Abstract
Background
Despite decades of research, little clarity exists regarding pathogenic mechanisms related to schizophrenia. Investigations on the disease biology of schizophrenia have primarily focused on neuronal alterations. However, there is substantial evidence pointing to a significant role for the brain's microvasculature in mediating neuroinflammation in schizophrenia.
Summary
Brain microvascular endothelial cells (BMEC) are a central element of the microvasculature that forms the blood-brain barrier (BBB) and shields the brain against toxins and immune cells via paracellular, transcellular, transporter, and extracellular matrix proteins. While evidence for BBB dysfunction exists in brain disorders, including schizophrenia, it is not known if BMEC themselves are functionally compromised and lead to BBB dysfunction.
Key Messages
Genome-wide association studies, postmortem investigations, and gene expression analyses have provided some insights into the role of the BBB in schizophrenia pathophysiology. However, there is a significant gap in our understanding of the role that BMEC play in BBB dysfunction. Recent advances differentiating human BMEC from induced pluripotent stem cells (iPSC) provide new avenues to examine the role of BMEC in BBB dysfunction in schizophrenia.
Keywords: Brain microvascular endothelial cells, Induced pluripotent stem cells, Paracellular junction, Efflux/influx transporter, Extracellular matrix, Schizophrenia
Introduction
Schizophrenia is a debilitating psychiatric disorder with complex genetic underpinnings as well as gene-environment interactions that play important roles in the disease process. Schizophrenia is characterized by positive symptoms such as hallucinations, delusions, and disorganized speech, by negative symptoms such as decreased motivation and decreased expression, and by cognitive deficits involving impaired executive functioning, memory, and processing speed [1], which together result in significant functional and social impairments. Current antipsychotic medications mainly target positive symptoms of psychosis, but they provide little relief from negative or cognitive symptoms for many patients [2]. Despite decades of research, little is known about the pathogenic mechanisms that lead to schizophrenia. Investigations have been hindered by our inability to access live brain tissue from patients for laboratory studies, especially before the development of disease progression, medication effects, and comorbidities [3, 4, 5]. Most theories and investigation on the pathophysiology of schizophrenia have been focused on neuronal differences. An early proponent of microvascular abnormalities in schizophrenia was Theodor Meynert [6], a prominent neuroanatomist who conducted pathological examinations of patient brains and proposed in 1884 that vasomotor problems depriving the brain of nutrients could be pathologic. In 1911, Eugene Bleuler, a psychiatrist recognized for coining the term “schizophrenia” [7], stated that “the fragility of the blood vessels which appears in many schizophrenics, both acute and chronic, seems to indicate a real vascular pathology” [8]. Later, Irving Gottesman, who pioneered studies of the genetic underpinnings of schizophrenia, proposed that damage to the cerebral microvasculature can result in a deteriorating course as seen in schizophrenia [9]. There has been increasing interest in the various facets of microvascular dysfunction in schizophrenia, including the role of early or late angiogenic abnormalities [10, 11], neurovascular unit (NVU) dysfunction [12, 13, 14] and blood-brain barrier (BBB) disruption [13, 14]. Here, we present a synthesis of the evidence and theories on the mechanistic underpinnings of brain microvascular endothelial cells (BMEC) in schizophrenia.
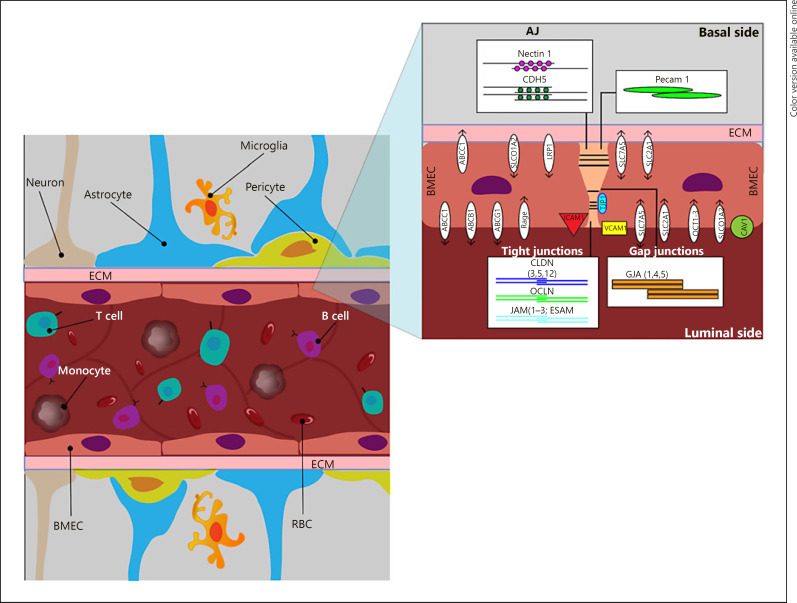
The BBB is composed of a monolayer of BMEC that line blood vessels which interact with neurons, microglia, pericytes, astrocytes, and the extracellular matrix (ECM) to form the NVU (Fig. 1). The NVU is dynamic and functions to regulate molecular and cellular trafficking between the bloodstream and brain parenchyma through physical, enzymatic, transport, and immunological processes that are vital for brain homeostasis. A core property of BMEC is the strict regulation of paracellular permeability due to junctional complexes (tight [TJ], adherens [AJ], and gap junctions [GJ]) between endothelial cells that limit passive diffusion. The BBB possess a high degree of trans-endothelial electrical resistance (TEER), which is a strong indicator of cellular barrier integrity and health [15, 16]. Regulation of brain parenchymal nourishment and waste removal is performed by selective BMEC transporters, which regulate signal transduction, endocytosis, transcytosis, and molecular transport through facilitated and active transporters [17]. Immune privilege in the brain is modulated by paracellular and transcellular proteins that assist with transmigration and extravasation of leukocytes during an immune response [17]. Maintenance of the BBB is also provided by ECM proteins, which hold the NVU together to prevent vulnerabilities in barrier function [17]. Lastly, an important vascular compartment linking the peripheral environment to the CNS is the choroid plexus, a region that is vulnerable to chronic stress and inflammatory responses leading to CNS disruption [10, 17].
Fig. 1.
Sagittal section of the NVU depicting BMEC and the proteins associated with paracellular, transcellular, transporter, and extracellular matrix function. Paracellular proteins: CLDN, OCLN, JAM, ESAM, TJP, NECTIN1, CDH5, GJA, and PECAM1. Transcellular proteins: ICAM1 and VCAM1. Transporter proteins: ABC, SLC, SLCO1A2, RAGE, LRP1, and CAV1. ECM proteins: consists of COL, FN1, and LAMA.
Convergent evidence for impaired microvascular function in schizophrenia arises from morphological, molecular, or physiological studies. In human studies, an increased CSF:serum albumin ratio in patients with schizophrenia suggests increased BBB permeability [18]. Postmortem brain studies show infiltration of CD3+ T lymphocytes and CD20+ B lymphocytes in the hippocampus, the frontal cortex, the thalamus, the medial temporal lobe, and the cingulate cortex in schizophrenia patients, which is indicative of BBB disruption [19]. In another postmortem study of hippocampal tissue, abnormally expressed immune and inflammatory pathway genes were found to be more likely to be present in BMEC [20]. Upregulation of immune and inflammatory genes in the choroid plexus of schizophrenia patients has been found to be correlated with peripheral inflammatory markers [21]. In support of this theory, we showed that the choroid plexus is enlarged in patients with psychosis and that this enlargement is related to increased plasma levels of the proinflammatory cytokine interleukin (IL)-6 [22]. However, hypoinflammatory states have also been proposed in schizophrenia, as evidenced by a downregulation of genes involved in cell adhesion, proliferation, and inflammation in BMEC located in the dorsolateral prefrontal cortex [23]. The evidence for BMEC transporters in schizophrenia is mixed. Levels of a major efflux transporter that carries antipsychotics out the brain, i.e., ABCB1, do not vary between schizophrenia patients and healthy control subjects [24], but there is increased ABCB1 activity in various brain regions [25]. In addition, differences in a variety of peripheral proinflammatory and angiogenic biomarkers have been implicated in different stages of psychosis [26, 27]. Taken together, these findings point towards a potential role for BMEC dysregulation in schizophrenia.
Human induced pluripotent stem cells (iPSC) provide us with the opportunity to develop physiologically relevant models of psychiatric disorders such as schizophrenia and bipolar disorder [28, 29, 30]. The use of iPSC-derived models to investigate schizophrenia pathophysiology has revealed disease-related changes in gene expression as well as deficits in specific neuronal subtypes [31, 32]. Recent technical advances that enable efficient differentiation of iPSC to BMEC provide an opportunity to culture and study BMEC with disease-specific genetic backgrounds. Here, we discuss the role of brain microvascular abnormalities in schizophrenia, focusing on BMEC paracellular and transcellular transport, as well as ECM dysfunction. We highlight existing models for studying iPSC-derived BMEC and discuss emerging approaches using 3-D and organ-on-a-chip models for investigating the role of the BBB in health and disease.
Brain Microvascular Abnormalities in Schizophrenia
BMEC Paracellular Function
Paracellular transport involves the transfer of substances from the blood across the endothelium via intercellular spaces. This process is controlled by a complex arrangement of TJ, GJ, and AJ that contribute to tissue integrity, barrier function, and cell-to-cell communication (Fig. 1).
The brain endothelial TJ are complex structures composed of multiple barriers and proteins involved in intracellular signaling. Claudins (CLDN), the primary sealing component of TJ, have tissue- or context-specific properties that can be expressed constitutively and/or through induction. CLDN5 is a major BBB component and it is linked to the cytoskeleton by zonula occludens family members (TJ protein [TJP]1–3). CLDN5 is regulated by WNT/β-catenin and it is crucial for angiogenesis and endothelial maintenance [17, 33]. CLDN3 and CLDN12 are also present in BMEC, but their role in BBB functioning is less clear; CLDN3, however, may be instrumental in maintaining the blood-CSF barrier in choroid plexus epithelial cells [34]. Occludin (OCLN) is involved in regulatory, rather than adhesive, functioning and interacts with CLDN-based strands and TJP1–3 to form the TJ complex [35]. A third complex connects TJ, allowing for regulation of leukocyte transmigration via endothelial cell-selective adhesion molecules (ESAM), junction adhesion molecules (JAM1–3), and nectins (NECTIN1) [17]. Lastly, tricellulin (MARVELD20) and lipolysis-stimulated lipoprotein receptors form tricellular junctions to stabilize junctional points between BMEC [16].
Gap junctions (GJ)are formed by members of the connexin family, which serve to connect the cytoplasms of 2 BMEC together and allow for the passage of various ions and small molecules (<1 kDa) [36]. GJ are crucial for intercellular communication, signal transduction, and hemichannel formation between neighboring cells. BMEC express GJA-1,-4,-5 [37].
AJ have a cytoplasmic aspect that links to the actin cytoskeleton to form TJ. BMEC-specific AJ include vascular endothelial (VE)-cadherin (CDH5) and N-cadherin (CDH2), which mediate pericyte and BMEC interactions [17]. AJ are responsible for cell adhesion, scaffolding, and regulation of out-in signaling [35]. Another AJ protein is platelet-endothelial cell adhesion molecule 1 (PECAM1), which makes up a large proportion of BMEC-specific intercellular junctions and is implicated in transendothelial migration [17].
BMEC Paracellular Abnormalities in Schizophrenia
TJ proteins involved in paracellular transport are vital for maintaining BMEC integrity. Several studies have described paracellular deficits in schizophrenia, particularly in CLDN5. For example, 22q11 deletion syndrome, which includes CLDN5 haploinsufficiency, confers a 30% risk of developing schizophrenia [38]. While 2 postmortem studies did not find significant differences in CLDN5 levels in schizophrenia [38, 39], 1 postmortem study reported a reduced CLDN5 expression [12]. Studies have also implicated CLDN5 in both BBB integrity and schizophrenia. A CLDN5 knockdown mouse model exhibited learning and memory impairment, anxiety-like behaviors, sensorimotor gating deficits, and contrast agent extravasation on MRI, suggesting that CLDN5 has a role in maintaining BBB integrity and schizophrenia phenotypes [38]. Dose-dependent increases in CLDN5 levels were observed with lithium or antipsychotics in rodent models, suggesting that the potential therapeutic effects of these agents may extend beyond neurotransmitter regulation [38]. In addition, a genetic variant of CLDN5 (rs10314) that results in a 50% reduction in BMEC-specific CLDN5 expression was found to be weakly associated with increased schizophrenia risk [38, 40, 41, 42, 43], though the association did not reach genome-wide significance (Table 1) [44, 45].
