Abstract
Aortic stenosis (AS) is one of the most common valvular diseases in developed countries. Transcatheter aortic valve implantation (TAVI) has emerged as alternative to medical treatment or surgical aortic valve replacement (SAVR) in all symptomatic patients with severe AS. In 2002, Cribier performed the first human TAVI through a trans-septal approach in a 57-year-old man with severe AS. Since then, several trials have compared TAVI vs. SAVR over the years. Today, it is superior in terms of mortality to medical therapy in extreme-risk patients, non-inferior or superior to surgery in high-risk patients, and non-inferior to surgery and even superior when transfemoral access is possible in intermediate-risk patients. Interesting results emerged from the latest multicentre trials involving patients with severe AS who were at low risk for death from surgery, demonstrating that this therapy will be offered to younger people in the next future.
Keywords: Aortic stenosis, TAVI, SAVR, SAPIEN, Evolut
Introduction
Aortic stenosis (AS) is the most frequent valvular heart disease in Western countries. Prevalence increases with age and is expected to increase due to demographic aging of the global population. If left untreated, symptomatic severe AS carries a poor prognosis.1 The introduction of transcatheter aortic valve implantation (TAVI) has raised the hope for an alternative, minimally invasive treatment for severe AS.
Transcatheter aortic valve implantation in inoperable and high-risk patients
In 1980s, the idea of TAVI emerged by the observation of the limits of balloon aortic valvuloplasty (BAV) to treat severe AS in adults. The insight had been the combination of a balloon-expandable stent with a high radial force that might be expanded within the native valve to prevent restenosis and a valvular structure inserted within the stent to mimic native valve function, by using mini-invasive catheterization techniques. After the first human percutaneous valve implantation in the pulmonary position by Bonhoeffer in a 12-year-old boy in 2000, in 2002, Cribier performed the first human TAVI through a trans-septal approach in a 57-year-old man with severe AS, cardiogenic shock, and a left ventricular ejection fraction of 12%. In 2004, Cribier reported the first case series of this novel technique in patients with end-stage calcific AS, not amenable to surgical aortic valve replacement (SAVR) demonstrating its feasibility1: success was achieved in five of six patients, with marked and sustained haemodynamic and clinical improvement. At first, the antegrade approach was used and then abandoned for the retrograde-transfemoral and transapical approach. A series of implants with the retrograde approach performed by Webb et al.1 showed an initial success rate of 78%, which rose to 96% after the first 25 cases, thus proving the importance of the learning curve. Thirty-day mortality was 12%, and there was no evidence of valve deterioration, embolization, or intraprosthetic failure at follow-up. Periprosthetic regurgitation was observed at 1 month in three cases, while in most patients there was a trivial paravalvular leak, with no significant hemodynamic consequences.
Commercial TAVI was then approved for clinical use in Europe in 2007, and in the USA in 2011.
During the years, ∼15 000 patients have been randomized in clinical trials of TAVI.
