Abstract
Supplemental Digital Content is available in the text.
Keywords: autopsy, coronavirus infections, heart failure, hypertension, stroke volume
The cause of death in patients with coronavirus disease 2019 (COVID-19) infection has not yet been completely clarified. In addition to lung failure, cardiac inflammation has also been suggested to occur. However, its source from a direct or indirect COVID-19 involvement, type of mechanism, that is, cytopathic versus immune-mediated, and compartments involved (vessels, nerves, and conduction tissue) are still undefined.
We report cardiac autopsy findings in 9 patients (aged 92 [male], 82 [female], 64 [female], 58 [male], 93 [male], 81 [male], 35 [male], 54 [male], and 70 [male] years) with COVID-19 infection (diagnosed with polymerase chain reaction of nasal secretion) and pneumonitis who died because of cardiogenic shock or sudden death, during hospitalization. The patients were under treatment with antiviral and antibiotics. In addition, all patients were treated with anticoagulants (enoxaparine/clexane). No systemic arterial hypertension was recorded in our cohort. ECG demonstrated sinus rhythm with nonspecific abnormalities of ST-T segments and normal QT duration in all patients. Echocardiogram showed severe compromise of cardiac contractility in 6 patients (92 [male], 82 [female], 64 [female], 58 [male], 93 [male], and 81 [male] years; left ventricular ejection fraction, ≤30%) and mild left ventricular dysfunction (ejection fraction between 40% and 48%) with ventricular tachyarrhythmias and then sudden death in 3 (35 [male], 54 [male], and 70 [male] years; Table).
Table.
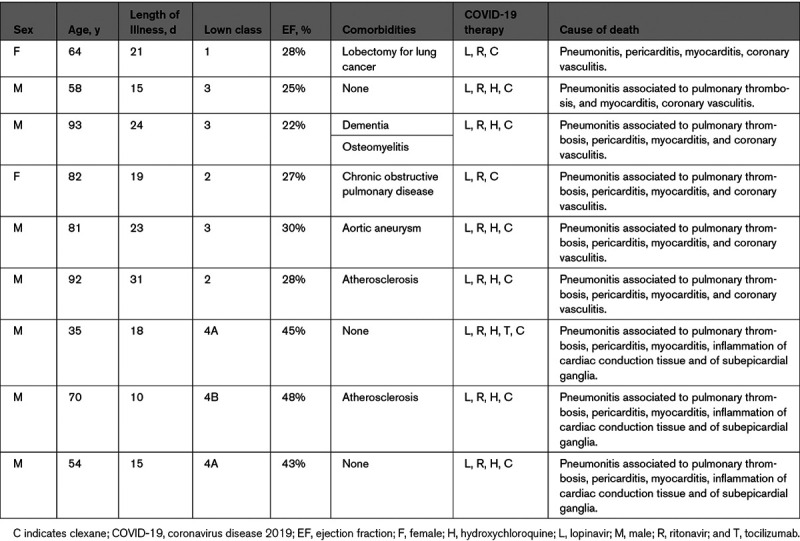
Clinical and Postmortem Data of 9 Patients With Coronavirus Infection
At macroscopic examination, the heart was always increased in weight (Figure 1A), pale and flabby (Figure 1B), and in 6, a pericardial effusion (Figure 1A) was also observed. No relevant (>70%) stenosis of epicardial coronary arteries was documented. At histology, lymphomononuclear infiltrates (CD68, CD45 Ro positive) were observed in the myocardium with focal necrosis of adjacent myocytes (Figure 1C and 1D), in all patients and in the pericardium (Figure 1E and 1F) and in intramural vessels with necrosis of vessels’ wall (Figure 1G and 1H) in the 6 subjects who died because of heart failure. Inflammatory infiltrates were mostly registered in the adventitia and outer vessel layer. Vasculitis of pulmonary vessels (Figures I and II in the Data Supplement) was commonly observed in our cohort. In the 3 patients with ventricular arrhythmias, who suffered sudden death, subepicardial ganglia were also involved by inflammation with degradation of neuron fibers (Figure 1I). In the last cohort, several sections including peripheral segments of His-Purkinje system presented lymphocytic infiltration and necrosis of conduction tissue (Figure 1J). The extensive involvement of intramural coronary vessels in these patients suggests to have been, in addition to cardiomyocyte necrosis, a major determinant of heart failure and cardiogenic shock. Inflammation of cardiac conduction tissue and of subepicardial ganglia documented in 3 patients gives reason for the occurrence of severe electrical instability and sudden death.