Table 1.
Polymorphisms related to BBB function as reported in the schizophrenia PGC database
| SNP | Gene | Function | Chromosome | A1 | A2 | OR | p value |
|---|---|---|---|---|---|---|---|
| rs10314 | CLDN5 | TJ | chr22 | C | G | 1.02 | 1.84E–01 |
| rs10791345 | JAM3 | TJ | chr11 | A | G | 0.95 | 4.10E–04 |
| rs55661361 | ESAM | TJ | chr11 | A | G | 0.92 | 3.68E–12 |
| rs989192 | GJA8 | GJ | chr1 | A | G | 1.01 | 4.85E–01 |
| rs4950495 | GJA8 | GJ | chr1 | T | C | 1.01 | 4.20E–01 |
| rs1045642 | ABCB1 | TPA | chr7 | A | G | 0.97 | 5.38E–03 |
| rs12814239 | LRP1 | TPA | chr12 | T | C | 0.86 | 1.48E–09 |
| rs324017 | LRP1 | TPA | chr12 | A | C | 0.94 | 2.13E–07 |
| rs11098403 | NDST3 | ECM | chr4 | A | G | 0.95 | 3.52E–05 |
| rs2445142 | HSPG2 | ECM | chr1 | C | G | 0.98 | 8.72E–02 |
Presented is a list of BBB gene polymorphisms that have been reported in smaller case-control studies, and those polymorphisms were cross-referenced with the findings described in the large schizophrenia PCG database. SNP, single-nucleotide polymorphism. Bold text indicates findings that are genome wide significant level.
CLDN3, a TJ protein, was found to be expressed at significantly higher levels in the frontal cortex of people with schizophrenia in a postmortem study, although there were no such differences for CLDN1, CLDN12, OCLN, MARVELD20, or CDH5 [39]. Higher CDH5 levels in the dorsolateral prefrontal cortex, however, have been described in schizophrenia [46]. These 2 studies used bulk cerebral tissue and did not differentiate between cell types (neuronal, glial, or endothelial) when determining differences in gene expression [39, 46]. Another postmortem study examining 15 cortical regions demonstrated that various critical BMEC genes, including CDH5 and PECAM1, were decreased in schizophrenia patients [12]. Pathway analysis of differentially expressed genes in schizophrenia has also shown them to be associated with downregulation of angiogenesis, migration, proliferation, and TJ and integrin signaling, as well as WNT, VEGF, IGF1, oncostatin M, angiopoietin, and ephrin receptor signaling [12, 47]. A small postmortem study in schizophrenia using laser capture dissection to measure gene expression in BMEC from the dorsolateral prefrontal cortex found a reduced expression of genes enriched in regulation of cell adhesion, the response to organic substance, and immune response [23].
ESAM plays an essential role in BBB permeability and leukocyte transmigration. A genome-wide association study (GWAS) testing the neuroimmune hypothesis in schizophrenia identified ESAM as an immune protein of interest from the 108 significant loci identified in the schizophrenia Psychiatric Genome Consortium (PGC) study (Table 1) [48]. In addition, de novo mutations resulting in a damaging frameshift deletion of the ESAM gene have been associated with schizophrenia [49, 50]. Since ESAM is normally expressed at lower levels in the temporal, occipital, and frontal lobes [48], we hypothesize that ESAM may play an important role in schizophrenia disease biology.
TJP1 is important for intercellular signal transduction and JAM3 is essential for cell-to-cell adhesion and limiting the passage of solutes and water through paracellular spaces [35].
In a GWAS examining treatment response in schizophrenia, researchers found a trio of TJP1 polymorphisms (i.e., rs711355, rs785423, and rs813676) to be associated with risperidone treatment efficacy [51]. A meta-analysis of JAM3 found that rs10791345 was associated with a significant risk of developing bipolar disorder [52]. A weak association has also been found between rs10791345 and an elevated risk of both bipolar disorder (p = 2.5 ×10−3) and schizophrenia (p = 4.1 × 10−4) (Table 1) [44, 45].
GJ proteins have also been implicated in schizophrenia pathology with copy number variations in GJA5 and GJA8 [53]. A GJA8 haplotype (rs989192 and rs4950495) is also associated with schizophrenia, although 4 other GJA5 polymorphisms tested were not found to be associated [54] and these variants were not genome-wide significant (Table 1) [44, 45]. While we do not currently understand the functional implications of GJ proteins in schizophrenia, there are animal studies showing that chronic treatment with clozapine increases, while haloperidol and lithium decrease, GJA1 expression in the prefrontal cortex, which could have potential clinical implications [55].
Peripheral blood markers associated with BBB dysfunction have also been examined in schizophrenia. For example, greater levels of CLDN5 and OCLN were observed in the blood of patients with deficit schizophrenia (syndrome consisting of primarily negative symptoms), compared to nondeficit schizophrenia or healthy controls, which may suggest a breakdown of BMEC [56]. In a serum proteomic study, increased CDH5 levels in schizophrenia patients were associated with greater positive symptoms compared to schizophrenia patients with greater negative symptoms [57].
BMEC Transcellular Function
BMEC contain proteins that regulate BBB permeability, leukocyte migration, integrin activation, and transcellular flow (Fig. 1). The plasmalemma vesicle-associated protein (PLVAP) forms the diaphragm that bridges endothelial fenestrae and regulates permeability, leukocyte transmigration, and angiogenesis [58]. During inflammation, adhesion molecules such as vascular cell adhesion molecule-1 (VCAM1) and intercellular adhesion molecule-1 (ICAM1) are elevated in the endothelium, which allows for attachment and transmigration of leukocytes across the BBB [17]. The translocator protein (TSPO), thought to be primarily expressed in microglia was recently demonstrated to have a strong endothelial-binding component [59] and this is consistent with evidence from experimental animals that TSPO expression is predominantly found in ependymal and endothelial cells [60]. Additionally, in cellular studies of BMEC, genetic knockdown of TSPO increased the expression of VCAM1 [61].
BMEC Transcellular Abnormalities in Schizophrenia
In a postmortem study of schizophrenia, there were higher levels of ICAM1 in the dorsolateral prefrontal cortex, which was associated with an increased number of perivascular and parenchymal macrophages [46]. Lower levels of ICAM2, a protein involved in lymphocyte recirculation and immune response and surveillance, have been reported in multiple cortical brain regions in schizophrenia [47]. While ICAM1 polymorphisms (e.g., G241A) are not associated with schizophrenia risk [62], allele carriers of G241A have lower levels of soluble ICAM1 [63]. In the peripheral blood of patients with deficit schizophrenia, greater levels of PLVAP have been observed compared to levels in nondeficit schizophrenia patients or healthy controls [56], with mixed results reported for ICAM1 and VCAM1 [64, 65]. In a transcriptomic analysis of postmortem tissue, ICAM1 was found to be highly upregulated in the brains for schizophrenia subjects, while VCAM1 was highly downregulated. However, it is unclear if these differences were specific to BMEC [66]. Interestingly, a recent study using stem cell-derived BMEC from schizophrenia patients with 22q11 deletion syndrome identified impaired barrier integrity and upregulation of endothelial ICAM1 that was associated with increased leukocyte transmigration [67]. However, further studies are required to determine which of the multiple genes deleted in this syndrome are associated with this BBB phenotype.
With regard to TSPO, the radioligand binding for TSPO has been shown to be reduced in the early stages of psychosis [68] and in relevant experimental rodent models, including in endothelial cells [60]. Thus, addressing the role of endothelial TSPO expression in schizophrenia using hiPSC models would be of interest to determine how much TSPO radioligand bindings reflect brain endothelial dysfunction.
BMEC Transporter Function
BMEC contain facilitated and active transporters (Fig. 1), as well as transcellular transporters that regulate signal transduction and molecular transport [17, 69, 70]. BMEC express multiple efflux transporters, including ATP binding cassettes (ABC) that consist of ABCB1, ABCG2, and ABCC1, all of which function to return substrates to the bloodstream [17, 35, 71, 72]. Influx transporters regulate the flow of molecules from the periphery to the CNS and include the solute carrier organic anion transporter family member 1A2 (SLCO1A2), solute carrier family 22 member 1 (SLC22A1), SLC22A2, SLC22A3, SLC7A5, SLC2A1 [69, 70], and SLC39A8 [73]. Low-density lipoprotein receptor-1 (LRP1) and the receptor for advanced glycation end-products (RAGE) are also expressed by BMEC and use the receptor-mediated transport system to carry peptides, neurotransmitters, hormones, growth factors, and other neuronal products into the CNS [69]. Other transcellular transporters include the major facilitator superfamily domain and caveolin-1 (CAV1), which regulate omega-3 fatty acid transport and lipid compositions of BMEC, respectively [17]. These transcellular and transporter proteins prevent CNS entry and facilitate removal of harmful molecules to maintain CNS homeostasis.
BMEC Transporter Abnormalities in Schizophrenia
Efflux Transporters. ABCB1 is of particular interest in schizophrenia since it is a major efflux transporter for antipsychotics, (e.g., aripiprazole, olanzapine, risperidone, and paliperidone) [74]. For example, tetrahydrocannabinol, which is associated with schizophrenia risk, increases the expression of ABCB1 without altering D2 or 5-HT2A receptor binding, which subsequently decreases the concentration of risperidone, but not clozapine (not a substrate of ABCB1), in the brain [75]. In a human PET imaging study measuring ABCB1 function in patients with chronic schizophrenia, increased ABCB1 activity was observed and it was hypothesized that this could be a factor in antipsychotic drug resistance [25]. A postmortem study in patients with chronic schizophrenia also showed a reduced expression of the efflux transporter ABCG2, though not of ABCB1 or ABCC1, and this effect was independent of antipsychotic (chlorpromazine equivalents) treatment when tested using immortalized BMEC from humans [46].
Genetic variants of ABCB1 have been studied as potential susceptibility factors for schizophrenia in relatively small samples and they have also been associated with treatment response (risperidone, olanzapine, paliperidone, and clozapine) or antipsychotic side effects (agranulocytosis, prolactin levels, weight gain, metabolic disturbances, and QT interval) [76, 77]. The ABCB1 polymorphism rs1045642 has been associated with a reduced P300 event-related amplitude, an intermediate phenotype of schizophrenia, and it is modestly associated with schizophrenia [78]. There is evidence to suggest that ABCB1 single-nucleotide polymorphisms (rs1128503, rs2032582, and rs1045642) are associated with schizophrenia, but evidence is inconclusive [74]. These commonly studied ABCB1 variants are considered “silent” in that they do not change amino acid sequence or expression levels. However, changes in amino acid codons and relative tRNA availability may result in altered conformation, stability, and function [79, 80]. In a study of the 16p12.11 copy number variation in 10,397 individuals with neurodevelopmental conditions that included schizophrenia, ABCC1 was reported as a potential core pathogenic gene [81]. High-depth targeted sequencing studies have also found that ABCC1 may be associated with psychosis and cognitive impairment [82]. To date, however, there are no definitive studies showing whether any of these transporter variants has functional consequences.
Influx Transporters. A reduced expression of SLC2A1 was described the postmortem brains of schizophrenia patients, which may have implications for insulin resistance and/or reduced glucose metabolism in the CNS [12]. There is early evidence associating SLC7A5 polymorphisms with schizophrenia risk; however, SLC7A5-mediated transport did not differ between controls and schizophrenia patients [83]. In addition to the above mentioned solute carriers, the missense variant Ala391Thr (rs13107325) for SLC39A8, a zinc/metal ion transporter, has also been found to be associated with schizophrenia [73]. Ala391Thr is a pleiotropic variant that affects the function of SLC39A8, including immune-related traits, such as Crohn disease and ulcerative colitis, in patients with schizophrenia [73, 84]. SLC39A8 codes for Zip8, which is a negative feedback mediator of the NF-κB pathway and is responsible for regulating the brain's immune response and neuronal development [73]. The Ala391Thr variant is hypothesized to decrease Zip8 function, leading to hyperstimulation of the NF-κB pathway and enhancing immune response in the brain [73].
