INTRODUCTION
Optical regulation provides unrivaled spatial and temporal precision for the control of protein function. We describe a strategy to engineer photosensitivity onto ion channels based on small photoisomerizable molecules. Photosensitivity is targeted to the ion channel of interest by combining the photoisomerizable moiety with a specific agonist or antagonist. Inclusion of a cysteine-reactive maleimide group enables covalent attachment of the photoswitch to a genetically engineered cysteine on the surface of the ion channel. Exposure to different wavelengths of light triggers changes in the properties of the photoswitch that enable control of protein function.
BACKGROUND INFORMATION
Optical control of ion channel function offers several advantages over traditional pharmacological regulation. Light can be turned on and off rapidly, allowing control of channel activity with exceptional temporal precision. Because light can be projected precisely in space, ion channel function can also be controlled at specific locations within a cell or tissue. In addition, the ability of light to penetrate tissue enables control of ion channel function from afar, minimizing invasiveness and potential damage to the structure under study.
Several strategies have been developed to control the function of ion channels and receptors with light, including the use of caged molecules (Ellis-Davies 2007) and the development of chimeric proteins that combine the protein of interest with a naturally light-sensitive protein (Airan et al. 2009). This article focuses on a third strategy: the use of small photoswitchable molecules to photosensitize genetically engineered or native ion channels (Fig. 1). These molecules contain distinct chemical moieties responsible for regulation of protein function (the ligand), photoisomerization (the photoswitch), and, if applicable, covalent attachment (the reactive moiety). Light absorption induces changes in the properties of the photoisomerizable moiety that are harnessed to trigger biophysical, and ultimately cellular, events. The modular nature of photoswitch molecules allows flexibility in the design of each functional group, yielding a combinatorial tool kit for optical regulation of ion channel function.
FIGURE 1.
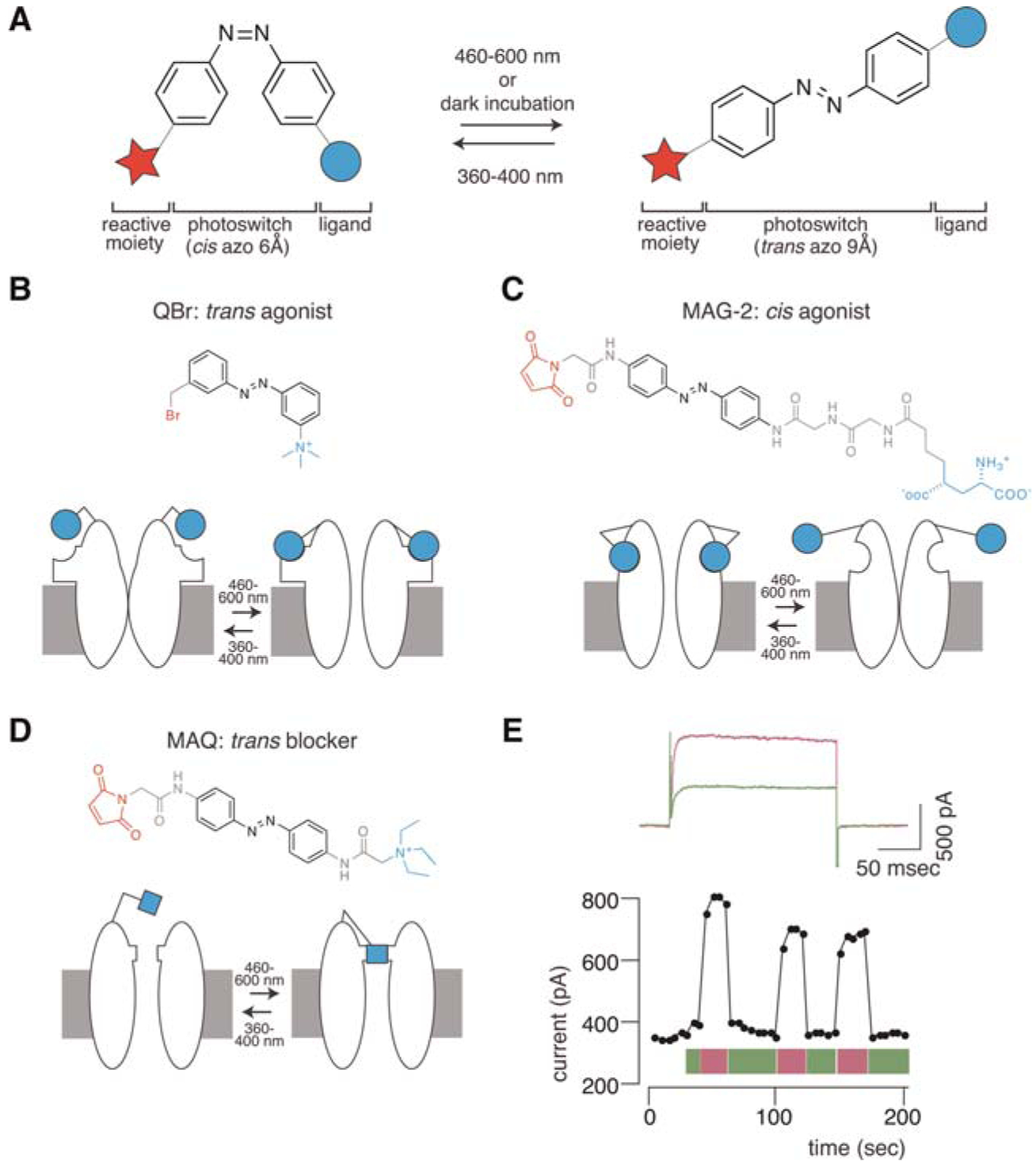
