Abstract
Background:
Delayed recognition of decompensation and failure-to-rescue on surgical wards are major sources of preventable harm. This review assimilates and critically evaluates available evidence and identifies opportunities to improve surgical ward safety.
Data Sources:
Fifty-eight articles from Cochrane Library, EMBASE, and PubMed databases were included.
Conclusions:
Only 15–20% of patients suffering ward arrest survive. In most cases, subtle signs of instability often occur prior to critical illness and arrest, and underlying pathology is reversible. Coarse risk assessments lead to under-triage of high-risk patients to wards, where surveillance for complications depends on time-consuming manual review of health records, infrequent patient assessments, prediction models that lack accuracy and autonomy, and biased, error-prone decision-making. Streaming electronic heath record data, wearable continuous monitors, and recent advances in deep learning and reinforcement learning can promote efficient and accurate risk assessments, earlier recognition of instability, and better decisions regarding diagnosis and treatment of reversible underlying pathology.
Keywords: Surgery, ward, cardiac arrest, decompensation, deterioration, machine learning
Summary
Delayed recognition of decompensation and failure-to-rescue on surgical wards are major sources of preventable harm. Ward safety is compromised by coarse risk assessments leading to under-triage of high-risk postoperative patients to wards, where understaffed providers make infrequent patient assessments and use cognitive shortcuts, leading to bias and error-prone decision-making. Streaming electronic heath record data, wearable continuous monitors, and recent machine learning advances can promote efficient and accurate risk assessments, earlier recognition of instability, and better decisions regarding diagnosis and treatment of reversible underlying pathology.
Introduction
Following surgery, traumatic injury, or the onset of a surgical disease, patients who are not critically ill but are too ill for discharge home are admitted to surgical wards. Their recovery may be complicated by hemorrhage, respiratory failure, opiate toxicity, sepsis, and other life-threatening conditions. On surgical wards, infrequent patient assessments may lead to unrecognized decompensation and progression to cardiac arrest. Only 15–20% of patients suffering ward arrest survive and survivors often require prolonged hospitalization and rehabilitation.1, 2
Some patients remain stable until suffering an acute event—like myocardial infarction and pulmonary embolism—where immediate recognition and rescue are the only paths to survival. In cases of gradual deterioration, subtle signs of physiologic instability often occur prior to organ failure and cardiac arrest, representing opportunities for prevention.3, 4 When risk for decompensation and arrest are underestimated, high-risk patients may be under-triaged to a ward rather than an intensive care unit (ICU).5 Traditional ward monitoring involves time-consuming, manual review of health records, infrequent patient assessments, high patient-to-provider ratios, outdated prediction models that lack accuracy and autonomy, and biased, error-prone decision-making.6–10 Surgical ward patients continue to incur preventable harm from unrecognized decompensation, resulting in failure-to-rescue.11, 12 Machine learning systems and emerging technologies have the potential to address weaknesses inherent to traditional approaches and improve surgical ward safety, but have not yet gained widespread recognition or clinical adoption.
The purpose of this article is to promote deeper understanding of problems and potential solutions associated with surgical ward safety. The objectives of this article are to (1) summarize available evidence regarding the epidemiology of hospital ward decompensation and arrest, unique aspects of surgical ward patients, and weaknesses inherent to traditional ward monitoring, and (2) propose a framework in which machine learning and emerging technologies address these weaknesses, while emphasizing the importance of bedside assessment and human intuition in recognizing and managing a decompensating patient.
Methods
Cochrane Library, EMBASE, and PubMed databases were searched from their inception to December 2018, using keywords and terms defined in Supplementary Figure 1. Three authors with medical degrees screened articles by reviewing abstracts for the following criteria: (1) published in English and (2) primary literature or a review article, including the following study types: cross-sectional study, Delphi consensus, observational study, prospective observational study, prospective randomized trial, retrospective study, review article, and systematic review. Ninety-three articles were excluded by these criteria. Articles were selected for inclusion by manually reviewing abstracts and full texts for the following criteria: (1) topical relevance, (2) methodological strength, and (3) novel or meritorious contribution to existing literature describing the epidemiology of ward decompensation, traditional approaches to ward monitoring, new technologies that address the deficits of traditional approaches, and the importance of integrating bedside assessment and human intuition with prediction models. One hundred and thirty-one articles were excluded by these criteria. The screening and selection process was unblinded. Due to heterogeneity among study populations, methods, and results from published literature, the authors assessed topical relevance, methodological strength, and novelty by subjective means. Unpublished articles and abstracts without an accompanying full text were not eligible for inclusion. The authors also performed a manual review of articles cited by articles identified in the initial search using the same selection criteria. Eight articles were included by these criteria. Therefore, fifty-eight studies were included and manually synthesized into categories pertinent to study objectives, which were determined a priori by the authors. The design, population, sample size, major findings pertinent to this review, sources of funding, and conflicts of interest for all included studies are listed in Table 1.
Table 1:
Summary of included studies.
| Primary Author | Study Design | Population | Sample Size | Major Findings Pertinent to this Scoping Review | Sources of Funding and Conflicts of Interest |
|---|---|---|---|---|---|
| Antink64 | Observational | Arrhythmia alarms | n=1250 | Machine learning techniques and multimodal rhythmicity estimation created a 95% TPR and 78% TNR for arrhythmia alarms | None reported |
| Arkin9 | Retrospective | Aortic valve replacements | n=410,157 | Higher nurse-to-patient ratios were protective against preventable complications (OR 0.94, 95% CI 0.90–0.99) | AHRQ |
| Bartkowiak47 | Retrospective | Ward patients | n=32,537 | Validation of the superiority of eCART vs. MEWS and NEWS; maximum respiratory rate was the most predictive vital sign for severe adverse events (AUC 0.67) | NHLBI, Philips Healthcare, Early Sense, Quant HC |
| Bechara71 | Cross-Sectional | Patients with prefrontal cortex damage, healthy controls | n=16 | In healthy individuals, activation of covert biases precedes overt reasoning, possibly reflecting unconscious access to previous experiences | None reported |
| Berlot4 | Retrospective | In-hospital arrests | n=263 | 86% of in-hospital arrests had recognizable anticipating events, survival without neurologic sequelae was 5% | None reported |
| Brown38 | Retrospective | Ward admissions | n=7,643 | Continuous monitoring was associated with shorter hospital LOS, ICU LOS, and fewer respiratory events | EarlySense |
| Chen65 | Retrospective | Hours of non-invasive vital sign monitoring | n=179,157 | ML models can be taught to discriminate clinically relevant changes in vital signs from artifacts (AUC>0.87) | NINR, NHLBI, NSF |
| Churpek46 | Retrospective | Ward admissions | n=269,999 | The eCART early warning score was more accurate than the MEWS for predicting cardiac arrest, ICU transfer, and death | NHLBI, NIA, Philips Healthcare, AHA, Laerdal Medical, EarlySense |
