Abstract
Introduction
International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) criteria are the most representative risk model for patients with metastatic renal cell carcinoma (mRCC). However, the intermediate-risk group of IMDC criteria is thought to include patients with different prognoses because many of the patients are classified into the intermediate-risk group. In this study, we investigated the impact of systemic immune-inflammation index (SII), which is calculated based on neutrophil count, platelet count, and lymphocyte count, on predicting the prognosis in patients with mRCC, and its usefulness for re-classification of patients with a more sophisticated risk model.
Methods
From January 2008 to January 2018, 179 mRCC patients with a pretreatment and SII were retrospectively investigated. All patients were classified into either a high-SII group or a low-SII group based on the cutoff value of a SII at 730, as reported in previous studies; the overall survival (OS) rates in each group were compared.
Results
The median age was 65 years old. Males and females comprised 145 and 34 cases, respectively. The categories of favorable-, intermediate-, and poor-risk groups in the IMDC model were assessed in 39, 102, and 38 cases, respectively. The median observation period was 24 months. The low-SII and high-SII groups consisted of 73 and 106 cases, respectively. The 50% OS in the high-SII group was 21.4 months, which was significantly worse than that in the low-SII group (49.7 months; p<0.0001). Multivariate analysis showed that a high SII was an independent predictive factor for a worse OS. Next, we constructed a modified IMDC risk model that included the SII instead of a neutrophil count and a platelet count. By using this modified IMDC model, all cases were re-classified into four groups of 33, 52, 81, and 13 cases with 50% OS of 88.8, 45.9, 29.4, and 4.8 months, respectively.
Conclusions
The SII is useful for establishing a more sophisticated prognostic model that can stratify mRCC patients into four groups with different prognoses.
Introduction
While increased screening has led to greater detection rates of clinically localized renal cell carcinoma (RCC), more than 30% of patients with RCC have metastases at initial presentation.1 The introduction of targeted agents, especially tyrosine kinase inhibitors (TKIs), has led to improved prognosis of metastatic RCC (mRCC) in the past decade.2 In addition, some randomized control trials showed the effectiveness of immune checkpoint inhibitors (ICIs) for mRCC patients.3,4 Several guidelines recommend the choice of a first-line agent based on risk model.5,6
The International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) risk model7 is one of the most widely used for mRCC; however, several patients with relatively different prognoses are thought to be classified into the intermediate-risk group. It is important to establish a more sophisticated prognostic model.
Several molecules or indexes related to the inflammation response derived from blood samples have been demonstrated as candidates of biomarkers predicting the effect of the treatment or prognosis of mRCC regardless of therapeutic option.8 Two of six parameters of the IMDC prognostic model, neutrophil and platelet count, are also involved in the inflammatory response. The systemic immune inflammation index (SII) was defined as follows: platelet count × neutrophil count / lymphocyte count.9 SII has been reported as a prognostic marker for several malignant diseases, and it can represent the balance of inflammation and immune response of hosts.10 We investigated the prognostic impact of SII in patients with mRCC treated with first-line TKI and assessed a modified IMDC risk model using SII.
Methods
Patients
From January 2008 to January 2018, 179 patients with pathologically diagnosed mRCC treated with TKI as first-line agents at our institute and affiliated hospitals in Hiroshima Prefecture in Japan were retrospectively investigated after approval by the Ethical Committee of Hiroshima University (allowance notification number E-45). Upon starting the prescription of first-line targeted agents, SII was calculated based on the data. The cutoff value of SII was determined to be 730, as reported in previous studies.10 Cases with pretreatment SII were 730 or higher, and the others were classified into a high-SII or low-SII group.
We compared the clinical and pathological data, including age, sex, histological finding, metastasis status, choice of drug, prior nephrectomy, Karnofsky performance status (KPS), anemia, serum calcium, neutrophil count, platelet count collected for all patients, and the distribution of these parameters in each group. The overall survival (OS) of each group classified according to these parameters was analyzed.
The modified IMDC model was determined using five poor prognostic factors, including KPS <80%, time from diagnosis to treatment <1 year, anemia, hypercalcemia, and SII >730. Cases were classified into four groups based on the presence of the number of these factors, 0, 1, 2–3, or 4–5.
Statistical analysis
The differences in the distribution of variables among groups were evaluated using a Chi-squared test for categorical variables and a Mann-Whitney test for continuous variables. Pearson correlation coefficients were assessed between SII and other inflammatory parameters. Tumor responses were determined using an investigator assessment according to the response evaluation criteria in solid tumor (RECIST), version 1.1. The OS was determined using the Kaplan-Meier method, and the differences between groups were analyzed using log-rank testing. Multivariate analyses of parameters associated with OS were evaluated using the Cox proportional hazards regression model. All statistical analyses were conducted using the JMP 10.0.0 (SAS Institute Inc., NC, U.S.), and p<0.05 was considered to be statistically significant.
