Abstract
Introduction
The optimal time to discontinue patients from mechanical ventilation is critical as premature discontinuation as well as delayed weaning can result in complications. The literature on diaphragm function assessment during the weaning process in the intriguing subpopulation of critically ill neuromuscular disease patients is lacking.
Methods
Patients with neuromuscular diseases, on mechanical ventilation for more than 7 days, and who were ready for weaning were studied. During multiple T-piece trials over days, diaphragm function using ultrasound and diaphragm electrical activity (Edi peaks using NAVA catheter) was measured every 30 min till a successful 2 h weaning.
Results
A total of 18 patients were screened for eligibility over 5-month period and eight patients fulfilled the inclusion criteria. Sixty-three data points in these 8 subjects were available for analysis. A successful breathing trial was predicted by Edi reduction (1.22 μV for every 30 min increase in weaning duration; 0.69 μV for every day of weaning) and increase in diaphragm excursion (2.81 mm for every 30 min increase in weaning duration; 2.18 mm for every day of weaning).
Conclusion
The Edi and diaphragm excursion changes can be used as additional objective tools in the decision-making of the weaning trials in neuromuscular disease.
Electronic supplementary material
The online version of this article (10.1007/s12028-020-01141-9) contains supplementary material, which is available to authorized users.
Keywords: Diaphragm ultrasound, Diaphragm excursion, Diaphragm thickening fraction, Neuromuscular disease, NAVA, Weaning
Introduction
The optimal time to discontinue patients from mechanical ventilation (MV) is critical. Premature liberation from MV may be followed by reinstitution of ventilator support in up to 25% of patients. On the other hand, delayed weaning may be associated with ventilator-induced diaphragm dysfunction [1, 2]. In fact, even a shorter duration of mechanical ventilation (18–96 h) has been shown, by histological examination, to induce disuse atrophy of diaphragm muscle fibers [3]. Both premature and delayed liberation from mechanical ventilation have been associated with increased mortality up to 36%, assuming that reintubation is not related to upper-airway obstruction [4, 5]. Early identification of patients who can successfully breathe spontaneously results in a shorter duration of mechanical ventilation and lower complication rate [1, 2].
Mechanical ventilation can be discontinued in the majority of the patients when the disease process that caused acute respiratory failure improves. However, a cohort of patients (20–30%) remains ventilator-dependent for prolonged periods [5, 6]. Diaphragm dysfunction is common in these patients and is responsible for delayed weaning as well as higher ICU and hospital mortality [7]. One of the significant determinants of weaning failure is the imbalance between the mechanical load imposed on the diaphragm and its ability to cope with it. Hence, evaluating the diaphragm function becomes important before any weaning attempt is performed [8]. Diaphragm dysfunction among patients requiring mechanical ventilation leads to prolonged weaning time and total ventilation time. However, despite growing evidence that diaphragm dysfunction plays a fundamental role in ventilator dependency, its function is still poorly monitored in intensive care units (ICUs) [9].
The diagnostic tools traditionally used to study diaphragm dysfunction, like fluoroscopy, phrenic nerve conduction study and trans-diaphragmatic pressure measurement, have limitations and disadvantages [10]. These include the use of ionizing radiation, non-availability of the facility to monitor, invasiveness, and the need for patient transportation and skilled or specifically trained operators [10].
Recently, ultrasound has been used to evaluate diaphragm function [6, 11, 12]. Ultrasound-based evaluation includes diaphragmatic excursion, diaphragm thickness and the speed of diaphragmatic contraction [11]. In fact, diaphragmatic thickening fraction (DTF) has been found to correlate with the respiratory workload. During spontaneous breathing trials, the diaphragm dysfunction can be documented at the bedside with ultrasound [8, 9]. Advantages of ultrasound include safety, avoidance of radiation hazards, possibility of repeated measurements and availability at the bedside.
Respiratory drive is an important factor in determining weaning success, especially in patients with neuromuscular disease. The electrical activity of the diaphragm (Edi) correlates positively with the respiratory drive and can be determined by measuring the electrical activity of the diaphragm in NAVA (Neurally adjusted ventilator assist) mode ventilation [12].
Though several studies have been published on diaphragm function assessment, none of them has been performed in the intriguing subpopulation of critically ill neuromuscular disease patients with difficult weaning [13, 14]. The present study aimed to determine the changes in diaphragm function during weaning in patients with neuromuscular diseases.
