Abstract
Total urinary 11-nor-9-carboxy-tetrahydrocannabinol (THCCOOH) concentrations are generally reported following cannabis administration. Few data are available for glucuronide and minor cannabinoid metabolite concentrations. All urine specimens from 11 frequent and 9 occasional cannabis users were analyzed for 11 cannabinoids for ~85 h by liquid chromatography with tandem mass spectrometry following controlled smoked, vaporized or oral 50.6 mg Δ9-tetrahydrocannabinol (THC) in a randomized, placebo-controlled, within-subject dosing design. No cannabidiol, cannabinol, cannabigerol, tetrahydrocannabivarin (THCV), THC, 11-OH-THC and Δ9-tetrahydrocannabinolic acid were detected in urine. Median THCCOOH-glucuronide maximum concentrations (Cmax) following smoked, vaporized and oral routes were 68.0, 26.7 and 360 μg/L for occasional and 378, 248 and 485 μg/L for frequent users, respectively. Median time to specific gravity-normalized Cmax (Tmax) was 5.1–7.9 h for all routes and all users. Median Cmax for THCCOOH, THC-glucuronide and 11-nor-9-carboxy-Δ9-THCV (THCVCOOH) were <7.5% of THCCOOH-glucuronide Cmax concentrations. Only THC-glucuronide mean Tmax differed between routes and groups, and was often present only in occasional users’ first urine void. Multiple THCCOOH-glucuronide and THCCOOH peaks were observed. We also evaluated these urinary data with published models for determining recency of cannabis use. These urinary cannabinoid marker concentrations from occasional and frequent cannabis users following three routes of administration provide a scientific database to assess single urine concentrations in cannabis monitoring programs. New target analytes (CBD, CBN, CBG, THCV and phase II metabolites) were not found in urine. The results are important to officials in drug treatment, workplace and criminal justice drug monitoring programs, as well as policy makers with responsibility for cannabis regulations.
Introduction
Cannabis is the most commonly abused drug in the United States after alcohol, with past month use in 26.0 million individuals age 12 and older (1). Medicinal cannabinoids are currently approved in 47 states and recreational use in 11 states and Washington, D.C. The prevalence of cannabis use is not expected to decline. More than 30 years of research, which was recently reviewed, provided important information on cannabinoids and their metabolism and pharmacokinetics (2).
Δ9-Tetrahydrocannabinol (THC) is metabolized to 11-hydroxy-THC (11-OH-THC) and 11-nor-9-carboxy-THC (THCCOOH), which are excreted primarily into urine as glucuronidated metabolites. Total THCCOOH in urine after alkaline hydrolysis was examined in six participants who smoked 15.8 and 33.8 mg THC cigarettes (3,4). Detection times were <2 days with a 50 μg/L cutoff, and maximum concentrations (Cmax) were 20.6–234 and 29.9–355 μg/L, respectively.
Smoking is the commonest route of cannabis administration, but oral ingestion is much more common with legalized edibles and oral medications. Gustafson et al. (5,6) reported mean positive screening rates following oral 7.5 mg Marinol or 14.8 mg hemp oil THC of 23.5–45.7%, with Cmax 19.0–436 μg/L. Mean elimination half-lives (T1/2) for urine THCCOOH to 15 μg/L was 24 ± 7.8 h for the 7.5 mg and 21 ± 4.3 h for the 14.8 mg THC doses, with a terminal T1/2 of 2–3 days. Vaporization of cannabis is also common, with 9.4% of cannabis users in the UK stating they vaped for recreational drug use (7). This route was recently investigated in controlled cannabis administration studies (8,9).
The importance of cannabis use history to predict urinary detection windows was recognized as early as 1985 when some of 86 chronic cannabis smokers were still immunoassay positive >20 μg/L after 30 days of abstinence (10). Many studies since then examined urinary excretion profiles of frequent cannabis users. Sixty frequent cannabis users were divided into three groups based on creatinine-normalized total THCCOOH concentrations in the first urine collected: 0–50, 51–150 and >150 μg/g (11). Mean days to last positive urine ≥ 50 μg/L immunoassay screen were 4.3, 9.7 and 15.4 days, respectively. Total THC was positive (LOQ = 2.5 μg/L) in the urine of seven frequent cannabis smokers for 3, 3, 4, 7, 7, 12 and 24 days of sustained abstinence (12). Two models for predicting new cannabis use between two urine specimens were developed, one for occasional users (13) and one for frequent users (14).
Urine cannabinoid excretion was generally reported as total THCCOOH after hydrolysis because direct THCCOOH-glucuronide measurement was not yet available. In 1992, THCCOOH-glucuronide was shown to be the primary urinary metabolite, with little free THCCOOH identified (15). Now liquid chromatography–tandem mass spectrometry (LC–MS-MS) directly quantifies free and glucuronide cannabinoids and metabolites (16). Desrosiers et al. (17) reported no measurable free THC, 11-OH-THC, cannabidiol (CBD) or cannabinol (CBN) in urine from occasional and frequent users following smoking of a 6.8% THC cigarette. Only 60% of occasional smokers’ urine specimens contained THCCOOH.
A randomized, double-blind, placebo-controlled, double-dummy, within-subject study of administration of 50.6 mg THC by the smoked, vaporized and oral routes was conducted (18). Free and glucuronide whole blood cannabinoid results for 11 frequent and 9 occasional cannabis smokers were reported. Here, we report 11 free and glucuronide urinary cannabinoids and metabolites collected in this study (19). Specific gravity (SG) and creatinine were also determined.
Materials and methods
This study was previously described in detail (18). Letters identifying participants here are the same as in that publication.
Participants
Healthy adults (18–50 years) were recruited for this study that was approved by National Institute on Drug Abuse Institutional Review Board, Food and Drug Administration and Drug Enforcement Administration. Inclusion criteria were a mean self-reported cannabis intake frequency ≥2×/month but ≤3×/week (occasional smokers) or ≥5×/week (frequent smokers) over the previous 3 months and a positive urine cannabinoid screen for frequent smokers. Participants provided written, informed consent to participate.
Study design
The study was randomized, double-blind and placebo-controlled with a crossover and double-dummy design. Participants entered the secure research unit ~19 h before dosing to preclude acute intoxication during baseline measures the following day. Cannabis cigarettes from the National Institute on Drug Abuse containing 50.6 mg THC, 1.5 mg CBD and 3.3 mg CBN or placebo were smoked, vaporized (210°C, Volcano Medic, Storz & Bickel) or ingested (Duncan Hines® Double Fudge brownie). Participants ingested a brownie within 10 min (Oral A) and either smoked or inhaled from a vaporizer in the next 10 min with only one active dose/session. In an optional fifth session, participants only ingested 50.6 mg THC in a brownie over 10 min (Oral B). The purpose of Oral B was to evaluate cannabinoids in oral fluid after oral cannabis, eliminating potential contribution from inhaled cannabis (20). Frequent smokers remained on the unit ~85 h post-dose and had to leave for ≥72 h to minimize withdrawal. Occasional smokers remained on the unit ~61 h post-dose and could remain on the unit for consecutive sessions, as long as they were not dosed more frequently than their self-reported intake frequency. During the Oral B protocol, all participants resided for 48 h post-dose. Every urine specimen was individually analyzed from admission to the end of all sessions. Urine specimens were stored at −20°C within 2 h.
