Abstract
The human brain is organized into segregated networks with strong within-network connections and relatively weaker between-network connections. This “small-world” organization may be essential for maintaining an energetically efficient system, crucial to the brain which consumes 20% of the body’s energy. Brain network segregation and glucose energy utilization both change throughout the lifespan. However, it remains unclear whether these processes interact to contribute to differences in cognitive performance with age. To address this, we examined fluorodeoxyglucose-positron emission tomography and resting-state functional magnetic resonance imaging from 88 participants aged 18–73 years old. Consistent with prior work, brain network segregation showed a negative association with age across both sensorimotor and association networks. However, relative glucose metabolism demonstrated an interaction with age, showing a negative slope in association networks but a positive slope in sensorimotor networks. Overall, brain networks with lower segregation showed significantly steeper age-related differences in glucose metabolism, compared with highly segregated networks. Sensorimotor network segregation mediated the association between age and poorer spatial cognition performance, and sensorimotor network metabolism mediated the association between age and slower response time. These data provide evidence that sensorimotor segregation and glucose metabolism underlie some age-related changes in cognition. Interventions that stimulate somatosensory networks could be important for treatment of age-related cognitive decline.
Keywords: brain glucose metabolism, brain networks, functional connectivity, modularity, oxidative phosphorylation
Introduction
The human brain is metabolically expensive, consuming far more oxygen and glucose than would be predicted based on its size (Mink et al. 1981). Much of the energetic demands are to support the electrochemical gradients of cell membranes needed for neuronal communication across large distances (Raichle 2015). To minimize the energetic cost of these processes, the brain is organized into discrete segregated networks—that is, clusters of regions with strong within-network connections and relatively weaker between-network connections (Bullmore and Sporns 2012). It was recently proposed that the healthy adult brain’s level of network segregation is optimized for energetically efficient communication—that is, abnormally high or low levels of segregation may be associated with impaired information flow across the brain or unnecessarily high amounts of energy consumption (Wig 2017). However, while this is just one of many prior suggestions that energy utilization and brain network organization are intrinsically linked (for more discussion, see Raichle 2015), there is little direct evidence in humans supporting this. This may be in part due to the difficulty of obtaining in vivo measurements of brain energy consumption. The primary method, positron emission tomography (PET) imaging of glucose or oxygen utilization, is expensive, and radiation exposure limits the number of times that an individual can be scanned. Therefore, it is hard to impose experimental manipulations and perform repeated measurements of energy utilization and brain network segregation in the same individuals.
An alternative method to assess the relationship between energy utilization and network segregation is to evaluate their association with aging cross-sectionally. Both measures demonstrate strong changes across the lifespan. Glucose metabolism and aerobic glycolysis decline with aging, although the cerebral metabolic rate of oxygen does not (De Santi et al. 1995; Aanerud et al. 2017; Goyal et al. 2017). Likewise, across the brain, network segregation generally shows a negative association with age (Chan et al. 2014; Grady et al. 2016; Wig 2017). It is theorized that these changes are associated with age-related decline in executive function, particularly in association cortices, which are thought to be more metabolically expensive and which show steeper age-related decreases in segregation than the sensorimotor cortices (Tomasi et al. 2013; van den Heuvel and Sporns 2013; Chan et al. 2014). Based on this set of observations, one could reasonably propose that loss of network segregation with age mediates age-related changes in brain glucose metabolism (or, conversely, that changes in brain glucose metabolism may mediate the age-related changes in network segregation). Alternatively, the most integrated networks, and therefore the most metabolically expensive, could “burn out” and be the most vulnerable to age-related changes in glucose metabolism. This would help validate simulations suggesting that highly integrated networks in the brain are the most sensitive to degradation in aging and neuropsychiatric disease (Crossley et al. 2014). Finally, network segregation and glucose metabolism may each mediate the association between age and differences in cognitive function across the lifespan.
However, to our knowledge, the relationships between aging, network segregation, energy utilization, and cognitive/motor function have not been systematically evaluated in the same cohort. To address this, we took advantage of a dataset previously collected in our laboratory to conduct exploratory analyses of regional glucose metabolism (using FDG-PET), brain network segregation (using resting fMRI) and executive function in a cohort of 88 adults aged 18–73 years old. Based on the extant theory (Bullmore and Sporns 2012; Wig 2017), we tested the following hypotheses: 1) brain network segregation would mediate the association between aging and glucose metabolism; 2) networks with lower segregation would be more vulnerable to age-related differences in glucose metabolism; and 3) segregation and glucose metabolism would mediate the association between age and (a) cognitive function (association cortices) and (b) motor response function (sensorimotor cortices).
Materials and Methods
Participants
Eighty-eight nonsmoking participants (aged 18–73 years old, 44 female, 44 male) were recruited and screened to exclude ferromagnetic implants, psychoactive medications and major medical problems, past or present history of substance abuse, and neurological or psychiatric disorders (including eating disorders) as assessed by an abbreviated Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV). The basic demographics of the study cohort are presented in Table 1. All participants tested negative on a urine drug screen panel on study days. Women were studied in the midfollicular phase and were neither pregnant nor breastfeeding. The study was approved by the Ethics Committee of the National Institutes of Health (Combined Neurosciences White Panel) and was in accordance with the Declaration of Helsinki. All participants gave informed written consent before participating in the study.
Table 1.
Sample demographics (n = 88)
| Age | 41.69 ± 14.12 |
| Sex | 44 females |
| Education (years)a | 16.10 ± 2.99 |
| IQ | 109.99 ± 16.46 |
| Body mass index | 27.15 ± 4.80 |
| Race | |
| White—non-Hispanic | 30 |
| Black/African-American | 46 |
| White—Hispanic | 6 |
| Asian | 5 |
| Mixed race | 1 |
Note: Values are reported as mean ± standard deviation (SD).
aEducation reported for only 66 participants of the larger sample (n = 88).
Behavioral Data: Executive Function
Participants completed a battery of assessments from the Cambridge Neuropsychological Test Automated Battery (CANTAB) suite (Robbins et al. 1994). Below is a brief summary of each task, with the main outcome measures highlighted in Supplementary Table 1.
Pattern Recognition Memory (PRM)
A participant views a series of patterns and is then presented with a pattern they have seen and a novel pattern. The participant must recognize and select the familiar pattern.
Reaction Time (RTI)
A participant looks at a white circle, waiting for a yellow spot to appear. When the yellow spot appears, the participant must remove their finger from a button to touch the yellow spot. Later, five circles are on the screen and the yellow spot can appear in any one of the five circles. The participant must touch the yellow spot in the circle in which it appears.
Stockings of Cambridge (SOC)
The participant sees an arrangement of balls hanging in stockings on the top of the screen. The participant must move the balls on the bottom of the screen to match the arrangement on the top in the least amount of moves possible.
Spatial Working Memory (SWM)
The participant sees an arrangement of colored squares on the screen. The computer a hides a yellow token in one of the squares, and the participant must find it. Once the participant finds the token, the computer will hide it in another square, and it will never use the same square twice. There is a black bar on the side of the screen, where the participant puts each token once he/she has found it. The task is complete when the participant has found enough tokens to fill the black bar.
Intra–Extra Dimensional Set Shift (IED)
This task is modeled after the Wisconsin Card Sorting Task. The participant sees two patterns on the screen, and one is correct. The participant must use the feedback provided by the computer to determine which pattern is correct; after six correct responses, the computer changes the rule. The first stage of the task (pre-ED) involves choosing between two simple patterns. The second stage of the task (EDS) involves 2D patterns (a pattern overlaid with another pattern), but only one of the dimensions determines the rule.
Spatial Span (SSP) Reverse Mode
Several white boxes are shown on a screen. One by one, a certain number of boxes will change color for a brief period. At the end of the sequence, a tone plays, and the participant must select the boxes that changed color in the reverse order that they originally changed in; that is, the first box that the participant selects is the last one he/she saw change on the screen.
Behavioral Measures of Interest: Principal Component Analysis
To first identify broad, aggregate measures of executive function, we z-transformed the data and reduced data dimensionality by performing probabilistic PCA on the data of all 88 participants (using all outcome measures listed above), as implemented with the ppca function in MATLAB, with missing datapoints imputed via an expectation–maximization algorithm. We included all measures available from the CANTAB suite for which >95% of participants had data.