Methodology
The study was initiated after obtaining ethical clearance from the institute ethics committee. The study was conducted in Neuro medical intensive care unit over a period of 5 months (January 2018–May 2018) in a tertiary care hospital. Patients with neuromuscular disease of either sex with Guillain–Barre syndrome (GBS) or myasthenia gravis, with age more than 18 years and who required mechanical ventilation for more than 7 days were included in the study. Oral consent was taken from the patient and a written informed consent from patient’s relative. Patients with thoracostomy, pneumothorax, or pneumomediastinum, baseline diaphragm thickness less than 1.5 mm, poor ultrasound window for diaphragm assessment, contraindication for nasogastric tube insertion, uncooperative, non-understanding and those did not agree to participate in the study were excluded from the study.
A subject data sheet was used to collect demographic data, including age, sex, diagnosis, and duration of mechanical ventilation before the initiation of spontaneous breathing trial. The decision to initiate the weaning trial and the duration of weaning was left to the treating intensivist. Once spontaneous breathing trial was planned, an Edi catheter (Maquet Critical Care AB, Solna, Sweden) was inserted, and the position was confirmed as per the manufacturer’s recommendation.
A standard weaning protocol was followed for spontaneous breathing trials (SBT). The patient was allowed to breathe spontaneously through a T-tube circuit with oxygen flow at 3–4 L/min for 30 min or one hour as tolerated during the SBT. The treating physician determined the subsequent weaning cycles. The nursing staff overseeing the t-piece trial assessed for symptoms of exhaustion and terminated the trial when deemed necessary [15]. A successful SBT was taken as when the patient tolerated 2 h of T-piece trial without interruptions. End-tidal carbon dioxide, hemodynamic parameters and SpO2 were monitored throughout the SBT.
Edi catheter was connected via a cable to the NAVA module of a SERVO-i ventilator (MAQUET, Solna, Sweden). The ventilator circuit was connected to a test lung, and ventilator was set to volume-controlled mode during the SBT. Edi peak values in µV were measured every minute during the weaning cycles till a successful SBT was achieved in the particular patient.
During each SBT, the diaphragm ultrasound measurements were recorded 5 min after disconnecting the patient from the ventilator (Baseline), at 30 min, 1 h, and end of weaning.
The examination was stopped immediately if patients showed any signs of discomfort or distress. All patients were evaluated in a supine position with head elevation of 45 degree. Ultrasound was performed using either an Esaote MyLab 40 ultrasound system (Esaote, Genova, Italy) or a Sonosite (Fujifilm Sonosite Inc, WA, USA) equipment with a 10-MHz linear probe. The right hemidiaphragm was insonated in all patients. Diaphragm thickness and excursion were measured, and the diaphragmatic thickness fraction (DTF) was calculated as a percentage with the following formula: (Thickness at the end of inspiration—Thickness at the end of expiration)/(Thickness at the end of expiration) [11]. The maneuvers were repeated three times, and the averages were taken. All the measurements were done by the same investigator.
Statistical Analysis
Data were collated offline in a spreadsheet format using Microsoft Excel version 2007. Analysis was conducted using R software version 3.5.0. Quantitative data are described as mean ± standard deviation, and qualitative data as numbers or percentage. Data were visualized using a line trend graphs for selecting appropriate modelling procedure. Due to an unequal number of days of weaning within the sample, linear mixed effect modelling with restricted maximum likelihood estimation was used for observing the effect of time points per day and the days of weaning on the study variables (package “lmerTest”). As baseline values and trends of change were widespread in our sample, a random intercept and random slope were incorporated. This was further tested by iterative inclusion and exclusion of the random parameters and comparing Akaike information criterion for each model. An unstructured correlation was deemed appropriate after visualizing the covariance matrix of the data points. Sixty-three data points in 8 subjects were available for analysis. Descriptive statistics for each time point are included in the supplement. A p value of < 0.05 was taken as significant.
Results
A total of 18 patients were assessed for eligibility over 5 months out of which 8 patients were included in the final analysis (Fig. 1). The demographic profile of these patients is given in Table 1. All 8 patients had successful weaning.
Fig. 1.