| Churpek51 | Retrospective | Ward patients | n=3,789 | A >6 hour interval between onset of critical illness and ICU transfer was associated with increased mortality (33 vs. 25%) | NHLBI, Philips Healthcare, AHA, ownership interest in Quant HC |
| DeVita52 | Retrospective | Rapid response team activations | n=3,269 | Rapid response team adoption was associated with fewer ward arrests (5.4 vs. 6.5 per 1,000 admissions) | None reported |
| DeVita54 | Consensus Conference | Experts | n=27 | Vital sign aberrations can be used to predict risk of decompensation and improved monitoring may impact outcome, however the burden and characteristics of intensive monitoring system have yet to adequately studied | AHA, AHRQ, CHEST, AACN, Italian Resuscitation Council, Unilink, Delmarva Foundation, VA, The Learning Clinic, Patientrack, Philips Medical systems |
| Douw32 | Systematic Review | Articles about clinical signs triggering nursing concern | n=18 | Qualitative, intuitional assessment of a patient’s condition can trigger concern prior to changes in vital signs | None reported |
| Dybowski6 | Retrospective | ICU patients | n=258 | An artificial neural network outperformed a regression model in predicting in-hospital mortality (AUC 0.86 vs. 0.75) | Special Trustees for St. Thomas’ Hospital |
| Eerikainen63 | Observational | Dysrhythmias | n=1,250 | Simultaneous use of ECG, arterial BP, and PPG signals, along with Random Forest models, increases TPR and TNR for dysrhythmia alarms | None reported |
| Fernando56 | Prospective | Ward patients | n=5,995 | Older patients decompensating on wards were more likely to have delayed rapid response team activation and worse outcomes | None reported |
| Franklin3 | Retrospective | Hospital admissions | n=21,505 | 66% of ward arrests were preceded by documented decompensation | None reported |
| Fry20 | Retrospective | Patients undergoing major surgery | n=702,268 | Decreased failure-to-rescue rates explained 64% of improvement in hospital mortality, a decrease in surgical complications accounted for 5% | NIH, AHRQ, ArborMetrix, PCORI |
| Ghaferi15 | Retrospective | Major surgeries | n=107,899 | Failure-to-rescue ranged from 6.8% for the top 20% of hospitals to 16.7% for the bottom 20% of hospitals | AHRQ, NCI |
| Ghaferi19 | Retrospective | Pancreatectomies | n=16,900 | Failure-to-rescue ranged from 6.4% for the top 20% of hospitals to 40.0% for the bottom 20% of hospitals | AHRQ, NCI, Robert Wood Johnson Clinical Scholars Program |
| Griffiths33 | Retrospective | Ward admissions | n=138,133 | The average patient-to-nurse ratio was 5.5, temporary staffing was associated with increased mortality | NIHR |
| Hassen34 | Delphi Consensus | Experts | n=27 | 100% agreement that inadequate staffing negatively impacts surgical ward safety | NIHR |
| Heller53 | Retrospective | Ward patients | n=3,827 | Automated EWS paging system implementation was associated with fewer ward arrests (2.1 vs. 5.3 per 1,000 admissions) | Philips Healthcare |
| Helling12 | Retrospective | Unexpected ICU transfers | n=111 | Multiple physician notifications were required prior to 29% of all unexpected ICU transfers | None reported |
| Karpman55 | Retrospective | Ward patients | n=20,745 | Rapid response team implementation was associated with more ICU admissions and no change in outcomes of transferred patients | None reported |
| Kim7 | Retrospective | ICU admissions | n=38,474 | Machine learning algorithms were as good as APACHE III in predicting ICU mortality | NCRR |
| Komorowski60 | Retrospective | Septic ICU patients | n=96,156 | A reinforcement learning model recommending intravenous fluid and vasopressor strategies outperformed human clinicians | Orion Pharma, Amomed Pharma, Ferring Pharma, Tenax Therapeutics, Baxter Healthcare, Bristol-Myers Squibb, GSK, HCA International |
| Kumar31 | Retrospective | Adult ICU patients with septic shock | n=2,731 | Each hour of delay in antimicrobial administration decreased survival by 7.6% | Eli-Lilly, Pfizer, Merck, Astra-Zeneca, GSK, Arginox, DeepBreeze, Minimitter, Edwards, OrthoBioTech |
| Martin66 | Review | Validation Studies for Wrist Actigraphy | n=15 | Wrist actigraphy can be used to estimate sleep parameters and provide a useful adjunct to sleep diaries, polysomnography, and clinical interviews in sleep disorders | NIH, NIA, Cedars Siani Sleep Medicine Fellowship, VA GLAHS GRECC |
| McGaughey43 | Systematic review | Trials about rapid response teams | n=2 | EWS and rapid response team adoption was not associated with decreased mortality | Ireland Cochrane Fellowship |
| McGinley45 | Review | - | - | Description of the National Early Warning Score and its application | None reported |
| McGloin11 | Retrospective | Ward deaths and ICU transfers | n=415 | 65% of all unexpected ward deaths followed gradual decompensation | None reported |
| McQuillan57 | Prospective | Unplanned ICU transfers | n=100 | Pre-transfer care was suboptimal for 54/100 patients, 69% of this group had delayed transfer, and their mortality was 48% | NHS Trust, Lilly Industries |
| Merchant17 | Cross-sectional | Adult IHCA with resuscitation response | n=209 million | Incidence study demonstrating ~200,000 cardiac arrests treated among US hospitalized patients annually | Philips Healthcare, Laerdal Medical, NIH, Cardiac Science, NHLBI, Medivance, Doris Duke Foundation, AHA, Lifebridge Medizintechnik, Gambro Renal |
| Mitchell50 | Prospective | Ward admissions | n=2,142 | Implementation of a ward monitoring system was associated with fewer unexpected ICU transfers and deaths | ACT Health |
| Mohammed Iddrisu23 | Retrospective | Surgical ward patients | n=100 | The most common reason for rapid response team activation was hypotension (26%) | None reported |
| Pearse5 | Retrospective | High-risk surgical patients in the UK | n=513,924 | High-risk patients accounted for 84% of deaths but for only 12.5% of procedures; highest mortality (39%) occurred in high-risk patients admitted to the ICU following initial admission to a standard ward | None reported |
| Peberdy14 | Retrospective | In-hospital cardiac arrests | n=14,720 | 44% of adult in-hospital cardiac arrest victims had ROSC; 17% survived to hospital discharge; 86% of those with CPC-1 at time of admission had post arrest CPC-1 at time of discharge | AHA |
| Perman16 | Retrospective | In-hospital cardiac arrests | n=85,201 | 41% of all in-hospital cardiac arrests occurred on wards with a rate of 0.1 events per 1,000 bed-days | NIH, Philips Healthcare, EarlySense, Quant HC, Physio-Control, Zoll Medical, Cardiac Science |
| Prgomet41 | Observational | Clinical staff | n=84 | Perceptions of hospital staff towards continuous monitoring is generally positive, but concerns remain for inappropriate escalations of care and patient discomfort | NHMRC |
| Rothman48 | Retrospective | Hospitalized patients | n=148,985 | Rothman Index predicted 24-hour mortality with AUC 0.93 or greater | Sarasota Memorial Healthcare Foundation, Greenfield Foundation |
| Rothman49 | Retrospective | Hospitalized patients | n=42,302 | 12 types of nursing assessments were significant predictors of mortality | Rothman Healthcare Corporation, Sarasota Memorial Healthcare Foundation, Greenfield Foundation |
| Sandroni2 | Review | Articles about in-hospital arrest | n=79 | In most studies, survival following in-hospital cardiac arrest ranges from 15–20% | None reported |
| Sanfey69 | Cross-Sectional | Players of a classic economic test in splitting sums of money | n=19 | fMRI imaging shows simultaneous heightened activity in emotion and cognition centers when deciding between fair and unfair offers | None reported |
| Schein18 | Prospective | Ward arrests | n=64 | The most common physiologic sign of decompensation prior to arrest was respiratory (38%) | None reported |
| Shickel59 | Retrospective | ICU admissions | n=85,164 | A deep learning model predicted in-hospital mortality more accurately than traditional acuity score calculation | NIGMS, NSF, UF CTSI, NCATS, J. Crayton Pruitt Family Department of Biomedical Engineering, NVIDIA |
| Silber10 | Retrospective | Hospital admissions | n=403,679 | Higher bed-to-nurse ratios were associated with increased odds of failure-to-rescue | NHLBI, Veterans Affairs Health Services Research and Development |