Transcellular Transporters. LRP1 has been strongly implicated in BBB function and in the etiopathology of developmental disorders, including schizophrenia. For example, 4 highly pathogenic de novo variants have been described in schizophrenia (2 variants), intellectual disability and autism spectrum disorder, demonstrating the pleiotropic effects of this gene [85, 86]. Three LRP1 nonsense mutations (rs75873762, rs79339212, and rs113087094) have been identified [87]. Additionally, 2 GWAS variants have been described and include rs324017 and rs12814239 (Table 1) [44]. However, there are also studies that fail to identify links between de novo mutations in LRP1 and schizophrenia risk [88]. A study examining genetic variation of myelination-related genes in schizophrenia using GWAS and exome sequencing identified rare variants for LRP1, but this effect did not survive multiple testing corrections [89]. Thus, LRP1 may be important not only for its role in lipid and lipoprotein metabolism but also for its involvement in regulation of BBB permeability, cell growth and migration, inflammation, and apoptosis.
While fewer studies have examined RAGE, 2 studies found that soluble RAGE protein levels were lower in patients with schizophrenia compared to controls. Conversely, 2 other studies reported elevated RAGE levels, and elevations in this protein are hypothesized to occur in the context of oxidative stress and inflammatory activation [90, 91, 92]. In a genetic study of Chinese schizophrenia patients, a RAGE functional polymorphism (rs1800625) was weakly associated with schizophrenia [93]. In a separate candidate gene study, the RAGE polymorphism rs2070600 was found to be associated with schizophrenia [94]. RAGE may also have important pathophysiologic implications as evidenced by altered RAGE levels due to vascular damage in both cardiovascular disease and diabetes [95, 96].
CAV1, a scaffolding protein that interacts with various cellular signaling molecules, has also been implicated in schizophrenia. In CAV1 knockout mice, there is an increased sensitivity to the psychomimetic actions of phencyclidine (an NMDA receptor antagonist), resulting in behavioral observations similar to those seen in schizophrenia patients, who were subsequently unresponsive to antipsychotic treatment [97]. Another study showed that neuron-specific overexpression of CAV1 increased the expression of proteins involved in synaptic plasticity, with CAV1 knockout having the opposite effect [98]. A microduplication of CAV1 has also been associated with schizophrenia, but the mechanism is unclear [99]. Therefore, disruptions in CAV1 may contribute to schizophrenia pathophysiology by impairing vascular and neuronal signaling.
BMEC ECM Function
The ECM (Fig. 1) interacts with cell-surface integrins and glycoproteins to hold NVU components together and to provide signaling cues [100, 101]. BMEC and pericytes produce ECM components such as collagen type IV (COL4A), fibronectin (FN), laminin 1 (LAMA1), and LAMA2 [17, 101], while astrocytes provide additional support by secreting LAMA1, LAMA2, and nidogen (NID1) [17]. Other ECM components include reelin, chondroitin sulfate proteoglycans, hyaluronic acid (HA), and heparan sulfate proteoglycans perlecan (HSPG2) and agrin (AGRN), which have surface receptors that sequester and interact with growth factors [100, 101, 102, 103]. Heparan sulfate proteoglycans are metabolized by N-deacetylase/N-sulfotransferase 3 (NDST3), a brain enzyme involved in leukocyte adhesion at the BBB [13, 104]. COL4A provides stability to the ECM by maintaining LAMA and HSPG2, while FN influences brain capillary endothelial cell proliferation in vitro [101]. Both COL4 and FN regulate BBB function by increasing TEER [101].
BMEC ECM Abnormalities in Schizophrenia
ECM abnormalities have been identified in postmortem investigations of brains of schizophrenia subjects. These include differences in reelin and chondroitin sulfate proteoglycans, which may lead to impairments in neuronal migration and the synaptic function of GABAgeric, glutamatergic, and dopaminergic connectivity [103]. HA is another major component of the ECM that binds to the cell adhesion molecule CD44 [102, 105]. In a postmortem study, CD44 was found to be upregulated in the dorsolateral prefrontal cortex of patients with schizophrenia [66]. However, it is unclear whether these differences were specifically related to BMEC as opposed to other brain cell types.
In other postmortem studies, a reduced brain expression of LAMA1, FN1, COL4A1, and COL4A2 has been previously reported in schizophrenia [12]. In a serum proteomic analysis of paranoid schizophrenia patients, researchers found reductions in COL4A1 but not COL4A2, although this effect was not significant [106]. De novo mutations have been identified in schizophrenia for LAMA1 (missense mutation) [87] and LAMA2 (frameshift and splice site mutations) [107]. FN1 polymorphisms were not associated with schizophrenia risk in a Japanese study [108]. In a study of whole blood gene expression, FN1 levels were reduced in chronic schizophrenia patients with greater symptoms of hallucinations or delusions [109]. However, no information is available on any possible association between the effect of antipsychotic medications on FN1 levels in these patients [109]. The NDST3 polymorphism (rs11098403) was associated (p < 6.6 ×10−9) with schizophrenia and bipolar disorder risk in an Ashkenazi Jewish population, and this was independently replicated in 6 schizophrenia and 5 bipolar disorder cohorts [110]. However, this association did not reach genome-wide significance (Table 1) [44] and a study evaluating NDST3 copy number variation did not find a significant association in a Japanese sample [111].
Ex Vivo Models of BMEC Function in Schizophrenia
Differentiation of BMEC from iPSC
Before the advent of somatic cell reprogramming, immortalized BMEC were used to study BBB function, but this method was limited by subphysiologic range TEER values [112]. Now, iPSC can be used to generate patient-derived BMEC that recapitulate the contribution of disease-specific genetic backgrounds. Recent methodological advances have led to robust and reliable protocols for the generation of homogeneous populations of iPSC-derived BMEC [15, 72, 113, 114, 115]. BMEC identity is confirmed by cytochemical analysis showing the coexpression of PECAM1, TJP1, OCLN, CLDN5 and SLC2A1, and the BBB phenotype is confirmed by the presence of physiologic range TEER values (2,000 Ω × cm2), angiogenic potential, and measurable transporter activity [72]. Coculturing BMEC with neural progenitor cells, pericytes, or astrocytes further enhances BBB function, with the combination of BMEC, pericytes, and astrocytes providing the greatest TEER values (∼5,000 Ω × cm2) [116]. These protocols have been used to generate BMEC from patients with Huntington disease and schizophrenia with 22q11 deletion syndrome [67, 71] and to test the effects of various infectious agents on BBB function [112, 117].
Two-Dimensional and Three-Dimensional Models for Studying BMEC Function
The capacity of BMEC to foster angiogenesis is of interest in elucidating proposed vascular pathologies in schizophrenia. Recently, methods have been developed for the generation of vasculature using iPSC-derived BMEC seeded within a fibrin gel, which is often used as a matrix for angiogenesis assays [118]. A multiculture microfluidic platform containing endothelial cells and fibroblasts encapsulated within a fibrin gel has been shown to provide a controlled environment to generate lumenized and perfusable vasculature [119]. Biomaterial scaffolds such as poly(L-lactic acid) and poly(lactic-co-glycolic acid) sponges used in combination with a fibrin gel have also been shown to support in vitro vascularization as well as in vivo graft neovascularization upon implantation [120].
Additional sophisticated fabrication methods are being developed that use complex scaffolds, such as the AngioChip, a fabricated device made from polycarbonate and poly(dimethylsiloxane). This device contains nanopores and micro-holes and supports endothelial cell seeding and subsequent vascularization into the matrix within which the device is encapsulated [121]. One templating strategy includes 3-D printing of a sacrificial carbohydrate lattice encased within a fibrin gel that can undergo hydrolysis to yield hollow channels. These channels support the attachment of endothelial cells along the wall, which can further sprout into the surrounding fibrin gel network [122]. Other approaches involve building the vasculature in a geometrically defined micropatterned template or developing biodegradable polymer scaffolds with a desired architecture using high-resolution projection micro stereolithography [123, 124, 125]. The limiting factor of these approaches to fabrication is the relatively low resolution of the resulting vascular constructs compared to the microcapillaries that form within hydrogels. Commercially available or naturally derived microbead-based cocultivation systems have also been adapted to in vitro angiogenesis assays. These assays provide a controlled environment for the rapid and reliable study of phenomenological events involved in microvascular network formation that are difficult to perform in fabricated structures [126, 127, 128, 129]. While 2-D Transwell/coculturing models are simple and less expensive than 3-D models, they cannot fully recapitulate the complexity of the multicellular system of the BBB [130]. Few studies have focused on the development of a 3-D ex vivomodel that faithfully recapitulates the properties of the BBB. When iPSC-derived BMEC were grown in hydrogel scaffolds made with an alginate/gelatin composite, they failed to attach and grow on the hydrogel coated with alginate, but they did grow on a hydrogel containing gelatin, promoting the development of better barrier phenotypes [131]. iPSC-derived BMEC grown using this 3-D model had more robust barrier properties and retained barrier function for up to 21 days [131].
Organ-chip Models for Studying BMEC Function
BBB chips and microfluidic devices are also being utilized for the study of BBB function [132, 133]. 3-D models allow for direct interaction between BMEC and other NVU cells and the shear force from laminar blood flow enhances BMEC maturation, proliferation, and angiogenesis [134, 135, 136] by increasing OCLN, CDH5, PECAM1, and caveolin expression [130]. Flow-induced vascularization has been demonstrated in organ-on-chip technologies for other systems such as kidney organoids [137]. BMEC have been combined with organ-chip technology to create a 3-D model of the BBB that provides a better representation of the microvascular environment in both healthy control and Huntington disease cell lines [130]. This model allows for real-time TEER measurement through vessel perfusion, as well as permeability measurement of targeted molecules across the BBB in both normal and disease conditions [130]. In a perfused BBB model, the effect of inflammatory cytokines can be observed through their pathophysiologic effects on BMEC. For instance, when TNF-α, IL-1β, and IL-8 were perfused through ex vivo blood vessels, TJP1 expression was reduced in response to TNF-α and IL-1β, but not IL-8, which consequently led to an increase in BBB leakage [130]. These BBB chip models have also been used for drug permeability studies and the metrics for permeability were comparable to in vivo values [138]. These studies show that BBB chips can be a valuable tool for the investigation of disease pathophysiology and for screening of novel drug candidates.
Brain Organoid Models for Studying BMEC Function
Endothelial coculture methods have been studied as a way to promote neovascularization to enhance brain organoid survival and development [139]. Transplantation of brain organoids into mice has shown progressive neuronal and glial maturation in multiple regions of the host brain [140]. However, while there was extensive infiltration of the host vasculature in the organoid, there was no evidence of graft-derived BMEC; this led to other studies attempting to coculture brain organoids with BMEC prior to transplantation. For example, when a human brain organoid is grown in the presence of BMEC from the same patient, there is greater vascularization and improved integration into a living mouse brain [141]. This early work is promising and future studies should examine the effects of vascularization on cytoarchitectural organization, neurophysiology, and BBB development.
Quality Control and Technical Consideration in the Use of iPSC and iPSC-Derived BMEC
The quality of iPSC can impact the variability of the phenotype of interest. Therefore, quality control is carried out by examining embryonic-like morphology, pluripotency, chromosomal stability, and contamination [142]. The pluripotency of iPSC is confirmed by testing for stem cell markers such as Nanog, SOX2, and OCT-3/4 [142, 143]. Karyotype analysis is used to determine the level of chromosomal stability and microbiological assessment is typically performed by screening for mycoplasma [142].
