Abstract
Background
Currently, no data are available on the burden of morbidity and mortality in people with HIV-1 (PWH) harboring a 4-class drug-resistant (4DR) virus (nucleoside reverse transcriptase inhibitors, non-nucleoside reverse transcriptase inhibitors, protease inhibitors, integrase strand transfer inhibitors). The study aimed to assess the incidence of clinical events and death in this population.
Methods
This was a cohort study on PWH from the PRESTIGIO Registry with a documented 4DR virus. Burden of disease was defined as the occurrence of any new event including an AIDS-defining event (ADE) or non-AIDS-defining event (NADE) or death from any cause after 4DR evidence (baseline). Cox regression models evaluated factors associated with the risk of new clinical events/death.
Results
Among 148 PWH followed for a median (interquartile range) of 47 (32–84) months after 4DR evidence, 38 PWH had 62 new events or died from any cause (incidence rate, 9.12/100 person-years of follow-up; 95% CI = 6.85–11.39): 12 deaths (6 AIDS-related and 6 non-AIDS-related), 18 ADEs, 32 NADEs; 20 of the 38 NADEs (45%) of the incident clinical events were malignancies. The 4-year cumulative incidence of death was 6% (95% CI, 3%–13%), and that of ≥1 event or death was 22% (95% CI, 16%–31%). A higher risk of new clinical events/death was more likely in PWH with previous clinical events (adjusted hazard ratio [aHR], 2.67; 95% CI, 1.07–6.67) and marginally associated with lower baseline CD4+/CD8+ ratio (aHR, 0.82; 95% CI, 0.65–1.02).
Conclusions
PWH harboring 4DR have a high burden of disease with a worrying incidence of malignancies, strongly advising for close prevention and monitoring interventions as well as access to innovative therapeutic strategies, especially in people with a history of clinical events and low CD4+/CD8+ ratio.
Keywords: AIDS-defining event, cancer, death, 4-class drug resistance, non-AIDS-defining event
Since 2007, antiretroviral therapy (ART) regimens based on an integrase strand transfer inhibitor (INSTI) have been introduced and largely used both in treatment-naïve and treatment-experienced people because of their safety and efficacy, as demonstrated in many clinical trials and observational studies [1, 2]. The widespread use of INSTI-based regimens, particularly first-generation agents, has led to the emergence of INSTI resistance in a limited proportion of people with HIV-1 infection (PWH) [3]. Italian data reported a 9% prevalence of multiclass drug resistance (MDR; defined as a virus with at least 1 major resistance mutation in at least 3 different drug classes [nucleoside reverse transcriptase inhibitors [NRTIs], non-nucleoside reverse transcriptase inhibitors [NNRTIs], protease inhibitors [PIs], INSTIs]) in ART-experienced HIV-1 infected people in the period 2011–2018 [4]. The prevalence of 4-class drug resistance (4DR; ie, with NRTI, NNRTI, PI, INSTI resistance) has recently risen from 1% in 2008–2013 to 3% and 2% in 2014–2016 and 2017–2018, respectively [4].
Previous studies have suggested that people with 3-class drug resistance have a particularly poor prognosis, with high morbidity and mortality. An Italian cohort study [5] including 623 people with HIV and triple-class virological failure (TCVF), followed in 1999–2002 for a median follow-up (interquartile range [IQR]) of 21 (7–37) months, reported an incidence rate (IR) of death from any cause equal to 11.9/100 person-years of follow-up (PYFU) and an IR of AIDS-related death of 9.4/100 PYFU. In addition, the 48-month probability of new AIDS events/death was estimated to be 35.9%.
In a previous European multicohort study [6] including PWH with TCVF, the estimated incidence rate of AIDS-defining events (1.2/100 PYFU in 2009) and the mortality rate (1.4/100 PYFU in 2008) did not decrease significantly in 9 years of follow-up.
To date, however, data on the burden of AIDS-related and non-AIDS-related morbidity in PWH harboring a 4DR virus are not available. Using the PRESTIGIO Registry, the aim of this analysis was to evaluate the incidence of AIDS-related and non-AIDS-related events and death in this population.
METHODS
The PRESTIGIO Registry (www.registroprestigio.com) is an observational, prospective, Italian, multicenter, annual collection of data on clinical, laboratory, treatment, and virological characteristics of PWH and 4DR viruses.
The registry includes people with the following characteristics: age >14 years; documented resistance to the 4 classes of antiretroviral drugs (NRTIs, NNRTIs, PIs, INSTIs), defined as intermediate or high-level resistance to at least 1 drug of each class according to the Stanford algorithm; if no INSTI genotypic resistance test is available, a documented virological failure to an INSTI-regimen was accepted as an inclusion criterion. The PRESTIGIO Registry is registered on the ClinicalTrials.gov website with the following identifier: NCT04098315.
In this analysis, we included people from the PRESTIGIO Registry with an available and verified clinical history and with ≥1 clinical visit after the date of the first evidence of a 4DR virus (baseline).
The outcomes for the present analysis were the incidence of (i) ≥1 new AIDS event or non-AIDS event or death from any cause; (ii) death from any cause; (iii) ≥1 new AIDS event or AIDS-related death; (iv) ≥1 new non-AIDS event or non-AIDS-related death.
AIDS events were defined according to the Centers for Disease Control and Prevention’s 1993 Revised Classification System for HIV Infection and Expanded Surveillance Case Definition for AIDS Among Adolescents and Adults [7].
Non-AIDS events included non-AIDS-related malignancies, major adverse cardiovascular events (MACEs), decompensated cirrhosis, chronic kidney disease (CKD), and diabetes. MACEs included acute myocardial infarction, coronary disease requiring invasive procedures, congestive heart failure, and stroke. CKD was defined as the occurrence of 2 consecutive (at least 3 months apart) estimated glomerular filtrate rate determinations <60 mL/min/1.73 m2 (using the CKD Epidemiology Collaboration Equation formula).
All incident clinical events that occurred from baseline to March 2019 (administrative censoring date) were considered in the analyses. Patients might have experienced a clinical event (AIDS-related or non-AIDS-related) before baseline.
Patient Consent Statement
The protocol of the PRESTIGIO Registry was approved (protocol number 41/int/December 2017) by the San Raffaele Ethical Committee (coordinating center) in December 2017. Forty Infectious Diseases clinical centers, located in almost all the Italian regions, are actively participating, and their ethics committees approved the registry protocol; all patients provided written informed consent to be enrolled.