Figure 1.
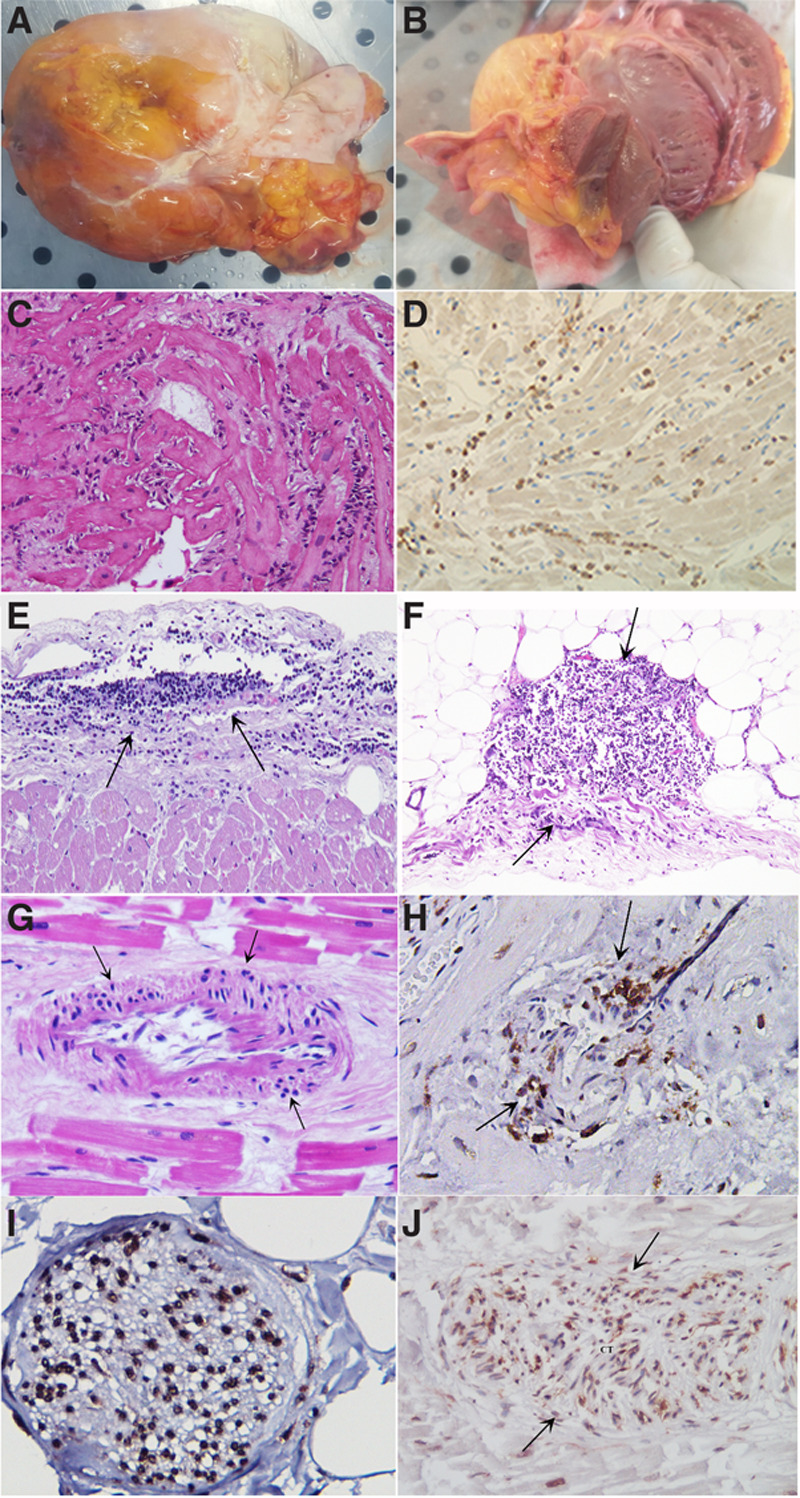
Cardiac postmortem findings in 9 patients affected by coronavirus disease 2019 (COVID-19) infection. A, Overweight heart (450 g) in a patient with coronavirus infection. Plaque-like fibrous whitish thickened pericardium denoting pericarditis. B, Grossly, the heart was dilated, and the ventricular myocardium was flabby and pale with thickened walls. C, Lymphocytic myocarditis (hematoxlin and eosin, 200×). D, Immunohistochemistry for cluster differentiation68 showing positive macrophages in the inflamed myocardium. E and F, Lymphocytic pericarditis (hematoxlin and eosin, 200×). G, Necrotizing vasculitis of an intramural coronary artery (hematoxlin and eosin, 200×). H, Immunohistochemistry for Cluster differentiation45Ro suggesting lymphocytic infiltration and necrosis of an intramural coronary artery (200×). I, Lymphocytic infiltration of a subepicardial ganglion (cluster differentiation45Ro, 400×). J, Severe infiltration with damage of a section of conduction tissue (hematoxlin and eosin, 200×).
Real-time polymerase chain reaction has been obtained from several frozen myocardial samples for the most common cardiotropic viral genomes, including adenovirus, cytomegalovirus, parvovirus B19, Epstein-Barr virus, human herpes virus 6, and herpes simplex virus 1 and 2, enterovirus, influenza virus A and B, hepatitis C virus, and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Molecular studies failed to find viral genomes, including SARS-CoV-2, in the myocardium of our cohort. Polymerase chain reaction for SARS-CoV-2 was always positive in the lung tissue.
These findings strongly suggest myocardial inflammation in COVID-19 infection contributes significantly to patients’ death. To this regard, all cardiac compartments may be involved including pericardium, myocardium, coronary vessels, conduction tissue, and neuron ganglia.