Results
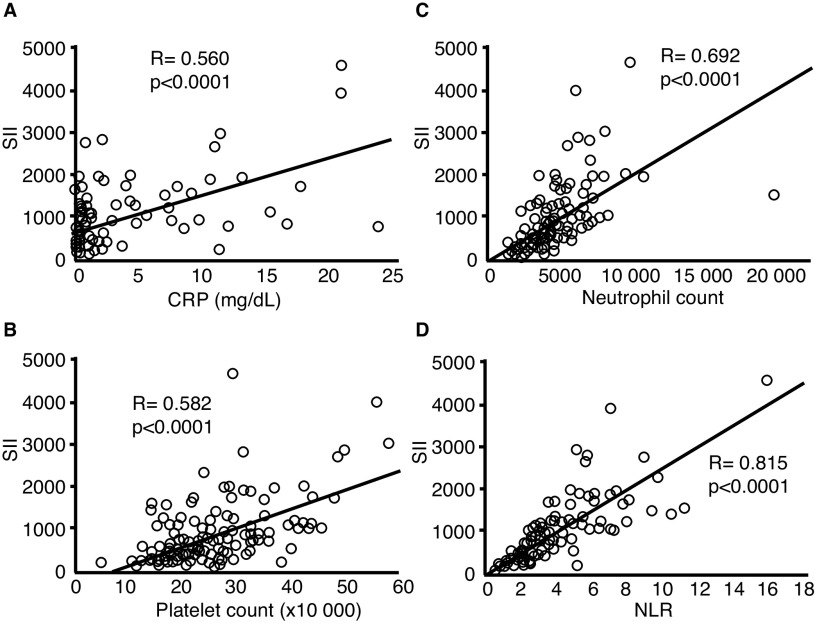
One hundred seventy-nine patients with mRCC treated with first-line TKI were investigated in this study. SII was 94.6–4603.1 (median 609.7), and positive correlations were found between SII and other inflammatory parameters, including C-reactive protein (CRP), neutrophil count, platelet count, and neutrophil-to-lymphocyte ratio (NLR) (Fig. 1). The characteristics of patients in this study cohort are listed in Table 1. The low-SII and high-SII groups consisted of 106 and 73 cases, respectively. The rate of cases with anemia, neutrophilia, thrombocytosis, hypercalcemia, time from diagnosis to treatment <1 year, and with IMDC poor-risk classification were higher in the high-SII group than in the low-SII group (Table 1).
Fig. 1.
Correlation between systemic immune inflammation index (SII) and other inflammatory parameters, including (A) C-reactive protein (CRP); (B) platelet count; (C) neutrophil count; and (D) neutrophil-to-lymphocyte ratio (NLR).
Table 1.
Characteristics of 179 patients with metastatic renal cell carcinoma who underwent targeted therapy
| High SII (≥730) | Low SII (<730) | p | ||
|---|---|---|---|---|
| No. of patients | (n=73) | (n=106) | (n=179) | |
| Age (median) | 64 (40–85) | 67 (40–85) | 0.1624 | 65 (40–85) |
| Sex (%) | ||||
| Male | 61 (83.6) | 84 (79.2) | 0.4694 | 145 (81.0) |
| Female | 12 (16.4) | 22 (20.8) | 34 (19.0) | |
| Histological type (%) | ||||
| Clear | 61 (83.6) | 96 (90.6) | 0.1607 | 157 (87.7) |
| Non-clear | 12 (16.4) | 10 (9.4) | 22 (12.3) | |
| KPS (%) | ||||
| ≥80% | 65 (89.0) | 101 (95.3) | 0.1138 | 166 (92.7) |
| <80% | 8 (11.0) | 5 (4.7) | 13 (7.3) | |
| Anemia (%) | ||||
| (−) | 45 (61.6) | 36 (34.0) | 0.0003 | 81 (45.3) |
| (+) | 28 (38.4) | 70 (66.0) | 98 (54.7) | |
| Neutrophilia (%) | ||||
