Abstract
COVID-19 is an evolving pandemic that has far reaching global effects, with a combination of factors that makes the virus difficult to contain. The symptoms of infection can be devastating or at the least very debilitating for vulnerable individuals. It is clear that the elderly are at most risk of the adverse impacts of the virus, including hospitalization and death. Others at risk are those with comorbidities such as cardiovascular disease and metabolic conditions and those with a hyper-excitable immune response. Treatment options for those with acute responses to the virus are limited and there is an urgent need for potential strategies that can mitigate these severe effects. One potential avenue for treatment that has not been explored is the microbiome gut/lung axis. In addition to those severely affected by their acute reaction to the virus, there is also a need for treatment options for those that are slow to recover from the effects of the infection and also those who have been adversely affected by the measures put in place to arrest the spread of the virus. One potential treatment option is photobiomodulation (PBM) therapy. PBM has been shown over many years to be a safe, effective, non-invasive and easily deployed adjunctive treatment option for inflammatory conditions, pain, tissue healing and cellular energy. We have also recently demonstrated the effectiveness of PBM to alter the gut microbiome. PBM therapy is worthy of consideration as a potential treatment for those most vulnerable to COVID-19, such as the elderly and those with comorbidities. The treatment may potentially be advantageous for those infected with the virus, those who have a slow recovery from the effects of the virus and those who have been denied their normal exercise/rehabilitation programs due to the isolation restrictions that have been imposed to control the COVID-19 pandemic.
Keywords: COVID-19, photobiomodulation, immunomodulation, mitochondrial dysfunction, microbiome
First reported in December 2019 (www.who.int/csr/don/05-january-2020-pneumonia-of-unkown-cause-china/en/), more than 21 million people have now been infected by the new Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). The infection per se, and its therapy, have claimed over half-a million deaths worldwide, which continues to rise daily www.worldometers.info/coronavirus/.
There are dozens if not hundreds of different types of coronaviruses, four of which have been shown to cause mild and mainly upper respiratory infections (the so called common cold); 2 strains cause severe and frequently lethal outbreaks of respiratory infections, one in 2002 referred to as SARs (severe acute respiratory syndrome), the other, MERs (Middle East Respiratory Syndrome) occurred in 2012. The third and latest coronavirus pandemic, raging since late 2019 is caused by yet another strain. The virus particle is around 100 nanometres in diameter and can be seen only through an electron microscope. The aerosol or droplet transmission of the virus to humans gains entry through mucosal surfaces of the mouth, nose and eyes [1]. Viral entry is mediated through the angiotensin converting enzyme 2 (ACE2) receptor, a membrane-associated enzyme expressed in vascular endothelia, renal and cardiovascular tissue as well as epithelia of the small intestine. The relationship between ACE2 expression and infection has not been determined [2]. The treatment of cardiovascular disease (CVD) with angiotensin-converting enzyme inhibitors and angiotensin receptor blockers have the additional effect to upregulate ACE2 expression, which may play a significant role in individual viral responses.
The median incubation period before symptoms might become evident is 4.9 - 5.8 days, with a range of 1 - 14 days [3]. Once infected and thus contagious, the relatively long incubation period in humans facilitates the spread of the infection [3]. Beyond the incubation period, symptoms of the infected vary widely, from none or minimal symptoms, to florid and rapidly progressive respiratory distress. The elderly are the most predisposed to the deleterious sequalae of COVID-19 [4-6], most probably due to an aging immune system, increased manifestations of inflammatory conditions and accumulated mitochondrial dysfunction [7]. In the USA, 77% of all deaths are in the over 65 years age group [5]. Those with chronic comorbidities that impact their immune system are the second group at risk, including CVD [8], type II diabetes (T2D) [9, 10], chronic respiratory disease [10], hypertension [10], cancer [11], metabolic syndrome [12] and obesity [13, 14]. It has been reported that less than 1% of all deaths from COVID-19 do not have a comorbidity [15]. A third susceptible group are those with a hyper-excitable neuro-immune axis, which affects the nervous system as well as endothelial and vascular responses leading to an over-intense immune response. These people are often younger and can have hyper-excitable physiological responses to environmental stressors. Risk factors for this response include genotypes for ion channelopathies such as migraine, erythromyalgia and epilepsy, resulting in these endothelial susceptibilities [16]. This group can be of higher intelligence [17] and are possibly over-represented in health care workers. Health care workers possibly have a higher morbidity and mortality compared to the general population [18], which may be influenced in some way by this immune hyper-excitability.
Those susceptible to the virus may succumb to dysregulated immunity, recalcitrant deoxygenation and respiratory distress, multi-organ failures and debility resulting from prolonged a catabolic state. Importantly, they also often suffer adverse reactions (ADRs) to therapeutic interventions including the established and potential cardiovascular ADRs related to the antibiotics and high dose corticosteroids, anti-viral and immune modulating drugs currently used in the management of COVID-19 infections [19, 20]. Other features of infection include cardiovascular injury [2], atrial fibrillation [21], central and peripheral nervous system symptoms [22], which affect over a third of the infected patients [23], mouth ulcers and hyposmia/anosmia (loss of smell). Rapid and fulminant progression to pneumonia can occur in the elderly and people with comorbidities. This is thought to occur as a result of an initial poor immune response followed by an inappropriate hyper-immune reaction or “cytokine storm”. The resulting unrelenting inflammation affects vital organs including pulmonary tissue and vascular structures [6]. This over-responsive immune response may be associated with neutrophil recruitment and activity [24]. The subsequent cellular damage and multi-organ failures heighten mortality risk more so than the infection itself and is particularly prevalent among the aged and those with comorbidities. Immunosuppressive therapies including corticosteroids https://clinicaltrials.gov/ct2/show/NCT04355247 to prevent or placate the cytokine storm [25, 26] in COVID-19 have so far only met with modest success. More targeted therapies are urgently needed.
Recovery from COVID-19 is frequently protracted and the long-term prognosis remains to be fully realized. An unknown percentage will have ongoing symptoms after discharge from hospital and/or recovery from overt respiratory and other life-threatening symptoms. It has been reported www.theguardian.com/australia-news/2020/jul/17/most-covid-19-patients-admitted-to-a-sydney-hospital-in-march-still-have-symptoms that at 3- or 4-months post hospital discharge, up to 80% of recovered COVID-19 patients continued to be symptomatic. Nonspecific aches and pain, dyspnoea, palpitations, joint and chest pain, dizziness or light-headedness, headaches, fatigability, hyposmia and anosmia are not infrequent [27]. More serious ongoing symptoms can include pulmonary hypertension and interstitial fibrosis, pericardial effusion and myocarditis, neurologic and neuropsychiatric sequalae including dysautonomia [28], myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) [29], depression [30] and autoimmune disease [31].