| Silver61 | Observational | Go boardgame | - | Deep reinforcement learning models provide high fidelity victories against previous Go algorithms and human experts | Google, Google DeepMind |
| Skogvoll1 | Retrospective | In-hospital deaths | n=4,927 | Survival after cardiopulmonary resuscitation outside of the ICU was 17% | None reported |
| Slight39 | Retrospective | Modeled ward admissions | n=5,000 | Costs and savings associated with a continuous ward monitoring system demonstrated favorable return on investment | EarlySense |
| Smith42 | Systematic review | Early warning systems | n=33 | AUC range for predicting adverse outcomes was 0.66–0.78 | The Learning Clinic Ltd. |
| Subbe36 | Review | Articles about ward decompensation | n=37 | Among high-risk ward patients, continuous monitoring may facilitate early detection of decompensation | NHS Trust |
| Subbe44 | Prospective | Medical emergency admissions | n=709 | Higher EWS were associated with increased odds of death and ICU transfer | NHS Trust |
| Taenzer37 | Prospective | Surgical ward admissions | n=13,398 | Continuous pulse oximetry was associated with fewer rescue events and unplanned ICU transfers | The Hitchcock Foundation, Masimo Corporation |
| Van den Bruel74 | Retrospective | Primary care patients | n=3,890 | Clinician intuition identified patients with illness severity that was underrepresented by clinical parameters | Research Foundation Flanders, Eurogenerics, NIHR |
| Van den Bruel75 | Systematic review | Articles about features of serious infections | n=30 | Traditional clinical parameters associated with serious infection are often absent among patients with serious infections | Health Technology Assessment, NIHR |
| Watkinson40 | Prospective randomized | Ward patients | n=402 | Major adverse events were similar between continuous and standard monitoring groups | Oxford BioSignals, NHS Trust |
| Weenk62 | Prospective | Ward patients | n=20 | EWS calculated with data from wearable continuous monitors were similar to EWS calculated from vitals obtained by nurses | Radboud University Medical Center |
| Wesnes35 | Prospective | Surgical house officers | n=10 | House officers had impaired concentration and memory after a weekend on call | None reported |
AAA = Abdominal Aortic Aneurysm; AHA: American Heart Association; AHRQ: Agency for Healthcare Research and Quality; APACHE: Acute Physiology and Chronic Health Evaluation; AUC: Area Under the Curve; CHEST: American College of Chest Physicians; AACN: American Association of Critical Care Nurses; CI: Confidence Interval; CPC: Cerebral Performance Category; eCART: electronic Cardiac Arrest Triage; EWS: Early Warning System; GSK: GlaxoSmithKline; HCA: Hospital Corporation of America; ICU: Intensive Care Unit; IHCA: In-hospital Cardiac Arrest; LOS: Length of Stay; MEWS: Modified Early Warning sSore; NCATS: National Center for Advancing Translational Science; NCI: National Cancer Institute; NCRR: National Center for Research Resources; NEWS: National Early Warning Score; NHLBI: National Heart, Lung, and Blood Institute; NHMRC: National Health and Medical Research Council; NHS: National Health Service; NIA: National Institute of Aging; NIGMS: National Institute for General Medical Sciences; NIH: National Institute of Health; NIHR: National Institute for Health Research; NINR: National Institute of Nursing Research; NSF: National Science Foundation; OR: Odds Ratio; PCORI: Patient-Centered Outcomes Research Institute; PPG: photoplethysmogram; TNR: True Negative Rate; TPR: True Positive Rate; UF CTSI: University of Florida Clinical and Translational Science Institute; VA GLAHS GRECC: Veteran’s Administration Greater Los Angeles Healthcare System Geriatric Research Education and Clinical Center
Results
Definitions and Epidemiology
Definitions of decompensation in published literature are heterogeneous and often subjective. An increase in the sequential organ failure assessment (SOFA) score by two or more can be used to identify the onset of organ dysfunction and critical illness, signifying decompensation.13 Cardiac arrest is defined as resuscitation requiring chest compressions and/or cardiac defibrillation.14 Failure-to-rescue is defined as the death of a patient following a complication.15
Approximately 41% of all in-hospital cardiac arrests occur on wards at a rate of 0.1 per 1,000 bed-days.16 On hospital wards, vital sign measurements and nursing assessments are often performed every four hours.1, 16, 17 This monitoring strategy may fail to identify early signs of physiologic deterioration, which often occur hours before arrest.3 In a review of 263 ward arrests, 86% were preceded by signs and symptoms of decompensation.4 The underlying disease process is often reversible.18
In an audit of unexpected ward deaths at a teaching hospital, almost two-thirds followed gradual decompensation.11 In a review of surgical ward decompensation requiring unplanned ICU transfer, nearly one in three episodes were characterized by delayed recognition of critical illness.12 When decompensation progresses to arrest, failure-to-rescue is the most likely outcome. In-hospital cardiac arrest occurring outside of the ICU is associated with 80–85% mortality.1, 2 Rates of failure-to-rescue for surgical patients following a complication is highly variable across US hospitals, suggesting that system-level factors contribute.15, 19 Although preventing complications is a laudable goal, decreasing failure-to-rescue may have a greater impact on mortality rates. In a recent analysis of Medicare beneficiary data, hospitals that performed well in reducing mortality after major surgery accomplished these reductions primarily by reducing failure-to-rescue rates rather than complications.20
Unique Characteristics of Surgical Patients Contributing to Decompensation and Arrest
Surgical patients are uniquely vulnerable to postoperative hemorrhage, respiratory failure, opiate toxicity, and sepsis.12, 21, 22 These conditions impart a second physiologic insult following surgery, traumatic injury, or acute illness requiring hospitalization. Traumatic injury and major surgery increase risk for the interval development of brisk hemorrhage. Up to 20% of all circulating blood may be lost before compensatory tachycardia is evident and up to one-third of all circulating blood may be lost before hypotension develops. When vital signs are measured every four hours, a patient may progress to hemorrhagic shock and organ dysfunction between assessments.23
Postoperative patients are susceptible to respiratory failure, especially following general anesthesia and painful thoracic and abdominal procedures. General anesthesia is associated with decreased functional residual capacity, alveolar macrophage dysregulation, increased alveolar capillary permeability, and increased tissue plasminogen activator (tPA) inhibitor levels. These factors predispose patients to atelectasis, pneumonia, deep vein thrombosis, and pulmonary embolism.24–27 In addition, painful thoracic and abdominal procedures impair patients’ ability to take deep breaths, cough, clear secretions, and participate in respiratory therapy exercises. In a review of surgical patients requiring unplanned ICU transfer, respiratory events accounted for 69% of all alerts.12 Respiratory depression, and eventual failure, among postoperative patients may be exacerbated by opiate consumption. Hospitalized surgical patients consume more opiates than hospitalized medical patients.21, 22 Patients with severe postoperative pain may receive high concentrations of opiates that saturate mu receptors and degradative enzymes, leading to dose-dependent respiratory depression.28, 29
Surgical ward patients are also vulnerable to sepsis, which accounts for one in four deaths following elective surgery.30 In a landmark 2006 study by Kumar et al.31 only half of all patients with septic shock received antibiotics within six hours of sepsis onset; each one-hour delay in antibiotic administration beyond six hours was associated with an 8% increase in mortality. Early diagnosis of postoperative sepsis may be confounded by difficulties in differentiating between sepsis and sterile inflammation. Vigilance and careful patient assessment are necessary to not only facilitate early recognition of postoperative sepsis but also avoid unnecessary intravenous fluid and antibiotic administration.