Urine cannabinoid analysis
Urine samples were simultaneously analyzed for THC, 11-OH-THC, THCCOOH, CBN, CBD, THCCOOH-glucuronide, THC-glucuronide, cannabigerol (CBG), ∆9-tetrahydrocannabinolic acid (THCAA), Δ9-tetrahydrocannabivarin (THCV) and 11-nor-9-carboxy-THCV (THCVCOOH) with a previously reported LC–MS-MS method (19). 11-OH-THC-glucuronide and THCAA-glucuronide were not examined because no reference standards are available. Linear ranges were 0.5–100 μg/L for THC and THCCOOH; 0.5–50 μg/L for 11-OH-THC, CBD, CBN, THCAA and THC-glucuronide; 1–100 μg/L for CBG, THCV and THCVCOOH; and 5–500 μg/L for THCCOOH-glucuronide (R2 > 0.99). Analytical biases were 88.3–113.7%, imprecisions 3.3–14.3%, extraction efficiencies 42.4–81.5% and matrix effects −10–32.5%. Matched deuterated internal standards were commercially available and utilized for the first six analytes.
Specific gravity analysis
SG was measured to three decimals using an Atago-10S 3-digit refractometer (Cole-Palmer, Vernon Hills, IL) as reported previously (21). Cannabinoid concentrations were normalized to SG (22).
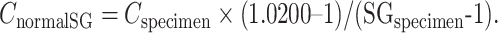
(CnormalSG, cannabinoid concentration normalized to SG; Cspecimen, specimen cannabinoid concentration; SGspecimen, specimen SG).
Creatinine analysis
Urine creatinine measurements were reported previously (21). The linear range was 0.006–6.00 g/L (R2 > 0.99), intraday imprecision 0.8–1.2% and interday imprecision 1.2–1.4%. Cannabinoid concentrations were normalized to creatinine concentrations as follows:
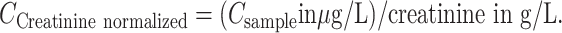
(CCreatinine normalized, creatinine-normalized analyte concentration)
Data analysis
Data were compartmentalized by each administration route session for a participant. After dosing, Cmax, time from start of dosing to Cmax (Tmax), first analyte concentration ≥ LOQ (Cfirst), time to Cfirst (Tfirst), last analyte concentration ≥ LOQ (Clast) and time of Clast (Tlast) were determined for each analyte and each session. Percent of specimens with analyte concentration ≥ LOQ, and percent positive for (THCCOOH-glucuronide + THCCOOH) ≥ 15 μg/L, the confirmation cutoff for workplace drug testing, were calculated. After determining each parameter, the mean (median) [range] was computed for the occasional and frequent smoker groups.
Statistics
C max, Tmax, C1st, T1st, Clast and Tlast were determined. A repeated-measures regression model was run for each parameter, with parameter as the dependent variable and route (oral, vapor or smoked) as the independent variable. Outcome variables were log10-transformed to correct skewness with the exceptions of Tlast and percent positive. Oral sessions A and B were combined. Routes were compared as follows: oral vs. vaporized, vaporized vs. smoked and oral vs. smoked. Frequent and occasional smokers were also compared on all parameters (Cmax, Tmax, C1st, T1st, Clast, Tlast, % ≥ LOQ) for each analyte with a common route of administration (oral, smoked, vaporized).
Pearson correlation coefficients were calculated to determine the degree of similarity between raw and specific gravity-normalized values for each analyte. Before calculating correlations, zeroes were removed from the dataset and non-zero concentrations were log10-transformed to make their right-skewed distributions more Gaussian. Because correlation coefficients do not account for within-subject data, which can lead to a Type I error, these four associations were also assessed using repeated-measures regression models to verify that conclusions did not change.
Results
Eleven frequent (nine males) and nine occasional (six males) cannabis smokers completed the study. Every urine void throughout all sessions was analyzed by LC–MS-MS for a total of 2232 specimens.
Analyte parameters
THC, 11-OH-THC, CBD, CBN, CBG, THCV and THCAA were not detected in any urine specimen. THCCOOH-glucuronide, THC-glucuronide, THCCOOH and THCVCOOH results are found in Table I. One occasional smoking participant (M) had no detectable analytes after an active smoking dose; these data were excluded from Cmax, Tmax, Cfirst, Tfirst, Clast and Tlast. The most likely explanation is that this occasional user ingested a low THC dose on this occasion due to an inadequate smoking topography or pattern of smoking that includes the number and length of puffs, time between puffs and depth of inhalation. Table II includes percent detected for each analyte in each session. Also displayed is the positive rate at the NLCP confirmation cutoff concentration of THCCOOH+THCCOOH-glucuronide ≥15 μg/L. Mean, median and range are presented for each parameter. The range in collection times is also shown because some frequent users remained above cutoff concentrations at the conclusion of the session.
Table I.
Urinary Parameters following Administration of 50.6 mg THC by Different Routes in Occasional and Frequent Cannabis Users
| THCCOOH-glucuronidea | |||||||
|---|---|---|---|---|---|---|---|
| C max (μg/L) | T max (h) | C first (μg/L) | T first (h) | C last (μg/L) | T last (h) | ||
| Occasional | n | Mean (median) [range] | Mean (median) [range] | Mean (median) [range] | Mean (median) [range] | Mean (median) [range] | Mean (median) [range] |
| Smoked | 8 | 55.2 (68.0) [14.4–80.6] | 10.2 (6.6) [5.0–23.0] | 19.4 (11.0) [7.29–78.3] | 2.0 (1.8) [0.2–4.3] | 9.7 (9.3) [5.8–13.9] | 50.2 (49.9) [39.2–60.6] |
| Vaped | 9 | 45.7 (26.7) [8.41–166] | 9.6 (8.0) [3.7–25.9] | 27.3 (16.5) [8.4–99.1) | 3.9 (3.7) [0.5–6.3] | 8.6 (9.0) [6.1–12.9] | 42.8 (46.7) [23.0–59.3) |