| (−) | 50 (68.5) | 100 (94.3) | <0.0001 | 150 (83.8) |
| (+) | 23 (31.5) | 6 (5.7) | 29 (16.2) | |
| Thrombocytosis (%) | ||||
| (−) | 51 (69.9) | 104 (98.1) | <0.0001 | 155 (86.6) |
| (+) | 22 (30.1) | 2 (1.9) | 24 (13.4) | |
| Hypercalcemia (%) | ||||
| (−) | 66 (90.4) | 106 (100) | 0.0011 | 172 (96.1) |
| (+) | 7 (9.6) | 0 (0) | 7 (3.9) | |
| Metastatic organs (%) | ||||
| 1 | 6 (42.9) | 8 (30.8) | 0.4446 | 14 (35.0) |
| ≥2 | 8 (57.1) | 18 (69.2) | 26 (65.0) | |
| Time from diagnosis to treatment (%) | ||||
| ≥1 year | 20 (27.4) | 45 (42.5) | 0.0396 | 65 (36.3) |
| <1 year | 53 (72.6) | 61 (57.5) | 114 (63.7) | |
| IMDC risk | ||||
| Favorable | 8 (11.0) | 31 (29.3) | 0.0036 | 39 (21.8) |
| Intermediate | 31 (42.5) | 71 (67.0) | 102 (57.0) | |
| Poor | 34 (46.6) | 4 (3.8) | <0.0001 | 38 (21.2) |
| Metastatic site | ||||
| Lung | 48 (65.8) | 80 (75.4) | 128 (71.5) | |
| Lymph node | 25 (34.3) | 30 (28.3) | 55 (30.7) | |
| Liver | 9 (12.3) | 13 (12.3) | 22 (12.3) | |
| Bone | 20 (27.4) | 26 (24,5) | 46 (25.7) | |
| Pancreas | 2 (2.7) | 7 (6.6) | 9 (5.0) | |
| Adrenal gland | 7 (9.6) | 7 (6.6) | 14 (7.8) | |
| Contralateral kidney | 5 (6.8) | 10 (9.4) | 15 (8.4) | |
| Brain | 3 (4.1) | 4 (3.7) | 7 (3.9) | |
| Soft tissue | 4 (5.5) | 4 (3.7) | 8 (4.5) | |
| Prior nephrectomy, n (%) | ||||
| Radical | 28 (38.4) | 65 (61.3) | 0.0025 | 93 (52.0) |
| Cytoreductive | 33 (45.2) | 34 (32.1) | 67 (37.4) | |
| None | 12 (16.4) | 7 (6.6) | 0.0358 | 19 (10.6) |
| First-line agents, n (%) | ||||
| Sunitinib | 55 (75.3) | 59 (55.7) | 114 (63.7) | |
| Pazopanib | 5 (6.8) | 7 (6.6) | 12 (6.7) | |
| Sorafenib | 13 (17.8) | 38 (35.9) | 51 (28.5) | |
| Axitinib | 0 (0) | 2 (1.9) | 2 (1.1) |
KPS: Karnofsky performance status, IMDC: International Metastatic Renal Cell Carcinoma Database Consortium; SII: systemic immune inflammation index.