Strategies for engineering light-regulated ion channels with azobenzene-containing molecules. (A) A photo-switchable tethered ligand (PTL) consists of a reactive moiety (red), a ligand (blue), and the photoswitch (here azobenzene [azo], black). Azobenzene undergoes trans to cis isomerization under 360- to 400-nm light. Visible light (>460 nm) or prolonged time in darkness returns the molecule to the trans state. The end-to-end distance between the para positions on the trans and cis azobenzenes is ~9 Å and 6 Å, respectively. (B) Tethered QBr is a trans-acting agonist of the nAChR. The receptor is activated when the agonist reaches its binding site under 460- to 600-nm light, whereas illumination with 360- to 400-nm light retracts the agonist, abrogating ion conduction through the channel. (C) MAG-2 is a cis-acting iGluR6 agonist that tethers to a genetically engineered cysteine. The agonist reached its binding site only in the cis configuration (i.e., under 360- to 400-nm light). (D) MAQ reacts with an engineered cysteine on the surface of a Shaker K+ channel and acts as a photoisomerizable tethered blocker, blocking ion conduction in the trans but not in the cis configuration. (E) (Top) Tethering MAQ to a genetically engineered cysteine (E422C) in a modified Shaker channel enables photoregulation of ion conduction. Under 500-nm light (green), MAQ is in the trans form; the QA group reaches the pore of the channel and blocks ion flow. Photoisomerization to the cis configuration with 380-nm light (violet) retracts the blocker, allowing ion flow. (Bottom) MAQ-treated Shaker channels can be regulated repetitively and persistently with light.
PHOTOSWITCHABLE TETHERED LIGANDS (PTLs)
The Photoswitch
Azobenzene is ideal for use as a photoswitch molecule because it can be photoisomerized repetitively with a high quantum yield and no detectable photobleaching (Renner and Moroder 2006). The cis and trans azobenzene isomers differ in length by as much as 3 Å (Fig. 1A); this can be exploited to use azobenzene as a mechanical lever that maneuvers a functional group between two different positions depending on the wavelength of illumination. The trans (i.e., extended) azobenzene isomer is predominant after prolonged incubation in darkness because it is more thermodynamically stable than the cis isomer. Exposure to near-ultraviolet (UV) light (~360–400 nm) triggers photoisomerization to the cis state. The reverse cis-to-trans conversion occurs spontaneously in darkness but can be accelerated by exposure to visible light (~460–600 nm). Photoconversion between isomers occurs within picoseconds (Lednev et al. 1996; Nägele et al. 1997; Ihalainen et al. 2007).