Additional considerations include whether iPSC-derived brain endothelial cell protocols recapitulate BBB formation/ontogeny, whether BMEC derivation is reliable/reproducible, and whether the developmental stage of BMEC influences the use/interpretation of these models. In the most recent iPSC-derived BMEC protocol, the maximum TEER reported was approximately 3,000 Ω × cm2 in a healthy control female iPSC line, with low levels of variability across 10 independent biological replicates [15]. The same was true for a healthy male iPSC line which had a maximal TEER of 2,000 Ω × cm2 and small SD across 4 independent biological replicates [15]. This new protocol was also successfully applied to 2 independent diseased lines, i.e., Huntington disease and tuberous sclerosis, with a small amount of variability across independent biological replicates [15]. In this protocol, the maximum TEER values are consistently observed after 1–2 days of culturing on collagen/fibronectin substrate and retinoic acid treatment [15, 115]. Retinoic acid regulates BMEC specification as indicated by a high expression of PECAM1, SLC2A1, and nuclear β-catenin, a sign of robust activation of the Wnt signaling pathway, which is necessary for inducing BBB properties in vivo [72, 144]. These results demonstrate the robustness of this protocol for establishing physiologic TEER values ex vivo. In the first week of BMEC culture, the variability of TEER values is low, with a small increase in variability thereafter. Also, the maximal TEER value is not generally maintained after day 2 (although in some cases a second peak is observed) and TEER values progressively decline over the course of 1–2 weeks [15, 115]. Therefore, this BMEC protocol is robust, reproducible, and reliable for generating BBB models ex vivo. However, more work is needed to determine the developmental stage of BMEC in these models.
Discussion
BBB disruption in one or more of the following components: paracellular, transcellular or extracellular matrix may be a final common pathological pathway in schizophrenia that results from genetic alterations that increase the vulnerability to psychosis. For example, CLDN5 knockdown in a mouse model leads to an increase in the extravasation of gadolinium into the brain [38], while a study using human iPSC-BMECs from schizophrenia patients with 22q deletion syndrome identified an increase in leukocyte transmigration into the brain that was meditated by the increased expression of ICAM1 [67]. GWAS studies have identified common risk variants for schizophrenia in ESAM and LRP1. ESAM induces interendothelial cell interaction to regulate vascular development and for the extravasation of immune cells, such as neutrophils, during the early phase of inflammation [37]. While there are no studies examining the pathophysiological role of LRP1 in schizophrenia, we know from the Alzheimer disease literature that LRP1, a protein involved in the transport of substrates across the BBB, is also important for removing Aβ from the brain of patients with Alzheimer [145, 146]. The loss of LRP1 inhibition on matrix metallopeptidase 9 (also implicated in schizophrenia) [147] subsequently leads to degradation of TJP (TJP1, OCLN, and CLDN5) and ECM (COL4) in the BBB [146]. Accelerated aging, as noted by progressive cognitive decline and brain loss, has been proposed in schizophrenia [148]. This literature could potentially be understood in the context of age-related cognitive and neuroanatomical impairments, which are thought be related to progressive BBB disruption mediated by increased TGF-β signaling [149]. Upregulation of TGF-β signaling occurs in the context of albumin infiltration into the aging brain (an observation noted in schizophrenia [18]), which in turn activates astrocytes to not only increase TGF-β signaling but also release inflammatory cytokines that cause further disruption of the BBB [149].
Environmental factors such as stress, peripheral inflammation, and infection also contribute to BBB disruption. In response to injury-related stress, exposure to infections, and medication-induced stress (e.g., type I interferon therapy for malignancies), BMEC release proinflammatory cytokines and adhesion molecules such as ICAM1 and VCAM1 to recruit immune cells into the brain [150, 151]. A recent study showed that social stress induced an increase in peripheral inflammation that led to the loss of CLDN5 expression in the TJ of BMEC and subsequent BBB breakdown [152, 153]. Another study suggested that ESAM may influence the susceptibility to schizophrenia by regulating the BBB in response to chronic low-grade inflammation [48]. The hypothesis that infection-induced BBB disruption increases the schizophrenia risk has been proposed for decades because many infections are associated with proinflammatory activation that increases the risk of developing schizophrenia or bipolar disorder [9, 104, 154]. A mechanistic study using iPSC-derived BMEC showed that Neisseria meningitidis disrupted the blood-CSF barrier by inducing the TJ transcriptional repressor gene known as Snail-1, which results in a decrease in TJP1, OCLN, and CLDN5 expression, as well as a decrease in TEER values [112]. This finding coincides with a previous study showing that Group B Streptococcus induces BBB deficits by decreasing TEER and TJ protein expression [117]. It is possible that environmental factors disrupt the BBB via paracellular and transcellular alterations, leading to extravasation of immune cells and activation of astrocytes and microglia, perpetuating proinflammatory cytokine release and subsequent BBB disruption.
Moreover, after BBB disruption occurs, compounds circulating in the blood such as albumin, toxins, cytokines, and immune cells are able to enter the brain, resulting in the continued activation of astrocytes and microglia. Peripheral proinflammatory cytokines, such as IFN-γ and TNF-α, can activate microglia to release other proinflammatory cytokines such as IL-12, IL-1β, IL-6, and IFN-γ, resulting in astrocyte activation and release of additional cytokines and chemokines [155]. IL-6, IL-1β, and TNF-α are also thought to inhibit GJA1-mediated GJ intercellular communication in astrocytes and contribute to inflammasome pathway activation by opening hemichannels [156]. Thus, either primary or secondary disruption of the BBB can trigger a neuroimmune cascade resulting in neuronal death.
In addition to cytokines, other immune response molecules such as VCAM1, ICAM1, and P-, E-, and L-selectins, can be found circulating in the blood of patients and may be indicators of BMEC dysfunction [104, 157, 158]. During inflammation, VCAM1 and ICAM1 are elevated to allow for the attachment and transmigration of leukocytes across the BBB [17, 150]. In schizophrenia, it is not clear whether ICAM1 and/or VCAM1 is elevated primarily due to induction of the immune response system or whether genetic variants directly cause their overexpression. Another potential pathogenic mechanism involves heparan sulfate, which is metabolized by NDST3 and has been weakly associated with schizophrenia and bipolar disorder [13, 104]. Heparan sulfate may facilitate ECM degradation through leukocyte extravasation and it is also expressed during inflammation to assist with the initial attachment of leukocytes to the inflamed endothelium [159]. Findings from existing studies suggest that BBB disruption induced by genetic or environmental factors can result in infiltration of harmful substances into the brain that activates microglia and astrocytes, which then release cytokines and other immune response molecules. The consequences of this cytokine release include transmigration and extravasation of leukocytes (Fig. 2) and immune cell stimulation causing the release of additional inflammatory cytokines, further exacerbating BBB dysfunction. We believe that BMEC are central to this process and identification of the major pathways involved in BBB disruption is key to identifying drug targets that could stop and/or reverse this process. For example, studies of age-related pathologies have shown that increased TGFβ signaling and BBB dysfunction secondary to age-related neurological changes can be reversed by TGFβ inhibition [149]. Before similar treatments can be designed for schizophrenia, we need to better understand the mechanisms of BBB dysfunction as they relate to this disorder. The knowledge gained from these studies will have the potential to change the way we understand and treat schizophrenia and other related disorders.
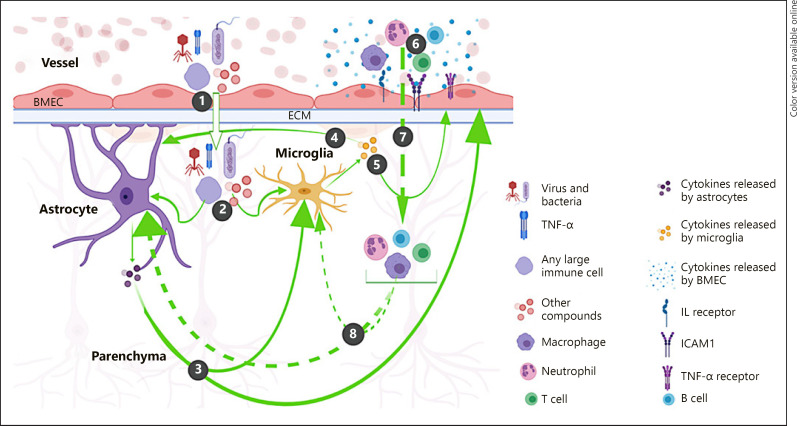
Fig. 2.
Conceptual model of the consequential pathways resulting from BMEC dysfunction in schizophrenia. (1) A disrupted paracellular structure allows blood materials including harmful substances to infiltrate into the brain parenchyma. (2) In the parenchyma, these blood materials activate astrocyte and/or microglia, causing them to release proinflammatory cytokines. (3) Astrocytic cytokines either activate microglia to release additional inflammatory cytokines or activate BMEC to release other proinflammatory cytokines, cytokine receptors, or adhesion molecules such as ICAM1. (4) Microglial cytokines stimulate the continued astrocytic release of proinflammatory cytokines that follow the positive feedback loop pathway described in (3). (5) Microglial cytokines also activate BMEC to release proinflammatory cytokines and other immune response molecules as described in (3). (6) Cytokines released by BMEC recruit other immune cells such as neutrophils, macrophages, B cells, and T cells. (7) Cytokines and adhesion molecules such as ICAM1 help immune cells to extravasate into the parenchyma. (8) Immune cells stimulate continued microglial and astrocytic activation and the subsequent release of proinflammatory cytokines, worsening BBB integrity.
While there is evidence for BMEC dysfunction in schizophrenia, some of these findings need to be considered in the context of the stage of illness, antipsychotic status, and treatment resistance. Many of the genetic studies involved moderately sized study samples from various countries, with the PGC study having the largest sample of patients with schizophrenia. We also discussed findings from 6 postmortem studies [12, 38, 39, 46, 66, 103], 3 studies with patients who had chronic schizophrenia [25, 46, 109], 1 study with subjects with acute paranoid schizophrenia [160], and 2 animal studies [38, 55]. There is limited information in terms of these finding in relation to exposure to antipsychotic medications. In one study, a dose-dependent increase in CLDN5 expression was observed in vitro in mouse BMEC after treatment with the antipsychotic medications haloperidol and chlorpromazine [38]. In a postmortem study of subjects who had chronic schizophrenia, there was evidence of a lower CLDN5 immunoreactivity in the parietal lobe of patients with schizophrenia compared to controls, but there was no information on the type and duration of antipsychotic treatment [38]. In another study of serum from patients with chronic schizophrenia and schizoaffective disorder, antipsychotic treatment was associated with elevated peripheral sICAM1 levels [46]. Experiments in immortalized human BMEC showed that exposure to a number of antipsychotics (clozapine, haloperidol, and risperidone) did not result in any changes in ICAM1 or any of the other BMEC biomarkers (CDH5, OCLN, and ABCG2) [46]. In patients with major depression, antipsychotic treatment was shown to reduce serum levels of sRAGE [90], while serum sRAGE was found to be increased in patients with acute paranoid schizophrenia after 6 weeks of antipsychotic treatment [160]. There is a gap in our understanding of how antipsychotic medications affect BBB function in the context of schizophrenia. The iPSC-derived BMEC can provide new ways to test how different antipsychotic medications alter TJ proteins and inflammatory cytokines and may provide further insights into understanding the pathophysiology of BBB disruption in schizophrenia.
Conclusion
BMEC are involved in a wide range of cellular processes (paracellular, transcellular, transporter, and extracellular matrix) that contribute to BBB and possibly neuroimmune dysfunction in schizophrenia. While existing studies have examined the relationship between BBB functionality and those biological processes associated with schizophrenia, no studies have delineated the role of BMEC in the etiopathogenesis of schizophrenia. Human iPSC-derived BMEC may allow us to clarify the role of BMEC and the BBB in neuropsychiatric disorders such as schizophrenia. Technological and methodological advances in 2-D and 3-D model generation using iPSC-derived BMEC from patients with schizophrenia may help to determine whether a causative relationship exists between BMEC dysfunction and schizophrenia.
Conflict of Interest Statement
The authors have no conflict of interests to declare.
Funding Sources
This work was supported by a Sydney R. Baer Jr. Foundation Grant (P.L.), the National Institutes of Health (KL2TR002542 to P.L.), BRAINS (R01MH113858 and K08MH086846 to R.K.), a Doris Duke Charitable Foundation Clinical Scientist Development Award (R.K.), the Ryan Licht Sang Bipolar Foundation (R.K.), the Phyllis and Jerome Lyle Rappaport Foundation (R.K.), the Harvard Stem Cell Institute (R.K.), and Steve Willis and Elissa Freud (R.K.).