PET Acquisition
For the PET imaging protocol, participants were asked to fast (except water) for at least 4 h prior to the PET session, which was performed using a high-resolution research tomography (HRRT) scanner (Siemens AG). Venous catheters were placed in the antecubital vein for radiotracer injection and in the dorsal hand vein for arterialized blood sampling (achieved by warming the hand to 44–50 °C) to measure the concentration of radioactivity in plasma (every minute from 1 to 10 min and then at 15, 20, 30, 40, 50, 60, and 75 min after FDG injection). After the patient was positioned in the scanner, a transmission scan was obtained with a 137 Cesium rotating pin source to correct emission images for attenuation. Commercially manufactured FDG (8 mCi) was injected intravenously over a period of approximately 1 min. Then a PET emission scan of the brain with ~2.7 mm isotropic point spread function was obtained using a 3D list mode starting immediately after FDG injection for up to 75 min. Fasting glucose levels were measured prior to FDG injection, 30 min after injection, and at the end of the PET scan. During the PET imaging procedures, the subjects rested quietly under dim illumination and minimal acoustic noise. To ensure that subjects did not fall asleep, they were monitored throughout the procedure and were asked to keep their eyes open. During the PET scan, a cap with small light reflectors was placed on the subject’s head to monitor head position with a Polaris Vicra head tracking system (Northern Digital Inc.). Information about head movement was used in the PET image reconstruction process to minimize motion-related image blurring.
MRI Acquisition
All subjects underwent MRI on a 3.0-T Magnetom Prisma scanner (Siemens Medical Solutions USA, Inc.). After collecting data from the first 27 participants, the head coil and MRI sequences changed; all statistical analyses controlled for the fact that there were two different MRI sequences across the participants.
For cohort A (n = 27), a 20-channel head coil and a single-shot gradient echo-planar (EPI) sequence (repetition time/echo time, TR/TE = 1500/30 ms; flip angle, FA = 70°; matrix = 64, 36 axial slices; 4 mm thickness; interleaved acquisition; no gap between slices; 3-mm in-plane resolution) covering the whole brain were used to acquire resting fMRI time series with 600 time points (15 min) while participants relaxed with their eyes open. A fixation cross was presented on a black background under dimmed room lighting using MRI-compatible goggles (Resonance Technology Inc.). T1-weighted 3D magnetization-prepared gradient-echo image (MP-RAGE) (TR/TE = 2200/4.25 ms; FA = 9°, 1-mm isotropic resolution) and T2-weighted spin-echo multislice (TR/TE = 8000/72 ms; 1.1-mm in-plane resolution; 94 slices, 1.7 mm slice thickness; matrix = 192) pulse sequences were used to acquire high-resolution anatomical brain images.
For cohort B (n = 61), a 32-channel head coil and a multiplexed EPI sequence were used with the same parameters as the Human Connectome Project resting fMRI sequence: multiband factor = 8, anterior–posterior phase encoding, TR/TE = 720/37 ms, FA = 52°, matrix = 104, and 72 slices were used to acquire resting fMRI time series with 2-mm isotropic voxels and 1238 time points while the participants relaxed with their eyes open. A fixation cross was presented on a black background under dimmed room lighting using a liquid-crystal display screen (BOLDscreen 32, Cambridge Research Systems). The 3D MP-RAGE (TR/TE = 2400/2.24 ms, FA = 8°) and variable-flip-angle turbo spin-echo (Siemens SPACE; TR/TE = 3200/564 ms) pulse sequences were used to acquire high-resolution anatomical brain images with 0.8-mm isotropic voxels field-of-view (FOV) = 240 × 256 mm, matrix = 300 × 320, and 208 sagittal slices.
We used the minimal preprocessing pipelines of the Human Connectome Project for the spatial normalization of the structural and functional scans. Specifically, FreeSurfer version 5.3.0 (http://surfer.nmr.mgh.harvard.edu) was used to automatically segment the anatomical MRI scans into cortical and subcortical gray matter regions of interest (ROIs) and for spatial normalization to the stereotactic space of the Montreal Neurological Institute (MNI).
PET Processing: Regional Glucose Metabolism
Voxelwise CMRglc was computed in PMOD v3.4 (PMOD Technologies), based on an autoradiographic solution for the two-tissue compartment model for the FDG summary image. The CMRglc maps in μmol/100 mL/min were aligned to the subject’s structural MRI and then normalized to the MNI template with a 2-mm isotropic resolution using the FSL Software Library (version 5.0; http://www.fmrib.ox.ac.uk/fsl) (Smith et al. 2004). To assess regional differences in energetics, we computed relative CMRglc (rCMRglc) images by normalizing the MRGlu maps to the whole brain mean (FSL’s “MNI_T1_2mm_brain_mask” image). We used rCMRglc instead of absolute glucose metabolism because across the cerebral cortex, absolute glucose metabolism is dominated by the global signal with very little regional variability: across the subjects the correlation between absolute metabolism in association and sensorimotor network is extremely high (r2 > 0.97). Since brain network segregation varies tremendously across the networks (see Results section), regional glucose metabolism lends itself to comparison with segregation more readily. We nevertheless report on the associations between absolute glucose metabolism and network segregation in the Supplementary material.
Brain Network Parcellation Scheme: Sensorimotor and Association Cortices
For the brain metrics (resting fMRI network segregation and rCMRglc), we followed procedures from the prior work (Chan et al. 2014) and extracted data from broad sets of sensorimotor and association cortices delineated using a popular resting fMRI parcellation scheme (Power et al. 2011) (Fig. 1a). We used the “consensus” parcellation scheme (264 regions of interest) and created a 5-mm sphere at each region from which we extracted data. After computing each measure of interest (see Processing sections below), we aggregated across networks by taking the average value across all “sensorimotor” and all “association” networks, as done previously (Chan et al. 2014): hand somato-motor, mouth somato-motor, visual, and auditory networks contributed to the sensorimotor system, and default-mode, frontoparietal, ventral attention, dorsal attention, cingulo-opercular, and salience networks contributed to the association system.
Figure 1.
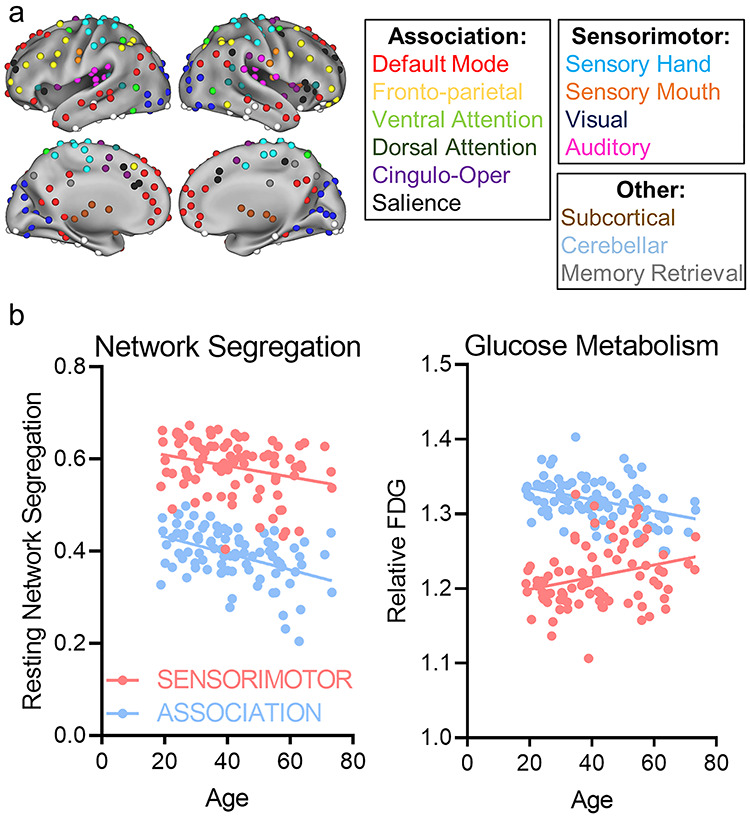
(a) Regions from the Power et al. parcellation were aggregated into sensorimotor and association networks, as in the previous work. (b) Scatterplots depicting the associations between age and the resting network segregation (left) as well as glucose metabolism (right) across the study population (n = 88). There was a significant interaction with aging between glucose metabolism in association networks and glucose metabolism in sensorimotor networks (z = −4.51, P < 0.0001).