Complete photoconversion to either cis or trans azobenzene cannot be achieved because the two isomers have overlapping absorption spectra. The relative amounts of cis and trans isomers at a given wavelength depend on the quantum yield for the isomerization processes and the lifetime of the cis isomer, both of which vary with chemical substitution to the azobenzene core, light intensity, temperature, and the environment surrounding the molecule (Yager and Barrett 2006). Typically, exposure to near-UV or visible light yields populations of azobenzene molecules containing up to ~85% cis or ~90% trans isomer, respectively (James et al. 2001). Near full conversion (>99%) to the trans state can be reached only after prolonged incubation in darkness (Renner and Moroder 2006).
The Reactive Moiety
Current strategies for photosensitizing ion channels with photoswitch-containing molecules rely on cysteine–maleimide chemistry. Surface-exposed endogenous cysteine(s) are first replaced with nonreactive amino acids using site-directed mutagenesis. Individual cysteine residues are then introduced on the surface of the protein and assayed for reactivity with the photoswitch-containing molecule. Maleimide reacts rapidly and irreversibly with cysteines, although the local environment of cysteines can influence their reactivity. The recent introduction of chemical approaches that target distinct genetically encoded protein tags (Marks and Nolan 2006) provides potential alternatives for orthogonal labeling if removing endogenous cysteines has a negative impact on ion channel function.
The Ligand
The ligand chosen in the design of the PTL defines which protein will become photosensitized. To be effective, there must be a “handle” on the ligand, such that it can be extended and conjugated to the azobenzene moiety without destroying the ability of the ligand to bind and activate the target protein. The success of affinity labeling as a technique to characterize enzyme function suggests that ligands can be modified with reactive groups and still maintain at least some biological activity (Silman and Karlin 1969; Wold 1977).
When designing PTLs, it can help to introduce flexible linkers between the different functional moieties to generate molecules slightly longer than the optimal distance between the ligand binding and attachment sites (Krishnamurthy et al. 2007). Flexible linkers impart conformational flexibility that might be necessary for tethering but can also compromise the effectiveness of the ligand by increasing the entropic cost of binding (Chung et al. 2009). The presence of flexible linkers can also introduce uncertainty by making the average length of the molecule different from its extended length (Kramer and Karpen 1998; Green et al. 2001). However, the increasing availability of computational tools, including molecular simulation and docking software, combined with the growing number of solved three-dimensional (3D) protein structures should aid in the rational design of PTL/target protein interactions.
TARGETED PROTEINS
Nicotinic Acetylcholine Receptor
Erlanger and colleagues were the first to use azobenzene-containing molecules to regulate the activity of the nicotinic acetylcholine receptor (nAChR) (Bartels et al. 1971). They synthesized 3-(α-bromomethyl)-3′-[α-(trimethylammonium)methyl]azobenzene bromide (QBr), a photoisomerizable agonist that attaches covalently to a previously reduced natively encoded cysteine on the surface of the nAChR. Once tethered, QBr activates the nAChR only in its trans configuration (Fig. 1B). The ability to toggle rapidly between activating and nonactivating QBr using light minimized nAChR desensitization allowing the kinetics of receptor activation to be studied with great temporal accuracy (Lester et al. 1980).