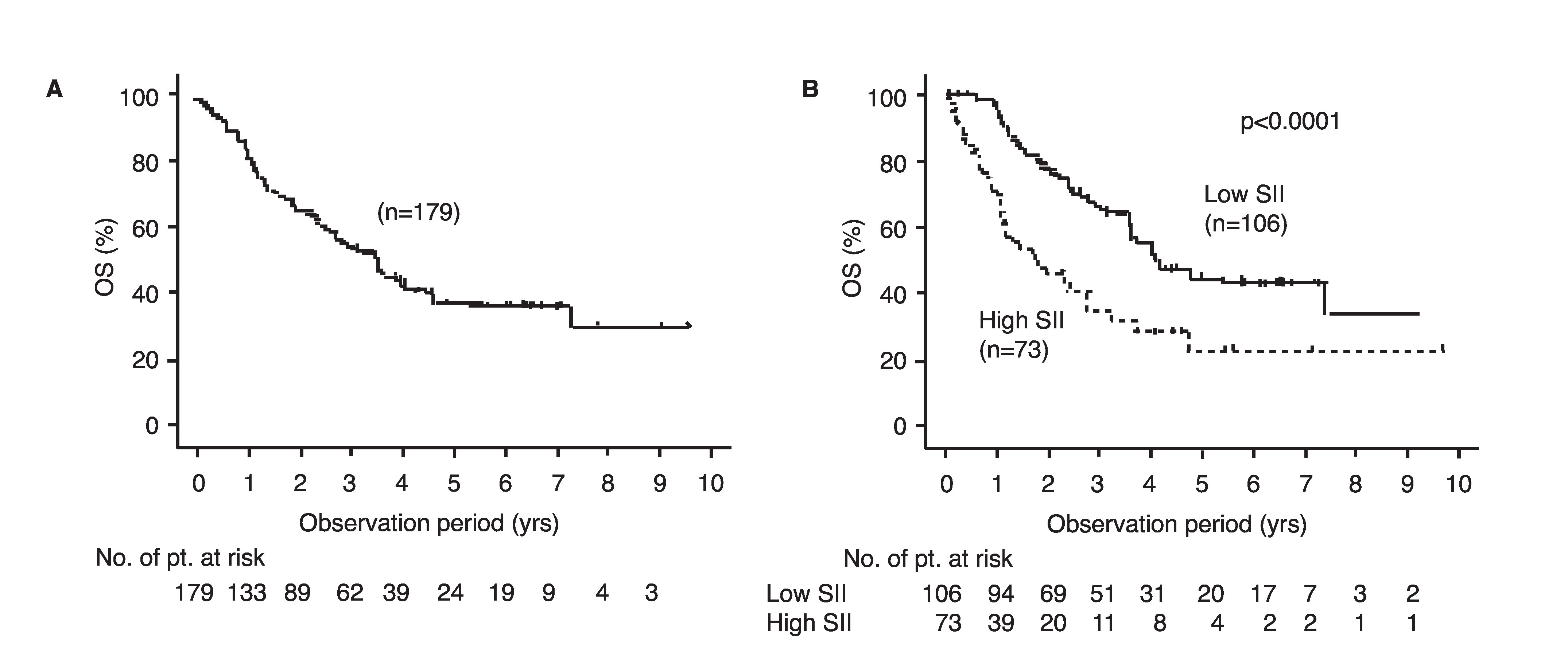
The median observation period was 24 months, and the 50% OS in the entire cohort was 43.3 months (Fig. 2A). Maximum effects of the first-line agents determined based on the RECIST criteria in each group are shown in Table 2. In the high-SII group, the rate of cases with complete response (CR) and partial response (PR) was significantly lower (p=0.0394), and that with progressive disease (PD) was significantly higher (p=0.0038) than in the low-SII group.
Fig. 2.
Overall survival (OS) (A) for all cases and (B) OS stratified based on pretreated systemic immune inflammation index (SII).
Table 2.
Best objective response of first-line agents in each group
| High SII | Low SII | Total | |
|---|---|---|---|
| No. of patients | (n=64) | (n=88) | (n=152) |
| CR/PR | 8 (12.5) | 23 (26.1) | 31 (20.4) |
| SD | 40 (62.5) | 58 (65.9) | 98 (64.5) |
| PD | 16 (25.0) | 7 (8.0) | 23 (15.1) |
CR: complete response; PD: progressive disease; PR: partial response; SD: stable disease; SII: systemic immune inflammation index.
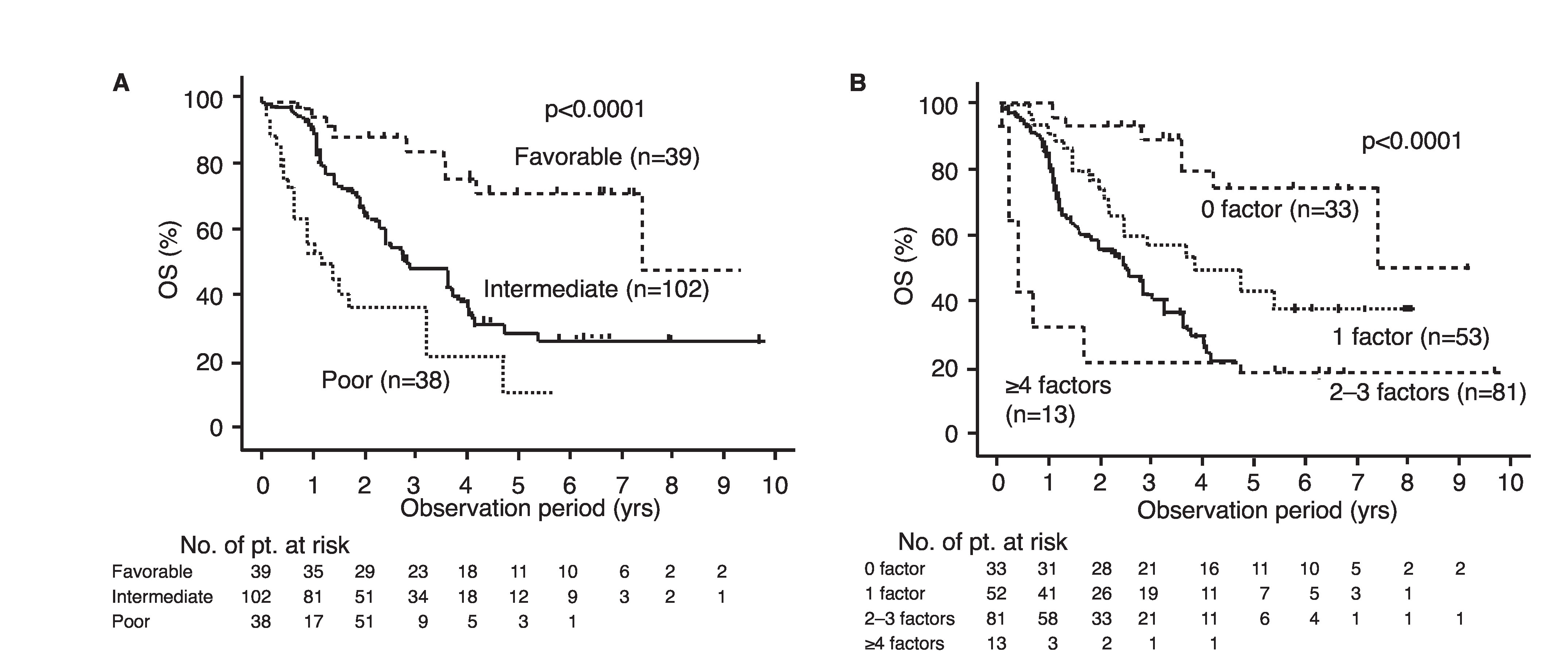
The 50% OS in the high-SII group was 21.4 months, which was significantly worse than that in the low-SII group (49.7 months, p<0.0001) (Fig. 2B). Multivariate analysis showed that a high SII, as well as non-clear histology, hypercalcemia, and time from diagnosis to treatment <1 year were independent predictive factors for worse OS (Table 3). Next, we constructed a modified IMDC risk model that included SII instead of neutrophil and platelet count. Using this modified IMDC model, all cases were re-classified into four groups of 33, 52, 81, and 13 cases with 50% OS of 88.8, 45.9, 29.4, and 4.8 months, respectively (p<0.0001); 102 cases (57% of total), were classified into the intermediate-risk group based on the conventional IMDC model (Fig. 3).