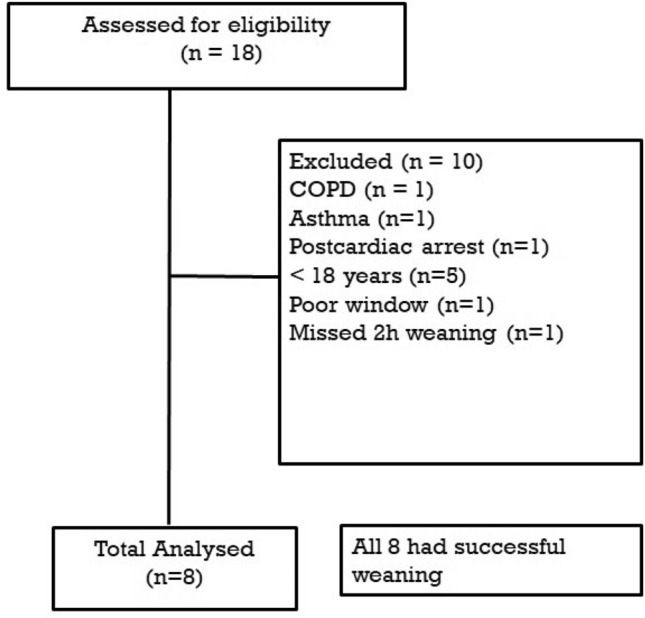
Consort diagram
Table 1.
Demographic profile of study participants
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | |
|---|---|---|---|---|---|---|---|---|
| Age (years) | 72 | 32 | 22 | 54 | 45 | 57 | 27 | 54 |
| Sex | Male | Male | Male | Male | Female | Male | Male | Male |
| Diagnosis | GBS | GBS | GBS | GBS | Myasthenia | Myasthenia | GBS | Myasthenia |
| Vasopressors/inotropes | No | No | No | No | Yes | No | No | No |
| Corticosteroids | Yes | No | No | No | Yes | Yes | Yes | Yes |
| Plasmapheresis cycles | 8 | 5 | 5 | 5 | 7 | 7 | 7 | 7 |
| Number of days of ventilation before inclusion (Days) | 25 | 7 | 10 | 14 | 32 | 12 | 20 | 16 |
| Number of days to successful SBT | 2 | 1 | 3 | 2 | 4 | 2 | 3 | 3 |
| Comorbidities |
Ischemic heart disease Hypertension Diabetes mellitus |
Nil | Nil | Diabetes mellitus | Hypertension | Nil | Nil | Nil |
| SOFA score | 2 | 2 | 2 | 2 | 4 | 2 | 2 | 2 |
| Ventilator setting | ||||||||
| PS (mm Hg) | 14 | 12 | 10 | 10 | 12 | 10 | 12 | 10 |
| PEEP(mm Hg) | 6 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| FiO2 (%) | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 |
Fio2 fractional oxygen concentration, GBS Guillain–Barre syndrome, PEEP peak end expiratory pressure, PS pressure support, SOFA sequential organ failure assessment
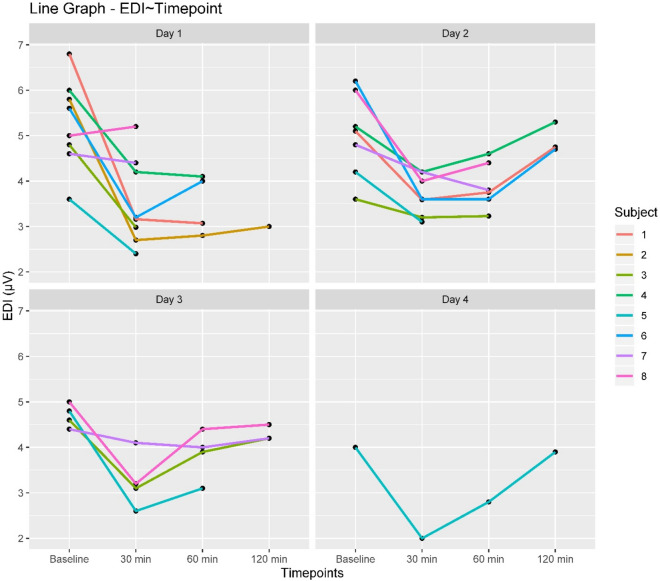
Edi values varied significantly over the weaning time points (Edi reduced by 1.22 µV for every 30 min of weaning) as well as over the days of weaning (Edi reduced by 0.69 µV for each day of weaning) (Table 2, Fig. 2). The interaction effect is significant meaning that weaning duration on each day had a considerable impact on the Edi values. The positive estimate (0.39) shows that as weaning progressed; the reduction in Edi for each weaning cycle was less with each passing day, thereby indicating a stabilization of Edi values as the patient progresses toward successful weaning.
