The dendrites of a retinal interneuron detect visual threats and initiate defensive responses through parallel pathways.
Abstract
Approaching predators cast expanding shadows (i.e., looming) that elicit innate defensive responses in most animals. Where looming is first detected and how critical parameters of predatory approaches are extracted are unclear. In mice, we identify a retinal interneuron (the VG3 amacrine cell) that responds robustly to looming, but not to related forms of motion. Looming-sensitive calcium transients are restricted to a specific layer of the VG3 dendrite arbor, which provides glutamatergic input to two ganglion cells (W3 and OFFα). These projection neurons combine shared excitation with dissimilar inhibition to signal approach onset and speed, respectively. Removal of VG3 amacrine cells reduces the excitation of W3 and OFFα ganglion cells and diminishes defensive responses of mice to looming without affecting other visual behaviors. Thus, the dendrites of a retinal interneuron detect visual threats, divergent circuits downstream extract critical threat parameters, and these retinal computations initiate an innate survival behavior.
INTRODUCTION
To survive, animals need to evade threats in their environments. How sensory systems detect threats quickly and how they measure critical parameters of a threat (e.g., the speed of an approaching predator) to select appropriate behavioral responses are central questions in neuroscience. Approaching objects cast expanding shadows (i.e., looming) that elicit innate defensive reactions in a wide range of animals, from insects to humans (1, 2). Mice use vision to avoid aerial predators (3, 4). The midbrain circuits that mediate looming responses in mice have been studied extensively (5–10), but where looming-selective signals first arise and how crucial parameters of predatory approaches are computed are unclear.
Early investigators suggested that the retina detects specific stimulus features and signals them to the brain through ganglion cell spike trains to trigger rapid behavioral responses (11, 12). Since then, a wide range of feature-selective responses has been identified in approximately 40 retinal ganglion cell types of mammals (13, 14). However, except for a link between direction-selective responses and a gaze-stabilizing reflex (15, 16), the behavioral significance of retinal feature detection remains uncertain. Furthermore, behavioral salience often depends on a combination of stimulus features (17). Whether and how the retina generates cooperative feature representations in ganglion cells are unknown.
The stimulus preferences of ganglion cells are shaped by amacrine cells, a diverse class of interneurons that encompasses more than 60 cell types (18–20). In part because of this diversity, the organization and function of circuits between amacrine and ganglion cells are poorly understood, a state that is emblematic of interneuron circuits throughout the nervous system (18, 21).
Most amacrine cells lack axons and receive input and provide output through their dendrites (18). By restricting the spread of input signals, different amacrine cell dendrites can compute different visual information and convey this information to separate targets (18, 22, 23). This enhances the computational power of amacrine cells and the complexity of retinal circuits. The sensory computations of amacrine cell dendrites, their influence on feature representations of ganglion cells, and contributions to vision are mostly unknown.
Here, we combine two-photon guided patch-clamp recordings, dendritic calcium imaging, optogenetics, anatomical circuit reconstructions, type-specific cell deletion, and behavioral assays to decipher the retinal processing of visual threats and its contributions to defensive responses.
RESULTS
Behavioral and neuronal responses to looming
We presented dark expanding disks (i.e., looming) on a monitor above a rectangular arena with virtual shelters on two sides (Fig. 1A; see Materials and Methods). After a period of acclimatization (>5 min), looming was triggered when mice crossed the center of the arena. Looming elicited stereotypic responses in which mice fled to a shelter and froze (Fig. 1, A and D, and movie S1). Consistent with previous observations (3), neither receding (i.e., dark contracting disks) nor white looming evoked similar responses (Fig. 1, B to D, and movies S2 and S3). How retinal circuits process looming and distinguish it from related forms of motion and which retinal circuits drive defensive behaviors are unclear.
Fig. 1. Behavioral and neuronal looming responses.
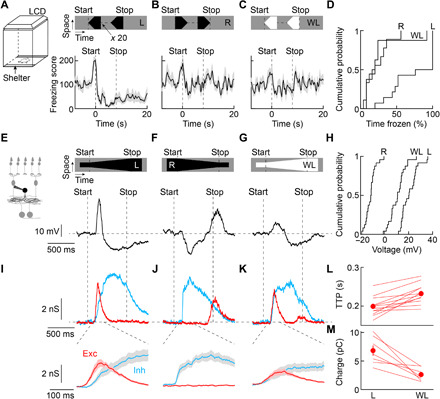
(A to C) Visual stimuli were shown to mice in a behavioral arena with virtual shelters (i.e., areas in which the monitor remained dark) on two sides. Freezing score responses of wild-type mice to looming (A; n = 14 mice), receding (B; n = 9 mice), and white looming (C; n = 8 mice). Lines (shaded areas) indicate mean (± SEM). LCD, liquid-crystal display. (D) Cumulative distributions of the time mice spent frozen from stimulus onset to 20 s later (stimulus duration: 8 s) for looming (L), receding (R), and white looming (WL). L versus R: P = 0.0033; L versus WL: P = 0.0028; R versus WL: P = 0.99; Kruskal-Wallis test. (E to G) Representative voltage traces of VG3-ACs (VG3-Cre Ai9 mice) during looming (E), receding (F), and white looming (G). Throughout this figure, the stimulus speed was 800 μm s−1. (H) Cumulative distributions of VG3 voltage responses to looming (L), receding (R), and white looming (WL). For all stimuli, n = 24 cells. L versus R: P = 9.7 × 10−10; L versus WL: P = 0.0015; R versus WL: P = 0.0015; Friedman’s test with Tukey-Kramer post hoc analysis. (I to K) Bottom traces show representative excitatory and inhibitory inputs to looming (I), receding (J), and white looming (K). Top traces (shaded areas) depict the mean (± SEM) responses starting 100 ms after the onset of motion. (L and M), Summary data comparing the time to peak (TTP) of excitation (L; n = 12 cells, P = 4.8 × 10−4, Wilcoxon signed-rank test) and the charge transferred during the window while excitation exceeds inhibition (M; n = 7 cells, P = 0.016, Wilcoxon signed-rank test).
Amacrine cells are an extraordinarily diverse class of interneurons that enable the retina to distinguish different forms of motion (18–20, 24, 25). On the basis of their receptive field properties (22, 23, 26, 27), we hypothesized that VGLUT3-expressing (VG3) amacrine cells contribute to the retinal processing of looming. In targeted patch-clamp recordings, we found that VG3 amacrine cells depolarized strongly to looming (Fig. 1, E and H, and fig. S1), hyperpolarized to receding (Fig. 1, F and H), and responded weakly to white looming (Fig. 1, G and H). In voltage-clamp recordings, excitatory inputs elicited by looming were consistently faster than those elicited by white looming (Fig. 1, I, K, and L). This created a window during which excitation exceeded inhibition, explaining the greater depolarization of VG3 amacrine cells to looming than white looming (Fig. 1, I, K, and M). Receding elicited strong synaptic inhibition with excitation relegated to the stimulus offset (Fig. 1J).
Dendritic processing of looming in VG3 amacrine cells
Amacrine cells send and receive signals through their dendrites. Thus, one amacrine cell can contain multiple input-output pathways that process information separately, bypassing the soma (22, 23, 28, 29). We used two-photon calcium imaging to analyze motion signals in VG3 dendrite arbors. Looming elicited robust calcium transients in VG3 dendrites in the outer part of the inner plexiform layer (Fig. 2, A and D). By contrast, receding did not evoke calcium transients during motion (Fig. 2, B and D), and the preference for looming versus receding was high throughout the VG3 dendrite arbor (Fig. 2E and fig. S2). White looming signals were weak and restricted to VG3 dendrites in the inner part of the inner plexiform layer (Fig. 2, C and D). Throughout the VG3 arbor, the preference for looming versus white looming was greater than the preference for stationary dark versus bright stimuli (Fig. 2F and fig. S2), and the preference for looming versus white looming in calcium signals was greater than that observed in voltage recordings (calcium, 0.75 ± 0.04; voltage, 0.35 ± 0.06; P = 8.92 × 10−7). Thus, looming-sensitive calcium signals are enhanced by dendritic nonlinearities and spatially segregated from weaker responses to related forms of motion.
Fig. 2. Dendritic processing of visual stimuli in VG3 amacrine cells.
