Abstract
Background
Moderate intensity exercise ranging 40–60% of maximum oxygen uptake is advised to promote energy expenditure and fat oxidation in overweight and obese people. Although fat oxidation has been shown to be highly variable among individual, there is still a relative uncertainty regarding exercise prescription for women specifically. This article aimed to determine whether indicators of body composition can be used to narrow the exercise intensity range for exercise prescription in women.
Methods
A total of 35 healthy women (age 30.8±9.5 yr) classified according to their BMI in normal weight (NOR; ≤24.9 kg·m2), overweight (OVW; 25–29.9 kg·m2) and obese groups (OBE; ≥30 kg·m2) completed a submaximal graded test (intensities eliciting ~30%, 40%, 50% and 60% of maximum oxygen uptake). Blood lactate, perceived exertion and absolute and relative substrate oxidation for fat (OXFAT) and carbohydrates (OXCHO) were measured at each stage.
Results
Perceived exertion and blood lactate increased as a function of exercise but did not differ across groups. There were no significant changes in absolute and relative OXFAT across groups, or as a function of exercise intensity. Peak OXFAT occurred at the 40%, 50% and 40% stages for NOR, OVW and OBE groups, respectively, with no significant differences across groups.
Conclusion
We measured no differences, but considerable inter-individual variation, in fat oxidation in women of different body composition. This result is in agreement with previous research based on exercise performed at constant rate and in independent participant groups. Our findings do not support the fat oxidation hypothesis, and further emphasise the perspective that exercise prescription should be individualised and likely be based on considerations other than substrate oxidation.
Introduction
Overweight and obesity is a metabolic condition currently affecting approximately 39% of the adult population worldwide, and between 60% and 65% in North America, Oceania, and most of Western Europe [1]. Overweight and obesity are associated with a substantial economic burden to society [2] from the increased risk of developing a number of chronic diseases, including diabetes, heart disease, hypertension, stroke [3], and an increased risk of mortality [4, 5], especially in the most severe forms of obesity [6]. Overweight and obesity are associated with insulin resistance [7–9] and increased intramuscular triglycerides [8]. A reduced ability to utilise free fatty acids (FFA) as a substrate at rest and during exercise [7, 9] has been cited as a contributing factor to further the progression of obesity [10, 11], although this fat oxidation hypothesis (the inability to utilise fat driving weight gain upward) is criticised [12].
Regular physical activity is advised by the American College of Sports Medicine (ACSM) to promote energy expenditure and contribute to optimal weight loss management in overweight and obese people, with current guidelines recommending up to 250–300 min of moderate-intensity aerobic exercise (40–60% of relative maximum oxygen uptake []) per week [13]. The same relatively broad intensity zone has also been shown to elicit the highest rates of fat oxidation, or peak fat oxidation (PFO), in sedentary and obese individuals [11, 14, 15], and moderate intensity exercise training has been shown to improve fat oxidation and lipolysis [16–19] in obese men [20] and women [21, 22].
Improving the definition of optimal exercise intensity for exercise prescription in overweight and obese people remains a challenge for practitioners. Although increasing total energy expenditure using a wide range of intensities currently is the main known factor informing exercise prescription, the effect of exercise intensity continues to be poorly understood. For instance, while increasing metabolic flexibility could be targeted as a benefit of exercise training, fat oxidation rates vary considerably among individuals and are not well predicted by fat mass [23] as they are influenced by pubertal status [24], training status [25], diet [10, 26], exercise intensity [10, 21, 27] and the mode of exercise that is performed [10, 28]. Furthermore, there is currently little consensus regarding the optimal training intensity specifically in women, despite the specific challenges pregnancy, menopause and contraception impose on cardiovascular health [29]. Despite normal heart rate responses during graded exercise performed at 30–60% of , significantly different blood pressure responses have been reported in obese compared to normal and overweight women [30]. Fat oxidation of independent groups of obese/overweight women has been reported to be lower [31], similar [32–35] or even greater [36] than lean women during constant work rate cycling at discrete exercise intensities from 50% to 65% of . Similarly, substrate oxidation of obese and normal weight women did not differ significantly during constant work rates treadmill exercise at 50%, 70% or 75% of [37, 38].
Still, there is currently a paucity of research measuring the dynamics of substrate oxidation at various submaximal exercise intensities, in subgroups of women matched for age and predictors of cardiovascular health but differing in body composition. Therefore, this study aimed to determine the dynamics of substrate utilisation as a function of body composition using a submaximal graded treadmill exercise with intensity ranging 30–60% of . From the available literature, it can be hypothesised that substrate oxidation would be characterised by high inter-individual variability, and that fat oxidation would be maximal at intensities 40–60%, with no differences between women of different body composition. Increasing our understanding of the responses to exercise intensity could produce instrumental information destined to clinicians, practitioners and those working in the health and fitness industry.
Materials & methods
Participants
Thirty-five healthy female participants were recruited for this study in the local community through the use of advertisements and e-mails (Table 1). Prior to inclusion, participants were informed of the procedures and risks of the study, screened for exclusion criteria (peri- or postmenopausal, currently smoking, pregnant or breast feeding, taking any prescribed medications which may affect heart rate or fat oxidation and excluding the oral contraceptive pill, diabetic, suffering from asthma) and provided written consent. Ethical clearance for this project was granted by the University of the Sunshine Coast ethics committee on human research (project S/11/317).
Table 1. Participants’ characteristics.