A less recognised consequence of the COVID-19 pandemic is the unintended effect that the measures taken to contain the pandemic have had on vulnerable individuals. Many of these individuals have been unable to socialise or to attend exercise and rehabilitation classes due to the lockdown period and the social isolation necessary to combat spread of the virus. The stress of this can have the effect of reducing the resilience and immunity of the very individuals (the elderly and those with comorbidities) that the measures are designed to protect [32], thus increasing the chances of an unfavourable outcome if infected.
A magic bullet solution for the current COVID-19 pandemic is unlikely in the near future, although much research is directed towards an effective vaccine. A wide range of social and lifestyle measures to reduce cross infection have been met with success in some but not other regions or countries [33]. Development and discovery of novel and effective pharmacotherapy, vaccines for disease prevention and repurposing existing drugs to fight the COVID-19 are being robustly pursued [34] but there is also a need for broad scale clinical trials of potential strategies, including interdisciplinary collaborations, aimed at mitigating the severe effects and side-effects of the COVID-19 pandemic [5].
The link to the gastrointestinal microbiome
There is a strong link between the gut microbiome and susceptibility to disease and most likely to COVID-19 [35]. The gut microbiota is well known to affect immunity [36, 37], interacting with the gut mucosa and stimulating the production of both pro and anti-inflammatory cytokines. Low inflammatory conditions support a healthy gut microbiota, which in turn contributes to maintaining non-inflammatory conditions. A cascade into dysbiosis leads to a disruption of the mucosal barrier, allowing microbial products of the dysregulated microbial population to leak into surrounding tissues and increase the inflammatory response, which further increases dysbiosis. This contributes to generally reduced immunity and to the comorbidities known to contribute to COVID-19 susceptibility (such as obesity, T2D, heart disease). Elderly individuals frequently suffer a decrease in microbial diversity in the gut, contributing to dysbiosis. In addition, there is a gut/lung axis that links the microbiome of the gut with lung health [38], with gut metabolites transferred to the lung [37] and the gut bacteria playing an important role in the response to acute respiratory distress syndrome (ARDS) [35]. Early results of the microbiome analysis of a small number of hospitalized COVID-19 patients indicated that the microbiome was adversely impacted, with depletion of bacteria representative of a healthy microbiome and enrichment of opportunistic pathogens [39]. These changes were correlated with disease severity and persisted throughout the hospitalization period.
Changing the composition of the gut microbiota with diet and supplements can improve immunity generally [40], while altering the microbiome (with soluble fibre) has been shown to reduce the severity of allergic lung inflammation [41]. Modulation of the gut microbiome (with diet, soluble fibre, probiotics) has been suggested as a potential way to assist viral respiratory infections generally [42] and SARS-Cov-2 in particular [35]. There has been speculation that the gut microbiota can have an influence on ACE2 receptors and cardiovascular health and are therefore a potential target for cardiopulmonary therapy [43]. Additionally, the ACE2 receptors are also expressed in the gut enterocytes [35]. The expression of ACE2 (in a mouse model) can be regulated by certain species of the gut microbiome (Bacteroides species), part of the population depleted during COVID-19 hospitalization [39]. The implications of this has yet to be investigated.
The oral microbiome is also an important component of immunity. A healthy microbial population can be disturbed by a dysregulated immune system, an inflammatory response or poor oral hygiene [44]. A disturbed microbiota is implicated in a number of diseases, such as periodontitis, CVD and Alzheimer’s disease [45] as well as being less able to prevent viral infection [46]. Interestingly, periodontal disease has a strong association with obesity, CVD, T2D as well as aging, the same comorbidities associated with a poor prognosis with COVID-19 and the potential of oral microbiome dysbiosis and susceptibility to COVID-19 has been raised [47, 48]. Improvement in oral hygiene is suggested as a way to maintain a healthy oral microbiome, which may be somewhat protective against viral infection [49].
Photobiomodulation (PBM) therapy in clinical medicine
Throughout the ages, light, including sunlight has been known for its wide-ranging health effects for myriad maladies. In 1903 Dr. Niels Ryberg Finsen, a Danish physician was awarded the Nobel Prize in Physiology or Medicine for his work in treating tuberculosis with ultraviolet or blue light and smallpox with red light [50]. The contemporary clinical practice of PBM therapy, often also referred to as low-level laser therapy, is the result of on-going evolution since its first application over half-a century ago, when the work of Dr. Endre Mester and colleagues at the Semmelweis Medical University in Hungary demonstrated its therapeutic benefits for wound healing [51].
PBM is the use of narrow wavelength bands of light (either LED or laser) to modulate cellular responses with no thermal effect. Putatively, PBM carries no risk to health [52-54], its safety profile equating that of ultrasound tests. Unlike much pharmaceutical therapy, PBM is free of serious deleterious side-effects and is, by its nature, non-invasive. PBM therapy is mostly delivered for no more than 10-20 minutes through portable, handheld or wearable devices and is safely repeatable. Measurable symptomatic and clinical benefits can result from a single treatment but PBM therapy is usually provided as a course of several treatment sessions.
The main target of PBM is considered to be the electron transport chain of the mitochondria, in particular complex IV, cytochrome-C-oxidase, which acts as a chromophore, absorbing red and near infrared light [55]. The effect of this absorption is thought to be the release of reactive oxygen species (ROS) from the complex, allowing increased membrane potential, increased ATP production and downstream cellular signalling via ATP, cAMP, ROS, Ca2+ and nitric oxide (NO) to influence gene transcription [55]. There are also many other chromophores capable of absorbing light with resulting physiological effects, such as opsins and light-activated ion channels. The most effective wavelength for delivery of PBM in immune modulation is likely to be in the red and near-infrared range, based on the cytochrome-C-oxidase and porphyrin absorption peaks being centred at 640 nm and HbO2 at 900nm [55]. The energy required for effective PBM is low, in the range of 1 to 16 joules/cm2. The PBM dose is biphasic, meaning that above a certain threshold (outside of the dose window) increasing the energy will not increase the therapeutic effect [56].