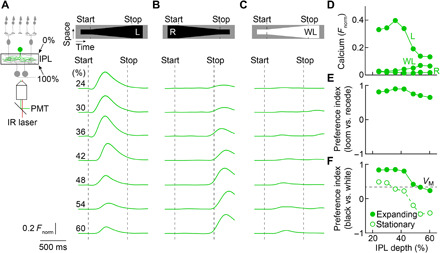
(A to C) Two-photon imaging of calcium transients in VG3 dendrites (VG3-Cre Ai148 mice) during looming (A), receding (B), and white looming (C). IR, infrared; PMT, photomultiplier tube. Green traces indicate the mean (shaded areas, almost indistinguishable from the green lines, indicate ± SEM) responses of dendritic regions of interest (ROIs) at different depths of the inner plexiform layer (IPL; 24%: n = 68 ROIs; 30%: n = 138 ROIs; 36%: n = 137 ROIs; 42%: n = 153 ROIs; 48%: 163 ROIs; 54%: n = 162 ROIs; 60%: n = 329 ROIs). (D) Average (± SEM) response amplitudes plotted as a function of IPL depth. Error bars indicating SEM are not visible because they are smaller than the circles. For IPL depths, 24 to 36% R and WL responses were not significantly different (P > 0.11); all other responses at all IPL depths were significantly different (P < 3.3 × 10−9, Friedman’s test with Tukey-Kramer post hoc analysis). (E and F) Summary data (means ± SEM) of preference indices for looming versus receding (E) and black versus white stimuli (F; expanding: filled circles; stationary: empty circles) across IPL depths. Error bars indicating SEM are not visible because they are smaller than the circles. For the preference index for looming versus receding (E), P = 1.3 × 10−21 for the main effect of IPL depth, Kruskal-Wallis test. By Tukey-Kramer post hoc analysis, pairwise comparisons showed statistically significant differences (P < 0.02) for 24 versus 54% and versus 60%, for 30 versus 48 to 60%, for 36 versus 48 to 60%, and for 42 versus 48 to 60%. For the preference index for looming versus white looming (F), P = 0 for the main effect of IPL depth, Kruskal-Wallis test. By Tukey-Kramer post hoc analysis, all pairwise comparisons revealed statistically significant differences (P < 0.02) except for 24 versus 30 to 42%, for 30 versus 36% and versus 42%, for 36 versus 42%, and for 54 versus 60%. For the preference index for black versus white stationary (F), P = 0 for the main effect of IPL depth, Kruskal-Wallis test. By Tukey-Kramer post hoc analysis, all pairwise comparisons revealed statistically significant differences (P < 0.02) except for 24 versus 30% and versus 36%, for 30 versus 36%, for 36 versus 42%, and for 54 versus 60%. At all depths, the preference index for black versus white looming was greater than that for stationary stimuli (P = 0 for a comparison across all depths and P < 10−9 for comparisons at each depth, Wilcoxon signed-rank test). In (F), the preference index for looming versus white looming for voltage responses (VM) is shown by a dashed line.
VG3 amacrine cells provide excitatory input to W3 and OFFα ganglion cells
The VG3 dendrites with the most robust looming responses overlap with the dendrites of two ganglion cell types that have been suggested to signal approaching aerial predators: W3 and OFFα (30–32). We used optogenetics to test the functional connectivity of VG3 amacrine cells with W3 and OFFα ganglion cells. VG3 amacrine cells are dual transmitter neurons that release glutamate and glycine (33, 34). We pharmacologically blocked transmission of photoreceptor signals to bipolar cells and matched Channelrhodopsin-2–mediated depolarizations of VG3 amacrine cells to their physiologic light responses (33). Optogenetic activation of VG3 amacrine cells elicited postsynaptic currents that reversed near 0 mV (Fig. 3, A and B), the reversal potential for cation-nonselective conductances in our recording conditions, in W3 and OFFα ganglion cells, indicating that VG3 amacrine cells provide purely glutamatergic input to both targets (27, 35).
Fig. 3. VG3 amacrine cells provide excitatory synaptic input to W3 and OFFα ganglion cells.
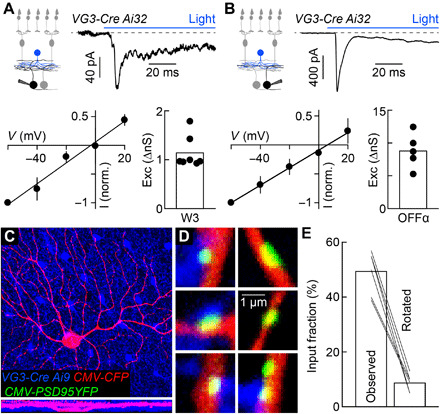
(A and B) Optogenetic stimulation of VG3 amacrine cells (VG3-Cre Ai32 mice) elicits postsynaptic currents in W3 (A; n = 7 cells) and OFFα (B; n = 5 cells) ganglion cells. Reversal near 0 mV identifies these as excitatory postsynaptic currents (EPSCs). (C and D) Overview projections (C) and single-plane excerpts (D) of a confocal image stack of an OFFα cell biolistically labeled with cyan fluorescent protein (CFP; C and D) and PSD95-YFP (D) in a retina, in which VG3 amacrine cells express tdTomato (VG3-Cre Ai9 mice). (E) Summary data of the fraction of PSD95-YFP puncta apposed by VG3 boutons in the obtained image stacks (observed) or the tdTomato channel was rotated by 90° (rotated, n = 6 cells; observed versus rotated: P = 0.03 by Wilcoxon signed-rank test). Exc, excitation; I, current; V, voltage.
To reconstruct anatomical connectivity patterns, we biolistically labeled OFFα ganglion cells with cytosolic cyan fluorescent protein and PSD95–yellow fluorescent protein (YFP), a marker of excitatory synapses (36), in mice in which VG3 amacrine cells express tdTomato (VG3-Cre Ai9; Fig. 3, C and D). In confocal image stacks, we found that approximately half the PSD95-YFP clusters on OFFα dendrites were apposed by VG3 boutons (Fig. 3E). The number of appositions markedly dropped when we rotated the tdTomato channel by 90° (Fig. 3E). Using a similar approach, we previously found that VG3 dendrites account for approximately half the excitatory synapses on W3 dendrites (26). Thus, VG3 amacrine cells provide a similar fraction (~1/2) of the excitatory input to W3 and OFFα ganglion cells.
Parallel parameter estimation in divergent VG3 circuits
To understand how looming signals are transformed from VG3 amacrine cells to W3 and OFFα ganglion cells, we analyzed the responses and underlying synaptic inputs of all three cells to dark disks expanding at different speeds. Responses of VG3 amacrine cells were restricted to the onset of motion (stimulus size at peak: 145 ± 8 μm, 4.3 ± 0.2°, n = 24 cells) and stable across looming speeds (Fig. 4, A and B). Responses were restricted to the onset of motion because transient excitation preceded sustained inhibition (Fig. 4, C and E). Responses were stable across looming speeds because the amplitudes of excitation and inhibition increased in parallel with stimulus speed, maintaining balance (Fig. 4D). W3 ganglion cells received a similar sequence of synaptic excitation and inhibition. Both inputs increased in amplitude together as a function of stimulus speed (Fig. 4, H to J). W3 ganglion cell responses, therefore, similar to those of VG3 amacrine cells, signaled the onset (i.e., critical size) of looming (stimulus size at peak: 154 ± 12 μm, 4.5 ± 0.4°, n = 5 cells) stably across different speeds of expansion (Fig. 4, F and G). By contrast, OFFα ganglion cell responses increased during looming motion and increased at higher stimulus speeds (Fig. 4, K and L) (31). Responses increased during motion because excitation coincided with disinhibition (Fig. 4, M and O). Responses increased at higher speeds as excitation and inhibition diverged in amplitudes (Fig. 4N). Stimulation with stationary dark spots revealed that the cell type–specific trajectories of excitation and inhibition during looming were temporal realizations of distinct spatial receptive field architectures by expanding motion (Fig. 4, E, J, and O, and fig. S3). W3 and OFFα ganglion cells strongly preferred expanding over contracting motion (fig. S4). Thus, by combining similar and, in part, shared excitatory input with dissimilar inhibition W3 and OFFα ganglion cells, which form parallel pathways from the retina to the brain, transform signals from VG3 amacrine cells to encode the onset (i.e., critical size) and speed of looming, respectively.
Fig. 4. Parallel parameter estimation in divergent circuits.