Resting measures of anthropometry and spirometry, and maximum cardiorespiratory and blood lactate measures during the incremental test to exhaustion, of participants classified according to BMI groups.
| Normal | Overweight | Obese | ANOVA | ||
|---|---|---|---|---|---|
| <24.9 kg·m2 (n = 15) | 25–29.9 kg·m2 (n = 13) | >30 kg·m2 (n = 7) | p | η2p | |
| Resting measures | |||||
| Age (yr) | 28.3 ± 10.1 | 33.4 ± 8.9 | 31.6 ± 8.8 | 0.362 | 0.062 |
| Height (m) | 1.65 ± 0.05 | 1.65 ± 0.05 | 1.68 ± 0.06 | 0.484 | 0.044 |
| Weight (kg) | 60.9 ± 7.8 | 72.3 ± 3.5 A C | 104.4 ± 18.4 B | <0.001 | 0.751 |
| W:H ratio | 0.74 ± 0.04 | 0.77 ± 0.05 | 0.78 ± 0.04 | 0.065 | 0.166 |
| Trunk fat (kg) | 5.0 ± 2.5 | 8.1 ± 2.2 A C | 18.7 ± 5.2 B | <0.001 | 0.760 |
| Body fat (kg) | 17.6 ± 5.7 | 25.6 ± 4.0 A C | 51.6 ± 10.2 B | <0.001 | 0.798 |
| Body fat (%) | 30.0 ± 7.0 | 36.8 ± 5.3 A C | 51.2 ± 2.5 B | <0.001 | 0.660 |
| LBM (kg) | 40.2 ± 4.3 | 43.9 ± 4.2 | 49.1 ± 8.5 A | 0.020 | 0.230 |
| Glucose (mmol·L-1) | 5.19 ± 0.49 | 5.32 ± 0.44 | 5.50 ± 0.41 | 0.348 | 0.064 |
| Cholesterol (mmol·L-1) | 4.33 ± 0.72 | 4.38 ± 0.55 | 4.44 ± 1.0 | 0.946 | 0.003 |
| FVC (L) | 3.79 ± 0.39 | 3.81 ± 0.47 | 3.76 ± 0.70 | 0.989 | 0.001 |
| FEV1 (%) | 84.3 ± 6.3 | 83.4 ± 5.9 | 84.1 ± 2.1 | 0.973 | 0.002 |
| Incremental test | |||||
| HR (bpm) | 188 ± 9 | 185 ± 7 | 187 ± 7 | 0.451 | 0.049 |
| [La] (mmol·L-1) | 11.0 ± 1.7 | 9.5 ± 2.1 | 8.4 ± 2.8 A | 0.026 | 0.205 |
| VO2MAX (L·min-1) | 2.34 ± 0.43 | 2.45 ± 0.49 | 2.46 ± 0.46 | 0.791 | 0.015 |
| VO2MAX (ml·kg·min-1) | 38.2 ± 7.2 | 33.3 ± 5.9 D | 23.8 ± 3.7 B | <0.001 | 0.451 |
| VEMAX (L·min-1) | 91.9 ± 13.9 | 93.3 ± 14.5 | 86.9 ± 13.1 | 0.613 | 0.030 |
| RER | 1.17 ± 0.06 | 1.16 ± 0.05 | 1.13 ± 0.08 | 0.397 | 0.056 |
Data are mean ± SD
A p < 0.05 from BMI <24.9 kg·m2
B p < 0.001 from BMI <24.9 kg·m2
C p < 0.001 from BMI >30 kg·m2
D p < 0.05 from BMI >30 kg·m2
Abbreviations used: W:H (waist-to-hip); LBM (lean body mass); FVC (forced vital capacity); FEV1 (forced expiratory volume in 1 s); HR (heart rate), [La] (blood lactate concentration), VO2MAX (maximum oxygen uptake), VEMAX (maximum minute ventilation), RER (respiratory exchange ratio).
Participants reported to the Sunshine Coast University’s exercise physiology laboratory initially for additional screening of medical history (Medical Health Questionnaires), resting measures of pulmonary function using spirometry with forced vital capacity (FVC) and forced expiratory volume (FEV1) manoeuvres, and for assessment of anthropometric characteristics of participants (height, weight, Body Mass Index [BMI; body weight divided by height squared], hip and waist circumferences, waist-to-hip ratio [W:H]), body composition (total and regional body fat, lean body mass [LBM], percentage body fat, bone mineral density and bone mineral content using dual-energy x-ray absorptiometry [DEXA; Lunar Prodigy Advance, GE Healthcare, Buckinghamshire, UK]).
Overview
This research project consisted of two testing sessions, with at least three days between sessions to minimise the effects of fatigue effects between tests. For both sessions, participants reported to the laboratory after a 10–12 hour overnight fast and no later than 10 am. Blood glucose (Accu-Chek Advantage, Roche Diagnostics, Indianapolis, IN, USA) and cholesterol (Cholestech LDX, Hayward, CA, USA), both measured in millimoles per litre (mmol·L-1), were collected using a small sample of capillary blood obtained at the fingertip while the participant was in a fasted state to ensure results were not affected by food ingestion. Exercise was performed using a treadmill (TMX425C, Trackmaster, Full Vision Inc., Newton, KS).