Table 2.
Analysis results for all variables
| Variables | Estimate (SE) | P value | |
|---|---|---|---|
| Edi | TimePoint | − 1.22 (0.29) | < 0.001* |
| Day | − 0.69 (0.26) | 0.009* | |
| TimePoint*Day | 0.39 (0.12) | 0.002* | |
| DTF | TimePoint | − 2.98 (1.31) | 0.026* |
| Day | − 0.98 (1.31) | 0.456 | |
| TimePoint*Day | 1.2 (0.55) | 0.032* | |
| Excursion | TimePoint | 2.81 (0.84) | 0.002* |
| Day | 2.18 (0.73) | 0.004* | |
| TimePoint*Day | − 0.73 (0.34) | 0.035* | |
| RR | TimePoint | 0.36 (0.77) | 0.638 |
| Day | − 0.69 (0.75) | 0.364 | |
| TimePoint*Day | 0.1 (0.32) | 0.747 | |
| EtCO2 | TimePoint | 0.19 (0.59) | 0.746 |
| Day | 0.95 (0.58) | 0.107 | |
| TimePoint*Day | − 0.13 (0.24) | 0.606 | |
| SBP | TimePoint | 0.58 (1.76) | 0.742 |
| Day | − 2.27 (1.71) | 0.191 | |
| TimePoint*Day | 0.62 (0.72) | 0.398 |
*P < 0.05
DTF diaphragm thickness fraction (%), Edi Diaphragm electrical activity (µV), EtCO2 end-tidal carbon dioxide(mmHg), RR respiratory rate (/min), SBP systolic blood pressure (mmHg)
Fig. 2.
Edi trend changes of individual subjects over weaning time points for each day of weaning. Edi Diaphragm electrical activity
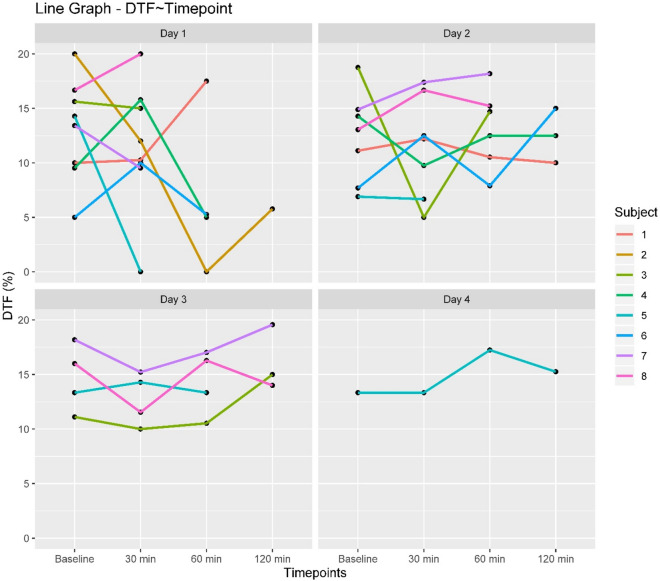
Among the diaphragm ultrasound parameters, DTF showed significant reduction by a magnitude of 2.98% for every 30 min of weaning but no change over days of weaning. A significant positive time point*day interaction (estimate = 1.2) was present indicating less reduction in DTF over the weaning time points as days of weaning progressed (Table 2, Fig. 3).
Fig. 3.