| Oral A | 9 | 359 (354) [117–667] | 10.8 (10.3) [5.5–19.0] | 130 (34.3) [6.9–566] | 4.0 (1.7) [0.5–10.3] | 21.8 (20.7) [10.8–34.1] | 54.0 (52.9) [48.9–59.9] |
| Oral B | 7 | 369 (372) [75.3–767] | 13.8 (14.2) [6.4–20.1] | 64.6 (20.1) [5.39–335) | 3.0 (1.8) [1.7–6.2] | 37.5 (33.5) [23.7–57.2] | 47.0 (46.8) [46.0–48.1] |
| Frequent | |||||||
| Smoked | 11 | 580 (378) [114–1680] | 11.5 (7.1) [3.8–24.2] | 478 (308) [34.3–1680] | 3.6 (1.9) [0.4–12.2] | 228 (79.2) [19.8–927] | 72.7 (71.0) [59.8–85.7] |
| Vaped | 11 | 517 (248) [54.1–1543] | 20.5 (6.3) [1.9–68.0] | 284 (123) [19.3–926] | 2.2 (1.3) [0.1–7.8] | 207 (47.0) [11.3–1540] | 69.1 (68.3) [50.8–82.1] |
| Oral A | 11 | 790 (537) [244–2010] | 10.3 (7.2) [5.2–30.0] | 412 (189) [13.5–1230] | 2.1 (1.6) [0.1–6.1] | 176 (135) [32.7–519] | 70.8 (71.1) [57.7–85.6] |
| Oral B | 8 | 792 (434) [134–3530] | 14.1 (8.0) [5.0–31.4] | 569 (106) [20.1–3530] | 3.8 (2.1) [0.1–14.6] | 204 (80.5) [22.0–897] | 46.7 (47.3) [43.9–47.6] |
| THC-glucuronide b | |||||||
| C max (μg/L) | T max (h) | C first (μg/L) | T first (h) | C last (μg/L) | Tlast (h) | ||
| Occasional | n | Mean (Median) [Range] | Mean (Median) [Range] | Mean (Median) [Range] | Mean (Median) [Range] | Mean (Median) [Range] | Mean (Median) [Range] |
| Smoked | 8 | 6.0 (5.1) [0.5–17.8] | 6.3 (1.8) [0.2–37.8] | 6.0 (5.1) [0.5–17.8] | 1.7 (1.5) [0.2–4.3] | 1.2 (0.8) [0.5–2.6] | 17.2 (13.2) [0.2–47.8] |
| Vaped | 9 | 3.4 (1.8) [0.9–12.8] | 1.3 (1.0) [0.5–3.2] | 3.4 (1.8) [0.9–12.8] | 1.3 (1.0) [0.5–3.2] | 1.0 (0.7) [0.5–2.3] | 10.1 (6.0) [1.2–25.9] |
| Oral A | 9 | 9.7 (4.8) [2.4–23.2] | 7.1 (5.5) [3.2–14.2] | 4.2 (1.7) [0.5–22.3] | 3.7 (2.0) [1.4–9.4] | 3.2 (1.0) [0.6–20.6] | 17.9 (19.9) [9.3–25.1] |
| Oral B | 7 | 8.1 (5.6) [2.0–28.9] | 8.3 (6.5) [3.1–20.0] | 5.6 (1.7) [0.9–28.9] | 3.6 (3.1) [1.7–6.2] | 1.3 (0.8) [0.5–3.7] | 24.0 (20.1) [8.2–46.7] |
| Frequent | |||||||
| Smoked | 11 | 13.8 (9.9) [4.6–40.3] | 10.7 (5.0) [1.8–42.6] | 12.3 (9.9) [2.0–40.3] | 3.6 (1.9) [0.4–12.2] | 5.9 (2.5) [0.5–19.5] | 71.5 (71.0) [50.0–85.7] |
| Vaped | 11 | 12.1 (7.1) [4.4–29.2] | 18.6 (2.4) [0.7–68.0] | 8.7 (6.1) [1.5–26.3] | 2.3 (1.3) [0.6–7.75] | 7.0 (1.7) [0.6–29.2] | 61.1 (68.0) [12.3–82.1] |
| Oral A | 11 | 17.0 (14.7) [3.8–37.1] | 11.7 (6.1) [3.9–44.0] | 9.6 (6.1) [0.6–37.1] | 3.0 (2.0) [0.4–6.7] | 3.4 (2.1) [0.7–10.2] | 70.8 (71.1) [57.7–85.6] |
| Oral B | 8 | 30.5 (19.1) [2.7–76.0) | 5.9 (5.0) [2.4–14.6] | 24.0 (14.6) [1.4–72.4] | 5.4 (4.6) [1.2–14.6] | 10.9 (3.3) [0.6–72.4] | 40.5 (45.7) [6.8–47.6] |
| THCCOOH c | |||||||
| C max (μg/L) | T max (h) | C first (μg/L) | T first (h) | C last (μg/L) | T last (h) | ||
| Occasional | n | Mean (Median) [Range] | Mean (Median) [Range] | Mean (Median) [Range] | Mean (Median) [Range] | Mean (Median) [Range] | Mean (Median) [Range] |
| Smoked | 5d | 1.6 (1.6) [0.8–2.8] | 20.6 (19.5) [4.3–43.9] | 1.3 (0.8) [0.7–2.8] | 9.9 (7.3) [4.3–18.0] | 1.1 (1.1) [0.5–1.8] | 35.7 (43.9) [19.5–47.6] |
| Vaped | 7e | 1.2 (0.8) [0.6–3.8] | 12.1 (9.9) [4.5–25.9] | 1.1 (0.8) [0.6–2.9] | 9.6 (6.3) [3.2–25.9] | 0.7 (0.6) [0.5–1.2] | 14.9 (11.9) [5.6–25.9] |
| Oral A | 9 | 10.2 (10.6) [1.6–28.2] | 12.8 (9.4) [5.5–33.2] | 6.2 (1.6) [0.5–28.3] | 6.9 (7.9) [1.2–12.9] | 1.0 (0.8) [0.6–1.7] | 47.3 (49.1) [30.2–55.3] |
| Oral B | 7 | 10.5 (10.2) [2.6–26.0] | 16.4 (19.5) [6.8–24.8] | 5.1 (4.0) [0.6–14.0] | 7.3 (6.4) [2.8–20.0] | 1.7 (1.5) [0.6–3.2] | 42.6 (46.7) [19.8–47.5] |
| Frequent | |||||||
| Smoked | 11 | 28.9 (16.1) [3.2–105] | 32.2 (44.0) [3.8–67.8] | 12.8 (9.0) [0.6–61.2] | 4.6 (3.8) [0.7–12.2] | 8.3 (1.8) [0.6–37.7] | 72.1 (70.1) [54.0–85.7] |
| Vaped | 11 | 35.9 (8.8) [1.4–218] | 24.0 (19.6) [3.6–68.0] | 6.8 (4.3) [0.8–21.0] | 3.3 (2.6) [0.7–7.8] | 30.0 (1.6) [0.5–218] | 66.0 (69.6) [20.0–82.1] |
| Oral A | 11 | 38.8 (12.8) [4.4–184] | 37.8 (46.9) [2.5–67.8] | 16.6 (9.4) [0.5–64.7] | 3.6 (2.5) [0.6–7.3] | 6.0 (2.7) [0.8–24.4] | 70.6 (70.6) [57.7–85.6] |
| Oral B | 8 | 34.1 (11.0) [3.5–155] | 23.6 (23.1) [6.8–44.0] | 27.6 (2.8) [0.8–155] | 5.1 (4.0) [0.5–14.6] | 22.6 (2.7) [0.7–101] | 40.7 (46.6) [6.8–47.6] |
| THCVCOOH f | |||||||
| C max (μg/L) | T max (h) | C first (μg/L) | T first (h) | C last (μg/L) | T last (h) | ||
| Occasional | n | Mean (Median) [Range] | Mean (Median) [Range] | Mean (Median) [Range] | Mean (Median) [Range] | Mean (Median) [Range] | Mean (Median) [Range] |
| Smoked | 0 | g | g | g | g | g | g |
| Vaped | 1 | 1.28i | 20.4i | i | i | i | i |
| Oral A | 9 | 3.8 (3.1) ]1.0–10.0] | 10.2 (9.4) [5.5–19.0] | 3.7 (2.6) [1.0–10.0] | 8.1 (7.1) [4.8–14.2] | 2.4 (1.4) [1.0–10.0] | 20.9 (20.4) [6.2–44.2] |
| Oral B | 7 | 3.9 (2.5) [1.4–10.9] | 14.2 (13.0) [6.0–24.8] | 3.3 (1.6) [1.1–10.9] | 8.5 (6.8) [2.8–20.0] | 3.6 (2.5) [1.4–10.9] | 17.2 (19.5) [6.8–24.8] |
| Frequent | |||||||
| Smoked | 8j | 3.7 (3.2) [1.4–10.0] | 234.6 (17.9) [1.8–50.8] | 2.5 (2.3) [1.4–4.6] | 9.6 (6.7) [0.7–24.2] | 2.2 (1.9) [1.1–3.9] | 61.1 (63.8) [19.9–85.7] |
| Vaped | 8k | 4.2 (2.9) [1.2–9.2] | 22.4 (20.2) [2.4–68.2] | 2.5 (1.8) [1.0–7.7] | 4.7 (5.5) [0.7–7.8] | 3.1 (1.8) [1.0–9.2] | 47.0 (57.4) [5.5–68.3] |
| Oral A | 11 | 3.5 (3.2) [1.1–7.1] | 22.6 (20.2) [3.9–47.8] | 2.4 (2.0) [1.0–4.1] | 8.6 (6.1) [0.6–27.1] | 2.0 (2.0) [1.0–3.1] | 47.3 (47.8) [20.2–74.9] |
| Oral B | 8 | 5.7 (2.6) [1.9–17.9] | 17.5 (14.4) [6.8–43.9] | 5.1 (2.4) [1.5–17.9] | 9.2 (6.9) [2.4–21.0] | 3.5 (2.2) [1.1–16.1] | 31.6 (30.9) [6.8–47.6] |
a≥5 μg/L.