Functional MRI Processing: Resting Network Segregation
For resting fMRI time series, the Human Connectome Project functional pipeline was used for gradient distortion correction, rigid body realignment, field map processing, and spatial normalization to the stereotactic MNI space. 0.008–0.09-Hz band-pass filtering was used to assess the low-frequency fluctuations in the resting fMRI data. Signals from the white matter and CSF were regressed out of the data. Framewise displacements (FD) were computed from head translations and rotations using a 50-mm radius to convert angle rotations to displacements. Scrubbing was used to remove time points excessively contaminated with motion. Specifically, time points were excluded if the root-mean-square change in the BOLD signal (DVARS) from volume to volume met the criteria: DVARS > 0.5% and FD > 0.5 mm.
Resting network segregation, a measure of the relative strength of within-network versus between-network connections, was calculated as in the prior work (Chan et al. 2014):
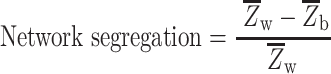 |
(1) |
where 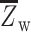 is the mean Fisher’s z-transformed correlation between regions within the same network and
is the mean Fisher’s z-transformed correlation between regions within the same network and 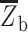 is the mean Fisher’s z-transformed correlation between regions of one network to all other networks. As in the prior work, we retained all positive connections (i.e., node pairs where the correlation Z value was greater than zero). Segregation was computed for each individual network separately, before averaging across the sensorimotor or association network sets.
is the mean Fisher’s z-transformed correlation between regions of one network to all other networks. As in the prior work, we retained all positive connections (i.e., node pairs where the correlation Z value was greater than zero). Segregation was computed for each individual network separately, before averaging across the sensorimotor or association network sets.
Statistical Analyses
We tested for associations between age, rCMRglc, resting network segregation, and executive and motor functions (principal component measures from CANTAB scores). For each brain measure (rCMRglc and segregation), each participant had two separate values (sensorimotor networks and association networks). This was the case for all analyses except hypothesis 2 (networks with lower segregation would be more vulnerable to differences in rCMRglc with age), in which we tested an association across all 13 resting networks in the Power et al. parcellation. First, we examined the zero-order correlations between aging and the brain measures. We then tested if sensorimotor and association networks were differentially associated with age: we tested if the slopes of these correlations were significantly different using Fisher’s z-test. For all analyses below, we controlled for the following covariates: sex, body mass index, and MRI scan sequence. We did not include years of education and IQ as covariates due to their known strong correspondence with performance on cognitive tasks (e.g., Anstey and Christensen 2000), which could remove substantial variance of interest, and because in our sample, the associations between age and years of education (r2 = 4 × 10−6) and age and IQ (r2 = 0.01) were close to zero.
Hypothesis 1: Resting Network Segregation Mediates the Age-rCMRglc Link
Following a recent hypothesis that network segregation is critical for brain energetics (Wig 2017), we tested whether resting network segregation mediated the association between age and rCMRglc, using the causal mediation analysis toolbox in R (Tingley et al. 2014) with 5000 permutations. We first tested whether the mediating variable (segregation) was significantly associated with both the predictor (age) and the outcome measure (rCMRglc) in a regression model controlling for all covariates, as a precondition for performing mediation analysis. We then conducted the causal mediation analysis, Bonferroni-correcting for two comparisons (sensorimotor and association networks), and therefore the effective significance threshold was P < 0.05/2 = 0.025. As a follow-up, we also tested the reverse mediation analysis (that rCMRglc would mediate the age-segregation link).
Hypothesis 2: Resting Networks with Low Segregation (Most Integrated) have the Steepest Negative Association between Age and Glucose Metabolism
We also sought to test whether the most highly integrated brain networks show the strongest negative correlations between age and glucose metabolism. Therefore, across all brain networks, we tested the correlation between the average network segregation and slope of age-related change in glucose metabolism. To test for significance, we used permutation testing. Rows representing the segregation for each network were randomly permuted 5000 times, and the correlation with the age-FDG slope was rerun after each permutation. The level of significance was the number of correlations stronger than the observed correlation, divided by the total number of permutations (P < 0.05).
Hypothesis 3: Glucose Metabolism and Resting Network Segregation Mediate the Link between Age and CANTAB Performance
To understand the contribution of these brain measures to differences in CANTAB performance across the lifespan, we again performed mediation analysis. As in the analysis for hypothesis 1, we first tested whether the mediating variable in each analysis was significantly associated with both the predictor (age) and the outcome measures (principal components reflecting spatial cognition and response time) in a regression model controlling for all covariates. In this instance, for the tests of cognitive/motor function, there were four possible mediators: network segregation and glucose metabolism for association and sensorimotor networks. Therefore, we Bonferroni-corrected for four comparisons, and the effective significance threshold was P < 0.05/4 = 0.0125. Then, for each potential mediator that showed a significant relationship with both the predictor and outcome, we performed the mediation analysis.
Exploratory Analysis
We performed a series of exploratory follow-up analyses related to the primary findings. These included testing associations between absolute glucose metabolism (CMRglu) and network segregation; interactions of segregation within and between the sensorimotor and association systems; and testing for variables independently associated with the PCs representing performance on CANTAB tests, including sex and age-by-sex interactions. These analyses and discussion are in the Supplementary material.
Power Analysis
We conducted power analysis for the mediation analyses in this study which required the most power of any of the primary analyses. We used the Monte Carlo Power Analysis for Indirect Effects toolbox in R (Schoemann et al. 2017). There are no consensus estimates on the strength of relationships between the variables in this study (i.e., age, network segregation, and CANTAB performance). Estimates from the recent work suggest bivariate associations are roughly r = |0.4–0.5| (Chan et al. 2014; Uresti-Cabrera et al. 2015); therefore, assuming association strengths at the lower end of this range of r = |0.4|, an n of 88 would yield a power of 0.76 to detect the indirect effect of a mediation (simulations conducted with 5000 replications, 20 000 Monte Carlo draws per replication, and a confidence level of 95%).
Results
Behavior: Principal Component Analysis
The first two components accounted for nearly 40% of the variance across all CANTAB measures and the 88 participants (Table 2). The first component (PC1: “spatial cognition”) loaded heavily on SWM, spatial span, and spatial planning processes. The second component (PC2: “response time”) loaded heavily on various measures of reaction time. Subsequent components were very mixed between the task domains and each accounted for <10% of the variance and were therefore not interpreted.
Table 2.