Factors contributing to Failure-to-Rescue
Limitations on Nursing and Physician Staffing
Low staffing levels on surgical wards are associated with failure-to-rescue. Nurses spend more time at the bedside than physicians and their intuitive sense of impending decompensation can precede objective signs of decompensation.32 This advantage may be abrogated by higher patient-to-nurse ratios, which averaged 5.5 in a review of over 138 thousand ward admissions.33 Higher patient-to-nurse ratios are associated with significantly increased odds of preventable postoperative complications and failure-to-rescue.9, 10 In a Delphi Consensus, there was 100% agreement that both inadequate staffing levels negatively impact patient safety on surgical wards and access to doctors at night promotes patient safety.34 However, the perceived benefits of access to doctors on call at night may be abrogated when low physician staffing levels and long hours on call negatively impact cognitive performance.35 When staffing levels are low, early identification of physiologic instability often depends on vital sign monitoring.
Continuous Monitoring of Vital Signs
On hospital wards, vital signs are typically measured every four hours. Among high-risk ward patients, continuous monitoring may facilitate early detection of decompensation.36 Continuous pulse oximetry has been associated with decreased frequency of rescue events and unplanned ICU transfers.37 Continuous monitoring of heart and respiratory rate has been associated with shorter hospital length of stay, fewer ICU days, and fewer respiratory decompensation events.38 Though continuous monitoring systems are expensive, the return on investment may be favorable.39
However, level 1 evidence to support continuous monitoring is lacking.40 Potential disadvantages of continuous monitoring include distracting false-positive alarms, patient discomfort, and impaired mobility.41 In addition, patient selection for continuous monitoring often depends on crude risk estimations using admission vital signs, time-consuming manual review of medical records, and initial impressions on clinical assessment. Current methods are inadequate for identifying high-risk, outlier patients that may benefit from close monitoring.34 The frequency of false alarms may be partially attributable to data dimensionality. As the number of vital sign data sources increases linearly, the combinations of alarm parameters that may indicate instability increase exponentially. Finally, reduced patient contact may be an unintended consequence of continuous monitoring.41
Early Warning Scores and Clinical Decision-Support Systems
Early warning scores facilitate identification of decompensating patients by considering vital signs and patient assessments in aggregate. Many early warning scores exist, but few perform well on external validation.42, 43 Additive scores like the Modified Early Warning Score (MEWS)44 and the National Early Warning Score (NEWS)45 are easy to interpret and apply, but their accuracy is hindered by use of static variable thresholds for a small number of parameters. The electronic Cardiac Arrest Risk Triage (eCART) score was developed using regression coefficients to identify cut-off values for patient demographics, vital signs, and 18 laboratory values.46 Notably, eCART outperformed MEWS and NEWS in a retrospective analysis of 32,537 postoperative surgical inpatients.47
The Rothman Index uses vital signs, cardiac rhythms, laboratory values, and nursing assessments for subtle signs of illness—like loss of appetite and mild confusion—and accurately predicts death or discharge to hospice within 24 hours (AUC 0.93).48, 49 Blood oxygen saturation is included, but the influence of supplemental oxygen is not considered. Laboratory values are blended with a linear decay function for 48 hours and while this seems appropriate for daily laboratory values, it is less useful for serum albumin or liver enzymes, which are often measured infrequently. Parametric regression modeling assumes linear relationships among variables. However, relationships among variables are often complex and non-linear, limiting the accuracy of regression models.6, 7
Decisions regarding ward patient management and ICU transfer may be affected by several forms of bias.8 The framing effect occurs when outcome predictions are affected by contextual information. A patient on a surgical ward who is mildly confused one day after an elective abdominal surgery, but has had stable vital signs since the operation, may be deemed appropriate for continued intermittent monitoring on a surgical ward. However, in the context of known liver dysfunction or history of crescendo transient ischemic attacks, this same patient may benefit from assessment of hepatic function, a focused neurologic exam, continuous monitoring, and consideration of ICU transfer. Overconfidence and optimistic bias occur when individuals perceive that things are better than they are and that adverse events disproportionately affect others. A surgeon may ignore signs that their patient is decompensating, and thus delay ICU transfer, due to overconfidence and unwarranted optimism in the likelihood of early recovery.
Anchoring bias, which occurs when outcome probability estimations gravitate toward an initial baseline value, may exacerbate the impact of overconfidence. A surgeon may observe that 95% of their patients recover uneventfully, leading them to believe that their next patient will also do well. However statistics mean nothing to an individual patient who is actively decompensating despite a 95% chance of uneventful recovery. Anchoring bias may be compounded when recent experiences have been positive, thus promoting recall bias in which outcome probability estimations are influenced by the ease with which prior experiences are recalled. Confirmation bias occurs when outcomes are predicted using personal beliefs rather than evidence. In the absence of high-quality evidence to guide surgical ward monitoring, personal beliefs may play a prominent role.
Rapid Response Teams
When ward patients develop acute organ dysfunction and decompensation, early ICU transfer is essential.50 In a review of 3,789 medical and surgical ward patients at five different hospitals, the median interval between onset of critical illness and ICU transfer was 5.4 hours.51 Each additional 1-hour delay in transfer was associated with 3% increased mortality. Delay in transfer more than 6 hours was also associated longer hospital length of stay among survivors (13 vs. 11 days, p=0.01). Many hospitals employ rapid response teams that receive alerts, travel to the bedside, and facilitate early diagnosis and treatment, including ICU transfer if necessary. Use of rapid response teams has been associated with decreased incidence of cardiac arrest and unplanned ICU transfers.52, 53 However, rapid response teams are only as good as their activation criteria, clinical judgement, and the quality of transitions from hospital wards to the ICU.54–56 In a review of medical and surgical patients with unplanned ICU admissions, pre-transfer care was suboptimal for 54%.57 Patients receiving suboptimal pre-transfer care had 56% mortality, almost double that of patients receiving appropriate care.
Future Directions: Preventing the Need to Rescue
By current methods, high-risk patients who would benefit from close monitoring in an ICU may be under-triaged to a ward, where care is compromised by time-consuming manual review of health records, infrequent patient assessments, high patient-to-provider ratios, prediction models that lack accuracy and autonomy, and biased, error-prone decision-making (Figure 1).5, 9, 10 Integrating new technologies with human intuition and bedside assessment have the potential to transform surgical ward monitoring.