At the first session, participants performed an incremental exercise test to volitional exhaustion to determine workloads associated with ~30%, 40%, 50% and 60% of . After a two minute warm-up walking at 4 km·h-1 or 6 km·h-1 (depending on the participant’s exercise history and physical characteristics), speed was increased every minute until the participant signalled they had reached a comfortable speed, whereupon the grade of the treadmill was increased by 1–2% every minute until exercise termination. At the second session, participants performed a graded submaximal test with five, 3-min stages (4.5 km·h-1 baseline, and treadmill speed×gradient workloads eliciting ~30%, 40%, 50% and 60% of ) interspersed with a short rest between stages to allow for the measure of blood lactate. The results of the initial baseline exercise stage are considered as a warmup and are not presented in the results. Although three minute stages may slightly overestimate fat oxidation [39], they have been used in previous research [23, 28] and permit minimising the carryover effects of fatigue.
Measurements
Ratings of perceived exertion (RPE) were determined at each stage of the graded submaximal test using the 6–20 linear Borg scale. Heart rate (HR) was measured during the incremental test and submaximal graded test at 0.2 Hz using a chest strap (Polar Electro, Kempele, Finland). Blood lactate concentration ([La]) was measured at the fingertip two minutes after exercise termination (session 1) and after each stage (session 2) using a portable analyser (Arkray, Lactate Pro, Kyoto, Japan).
For both tests, the rate of oxygen utilization (), carbon dioxide production (), minute ventilation (), and the respiratory exchange ratio (RER = ) were measured using a two-way, non-rebreathing valve (series 2700, Hans-Rudolph, Kansas City, USA) and an automated open-circuit spirometry metabolic analysis system (True One 2400, Parvo Medics, Sandy UT) with data averaged over 15-s periods. The highest value of was used as provided two or more of the following criteria were met: 1) <2.1 ml·kg-1·min-1 increase in with increasing workload, 2) [La] ≥8 mmol·l-1, 3) maximum HR within 10 bpm of their age-predicted maximum HR (based on 220-age), and 4) RER >1.10. All equipments were initially calibrated following standard procedures.
For each intensity of the graded submaximal exercise, absolute (g·min-1) and relative (i.e. scaled for LBM: mg·kg LBM-1·min-1) rates of fat (OXFAT) and carbohydrate oxidation (OXCHO) were calculated during the last 60-s of each stage using stoichiometric equations [40], based on the assumption that the excretion of urinary nitrogen was negligible. Peak OXFAT is abbreviated as PFO in the following analysis as is customary in the specific literature. Crossover points [41] for each groups were estimated after calculating absolute (kcal·min-1) and relative (%) contributions of fat (EEFAT) and carbohydrate oxidation (EECHO) to total energy expenditure (EE) using Atwater factors for fat (9 kcal·g-1) and carbohydrate (4 kcal·g-1).
Statistical analyses
Participants were classified into three groups based on BMI (normal weight (NOR) ≤24.9 kg·m2; overweight (OVW) 25–29.9 kg·m2; obese (OBE) ≥30 kg·m2). For comparison purposes, participants were also grouped into tertiles of percentage of body fat. Contingency tables showed strong association (p<0.001) between the two approaches and BMI groups were used for analyses.
Statistical tests were performed using the open-access statistical package jamovi [42], with data expressed as mean ± standard deviation (SD), and the level of significance set at p<0.05. An a-priori sample-size analysis revealed the study design could reliably detect significant differences between independent groups of n = 7 with moderate to large effect sizes (d>0.5) with a probability greater than 0.8, assuming a two-sided criterion allowing for a maximum Type I error rate of α = 0.05. Differences between groups (NOR, OVW, OBE) in resting characteristics (anthropometry, body composition, blood glucose and cholesterol, spirometry), in cardiorespiratory and [La] measures during the incremental test, in absolute and relative PFO and crossover points during the submaximal graded test, were determined using one-way ANOVAs with Bonferroni post-hoc tests. The effect of exercise intensity (30%, 40%, 50% and 60%), BMI group (NOR, OVW, OBE) and their interaction (intensity×group) on RPE, [La], absolute and relative OXFAT were assessed using 2-way, repeated measures ANOVAs, with Bonferroni post-hoc tests. Pearson’s r was used to assess relationships between PFO (absolute and relative), age, body fat, LBM and absolute . To identify the main predictors of substrate utilisation, a multiple linear regression was then performed between absolute OXFAT and covariates age, body fat, LBM, and absolute . For ANOVAs, asssumptions of equality of variances were initially checked using Levene’s test, and assumptions of sphericity were checked using Mauchly’s W with Greenhouse-Geisser corrections if significant. For correlations, assumptions of normality were initially checked. We reported effect sizes for ANOVAs using partial eta-squared (η2p) interpreted according to Cohen’s scale (small effect: 0.01<η2p<0.06, medium effect: 0.06<η2p<0.14, and large effect: η2p>0.14).
Results
All participants completed the study procedures. There were no significant differences between participant groups in age, height, and pulmonary function (Table 1). Participants in OVW group were characterised by higher weight, body and trunk fat compared to NOR (Table 1). Participants in OBE group were characterised by higher weight, body fat and trunk fat compared to both OVW and NOR, and higher LBM compared to NOR only (Table 1). During the incremental test, participants in OBE group had significantly lower maximum [La] concentrations compared to NOR, and significantly lower relative compared to NOR and OVW (Table 1). There were no other significant differences in maximum absolute , VE or HR across participant groups (Table 1).