Table 3.
Multivariate analyses of association between various parameters and overall survival
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| HR | p | HR | 95% CI | p | ||
| Age | >65 | Reference | 0.0641 | |||
| ≤65 | 1.539 | |||||
| Sex | Male | Reference | 0.4369 | |||
| Female | 1.257 | |||||
| Pathology | Non-clear | Reference | 0.0035 | Reference | 0.0110 | |
| Clear | 0.432 | 0.477 | 0.269–0.844 | |||
| KPS | ≥80% | Reference | 0.2527 | |||
| <80% | 1.573 | |||||
| Anemia | (−) | Reference | 0.0004 | Reference | 0.0658 | |
| (+) | 2.201 | 1.557 | 0.971–2.495 | |||
| Hypercalcemia | (−) | Reference | <0.0001 | Reference | 0.0005 | |
| (+) | 10.315 | 5.233 | 2.074–13.203 | |||
| Time from diagnosis to treatment | <1 year | Reference | 0.0002 | Reference | 0.0072 | |
| ≥1 year | 0.407 | 0.479 | 0.280–0.819 | |||
| Liver metastasis | (+) | Reference | 0.2816 | |||
| (−) | 0.705 | |||||
| Bone metastasis | (+) | Reference | 0.3267 | |||
| (−) | 0.793 | |||||
| SII | ≤730 | Reference | <0.0001 | Reference | 0.0259 | |
| >730 | 2.424 | 1.721 | 1.067–2.774 | |||
CI: confidence interval; HR: hazard ratio; KPS: Karnofsky Performance Status; SII: systemic immune inflammation index.
Fig. 3.
Overall survival (OS) stratified based on (A) International Metastatic Renal Cell Carcinoma Database consortium (IMDC) risk model and (B) modified version of IMDC risk model consisting of five factors including systemic immune inflammation index (SII).
Discussion
In this study, we assessed the impact of SII for predicting the prognosis of cases with mRCC treated with TKI, and we showed the usefulness of SII to modify the IMDC risk model. To the best of our knowledge, this study is the first report on the impact of SII for establishing a modified prognostic model.