Statistical Analysis
Results are described as median (interquartile range) and frequency (%).
Distributions of laboratory parameters were compared between PWH with vs without events using the Wilcoxon rank-sum test for continuous variables and the chi-square/Fisher exact test for categorical variables; we considered the nearest values to the date of the first event (within 90 days before the event) for people with events and the last available value for people without events. Incidence rates (IRs) were estimated by univariate Poisson regression as number of events recorded after baseline and PYFU and expressed as rate per 100 PYFU, with 95% confidence intervals. As participants could experience >1 incident event, in measuring incidence the occurrence of different types of clinical events was allowed, while recurrences of the same type of event were excluded. In these analyses, follow-up started at BL and ended at the date of the incident clinical events, date of death, or date of the last available visit.
For time-to-event analyses, Kaplan-Meier curves were used to estimate the cumulative probability of the first incident: (i) clinical event (AIDS- or non-AIDS-related or death from any cause, whichever occurred first); (ii) AIDS-related event or AIDS-related death; (iii) non-AIDS-related event or non-AIDS-related death after 4DR evidence. In these analyses, follow-up started at baseline and ended at the date of first event, date of death, or last available visit (whichever occurred first) and was right-censored 96 months after baseline due to the low number of cases observed thereafter; there were no competing events.
Time to first clinical event (either AIDS- or non-AIDS-related) or death from any cause was estimated by use of Kaplan-Meier curves.
Multivariable Cox regression models were fitted to determine the factors associated with the risk of ≥1 clinical event (either AIDS- or non-AIDS-related) or death from any cause; the effect estimate was reported as the adjusted hazard ratio (aHR) with corresponding 95% CI, estimated according to Wald approximation. The multivariable model included covariates with a P value <.2 on univariable analysis. To define the final multivariable model, we fitted different models and assessed for collinearity. The multivariable models included the correlated variables, first individually and then in combination; the additional contribution of each covariate was assessed with the Akaike information criterion. The variables included in the models were fitted as time-fixed and measured at baseline.
For the analyses, 2-sided P values <.05 were considered statistically significant. All analyses were performed using SAS, release 9.4 (SAS Institute, Cary, NC, USA).
RESULTS
Overall, 148 PWH harboring a 4DR virus were evaluated and contributed to 680 PYFU. They were predominantly males (78%), with a median age (IQR) of 49 (44–53) years; 21% were co-infected with hepatitis C virus (HCV) and treated with ART for 16.8 (14.0–19.7) years. Thirty-nine percent had experienced previous AIDS events, and 21% had previous non-AIDS events, mostly a non-AIDS malignancy (16%); at baseline, the RGSS score (IQR) was 5.5 (3.75–9.5), 93% of people had plasma HIV-1 RNA >50 copies/mL with a median (IQR) of 3.49 (2.37–4.48) log10 copies/mL, the CD4+ cell count was 346 (169–600) cells/µL, and the CD4+/CD8+ ratio was 0.35 (0.20–0.61). The other characteristics of the 148 people considered in this study are provided in Table 1.
Table 1.
Baselinea Characteristics of the 148 PWH and 4-Class Drug-Resistant Virus
| Baseline Characteristics | Median (IQR) or Frequency (%) |
|---|---|
| Age, y | 49 (44–53) |
| Male gender | 115 (78) |
| HCVAb positive | 31 (21) |
| HBsAg positive | 13 (9) |
| Years from HIV diagnosis | 20.4 (16.2–24.5) |
| Years of ART | 16.8 (14.0–19.7) |
| Pre-ART plasma HIV-RNA, log10 copies/mL | 5.18 (4.95–5.67) |
| Nadir CD4+ cell count, cells/µL | 92 (18–203) |
| Previous AIDS events | 58 (39) |
| Previous non-AIDS events | 31 (21) |
| Diabetes | 9 (6) |
| Decompensated cirrhosis | 1 (1) |
| Malignancies | 24 (16) |
| MACE | 4 (3) |
| CKD, eGFR <60 mL/min/1.73 m2 | 3 (2) |
| Number of available genotypic resistance tests per individual | 3 (2–6) |
| Residual genotypic susceptibility score, RGSS scoreb | 5.5 (3.75–9.5) |
| NRTI RGSS | 0.5 (0–2) |
| NNRTI RGSS | 1 (0.5–2.5) |
| PI RGSS | 1 (0–4) |
| INSTI RGSS | 1 (0.5–2) |
| Previous exposure to maraviroc | 50 (34) |
| Previous exposure to enfurvitide | 56 (38) |
| Most frequent ART regimens | |
| DRV/b+DTG | 21 (14.2) |
| DRV/b+DTG+MVC | 9 (6.1) |
| DRV/b+DTG+ETV | 8 (5.4) |
| FTC/TAF+DRV/b+DTG | 8 (5.4) |
| 3TC+DRV/b+DTG | 6 (4.1) |
| DRV/b+DTG+RPV | 5 (3.4) |
| INSTI-including regimen | 128 (86.5) |
| Plasma HIV-1 RNA ≥50 copies/mL | 137 (93) |
| Plasma HIV-RNA, log10 copies/mL | 3.49 (2.37–4.48) |
| CD4+ cell count, cells/µL | 346 (169–600) |
| CD8+ cell count, cells/µL | 946 (629–1284) |
| CD4+/CD8+ ratio | 0.35 (0.20–0.61) |
Abbreviations: 4DR, 4-class drug-resistant; ART, antiretroviral therapy; chronic CKD, kidney disease; eGFR, estimated glomerular filtration rate; INSTI, integrase strand transfer inhibitor; MACE, major adverse cardiovascular event; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; RGSS, Residual Genotypic Susceptibility Score.
aBaseline is defined as the date with the first evidence of a 4DR virus.
bThe RGSS was estimated according to the cumulative data of the available plasma genotyping resistance tests recorded for each patient until baseline. Based on the patient’s cumulative resistance mutations, the level of resistance of each of 24 antiretroviral drugs (excluding maraviroc, enfuvirtide) was calculated using the Genotypic Resistance Interpretation Algorithm of the Stanford HIV Drug Resistance Database Program (version 8.8, last updated on 2019-02-13; http://hivdb.stanford.edu). For each of the 24 Food and Drug Administration–approved antiretroviral drugs, a drug penalty score was assigned according to the degree of resistance. Antiretroviral drug resistance was scored as 1 point if classified as “susceptible” or “potential low-level or low-level resistance,” as 0.5 points if classified as “intermediate resistance,” and as 0 points if classified as “high-level resistance.” In people with a documented virological failure to an INSTI regimen (n = 21; 11 individuals failed raltegravir, 8 dolutegravir, and 2 elvitegravir), antiretroviral drug resistance (of the failed drug) was scored as 0.5 points; similarly, people previously failing maraviroc-containing (n = 50) or enfuvirtide-containing (n = 56) regimens were labeled resistant to these agents and scored as 0.5 points.