Ionotropic Glutamate Receptor
If available, protein structure information can guide the development of PTLs and engineering of target ion channels. For example, a light-activated ionotropic glutamate receptor (LiGluR) was designed based on the X-ray structure of the ligand-binding domain of the ionotropic glutamate receptor-6 (iGluR6) bound to the agonist (2S,4R)-4-methyl glutamate (Mayer 2005). Binding of an agonist causes the ligand-binding domain to engulf the ligand, except for a small cavity through which a molecular tether that includes an azobenzene moiety and a maleimide reactive group can be threaded (Volgraf et al. 2006). A cysteine was engineered into iGluR6 such that maleimide–azobenzene–glutamate-2 (MAG-2), a PTL for this receptor, reaches the ligand-binding domain and activates the receptor only in its cis configuration (Fig. 1C; Volgraf et al. 2006). Activation of the resulting LiGluR with near-UV light depolarizes neurons and induces action potential firing (Szobota et al. 2007). A shorter PTL, MAG0, activates iGluR in its cis and trans configurations, depending on the position of the cysteine attachment site. Molecular dynamics simulations revealed that the site of attachment for MAG0 influences the occupancy of the ligand-binding domain and its closure after binding of the PTL, thus determining which photo-isomer is “active” (Numano et al. 2009).
Voltage-Gated Potassium Channel
The engineering of the synthetic photoisomerizable azobenzene-regulated K+ (SPARK) channel (Banghart et al. 2004) was also guided by the abundant biophysical and structural information available on voltage-gated K+ channels. In particular, a 4 Å difference in the length of tethered tetraethyl-ammonium pore blockers (11 Å vs. 15 Å) attached to an engineered cysteine (E422C) made the distinction between ineffective or complete blockage of the channel (Blaustein et al. 2000). Based on this, the PTL maleimide–azobenzene–quaternary ammonium (MAQ) was synthesized to photoregulate the activity of a Shaker K+ channel. Once MAQ is tethered to its target cysteine (E422C), the blocking quaternary ammonium can reach the pore of the channel only in its extended (~17 Å) trans configuration but not in its short (~10 Å) cis form (Fig. 1D,E). Additional mutations were engineered into the channel to abolish fast inactivation (Hoshi et al. 1990) and shift the voltage sensitivity of activation (Lopez et al. 1991), generating a channel with a high resting K+ conductance that hyperpolarizes and silences neurons illuminated with near-UV light. Illumination with visible light induces photoisomerization to the trans configuration and closes the chemical gate, restoring normal membrane potential and action potential firing (Banghart et al. 2004). Mutation of another amino acid in SPARK (V443Q) generates a nonspecific depolarizing cation channel (D-SPARK) by altering the relative permeability of the channel for Na+ ions (Heginbotham and MacKinnon 1992). Exposure to near-UV light triggers action potential firing in MAQ-treated neurons expressing D-SPARK that can then be halted by illumination with visible light (Chambers et al. 2006).
MODIFICATIONS TO EXISTING LIGHT-GATED CHANNELS
Tuning the Photoswitch
Cis azobenzene reverts spontaneously to the trans configuration in the dark, a process known as thermal relaxation. Thus, continuous illumination is required to maintain the cis configuration on modified proteins. In the case of LiGluR, the protein environment surrounding the PTL stabilizes the cis state (Gorostiza et al. 2007). Because this might not be the case for other proteins, it can be desirable to design azobenzene-based molecules with enhanced thermal stability. Modification of the nature and position of substituents on the azobenzene core can decrease the rate of thermal relaxation from milliseconds to days (Forber et al. 1985; Pozhidaeva et al. 2004; Chi et al. 2006; Sadovski et al. 2009).
Existing PTLs undergo maximal photoisomerization to the cis configuration with exposure to near-UV light. Because short-wavelength light is scattered by living tissue, it can be advantageous to shift the action spectra of PTLs from near-UV toward longer wavelengths by modifying the azobenzene core to shift its spectral sensitivity (Chi et al. 2006; Sadovski et al. 2009). Similarly, it can be useful to modify current PTLs for use in deep tissue by improving their two-photon absorption cross-section. Although the two-photon absorption cross-sections of several azobenzene-containing molecules have been investigated (De Boni et al. 2005), these photoswitches have not been incorporated into the design of PTLs. Also, nonlinear optics have not yet been used to regulate ion channels modified with azobenzene-containing PTLs, but this is an important future goal.