Inoperable and high-risk patients
The PARTNER (Placement of Aortic Transcatheter Valves) study was the first prospective randomized comparative study between TAVI and traditional medical and surgical therapy. The primary endpoint was all-cause mortality at 1 year. The USA and Canadian patients were enrolled and divided into two treatment arms:
The first arm (Cohort B), of about 350 inoperable patients, comparing optimized medical therapy and BAV vs. TAVI in patients with absolute contraindication to aortic valve surgery.1
The second arm (Cohort A), of about 700 patients, analysing equivalence in terms of non-inferiority between TAVI and SAVR in high-risk surgical patients (STS score >10%).1
The implanted device was the first-generation Edwards SAPIEN valve (Edwards Lifesciences, Inc., Irvine, CA, USA) via transfemoral and transapical approach. The results of Cohort B showed that TAVI can improve survival compared with conventional treatment. At 30-day follow-up, stroke and major vascular events were higher in the TAVI group but statistically significant only for the major vascular events (P < 0.001). However, at 1 year, the incidence of death from any cause was 30.7% in the TAVI group, compared with 50.7% in the standard therapy group (P < 0.001). Repeat hospitalization was also much lower in the TAVI group (P < 0.001). Among survivors at 1 year, the most advanced New York Heart Association (NYHA) classes were lower in the TAVI group. It was also seen that the quality of life, investigated using the Kansas City Cardiomyopathy Questionnaire (KCCQ) and the Medical Outcomes Study Short Form-12 (SF-12) Health Survey, registered a significant increase in scores at 12 months only in the TAVI group while improving significantly compared with the baseline in both populations at 1 month and 6 months. These results were confirmed at 2-year follow-up. At 5 years, 42 (86%) of 49 survivors in the TAVI group had NYHA class 1 or 2 symptoms compared with 3 of 5 (60%) in the standard treatment group1 (Figure 1). Echocardiography after TAVI showed durable haemodynamic benefit, with no evidence of structural valve deterioration (SVD).
Figure 1.

Primary composite endpoint from PARTNER 1B trial.
Data on Cohort A led to the conclusion that TAVI is not inferior to SAVR in high-risk patients (30-day mortality, 3.4% vs. 6.5%, P = 0.07; 1-year mortality, 24.2% vs. 26.8%; P = 0.44). At 2-year follow-up, the two treatments were similar with respect to mortality (33.9% vs. 35.0% P = 0.78), reduction in symptoms, and improved valve haemodynamics, but paravalvular regurgitation was more frequent after TAVI and was associated with increased late mortality.1 In 2015, data on 5-year follow-up were published2: risk of death was 67.8% in the TAVI group compared with 62.4% in the SAVR group (P = 0.76) (Figure 2). No SVD requiring surgical valve replacement was recorded in either group. Moderate or severe aortic regurgitation occurred in 40 (14%) of 280 patients in the TAVI group and 2 (1%) of 228 in the SAVR group (P < 0.0001) and was associated with increased 5-year risk of mortality in the TAVI group (72.4% for moderate or severe aortic regurgitation vs. 56.6% for those with mild aortic regurgitation or less; P = 0.003).
Figure 2.

Primary composite endpoint from PARTNER 1A trial.
The CoreValve High-Risk Study was a randomized trial comparing TAVI with a self-expanding transcatheter aortic valve bioprosthesis (Medtronic CoreValve) with SAVR in patients with severe AS and an increased risk of death during surgery.1 A total of 795 patients underwent randomization at 45 centres in the USA. The rate of death from any cause at 1 year was significantly lower in the TAVI group than in the surgical group (14.2% vs. 19.1%), with an absolute reduction in risk of 4.9%. TAVI was non-inferior with respect to echocardiographic indexes of valve stenosis, functional status, and quality of life. The rates of major adverse cardiovascular and cerebrovascular events were lower in the TAVI group and there was no increase in the risk of stroke. In 2018, data on 5-year follow-up were published3: risk of death and major stroke was 55.3% and 12.3% for TAVI and 55.4% and 13.2% for SAVR, respectively. No clinical valve thrombosis was observed. Freedom from severe SVD was 99.2% for TAVI and 98.3% for SAVR (P = 0.32), and freedom from valve reintervention was 97.0% for TAVI and 98.9% for SAVR (P = 0.04). A permanent pacemaker was implanted in 33.0% of TAVI and 19.8% of surgery patients at 5 years (P < 0.001)3 (Figure 3).
Figure 3.

Primary composite endpoint from CoreValve High-Risk trial.
Intermediate-risk patients
The increasing operators experience and the improvement of the transcatheter valve systems led to a general trend to extend the use of TAVI in patients who were at lower risk for surgery. The following expansion of the use of TAVI in this cluster of patients was validated by rigorous clinical trials.