DTF trend changes of DTF of individual subjects over weaning time points for each day of weaning. DTF Diaphragm thickness fraction
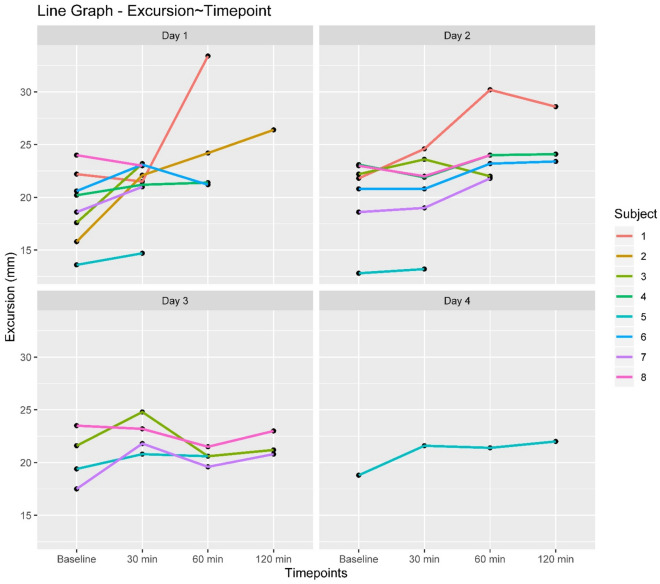
Diaphragm excursions increased by 2.81 mm for every 30 min increase in weaning duration and by 2.18 mm for every day of weaning toward a successful breathing trial. The significant estimate for Time point*Day interaction can be interpreted as a lesser increase in excursion over weaning time points, for each successive weaning day. (Table 2, Fig. 4).
Fig. 4.
Trend changes in diaphragmatic excursions of individual subjects over weaning time points for each day of weaning
The hemodynamic variables like heart rate and blood pressure did not change significantly over weaning time points or weaning days. The clinical respiratory variables monitored during weaning like respiratory rate, EtCO2 also did not reveal any significant change over the weaning period. (Table 2).
Discussion
This study assessed the functional and electrical component of diaphragm function in patients with neuromuscular disease during spontaneous breathing trial.
The role of diaphragm function assessment in predicting weaning success has been previously described. The routine use of ultrasound in the ICU has enhanced its applicability in the assessment of diaphragm function at the bedside. Repeated bedside assessment of diaphragm allows the clinician to decide the weaning process. Most of the studies attribute diaphragm dysfunction, as measured by ultrasound, as a cause of weaning failure [12]. Patients with weaning failure showed lower DTF and excursions compared to patients with successful weaning [8]. Savino et al. in his study of 51 patients requiring mechanical ventilation for more than 48 h showed that diaphragm displacement measured during SBT predicted successful weaning and extubation better than rapid shallow breath index [11]. Dinino et al. in his study on patients undergoing weaning in a medical ICU showed that a change in diaphragm thickness fraction percentage of more than 30% was associated with successful extubation [16]. On the other hand, Rittayamai et al. in his study of diaphragm ultrasound during SBT concluded that DTF could not distinguish between patients with SBT success and failure [17].
Our study showed a decrease in the DTF with the increase in weaning duration and this decrease was less with the increase in weaning days. DTF has been shown to correlate with pressure generating capacity of the diaphragm. When compared to healthy individuals, neuromuscular disease patients have an inherent reduction in diaphragm thickness and excursion during spontaneous respiration. This could be one of the reasons for lower DTF values observed in the present study. In neuromuscular disease, this mechanism might not be the only factor contributing to successful weaning [9]. Hence, an increase in DTF by 25–30% as a cutoff to predict successful weaning, though suitable in other patient population, might not hold true for patients with neuromuscular disease [16]. In this patient population, a trend change of DTF values and stability of the value might be a better indicator of successful weaning. Similar to the published study, the patients in the current study showed increased diaphragm excursions with increased duration and days of weaning, and all had successful weaning [9]. Whether respiratory muscle weakness represents a part of ICU acquired weakness leading to prolonged weaning, is still debatable [18, 19]. In our patient population, muscle weakness was due to the disease process (myasthenia, GBS) requiring ICU admission rather than to systemic illnesses resulting in muscle weakness. However, it is difficult to differentiate these two entities clinically. None of our patients had sepsis during the study period.
In all patients in the current study, who were weaned and had completed a successful spontaneous breathing trial of 2 h, the Edi values decreased uniformly. The normal range of values quoted for Edi is between 5 and 30 µV in spontaneously breathing healthy patients, but usually around 15 µV. Our patients had lower values (< 6 µV) when compared to other studies [12]. This could be due to the reduced muscle mass seen in neuromuscular disease patients. Five of the eight study patients were on long-term steroid therapy which could have also affected the muscle mass.