Author Contributions
P.L. and R.K. conceived the idea. S.P. wrote the first draft of this paper with guidance and supervision from P.L. and R.K. J.R.B. and M.S. contributed to the writing of this paper and provided guidance on the genetic and engineering components of this work, respectively.
Acknowledgment
The authors wish to acknowledge the help of Nick Raymond of Beth Israel Deaconess Medical Center for creating the images used in this paper. The authors would also like to thank Dr. Meredith Gansner for proofreading.
References
- 1.Fleischhacker WW, Arango C, Arteel P, Barnes TR, Carpenter W, Duckworth K, et al. Schizophrenia—time to commit to policy change. Schizophr Bull. 2014 Apr;40(Suppl 3):S165–94. doi: 10.1093/schbul/sbu006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haller CS, Padmanabhan JL, Lizano P, Torous J, Keshavan M. Recent advances in understanding schizophrenia. F1000Prime Rep. 2014 Jul;6:57. doi: 10.12703/P6-57. Available from: http://www.f1000.com/reports/m/6/57/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Readhead B, Hartley BJ, Eastwood BJ, Collier DA, Evans D, Farias R, et al. Expression-based drug screening of neural progenitor cells from individuals with schizophrenia. Nat Commun. 2018 Oct;9((1)):4412. doi: 10.1038/s41467-018-06515-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wen Z, Christian KM, Song H, Ming GL. Modeling psychiatric disorders with patient-derived iPSCs. Curr Opin Neurobiol. 2016 Feb;36:118–27. doi: 10.1016/j.conb.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watmuff B, Berkovitch SS, Huang JH, Iaconelli J, Toffel S, Karmacharya R. Disease signatures for schizophrenia and bipolar disorder using patient-derived induced pluripotent stem cells. Mol Cell Neurosci. 2016 Jun;73:96–103. doi: 10.1016/j.mcn.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meynert T. Psychiatrie: Klinik der Erkrankungen des Vorderhirns begründet auf dessen Bau, Leistungen und Ernährung [Internet] Wilhelm Braumüller; 1884 Available from: https://books.google.com/books?id= bZdPAAAAYAAJ. [Google Scholar]
- 7.Ashok AH, Baugh J, Yeragani VK. Paul Eugen Bleuler and the origin of the term schizophrenia (SCHIZOPRENIEGRUPPE) Indian J Psychiatry. 2012 Jan;54((1)):95–6. doi: 10.4103/0019-5545.94660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bleuler E. Dementia Praecox or the Group of Schizophrenias. New York: International Univ. Press; 1950. [Google Scholar]
- 9.Hanson DR, Gottesman II. Theories of schizophrenia: a genetic-inflammatory-vascular synthesis. BMC Med Genet. 2005 Feb;6((1)):7. doi: 10.1186/1471-2350-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baruah J, Vasudevan A. The Vessels Shaping Mental Health or Illness. Open Neurol J. 2019;13((1)):1–9. doi: 10.2174/1874205X01913010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lopes R, Soares R, Coelho R, Figueiredo-Braga M. Angiogenesis in the pathophysiology of schizophrenia - a comprehensive review and a conceptual hypothesis. Life Sci. 2015 May;128:79–93. doi: 10.1016/j.lfs.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 12.Katsel P, Roussos P, Pletnikov M, Haroutunian V. Microvascular anomaly conditions in psychiatric disease. Schizophrenia - angiogenesis connection. Neurosci Biobehav Rev. 2017 Jun;77:327–39. doi: 10.1016/j.neubiorev.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Najjar S, Pahlajani S, De Sanctis V, Stern JN, Najjar A, Chong D. Neurovascular Unit Dysfunction and Blood-Brain Barrier Hyperpermeability Contribute to Schizophrenia Neurobiology: A Theoretical Integration of Clinical and Experimental Evidence. Front Psychiatry. 2017 May;8:83. doi: 10.3389/fpsyt.2017.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pollak TA, Drndarski S, Stone JM, David AS, McGuire P, Abbott NJ. The blood-brain barrier in psychosis. Lancet Psychiatry. 2018 Jan;5((1)):79–92. doi: 10.1016/S2215-0366(17)30293-6. [DOI] [PubMed] [Google Scholar]
- 15.Neal EH, Marinelli NA, Shi Y, McClatchey PM, Balotin KM, Gullett DR, et al. A Simplified, Fully Defined Differentiation Scheme for Producing Blood-Brain Barrier Endothelial Cells from Human iPSCs. Stem Cell Reports. 2019 Jun;12((6)):1380–8. doi: 10.1016/j.stemcr.2019.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith QR, Rapoport SI. Cerebrovascular permeability coefficients to sodium, potassium, and chloride. J Neurochem. 1986 Jun;46((6)):1732–42. doi: 10.1111/j.1471-4159.1986.tb08491.x. [DOI] [PubMed] [Google Scholar]
- 17.Liebner S, Dijkhuizen RM, Reiss Y, Plate KH, Agalliu D, Constantin G. Functional morphology of the blood-brain barrier in health and disease. Acta Neuropathol. 2018 Mar;135((3)):311–36. doi: 10.1007/s00401-018-1815-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bechter K, Reiber H, Herzog S, Fuchs D, Tumani H, Maxeiner HG. Cerebrospinal fluid analysis in affective and schizophrenic spectrum disorders: identification of subgroups with immune responses and blood-CSF barrier dysfunction. J Psychiatr Res. 2010 Apr;44((5)):321–30. doi: 10.1016/j.jpsychires.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 19.Busse S, Busse M, Schiltz K, Bielau H, Gos T, Brisch R, et al. Different distribution patterns of lymphocytes and microglia in the hippocampus of patients with residual versus paranoid schizophrenia: further evidence for disease course-related immune alterations? Brain Behav Immun. 2012 Nov;26((8)):1273–9. doi: 10.1016/j.bbi.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 20.Hwang Y, Kim J, Shin JY, Kim JI, Seo JS, Webster MJ, et al. Gene expression profiling by mRNA sequencing reveals increased expression of immune/inflammation-related genes in the hippocampus of individuals with schizophrenia. Transl Psychiatry. 2013 Oct;3((10)):e321–321. doi: 10.1038/tp.2013.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim S, Hwang Y, Lee D, Webster MJ. Transcriptome sequencing of the choroid plexus in schizophrenia. Transl Psychiatry. 2016 Nov;6((11)):e964. doi: 10.1038/tp.2016.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lizano P, Lutz O, Ling G, Lee AM, Eum S, Bishop JR, et al. Association of Choroid Plexus Enlargement With Cognitive, Inflammatory, and Structural Phenotypes Across the Psychosis Spectrum. Am J Psychiatry. 2019 Jul;176((7)):564–72. doi: 10.1176/appi.ajp.2019.18070825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harris LW, Wayland M, Lan M, Ryan M, Giger T, Lockstone H, et al. The Cerebral Microvasculature in Schizophrenia: A Laser Capture Microdissection Study. In: Hashimoto K, editor. PLoS ONE. (12) Vol. 3. 2008. Dec 17. p. e3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bernstein HG, Hildebrandt J, Dobrowolny H, Steiner J, Bogerts B, Pahnke J. Morphometric analysis of the cerebral expression of ATP-binding cassette transporter protein ABCB1 in chronic schizophrenia: circumscribed deficits in the habenula. Schizophr Res. 2016 Nov;177((1-3)):52–8. doi: 10.1016/j.schres.2016.02.036. [DOI] [PubMed] [Google Scholar]
- 25.de Klerk OL, Willemsen AT, Bosker FJ, Bartels AL, Hendrikse NH, den Boer JA, et al. Regional increase in P-glycoprotein function in the blood-brain barrier of patients with chronic schizophrenia: a PET study with [(11)C]verapamil as a probe for P-glycoprotein function. Psychiatry Res. 2010 Aug;183((2)):151–6. doi: 10.1016/j.pscychresns.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 26.Goldsmith DR, Rapaport MH, Miller BJ. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry. 2016 Dec;21((12)):1696–709. doi: 10.1038/mp.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lizano PL, Keshavan MS, Tandon N, Mathew IT, Mothi SS, Montrose DM, et al. Angiogenic and immune signatures in plasma of young relatives at familial high-risk for psychosis and first-episode patients: A preliminary study. Schizophr Res. 2016 Jan;170((1)):115–22. doi: 10.1016/j.schres.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kathuria A, Lopez-Lengowski K, Vater M, McPhie D, Cohen BM, Karmacharya R. Transcriptome analysis and functional characterization of cerebral organoids in bipolar disorder. Genome Med. 2020;19(12(1)):34. doi: 10.1186/s13073-020-00733-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karmacharya R, Haggarty SJ. Stem cell models of neuropsychiatric disorders. Mol Cell Neurosci. 2016 Jun;73:1–2. doi: 10.1016/j.mcn.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 30.Watmuff B, Liu B, Karmacharya R. Stem cell-derived neurons in the development of targeted treatment for schizophrenia and bipolar disorder. Pharmacogenomics. 2017 Apr;18((5)):471–9. doi: 10.2217/pgs-2016-0187. [DOI] [PubMed] [Google Scholar]
- 31.Kathuria A, Lopez-Lengowski K, Watmuff B, McPhie D, Cohen BM, Karmacharya R. Synaptic deficits in iPSC-derived cortical interneurons in schizophrenia are mediated by NLGN2 and rescued by N-acetylcysteine. Transl Psychiatry. 2019 Nov;9((1)):321. doi: 10.1038/s41398-019-0660-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kathuria A, Lopez-Lengowski K, Jagtap SS, McPhie D, Perlis RH, Cohen BM, et al. Transcriptomic Landscape and Functional Characterization of Induced Pluripotent Stem Cell–Derived Cerebral Organoids in Schizophrenia. JAMA Psychiatry [Internet] 2020 Mar 18; doi: 10.1001/jamapsychiatry.2020.0196. Available from: https://jamanetwork.com/journals/jamapsychiatry/fullarticle/2762982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benz F, Wichitnaowarat V, Lehmann M, Germano RF, Mihova D, Macas J, et al. Low wnt/b-catenin signaling determines leaky vessels in the subfornical organ and affects water homeostasis in mice. Dev Biol. 2019;•••:29. doi: 10.7554/eLife.43818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tietz S, Engelhardt B. Brain barriers: Crosstalk between complex tight junctions and adherens junctions. 2015:14. doi: 10.1083/jcb.201412147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stamatovic SM, Keep RF, Andjelkovic AV. Brain endothelial cell-cell junctions: how to “open” the blood brain barrier. Curr Neuropharmacol. 2008 Sep;6((3)):179–92. doi: 10.2174/157015908785777210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simpson I, Rose B, Loewenstein WR. Size limit of molecules permeating the junctional membrane channels. Science. 1977 Jan;195((4275)):294–6. doi: 10.1126/science.831276. [DOI] [PubMed] [Google Scholar]
- 37.Stamatovic SM, Johnson AM, Keep RF, Andjelkovic AV. Junctional proteins of the blood-brain barrier: new insights into function and dysfunction. Tissue Barriers. 2016 Feb;4((1)):e1154641. doi: 10.1080/21688370.2016.1154641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Greene C, Kealy J, Humphries MM, Gong Y, Hou J, Hudson N, et al. Dose-dependent expression of claudin-5 is a modifying factor in schizophrenia. Mol Psychiatry. 2018 Nov;23((11)):2156–66. doi: 10.1038/mp.2017.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fiorentino M, Sapone A, Senger S, Camhi SS, Kadzielski SM, Buie TM, et al. Blood-brain barrier and intestinal epithelial barrier alterations in autism spectrum disorders. Mol Autism. 2016 Nov;7((1)):49. doi: 10.1186/s13229-016-0110-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun ZY, Wei J, Xie L, Shen Y, Liu SZ, Ju GZ, et al. The CLDN5 locus may be involved in the vulnerability to schizophrenia. Eur Psychiatry. 2004 Sep;19((6)):354–7. doi: 10.1016/j.eurpsy.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 41.Ye L, Sun Z, Xie L, Liu S, Ju G, Shi J, et al. Further study of a genetic association between the CLDN5 locus and schizophrenia. Schizophr Res. 2005 Jun;75((1)):139–41. doi: 10.1016/j.schres.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 42.Wu N, Zhang X, Jin S, Liu S, Ju G, Wang Z, et al. A weak association of the CLDN5 locus with schizophrenia in Chinese case-control samples. Psychiatry Res. 2010 Jun;178((1)):223. doi: 10.1016/j.psychres.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 43.Omidinia E, Mazar FM, Shahamati P. Polymorphism of the CLDN5 gene and Schizophrenia in an Iranian Population. 2014;43:5. [PMC free article] [PubMed] [Google Scholar]