Several prognostic classifications are available for mRCC.11 Of these, the Memorial Sloan Kettering Cancer Center (MSKCC) and the IMDC risk models are two of the most widely used ones. The former was based on data from patients who were enrolled in clinical trials of cytokine therapy, and the latter was derived from patients treated with targeted agents. In both models, patients are stratified into three categories: favorable-, intermediate-, and poor-risk groups. However, because many patients with different prognoses are thought to be included in the same intermediate-risk group, improvement in risk stratification, especially in the intermediate-risk group, is required for both models.
Some investigators focused on the relationship between the number of positive risk factors. Tamada et al12 and Sella et al13 reported that mRCC patients treated with targeted agents in the intermediate-risk group of the MSKCC model could be divided into two groups with different prognoses. Others were re-stratified into intermediate-risk group patients based on the CRP level,14,15 which has been demonstrated as a predictive factor for prognosis and therapeutic efficacy in many reports.16,17 In addition to CRP, various reports have demonstrated the association of enhanced inflammatory response with the progression of RCC, including peripheral blood markers and indexes constructed from these components. Neutrophils can secrete various growth factors and cytokines, and they are associated with the stimulation of the tumor microenvironment.18 Lymphocytes can show an anti-tumoral role through the induction of cytotoxic cell death,19,20 and perioperative lymphopenia was reported to be associated with inferior prognosis in patients with mRCC.21 NLR is one of the most representative indexes that has been reported in terms of its prognostic impact on mRCC.18,22,23 Tanaka et al24 established a modified IMDC model using NLR. Platelets have been reported to be capable of inducing epithelial-to mesenchymal transition, promoting migration and metastasis, and protecting the autoimmune system from cancer cells.25 SII can represent these three peripheral blood parameters involving different molecular mechanisms of cancer cells. Also, previous studies have reported the prognostic impact of SII for mRCC treated with targeted agents and ICIs.26 As shown in Table 1, the rates of cases with parameters for poor prognosis were significantly higher than those in the high-SII group. Moreover, according to multivariate analysis, high SII was an independent predictive factor for OS (Table 3). These were consistent with the data from previous studies that have reported the association of elevated SII with poor prognosis of patients with malignant diseases, including mRCC.9,10
The simplicity of any risk model is important for use in the real world of clinical practice. Because SII is calculated using neutrophils and platelets, which are parameters in the IMDC model, and lymphocytes, which are measured alongside neutrophils, the modified risk model including SII can be used without any additional examination. Recent randomized controlled trials have demonstrated the effectiveness of combination regimens that consists of one TKI and one ICI (with the exception of one trial that included two ICIs4).27,28 While options for first-line therapy for mRCC have increased, it is still unclear how to decide on the best one. The IMDC model was established based on the data of patients who underwent target therapy, therefore, patients with higher risk in the IMDC model are thought to represent resistance to targeted agents. As such, re-stratification to more subgroups of this model can help guide therapeutic decisions.
Investigators have previously focused on many molecules as candidates for serum biomarkers, including growth factors29,30, microRNA,31 and cell-free DNA.32 However, clinically applying these molecules as biomarkers for mRCC is difficult. Alternatively, components of SII are routinely measured in real clinical practice during the management of patients with mRCC undergoing systemic therapy. Our data indicate the possibility of SII as a biomarker to enable us to improve the prognostic model for mRCC in the near future.
The limitation of the present report is that it is a relatively small, retrospective study. Because the data were derived from real-world clinical practice, some selection bias of patients or therapeutic options is inevitable. In addition, inflammatory factors do not represent the sensitivity of targeted agents but the status of progressiveness of mRCC; therefore, they can be affected by other therapeutic options.26,33,34 Further prospective study with higher patient volume is required to confirm the impact of the SII for predicting the prognosis of patients with mRCC.
Conclusions
We demonstrated the impact of SII for the prediction of the prognosis and modification of risk model in patients with mRCC treated with first-line TKI. SII is promising as a prognostic factor for mRCC patients, and this finding might lead to the establishment of novel therapeutic strategies and multiple options for mRCC.
Acknowledgement
In addition to the authors, the following investigators participated in the study: Shuntaro Koda, Kure Medical Center; Hiroshi Masumoto, Fukuyama Medical Center; Shinya Ohara and Kosuke Sadahide, Hiroshima Prefectural Hospital; Koichi Shoji, Mazda Hospital; Hideo Iwamoto, Higashi-Hiroshima Medical Center; Hideki Mochizuki and Daiki Murata, Hiroshima City Asa Citizens Hospital; and Yuichi Kadonishi, Onomichi General Hospital.
Footnotes
Competing interests: The authors report no competing personal or financial interests related to this work.
Funding: This study was supported by Pfizer Inc. and Novartis Pharma Inc.