In a study on difficult to wean neurological patients following stroke and traumatic brain injury, Trapp et al. showed that rising Edi levels were associated with failed weaning trial and this was seen even before the protocol-based measurements could detect weaning failure [20]. These authors showed that a rise in Edi by 12% indicated patient exhaustion. Patients who failed to wean could not overcome the respiratory workload resulting in increased respiratory drive and this manifested as high Edi [20]. In our study, none of the patients had increased Edi values during weaning and all completed successful weaning trial. However, one must be cautious about the fact that these patients might not be able to generate greater Edi values due to poor muscle strength, and this should not be interpreted as a successful weaning parameter.
Weaning from mechanical ventilation in neurological disease requires close monitoring and proper timing to begin the process. In patients with neuromuscular diseases, there is always a risk of weaning failure due to fatigue, co-existing autonomic dysfunction and diaphragmatic paralysis either due to the primary disease process or to prolonged ventilator-induced diaphragm dysfunction [20]. By convention, clinical signs and parameters derived from respiratory mechanics are used as indirect measures of diaphragm function and readiness to wean patients from mechanical ventilation. In the present study, neither the systemic hemodynamic variables nor the carbon dioxide values could predict successful weaning. The present study demonstrates the utility of Edi trend values in addition to diaphragm ultrasound for assessing patients with neuromuscular disease who undergo weaning trial. This technique may prove useful in assessing patients who are prone to exhaustion and where clinical signs of exhaustion may not be readily evident due to muscle weakness.
The study, however, had considerable limitations. Firstly, the study population included only patients with neuromuscular disease and hence the Edi values need further validation. Secondly, the population size is small, which renders the results less robust. Thirdly, since there were no failures of SBT in our study, cutoff values for failed weaning could not be demonstrated.
Conclusion
The present study investigated the changes in Edi values and diaphragm ultrasound parameters during the course of weaning in neurologically ill patients. It seems to be prudent to recommend multimodality monitoring incorporating diaphragm function to assess readiness to wean in this subgroup of a population where there is a risk of weaning failure.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author Contributions
MKK participated in conduct, data collection, manuscript preparation, review. RM participated in Design, manuscript preparation, manuscript review. DC participated in Statistical analysis, manuscript review.
Source of Support
Nil
Conflict of interest
Nil
Ethical Approval
The study was initiated after obtaining ethical clearance from the institute ethics committee. The study was conducted in Neuro medical intensive care unit over a period of 5 months (January 2018–May 2018)in a tertiary care hospital. Patients with neuromuscular disease of either sex with Guillain–Barre syndrome (GBS) or myasthenia gravis, with age more than 18 years and who required mechanical ventilation for more than 7 days were included in the study.
Informed consent
Oral consent was taken from the patient and a written informed consent from patient’s relative. Patients with thoracostomy, pneumothorax, or pneumomediastinum, baseline diaphragm thickness less than 1.5 mm, poor ultrasound window for diaphragm assessment, contraindication for nasogastric tube insertion, uncooperative, non-understanding and those did not agree to participate in the study were excluded from the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vassilakopoulos T, Petrof BJ. Ventilator-induced diaphragmatic dysfunction. Am J Respir Crit Care Med. 2004;169(3):336–341. doi: 10.1164/rccm.200304-489CP. [DOI] [PubMed] [Google Scholar]
- 2.Penuelas O, Keough E, Rodriguez LL, Carriedo D, Goncalves G, Barreiro E, Lorente JA. Ventilator-induced diaphragm dysfunction: translational mechanisms lead to therapeutical alternatives in the critically ill. Inten Care Med Exp. 2019;7(48):1–25. doi: 10.1186/s40635-019-0259-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levine S, Nguyen T, Taylor N, Friscia ME, Budak MT, Rothenberg P, et al. Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N Engl J Med. 2008;358(13):1327–1335. doi: 10.1056/NEJMoa070447. [DOI] [PubMed] [Google Scholar]