Speed, gradient, RPE, blood [La] and absolute and relative OXFAT measured during each stage of the submaximal test are provided in Table 2. There was a significant and large main effect of exercise on RPE (p<0.001; η2p = 0.803), with no effect of group (p = 0.799; η2p = 0.014) or interaction effects (p = 0.728; η2p = 0.036). There was also a significant and large main effect of exercise on [La] (p<0.001; η2p = 0.490), with no group (p = 0.560; η2p = 0.036) or interaction effects (p = 0.206; η2p = 0.083). For absolute PFO, there were no significant effects or exercise (p = 0.522; η2p = 0.026), group (p = 0.124; η2p = 0.139) or interaction (p = 0.861; η2p = 0.029). For relative OXFAT, there were also no significant effects of exercise (p = 0.765; η2p = 0.015), group (p = 0.162; η2p = 0.131) or interaction (p = 0.998; η2p = 0.006). PFO occurred at 50%, 40% and 50% for NOR, OVW and OBE groups, respectively, with no statistically significant effect of group for absolute (p = 0.113; η2p = 0.127) or relative measures (p = 0.283; η2p = 0.081).
Table 2. Physiological responses during the submaximal graded test.
Speed, gradient, ratings of perceived exertion, blood lactate concentration and absolute and relative substrate oxidation of participants classified according to BMI groups.
| Normal | Overweight | Obese | |
|---|---|---|---|
| <24.9 kg·m2 (n = 15) | 25–29.9 kg·m2 (n = 13) | >30 kg·m2 (n = 7) | |
| Speed (km·hr-1) | |||
| 30%VO2MAX | 4.6 ± 1.0 | 4.0 ± 0.8 | 2.9 ± 0.5 |
| 40%VO2MAX | 5.6 ± 0.9 | 5.1 ± 0.8 | 3.7 ± 1.0 |
| 50%VO2MAX | 6.6 ± 1.0 | 6.1 ± 0.9 | 4.9 ± 0.7 |
| 60%VO2MAX | 7.5 ± 1.2 | 6.9 ± 0.7 | 5.7 ± 0.6 |
| Gradient (%) | |||
| 30%VO2MAX | 0.5 ± 0 | 0.5 ± 0 | 0.5 ± 0 |
| 40%VO2MAX | 0.5 ± 0 | 0.5 ± 0 | 0.5 ± 0 |
| 50%VO2MAX | 0.7 ± 0.5 | 0.5 ± 0 | 0.5 ± 0 |
| 60%VO2MAX | 1.1 ± 1.4 | 1.0 ± 0.9 | 0.5 ± 0 |
| RPE | |||
| 30%VO2MAX | 7.2 ± 1.4 | 7.3 ± 1.8 | 6.1 ± 0.4 |
| 40%VO2MAX | 9.0 ± 2.2 | 9.3 ± 2.0 | 8.3 ± 1.9 |
| 50%VO2MAX | 11.0 ± 2.0 | 11.1 ± 2.1 | 10.9 ± 2.4 |
| 60%VO2MAX | 12.7 ± 2.2 | 12.9 ± 2.4 | 13.5 ± 1.4 |
| [La] (mmol·L-1) | |||
| 30%VO2MAX | 1.3 ± 0.3 | 1.3 ± 0.4 | 1.5 ± 0.7 |
| 40%VO2MAX | 1.4 ± 0.4 | 1.4 ± 0.4 | 1.5 ± 0.7 |
| 50%VO2MAX | 2.0 ± 0.6 | 1.6 ± 0.6 | 1.7 ± 1.0 |
| 60%VO2MAX | 2.9 ± 0.9 | 2.5 ± 1.0 | 2.2 ± 1.3 |
| OXFAT (g·min-1) | |||
| 30%VO2MAX | 0.301 ± 0.093 | 0.262 ± 0.093 | 0.322 ± 0.103 |
| 40%VO2MAX | 0.312 ± 0.103 | 0.272 ± 0.106 | 0.359 ± 0.072 |
| 50%VO2MAX | 0.319 ± 0.087 | 0.254 ± 0.133 | 0.387 ± 0.032 |
| 60%VO2MAX | 0.298 ± 0.145 | 0.258 ± 0.206 | 0.379 ± 0.112 |
| OXFAT (mg·kg LBM-1·min-1) | |||
| 30%VO2MAX | 7.41 ± 2.08 | 5.94 ± 2.00 | 6.69 ± 1.86 |
| 40%VO2MAX | 7.71 ± 2.49 | 6.20 ± 2.17 | 7.28 ± 1.67 |
| 50%VO2MAX | 7.86 ± 1.91 | 5.60 ± 2.90 | 8.25 ± 1.73 |
| 60%VO2MAX | 6.84 ± 3.75 | 5.90 ± 4.42 | 8.05 ± 2.11 |
Data is displayed as mean ± SD
Abbreviations used: RPE (ratings of perceived exertion), [La] (blood lactate concentration), OXFAT (fat oxidation), EEFAT (relative contribution of fat oxidation to total energy expenditure)
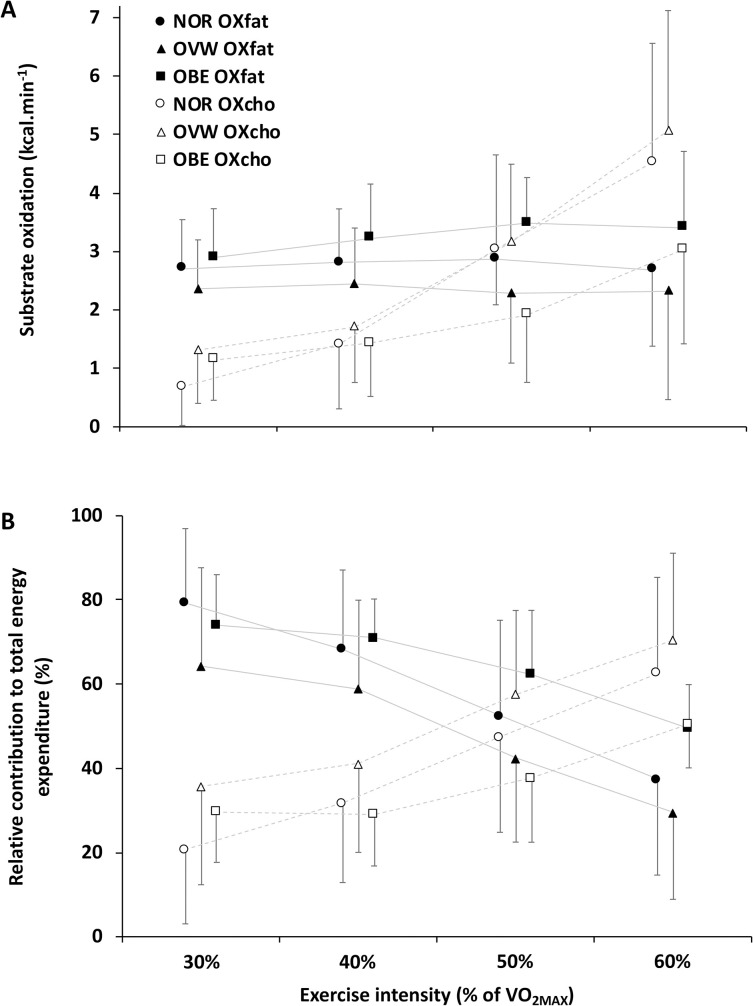
Absolute (kcal·min-1) and relative (%) EEFAT and EECHO are presented (Fig 1). Crossover point was estimated to occur at 50.6±6.8%, 48.3±8.2% and 55.1±8.2% of for NOR, OVW and OBE groups, respectively, with no statistically significant differences across groups (p = 0.226; η2p = 0.108).
Fig 1. Substrate oxidation and energy expenditure.
Absolute substrate oxidation (panel A) and relative contributions to total energy expenditure (panel B) for fat (OXFAT) and carbohydrate oxidation (OXCHO) during the submaximal graded test of participants classified according to BMI groups (NOR <24.9 kg·m2; OVW = 25–29.9 kg·m2; OBE >30 kg·m2).