Figure 1:
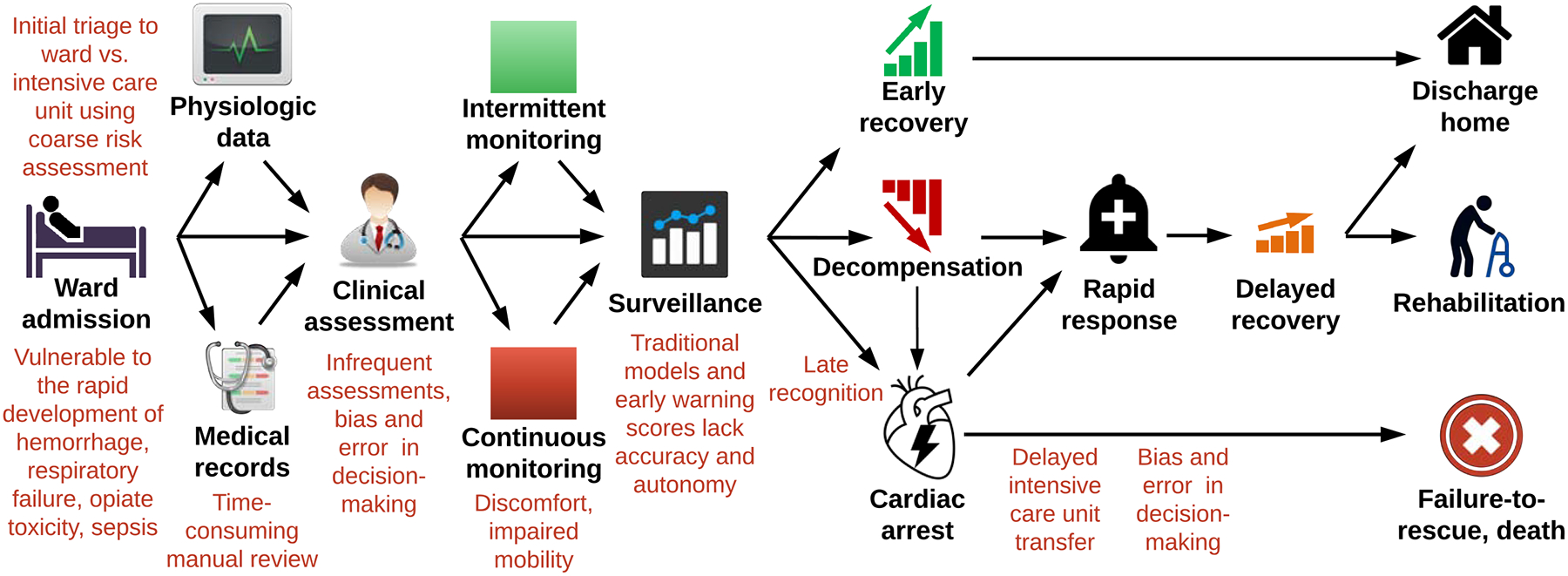
Traditional approaches to surgical ward monitoring have several pitfalls and opportunities for improvement.
Use of Machine Learning for Phenotyping and Decision Support
Deep learning is an machine learning extension of regression-based techniques that is adept at learning and representing highly-dimensional data with non-linear functions.58 The initial and final layers are inputs and outputs, respectively. The middle layers contain hidden nodes. A multilayer perceptron is a standard neural network with a single middle layer; deep models can have hundreds or thousands of middle layers. Each link connecting two nodes is assigned a weight that is influenced by previous layers and affects the output from that node. Weights are continually updated by an algorithm that optimizes the relationships between input and output layers, allowing accurate representation of complex, non-linear relationships among input variables. Deep models automatically learn optimal feature representations from raw data: a major advantage over models that require time-intensive, hand-crafted feature engineering. However, deep learning is difficult to interpret and is susceptible to overfitting and vanishing gradients unless corrective measures—like dropout and rectified linear units—are employed.
Deep models like the recurrent neural network—with its variants the Long Short-Term Memory (LSTM) and Gated Recurrent Unit (GRU)—capitalize on the availability of vast amounts of sequentially ordered, time-series vital sign data in electronic health records (EHRs). Recurrent neural networks update weights according to both the current inputs and previous states of each hidden unit.58 GRU modeling has been used to predict in-hospital mortality for ICU patients using SOFA score variables from the electronic health record, and these models exhibit greater accuracy than the SOFA score itself.59 A similar model could predict the onset of critical illness, unplanned ICU transfer, and death among surgical ward patients. Despite these capabilities, deep learning cannot suggest a course of action for a given clinical scenario, but reinforcement learning can.
In reinforcement learning, an algorithm learns that certain policies applied to given states lead to defined outcomes. This occurs in the context of a Markov decision process (MDP). Consisting of a finite set of states and actions, the MDP uses both the probability that a given action within a given state will lead to a new state along with the reward that results from the new state. Reinforcement learning derives a policy comparing the predicted utility of different actions under certain circumstances, identifying the choice or action with the highest probability of a defined outcome.
The Artificial Intelligence (AI) Clinician is a reinforcement learning model capable of recommending fluid resuscitation and vasopressor administration strategies among septic patients. This model was built on an MDP framework trained on Medical Information Mart for Intensive Care (MIMIC-III) data, and validated with Philips eICU data, including 96,156 ICU admissions.60 Forty-eight variables—including vital signs, laboratory values, and comorbidities—were tracked by 4-hour increments over 72 hours and clustered into 750 distinct states. The model was then trained to predict the probability that, from any of the states, a given treatment would result in transition to another state. To establish the reward principle, positive scores were assigned to cases leading to survival. The model then experimented with fluid resuscitation and vasopressor administration strategies, learning which single strategy was associated with the highest probability of survival in each state. Mortality was lowest when actions taken by clinicians matched actions recommended by AI Clinician.
Combining deep and reinforcement learning offers unique advantages for complex scenarios, as demonstrated in the gaming industry.61 A similar approach could be used to augment surgical ward monitoring (Figure 2). Given the rarity of cardiac arrest on wards and the inherent difficulties associated with expeditious recognition and appropriate management of ward arrest, it seems prudent to seek prevention as a primary objective. Initial patient triage presents an important opportunity to achieve this objective. Rather than depending solely on the accuracy and reliability of triage decisions made by clinicians in the emergency department, operating room, or post-anesthesia care unit, deep models would identify high-risk patients who are likely to benefit from initial triage to a high level of care with continuous or frequent monitoring and patient assessments, and offer recommendations to clinicians. The same models could be used to avoid unnecessary over-triage of low-risk patients to an ICU. Continuous vital sign, actigraphy data from wearable devices, and live-streaming EHR data would fuel real-time, deep learning predictions of impending critical illness and in-hospital mortality with automated activation of rapid response teams. Reinforcement learning models would augment decisions regarding initial treatment of underlying pathology and ICU transfer.
Figure 2:
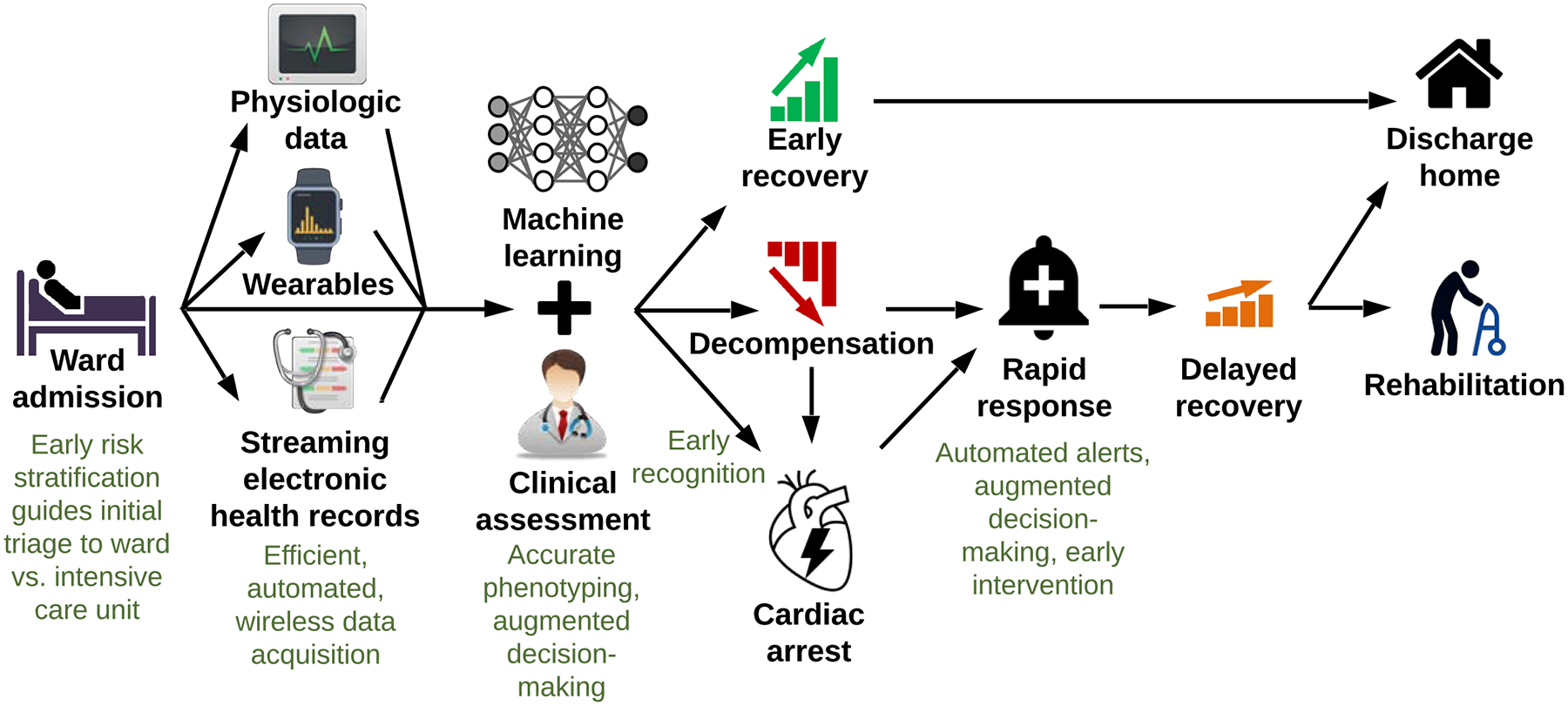
Wearable monitors, streaming electronic health record data, and machine learning can improve surgical ward monitoring.
