. Endocrinology, 152, 3165–71. [DOI] [PubMed] [Google Scholar]
- SYED M, COZART M, HANEY AC, AKHTER N, ODLE AK, ALLENSWORTH-JAMES M, CRANE C, SYED FM & CHILDS GV 2013. Ghrelin restoration of function in vitro in somatotropes from male mice lacking the Janus kinase (JAK)-binding site of the leptin receptor. Endocrinology, 154, 1565–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAO YX 2010. The melanocortin-4 receptor: physiology, pharmacology, and pathophysiology. Endocr Rev, 31, 506–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WADA N, HIRAKO S, TAKENOYA F, KAGEYAMA H, OKABE M & SHIODA S 2014. Leptin and its receptors. J Chem Neuroanat, 61–62, 191–9. [DOI] [PubMed]
- WANG G, LEE HM, ENGLANDER E & GREELEY GH JR. 2002. Ghrelin--not just another stomach hormone. Regul Pept, 105, 75–81. [DOI] [PubMed] [Google Scholar]
- WAUMAN J, ZABEAU L & TAVERNIER J 2017. The Leptin Receptor Complex: Heavier Than Expected? Front Endocrinol (Lausanne), 8, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WORLD HEALTH ORGANIZATION. 2018. Malnutrition [Online]. Available: https://www.who.int/en/news-room/fact-sheets/detail/malnutrition [Accessed 22 Jan 2020].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