This paper has been peer-reviewed
References
- 1.Vera-Badillo FE, Templeton AJ, Duran I, et al. Systemic therapy for non-clear cell renal cell carcinomas: A systematic review and meta-analysis. Eur Urol. 2015;67:740–9. doi: 10.1016/j.eururo.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 2.Pal SK, Nelson RA, Vogelzang N. Disease-specific survival in de novo metastatic renal cell carcinoma in the cytokine and targeted therapy era. PLoS One. 2013;8:e63341. doi: 10.1371/journal.pone.0063341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab vs. everolimus in advanced renal cell carcinoma. N Engl J Med. 2015;373:1803–13. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab vs. sunitinib in advanced renal cell carcinoma. N Engl J Med. 2018;378:1277–90. doi: 10.1056/NEJMoa1712126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.NCCN. [Accessed June 5, 2020]. Available at: https://www.nccn.org.
- 6.Ljungberg B, Bensalah K, Canfield S, et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol. 2015;67:913–24. doi: 10.1016/j.eururo.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Heng DY, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: Results from a large, multicenter study. J Clin Oncol. 2009;27:5794–9. doi: 10.1200/JCO.2008.21.4809. [DOI] [PubMed] [Google Scholar]
- 8.Teishima J, Inoue S, Hayashi T, et al. Current status of prognostic factors in patients with metastatic renal cell carcinoma. Int J Urol. 2019;26:608–17. doi: 10.1111/iju.13956. [DOI] [PubMed] [Google Scholar]
- 9.Zhong JH, Huang DH, Chen ZY. Prognostic role of systemic immune-inflammation index in solid tumors: A systematic review and meta-analysis. Oncotarget. 2017;8:75381–8. doi: 10.18632/oncotarget.18856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lolli C, Basso U, Derosa L, et al. Systemic immune-inflammation index predicts the clinical outcome in patients with metastatic renal cell cancer treated with sunitinib. Oncotarget. 2016;7:54564–71. doi: 10.18632/oncotarget.10515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Motzer RJ, Bacik J, Murphy BA, et al. Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol. 2002;20:289–96. doi: 10.1200/JCO.2002.20.1.289. [DOI] [PubMed] [Google Scholar]
- 12.Tamada S, Iguchi T, Yasuda S, et al. The difference in the survival rate of patients with metastatic renal cell carcinoma in the intermediate-risk group of the Memorial Sloan Kettering Cancer Center criteria. Oncotarget. 2018;9:27752–9. doi: 10.18632/oncotarget.25554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sella A, Michaelson MD, Matczak E, et al. Heterogeneity of patients with intermediate-prognosis metastatic renal cell carcinoma treated with sunitinib. Clin Genitourin Cancer. 2017;15:291–9.e1. doi: 10.1016/j.clgc.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 14.Teishima J, Kobatake K, Hayashi T, et al. Prognostic significance of C-reactive protein in patients with intermediate-risk metastatic renal cell carcinoma treated with molecular targeted therapy. Oncol Lett. 2014;8:881–5. doi: 10.3892/ol.2014.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takamatsu K, Mizuno R, Omura M, et al. Prognostic value of baseline serum C-reactive protein level in intermediate-risk group patients with metastatic renal cell carcinoma treated by first-line vascular endothelial growth factor-targeted therapy. Clin Genitourin Cancer. 2018;16:e927–e33. doi: 10.1016/j.clgc.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 16.Teishima J, Kobatake K, Kitano H, et al. The impact of change in serum C-reactive protein level on the prediction of effects of molecular targeted therapy in patients with metastatic renal cell carcinoma. BJU Int. 2016;117:E67–74. doi: 10.1111/bju.13260. [DOI] [PubMed] [Google Scholar]
- 17.Teishima J, Kobatake K, Shinmei S, et al. The effect of kinetics of C-reactive protein in the prediction of overall survival in patients with metastatic renal cell carcinoma treated with tyrosine kinase inhibitor. Urol Oncol. 2017;35:662.e1–e7. doi: 10.1016/j.urolonc.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 18.Templeton AJ, McNamara MG, Šeruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: A systematic review and meta-analysis. J Natl Cancer Inst. 2014;106 doi: 10.1093/jnci/dju124. [DOI] [PubMed] [Google Scholar]