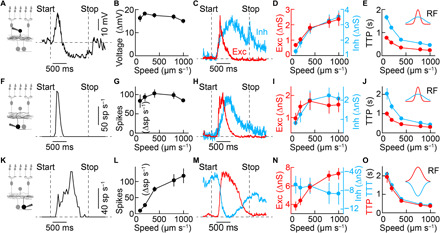
(A and B) Representative trace (A) and summary data (B) of VG3 amacrine cell voltage responses to looming with varying speeds of expansion. The speed for all representative traces in this figure is 400 μm s−1. VG3 amacrine cells responses varied slightly with speed (n = 8 cells; P = 0.038, Friedman’s test). (C to E) Representative traces (C) of excitatory and inhibitory inputs to VG3 amacrine cells and summary data of the amplitude (D) and latency (E; time to peak) of excitatory (n = 11 cells) and inhibitory (n = 12 cells) conductances at varying speeds of expansion. Excitation and inhibition increased in amplitude (excitation: P = 9.3 × 10−7; inhibition: P = 5 × 10−9; Friedman’s test) and decreased in latency (excitation: P = 1.3 × 10−8; inhibition: P = 2.8 × 10−9; Friedman’s test) with increasing stimulus speeds. Across speeds, inhibition was slower than excitation (P = 1.5 × 10−5, bootstrapping). (F and G) Representative trace (F) and summary data (G) for W3 spike responses, which did not vary significantly as a function of stimulus speed (n = 5 cells; P = 0.31, Friedman’s test). (H to J) Representative traces (H) of excitatory and inhibitory inputs to W3 ganglion cells and summary data of the amplitude (I) and latency (J) of excitatory and inhibitory conductances at varying speeds of expansion (n = 5 cells). Inhibition, but not excitation, increased significantly in amplitude (excitation: P = 0.14; inhibition: P = 0.032; Friedman’s test), and both decreased in latency (excitation: P = 5 × 10−4; inhibition: P = 5 × 10−4; Friedman’s test) with increasing stimulus speed. Across speeds, inhibition was slower than excitation (P = 0.01, bootstrapping). (K and L) Representative trace (K) and summary data (L) for OFFα spike responses, increasing with stimulus speed (n = 4 cells; P = 0.0086, Friedman’s test). (M to O) Representative traces (M) of excitatory and inhibitory inputs to OFFα ganglion cells and summary data of the amplitude (N) and latency (O) of excitatory and inhibitory conductances. Excitation increased and inhibition decreased in amplitude with increasing stimulus speed (excitation: n = 4 cells, P = 0.0016; inhibition: n = 5 cells, P = 0.0037; Friedman’s test). The latency of excitation was not significantly different from that of inhibition (P = 0.45, bootstrapping), and both decreased with stimulus speed (excitation: P = 0.003; inhibition: P = 5 × 10−4; Friedman’s test). Inset schematics in (E), (J), and (O) illustrate excitatory and inhibitory spatial receptive fields mapped with stationary dark spots (fig. S3). TTT, time to trough; TTP, time to peak; Exc, excitation; Inh, inhibition.
Responses of W3 and OFFα ganglion cells to looming depend on VG3 amacrine cells
To test the contributions of VG3 amacrine cells to the looming responses of W3 and OFFα ganglion cells, we selectively removed VG3 amacrine cells from the adult retina by injecting VG3-DTR mice intraperitoneally with diphtheria toxin (DT; fig. S5) (33, 35). We first analyzed the effect of this manipulation on ganglion cell dendrites and their synapses. Using biolistic labeling and confocal reconstructions, we found that VG3 removal did not affect the size of W3 and OFFα dendrite arbors but approximately halved the density of excitatory synapses (Fig. 5, A to D). This synapse loss matched the estimated fraction of excitatory synapses from VG3 amacrine cells in control retinas (Fig. 3E) (36), indicating that ganglion cells do not shift connections to other input partners in the adult retina as they do during development (37, 38).
Fig. 5. VG3 removal reduces the density of excitatory synapses on W3 and OFFα ganglion cells.
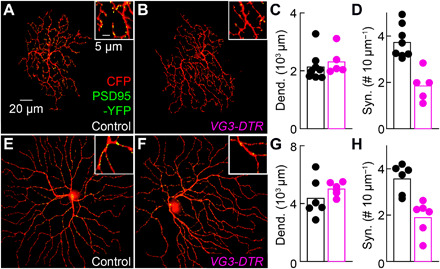
(A and B) Overview projections and excerpts (insets) of W3 dendrites biolistically labeled with CFP and PSD95-YFP in VG3-DTR mice (B) and littermate controls (A). (C and D) Summary data show that W3 dendrite length was unchanged (C; control: n = 8 cells; VG3-DTR: n = 5 cells, P = 0.35, Mann-Whitney U test), but excitatory synapse density was reduced (D; P = 0.0016, by Wilcoxon rank-sum test) by VG3 amacrine cell removal. (E and F) Overview projections and excerpts of OFFα dendrites biolistically labeled as in (A). (G and H) Summary data indicate that OFFα dendrite length was unchanged (G; control: n = 6 cells; VG3-DTR: n = 6 cells, P = 0.24, Mann-Whitney U test), but excitatory synapse density was reduced (H; P = 0.0022, Mann-Whitney U test) by VG3 amacrine cell removal.
In patch-clamp recordings, we found that the responses of W3 (Fig. 6, A to C) and OFFα ganglion cells (Fig. 6, D to F) to looming were attenuated by the removal of VG3 amacrine cells across a wide range of stimulus contrasts and speeds. For both ganglion cell types, this was caused by reduced synaptic excitation during looming (Fig. 6, G to L), whereas inhibition of W3 (Fig. 6, M and O) and disinhibition of OFFα ganglion cells (Fig. 6, P and R) were unchanged. Thus, looming signals from the retina to the brain depend on VG3 amacrine cells.
Fig. 6. Looming responses of W3 and OFFα ganglion cells depend on VG3 amacrine cells.
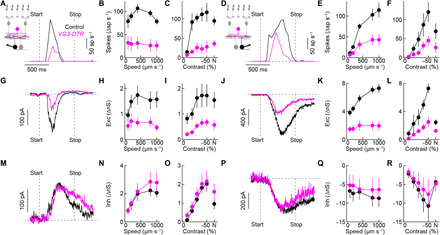
(A to F) Representative traces and summary data of W3 (A to C) and OFFα (D to F) ganglion cell spike responses to looming. The speed for all representative traces in this figure is 800 μm s−1. Across stimulus speeds and contrasts (including luminance-neutral approach motion; N), spike responses of W3 (control: n = 6 cells; VG3-DTR: n = 6 cells; speed: P = 4 × 10−5; contrast: P = 0.0017, bootstrapping) and OFFα (control: n = 4 to 5 cells; VG3-DTR: n = 6 to 8 cells; speed: P = 0.048; contrast: P = 0.047, bootstrapping) ganglion cells were attenuated by VG3 amacrine cell removal. (G to L) Synaptic excitation was reduced by VG3 amacrine cell removal in W3 (G to I; control: n = 5 cells; VG3-DTR: n = 7 to 8 cells; speed: P = 0.0024; contrast: P = 0.0021, bootstrapping) and OFFα (J to L; control: n = 4 cells; VG3-DTR: n = 4 cells; speed: P = 3 × 10−5; contrast: P = 0.0013, bootstrapping) ganglion cells. (M to R) Looming-evoked synaptic inhibition was unaffected by VG3 amacrine cell removal in W3 (M to O; control: n = 5 cells; VG3-DTR: n = 6 to 7 cells; speed: P = 0.77; contrast: P = 0.82, bootstrapping) and OFFα (P to R; control: n = 5 cells; VG3-DTR: n = 4 cells; speed: P = 0.91; contrast: P = 0.83, bootstrapping) ganglion cells. The control data for different looming speeds in this figure are the same as those in Fig. 4. Exc, excitation; Inh, inhibition.
Defensive responses to looming stimuli depend on VG3 amacrine cells
We next wanted to test how the removal of VG3 amacrine cells affects the behavioral responses to looming stimuli. In VG3-DTR mice, VG3 neurons in the retina and the brain express the DT receptor. Using tdTomato labeling in VG3-DTR Ai9 mice as a proxy, we found that a subset of midbrain areas involved in defensive responses to looming contain VG3 neurons (fig. S5) (5–10). To our surprise, these neurons were unaffected by intraperitoneal injections of DT that ablated most VG3 amacrine cells (fig. S5). Thus, the systemic administration of DT selectively removed VG3 neurons in the retina. To ensure further that cell removal was restricted to the retina, we alternatively injected DT directly into both eyes (i.e., intraocularly) of VG3-DTR mice (fig. S5).