During a median follow-up (IQR) of 47 (32–84) months after the first 4DR evidence, 38 PWH had 62 new events or died from any cause (12 deaths from any cause [6 AIDS-related deaths and 6 non-AIDS-related deaths], 17 primary non-AIDS-defining malignancies, 3 primary AIDS-defining malignancies, 15 AIDS events, 6 MACE, 6 CKD, and 3 cirrhosis).
The incidence rate of any new event or death from any cause was 9.12/100 PYFU (95% CI, 6.85–11.39). Fifteen PWH developed 24 new AIDS events or had AIDS-related death (IR, 3.52/100 PYFU; 95% CI, 2.12–4.94); the incidence rate of AIDS-related events was 2.65/100 PYFU (95% CI, 1.57–4.01). Twenty-six PWH developed 38 non-AIDS events or died a non-AIDS-related death (IR, 5.59/100 PYFU; 95% CI, 3.81–7.37); the incidence rate of non-AIDS-related events was 4.71/100 PYFU (95% CI, 3.08–6.34).
Interestingly, 20 (32% in 16 PWH) of the incident clinical events were malignancies, and among the incident non-AIDS events, 17 (45%) were non-AIDS-defining malignancies. Among the 38 people who had a first primary malignancy (22 before, 14 after, and 2 both before and after the date of 4DR evidence), 1 (3%) individual had a relapse of the same malignancy (primary diagnosis of hepatocellular carcinoma in 2016 with relapse in 2018) and 4 (11%) individuals had a second diagnosis of cancer: 1 anal cancer in 2013 (before 4DR date) followed by cutaneous Kaposi sarcoma (KS) in 2019; 1 cutaneous KS in 1997 (before 4DR date) followed by squamous cell skin cancer in 2016; 1 Hodgkin lymphoma in 2010 followed by non-Hodgkin lymphoma in 2017; 1 Hodgkin lymphoma in 2014 followed by lung cancer in 2017. At the time of this study, 4/38 (11%) had died.
Kaplan-Meier analysis showed that the estimated probabilities of a clinical event (AIDS event or non-AIDS event or death from any cause, whichever occurred first) were 10% (95% CI, 6%–16%), 13% (95% CI, 9%–20%), 17% (95% CI, 11%–24%), and 22% (95% CI, 16%–31%) at 12, 24, 36, and 48 months since BL, respectively. People with incident clinical events or death from any cause, compared with those without events/death, had lower baseline CD4+ cell counts (IQR) (210 [49–443] vs 436 [223–629] cells/µL; P = .003), lower baseline CD4+/CD8+ ratios (IQR) (0.20 [0.09–0.44] vs 0.45 [0.24–0.69]; P = .002), and RGSS scores (IQR) that were marginally lower (4.8 [2.5–7.5] vs 6 [4–10]; P = .072). Baseline HIV-1 RNA was >50 copies/mL in 95% of people with incident clinical events/death and in 92% of people without events/death (P = .792).
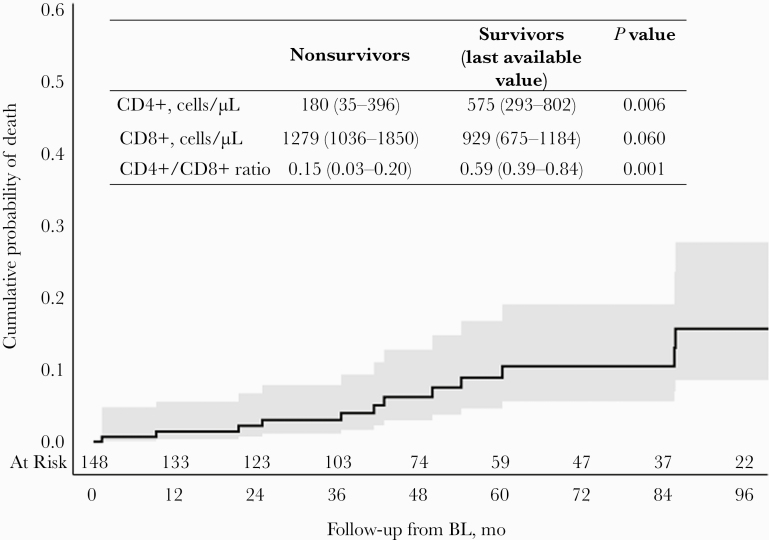
Overall, the incidence rate of death from any cause was 1.76/100 PYFU (95% CI, 0.91–2.90). The cumulative probabilities of death were 1.4% (95% CI, 0.4%–5.6%), 2.2% (95% CI, 0.7%–6.7%), 3.0% (95% CI, 1.1%–7.9%), and 6.2% (95% CI, 3.0%–12.8%) at 12, 24, 36, and 48 months since BL, respectively (Figure 1).
Figure 1. .
Time to death from any cause. Twelve people with HIV died (incidence rate, 1.76/100 person-years of follow-up; 95% CI, 0.91–2.90): 6 AIDS-related deaths (1 wasting syndrome, 1 non-Hodgkin lymphoma [Burkitt], 1 tuberculous meningoencephalitis, 1 Pneumocystis jirovecii¸ 1 mycobacteriosis, 1 extrapulmonary Mycobacterium tuberculosis) and 6 non-AIDS-related deaths (2 major adverse cardiovascular events, 1 Hodgkin lymphoma, 1 bacterial pneumonia, 1 suicide, 1 unspecified non-AIDS event). Abbreviation: BL, baseline.