- 19.De Giorgi U, Mego M, Scarpi E, et al. Relationship between lymphocytopenia and circulating tumor cells as prognostic factors for overall survival in metastatic breast cancer. Clin Breast Cancer. 2012;12:264–9. doi: 10.1016/j.clbc.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 20.Minami T, Minami T, Shimizu N, et al. Identification of programmed death ligand 1-derived peptides capable of inducing cancer-reactive cytotoxic T lymphocytes from HLA-A24+ patients with renal cell carcinoma. J Immunother. 2015;38:285–91. doi: 10.1097/CJI.0000000000000090. [DOI] [PubMed] [Google Scholar]
- 21.Saroha S, Uzzo RG, Plimack ER, et al. Lymphopenia is an independent predictor of inferior outcome in clear-cell renal carcinoma. J Urol. 2013;189:454–61. doi: 10.1016/j.juro.2012.09.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Templeton AJ, Knox JJ, Lin X, et al. Change in neutrophil-to-lymphocyte ratio in response to targeted therapy for metastatic renal cell carcinoma as a prognosticator and biomarker of efficacy. Eur Urol. 2016;70:358–64. doi: 10.1016/j.eururo.2016.02.033. [DOI] [PubMed] [Google Scholar]
- 23.Teishima J, Ohara S, Sadahide K, et al. Impact of neutrophil-to-lymphocyte ratio on effects of targeted therapy for metastatic renal cell carcinoma patients with extrapulmonary metastasis. Can Urol Assoc J. 2017;11:E207–14. doi: 10.5489/cuaj.4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanaka N, Mizuno R, Yasumizu Y, et al. Prognostic value of neutrophil-to-lymphocyte ratio in patients with metastatic renal cell carcinoma treated with first-line and subsequent second-line targeted therapy: A proposal of the modified-IMDC risk model. Urol Oncol. 2017;35:39.e19–e28. doi: 10.1016/j.urolonc.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 25.Schumacher D, Strilic B, Sivaraj KK, et al. Platelet-derived nucleotides promote tumor-cell transendothelial migration and metastasis via P2Y2 receptor. Cancer Cell. 2013;24:130–7. doi: 10.1016/j.ccr.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 26.De Giorgi U, Procopio G, Giannarelli D, et al. Association of systemic inflammation index and body mass index with survival in patients with renal cell cancer treated with nivolumab. Clin Cancer Res. 2019;25:3839–46. doi: 10.1158/1078-0432.CCR-18-3661. [DOI] [PubMed] [Google Scholar]
- 27.Motzer RJ, Penkov K, Haanen J, et al. Avelumab plus axitinib vs. sunitinib for advanced renal cell carcinoma. N Engl J Med. 2019;380:1103–15. doi: 10.1056/NEJMoa1816047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus axitinib vs. sunitinib for advanced renal cell carcinoma. N Engl J Med. 2019;380:1116–27. doi: 10.1056/NEJMoa1816714. [DOI] [PubMed] [Google Scholar]
- 29.Hegde PS, Jubb AM, Chen D, et al. Predictive impact of circulating vascular endothelial growth factor in four phase 3 trials evaluating bevacizumab. Clin Cancer Res. 2013;19:929–37. doi: 10.1158/1078-0432.CCR-12-2535. [DOI] [PubMed] [Google Scholar]
- 30.Peña C, Lathia C, Shan M, et al. Biomarkers predicting outcome in patients with advanced renal cell carcinoma: Results from sorafenib phase 3 Treatment Approaches in Renal Cancer Global Evaluation Trial. Clin Cancer Res. 2010;16:4853–63. doi: 10.1158/1078-0432.CCR-09-3343. [DOI] [PubMed] [Google Scholar]
- 31.Gu L, Li H, Cken K, et al. Micro RNAs as prognostic molecular signatures in renal cell carcinoma: A systematic review and meta-analysis. Oncotarget. 2015;6:32545–60. doi: 10.18632/oncotarget.5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Martino M, Klatte T, Haitel A, et al. Serum cell-free DNA in renal cell carcinoma: A diagnostic and prognostic marker. Cancer. 2012;118:82–90. doi: 10.1002/cncr.26254. [DOI] [PubMed] [Google Scholar]
- 33.Teishima J, Ohara S, Shinmei S, et al. Normalization of C-reactive protein levels following cytoreductive nephrectomy in patients with metastatic renal cell carcinoma treated with tyrosine kinase inhibitors is associated with improved overall survival. Urol Oncol. 2018;36:339.e9–e15. doi: 10.1016/j.urolonc.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 34.Teishima J, Murata D, Hasegawa Y, et al. C-reactive protein can be used to predict the therapeutic effects of nivolumab in patients with metastatic renal cell carcinoma. Int J Urol. 2019;26:1076–7. doi: 10.1111/iju.14084. [DOI] [PubMed] [Google Scholar]