Loadings for the top two principal components (PCs)
| PC1 (cognition) loadings | Measure | PC2 (motor) loadings | Measure |
|---|---|---|---|
| 0.202 | SSP span length | 0.308 | RTI mean simple movement time |
| 0.200 | SOC problems solved in minimum moves | 0.299 | RTI median simple movement time |
| 0.167 | SOC problems solved in minimum moves, three moves | 0.299 | RTI mean five-choice movement time |
| 0.159 | IED stages completed | 0.298 | RTI median five-choice movement time |
| 0.153 | SOC problems solved in minimum moves, four moves | 0.259 | RTI five-choice movement time |
| 0.147 | SOC problems solved in minimum moves, five moves | 0.257 | RTI five-choice reaction time |
| 0.137 | PRM percent correct | 0.243 | RTI median simple reaction time |
| 0.116 | SOC problems solved in minimum moves, two moves | 0.242 | RTI mean simple reaction time |
| 0.111 | SOC mean initial thinking time five moves | 0.172 | RTI SD five-choice reaction time |
| 0.086 | SSP total errors | 0.169 | RTI SD simple movement time |
| 0.036 | SOC mean initial thinking time five moves | 0.169 | RTI SD simple reaction time |
| 0.011 | RTI simple accuracy score | 0.167 | RTI SD five-choice movement time |
| −0.001 | SOC mean initial thinking time two moves | 0.122 | RTI simple accuracy score |
| −0.009 | RTI SD simple movement time | 0.110 | SOC problems solved in minimum moves, five moves |
| −0.020 | RTI five-choice accuracy score | 0.110 | SOC problems solved in minimum moves |
| −0.072 | RTI SD five-choice movement time | 0.100 | IED stages completed |
| −0.072 | SWM within errors | 0.084 | SOC problems solved in minimum moves, three moves |
| −0.085 | SWM double errors | 0.076 | SOC problems solved in minimum moves, four moves |
| −0.086 | SOC mean initial thinking time three moves | 0.073 | RTI five-choice accuracy score |
| −0.095 | IED EDS errors | 0.048 | SSP span length |
| −0.101 | SOC mean subsequent thinking time two moves | 0.046 | SSP total errors |
| −0.106 | SWM mean time to first response | 0.045 | PRM percent correct |
| −0.115 | SOC mean moves two moves | 0.035 | SOC mean moves, two moves |
| −0.125 | RTI mean simple movement time | 0.027 | SWM mean time to the first response |
| −0.131 | RTI median simple movement time | 0.013 | SOC mean subsequent thinking time two moves |
| −0.134 | RTI median five-choice movement time | 0.008 | SOC mean initial thinking time four moves |
| −0.136 | RTI mean five-choice movement time | -0.023 | IED pre-ED errors |
| −0.137 | RTI median simple reaction time | -0.026 | SOC mean initial thinking time two moves |
| −0.138 | SOC mean subsequent thinking time three moves | -0.034 | SOC problems solved in minimum moves, two moves |
| −0.146 | RTI five-choice reaction time | -0.037 | IED total latency |
| −0.150 | RTI SD simple reaction time | -0.044 | SOC mean initial thinking time five moves |
| −0.151 | IED pre-ED errors | -0.054 | SOC mean moves, four moves |
| −0.151 | SOC mean subsequent thinking time five moves | -0.063 | SOC mean moves, three moves |
| −0.152 | SOC mean moves four moves | -0.071 | SWM mean time to the last response |
| −0.154 | RTI mean simple reaction time | -0.085 | SOC mean subsequent thinking time three moves |
| −0.157 | RTI five-choice movement time | -0.090 | SWM within errors |
| −0.158 | SOC mean moves five moves | -0.093 | IED EDS errors |
| −0.171 | SOC mean moves three moves | -0.097 | SWM between errors |
| −0.172 | RTI SD five-choice reaction time | -0.097 | SWM total errors |
| −0.175 | IED total errors | -0.100 | SOC mean initial thinking time three moves |
| −0.176 | IED total latency | -0.100 | SWM strategy |
| −0.176 | IED total trials | -0.103 | SWM double errors |
| −0.181 | SOC mean subsequent thinking time four moves | -0.106 | SOC mean subsequent thinking time five moves |
| −0.183 | IED total errors adjusted | -0.115 | IED total errors adjusted |
| −0.187 | IED total trials adjusted | -0.116 | IED total trials adjusted |
| −0.188 | SWM mean time to the last response | -0.119 | SOC mean subsequent thinking time four moves |
| −0.193 | SWM strategy | -0.124 | IED total errors |
| −0.200 | SWM total errors | -0.127 | IED total trials |
| −0.202 | SWM between errors | -0.148 | SOC mean moves, five moves |
Note: PC1 loaded heavily on SWM, spatial span, and spatial planning processes. PC2 loaded heavily on simple reaction time measures.
Associations between Age, Resting Segregation, and Glucose Metabolism
The average number of frames removed due to motion in each scan cohort was as follows: cohort A, 11.0 (1.8%), and cohort B, 47.9 (3.8%). The sensorimotor networks were significantly more segregated than the association networks (t(87) = 34.46, P < 0.0001). In contrast, the sensorimotor networks showed significantly lower relative glucose metabolism than the association networks (t(87) = 19.99, P < 0.0001). The scatterplots showing the association between age and the brain measures (for both association and sensorimotor networks) are shown in Figure 1b. There was a negative correlation between age and brain network segregation in association networks (r = −0.448, P < 0.0001), and this negative correlation was observed to a lesser extent in sensorimotor networks (r = −0.281, P = 0.008). However, rCMRglc only showed a negative association with age in association networks (r = −0.384, P = 0.0002), while we observed a positive association between age and rCMRglc in sensorimotor networks (r = 0.279, P = 0.009). There was a significant interaction with aging between rCMRglc in association networks and rCMRglc in sensorimotor networks (z = −4.51, P < 0.0001) indicative of a distinct effect of age in relative glucose metabolism between association and sensorimotor networks. The associations between age and segregation/rCMRglc were fairly consistent across the individual networks within the sensorimotor and association systems (see Supplementary Fig. 1).
Hypothesis 1: Resting Network Segregation Mediates the Age-Glucose Metabolism Link
We first performed regression analysis to determine if the mediating variable was significantly associated with the predictor and the outcome while controlling for all covariates. While segregation was significantly associated with age (association, t = −4.50, P < 0.001; sensorimotor, t = −3.31, P = 0.001), it was not significantly associated with relative glucose metabolism in either of the networks (association, t = −0.85, uncorrected P = 0.395; sensorimotor, t = 1.44, uncorrected P = 0.155). Nevertheless, we still performed mediation analysis to confirm the lack of a mediating effect (Zhao et al. 2010). There was a significant direct effect of age on glucose metabolism in association networks (direct effect estimate = −0.0006, 95% CI = [−0.00114, −0.00014], Bonferroni-corrected P = 0.024) and sensorimotor networks (direct effect estimate = 0.0009, 95% CI = [0.00038, 0.00156], Bonferroni-corrected P = 0.002). However, the mediation analysis did not support a significant mediating effect of network segregation on age and glucose metabolism in association or sensorimotor networks (mediation effects: uncorrected P’s > 0.20). Likewise, the reverse mediation analysis (that rCMRglc mediates the age-segregation link) was also not significant: (sensory networks, mediation effect estimate = −0.00093, 95% CI = [−0.00035, 0.000564], P = 0.630; association networks, mediation effect estimate = −0.00053, 95% CI = [−0.00027, 0.000169], P = 0.648).
Hypothesis 2: Resting Networks with Low Segregation have the Steepest Age-Related Differences in Glucose Metabolism
We confirmed that the level of segregation differed significantly across the 13 networks of the Power et al. parcellation, with the sensory mouth and the visual networks having the greatest segregation and the salience network having the least segregation (F(6.4, 558.0) = 125.7, P < 0.0001). rCMRglc also differed significantly across the 13 networks, with the memory retrieval and frontoparietal networks having the highest relative metabolism and the cerebellar network having the lowest metabolism (F(6.7, 583.0) = 442.4, P < 0.0001). There was a positive correlation between-network segregation and the slope of age–rCMRglc association across the 88 study participants (r = 0.675, P = 0.010, 5000 permutations). Importantly, networks with the lowest segregation tended to show a negative association between age and rCMRglc. The same general association was also observed at the level of individual nodes of the Power et al. parcellation (Supplementary Fig. 2; r = 0.30). In other words, networks that were least segregated (or most integrated) showed the strongest age-related differences in glucose metabolism.
Hypothesis 3: Glucose Metabolism and Resting Network Segregation Mediate the Link between Age and CANTAB Performance
As in hypothesis 1, we first performed regression analysis to determine if the mediating variables were significantly associated with the predictor and the outcome while controlling for all covariates. Of the four measures, only resting segregation of the sensorimotor networks was significantly associated with age (t = −4.478, P < 0.0001) and the spatial cognition PC (PC1; t = 3.989, P = 0.0001). Further, only rCMRglc of the sensorimotor networks was significantly associated with age (t = 2.646, P = 0.0097) and the response time PC (PC2; t = 3.989, P = 0.0001). Of note, resting segregation of the association networks was significantly associated with age (t = −5.020, P < 0.0001), but its association with the “cognition” PC only showed a trend-level association that did not reach significance after Bonferroni correction (t = 2.065, uncorrected P = 0.0421).