Engineering Ion Channel Targets
The introduction of previously characterized trafficking motifs in light-regulated ion channels can enable better control of biological function. For example, SPARK channels localize to the cell body and axon when expressed heterologously in neurons. This localization is adequate for the photocontrol of action potential firing (Banghart et al. 2004). However, depending on the study, it might be more efficacious to target SPARK to dendrites, using a dendritic localization motif from Kv4.2 (Rivera et al. 2003) or a myosin-binding domain (Lewis et al. 2009), to silence inputs to the SPARK-expressing neuron. The cytoplasmic loop linking domains II and III of voltage-gated Na+ channels (Garrido et al. 2003; Lemaillet et al. 2003) can be used to target proteins to the axon initial segment. Targeting light-regulated depolarizing channels such as D-SPARK and LiGluR to the axon initial segment will mimic the way spikes are initiated naturally in vivo and could be more likely to induce normal downstream events, such as synaptic plasticity.
In addition to providing a means to photoregulate biological functions (e.g., neuronal firing), the use of PTLs also enables functional studies of target proteins in cells and tissues. This can be particularly useful in cases in which few or no specific pharmacological regulators of the protein of interest exist (e.g., between different members of a K+ channel subfamily). Traditional gene knockouts have provided a wealth of information about ion channel function but sometimes result in unintended lethality or induce compensation from related channels. Replacement of a native ion channel with a cysteine-containing variant in combination with a PTL provides a “chemical knockout” strategy. As a proof-of-concept for this method, we are using the previously characterized MAQ to photosensitize various K+ channels containing a cysteine at a position analogous to E422C in Shaker. Because of the divergence of loop sequences between different K+ channels, cysteine scanning might be required to identify the optimal tethering position. The chemical knockout strategy is not limited to K+ channels; the modular nature of PTLs allows flexibility in the design of each functional group. The choice of ligand and the introduction of the cysteine attachment site will determine which ion channel/receptor is targeted.
Photochromic Ligands
The use of PTLs involves engineering target proteins to contain a cysteine attachment site followed by heterologous expression of the protein in cells. However, if genetic manipulation is not available or not desirable, photosensitive chemicals that target native proteins can provide an alternative one-component system for photosensitization. We have described photoswitchable molecules that confer photosensitivity to endogenously expressed K+ channels in genetically unadulterated cells or tissues. These molecules contain a pore-blocking quaternary ammonium, a photoisomerizable azobenzene and an electrophilic group for attachment to the channel. In particular, acryl–azobenzene–quaternary ammonium (AAQ) efficiently photosensitizes K+ channels expressed natively in neurons and enables control of neuronal excitability in dissociated cultures, slices, and semi-intact neuronal preparations (Fortin et al. 2008). Further characterization suggested that AAQ acts as an open channel blocker and thus at the internal tetraethylammonium-binding site of K+ channels (Banghart et al. 2009). Replacement of the acryl moiety with a series of nonreactive aliphatic tails results in nontethered photoswitch-containing molecules whose potency for K+ channel blocking generally correlates with tail length and hydrophobicity (Banghart et al. 2009). Similar nontethered agonists have been generated for the control of the nAChR and iGluRs (Deal et al. 1969; Bartels et al. 1971; Volgraf et al. 2007). Unlike photolysis of caged molecules, which consumes the starting material and cannot be reversed, nontethered azobenzene-containing ligands can be toggled repeatedly between cis and trans configurations to turn ion channel function on and off without genetic modification.