- 44.Schizophrenia Working Group of the Psychiatric Genomics Consortium Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014 Jul;511((7510)):421–7. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Psychiatric GWAS Consortium Bipolar Disorder Working Group Large-scale genome-wide association analysis of bipolar disorder identifies a new susceptibility locus near ODZ4. Nat Genet. 2011 Sep;43((10)):977–83. doi: 10.1038/ng.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cai HQ, Catts VS, Webster MJ, Galletly C, Liu D, O'Donnell M, et al. Increased macrophages and changed brain endothelial cell gene expression in the frontal cortex of people with schizophrenia displaying inflammation. Mol Psychiatry. 2018 Sep 13; doi: 10.1038/s41380-018-0235-x. Available from: http://www.nature.com/articles/s41380–018–0235-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Katsel P, Davis KL, Gorman JM, Haroutunian V. Variations in differential gene expression patterns across multiple brain regions in schizophrenia. Schizophr Res. 2005 Sep;77((2-3)):241–52. doi: 10.1016/j.schres.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 48.Pouget JG, Gonçalves VF, Spain SL, Finucane HK, Raychaudhuri S, Kennedy JL, et al. Schizophrenia Working Group of the Psychiatric Genomics Consortium Genome-Wide Association Studies Suggest Limited Immune Gene Enrichment in Schizophrenia Compared to 5 Autoimmune Diseases. Schizophr Bull. 2016 Sep;42((5)):1176–84. doi: 10.1093/schbul/sbw059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu B, Roos JL, Dexheimer P, Boone B, Plummer B, Levy S, et al. Exome sequencing supports a de novo mutational paradigm for schizophrenia. Nat Genet. 2011 Aug;43((9)):864–8. doi: 10.1038/ng.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jia P, Chen X, Fanous AH, Zhao Z. Convergent roles of de novo mutations and common variants in schizophrenia in tissue-specific and spatiotemporal co-expression network. Transl Psychiatry. 2018 May;8((1)):105. doi: 10.1038/s41398-018-0154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clark SL, Souza RP, Adkins DE, Åberg K, Bukszár J, McClay JL, et al. Genome-wide association study of patient-rated and clinician-rated global impression of severity during antipsychotic treatment. Pharmacogenet Genomics. 2013 Feb;23((2)):69–77. doi: 10.1097/FPC.0b013e32835ca260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baum AE, Hamshere M, Green E, Cichon S, Rietschel M, Noethen MM, et al. Meta-analysis of two genome-wide association studies of bipolar disorder reveals important points of agreement. Mol Psychiatry. 2008 May;13((5)):466–7. doi: 10.1038/mp.2008.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Luo X, Huang L, Han L, Luo Z, Hu F, Tieu R, et al. Systematic Prioritization and Integrative Analysis of Copy Number Variations in Schizophrenia Reveal Key Schizophrenia Susceptibility Genes. 2014:15. doi: 10.1093/schbul/sbu045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ni X, Valente J, Azevedo MH, Pato MT, Pato CN, Kennedy JL. Connexin 50 gene on human chromosome 1q21 is associated with schizophrenia in matched case control and family-based studies. J Med Genet. 2007 Aug;44((8)):532–6. doi: 10.1136/jmg.2006.047944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fatemi SH, Folsom TD, Reutiman TJ, Pandian T, Braun NN, Haug K. Chronic psychotropic drug treatment causes differential expression of connexin 43 and GFAP in frontal cortex of rats. Schizophr Res. 2008 Sep;104((1-3)):127–34. doi: 10.1016/j.schres.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 56.Maes M, Sirivichayakul S, Kanchanatawan B, Vodjani A. Breakdown of the Paracellular Tight and Adherens Junctions in the Gut and Blood Brain Barrier and Damage to the Vascular Barrier in Patients with Deficit Schizophrenia. Neurotox Res. 2019 Aug;36((2)):306–22. doi: 10.1007/s12640-019-00054-6. [DOI] [PubMed] [Google Scholar]
- 57.Smirnova L, Seregin A, Boksha I, Dmitrieva E, Simutkin G, Kornetova E, et al. The difference in serum proteomes in schizophrenia and bipolar disorder. BMC Genomics. 2019 Jul;20((S7 Suppl 7)):535. doi: 10.1186/s12864-019-5848-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guo L, Zhang H, Hou Y, Wei T, Liu J. Plasmalemma vesicle-associated protein: A crucial component of vascular homeostasis. Exp Ther Med. 2016 Sep;12((3)):1639–44. doi: 10.3892/etm.2016.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rizzo G, Veronese M, Tonietto M, Bodini B, Stankoff B, Wimberley C, et al. Generalization of endothelial modelling of TSPO PET imaging: considerations on tracer affinities. J Cereb Blood Flow Metab. 2019 May;39((5)):874–85. doi: 10.1177/0271678X17742004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Notter T, Coughlin JM, Gschwind T, Weber-Stadlbauer U, Wang Y, Kassiou M, et al. Translational evaluation of translocator protein as a marker of neuroinflammation in schizophrenia. Mol Psychiatry. 2018 Feb;23((2)):323–34. doi: 10.1038/mp.2016.248. [DOI] [PubMed] [Google Scholar]
- 61.Joo HK, Lee YR, Kang G, Choi S, Kim CS, Ryoo S, et al. The 18-kDa Translocator Protein Inhibits Vascular Cell Adhesion Molecule-1 Expression via Inhibition of Mitochondrial Reactive Oxygen Species. Mol Cells. 2015 Dec;38((12)):1064–70. doi: 10.14348/molcells.2015.0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Riedel M, Krönig H, Schwarz MJ, Engel RR, Sikorski C, Kühn KU, et al. Investigation of the ICAM-1 G241A and A469G gene polymorphisms in schizophrenia. Mol Psychiatry. 2003 Mar;8((3)):257–8. doi: 10.1038/sj.mp.4001320. [DOI] [PubMed] [Google Scholar]
- 63.Krönig H, Riedel M, Schwarz MJ, Strassnig M, Möller HJ, Ackenheil M, et al. ICAM G241A polymorphism and soluble ICAM-1 serum levels: evidence for an active immune process in schizophrenia. Neuroimmunomodulation. 2005;12((1)):54–9. doi: 10.1159/000082364. [DOI] [PubMed] [Google Scholar]
- 64.Kavzoglu SO, Hariri AG. Intracellular Adhesion Molecule (ICAM-1), Vascular Cell Adhesion Molecule (VCAM-1) and E-Selectin Levels in First Episode Schizophrenic Patients. Klinik Psikofarmakol BBülteni. 2013 Sep;23((3)):205–14. [Google Scholar]
- 65.Nguyen TT, Dev SI, Chen G, Liou SC, Martin AS, Irwin MR, et al. Abnormal levels of vascular endothelial biomarkers in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2018 Dec;268((8)):849–60. doi: 10.1007/s00406-017-0842-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fillman SG, Cloonan N, Miller LC, Weickert CS. Markers of inflammation in the prefrontal cortex of individuals with schizophrenia. Mol Psychiatry. 2013 Feb;18((2)):133–133. doi: 10.1038/mp.2012.199. [DOI] [PubMed] [Google Scholar]
- 67.Crockett AM, Ryan SK, Vasquez AH, Canning C, Kanyuch N, Kebir H, et al. Disruption of the Blood-Brain Barrier in 22q11.2 Deletion Syndrome. Neuroscience; 2019 Nov; doi: 10.1093/brain/awab055. Available from: http://biorxiv.org/lookup/doi/10.1101/824987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Plavén-Sigray P, Cervenka S. Meta-analytic studies of the glial cell marker TSPO in psychosis - a question of apples and pears?: A commentary on ‘Neuroinflammation in schizophrenia: metaanalysis of in-vivo microglial imaging’ by Marques et al. - ERRATUM. Psychol Med. 2019 Jul;49((9)):1583–4. doi: 10.1017/S0033291719000746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Qosa H, Mohamed LA, Alqahtani S, Abuasal BS, Hill RA, Kaddoumi A. Transporters as Drug Targets in Neurological Diseases. Clin Pharmacol Ther. 2016 Nov;100((5)):441–53. doi: 10.1002/cpt.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sanchez-Covarrubias L, Slosky LM, Thompson BJ, Davis TP, Ronaldson PT. Transporters at CNS barrier sites: obstacles or opportunities for drug delivery? Curr Pharm Des. 2014;20((10)):1422–49. doi: 10.2174/13816128113199990463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lim RG, Quan C, Reyes-Ortiz AM, Lutz SE, Kedaigle AJ, Gipson TA, et al. Huntington's Disease iPSC-Derived Brain Microvascular Endothelial Cells Reveal WNT-Mediated Angiogenic and Blood-Brain Barrier Deficits. Cell Rep. 2017 May;19((7)):1365–77. doi: 10.1016/j.celrep.2017.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lippmann ES, Azarin SM, Kay JE, Nessler RA, Wilson HK, Al-Ahmad A, et al. Derivation of blood-brain barrier endothelial cells from human pluripotent stem cells. Nat Biotechnol. 2012 Aug;30((8)):783–91. doi: 10.1038/nbt.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Costas J. The highly pleiotropic gene SLC39A8 as an opportunity to gain insight into the molecular pathogenesis of schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2018 Mar;177((2)):274–83. doi: 10.1002/ajmg.b.32545. [DOI] [PubMed] [Google Scholar]
- 74.Eum S, Lee AM, Bishop JR. Pharmacogenetic tests for antipsychotic medications: clinical implications and considerations. Dialogues Clin Neurosci. 2016 Sep;18((3)):323–37. doi: 10.31887/DCNS.2016.18.3/jbishop. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brzozowska NI, de Tonnerre EJ, Li KM, Wang XS, Boucher AA, Callaghan PD, et al. The Differential Binding of Antipsychotic Drugs to the ABC Transporter P-Glycoprotein Predicts Cannabinoid-Antipsychotic Drug Interactions. Neuropsychopharmacology. 2017 Oct;42((11)):2222–31. doi: 10.1038/npp.2017.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hoosain FG, Choonara YE, Tomar LK, Kumar P, Tyagi C, du Toit LC, et al. Bypassing P-Glycoprotein Drug Efflux Mechanisms: Possible Applications in Pharmacoresistant Schizophrenia Therapy. BioMed Res Int. 2015;2015:484963. doi: 10.1155/2015/484963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lin YC, Ellingrod VL, Bishop JR, Miller DD. The relationship between P-glycoprotein (PGP) polymorphisms and response to olanzapine treatment in schizophrenia. Ther Drug Monit. 2006 Oct;28((5)):668–72. doi: 10.1097/01.ftd.0000246761.82377.a6. [DOI] [PubMed] [Google Scholar]
- 78.Decoster J, De Hert M, Viechtbauer W, Nagels G, Myin-Germeys I, Peuskens J, et al. Genetic association study of the P300 endophenotype in schizophrenia. Schizophr Res. 2012 Oct;141((1)):54–9. doi: 10.1016/j.schres.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 79.Kimchi-Sarfaty C, Oh JM, Kim IW, Sauna ZE, Calcagno AM, Ambudkar SV, et al. A “silent” polymorphism in the MDR1 gene changes substrate specificity. Science. 2007 Jan;315((5811)):525–8. doi: 10.1126/science.1135308. [DOI] [PubMed] [Google Scholar]