Pervasive Sensing
The multi-billion dollar market for wearable fitness devices has fostered interest in medical applications, including continuous monitoring devices. Older wearable monitors used rigid, silicon-based hardware; newer devices use lightweight, flexible plastics and elastomers that interface well with human skin. Wearable devices have the potential to transform continuous monitoring of ward patients by streaming vital sign data to bedside and central monitoring systems while simultaneously minimizing patient discomfort and promoting mobility. Weenk et al.62 performed a pilot study in which 20 ward patients wore one of two devices. Vital sign measurements from the wearable devices were consistent with manual vital sign measurements by nurses. Data was missing or invalid 13–16% of the time, usually due to wireless connection failure and loss of skin contact. Data artifact can be mitigated by machine learning algorithms.63–65
Modern physical activity and functional status assessments often rely on questionnaire-based evaluations along with daily interactions with nurses and physical and occupational therapists. Physical activity can also be monitored with non-invasive actigraphy devices that continuously collect activity data over long periods of time.66 Beyond guiding mobilization interventions, these data could be used in concert with other vital sign and clinical assessments to identify patients at increased risk for decompensation and arrest.67, 68 In addition, incorporating data from operating room monitors may improve model accuracy by capturing important intraoperative events and physiologic changes.
Barriers to Implementation
Machine learning models are only as good as the quality and quantity of data used to train them.58 Poorly trained models could provide a false sense of security, promote over-triage, or recommend ineffective or harmful treatments. Any of these scenarios could occur on a large scale. A clinician makes mistakes one patient at a time, but an errant model could harm hundreds or thousands of patients all over the world in a short period of time.
Traditional early warning scores, like the MEWS and NEWS, are easy to interpret by simple calculations using defined cut-off values.44–46 Deep learning models provide limited insight regarding the relative importance of model inputs in determining model outputs. In discussing end-of-life care with patients and their caregivers, it would be inadequate to explain that the likelihood of functional recovery is low according to a computer, and that it is unclear why the computer reached this conclusion.
A deep reinforcement learning platform requires information technology infrastructure and expertise. These resources are not available in many clinical settings. Careful analysis of costs and savings would be necessary to determine whether this platform could be broadly applied.
Human Intuition
Bedside evaluation by an astute clinician is the most important element of patient assessment. Face-to-face interactions elicit signs and symptoms of worsening clinical status like diaphoresis, confusion, malaise, pain, and tenderness. Computer programs can also elicit some signs and symptoms of illness and their ability to do so will improve over time. However, human interactions will remain essential because they incite one of the greatest assets in surgical decision-making: human intuition, which appears to emerge from predictions made by flexible limbic system dopaminergic neurons that adjust their connections in response to positive and negative stimuli.69, 70
Purely rational, evidence-based decision-making which uses a limitless supply of infallible data would be well suited to deep reinforcement learning. However, data and evidence are often lacking, particularly for surgical emergencies. In addition, optimal decision-making occurs under the influence of human intuition, as evident in decisions regarding card games, fight or flight survival responses, and naval warfare.71–73 Intuition can also identify patients with severe, life-threatening conditions that would otherwise remain unrecognized by traditional clinical parameters.74, 75 Among ward patients, nursing intuition of imminent decompensation may precede objective signs of decompensation.32
Conclusions
Early warning scores, continuous monitoring of high-risk patients, rapid response teams, and clinical decision-support systems promote early identification and appropriate management of decompensating surgical ward patients. Despite these advances, patients continue to incur preventable harm from delayed recognition of worsening clinical status, suboptimal decision-making, and failure-to-rescue. There is an urgent need for workflow-integrated decision-support tools to 1) ensure that high-risk patients are triaged to an ICU or continuously monitored on a ward, 2) predict decompensation prior to organ failure and arrest, and 3) recommend treatments for underlying pathology and ICU transfer for decompensating patients. Live-streaming EHR data, wearable continuous monitoring devices, and machine learning models have the potential to transform ward monitoring by providing accurate, autonomous, real-time patient assessments to augment these error-prone tasks and decision-making processes. The limitations of machine learning and the importance of human intuition should be emphasized throughout the clinical adoption process.
Supplementary Material
Highlights.
Delayed recognition of decompensation on surgical wards is a major source of preventable harm
Coarse risk assessments lead to under-triage of high-risk postoperative patients to wards
Subtle signs of instability often precede ward arrest, underlying pathology is often reversible
Understaffed providers use cognitive shortcuts, leading to bias and poor decisions
Wearable monitors, streaming EHR data, and machine learning can improve ward safety
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors have no relevant conflicts of interest. AB and PR were supported by R01 GM110240 from the NIGMS. AB was supported by P50 GM-111152 from the NIGMS. PR was supported by CAREER award, NSF-IIS 1750192, from the National Science Foundation (NSF), Division of Information and Intelligent Systems (IIS). PTJ was supported by R01GM114290 from the NIGMS. TJL was supported by a post-graduate training grant (T32 GM-008721) in burns, trauma, and perioperative injury from the NIGMS.