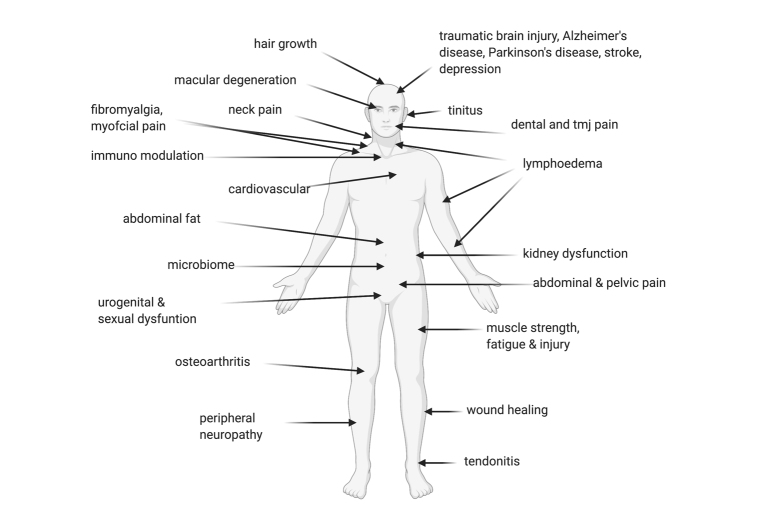
PBM has a multitude of effects on the body, in many organ systems and is able to treat various disorders (Figure 1), through its action at the cellular and mitochondrial level [55]. In experimental models, the degree and type of immune responses to PBM are influenced by the anatomic surface where treatment is applied. For example, immunomodulatory effects appeared to be more effective when applied on the thymus area compared to limbs, with a favourable rise in interleukin (IL)-2, NO and heat shock protein 70 production. Treatment dose, cumulative dose and duration of exposure also appear to pay a role where unduly prolonged treatment duration may even cause attenuation and reversal of treatment efficacy toward immunosuppression [57].
Figure 1.
Conditions that have been shown to be successfully treated using photobiomodulation therapy.
The literature is replete with experimental and clinical trials demonstrating therapeutic efficacy of PBM in a multitude of disease process, including inflammation. PBM therapy has recently been recommended as standard care for the treatment of oral mucositis following chemo or radiation therapy in the MSCC/ISOO guidelines www.mascc.org/mucositis-guidelines.
Photobiomodulation therapy and general health
PBM has been shown to improve the general health and resilience of cells and tissues. The effect of PBM to improve mitochondrial metabolism and ATP generation leads to increased muscle strength and performance in sports and athletics [58, 59] and to reduced muscle wasting and degeneration in animal and cell culture models [60-62]. This makes it a candidate for treatment of COVID-19 cases under respiratory distress. PBM has been demonstrated to be more effective in damaged or diseased cells, tissues and individuals [55]. PBM has also been shown to have an effect in chronic obstructive airway disease when the muscles of the chest wall are treated [63]. Efficient mitochondria are also important in overcoming disease and in the recovery process. Often, mitochondrial dysfunction increases with age and may not be sufficient to enable recovery after infective disease and other immune insults [64]. PBM is known to enhance mitochondrial function, but the positive effect of PBM on the aging process in animal models has yet to be demonstrated in humans [65]. The action of PBM on the mitochondria also has the effect of activating transcription factors, which can lead to increased expression of genes involved in inflammatory signalling [66].
PBM is effective at reducing myocardial infarct size and reducing inflammation in animal models [67], has been suggested as therapy in human CVD [68] and has been shown to modulate the expression of ACE2 [67]. PBM has also been shown to improve blood flow and oxygenation [69, 70], both peripherally and in the CNS most probably due to the release of NO, an important vasodilator. PBM therapy has been used to treat post-viral and chronic fatigue, as well as fibromyalgia and other instances of centrally mediated pain [71].
Evidence and potential mechanisms of PBM in immunomodulation
PBM appears to exert pluripotent effects in the modulation of inflammation and immunity [72]. Many studies have demonstrated that PBM modulates inflammation by reducing the pro-inflammatory cytokines (such as IL-1β, IL-6, IL-8, TNF-α) and other inflammatory markers released from activated inflammatory cells, while increasing the anti-inflammatory cytokines (IL-10) [72]. The immuno-modulatory effect of PBM on cytokines regulation and the complement cascade occurs via the POMC pathway, involving regulation of the hypothalamic pituitary axis through the direct modulation of the POMC/melanocortin signalling pathway including a-MSH, a potent anti-inflammatory molecule. The POMC pathway is regulated by PBM [73], which in turn modulates both ACTH and β-opioid, as well as, interestingly, ACE activity [74].
One of the central effects of PBM on the immune response is via the modulation of neutrophil function [75] by balancing neutrophil numbers, improving neutrophil efficiency and modulating the neutrophil extracellular trap formation [76]. Reducing over-accumulation of neutrophils is a major mechanism for the effect of PBM in reducing acute lung inflammation [77]. This is crucial in preventing the cytokine storm cascade in autoimmune diseases. PBM also modulates the ratio of M1 and M2 macrophage phenotypes, reducing pro-inflammatory cytokines and chemokines and increasing anti-inflammatory cytokines and thus balance the inflammatory process [78].
These inflammatory changes facilitated by PBM have profound effects on many body processes. For example, PBM therapy has been shown to modulate peripheral blood mononuclear cells and CD4+ cells to reduce inflammatory effects in multiple sclerosis patients and healthy adults by increasing IL-10 and reducing IFN-γ [79, 80]. PBM reduces the number of inflammatory cells, pro-inflammatory cytokines as well as fibrotic tissue in a mouse model of lung fibrosis [81]. Acute lung inflammation in rats is reduced with PBM to reduce oedema, neutrophil influx and TNF-α, while reducing IL-10 in rats [82].
In an experimental model of induced acute peritonitis in rats, Yu and co-workers [83] showed PBM resulted in lymphocyte proliferation and enhanced lymphocyte ATP synthesis compared to controls, and the 60-day survival rate of the PBM group was double that of the control group (p<0.001). Assis et al [84] further demonstrated the immune modulation capability of PBM, with septic rats treated with PBM exhibiting lower IL-6 activity and decreased atrogin-1 and MuRF-1 immuno-expression (markers of sepsis related muscle catabolic states).