In behavioral experiments, we found that the stereotypic behavioral responses to looming consisting of flight and prolonged freezing were preserved in control mice injected with DT intraperitoneally (Fig. 7, A and C, and movie S4) or i.o. (Fig. 7, E and G, and movie S5) but diminished by the same injections in VG3-DTR mice (Fig. 7, B, C, F, and G, and movies S6 and S7). In contrast, all four groups of mice performed indistinguishably in a visual cliff test (Fig. 7, D and H). Furthermore, visually evoked potentials, which measure the signals propagated along the retino-geniculo-cortical pathway, were not significantly different between VG3-DTR mice and littermate controls irrespective of the DT injection site (Fig. 7, I to P). Thus, VG3 amacrine cells are required selectively for the innate defensive responses of mice to looming.
Fig. 7. Innate defensive responses to looming selectively depend on VG3 amacrine cells.
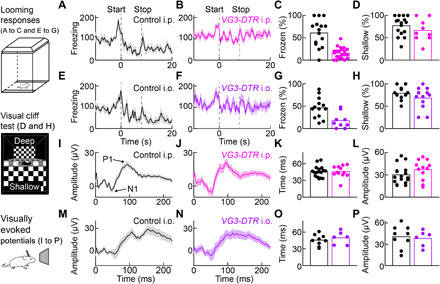
(A and B) Lines (shaded areas) indicate the mean (±SEM) freezing score traces in response to looming for VG3-DTR mice (B; n = 22 mice) and control littermates (A; n = 15 mice) injected intraperitoneally with DT. (C) Summary data comparing the fraction of time frozen between these groups of mice (VG3-DTR intraperitoneal and Ctrl intraperitoneal) from stimulus onset to 20 s later (stimulus duration: 8 s). P = 3.5 × 10−5, Mann-Whitney U test. (D) Summary data of the performance of VG3-DTR intraperitoneal (n = 9 mice) and Ctrl intraperitoneal mice (n = 15 mice) in a visual cliff test. For each mouse, the percentage of shallow-side choices in 10 trials was measured. P = 0.34, Mann-Whitney U test. (E to H) Analogous to (A to D), comparing VG3-DTR mice and control littermates injected intraocular with DT. For looming responses (E to G), VG3-DTR intraocular: n = 9 mice; Ctrl intraocular: n = 13 mice, P = 0.0066, Mann-Whitney U test. For the visual cliff test (H), VG3-DTR intraocular: n = 12 mice; Ctrl intraocular: n = 11 mice, P = 0.35, Mann-Whitney U test. (I and J) Lines (shaded areas) indicate the mean (±SEM) of visually evoked potentials recorded on skull electrodes above primary visual cortex in control (I; n = 17 mice) and VG3-DTR mice (J; n = 12 mice) 2 weeks after intraperitoneal injection of DT. (K and L) Summary data comparing implicit times (i.e., time to N1, P = 0.89, Mann-Whitney U test) and response amplitudes (i.e., P1-N1, P = 0.16, Mann-Whitney U test). (M to P) Analogous to (I) to (L) for control (n = 9 mice) and VG3-DTR mice (n = 6 mice) injected 2 weeks after bilateral intraocular injections of DT (implicit time: P = 0.52; amplitude: P = 0.78, Mann-Whitney U test). i.p., intraperitoneal; i.o., intraocular.
DISCUSSION
Threat detection and assessment are essential functions of nervous systems. Yet, where threats are detected and how critical threat parameters are measured to guide behavioral responses are unclear. Here, we characterize circuits in the mouse retina that process looming, a visual threat that elicits innate defensive responses in a wide range of animals, including humans (1, 2). We reach six main conclusions. First, a retinal interneuron (the VG3 amacrine cell) detects looming through stimulus-specific delays between synaptic excitation and inhibition. Second, the dendrites of VG3 amacrine cells enhance and spatially isolate looming signals. Third, the looming-sensitive dendrites of VG3 amacrine cells provide excitatory input to two ganglion cell types: W3 and OFFα. Fourth, W3 and OFFα ganglion cells combine shared excitation with dissimilar inhibition to encode the onset and speed of looming, respectively. Fifth, looming responses of W3 and OFFα ganglion cells depend on VG3 amacrine cells. Sixth, innate defensive responses to looming, but not other visual behaviors, depend on VG3 amacrine cells.
A contrast-specific delay in synaptic inputs generates feature selectivity
VG3 amacrine cells receive excitatory input from ON and OFF bipolar cells, which signal light increments (i.e., positive contrast) and decrements (i.e., negative contrast), respectively (26, 27, 39). We find that VG3 amacrine cells respond more strongly to looming than white looming because of a contrast-specific delay between excitation and inhibition. Whereas excitation and inhibition coincide during white looming, excitation precedes inhibition during looming (Fig. 1). This could either be due to differences in the size or arrangement of ON and OFF excitatory and inhibitory receptive fields, differences in the kinetics of the underlying mechanisms, or a combination of both. Maps constructed from responses to stationary stimuli indicate that OFF receptive fields of VG3 amacrine cells are smaller than their ON counterparts (22, 26, 27). The smaller OFF receptive fields explain the faster time to peak excitation for looming versus white looming (Fig. 1). However, the size ratios of the excitatory and inhibitory components do not differ significantly between the ON and OFF receptive fields of VG3 amacrine cells (ON: 0.53 ± 0.08; OFF: 0.56 ± 0.08; P = 0.79), and both are centrally aligned (26, 27). Therefore, we hypothesize that differences in the kinetics of receptive field mechanisms underlie the disparities in the relative timing of excitation and inhibition during looming versus white looming. The amacrine cells that inhibit VG3 amacrine cells during white looming may be driven by gap-junctional input from ON bipolar cells (40), whereas those that inhibit VG3 amacrine cells during looming are likely driven by glutamate release from OFF bipolar cells. Identification of the amacrine cell types that inhibit VG3 amacrine cells will enable tests of this hypothesis.
Dendritic processing enhances feature selectivity
The dendrites of many neurons process synaptic inputs locally (41). Yet, the mechanisms of dendritic processing and its contributions to sensory feature detection remain mostly unknown (28, 29, 42). We combined patch-clamp recordings and two-photon calcium imaging to analyze how the dendrites of VG3 amacrine cells process visual threats. We found that dendritic processing enhances the looming preferences of VG3 amacrine cells via (i) thresholding and (ii) spatial segregation. The preference of VG3 amacrine cells for looming versus white looming was greater for dendritic calcium transients than voltage responses (Fig. 2). This was true across regions of interest (ROIs) with different baseline fluorescence, suggesting that it is not an effect of the calcium indicator (22). Instead, the higher selectivity of dendritic calcium signals likely reflects activation thresholds of voltage-gated calcium channels, which are expressed in VG3 amacrine cells and contribute to transmitter release (27, 39). These activation thresholds sharpen the tuning of dendritic signals (43, 44). The cooperativity of calcium in promoting vesicle fusion likely further enhances looming preferences at the level of dendritic transmitter release (45). Thus, layered thresholding nonlinearities help VG3 dendrites detect visual threats.
VG3 dendrites limit the spread of synaptic inputs vertically and horizontally. Restrictions on vertical signal propagation segregate the processing of negative- and positive-contrast stimuli, which are relayed to different layers of their arbor by ON and OFF bipolar cells, respectively (22, 23). Vertical segregation enhances the looming preferences of the proximal layer of the VG3 arbor by preventing contamination from the white looming signals in the distal layer. In the VG3 plexus, the arbors of seven neighboring cells overlap at any point (26). Restrictions on horizontal signal spread (22, 23) increase the spatial resolution of looming signals and impose activity patterns with subcellular precision on the VG3 plexus irrespective of the cell identities of its constituent dendrites. Thus, spatial separation enhances the feature selectivity and topographic precision of looming responses in VG3 dendrites. Locally processing dendrites that receive input and provide output are a conserved feature of interneurons at the first three synapses of the visual system (i.e., horizontal cells, amacrine cells, and local interneurons of the thalamus) (18, 46, 47). In each case, they enhance spatial resolution and enable individual neurons to make different contributions to parallel pathways that generate and propagate feature-selective signals.