References
- 1.Skogvoll E, Isern E, Sangolt GK, Gisvold SE. In-hospital cardiopulmonary resuscitation. 5 years’ incidence and survival according to the Utstein template. Acta Anaesthesiol Scand, 1999. February;43(2):177–84. [DOI] [PubMed] [Google Scholar]
- 2.Sandroni C, Nolan J, Cavallaro F, Antonelli M. In-hospital cardiac arrest: incidence, prognosis and possible measures to improve survival. Intensive Care Med, 2007. February;33(2):237–45. [DOI] [PubMed] [Google Scholar]
- 3.Franklin C, Mathew J. Developing strategies to prevent inhospital cardiac arrest: analyzing responses of physicians and nurses in the hours before the event. Crit Care Med, 1994. February;22(2):244–7. [PubMed] [Google Scholar]
- 4.Berlot G, Pangher A, Petrucci L, et al. Anticipating events of in-hospital cardiac arrest. Eur J Emerg Med, 2004. February;11(1):24–8. [DOI] [PubMed] [Google Scholar]
- 5.Pearse RM, Harrison DA, James P, et al. Identification and characterisation of the high-risk surgical population in the United Kingdom. Crit Care, 2006;10(3):R81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dybowski R, Weller P, Chang R, Gant V. Prediction of outcome in critically ill patients using artificial neural network synthesised by genetic algorithm. Lancet, 1996. April 27;347(9009):1146–50. [DOI] [PubMed] [Google Scholar]
- 7.Kim S, Kim W, Park RW. A Comparison of Intensive Care Unit Mortality Prediction Models through the Use of Data Mining Techniques. Healthc Inform Res, 2011. December;17(4):232–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Groopman JE. How doctors think. Boston: Houghton Mifflin; 2007. 307 p. p. [Google Scholar]
- 9.Arkin N, Lee PH, McDonald K, Hernandez-Boussard T. Association of Nurse-to-Patient Ratio with mortality and preventable complications following aortic valve replacement. J Card Surg, 2014. March;29(2):141–8. [DOI] [PubMed] [Google Scholar]
- 10.Silber JH, Romano PS, Rosen AK, et al. Failure-to-rescue: comparing definitions to measure quality of care. Med Care, 2007. October;45(10):918–25. [DOI] [PubMed] [Google Scholar]
- 11.McGloin H, Adam SK, Singer M. Unexpected deaths and referrals to intensive care of patients on general wards. Are some cases potentially avoidable? J R Coll Physicians Lond, 1999. May-Jun;33(3):255–9. [PMC free article] [PubMed] [Google Scholar]
- 12.Helling TS, Martin LC, Martin M, Mitchell ME. Failure events in transition of care for surgical patients. J Am Coll Surg, 2014. April;218(4):723–31. [DOI] [PubMed] [Google Scholar]
- 13.Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA, 2016. February 23;315(8):801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peberdy MA, Kaye W, Ornato JP, et al. Cardiopulmonary resuscitation of adults in the hospital: a report of 14720 cardiac arrests from the National Registry of Cardiopulmonary Resuscitation. Resuscitation, 2003. September;58(3):297–308. [DOI] [PubMed] [Google Scholar]
- 15.Ghaferi AA, Birkmeyer JD, Dimick JB. Complications, failure to rescue, and mortality with major inpatient surgery in medicare patients. Ann Surg, 2009. December;250(6):1029–34. [DOI] [PubMed] [Google Scholar]
- 16.Perman SM, Stanton E, Soar J, et al. Location of In-Hospital Cardiac Arrest in the United States-Variability in Event Rate and Outcomes. J Am Heart Assoc, 2016. September 29;5(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merchant RM, Yang L, Becker LB, et al. Incidence of treated cardiac arrest in hospitalized patients in the United States. Crit Care Med, 2011. November;39(11):2401–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schein RM, Hazday N, Pena M, et al. Clinical antecedents to in-hospital cardiopulmonary arrest. Chest, 1990. December;98(6):1388–92. [DOI] [PubMed] [Google Scholar]
- 19.Ghaferi AA, Osborne NH, Birkmeyer JD, Dimick JB. Hospital characteristics associated with failure to rescue from complications after pancreatectomy. J Am Coll Surg, 2010. September;211(3):325–30. [DOI] [PubMed] [Google Scholar]
- 20.Fry BT, Smith ME, Thumma JR, et al. Ten-year Trends in Surgical Mortality, Complications, and Failure to Rescue in Medicare Beneficiaries. Ann Surg, 2019. January 23. [DOI] [PubMed] [Google Scholar]
- 21.Calcaterra SL, Yamashita TE, Min SJ, et al. Opioid Prescribing at Hospital Discharge Contributes to Chronic Opioid Use. J Gen Intern Med, 2016. May;31(5):478–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun EC, Darnall BD, Baker LC, Mackey S. Incidence of and Risk Factors for Chronic Opioid Use Among Opioid-Naive Patients in the Postoperative Period. JAMA Intern Med, 2016. September 1;176(9):1286–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohammed Iddrisu S, Considine J, Hutchinson A. Frequency, nature and timing of clinical deterioration in the early postoperative period. J Clin Nurs, 2018. October;27(19–20):3544–53. [DOI] [PubMed] [Google Scholar]
- 24.Reber A, Nylund U, Hedenstierna G. Position and shape of the diaphragm: implications for atelectasis formation. Anaesthesia, 1998. November;53(11):1054–61. [DOI] [PubMed] [Google Scholar]
- 25.Pelosi P, Croci M, Ravagnan I, et al. The effects of body mass on lung volumes, respiratory mechanics, and gas exchange during general anesthesia. Anesth Analg, 1998. September;87(3):654–60. [DOI] [PubMed] [Google Scholar]
- 26.Kotani N, Lin CY, Wang JS, et al. Loss of alveolar macrophages during anesthesia and operation in humans. Anesth Analg, 1995. December;81(6):1255–62. [DOI] [PubMed] [Google Scholar]
- 27.ChangLai SP, Hung WT, Liao KK. Detecting alveolar epithelial injury following volatile anesthetics by (99m)Tc DTPA radioaerosol inhalation lung scan. Respiration, 1999. Nov-Dec;66(6):506–10. [DOI] [PubMed] [Google Scholar]
- 28.Hoffman JR, Schriger DL, Luo JS. The empiric use of naloxone in patients with altered mental status: a reappraisal. Ann Emerg Med, 1991. March;20(3):246–52. [DOI] [PubMed] [Google Scholar]
- 29.Boyer EW. Management of opioid analgesic overdose. N Engl J Med, 2012. July 12;367(2):146–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vogel TR, Dombrovskiy VY, Lowry SF. Trends in postoperative sepsis: are we improving outcomes? Surg Infect (Larchmt), 2009. February;10(1):71–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med, 2006. June;34(6):1589–96. [DOI] [PubMed] [Google Scholar]
- 32.Douw G, Schoonhoven L, Holwerda T, et al. Nurses’ worry or concern and early recognition of deteriorating patients on general wards in acute care hospitals: a systematic review. Crit Care, 2015. May 20;19:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Griffiths P, Ball J, Bloor K, et al. Nurse staffing levels, missed vital signs and mortality in hospitals: retrospective longitudinal observational study. Southampton (UK)2018. [PubMed] [Google Scholar]
- 34.Hassen YAM, Johnston MJ, Singh P, et al. Key Components of the Safe Surgical Ward: International Delphi Consensus Study to Identify Factors for Quality Assessment and Service Improvement. Ann Surg, 2018. February 20. [DOI] [PubMed] [Google Scholar]
- 35.Wesnes KA, Walker MB, Walker LG, et al. Cognitive performance and mood after a weekend on call in a surgical unit. Br J Surg, 1997. April;84(4):493–5. [PubMed] [Google Scholar]
- 36.Subbe CP, Williams E, Fligelstone L, Gemmell L. Does earlier detection of critically ill patients on surgical wards lead to better outcomes? Ann R Coll Surg Engl, 2005. July;87(4):226–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taenzer AH, Pyke JB, McGrath SP, Blike GT. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology, 2010. February;112(2):282–7. [DOI] [PubMed] [Google Scholar]
- 38.Brown H, Terrence J, Vasquez P, et al. Continuous monitoring in an inpatient medical-surgical unit: a controlled clinical trial. Am J Med, 2014. March;127(3):226–32. [DOI] [PubMed] [Google Scholar]
- 39.Slight SP, Franz C, Olugbile M, et al. The return on investment of implementing a continuous monitoring system in general medical-surgical units. Crit Care Med, 2014. August;42(8):1862–8. [DOI] [PubMed] [Google Scholar]