Therefore, we proceeded to perform two mediation analyses: one where age was the predictor, sensorimotor network resting segregation was the mediator, and PC1 was the outcome variable and another where age was the predictor, sensorimotor network rCMRglc was the mediator, and PC2 was the outcome variable. Both analyses showed significant mediation effects. The former analysis revealed that sensorimotor network segregation significantly mediated the age–PC1 link (mediation effect estimate = −0.0355, 95% CI = [−0.0690, −0.0050], P = 0.023; direct effect estimate = −0.033, 95% CI = [−0.0880, 0.0226], P = 0.251). The latter analysis revealed that sensorimotor network rCMRglc significantly mediated the age–PC2 link (mediation effect estimate = −0.013, 95% CI = [−0.0292, −0.0019], P = 0.011; direct effect estimate = −0.016, 95% CI = [−0.0553, 0.0209], P = 0.251). Since we observed a trend-level association between association network segregation and PC1 (uncorrected P = 0.04), we also performed an exploratory mediation analysis where association network segregation was the mediator, age the predictor, and PC1 the outcome. Results suggested that association network segregation did not significantly mediate the age–PC1 link (mediation effect estimate = −0.012, 95% CI = [−0.0419, 0.0150], P = 0.35).
Although we controlled for different scanner sequences in cohort A versus cohort B in the latter analyses, we also re-ran these analyses only using the data from cohort B (n = 61) to confirm that findings remained significant. Indeed, results were consistent with the larger analysis; for sensorimotor network segregation mediating the age–PC1 link, the mediation effect estimate = −0.0205, 95% CI = [−0.0470, −0.0019], P = 0.026 and direct effect estimate = −0.035, 95% CI = [−0.0789, 0.00826], P = 0.116). Likewise, for sensorimotor rCMRglc mediating the age–PC2 link, the mediation effect estimate = −0.0122, 95% CI = [−0.0301, −0.0016], P = 0.015 and direct effect estimate = −0.019, 95% CI = [−0.0601, 0.0178], P = 0.321) (Figs 2 and 3).
Figure 2.
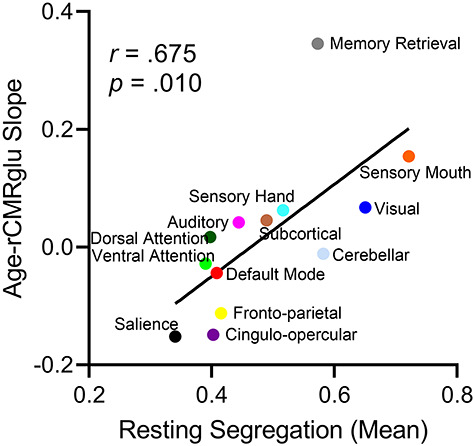
The association between average resting segregation (x-axis) and the slope of age-related differences in relative glucose metabolism (rCMRglu) in that network (y-axis). The networks with the lowest segregation (or highest level of integration) tended to show a negative association between age and glucose metabolism.
Figure 3.
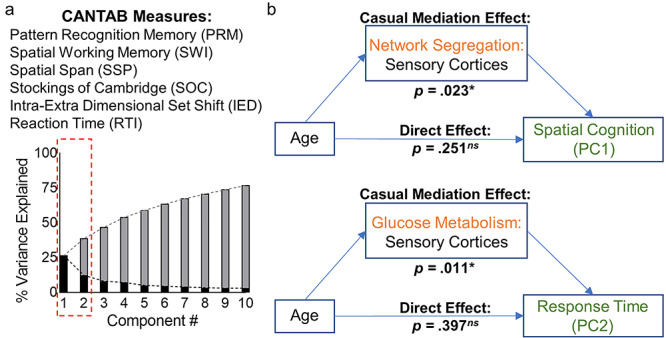
(a) Principal component analysis of CANTAB executive function tasks. The top two principal components had strong loadings for SWM processes (PC1; spatial cognition) and simple reaction time (PC2; response time). (b) Mediation analyses. Sensorimotor network segregation significantly mediated the relationship between age and spatial cognition performance (top). Sensorimotor network glucose metabolism significantly mediated the relationship between age and response time (bottom). In both analyses, when accounting for the mediation effects, the direct effects of age on spatial cognition and response time were no longer significant.
Discussion
Here we investigated the associations between brain functional network segregation and glucose energy utilization as a function of age and its relevance for mediating age-related cognitive/motor differences. We discuss these findings below.
Brain Network Segregation and Glucose Metabolism: Associations with Age
When parcellating the brain based on resting fMRI data (Power et al. 2011), network segregation and relative glucose metabolism showed contrasting patterns with age. Whereas segregation showed age-related decreases in both sensorimotor and association networks, relative glucose metabolism only showed age-related decreases in association networks with relative increases in metabolism in sensorimotor networks. There was a significant aging interaction that indicated distinct effects of age in relative glucose metabolism between association and sensorimotor networks. The segregation findings replicate those of many recent studies (e.g., Chan et al. 2014; Geerligs et al. 2014; Ng et al. 2016; Spreng et al. 2016; Monteiro et al. 2019) and reiterate that the age-related differences are more pronounced in association as compared with sensorimotor networks (Wig 2017). The glucose metabolism findings provide a novel perspective on previous studies examining individual regions. For instance, strong age-related decreases in relative glucose metabolism have been observed in medial frontal and cingulate gyri (Volkow et al. 2000), whereas age-related increases have been observed in thalamus and sensorimotor cortex, at least in males (Shen et al. 2012). These findings might reflect that the most recently phylogenetically developed networks (i.e., association cortices) are the most vulnerable to age-related differences in regional energetics (Kalpouzos et al. 2009) and these networks appear to be sensitive to neurological insult more generally (de Lange et al. 2019). However, MRI-based measurements of brain structure do not always appear to follow this pattern; Taubert et al. (2020) recently observed age-related differences in brain volume were proportionally steeper in sensorimotor and subcortical brain regions compared with the rest of the brain. Possible explanations for this discrepancy include methodological differences (e.g., proportional scaling to remove global effects of age-related volume loss); age range of participants, which was 44–86 (Taubert et al. 2020); or differences in the functional consequences of age-related volume loss across the brain regions.
Contrary to the working hypothesis (Wig 2017), brain network segregation did not mediate the age-related differences in relative glucose metabolism (nor the reverse: relative glucose metabolism did not mediate age-related differences in network segregation). Hence individual differences in network segregation do not appear to be a major factor behind the age-related differences in regional glucose metabolism. Although studies have documented a strong correspondence between resting connectivity and glucose metabolism (Tomasi et al. 2013) including in the default-mode and salience networks (Riedl et al. 2014; Passow et al. 2015), the recent work suggests that there are brain networks where glucose metabolism and functional connectivity are mismatched (Shokri-Kojori et al. 2019). It has been hypothesized that the segregated organization of the brain minimizes “wiring costs,” which should theoretically have relevance for energetics including glucose metabolism. However, it is possible that structural rather than functional connectivity organization mediates these processes (Bullmore and Sporns 2012). Alternatively, other measures of brain energy consumption, including aerobic glycolysis, may have better correspondence with network connectivity organization (Raichle 2015). Nevertheless, networks which were on average the least segregated at rest (e.g., salience network) tended to show the steepest age-related differences in glucose metabolism. Future work is needed to determine if these findings are merely epiphenomena or if they are in fact intrinsically linked.