b≥0.5 μg/L.
c≥0.5 μg/L.
dParticipants M, P, R & T had no THCCOOH, zero values not included in statistics.
eParticipants M & N had no THCCOOH, zero values not included in statistics.
f≥1 μg/L.
gNo participant had detectable THCVCOOH.
hParticipant Q had 2 THCVCOOH-positive samples (18.2%). All other subjects had none detected.
iParticipants B, G & K had no THCVCOOH ≥ LOQ, zero values not included in statistics.
jParticipants G, H & K had no THCVCOOH ≥ LOQ, zero values not included in statistics.
Table II.
Detection Rates at the LOQ following Administration of 50.6 mg THC by the Smoked, Vaporized and Oral Routes in Occasional and Frequent Cannabis Users*
| THCCOOH-glucuronide | |||||
|---|---|---|---|---|---|
| % Detecteda | Last collection (h) | % Positive | |||
| Occasional | n | Mean (median) [range] | Mean (median) [range] | Mean (median) [range] | |
| Smoked | 9 | 68.6 (77.4) [0–100] | 56.3 (53.3) [47.3–78.4] | 27.9 (26.9) [0–57.1] | |
| Vaporized | 9 | 45.9 (36.8) [11.5–100] | 53.9 (53.1) [48.7–61.8] | 21.5 (10.5) [0–81.8] | |
| Oral A | 9 | 94.5 (100) [63.6–100] | 54.1 (52.9) [49.7–59.9] | 80.9 (86.2) [27.3–100] | |
| Oral B | 7 | 96.9 (100) [90.0–100] | 47.0 (46.8) [46.0–48.1] | 81.1 (88.2) [50.0–100] | |
| Frequent | |||||
| Smoked | 11 | 99.1 (100) [90.0–100] | 72.7 (71.0) [59.8–85.7] | 96.0 (100) [63.3–100] | |
| Vaporized | 11 | 100 | 69.1 (68.3) [50.8–82.1] | 92.7 (100) [73.7–100] | |
| Oral A | 11 | 100 | 70.8 (71.1) 57.7–85.6] | 98.0 (100) [82.9–100] | |
| Oral B | 8 | 100 | 46.7 (47.3) [43.9–47.6] | 99.1 (100) [92.9–100] | |
| THC-Glucuronide | |||||
| % Detectedb | Last collection (h) | ||||
| Occasional | n | Mean (Median) [Range] | Mean (Median) [Range] | ||
| Smoked | 9 | 14.5 (7.7) [3.2–38.5] | 56.3 (53.3) [47.3–78.4] | ||
| Vaporized | 9 | 12.6 (11.8) [0–36.4] | 53.9 (53.1) [48.7–61.8] | ||
| Oral A | 9 | 24.6 (23.1) [17.2–42.9] | 54.1 (52.9) [49.7–59.9] | ||
| Oral B | 7 | 31.6 (27.3) [17.6–55.6] | 47.0 (46.8) [46.0–48.1] | ||
| Frequent | |||||
| Smoked | 11 | 85.9 (100) [20.0–100] | 72.7 (71.0) [59.8–85.7] | ||
| Vaporized | 11 | 75.7 (84.6) [22.2–100] | 69.1 (68.3) [50.8–82.1] | ||
| Oral A | 11 | 85.7 (100) [31.4–100] | 70.8 (71.1) 57.7–85.6] | ||
| Oral B | 8 | 90.4 (95.0) [64.7–100] | 46.7 (47.3) [43.9–47.6] | ||
| THCCOOH | |||||
| % Detectedb | Last collection (h) | ||||
| Occasional | n | Mean (Median) [Range] | Mean (Median) [Range] | ||
| Smoked | 9 | 10.7 (11.5) [0–36.4]c | 56.3 (53.3) [47.3–78.4] | ||
| Vaporized | 9 | 10.7 (5.26) [0–54.5]d | 53.9 (53.1) [48.7–61.8] | ||
| Oral A | 9 | 50.3 (44.8) [18.2–84.2] | 54.1 (52.9) [49.7–59.9] | ||
| Oral B | 7 | 56.0 (50.0) [29.4–90.9] | 47.0 (46.8) [46.0–48.1] | ||
| Frequent | |||||
| Smoked | 11 | 80.5 (93.8) [26.7–100] | 72.7 (71.0) [59.8–85.7] | ||
| Vaporized | 11 | 65.7 (83.3) [0–100] | 69.1 (68.3) [50.8–82.1] | ||
| Oral A | 11 | 83.4 (100) [45.7–100] | 70.8 (71.1) 57.7–85.6] | ||
| Oral B | 8 | 91.0 (96.2) [53.6–100] | 46.7 (47.3) [43.9–47.6] | ||
| THCVCOOH | |||||
| % Detectede | Last collection (h) | ||||
| Occasional | n | Mean (Median) [Range] | Mean (Median) [Range] | ||
| Smoked | 9 | 0 (0) [0]f | 56.3 (53.3) [47.3–78.4] | ||
| Vaporized | 9 | 2.02 (0) [0–18.2]g | 53.9 (53.1) [48.7–61.8] | ||
| Oral A | 9 | 13.5 (11.8) [0–36.8] | 54.1 (52.9) [49.7–59.9] | ||
| Oral B | 7 | 13.9 (12.5) [5.88–27.3] | 47.0 (46.8) [46.0–48.1] | ||
| Frequent | |||||
| Smoked | 11 | 30.5 (20.0) [0–100] | 72.7 (71.0) [59.8–85.7] | ||
| Vaporized | 11 | 25.3 (14.3) [0–78.6] | 69.1 (68.3) [50.8–82.1] | ||
| Oral A | 11 | 30.4 (18.2) [2.94–91.7] | 70.8 (71.1) 57.7–85.6] | ||
| Oral B | 8 | 42.3 (35.8) [10.7–62.5] | 46.7 (47.3) [43.9–47.6] |
*The % positive rates refer only to the 15 μg/L cutoff for total THCCOOH (THCCOOH and THCCOOH-glucuronide) utilized by the National Laboratory Certification Program and most drug testing monitoring programs.
a≥ 5 μg/L.
b≥0.5 μg/L.
cParticipants M, P, R & T had no THCCOOH, all other subjects had 11.5–36.4% detected.
dParticipants M & N had no detectable THCCOOH; L, P, R, S & T one; O 18.75%; Q 54.5%.
e≥1 μg/L.
fNo participant had detectable THCVCOOH.
gParticipant Q had 2 THCVCOOH detected (18.2%); all others had no THCVCOOH detected.