PBM causes mitogenic stimulation responsive lymphocyte proliferation and enhanced lymphocyte ATP synthesis [83]. A plausible mechanism for PBM induced lymphocytic proliferation is through the reaction of light with haemoglobin, resulting in oxygen radical production [85]. Indeed, in immunological cells, PBM induces production of reactive oxygen species, NO or interleukins most often, leading to an anti-inflammatory effect [85]. It is well documented that various immune response processes are highly dependent on cellular energy, the latter being depressed in sepsis and septic shock cases [86, 87]. The mitochondria probably act as photo-acceptors for PBM and robustly reactivate cellular energy synthesis to re-establish ATP levels in a variety of cells including lymphocytes and macrophages, and through several pathways that trigger activation of nucleic acid synthesis and cellular proliferation [88, 89].
PBM in airway inflammation, gut microbiome and dysautonomia
PBM has been shown to be effective in controlling neutrophil activation, thus restoring the balance between pro and antioxidant mediators by reducing pro-inflammatory cytokines (IL-6, TNF-α) and increasing anti-inflammatory cytokines (IL-10) in a mouse model of acute lung injury induced by gut ischemia and reperfusion [82, 90]. This has also been shown in mouse models of pulmonary fibrosis [91] and chronic obstructive airway disease induced by tobacco smoke [92]. The infiltration of neutrophils into the lungs, which contributes to inflammation, is also reduced by PBM [78].
The efficacy of PBM therapy to treat pneumonia has been reported in 48 infants treated with conventional therapy who also received laser therapy with “Vostok” laser therapeutic devices for 2-3 days, compared to 45 infants receiving conventional therapy alone and another 18 healthy newborns as controls. In a trial of using red light therapy to treat retinopathy of prematurity [93], one notable side-effect was the survival of all 21 premature infants in the treatment group, while 4 infants in the non-treatment group died from lung complications (pers. com. Prof Krisztina Valter). It has also been reported that ARDS can be successfully treated with PBM therapy [94].
We have previously shown [95] that PBM can alter the gut microbiome in a favourable way in a mouse model. We have also demonstrated favourable changes in the gut microbiome in a number of human trials (manuscript in preparation) and are currently investigating the potential of PBM to alter the oral microbiome. One potential mechanism for the effect on gut microbiota is the reduction of inflammation in the adipose tissue of the abdomen afforded by PBM. Improving the gut microbiome from a dysbiotic state, whether by diet, prebiotics, exercise or PBM, will reduce inflammatory processes, improve general health and protect against future immunological insults [96], including, perhaps, a future cytokine storm.
Recently there has been much interest in the use of transcranial PBM to address many symptoms of neurological and neuropsychiatric disorders [97]. Transcranial devices have been shown to modulate neural oscillations [70, 98], improve cognition in healthy adults, improve cognitive performance of people with TBIs [99] and improve symptoms of depression [100]. We have demonstrated a positive effect of PBM therapy in improvement of cognition scores in individuals with Parkinson’s disease (manuscript in preparation).
The potential of PBM for COVID-19
A number of recent publications have suggested that PBM therapy may be of benefit in the treatment and/or recovery of COVID-19 [101, 102] by targeting the blood, either directly or trans-dermally [102, 103] and/or targeting the lungs [104]. At least one trial of PBM therapy to the respiratory muscles is underway https://clinicaltrials.gov/ct2/show/NCT04386694 and PBM therapy is being trialled as a therapy for COVID-19 in Russia www.lazmik.ru/assets/templates/docs/Coronaviridae_SARS_COVID-19_LLLT_protocol_eng1.pdf. There has been one recent case report of the effectiveness of PBM therapy to treat a patient with severe COVID-19 pneumonia [105].
There are a number of areas in the COVID-19 crisis that may benefit from PBM therapy, especially among the elderly and other individuals with comorbidities or conditions that make them especially vulnerable to the virus:
-
1.
Individuals infected with the virus and who are admitted to intensive care units may benefit from PBM therapy to the chest to help improve airways, improve blood oxygenation and increase muscle performance to assist with breathing. PBM may also help to balance the immune system and reduce immune hyperactivity to resist progression to a cytokine storm. The same mechanisms may help vulnerable individuals infected with the virus to avoid the worsening of symptoms that would otherwise lead to admission to hospital.
-
2.
The main clinical benefit of PBM therapy in COVID-19, however, appears to be for patients who continue to be chronically symptomatic in convalescence, including the elderly, those with multiple comorbidities and the hyper immune-excitable. These groups are particularly susceptible to serious infection with protracted recovery. PBM therapy is likely to improve cellular energy and general health status, lung immune function, gut microbiome/immune status, brain function and reduce muscle fatigue. We have also shown that PBM readily reverses anosmia in participants with Parkinson’s disease (manuscript in preparation). In the short term PBM therapy could improve recovery from COVID-19 and reduce the risk of post-infection sequalae. In the longer term PBM therapy could improve the comorbidities that increase vulnerability to viral infection in these populations. It would also be important to identify those younger hyper-excitable individuals who are at greater risk of an over-reaction to the viral infection.
-
3.
Individuals who have been adversely affected by the lockdown and social isolation strategies in that they have been unable to regularly exercise and/or attend their normal rehabilitation session are likely to also benefit greatly from PBM therapy in the same ways as detailed above.
-
4.
An additional benefit of PBM is as an adjuvant to vaccination. The elderly and those with comorbidity are most prone to be non- or under-responders to vaccines [106]. Kashiwagi et al [107] demonstrated that near infrared laser acts as an adjuvant to vaccination and significantly increases immune responses to intradermal influenza vaccination without augmenting Immunoglobulin-E. This conferred increased protection compared to an unadjuvanted vaccine control in a mouse influenza lethal challenge model. Thus, it is an exciting hypothesis for PBM to act as a non-invasive, risk-free and easily deployed adjuvant therapy, especially for at-risk populations.