- 80.Fung KL, Pan J, Ohnuma S, Lund PE, Pixley JN, Kimchi-Sarfaty C, et al. MDR1 synonymous polymorphisms alter transporter specificity and protein stability in a stable epithelial monolayer. Cancer Res. 2014 Jan;74((2)):598–608. doi: 10.1158/0008-5472.CAN-13-2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tropeano M, Ahn JW, Dobson RJ, Breen G, Rucker J, Dixit A, et al. Male-Biased Autosomal Effect of 16p13.11 Copy Number Variation in Neurodevelopmental Disorders. Liu C, editor. PLoS ONE. 2013 Apr 18;8((4)):e61365. doi: 10.1371/journal.pone.0061365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Martínez-Magaña JJ, Genís-Mendoza AD, González-Covarrubias V, Jiménez-Guenchi J, Galindo-Chávez AG, Roche-Bergua A, et al. Exploratory Analysis of Rare and Novel Variants in Mexican Patients Diagnosed with Schizophrenia and Dementia. Rev Invest Clin. 2019;71((4)):246–54. doi: 10.24875/RIC.19002923. [DOI] [PubMed] [Google Scholar]
- 83.Comasco E, Vumma R, Toffoletto S, Johansson J, Flyckt L, Lewander T, et al. Genetic and Functional Study of L-Type Amino Acid Transporter 1 in Schizophrenia. Neuropsychobiology. 2016;74((2)):96–103. doi: 10.1159/000455234. [DOI] [PubMed] [Google Scholar]
- 84.Pickrell JK, Berisa T, Liu JZ, Ségurel L, Tung JY, Hinds DA. Detection and interpretation of shared genetic influences on 42 human traits. Nat Genet. 2016 Jul;48((7)):709–17. doi: 10.1038/ng.3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Torrico B, Shaw AD, Mosca R, Vivó-Luque N, Hervás A, Fernàndez-Castillo N, et al. Truncating variant burden in high-functioning autism and pleiotropic effects of LRP1 across psychiatric phenotypes. J Psychiatry Neurosci. 2019 Sep;44((5)):350–9. doi: 10.1503/jpn.180184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang Q, Li M, Yang Z, Hu X, Wu HM, Ni P, et al. Increased co-expression of genes harboring the damaging de novo mutations in Chinese schizophrenic patients during prenatal development. Sci Rep. 2015 Dec;5((1)):18209. doi: 10.1038/srep18209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Girard SL, Gauthier J, Noreau A, Xiong L, Zhou S, Jouan L, et al. Increased exonic de novo mutation rate in individuals with schizophrenia. Nat Genet. 2011 Jul;43((9)):860–3. doi: 10.1038/ng.886. [DOI] [PubMed] [Google Scholar]
- 88.Jouan L, Girard SL, Dobrzeniecka S, Ambalavanan A, Krebs MO, Joober R, et al. Investigation of rare variants in LRP1, KPNA1, ALS2CL and ZNF480 genes in schizophrenia patients reflects genetic heterogeneity of the disease. Behav Brain Funct. 2013 Feb;9((1)):9. doi: 10.1186/1744-9081-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Stokowy T, Polushina T, Sønderby IE, Karlsson R, Giddaluru S, Le Hellard S, et al. Genetic variation in 117 myelination-related genes in schizophrenia: replication of association to lipid biosynthesis genes. Sci Rep. 2018 May;8((1)):6915. doi: 10.1038/s41598-018-25280-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Emanuele E, Martinelli V, Carlin MV, Fugazza E, Barale F, Politi P. Serum levels of soluble receptor for advanced glycation endproducts (sRAGE) in patients with different psychiatric disorders. Neurosci Lett. 2011 Jan;487((1)):99–102. doi: 10.1016/j.neulet.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 91.Takeda M, Ohnuma T, Takeuchi M, Katsuta N, Maeshima H, Takebayashi Y, et al. Altered serum glyceraldehyde-derived advanced glycation end product (AGE) and soluble AGE receptor levels indicate carbonyl stress in patients with schizophrenia. Neurosci Lett. 2015 Apr;593:51–5. doi: 10.1016/j.neulet.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 92.Dwir D, Giangreco B, Xin L, Tenenbaum L, Cabungcal JH, Steullet P, et al. MMP9/RAGE pathway overactivation mediates redox dysregulation and neuroinflammation, leading to inhibitory/excitatory imbalance: a reverse translation study in schizophrenia patients. Mol Psychiatry. 2019 Mar 25; doi: 10.1038/s41380-019-0393-5. Available from: http://www.nature.com/articles/s41380–019–0393–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fu J, Zuo X, Yin J, Luo X, Li Z, Lin J, et al. Association of Polymorphisms of the Receptor for Advanced Glycation Endproducts Gene with Schizophrenia in a Han Chinese Population. BioMed Res Int. 2017;2017:6379639. doi: 10.1155/2017/6379639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Suchankova P, Klang J, Cavanna C, Holm G, Nilsson S, Jönsson EG, et al. Is the Gly82Ser polymorphism in the RAGE gene relevant to schizophrenia and the personality trait psychoticism? J Psychiatry Neurosci. 2012 Feb;37((2)):122–8. doi: 10.1503/jpn.110024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Prasad A, Bekker P, Tsimikas S. Advanced glycation end products and diabetic cardiovascular disease. Cardiol Rev. 2012 Jul-Aug;20((4)):177–83. doi: 10.1097/CRD.0b013e318244e57c. [DOI] [PubMed] [Google Scholar]
- 96.Srikanth V, Maczurek A, Phan T, Steele M, Westcott B, Juskiw D, et al. Advanced glycation endproducts and their receptor RAGE in Alzheimer's disease. Neurobiol Aging. 2011 May;32((5)):763–77. doi: 10.1016/j.neurobiolaging.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 97.Allen JA, Yadav PN, Setola V, Farrell M, Roth BL. Schizophrenia risk gene CAV1 is both pro-psychotic and required for atypical antipsychotic drug actions in vivo. Transl Psychiatry. 2011 Aug;1((8)):e33–33. doi: 10.1038/tp.2011.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kassan A, Egawa J, Zhang Z, Almenar-Queralt A, Nguyen QM, Lajevardi Y, et al. Caveolin-1 regulation of disrupted-in-schizophrenia-1 as a potential therapeutic target for schizophrenia. J Neurophysiol. 2017 Jan;117((1)):436–44. doi: 10.1152/jn.00481.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Walsh T, McClellan JM, McCarthy SE, Addington AM, Pierce SB, Cooper GM, et al. Rare Structural Variants Disrupt Multiple Genes in Neurodevelopmental Pathways in Schizophrenia. 2008;320:6. doi: 10.1126/science.1155174. [DOI] [PubMed] [Google Scholar]
- 100.Witjas FM, van den Berg BM, van den Berg CW, Engelse MA, Rabelink TJ. Concise Review: The Endothelial Cell Extracellular Matrix Regulates Tissue Homeostasis and Repair. Stem Cells Transl Med. 2019 Apr;8((4)):375–82. doi: 10.1002/sctm.18-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Baeten KM, Akassoglou K. Extracellular matrix and matrix receptors in blood-brain barrier formation and stroke. Dev Neurobiol. 2011 Nov;71((11)):1018–39. doi: 10.1002/dneu.20954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fowke TM, Karunasinghe RN, Bai JZ, Jordan S, Gunn AJ, Dean JM. Hyaluronan synthesis by developing cortical neurons in vitro. Sci Rep. 2017 Mar;7((1)):44135. doi: 10.1038/srep44135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Berretta S. Extracellular matrix abnormalities in schizophrenia. Neuropharmacology. 2012 Mar;62((3)):1584–97. doi: 10.1016/j.neuropharm.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Khandaker GM, Dantzer R. Is there a role for immune-to-brain communication in schizophrenia? Psychopharmacology (Berl) 2016 May;233((9)):1559–73. doi: 10.1007/s00213-015-3975-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang AW, Avramopoulos D, Lori A, Mulle J, Conneely K, Powers A, et al. Genome-wide association study in two populations to determine genetic variants associated with Toxoplasma gondii infection and relationship to schizophrenia risk. Prog Neuropsychopharmacol Biol Psychiatry. 2019 Jun;92:133–47. doi: 10.1016/j.pnpbp.2018.12.019. [DOI] [PubMed] [Google Scholar]
- 106.Xu R, Liang J, Luo Y, Wan X, Li K, Qi L, et al. Mass spectrometry identification of potential biomarker proteins in the 150-kD electrophoretic band in patients with schizophrenia. Medicine (Baltimore) 2018 Dec;97((51)):e13553. doi: 10.1097/MD.0000000000013553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Xu B, Ionita-Laza I, Roos JL, Boone B, Woodrick S, Sun Y, et al. De novo gene mutations highlight patterns of genetic and neural complexity in schizophrenia. Nat Genet. 2012 Dec;44((12)):1365–9. doi: 10.1038/ng.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nakata K, Ujike H, Sakai A, Takaki M, Imamura T, Tanaka Y, et al. Association study between the fibronectin gene and schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2003 Jan;116B((1)):41–4. doi: 10.1002/ajmg.b.10796. [DOI] [PubMed] [Google Scholar]
- 109.Kurian SM, Le-Niculescu H, Patel SD, Bertram D, Davis J, Dike C, et al. Identification of blood biomarkers for psychosis using convergent functional genomics. Mol Psychiatry. 2011 Jan;16((1)):37–58. doi: 10.1038/mp.2009.117. [DOI] [PubMed] [Google Scholar]
- 110.Prata DP, Costa-Neves B, Cosme G, Vassos E. Unravelling the genetic basis of schizophrenia and bipolar disorder with GWAS: A systematic review. J Psychiatr Res. 2019 Jul;114:178–207. doi: 10.1016/j.jpsychires.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 111.Sakai M, Watanabe Y, Someya T, Araki K, Shibuya M, Niizato K, et al. Assessment of copy number variations in the brain genome of schizophrenia patients. Mol Cytogenet. 2015 Jul;8((1)):46. doi: 10.1186/s13039-015-0144-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Martins Gomes SF, Westermann AJ, Sauerwein T, Hertlein T, Förstner KU, Ohlsen K, et al. Induced Pluripotent Stem Cell-Derived Brain Endothelial Cells as a Cellular Model to Study Neisseria meningitidis Infection. Front Microbiol. 2019 May;10:1181. doi: 10.3389/fmicb.2019.01181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lippmann ES, Al-Ahmad A, Azarin SM, Palecek SP, Shusta EV. A retinoic acid-enhanced, multicellular human blood-brain barrier model derived from stem cell sources. Sci Rep. 2014 Feb;4((1)):4160. doi: 10.1038/srep04160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Katt ME, Xu ZS, Gerecht S, Searson PC, Human Brain Microvascular Endothelial Cells Derived from the BC1 iPS Cell Line Exhibit a Blood-Brain Barrier Phenotype Deli MA, editor. PLOS ONE. 2016 Apr 12;11((4)):e0152105. doi: 10.1371/journal.pone.0152105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hollmann EK, Bailey AK, Potharazu AV, Neely MD, Bowman AB, Lippmann ES. Accelerated differentiation of human induced pluripotent stem cells to blood-brain barrier endothelial cells. Fluids Barriers CNS. 2017 Apr;14((1)):9. doi: 10.1186/s12987-017-0059-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Srinivasan B, Kolli AR. Transepithelial/Transendothelial Electrical Resistance (TEER) to Measure the Integrity of Blood-Brain Barrier. In: Barichello T, editor. Blood-Brain Barrier. New York (NY): Springer New York; 2019. pp. pp. 99–114. Available from http://link.springer.com/10.1007/978–1-4939–8946–1_6. [Google Scholar]