For absolute PFO, correlations were statistically significant with absolute (r = 0.490; p<0.001), and LBM (r = 0.374; p = 0.032), but not with age (r = -0.162; p = 0.353) and fat mass (r = 0.190; p = 0.290). For relative PFO, correlations were not statistically significant with absolute (r = 0.219; p = 0.221), age (r = -0.172; p = 0.339), LBM (r = -0.047; p = 0.797) and fat mass (r = -0.049; p = 0.785). Significant relationships were also observed between age and absolute (r = -0.409; p = 0.015) and body fat (r = -0.791; p<0.001). The multiple linear regression for absolute peak OXFAT with the covariates absolute (standardised estimate [SE] = 0.588; p = 0.039), age (SE = 0.006; p = 0.997), body fat (SE = -0.158; p = 0.615) and LBM (SE = 0.291; p = 0.228) was not significant (p = 0.905; r = 0.529, R2 = 0.280).
Discussion
The aim of this study was to compare substrate utilization during submaximal treadmill exercise in women classified as normal, overweight and obese BMI. The main finding of this project was that there were no differences in fat oxidation in 35 women of normal, overweight and obese BMI, aged 18–50 years, with no underlying health conditions.
In agreement with previous literature [23], we observed very large inter-individual variation in substrate oxidation rates (both absolute and relative to lean body mass) independent of body composition in a graded exercise ranging 30–60% of maximum aerobic capacity. While no previous study has specifically compared PFO between normal, overweight and obese women 18–50 years of age, our results are consistent with the absolute [11, 38] and relative fat oxidation rates [23] observed in similar participant groups independently. Similarly, substrate oxidation relative to total energy expenditure was not different across groups of BMI, albeit with important inter-individual variability. Furthermore, while the relative contribution of fat oxidation to total energy expenditure decreased after 50% in all groups, absolute fat oxidation remained unaffected by exercise intensity, including at 60%.
A significant strength of the current study is the assessment of body composition using DEXA to ensure all participants were appropriately grouped into the correct BMI category: while average age and height of each BMI category were not different across groups, weight was significantly greater in OVW compared to NOR, and greater in OBE compared to OVW. In agreement with previous research [11], we also measured higher body fat (absolute and relative) in OBE compared to NOR, and in OVE compared to NOR. We also measured higher trunk fat in OBE compared to NOR, and in OVE compared to NOR, although W:H ratio was not significantly different across groups. Importantly, both body fat and trunk fat are associated with increase the risk people to develop further health problems such as metabolic syndrome and cardiovascular disease [13].
In this study, simple linear regressions indicate that the main determinants of absolute PFO were and LBM, although the regression model failed to reach significance. While was negatively correlated with age and body fat, we did not find age to be a significant predictor of OXFAT during exercise, which could differ from conclusions drawn from OXFAT measured at rest [43]. We also measured no differences between groups in blood glucose and cholesterol at rest, or differences between groups in blood lactate concentration or in ratings of perceived exertion during the graded treadmill exercise, despite differences in relative and lactate concentration during the incremental test. As such, our results confirm the exercise was truly submaximal, and are consistent with recent literature having determined that aerobic fitness (here represented as ) is a greater contributor to PFO than dietary intake [44].