Conclusions
COVID-19 is not only a major challenge in people with comorbidities that affect their immune and inflammatory status, but is also particularly aggressive in the elderly, who have the compounding problems of an aging immune response, increased baseline inflammation, increased mitochondrial dysfunction and decreasing microbial diversity in their gut microbiome. PBM therapy is worthy of further rapid evaluation and could offer a safe, non-invasive, side-effect free and easily deployed adjunctive treatment and prevention, particularly suited for the most at-risk populations. Studies to evaluate the role of PBM in combatting COVID-19 infection and disease prevention may ultimately not only benefit the elderly and chronically sick but could have larger ramifications as a low risk, low-cost intervention in the prevention, treatment and healing of a variety of conditions. This may have implications for the most vulnerable individuals impacted by COVID-19, especially the elderly, infected with the virus, those who are slow to recover from the effects of the infection and those who have been denied their normal exercise/rehabilitation programs due to the necessary isolation restrictions.
Footnotes
Conflict of Interest
AL, BB and WM are founders and employees of SYMBYX Pty Ltd, a medical technology company that develops photobiomodulation devices to treat metabolic and neurodegenerative conditions.
References
- [1].Zhang R, Li Y, Zhang AL, Wang Y, Molina MJ (2020). Identifying airborne transmission as the dominant route for the spread of COVID-19. Proc Natl Acad of Sci, 117:14857-14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Guo J, Huang Z, Lin L, Lv J (2020). Coronavirus Disease 2019 (COVID-19) and Cardiovascular Disease: A Viewpoint on the Potential Influence of Angiotensin-Converting Enzyme Inhibitors/Angiotensin Receptor Blockers on Onset and Severity of Severe Acute Respiratory Syndrome Coronavirus 2 Infection. J Am Heart Assoc, 9:e016219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lauer SA, Grantz KH, Bi Q, Jones FK, Zheng Q, Meredith HR, et al. (2020). The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med, 172:577-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wu Z, McGoogan JM (2020). Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA, 323:1239-1242. [DOI] [PubMed] [Google Scholar]
- [5].Barzilai N, Appleby JC, Austad SN, Cuervo AM, Kaeberlein M, Gonzalez-Billault C, et al. (2020). Geroscience in the Age of COVID-19. Aging Dis, 11:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kochi AN, Tagliari AP, Forleo GB, Fassini GM, Tondo C (2020). Cardiac and arrhythmic complications in patients with COVID-19. J Cardiovasc Electrophysiol, 31:1003-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Picca A, Lezza AMS, Leeuwenburgh C, Pesce V, Calvani R, Landi F, et al. (2017). Fueling inflamm-aging through mitochondrial dysfunction: mechanisms and molecular targets. Int J Mol Sci, 18:933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Clerkin KJ, Fried JA, Raikhelkar J, Sayer G, Griffin JM, Masoumi A, et al. (2020). COVID-19 and cardiovascular disease. Circulation, 141:1648-1655. [DOI] [PubMed] [Google Scholar]
- [9].Filardi T, Morano S (2020). COVID-19: is there a link between the course of infection and pharmacological agents in diabetes? J Endocrinol Invest: 1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Jordan RE, Adab P, Cheng K 2020. Covid-19: risk factors for severe disease and death. Bri Med J, 368:m1198. [DOI] [PubMed] [Google Scholar]
- [11].Liang W, Guan W, Chen R, Wang W, Li J, Xu K, et al. (2020). Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol, 21:335-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Rebelos E, Moriconi D, Virdis A, Taddei S, Foschi D, Nannipieri M (2020). Importance of metabolic health in the era of COVID-19. Metabolism, 108:154247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Finer N, Garnett SP, Bruun JM (2020). COVID-19 and obesity. Clin Obes, 10:e12365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Butler MJ, Barrientos RM (2020). The impact of nutrition on COVID-19 susceptibility and long-term consequences. Brain Behav Immun, 87:53-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Saghazadeh A, Rezaei N (2020). Immune-epidemiological parameters of the novel coronavirus-a perspective. Expert Rev Clin Immunol, 16:465-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Cheng J, Wen J, Wang N, Wang C, Xu Q, Yang Y (2019). Ion channels and vascular diseases. Arterioscler Thromb and Vasc Biol, 39:e146-e156. [DOI] [PubMed] [Google Scholar]
- [17].Karpinski RI, Kinase Kolb AM, Tetreault NA, Borowski TB (2018). High intelligence: A risk factor for psychological and physiological overexcitabilities. Intelligence, 66:8-23. [Google Scholar]
- [18].Kursumovic E, Lennane S, Cook TM (2020). Deaths in healthcare workers due to COVID-19: the need for robust data and analysis. Anaesth, 75:989-992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Yu W-L, Toh HS, Liao C-T, Chang W-T (2020). A Double-Edged Sword—Cardiovascular Concerns of Potential Anti-COVID-19 Drugs. Cardiovasc Drugs Ther, 17:1-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Roy S (2020). Ventricular Arrhythmia Risk Based on Ethnicity in COVID-19 Patients on Hydroxychloroquine and Azithromycin Combination. SN Compr Clin Med, 2:1019-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Stone E, Kiat H, McLachlan CS (2020). Atrial fibrillation in COVID-19: A review of possible mechanisms. FASEB J, 34,9:11347-11354 [DOI] [PubMed] [Google Scholar]