- 117.Kim BJ, Bee OB, McDonagh MA, Stebbins MJ, Palecek SP, Doran KS, et al. Modeling Group B Streptococcus and Blood-Brain Barrier Interaction by Using Induced Pluripotent Stem Cell-Derived Brain Endothelial Cells. D'Orazio SEF, editor. mSphere. 2017 Nov 1;2((6)):e00398–17. doi: 10.1128/mSphere.00398-17. /msphere/2/6/mSphere0398–17.atom. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Belair DG, Whisler JA, Valdez J, Velazquez J, Molenda JA, Vickerman V, et al. Human vascular tissue models formed from human induced pluripotent stem cell derived endothelial cells. Stem Cell Rev Rep. 2015 Jun;11((3)):511–25. doi: 10.1007/s12015-014-9549-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Whisler JA, Chen MB, Kamm RD. Control of perfusable microvascular network morphology using a multiculture microfluidic system. Tissue Eng Part C Methods. 2014 Jul;20((7)):543–52. doi: 10.1089/ten.tec.2013.0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lesman A, Koffler J, Atlas R, Blinder YJ, Kam Z, Levenberg S. Engineering vessel-like networks within multicellular fibrin-based constructs. Biomaterials. 2011 Nov;32((31)):7856–69. doi: 10.1016/j.biomaterials.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 121.Zhang B, Montgomery M, Chamberlain MD, Ogawa S, Korolj A, Pahnke A, et al. Biodegradable scaffold with built-in vasculature for organ-on-a-chip engineering and direct surgical anastomosis. Nat Mater. 2016 Jun;15((6)):669–78. doi: 10.1038/nmat4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Miller JS, Stevens KR, Yang MT, Baker BM, Nguyen DH, Cohen DM, et al. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat Mater. 2012 Sep;11((9)):768–74. doi: 10.1038/nmat3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chaturvedi RR, Stevens KR, Solorzano RD, Schwartz RE, Eyckmans J, Baranski JD, et al. Patterning vascular networks in vivo for tissue engineering applications. Tissue Eng Part C Methods. 2015 May;21((5)):509–17. doi: 10.1089/ten.tec.2014.0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Xia C, Fang NX. 3D microfabricated bioreactor with capillaries. Biomed Microdevices. 2009 Dec;11((6)):1309–15. doi: 10.1007/s10544-009-9350-4. [DOI] [PubMed] [Google Scholar]
- 125.Grigoryan B, Paulsen SJ, Corbett DC, Sazer DW, Fortin CL, Zaita AJ, et al. Multivascular networks and functional intravascular topologies within biocompatible hydrogels. Science. 2019 May;364((6439)):458–64. doi: 10.1126/science.aav9750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Griffith CK, George SC. The effect of hypoxia on in vitro prevascularization of a thick soft tissue. Tissue Eng Part A. 2009 Sep;15((9)):2423–34. doi: 10.1089/ten.tea.2008.0267. [DOI] [PubMed] [Google Scholar]
- 127.Griffith CK, Miller C, Sainson RC, Calvert JW, Jeon NL, Hughes CC, et al. Diffusion limits of an in vitro thick prevascularized tissue. Tissue Eng. 2005 Jan-Feb;11((1-2)):257–66. doi: 10.1089/ten.2005.11.257. [DOI] [PubMed] [Google Scholar]
- 128.Nehls V, Drenckhahn D. A microcarrier-based cocultivation system for the investigation of factors and cells involved in angiogenesis in three-dimensional fibrin matrices in vitro. Histochem Cell Biol. 1995 Dec;104((6)):459–66. doi: 10.1007/BF01464336. [DOI] [PubMed] [Google Scholar]
- 129.Nakatsu MN, Sainson RC, Aoto JN, Taylor KL, Aitkenhead M, Pérez-del-Pulgar S, et al. Angiogenic sprouting and capillary lumen formation modeled by human umbilical vein endothelial cells (HUVEC) in fibrin gels: the role of fibroblasts and Angiopoietin-1. Microvasc Res. 2003 Sep;66((2)):102–12. doi: 10.1016/s0026-2862(03)00045-1. [DOI] [PubMed] [Google Scholar]
- 130.Vatine GD, Barrile R, Workman MJ, Sances S, Barriga BK, Rahnama M, et al. Human iPSC-Derived Blood-Brain Barrier Chips Enable Disease Modeling and Personalized Medicine Applications. Cell Stem Cell. 2019 Jun;24((6)):995–1005.e6. doi: 10.1016/j.stem.2019.05.011. [DOI] [PubMed] [Google Scholar]
- 131.Faley SL, Neal EH, Wang JX, Bosworth AM, Weber CM, Balotin KM, et al. iPSC-Derived Brain Endothelium Exhibits Stable, Long-Term Barrier Function in Perfused Hydrogel Scaffolds. Stem Cell Reports. 2019 Mar;12((3)):474–87. doi: 10.1016/j.stemcr.2019.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Park TE, Mustafaoglu N, Herland A, Hasselkus R, Mannix R, FitzGerald EA, et al. Hypoxia-enhanced Blood-Brain Barrier Chip recapitulates human barrier function and shuttling of drugs and antibodies. Nat Commun. 2019 Jun;10((1)):2621. doi: 10.1038/s41467-019-10588-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Campisi M, Shin Y, Osaki T, Hajal C, Chiono V, Kamm RD. 3D self-organized microvascular model of the human blood-brain barrier with endothelial cells, pericytes and astrocytes. Biomaterials. 2018 Oct;180:117–29. doi: 10.1016/j.biomaterials.2018.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Liu Y, Chen BP, Lu M, Zhu Y, Stemerman MB, Chien S, et al. Shear stress activation of SREBP1 in endothelial cells is mediated by integrins. Arterioscler Thromb Vasc Biol. 2002 Jan;22((1)):76–81. doi: 10.1161/hq0102.101822. [DOI] [PubMed] [Google Scholar]
- 135.White CR, Haidekker M, Bao X, Frangos JA. Temporal gradients in shear, but not spatial gradients, stimulate endothelial cell proliferation. Circulation. 2001 May;103((20)):2508–13. doi: 10.1161/01.cir.103.20.2508. [DOI] [PubMed] [Google Scholar]
- 136.Conklin BS, Zhong DS, Zhao W, Lin PH, Chen C. Shear stress regulates occludin and VEGF expression in porcine arterial endothelial cells. J Surg Res. 2002 Jan;102((1)):13–21. doi: 10.1006/jsre.2001.6295. [DOI] [PubMed] [Google Scholar]
- 137.Homan KA, Gupta N, Kroll KT, Kolesky DB, Skylar-Scott M, Miyoshi T, et al. Flow-enhanced vascularization and maturation of kidney organoids in vitro. Nat Methods. 2019 Mar;16((3)):255–62. doi: 10.1038/s41592-019-0325-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang YI, Abaci HE, Shuler ML. Microfluidic blood-brain barrier model provides in vivo-like barrier properties for drug permeability screening. Biotechnol Bioeng. 2017 Jan;114((1)):184–94. doi: 10.1002/bit.26045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Song L, Yuan X, Jones Z, Griffin K, Zhou Y, Ma T, et al. Assembly of Human Stem Cell-Derived Cortical Spheroids and Vascular Spheroids to Model 3-D Brain-like Tissues. Sci Rep. 2019 Apr;9((1)):5977. doi: 10.1038/s41598-019-42439-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mansour AA, Gonçalves JT, Bloyd CW, Li H, Fernandes S, Quang D, et al. An in vivo model of functional and vascularized human brain organoids. Nat Biotechnol. 2018 Jun;36((5)):432–41. doi: 10.1038/nbt.4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pham MT, Pollock KM, Rose MD, Cary WA, Stewart HR, Zhou P, et al. Generation of human vascularized brain organoids. Neuroreport. 2018 May;29((7)):588–93. doi: 10.1097/WNR.0000000000001014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Huang CY, Liu CL, Ting CY, Chiu YT, Cheng YC, Nicholson MW, et al. Human iPSC banking: barriers and opportunities. J Biomed Sci. 2019 Oct;26((1)):87. doi: 10.1186/s12929-019-0578-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wakui T, Matsumoto T, Matsubara K, Kawasaki T, Yamaguchi H, Akutsu H. Method for evaluation of human induced pluripotent stem cell quality using image analysis based on the biological morphology of cells. J Med Imaging (Bellingham) 2017 Oct;4((4)):044003. doi: 10.1117/1.JMI.4.4.044003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Qian T, Maguire SE, Canfield SG, Bao X, Olson WR, Shusta EV, et al. Directed differentiation of human pluripotent stem cells to blood-brain barrier endothelial cells. Sci Adv. 2017 Nov;3((11)):e1701679. doi: 10.1126/sciadv.1701679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Patel JP, Frey BN. Disruption in the Blood-Brain Barrier: The Missing Link between Brain and Body Inflammation in Bipolar Disorder? Neural Plast. 2015;2015:708306. doi: 10.1155/2015/708306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Halliday MR, Rege SV, Ma Q, Zhao Z, Miller CA, Winkler EA, et al. Accelerated pericyte degeneration and blood-brain barrier breakdown in apolipoprotein E4 carriers with Alzheimer's disease. J Cereb Blood Flow Metab. 2016 Jan;36((1)):216–27. doi: 10.1038/jcbfm.2015.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bitanihirwe BK, Woo TW. A conceptualized model linking matrix metalloproteinase-9 to schizophrenia pathogenesis. Schizophr Res. 2020 Apr;218:28–35. doi: 10.1016/j.schres.2019.12.015. [DOI] [PubMed] [Google Scholar]
- 148.Schnack HG, van Haren NEM, Nieuwenhuis M, Hulshoff Pol HE, Cahn W, Kahn RS. Accelerated Brain Aging in Schizophrenia: A Longitudinal Pattern Recognition Study. Am J Psychiatry. 2016 Jan;173((6)):607–616. doi: 10.1176/appi.ajp.2015.15070922. [DOI] [PubMed] [Google Scholar]
- 149.Jr S. Blood-brain barrier dysfunction in aging induces hyperactivation of TGFβ signaling and chronic yet reversible neural dysfunction. Sci Transl Med. 2019;•••:15. doi: 10.1126/scitranslmed.aaw8283. [DOI] [PubMed] [Google Scholar]
- 150.Müller N. The Role of Intercellular Adhesion Molecule-1 in the Pathogenesis of Psychiatric Disorders. Front Pharmacol. 2019 Nov;10:1251. doi: 10.3389/fphar.2019.01251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Blank T, Detje CN, Spieß A, Hagemeyer N, Brendecke SM, Wolfart J, et al. Brain Endothelial- and Epithelial-Specific Interferon Receptor Chain 1 Drives Virus-Induced Sickness Behavior and Cognitive Impairment. Immunity. 2016 Apr;44((4)):901–12. doi: 10.1016/j.immuni.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 152.Dudek KA, Dion-Albert L, Lebel M, LeClair K, Labrecque S, Tuck E, et al. Molecular adaptations of the blood-brain barrier promote stress resilience vs. depression. Proc Natl Acad Sci USA. 2020 Feb;117((6)):3326–36. doi: 10.1073/pnas.1914655117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Menard C, Pfau ML, Hodes GE, Kana V, Wang VX, Bouchard S, et al. Social stress induces neurovascular pathology promoting depression. Nat Neurosci. 2017 Dec;20((12)):1752–60. doi: 10.1038/s41593-017-0010-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Falcone T, Carlton E, Lee C, Janigro M, Fazio V, Forcen FE, et al. Does Systemic Inflammation Play a Role in Pediatric Psychosis? Clin Schizophr Relat Psychoses. 2015;9((2)):65–78B. doi: 10.3371/CSRP.FACA.030813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Trépanier MO, Hopperton KE, Mizrahi R, Mechawar N, Bazinet RP. Postmortem evidence of cerebral inflammation in schizophrenia: a systematic review. Mol Psychiatry. 2016 Aug;21((8)):1009–26. doi: 10.1038/mp.2016.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sarrouilhe D, Dejean C, Mesnil M. Connexin43- and Pannexin-Based Channels in Neuroinflammation and Cerebral Neuropathies. Front Mol Neurosci. 2017 Oct;10:320. doi: 10.3389/fnmol.2017.00320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2011 Oct;70((7)):663–71. doi: 10.1016/j.biopsych.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mohite S, Yang F, Amin PA, Zunta-Soares G, Colpo GD, Stertz L, et al. Plasma soluble L-selectin in medicated patients with schizophrenia and healthy controls. In: Zhang XY, editor. PLOS ONE. (3) Vol. 12. 2017. Mar 23. p. e0174073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Parish CR. The role of heparan sulphate in inflammation. Nat Rev Immunol. 2006 Sep;6((9)):633–43. doi: 10.1038/nri1918. [DOI] [PubMed] [Google Scholar]
- 160.Steiner J, Walter M, Wunderlich MT, Bernstein HG, Panteli B, Brauner M, et al. A new pathophysiological aspect of S100B in schizophrenia: potential regulation of S100B by its scavenger soluble RAGE. Biol Psychiatry. 2009 Jun;65((12)):1107–10. doi: 10.1016/j.biopsych.2008.10.044. [DOI] [PubMed] [Google Scholar]