PRACTICAL CONSIDERATIONS FOR USING PTL-MODIFIED ION CHANNELS
Light
We have successfully regulated the activity of PTL-modified ion channels using lasers and other light sources such as mercury and xenon arc lamps equipped with band-pass filters. Our typical light output is in the low milliwatts per square centimeter (mW/cm2) range or below (measured with a Newport light power meter and normalized to the illuminated area at the sample plane). For azobenzene-based PTLs, the intensity of light influences the relative amount of cis and trans photo-isomers generated at a given wavelength and the rate at which photoequilibrium is reached.
Cysteine–Maleimide Reactivity
Maleimides react specifically and rapidly with cysteine residues; for example, N-ethyl maleimide reacts within seconds at neutral pH (Gregory 1955). Cysteine residues are deprotonated and thus most reactive at slightly alkaline pH, which can, however, accelerate the nonspecific hydrolysis of maleimide. For efficient labeling of cysteine-containing ion channels with PTLs, we typically apply the PTL in pH 7.4–8 saline solution. The redox state of cysteine residues will also affect the efficiency and extent of maleimide–cysteine labeling. When photoregulating K+ channels expressed in HEK293 cells, pretreatment with a reducing agent (1 mmothreitol or 1 mm tris (2-carboxyethyl)phosphine) is essential for efficient tethering, presumably because the high levels of cystine in Dulbecco’s minimum essential-based growth media oxidize surface-exposed cysteine residues. If ion channel function requires disulfide bonds, follow the reducing treatment with a “resting” incubation in normal saline solution to allow native disulfide pairs to reform before PTL treatment.
CONCLUSION
The method described here for engineering light-regulated ion channels and receptors is based on exogenously applied synthetic photoisomerizable molecules. The versatility of the photoswitch approach has already been demonstrated with the development of PTLs and photochromic ligands for different classes of proteins, including an ionotropic glutamate receptor (Volgraf et al. 2006) and voltage-gated K+ channels (Banghart et al. 2004; Fortin et al. 2008). Based on the increasing availability of structural data and extensive pharmacological knowledge of various ion channels and receptors, extensions of this approach to other proteins are likely forthcoming.
ACKNOWLEDGMENTS
This work was supported by the NIH Eye Institute (R01EY018957) and the Nanomedicine Development Center for the Optical Control of Biological Function (PN2EY018241). We thank the UC Berkeley College of Chemistry for use of computing resources.
REFERENCES
- Airan RD, Thompson KR, Fenno LE, Bernstein H, Deisseroth K. 2009. Temporally precise in vivo control of intracellular signalling. Nature 458: 1025–1029. [DOI] [PubMed] [Google Scholar]
- Banghart M, Borges K, Isacoff E, Trauner D, Kramer RH. 2004. Light-activated ion channels for remote control of neuronal firing. Nat Neurosci 7: 1381–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banghart MR, Mourot A, Fortin DL, Yao JZ, Kramer RH, Trauner D. 2009. Photochromic blockers of voltage-gated potassium channels. Angew Chem Int Ed Engl 48: 9097–9101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels E, Wassermann NH, Erlanger BF. 1971. Photochromic activators of the acetylcholine receptor. Proc Natl Acad Sci 68: 1820–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein RO, Cole PA, Williams C, Miller C. 2000. Tethered blockers as molecular “tape measures” for a voltage-gated K+ channel. Nat Struct Mol Biol 7: 309–311. [DOI] [PubMed] [Google Scholar]