Sensorimotor Network Segregation Mediates Age-Related Differences in Spatial Cognition
The mediation analysis suggested that resting segregation of the sensorimotor networks mediated age-related differences in spatial cognition. A recent study also observed that segregation of the sensorimotor networks was associated with age-related differences in cognitive performance among adults 55–85 years old (Stumme et al. 2020). Based on the prior work (Chan et al. 2014; Nashiro et al. 2017), we expected association network segregation to be more strongly linked with cognitive performance than sensorimotor network segregation. However, association network segregation showed significant mediation only at an uncorrected threshold. This may reflect the specific tasks from the CANTAB battery used in this study: the principal component was heavily associated with SWM, spatial span, and spatial planning performance. Prior studies found that association network segregation was most strongly associated with long-term episodic memory (Chan et al. 2014) and verbal fluency (Nashiro et al. 2017). Another recent study using different cognitive tasks during scanning found that reduced frontoparietal/default-mode network segregation was associated with poorer vocabulary performance whereas reduced dorsal attention network/memory network segregation was associated with poorer fluid reasoning (Varangis et al. 2019). Further, features of specific tasks, such as their complexity, appear to have a critical association with system segregation (Yue et al. 2017). Thus, the discrepant findings here might relate to either the domain or the complexity of the tasks in the CANTAB battery. It is also possible that motor responses (apart from response time, which was largely captured in the orthogonal PC2) in the spatial cognition tasks could be a common feature that links sensorimotor system segregation to task performance across the lifespan. Accordingly, Carson (2018) has suggested that since cognition and motor control decline in parallel with aging, it is likely that neurodegeneration of common brain regions underlies this effect. In this context, lifespan differences in sensorimotor system communication might represent a common feature that links lifespan differences in cognitive and motor behavior.
Regardless, since spatial processes including working memory are a fundamental building block of cognition in humans (Baddeley 1986), the association with sensorimotor network segregation is notable. Functional reorganization of sensorimotor systems has been observed in association with cognitive decline in healthy senescence (Romero-Garcia et al. 2014; He et al. 2017) and in mild cognitive impairment and Alzheimer’s disease (Agosta et al. 2010). It is well documented that sensory impairments, especially vision and hearing, seem to be associated with cognitive decline in aging (Humes and Young 2016), including episodic memory (Maharani et al. 2018) and processing speed (Lindenberger and Baltes 1994), and these associations do not appear to be driven by social isolation from sensory loss (Hämäläinen et al. 2019). Sensory loss may be a top modifiable risk factor for cognitive decline (Livingston et al. 2017), and indeed the use of hearing aids for 6 months improved working memory performance in a senescent cohort (Karawani et al. 2018). Baseline sensorimotor functional connectivity predicts task learning rate over time (Mattar et al. 2018), and network segregation has recently been posited as a key target for “brain training” interventions to improve cognition in aging (Gallen and D’Esposito 2019). Emerging tools such as fMRI neurofeedback could be used to promote resting network segregation and test whether this improves cognitive performance.
The biological origins of these network segregation changes are unclear, which remains a major obstacle toward developing effective interventions. The recent work has attempted to find the neurochemical underpinnings of the brain’s network organization (Shine and Poldrack 2018). Based on preclinical and clinical evidence, it is suggested that noradrenaline, acetylcholine, and dopamine modulate the topological configuration (segregation or integration) of functional networks during rest and task conditions (Shine et al. 2019). The loss of sensorimotor network segregation in aging is associated with reductions in GABAergic transmission (Cassady et al. 2019; Lalwani et al. 2019). This mirrors work showing that GABAergic degradation in aging rodents and cats is associated with “dedifferentiation” (reduced response selectivity) of a single-neuron activity in sensory cortices (Hua et al. 2008; Ding et al. 2017). In addition, the NMDA-receptor antagonist ketamine boosts network segregation in rats (Becker et al. 2016). Together these data suggest that preserving neurotransmitter signaling mechanisms (and thereby augmenting a segregated network structure while providing for an integrated network structure when it is required by specific tasks) may be a fruitful area of investigation for the prevention of age-related cognitive/motor decline. Interestingly, visual restoration after cataract surgery has been associated with improved structure and function of visual and cognitive brain regions (Lin et al. 2018). Thus studies that evaluate the effects of sensorimotor and cognitive stimulation in elderly individuals are needed to assess if they can help recover the age-associated loss of network segregation.
Sensorimotor Network Glucose Metabolism Mediates Age-Related Differences in Response Time
Finally, sensorimotor network metabolism mediated the age-related differences in a principal component that largely reflected motor slowing on a simple reaction time test. It is well-established that brain glucose metabolism changes dramatically with age, including the sensorimotor regions (Moeller et al. 1996; Garraux et al. 1999). These changes likely have relevance for motor function: hypometabolism in supplementary motor area is associated with a fear of falling in healthy aging (Sakurai et al. 2017). Further, sensorimotor hypometabolism is implicated in the motor deficits observed in Parkinson’s disease (Lee et al. 2011; Wu et al. 2018). The current data add to the existing literature and suggest that relative glucose metabolism in a broader network of sensorimotor regions mediates age-related differences in motor performance, particularly response time.
Interestingly, it was age-related increases, rather than decreases, in rCMRglu that were associated with the longer response times among the elderly. To our knowledge, this is the first study to observe this pattern. Berman et al. (2008) tested people with chronic methamphetamine use both 1 week after initiating abstinence and again 4 weeks later and found that decreases in rCMRglu in ventromedial prefrontal cortex were positively associated with slowing of RT in a vigilance task. Another group observed that, among healthy adults aged 51–71 years old, lower glucose metabolism in an “Alzheimer signature” meta-ROI (inferior temporal cortex, posterior cingulate cortex, angular gyrus) was associated with slower RT in one-back and choice RT tasks, although maps were normalized to the cerebellum rather than the whole brain (Mielke et al. 2014). More generally, higher sensorimotor rCMRglu might be related to poorer performance on neuropsychological tests in aging. In a cohort of 70 healthy adults aged 20–87 years old, Brickman et al. (2003) observed that higher caudate rCMRglu was associated with a better verbal learning performance but higher putamen rCMRglu was associated with a worse performance across the lifespan. Although speculative, these data may be relevant inasmuch as the caudate is heavily connected with associative cortices whereas the putamen is more connected with sensorimotor cortices (Haber et al. 2020).
Putting the network segregation and rCMRglu findings together, these data broadly fit with a study showing that internetwork segregation is more associated with the performance on complex cognitive tasks whereas local activity is more associated with the function on simple motor tasks (Cohen and D’Esposito 2016). This provides a possible parsimonious explanation for the set of findings observed here, where network segregation was more associated with spatial cognition performance and glucose metabolism was more associated with response time.
Limitations
There were several limitations to the current study, most notably that the data are cross-sectional and correlational. Although cross-sectional mediation analysis provides a mathematical framework for examining potential underlying mechanisms, it can over- or underestimate true temporal effects, and longitudinal studies would provide a more direct insight into age-related processes (Maxwell et al. 2011). Second, rCMRglu is only one aspect of brain energetics, and combining other measures such as aerobic glycolysis would provide a more complete picture on how energy consumption relates to network segregation (Raichle 2015). Relatedly, absolute glucose metabolism has little regional variability, necessitating the use of rCMRglu (whole-brain averaged signals), which means those values were inherently handled differently than the network segregation signals (not whole-brain averaged). Nevertheless, testing for associations between absolute glucose metabolism and network segregation (see Supplementary material) yielded similar nonsignificant findings. Third, we did not have measures of peripheral sensory ability in this study, which may covary with both age and CANTAB task performance. Finally, a more diverse battery of cognitive/motor tests and more imaging sessions (e.g., comparing task vs. resting state; Gallen et al. 2016) will be needed to provide a more global perspective on the current findings.
In summary, we studied the associations between brain network segregation, regional brain glucose utilization, and cognitive/motor performance across the adult lifespan. Brain network segregation did not mediate age-related changes in glucose metabolism. However, networks that were on average the least segregated (e.g., salience network) showed the strongest age-related differences in regional metabolism. Sensorimotor network segregation mediated age-related differences in spatial cognition, and sensorimotor glucose metabolism mediated age-related differences in response time. Our findings are broadly consistent with studies suggesting enriched environments that include somatosensory stimulation in the elderly might help prevent age-associated cognitive decline (Leon and Woo 2018).
Supplementary Material
Funding
National Institute on Alcohol Abuse and Alcoholism (Y1AA-3009).
Notes
We thank Elizabeth Cabrera, Clara Freeman, Elsa Lindgren, Gregg Miller, Veronica Ramirez, Jamie Burns, Christopher Kure Liu, Karen Torres, Christopher Wong, Amna Zehra, Lori Talagala, and Minoo McFarland for their contributions. Conflict of Interest: None declared.