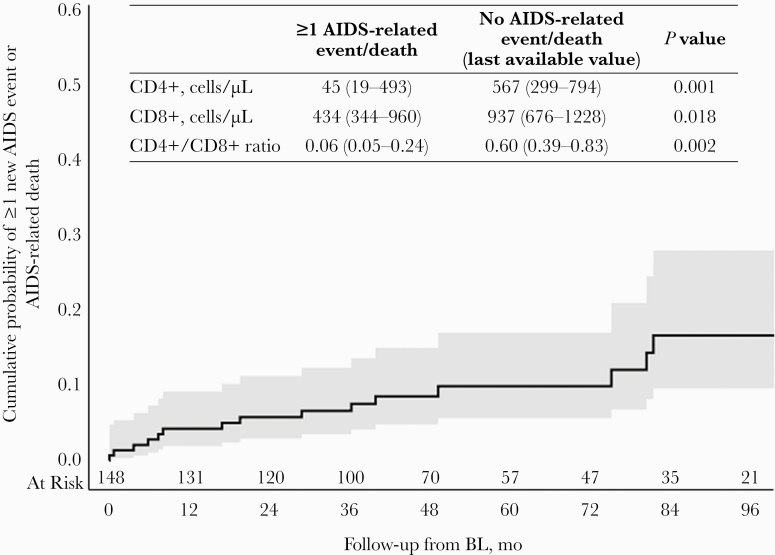
Twelve months after baseline, 4% (95% CI, 2%–9%) of PWH were estimated to have developed an AIDS event or died an AIDS-related death, increasing to 6% (95% CI, 3%–11%), 7% (95% CI, 4%–12%), and 9% (95% CI, 5%–15%) at 24, 36, and 48 months, respectively (Figure 1). People with incident AIDS events/death, compared with people without AIDS events/death, had a similar baseline virological profile (HIV-1 RNA >50 copies/mL: 100% vs 92%; P = .604) and a poor immunological profile (CD4+ cell count [IQR], 55 [20–328] vs 416 [204–629] cells/µL; P = .0001; CD4+/CD8+ ratio [IQR], 0.10 [0.06–0.24] vs 0.38 [0.22–0.69]; P = .0006). At the event’s occurrence or last available visit, HIV-1 RNA >50 copies/mL was recorded in 70% of people with incident AIDS events/death vs 24% of people without AIDS events/death (P = .004), and a poor immunological profile was significantly associated with the occurrence of incident AIDS events or AIDS-related death (Figure 2).
Figure 2. .
Time to occurrence of new AIDS-related events or AIDS-related death. Fifteen people with HIV developed 24 new AIDS events or died an AIDS-related death (incidence rate, 3.52/100 person-years of follow-up; 95% CI, 2.12–4.94): 3 atypical mycobacteriosis, 3 pulmonary tuberculosis, 1 tuberculous meningoencephalitis, 2 non-Hodgkin lymphoma, 1 cutaneous Kaposi sarcoma, 2 esophageal candidiasis, 2 Pneumocystis jirovecii pneumonia, 1 disseminated herpes simplex, 1 wasting syndrome, 1 cryptococcal meningitis, 1 cytomegalovirus encephalitis, and 6 AIDS-related deaths. Abbreviation: BL, baseline.
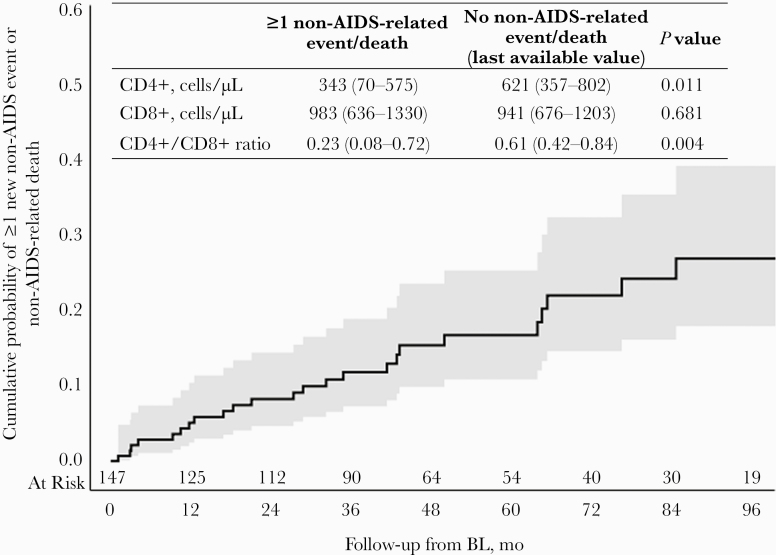
The estimated probabilities of non-AIDS events or non-AIDS-related deaths were 5% (95% CI, 3%–10%), 8% (95% CI, 5%–15%), 12% (95% CI, 7%–19%), and 15% (95% CI, 10%–24%) at 12, 24, 36, and 48 months since BL, respectively (Figure 2). People with incident non-AIDS events/death, compared with people without non-AIDS events/death, had a similar baseline virological profile (HIV-1 RNA >50 copies/mL: 92% vs 93%; P = .994) and immunological profile (CD4+ cell count [IQR], 282 [168–501] vs 354 [170–601] cells/µL; P = .417; CD4+/CD8+ ratio [IQR], 0.26 [0.15–0.54] vs 0.37 [0.21–0.61]; P = .266). On the contrary, at the event’s occurrence or last available visit, people with incident non-AIDS events or non-AIDS-related death more frequently had HIV-1 RNA >50 copies/mL (59% of people with events vs 24% of people without events; P = .002) and a poor immunological profile (Figure 3).
Figure 3. .
Time to occurrence of new non-AIDS-related events or non-AIDS-related death. Twenty-six people with HIV developed 38 non-AIDS events or died a non-AIDS related death (incidence rate, 5.59/100 person-years of follow-up; 95% CI, 3.81–7.37): 3 Hodgkin lymphoma, 4 anal cancer, 3 hepatocellular carcinoma, 2 prostate cancer, 1 lung cancer, 1 breast cancer, 1 laryngeal cancer, 1 conjunctival spinocellular carcinoma, 1 squamous cell skin cancer, 6 major adverse cardiovascular events, 6 chronic kidney disease, 3 cirrhosis, and 6 non-AIDS-related deaths. Abbreviation: BL, baseline.