Statistical analyses
Statistical results are shown in Tables III and IV. To summarize, compared to oral, smoked had lower Cmax, lower Clast and lower percent detected and percent positive. Compared to oral, most vaporized parameters were lower. Smoked parameters were higher or longer than vaporized for Cmax, Tlast and percent positive.
Table III.
Statistical Comparison of Routes and of Groups, Holding Route Constant for Each Combination of Analyte and Parameter*
| Analyte | Parameter | Comparison of routes | Comparison of groups, Holding route constant | |
|---|---|---|---|---|
| Smoked vs oral | Vapor vs oral | Frequent vs occasional | ||
| THCCOOH-glucuronide | C max | –0.43 ± 0.10 | –0.58 ± 0.12 | 0.75 ± 0.13 |
| T max | –0.06 ± 0.06 | –0.03 ± 0.09 | 0.02 ± 0.09 | |
| C first | –0.06 ± 0.10 | –0.11 ± 0.12 | 0.91 ± 0.19 | |
| T first | 0.02 ± 0.10 | 0.03 ± 0.09 | –0.21 ± 0.15 | |
| C last | –0.27 ± 0.06 | –0.41 ± 0.10 | 0.91 ± 0.13 | |
| T last | 0.08 ± 0.04 | 0.03 ± 0.04 | 0.16 ± 0.04 | |
| % Detected | –12.8 ± 5.9 | –22.5 ± 7.0 | 34.1 ± 3.8 | |
| THC-glucuronide | C max | –0.19 ± 0.09 | –0.32 ± 0.08 | 0.53 ± 0.13 |
| T max | –0.23 ± 0.11 | –0.40 ± 0.17 | 0.29 ± 0.10 | |
| C first | 0.11 ± 0.11 | –0.05 ± 0.08 | 0.50 ± 0.13 | |
| T first | –0.22 ± 0.10 | –0.34 ± 0.09 | 0.03 ± 0.10 | |
| C last | –0.03 ± 0.10 | –0.05 ± 0.09 | 0.48 ± 0.15 | |
| T last | –0.12 ± 0.14 | –0.16 ± 0.09 | 0.72 ± 0.09 | |
| % Detected | –7.3 ± 3.8 | –13.9 ± 4.1 | 68.4 ± 6.0 | |
| THCCOOH | C max | –0.32 ± 0.11 | –0.47 ± 0.12 | 0.73 ± 0.16 |
| T max | 0.03 ± 0.11 | –0.13 ± 0.10 | 0.19 ± 0.10 | |
| C first | –0.13 ± 0.12 | –0.28 ± 0.10 | 0.54 ± 0.18 | |
| T first | 0.10 ± 0.09 | –0.01 ± 0.10 | –0.35 ± 0.10 | |
| C last | –0.07 ± 0.07 | –0.06 ± 0.07 | 0.61 ± 0.18 | |
| T last | 0.05 ± 0.05 | –0.17 ± 0.09 | 0.26 ± 0.04 | |
| % Detected | –23.3 ± 5.4 | –31.4 ± 6.2 | 49.0 ± 8.1 | |
| THCVCOOH | C max | –0.10 ± 0.08 | –0.10 ± 0.07 | 0.10 ± 0.10 |
| T max | –0.01 ± 0.12 | –0.10 ± 0.14 | 0.11 ± 0.11 | |
| C first | –0.04 ± 0.05 | –0.14 ± 0.10 | 0.02 ± 0.10 | |
| T first | –0.06 ± 0.18 | –0.20 ± 0.13 | –0.10 ± 0.10 | |
| C last | –0.05 ± 0.07 | 0.02 ± 0.09 | 0.01 ± 0.09 | |
| T last | 0.25 ± 0.09 | 0.07 ± 0.12 | 0.32 ± 0.08 | |
| % Detected | –9.4 ± 4.3 | –11.4 ± 3.2 | 23.4 ± 7.6 | |
*Repeated measures linear regression parameter estimates ± SE are shown; bold type indicates statistical significance (P < 0.05). A positive parameter estimate indicates that for the first route or group shown the parameter is greater than for the second. A negative parameter estimate is the opposite.
Table IV.
Parameter Statistics for Comparison of Routes of Administration
| C max – all three comparisons were significantly different: smoked vs. oral (–0.28 ± 0.05, P < 0.0001), smoked vs. vapor (0.10 ± 0.04, P = 0.02) and vapor vs. oral (–0.40 ± 0.06, P < 0.0001) |
| T max – the only significant difference was vapor vs. oral (–0.18 ± 0.07, P = 0.0082) |
| C first – the only significant difference was vapor vs. oral (–0.13 ± 0.05, P = 0.018) |
| T first – the only significant difference was vapor vs. oral (–0.13 ± 0.05, P = 0.019) |
| C last – significant comparisons were smoked vs. oral (–0.12 ± 0.04, P = 0.0058) and vapor vs. oral (–0.15 ± 0.05, P = 0.0039) |
| T last – significant comparisons were smoked vs. oral (11.21 ± 2.53, P < 0.0001) and smoked vs. vapor (8.37 ± 2.35, P = 0.00038) |
| % positive – all three comparisons were significantly different: smoked vs. oral (–13.0 ± 2.57, P < 0.0001), smoked vs. vapor (6.62 ± 2.58, P = 0.01) and vapor vs. oral (–19.94 ± 2.82, P < 0.0001) |
T max was examined for groups as a whole and for occasional and frequent smokers separately. A repeated-measures regression model of log10-transformed data, with Tmax as the dependent variable and analyte as the independent variable showed a statistically significant effect of analyte (chi square3 = 28.1, P < 0.0001) for all participants. Using THCCOOH as the reference category, Tmax was significantly greater than for THC-glucuronide (z = 25.9, P < 0.0001) and THCCOOH-glucuronide (z = 9.6, P = 0.002) but not different from THCVCOOH (z = 1.19, P = 0.28). Repeating this analysis for occasional smokers showed a similar pattern of results, with a statistically significant effect of analyte (chi square3 = 49.3, P < 0.0001), as well as significant differences between THCCOOH and THC-glucuronide (z = 43.5, P < 0.001) but not THCCOOH and THCCOOH-glucuronide (z = 3.07, P = 0.08) or THCVCOOH (z = 0.31, P = 0.58). Repeating this analysis for frequent smokers also showed a significant effect of analyte (chi square3 = 11.7, P = 0.0085) and significant differences between THCCOOH and THC-glucuronide (z = 10.4, P = 0.0013) and THCCOOH-glucuronide (z = 7.07, P = 0.0079) but not THCVCOOH (z = 1.19, P = 0.27).