Our autopsy series demonstrated myocardial inflammation in the absence of viral genomes most likely related to an abnormal immunologic response.1 Its severity is probably linked to the specificity of human leukocyte antigen (HLA) system and explains the different clinical response to SARS-CoV-2 infection: indeed, some patients have a mild or a moderate form of myocarditis and recover spontaneously, whereas others may have a severe manifestation and die.
A recent report demonstrates that HLA variation affects the cellular immune response to peptides from human-infecting coronaviruses with HLA-B*46:01 causing increased and HLA-B*15:01 reduced vulnerability to viral infection.2
Lack of detection of SARS-CoV-2 in the myocardium is at variance with another previously published case report3 showing ultrastructural evidence of viral particles in cardiac macrophages. This probably represents a hyperacute and highly viremic phase of the COVID-19 disease, with a direct viral damage, which is likely followed after some days, as in our cases, by an immune-mediated insult.
Differently from recent observation of endothelitis4,5 caused by SARS-CoV-2, coronary vasculitis in our study proceeded from adventitia to internal vessel layers (Figure 1C and 1D) suggesting the immunologic damage coming from vasa vasorum. We can speculate that immunocytes and circulating immunocomplexes might participate in vessel inflammation as recently suggested.5 Pathological implication in this instance is the loss of pericytes leading to additional impairment of angiogenesis.6
Clinical implications from these observations are that these patients may respond to immune-modulation therapy, including steroids, inhibitors of interleukin-1β, and inhibitors of receptors for interleukin-6, in addition to an antiviral therapy. The presence of extensive necrotizing vasculitis of intramural coronary vessels solicits the administration of high-dosage immune-modulating agents.