References
- Aanerud J, Borghammer P, Rodell A, Jónsdottir KY, Gjedde A. 2017. Sex differences of human cortical blood flow and energy metabolism. J Cereb Blood Flow Metab. 37:2433–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agosta F, Rocca MA, Pagani E, Absinta M, Magnani G, Marcone A, Falautano M, Comi G, Gorno-Tempini ML, Filippi M. 2010. Sensorimotor network rewiring in mild cognitive impairment and Alzheimer’s disease. Hum Brain Mapp. 31:515–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anstey K, Christensen H. 2000. Education, activity, health, blood pressure and apolipoprotein E as predictors of cognitive change in old age: a review. Gerontology. 46:163–177. [DOI] [PubMed] [Google Scholar]
- Baddeley AD 1986. Working memory, Oxford psychology series; No. 11.
- Becker R, Braun U, Schwarz AJ, Gass N, Schweiger JI, Weber-Fahr W, Schenker E, Spedding M, Clemm von Hohenberg C, Risterucci C, et al. 2016. Species-conserved reconfigurations of brain network topology induced by ketamine. Transl Psychiatry. 6:e786–e786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman SM, Voytek B, Mandelkern MA, Hassid BD, Isaacson A, Monterosso J, Miotto K, Ling W, London ED. 2008. Changes in cerebral glucose metabolism during early abstinence from chronic methamphetamine abuse. Mol Psychiatry. 13:897–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman AM, Buchsbaum MS, Shihabuddin L, Hazlett EA, Borod JC, Mohs RC. 2003. Striatal size, glucose metabolic rate, and verbal learning in normal aging. Cogn Brain Res. 17:106–116. [DOI] [PubMed] [Google Scholar]
- Bullmore E, Sporns O. 2012. The economy of brain network organization. Nat Rev Neurosci. 13:336–349. [DOI] [PubMed] [Google Scholar]
- Carson RG 2018. Get a grip: individual variations in grip strength are a marker of brain health. Neurobiol Aging. 71:189–222. [DOI] [PubMed] [Google Scholar]
- Cassady K, Gagnon H, Lalwani P, Simmonite M, Foerster B, Park D, Peltier SJ, Petrou M, Taylor SF, Weissman DH, et al. 2019. Sensorimotor network segregation declines with age and is linked to GABA and to sensorimotor performance. NeuroImage. 186:234–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan MY, Park DC, Savalia NK, Petersen SE, Wig GS. 2014. Decreased segregation of brain systems across the healthy adult lifespan. Proc Natl Acad Sci U S A. 111:E4997–E5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JR, D’Esposito M. 2016. The segregation and integration of distinct brain networks and their relationship to cognition. J Neurosci. 36:12083–12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossley NA, Mechelli A, Scott J, Carletti F, Fox PT, Mcguire P, Bullmore ET. 2014. The hubs of the human connectome are generally implicated in the anatomy of brain disorders. Brain. 137:2382–2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lange SC, Scholtens LH, van den Berg LH, Boks MP, Bozzali M, Cahn W, Dannlowski U, Durston S, Geuze E, van Haren NEM, et al. 2019. Shared vulnerability for connectome alterations across psychiatric and neurological brain disorders. Nat Hum Behav. 3:988–998. [DOI] [PubMed] [Google Scholar]
- De Santi S, de Leon MJ, Convit A, Tarshish C, Rusinek H, Tsui WH, Sinaiko E, Wang G-J, Bartlet E, Volkow ND. 1995. Age-related changes in brain: II. Positron emission tomography of frontal and temporal lobe glucose metabolism in normal subject's. Psychiatry Q. 66:357–370. [DOI] [PubMed] [Google Scholar]
- Ding Y, Zheng Y, Liu T, Chen T, Wang C, Sun Q, Hua M, Hua T. 2017. Changes in GABAergic markers accompany degradation of neuronal function in the primary visual cortex of senescent rats. Sci Rep. 7:14897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallen CL, D’Esposito M. 2019. Brain modularity: a biomarker of intervention-related plasticity. Trends Cogn Sci. 23:293–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallen CL, Turner GR, Adnan A, D’Esposito M. 2016. Reconfiguration of brain network architecture to support executive control in aging. Neurobiol Aging. 44:42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garraux G, Salmon E, Degueldre C, Lemaire C, Laureys S, Franck G. 1999. Comparison of impaired subcortico-frontal metabolic networks in normal aging, subcortico-frontal dementia, and cortical frontal dementia. NeuroImage. 10:149–162. [DOI] [PubMed] [Google Scholar]
- Geerligs L, Maurits NM, Renken RJ, Lorist MM. 2014. Reduced specificity of functional connectivity in the aging brain during task performance. Hum Brain Mapp. 35:319–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal MS, Vlassenko AG, Blazey TM, Su Y, Couture LE, Durbin TJ, Bateman RJ, Benzinger TLS, Morris JC, Raichle ME. 2017. Loss of brain aerobic glycolysis in normal human aging. Cell Metab. 26:353–360.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, Sarraf S, Saverino C, Campbell K. 2016. Age differences in the functional interactions among the default, Frontoparietal control and dorsal attention networks. Neurobiol Aging. 41:159–172. [DOI] [PubMed] [Google Scholar]
- Haber SN, Tang W, Choi EY, Yendiki A, Liu H, Jbabdi S, Versace A, Phillips M. 2020. Circuits, networks, and neuropsychiatric disease: transitioning from anatomy to imaging. Biol Psychiatry. 87:318–327. [DOI] [PubMed] [Google Scholar]
- Hämäläinen A, Phillips N, Wittich W, Pichora-Fuller MK, Mick P. 2019. Sensory-cognitive associations are only weakly mediated or moderated by social factors in the Canadian longitudinal study on aging. Sci Rep. 9:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H, Luo C, Chang X, Shan Y, Cao W, Gong J, Klugah-Brown B, Bobes MA, Biswal B, Yao D. 2017. The functional integration in the sensory-motor system predicts aging in healthy older adults. Front Aging Neurosci. 8:306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua T, Kao C, Sun Q, Li X, Zhou Y. 2008. Decreased proportion of GABA neurons accompanies age-related degradation of neuronal function in cat striate cortex. Brain Res Bull. 75:119–125. [DOI] [PubMed] [Google Scholar]
- Humes LE, Young LA. 2016. Sensory–cognitive interactions in older adults. Ear Hear. 37:52S–61S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalpouzos G, Chételat G, Baron J-C, Landeau B, Mevel K, Godeau C, Barré L, Constans J-M, Viader F, Eustache F, et al. 2009. Voxel-based mapping of brain gray matter volume and glucose metabolism profiles in normal aging. Neurobiol Aging. 30:112–124. [DOI] [PubMed] [Google Scholar]
- Karawani H, Jenkins K, Anderson S. 2018. Restoration of sensory input may improve cognitive and neural function. Neuropsychologia. 114:203–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalwani P, Gagnon H, Cassady K, Simmonite M, Peltier S, Seidler RD, Taylor SF, Weissman DH, Polk TA. 2019. Neural distinctiveness declines with age in auditory cortex and is associated with auditory GABA levels. NeuroImage. 201:116033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MS, Lyoo CH, Ryu YH, Lim HS, Nam CM, Kim HS, Rinne JO. 2011. The effect of age on motor deficits and cerebral glucose metabolism of Parkinson’s disease. Acta Neurol Scand. 124:196–201. [DOI] [PubMed] [Google Scholar]
- Leon M, Woo C. 2018. Environmental enrichment and successful aging. Front Behav Neurosci. 12:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, Zhang L, Lin D, Chen W, Zhu Y, Chen C, Chan KC, Liu Y, Chen W. 2018. Visual restoration after cataract surgery promotes functional and structural brain recovery. EBioMedicine. 30:52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenberger U, Baltes PB. 1994. Sensory functioning and intelligence in old age: a strong connection. Psychol Aging. 9:339–355. [DOI] [PubMed] [Google Scholar]
- Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, Ballard C, Banerjee S, Burns A, Cohen-Mansfield J, et al. 2017. Dementia prevention, intervention, and care. Lancet. 390:2673–2734. [DOI] [PubMed] [Google Scholar]