Repeated-measures regression models with Tmax as the dependent variable and group as the independent variable run separately for each of the four analytes showed the groups differed only in terms of Tmax for THC-glucuronide, with occasional users shorter than frequent users (chi square1 = 15.3, P < 0.0001); Tmax for the other three analytes did not differ by group (P > 0.05). This statistical analysis indicates that the THC-glucuronide median Tmax range, especially for the smoking and vaporization routes in occasional users seen in Table I of 1.8 h and 1.0 h, is significantly shorter than that for frequent users, 5.0 h and 2.4 h.
Normalized data results
The correlation coefficients between raw and specific gravity-normalized concentrations were high, ranging from 0.81 for THCVCOOH to 0.93 for THCCOOH. The correlations were all highly significant (P < 0.0001) and these conclusions did not change when the associations were assessed using repeated-measures regression. The parameters derived from normalized concentrations did not differ substantively from those derived from raw concentrations (Tables I and II).
Although normalized Tmax for THCCOOH-glucuronide did not differ significantly from raw data results, the range of median values was smaller; for all routes, all users 5.1–7.9 h. A boxplot of specific gravity-normalized Tmax results is displayed in Figure 1. The crossbar in each plot represents the median value. THC-glucuronide Tmax results following smoking and vaporization are displayed separately from those for oral routes since they were statistically different (Table III).
Figure 1.
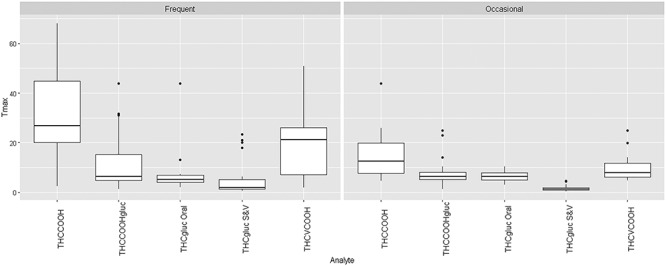
Time to specific gravity-normalized maximum urine concentrations (Tmax) for free and glucuronidated (gluc) analytes from frequent and occasional cannabis users. THC-glucuronide oral route results are presented separately from the smoking and vaporization (S & V) routes as they were statistically different (see Table III). Crossbars represent median concentrations and individual points outside a box are outliers.
A second important observation was that normalized results clearly demonstrated two or more THCCOOH peaks following each route of administration, with variability more pronounced following oral administration and more often in frequent users (Figure 2). The boxplot in Figure 1 displays the THCCOOH Tmax, the maximum normalized concentration, that was often a second peak. THCCOOH-glucuronide also occasionally had a second peak but generally it was lower in concentration than the first.
Figure 2.
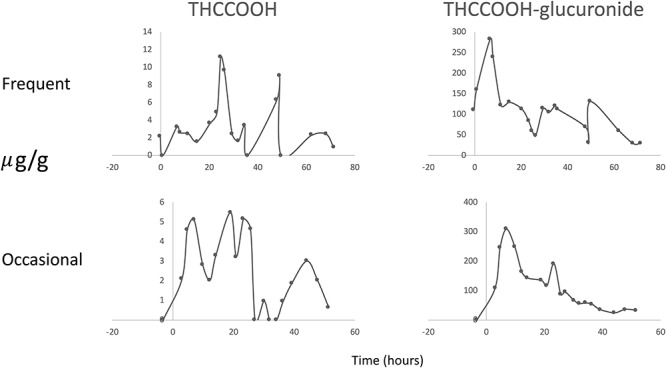
Creatinine normalized 9-carboxy-Δ9-tetrahydrocannabinol (THCCOOH) and THCCOOH-glucuronide urine concentration time profiles after receiving a 50.6 mg oral dose of Δ9-tetrahydrocannabinol for one frequent cannabis user (Participant A) and one occasional user (Participant Q) demonstrating more than one peak concentration.
Discussion
This controlled cannabis administration study had many strengths, including a within-subject design of the same THC dose by three routes of administration, and evaluation in occasional and frequent cannabis users. The study was conducted on a closed residential unit for multiple days to eliminate self-administered drug, every urine void was analyzed to fully capture individual variability, and samples were analyzed for 11 cannabinoids and metabolites with low limits of quantification. One limitation was not following some urine cannabinoid markers until they were no longer detectable; however, the high expense for a residential study of longer length was not possible.
Free THC and six metabolites were not present in frequent or occasional cannabis users’ urine following THC by three routes of administration. THCCOOH-glucuronide was the predominant analyte in urine. THCCOOH concentrations for occasional users were 2.4–3.0% and for frequent smokers 2.4–4.3% of those for THCCOOH-glucuronide at median Cmax. Three of eight occasional users had no detectable THCCOOH after smoking and detection rates in the others were only 11.5–36.4%. Results were similar following vaporization with two having no THCCOOH and five having it detected in only one specimen each. The median THCCOOH percent detected were 44.8% and 50.0% in occasional users in Oral A and B, respectively. Excretion patterns for THCCOOH were similar to those of THCCOOH-glucuronide but more variable.
THC-glucuronide concentrations were 1.4–7.5% of those for THCCOOH-glucuronide at Cmax for occasional users and 2.6–4.4% for frequent smokers. For seven of eight occasional group participants with THC-glucuronide present, Cmax was in the first urine void. As with THCCOOH, THC-glucuronide Cmax was significantly higher following the two oral routes compared to smoking. Tmax following smoking and vaporization also differed significantly from oral ingestion, with the oral route reaching maximum concentration later for occasional users. Concentrations decreased rapidly after Tmax with none detected after a median Tlast of 6.0–20.1 h for occasional users at the 0.5 μg/L LOQ. For frequent users, the median percent of specimens with detectable THC-glucuronide was 100% across all routes of administration, and for many participants, could be identified in the last specimen collected. Cmax for frequent users was significantly larger than for occasional users as expected. Since this was more significant for the smoking and vaporization routes of administration, the explanation again is most likely related to occasional users ingesting a smaller amount of THC due to poor smoking topography.
As demonstrated in past studies THCCOOH-glucuronide was the predominant metabolite for all sessions (17). For occasional users the mean (median) [Range] detection rates for THCCOOH-glucuronide (LOQ = 5 μg/L) were 68.6% (77.4) [0–100] for smoking, 45.9% (36.8) [11.5–100] for vaporization and 100% for oral dosing. Median detection rates for all routes in the frequent user group were 100%. Occasional users median Cmax were significantly lower for smoking and vaporization than following the oral routes. Tmax were not significantly different between administration routes within a group or between occasional and frequent users. Total THCCOOH Tmax was reported as longer following oral daily 7.5 mg THC Marinol doses (5) (mean Tmax ± SD 97.8 ± 24.2 h) compared to 7.7 ± 0.8 and 13.9 ± 3.5 h following smoking a single 1.75% and 3.5% THC cigarette, respectively (4). Others reported a Tmax of 6–9 h following single 10–50 mg THC doses in a brownie (23). The median THCCOOH-glucuronide Tmax found here is consistent with the latter publication, and when concentrations were normalized to reduce dilution effect Tmax was 5.1–7.9 h. For the frequent user group, there was little difference between routes of administration for any THCCOOH-glucuronide parameter.