The estimated probabilities of any clinical event (AIDS event or non-AIDS event) or death from any cause, AIDS event or AIDS-related death, non-AIDS events or non-AIDS-related deaths, according to according to age (<50 vs ≥50 years), baseline CD4+ cell count (≤350 vs >350 cells/µL), and baseline viral load (<50 vs ≥50 copies/mL and ≤200 vs >200 copies/mL) are reported in Supplementary Figures 1–12). Age was associated only with the occurrence of non-AIDS-related events or non-AIDS-related death, while baseline CD4+ cell count influenced either the burden of disease or the occurrence of AIDS-related events or AIDS-related death; viral load stratified on the basis of both cutoffs was not found to be associated with any of the study outcomes.
At multivariate analysis (Table 2), the occurrence of previous clinical events (AIDS or non-AIDS) before baseline was associated with an increased risk of incident clinical events/death (aHR [≥1 vs none], 2.674; 95% CI, 1.067–6.667; P = .036), while higher values of BL CD4+/CD8+ ratio were marginally associated with a lower risk of incident clinical events/death from any cause (aHR [per 0.1-unit higher], 0.816; 95% CI, 0.654–1.019; P = .072).
Table 2. .
Univariable and Multivariable Hazard Ratios for Incident Clinical Events or Death From Any Cause Among the 148 PWH and 4-Class Drug-Resistant Virus
| Univariable Analysis | Multivariable Analysisa | ||||
|---|---|---|---|---|---|
| Covariate | Category | Hazard Ratio (95% CI) | P Value | Adjusted Hazard Ratio (95% CI) | P Value |
| Age | Per 1 y older | 1.036 (0.993–1.080) | .102 | 1.022 (0.972–1.073) | .397 |
| Gender | Female vs male | 0.546 (0.210–1.418) | .194 | 0.773 (0.264–2.262) | .639 |
| HCVAb | Positive vs negative | 1.227 (0.546–2.758) | .621 | Not included | - |
| HBsAg | Positive vs negative | 2.856 (0.377–21.634) | .310 | Not included | - |
| Years since HIV diagnosis | Per 1 y longer | 1.003 (0.906–1.024) | .230 | Not included | - |
| Years of ART | Per 1 y longer | 0.967 (0.898–1.041) | .369 | Not included | - |
| Pre-ART HIV-RNA | Per 1 log10 copies/mL higher | 0.628 (0.181–2.178) | .464 | Not included | - |
| Nadir CD4+ cell count | Per 100 cells/µL higher | 0.531 (0.346–0.816) | .004 | 0.756 (0.487–1.173) | .212 |
| Previous AIDS events | ≥1 vs none | 2.313 (1.141–4.688) | .020 | Not included | - |
| Previous non-AIDS event | ≥1 vs none | 2.724 (1.327–5.593) | .006 | Not included | - |
| Previous AIDS or non-AIDS event | ≥1 vs none | 3.644 (1.760–7.547) | 0.0005 | 2.674 (1.067–6.667) | .036 |
| BL plasma HIV-1 RNA | ≥50 vs <50 copies/mL | 2.209 (0.300–16.250) | .436 | Not included | - |
| BL plasma HIV-1 RNA | Per 1 log10 copies/mL higher | 1.299 (0.967–1.745) | .083 | 0.992 (0.615–1.600) | .973 |
| BL CD4+ cell count | Per 100 cells/µL higher | 0.802 (0.687–0.936) | .005 | Not included | - |
| BL CD8+ cell count | Per 100 cells/µL higher | 1.031 (0.965–1.101) | .363 | Not included | - |
| BL CD4+/CD8+ ratio | Per 0.1 unit higher | 0.729 (0.602–0.885) | .001 | 0.816 (0.654–1.019) | .072 |
| Burden of resistance (RGSS score) | ≤5.5 vs >5.5b | 1.630 (0.830–3.204) | .176 | Not included | - |
| Burden of resistance (RGSS score) | Per 1 point higher | 0.962 (0.882–1.049) | .179 | 1.051 (0.936–1.180) | .400 |
Abbreviations: 4DR, 4-class drug-resistant; ART, antiretroviral therapy; BL, baseline (date with the first evidence of a 4DR virus); RGSS, Residual Genotypic Susceptibility Score.
aCovariates with univariable P value <.20 were included in the final multivariable model.
bMedian value.
DISCUSSION
In this analysis, we reported that a substantial proportion of the 148 HIV-1 adults with 4DR had new clinical events or death following 4DR evidence, mainly non-AIDS-related (61%); 32% of the recorded clinical events were malignancies, and among the non-AIDS events, 45% were non-AIDS-defining malignancies. The occurrence of new clinical events or death from any cause was affected by the immunological profile at 4DR and marginally by a history of clinical events.
While the implication of multidrug resistance on clinical progression has been previously studied in people with TCVF [5, 6, 8, 9], no data have been yet reported in HIV-infected people with 4DR. Data from the PLATO II project [6], including 91 764 individuals, showed that the crude incidence of AIDS-defining events was 1.2/100 PYFU in 2009, and the crude rate of death in 2008 was 1.4/100 PYFU. These rates were derived around 10 years earlier than our study, when treatment options were less effective. However, the rates of AIDS-related events and death in our 4DR cohort are definitely higher (2.65 and 1.76/100-PYFU, respectively). Although the estimates obtained from the PRESTIGIO Registry derive from a smaller sample size, our findings highlight an excess of clinical events and mortality among PWH and 4DR virus, despite the availability of modern and highly effective antiretroviral drugs.
Another relevant finding from our study is the high incidence of non-AIDS events or non-AIDS-related death, with a rate much higher than that of the AIDS-related events. Similar findings were previously observed in a study of 12 844 PWH by Mocroft et al. [10] reporting that non-AIDS events are common among PWH in the highly active antiretroviral therapy era, that they are associated with considerable mortality, and that there is no relationship between the incidence of these events and CD4+ levels, especially CD4+ levels >350 cells/µL; the authors also observed that the incidence of non-AIDS events was similar to, or exceeded, the incidence of AIDS events at CD4 counts ≥200 cells/µL. We indeed estimated a higher incidence rate of non-AIDS events in our study, likely reflecting the higher degree of fragility of people with 4DR compared with the PWH population in general.