- [22].Asadi-Pooya AA, Simani L (2020). Central nervous system manifestations of COVID-19: A systematic review. J Neurol Sci, June 15:116832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Jahanshahlu L, Rezaei N (2020). Central Nervous System Involvement in COVID-19. Arch Med Res, May 22:S0188-4409(20)30797-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Didangelos A (2020). COVID-19 Hyperinflammation: What about Neutrophils? mSphere, 5:e00367-00320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Schett G, Sticherling M, Neurath MF (2020). COVID-19: risk for cytokine targeting in chronic inflammatory diseases? Nat Rev Immunol, 20:271-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, et al. (2020). COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet, 395:1033-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Carfì A, Bernabei R, Landi F (2020). Persistent symptoms in patients after acute covid-19. JAMA, 324:603-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Varatharaj A, Thomas N, Ellul MA, Davies NW, Pollak TA, Tenorio EL, et al. (2020). Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry, June 25:S2215-0366(20)30287-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Islam MF, Cotler J, Jason LA (2020). Post-viral fatigue and COVID-19: lessons from past epidemics. Fatigue, 8:61-69. [Google Scholar]
- [30].Rogers JP, Chesney E, Oliver D, Pollak TA, McGuire P, Fusar-Poli P, et al. (2020). Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry, 7:611-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Dalakas MC (2020). Guillain-Barré syndrome: The first documented COVID-19-triggered autoimmune neurologic disease. Neurol Neuroimmunol Neuroinflamm, 7:e781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Schippers M, Kompanje E (2020). For the Greater Good? The Devastating Ripple Effects of the Covid-19 Crisis. The Devastating Ripple Effects of the Covid-19 Crisis https://ideas.repec.org/p/ems/eureri/127236.html. [DOI] [PMC free article] [PubMed]
- [33].Khosrawipour V, Lau H, Khosrawipour T, Kocbach P, Ichii H, Bania J, et al. (2020). Failure in initial stage containment of global COVID-19 epicenters. J Med Virol, 92:863-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Mahase E (2020). Covid-19: what treatments are being investigated? Bri Med J, 368:m1252. [DOI] [PubMed] [Google Scholar]
- [35].Dhar D, Mohanty A (2020). Gut microbiota and Covid-19- possible link and implications. Virus Res, 285:198018-198018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI (2011). Human nutrition, the gut microbiome, and immune system: envisioning the future. Nature, 474:327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zheng D, Liwinski T, Elinav E (2020). Interaction between microbiota and immunity in health and disease. Cell Res, 30:492-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Marsland BJ, Trompette A, Gollwitzer ES (2015). The gut-lung axis in respiratory disease. Ann Am Thorac Soc, 12:S150-S156. [DOI] [PubMed] [Google Scholar]
- [39].Zuo T, Zhang F, Lui GCY, Yeoh YK, Li AYL, Zhan H, et al. (2020). Alterations in Gut Microbiota of Patients With COVID-19 During Time of Hospitalization. Gastroenterology, May 20:S0016- 5085(20)34701-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Lazar V, Ditu L-M, Pircalabioru GG, Gheorghe I, Curutiu C, Holban AM, et al. (2018). Aspects of Gut Microbiota and Immune System Interactions in Infectious Diseases, Immunopathology, and Cancer. Front Immunol, 9:1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, et al. (2014). Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med, 20:159-166. [DOI] [PubMed] [Google Scholar]
- [42].Osamu K, Akira A, Sazaly A, Naoki Y (2018). Probiotics and Paraprobiotics in Viral Infection: Clinical Application and Effects on the Innate and Acquired Immune Systems. Curr Pharm Des, 24:710-717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Cole-Jeffrey CT, Liu M, Katovich MJ, Raizada MK, Shenoy V (2015). ACE2 and microbiota: emerging targets for cardiopulmonary disease therapy. J Cardiovasc Pharmacol, 66:540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Idris A, Hasnain SZ, Huat LZ, Koh D (2017). Human diseases, immunity and the oral microbiota—Insights gained from metagenomic studies. Oral Sci Int, 14:27-32. [Google Scholar]
- [45].Kumar PS (2017). From focal sepsis to periodontal medicine: a century of exploring the role of the oral microbiome in systemic disease. J Physiol, 595:465-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Ma W-T, Pang M, Fan Q-L, Chen D-K (2019). The commensal microbiota and viral infection: a comprehensive review. Front Immunol, 10:1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Pitones-Rubio V, Chávez-Cortez EG, Hurtado-Camarena A, González-Rascón A, Serafín-Higuera N (2020). Is periodontal disease a risk factor for severe COVID-19 illness? Med Hypotheses, 144:109969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Patel J, Sampson V (2020). The role of oral bacteria in COVID-19. Lancet Microbe, 1:e105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Sampson V (2020). Oral hygiene risk factor. Br Dent J, 228:569-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Grzybowski A, Pietrzak K (2012). From patient to discoverer—Niels Ryberg Finsen (1860-1904)—the founder of phototherapy in dermatology. Clinics Dermatol, 30:451-455. [DOI] [PubMed] [Google Scholar]
- [51].Mester E, Ludany G, Selyei M, Szende B 1968. The stimulating effect of low power laser rays on biological systems. Laser Rev, 1:3 [Google Scholar]
- [52].Khan I, Tang E, Arany P (2015). Molecular pathway of near-infrared laser phototoxicity involves ATF-4 orchestrated ER stress. Sci Rep, 5:10581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Moro C, Torres N, Arvanitakis K, Cullen K, Chabrol C, Agay D, et al. (2017). No evidence for toxicity after long-term photobiomodulation in normal non-human primates. Exp Brain Res, 235:3081-3092. [DOI] [PubMed] [Google Scholar]
- [54].Cassano P, Caldieraro MA, Norton R, Mischoulon D, Trinh N-H, Nyer M, et al. (2019). Reported Side Effects, Weight and Blood Pressure, After Repeated Sessions of Transcranial Photobiomodulation. Photobiomodul Photomed Laser Surg, 37:651-656. [DOI] [PubMed] [Google Scholar]
- [55].Hamblin MR (2018). Mechanisms and Mitochondrial Redox Signaling in Photobiomodul Photomed Laser Surg, 94:199-212. [Google Scholar]
- [56].Huang YY, Sharma SK, Carroll J, Hamblin MR (2011). Biphasic dose response in low level light therapy - an update. Dose Response, 9:602-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Kandolf-Sekulovic L, Kataranovski M, Pavlovic MD (2003). Immunomodulatory effects of low-intensity near-infrared laser irradiation on contact hypersensitivity reaction. Photodermatol, Photoimmunol Photomed, 19:203-212. [DOI] [PubMed] [Google Scholar]