The findings of the current study are therefore in agreement with an existing body of literature underscoring that marked inter-individual variations in fat oxidation exist between healthy women of different body composition, and that establishing a substrate oxidation profile by increasing the number of exercise intensities tested did no modify the expected outcomes that can be inferred from the literature. Further, low power correlations existed between fat oxidation and typical predictors of cardiorespiratory fitness. Lastly, exercise intensity had no significant bearing on fat oxidation and therefore, exercise prescription should be individualised and likely be based on considerations other than substrate oxidation (at least during submaximal exercise). As such, the popularity of high-intensity interval exercise provides interesting avenues for further research in substrate oxidation as a function of body composition, especially considering the effect of exercise intensity of post-exercise energy consumption and thermogenesis.
It is important to note that our study had four main limitations. The first limitation is that menstrual phase was not accounted for in the present investigation, however, all participants were required to have regular menstruation and not present with any symptoms suggesting menopause. Menstrual phase is a contentious issue regarding substrate utilisation, with conflicting findings being reported throughout the literature, and should be further investigated. The second limitation is that the obese BMI group counted only 7 women in the current study, which could have blunted true population variance. However, inter-individual variations were similar across the three BMI groups accounting for 35 women in total, and we measured high association between groups of BMI and participants grouped by tertiles of body mass. Therefore, and in agreement with previous studies using constant work rates in independent groups, it is unlikely that increasing our participant pool would have affected our main finding. Thirdly, the graded submaximal test used a warmup stage followed by consecutive stages of increasing difficulty as priming exercises to reach steady state as quickly as possible. Although we mitigated the risk of fatigue carryover between stages by including a rest period between each stage (all inferior to 2-min), it is possible that the later stages were affected by exercise-induced fatigue. Additionally, there is evidence 3-min tests may be too short in very sedentary participants to establish a reliable measure of fat oxidation [39]. Nonetheless, the graded test was tolerated by all participants, performed at truly submaximal intensities, and all our results are consistent with previous literature. Still, randomized intensity stages may be a useful addition to future studies using graded tests for similar purposes. Lastly, we did not quantitatively control for habitual diet and physical activity before the submaximal test, which are factors known to affect measures of macronutrient contribution to total energy energy expenditure to some extent [12].
Conclusions
In agreement with the literature, this study reports that there were no differences in fat oxidation (absolute and relative to lean body mass) during submaximal exercise performed in a fasted state in women differing in body composition, with peak fat oxidation occurring at 40–50% and crossover point estimated at 48–55% of . Therefore, specific exercise intensities are not influenced by body composition and our findings do not support the fat oxidation hypothesis. Appropriate exercise prescription should emphasize individualizing testing responses and likely be based on considerations other than substrate oxidation for efficient weight management.
Supporting information
(TXT)
(TXT)
Acknowledgments
The authors would like to thank all the participants for their effort and valuable time, and the university’s laboratory technicians (Stephen Bishop, Alysha Hyde, Darren Morrow, Ambrose Molinaro and Ava Kerr) for their technical support and expertise.
Data Availability
The data underlying the results presented in the study are available at the following address: doi.org/10.6084/m9.figshare.12783347.v1doi.org/10.6084/m9.figshare.13043009.v1.
Funding Statement
This study has received no specific funding.
References
- 1.World Health Organization. Global Health Observatory (GHO) data. Overweight and obesity. 2015. [Google Scholar]
- 2.Nguyen DM, El-Serag HB. The epidemiology of obesity. Gastroenterology Clinics of North America. 2010;39(1):1–7. 10.1016/j.gtc.2009.12.014 PubMed Central PMCID: PMC2833287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Field AE, Coakley EH, Must A, Spadano JL, Laird N, Dietz WH, et al. Impact of overweight on the risk of developing common chronic diseases during a 10-year period. Archives of Internal Medicine. 2001;161(13):1581–6. 10.1001/archinte.161.13.1581 [DOI] [PubMed] [Google Scholar]
- 4.Adams KF, Schatzkin A, Harris TB, Kipnis V, Mouw T, Ballard-Barbash R, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. The New England Journal of Medicine. 2006;355(8):763–78. 10.1056/NEJMoa055643 [DOI] [PubMed] [Google Scholar]