In our study, one-third of these events were malignancies (45% among the incident non-AIDS events), at least in part virus-related cancers (Epstein-Barr virus–related, human papillomavirus–related, and HCV-related) followed by cardiovascular events, and we found that the occurrence of these events or death is more likely in people with a history of previous clinical events (48% of people had ≥1 clinical event before evidence of 4DR). Most of the observed non-AIDS events are similar to those experienced by the general population and, at least in part, potentially associated with modifiable lifestyle factors, such as smoking.
It is also of interest to note that, in our study, among the 24 people who survived a first primary malignancy, 4 (17%) individuals had a diagnosis of a second primary cancer. Although the limited sample size of our study might have negatively affected the accuracy of this estimate, this proportion slightly exceeds that estimated in a recent population study by Hessol and colleagues [11] showing that 9% of all the identified primary cancers among people with HIV were second or later primary cancers, a proportion similar to that of the general population of people aged 20–64 years (11%) [12]. Tenorio and colleagues reported that in people on suppressive ART inflammation and coagulation markers are associated with higher risk of non-AIDS-defining events and mortality [13]. Borges and colleagues [14] also found that activated inflammation and coagulation pathways are associated with increased cancer risk during HIV infection. We may argue that these conditions are even truer for people with a 4DR virus, are frequently characterized by persistent viremia (possibly associated with nonadherence), and are likely affected by a higher burden of inflammation [15].
Another finding from this study is that low CD4+/CD8+ ratio values are marginally associated with a higher risk of new clinical events or death. Low CD4+/CD8+ ratio cell counts have long been established as a risk factor for the development of clinical events and death, either AIDS-related [16] or non-AIDS-related [17–19]. In consideration of the fact that most of the non-AIDS-related events in our study were malignancies, the study by Hema et al. [19] is of particular interest; in fact, the authors reported that the risk of cancer was twice as high in the subset of patients who had a CD4+/CD8+ ratio <0.5, independent of CD4+ cell count. This finding is in line with the median CD4+/CD8+ ratio we observed in people with 4DR virus either at baseline or at the event’s occurrence.
There are some limitations to our study that are important to highlight. Although clinical records were carefully monitored, we cannot exclude a potential underestimation of clinical events, especially of non-AIDS-related events and mortality for which a link with the national death registry is not available in Italy. Another issue is related to the recent establishment of the PRESTIGIO Registry: Recorded people are those who survived a previous potentially fatal event, and for this reason, we might have underestimated the true incidences of AIDS- and non-AIDS-related events and death.
The absence of a control group is an additional study limitation that impairs the assessment of whether the estimated incidence rates are similar, lower than, or greater than those observed in other individuals. Unfortunately, a study that evaluated the same outcomes in individuals with similar characteristics and without extensive drug resistance is lacking, and at this time, any comparator group can only be inadequate. Another limitation is the date we considered baseline for these analyses: This is the date on which 4-drug resistance was highlighted for the first time, evidence that might be late compared with the time when this condition actually occurred. Finally, predictive factors were estimated on the composite end point including AIDS- and non-AIDS-related events and deaths from any cause; this analysis allowed us to increase power, although the potential risk factors may differ for each single component of the composite end point.
In conclusion, among PWH and 4-class drug-resistant virus, the occurrence of clinical events and deaths from any cause was particularly high and the incidence of new non-AIDS events/non-AIDS-related death was almost twice that of new AIDS events/AIDS-related death, with a worrying incidence of malignancies. Because of the high burden of disease in PWH with 4DR virus, close prevention and monitoring interventions are highly recommended, especially in people with a history of clinical events and a low CD4+/CD8+ ratio. In PWH with 4DR virus, efforts to provide access to drugs with new mechanisms of action are also urgently needed in order to achieve undetectable HIV-RNA load, preserve the immune system, prevent clinical progression, and ultimately improve quality of life.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
The authors wish to thank the PRESTIGIO Study Group:
Steering committee. Antonella Castagna (Coordinator), Nicola Gianotti, Laura Galli, Franco Maggiolo, Leonardo Calza, Emanuele Focà, Gaetana Sterrantino, Giovanni Cenderello, Antonio Di Biagio, Stefano Rusconi, Cristina Mussini, Marianna Menozzi, Andrea Antinori, Roberta Gagliardini, Stefano Bonora, Micol Ferrara, Maurizio Zazzi, Maria Santoro, Giulio Maria Corbelli.
Virology team and biological bank. Maurizio Zazzi, Maria Mercedes Santoro, Andrea Galli.
Study coordinators. Elisabetta Carini, Maria Rita Parisi.
Statistical and monitoring team. Laura Galli, Andrea Poli, Alba Bigoloni.