- Chambers JJ, Banghart MR, Trauner D, Kramer RH. 2006. Light-induced depolarization of neurons using a modified Shaker K+ channel and a molecular photoswitch. J Neurophysiol 96: 2792–2796. [DOI] [PubMed] [Google Scholar]
- Chi L, Sadovski O, Woolley GA. 2006. A blue-green absorbing cross-linker for rapid photoswitching of peptide helix content. Bioconjug Chem 17: 670–676. [DOI] [PubMed] [Google Scholar]
- Chung S, Parker JB, Bianchet M, Amzel LM, Stivers JT. 2009. Impact of linker strain and flexibility in the design of a fragment-based inhibitor. Nat Chem Biol 5: 407–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deal WJ, Erlanger BF, Nachmansohn D. 1969. Photoregulation of biological activity by photochromic reagents, III. Photoregulation of bioelectricity by acetylcholine receptor inhibitors Proc Natl Acad Sci 64: 1230–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Boni L, Misoguti L, Zílio SC, Mendonça CR. 2005. Degenerate two-photon absorption spectra in azoaromatic compounds. Chemphyschem 6: 1121–1125. [DOI] [PubMed] [Google Scholar]
- Ellis-Davies GCR. 2007. Caged compounds: Photorelease technology for control of cellular chemistry and physiology. Nat Methods 4: 619–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forber CL, Kelusky EC, Bunce NJ, Zerner MC. 1985. Electronic spectra of cis- and trans-azobenzenes: Consequences of ortho substitution. J Am Chem Soc 107: 5884–5890. [Google Scholar]
- Fortin DL, Banghart MR, Dunn TW, Borges K, Wagenaar DA, Gaudry Q, Karakossian MH, Otis TS, Kristan WB, Trauner D, et al. 2008. Photochemical control of endogenous ion channels and cellular excitability. Nat Methods 5: 331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido JJ, Giraud P, Carlier E, Fernandes F, Moussif A, Fache M-P, Debanne D, Dargent B. 2003. A targeting motif involved in sodium channel clustering at the axonal initial segment. Science 300: 2091–2094. [DOI] [PubMed] [Google Scholar]
- Gorostiza P, Volgraf M, Numano R, Szobota S, Trauner D, Isacoff EY. 2007. Mechanisms of photoswitch conjugation and light activation of an ionotropic glutamate receptor. Proc Natl Acad Sci 104: 10865–10870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green NS, Reisler E, Houk KN. 2001. Quantitative evaluation of the lengths of homobifunctional protein cross-linking reagents used as molecular rulers. Protein Sci 10: 1293–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory JD. 1955. The stability of N-ethylmaleimide and its reaction with sulfhydryl groups. J Am Chem Soc 77: 3922–3923. [Google Scholar]
- Heginbotham L, MacKinnon R. 1992. The aromatic binding site for tetraethylammonium ion on potassium channels. Neuron 8: 483–491. [DOI] [PubMed] [Google Scholar]
- Hoshi T, Zagotta WN, Aldrich RW. 1990. Biophysical and molecular mechanisms of Shaker potassium channel inactivation. Science 250: 533–538. [DOI] [PubMed] [Google Scholar]
- Ihalainen JA, Bredenbeck J, Pfister R, Helbing J, Chi L, van Stokkum IHM, Woolley GA, Hamm P. 2007. Folding and unfolding of a photo-switchable peptide from picoseconds to microseconds. Proc Natl Acad Sci 104: 5383–5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James DA, Burns DC, Woolley GA. 2001. Kinetic characterization of ribonuclease S mutants containing photoisomerizable phenylazophenylalanine residues. Protein Eng 14: 983–991. [DOI] [PubMed] [Google Scholar]
- Kramer RH, Karpen JW. 1998. Spanning binding sites on allosteric proteins with polymer-linked ligand dimers. Nature 395: 710–713. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy VM, Semetey V, Bracher PJ, Shen N, Whitesides GM. 2007. Dependence of effective molarity on linker length for an intramolecular protein–ligand system. J Am Chem Soc 129: 1312–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lednev IK, Ye T-Q, Hester RE, Moore JN. 1996. Femtosecond time-resolved UV-visible absorption spectroscopy of trans-azobenzene in solution. J Phys Chem 100: 13338–13341. [Google Scholar]