Synaptic mechanisms of parallel parameter estimation in divergent circuits
Using optogenetics and anatomical circuit reconstructions, we show that VG3 amacrine cells form glutamatergic synapses with W3 and OFFα ganglion cells (Fig. 2) (26, 27, 35). Analyses of synaptic contacts in control mice (Fig. 2) (26) and synapse loss in VG3-DTR mice suggest that VG3 amacrine cells provide approximately half of the excitatory input to W3 and OFFα ganglion cells (Fig. 5); bipolar cells likely account for the remainder. W3 and OFFα ganglion cells combine shared excitation with categorically different inhibition to encode distinct parameters of looming (Fig. 4). During looming, W3 ganglion cells receive delayed inhibition (Fig. 4) from TH2 and other wide-field GABAergic amacrine cells (32, 35, 48). In this feedforward circuit, inhibitory and excitatory receptive fields have the same sign but differ in size (inhibition > excitation). Expanding motion, therefore, activates excitation and inhibition sequentially, and the response of W3 ganglion cells peaks at a critical stimulus size (~154 μm or ~4.5°) independent of the speed of motion.
In contrast, OFFα ganglion cells receive tonic inhibition from AII amacrine cells, a narrow-field glycinergic ON responsive amacrine cell (31). During looming, inhibition from AII amacrine to OFFα ganglion cells is relieved (Fig. 4). In this crossover circuit, inhibitory and excitatory receptive fields have the opposite sign but similar size. As a result, OFFα ganglion cells’ firing ramps up gradually during looming. Because excitation increases unopposed with stimulus speed, OFFα ganglion cells encode the speed of expanding motion. Together, these findings highlight the modularity of interneuron circuits in the retina, in which a single amacrine cell can distribute signals to multiple targets (i.e., divergence modularity), which combine them with input from other amacrine cells that make distinct and separable contributions to the overall computation (i.e., convergence modularity). Modular circuits transform signals from VG3 dendrites into parallel retinal outputs that encode the onset (or critical size) and speed of looming, respectively. It will be interesting to see how modular combinations of amacrine cells generate other feature representations in the retinal output and whether the principle of modularity generalizes to interneuron circuits elsewhere in the nervous system (49).
Behavioral significance of retinal feature detection
More than 60 years ago, Lettvin et al. (11) suggested that the frog retina explicitly reports salient events in the visual world to the frog brain. The behavioral significance of this retinal event detection, however, remained untested. Here, we show that VG3 amacrine cells and downstream ganglion cells signal looming and drive defensive responses by which mice evade aerial predators (Fig. 7). We find that W3 and OFFα ganglion cells signal different aspects of looming, critical size, and speed, respectively (Fig. 4). Similar response types have been identified in the looming-sensitive tectal neurons of pigeons (17), indicating a conserved strategy in assessing predatory approaches.
In the taxonomy of visual motion, the first distinction is between (local) object motion and (global) self-generated motion (50). Looming is a specific form of object motion. VG3 amacrine cells distinguish object motion from self-generated motion and respond strongly to looming (Fig. 1) (26). Object motion sensitivity is tested with patterned stimuli that balance bright and dark regions (26, 32, 51), whereas looming consists of a radially expanding darkness (3, 50). The VG3 amacrine cell responses to balanced object motion and looming differ in their subcellular distribution. All VG3 dendrites respond to balanced object motion (22), whereas looming responses are restricted to the proximal layer of the VG3 arbor (Fig. 2). These stimulus-specific dendritic response distributions likely cause VG3 amacrine cells to activate (and suppress) different ganglion cell complements and contribute to the different behavioral responses to looming and balanced object motion.
We characterize the VG3-dependent looming responses of W3 and OFFα ganglion cells (Figs. 4 and 6). How other ganglion cell types, including suppressed-by-contrast ganglion cells, which receive stimulus-specific inhibition from VG3 amacrine cells (33, 34), respond to looming and how their responses are shaped by VG3 amacrine cells remains to be tested. Furthermore, how the VG3-dependent signals of W3, OFFα, and other ganglion cells are processed in downstream pathways that mediate defensive responses to looming is an exciting area for future investigation (5–10, 52). VG3 amacrine cells are conserved from rodents to primates (53, 54). We speculate that VG3 amacrine cells contribute to the innate defensive responses of infants to expanding shadows (55).
MATERIALS AND METHODS
Animals
To genetically target VG3 amacrine cells, we used bacterial artificial chromosome (BAC) transgenic mice expressing Cre recombinase under the control of regulatory sequences of the Slc17a8 gene, which encodes VG3 (VG3-Cre mice) (39). We crossed VG3-Cre mice to a fluorescent reporter (Ai9) (56) and optogenetic actuator (Ai32) (57) 57 lines to enable targeted recording and connectivity mapping. For subcellular analyses of visual processing by two-photon imaging, we crossed VG3-Cre mice to a GCaMP6f reporter line (Ai148) (22, 58). To selectively remove VG3 amacrine cells, we crossed VG3-Cre mice to a line expressing the DT receptor in a Cre-dependent manner (DTR mice) (59). We injected double-positive offspring (VG3-DTR mice) and their Cre− and/or DTR− littermates (control mice) intraperitoneally or intraocularly (i.o.) with DT. For intraperitoneal administration, mice were injected four times with DT (1 mg per 50 g body weight) once a day every other day starting at postnatal day 30 (P30). For i.o. administration, mice were injected once with 10 to 15 ng of DT in each eye at P30. Experiments were performed 1 to 2 weeks after the last injection. All procedures in this study were approved by the Animal Studies Committee of Washington University School of Medicine (protocol nos. 20170033 and 20-0055) and performed in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Patch-clamp recordings and optogenetics
We obtained patch-clamp recordings of VG3 amacrine cells and W3 and OFFα ganglion cells in retinal flat-mount preparations. Retinas from dark-adapted (>2 hours) mice were isolated under infrared illumination, mounted on membrane disk (Anodisc, Whatman), and continually perfused (~7 ml min−1) with warm (~33°C) bicarbonate-buffered mouse artificial cerebrospinal fluid (mACSFNaHCO3) containing 125 mM NaCl, 2.5 mM KCl, 1 mM MgCl2, 1.25 mM NaH2PO4, 2 mM CaCl2, 20 mM glucose, 26 mM NaHCO3, and 0.5 mM l-glutamine equilibrated with 95% O2/5% CO2. For optogenetics, l-2-amino-4-phosphonobutyric acid (AP4; 20 mM) and (S)-1-(2-Amino-2-carboxyethyl)-3-(2-carboxy-5-phenylthiophene-3-yl-methyl)-5-methylpyrimidine-2,4-dione (ACET) (10 mM) were added to mACSFNaHCO3 to block transmission of photoreceptor signals to ON and OFF bipolar cells, respectively. The intracellular solution for current-clamp recordings contained 125 mM K-gluconate, 10 mM NaCl, 1 mM MgCl2, 10 mM EGTA, 5 mM Hepes, 5 mM adenosine 5′-triphosphate–Na, and 0.1 mM guanosine 5′-triphosphate–Na (pH adjusted to 7.2 with KOH). The intracellular solution for voltage-clamp recordings contained 120 mM Cs-gluconate, 1 mM CaCl2, 1 mM MgCl2, 10 mM Na-Hepes, 11 mM EGTA, 10 mM tetraethylammonium (TEA)-Cl, and 2 mM Qx314 (pH adjusted to 7.2 with CsOH). Patch pipettes had resistances of 4 to 7 MΩ (borosilicate glass). Signals were amplified with a Multiclamp 700B amplifier (Molecular Devices), filtered at 3 kHz (8-pole Bessel low-pass), and sampled at 10 kHz (Digidata 1440A, Molecular Devices). In voltage-clamp recordings, series resistance (10 to 15 MΩ) was compensated electronically by ~75%. Excitatory postsynaptic currents and inhibitory postsynaptic currents were isolated by holding cells at the reversal potential of inhibitory (−60 mV) and excitatory (0 mV) conductances, respectively. In current-clamp recordings, no bias currents were injected. We targeted VG3 amacrine cells under two-photon guidance and recorded W3 and OFFα ganglion cells under conventional infrared illumination. We identified ganglion cell types and confirmed VG3 amacrine cell identity by including Alexa 488 or Alexa 568 (0.1 mM) in the intracellular solution and acquiring two-photon image stacks at the end of each recording.