Participating centers. Ancona: Marcello Tavio, Luca Butini, Andrea Giacometti; Aviano: Emanuela Vaccher, Ferdinando Martellotta, Valentina Da Ros; Bari: Gioacchino Angarano, Annalisa Saracino, Flavia Balena; Bergamo: Franco Maggiolo, Laura Comi, Elisa Di Filippo, Daniela Valenti, Claudia Suardi, Barbara Mazzola; Bologna: Pierluigi Viale, Leonardo Calza, Elena Rosselli del Turco, Marta Vacas Ramirez; Brescia: Francesco Castelli, Emanuele Focà, Anna Celotti, Francesca Brognoli; Busto Arsizio: Guido Bonoldi, Barbara Menzaghi, Clara Abeli, Maddalena Farinazzo; Cagliari: Francesco Ortu, Marco Campus; Catania: Bruno Cacopardo, Maurizio Celesia; Cremona: Angelo Pan, Chiara Fornabaio; Firenze: Alessandro Bartoloni, Gaetana Sterrantino, Francesca Rinaldi, Susanna Giachè, Blanc Pierluigi, Francesca Vichi, Francesco Maria Fusco; Foggia: Teresa Santantonio, Sergio Ferrara, Serena Rita Bruno; Genova: Giovanni Cassola, Giovanni Cenderello, Feasi Marcello, Francesca Calautti, Matteo Bassetti, Antonio Di Biagio, Bianca Bruzzone; La Spezia: Stefania Artioli; Milano: Adriano Lazzarin, Antonella Castagna, Nicola Gianotti, Elisabetta Carini, Maria Rita Parisi, Laura Galli, Andrea Poli, Andrea Galli, Diana Canetti, Massimo Galli, Stefano Rusconi, Tiziana Formenti, Valentina Morena, Arianna Gabrieli, Antonella d’Arminio Monforte, Lidia Gazzola, Esther Merlini, Valentina Minieri, Andrea Gori, Alessandra Bandera, Valeria Pastore, Valentina Ferroni, Massimo Puoti, Cristina Moioli; Sara Vassalli; Modena: Cristina Mussini, Marianna Menozzi, Roncaglia Enrica, Nardini Giulia, Barbara Beghetto; Napoli: Elio Manzillo, Alfredo Franco; Padova: Anna Maria Cattelan, Serena Marinello, Silvia Cavinato, Annamaria Macario; Palermo: Antonio Cascio, Giovanni Mazzola; Parma: Anna Maria degli Antoni, Carlo Ferrari, Diletta Laccabue; Pavia: Gaetano Filice, Roberto Gulminetti, Layla Pagnucco, Annalia Asti; Perugia/Terni: Daniela Francisci, Pasticci, Elisabetta Schiaroli, Chiara Papalini, Francesca Italiani; Pistoia: Massimo Di Pietro; Reggio Emilia: Giacomo Magnani, Garlassi Elisa, Enrico Barchi, Romina Corsini; Roma: Andrea Antinori, Roberta Gagliardini, Alessandra Vergori, Stefania Cicalini, Giovanna Onnelli, Alberto Giannetti, Roberto Cauda, Arturo Ciccullo, Silvia La Monica, Vincenzo Vullo, Gabriella Dettorre, Eugenio Nelson Cavallari, Massimo Andreoni, Vincenzo Malagnino, Laura Ceccarelli; Rovigo: Filippo Viviani, Lolita Sasset; Sanremo: Chiara Dentone; Siena: Barbara Rossetti, Sara Modica, Valentina Borgo; Torino: Giovanni Di Perri, Stefano Bonora, Micol Ferrara, Chiara Carcieri; Verona: Marina Malena, Marta Fiscon, Barbara Padovani; Trieste: Roberto Luzzati, Sandro Centonze, Romina Valentinotti.
Financial support. This work and the PRESTIGIO Registry were supported by ViiV Healthcare and Theratechnologies. The funding source had no involvement in the study.
Potential conflicts of interest. All authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions. A.C., L.G., and A.P. had full access to all the data in the study and take final responsibility for the integrity of the data, the accuracy of the data analysis, and the decision to submit for publication. Study concept: A.C. Data collection, administrative or material support: A.P., M.R.P., M.M., M.F., E.G., D.F., A.D.B., G.S., C.F., A.D.A., G.A., F.M.F., A.D.M., G.M.C., M.M.S., M.Z. Statistical analysis: L.G., A.P. Drafting of the manuscript: L.G., M.R.P., A.C. Data interpretation and critical revision of the manuscript: all authors.
Ethical approval. This retrospective, longitudinal study was approved by the Ethics Committee of the San Raffaele Scientific Institute (approval No. 34–2017) and evaluated data from the HIV Clinic of San Luigi Center, San Raffaele Hospital, Milan, Italy.
Prior presentation. Preliminary results of this work were presented at the European AIDS Conference, November 6–9 2019, Basel, Switzerland. Abstract number PE20/11.
Contributor Information
PRESTIGIO Study Group:
Antonella Castagna, Nicola Gianotti, Laura Galli, Franco Maggiolo, Leonardo Calza, Emanuele Focà, Gaetana Sterrantino, Giovanni Cenderello, Antonio Di Biagio, Stefano Rusconi, Cristina Mussini, Marianna Menozzi, Andrea Antinori, Roberta Gagliardini, Stefano Bonora, Micol Ferrara, Maurizio Zazzi, Maria Santoro, Giulio Maria Corbelli, Maurizio Zazzi, Maria Mercedes Santoro, Andrea Galli, Elisabetta Carini, Maria Rita Parisi, Laura Galli, Andrea Poli, Alba Bigoloni, Marcello Tavio, Luca Butini, Andrea Giacometti, Emanuela Vaccher, Ferdinando Martellotta, Valentina Da Ros, Gioacchino Angarano, Annalisa Saracino, Flavia Balena, Franco Maggiolo, Laura Comi, Elisa Di Filippo, Daniela Valenti, Claudia Suardi, Barbara Mazzola, Pierluigi Viale, Leonardo Calza, Elena Rosselli del Turco, Marta Vacas Ramirez, Francesco Castelli, Emanuele Focà, Anna Celotti, Francesca Brognoli, Guido Bonoldi, Barbara Menzaghi, Clara Abeli, Maddalena Farinazzo, Francesco Ortu, Marco Campus, Bruno Cacopardo, Maurizio Celesia, Angelo Pan, Chiara Fornabaio, Alessandro Bartoloni, Gaetana Sterrantino, Francesca Rinaldi, Susanna Giachè, Blanc Pierluigi, Francesca Vichi, Francesco Maria Fusco, Teresa Santantonio, Sergio Ferrara, Serena Rita Bruno, Giovanni Cassola, Giovanni Cenderello, Feasi Marcello, Francesca Calautti, Matteo Bassetti, Antonio Di Biagio, Bianca Bruzzone, Stefania Artioli, Adriano Lazzarin, Antonella Castagna, Nicola Gianotti, Elisabetta Carini, Maria Rita Parisi, Laura Galli, Andrea Poli, Andrea Galli, Diana Canetti, Massimo Galli, Stefano Rusconi, Tiziana Formenti, Valentina Morena, Arianna Gabrieli, Antonella d’Arminio Monforte, Lidia Gazzola, Esther Merlini, Valentina Minieri, Andrea Gori, Alessandra Bandera, Valeria Pastore, Valentina Ferroni, Massimo Puoti, Cristina Moioli, Sara Vassalli, Cristina Mussini, Marianna Menozzi, Roncaglia Enrica, Nardini Giulia, Barbara Beghetto, Elio Manzillo, Alfredo Franco, Anna Maria Cattelan, Serena Marinello, Silvia Cavinato, Annamaria Macario, Antonio Cascio, Giovanni Mazzola, Anna Maria degli Antoni, Carlo Ferrari, Diletta Laccabue, Gaetano Filice, Roberto Gulminetti, Layla Pagnucco, Annalia Asti, Daniela Francisci, Elisabetta Schiaroli, Chiara Papalini, Francesca Italiani, Massimo Di Pietro, Giacomo Magnani, Garlassi Elisa, Enrico Barchi, Romina Corsini, Andrea Antinori, Roberta Gagliardini, Alessandra Vergori, Stefania Cicalini, Giovanna Onnelli, Alberto Giannetti, Roberto Cauda, Arturo Ciccullo, Silvia La Monica, Vincenzo Vullo, Gabriella Dettorre, Eugenio Nelson Cavallari, Massimo Andreoni, Vincenzo Malagnino, Laura Ceccarelli, Filippo Viviani, Lolita Sasset, Chiara Dentone, Barbara Rossetti, Sara Modica, Valentina Borgo, Giovanni Di Perri, Stefano Bonora, Micol Ferrara, Chiara Carcieri, Marina Malena, Marta Fiscon, Barbara Padovani, Roberto Luzzati, Sandro Centonze, and Romina Valentinotti