Figure 2.
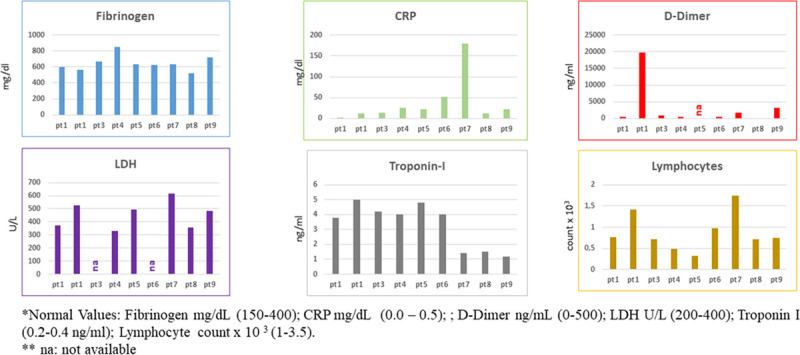
Biomarker data of 9 patients (pts) with coronavirus infection. The levels of fibrinogen, CRP, d-dimer, lactate dehydrogenase (LDH), troponin I, and lymphocytes were evaluated by diagnostic tools in 9 pts with coronavirus infection. CRP indicates C-reactive protein; and na, not available.
Sources of Funding
This work was supported by Line 1, Ricerca Corrente of the Italian Ministry of Health, on emerging and reemerging infections and by Progetto COVID 2020 12371675, 12371817 both funded by the Italian Ministry of Health and European Project-Transnational Research Projects on Cardiovascular Diseases ERA-CVD.
Disclosures
None.
Supplemental Materials
Figures I and II
Supplementary Material
Footnotes
Drs del Nonno and Frustaci contributed equally to this work.
The Data Supplement is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCHEARTFAILURE.120.007636.
For Sources of Funding and Disclosures, see page 695.
Contributor Information
Franca del Nonno, Email: franca.delnonno@inmi.it.
Romina Verardo, Email: romina.verardo@inmi.it.
Cristina Chimenti, Email: cristinachimenti@libero.it.
Emanuele Nicastri, Email: emanuele.nicastri@inmi.it.
Andrea Antinori, Email: biocard@inmi.it.
Nicola Petrosillo, Email: nicola.petrosillo@inmi.it.
Eleonora Lalle, Email: eleonora.lalle@inmi.it.
Chiara Agrati, Email: chiara.agrati@inmi.it.
Giuseppe Ippolito, Email: giuseppe.ippolito@inmi.it.
References
- 1.Sala S, Peretto G, Gramegna M, Palmisano A, Villatore A, Vignale D, De Cobelli F, Tresoldi M, Cappelletti AM, Basso C, et al. Acute myocarditis presenting as a reverse Tako-Tsubo syndrome in a patient with SARS-CoV-2 respiratory infection. Eur Heart J. 2020;41:1861–1862. doi: 10.1093/eurheartj/ehaa286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nguyen A, David JK, Maden SK, Wood MA, Weeder BR, Nellore A, Thompson RF. Human leukocyte antigen susceptibility map for severe acute respiratory syndrome coronavirus 2. J Virol. 2020;94:e00510–e00520. doi: 10.1128/JVI.00510-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tavazzi G, Pellegrini C, Maurelli M, Belliato M, Sciutti F, Bottazzi A, Sepe PA, Resasco T, Camporotondo R, Bruno R, et al. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail. 2020;22:911–915. doi: 10.1002/ejhf.1828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Magro C, Mulvey JJ, Berlin D, Nuovo G, Salvatore S, Harp J, Baxter-Stoltzfus A, Laurence J. Complement associated microvascular injury and thrombosis in the pathogenesis of severe Covid-19 infection: a report of five case. Transl Res. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergers G, Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol. 2005;7:452–464. doi: 10.1215/S1152851705000232 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