- Lemaillet G, Walker B, Lambert S. 2003. Identification of a conserved ankyrin-binding motif in the family of sodium channel α subunits. J Biol Chem 278: 27333–27339. [DOI] [PubMed] [Google Scholar]
- Lester HA, Krouse ME, Nass MM, Wassermann NH, Erlanger BF. 1980. A covalently bound photoisomerizable agonist: Comparison with reversibly bound agonists at Electrophorus electroplaques. J Gen Physiol 75: 207–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis TL Jr, Mao T, Svoboda K, Arnold DB. 2009. Myosin-dependent targeting of transmembrane proteins to neuronal dendrites. Nat Neurosci 12: 568–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez GA, Ian YN, Ian LY. 1991. Hydrophobic substitution mutations in the S4 sequence alter voltage-dependent gating in Shaker K+ channels. Neuron 7: 327–336. [DOI] [PubMed] [Google Scholar]
- Marks KM, Nolan GP. 2006. Chemical labeling strategies for cell biology. Nat Methods 3: 591–596. [DOI] [PubMed] [Google Scholar]
- Mayer ML. 2005. Crystal structures of the GluR5 and GluR6 ligand binding cores: Molecular mechanisms underlying kainate receptor selectivity. Neuron 45: 539–552. [DOI] [PubMed] [Google Scholar]
- Nägele T, Hoche R, Zinth W, Wachtveitl J. 1997. Femtosecond photo-isomerization of cis-azobenzene. Chem Phys Lett 272: 489–495. [Google Scholar]
- Numano R, Szobota S, Lau AY, Gorostiza P, Volgraf M, Roux B, Trauner D, Isacoff EY. 2009. Nanosculpting reversed wavelength sensitivity into a photoswitchable iGluR. Proc Natl Acad Sci 106: 6814–6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozhidaeva N, Cormier M-E, Chaudhari A, Woolley GA. 2004. Reversible photocontrol of peptide helix content: Adjusting thermal stability of the cis state. Bioconjug Chem 15: 1297–1303. [DOI] [PubMed] [Google Scholar]
- Renner C, Moroder L. 2006. Azobenzene as conformational switch in model peptides. Chembiochem 7: 868–878. [DOI] [PubMed] [Google Scholar]
- Rivera JF, Ahmad S, Quick MW, Liman ER, Arnold DB. 2003. An evolutionarily conserved dileucine motif in Shal K+ channels mediates dendritic targeting. Nat Neurosci 6: 243–250. [DOI] [PubMed] [Google Scholar]
- Sadovski O, Beharry AA, Zhang F, Woolley GA. 2009. Spectral tuning of azobenzene photoswitches for biological applications. Angew Chem Int Ed Engl 48: 1484–1486. [DOI] [PubMed] [Google Scholar]
- Silman I, Karlin A. 1969. Acetylcholine receptor: Covalent attachment of depolarizing groups at the active site. Science 164: 1420–1421. [DOI] [PubMed] [Google Scholar]
- Szobota S, Gorostiza P, Del Bene F, Wyart C, Fortin DL, Kolstad KD, Tulyathan O, Volgraf M, Numano R, Aaron HL, et al. 2007. Remote control of neuronal activity with a light-gated glutamate receptor. Neuron 54: 535–545. [DOI] [PubMed] [Google Scholar]
- Volgraf M, Gorostiza P, Numano R, Kramer RH, Isacoff EY, Trauner D. 2006. Allosteric control of an ionotropic glutamate receptor with an optical switch. Nat Chem Biol 2: 47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volgraf M, Gorostiza P, Szobota S, Helix MR, Isacoff EY, Trauner D. 2007. Reversibly caged glutamate: A photochromic agonist of ionotropic glutamate receptors. J Am Chem Soc 129: 260–261. [DOI] [PubMed] [Google Scholar]
- Wold F 1977. Affinity labeling—An overview. Methods Enzymol 46: 3–14. [DOI] [PubMed] [Google Scholar]
- Yager KG, Barrett CJ. 2006. Novel photo-switching using azobenzene functional materials. J Photochem Photobiol A Chem 182: 250–261. [Google Scholar]