- 5.Wei M, Kampert JB, Barlow CE, Nichaman MZ, Gibbons LW, Paffenbarger J, et al. Relationship between low cardiorespiratory fitness and mortality in normal-weight, overweight, and obese men. JAMA. 1999;282(16):1547–53. 10.1001/jama.282.16.1547 [DOI] [PubMed] [Google Scholar]
- 6.Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. 2013;309(1):71–82. 10.1001/jama.2012.113905 PubMed Central PMCID: PMC4855514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colberg SR, Simoneau J-A, Thaete FL, Kelley DE. Skeletal muscle utilization of free fatty acids in women with visceral obesity. Journal of Clinical Investigation. 1995;95(4):1846–53. 10.1172/JCI117864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelley DE, Goodpaster BH, Wing RR, Simoneau J-A. Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. American Journal of Physiology—Endocrinology And Metabolism. 1999;277(6 pt. 1):E1130–41. [DOI] [PubMed] [Google Scholar]
- 9.Kelley DE, Williams KV, Price JC, McKolanis TM, Goodpaster BH, Thaete FL. Plasma fatty acids, adiposity, and variance of skeletal muscle insulin resistance in type 2 diabetes mellitus. The Journal of Clinical Endocrinology & Metabolism. 2001;86(11):5412–9. 10.1210/jcem.86.11.8027 [DOI] [PubMed] [Google Scholar]
- 10.Achten J, Jeukendrup AE. Optimizing fat oxidation through exercise and diet. Nutrition. 2004;20(7–8):716–27. 10.1016/j.nut.2004.04.005 [DOI] [PubMed] [Google Scholar]
- 11.Lazzer S, Busti C, Agosti F, De Col A, Pozzo R, Sartorio A. Optimizing fat oxidation through exercise in severely obese Caucasian adolescents. Clinical Endocrinology. 2007;36(4):582–8. 10.1111/j.1365-2265.2007.02929.x [DOI] [PubMed] [Google Scholar]
- 12.Péronnet F, Haman F. Low capacity to oxidize fat and body weight. Obesity Reviews. 2019;20(10):1367–83. 10.1111/obr.12910 [DOI] [PubMed] [Google Scholar]
- 13.Pescatello LS, Arena R, Riebe D, Thompson PD. ACSM's guidelines for exercise testing and prescription. 9th edition ed Medicine ACoS, editor. Baltimore, MD: Lippincott Williams & Wilkins; 2014. [Google Scholar]
- 14.Bogdanis GC, Vangelakoudi A, Maridaki M. Peak fat oxidation rate during walking in sedentary overweight men and women. Journal of Sports Science and Medicine. 2008;7(4):525–31. PubMed Central PMCID: PMC3761910. [PMC free article] [PubMed] [Google Scholar]
- 15.Bircher S, Knechtle B. Relationship between fat oxidation and lactate threshold in athletes and obese women and men. Journal of Sports Science and Medicine. 2004;3(3):174–81. PubMed Central PMCID: PMC3905300. [PMC free article] [PubMed] [Google Scholar]
- 16.Tunstall RJ, Mehan KA, Wadley GD, Collier GR, Bonen A, Hargreaves M, et al. Exercise training increases lipid metabolism gene expression in human skeletal muscle. American Journal of Physiology-Endocrinology and Metabolism. 2002;283(1):E66–E72. 10.1152/ajpendo.00475.2001 . [DOI] [PubMed] [Google Scholar]
- 17.Hurley BF, Nemeth PM, Martin WHr, Hagberg JM, Dalsky GP, Holloszy JO. Muscle triglyceride utilization during exercise: effect of training. Journal of Applied Physiology. 1986;60(2):562–7. 10.1152/jappl.1986.60.2.562 . [DOI] [PubMed] [Google Scholar]
- 18.Martin WHr Dalsky GP, Hurley BF Matthews DE, Bier DM Hagberg JM, et al. Effect of endurance training on plasma free fatty acid turnover and oxidation during exercise. American Journal of Physiology-Endocrinology and Metabolism. 1993;265(5):E708–E14. 10.1152/ajpendo.1993.265.5.E708 . [DOI] [PubMed] [Google Scholar]
- 19.Phillips SM, Green HJ, Tarnopolsky MA, Heigenhauser GJF, Hill RE, Grant SM. Effects of training duration on substrate turnover and oxidation during exercise. Journal of Applied Physiology. 1996;81(5):2182–91. 10.1152/jappl.1996.81.5.2182 . [DOI] [PubMed] [Google Scholar]
- 20.van Aggel-Leijssen DPC, Saris WHM, Wagenmakers AJM, Senden JM, Van Baak MA. Effect of exercise training at different intensities on fat metabolism of obese men. Journal of Applied Physiology. 2002;92(3):1300–9. 10.1152/japplphysiol.00030.2001 . [DOI] [PubMed] [Google Scholar]
- 21.Friedlander AL, Casazza GA, Horning MA, Buddinger TF, Brooks GA. Effects of exercise intensity and training on lipid metabolism in young women. American Journal of Physiology-Endocrinology and Metabolism. 1998;275(5):E853–E63. 10.1152/ajpendo.1998.275.5.E853 . [DOI] [PubMed] [Google Scholar]
- 22.van Aggel-Leijssen DP, Saris WH, Wagenmakers AJ, Hul GB, van Baak MA. The effect of low-intensity exercise training on fat metabolism of obese women. Obesity Research. 2001;9(2):86–96. 10.1038/oby.2001.11 [DOI] [PubMed] [Google Scholar]
- 23.Venables MC, Achten J, Jeukendrup AE. Determinants of fat oxidation during exercise in healthy men and women: a cross-sectional study. Journal of Applied Physiology. 2005;98(1):160–7. 10.1152/japplphysiol.00662.2003 [DOI] [PubMed] [Google Scholar]
- 24.Riddell MC, Jamnik VK, Iscoe KE, Timmons BW, Gledhill N. Fat oxidation rate and the exercise intensity that elicits maximal fat oxidation decreases with pubertal status in young male subjects. Journal of Applied Physiology. 2008;105(2):742–8. 10.1152/japplphysiol.01256.2007 . [DOI] [PubMed] [Google Scholar]