- 40.Watkinson PJ, Barber VS, Price JD, et al. A randomised controlled trial of the effect of continuous electronic physiological monitoring on the adverse event rate in high risk medical and surgical patients. Anaesthesia, 2006. November;61(11):1031–9. [DOI] [PubMed] [Google Scholar]
- 41.Prgomet M, Cardona-Morrell M, Nicholson M, et al. Vital signs monitoring on general wards: clinical staff perceptions of current practices and the planned introduction of continuous monitoring technology. Int J Qual Health Care, 2016. September;28(4):515–21. [DOI] [PubMed] [Google Scholar]
- 42.Smith GB, Prytherch DR, Schmidt PE, Featherstone PI. Review and performance evaluation of aggregate weighted ‘track and trigger’ systems. Resuscitation, 2008. May;77(2):170–9. [DOI] [PubMed] [Google Scholar]
- 43.McGaughey J, Alderdice F, Fowler R, et al. Outreach and Early Warning Systems (EWS) for the prevention of intensive care admission and death of critically ill adult patients on general hospital wards. Cochrane Database Syst Rev, 2007. July 18(3):CD005529. [DOI] [PubMed] [Google Scholar]
- 44.Subbe CP, Kruger M, Rutherford P, Gemmel L. Validation of a modified Early Warning Score in medical admissions. QJM, 2001. October;94(10):521–6. [DOI] [PubMed] [Google Scholar]
- 45.McGinley A, Pearse RM. A national early warning score for acutely ill patients. BMJ, 2012. August 8;345:e5310. [DOI] [PubMed] [Google Scholar]
- 46.Churpek MM, Yuen TC, Winslow C, et al. Multicenter development and validation of a risk stratification tool for ward patients. Am J Respir Crit Care Med, 2014. September 15;190(6):649–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bartkowiak B, Snyder AM, Benjamin A, et al. Validating the Electronic Cardiac Arrest Risk Triage (eCART) Score for Risk Stratification of Surgical Inpatients in the Postoperative Setting: Retrospective Cohort Study. Ann Surg, 2019. June;269(6):1059–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rothman MJ, Rothman SI, Beals Jt. Development and validation of a continuous measure of patient condition using the Electronic Medical Record. J Biomed Inform, 2013. October;46(5):837–48. [DOI] [PubMed] [Google Scholar]
- 49.Rothman MJ, Solinger AB, Rothman SI, Finlay GD. Clinical implications and validity of nursing assessments: a longitudinal measure of patient condition from analysis of the Electronic Medical Record. BMJ Open, 2012;2(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mitchell IA, McKay H, Van Leuvan C, et al. A prospective controlled trial of the effect of a multi-faceted intervention on early recognition and intervention in deteriorating hospital patients. Resuscitation, 2010. June;81(6):658–66. [DOI] [PubMed] [Google Scholar]
- 51.Churpek MM, Wendlandt B, Zadravecz FJ, et al. Association between intensive care unit transfer delay and hospital mortality: A multicenter investigation. J Hosp Med, 2016. November;11(11):757–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.DeVita MA, Braithwaite RS, Mahidhara R, et al. Use of medical emergency team responses to reduce hospital cardiopulmonary arrests. Qual Saf Health Care, 2004. August;13(4):251–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heller AR, Mees ST, Lauterwald B, et al. Detection of Deteriorating Patients on Surgical Wards Outside the ICU by an Automated MEWS-Based Early Warning System With Paging Functionality. Ann Surg, 2018. May 16. [DOI] [PubMed] [Google Scholar]
- 54.DeVita MA, Smith GB, Adam SK, et al. “Identifying the hospitalised patient in crisis”--a consensus conference on the afferent limb of rapid response systems. Resuscitation, 2010. April;81(4):375–82. [DOI] [PubMed] [Google Scholar]
- 55.Karpman C, Keegan MT, Jensen JB, et al. The impact of rapid response team on outcome of patients transferred from the ward to the ICU: a single-center study. Crit Care Med, 2013. October;41(10):2284–91. [DOI] [PubMed] [Google Scholar]
- 56.Fernando SM, Reardon PM, McIsaac DI, et al. Outcomes of Older Hospitalized Patients Requiring Rapid Response Team Activation for Acute Deterioration. Crit Care Med, 2018. September 18. [DOI] [PubMed] [Google Scholar]
- 57.McQuillan P, Pilkington S, Allan A, et al. Confidential inquiry into quality of care before admission to intensive care. BMJ, 1998. June 20;316(7148):1853–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goodfellow I, Bengio Y, Courville A. Deep Learning. MIT Press, 2016. [Google Scholar]
- 59.Shickel B, Loftus TJ, Adhikari L, et al. DeepSOFA: A Continuous Acuity Score for Critically Ill Patients using Clinically Interpretable Deep Learning. Sci Rep, 2019. February 12;9(1):1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Komorowski M, Celi LA, Badawi O, et al. The Artificial Intelligence Clinician learns optimal treatment strategies for sepsis in intensive care. Nat Med, 2018. October 22;24(11):1716–20. [DOI] [PubMed] [Google Scholar]
- 61.Silver D, Huang A, Maddison CJ, et al. Mastering the game of Go with deep neural networks and tree search. Nature, 2016. January 28;529(7587):484–9. [DOI] [PubMed] [Google Scholar]
- 62.Weenk M, van Goor H, Frietman B, et al. Continuous Monitoring of Vital Signs Using Wearable Devices on the General Ward: Pilot Study. JMIR Mhealth Uhealth, 2017. July 5;5(7):e91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eerikainen LM, Vanschoren J, Rooijakkers MJ, et al. Reduction of false arrhythmia alarms using signal selection and machine learning. Physiol Meas, 2016. August;37(8):1204–16. [DOI] [PubMed] [Google Scholar]
- 64.Antink CH, Leonhardt S, Walter M. Reducing false alarms in the ICU by quantifying self-similarity of multimodal biosignals. Physiol Meas, 2016. August;37(8):1233–52. [DOI] [PubMed] [Google Scholar]
- 65.Chen L, Dubrawski A, Wang D, et al. Using Supervised Machine Learning to Classify Real Alerts and Artifact in Online Multisignal Vital Sign Monitoring Data. Crit Care Med, 2016. July;44(7):e456–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Martin JL, Hakim AD. Wrist actigraphy. Chest, 2011. June;139(6):1514–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hodgson CL, Berney S, Harrold M, et al. Clinical review: Early patient mobilization in the ICU. Critical Care, 2013. February/28;17(1):207-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Green M, Marzano V, Leditschke IA, et al. Mobilization of intensive care patients: a multidisciplinary practical guide for clinicians. Journal of Multidisciplinary Healthcare, 2016. May/25;9:247–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sanfey AG, Rilling JK, Aronson JA, et al. The neural basis of economic decision-making in the Ultimatum Game. Science, 2003. June 13;300(5626):1755–8. [DOI] [PubMed] [Google Scholar]
- 70.Kahneman D. Thinking, fast and slow. 1st pbk ed. New York: Farrar, Straus and Giroux; 2013. 499 p. p. [Google Scholar]
- 71.Bechara A, Damasio H, Tranel D, Damasio AR. Deciding advantageously before knowing the advantageous strategy. Science, 1997. February 28;275(5304):1293–5. [DOI] [PubMed] [Google Scholar]
- 72.Seymour B, Dolan R. Emotion, decision making, and the amygdala. Neuron, 2008. June 12;58(5):662–71. [DOI] [PubMed] [Google Scholar]
- 73.LeDoux J. Rethinking the emotional brain. Neuron, 2012. February 23;73(4):653–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Van den Bruel A, Thompson M, Buntinx F, Mant D. Clinicians’ gut feeling about serious infections in children: observational study. BMJ, 2012. September 25;345:e6144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Van den Bruel A, Haj-Hassan T, Thompson M, et al. Diagnostic value of clinical features at presentation to identify serious infection in children in developed countries: a systematic review. Lancet, 2010. March 6;375(9717):834–45. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