References
- 1. Snedecor SJ, Radford M, Kratochvil D, et al. Comparative efficacy and safety of dolutegravir relative to common core agents in treatment-naïve patients infected with HIV-1: a systematic review and network meta-analysis. BMC Infect Dis 2019; 19:484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Raffi F, Esser S, Nunnari G, et al. Switching regimens in virologically suppressed HIV-1-infected patients: evidence base and rationale for integrase strand transfer inhibitor (INSTI)-containing regimens. HIV Med 2016; 17(Suppl 5):3–16. [DOI] [PubMed] [Google Scholar]
- 3. Nakagawa F, Lodwick R, Costagliola D, et al. Calendar time trends in the incidence and prevalence of triple-class virologic failure in antiretroviral drug-experienced people with HIV in Europe. J Acquir Immune Defic Syndr 2012; 59:294–9. [DOI] [PubMed] [Google Scholar]
- 4. Lombardi F, Giacomelli A, Armenia D, et al. Evaluation of multidrug resistance over the last two decades in art-experienced HIV-1 infected patients in the ARCA database. Paper presented at: Italian Conference on AIDS and Antiviral Research (ICAR); 5–7 June, 2019; Milan, Italy. [Google Scholar]
- 5. Zaccarelli M, Tozzi V, Lorenzini P, et al. ; Collaborative Group for Clinical Use of HIV Genotype Resistance Test (GRT) at National Institute for Infectious Diseases Lazzaro Spallanzani Multiple drug class-wide resistance associated with poorer survival after treatment failure in a cohort of HIV-infected patients. AIDS 2005; 19:1081–9. [DOI] [PubMed] [Google Scholar]
- 6. Pursuing Later Treatment Option II (PLATO II) project team, Observational HIV Epidemiological Research Europe (COHERE) Group, Costagliola D, et al. Trends in virological and clinical outcomes in individuals with HIV-1 infection and virological failure of drugs from three antiretroviral drug classes: a cohort study. Lancet Infect Dis 2012; 12:119–27. [DOI] [PubMed] [Google Scholar]
- 7. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adult. MMWR Recomm Rep 1992; 41(RR-17):1–19. [PubMed] [Google Scholar]
- 8. Cozzi-Lepri A, Phillips AN, Clotet B, et al. ; EuroSIDA Study Group Detection of HIV drug resistance during antiretroviral treatment and clinical progression in a large European cohort study. AIDS 2008; 22:2187–98. [DOI] [PubMed] [Google Scholar]
- 9. Lohse N, Jørgensen LB, Kronborg G, et al. ; Danish HIV Cohort Study Genotypic drug resistance and long-term mortality in patients with triple-class antiretroviral drug failure. Antivir Ther 2007; 12:909–17. [PubMed] [Google Scholar]
- 10. Mocroft A, Reiss P, Gasiorowski J, et al. ; EuroSIDA Study Group Serious fatal and nonfatal non-AIDS-defining illnesses in Europe. J Acquir Immune Defic Syndr 2010; 55:262–70. [DOI] [PubMed] [Google Scholar]
- 11. Hessol NA, Whittemore H, Vittinghoff E, et al. Incidence of first and second primary cancers diagnosed among people with HIV, 1985–2013: a population-based, registry linkage study. Lancet HIV 2018; 5: e647–55. [DOI] [PubMed] [Google Scholar]
- 12. Murphy CC, Gerber DE, Pruitt SL. Prevalence of prior cancer among persons newly diagnosed with cancer: an initial report from the surveillance, epidemiology, and end results program. JAMA Oncol 2018; 4:832–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tenorio AR, Zheng Y, Bosch RJ, et al. Soluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment. J Infect Dis 2014; 210:1248–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Borges ÁH, Silverberg MJ, Wentworth D, et al. ; INSIGHT SMART; ESPRIT; SILCAAT Study Groups Predicting risk of cancer during HIV infection: the role of inflammatory and coagulation biomarkers. AIDS 2013; 27:1433–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Spagnuolo V, Clemente T, Caccia R, et al. ; on behalf of the PRESTIGIO Study Group. Inflammatory burden in people living with four-class drug resistant HIV: data from the PRESTIGIO Registry. Paper presented at: AIDS 2020: 23rd International AIDS Conference; 6–10 July 2020. Poster PEB0250. [Google Scholar]
- 16. Lundgren JD, Babiker AG, Gordin F, et al. ; for the INSIGHT START Study Group Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 2015; 373:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mussini C, Lorenzini P, Cozzi-Lepri A, et al. ; Icona Foundation Study Group CD4/CD8 ratio normalisation and non-AIDS-related events in individuals with HIV who achieve viral load suppression with antiretroviral therapy: an observational cohort study. Lancet HIV 2015; 2:e98–106. [DOI] [PubMed] [Google Scholar]
- 18. Monforte Ad, Abrams D, Pradier C, et al. ; Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) Study Group HIV-induced immunodeficiency and mortality from AIDS-defining and non-AIDS-defining malignancies. AIDS 2008; 22:2143–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hema MN, Ferry T, Dupon M, et al. ; ANRS CO 8 (APROCO/COPILOTE) study group Low CD4/CD8 ratio is associated with non AIDS-defining cancers in patients on antiretroviral therapy: ANRS CO8 (Aproco/Copilote) prospective cohort study. PLoS One 2016; 11:e0161594. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.