Table II displays percent positive specimens at the 15 μg/L NLCP urine confirmation cutoff for total THCCOOH+THCCOOH-glucuronide. Median percent positive for occasional users following smoking (26.9%) and vaporization (10.5%) routes were significantly lower than after oral dosing (>85%). Some participants had no positive results following inhalation of 50.6 mg THC. Percent positive for frequent users approached 100%, reflecting their better smoking topography and long-term analyte excretion.
Although no urinary THCV was detected, its metabolite THCVCOOH was present. For occasional users the median detection rate after oral dosing was about 12% [0–36.8%], with most concentrations near 1 μg/L [1.0–10.9] and no obvious elimination pattern. No urine specimens were positive after the smoked dose and only two from one participant (Q) after vaporization. Each participant in the occasional use group had at least one specimen with THCVCOOH following oral dosing. After a smoked dose, frequent users had a median 3.2 μg/L [range 1.4–10.0] THCVCOOH Cmax, 1.8–68.2 h Tmax and 5.5–85.7 h Tlast. THCVCOOH for frequent users was participant-dependent, with three having no THCVCOOH and one (F) with 100% positive specimens. THCVCOOH parameters following vaporization were similar to those following smoking with three participants having none and one (C) with a 78.6% detection rate. Each frequent user had at least one specimen with detectable THCVCOOH following oral administration. Median Cmax was similar to the other administration routes at 3 μg/L, but with a larger range [1.2–17.9 μg/L]; detection rates ranged from 2.9–91.7%.
Application of models for recent and new use
Desrosiers et al. (17) presented a model for determining recent cannabis use employing sequential creatinine-normalized urine THC-glucuronide concentrations. We applied this formula to the current study data and found good predictability for the smoking/vaporization routes, 88.9% correct for occasional and 90.9% for frequent users, but not after the oral route, where predictability was poor at 28.6% and 37.5%, respectively. For the oral route where concentrations are much lower and peak later, there were several instances of paired specimens meeting the criteria for recent use late in the excretion process, in one case at 31.9 h. Also, two frequent users receiving placebo cannabis had residual THC-glucuronide from previously self-administered cannabis that met the THC-glucuronide criteria at 11.3 and 50.6 h after dosing.
Smith et al. (13) designed a urine model for predicting new cannabis use using sequential creatinine-normalized total THCCOOH concentrations and time between collections in occasional users. We applied the model to occasional user data in the present study using 95% confidence limits where there was no new cannabis use. Of 334 urine pairs there were 311 correct predictions of no new use (93%) and 23 incorrect (Table V). We also applied the model to predict new use in 770 urine pairs from occasional users in the current study when participants remained on the unit after a dosing session to begin their next session. The model correctly predicted 606 cases of new cannabis use and incorrectly predicted no new use for 164 cases (Table V). Most incorrect predictions occurred when subsequent urine specimen concentrations (U2) followed dosing (new use) prior to Cmax, that is, during the absorption phase. As Table V displays, 92 of 164 incorrect predictions occurred in one individual with an oral session followed by a vaporization session with respective median Cmaxs of 265.1 (oral) and 65.3 (vaporization) μg/L. These results indicate that individuals may avoid detection of new use with this model if their subsequent drug dose is much less than usual.
Table V.
Application of the New Use Model for Occasional Smokers (13)
| a. Predicting no new use when it did not occur. All nine participants had some sessions with data meeting criteria | |||
| Correct = correctly predicting no new use. Incorrect = predicting new use when it did not occur | |||
| Criteria | Total THCCOOH ≥ 15 μg/L. U1 > 24 h after dosing. U2 > U1 by ≥24 h. 95% confidence limits | ||
| Result | 311 correct findings | 23 incorrect findings | |
| b. Predicting new use when it did occur. Six of nine participants had eight instances of consecutive dosing sessions | |||
| Correct = correctly predicting new use when it occurred. Incorrect = predicting no new use when use occurred | |||
| Criteria | Total THCCOOH ≥ 15 μg/L. U2 > U1 by 24 h. U1 begins at peak. U2 all values post second dose. 95% confidence limits | ||
| PARTICIPANT | Correct | Incorrect | |
| L | Smoked followed by oral B | 144 | 0 |
| N | Oral A followed by oral B | 230 | 58 |
| O | Oral A followed by oral B | 186 | 12* |
| P | Smoked followed by vaped | 5 | 0 |
| Vaped followed by oral B | 15 | 0 | |
| R | Smoked followed by vaped | 1 | 2 |
| Vaped followed by oral A | 22 | 0 | |
| S | Oral A followed by vaped | 3 | 92 |
| Totals | 606 | 164 | |
*Eleven incorrect at U2 = 0.6 hours post new dose.
Schwilke et al. (14) published a model for predicting new cannabis use in frequent cannabis smokers but in our study frequent users were required to leave the unit between sessions, providing no new use scenarios for applying the model.
Conclusions
These urine cannabinoid marker concentrations from occasional and frequent cannabis users following three routes of administration provide a scientific database to assess single urine concentrations in cannabis monitoring programs. Unlike many previous pharmacokinetic studies, we include many new target analytes (CBD, CBN, CBG, THCV and phase II metabolites), and include data from chronic frequent cannabis users, as well as occasional users. Some results were expected, such as THCCOOH-glucuronide being the predominant analyte and frequent users having higher urine concentrations with longer detection times. Some results were previously reported following smoking (17) but not after vaporization or oral routes of administration. Although free THCCOOH was measurable in many specimens, concentrations were generally <5% those of THCCOOH-glucuronide. Other findings were not expected, i.e. median Tmax for THCCOOH-glucuronide, 5.1–7.9 h, was not different between routes or between frequent and occasional users. There were frequently multiple peaks for THCCOOH and less often for THCCOOH-glucuronide, especially in frequent users’ urine. Median Tmax were not statistically different when determined from raw vs specific gravity-normalized data. Only THC-glucuronide had mean Tmax values that differed between routes and groups. The results of this well-controlled study demonstrated excellent performance (93% within 95% CI) of a model for predicting no new use in occasional smokers based on creatinine-normalized THCCOOH+THCCOOH-glucuronide concentrations (13). However, the results demonstrated that new use could be missed with this model if the occasional user’s subsequent dose was much smaller than usual. The results also permitted evaluation of a different published model for predicting recent use based on normalized THC-glucuronide concentrations and demonstrated that the model was valid following smoking and vaporization but did not perform as well after oral ingestion. These results are important to officials in drug treatment and workplace and criminal justice drug monitoring programs, as well as policy makers with responsibility for cannabis control regulations.
Acknowledgements
The authors wish to thank Dr. David A. Gorelick, Chemistry and Drug Metabolism Section, Intramural Research Program, National Institute on Drug Abuse, for contributing to study design and Dr. Karl B. Scheidweiler for excellent oversight of data analysis and quality control.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position or policy of the National Institutes of Health or Centers for Disease Control and Prevention.