- 4.Esteban A, Anzueto A, Frutos F, Alia I, Brochard L, Stewart TE, et al. Characteristics and outcomes in adult patients receiving mechanical ventilation: a 28-day international study. JAMA. 2002;287(3):345–355. doi: 10.1001/jama.287.3.345. [DOI] [PubMed] [Google Scholar]
- 5.Funk GC, Anders S, Breyer MK, Burghuber OC, Edelmann G, Heindl W, et al. Incidence and outcome of weaning from mechanical ventilation according to new categories. Eur Respir J. 2010;35(1):88–94. doi: 10.1183/09031936.00056909. [DOI] [PubMed] [Google Scholar]
- 6.Farghaly S, Hasan AA. Diaphragm ultrasound as a new method to predict extubation outcome in mechanically ventilated patients. Aust Crit Care. 2017;30(1):37–43. doi: 10.1016/j.aucc.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 7.El-Khatib MF, Bou-Khalil P. Clinical review: liberation from mechanical ventilation. Crit Care. 2008;12(4):221. doi: 10.1186/cc6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spadaro S, Grasso S, Mauri T, Dalla Corte F, Alvisi V, Ragazzi R, et al. Can diaphragmatic ultrasonography performed during the T-tube trial predict weaning failure? The role of diaphragmatic rapid shallow breathing index. Crit Care. 2016;20(1):305. doi: 10.1186/s13054-016-1479-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dres M, Demoule A. Diaphragm dysfunction during weaning from mechanical ventilation: an underestimated phenomenon with clinical implications. Crit Care. 2018;22(1):73. doi: 10.1186/s13054-018-1992-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American Thoracic Society ATS/ERS Statement on Respiratory Muscle Testing: joint statement of the american thoracic society (ats), and the european respiratory society (ERS) Am J Respir Crit Care Med. 2002;166(4):518–624. doi: 10.1164/rccm.166.4.518. [DOI] [PubMed] [Google Scholar]
- 11.Matamis D, Soilemezi E, Tsagourias M, Akoumianaki E, Dimassi S, Boroli F, et al. Sonographic evaluation of the diaphragm in critically ill patients: technique and clinical applications. Intens Care Med. 2013;39(5):801–810. doi: 10.1007/s00134-013-2823-1. [DOI] [PubMed] [Google Scholar]
- 12.Dres M, Demoule A. Monitoring diaphragm function in the ICU. Curr Opin Crit Care. 2020;26(1):18–25. doi: 10.1097/MCC.0000000000000682. [DOI] [PubMed] [Google Scholar]
- 13.Li C, Li X, Han H, Cui H, Wang G, Wang Z. Diaphragmatic ultrasonography for predicting ventilator weaning: a meta-analysis. Medicine (Baltimore) 2018;97(22):e10968. doi: 10.1097/MD.0000000000010968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Llamas-Álvarez AM, Tenza-Lozano EM, Latour-Pérez J. Diaphragm and lung ultrasound to predict weaning outcome: systematic review and meta-analysis. Chest. 2017;152(6):1140–1150. doi: 10.1016/j.chest.2017.08.028. [DOI] [PubMed] [Google Scholar]
- 15.Esteban A, Alia I, Tobin MJ, et al. Effects of spontaneous breathing trial duration on outcome of attempts to discontinue mechanical ventilation. Spanish Lung Failure Collaborative Group. Am J Respir Crit Care Med. 1999;159(2):512–518. doi: 10.1164/ajrccm.159.2.9803106. [DOI] [PubMed] [Google Scholar]
- 16.DiNino E, Gartman EJ, Sethi JM, McCool FD. Diaphragm ultrasound as a predictor of successful extubation from mechanical ventilation. Thorax. 2014;69(5):423–427. doi: 10.1136/thoraxjnl-2013-204111. [DOI] [PubMed] [Google Scholar]
- 17.Rittayamai N, Hemvimon S, Chierakul N. The evolution of diaphragm activity and function determined by ultrasound during spontaneous breathing trials. J Crit Care. 2019;51:133–138. doi: 10.1016/j.jcrc.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 18.Dres M, Jung B, Molinari N, Manna F, Dube BP, Chanques G, et al. Respective contribution of intensive care unit-acquired limb muscle and severe diaphragm weakness on weaning outcome and mortality: a post hoc analysis of two cohorts. Crit Care. 2019;23:370. doi: 10.1186/s13054-019-2650-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeong BH, Nam J, Ko MG, Chung CR, Suh GY, Jeon K. Impact of limb weakness on extubation failure after planned extubation in medical patients. Respirology. 2018;23(9):842–850. doi: 10.1111/resp.13305. [DOI] [PubMed] [Google Scholar]
- 20.Trapp O, Fiedler M, Hartwich M, Schorl M, Kalenka A. Monitoring of electrical activity of the diaphragm shows failure of T-piece trial earlier than protocol-based parameters in prolonged weaning in non-communicative neurological patients. Neurocrit Care. 2017;27(1):35–43. doi: 10.1007/s12028-016-0360-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.