The PARTNER 2 (Placement of Aortic Transcatheter Valves) trial is a prospective, multicentre, randomized clinical trial in which TAVI with SAPIEN XT valve system (Edwards Lifesciences) was compared with conventional surgery in patients with severe symptomatic AS and intermediate risk for surgery.4 The primary endpoint was a non-hierarchical composite of death from any cause or disabling stroke at 2 years. In total, 2032 intermediate-risk patients with severe AS were enrolled. On the basis of clinical and imaging findings, patients were divided into two different cohorts, according to access route (TF or transthoracic) and were than randomly assigned (in a 1:1 ratio) to undergo either TAVI or surgical aortic-valve replacement.
The rate of death from any cause or disabling stroke at 2 years was similar in the TAVI and the surgery group (P = 0.001 for non-inferiority). At 2 years, the Kaplan–Meier event rates were 19.3% in the TAVI group and 21.1% in the surgery group (hazard ratio in the TAVI group 0.89, 95% confidence interval 0.73–1.09; P = 0.25). In the TF-access cohort, TAVI resulted in a lower rate of death or disabling stroke than surgery (hazard ratio 0.79, 95% confidence interval 0.62–1.00; P = 0.05), whereas in the transthoracic-access cohort outcomes were similar in the two groups. The results with respect to the individual components of the primary endpoint, death or stroke, were also similar in the two groups (death: 16.7% in the TAVI group vs. 18.0% in the surgery group; disabling stroke: 6.2% in the TAVI group vs. 6.4% in the surgery group) (Figure 4). Earlier outcomes at 30 days and 1 year similarly showed no significant differences between TAVI and surgery groups. TAVI resulted in larger aortic valve areas than did surgery and also resulted in lower rates of acute kidney injury, severe bleeding, and new-onset atrial fibrillation; surgery resulted in fewer major vascular complications and less paravalvular aortic regurgitation. In conclusion, PARTNER 2 trial found that in intermediate-risk patients with severe symptomatic AS, surgical and transcatheter valve replacement were similar with respect to the primary endpoint of death or disabling stroke for up to 2 years and resulted in a similar degree of lessening of cardiac symptoms.
Figure 4.

Primary composite endpoint from PARTNER 2 trial.
The SURTAVI (Surgical Replacement and Transcatheter Aortic Valve Implantation) trial5 is a multinational, randomized, non-inferiority clinical trial designed to compare safety and efficacy of TAVI, using self-expanding bioprostheses, and surgery in patients with symptomatic, severe AS at intermediate surgical risk. The primary endpoint was a composite of death from any cause or disabling stroke at 2 years. A total of 1746 patients underwent randomization. Of these patients, 1660 underwent TAVI or surgery. At 2 years, the estimated incidence of the primary endpoint was 12.6% in the TAVI group and 14.0% in the surgery group (Figure 5). Surgery was associated with higher rates of acute kidney injury, atrial fibrillation, and transfusion requirements, whereas TAVI had higher rates of residual aortic regurgitation, major vascular complications, and need for pacemaker implantation. TAVI resulted in lower mean gradients and larger aortic-valve areas than surgery, but a higher number of patients showed moderate or severe residual paravalvular regurgitation at 1 year (5.3% in the TAVI group vs. 0.6% in the surgery group). Structural valve deterioration at 2 years did not occur in either group. Cardiac symptoms and quality of life improved significantly in the two groups from baseline and the improvement persisted throughout the 2 years of follow-up period. In conclusion, the SURTAVI trial demonstrated that TAVI is a statistically non-inferior alternative to surgery with respect to death from any cause or disabling stroke at 2 years.
Figure 5.

Primary composite endpoint from SURTAVI trial.
In conclusion, although a comparison between two different randomized clinical trials carries inherent risks, both the PARTNER 2A trial and the SURTAVI trial achieved their non-inferiority endpoints of death from any cause or disabling stroke in the intermediate-risk populations.