- Maharani A, Dawes P, Nazroo J, Tampubolon G, Pendleton N. 2018. Visual and hearing impairments are associated with cognitive decline in older people. Age Ageing. 47:575–581. [DOI] [PubMed] [Google Scholar]
- Mattar MG, Wymbs NF, Bock AS, Aguirre GK, Grafton ST, Bassett DS. 2018. Predicting future learning from baseline network architecture. NeuroImage. 172:107–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell SE, Cole DA, Mitchell MA. 2011. Bias in cross-sectional analyses of longitudinal mediation: partial and complete mediation under an autoregressive model. Multivar Behav Res. 46:816–841. [DOI] [PubMed] [Google Scholar]
- Mielke MM, Weigand SD, Wiste HJ, Vemuri P, Machulda MM, Knopman DS, Lowe V, Roberts RO, Kantarci K, Rocca WA, et al. 2014. Independent comparison of CogState computerized testing and a standard cognitive battery with neuroimaging. Alzheimers Dement. 10:779–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mink JW, Blumenschine RJ, Adams DB. 1981. Ratio of central nervous system to body metabolism in vertebrates: its constancy and functional basis. Am J Physiol Integr Comp Physiol. 241:R203–R212. [DOI] [PubMed] [Google Scholar]
- Moeller JR, Ishikawa T, Dhawan V, Spetsieris P, Mandel F, Alexander GE, Grady C, Pietrini P, Eidelberg D. 1996. The metabolic topography of normal aging. J Cereb Blood Flow Metab. 16:385–398. [DOI] [PubMed] [Google Scholar]
- Monteiro TS, King BR, Zivari Adab H, Mantini D, Swinnen SP. 2019. Age-related differences in network flexibility and segregation at rest and during motor performance. NeuroImage. 194:93–104. [DOI] [PubMed] [Google Scholar]
- Nashiro K, Sakaki M, Braskie MN, Mather M. 2017. Resting-state networks associated with cognitive processing show more age-related decline than those associated with emotional processing. Neurobiol Aging. 54:152–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng KK, Lo JC, Lim JKW, Chee MWL, Zhou J. 2016. Reduced functional segregation between the default mode network and the executive control network in healthy older adults: a longitudinal study. NeuroImage. 133:321–330. [DOI] [PubMed] [Google Scholar]
- Passow S, Specht K, Adamsen TC, Biermann M, Brekke N, Craven AR, Ersland L, Grüner R, Kleven-Madsen N, Kvernenes O-H, et al. 2015. Default-mode network functional connectivity is closely related to metabolic activity. Hum Brain Mapp. 36:2027–2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, Vogel AC, Laumann TO, Miezin FM, Schlaggar BL, et al. 2011. Functional network Organization of the Human Brain. Neuron. 72:665–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME 2015. The restless brain: how intrinsic activity organizes brain function. Philos Trans R Soc Lond B Biol Sci. 370:20140172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedl V, Bienkowska K, Strobel C, Tahmasian M, Grimmer T, Forster S, Friston KJ, Sorg C, Drzezga A. 2014. Local activity determines functional connectivity in the resting human brain: a simultaneous FDG-PET/fMRI study. J Neurosci. 34:6260–6266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, James M, Owen AM, Sahakian BJ, McInnes L, Rabbitt P. 1994. Cambridge neuropsychological test automated battery (CANTAB): a factor analytic study of a large sample of normal elderly volunteers. Dementia. 5:266–281. [DOI] [PubMed] [Google Scholar]
- Romero-Garcia R, Atienza M, Cantero JL. 2014. Predictors of coupling between structural and functional cortical networks in normal aging. Hum Brain Mapp. 35:2724–2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai R, Fujiwara Y, Yasunaga M, Suzuki H, Kanosue K, Montero-Odasso M, Ishii K. 2017. Association between hypometabolism in the supplementary motor area and fear of falling in older adults. Front Aging Neurosci. 9:251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoemann AM, Boulton AJ, Short SD. 2017. Determining power and sample size for simple and complex mediation models. Soc Psychol Personal Sci. 8:379–386. [Google Scholar]
- Shen X, Liu H, Hu Z, Hu H, Shi P. 2012. The relationship between cerebral glucose metabolism and age: report of a large brain PET data set. PLoS One. 7:e51517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine JM, Breakspear M, Bell PT, Ehgoetz Martens K, Shine R, Koyejo O, Sporns O, Poldrack RA. 2019. Human cognition involves the dynamic integration of neural activity and neuromodulatory systems. Nat Neurosci. 22:289–296. [DOI] [PubMed] [Google Scholar]
- Shine JM, Poldrack RA. 2018. Principles of dynamic network reconfiguration across diverse brain states. NeuroImage. 180:396–405. [DOI] [PubMed] [Google Scholar]
- Shokri-Kojori E, Tomasi DG, Alipanahi B, Wiers CE, Wang G-J, Volkow ND. 2019. Correspondence between cerebral glucose metabolism and BOLD reveals relative power and cost in human brain. Nat Commun. 10:690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, et al. 2004. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 23:S208–S219. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Stevens WD, Viviano JD, Schacter DL. 2016. Attenuated anticorrelation between the default and dorsal attention networks with aging: evidence from task and rest. Neurobiol Aging. 45:149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumme J, Jockwitz C, Hoffstaedter F, Amunts K, Caspers S. 2020. Functional network reorganization in older adults: graph-theoretical analyses of age, cognition and sex. NeuroImage. 214:116756. [DOI] [PubMed] [Google Scholar]
- Taubert M, Roggenhofer E, Melie-Garcia L, Muller S, Lehmann N, Preisig M, Vollenweider P, Marques-Vidal P, Lutti A, Kherif F, et al. 2020. Converging patterns of aging-associated brain volume loss and tissue microstructure differences. Neurobiol Aging. 88:108–118. [DOI] [PubMed] [Google Scholar]
- Tingley D, Yamamoto T, Hirose K, Keele L, Imai K. 2014. Mediation: R package for causal mediation analysis. J Stat Softw. 59:1–38.26917999 [Google Scholar]
- Tomasi DG, Wang G-J, Volkow ND. 2013. Energetic cost of brain functional connectivity. Proc Natl Acad Sci U S A. 110:13642–13647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uresti-Cabrera LA, Diaz R, Vaca-Palomares I, Fernandez-Ruiz J. 2015. The effect of spatial working memory deterioration on strategic Visuomotor learning across aging. Behav Neurol. 2015:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Sporns O. 2013. Network hubs in the human brain. Trends Cogn Sci. 17:683–696. [DOI] [PubMed] [Google Scholar]
- Varangis E, Razlighi Q, Habeck CG, Fisher Z, Stern Y. 2019. Between-network functional connectivity is modified by age and cognitive task domain. J Cogn Neurosci. 31:607–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Logan J, Fowler JS, Wang G-J, Gur RC, Wong C, Felder C, Gatley SJ, Ding YS, Hitzemann R, et al. 2000. Association between age-related decline in brain dopamine activity and impairment in frontal and cingulate metabolism. Am J Psychiatry. 157:75–80. [DOI] [PubMed] [Google Scholar]
- Wig GS 2017. Segregated systems of human brain networks. Trends Cogn Sci. 21:981–996. [DOI] [PubMed] [Google Scholar]
- Wu L, Liu F, Ge J, Zhao J, Tang Y, Yu W, Yu H, Anderson T, Zuo C, Chen L, et al. 2018. Clinical characteristics of cognitive impairment in patients with Parkinson’s disease and its related pattern in 18 F-FDG PET imaging. Hum Brain Mapp. 39:4652–4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Q, Martin RC, Fischer-Baum S, Ramos-Nuñez AI, Ye F, Deem MW. 2017. Brain modularity mediates the relation between task complexity and performance. J Cogn Neurosci. 29:1532–1546. [DOI] [PubMed] [Google Scholar]
- Zhao X, Lynch JG, Chen Q. 2010. Reconsidering Baron and Kenny: myths and truths about mediation analysis. J Consum Res. 37:197–206. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