Nonstandard abbreviations
C first, first concentration ≥ LOQ appearing after dosing; Clast, last concentration ≥ LOQ after dosing; Cmax, maximum concentration after dosing; CBD, cannabidiol; CBG, cannabigerol; CBN, cannabinol; THC, Δ9-tetrahydrocannabinol; 11-OH-THC, 11-hydroxy-THC; THCAA, ∆9-tetrahydrocannabinolic acid; THCCOOH, 11-nor-9-carboxy-THC; THCV, Δ9-tetrahydrocannabivarin; THCVCOOH, 11-nor-9-carboxy-THCV; Tfirst, time of Cfirst; Tlast, time of Clast; Tmax, time of Cmax; T1/2 half-life of a drug or metabolite; NLCP, United States National Laboratory Certification Program
References
- 1. Substance Abuse and Mental Health Services Administration Key Substance Use and Mental Health Indicators in the United States: Results from the 2017 National Survey on Drug Use and Health (HHS Publication No. SMA 18-5068, NSDUH Series H-53) Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration; (2018). https://www.samhsa.gov/data (accessed Oct 10, 2019). [Google Scholar]
- 2. Huestis M.A., Smith M.L. (2018) Cannabinoid markers in biological fluids and tissues: Revealing intake. Trends in Molecular Medicine, 24, 156–172 Review. [DOI] [PubMed] [Google Scholar]
- 3. Huestis M.A., Mitchell J.M., Cone E.J. (1995) Detection times of marijuana metabolites in urine by immunoassay and GC–MS. Journal of Analytical Toxicology, 19, 443–449. [DOI] [PubMed] [Google Scholar]
- 4. Huestis M.A., Mitchell J.M., Cone E.J. (1996) Urinary excretion profiles of 11-nor-9-carboxy-delta 9-tetrahydrocannabinol in humans after single smoked doses of marijuana. Journal of Analytical Toxicology, 20, 441–452. [DOI] [PubMed] [Google Scholar]
- 5. Gustafson R.A., Levine B., Stout P.R., Klette K.L., George M.P., Moolhan E.T., et al. (2003) Urinary cannabinoid detection times after controlled oral administration of delta9-tetrahydrocannabinol to humans. Clinical Chemistry, 49, 1114–1124. [DOI] [PubMed] [Google Scholar]
- 6. Gustafson R.A., Kim I., Stout P.R., Klette K.L., George M.P., Moolchan E.T., et al. (2004) Urinary pharmacokinetics of 11-nor-9-carboxy-delta9-tetrahydrocannabinol after controlled oral delta9-tetrahydrocannabinol administration. Journal of Analytical Toxicology, 28, 160–167. [DOI] [PubMed] [Google Scholar]
- 7. Blundell M.S., Dargan P.I., Wood D.M. (2018) A dark cloud of recreational drugs and vaping. QJM, 111, 145–148. [DOI] [PubMed] [Google Scholar]
- 8. Hartman R.L., Brown T.L., Milavetz G., Spurgin A., Gorelick D.A., Gaffney G., et al. (2015) Controlled cannabis vaporizer administration: Blood and plasma cannabinoids with and without alcohol. Clinical Chemistry, 61, 850–869. [DOI] [PubMed] [Google Scholar]
- 9. Swortwood M.J., Newmeyer M.N., Andersson M., Abulseoud O.A., Scheidweiler K.B., Huestis M.A. (2017) Cannabinoid disposition in oral fluid after controlled smoked, vaporized and oral cannabis administration. Drug Testing and Analysis, 9, 905–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ellis G.M. Jr., Mann M.A., Judson B.A., Schramm N.T., Tashchian A. (1985) Execretion patterns of cannabinoid metabolites after last use in a group of chronic users. Clinical Pharmacology and Therapeutics, 38, 572–578. [DOI] [PubMed] [Google Scholar]
- 11. Goodwin R.S., Darwin W.D., Chiang C.N., Shih M., Li S.H., Huestis M.A. (2008) Urinary elimination of 11-nor-9-carboxy-delta9-tetrahydrocannabinol in cannabis users during continuously monitored abstinence. Journal of Analytical Toxicology, 32, 562–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lowe R.H., Abraham T.T., Darwin W.D., Herning R., Cadet J.L., Huestis M.A. (2009) Extended urinary delta9-tetrahydrocannabinol excretion in chronic cannabis users precludes use as a biomarker of new drug exposure. Drug and Alcohol Dependence, 105, 24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Smith M.L., Barnes A.J., Huestis M.A. (2009) Identification of new cannabis use with urine creatinine-normalized THCCOOH concentrations and time intervals between specimen collections. Journal of Analytical Toxicology, 33, 185–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schwilke E.W., Gullberg R.G., Darwin W.D., Chiang C.N., Cadet J.L., Gorelick D.A., et al. (2011) Differentiating new cannabis use from residual urinary cannabinoid excretion in chronic, daily cannabis users. Addiction, 106, 499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kelly P., Jones R.T. (1992) Metabolism of tetrahydrocannabinol in frequent and infrequent marijuana users. Journal of Analytical Toxicology, 16, 228–235. [DOI] [PubMed] [Google Scholar]
- 16. Scheidweiler K.B., Desrosiers N.A., Huestis M.A. (2012) Simultaneous quantification of free and glucuronidated cannabinoids in human urine by liquid chromatography tandem mass spectrometry. Clinica Chimica Acta, 413, 1839–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Desrosiers N.A., Lee D., Concheiro-Guisan M., Scheidweiler K.B., Gorelick D.A., Huestis M.A. (2014) Urinary cannabinoid disposition in occasional and frequent smokers: Is THC-glucuronide in sequential urine samples a marker of recent use in frequent smokers? Clinical Chemistry, 60, 361–372. [DOI] [PubMed] [Google Scholar]
- 18. Newmeyer M.N., Swortwood M.J., Barnes A.J., Abulseoud O.A., Scheidweiler K.B., Huestis M.A. (2016) Free and glucuronide whole blood cannabinoids’ pharmacokinetics after controlled smoked, vaporized, and oral cannabis administration to frequent and occasional cannabis users: Identification of recent cannabis use. Clinical Chemistry, 62, 1579–1592. [DOI] [PubMed] [Google Scholar]
- 19. Andersson M., Scheidweiler K.B., Sempio C., Barnes A.J., Huestis M.A. (2016) Simultaneous quantification of 11 cannabinoids and metabolites by liquid chromatography tandem mass spectrometry using WAX-S tips. Analytical and Bioanalytical Chemistry, 408, 6461–6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Newmeyer M.N., Swortwood M.J., Andersson M., Abulseoud O.A., Scheidweiler K.B., Huestis M.A. (2017) Cannabis edibles: Blood and oral fluid cannabinoid pharmacokinetics and evaluation of oral fluid screening devices for predicting Δ9-tetrahydrocannabinol in blood and oral fluid following cannabis brownie administration. Clinical Chemistry, 63, 647–662. [DOI] [PubMed] [Google Scholar]
- 21. Huestis M.A., Blount B.C., Milan D.F., Newmeyer M.N., Schroeder J., Smith M.L. (2019) Correlation of creatinine- and specific gravity-normalized free & glucuronide urine cannabinoid concentrations following smoked, vaporized and oral cannabis in frequent and occasional cannabis users. Drug Testing and Analysis, 11, 968–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. World Anti-Doping Agency Decision limits for the confirmatory quantification of threshold substances. Technical Document, TD2018DL. 1 March 2018. [Google Scholar]
- 23. Schlienz N.J., Cone E.J., Herrmann E.S., Lembeck N.A., Mitchell J.M., Bigelow G.E., et al. (2018) Pharmacokinetic characterization of 11-nor-9-carboxy-Δ9-tetrahydrocannabinol in urine following acute oral cannabis ingestion in healthy adults. Journal of Analytical Toxicology, 42, 232–247. [DOI] [PMC free article] [PubMed] [Google Scholar]