- [58].Toma RL, Oliveira MX, Renno ACM, Laakso E-L (2018). Photobiomodulation (PBM) therapy at 904 nm mitigates effects of exercise-induced skeletal muscle fatigue in young women. Lasers Med Sci, 33:1197-1205. [DOI] [PubMed] [Google Scholar]
- [59].Ferraresi C, Huang YY, Hamblin MR (2016). Photobiomodulation in human muscle tissue: an advantage in sports performance? J Biophotonics, 9:1273-1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Trajano LAdSN, Stumbo AC, Da Silva CL, Mencalha AL, Fonseca AS (2016). Low-level infrared laser modulates muscle repair and chromosome stabilization genes in myoblasts. Lasers Med Sci, 31:1161-1167. [DOI] [PubMed] [Google Scholar]
- [61].de Brito A, Alves AN, Ribeiro BG, Barbosa DVDE, Magalhaes EMR, Fernandes KPS, et al. (2018). Effect of photobiomodulation on connective tissue remodeling and regeneration of skeletal muscle in elderly rats. Lasers Med Sci, 33:513-521. [DOI] [PubMed] [Google Scholar]
- [62].Rodrigues NC, Brunelli R, de Araújo HSS, Parizotto NA, Renno ACM (2013). Low-level laser therapy (LLLT)(660 nm) alters gene expression during muscle healing in rats. J Photochem Photobiol B, 120:29-35. [DOI] [PubMed] [Google Scholar]
- [63].de Souza GHM, Ferraresi C, Moreno MA, Pessoa BV, Damiani APM, Gasparotto Filho V, et al. (2020). Acute effects of photobiomodulation therapy applied to respiratory muscles of chronic obstructive pulmonary disease patients: a double-blind, randomized, placebo-controlled crossover trial. Lasers Med Sci, 35:1055-1063. [DOI] [PubMed] [Google Scholar]
- [64].Prolla TA, Denu JM (2014). NAD+ deficiency in age-related mitochondrial dysfunction. Cell Met, 19:178-180. [DOI] [PubMed] [Google Scholar]
- [65].Mitrofanis J, Jeffery G (2018). Does photobiomodulation influence ageing? Aging, 10:2224-2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].de Freitas LF, Hamblin MR (2016). Proposed Mechanisms of Photobiomodulation or Low-Level Light Therapy. IEEE J Sel Top Quantum Electron, 22:7000417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Manchini MT, Serra AJ, dos Santos Feliciano R, Santana ET, Antonio EL, de Carvalho PdTC, et al. (2014). Amelioration of cardiac function and activation of anti-inflammatory vasoactive peptides expression in the rat myocardium by low level laser therapy. PLoS One, 9:e101270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Liebert A, Krause A, Goonetilleke N, Bicknell B, Kiat H (2017). A Role for Photobiomodulation in the Prevention of Myocardial Ischemic Reperfusion Injury: A Systematic Review and Potential Molecular Mechanisms. Sci Rep, 7:42386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Wang X, Tian F, Soni SS, Gonzalez-Lima F, Liu H (2016). Interplay between up-regulation of cytochrome-c-oxidase and hemoglobin oxygenation induced by near-infrared laser. Sci Rep, 6:30540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Zomorrodi R, Loheswaran G, Pushparaj A, Lim L (2019). Pulsed near infrared transcranial and intranasal photobiomodulation significantly modulates neural oscillations: a pilot exploratory study. Sc Rep, 9:6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].da Silva MM, Albertini R, de Carvalho PdTC, Leal-Junior ECP, Bussadori SK, Vieira SS, et al. (2018). Randomized, blinded, controlled trial on effectiveness of photobiomodulation therapy and exercise training in the fibromyalgia treatment. Lasers Med Sci, 33:343-351. [DOI] [PubMed] [Google Scholar]
- [72].Hamblin MR (2017). Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS Biophys, 4:337-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Laakso L, Cramond T, Richardson C, Galligan JP (1994). Plasma ACTH and betaendorphin levels in response to low level laser therapy (LLLT) in myofascial trigger points. Laser Ther, 6:133-142. [Google Scholar]
- [74].Scholzen TE, Ko¨nig S, Fastrich M, Bo¨hm M, Luger TA (2007). Terminating the stress: peripheral peptidolysis of proopiomelanocortin-derived regulatory hormones by the dermal microvascular endothelial cell extracellular peptidases neprilysin and angiotensin-converting enzyme. Endocrinology, 148:2793-2805. [DOI] [PubMed] [Google Scholar]
- [75].Cerdeira CD, Lima Brigagão MRP, Carli ML, de Souza Ferreira C, de Oliveira Isac Moraes G, Hadad H, et al. (2016). Low-level laser therapy stimulates the oxidative burst in human neutrophils and increases their fungicidal capacity. J Biophotonics, 9:1180-1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Neubert E, Bach K, Busse J, Bogeski I, Schön MP, Kruss S, et al. (2019). Blue and long-wave ultraviolet light induce in vitro Neutrophil Extracellular Trap (NET) formation. Front Immunol, 10:2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Aimbire F, De Oliveira AL, Albertini R, Correa J, De Campos CL, Lyon J, et al. (2008). Low level laser therapy (LLLT) decreases pulmonary microvascular leakage, neutrophil influx and IL-1β levels in airway and lung from rat subjected to LPS-induced inflammation. Inflammation, 31:189. [DOI] [PubMed] [Google Scholar]
- [78].de Brito Sousa K, Rodrigues M, de Souza Santos D, Mesquita-Ferrari RA, Nunes FD, de Fátima Teixeira da Silva D, et al. (2020). Differential expression of inflammatory and anti-inflammatory mediators by M1 and M2 macrophages after photobiomodulation with red or infrared lasers. Lasers Med Sci, 35:337-343. [DOI] [PubMed] [Google Scholar]
- [79].Tolentino M, Cho CC, Lyons J-A (2019). Photobiomodulation therapy (PBMT) regulates the production of IL-10 and IFN-Ɣ by peripheral blood mononuclear cells (PBMC) and CD4+ T cells isolated from subjects with Multiple Sclerosis (MS). J Immunol, 202(1 Supp):193.16. [Google Scholar]
- [80].Tolentino M, Cho CC, Lyons J-A (2020). Photobiomodulation (PBM) regulates nitric oxide (NO) production by peripheral blood mononuclear cells (PBMC) isolated from Multiple Sclerosis (MS) patients. J Immunol, 204:160.8. [Google Scholar]