Low-risk patients
During the last American Congress of Cardiology annual scientific session (ACC19) have been released data from two major new studies that demonstrate TAVI role in low-risk patients. These studies increase the possibility that TAVI use will be approved in these patients.
The PARTNER 3 was a multicentre, randomized trial6 in which randomly assigned patients with severe AS and low surgical risk to undergo either TAVI with TF placement of a third-generation balloon-expandable valve or standard surgical aortic-valve replacement. The primary endpoint was a composite of death from any cause, stroke, or rehospitalization at 1 year after the procedure. A total of 1000 patients were enrolled at 71 sites and underwent randomization; 503 were assigned to TAVR; and 497 were assigned to surgery. These patients were younger (mean age, 73 years), were more men (69.3%), and had lower STS-PROM scores (mean score 1.9%) and fewer comorbidities than patients enrolled in previous randomized trials of TAVI.4,7,8 Results have been recently published showing that TAVI, performed by means of TF placement of the balloon-expandable SAPIEN 3 system, was superior to surgery with regard to the primary composite endpoint of death from any cause, stroke, or rehospitalization at 1 year after the procedure (Figure 6). Furthermore, TAVI was associated with a significantly a shorter index hospitalization, lower rate of new-onset atrial fibrillation at 30 days, a lower risk of a poor treatment outcome (death or a low KCCQ score) at 30 days than surgery and had more rapid improvements in the 6-min walk-test distance, NYHA class, and KCCQ score than those who underwent surgery. The most important limitation of PARTNER trial is that these results reflect only 1-year outcomes after the procedure and do not address the problem of long-term SVD .9,10
Figure 6.

Primary composite endpoint from PARTNER 3 trial.
The EVOLUT LOW RISK trial11 is a randomized non-inferiority trial in which TAVI, with a self-expanding supra-annular bioprosthesis, was compared with surgical aortic-valve replacement in patients at low surgical risk with severe AS. The primary safety and effectiveness endpoint was a composite of death from any cause or disabling stroke at 24 months after the procedure. A total of 1468 patients were randomly assigned to undergo either TAVI (734) or surgery (734). The mean age of the patients was 74 years, all the patients were at low surgical risk and 34.9% were women. Results showed that among patients deemed to be at a low risk for death from surgery, TAVI with a self-expanding supra-annular bioprosthesis was non-inferior to surgery with respect to the risk of disabling stroke or death at 24 months after the procedure (Figure 7). Furthermore, it was associated with a lower incidence of atrial fibrillation, acute kidney injury, bleeding events, and disabling stroke than surgery but with a higher incidence of permanent pacemaker use and aortic regurgitation. Both surgery and TAVI provided functional improvement at 12 months, but the TAVI group had better recovery at 30 days, as indicated by the KCCQ score.
Figure 7.
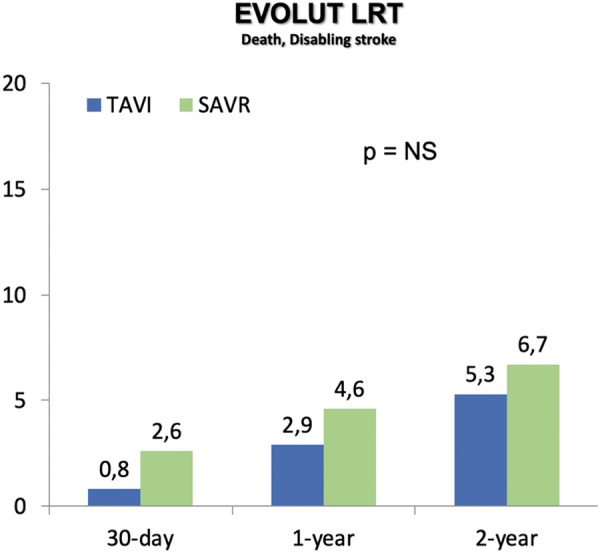
Primary endpoint from EVOLUT LOW RISK trial.