Visual stimuli were presented on an organic light-emitting display (eMagin) and projected onto the photoreceptor side of the dorsal retina via the substage condenser. In looming stimuli, a 20-μm (0.6°) disk appeared on a gray background, remained stable for 1 s, then expanded to 600 μm (17.6°), remained at this size for 1 s, and disappeared (Fig. 1). The contrast of the disk relative to the background was varied (−100 to 100% Weber contrast), as was the speed of expansion (100 to 1000 μm s−1 or 2.9 to 29° s−1). In receding stimuli, the inverse sequence to looming stimuli was shown. In luminance-neutral approaching motion stimuli, the summed intensity in a 600-μm-diameter disk was kept constant as the central disk expanded (Fig. 6 and fig. S1). Response amplitudes were measured as the mean of the respective traces in 100-ms windows centered on their extrema.
To activate Channelrhodopsin-2 in VG3-Cre Ai32 retinas, light stimuli were presented from the ganglion cell side through a 20 × 0.95 numerical aperture (NA) water immersion objective. Light from a mercury bulb (Olympus) was bandpass-filtered (426 to 446 nm, Chroma) and attenuated by neutral density filters (Chroma). In targeted recordings from VG3 amacrine cells, we previously identified an optogenetic stimulus intensity (3.15 × 10−4 W mm−2) that matches photoreceptor-mediated light responses of VG3 amacrine cells (33). Stimulus timing was controlled by a Uniblitz shutter (Vincent Associates).
Two-photon calcium imaging
The retina was isolated and flat-mounted as for patch-clamp recordings. A custom-built upright two-photon microscope (Scientifica) controlled by the Scanimage r3.8 MATLAB toolbox was used to acquire images via a DAQ NI PCI6110 data acquisition board (National Instruments). In VG3-Cre Ai148 mice, GCaMP6f was excited with a Mai-Tai laser (Spectra-Physics) tuned to 930 nm (laser power: <7 mW at the sample), and fluorescence emission was collected via a 60 × 1.0 NA water immersion objective (Olympus) filtered through consecutive 450-nm long-pass (Thorlabs) and 513- to 528-nm bandpass filters (Chroma). This blocked visual stimulus light (385 nm) from reaching the PMT. We acquired images throughout this study at 9.5 Hz with a pixel density of 4.7 pixels μm−2. Imaging depths were registered by their relative distances to the borders between the inner plexiform layer (IPL) and the inner nuclear layer (IPL depth: 0%) and between the IPL and the ganglion cell layer (IPL depth: 100%). Borders were detected in transmitted light images. Scan fields at different IPL depths were imaged in pseudorandom order, and for each scan, the retina was adapted to the laser light for 30 s before the presentation of visual stimuli. Throughout the experiments, retinas we perfused at ~7 ml min−1 with 33°C mACSFNaHCO3 equilibrated with 95% O2/5% CO2. Images were denoised, registered, and segmented into functionally distinct ROIs as described previously (22).
Visual stimuli were presented from a ultraviolet E4500 MKII PLUS II projector illuminated by a 385-nm light-emitting diode (EKB Technologies) and focused onto the photoreceptors of the ventral retina, where S-opsin dominates (60), via the substage condenser. We used neutral density filters (Thorlabs, FW102CNEB) to attenuate the output of the projector. Stimuli were centered on the two-photon scan field, and their average intensity kept constant at ~1600 S-opsin isomerizations S-cone−1 s−1. The parameters of looming, receding, and white looming stimuli were identical to those used for patch-clamp recordings. Stimulus speeds were limited to 800 μm s−1 (24° s−1). In addition, we presented stationary stimuli, in which the light intensity was square-wave modulated (1.5 s ON and 1.5 s OFF) in a 100-μm disk. Stationary stimuli were interleaved with looming, receding, and white looming stimuli to facilitate comparisons of the responses of individual ROIs. We calculated the following preference indices: Preference index (loom versus recede) = (L − R)/(L + R), where L and R indicate responses to looming and receding, respectively; Preference index (black versus white)expanding = (L − WL)/(L + WL), where L and WL indicate responses to looming and white looming, respectively; and Preference index (black versus white)stationary = (OFF − ON)/(OFF + ON), where OFF and ON indicate responses to light decrements and increments, respectively, of stationary disks.
Anatomy
To map excitatory connections of VG3 amacrine cells with W3 and OFFα ganglion cells, we biolistically labeled flat-mounted retinas with cytosolic tdTomato and PSD95-YFP. Retinas were isolated in Hepes-buffered mouse artificial cerebrospinal fluid (mACSFHEPES)—containing 119 mM NaCl, 2.5 mM KCl, 2.5 mM CaCl2, 1.3 mM MgCl2, 1 mM NaH2PO4, 11 mM glucose, and 20 mM Hepes (pH adjusted to 7.37 with NaOH)—and mounted on membrane disks (HABGO1300, Millipore). Gold particles (1.6-μm diameter; Bio-Rad) were coated with plasmids encoding tdTomato and PSD95-YFP as previously described and were delivered to ganglion cells from a helium-pressurized gun (Bio-Rad) at 40 psi. Retinas were then incubated in a humid oxygenated chamber at 33° to 35°C for 14 to 18 hours in mACSFHepes, fixed for 30 min in 4% paraformaldehyde in mACSFHepes, and washed in PBS (three times, 10 min) before mounting and imaging.
Confocal image stacks (voxel size: 0.103 μm x/y, 0.3 μm z) of biolistically labeled W3 and OFFα ganglion cells were acquired on an Fv1000 laser scanning microscope (Olympus) using a 60 × 1.35 NA oil immersion objective. We skeletonized dendrites and synapses identified in confocal image stacks using previously described algorithms (36) to measure dendrite lengths and synapse densities (Fig. 5). To determine the fraction of excitatory synapses on OFFα dendrites apposed by VG3 boutons (Fig. 3), we separately masked OFFα dendrites, PSD95-YFP puncta, and VG3 dendrites in Amira (Thermo Fisher Scientific). Excitatory synapses on OFFα dendrites formed by VG3 amacrine cells were defined as PSD95 clusters with a center of mass within 0.5 μm of a VG3 dendrite. We confirmed that varying this distance from 0.25 to 1 μm did not qualitatively change the results. We repeated the same analysis for each cell in image stacks in which the amacrine cell channel was rotated by 90° to compare the fraction of PSD95-YFP apposed by VG3 dendrites to that caused by random signal overlap.
To analyze the number and distribution of VG3 neurons, we stained retinal flat mounts and vibratome slices (thickness: 100 μm) of different brain regions in VG3-Cre Ai9 mice and VG3-DTR Ai9 mice were injected with DT intraperitoneally or i.o. for tdTomato (primary antibody: rabbit anti-DsRed, 1:1000; BD Biosciences, RRID:AB_394264; secondary antibody: donkey anti-rabbit immunoglobulin G Alexa 568, 1:1000; Thermo Fisher Scientific, RRID:AB_253401) and obtained confocal images stacks (voxel size: 0.62 to 1.24 μm x/y and 1 to 5 μm z) on an Fv1000 laser scanning microscope (Olympus) using 20 × 1.35 NA oil immersion and 10× objective.
Visually evoked potential recordings
We recorded flash visually evoked potentials on the UTAS-E3000 Visual Electrodiagnostic System (LKC Technologies). Mice were dark-adapted for >2 hours and anesthetized with ketamine (80 mg kg−1) and xylazine (15 mg kg−1) cocktail under dim red light. We dilated pupils with 1% atropine sulfate and applied hypromellose ophthalmic ointment (Gonak) to the cornea. A platinum wire (diameter: 0.25 mm; Alfa Aesar) was inserted subcutaneously over the right side of visual cortex (~8 mm from the midline), and mice were placed on a heating pad with a reference needle electrode pinned in the left ear and a ground needle electrode inserted under the tail skin. The platinum wire was connected to the system via a clamp electrode. Mice were in the complete darkness for 3 min before recordings. For each recording, responses of 80 repeated flashes (intensity: 2 cdS m−2, frequency: 2 Hz) were averaged.