- 25.Holloszy JO, Coyle EF. Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. Journal of Applied Physiology. 1984;56(4):831–8. 10.1152/jappl.1984.56.4.831 . [DOI] [PubMed] [Google Scholar]
- 26.Melanson EL, Sharp TA, Schneider J, Donahoo WT, Grunwald GK, Hill JO. Relation between calcium intake and fat oxidation in adult humans. International Journal of Obesity. 2003;27(2):196–203. 10.1038/sj.ijo.802202 [DOI] [PubMed] [Google Scholar]
- 27.Romijn JA, Coyle EF, Sidossis LS, Gastaldelli A, Horowitz JF, Endert E, et al. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. American Journal of Physiology-Endocrinology and Metabolism. 1993;265(3):E380–E91. 10.1152/ajpendo.1993.265.3.E380 . [DOI] [PubMed] [Google Scholar]
- 28.Achten J, Venables MC, Jeukendrup AE. Fat oxidation rates are higher during running compared with cycling over a wide range of intensities. Metabolism—Clinical and Experimental. 2003;52(6):747–52. 10.1016/s0026-0495(03)00068-4 [DOI] [PubMed] [Google Scholar]
- 29.Woodward M. Cardiovascular Disease and the Female Disadvantage. International Journal of Environmental Research and Public Health. 2019;16(7):1165 10.3390/ijerph16071165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kerhervé HA, Harvey LM, Eagles AN, McLellan C, Lovell D. Body composition influences blood pressure during submaximal graded test in women. Obesity Research & Clinical Practice. 2020;In Press. 10.1016/j.orcp.2020.08.008 [DOI] [PubMed] [Google Scholar]
- 31.Hickner RC, Privette J, McIver K, Barakat H. Fatty acid oxidation in African-American and Caucasian women during physical activity. Journal of Applied Physiology. 2001;90(6):2319–24. 10.1152/jappl.2001.90.6.2319 . [DOI] [PubMed] [Google Scholar]
- 32.Devries MC, Samjoo IA, Hamadeh MJ, McCready C, Raha S, Watt MJ, et al. Endurance Training Modulates Intramyocellular Lipid Compartmentalization and Morphology in Skeletal Muscle of Lean and Obese Women. The Journal of Clinical Endocrinology & Metabolism. 2013;98(12):4852–62. 10.1210/jc.2013-2044 [DOI] [PubMed] [Google Scholar]
- 33.Ezell DM, Geiselman PJ, Anderson AM, Dowdy ML, Womble LG, Greenway FL, et al. Substrate oxidation and availability during acute exercise in non‐obese, obese, and post‐obese sedentary females. International Journal of Obesity. 1999;23(10):1047–56. 10.1038/sj.ijo.0801037 [DOI] [PubMed] [Google Scholar]
- 34.Kanaley JA, Cryer PE, Jensen MD. Fatty acid kinetic responses to exercise. Effects of obesity, body fat distribution, and energy-restricted diet. J Clin Invest. 1993;92(1):255–61. 10.1172/JCI116559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thyfault JP, Kraus RM, Hickner RC, Howell AW, Wolfe RR, Dohm GL. Impaired plasma fatty acid oxidation in extremely obese women. American Journal of Physiology-Endocrinology and Metabolism. 2004;287(6):E1076–E81. 10.1152/ajpendo.00177.2004 . [DOI] [PubMed] [Google Scholar]
- 36.Horowitz JF, Klein S. Oxidation of nonplasma fatty acids during exercise is increased in women with abdominal obesity. Journal of Applied Physiology. 2000;89(6):2276–82. 10.1152/jappl.2000.89.6.2276 . [DOI] [PubMed] [Google Scholar]
- 37.Kanaley J, Weatherup-Dentes M, Alvarado C, Whitehead G. Substrate oxidation during acute exercise and with exercise training in lean and obese women. European Journal of Applied Physiology. 2001;85(1):68–73. 10.1007/s004210100404 [DOI] [PubMed] [Google Scholar]
- 38.Steffan HG, Elliott W, Miller WC, Fernhall B. Substrate utilization during submaximal exercise in obese and normal-weight women. European Journal of Applied Physiology and Occupational Physiology. 1999;80(3):233–9. 10.1007/s004210050587 [DOI] [PubMed] [Google Scholar]
- 39.Bordenave S, Flavier S, Fédou C, Brun JF, Mercier J. Exercise calorimetry in sedentary patients: procedures based on short 3 min steps underestimate carbohydrate oxidation and overestimate lipid oxidation. Diabetes & Metabolism. 2007;33(5):379–84. 10.1016/j.diabet.2007.04.003 [DOI] [PubMed] [Google Scholar]
- 40.Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. Journal of Applied Physiology. 1983;55(2):628–34. 10.1152/jappl.1983.55.2.628 [DOI] [PubMed] [Google Scholar]
- 41.Brooks GA, Mercier J. Balance of carbohydrate and lipid utilization during exercise: the "crossover" concept. Journal of Applied Physiology. 1994;76(6):2253–61. 10.1152/jappl.1994.76.6.2253 . [DOI] [PubMed] [Google Scholar]
- 42.jamovi project. jamovi (Version 0.9). Retrieved from https://www.jamovi.org2018.
- 43.Calles-Escandon J, Arciero PJ, Gardner AW, Bauman C, Poehlman ET. Basal fat oxidation decreases with aging in women. Journal of Applied Physiology. 1995;78(1):266–71. 10.1152/jappl.1995.78.1.266 . [DOI] [PubMed] [Google Scholar]
- 44.Fletcher G, Eves FF, Glover EI, Robinson SL, Vernooij CA, Thompson JL, et al. Dietary intake is independently associated with the maximal capacity for fat oxidation during exercise. American Journal of Clinical Nutrition. 2017;105(4):864–872. 10.3945/ajcn.116.133520 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TXT)
(TXT)
Data Availability Statement
The data underlying the results presented in the study are available at the following address: doi.org/10.6084/m9.figshare.12783347.v1doi.org/10.6084/m9.figshare.13043009.v1.