In the NOTION (Nordic Aortic Valve Intervention) trial,12 all-comer patients with severe AS and lower surgical risk for mortality were randomized 1:1 to TAVI (139) or surgery (135). The aim of this study was to compare the durability of transcatheter and surgical bioprosthetic aortic valves using standardized criteria. At 6 years, the Kaplan–Meier rates of death from any cause were similar for TAVI (42.5%) and surgery (37.7%) patients (log rank P = 0.58). There were no significant differences between surgery and TAVI patients for bioprosthetic valve dysfunction, while there was significant more moderate/severe SVD for surgery group than the TAVI group (24.0% vs. 4.8%; P < 0.001) (Figure 8).
Figure 8.

Cumulative incidence of structural valve deterioration at 6 years from NOTION trial.
Conclusions
The development of TAVI has been a long 20-year process from concept to the real world. Since Cribier performed the first-in-human TAVI, in 2002, several trials had compared TAVI vs. SAVR (Figure 9). In the past decade, TAVI was reserved only for patients considered non-operable or at high risk for surgery. Today, TAVI has become the standard of care in inoperable and at high or intermediate surgical risk patients. Recently, several trials demonstrated the non-inferiority or superiority of TAVI vs. surgery in low-risk patients, so it is increasingly being performed in this cluster of patients. However, before broad expansion of TAVI indications to younger and lower risk patients, several challenges, such as valve durability, need to be addressed. Ongoing trials are expected to investigate these issues.
Figure 9.
Timeline of the transcatheter aortic valve implantation clinical trials.
Conflict of interest: none declared.
References
- 1. Tamburino C, Barbanti M, Capodanno D. Percutaneous Treatment of Left Side Cardiac Valves. Third Edition: A Practical Guide for the Interventional Cardiologist. Springer International Publishing, 2018. [Google Scholar]
- 2. Mack MJ, Leon MB, Smith CR, Miller DC, Moses JW, Tuzcu EM, Webb JG, Douglas PS, Anderson WN, Blackstone EH, Kodali SK, Makkar RR, Fontana GP, Kapadia S, Bavaria J, Hahn RT, Thourani VH, Babaliaros V, Pichard A, Herrmann HC, Brown DL, Williams M, Davidson MJ, Svensson LG, Akin J. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet 2015;385:2477–2484. [DOI] [PubMed] [Google Scholar]
- 3. Gleason TG, Reardon MJ, Popma JJ, Deeb GM, Yakubov SJ, Lee JS, Kleiman NS, Chetcuti S, Hermiller JB, Heiser J, Merhi W, Zorn GL, Tadros P, Robinson N, Petrossian G, Hughes GC, Harrison JK, Conte JV, Mumtaz M, Oh JK, Huang J, Adams DH. 5-year outcomes of self-expanding transcatheter versus surgical aortic valve replacement in high-risk patients. J Am Coll Cardiol 2018;72:2687–2696. [DOI] [PubMed] [Google Scholar]
- 4. Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, Thourani VH, Tuzcu EM, Miller DC, Herrmann HC, Doshi D, Cohen DJ, Pichard AD, Kapadia S, Dewey T, Babaliaros V, Szeto WY, Williams MR, Kereiakes D, Zajarias A, Greason KL, Whisenant BK, Hodson RW, Moses JW, Trento A, Brown DL, Fearon WF, Pibarot P, Hahn RT, Jaber WA, Anderson WN, Alu MC, Webb JG. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med 2016;374:1609–1620. [DOI] [PubMed] [Google Scholar]