Behavior
To analyze responses to looming stimuli, mice were placed in a 45 cm by 27 cm by 31 cm box (width × depth × height) with three opaque walls and one transparent wall (Fig. 1A). We recorded videos through the transparent wall with a universal serial bus (USB) camera (720p, ELP, or C310, Logitech) and presented stimuli on an LCD monitor (32 cm by 24 cm display area; mean stimulus intensity: ~1600 M-opsin isomerizations M-cone−1 s−1; refresh rate: 60 Hz), which formed the ceiling of the box. Looming stimuli consisted of a 2° (diameter) dark disk on a gray background that expanded to a 20° in 0.25 to 1 s (18 to 72° s−1), remained this size for 0.25 s, before starting again at 2° for a sequence that repeated 20 times without gaps (Fig. 1A). Stimuli were started when mice entered the center of the arena. Alternatively, mice were shown a bright disk expanding on a gray background or a dark disk shrinking with kinetic and size parameters matching the looming stimulus (Fig. 1, B and C). Mice were acclimatized to the behavioral arena for at least 5 min before stimulus presentation. Because mice habituate to these stimuli, only the first presentation of each stimulus for an animal was included in our analysis. We recorded and analyzed mouse behavior automatically using ANY-maze tracking software (Stoelting). Consistent with previous studies (8), we defined mice as frozen when the freezing score was <30. We report the percentage of time mice spent in this state from stimulus onset to 20 s later as a summary parameter. We confirmed that varying the freezing score threshold (±50%) did not qualitatively change our results.
Visual cliff tests were performed on a 56-cm by 41-cm platform (width × depth) with a 3.8-cm by 1.7-cm ridge (height × width) across its center. On one side of the ridge, a checkered pattern was immediately below the platform (i.e., the shallow side), and on the other side, an identical checkered pattern was 61 cm below the platform (i.e., the cliff side). Mice were placed on the ridge and filmed via a USB camera (720p, ELP, or C310, Logitech). For each mouse, we measured the percentage of shallow-side choices in at least 10 trials.
Statistical analyses
Data were analyzed using scripts written in MATLAB (The MathWorks). The code will be made available upon request. Summary data are presented as means ± SEM. Nonparametric tests (Mann-Whitney U, Wilcoxon signed-rank, Friedman’s, and Kruskal-Wallis) and bootstrapping were used to compare data from different experimental groups as specified in the figure legends. Statistical significance was considered when P < 0.05.
Supplementary Material
Acknowledgments
We thank all members of the Kerschensteiner laboratory for discussions throughout the project; B. Wang and L. Zhao for expert technical assistance; and M. Bagnall, M. Kerschensteiner, J.L. Morgan, and F. Soto for critical reading of the manuscript. Funding: This work was supported by the NIH (EY023341, EY026978, EY027411, and EY030623 to D.K. and the Vision Core grant EY0268), the Grace Nelson Lacy Research Fund (director: D.K.), and an unrestricted grant to the Department of Ophthalmology and Visual Sciences from Research to Prevent Blindness. Author contributions: T.K. and D.K. conceived of this study. T.K. and K.P.J. performed patch-clamp recordings. T.K. and D.K. performed anatomy experiments. J.-C.H. performed two-photon imaging experiments. T.K., N.S., and D.K. performed behavioral experiments. All authors analyzed data and contributed to the experimental design. D.K. wrote the manuscript with input from all authors. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/6/47/eabc9920/DC1
REFERENCES AND NOTES
- 1.Fotowat H., Gabbiani F., Collision detection as a model for sensory-motor integration. Annu. Rev. Neurosci. 34, 1–19 (2011). [DOI] [PubMed] [Google Scholar]
- 2.Peek M. Y., Card G. M., Comparative approaches to escape. Curr. Opin. Neurobiol. 41, 167–173 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Yilmaz M., Meister M., Rapid innate defensive responses of mice to looming visual stimuli. Curr. Biol. 23, 2011–2015 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Franceschi G., Vivattanasarn T., Saleem A. B., Solomon S. G., Vision guides selection of freeze or flight defense strategies in mice. Curr. Biol. 26, 2150–2154 (2016). [DOI] [PubMed] [Google Scholar]
- 5.Shang C., Liu Z., Chen Z., Shi Y., Wang Q., Liu S., Li D., Cao P., A parvalbumin-positive excitatory visual pathway to trigger fear responses in mice. Science 348, 1472–1477 (2015). [DOI] [PubMed] [Google Scholar]
- 6.Shang C., Chen Z., Liu A., Li Y., Zhang J., Qu B., Yan F., Zhang Y., Liu W., Liu Z., Guo X., Li D., Wang Y., Cao P., Divergent midbrain circuits orchestrate escape and freezing responses to looming stimuli in mice. Nat. Commun. 9, 1232 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang L., Yuan T., Tan M., Xi Y., Hu Y., Tao Q., Zhao Z., Zheng J., Han Y., Xu F., Luo M., Sollars P. J., Pu M., Pickard G. E., So K.-F., Ren C., A retinoraphe projection regulates serotonergic activity and looming-evoked defensive behaviour. Nat. Commun. 8, 14908 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wei P., Liu N., Zhang Z., Liu X., Tang Y., He X., Wu B., Zhou Z., Liu Y., Li J., Zhang Y., Zhou X., Xu L., Chen L., Bi G., Hu X., Xu F., Wang L., Processing of visually evoked innate fear by a non-canonical thalamic pathway. Nat. Commun. 6, 6756 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salay L. D., Ishiko N., Huberman A. D., A midline thalamic circuit determines reactions to visual threat. Nature 557, 183–189 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evans D. A., Stempel A. V., Vale R., Ruehle S., Lefler Y., Branco T., A synaptic threshold mechanism for computing escape decisions. Nature 558, 590–594 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lettvin J. Y., Maturana H. R., McCulloch W. S., Pitts W. H., What the frog’s eye tells the frog’s brain. Proc. IRE. 47, 1940–1951 (1959). [Google Scholar]
- 12.H. Barlow, Possible Principles Underlying the Transformations of Sensory Messages in Sensory Communication (MIT Press, 1961); 10.7551/mitpress/9780262518420.003.0013.
- 13.Gollisch T., Meister M., Eye smarter than scientists believed: Neural computations in circuits of the retina. Neuron 65, 150–164 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanes J. R., Masland R. H., The types of retinal ganglion cells: Current status and implications for neuronal classification. Annu. Rev. Neurosci. 38, 221–246 (2015). [DOI] [PubMed] [Google Scholar]
- 15.Yoshida K., Watanabe D., Ishikane H., Tachibana M., Pastan I., Nakanishi S., A key role of starburst amacrine cells in originating retinal directional selectivity and optokinetic eye movement. Neuron 30, 771–780 (2001). [DOI] [PubMed] [Google Scholar]
- 16.Yonehara K., Fiscella M., Drinnenberg A., Esposti F., Trenholm S., Krol J., Franke F., Scherf B. G., Kusnyerik A., Müller J., Szabo A., Jüttner J., Cordoba F., Reddy A. P., Németh J., Nagy Z. Z., Munier F., Hierlemann A., Roska B., Congenital nystagmus gene FRMD7 is necessary for establishing a neuronal circuit asymmetry for direction selectivity. Neuron 89, 177–193 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun H., Frost B. J., Computation of different optical variables of looming objects in pigeon nucleus rotundus neurons. Nat. Neurosci. 1, 296–303 (1998). [DOI] [PubMed] [Google Scholar]
- 18.Diamond J. S., Inhibitory interneurons in the retina: Types, circuitry, and function. Annu. Rev. Vis. Sci. 3, 1–24 (2017). [DOI] [PubMed] [Google Scholar]
- 19.Helmstaedter M., Briggman K. L., Turaga S. C., Jain V., Seung H. S., Denk W., Connectomic reconstruction of the inner plexiform layer in the mouse retina. Nature 500, 168–174 (2013). [DOI] [PubMed] [Google Scholar]
- 20.Yan W., Laboulaye M. A., Tran N. M., Whitney I. E., Benhar I., Sanes J. R., Mouse retinal cell atlas: Molecular identification of over sixty amacrine cell types. J. Neurosci. 40, 5177–5195 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang Z. J., Paul A., The diversity of GABAergic neurons and neural communication elements. Nat. Rev. Neurosci. 20, 563–572 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsiang J.-C., Johnson K. P., Madisen L., Zeng H., Kerschensteiner D., Local processing in neurites of VGluT3-expressing amacrine cells differentially organizes visual information. eLife 6, e31307 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen M., Lee S., Zhou Z. J., Local synaptic integration enables ON-OFF asymmetric and layer-specific visual information processing in vGluT3 amacrine cell dendrites. Proc. Natl. Acad. Sci. U.S.A. 114, 11518–11523 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mauss A. S., Vlasits A., Borst A., Feller M., Visual circuits for direction selectivity. Annu. Rev. Neurosci. 40, 211–230 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wei W., Neural mechanisms of motion processing in the mammalian retina. Annu. Rev. Vis. Sci. 4, 165–192 (2018). [DOI] [PubMed] [Google Scholar]