- [81].Brochetti RA, Leal MP, Rodrigues R, Da Palma RK, de Oliveira LVF, Horliana ACRT, et al. (2017). Photobiomodulation therapy improves both inflammatory and fibrotic parameters in experimental model of lung fibrosis in mice. Lasers Med Sci, 32:1825-1834. [DOI] [PubMed] [Google Scholar]
- [82].de Lima FM, Villaverde A, Albertini R, Corrêa J, Carvalho R, Munin E, et al. (2011). Dual Effect of low-level laser therapy (LLLT) on the acute lung inflammation induced by intestinal ischemia and reperfusion: Action on anti-and pro-inflammatory cytokines. Lasers Surg Med, 43:410-420. [DOI] [PubMed] [Google Scholar]
- [83].Yu W, Chi LH, Naim JO, Lanzafame RJ (1997). Improvement of host response to sepsis by photobiomodulation. Lasers Med Sci, 21:262-268. [DOI] [PubMed] [Google Scholar]
- [84].Assis L, Tim C, Magri A, Fernandes KR, Vassão PG, Renno ACM (2018). Interleukin-10 and collagen type II immunoexpression are modulated by photobiomodulation associated to aerobic and aquatic exercises in an experimental model of osteoarthritis. Lasers Med Sci, 33:1875-1882. [DOI] [PubMed] [Google Scholar]
- [85].Stadler I, Evans R, Kolb B, Naim JO, Narayan V, Buehner N, et al. (2000). In vitro effects of low-level laser irradiation at 660 nm on peripheral blood lymphocytes. Lasers Surg Med, 27:255-261. [DOI] [PubMed] [Google Scholar]
- [86].Cerra FB (1990). Multiple organ failure syndrome. Perspect Vasc Surg Endovasc Ther, 3:139-160. [Google Scholar]
- [87].Meldrum DR, Ayala A, Chaudry IH (1994). Energetics of lymphocyte" burnout" in late sepsis: adjuvant treatment with ATP-MgCl2 improves energetics and decreases lethality. J Surg Res, 56:537-542. [DOI] [PubMed] [Google Scholar]
- [88].Karu T (1989). Photobiology of low-power laser effects. Health Phys, 56:691-704. [DOI] [PubMed] [Google Scholar]
- [89].Yu W, McGowan M, Naim J, Lanzafame R 1996. Mechanism of low level laser biostimulatory effects. In 16th Annual Meeting American Society for Laser Medicine and Surgery; Lake Buena Vista, Florida (April 15-17, 1996). [Google Scholar]
- [90].Mafra de Lima F (2013). Low-level laser therapy restores the oxidative stress balance in acute lung injury induced by gut ischemia and reperfusion. Photochem and Photobiol, 89:179-188. [DOI] [PubMed] [Google Scholar]
- [91].De Brito Léia AA, Herculano KZ, Santos TG, Rigonato-Oliveira NC, Alves CE, Palma RK, et al. (2019). Effect of photobiomodulation on inflammation and production of TGF-ß in experimental model of pulmonary fibrosis. Eur Respir J, 54:PA5199. [Google Scholar]
- [92].da Cunha Moraes G, Vitoretti LB, de Brito AA, Alves CE, de Oliveira NCR, dos Santos Dias A, et al. (2018). Low-Level Laser Therapy Reduces Lung Inflammation in an Experimental Model of Chronic Obstructive Pulmonary Disease Involving P2X7 Receptor. Oxid Med Cell Longev, 2018:6798238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Kent AL, Broom M, Parr V, Essex RW, Abdel-Latif ME, Dahlstrom JE, et al. (2015). A safety and feasibility study of the use of 670[thinsp]nm red light in premature neonates. J Perinat, 35:493-496. [DOI] [PubMed] [Google Scholar]
- [94].Gunn C (2005). Acute respiratory distress syndrome successfully treated with low level laser therapy. J Complement Integr Med, 2:1 [Google Scholar]
- [95].Bicknell B, Liebert A, Johnstone D, Kiat H (2018). Photobiomodulation of the microbiome: implications for metabolic and inflammatory diseases. Lasers Med Sci, 34:317-327. [DOI] [PubMed] [Google Scholar]
- [96].Rooks MG, Garrett WS (2016). Gut microbiota, metabolites and host immunity. Nat Rev Immunol, 16:341-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Hamblin MR (2016). Shining light on the head: photobiomodulation for brain disorders. BBA Clin, 6:113-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].El Khoury H, Mitrofanis J, Henderson LA (2019). Exploring the Effects of Near Infrared Light on Resting and Evoked Brain Activity in Humans Using Magnetic Resonance Imaging. Neuroscience, 422:161-171. [DOI] [PubMed] [Google Scholar]
- [99].Naeser MA, Zafonte R, Krengel MH, Martin PI, Frazier J, Hamblin MR, et al. (2014). Significant improvements in cognitive performance post-transcranial, red/near-infrared light-emitting diode treatments in chronic, mild traumatic brain injury: open-protocol study. J Neurotrauma, 31:1008-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Schiffer F, Johnston AL, Ravichandran CT, Polcari A, Teicher MH, Webb RH, et al. (2009). Psychological benefits 2 and 4 weeks after a single treatment with near infrared light to the forehead: a pilot study of 10 patients with major depression and anxiety. Behav Brain Funct, 5:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Buchaim RL, Barbalho SM, Hamzé AL, de Alvares Goulartoulart R, Rocha KTP, Reis CHB, et al. (2020). Loss of smell and COVID-19: Anatomical and physiological considerations. Int J Adv Eng Res Sci, 7:278-280. [Google Scholar]
- [102].Fernandes AB, Lima CJd, Villaverde AGB, Pereira PC, Carvalho HC, Zângaro RA (2020). Photobiomodulation: Shining Light on COVID-19. Photobiomodul Photomed and Laser Surg, 38:395-397. [DOI] [PubMed] [Google Scholar]
- [103].Domínguez A, Velásquez SA, David MA (2020). Can Transdermal Photobiomodulation Help Us at the Time of COVID-19? Photobiomodul Photomed Laser Surg, 38:258-259. [DOI] [PubMed] [Google Scholar]
- [104].Fekrazad R 2020. Photobiomodulation and antiviral photodynamic therapy as a possible novel approach in COVID-19 management. Photobiomodul, Photomed, and Laser Surg, 38:255-257. [DOI] [PubMed] [Google Scholar]
- [105].Sigman SA, Soheila M, Monica M, Vetrici MA (2020). A 57-Year-Old African American Man with Severe COVID-19 Pneumonia Who Responded to Supportive Photobiomodulation Therapy (PBMT): First Use of PBMT in COVID-19. Am J Case Rep, 21:e926779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Derhovanessian E, Pawelec G (2012). Vaccination in the elderly. Microb Biotechnol, 5:226-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Kashiwagi S, Yuan J, Forbes B, Hibert ML, Lee EL, Whicher L, et al. (2013). Near-infrared laser adjuvant for influenza vaccine. PloS One, 8:e82899. [DOI] [PMC free article] [PubMed] [Google Scholar]