- 5. Reardon MJ, Van Mieghem NM, Popma JJ, Kleiman NS, Søndergaard L, Mumtaz M, Adams DH, Deeb GM, Maini B, Gada H, Chetcuti S, Gleason T, Heiser J, Lange R, Merhi W, Oh JK, Olsen PS, Piazza N, Williams M, Windecker S, Yakubov SJ, Grube E, Makkar R, Lee JS, Conte J, Vang E, Nguyen H, Chang Y, Mugglin AS, Serruys PWJC, Kappetein AP. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med 2017;376:1321–1331. [DOI] [PubMed] [Google Scholar]
- 6. Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M, Kapadia SR, Malaisrie SC, Cohen DJ, Pibarot P, Leipsic J, Hahn RT, Blanke P, Williams MR, McCabe JM, Brown DL, Babaliaros V, Goldman S, Szeto WY, Genereux P, Pershad A, Pocock SJ, Alu MC, Webb JG, Smith CR. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med 2019;380:1695–1705. [DOI] [PubMed] [Google Scholar]
- 7. Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Brown DL, Block PC, Guyton RA, Pichard AD, Bavaria JE, Herrmann HC, Douglas PS, Petersen JL, Akin JJ, Anderson WN, Wang D, Pocock S. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010;363:1597–1607. [DOI] [PubMed] [Google Scholar]
- 8. Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Williams M, Dewey T, Kapadia S, Babaliaros V, Thourani VH, Corso P, Pichard AD, Bavaria JE, Herrmann HC, Akin JJ, Anderson WN, Wang D, Pocock SJ. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011;364:2187–2198. [DOI] [PubMed] [Google Scholar]
- 9. Capodanno D, Petronio AS, Prendergast B, Eltchaninoff H, Vahanian A, Modine T, Lancellotti P, Sondergaard L, Ludman PF, Tamburino C, Piazza N, Hancock J, Mehilli J, Byrne RA, Baumbach A, Kappetein AP, Windecker S, Bax J, Haude M. Standardized definitions of structural deterioration and valve failure in assessing long-term durability of transcatheter and surgical aortic bioprosthetic valves: a consensus statement from the European Association of Percutaneous Cardiovasc Intervention. Eur Heart J 2017;38:3382–3390. [DOI] [PubMed] [Google Scholar]
- 10. Dvir D, Bourguignon T, Otto CM, Hahn RT, Rosenhek R, Webb JG, Treede H, Sarano ME, Feldman T, Wijeysundera HC, Topilsky Y, Aupart M, Reardon MJ, Mackensen GB, Szeto WY, Kornowski R, Gammie JS, Yoganathan AP, Arbel Y, Borger MA, Simonato M, Reisman M, Makkar RR, Abizaid A, McCabe JM, Dahle G, Aldea GS, Leipsic J, Pibarot P, Moat NE, Mack MJ, Kappetein AP, Leon MB. Standardized definition of structural valve degeneration for surgical and transcatheter bioprosthetic aortic valves. Circulation 2018;137:388–399. [DOI] [PubMed] [Google Scholar]
- 11. Popma JJ, Deeb GM, Yakubov SJ, Mumtaz M, Gada H, O’Hair D, Bajwa T, Heiser JC, Merhi W, Kleiman NS, Askew J, Sorajja P, Rovin J, Chetcuti SJ, Adams DH, Teirstein PS, Zorn GL, Forrest JK, Tchétché D, Resar J, Walton A, Piazza N, Ramlawi B, Robinson N, Petrossian G, Gleason TG, Oh JK, Boulware MJ, Qiao H, Mugglin AS, Reardon MJ. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med 2019;380:1706–1715. [DOI] [PubMed] [Google Scholar]
- 12. Søndergaard L, Ihlemann N, Capodanno D, Jørgensen TH, Nissen H, Kjeldsen BJ, Chang Y, Steinbrüchel DA, Olsen PS, Petronio AS, Thyregod HGH. Durability of transcatheter and surgical bioprosthetic aortic valves in patients at lower surgical risk. J Am Coll Cardiol 2019;73:546–553. [DOI] [PubMed] [Google Scholar]