- 26.Kim T., Soto F., Kerschensteiner D., An excitatory amacrine cell detects object motion and provides feature-selective input to ganglion cells in the mouse retina. eLife 4, e08025 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee S., Chen L., Chen M., Ye M., Seal R. P., Zhou Z. J., An unconventional glutamatergic circuit in the retina formed by vGluT3 amacrine cells. Neuron 84, 708–715 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Euler T., Detwiler P. B., Denk W., Directionally selective calcium signals in dendrites of starburst amacrine cells. Nature 418, 845–852 (2002). [DOI] [PubMed] [Google Scholar]
- 29.Grimes W. N., Zhang J., Graydon C. W., Kachar B., Diamond J. S., Retinal parallel processors: More than 100 independent microcircuits operate within a single interneuron. Neuron 65, 873–885 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bae J. A., Mu S., Kim J. S., Turner N. L., Tartavull I., Kemnitz N., Jordan C. S., Norton A. D., Silversmith W. M., Prentki R., Sorek M., David C., Jones D. L., Bland D., Sterling A. L. R., Park J., Briggman K. L., Seung H. S.; Eyewirers , Digital museum of retinal ganglion cells with dense anatomy and physiology. Cell 173, 1293–1306.e19 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Münch T. A., da Silveira R. A., Siegert S., Viney T. J., Awatramani G. B., Roska B., Approach sensitivity in the retina processed by a multifunctional neural circuit. Nat. Neurosci. 12, 1308–1316 (2009). [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y., Kim I.-J., Sanes J. R., Meister M., The most numerous ganglion cell type of the mouse retina is a selective feature detector. Proc. Natl. Acad. Sci. U.S.A. 109, E2391–E2398 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tien N.-W., Kim T., Kerschensteiner D., Target-specific glycinergic transmission from VGluT3-expressing amacrine cells shapes suppressive contrast responses in the retina. Cell Rep. 15, 1369–1375 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee S., Zhang Y., Chen M., Zhou Z. J., Segregated glycine-glutamate co-transmission from vGluT3 amacrine cells to contrast-suppressed and contrast-enhanced retinal circuits. Neuron 90, 27–34 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krishnaswamy A., Yamagata M., Duan X., Hong Y. K., Sanes J. R., Sidekick 2 directs formation of a retinal circuit that detects differential motion. Nature 524, 466–470 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kerschensteiner D., Morgan J. L., Parker E. D., Lewis R. M., Wong R. O. L., Neurotransmission selectively regulates synapse formation in parallel circuits in vivo. Nature 460, 1016–1020 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tien N.-W., Soto F., Kerschensteiner D., Homeostatic plasticity shapes cell-type-specific wiring in the retina. Neuron 94, 656–665.e4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okawa H., Della Santina L., Schwartz G. W., Rieke F., Wong R. O. L., Interplay of cell-autonomous and nonautonomous mechanisms tailors synaptic connectivity of converging axons in vivo. Neuron 82, 125–137 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grimes W. N., Seal R. P., Oesch N., Edwards R. H., Diamond J. S., Genetic targeting and physiological features of VGLUT3+ amacrine cells. Vis. Neurosci. 28, 381–392 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Farrow K., Teixeira M., Szikra T., Viney T. J., Balint K., Yonehara K., Roska B., Ambient illumination toggles a neuronal circuit switch in the retina and visual perception at cone threshold. Neuron 78, 325–338 (2013). [DOI] [PubMed] [Google Scholar]
- 41.Branco T., Häusser M., The single dendritic branch as a fundamental functional unit in the nervous system. Curr. Opin. Neurobiol. 20, 494–502 (2010). [DOI] [PubMed] [Google Scholar]
- 42.Jia H., Rochefort N. L., Chen X., Konnerth A., Dendritic organization of sensory input to cortical neurons in vivo. Nature 464, 1307–1312 (2010). [DOI] [PubMed] [Google Scholar]
- 43.Isaacson J. S., Scanziani M., How inhibition shapes cortical activity. Neuron 72, 231–243 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oesch N., Euler T., Taylor W. R., Direction-selective dendritic action potentials in rabbit retina. Neuron 47, 739–750 (2005). [DOI] [PubMed] [Google Scholar]
- 45.Dodge F. A. Jr., Rahamimoff R., Co-operative action of calcium ions in transmitter release at the neuromuscular junction. J. Physiol. 193, 419–432 (1967). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morgan J. L., Lichtman J. W., An individual interneuron participates in many kinds of inhibition and innervates much of the mouse visual thalamus. Neuron 106, 468–481.e2 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chapot C. A., Euler T., Schubert T., How do horizontal cells “talk” to cone photoreceptors? Different levels of complexity at the cone-horizontal cell synapse. J. Physiol. 595, 5495–5506 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim T., Kerschensteiner D., Inhibitory control of feature selectivity in an object motion sensitive circuit of the retina. Cell Rep. 19, 1343–1350 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pfeffer C. K., Xue M., He M., Huang Z. J., Scanziani M., Inhibition of inhibition in visual cortex: The logic of connections between molecularly distinct interneurons. Nat. Neurosci. 16, 1068–1076 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Frost B. J., A taxonomy of different forms of visual motion detection and their underlying neural mechanisms. Brain Behav. Evol. 75, 218–235 (2010). [DOI] [PubMed] [Google Scholar]
- 51.Ölveczky B. P., Baccus S. A., Meister M., Segregation of object and background motion in the retina. Nature 423, 401–408 (2003). [DOI] [PubMed] [Google Scholar]
- 52.Reinhard K., Li C., Do Q., Burke E. G., Heynderickx S., Farrow K., A projection specific logic to sampling visual inputs in mouse superior colliculus. eLife 8, e50697 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haverkamp S., Wässle H., Characterization of an amacrine cell type of the mammalian retina immunoreactive for vesicular glutamate transporter 3. J. Comp. Neurol. 468, 251–263 (2004). [DOI] [PubMed] [Google Scholar]
- 54.Peng Y.-R., Shekhar K., Yan W., Herrmann D., Sappington A., Bryman G. S., van Zyl T., Do M. T. H., Regev A., Sanes J. R., Molecular classification and comparative taxonomics of foveal and peripheral cells in primate retina. Cell 176, 1222–1237.e22 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ball W., Tronick E., Infant responses to impending collision: Optical and real. Science 171, 818–820 (1971). [DOI] [PubMed] [Google Scholar]
- 56.Madisen L., Zwingman T. A., Sunkin S. M., Oh S. W., Zariwala H. A., Gu H., Ng L. L., Palmiter R. D., Hawrylycz M. J., Jones A. R., Lein E. S., Zeng H., A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 13, 133–140 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Madisen L., Mao T., Koch H., Zhuo J.-M., Berenyi A., Fujisawa S., Hsu Y.-W. A., Garcia A. J. III, Gu X., Zanella S., Kidney J., Gu H., Mao Y., Hooks B. M., Boyden E. S., Buzsáki G., Ramirez J. M., Jones A. R., Svoboda K., Han X., Turner E. E., Zeng H., A toolbox of Cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nat. Neurosci. 15, 793–802 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Daigle T. L., Madisen L., Hage T. A., Valley M. T., Knoblich U., Larsen R. S., Takeno M. M., Huang L., Gu H., Larsen R., Mills M., Bosma-Moody A., Siverts L. A., Walker M., Graybuck L. T., Yao Z., Fong O., Nguyen T. N., Garren E., Lenz G. H., Chavarha M., Pendergraft J., Harrington J., Hirokawa K. E., Harris J. A., Nicovich P. R., McGraw M. J., Ollerenshaw D. R., Smith K. A., Baker C. A., Ting J. T., Sunkin S. M., Lecoq J., Lin M. Z., Boyden E. S., Murphy G. J., da Costa N. M., Waters J., Li L., Tasic B., Zeng H., A suite of transgenic driver and reporter mouse lines with enhanced brain-cell-type targeting and functionality. Cell 174, 465–480.e22 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Buch T., Heppner F. L., Tertilt C., Heinen T. J. A. J., Kremer M., Wunderlich F. T., Jung S., Waisman A., A Cre-inducible diphtheria toxin receptor mediates cell lineage ablation after toxin administration. Nat. Methods 2, 419–426 (2005). [DOI] [PubMed] [Google Scholar]
- 60.Wang Y. V., Weick M., Demb J. B., Spectral and temporal sensitivity of cone-mediated responses in mouse retinal ganglion cells. J. Neurosci. 31, 7670–7681 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/6/47/eabc9920/DC1


